Monitoring Lead–Phosphorus Interactions Through 31P-NMR Used as a Sensor in Phosphine Functionalized Silica Gel Adsorbent
Abstract
1. Introduction
2. Results and Discussion
2.1. Lead Adsorption Test
2.2. Surface Textural Properties
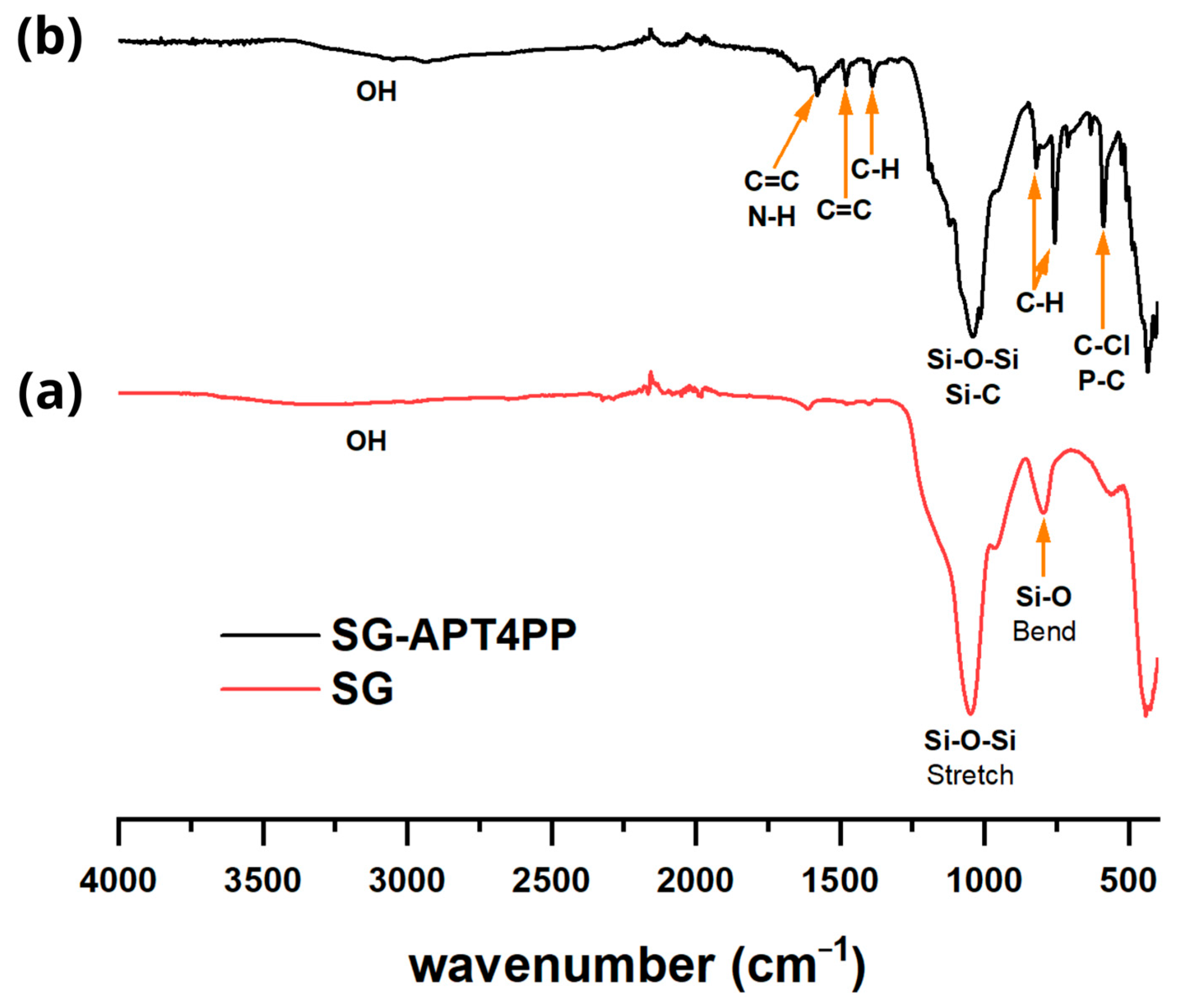
2.3. FTIR Analysis
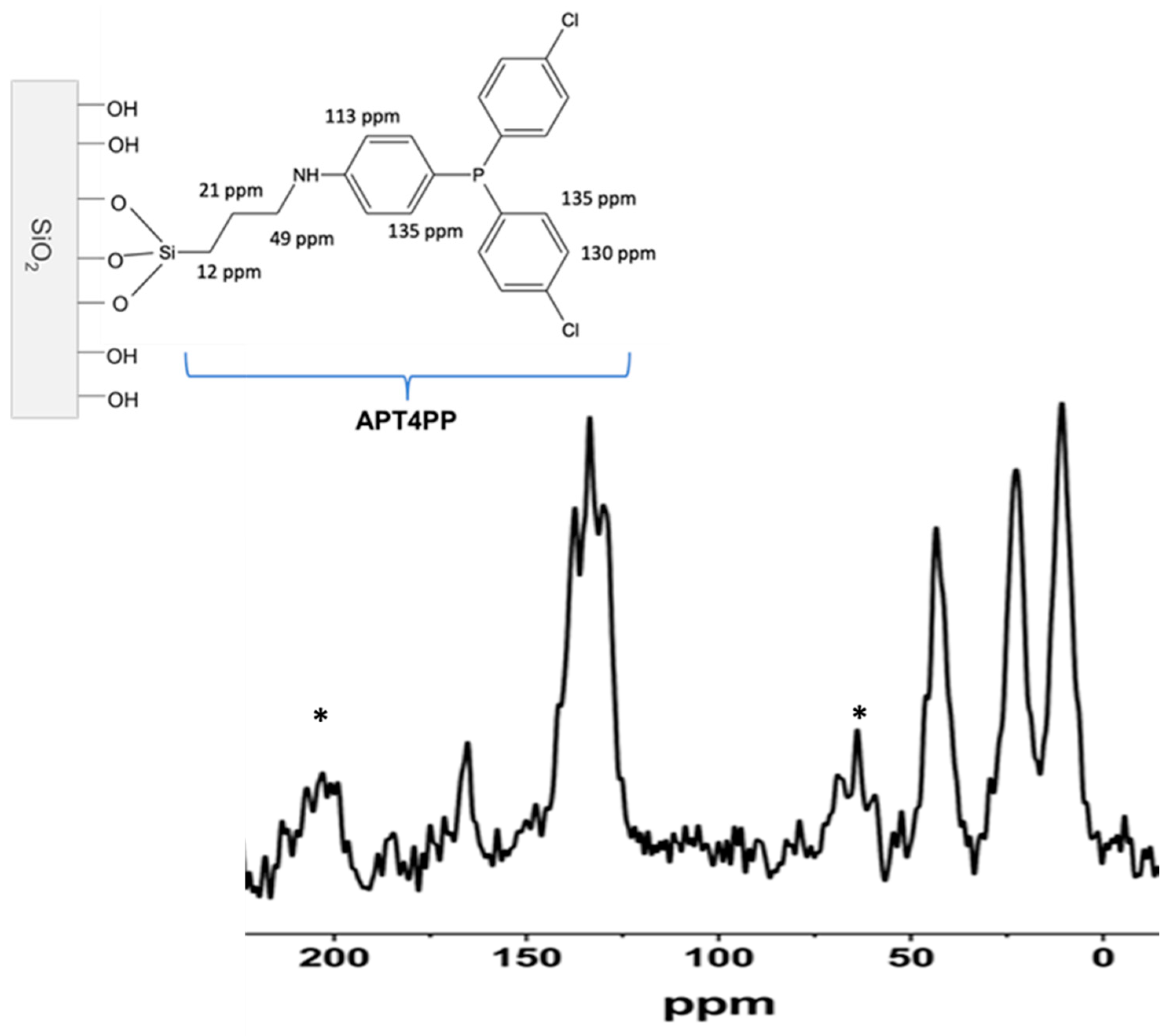
2.4. NMR Analysis
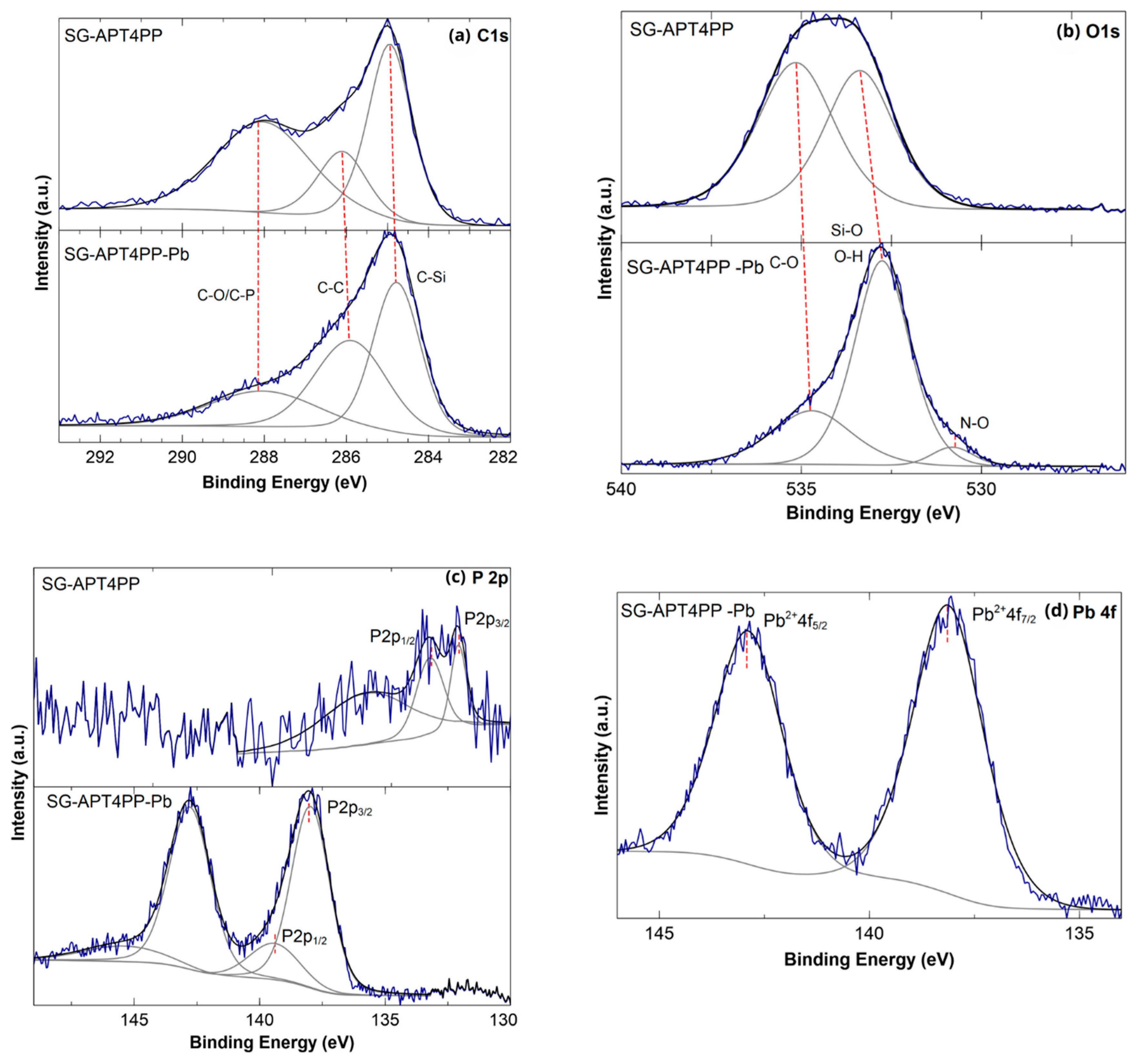
2.5. XPS Analysis Results
2.6. Evaluation of Lead Interaction by 31P NMR
3. Conclusions
4. Materials and Methods
4.1. Materials
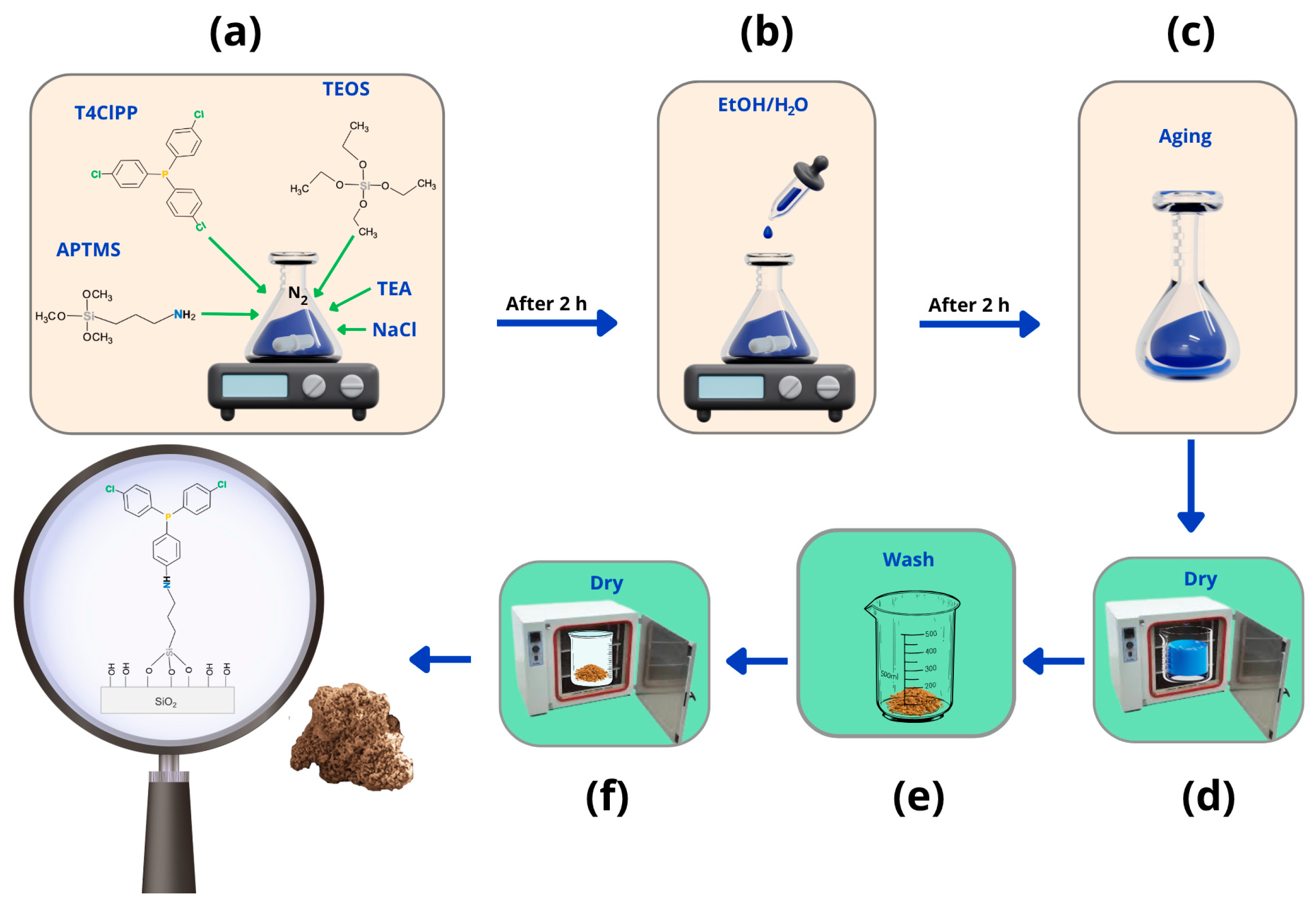
4.2. Synthesis of SG-APT4PP
4.3. SG-APT4PP Characterization
4.4. Lead Removal Test
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mushak, P. A Brief Early History of Lead as an Evolving Global Pollutant and Toxicant. Trace Met. Other Contam. Environ. 2011, 10, 23–39. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of Heavy Metal Ions from Wastewaters: A Review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Costa-Böddeker, S.; Hoelzmann, P.; Thuyên, L.X.; Huy, H.D.; Nguyen, H.A.; Richter, O.; Schwalb, A. Ecological Risk Assessment of a Coastal Zone in Southern Vietnam: Spatial Distribution and Content of Heavy Metals in Water and Surface Sediments of the Thi Vai Estuary and Can Gio Mangrove Forest. Mar. Pollut. Bull. 2017, 114, 1141–1151. [Google Scholar] [CrossRef]
- Collin, M.S.; Venkatraman, S.K.; Vijayakumar, N.; Kanimozhi, V.; Arbaaz, S.M.; Stacey, R.G.S.; Anusha, J.; Choudhary, R.; Lvov, V.; Tovar, G.I.; et al. Bioaccumulation of Lead (Pb) and Its Effects on Human: A Review. J. Hazard. Mater. Adv. 2022, 7, 100094. [Google Scholar] [CrossRef]
- Strandberg, J. Lead in Drinking-Water, Health Risks, Monitoring and Corrective Actions—Technical Brief; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of Heavy Metal Ions from Wastewater: A Comprehensive and Critical Review. NPJ Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Gahrouei, A.E.; Rezapour, A.; Pirooz, M.; Pourebrahimi, S. From Classic to Cutting-Edge Solutions: A Comprehensive Review of Materials and Methods for Heavy Metal Removal from Water Environments. Desalin. Water Treat. 2024, 319, 100446. [Google Scholar] [CrossRef]
- Lupa, L.; Cocheci, L. Heavy Metals Removal from Water and Wastewater. In Heavy Metals—Recent Advances; Almayyahi, B.A., Ed.; IntechOpen: Rijeka, Croatia, 2023; ISBN 978-1-83768-515-8. [Google Scholar]
- Chowdhury, I.R.; Chowdhury, S.; Mazumder, M.A.J.; Al-Ahmed, A. Removal of Lead Ions (Pb2+) from Water and Wastewater: A Review on the Low-Cost Adsorbents. Appl. Water Sci. 2022, 12, 185. [Google Scholar] [CrossRef]
- Wieszczycka, K.; Staszak, K.; Woźniak-Budych, M.J.; Litowczenko, J.; Maciejewska, B.M.; Jurga, S. Surface Functionalization—The Way for Advanced Applications of Smart Materials. Coord. Chem. Rev. 2021, 436, 213846. [Google Scholar] [CrossRef]
- Kumar, V.; Dwivedi, S.K.; Oh, S. A Critical Review on Lead Removal from Industrial Wastewater: Recent Advances and Future Outlook. J. Water Process Eng. 2022, 45, 102518. [Google Scholar] [CrossRef]
- Rigoletto, M.; Calza, P.; Gaggero, E.; Laurenti, E. Hybrid Materials for the Removal of Emerging Pollutants in Water: Classification, Synthesis, and Properties. Chem. Eng. J. Adv. 2022, 10, 100252. [Google Scholar] [CrossRef]
- Quirarte-Escalante, C.A.; Soto, V.; De La Cruz, W.; Porras, G.R.; Manríquez, R.; Gomez-Salazar, S. Synthesis of Hybrid Adsorbents Combining Sol-Gel Processing and Molecular Imprinting Applied to Lead Removal from Aqueous Streams. Chem. Mater. 2009, 21, 1439–1450. [Google Scholar] [CrossRef]
- Bakry, A.M.; Amri, N.; Adly, M.S.; Alamri, A.A.; Salama, R.S.; Jabbari, A.M.; El-Shall, M.S.; Awad, F.S. Remediation of Water Containing Lead(II) Using (3-Iminodiacetic Acid) Propyltriethoxysilane Graphene Oxide. Sci. Rep. 2024, 14, 18848. [Google Scholar] [CrossRef]
- Liu, B.; Azmatullah, K.; Ki-Hyun, K.; Deepak, K.; Zhang, M. The Adsorptive Removal of Lead Ions in Aquatic Media: Performance Comparison between Advanced Functional Materials and Conventional Materials. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2441–2483. [Google Scholar] [CrossRef]
- Samuel, M.S.; Shang, M.; Klimchuk, S.; Niu, J. Novel Regenerative Hybrid Composite Adsorbent with Improved Removal Capacity for Lead Ions in Water. Ind. Eng. Chem. Res. 2021, 60, 5124–5132. [Google Scholar] [CrossRef]
- Blair, S.M.; Brodbelt, J.S.; Marchand, A.P.; Kumar, K.A.; Chong, H.-S. Evaluation of Binding Selectivities of Caged Crown Ligands toward Heavy Metals by Electrospray Ionization/Quadrupole Ion Trap Mass Spectrometry. Anal. Chem. 2000, 72, 2433–2445. [Google Scholar] [CrossRef]
- Deblonde, G.J.-P.; Lohrey, T.D.; An, D.D.; Abergel, R.J. Toxic Heavy Metal—Pb, Cd, Sn—Complexation by the Octadentate Hydroxypyridinonate Ligand Archetype 3,4,3-LI(1,2-HOPO). N. J. Chem. 2018, 42, 7649–7658. [Google Scholar] [CrossRef]
- Deng, H.; Tian, C.; Li, L.; Liang, Y.; Yan, S.; Hu, M.; Xu, W.; Lin, Z.; Chai, L. Microinteraction Analysis between Heavy Metals and Coexisting Phases in Heavy Metal Containing Solid Wastes. ACS EST Eng. 2022, 2, 547–563. [Google Scholar] [CrossRef]
- Aztatzi-Pluma, D.; Castrejón-González, E.O.; Almendarez-Camarillo, A.; Alvarado, J.F.J.; Durán-Morales, Y. Study of the Molecular Interactions between Functionalized Carbon Nanotubes and Chitosan. J. Phys. Chem. C 2016, 120, 2371–2378. [Google Scholar] [CrossRef]
- Shenderovich, I.G. Effect of Noncovalent Interactions on the 31P Chemical Shift Tensor of Phosphine Oxides, Phosphinic, Phosphonic, and Phosphoric Acids, and Their Complexes with Lead(II). J. Phys. Chem. C 2013, 117, 26689–26702. [Google Scholar] [CrossRef]
- Gutiérrez-Ortega, J.A.; Gómez-Salazar, S.; Shenderovich, I.G.; Manríquez-González, R. Efficiency and Lead Uptake Mechanism of a Phosphonate Functionalized Mesoporous Silica through P/Pb Association Ratio. Mater. Chem. Phys. 2020, 239, 122037. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Landers, J.; Gor, G.Y.; Neimark, A.V. Density Functional Theory Methods for Characterization of Porous Materials. Colloids Surf. A Physicochem. Eng. Asp. 2013, 437, 3–32. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Springer: Berlin/Heidelberg, Germany, 2006; Volume 16. [Google Scholar]
- Niu, F.; Tao, L.-M.; Deng, Y.-C.; Wang, Q.-H.; Song, W.-G. Phosphorus Doped Graphene Nanosheets for Room Temperature NH3 Sensing. N. J. Chem. 2014, 38, 2269–2272. [Google Scholar] [CrossRef]
- Peng, K.-H.; Yang, S.-H.; Wu, Z.-Y.; Hsu, H.-C. Synthesis of Red Cesium Lead Bromoiodide Nanocrystals Chelating Phenylated Phosphine Ligands with Enhanced Stability. ACS Omega 2021, 6, 10437–10446. [Google Scholar] [CrossRef]
- Aziz, I.; Sirajuddin, M.; Nadeem, S.; Tirmizi, S.; Sajjad, W. Synthesis, Spectral Characterization and Evaluation of Biological Properties of Pd(II) Tris-4-Cholorophenyl Phosphine Complexes of Thioureas and Heterocyclic Thiones. J. Chem. Soc. Pak. 2018, 40, 171–181. [Google Scholar]
- Schimming, V.; Hoelger, C.-G.; Buntkowsky, G.; Sack, I.; Fuhrhop, J.-H.; Rocchetti, S.; Limbach, H.-H. Evidence by 15N CPMAS and 15N−13C REDOR NMR for Fixation of Atmospheric CO2 by Amino Groups of Biopolymers in the Solid State. J. Am. Chem. Soc. 1999, 121, 4892–4893. [Google Scholar] [CrossRef]
- Mauder, D.; Akcakayiran, D.; Lesnichin, S.B.; Findenegg, G.H.; Shenderovich, I.G. Acidity of Sulfonic and Phosphonic Acid-Functionalized SBA-15 under Almost Water-Free Conditions. J. Phys. Chem. C 2009, 113, 19185–19192. [Google Scholar] [CrossRef]
- Ek, S.; Iiskola, E.I.; Niinistö, L.; Vaittinen, J.; Pakkanen, T.T.; Root, A. A 29Si and 13C CP/MAS NMR Study on the Surface Species of Gas-Phase-Deposited γ-Aminopropylalkoxysilanes on Heat-Treated Silica. J. Phys. Chem. B 2004, 108, 11454–11463. [Google Scholar] [CrossRef]
- Casserly, T.B.; Gleason, K.K. Density Functional Theory Calculation of 29Si NMR Chemical Shifts of Organosiloxanes. J. Phys. Chem. B 2005, 109, 13605–13610. [Google Scholar] [CrossRef]
- Brückner, R.; Chun, H.-U.; Goretzki, H.; Sammet, M. XPS Measurements and Structural Aspects of Silicate and Phosphate Glasses. J. Non-Cryst. Solids 1980, 42, 49–60. [Google Scholar] [CrossRef]
- Chen, D.A.; Ratliff, J.S.; Hu, X.; Gordon, W.O.; Senanayake, S.D.; Mullins, D.R. Surface Science Dimethyl Methylphosphonate Decomposition on Fully Oxidized and Partially Reduced Ceria Thin Films. Surf. Sci. 2010, 604, 574–587. [Google Scholar] [CrossRef]
- Tosatti, S.; Michel, R.; Textor, M.; Spencer, N.D. Self-Assembled Monolayers of Dodecyl and Hydroxy-Dodecyl Phosphates on Both Smooth and Rough Titanium and Titanium Oxide Surfaces. Langmuir 2002, 18, 3537–3548. [Google Scholar] [CrossRef]
- Melhi, S.; Alosaimi, E.H.; El-Gammal, B.; Alshahrani, W.A.; El-Aryan, Y.F.; Al-Shamiri, H.A.; Elhouichet, H. Novel Porous Organic Polymer for High-Performance Pb(II) Adsorption from Water: Synthesis, Characterization, Kinetic, and Isotherm Studies. Crystals 2023, 13, 956. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Jin, C.; Wu, G.; Liu, G.; Kong, Z. Efficient and Selective Removal of Pb(Ii) from Aqueous Solution by a Thioether-Functionalized Lignin-Based Magnetic Adsorbent. RSC Adv. 2022, 12, 1130–1140. [Google Scholar] [CrossRef]
- Baltrusaitis, J.; Chen, H.; Rubasinghege, G.; Grassian, V.H. Heterogeneous Atmospheric Chemistry of Lead Oxide Particles with Nitrogen Dioxide Increases Lead Solubility: Environmental and Health Implications. Environ. Sci. Technol. 2012, 46, 12806–12813. [Google Scholar] [CrossRef]
- Aliev, V.S.; Badmaeva, I.A.; Pokrovsky, L.D. Lead Phthalocyanine Films Deposited by ECR Plasma-Induced Sublimation. J. Phys. D Appl. Phys. 2012, 45, 305202. [Google Scholar] [CrossRef]
- Wakiya, N.; Kuroyanagi, K.; Xuan, Y.; Shinozaki, K.; Mizutani, N. An XPS Study of the Nucleation and Growth Behavior of an Epitaxial Pb(Zr,Ti)O3/MgO(100) Thin Film Prepared by MOCVD. Thin Solid Film. 2000, 372, 156–162. [Google Scholar] [CrossRef]
- Ai, S.; Huang, Y.; Xie, T.; Zhang, X.; Huang, C. Fabrication of Composites with Ultra-Low Chitosan Loadings and the Adsorption Mechanism for Lead Ions. Environ. Sci. Pollut. Res. 2020, 27, 37927–37937. [Google Scholar] [CrossRef]
- Aklil, A.; Mouflih, M.; Sebti, S. Removal of Heavy Metal Ions from Water by Using Calcined Phosphate as a New Adsorbent. J. Hazard. Mater. 2004, 112, 183–190. [Google Scholar] [CrossRef]
- Shenderovich, I.G. Experimentally Established Benchmark Calculations of 31P NMR Quantities. Chem.–Methods 2021, 1, 61–70. [Google Scholar] [CrossRef]
- Shenderovich, I.G. For Whom a Puddle Is the Sea? Adsorption of Organic Guests on Hydrated MCM-41 Silica. Langmuir 2020, 36, 11383–11392. [Google Scholar] [CrossRef]
- Shenderovich, I.G. 1,3,5-Triaza-7-Phosphaadamantane (PTA) as a 31P NMR Probe for Organometallic Transition Metal Complexes in Solution. Molecules 2021, 26, 1390. [Google Scholar] [CrossRef]
- Ransing, A.A.; Dhavale, R.P.; Parale, V.G.; Bangi, U.K.H.; Choi, H.; Lee, W.; Kim, J.; Wang, Q.; Phadtare, V.D.; Kim, T.; et al. One-Pot Sol–Gel Synthesis of Highly Insulative Hybrid P(AAm-CO-AAc)-Silica Aerogels with Improved Mechanical and Thermal Properties. Gels 2023, 9, 651. [Google Scholar] [CrossRef]
- Moran-Salazar, R.G.; Carbajal-Arizaga, G.G.; Gutierréz-Ortega, J.A.; Badillo-Camacho, J.; Manríquez-González, R.; Shenderovich, I.G.; Gómez-Salazar, S. As(V) Removal from Aqueous Media Using an Environmentally Friendly Zwitterion L-Cysteine Functionalized Silica Adsorbent. Chem. Eng. Sci. 2023, 278, 118879. [Google Scholar] [CrossRef]




| Sample | BET Surface Area | Vp | Dp |
|---|---|---|---|
| SBET (m2g−1) | cm3g−1 | nm | |
| SG | 406.09 | 0.70 | 5.22 |
| SG-APT4PP | 21.12 | 0.41 | 19.74 |
| SG-APT4PP-Pb | 12.78 | 0.38 | 21.66 |
| Sample | Chemical Shift (ppm) | Q/T | ||||
|---|---|---|---|---|---|---|
| T2 | T3 | Q2 | Q3 | Q4 | ||
| SG-APT4PP | −63.0 (2.7, 13%) a | −67.6 (1.9, 9%) a | −90.8 (0.8, 4%) a | −99.5 (6.4, 30%) a | −109.6 (9.4, 44%) a | 3.6 |
| SG-APT4PP-Pb | −61.9 (1.2, 6%) a | −67.1 (2.8, 14%) a | −93.2 (1.3, 6%) a | −99.1 (4.5, 23%) a | −109.1 (10.1, 51%) a | 3.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badillo-Camacho, J.; Gutiérrez-Ortega, J.A.; Shenderovich, I.G.; Velázquez-Galván, Y.G.; Manríquez-González, R. Monitoring Lead–Phosphorus Interactions Through 31P-NMR Used as a Sensor in Phosphine Functionalized Silica Gel Adsorbent. Gels 2025, 11, 580. https://doi.org/10.3390/gels11080580
Badillo-Camacho J, Gutiérrez-Ortega JA, Shenderovich IG, Velázquez-Galván YG, Manríquez-González R. Monitoring Lead–Phosphorus Interactions Through 31P-NMR Used as a Sensor in Phosphine Functionalized Silica Gel Adsorbent. Gels. 2025; 11(8):580. https://doi.org/10.3390/gels11080580
Chicago/Turabian StyleBadillo-Camacho, Jessica, José A. Gutiérrez-Ortega, Ilya G. Shenderovich, Yenni G. Velázquez-Galván, and Ricardo Manríquez-González. 2025. "Monitoring Lead–Phosphorus Interactions Through 31P-NMR Used as a Sensor in Phosphine Functionalized Silica Gel Adsorbent" Gels 11, no. 8: 580. https://doi.org/10.3390/gels11080580
APA StyleBadillo-Camacho, J., Gutiérrez-Ortega, J. A., Shenderovich, I. G., Velázquez-Galván, Y. G., & Manríquez-González, R. (2025). Monitoring Lead–Phosphorus Interactions Through 31P-NMR Used as a Sensor in Phosphine Functionalized Silica Gel Adsorbent. Gels, 11(8), 580. https://doi.org/10.3390/gels11080580









