Preparation and Failure Behavior of Gel Electrolytes for Multilayer Structure Lithium Metal Solid-State Batteries
Abstract
1. Introduction
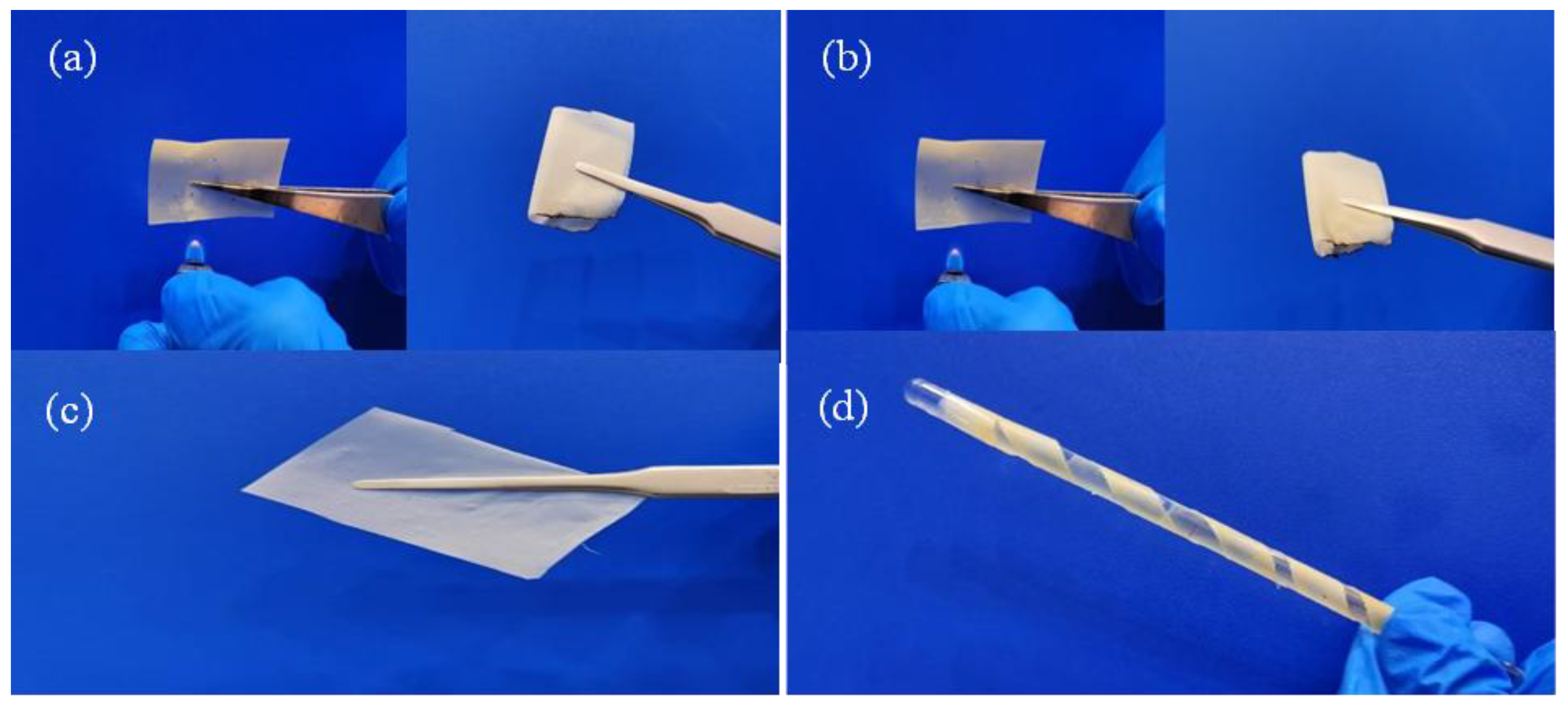
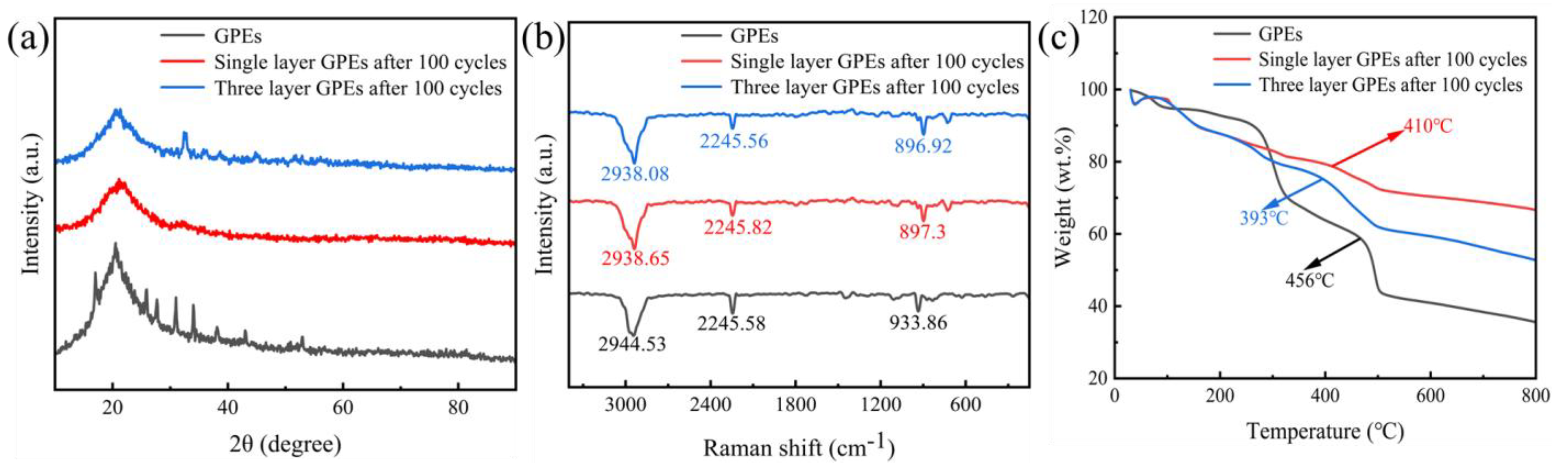
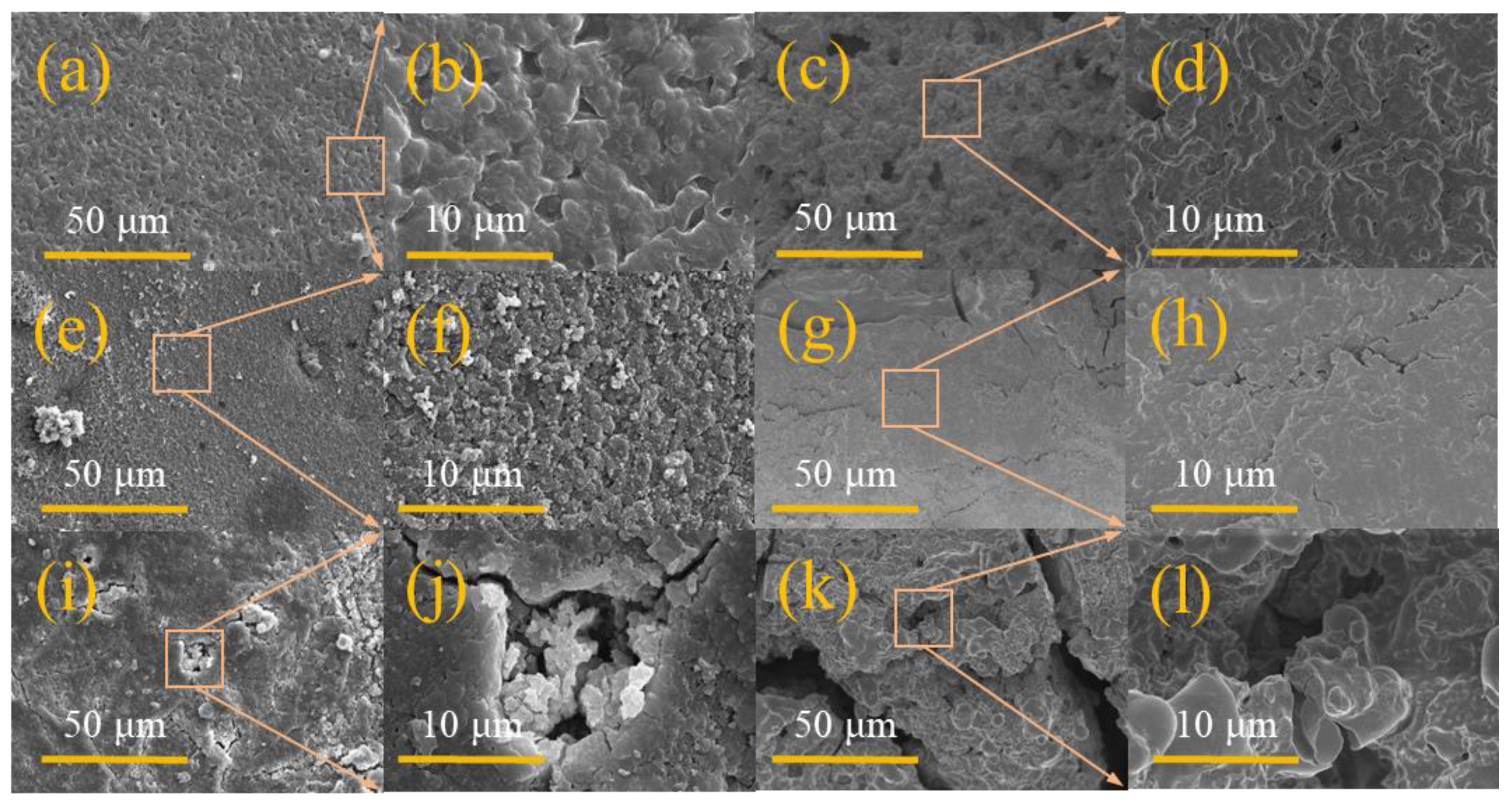
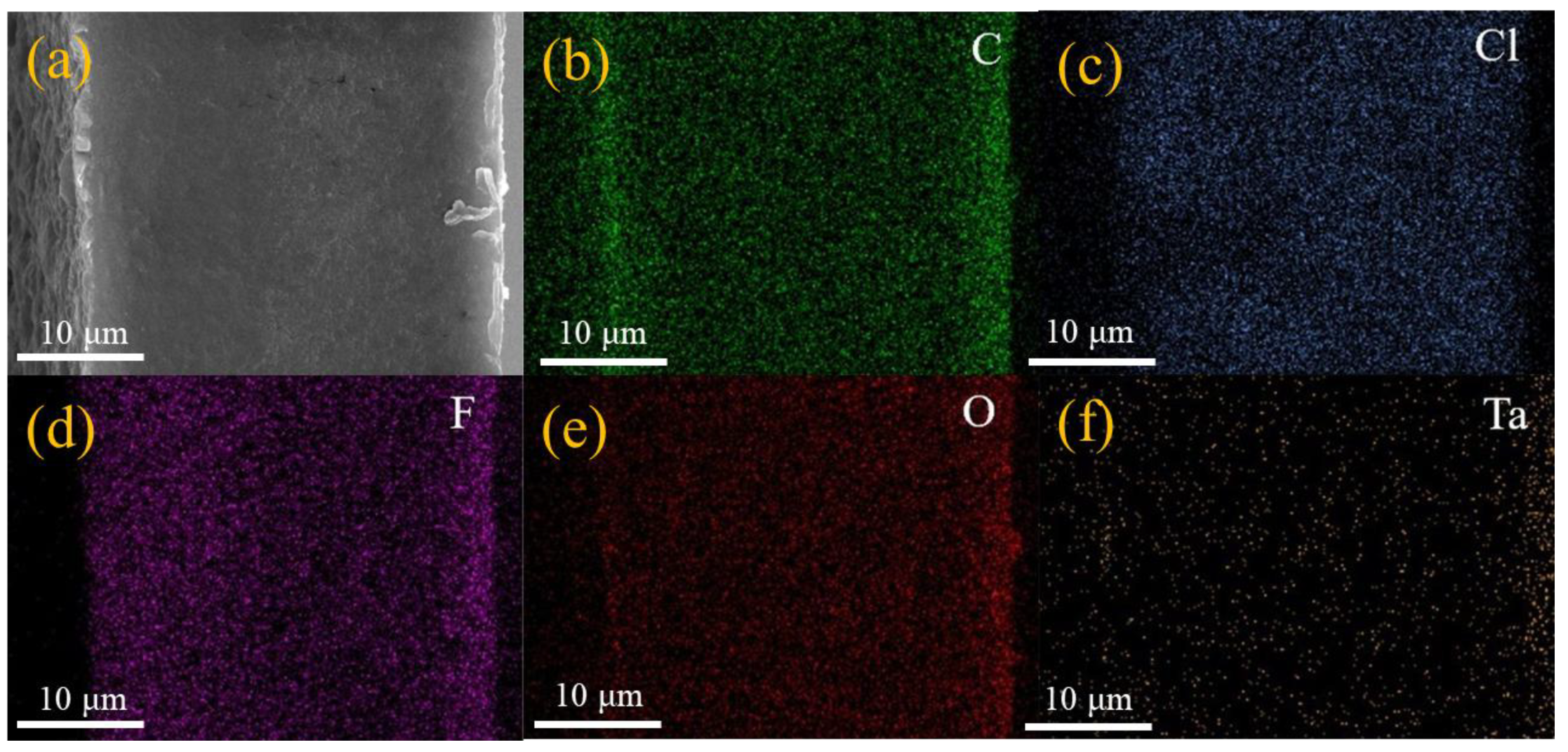
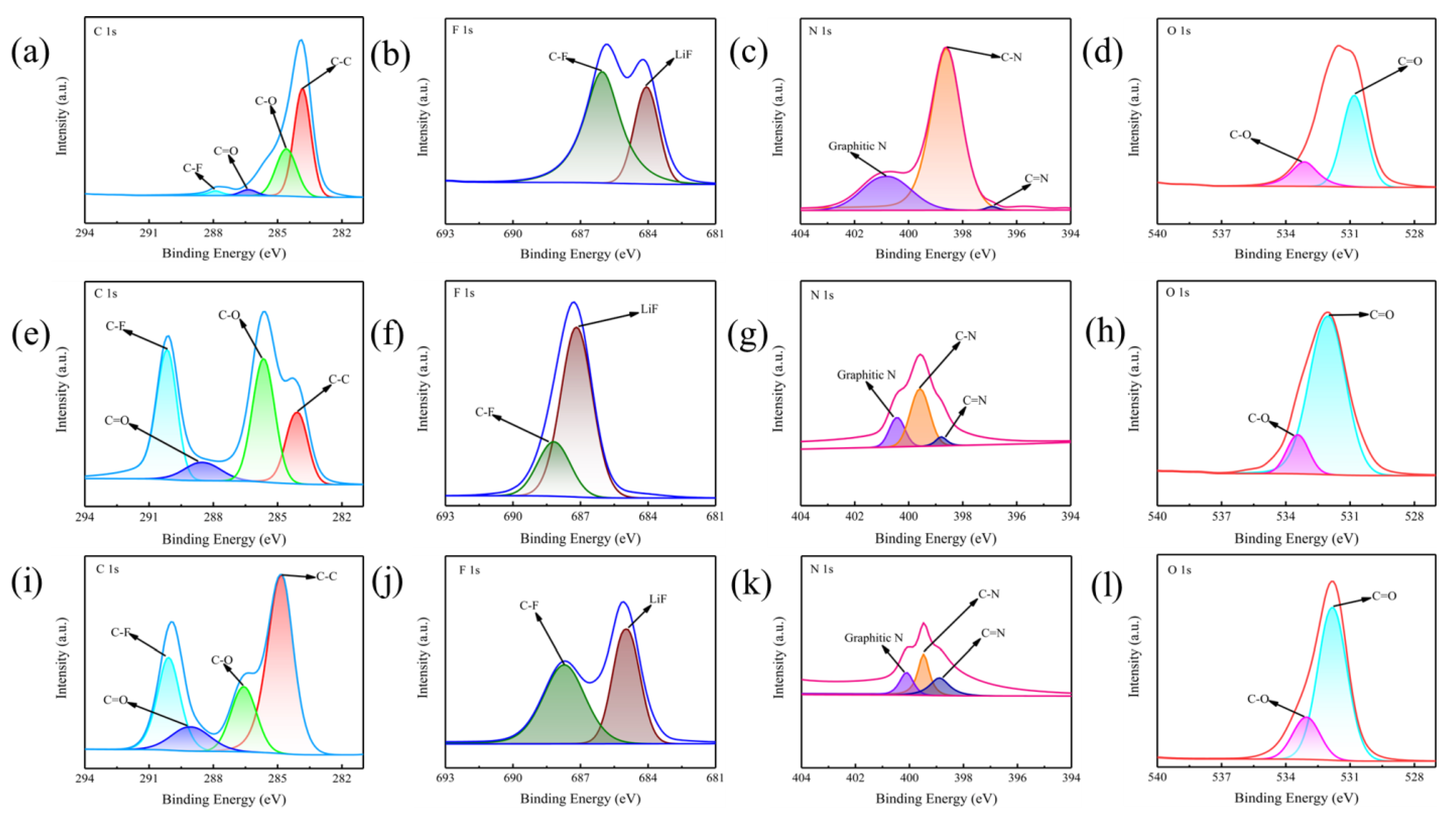
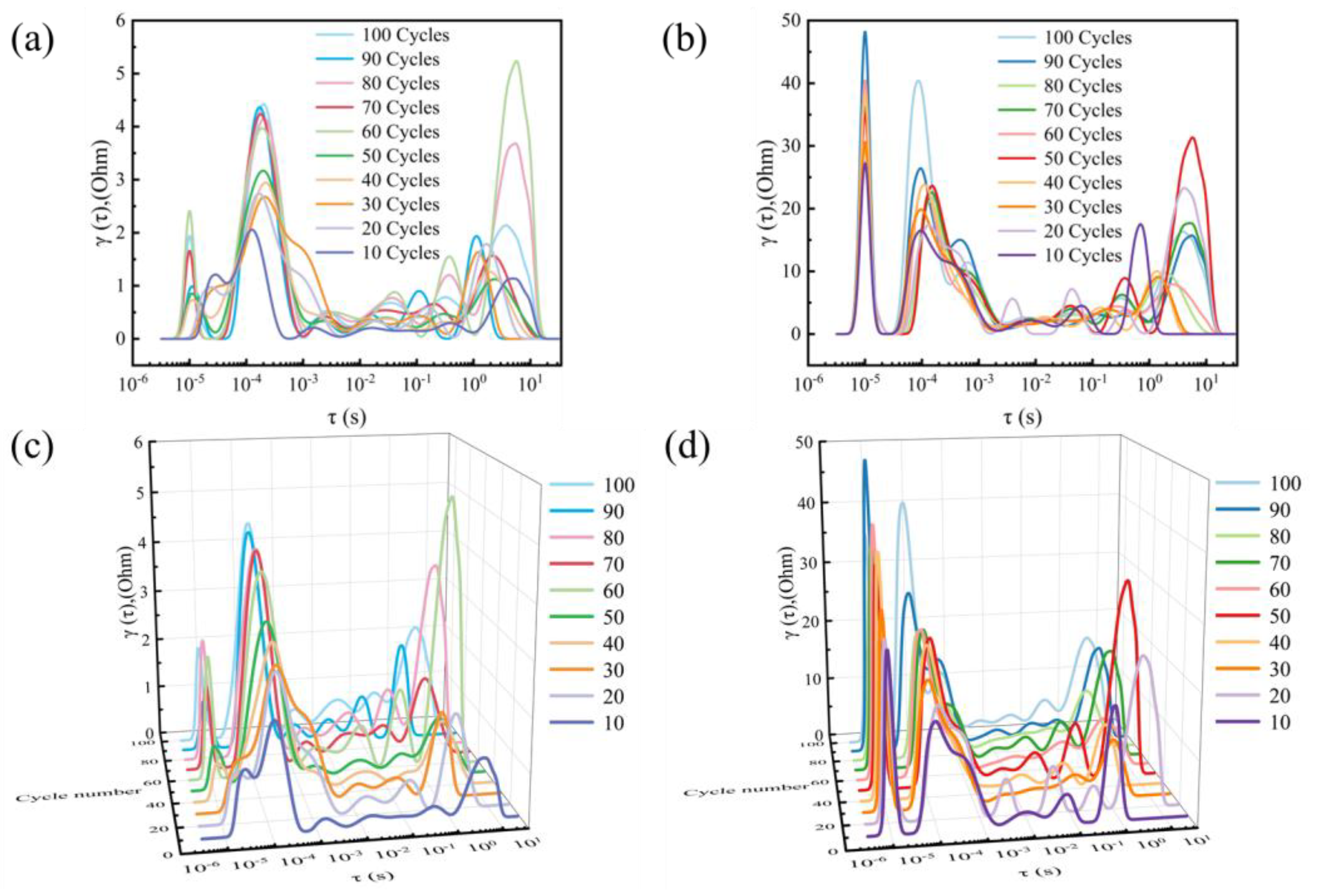
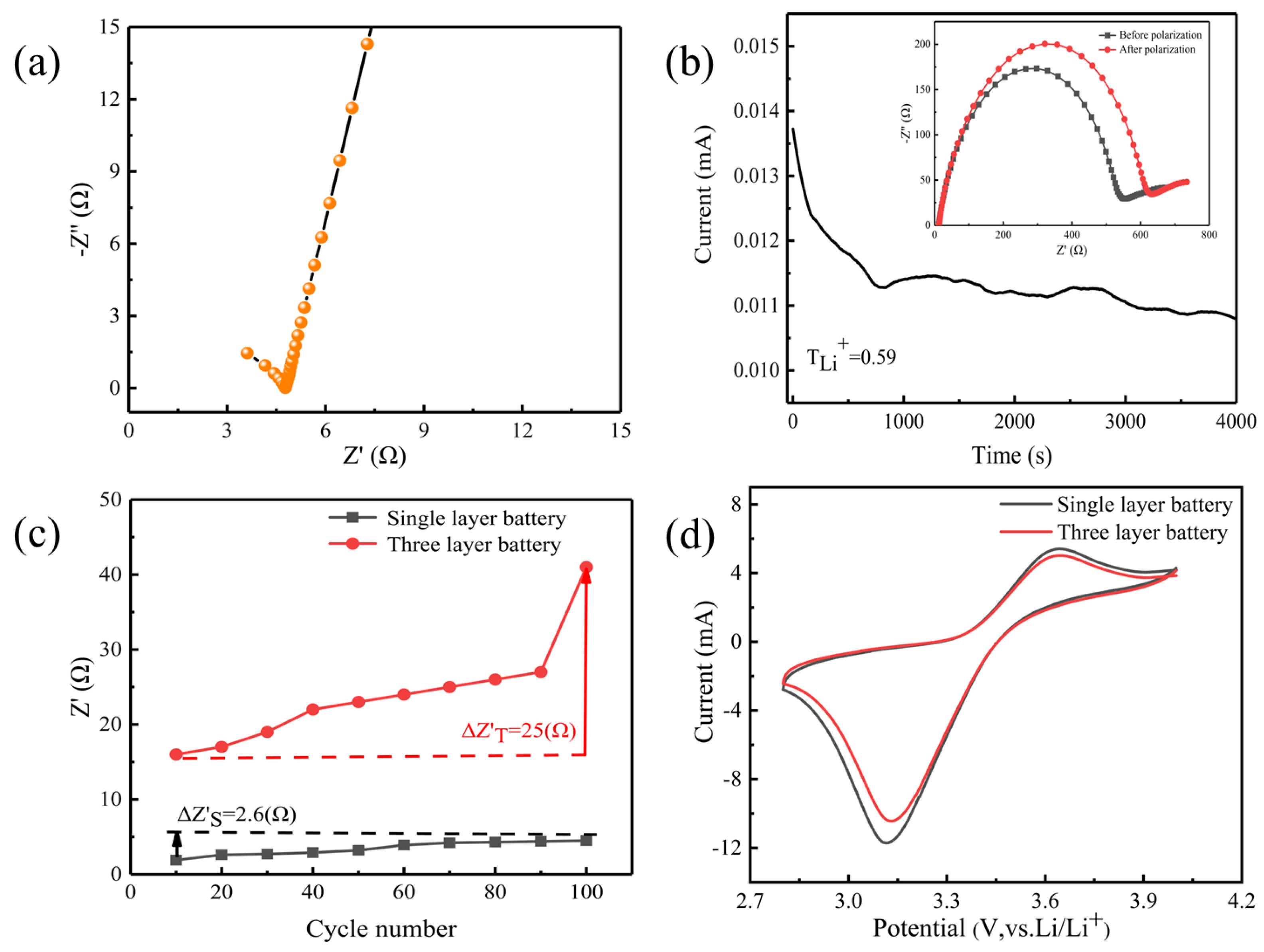
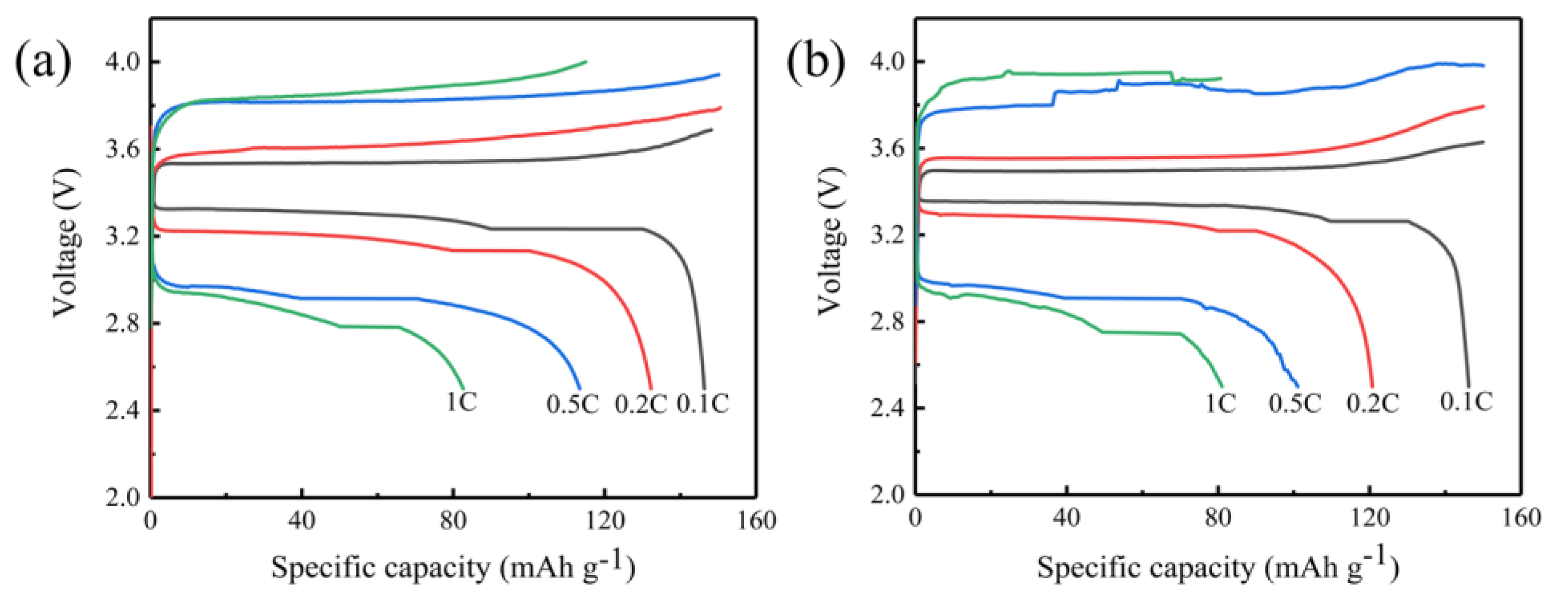
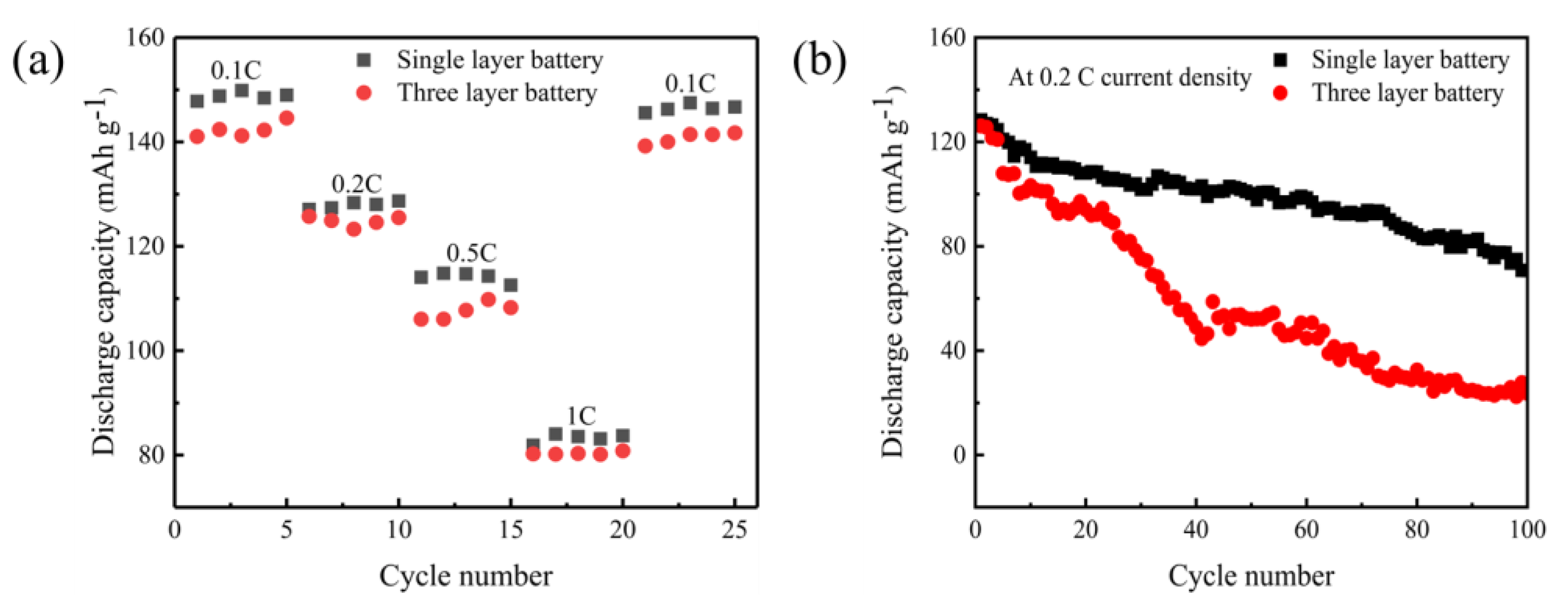
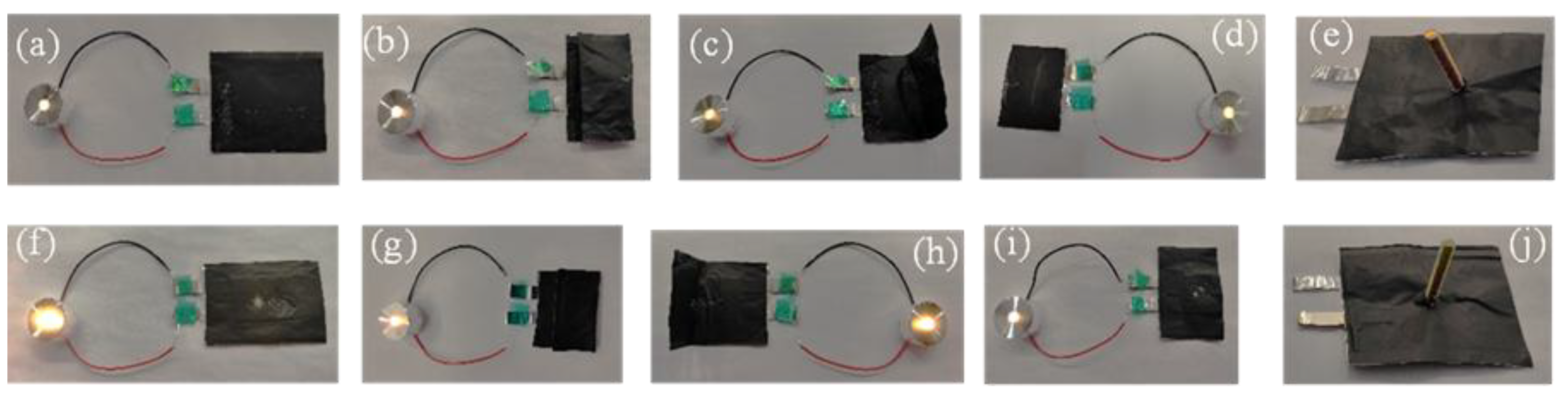
2. Results and Discussion
3. Conclusions
4. Materials and Methods
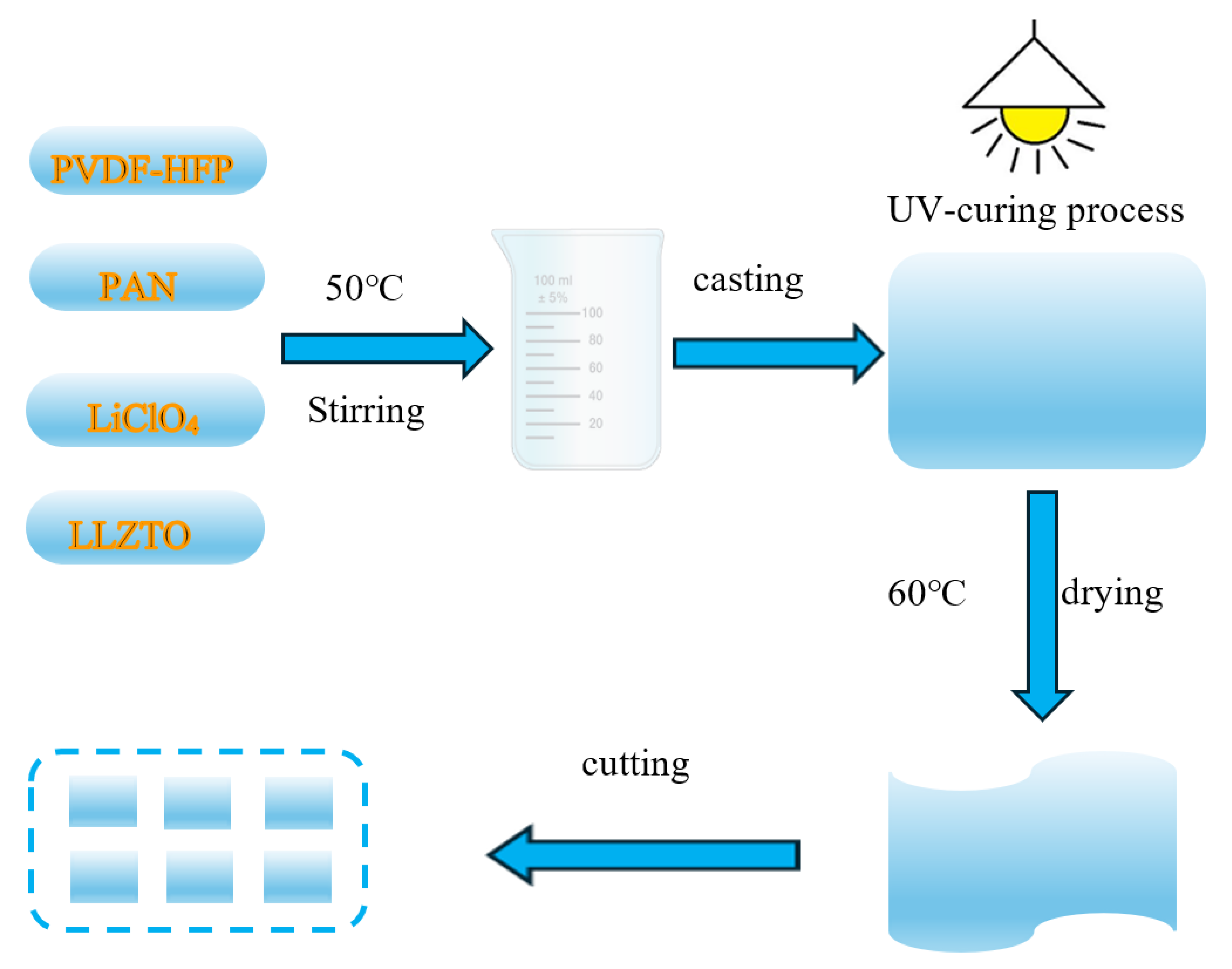
4.1. Preparation of PVDF-HFP/PAN-LiClO4-LLZTO Gel Polymer Electrolyte
4.2. Material Characterization
4.3. Electrode Preparation
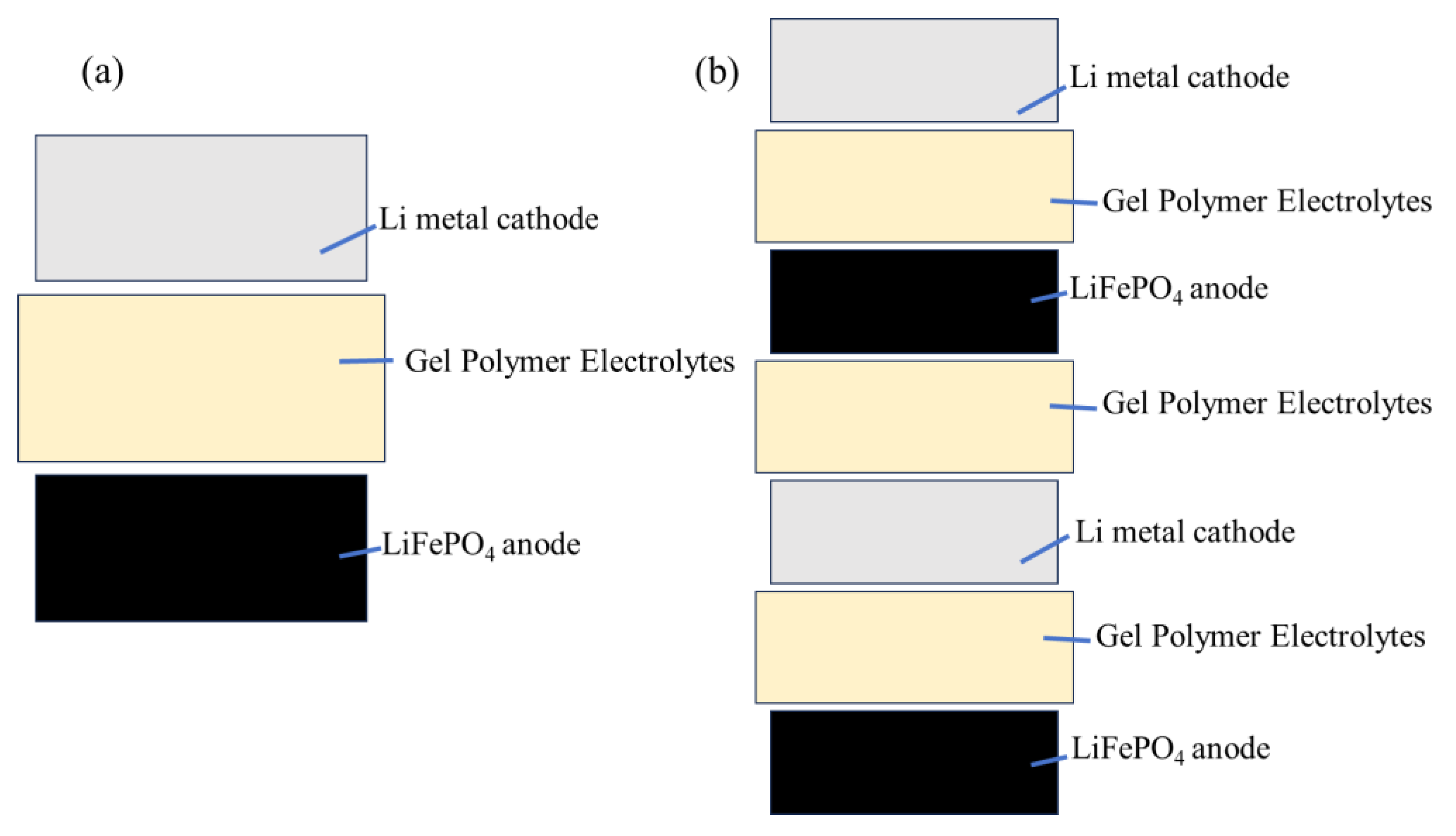
4.4. Battery Assembly
4.5. Electrochemical Properties Test
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, B.; Wen, K.; Chen, D.; Liu, Y.; Dong, Y.; Feng, C.; Han, Y.; Han, J.; Zhang, Y.; Xia, C.; et al. Composite separators for robust high rate lithium ion batteries. Adv. Funct. Mater. 2021, 31, 2101420. [Google Scholar] [CrossRef]
- Zhou, S.; Zhong, S.; Dong, Y.; Liu, Z.; Dong, L.; Yuan, B.; Xie, H.; Liu, Y.; Qiao, L.; Han, J.; et al. Composition and structure design of poly (vinylidene fluoride)-based solid polymer electrolytes for lithium batteries. Adv. Funct. Mater. 2023, 33, 2214432. [Google Scholar] [CrossRef]
- Li, Z.; Dong, Y.; Feng, J.; Xu, T.; Ren, H.; Gao, C.; Li, Y.; Cheng, M.; Wu, W.; Wu, M. Controllably enriched oxygen vacancies through polymer assistance in titanium pyrophosphate as a super anode for Na/K-ion batteries. ACS Nano 2019, 13, 9227–9236. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B. How we made the Li-ion rechargeable battery. Nat. Electron. 2018, 1, 204. [Google Scholar] [CrossRef]
- Zhong, S.; Yuan, B.; Guang, Z.; Chen, D.; Li, Q.; Dong, L.; Ji, Y.; Dong, Y.; Han, J.; He, W. Recent progress in thin separators for upgraded lithium ion batteries. Energy Storage Mater. 2021, 41, 805–841. [Google Scholar] [CrossRef]
- Lv, Z.; Kang, Y.; Tang, R.; Yang, J.; Chen, G.; Hu, Y.; Lin, P.; Yang, H.; Wu, Q.; Zhang, M.; et al. Stabilizing layered superlattice MoSe2 anodes by the rational solvation structure design for low-temperature aqueous zinc-ion batteries. Electron 2023, 1, e5. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Wang, Y.; Liu, Q.; Chen, Q.; Chen, M. Advances and prospects of PVDF based polymer electrolytes. J. Energy Chem. 2022, 64, 62–84. [Google Scholar] [CrossRef]
- Chattopadhyay, J.; Pathak, T.S.; Santos, D.M. Applications of polymer electrolytes in lithium-ion batteries: A review. Polymers 2023, 15, 3907. [Google Scholar] [CrossRef]
- Liu, X.; Jia, H.; Li, H. Flame-retarding quasi-solid polymer electrolytes for high-safety lithium metal batteries. Energy Storage Mater. 2024, 67, 103263. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, Y.; Yu, L.; Qi, L.; Oh, K.; Hu, P.; Lee, S.; Chen, C. Gel polymer electrolytes for rechargeable batteries toward wide-temperature applications. Chem. Soc. Rev. 2024, 53, 5291–5337. [Google Scholar] [CrossRef]
- Zou, S.; Yang, Y.; Wang, J.; Zhou, X.; Wan, X.; Zhu, M.; Liu, J. In-situ polymerization of solid-state polymer electrolytes for lithium metal batteries: A review. Energy Environ. Sci. 2024, 17, 4426–4460. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, G.; Li, X.; Zhang, S.; Chen, R.; Wang, X.; Gao, Y.; Wang, Z.; Chen, L. Polymer electrolytes based on interactions between [solvent-Li+] complex and solvent-modified polymer. Energy Storage Mater. 2022, 51, 443–452. [Google Scholar] [CrossRef]
- An, Y.; Han, X.; Liu, Y.; Azhar, A.; Na, J.; Nanjundan, A.K.; Wang, S.; Yu, J.; Yamauchi, Y. Progress in solid polymer electrolytes for lithium-ion batteries and beyond. Small 2022, 18, 2103617. [Google Scholar] [CrossRef]
- Sand, S.C.; Rupp, J.L.; Yildiz, B. A critical review on Li-ion transport, chemistry and structure of ceramic–polymer composite electrolytes for solid state batteries. Chem. Soc. Rev. 2024, 54, 178–200. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Zhao, N.; Huo, H.; Guo, X. Comprehensive investigation into garnet electrolytes toward application-oriented solid lithium batteries. Electrochem. Energy Rev. 2020, 3, 656–689. [Google Scholar] [CrossRef]
- Yan, B.; Li, X.; Xiao, W.; Hu, J.; Zhang, L.; Yang, X. Design, synthesis, and application of metal sulfides for Li–S batteries: Progress and prospects. J. Mater. Chem. A 2020, 8, 17848–17882. [Google Scholar] [CrossRef]
- Xu, R.; Han, F.; Ji, X.; Fan, X.; Tu, J.; Wang, C. Interface engineering of sulfide electrolytes for all-solid-state lithium batteries. Nano Energy 2018, 53, 958–966. [Google Scholar] [CrossRef]
- Bi, Z.; Zhao, N.; Ma, L.; Fu, Z.; Xu, F.; Wang, C.; Guo, X. Interface engineering on cathode side for solid garnet batteries. Chem. Eng. J. 2020, 387, 124089. [Google Scholar] [CrossRef]
- Huang, X.; Lu, Y.; Song, Z.; Rui, K.; Wang, Q.; Xiu, T.; Badding, M.E.; Wen, Z. Manipulating Li2O atmosphere for sintering dense Li7La3Zr2O12 solid electrolyte. Energy Storage Mater. 2019, 22, 207–217. [Google Scholar] [CrossRef]
- Lu, W.; Xue, M.; Zhang, C. Modified Li7La3Zr2O12 (LLZO) and LLZO-polymer composites for solid-state lithium batteries. Energy Storage Mater. 2021, 39, 108–129. [Google Scholar] [CrossRef]
- Dirican, M.; Yan, C.; Zhu, P.; Zhang, X. Composite solid electrolytes for all-solid-state lithium batteries. Mater. Sci. Eng. R Rep. 2019, 136, 27–46. [Google Scholar] [CrossRef]
- Xu, L.; Li, G.; Guan, J.; Wang, L.; Chen, J.; Zheng, J. Garnet-doped composite polymer electrolyte with high ionic conductivity for dendrite-free lithium batteries. J. Energy Storage 2019, 24, 100767. [Google Scholar] [CrossRef]
- Keller, M.; Varzi, A.; Passerini, S. Hybrid electrolytes for lithium metal batteries. J. Power Sources 2018, 392, 206–225. [Google Scholar] [CrossRef]
- Li, Y.; Xu, B.; Xu, H.; Duan, H.; Lü, X.; Xin, S.; Zhou, W.; Xue, L.; Fu, G.; Manthiram, A.; et al. Hybrid polymer/garnet electrolyte with a small interfacial resistance for lithium-ion batteries. Angew. Chem. Int. Ed. 2017, 56, 753–756. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Deng, Y.; Duan, H.; Qian, Y.; Chen, G. LiF and LiNO3 as synergistic additives for PEO-PVDF/LLZTO-based composite electrolyte towards high-voltage lithium batteries with dual-interfaces stability. J. Energy Chem. 2022, 65, 319–328. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.Q.; Shen, L.; Liu, Q.; Ma, J.B.; Lv, W.; He, Y.B.; Yang, Q.H. Progress and perspective of ceramic/polymer composite solid electrolytes for lithium batteries. Adv. Sci. 2020, 7, 1903088. [Google Scholar] [CrossRef]
- Zheng, Y.; Yao, Y.; Ou, J.; Li, M.; Luo, D.; Dou, H.; Li, Z.; Amine, K.; Yu, A.; Chen, Z. A review of composite solid-state electrolytes for lithium batteries: Fundamentals, key materials and advanced structures. Chem. Soc. Rev. 2020, 49, 8790–8839. [Google Scholar] [CrossRef]
- Yang, S.J.; Jiang, F.N.; Hu, J.K.; Yuan, H.; Cheng, X.B.; Kaskel, S.; Zhang, Q.; Huang, J.Q. Life cycle safety issues of lithium metal batteries: A perspective. Electron 2023, 1, e8. [Google Scholar] [CrossRef]
- Zhong, L.; Li, Z.; Wang, S.; Huang, S. Ultra-Thin Solid Electrolyte in Lithium-Ion Batteries. Sustain. Polym. Energy 2023, 1, 10004. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, S.; Lu, T.; Guan, L.; Hou, L.; Du, H.; Wei, H.; Liu, X.; Wei, Y.; Zhou, H. Ultrathin Solid Polymer Electrolyte Design for High-Performance Li Metal Batteries: A Perspective of Synthetic Chemistry. Adv. Sci. 2023, 10, 2205233. [Google Scholar] [CrossRef]
- Chen, D.; Liu, Y.; Feng, C.; He, Y.; Zhou, S.; Yuan, B.; Dong, Y.; Xie, H.; Zeng, G.; Han, J.; et al. Unified throughout-pore microstructure enables ultrahigh separator porosity for robust high-flux lithium batteries. Electron 2023, 1, e1. [Google Scholar] [CrossRef]
- Shi, P.; Ma, J.; Liu, M.; Guo, S.; Huang, Y.; Wang, S.; Zhang, L.; Chen, L.; Yang, K.; Liu, X.; et al. A dielectric electrolyte composite with high lithium-ion conductivity for high-voltage solid-state lithium metal batteries. Nat. Nanotechnol. 2023, 18, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Wen, P.; Wang, H.; Zhou, Y.; Gu, Y.; Zhang, L.; Shao-Horn, Y.; Lin, X.; Chen, M. Sequencing polymers to enable solid-state lithium batteries. Nat. Mater. 2023, 22, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, T.; Zhang, S.; Huang, X.; Xu, B.; Lin, Y.; Xu, B.; Li, L.; Nan, C.; Shen, Y. Synergistic coupling between Li6.75La3Zr1.75Ta0.25O12 and poly (vinylidene fluoride) induces high ionic conductivity, mechanical strength, and thermal stability of solid composite electrolytes. J. Am. Chem. Soc. 2017, 139, 13779–13785. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; She, S.; Hong, Z.; Huang, Y.; Wu, Y. Enabling lithium metal battery with flexible polymer/garnet type solid oxide composite electrolyte. Solid State Ion. 2021, 368, 115710. [Google Scholar] [CrossRef]
- Li, F.; Ren, K.; Hou, M.; Lin, M.; Yang, X.; Zhou, Y.; Xiong, S.; Liang, F. Ultrafast UV Curing Enabling A Stable Interphase and Interface for Solid-State Sodium–Metal Batteries. ACS Energy Lett. 2024, 10, 195–204. [Google Scholar] [CrossRef]
- Galos, J.; Pattarakunnan, K.; Best, A.S.; Kyratzis, I.L.; Wang, C.H.; Mouritz, A.P. Energy storage structural composites with integrated lithium-ion batteries: A review. Adv. Mater. Technol. 2021, 6, 2001059. [Google Scholar] [CrossRef]
- Shateri, N.; Auger, D.J.; Fotouhi, A.; Brighton, J.; Du, W.; Owen, R.E.; Brett, D.J.; Shearing, P.R. Investigation of the effect of temperature on lithium-sulfur cell cycle life performance using system identification and x-ray tomography. Batter. Supercaps 2022, 5, e202200035. [Google Scholar] [CrossRef]
- Stock, S.; Diller, F.; Böhm, J.; Hille, L.; Hagemeister, J.; Sommer, A.; Daub, R. Operando analysis of the gassing and swelling behavior of lithium-ion pouch cells during formation. J. Electrochem. Soc. 2023, 170, 60539. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, S.; Zhang, K.; Lu, G.; Li, Y. Extremely high heat flux dissipation and hotspots removal with nature-inspired single-phase microchannel heat sink designs. Appl. Therm. Eng. 2023, 234, 121282. [Google Scholar] [CrossRef]
- Wang, Q.; Tao, J.; Cui, Z.; Zhang, T.; Chen, G. Passive enhanced heat transfer, hotspot management and temperature uniformity enhancement of electronic devices by micro heat sinks: A review. Int. J. Heat Fluid Flow 2024, 107, 109368. [Google Scholar] [CrossRef]
- Hun, Q.; Lan, L.; Lu, X.; Hu, Q.; Liang, X.; Guo, Y.; Wang, Y. Bilayer Heterostructure Electrolytes Were Prepared by a UV-Curing Process for High Temperature Lithium-Ion Batteries. Polymers 2024, 16, 2972. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhang, Y.; Ning, Y.; Huang, D.; Lan, L.; Li, S. Quasi-solid-state lithium-sulfur batteries assembled by composite polymer electrolyte and nitrogen doped porous carbon fiber composite cathode. Nanomaterials 2022, 12, 2614. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Ning, Y.; Lan, L.; Yang, G.; Li, M.; Tang, S.; Huang, J. Electrochemical performance of a PVDF-HFP-LiClO4-Li6.4La3.0Zr1.4Ta0.6O12 composite solid electrolyte at different temperatures. Nanomaterials 2022, 12, 3390. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Wu, Y.; Li, Y.; Yue, Z.; Chen, M. PVDF-HFP/PAN/PDA@ LLZTO composite solid electrolyte enabling reinforced safety and outstanding low-temperature performance for quasi-solid-state lithium metal batteries. ACS Appl. Mater. Interfaces 2023, 15, 21526–21536. [Google Scholar] [CrossRef]
- Mallaiah, Y.; Jeedi, V.R.; Swarnalatha, R.; Raju, A.; Reddy, S.N.; Chary, A.S. Impact of polymer blending on ionic conduction mechanism and dielectric properties of sodium based PEO-PVdF solid polymer electrolyte systems. J. Phys. Chem. Solids 2021, 155, 110096. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, T.; Lv, Y.; Fang, J.; Dong, S.; Yao, S. “Polymer in ceramic” type LLZTO/PEO/PVDF composite electrolyte with high lithium migration number for solid-state lithium batteries. Ionics 2024, 30, 787–798. [Google Scholar] [CrossRef]
- Nguyen, M.C.; Nguyen, H.L.; Duong, T.P.M.; Kim, S.H.; Kim, J.Y.; Bae, J.H.; Kim, H.K.; Lim, S.N.; Ahn, W. Highly Safe, Ultra-Thin MOF-Based Solid Polymer Electrolytes for Superior All-Solid-State Lithium-Metal Battery Performance. Adv. Funct. Mater. 2024, 34, 2406987. [Google Scholar] [CrossRef]
- Liang, X.; Huang, D.; Lan, L.; Yang, G.; Huang, J. Enhancement of the Electrochemical Performances of Composite Solid-State Electrolytes by Doping with Graphene. Nanomaterials 2022, 12, 3216. [Google Scholar] [CrossRef]
- Zou, K.; Cai, Z.; Ke, X.; Wang, K.; Tan, X.; Luo, D.; Huang, F.; Wang, C.; Cheng, J.; Xiao, R. Electrochemical properties of LATP ceramic electrolyte doped with LiBiO3 sintering additive and its derived sandwich structure composite solid electrolyte. Ionics 2023, 29, 2665–2678. [Google Scholar] [CrossRef]
- Xu, C.; Xiang, W.; Wu, Z.; Xu, Y.; Li, Y.; Wang, Y.; Xiao, Y.; Guo, X.; Zhong, B. Highly stabilized Ni-rich cathode material with Mo induced epitaxially grown nanostructured hybrid surface for high-performance lithium-ion batteries. ACS Appl. Mater. Interfaces 2019, 11, 16629–16638. [Google Scholar] [CrossRef]
- Shen, X.; Tian, Z.; Fan, R.; Shao, L.; Zhang, D.; Cao, G.; Kou, L.; Bai, Y. Research progress on silicon/carbon composite anode materials for lithium-ion battery. J. Energy Chem. 2018, 27, 1067–1090. [Google Scholar] [CrossRef]
- Lin, L.; Chen, C. Accurate characterization of transference numbers in electrolyte systems. J. Power Sources 2024, 603, 234236. [Google Scholar] [CrossRef]
- Meng, J.; You, Y.; Lin, M.; Wu, J.; Song, Z. Multi-scenarios transferable learning framework with few-shot for early lithium-ion battery lifespan trajectory prediction. Energy 2024, 286, 129682. [Google Scholar] [CrossRef]
| GPEs | Ionic Conductivity (×10−4 S/cm) | Ref. |
|---|---|---|
| PAP/PEP | 4.27 (RT) | [42] |
| CEPs | 0.81(25 °C) | [43] |
| CSE (15 wt.% LLZTO) | 0.55 (30 °C) | [44] |
| PPPL-10 | 4 (25 °C) | [45] |
| PEO/PVDF/NaNO3 | 0.93 (25 °C) | [46] |
| PEO-PVDF-LITFSI | 2.46 (25 °C) | [47] |
| SPEs (PLiZ) | 1.19 (25 °C) | [48] |
| PPLG (5 wt.% graphene) | 3.39 (25 °C) | [49] |
| SSCEs | 2.91 (25 °C) | [50] |
| GPEs | 3.86 (RT) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Qin, W.; Hun, Q.; Wang, Y.; Liang, X.; Tan, R.; Li, J.; Guo, Y. Preparation and Failure Behavior of Gel Electrolytes for Multilayer Structure Lithium Metal Solid-State Batteries. Gels 2025, 11, 573. https://doi.org/10.3390/gels11080573
Chen C, Qin W, Hun Q, Wang Y, Liang X, Tan R, Li J, Guo Y. Preparation and Failure Behavior of Gel Electrolytes for Multilayer Structure Lithium Metal Solid-State Batteries. Gels. 2025; 11(8):573. https://doi.org/10.3390/gels11080573
Chicago/Turabian StyleChen, Chu, Wendong Qin, Qiankun Hun, Yujiang Wang, Xinghua Liang, Renji Tan, Junming Li, and Yifeng Guo. 2025. "Preparation and Failure Behavior of Gel Electrolytes for Multilayer Structure Lithium Metal Solid-State Batteries" Gels 11, no. 8: 573. https://doi.org/10.3390/gels11080573
APA StyleChen, C., Qin, W., Hun, Q., Wang, Y., Liang, X., Tan, R., Li, J., & Guo, Y. (2025). Preparation and Failure Behavior of Gel Electrolytes for Multilayer Structure Lithium Metal Solid-State Batteries. Gels, 11(8), 573. https://doi.org/10.3390/gels11080573








