Micro-Gas Flow Sensor Utilizing Surface Network Density Regulation for Humidity-Modulated Ion Transport
Abstract
1. Introduction
2. Results and Discussion
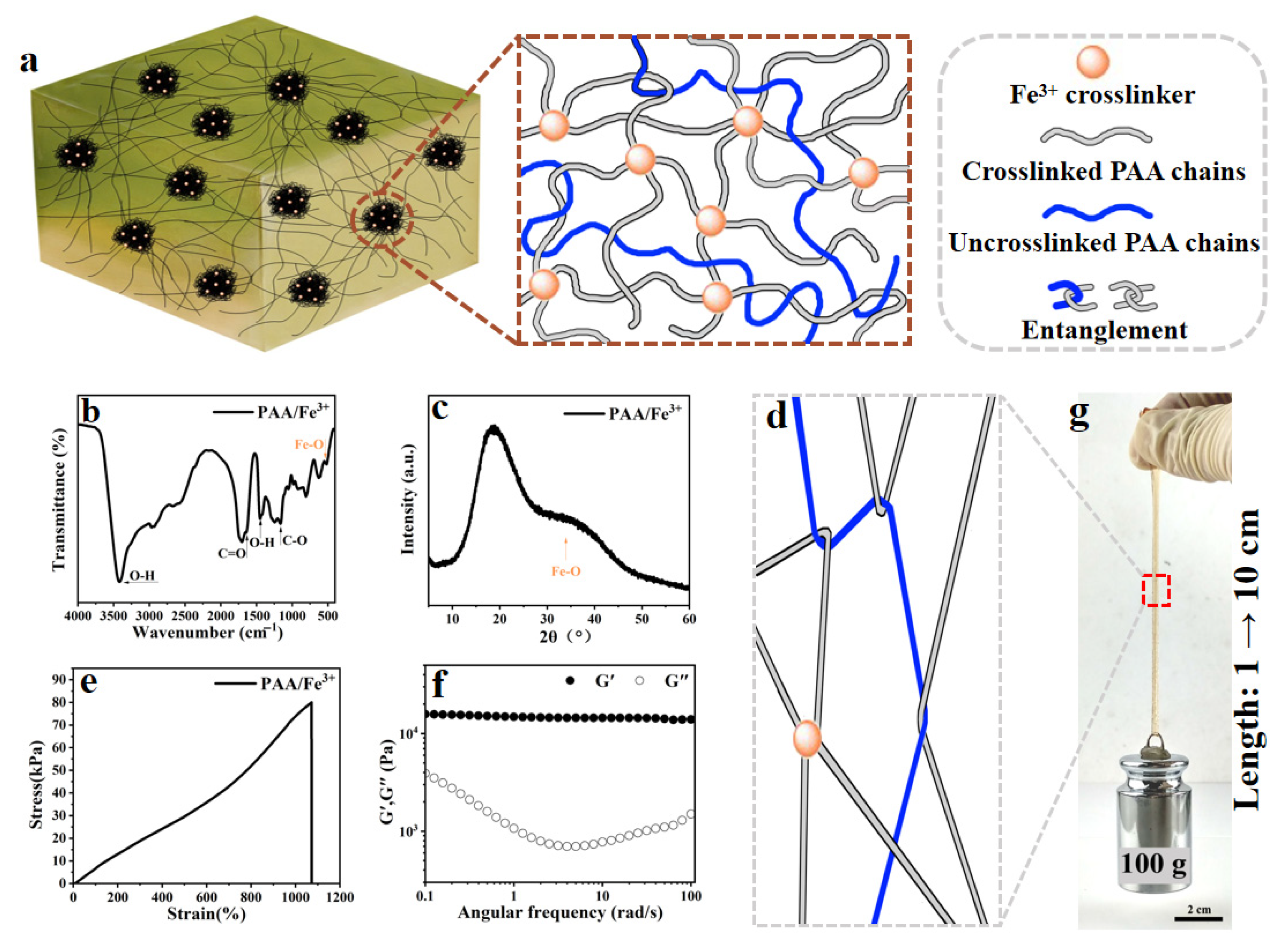
2.1. PAA/Fe3+ Hydrogel Design and Preparation
2.2. Airflow Sensitiviy of PAA/Fe3+ Hydrogel
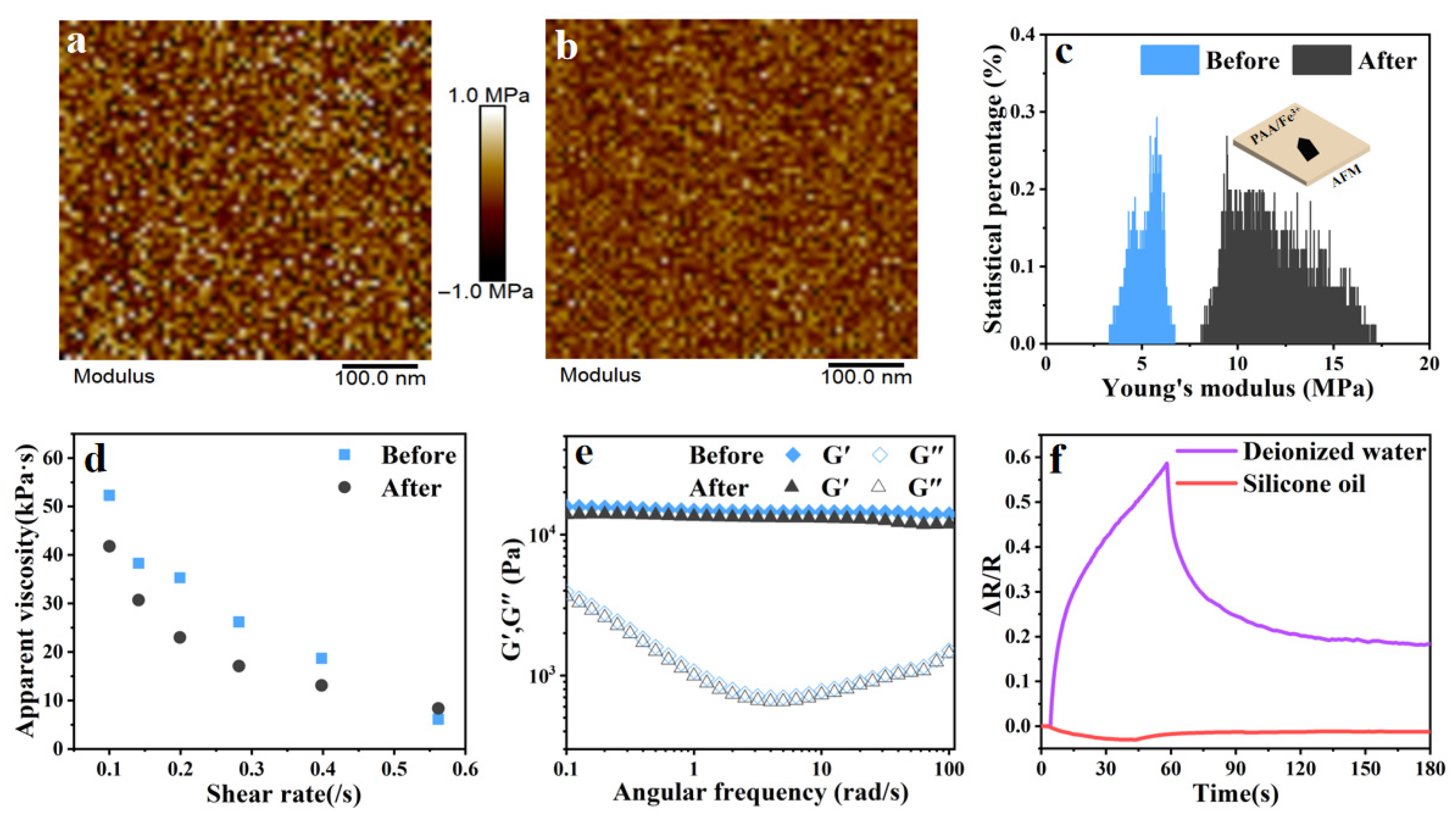
2.3. Ion Transport Mechanisms of PAA/Fe3+ Hydrogel
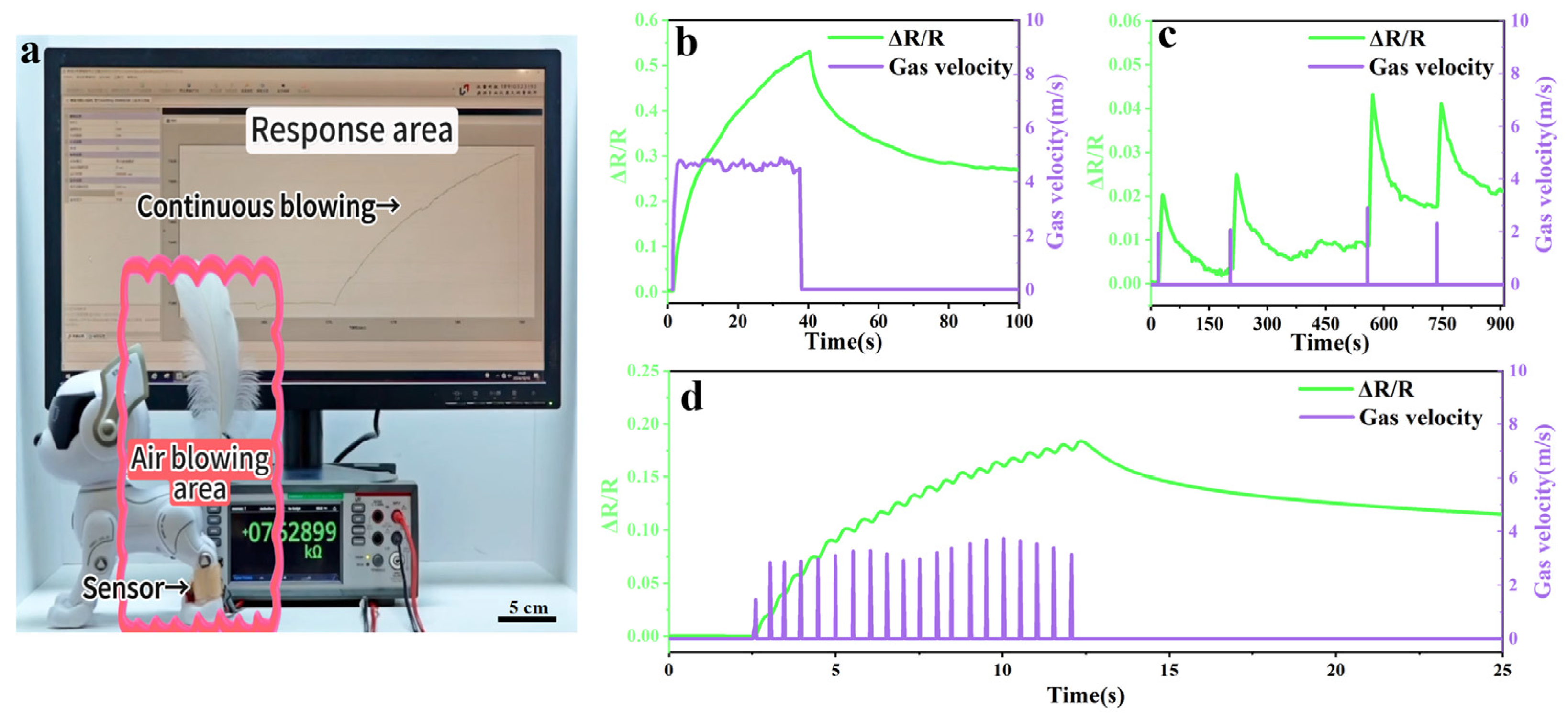
2.4. Application of PAA/Fe3+ Hydrogel
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Fabrication of PAA/Fe3+ Hydrogel
4.3. Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Acrylic acid |
| PAA | Polyacrylic acid |
| PAA/Fe3+ | Polyacrylic acid/ferric ion hydrogel |
References
- Larson, C.; Peele, B.; Li, S.; Robinson, S.; Totaro, M.; Beccai, L.; Mazzolai, B.; Shepherd, R. Highly stretchable electroluminescent skin for optical signaling and tactile sensing. Science 2016, 351, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Yuan, F.Y.; Liu, Z.M.; Sun, Z.N.; Knubben, E.M.; Wen, L. A Proprioceptive soft tentacle gripper based on crosswise stretchable sensors. IEEE/ASME Trans. Mechatron. 2020, 25, 1841–1850. [Google Scholar] [CrossRef]
- Truby, R.L.; Wehner, M.; Grosskopf, A.K.; Vogt, D.M.; Uzel, S.G.M.; Wood, R.J.; Lewis, J.A. Soft somatosensitive actuators via embedded 3D printing. Adv. Mater. 2018, 30, 1706383. [Google Scholar] [CrossRef] [PubMed]
- Wall, V.; Zöller, G.; Brock, O. A method for sensorizing soft actuators and its application to the RBO hand 2. In Proceedings of the 2017 IEEE International Conference on Robotics and Automation (ICRA), Singapore, 29 May–3 June 2017. [Google Scholar]
- Wang, P.Y.; Xie, Z.X.; Xin, W.C.; Tang, Z.Q.; Yang, X.H.; Mohanakrishnan, M.; Guo, S.; Laschi, C. Sensing expectation enables simultaneous proprioception and contact detection in an intelligent soft continuum robot. Nat. Commun. 2024, 15, 9978. [Google Scholar] [CrossRef] [PubMed]
- Flavin, M.T.; Ha, K.H.; Guo, Z.R.; Li, S.P.; Kim, J.T.; Saxena, T.; Simatos, D.; Al-Najjar, F.; Mao, Y.X.; Bandapalli, S.; et al. Bioelastic state recovery for haptic sensory substitution. Nature 2024, 635, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.C.; Jiang, Y.; Zhong, D.; Zhang, Z.; Choudhury, S.; Lai, J.C.; Gong, H.; Niu, S.; Yan, X.; Zheng, Y.; et al. Neuromorphic sensorimotor loop embodied by monolithically integrated, low-voltage, soft e-skin. Science 2023, 380, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Lee, J.; Beak, C.J.; Biswas, S.; Lee, S.H.; Kim, H. Flexible neuromorphic electronics for wearable near-sensor and in-sensor computing systems. Adv. Mater. 2025, 37, 2416073. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Lee, Y.; Kim, N.; Seo, D.G.; Go, G.T.; Lee, T.W. Flexible neuromorphic electronics for computing, soft robotics, and neuroprosthetics. Adv. Mater. 2020, 32, 1903558. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Li, H.; Duan, Z.H.; Huang, Q.; Zhang, M.X.; Zhang, H.X.; Guo, J.H.; Jiang, Y.D.; Tai, H.L. High sensitivity, wide range and waterproof strain sensor with inner surface sensing layer for motion detection and gesture reconstruction. Sens. Actuators A Phys. 2024, 369, 115202. [Google Scholar] [CrossRef]
- Qin, Z.H.; Sun, X.; Yu, Q.Y.; Zhang, H.T.; Wu, X.J.; Yao, M.M.; Liu, W.W.; Yao, F.L.; Li, J.J. Carbon nanotubes/hydrophobically associated hydrogels as ultrastretchable, highly sensitive, stable strain, and pressure sensors. ACS Appl. Mater. Interfaces 2020, 12, 4944–4953. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Q.; Xi, L.; Li, T.Y.; Du, X.N.; Zhang, X.Y.; Hou, L.L. Highly sensitive low-frequency acoustic sensor based on functionalized graphene oxide. Small 2024, 2409043. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Cao, J.J.; Li, Y.R.; Sun, G.F.; Shao, H.Y.; Meng, C.Z. Highly breathable and sensitive iontronic wearable sensor based on porous ionic electrolyte and microstructure for human movement sensing. Chem. Eng. J. 2024, 495, 153525. [Google Scholar] [CrossRef]
- Lv, Y.; Wei, J.; Wang, W.F.; Deng, H.Y.; Huang, Z.; Zhou, J.; Chen, Z.L.; Xie, J.M.; Huang, X.W.; Guo, Y.; et al. Superelastic bamboo cellulose nanofiber based carbon aerogel with layered network microstructure for high sensitivity piezoresistive sensor. Int. J. Biol. Macromol. 2025, 295, 139553. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.H.; Liu, T.C.; Tang, Y.M.; Li, W.; Liu, L.; Wang, D.; Zhang, Y.F.; Zhang, T.X.; Zhu, X.W.; Guan, Y.X.; et al. Bioinspired low hysteresis flexible pressure sensor using nanocomposites of multiwalled carbon nanotubes, silicone rubber, and carbon nanofiber for human–computer interaction. ACS Appl. Nano Mater. 2024, 7, 15626–15639. [Google Scholar] [CrossRef]
- Vo, T.H.; Lam, P.K.; Sheng, Y.J.; Tsao, H.K. Jammed microgels in deep eutectic solvents as a green and low-cost ink for 3D printing of reliable auxetic strain sensors. ACS Appl. Mater. Interfaces 2023, 15, 33109–33118. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liang, W.; Zhang, L.; Ren, F.C.; Xuan, F.Z. TPU/CNTs flexible strain sensor with auxetic structure via a novel hybrid manufacturing process of fused deposition modeling 3D printing and ultrasonic cavitation-enabled treatment. Sens. Actuators A Phys. 2022, 340, 113526. [Google Scholar] [CrossRef]
- Pal, S.; Su, Y.Z.; Chen, Y.W.; Yu, C.H.; Kung, C.W.; Yu, S.S. 3D printing of metal−organic framework-based ionogels: Wearablesensors with colorimetric and mechanical responses. ACS Appl. Mater. Interfaces 2022, 14, 28247–28257. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.D.; Lv, C.H.; Ye, X.L.; Ping, J.F.; Ying, Y.B.; Lan, L.Y. Hydrogel-based pressure sensors for electronic skin systems. Matter 2025, 8, 101992. [Google Scholar] [CrossRef]
- Bai, N.; Wang, L.; Wang, Q.; Deng, J.; Wang, Y.; Lu, P.; Huang, J.; Li, G.; Zhang, Y.; Yang, J.L.; et al. Graded intrafillable architecture-based iontronic pressure sensor with ultra-broad-range high sensitivity. Nat. Commun. 2020, 11, 209. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Q.; Zhang, Y.T.; Li, S.N.; Zheng, J.X.; Yang, H.L.; Ren, J.Y.; Zhu, C.J.; Zhou, Y.C.; Chen, Y.M.; Fu, J. Flexible artificial tactility with excellent robustness and temperature tolerance based on organohydrogel sensor array for robot motion detection and object shape recognition. Adv. Mater. 2024, 36, 2408193. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Song, Q.; Liu, K.; Liu, H.Y.; Pan, J.J.; Liu, W.; Dai, L.; Zhang, M.; Wang, Y.X.; Si, C.L.; et al. Nanocellulose-assisted construction of multifunctional mxene-based aerogels with engineering biomimetic texture for pressure sensor and compressible electrode. Nano-Micro Lett. 2023, 15, 98. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Peng, Q.Y.; Thundat, T.; Zeng, H.B. Stretchable, injectable, and self-healing conductive hydrogelenabled by multiple hydrogen bonding toward wearableelectronics. Chem. Mater. 2019, 31, 4553–4563. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Yang, J.W.; Wang, H.Y.; Wang, C.Y.; Gu, Y.H.; Xu, Y.M.; Lee, S.; Yokota, T.; Haick, H.; Someya, T.; et al. A 10-micrometer-thick nanomesh-reinforced gas-permeable hydrogel skin sensor for long-term electrophysiological monitoring. Sci. Adv. 2024, 10, eadj5389. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.Q.; Su, P.P.; Jin, M.H.; Huang, X.C.; Wang, Z.B.; Zhang, R.H.; Xu, H.; Liu, W.N.; Ye, Y.M. Ultrathin hierarchical hydrogel-carbon nanocomposite for highly stretchable fast-response water-proof wearable humidity sensors. Mater. Horiz. 2023, 10, 5263–5276. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Ding, Q.L.; Wang, H.; Wu, Z.X.; Li, J.Y.; Li, Z.Y.; Tao, K.; Gui, X.C.; Wu, J. Humidity sensing of stretchable and transparent hydrogel films for wireless respiration monitoring. Nano-Micro Lett. 2022, 14, 183. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.L.; Wang, H.; Zhou, Z.J.; Wu, Z.X.; Tao, K.; Gui, X.C.; Liu, C.A.; Shi, W.X.; Wu, J. Stretchable, self-healable, and breathable biomimetic iontronics with superior humidity-sensing performance for wireless respiration monitoring. SmartMat 2023, 4, 1147. [Google Scholar] [CrossRef]
- Qin, S.Y.; Yang, P.; Liu, Z.Q.; Hu, J.; Li, N.; Ding, L.M.; Chen, X.Y. Triboelectric sensor with ultra-wide linear range based on water-containing elastomer and ion-rich interface. Nat. Commun. 2024, 15, 10640. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Dai, P.H.; Li, X.R.; Cong, Z.C.; Dong, T.J.; Sun, Y.; Liu, X.K.; Sui, Y.; Chen, P.; Yu, X.L.; et al. Flexible wearable microfiber respiratory sensor based on microspheres coupling. IEEE Sens. J. 2023, 23, 27324–27330. [Google Scholar] [CrossRef]
- Zhang, Q.; Ruan, W.Z.; Wang, H.; Zhou, Y.Z.; Wang, Z.Y.; Liu, L.T. A self-bended piezoresistive microcantilever flow sensor for low flow rate measurement. Sens. Actuators A Phys. 2010, 158, 273–279. [Google Scholar] [CrossRef]
- Lin, J.; Li, J.W.; Li, W.; Chen, S.T.; Lu, Y.Y.; Ma, L.J.; He, X.H.; Zhao, Q.L. Multifunctional polyimide nanofibrous aerogel sensor for motion monitoring and airflow perception. Compos. Part A Appl. Sci. Manuf. 2024, 178, 108003. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, R.; Wang, F.; Shi, X.F.; Chen, F.X.; Huang, Y.; Wang, B.S.; Zhang, W.S.; Wu, X.K.; Wei, F.; et al. Ultrasensitive airflow sensors based on suspended carbon nanotube networks. Adv. Mater. 2022, 34, 2107062. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Huang, T.R.; Liang, K.; Xie, W.J.; Chen, Y.; Li, J.Y. Small-volume omnidirectional intelligent airflow sensor with differential pressure measurement. Measurement 2025, 242, 116113. [Google Scholar] [CrossRef]
- Gong, Z.; Di, W.C.; Jiang, Y.G.; Dong, Z.H.; Yang, Z.; Ye, H.; Zhang, H.R.; Liu, H.J.; Wei, Z.X.; Tu, Z.; et al. Flexible calorimetric flow sensor with unprecedented sensitivity and directional resolution for multiple flight parameter detection. Nat. Commun. 2024, 15, 3091. [Google Scholar] [CrossRef] [PubMed]
- Talbi, A.; Gimeno, L.; Gerbedoen, J.C.; Viard, R.; Soltani, A.; Mortet, V.; Preobrazhensky, V.; Merlen, A.; Pernod, P. A micro-scale hot wire anemometer based on low stress (Ni/W) multi-layers deposited on nano-crystalline diamond for air flow sensing. J. Micromech. Microeng. 2015, 25, 125029. [Google Scholar] [CrossRef]
- Sadeghi, M.M.; Peterson, R.L.; Najafi, K. Air flow sensing using micro-wire-bonded hair-like hot-wire anemometry. J. Micromech. Microeng. 2013, 23, 085017. [Google Scholar] [CrossRef]
- Liu, H.B.; Lin, N.; Pan, S.; Miao, J.; Norford, L.K. High sensitivity, miniature, full 2-D anemometer based on mems hot-film sensors. IEEE Sens. J. 2013, 13, 1914–1920. [Google Scholar] [CrossRef]
- Al-Salaymeh, A.; Ashhab, M.S. Modelling of a novel hot-wire thermal flow sensor with neural nets under different operating conditions. Sens. Actuators A Phys. 2006, 126, 7–14. [Google Scholar] [CrossRef]
- Park, J.; Lee, Y.; Hong, J.; Ha, M.; Jung, Y.D.; Lim, H.; Kim, S.Y.; Ko, H. Giant tunneling piezoresistance of composite elastomers with interlocked microdome arrays for ultrasensitive and multimodal electronic skins. ACS Nano 2014, 8, 4689–4697. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Liu, Y.; Li, G.; Song, Y.; Su, J.J.; Cheng, L.; Guo, W.H.; Zhao, G.G.; Shen, H.C.; Yan, Z.; et al. Ultrasensitive, fast-responsive, directional airflow sensing by bioinspired suspended graphene fibers. Nano Lett. 2023, 23, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Duan, L.; Liu, G.; Sun, J.F.; Shahbazi, M.A.; Kundu, S.C.; Reis, R.L.; Xiao, B.; Yang, X. Bioinspired polyacrylic acid-based dressing: Wet adhesive, self-healing, and multi-biofunctional coacervate hydrogel accelerates wound healing. Adv. Sci. 2023, 10, 2207352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, J.; Chen, Y.Y.; Zhao, S.L.; Zhang, Y.P.; Wang, R.M.; He, Y.F.; Song, P.F. Combination of recycled cellulose with nano-thick flower layered double hydroxides: Biomass water retention slow-release fertilizer for sustainable agriculture. Colloids Surf. A Physicochem. Eng. Asp. 2024, 702, 135144. [Google Scholar] [CrossRef]
- Wu, H.; Yu, G.H.; Pan, L.J.; Liu, N.A.; McDowell, M.T.; Bao, Z.A.; Cui, Y. Stable Li-ion battery anodes by in-situ polymerization of conducting hydrogel to conformally coat silicon nanoparticles. Nat. Commun. 2013, 4, 1943. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.C.; Wu, K.L.; Wu, J.N.; Meng, G.H.; Liu, Z.Y.; Guo, X.H. Synthesis of cellulose-based double-network hydrogels demonstrating high strength, self-healing, and antibacterial properties. Carbohydr. Polym. 2017, 168, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.L.; Li, J.W.; Li, Y.; Wang, H.L.; Zhang, Y.C.; Guo, B.B.; Yu, J.; Wang, Y.F. 3D printed silk fibroin-based hydrogels with tunableadhesion and stretchability for wearable sensing. Adv. Funct. Mater. 2024, 34, 2404451. [Google Scholar] [CrossRef]
- Zhang, X.M.; Yao, Y.Y.; Wu, Y.; Liu, W.J.; Wang, X.W.; Feng, P.Z.; Zhang, J.M.; Hu, W.; Shang, E.D. Enhancement and mechanism of mechanical properties and functionalities of polyacrylamide/polyacrylic acid hydrogels by 1D and 2D nanocarbon. J. Colloid Interface Sci. 2025, 679, 79–90. [Google Scholar] [CrossRef] [PubMed]
- You, I.S.; Mackanic, D.G.; Matsuhisa, N.; Kang, J.; Kwon, J.; Beker, L.; Mun, J.; Suh, W.; Kim, T.Y.; Tok, J.B.H.; et al. Artificial multimodal receptors based on ion relaxation dynamics. Science 2020, 370, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Xie, Z.; Yu, Y.; Lee, J.; Vazquez-Guardado, A.; Luan, H.; Ruban, J.; Ning, X.; Akhtar, A.; Li, D.; et al. Skin-integrated wireless haptic interfaces for virtual and augmented reality. Nature 2019, 575, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, X.Y.; Liu, Y.; Yang, C.Q.; Liu, W.Z.; Qi, M.Y.; Zhang, D.Z. PDMS film-based flexible pressure sensor array with surface protruding structure for human motion detection and wrist posture recognition. ACS Appl. Mater. Interfaces 2024, 16, 2554–2563. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Wu, Y.H.; Ke, Y.; He, X.L.; Sun, Z.Z.; Xu, J. Waterproof flexible strain sensors with high sensitivity for detecting human movement, airflow size and water ripples prepared by demulsification. Sens. Actuators A Phys. 2024, 377, 115775. [Google Scholar] [CrossRef]
- Li, D.F.; He, J.H.; Song, Z.; Yao, K.M.; Wu, M.G.; Fu, H.R.; Liu, Y.M.; Gao, Z.; Zhou, J.K.; Wei, L.; et al. Miniaturization of mechanical actuators in skin-integrated electronics for haptic interfaces. Microsyst. Nanoeng. 2021, 7, 85. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Liu, Z. Micro-Gas Flow Sensor Utilizing Surface Network Density Regulation for Humidity-Modulated Ion Transport. Gels 2025, 11, 570. https://doi.org/10.3390/gels11080570
Liu C, Liu Z. Micro-Gas Flow Sensor Utilizing Surface Network Density Regulation for Humidity-Modulated Ion Transport. Gels. 2025; 11(8):570. https://doi.org/10.3390/gels11080570
Chicago/Turabian StyleLiu, Chuanjie, and Zhihong Liu. 2025. "Micro-Gas Flow Sensor Utilizing Surface Network Density Regulation for Humidity-Modulated Ion Transport" Gels 11, no. 8: 570. https://doi.org/10.3390/gels11080570
APA StyleLiu, C., & Liu, Z. (2025). Micro-Gas Flow Sensor Utilizing Surface Network Density Regulation for Humidity-Modulated Ion Transport. Gels, 11(8), 570. https://doi.org/10.3390/gels11080570







