O2-Generated Electrical and Mechanical Properties of Polyphenol-Mediated Hydrogel Sensor
Abstract
1. Introduction
2. Results and Discussion
2.1. Fabrication and Characterization of the O2-Responsive HDP–PVA Hydrogel
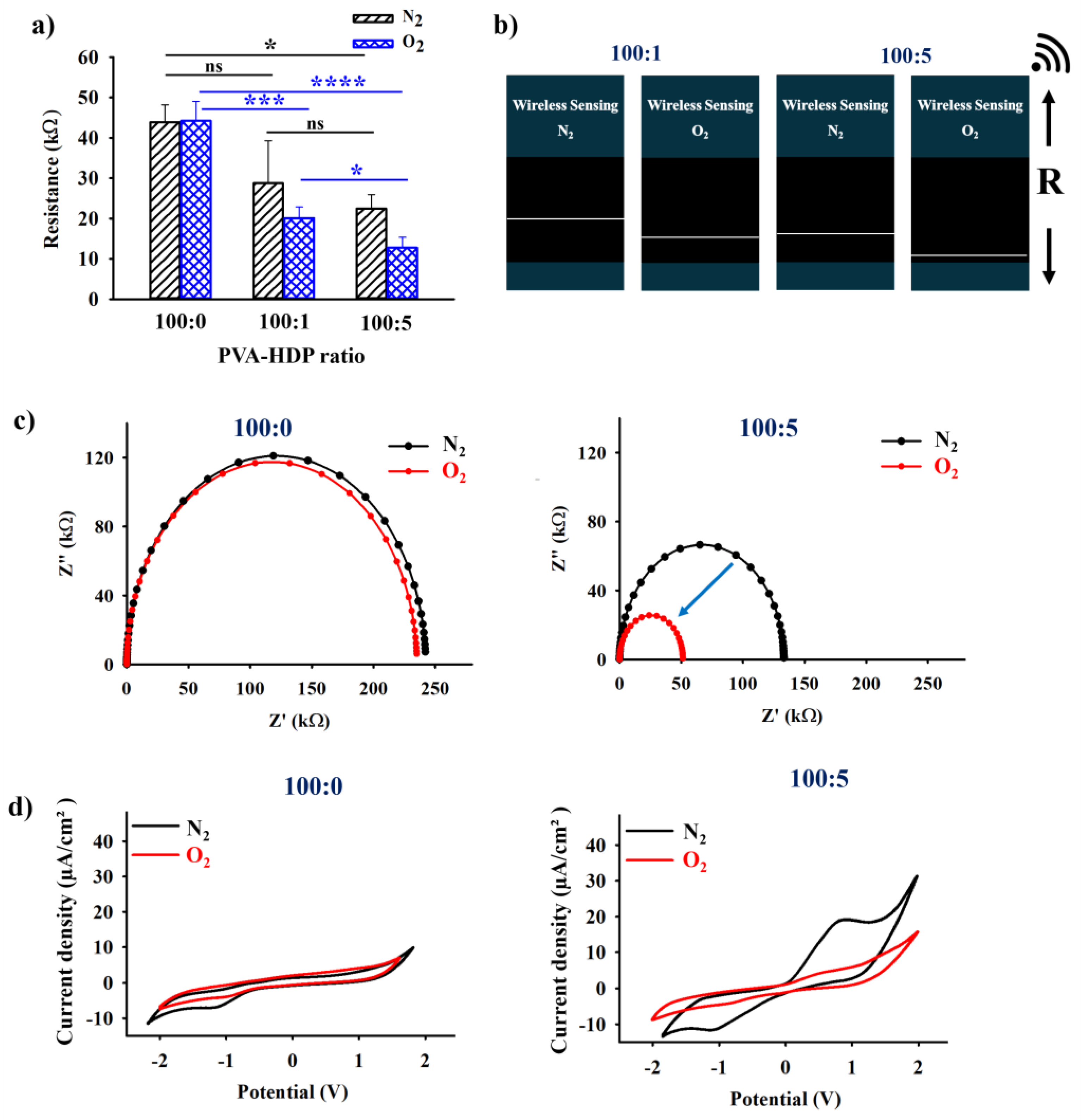
2.2. Electrical and Wireless Output Analysis of the HDP–PVA Hydrogel
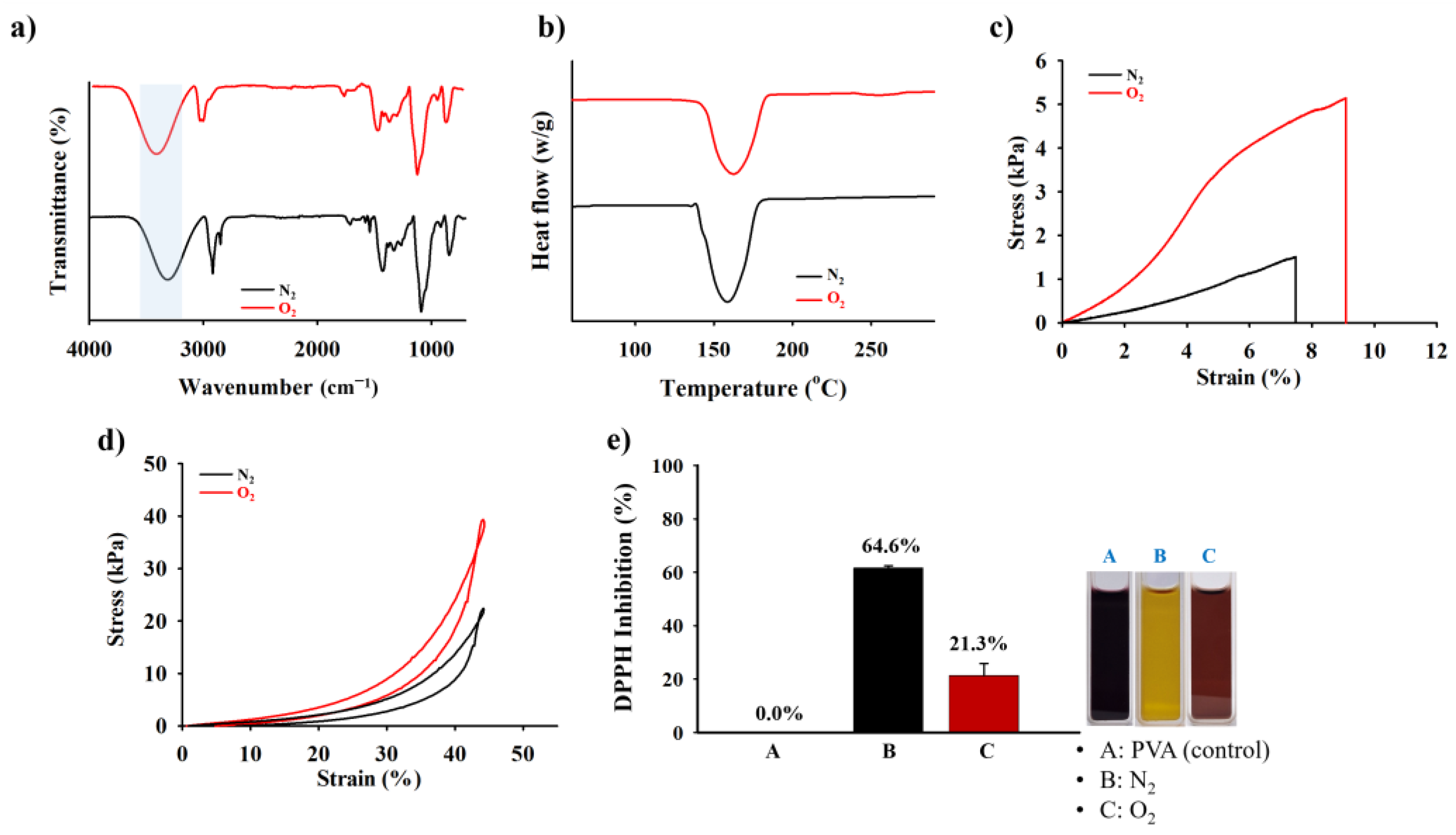
2.3. Mechanical Compressive, Tensile Test, and ROS Scavenging Capability of the HDP–PVA Hydrogel
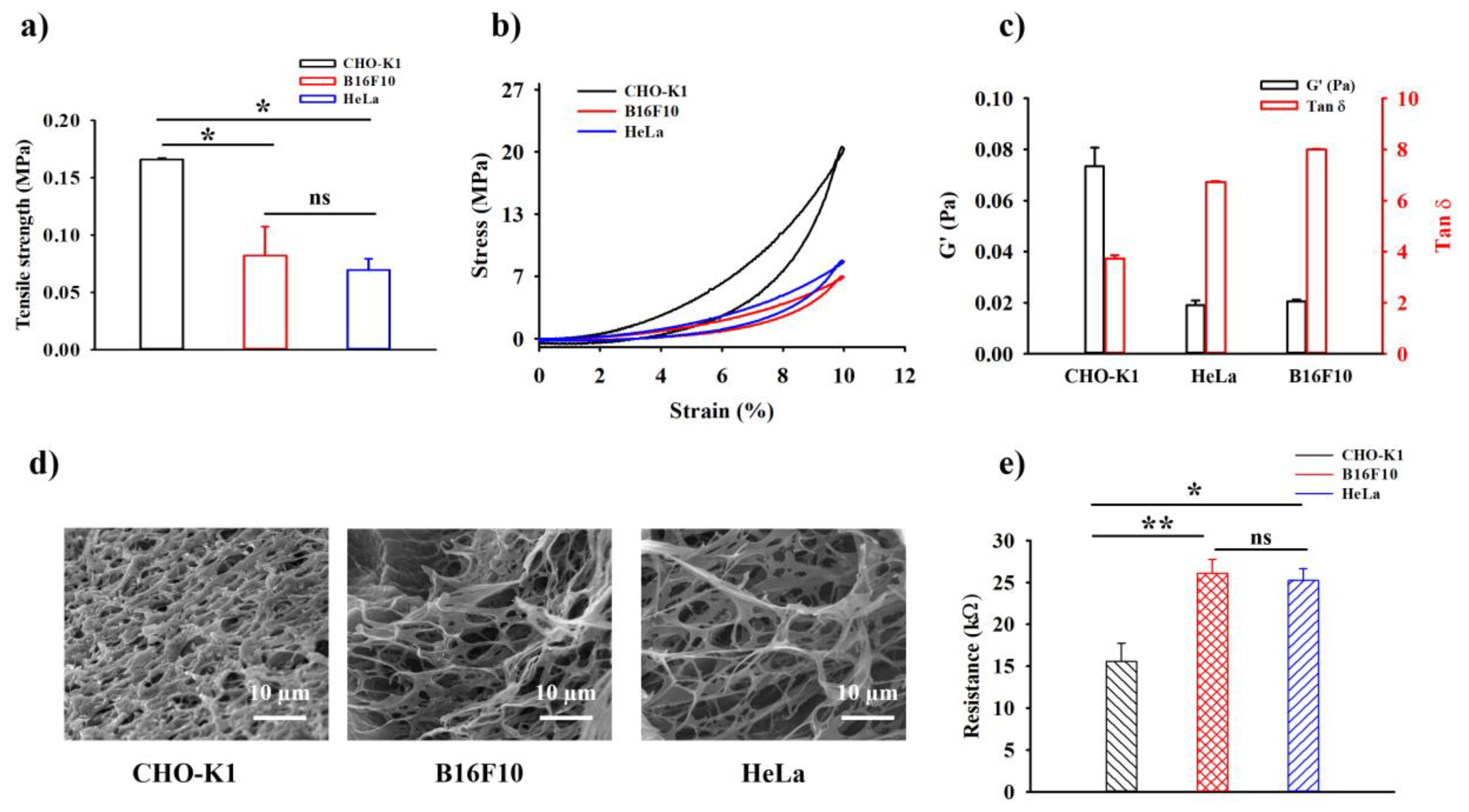
2.4. In Vitro Rheological, Mechanical Compressive Test, and Electrical Analysis of the HDP–PVA Hydrogel
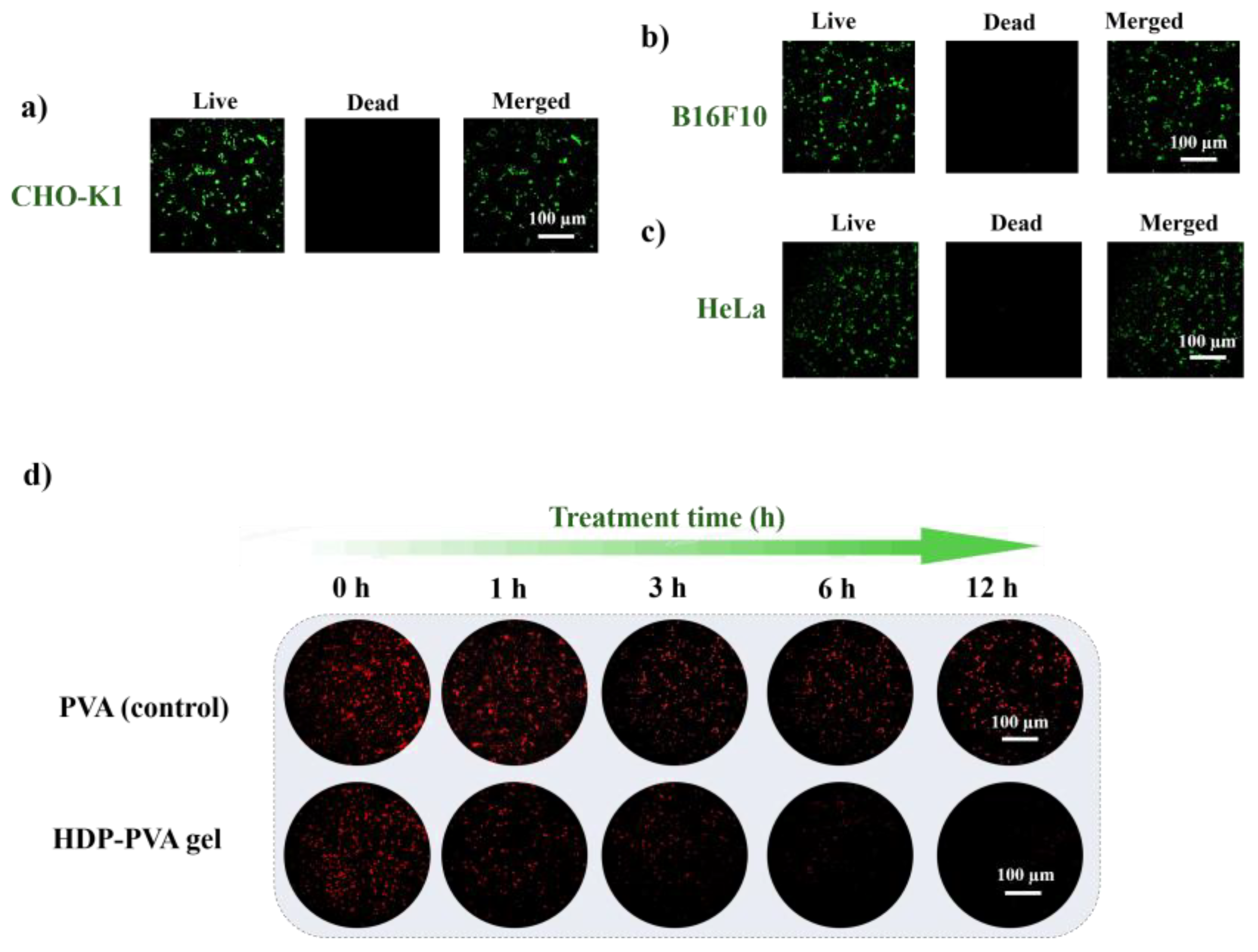
2.5. In Vitro Biocompatibility and ROS-Scavenging Assessment of the HDP–PVA Hydrogel
3. Conclusions
4. Materials and Methods
4.1. Materials and Characterizations
4.2. Fabrication of O2-Responsive HDP–PVA Hydrogel
4.3. Physical and Mechanical Assessment of the HDP–PVA Hydrogel
4.4. Evaluation of Electrochemical and Wireless Analysis of HDP–PVA Hydrogel
4.5. Evaluation of Radical Scavenging Performance of the HDP–PVA Hydrogel
4.6. In Vitro Analysis of the Mechanical and Electrical Properties of HDP–PVA Hydrogel
4.7. In Vitro ROS Scavenging Capability of HDP–PVA Hydrogel
4.8. Live and Dead Assessment of HDP–PVA Hydrogel
4.9. MTT Assay
4.10. RNA Isolation and Quantitative Real-Time (qRT)-PCR
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, P.S.; Chiu, W.T.; Hsu, P.L.; Lin, S.C.; Peng, I.C.; Wang, C.Y.; Tsai, S.J. Pathophysiological Implications of Hypoxia in Human Diseases. J. Biomed. Sci. 2020, 27, 63. [Google Scholar] [CrossRef]
- Khan, B.D.; Nhan, T.N.T.; Chau, H.N.M.; Nhi, N.T.Y. Roles of Hypoxia in Tumor Progression and Novel Strategies for Cancer Treatment. Biomed. Res. Ther. 2022, 9, 5361–5374. [Google Scholar] [CrossRef]
- Lewis, D.M.; Blatchley, M.R.; Park, K.M.; Gerecht, S. O2-Controllable Hydrogels for Studying Cellular Responses to Hypoxic Gradients in Three Dimensions in Vitro and in Vivo. Nat. Protoc. 2017, 12, 1620–1638. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Shi, R.; Zhang, Q. Hypoxia and Oxygen-Sensing Signaling in Gene Regulation and Cancer Progression. Int. J. Mol. Sci. 2020, 21, 8162. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.K.; Go, K.; Lee, E.; Kim, H.; Kim, S.; Kim, J.; Chae, M.S.; Jeong, J. Multifunctional Hydrogels for Advanced Cancer Treatment: Diagnostic Imaging and Therapeutic Modalities. Gels 2025, 11, 426. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Liao, M.; Wang, Y.; Lu, J.R. Advances in Composite Stimuli-Responsive Hydrogels for Wound Healing: Mechanisms and Applications. Gels 2025, 11, 420. [Google Scholar] [CrossRef]
- Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Versatility of Hydrogels: From Synthetic Strategies, Classification, and Properties to Biomedical Applications. Gels 2022, 8, 167. [Google Scholar] [CrossRef]
- Chen, J.; Pan, C.; Gao, Y.; Chen, Q.; An, X.; Liu, Z. Reactive Oxygen Species Scavenging Injectable Hydrogel Potentiates the Therapeutic Potential of Mesenchymal Stem Cells in Skin Flap Regeneration. ACS Appl. Mater. Interfaces 2024, 16, 17120–17128. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Fei, Y.; Zhang, J.; Yue, O.; Wang, X.; Jiang, H. Locally Injectable, ROS-Scavenging, and ROS-/PH-Responsive Polymeric-Micelles-Embedded Hydrogels for Precise Minimally Invasive and Long-Lasting Rheumatoid Therapy. Adv. Healthc. Mater. 2025, 14, 2403579. [Google Scholar] [CrossRef]
- Zhang, J.; Zhong, J.; Liang, B.; Liu, W.; Zhang, H.; Wang, T.; Huang, L.; Yang, L.; Gu, Z.; Li, Y. Robust Injectable Hydrogels for Hemophilic Arthropathy via Anti-Inflammation, Iron Removal and Cartilage Protection. Adv. Funct. Mater. 2025, 2500271, 1–14. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, S.; Tang, Z.; Zhong, Q.; Chen, R.; Wang, P.; Fu, J.; Xie, J.; Ning, Y.; Lei, M.; et al. Injectable, Oxygen-Releasing, Thermosensitive Hydrogel Promotes Vascularized Bone Formation with Prolonged Oxygen Delivery and Improved Osteoinductivity. Mater. Today Bio 2024, 29, 101267. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Roy, K.; Subba, S.H.; Lee, G.; Park, S.Y. MXene/Polymer Dot-Decorated Flexible Sensor for Cancer Cell-Responsive Hydrogel with Tunable Elastic Modulus, Porosity, and Conductivity. Talanta 2025, 281, 126874. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.R.; Kim, J.H. Biodegradable Thermo- and PH-Responsive Hydrogels Based on Amphiphilic Poly-Aspartamide Derivatives Containing N,N-Diisopropylamine Pendants. Macromol. Res. 2008, 16, 489–491. [Google Scholar] [CrossRef]
- Völlmecke, K.; Afroz, R.; Bierbach, S.; Brenker, L.J.; Frücht, S.; Glass, A.; Giebelhaus, R.; Hoppe, A.; Kanemaru, K.; Lazarek, M.; et al. Hydrogel-Based Biosensors. Gels 2022, 8, 768. [Google Scholar] [CrossRef]
- Liu, D.; Huyan, C.; Wang, Z.; Guo, Z.; Zhang, X.; Torun, H.; Mulvihill, D.; Xu, B.; Chen, F. Conductive Polymer Based Hydrogels and Their Application in Wearable Sensors: A Review. Mater. Horiz. 2023, 10, 2800–2823. [Google Scholar] [CrossRef]
- Kougkolos, G.; Golzio, M.; Laudebat, L.; Valdez-Nava, Z.; Flahaut, E. Hydrogels with Electrically Conductive Nanomaterials for Biomedical Applications. J. Mater. Chem. B 2023, 11, 2036–2062. [Google Scholar] [CrossRef]
- Rao, K.M.; Uthappa, U.T.; Kim, H.J.; Han, S.S. Tissue Adhesive, Biocompatible, Antioxidant, and Antibacterial Hydrogels Based on Tannic Acid and Fungal-Derived Carboxymethyl Chitosan for Wound-Dressing Applications. Gels 2023, 9, 354. [Google Scholar] [CrossRef]
- Dong, L.; Jia, R.; Liu, Z.; Aiyiti, W.; Shuai, C.; Li, Z.; Fu, Q.; Li, X. Tannic Acid Based Multifunctional Hydrogels with Mechanical Stability for Wound Healing. Colloids Surf. B Biointerfaces 2024, 243, 114127. [Google Scholar] [CrossRef]
- Kim, T.M.; Won, H.J.; Yang, J.H.; Jo, H.; Kim, A.H.; Nam, D.; Kim, S.G.; Jin, E.J.; Bae, H.J.; Park, S.Y. Multicolor Hair Dyeing with Biocompatible Dark Polyphenol Complex-Integrated Shampoo with Reactive Oxygen Species Scavenging Activity. Biomimetics 2023, 8, 469. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H. Bin Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef]
- Rahman Khan, M.M.; Rumon, M.M.H. Synthesis of PVA-Based Hydrogels for Biomedical Applications: Recent Trends and Advances. Gels 2025, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhong, H.-J.; Ding, H.; Yu, B.; Ma, X.; Liu, X.; Chong, C.-M. Polyvinyl alcohol (PVA)-based hydrogels: Recent progress in fabrication, properties, and multifunctional applications. Polymers 2024, 16, 2755. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Li, C.; Guan, Y.; Zhang, Y.; Zhu, X.X. Enzymatically Crosslinked Alginate Hydrogels with Improved Adhesion Properties. Polym. Chem. 2015, 6, 2204–2213. [Google Scholar] [CrossRef]
- Xia, Q.; Liang, Y.; Cao, A.; Cao, Y.; Cai, L. Preparation and Characterization of PH-Responsive Metal-Polyphenol Structure Coated Nanoparticles. Food Sci. Hum. Wellness 2024, 13, 1303–1310. [Google Scholar] [CrossRef]
- Tian, Z.; Wu, G.; Libby, M.; Wu, K.; Jeong, K.J.; Kim, Y.J. Synthesis of Biologically Derived Poly(Pyrogallol) Nanofibers for Antibacterial Applications. J. Mater. Chem. B 2023, 11, 3356–3363. [Google Scholar] [CrossRef]
- Han, N.; Xu, Z.; Cui, C.; Li, Y.; Zhang, D.; Xiao, M.; Fan, C.; Wu, T.; Yang, J.; Liu, W. A Fe3+-Crosslinked Pyrogallol-Tethered Gelatin Adhesive Hydrogel with Antibacterial Activity for Wound Healing. Biomater. Sci. 2020, 8, 3164–3172. [Google Scholar] [CrossRef]
- Subba, S.H.; Jiang, S.; Jin, E.J.; Park, S.Y. Hypoxia-Sensitive Smart Hydrogel Biosensor for Distinct Mechanical and Electrical Signals with Muscle Ischemia Regeneration. Adv. Funct. Mater. 2025, 2417935, 1–13. [Google Scholar] [CrossRef]
- Shen, J.; Gao, G.; Liu, X.; Fu, J. Natural Polyphenols Enhance Stability of Crosslinked UHMWPE for Joint Implants. Clin. Orthop. Relat. Res. 2015, 473, 760–766. [Google Scholar] [CrossRef]
- Lee, F.; Chung, J.E.; Xu, K.; Kurisawa, M. Injectable Degradation-Resistant Hyaluronic Acid Hydrogels Cross-Linked via the Oxidative Coupling of Green Tea Catechin. ACS Macro Lett. 2015, 4, 957–960. [Google Scholar] [CrossRef]
- Gijutsu, S.; Kenkyujo, S.; Chuo, T.; Technologies, S. Catechol-Functionalized Hydrogels: Biomimetic Design, Adhesion Mechanism, and Biomedical Applications. Chem. Soc. Rev. 2019, 49, 433–464. [Google Scholar]
- An, S.; Jeon, E.J.; Kim, M.; Han, S.Y.; Song, Y.S.; Jeon, J.; Park, J.U.; Cho, S.W. Endomysium-Permeable Muscle Extracellular Matrix Composite Hydrogel for Promoting Functional Muscle Recovery in Muscle Atrophy. Chem. Eng. J. 2024, 485, 149906. [Google Scholar] [CrossRef]
- Shin, M.; Park, E.; Lee, H. Plant-Inspired Pyrogallol-Containing Functional Materials. Adv. Funct. Mater. 2019, 29, 1903022. [Google Scholar] [CrossRef]
- Son, E.J.; Kim, J.H.; Kim, K.; Park, C.B. Quinone and Its Derivatives for Energy Harvesting and Storage Materials. J. Mater. Chem. A 2016, 4, 11179–11202. [Google Scholar] [CrossRef]
- Han, X.; Kong, H.; Chen, T.; Gao, J.; Zhao, Y.; Sang, Y.; Hu, G. Effect of π–π Stacking Interfacial Interaction on the Properties of Graphene/Poly(Styrene-b-Isoprene-b-Styrene) Composites. Nanomaterials 2021, 11, 2158. [Google Scholar] [CrossRef]
- Su, Z.; Wang, H.; Tian, K.; Huang, W.; Xiao, C.; Guo, Y.; He, J.; Tian, X. The Combination of π-π Interaction and Covalent Bonding Can Synergistically Strengthen the Flexible Electrical Insulating Nanocomposites with Well Adhesive Properties and Thermal Conductivity. Compos. Sci. Technol. 2018, 155, 1–10. [Google Scholar] [CrossRef]
- Jo, H.J.; Shit, A.; Jhon, H.S.; Park, S.Y. Highly Sensitive Non-Enzymatic Wireless Glucose Sensor Based on Ni–Co Oxide Nanoneedle-Anchored Polymer Dots. J. Ind. Eng. Chem. 2020, 89, 485–493. [Google Scholar] [CrossRef]
- Lemaitre, L.; Moors, M.; Van Peteghem, A.P. The Estimation of the Charge Transfer Resistance by Graphical Analysis of Inclined Semicircular Complex Impedance Diagrams. J. Appl. Electrochem. 1983, 13, 803–806. [Google Scholar] [CrossRef]
- Laschuk, N.O.; Easton, E.B.; Zenkina, O.V. Reducing the Resistance for the Use of Electrochemical Impedance Spectroscopy Analysis in Materials Chemistry. RSC Adv. 2021, 11, 27925–27936. [Google Scholar] [CrossRef]
- Behboodi-Sadabad, F.; Zhang, H.; Trouillet, V.; Welle, A.; Plumeré, N.; Levkin, P.A. UV-Triggered Polymerization, Deposition, and Patterning of Plant Phenolic Compounds. Adv. Funct. Mater. 2017, 27, 1700127. [Google Scholar] [CrossRef]
- Ball, V. Electrodeposition of Pyrogallol versus Pyrocatechol Using Cyclic Voltammetry and Chronoamperometry. J. Electroanal. Chem. 2022, 909, 116142. [Google Scholar] [CrossRef]
- Astaf’eva, T.V.; Arsenyev, M.V.; Rumyantcev, R.V.; Fukin, G.K.; Cherkasov, V.K.; Poddelsky, A.I. Imine-Based Catechols and o-Benzoquinones: Synthesis, Structure, and Features of Redox Behavior. ACS Omega 2020, 5, 22179–22191. [Google Scholar] [CrossRef]
- Randolph, C.; Lahive, C.W.; Sami, S.; Havenith, R.W.A.; Heeres, H.J.; Deuss, P.J. Biobased Chemicals: 1,2,4-Benzenetriol, Selective Deuteration and Dimerization to Bifunctional Aromatic Compounds. Org. Process. Res. Dev. 2018, 22, 1663–1671. [Google Scholar] [CrossRef]
- Chen, C.; Li, D.; Yano, H.; Abe, K. Bioinspired Hydrogels: Quinone Crosslinking Reaction for Chitin Nanofibers with Enhanced Mechanical Strength via Surface Deacetylation. Carbohydr. Polym. 2019, 207, 411–417. [Google Scholar] [CrossRef]
- Jia, Z.; Gong, J.; Zeng, Y.; Ran, J.; Liu, J.; Wang, K.; Xie, C.; Lu, X.; Wang, J. Bioinspired Conductive Silk Microfiber Integrated Bioelectronic for Diagnosis and Wound Healing in Diabetes. Adv. Funct. Mater. 2021, 31, 2010461. [Google Scholar] [CrossRef]
- Kapiszewska, M.; Soltys, E.; Visioli, F.; Cierniak, A.; Zajac, G. The protective ability of the Mediterranean plant extracts against the oxidative DNA damage. The role of the radical oxygen species and the polyphenol content. J. Physiol. Pharmacol. Suppl. 2005, 56, 183–197. [Google Scholar]
- Luo, N. Editorial: Tumor Microenvironment in Cancer Hallmarks and Therapeutics. Front. Mol. Biosci. 2022, 9, 1019830. [Google Scholar] [CrossRef] [PubMed]
- Petrova, V.; Annicchiarico-Petruzzelli, M.; Melino, G.; Amelio, I. The Hypoxic Tumour Microenvironment. Oncogenesis 2018, 7, 10. [Google Scholar] [CrossRef]
- D’Aiuto, N.; Hochmann, J.; Millán, M.; Di Paolo, A.; Bologna-Molina, R.; Sotelo Silveira, J.; Arocena, M. Hypoxia, Acidification and Oxidative Stress in Cells Cultured at Large Distances from an Oxygen Source. Sci. Rep. 2022, 12, 21699. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The Mtt Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef]
- Caló, E.; Barros, J.; Ballamy, L.; Khutoryanskiy, V.V. Poly(Vinyl Alcohol)-Gantrez® AN Cryogels for Wound Care Applications. RSC Adv. 2016, 6, 105487–105494. [Google Scholar] [CrossRef]
- Scholz, C.C.; Cavadas, M.A.S.; Tambuwala, M.M.; Hams, E.; Rodríguez, J.; Von Kriegsheim, A.; Cotter, P.; Bruning, U.; Fallon, P.G.; Cheong, A.; et al. Regulation of IL-1β-Induced NF-kB by Hydroxylases Links Key Hypoxic and Inflammatory Signaling Pathways. Proc. Natl. Acad. Sci. USA 2013, 110, 18490–18495. [Google Scholar] [CrossRef]
- Xia, Y.; Shen, S.; Verma, I.M. NF-ΚB, an Active Player in Human Cancers. Cancer Immunol. Res. 2014, 2, 823–830. [Google Scholar] [CrossRef]
- Min Kim, T.; Ryplida, B.; Lee, G.; Young Park, S. Cancer Cells Targeting H2O2-Responsive MXene-Integrated Hyaluronic Acid Polymer Dots Coated Sensor. J. Ind. Eng. Chem. 2023, 120, 188–194. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subba, S.H.; Kim, A.H.; Dey, A.; Lee, B.C.; Park, S.Y. O2-Generated Electrical and Mechanical Properties of Polyphenol-Mediated Hydrogel Sensor. Gels 2025, 11, 566. https://doi.org/10.3390/gels11080566
Subba SH, Kim AH, Dey A, Lee BC, Park SY. O2-Generated Electrical and Mechanical Properties of Polyphenol-Mediated Hydrogel Sensor. Gels. 2025; 11(8):566. https://doi.org/10.3390/gels11080566
Chicago/Turabian StyleSubba, Sunu Hangma, A Hyeon Kim, Anneshwa Dey, Byung Chan Lee, and Sung Young Park. 2025. "O2-Generated Electrical and Mechanical Properties of Polyphenol-Mediated Hydrogel Sensor" Gels 11, no. 8: 566. https://doi.org/10.3390/gels11080566
APA StyleSubba, S. H., Kim, A. H., Dey, A., Lee, B. C., & Park, S. Y. (2025). O2-Generated Electrical and Mechanical Properties of Polyphenol-Mediated Hydrogel Sensor. Gels, 11(8), 566. https://doi.org/10.3390/gels11080566






