In Vitro Release and In Vivo Study of Recombinant TGF-β and EGCG from Dual Self-Cross-Linked Alginate-Di-Aldehyde In Situ Injectable Hydrogel for the Repair of a Degenerated Intervertebral Disc in a Rat Tail
Abstract
1. Introduction
2. Results and Discussion
2.1. Oxidation of Sodium Alginate and UV–Vis-Based Determination of Degree of Oxidation
2.2. Gelation Profile and Rheological Evaluation of the Hydrogel Formulation
2.3. In Vitro Degradation Behaviour of the Hydrogel
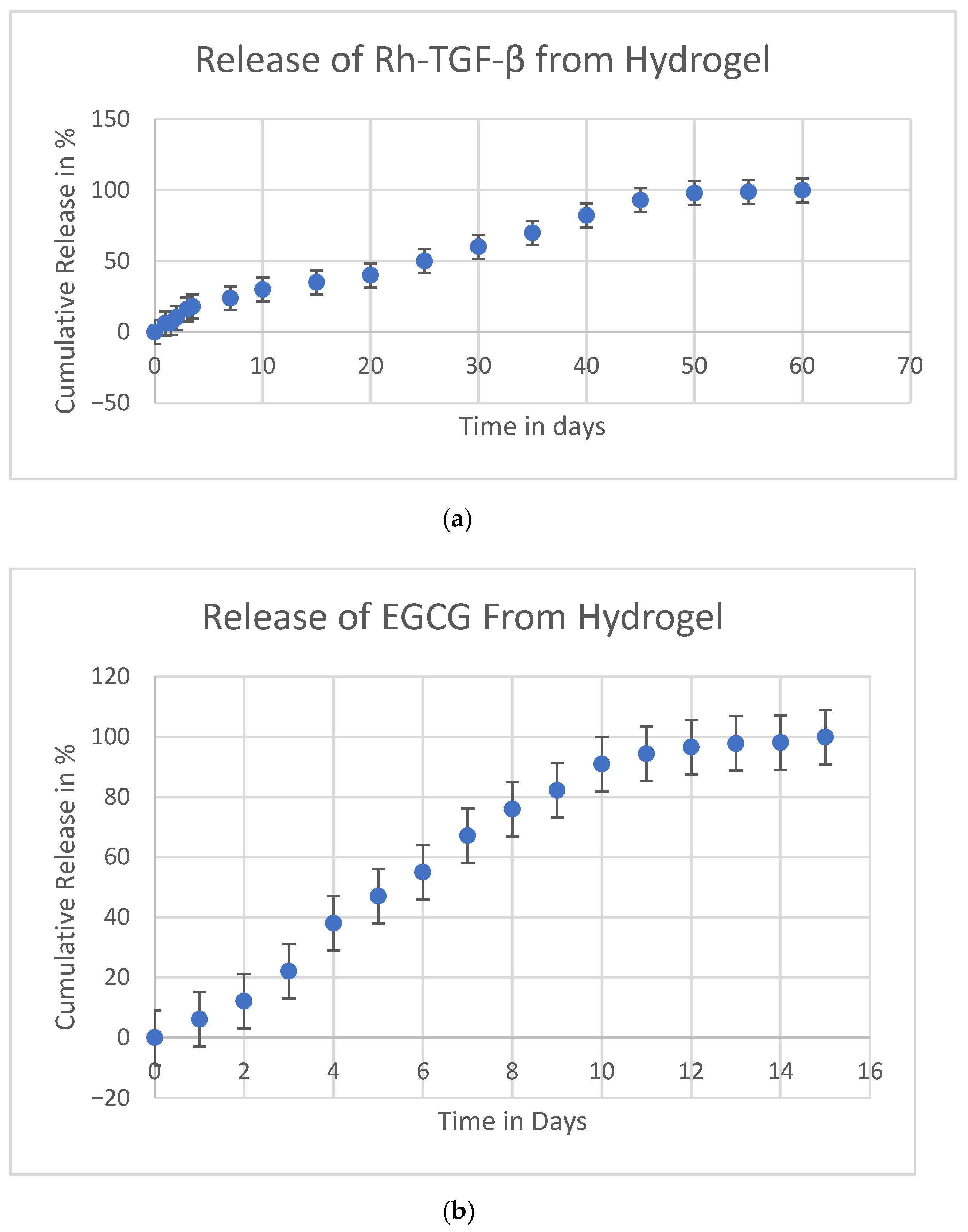
2.4. In Vitro Release of (Rh-TGF-β) Recombinant Transforming Growth Factor-Beta and Epigallocatechin-3-Gallate (EGCG)
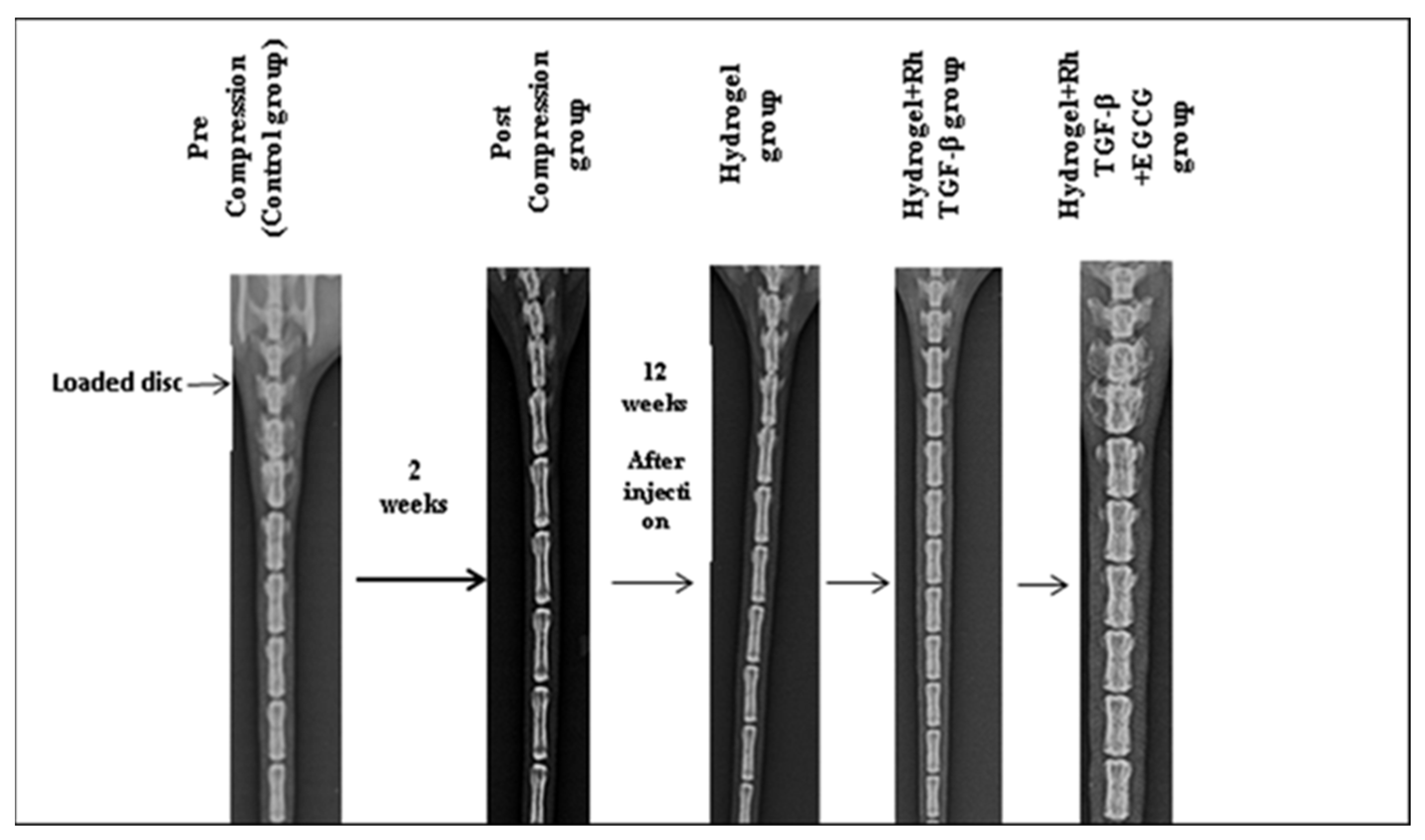
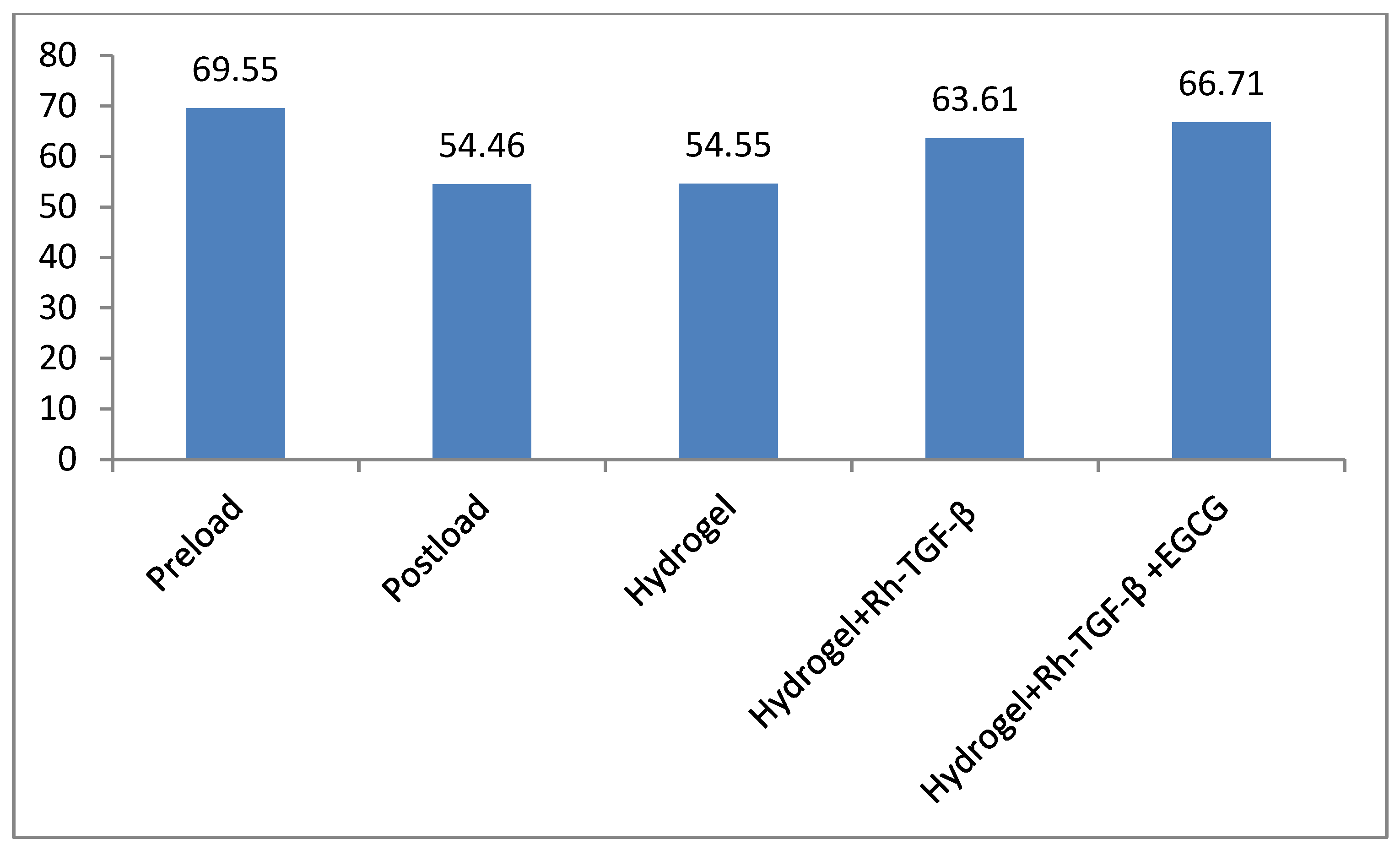
2.5. Radiological Results
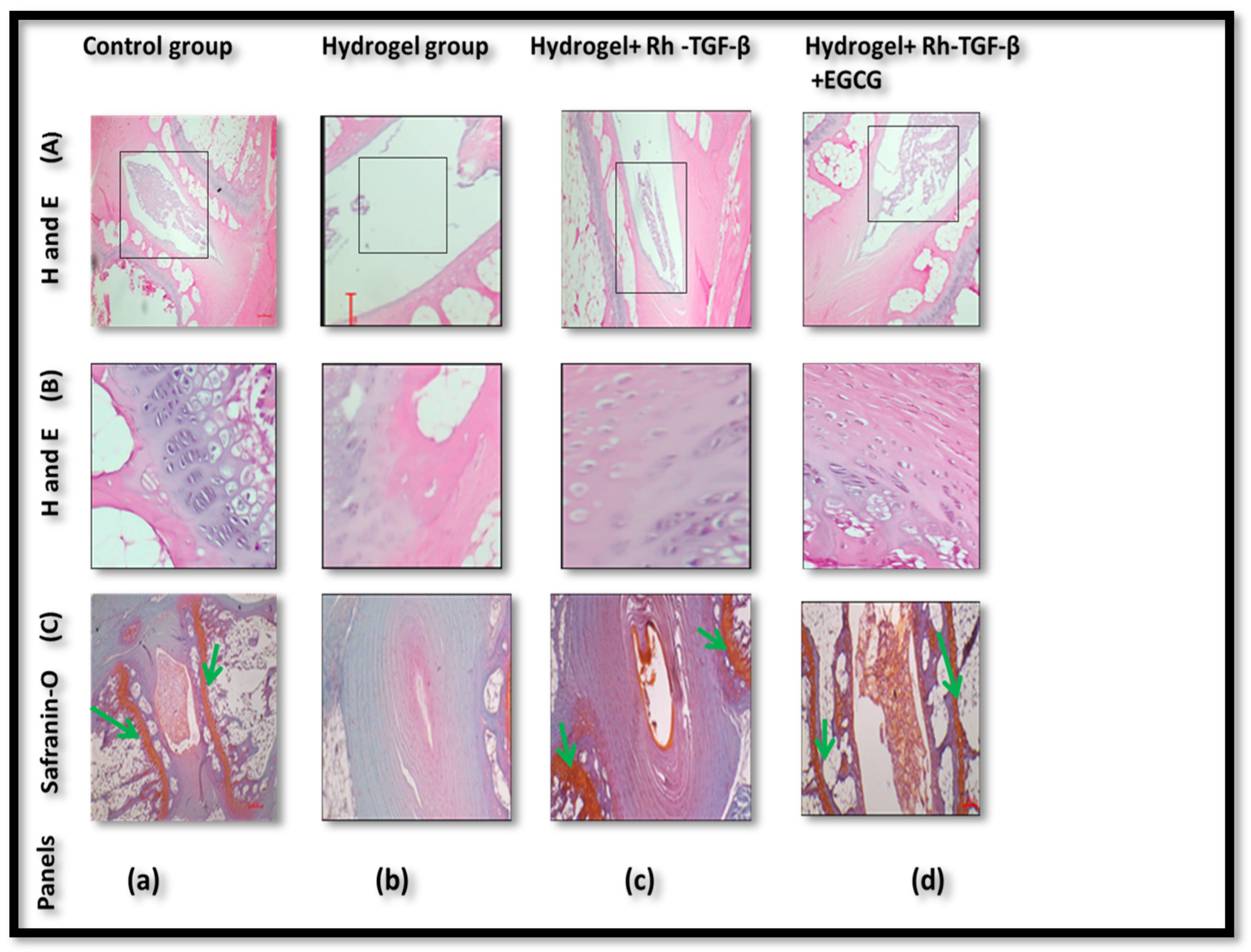
2.6. Histologic Results
2.7. Immunohistochemistry Results
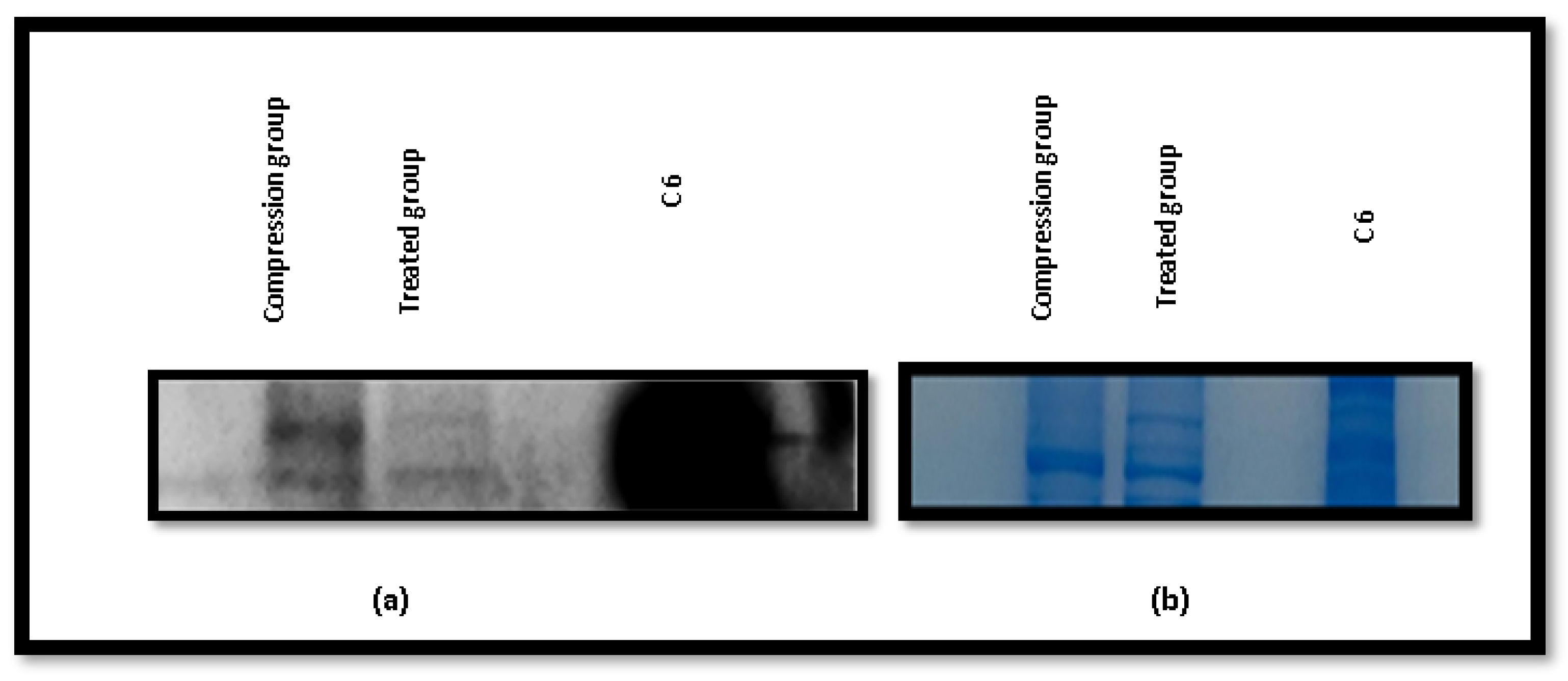
2.8. Western Blotting Results
2.9. Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Animal Care and Use
4.3. Preparation and Determination of Alginate-Di-Aldehyde (ADA)
4.4. Preparation of Injectable Hydrogel
4.5. Gelation Temperature, Gelling Time, and Rheological Measurements
4.6. Degradation
4.7. Preparation of ADA Hydrogel by Incorporating Rh-TGF-β (Recombinant Transforming Growth Factor-β) for In Vitro Release
4.8. Preparation of ADA Hydrogel by Incorporating EGCG (Epigallocatechin-3-Gallate) Injection for In Vitro Release
4.9. In Vivo Study
4.9.1. Disc Degeneration Model
4.9.2. Radiological Analysis
4.9.3. Tissue Processing
4.9.4. Histologic Preparation
4.9.5. Immunohistochemistry (IHC)
4.9.6. Western Blotting
4.9.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADA | Alginate Di-Aldehyde |
| AF | Annulus Fibrosis |
| ANOVA | Analysis of Variance |
| BCA | Bicinchoninic Acid |
| BMPs | Bone Morphogenetic Proteins |
| BSA | Bovine Serum Albumin |
| C7 | Caudal disc-7 vertebra |
| CEP | Cartilage Endplate |
| DAB | 3,3-Diaminobenzidine |
| DI | Deionized |
| DPX | Digital Picture Exchange |
| ECL | Enhanced Chemiluminescence |
| ECM | Extracellular Matrix |
| EDTA | Ethylenediaminetetraacetic Acid |
| EGCG | Epigallocatechin Gallate |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| GF | Growth Factor |
| H&E | Haematoxylin and Eosin |
| H2O2 | Hydrogen Peroxide |
| HSPs | Heat Shock Proteins |
| IDD | Intervertebral Disc Degeneration |
| IgG | Immunoglobulin G |
| IHC | Immunohistochemistry |
| IVD | Intervertebral Disc |
| IVDD | Intervertebral Disc Degeneration |
| L | Litre |
| M | Molar |
| ml | Millilitre |
| mm | Millimetre |
| mM | Millimolar |
| MW | Molecular Weight |
| nm | Nanometre |
| NP and NPCs | Nucleus Pulpous and Nucleus Pulpous cells |
| NP–EP junction | Nucleus Pulpous–End plate Junction |
| PBS | Phosphate-buffered saline |
| PEG | Polyethylene Glycol |
| PVDF | Polyvinylidene Fluoride |
| Rh-TGF-β | Recombinant Transforming Growth Factor-Beta |
| RIPA | Radio-Immunoprecipitation Assay |
| RPM | Rotation Per Minute |
| SDS | Sodium Dodecyl Sulphate |
| SDS PAGE gel | sodium dodecyl sulphate |
| TBST | Tris-buffered saline with Tween 20 |
| TGF-β | Transforming Growth Factor-Β |
| U | Numerical units refer to the grayscale intensity values obtained from the radiographic images, which are measured using image analysis software version 1.53a. These values represent the relative radio density of the intervertebral disc region. |
| UV | Ultraviolet |
| WB | Western Blot |
References
- Yang, F.; Xiao, D.; Zhao, Q.; Chen, Z.; Liu, K.; Chen, S.; Sun, X.; Yue, Q.; Zhang, R.; Feng, G. Fabrication of a Novel Whole Tissue-Engineered Intervertebral Disc for Intervertebral Disc Regeneration in the Porcine Lumbar Spine. RSC Adv. 2018, 8, 39013–39021. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-z.; Chang, Q.; Lu, J.; Wang, C. Growth Factors and Platelet-Rich Plasma: Promising Biological Strategies for Early Intervertebral Disc Degeneration. Int. Orthop. 2015, 39, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Whatley, B.R.; Wen, X. Intervertebral Disc (IVD): Structure, Degeneration, Repair and Regeneration. Mater. Sci. Eng. C 2012, 32, 61–77. [Google Scholar] [CrossRef]
- Tang, G.; Zhou, B.; Li, F.; Wang, W.; Liu, Y.; Wang, X.; Liu, C.; Ye, X. Advances of Naturally Derived and Synthetic Hydrogels for Intervertebral Disk Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 745. [Google Scholar] [CrossRef] [PubMed]
- Risbud, M.V.; Shapiro, I.M. Notochordal Cells in the Adult Intervertebral Disc: New Perspective on an Old Question. Crit. Rev. Eukaryot. Gene Expr. 2011, 21, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Erwin, W.M. Biologically Based Therapy for the Intervertebral Disk: Who Is the Patient? Glob. Spine J. 2013, 3, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Kadow, T.; Sowa, G.; Vo, N.; Kang, J.D. Molecular Basis of Intervertebral Disc Degeneration and Herniations: What Are the Important Translational Questions? Clin. Orthop. Relat. Res. 2015, 473, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K. Biological Repair of the Degenerated Intervertebral Disc by the Injection of Growth Factors. Eur. Spine J. 2008, 17 (Suppl. 4), 441–451. [Google Scholar] [CrossRef] [PubMed]
- Fortier, L.A.; Barker, J.U.; Strauss, E.J.; McCarrel, T.M.; Cole, B.J. The Role of Growth Factors in Cartilage Repair. Clin. Orthop. Relat. Res. 2011, 469, 2706–2715. [Google Scholar] [CrossRef] [PubMed]
- Finnson, K.W.; Chi, Y.; Bou-Gharios, G.; Leask, A.; Philip, A. TGF-b Signaling in Cartilage Homeostasis and Osteoarthritis. Front. Biosci. (Sch. Ed.) 2012, 4, 251–268. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.F.; Ning, B.; Zhang, H.; Wang, D.C.; Hu, Y.L.; Qiao, G.X.; Zhao, Y.P.; Hu, Y.G. Effect of RAAV2-HTGFβ1 Gene Transfer on Matrix Synthesis in an In Vivo Rabbit Disk Degeneration Model. Clin. Spine Surg. 2016, 29, E127–E134. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, T.; Wu, L.; Chen, C.; Jia, Z.; Bai, X.; Ruan, D. Blocking the Function of Inflammatory Cytokines and Mediators by Using IL-10 and TGF-β: A Potential Biological Immunotherapy for Intervertebral Disc Degeneration in a Beagle Model. Int. J. Mol. Sci. 2014, 15, 17270–17283. [Google Scholar] [CrossRef] [PubMed]
- Morales, T.I.; Roberts, A.B. Transforming Growth Factor Beta Regulates the Metabolism of Proteoglycans in Bovine Cartilage Organ Cultures. J. Biol. Chem. 1988, 263, 12828–12831. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, M.J.; Quirk, R.A.; Howdle, S.M.; Shakesheff, K.M. Growth Factor Release from Tissue Engineering Scaffolds. J. Pharm. Pharmacol. 2001, 53, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.; Lu, W.W.; Zhen, W.; Yang, D.; Peng, S. Novel Biomaterial Strategies for Controlled Growth Factor Delivery for Biomedical Applications. NPG Asia Mater. 2017, 9, e435. [Google Scholar] [CrossRef]
- Silva, A.K.A.; Richard, C.; Bessodes, M.; Scherman, D.; Merten, O.W. Growth Factor Delivery Approaches in Hydrogels. Biomacromolecules 2009, 10, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Bahram, M.; Mohseni, N.; Moghtader, M. An Introduction to Hydrogels and Some Recent Applications. In Emerging Concepts in Analysis and Applications of Hydrogels; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Frith, J.E.; Cameron, A.R.; Menzies, D.J.; Ghosh, P.; Whitehead, D.L.; Gronthos, S.; Zannettino, A.C.W.; Cooper-White, J.J. An Injectable Hydrogel Incorporating Mesenchymal Precursor Cells and Pentosan Polysulphate for Intervertebral Disc Regeneration. Biomaterials 2013, 34, 9430–9440. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, Y.; Alimi, M.; Khair, T.; Manolarakis, G.; Berlin, C.; Bonassar, L.J.; Härtl, R. Biological Treatment Approaches for Degenerative Disk Disease: A Literature Review of In Vivo Animal and Clinical Data. Glob. Spine J. 2016, 6, 497–518. [Google Scholar] [CrossRef] [PubMed]
- Tessmar, J.K.; Göpferich, A.M. Matrices and Scaffolds for Protein Delivery in Tissue Engineering. Adv. Drug Deliv. Rev. 2007, 59, 274–291. [Google Scholar] [CrossRef] [PubMed]
- Georgopoulos, C.; Welch, W.J. Role of the Major Heat Shock Proteins as Molecular Chaperones. Annu. Rev. Cell Biol. 1993, 9, 601–634. [Google Scholar] [CrossRef] [PubMed]
- Chooi, W.H.; Chan, S.C.W.; Gantenbein, B.; Chan, B.P. Correction: Loading-Induced Heat-Shock Response in Bovine Intervertebral Disc Organ Culture. PLoS ONE 2016, 11, e0161615. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhang, S.; Liu, W.; Wang, P.; Chen, S.; Lv, X.; Shi, D.; Ma, K.; Wang, B.; Wu, Y.; et al. Inhibiting Heat Shock Protein 90 Protects Nucleus Pulposus-Derived Stem/Progenitor Cells From Compression-Induced Necroptosis and Apoptosis. Front. Cell Dev. Biol. 2020, 8, 685. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Kubo, T.; Goomer, R.S.; Amiel, D.; Kobayashi, K.; Imanishi, J.; Teshima, R.; Hirasawa, Y. Analysis of Heat Shock Proteins and Cytokines Expressed during Early Stages of Osteoarthritis in a Mouse Model. Osteoarthr. Cartil. 1997, 5, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Amolins, M.W.; Blagg, B.S.J. Natural Product Inhibitors of Hsp90: Potential Leads for Drug Discovery. Mini Rev. Med. Chem. 2009, 9, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Begum, B.; Koduru, T.S.; Madni, S.N.; Fathima Anjum, N.; Seetharaman, S.; Veeranna, B.; Gupta, V.K. Dual-Self-Crosslinking Effect of Alginate-Di-Aldehyde with Natural and Synthetic Co-Polymers as Injectable In Situ-Forming Biodegradable Hydrogel. Gels 2024, 10, 649. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Zheng, Y.; Ma, T.; Xia, B.; Gao, X.; Hao, Y.; Luo, Z.; Huang, J. (-)-Epigallocatechin-3-Gallate Ameliorates Intervertebral Disc Degeneration Through Reprogramming of the Circadian Clock. Front. Pharmacol. 2021, 12, 753548. [Google Scholar] [CrossRef] [PubMed]
- Freeman, I.; Cohen, S. The Influence of the Sequential Delivery of Angiogenic Factors from Affinity-Binding Alginate Scaffolds on Vascularization. Biomaterials 2009, 30, 2122–2131. [Google Scholar] [CrossRef] [PubMed]
- Krupkova, O.; Handa, J.; Hlavna, M.; Klasen, J.; Ospelt, C.; Ferguson, S.J.; Wuertz-Kozak, K. The Natural Polyphenol Epigallocatechin Gallate Protects Intervertebral Disc Cells from Oxidative Stress. Oxidative Med. Cell. Longev. 2016, 2016, 7031397. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Jayakrishnan, A. Self-Cross-Linking Biopolymers as Injectable in Situ Forming Biodegradable Scaffolds. Biomaterials 2005, 26, 3941–3951. [Google Scholar] [CrossRef] [PubMed]
- Bakker, A.C.; Van De Loo, F.A.; Van Beuningen, H.M.; Sime, P.; Van Lent, P.L.; Van Der Kraan, P.M.; Richards, C.D.; Van Den Berg, W.B. Overexpression of active TGF-beta-1 in the murine knee joint: Evidence for synovial-layer-dependent chondro-osteophyte formation. Osteoarthr. Cartil. 2001, 2, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.J.L.; Bradford, D.S.; Lotz, J.C. In Vivo Growth Factor Treatment of Degenerated Intervertebral Discs. Spine 2004, 29, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, P.; Hu, B.; Liu, W.; Lv, X.; Chen, S.; Shao, Z. HSP90 Inhibitor 17-AAG Attenuates Nucleus Pulposus Inflammation and Catabolism Induced by M1-Polarized Macrophages. Front. Cell Dev. Biol. 2022, 9, 796974. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Diao, L.; Lu, Y.; Liu, C.; Li, J.; Chen, Y.; Chen, J.; Lv, G.; Chen, X. Heparin-Based Sericin Hydrogel-Encapsulated Basic Fibroblast Growth Factor for in Vitro and in Vivo Skin Repair. Heliyon 2023, 9, e13554. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, M.Y.; Lin, A.C.; Liao, W.H.; Wang, T.G.; Hsu, C.H.; Chen, W.S.; Lin, F.H. Drug-Loaded Hyaluronic Acid Hydrogel as a Sustained-Release Regimen with Dual Effects in Early Intervention of Tendinopathy. Sci. Rep. 2019, 9, 4784. [Google Scholar] [CrossRef] [PubMed]
- Grivas, T.B.; Vasiliadis, E.S.; Kaspiris, A.; Khaldi, L.; Kletsas, D. Expression of Matrix Metalloproteinase-1 (MMP-1) in Wistar Rat’s Intervertebral Disc after Experimentally Induced Scoliotic Deformity. Scoliosis 2011, 6, 9. [Google Scholar] [CrossRef] [PubMed]
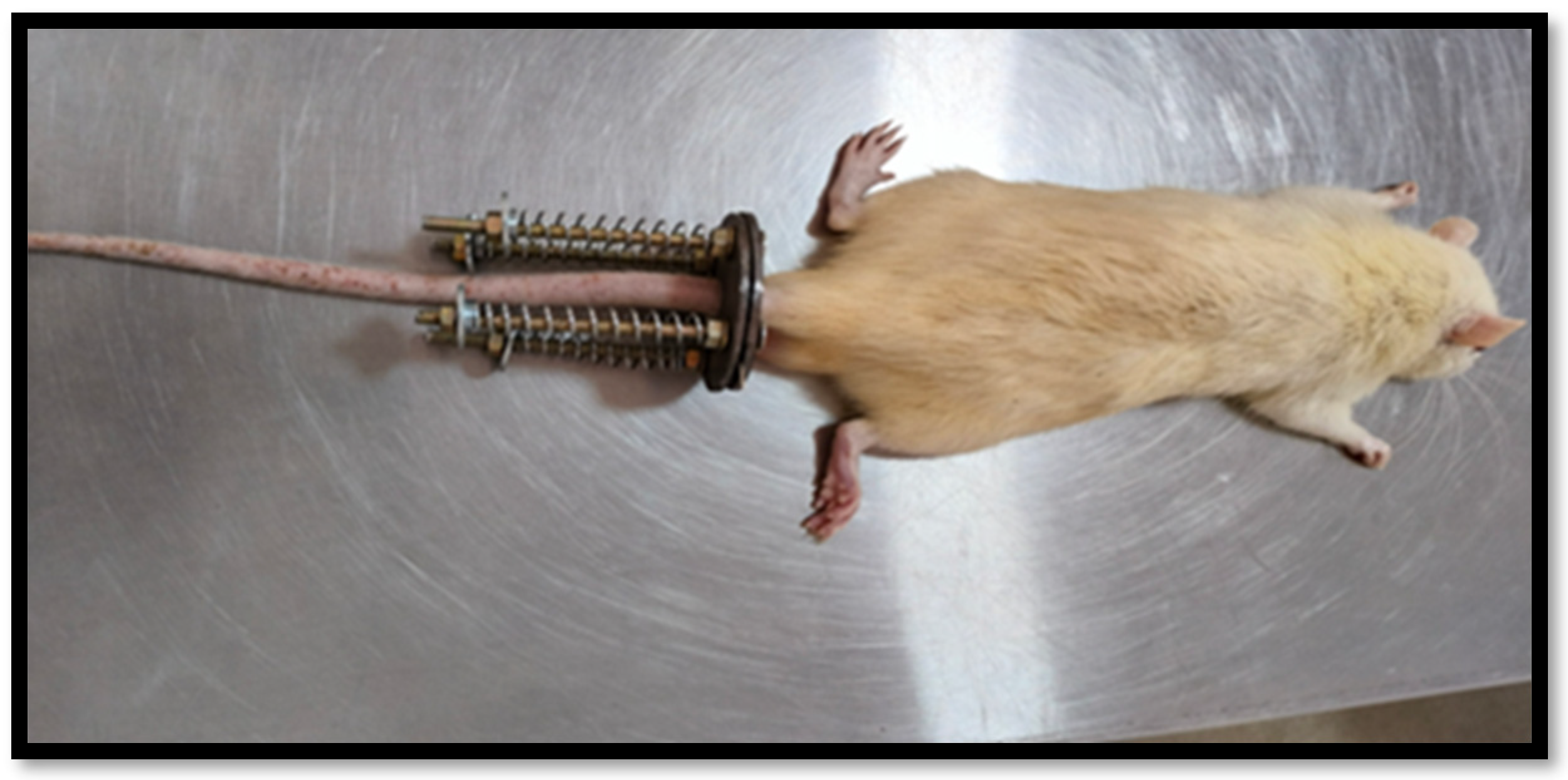
- Hirata, H.; Yurube, T.; Kakutani, K.; Maeno, K.; Takada, T.; Yamamoto, J.; Kurakawa, T.; Akisue, T.; Kuroda, R.; Kurosaka, M.; et al. A rat tail temporary static compression model reproduces different stages of intervertebral disc degeneration with decreased notochordal cell phenotype. J. Orthop. Res. 2014, 32, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Chujo, T.; An, H.S.; Akeda, K.; Miyamoto, K.; Muehleman, C.; Attawia, M.; Andersson, G.; Masuda, K. Effects of Growth Differentiation Factor-5 on the Intervertebral Disc--in Vitro Bovine Study and in Vivo Rabbit Disc Degeneration Model Study. Spine 2006, 31, 2909–2917. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Nan, L.-P.; Zhou, S.-F.; Liu, Y.; Wang, Z.-Y.; Wang, J.-C.; Feng, X.-M.; Zhang, L. Injectable Hydrogel Combined with Nucleus Pulposus-Derived Mesenchymal Stem Cells for the Treatment of Degenerative Intervertebral Disc in Rats. Stem Cells Int. 2019, 2019, 8496025. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Aota, Y.; Muehleman, C.; Imai, Y.; Okuma, M.; Thonar, E.J.; Andersson, G.B.; An, H.S. A Novel Rabbit Model of Mild, Reproducible Disc Degeneration by an Anulus Needle Puncture: Correlation between the Degree of Disc Injury and Radiological and Histological Appearances of Disc Degeneration. Spine 2005, 30, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Siebelt, M.; Jahr, H.; Groen, H.C.; Sandker, M.; Waarsing, J.H.; Kops, N.; Müller, C.; Van Eden, W.; De Jong, M.; Weinans, H. Hsp90 Inhibition Protects Against Biomechanically Induced Osteoarthritis in Rats. Arthritis Rheum. 2013, 65, 2102–2112. [Google Scholar] [CrossRef] [PubMed]
- Madhunapantula, S.R.V.; Sharma, A.; Robertson, G.P. PRAS40 Deregulates Apoptosis in Malignant Melanoma. Cancer Res. 2007, 67, 3626–3636. [Google Scholar] [CrossRef] [PubMed]

| Group | Loading Protocol | Vertebral Body (Caudal Vertebrae) | No. of Animals | |
|---|---|---|---|---|
| Control (Group-1) | Normal condition | Healthy | 3 | |
| Static Load Compression (Group-2) | Instrumented with rings, rods, and calibrated springs to immobilize and apply axial forces to the instrumented discs | C7 | 21 | |
| Post compression | 3 | |||
| Injectable Treated Subgroups unloaded | Subgroups | Dose | A total of 18 animals were selected from the group of 21. | |
| (1) Hydrogel | 15 µL hydrogel | C7 | 6 | |
| (2) Hydrogel + Recombinant TGF-β | 15 µL hydrogel + 500 ng Rh TGF-β | 6 | ||
| (3) Hydrogel + Recombinant TGF-β + EGCG | 15 µL hydrogel + 500 ng Rh TGF-β + 50 µM EGCG | 6 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Begum, B.; Mudhol, S.; Begum, B.; Madni, S.N.; Honganoor Padmanabha, S.; Ahmed, V.A.; Gupta, N.V. In Vitro Release and In Vivo Study of Recombinant TGF-β and EGCG from Dual Self-Cross-Linked Alginate-Di-Aldehyde In Situ Injectable Hydrogel for the Repair of a Degenerated Intervertebral Disc in a Rat Tail. Gels 2025, 11, 565. https://doi.org/10.3390/gels11080565
Begum B, Mudhol S, Begum B, Madni SN, Honganoor Padmanabha S, Ahmed VA, Gupta NV. In Vitro Release and In Vivo Study of Recombinant TGF-β and EGCG from Dual Self-Cross-Linked Alginate-Di-Aldehyde In Situ Injectable Hydrogel for the Repair of a Degenerated Intervertebral Disc in a Rat Tail. Gels. 2025; 11(8):565. https://doi.org/10.3390/gels11080565
Chicago/Turabian StyleBegum, Bushra, Seema Mudhol, Baseera Begum, Syeda Noor Madni, Sharath Honganoor Padmanabha, Vazir Ashfaq Ahmed, and N. Vishal Gupta. 2025. "In Vitro Release and In Vivo Study of Recombinant TGF-β and EGCG from Dual Self-Cross-Linked Alginate-Di-Aldehyde In Situ Injectable Hydrogel for the Repair of a Degenerated Intervertebral Disc in a Rat Tail" Gels 11, no. 8: 565. https://doi.org/10.3390/gels11080565
APA StyleBegum, B., Mudhol, S., Begum, B., Madni, S. N., Honganoor Padmanabha, S., Ahmed, V. A., & Gupta, N. V. (2025). In Vitro Release and In Vivo Study of Recombinant TGF-β and EGCG from Dual Self-Cross-Linked Alginate-Di-Aldehyde In Situ Injectable Hydrogel for the Repair of a Degenerated Intervertebral Disc in a Rat Tail. Gels, 11(8), 565. https://doi.org/10.3390/gels11080565







