Recent Evidence for Orthobiologics Combined with Hydrogels for Joint Tissue Regeneration: Focus on Osteoarthritis
Abstract
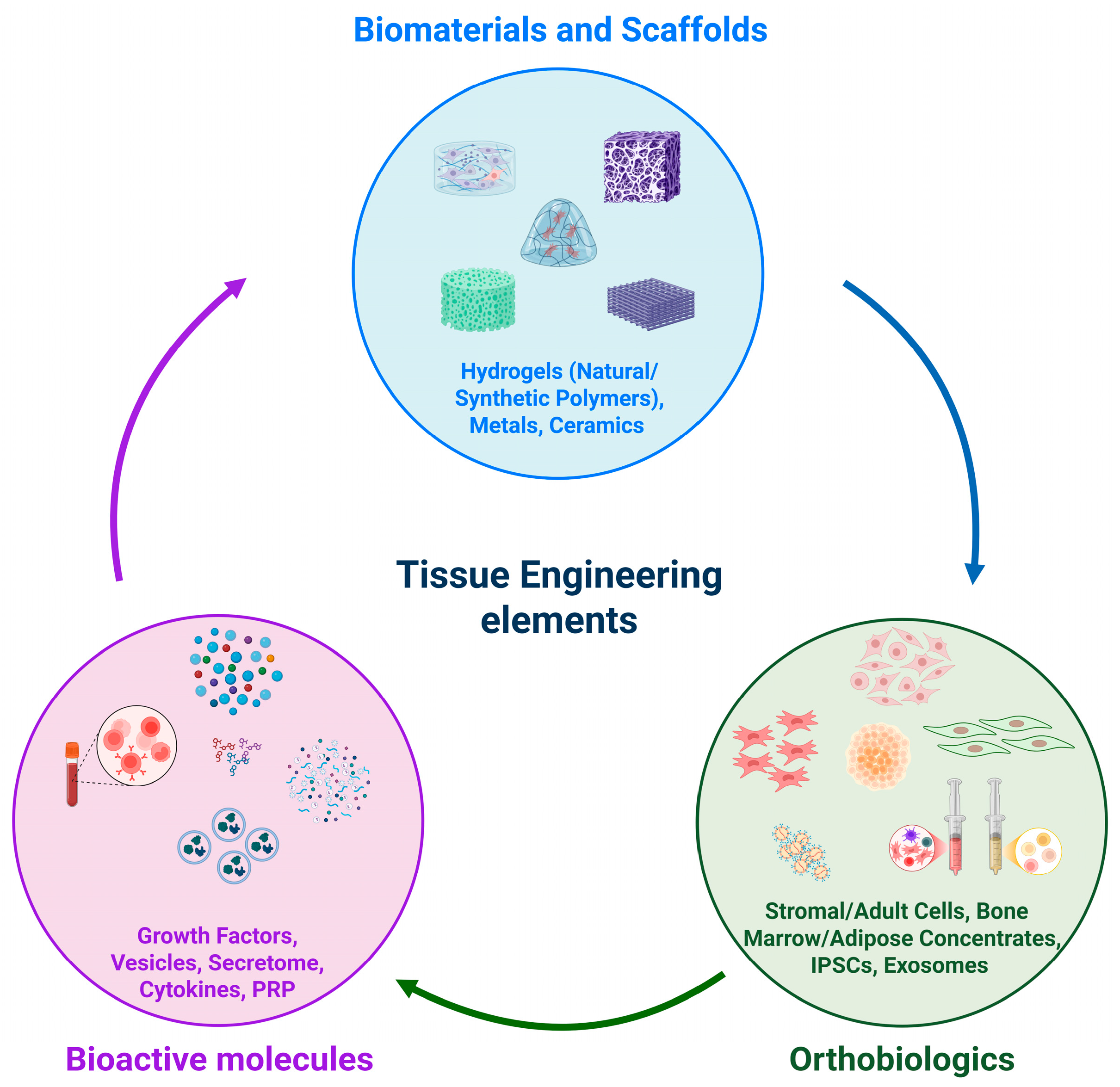
1. Introduction
2. Orthobiologics
| Biomarkers | Type | Clinical Significance | Ref. |
|---|---|---|---|
| CD44, CD151, CD105, CD271 | Surface chondrogenic markers | Present on the surface of cells, help to identify cells with chondrogenic potential | [19] |
| SOX-6, SOX-9 | Intracellular chondrogenic markers | Transcription factors that play key roles in cartilage development | [20] |
| Collagen type II, proteoglycans | Secreted chondrogenic markers | Principal components of hyaline cartilage | [19] |
| Procollagen type II C-terminal propeptide (PIICP) | Secreted chondrogenic marker | A biomarker of collagen type II synthesis released when cartilage cells produce new collagen | [19,21] |
| COMP (Cartilage oligomeric matrix protein) | Cartilage metabolism | A marker of cartilage synthesis and degradation, reflecting overall cartilage health | [19] |
| Alkaline Phosphatase, Collagen Type I, Bone Sialoprotein | Early osteogenic markers | Osteoblast differentiation and activity | [22] |
| Osterix, Osteocalcin, Osteopontin | Late osteogenic markers | Late marker of bone formation, associated with mineralization and bone remodeling | [22] |
| Runx-2, TGF-β, BMP (Bone Morphogenetic protein) | Osteogenic markers | Signaling molecules that induce osteogenic differentiation | [19] |
| VEGF (Vascular endothelial growth factor) | Cell growth, differentiation | Important for promoting angiogenesis and tissue repair | [23] |
| Procollagen Type I N-terminal Propeptide (P1NP) | Bone formation | A peptide released during collagen synthesis by osteoblasts, indicating bone formation | [22] |
| hs-CRP (High-sensitivity C-reactive protein) | Inflammation | Elevated levels are associated with inflammation and can predict cartilage loss in OA | [24] |
| IL-6, TNF-alpha | Inflammation | Cytokines that play a role in inflammation and can be measured in synovial fluid or serum of OA patients | [25] |
| Chemokines (MCPs, MIP-1α, RANTES, Eotaxins and their receptors) | Inflammation | Molecules involved in the OA pathogenesis | [26] |
3. Hydrogels
3.1. Properties of Hydrogels
3.1.1. Biodegradability
3.1.2. Biocompatibility
3.1.3. Controlled Drug Delivery Rate
3.1.4. Thermoplasticity and Viscoelasticity
3.1.5. Non-Toxic By-Products
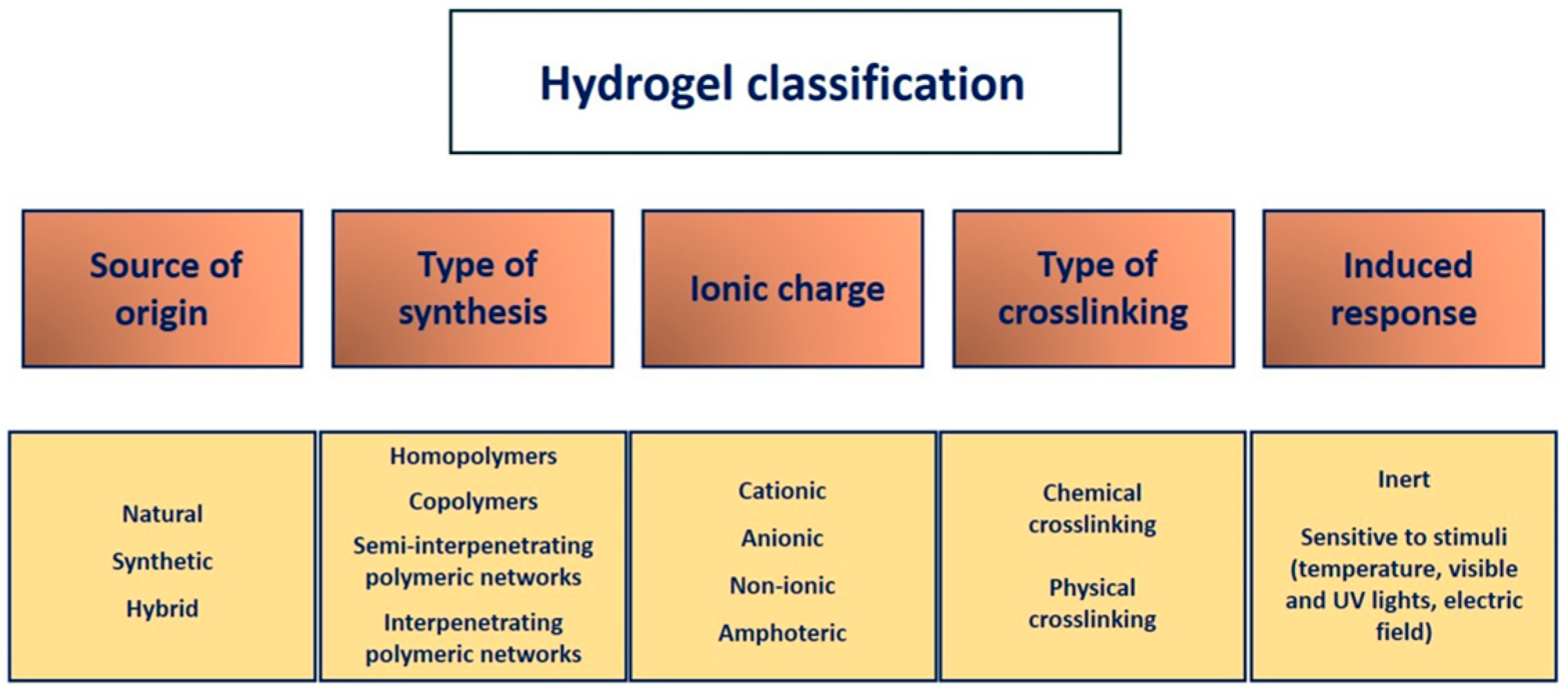
3.2. Classification of Hydrogels
3.2.1. Source of Origin
3.2.2. Type of Synthesis
3.2.3. Type of Crosslinking
3.2.4. Ionic Charge
3.2.5. Induced Response
4. Hydrogels for OA Treatment
4.1. Expanded Cells Combined with Hydrogels for OA Treatment
4.2. Concentrated Cells Combined with Hydrogels for OA Treatment
4.3. MSCs-Derived Molecules Combined with Hydrogels for OA Treatment
4.4. Exosomes Combined with Hydrogels for OA Treatment
4.5. Growth Factors Combined with Hydrogels for OA Treatment
Platelet-Rich Plasma
5. 3D/4D Bioprinting
6. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.J. Osteoarthritis: Toward a Comprehensive Understanding of Pathological Mechanism. Bone Res. 2017, 5, 16044. [Google Scholar] [CrossRef] [PubMed]
- Rezuș, E.; Cardoneanu, A.; Burlui, A.; Luca, A.; Codreanu, C.; Tamba, B.I.; Stanciu, G.D.; Dima, N.; Bădescu, C.; Rezuș, C. The Link between Inflammaging and Degenerative Joint Diseases. Int. J. Mol. Sci. 2019, 20, 614. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F. Aging and Osteoarthritis: The Role of Chondrocyte Senescence and Aging Changes in the Cartilage Matrix. Osteoarthr. Cartil. 2009, 17, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A Disease of the Joint as an Organ. Arthritis Rheumatol. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Goldring, S.R. Osteoarthritis. J. Cell. Physiol. 2007, 213, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-Grade Inflammation as a Key Mediator of the Pathogenesis of Osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Anandacoomarasamy, A.; March, L. Current Evidence for Osteoarthritis Treatments. Ther. Adv. Musculoskelet. Dis. 2010, 2, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Luyten, F.P.; Vanlauwe, J. Tissue Engineering Approaches for Osteoarthritis. Bone 2012, 51, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Sahin, N.; Yesil, H. Regenerative Methods in Osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2023, 37, 101824. [Google Scholar] [CrossRef] [PubMed]
- Paul, W.; Sharma, C.P. Tissue and Organ Regeneration: An Introduction. In Regenerated Organs: Future Perspectives; Elsevier: Amsterdam, The Netherlands, 2021; pp. 3–9. ISBN 9780128210857. [Google Scholar]
- Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Unlike Bone, Cartilage Regeneration Remains Elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.K.; Soker, S.; Atala, A. Tissue Engineering: Current Status and Future Perspectives. In Principles of Tissue Engineering; Academic Press: Cambridge, MA, USA, 2020; pp. 1–35. [Google Scholar] [CrossRef]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the Potential of Hydrogels for Advanced Therapeutic Applications: Current Achievements and Future Directions. Signal Transduct. Target. Ther. 2024, 9, 166. [Google Scholar] [CrossRef] [PubMed]
- Mavrogenis, A.F.; Karampikas, V.; Zikopoulos, A.; Sioutis, S.; Mastrokalos, D.; Koulalis, D.; Scarlat, M.M.; Hernigou, P. Orthobiologics: A Review. Int. Orthop. 2023, 47, 1645–1662. [Google Scholar] [CrossRef] [PubMed]
- Trapana, J.; Weinerman, J.; Lee, D.; Sedani, A.; Constantinescu, D.; Best, T.M.; Hornicek, F.J.; Hare, J.M. Cell-Based Therapy in the Treatment of Musculoskeletal Diseases. Stem Cells Transl. Med. 2024, 13, 959–978. [Google Scholar] [CrossRef] [PubMed]
- Vinatier, C.; Guicheux, J. Cartilage Tissue Engineering: From Biomaterials and Stem Cells to Osteoarthritis Treatments. Ann. Phys. Rehabil. Med. 2016, 59, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Rodeo, S.A. Orthobiologics: Current Status in 2023 and Future Outlook. J. Am. Acad. Orthop. Surg. 2023, 31, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Fernandez, P.; Rodríguez-Nogales, C.; Jordan, O.; Allémann, E. Combination of Mesenchymal Stem Cells and Bioactive Molecules in Hydrogels for Osteoarthritis Treatment. Eur. J. Pharm. Biopharm. 2022, 172, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Hissnauer, T.N.; Baranowsky, A.; Pestka, J.M.; Streichert, T.; Wiegandt, K.; Goepfert, C.; Beil, F.T.; Albers, J.; Schulze, J.; Ueblacker, P.; et al. Identification of Molecular Markers for Articular Cartilage. Osteoarthr. Cartil. 2010, 18, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- Baran, K.; Brzeziańska-Lasota, E.; Kryczka, J.; Boncela, J.; Czechowska, A.; Kopacz, K.; Padula, G.; Nowak, K.; Domżalski, M. The Expression Level of SOX Family Transcription Factors’ MRNA as a Diagnostic Marker for Osteoarthritis. J. Clin. Med. 2025, 14, 1176. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Korntner, S.H.; Mullen, A.M.; Zeugolis, D.I. Collagen Type II: From Biosynthesis to Advanced Biomaterials for Cartilage Engineering. Biomater. Biosyst. 2021, 4, 100030. [Google Scholar] [CrossRef] [PubMed]
- Schini, M.; Vilaca, T.; Gossiel, F.; Salam, S.; Eastell, R. Bone Turnover Markers: Basic Biology to Clinical Applications. Endocr. Rev. 2023, 44, 417–473. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Olsen, B.R. The Roles of Vascular Endothelial Growth Factor in Bone Repair and Regeneration. Bone 2016, 91, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Daghestani, H.N.; Kraus, V.B. Inflammatory Biomarkers in Osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1890–1896. [Google Scholar] [CrossRef] [PubMed]
- Coleman, L.J.; Byrne, J.L.; Edwards, S.; O’Hara, R. Evaluating Interleukin-6, Tumour Necrosis Factor Alpha, and Myeloperoxidase as Biomarkers in Severe Osteoarthritis Patients: A Biostatistical Perspective. LabMed 2025, 2, 8. [Google Scholar] [CrossRef]
- Scanzello, C.R. Chemokines and Inflammation in Osteoarthritis: Insights from Patients and Animal Models. J. Orthop. Res. 2017, 35, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Wichterle, O.; Lím, D. Hydrophilic Gels for Biological Use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical Applications of Hydrogels: A Review of Patents and Commercial Products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Ullah, A.; Ullah, K.; Rehman, N.U. Insight into Hydrogels. Des. Monomers Polym. 2016, 19, 456–478. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magaña, H.; Bucio, E. Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef] [PubMed]
- Ganji, F.; Farahani, S.V.; Vasheghani-Farahani, E.; Vasheghani-Farahani, S. Theoretical Description of Hydrogel Swelling: A Review. Iran. Polym. J. 2010, 19, 375–398. [Google Scholar]
- Mehta, P.; Sharma, M.; Devi, M. Hydrogels: An Overview of Its Classifications, Properties, and Applications. J. Mech. Behav. Biomed. Mater. 2023, 147, 106145. [Google Scholar] [CrossRef] [PubMed]
- Visan, A.I.; Negut, I. Development and Applications of PLGA Hydrogels for Sustained Delivery of Therapeutic Agents. Gels 2024, 10, 497. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K.; Jung, E.; Priya, S.; Won, S.Y.; Han, S.S. Recent Advances in Biopolymer-Based Hydrogels and Their Potential Biomedical Applications. Carbohydr. Polym. 2024, 323, 121408. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Ajazuddin; Khan, J.; Saraf, S.; Saraf, S. Polyethylene Glycol (PEG)-Poly(N-Isopropylacrylamide) (PNIPAAm) Based Thermosensitive Injectable Hydrogels for Biomedical Applications. Eur. J. Pharm. Biopharm. 2014, 88, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Blanco-Fernandez, B.; Puga, A.M.; Concheiro, A. Crosslinked Ionic Polysaccharides for Stimuli-Sensitive Drug Delivery. Adv. Drug Deliv. Rev. 2013, 65, 1148–1171. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ma, X.; Liu, J.; Zhang, H.; Fu, D. Advances in the Application of Natural/Synthetic Hybrid Hydrogels in Tissue Engineering and Delivery Systems: A Comprehensive Review. Int. J. Pharm. 2025, 672, 125323. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Chen, G. Research Progress of Natural Tissue-Derived Hydrogels for Tissue Repair and Reconstruction. Int. J. Biol. Macromol. 2022, 214, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhao, H.; Li, Y.; Lee, A.L.; Li, Z.; Fu, M.; Li, C.; Yang, Y.Y.; Yuan, P. Synthetic Peptide Hydrogels as 3D Scaffolds for Tissue Engineering. Adv. Drug Deliv. Rev. 2020, 160, 78–104. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.M.; De la Hoz Siegler, H. Evolution of Hybrid Hydrogels: Next-Generation Biomaterials for Drug Delivery and Tissue Engineering. Gels 2024, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Chelu, M.; Musuc, A.M. Polymer Gels: Classification and Recent Developments in Biomedical Applications. Gels 2023, 9, 161. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.A.; Aslam, M.A.; Abdullah, M.F.B.; Al-Arjan, W.S.; Stojanovic, G.M.; Hasan, A. Hydrogels: Classifications, Fundamental Properties, Applications, and Scopes in Recent Advances in Tissue Engineering and Regenerative Medicine—A Comprehensive Review. Arab. J. Chem. 2024, 17, 105968. [Google Scholar] [CrossRef]
- Kumi, M.; Ejeromedoghene, O.; Sudane, W.D.; Zhang, Z. Unlocking the Biological Response of Smart Stimuli-Responsive Hydrogels and Their Application in Biological Systems. Eur. Polym. J. 2024, 209, 112906. [Google Scholar] [CrossRef]
- Gan, X.; Wang, X.; Huang, Y.; Li, G.; Kang, H. Applications of Hydrogels in Osteoarthritis Treatment. Biomedicines 2024, 12, 923. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, J.Y.; Meng, T.; Ma, X.W.; Li, H.; Li, K. Role of Hydrogels in Osteoarthritis: A Comprehensive Review. Int. J. Rheum. Dis. 2023, 26, 2390–2401. [Google Scholar] [CrossRef] [PubMed]
- Ferkel, E.; Manjoo, A.; Martins, D.; Bhandari, M.; Sethi, P.; Nicholls, M. Intra-Articular Hyaluronic Acid Treatments for Knee Osteoarthritis: A Systematic Review of Product Properties. Cartilage 2023, 14, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Ahearne, M. Introduction to Cell–Hydrogel Mechanosensing. Interface Focus 2014, 4, 20130038. [Google Scholar] [CrossRef] [PubMed]
- Heath, C.A. Cells for Tissue Engineering. Trends Biotechnol. 2000, 18, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Man, Z.; Hu, X.; Liu, Z.; Huang, H.; Meng, Q.; Zhang, X.; Dai, L.; Zhang, J.; Fu, X.; Duan, X.; et al. Transplantation of Allogenic Chondrocytes with Chitosan Hydrogel-Demineralized Bone Matrix Hybrid Scaffold to Repair Rabbit Cartilage Injury. Biomaterials 2016, 108, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Stampoultzis, T.; Rana, V.K.; Guo, Y.; Pioletti, D.P. Impact of Molecular Dynamics of Polyrotaxanes on Chondrocytes in Double-Network Supramolecular Hydrogels under Physiological Thermomechanical Stimulation. Biomacromolecules 2024, 25, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Köck, H.; Striegl, B.; Kraus, A.; Zborilova, M.; Christiansen, S.; Schäfer, N.; Grässel, S.; Hornberger, H. In Vitro Analysis of Human Cartilage Infiltrated by Hydrogels and Hydrogel-Encapsulated Chondrocytes. Bioengineering 2023, 10, 767. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shao, J.Z.; Xiang, L.X.; Dong, X.J.; Zhang, G.R. Mesenchymal Stem Cells: A Promising Candidate in Regenerative Medicine. Int. J. Biochem. Cell Biol. 2008, 40, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Li, S.; Yu, Q.; Chen, T.; Liu, D. Application of Stem Cells in Regeneration Medicine. MedComm 2023, 4, e291. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, L.; Liu, K.; Gao, F. Hydrogel Scaffolds in Bone Regeneration: Their Promising Roles in Angiogenesis. Front. Pharmacol. 2023, 14, 1050954. [Google Scholar] [CrossRef] [PubMed]
- Wagenbrenner, M.; Mayer-Wagner, S.; Rudert, M.; Holzapfel, B.M.; Weissenberger, M. Combinations of Hydrogels and Mesenchymal Stromal Cells (Mscs) for Cartilage Tissue Engineering—A Review of the Literature. Gels 2021, 7, 217. [Google Scholar] [CrossRef] [PubMed]
- Roseti, L.; Grigolo, B. Current Concepts and Perspectives for Articular Cartilage Regeneration. J. Exp. Orthop. 2022, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Modi, U.; Sharma, R.; Bhatia, D.; Solanki, R. Biochemical and Biophysical Cues of the Extracellular Matrix Modulates Stem Cell Fate: Progress and Prospect in Extracellular Matrix Mimicking Biomaterials. Biomed. Eng. Adv. 2025, 9, 100143. [Google Scholar] [CrossRef]
- Park, S.-s.; Park, M.; Lee, B.T. Autologous Stromal Vascular Fraction-Loaded Hyaluronic Acid/Gelatin-Biphasic Calcium Phosphate Scaffold for Bone Tissue Regeneration. Mater. Sci. Eng. C 2022, 132, 112533. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal Stem Cell Perspective: Cell Biology to Clinical Progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Mastrolia, I.; Foppiani, E.M.; Murgia, A.; Candini, O.; Samarelli, A.V.; Grisendi, G.; Veronesi, E.; Horwitz, E.M.; Dominici, M. Challenges in Clinical Development of Mesenchymal Stromal/Stem Cells: Concise Review. Stem Cells Transl. Med. 2019, 8, 1135–1148. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Rafieezadeh, D. Extracellular Vesicles and Their Therapeutic Applications: A Review Article (Part1). Int. J. Physiol. Pathophysiol. Pharmacol. 2024, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Daneshmandi, L.; Shah, S.; Jafari, T.; Bhattacharjee, M.; Momah, D.; Saveh-Shemshaki, N.; Lo, K.W.H.; Laurencin, C.T. Emergence of the Stem Cell Secretome in Regenerative Engineering. Trends Biotechnol. 2020, 38, 1373–1384. [Google Scholar] [CrossRef] [PubMed]
- González-González, A.; García-Sánchez, D.; Dotta, M.; Rodríguez-Rey, J.C.; Pérez-Campo, F.M. Mesenchymal Stem Cells Secretome: The Cornerstone of Cell-Free Regenerative Medicine. World J. Stem Cells 2020, 12, 1439–1690. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, Y.; Hu, S.; Liu, X. Insights into the Function of ESCRT and Its Role in Enveloped Virus Infection. Front. Microbiol. 2023, 14, 1261651. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.B.; Kumar, R.; et al. Extracellular Vesicles as Tools and Targets in Therapy for Diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Wang, S.; Wu, W.; Shan, P.; Chen, Y.; Meng, J.; Xing, L.; Yun, J.; Hao, L.; Wang, X.; et al. Mechanisms of CircRNA/LncRNA-MiRNA Interactions and Applications in Disease and Drug Research. Biomed. Pharmacother. 2023, 162, 114672. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yin, Z.; Wang, X.; Su, J. Exosome-Laden Hydrogels: A Novel Cell-Free Strategy for In-Situ Bone Tissue Regeneration. Front. Bioeng. Biotechnol. 2022, 10, 866208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiao, G.; Ren, S.; Zhang, X.; Li, C.; Wu, W.; Wang, H.; Liu, H.; Zhou, H.; Chen, Y. Exosomes from Bone Marrow Mesenchymal Stem Cells Enhance Fracture Healing through the Promotion of Osteogenesis and Angiogenesis in a Rat Model of Nonunion. Stem Cell Res. Ther. 2020, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Ni, J.; Witherel, C.E.; Yang, M.; Burdick, J.A.; Wen, C.; Dexter Wong, S.H. Harnessing Tissue-Derived Extracellular Vesicles for Osteoarthritis Theranostics. Theranostics 2022, 27, 207–231. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Sun, M.L.; Zhang, X.A.; Wang, X.Q. Crosstalk Among CircRNA/LncRNA, MiRNA, and MRNA in Osteoarthritis. Front. Cell Dev. Biol. 2021, 9, 774370. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Zhou, S.; Li, S.; Kuang, L.; Chen, H.; Luo, X.; Ouyang, J.; He, M.; Du, X.; Chen, L. Exosomes: Roles and Therapeutic Potential in Osteoarthritis. Bone Res. 2020, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; He, Y.; Yao, Y.; Yang, H.; Lu, F. Exosomes Treating Osteoarthritis: Hope with Challenge. Heliyon 2023, 9, e13152. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Liao, H.Y.; Huang, Y.C.; Shen, H.L. Application of Injectable Hydrogels as Delivery Systems in Osteoarthritis and Rheumatoid Arthritis. Br. J. Hosp. Med. 2024, 85, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.X.; Liu, P.; Ding, W.; Meng, Q.B.; Su, D.H.; Zhang, Q.C.; Lian, R.X.; Yu, B.Q.; Zhao, M.D.; Dong, J.; et al. Injectable Mussel-Inspired Highly Adhesive Hydrogel with Exosomes for Endogenous Cell Recruitment and Cartilage Defect Regeneration. Biomaterials 2021, 278, 121169. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Qin, H.; Wang, Z.; Yu, M.; Liu, Z.; Peng, H.; Liang, L.; Zhang, C.; Wei, X. Bone Mesenchymal Stem Cell-Derived SEV-Encapsulated Thermosensitive Hydrogels Accelerate Osteogenesis and Angiogenesis by Release of Exosomal MiR-21. Front. Bioeng. Biotechnol. 2022, 9, 829136. [Google Scholar] [CrossRef] [PubMed]
- Kumarswamy, R.; Volkmann, I.; Thum, T. Regulation and Function of MiRNA-21 in Health and Disease. RNA Biol. 2011, 8, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Cao, T.; Zhao, X.; Lu, W.; Miao, S.; Ning, F.; Wang, D.; Gao, Y.; Wang, L.; Pei, G.; et al. Nidogen1-Enriched Extracellular Vesicles Accelerate Angiogenesis and Bone Regeneration by Targeting Myosin-10 to Regulate Endothelial Cell Adhesion. Bioact. Mater. 2022, 12, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, J.; Alahdal, M. Exosomes Loaded with Chondrogenic Stimuli Agents Combined with 3D Bioprinting Hydrogel in the Treatment of Osteoarthritis and Cartilage Degeneration. Biomed. Pharmacother. 2023, 168, 115715. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhao, M.; Lash, B.; Martino, M.M.; Julier, Z. Growth Factor Engineering Strategies for Regenerative Medicine Applications. Front. Bioeng. Biotechnol. 2020, 7, 469. [Google Scholar] [CrossRef] [PubMed]
- Gillman, C.E.; Jayasuriya, A.C. FDA-Approved Bone Grafts and Bone Graft Substitute Devices in Bone Regeneration. Mater. Sci. Eng. C 2021, 130, 112466. [Google Scholar] [CrossRef] [PubMed]
- Zara, J.N.; Siu, R.K.; Zhang, X.; Shen, J.; Ngo, R.; Lee, M.; Li, W.; Chiang, M.; Chung, J.; Kwak, J.; et al. High Doses of Bone Morphogenetic Protein 2 Induce Structurally Abnormal Bone and Inflammation in Vivo. Tissue Eng. Part A 2011, 17, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.H.; Wu, F.G. Hydrogel-Based Growth Factor Delivery Platforms: Strategies and Recent Advances. Adv. Mater. 2024, 36, e2210707. [Google Scholar] [CrossRef] [PubMed]
- Lowery, J.W.; Rosen, V. Bone Morphogenetic Protein–Based Therapeutic Approaches. Cold Spring Harb. Perspect. Biol. 2018, 10, a022327. [Google Scholar] [CrossRef] [PubMed]
- Wrana, J.L. Signaling by the TGFβ Superfamily. Cold Spring Harb. Perspect. Biol. 2013, 5, a011197. [Google Scholar] [CrossRef] [PubMed]
- van Brakel, F.; Zhao, Y.; van der Eerden, B.C.J. Fueling Recovery: The Importance of Energy Coupling between Angiogenesis and Osteogenesis during Fracture Healing. Bone Rep. 2024, 21, 101757. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, T.; Jain, K.; Gu, S.X.; Qiu, M.; Gu, V.W.; Melchinger, H.; Rinder, H.; Martin, K.A.; Gardiner, E.E.; Lee, A.I.; et al. A Guide to Molecular and Functional Investigations of Platelets to Bridge Basic and Clinical Sciences. Nat. Cardiovasc. Res. 2022, 1, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Giannotti, L.; Di Chiara Stanca, B.; Spedicato, F.; Nitti, P.; Damiano, F.; Demitri, C.; Calabriso, N.; Carluccio, M.A.; Palermo, A.; Siculella, L.; et al. Progress in Regenerative Medicine: Exploring Autologous Platelet Concentrates and Their Clinical Applications. Genes 2023, 14, 1669. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhao, Z.; Li, D.; Wang, X.; Dai, D.; Fu, H. Effect Study of Exosomes Derived from Platelet-Rich Plasma in the Treatment of Knee Cartilage Defects in Rats. J. Orthop. Surg. Res. 2023, 18, 160. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, V.; Ciric, M.; Jovanovic, V.; Trandafilovic, M.; Stojanovic, P. Platelet-Rich Fibrin: Basics of Biological Actions and Protocol Modifications. Open Med. 2021, 16, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Jiraboonsri, S.; Jitpibull, J.; Itthipanichpong, T.; Ratanavaraporn, J. Silk Fibroin-Platelet Rich Plasma Injectable Hydrogel with Controlled-Release of Growth Factors for Knee Osteoarthritis. J. Drug Deliv. Sci. Technol. 2025, 105, 106625. [Google Scholar] [CrossRef]
- Mirsky, N.A.; Ehlen, Q.T.; Greenfield, J.A.; Antonietti, M.; Slavin, B.V.; Nayak, V.V.; Pelaez, D.; Tse, D.T.; Witek, L.; Daunert, S.; et al. Three-Dimensional Bioprinting: A Comprehensive Review for Applications in Tissue Engineering and Regenerative Medicine. Bioengineering 2024, 11, 777. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Schilling, A.F.; Yonezawa, T.; Wang, J.; Dai, G.; Cui, X. Bioactive Nanoparticles Stimulate Bone Tissue Formation in Bioprinted Three-Dimensional Scaffold and Human Mesenchymal Stem Cells. Biotechnol. J. 2014, 9, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Ye, X.; Liu, J.; Wang, C.; Li, J.; Wang, X.; Ma, M.; Wang, M. 4D Printing of Highly Printable and Shape Morphing Hydrogels Composed of Alginate and Methylcellulose. Mater. Des. 2021, 205, 109699. [Google Scholar] [CrossRef]
- Dell, A.C.; Wagner, G.; Own, J.; Geibel, J.P. 3D Bioprinting Using Hydrogels: Cell Inks and Tissue Engineering Applications. Pharmaceutics 2022, 14, 2596. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, Q.; Deng, C.; Xu, B.; Zhang, Z.; Yang, Y.; Lu, T. Exquisite Design of Injectable Hydrogels in Cartilage Repair. Theranostics 2020, 10, 9843–9864. [Google Scholar] [CrossRef] [PubMed]
- Boere, K.W.M.; Visser, J.; Seyednejad, H.; Rahimian, S.; Gawlitta, D.; Van Steenbergen, M.J.; Dhert, W.J.A.; Hennink, W.E.; Vermonden, T.; Malda, J. Covalent Attachment of a Three-Dimensionally Printed Thermoplast to a Gelatin Hydrogel for Mechanically Enhanced Cartilage Constructs. Acta Biomater. 2014, 10, 2602–2611. [Google Scholar] [CrossRef] [PubMed]
- Koh, R.H.; Jin, Y.; Kim, J.; Hwang, N.S. Inflammation-Modulating Hydrogels for Osteoarthritis Cartilage Tissue Engineering. Cells 2020, 9, 419. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Ozhava, D.; Mao, Y. Expansion and Delivery of Human Chondrocytes on Gelatin-Based Cell Carriers. Gels 2025, 11, 199. [Google Scholar] [CrossRef] [PubMed]
- Ogéus, T. Hydrogel Alone or in Combination with Regenerative Interventions for Knee Osteoarthritis, A Case Series. J. Orthop. Sports Med. 2025, 7, 16–22. [Google Scholar] [CrossRef]
- Yao, J.; Huo, Z.; Xu, J.; Shang, J.; Weng, Y.; Xu, D.; Liu, T.; Huang, Y.; Zhou, X. Enhanced Surface Immunomodification of Engineered Hydrogel Materials through Chondrocyte Modulation for the Treatment of Osteoarthritis. Coatings 2024, 14, 308. [Google Scholar] [CrossRef]
- Duan, W.L.; Zhang, L.N.; Bohara, R.; Martin-Saldaña, S.; Yang, F.; Zhao, Y.Y.; Xie, Y.; Bu, Y.Z.; Pandit, A. Adhesive Hydrogels in Osteoarthritis: From Design to Application. Mil. Med. Res. 2023, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ran, C.; Zhao, D.; Yang, F.; Guo, Q.; Yang, J.; Zhang, X. Mesenchymal Stem Cells and Their Exosomes Mitigate Osteoarthritis by Restoring the Balance between Proinflammatory Teffs and Tregs. Front. Aging 2024, 5, 1509014. [Google Scholar] [CrossRef] [PubMed]
- Goulian, A.J.; Goldstein, B.; Saad, M.A. Advancements in Regenerative Therapies for Orthopedics: A Comprehensive Review of Platelet-Rich Plasma, Mesenchymal Stem Cells, Peptide Therapies, and Biomimetic Applications. J. Clin. Med. 2025, 14, 2061. [Google Scholar] [CrossRef] [PubMed]
- Hashemi-Afzal, F.; Fallahi, H.; Bagheri, F.; Collins, M.N.; Eslaminejad, M.B.; Seitz, H. Advancements in Hydrogel Design for Articular Cartilage Regeneration: A Comprehensive Review. Bioact. Mater. 2025, 43, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.R.; Pires, L.; Martins, R.A.; Santos, M.; Santos, G.S.; Lana, J.V.; Costa, B.R.; Santos, N.; de Macedo, A.P.; Kruel, A.; et al. Orthobiologics Revisited: A Concise Perspective on Regenerative Orthopedics. Curr. Issues Mol. Biol. 2025, 47, 247. [Google Scholar] [CrossRef]
- Rahman, E.; Rao, P.; Abu-Farsakh, H.N.; Thonse, C.; Ali, I.; Upton, A.E.; Baratikkae, S.Y.; Carruthers, J.D.A.; Mosahebi, A.; Heidari, N.; et al. Systematic Review of Platelet-Rich Plasma in Medical and Surgical Specialties: Quality, Evaluation, Evidence, and Enforcement. J. Clin. Med. 2024, 13, 4571. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Zhang, S.; Liu, X.; Zhao, Y.; Yang, J.; Chai, G.; Wang, N.; Ma, S.; Liu, W.; Ding, C. Hydrogel Tissue Bioengineered Scaffolds in Bone Repair: A Review. Molecules 2023, 28, 7039. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Nan, D.; Jin, H.; Qu, X. Recent Advances of Injectable Hydrogels for Drug Delivery and Tissue Engineering Applications. Polym. Test. 2020, 81, 106283. [Google Scholar] [CrossRef]
- Haq-Siddiqi, N.A.; Britton, D.; Kim Montclare, J. Protein-Engineered Biomaterials for Cartilage Therapeutics and Repair. Adv. Drug Deliv. Rev. 2023, 192, 114647. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, Q.; Zhang, H.; Liu, H.; Ji, X.; Tang, B.Z. Codes in Code: AIE Supramolecular Adhesive Hydrogels Store Huge Amounts of Information. Adv. Mater. 2021, 33, e2105418. [Google Scholar] [CrossRef] [PubMed]



| Orthobiologic | Hydrogel | Preparation Technique | Results | Ref. |
|---|---|---|---|---|
| Chondrocytes | Chitosan hydrogel–demineralized bone matrix. | Chitosan powder is dissolved in 0.1 M acetic acid to yield 2.5 2.5% (w/v) aqueous solution and then sterilized in an autoclave. The CS solution is mixed with filter-sterilized and pre-cooled 60% (w/v) GP at a ratio of 9:1 to obtain homogeneous CS/GP solutions | The study demonstrated that the transplantation of allogenic chondrocytes with chitosan/DBM scaffold successfully repaired rabbit cartilage injury with only one-step operation | [52] |
| Chondrocytes | α-cyclodextrin and polyethylene glycol (PEG) incorporated into a gelatin covalent matrix | The preparation of PEG/α-CD polyrotaxanes involves dissolving two different concentrations of α-cyclodextrin (α-CD) (12 and 36 mg) in 400 μL of PBS. This solution is then added to a 600 μL PEG/PBS solution (6.5 wt%) | By incorporating supramolecular motifs into a covalent-based hydrogel system, it is possible to mimic the intricate dynamic and noncovalent interactions present in natural cartilage | [53] |
| Chondrocytes | Photo-crosslinking, zwitterionic | Three different monomer mixtures were prepared and are denoted as follows: MPC60, MPC30, and SBMA60. The monomer solutions were polymerized into hydrogels for 10 min using a Bluephase G4 polymerization lamp | The results demonstrated that zwitterionic cartilage–hydrogel networks are formed by infiltration The applied hydrogels could completely infiltrate the human cartilage explants and still be polymerized by visible light | [54] |
| MSCs (from bone marrow, adipose tissues, or other sources) | HA, agarose, alginate, glycosaminoglycans, and chitosan, proteins like collagen, elastin, gelatin, polyvinyl alcohol (PVA), or Poly(lactide-co-glycolide) (PLGA) | Conventional subtractive or innovative additive manufacturing (3D printing) | Choncrogenic/osteogenic effects | [57,58] |
| SVF | HA/gelatin-biphasic calcium phosphate (HA-Gel/BCP) | The BCP scaffold was prepared by the sponge replicate method. The gelatin was dissolved and mixed with HA. Then, the Ha-Gel solution was added to the BCP. Finally, the HA-Gel-loaded BCP scaffold was crosslinked using 1-ethyl-3(3-dimethylaminopropyl)carbodiimide hydrochloride and N-hydroxyl succinimide | The isolated SVF showed osteogenic differentiation ability. In vivo implantation of autologous SVF-hydrogel complex showed excellent bone regeneration in a rat skull critical-size defect model | [61] |
| Exosomes (MSC-derived) | Alginate–dopamine and chondroitin sulphate with a silk fibroin hydrogel sponge (AD/CS/RSF) | Hydrogel preparation encompasses using a crosslinked network | The hydrogel with encapsulated exosomes recruited BMSCs and promoted cartilage regeneration | The AD/CS/RSF/EXO hydrogel |
| Exosomes containing miR-21targeted SPRY2 (Bone-MSC-derived) | Thermosensitive β-glycerophosphate/chitosan | Chitosan was added to an acetic acid solution, followed by the dropwise addition of β- glycerophosphate solution | The exosome–hydrogel complexes were biocompatible, exhibiting excellent thermosensitive properties, and enhancing bone regeneration | [80] |
| Nidogen1-enriched Exosomes (Bone marrow-MSC-derived) | Alginate/PEG2000/gelatin composite | A mixed prepolymer solution (Sodium alginate, PEG2000, and gelatin) was added with exosomes carrying Nidogen-1 and transferred to a mold and solidified. Then, 2% CaCl2 solution was used to crosslink | Nidogen1-enriched Exosomes derived from BMSCs promote angiogenesis and bone regeneration in rat femoral defects | [82] |
| Krthogenin-enriched Exosomes (Umbilical cord-MSC-derived) | In situ HA hydrogels achieving gelation by imine-mediated ortho-nitrobenzyl alcohol- photo crosslinking | Bioprinting | Krthogenin-enriched exosomes reduced intracellular inflammasome formation and the release of inflammatory agents, while preventing chondrocyte apoptosis and enhancing chondrogenesis in synovial MSCs, osteoprogenitor cells, and osteoclasts in OA | [83] |
| Growth factors (BMP-2, BMP-6, BMP-7, TGF-β1, TGF-β3, IGF-1, VEGF, PDGF) | Natural, synthetic, and composites | Recombinant process | Increase cell proliferation and differentiation; promote ECM synthesis | [87] |
| PRP | Silk fibroin | A fibroin solution of 4% aseptically prepared medical grade Thai silk was sonicated and solutions were mixed with PRP at the ratio of 1:1, giving the final con-centrations of SF. The mixtures were then left to gelate | Silk Fibroin-PRP complex can be easily injected, releasing GFs in a sustained manner. It is biocompatible, able to promote chondrocyte proliferation, and is not cytotoxic | [95] |
| Pros | Cons |
|---|---|
| Enhanced Tissue Regeneration Hydrogels provide a 3D scaffold for orthobiologics, mimicking matrix structure, that supports cell survival, differentiation, and integration into the damaged tissue, and potentially creates the condition for the regeneration of damaged joint tissues regeneration [100]. | Degradation and Integration Hydrogels can precociously or partially integrate with the surrounding joint tissue, affecting long-term effectiveness [101]. |
| Controlled Delivery Hydrogels’ design can be tailored to control orthobiologics release, and therefore their therapeutic effect [102]. | Cell Limitations Chondrocytes can be difficult to culture and expand in sufficient numbers, and they may not always differentiate into the desired hyaline cartilage [103]. MSCs can undergo hypertrophic differentiation During the physiological process of cartilage formation, MSCs differentiate into chondrocytes, which then differentiate into hypertrophic chondrocytes that produce Type X collagen and mineralize. In the context of cartilage regeneration using MSCs, an undesired hypertrophic differentiation can happen, leading to bone, but not cartilage formation. |
| Minimally Invasiveness Injectable hydrogels offer a less invasive approach compared to some surgical procedures for joint tissue repair [104]. | Immunogenicity Some hydrogel materials may trigger an immune response, leading to inflammation and rejection [105]. |
| Improved Adhesion Some hydrogels can adhere to joint tissues, improving their retention at the treatment site [106]. | Cost and Complexity Developing and manufacturing hydrogels and cell-based therapies can be expensive and complex [13]. |
| Reduced Inflammation MSCs, concentrates, exosomes, and growth factors possess immunomodulatory properties and reduce inflammation in the joint, a key factor in OA progression [107]. | Limited Long-term Results Clinical studies on safety and efficacy orthobiologic–hydrogel combinations mostly concern short term evaluations. Long-term clinical results are still needed to support the use of those strategies [108]. |
| Targeted Delivery Hydrogels can be engineered to deliver Orthobiologics to the site of joint tissue damage, maximizing their therapeutic effect and minimizing potential side effects due to systemic administration [13,100]. | Cell Viability and Survival Cells can suffer from the hostile, inflamed OA joint, thus hampering a proper engraftment in the site of damage [13]. |
| Improved Mechanical Support Hydrogels must mimic the mechanical properties of joint tissue to improve weight bearing and mobility [109]. | Hydrogel Degradation Hydrogels can degrade over time, potentially leading to the release of cells before they have fully integrated into the tissue [109]. |
| Potential for Long-Term Results While more research is needed, some studies indicate that orthobiologics therapy, particularly when combined with hydrogels, may offer long-term benefits for OA patients by promoting sustained joint tissue repair and reducing disease progression [47]. | Mechanical Properties The mechanical properties of hydrogels must ensure the engraftment to support the stresses and strains of the joint [109]. |
| Improved Pain Relief The combination of orthobiologics with hydrogels can reduce pain associated with OA, likely due to their ability to modulate the inflammatory environment and promote tissue regeneration [110]. | Need for Standardization Standardized protocols for orthobiologic preparation and delivery are still scarce, which can affect the consistency and reproducibility of the results [111]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavallo, C.; Desando, G.; D’Alessandro, M.; Grigolo, B.; Roseti, L. Recent Evidence for Orthobiologics Combined with Hydrogels for Joint Tissue Regeneration: Focus on Osteoarthritis. Gels 2025, 11, 551. https://doi.org/10.3390/gels11070551
Cavallo C, Desando G, D’Alessandro M, Grigolo B, Roseti L. Recent Evidence for Orthobiologics Combined with Hydrogels for Joint Tissue Regeneration: Focus on Osteoarthritis. Gels. 2025; 11(7):551. https://doi.org/10.3390/gels11070551
Chicago/Turabian StyleCavallo, Carola, Giovanna Desando, Martina D’Alessandro, Brunella Grigolo, and Livia Roseti. 2025. "Recent Evidence for Orthobiologics Combined with Hydrogels for Joint Tissue Regeneration: Focus on Osteoarthritis" Gels 11, no. 7: 551. https://doi.org/10.3390/gels11070551
APA StyleCavallo, C., Desando, G., D’Alessandro, M., Grigolo, B., & Roseti, L. (2025). Recent Evidence for Orthobiologics Combined with Hydrogels for Joint Tissue Regeneration: Focus on Osteoarthritis. Gels, 11(7), 551. https://doi.org/10.3390/gels11070551








