Succinimidyl Alginate-Modified Fibrin Hydrogels from Human Plasma for Skin Tissue Engineering
Abstract
1. Introduction
2. Results and Discussion
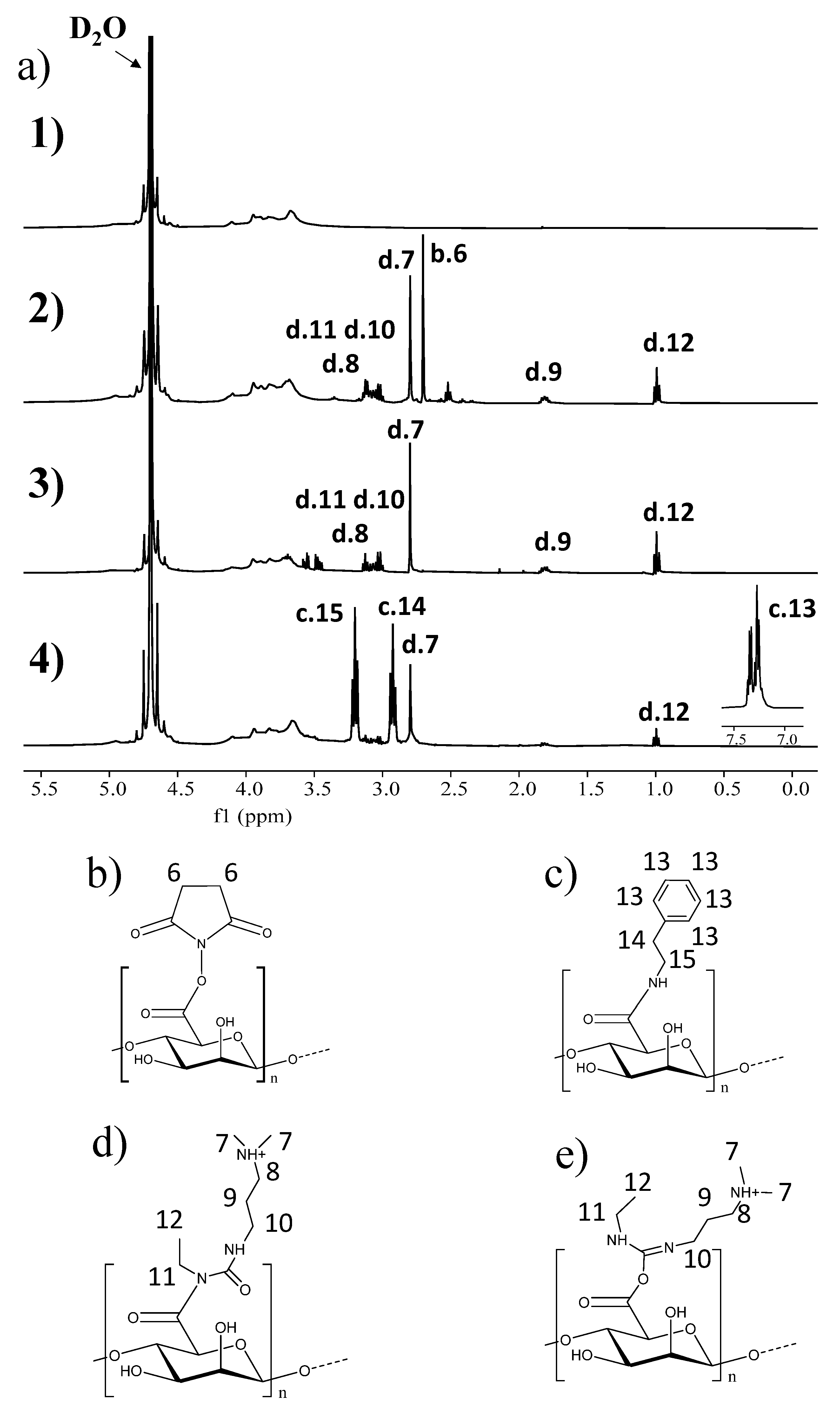
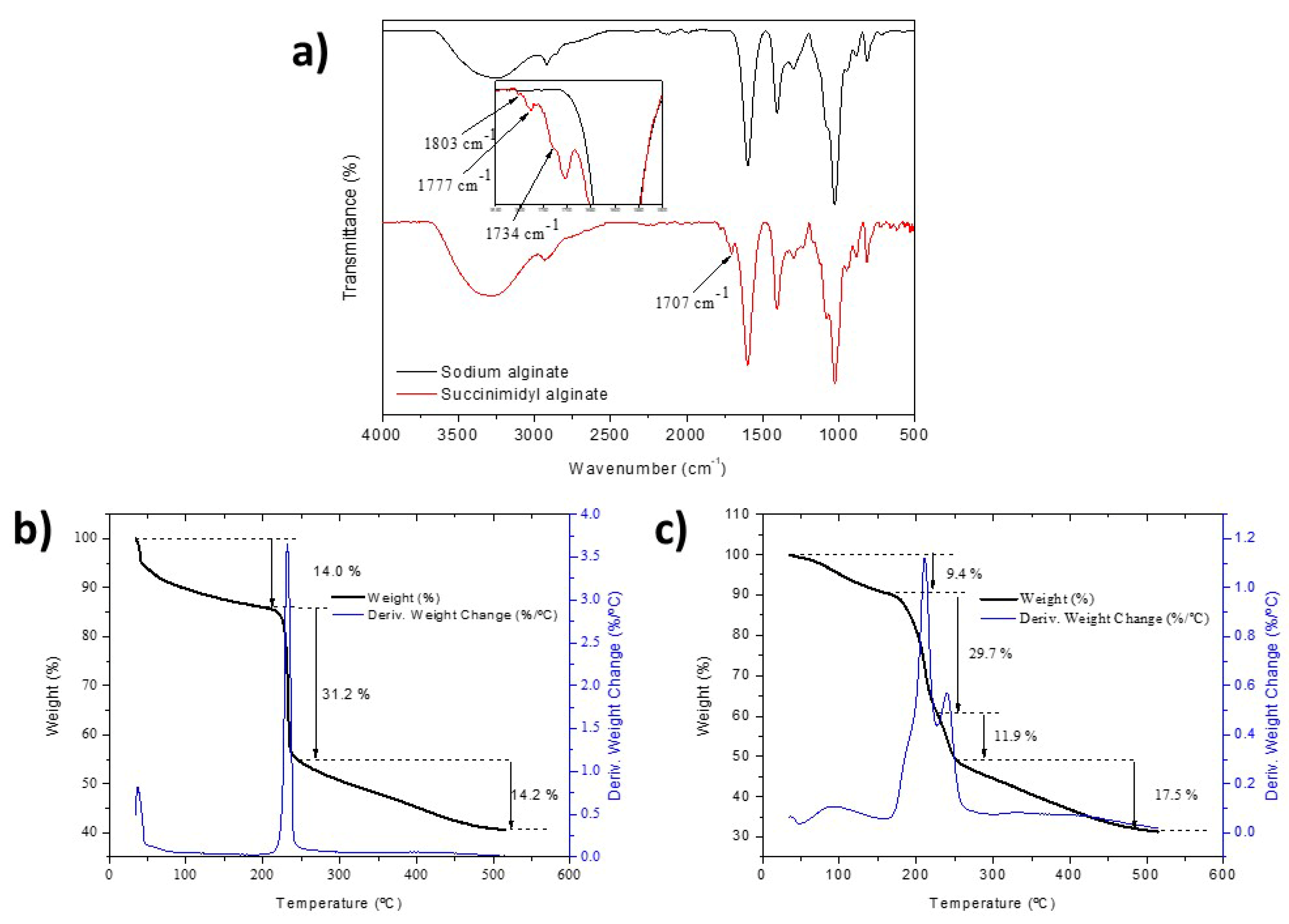
2.1. Synthesis and Characterization of Succinimidyl Alginate (SA)
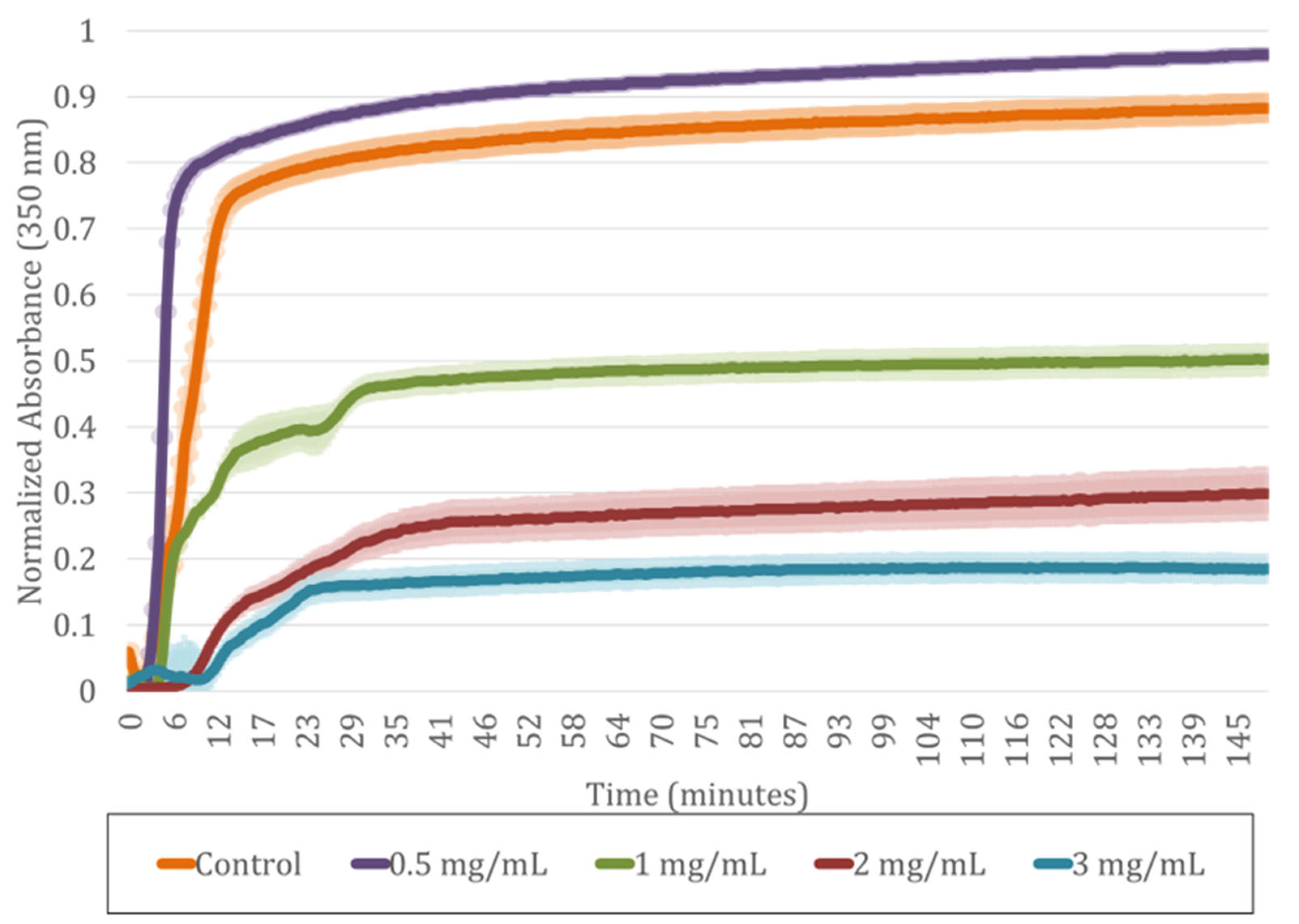
2.2. Incorporation of SA into Human Plasma-Derived Fibrin Hydrogels
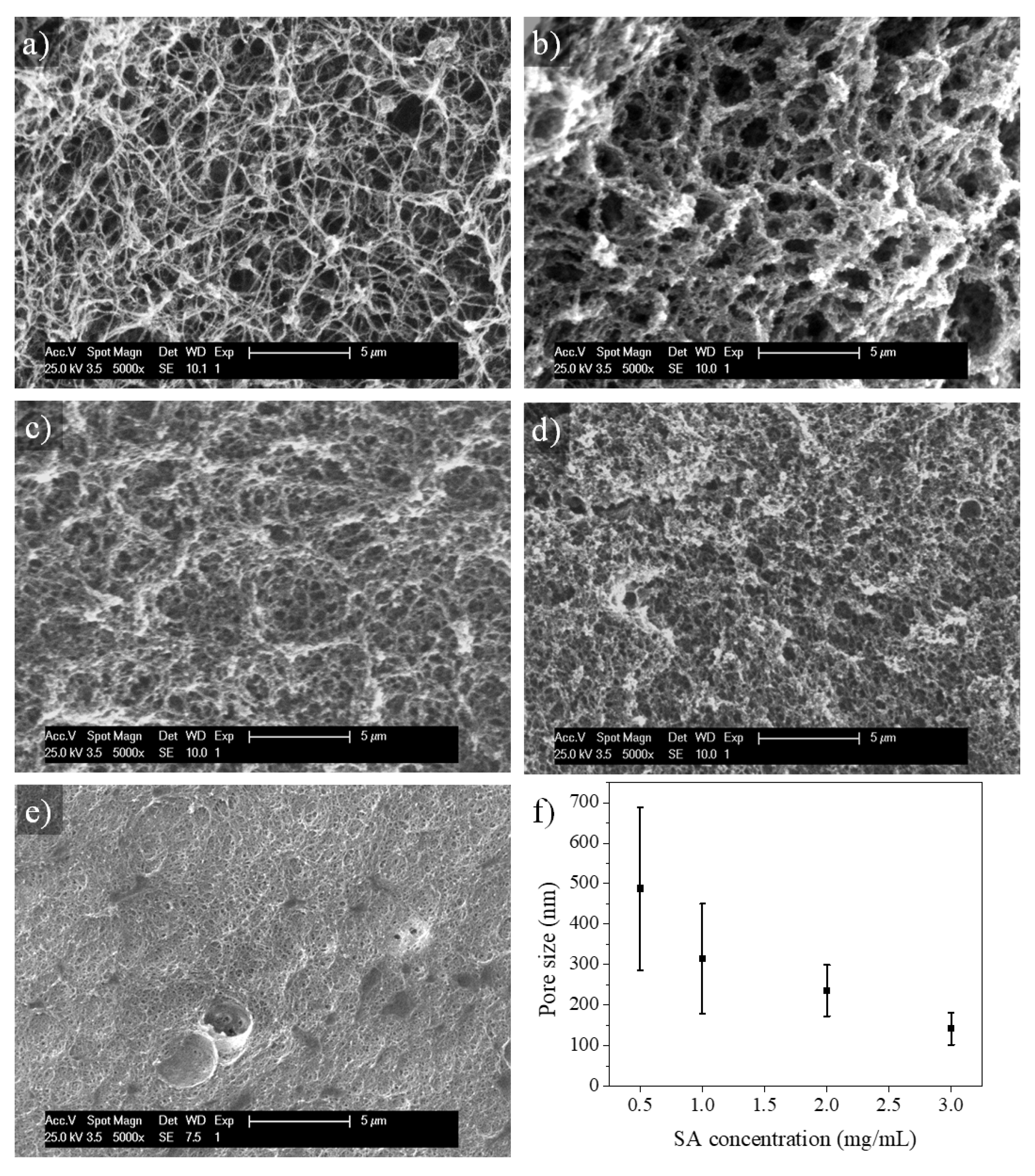
2.3. Microstructure of SA-Modified Plasma-Derived Fibrin Hydrogels
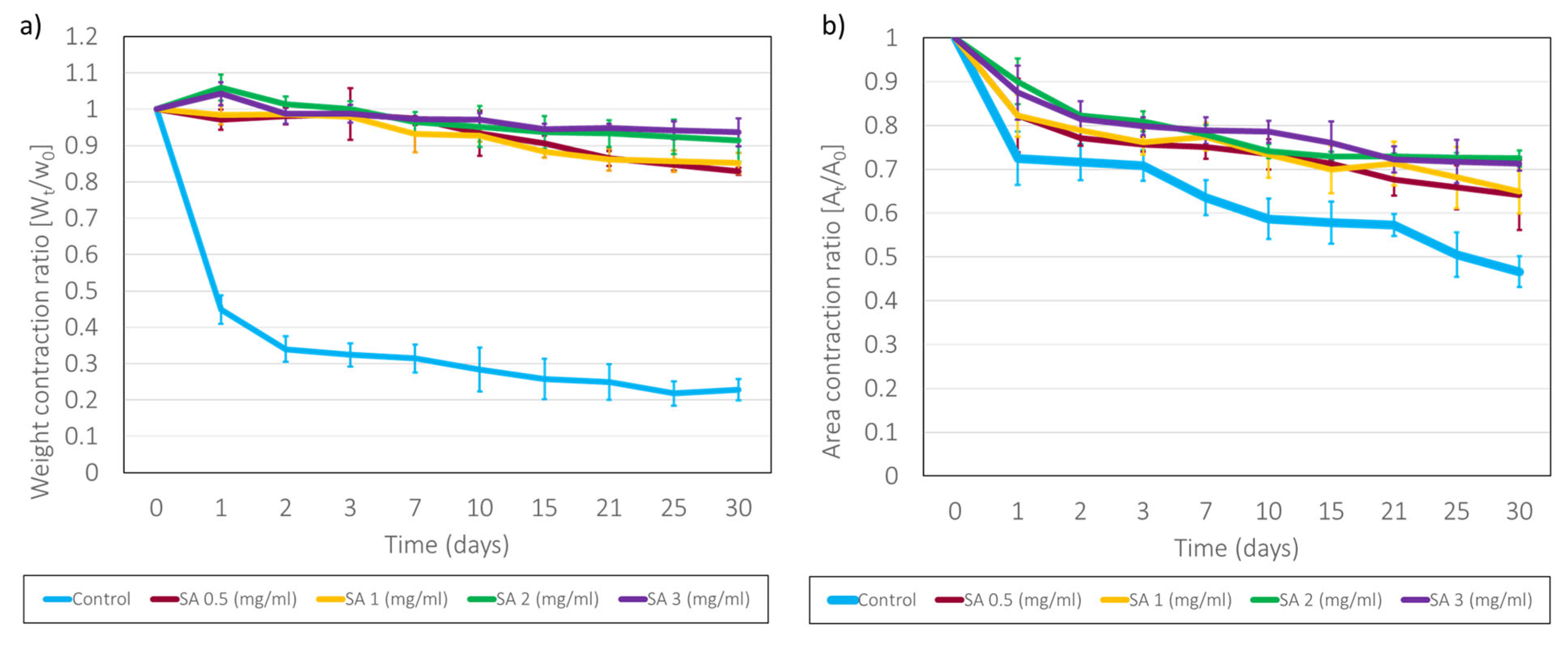
2.4. Long-Term Stability of SA-Modified Plasma-Derived Fibrin Hydrogels
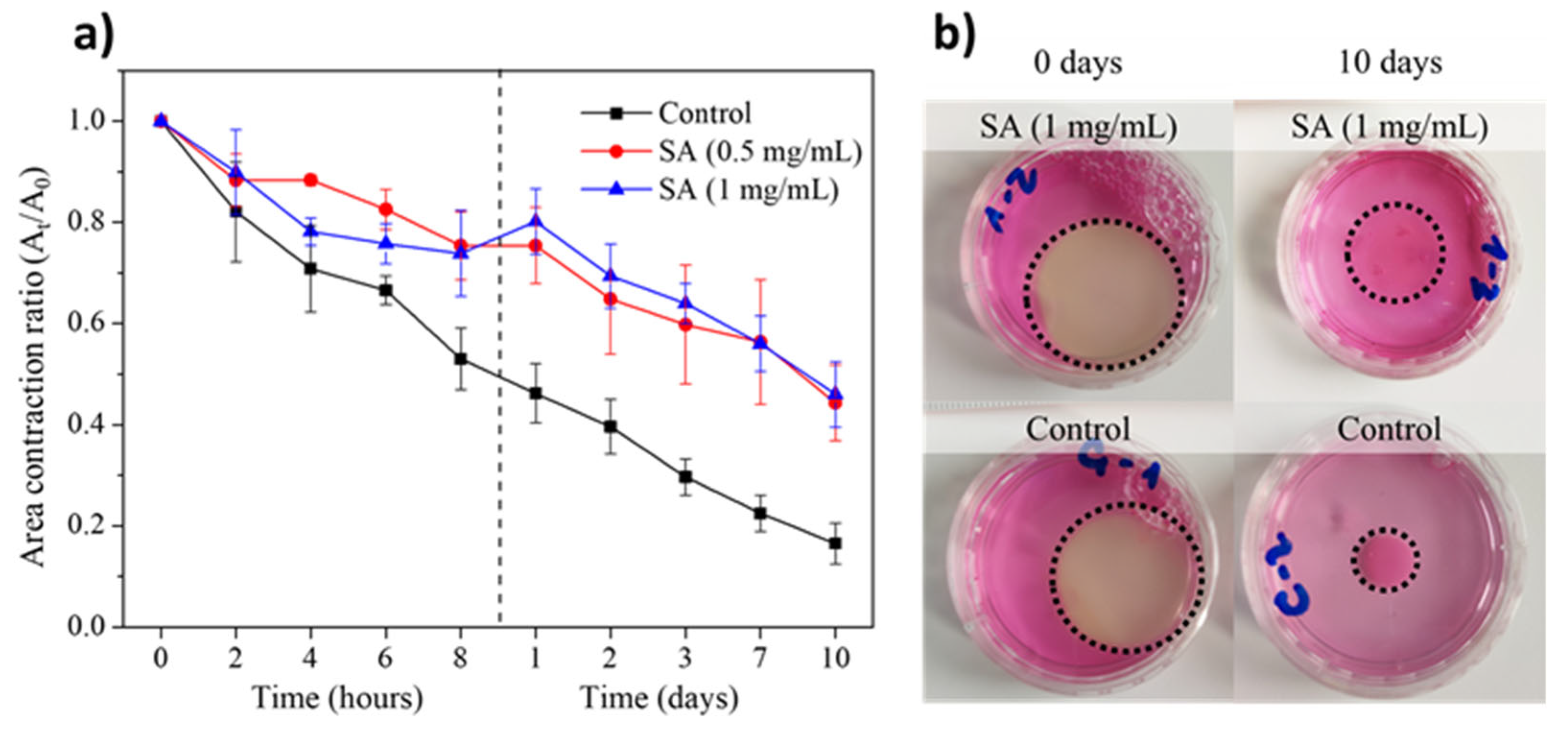
2.5. Stability of SA-Modified Plasma-Derived Fibrin Hydrogels Containing hFBs
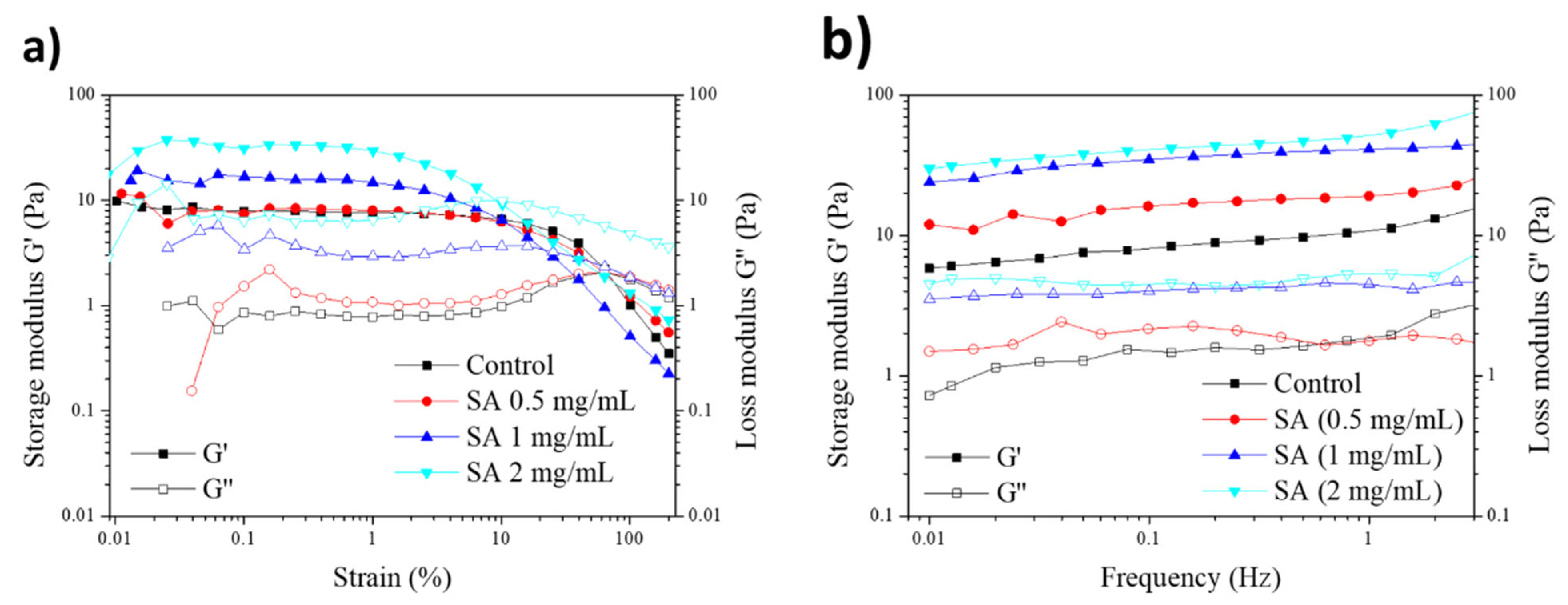
2.6. Rheological Properties of SA-Modified Plasma-Derived Fibrin Hydrogels
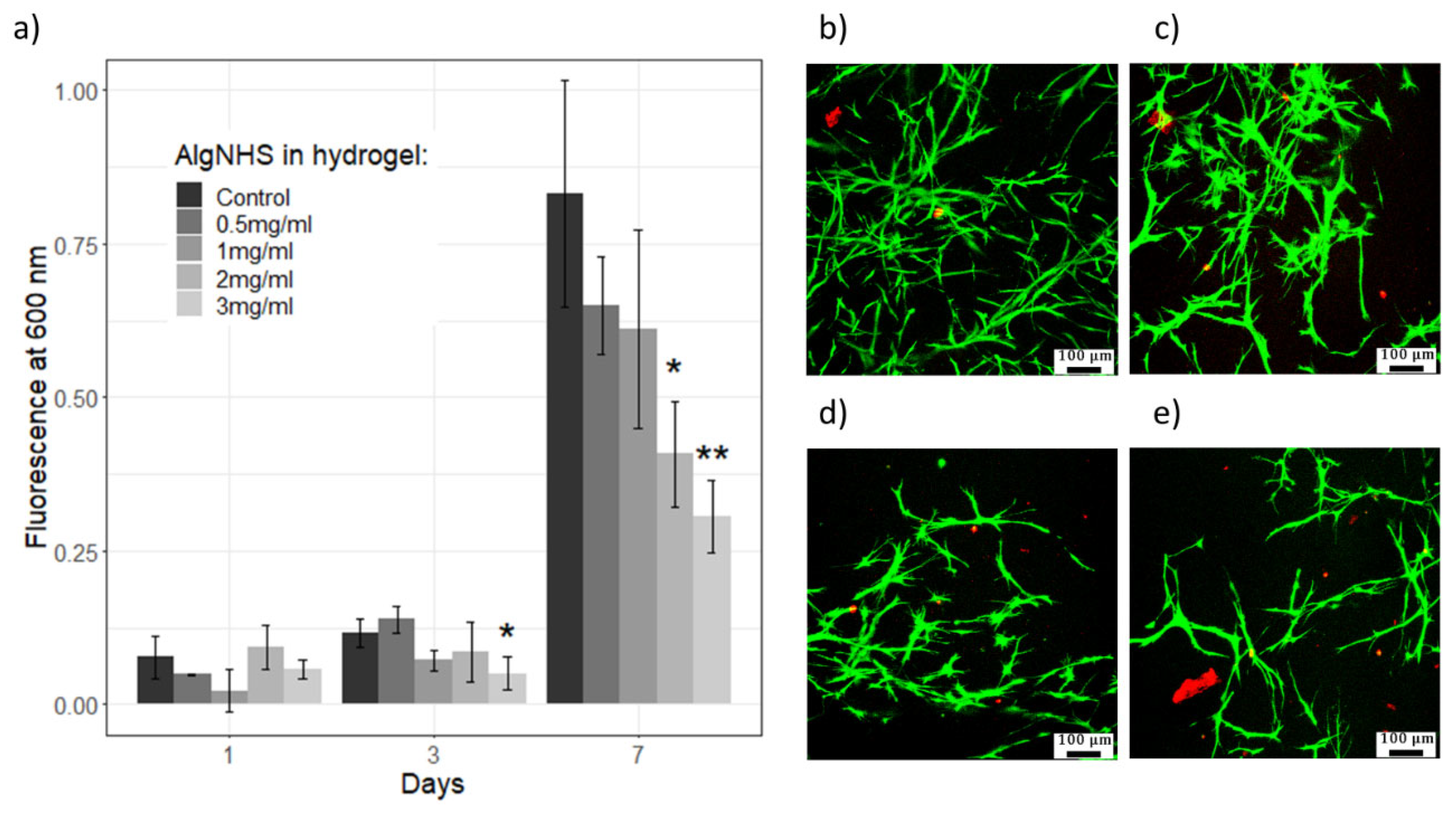
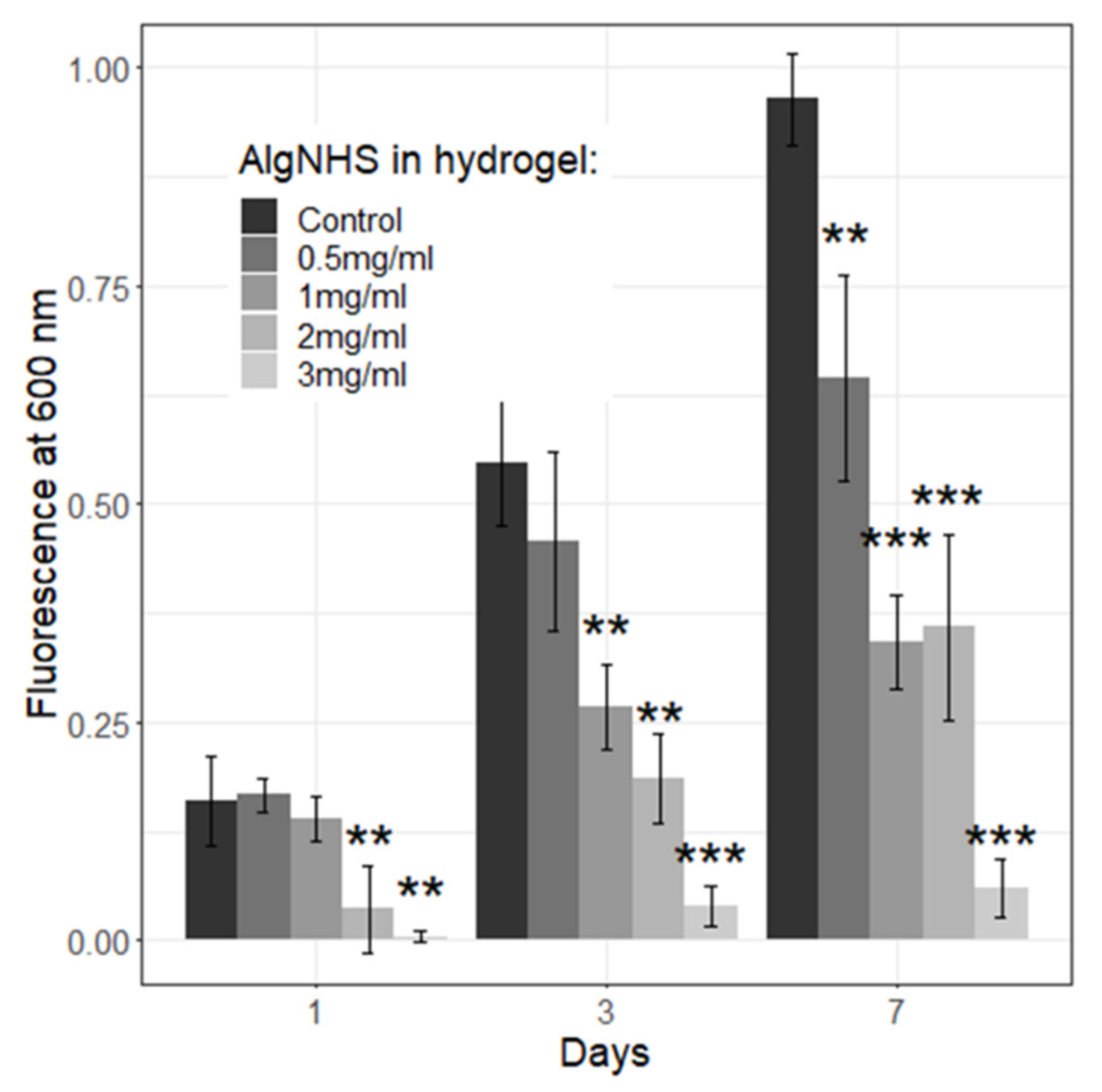
2.7. Viability and Proliferation of Primary Human Fibroblasts (hFBs) and Immortalized Human Keratinocytes (HaCaT) in Plasma-Derived Fibrin/SA Hydrogels
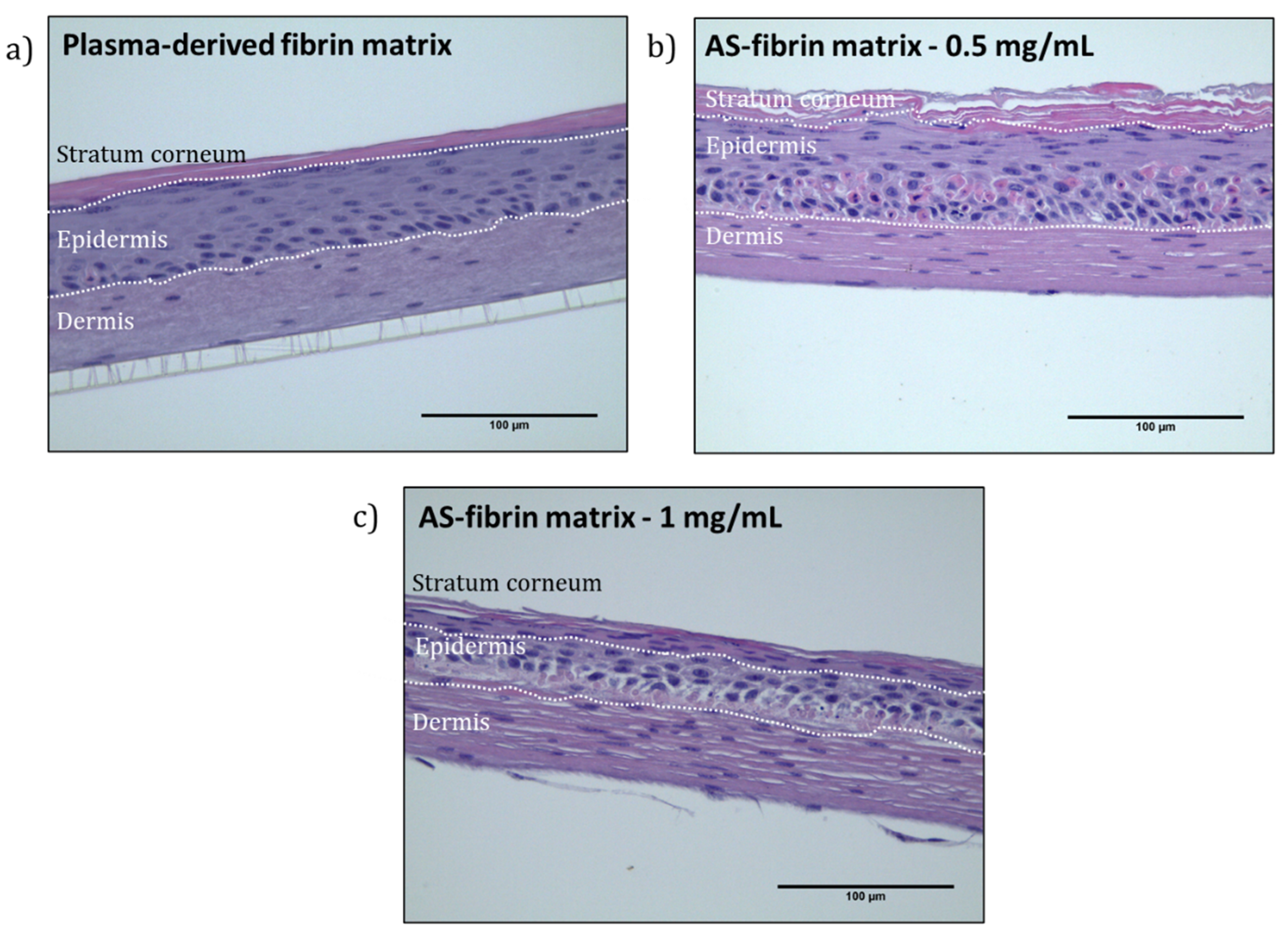
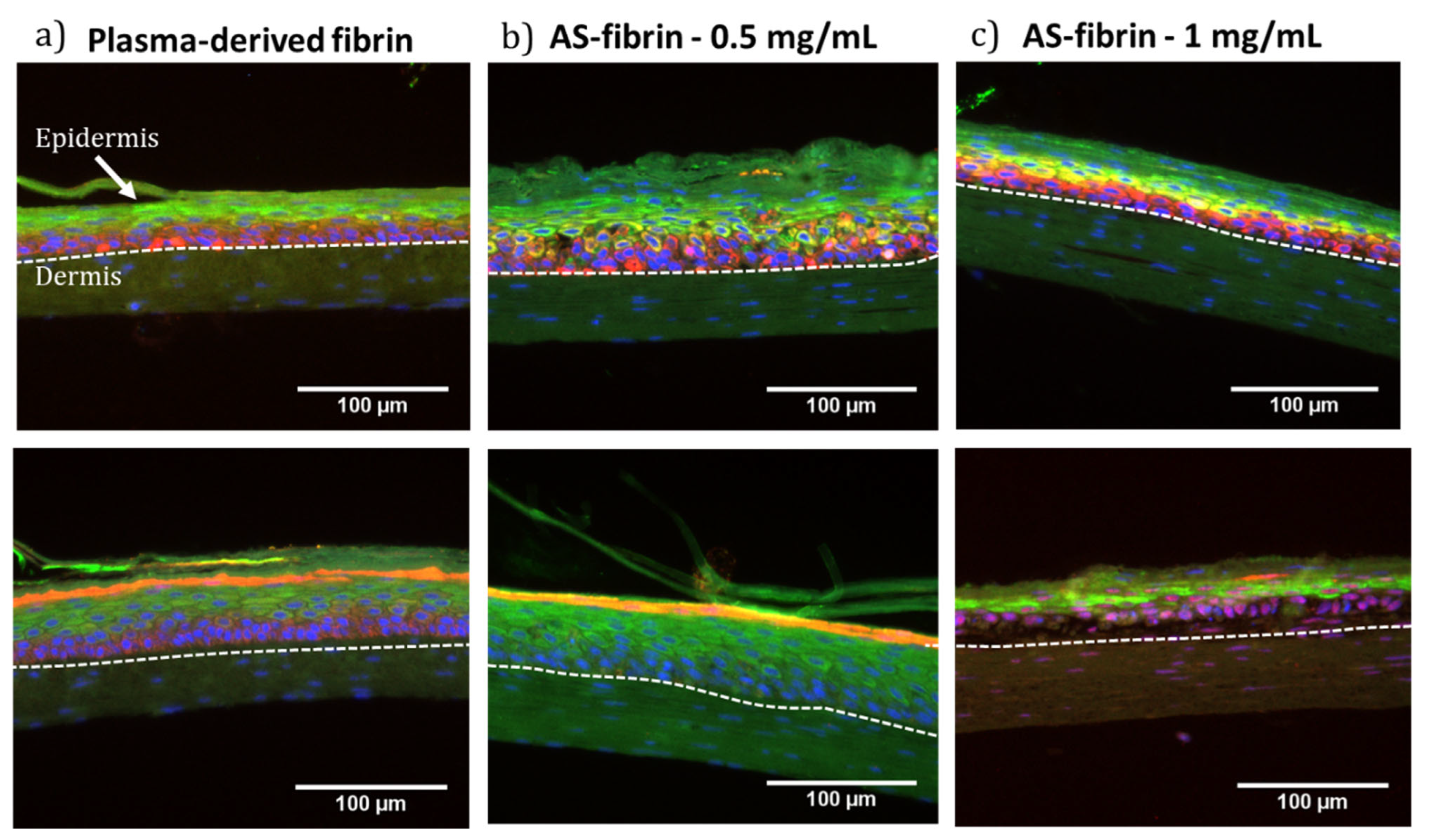
2.8. Plasma-Derived Fibrin/SA Dermo-Epidermal Skin Equivalents Containing Human Primary Cells
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Synthesis of Succinimidyl Alginate (SA)
4.2.2. Nuclear Magnetic Resonance Spectroscopy
4.2.3. FTIR-ATR
4.2.4. Thermogravimetric Analysis (TGA)
4.2.5. Gel Permeation Chromatography (GPC)
4.2.6. Preparation of SA-Modified Plasma-Derived Fibrin Hydrogels
4.2.7. Time and Kinetics of Gelation
4.2.8. Analysis of the Hydrogel Microstructure (SEM)
4.2.9. Rheological Properties
4.2.10. Contraction of Plasma-Derived Fibrin/SA Hydrogels
4.2.11. Biological Tests and Culture of Primary Human Fibroblasts, Primary Human Keratinocytes, and Immortal Human Skin Keratinocytes (HaCaT Cells)
4.2.12. Area Contraction of Plasma-Derived Fibrin/SA Hydrogels with hFBs
4.2.13. Live/Dead Fluorescence Assays
4.2.14. AlamarBlue Assay
4.2.15. Dermo-Epidermal Skin Constructs. H&E Staining and Immunostaining Assays
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SA | Succinimidyl alginate |
| NHS | N-hydroxysuccinimide |
| H&E | Hematoxylin and eosin |
| EDC | Carbodiimide |
| hFBs | Human fibroblast |
| hKCs | Keratinocye |
References
- Fan, L.; Cao, M.; Gao, S.; Wang, T.; Wu, H.; Peng, M.; Zhou, X.; Nie, M. Preparation and characterization of sodium alginate modified with collagen peptides. Carbohydr. Polym. 2013, 93, 380–385. [Google Scholar] [CrossRef]
- Zhang, X.; Wan, H.; Lan, W.; Miao, F.; Qin, M.; Wei, Y.; Hu, Y.; Liang, Z.; Huang, D. Fabrication of adhesive hydrogels based on poly (acrylic acid) and modified hyaluronic acid. J. Mech. Behav. Biomed. Mater. 2022, 126, 105044. [Google Scholar] [CrossRef] [PubMed]
- Strehin, I.; Ambrose, W.M.I.; Schein, O.; Salahuddin, A.; Elisseeff, J. Synthesis and characterization of a chondroitin sulfate-polyethylene glycol corneal adhesive. J. Cataract Refract. Surg. 2009, 35, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Horta, R.; Matesanz, A.; Gallardo, A.; Reinecke, H.; Jorcano, J.L.; Acedo, P.; Velasco, D.; Elvira, C. Technological advances in fibrin for tissue engineering. J. Tissue Eng. 2023, 14, 20417314231190288. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Steck, J.; Suo, Z. Gelation kinetics of alginate chains through covalent bonds. Extrem. Mech. Lett. 2020, 40, 100898. [Google Scholar] [CrossRef]
- Lee, J.M.; Edwards, H.H.L.; Pereira, C.A.; Samii, S.I. Crosslinking of tissue-derived biomaterials in 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC). J. Mater. Sci. Mater. Med. 1996, 7, 531–541. [Google Scholar] [CrossRef]
- Bax, D.V.; Nair, M.; Weiss, A.S.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Tailoring the biofunctionality of collagen biomaterials via tropoelastin incorporation and EDC-crosslinking. Acta Biomater. 2021, 135, 150–163. Available online: https://ora.ox.ac.uk/objects/uuid:5c688723-495e-4bd8-a1ce-2a0079b0d5e2 (accessed on 1 May 2025). [CrossRef]
- Yang, Y. Chapter 5—Side Reactions Upon Amino Acid/Peptide Carboxyl Activation. In Side Reactions in Peptide Synthesis; Yang, Y., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 95–118. [Google Scholar] [CrossRef]
- Adamiak, K.; Sionkowska, A. Current methods of collagen cross-linking: Review. Int. J. Biol. Macromol. 2020, 161, 550–560. [Google Scholar] [CrossRef]
- Golunova, A.; Velychkivska, N.; Mikšovská, Z.; Chochola, V.; Jaroš, J.; Hampl, A.; Pop-Georgievski, O.; Proks, V. Direct and Indirect Biomimetic Peptide Modification of Alginate: Efficiency, Side Reactions, and Cell Response. Int. J. Mol. Sci. 2021, 22, 11. [Google Scholar] [CrossRef]
- Kim, Y.S.; Guo, J.L.; Lam, J.; Grande-Allen, K.J.; Engel, P.S.; Mikos, A.G. Synthesis of Injectable, Thermally Responsive, Chondroitin Sulfate-Cross-Linked Poly(N-isopropylacrylamide) Hydrogels. ACS Biomater. Sci. Eng. 2019, 5, 6405–6413. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Li, Q.; Neufeld, C.I.; Pouli, D.; Sun, S.; Yang, L.; Deng, P.; Wang, M.; Georgakoudi, I.; et al. Hyaluronic acid modification of RNase A and its intracellular delivery using lipid-like nanoparticles. J. Control. Release Off. J. Control. Release Soc. 2017, 263, 39–45. [Google Scholar] [CrossRef]
- Chan, A.T.; Karakas, M.F.; Vakrou, S.; Afzal, J.; Rittenbach, A.; Lin, X.; Wahl, R.L.; Pomper, M.G.; Steenbergen, C.J.; Tsui, B.M.W.; et al. Hyaluronic acid-serum hydrogels rapidly restore metabolism of encapsulated stem cells and promote engraftment. Biomaterials 2015, 73, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Khadka, R.; Carraher, C.; Hamiaux, C.; Travas-Sejdic, J.; Kralicek, A. Synergistic improvement in the performance of insect odorant receptor based biosensors in the presence of Orco. Biosens. Bioelectron. 2020, 153, 112040. [Google Scholar] [CrossRef] [PubMed]
- Strehin, I.; Nahas, Z.; Arora, K.; Nguyen, T.; Elisseeff, J. A versatile pH sensitive chondroitin sulfate–PEG tissue adhesive and hydrogel. Biomaterials 2010, 31, 2788–2797. [Google Scholar] [CrossRef]
- Chang, C.Y.; Chan, A.T.; Armstrong, P.A.; Luo, H.-C.; Higuchi, T.; Strehin, I.A.; Vakrou, S.; Lin, X.; Brown, S.N.; O’Rourke, B.; et al. Hyaluronic acid-human blood hydrogels for stem cell transplantation. Biomaterials 2012, 33, 8026–8033. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ma, Z.; Ke, Y.; Xia, Y.; Xu, X.; Liu, J.; Gong, Y.; Shi, Q.; Yin, J. An injectable serotonin–chondroitin sulfate hydrogel for bio-inspired hemostatic adhesives with high wound healing capability. Mater. Adv. 2021, 2, 5150–5159. [Google Scholar] [CrossRef]
- Vig, K.; Chaudhari, A.; Tripathi, S.; Dixit, S.; Sahu, R.; Pillai, S.; Shree, R.S. Advances in skin regeneration using tissue engineering. Int. J. Mol. Sci. 2017, 18, 789. [Google Scholar] [CrossRef]
- Jeong, K.H.; Park, D.; Lee, Y.C. Polymer-Based Hydrogel Scaffolds for Skin Tissue Engineering Applications: A Mini-Review. J. Polym. Res. 2017, 24, 112. [Google Scholar] [CrossRef]
- Stojic, M.; López, V.; Montero, A.; Quílez, C.; de Aranda, I.G.; Vojtova, L.; Velasco, D. Skin Tissue Engineering. Biomaterials for Skin Repair and Regeneration; Woodhead Publishing: Cambridge, UK, 2019; pp. 59–99. Available online: https://www.sciencedirect.com/science/article/pii/B9780081025468000030 (accessed on 1 May 2025).
- Choi, J.; Kim, H.; Choi, J.; Oh, S.M.; Park, J.; Park, K. Skin corrosion and irritation test of sunscreen nanoparticles using reconstructed 3D human skin model. Environ. Health Toxicol. 2014, 29, e2014004. [Google Scholar] [CrossRef]
- Reddy, M.S.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A comparative review of natural and synthetic biopolymer composite scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Llames, S.G.; Del Rio, M.; Larcher, F.; Garcia EGarcia MEscamez, M.J.; Jorcano, J.L.; Holguin, P.; Meana, A. Human plasma as a dermal scaffold for the generation of a completely autologous bioengineered skin. Transplantation 2004, 77, 350–355. [Google Scholar] [CrossRef]
- Kuo, J.W.; Swann, D.A.; Prestwich, G.D. Chemical modification of hyaluronic acid by carbodiimides. Bioconj. Chem. 1991, 2, 232–241. [Google Scholar] [CrossRef]
- McDonagh, B.H. Optimalised Carbodiimide Chemistry for RGD-Coupled Alginate. Master’s Thesis, Norwegian University of Science and Technology (NTNU), Trondheim, Norway, 2012. Available online: https://ntnuopen.ntnu.no/ntnu-xmlui/handle/11250/245812 (accessed on 1 May 2025).
- Li, M.; Shi, X.; Yang, B.; Qin, J.; Han, X.; Peng, W.; He, Y.; Mao, H.; Kong, D.; Gu, Z. Single-component hyaluronic acid hydrogel adhesive based on phenylboronic ester bonds for hemostasis and wound closure. Carbohydr. Polym. 2022, 296, 119953. [Google Scholar] [CrossRef]
- Iwasawa, T.; Wash, P.; Gibson, C.; Rebek, J. Reaction of an Introverted Carboxylic Acid with Carbodiimide. Tetrahedron 2007, 63, 6506–6511. [Google Scholar] [CrossRef]
- Saeger, M. Effect of Carbodimide Functionalization Chemistry on Alginate Structure and Hydrogel Properties. Master’s Thesis, Tufts University, Medford, MA, USA, 2016. [Google Scholar]
- Schulz, A.; Gepp, M.M.; Stracke, F.; von Briesen, H.; Neubauer, J.C.; Zimmermann, H. Tyramine-conjugated alginate hydrogels as a platform for bioactive scaffolds. J. Biomed. Mater. Res. Part A 2019, 107, 114–121. [Google Scholar] [CrossRef]
- Kamimura, W.; Hattori, R.; Koyama, H.; Miyata, T.; Takato, T. A calcium-cross-linked hydrogel based on alginate-modified atelocollagen functions as a scaffold material. J. Biomater. Sci. Polym. Ed. 2012, 23, 609–628. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, J.; Deng, F.; Guo, C.; Yang, Q.; Wu, H.; Ni, Y.; Huang, L.; Chen, L.; Ding, C. Dual-functionalized hyaluronic acid as a facile modifier to prepare polyanionic collagen. Carbohydr. Polym. 2019, 215, 358–365. [Google Scholar] [CrossRef]
- Hua, J.; Li, Z.; Xia, W.; Yang, N.; Gong, J.; Zhang, J.; Qiao, C. Preparation and properties of EDC/NHS mediated crosslinking poly (gamma-glutamic acid)/epsilon-polylysine hydrogels. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 879–892. [Google Scholar] [CrossRef]
- Fernandes-Cunha, G.M.; Chen, K.M.; Chen, F.; Le, P.; Han, J.H.; Mahajan, L.A.; Lee, H.J.; Na, K.S.; Myung, D. In situ-forming collagen hydrogel crosslinked via multi-functional PEG as a matrix therapy for corneal defects. Sci. Rep. 2020, 10, 16671. [Google Scholar] [CrossRef]
- Shpichka, A.I.; Koroleva, A.V.; Deiwick, A.; Timashev, P.S.; Semenova, E.F.; Moiseeva IYa Konoplyannikov, M.A.; Chichkov, B.N. Evaluation of the vasculogenic potential of hydrogels based on modified fibrin. Cell Tissue Biol. 2017, 11, 81–87. [Google Scholar] [CrossRef]
- Shpichka, A.I.; Konarev, P.V.; Efremov, Y.M.; Kryukova, A.E.; Aksenova, N.A.; Kotova, S.L.; Frolova, A.A.; Kosheleva, N.V.; Zhigalina, O.M.; Yusupov, V.I.; et al. Digging deeper: Structural background of PEGylated fibrin gels in cell migration and lumenogenesis. RSC Adv. 2020, 10, 4190–4200. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Q.; Li, J.; Du, X. Morphing-to-Adhesion Polysaccharide Hydrogel for Adaptive Biointerfaces. ACS Appl. Mater. Interfaces 2022, 14, 42420–42429. [Google Scholar] [CrossRef]
- Dadashzadeh, A.; Moghassemi, S.; Amorim, C.A. Evaluation of PEGylated fibrin as a three-dimensional biodegradable scaffold for ovarian tissue engineering. Mater. Today Chem. 2021, 22, 100626. [Google Scholar] [CrossRef]
- Jiang, L.; Dong, X.; Chen, L.; Han, R.; Hao, P.; Wang, L.; Gao, J.; Chen, X.; Li, X. A composite hydrogel membrane with shape and water retention for corneal tissue engineering. Heliyon 2023, 9, e17950. [Google Scholar] [CrossRef]
- Kavitha, N.; Karunya, T.P.; Kanchana, S.; Mohan, K.; Sivaramakrishnan, R.; Uthra, S.; Kapilan, K.; Yuvaraj, D.; Arumugam, M. Formulation of alginate based hydrogel from brown seaweed, Turbinaria conoides for biomedical applications. Heliyon 2019, 5, e02916. [Google Scholar] [CrossRef]
- Hu, C.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Ions-induced gelation of alginate: Mechanisms and applications. Int. J. Biol. Macromol. 2021, 177, 578–588. [Google Scholar] [CrossRef]
- Vorwald, C.E.; Gonzalez-Fernandez, T.; Joshee, S.; Sikorski, P.; Leach, J.K. Tunable Fibrin-Alginate Interpenetrating Network Hydrogels to Support Cell Spreading and Network Formation. Acta Biomater. 2020, 108, 142–152. [Google Scholar] [CrossRef]
- Sanz-Horta, R.; Matesanz, A.; Jorcano, J.L.; Velasco, D.; Acedo, P.; Gallardo, A.; Reinecke, H.; Elvira, C. Preparation and Characterization of Plasma-Derived Fibrin Hydrogels Modified by Alginate di-Aldehyde. Int. J. Mol. Sci. 2022, 23, 4296. [Google Scholar] [CrossRef]
- Natesan, S.; Stone, R.; Coronado, R.E.; Wrice, N.L.; Kowalczewski, A.C.; Zamora, D.O.; Christy, R.J. PEGylated Platelet-Free Blood Plasma-Based Hydrogels for Full-Thickness Wound Regeneration. Adv. Wound Care 2019, 8, 323–340. [Google Scholar] [CrossRef]
- Montalbetti, C.; Falque, V. Amide Bond Formation and Peptide Coupling. Tetrahedon 2005, 61, 10827–10852. [Google Scholar] [CrossRef]
- Montero, A.; Acosta, S.; Hernández, R.; Elvira, C.; Jorcano, J.L.; Velasco, D. Contraction of fibrin-derived matrices and its implications for in vitro human skin bioengineering. J. Biomed. Mater. Res. Part A 2021, 109, 500–514. [Google Scholar] [CrossRef]
- Lee, F.; Kurisawa, M. Formation and stability of interpenetrating polymer network hydrogels consisting of fibrin and hyaluronic acid for tissue engineering. Acta Biomater. 2013, 9, 5143–5152. [Google Scholar] [CrossRef]
- Burmeister, D.M.; Roy, D.C.; Becerra, S.C.; Natesan, S.; Christy, R.J. In Situ Delivery of Fibrin-Based Hydrogels Prevents Contraction and Reduces Inflammation. J. Burn Care Res. Off. Publ. Am. Burn Assoc. 2018, 39, 40–53. [Google Scholar] [CrossRef]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef]
- Murphy, K.C.; Whitehead, J.; Zhou, D.; Ho, S.S.; Leach, J.K. Engineering fibrin hydrogels to promote the wound healing potential of mesenchymal stem cell spheroids. Acta Biomater. 2017, 64, 176–186. [Google Scholar] [CrossRef]
- Somasekharan, L.; Kasoju, N.; Raju, R.; Bhatt, A. Formulation and Characterization of Alginate Dialdehyde, Gelatin, and Platelet-Rich Plasma-Based Bioink for Bioprinting Applications. Bioengineering 2020, 7, 108. [Google Scholar] [CrossRef]
- Sarker, B.; Singh, R.; Silva, R.; Roether, J.A.; Kaschta, J.; Detsch, R.; Schubert, D.W.; Cicha, I.; Boccaccini, A.R. Evaluation of Fibroblasts Adhesion and Proliferation on Alginate-Gelatin Crosslinked Hydrogel. PLoS ONE 2014, 9, e107952. [Google Scholar] [CrossRef]
- Li, S.; Nih, L.R.; Bachman, H.; Fei, P.; Li, Y.; Nam, E.; Dimatteo, R.; Carmichael, S.T.; Barker, T.H.; Segura, T. Hydrogels with precisely controlled integrin activation dictate vascular patterning and permeability. Nat. Mater. 2017, 16, 953–961. [Google Scholar] [CrossRef]
- Liu, J.C.; Tirrell, D.A. Cell Response to RGD Density in Cross-Linked Artificial Extracellular Matrix Protein Films. Biomacromolecules 2008, 9, 2984–2988. [Google Scholar] [CrossRef][Green Version]
- Tan, J.J.Y.; Nguyen, D.-V.; Common, J.E.; Wu, C.; Ho, P.C.L.; Kang, L. Investigating PEGDA and GelMA Microgel Models for Sustained 3D Heterotypic Dermal Papilla and Keratinocyte Co-Cultures. Int. J. Mol. Sci. 2021, 22, 4. [Google Scholar] [CrossRef]
- Chen, X.; Liu, C.; McDaniel, G.; Zeng, O.; Ali, J.; Zhou, Y.; Wang, X.; Driscoll, T.; Zeng, C.; Li, Y. Viscoelasticity of Hyaluronic Acid Hydrogels Regulates Human Pluripotent Stem Cell-derived Spinal Cord Organoid Patterning and Vascularization. Adv. Healthc. Mater. 2024, 13, e2402199. [Google Scholar] [CrossRef]
- Kong, D.; Nguyen, K.D.Q.; Megone, W.; Peng, L.; Gautrot, J.E. The culture of HaCaT cells on liquid substrates is mediated by a mechanically strong liquid–liquid interface. Faraday Discuss 2017, 204, 367–381. [Google Scholar] [CrossRef]
- Song, J.; Shea, C.R. Benign versus malignant parakeratosis: A nuclear morphometry study. Mod. Pathol. 2010, 23, 799–803. [Google Scholar] [CrossRef]
- Berger, F.M.; Ludwig, B.J.; Wielich, K.H. The hydrophilic and acid binding properties of alginates. Am. J. Dig. Dis. 1953, 20, 39–42. [Google Scholar] [CrossRef]
- Savić Gajić, I.M.; Savić, I.M.; Svirčev, Z. Preparation and Characterization of Alginate Hydrogels with High Water-Retaining Capacity. Polymers 2023, 15, 12. [Google Scholar] [CrossRef]
- Montero, A.; Quílez, C.; Valencia, L.; Girón, P.; Jorcano, J.L.; Velasco, D. Effect of Fibrin Concentration on the In Vitro Production of Dermo-Epidermal Equivalents. Int. J. Mol. Sci. 2021, 22, 13. [Google Scholar] [CrossRef]
- Meana, A.; Iglesias, J.; Del Rio, M.; Larcher, F.; Madrigal, B.; Fresno, M.F.; Martin, C.; San Roman, F.; Tevar, F. Large surface of cultured human epithelium obtained on a dermal matrix based on live fibroblast-containing fibrin gels. Burn J. Int. Soc. Burn Inj. 1998, 24, 621–630. [Google Scholar] [CrossRef]
- Del Rio, M.; Larcher, F.; Serrano, F.; Meana, A.; Muñoz, M.; Garcia, M.; Muñoz, E.; Martin, C.; Bernad, A.; Jorcano, J.L. A preclinical model for the analysis of genetically modified human skin in vivo. Hum. Gene Ther. 2002, 13, 959–968. [Google Scholar] [CrossRef]
- Rheinwald, J.G.; Green, H. Serial cultivation of strains of human epidermal keratinocytes: The formation of keratinizing colonies from single cells. Cell 1975, 6, 331–343. [Google Scholar] [CrossRef]
- Gantenbein-Ritter, B.; Sprecher, C.M.; Chan, S.; Illien-Jünger, S.; Grad, S. Confocal imaging protocols for live/dead staining in three-dimensional carriers. Methods Mol. Biol. 2011, 740, 127–140. [Google Scholar] [CrossRef]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008, 2008, pdb.prot4986. [Google Scholar] [CrossRef]
| Precipitation Solvent | Reaction Time (min) | % NHS |
|---|---|---|
| Ethanol | 30 | 14.8 |
| Ethanol | 60 | 13.0 |
| Dioxane | 30 | 11.1 |
| DMF | 30 | 13.6 |
| Isopropanol | 30 | 13.0 |
| Isopropanol | 120 | 26.8 |
| SA Final Concentration in Hydrogel (mg/ML) | Gelification Time (Every 5 Min) | Gelification (Yes/No) |
|---|---|---|
| Control (0 mg/mL) | 15 | Yes |
| 0.5 | 10 | Yes |
| 1.0 | 10 | Yes |
| 1.5 | 15 | Yes |
| 2.0 | 15 | Yes |
| 2.5 | 15 | Yes |
| 3.0 | 15 | Yes |
| 3.5 | 20 | Yes |
| 4.0 | 20 | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matesanz, A.; Sanz-Horta, R.; Gallardo, A.; Quílez, C.; Reinecke, H.; Acedo, P.; Velasco, D.; Martínez-Campos, E.; Jorcano, J.L.; Elvira, C. Succinimidyl Alginate-Modified Fibrin Hydrogels from Human Plasma for Skin Tissue Engineering. Gels 2025, 11, 540. https://doi.org/10.3390/gels11070540
Matesanz A, Sanz-Horta R, Gallardo A, Quílez C, Reinecke H, Acedo P, Velasco D, Martínez-Campos E, Jorcano JL, Elvira C. Succinimidyl Alginate-Modified Fibrin Hydrogels from Human Plasma for Skin Tissue Engineering. Gels. 2025; 11(7):540. https://doi.org/10.3390/gels11070540
Chicago/Turabian StyleMatesanz, Ana, Raúl Sanz-Horta, Alberto Gallardo, Cristina Quílez, Helmut Reinecke, Pablo Acedo, Diego Velasco, Enrique Martínez-Campos, José Luis Jorcano, and Carlos Elvira. 2025. "Succinimidyl Alginate-Modified Fibrin Hydrogels from Human Plasma for Skin Tissue Engineering" Gels 11, no. 7: 540. https://doi.org/10.3390/gels11070540
APA StyleMatesanz, A., Sanz-Horta, R., Gallardo, A., Quílez, C., Reinecke, H., Acedo, P., Velasco, D., Martínez-Campos, E., Jorcano, J. L., & Elvira, C. (2025). Succinimidyl Alginate-Modified Fibrin Hydrogels from Human Plasma for Skin Tissue Engineering. Gels, 11(7), 540. https://doi.org/10.3390/gels11070540









