Recent Achievements of Epicardial Patch Electronics Using Adhesive and Conductive Hydrogels
Abstract
1. Introduction
2. Adhesive Hydrogel
2.1. Physically Adhesive Hydrogel
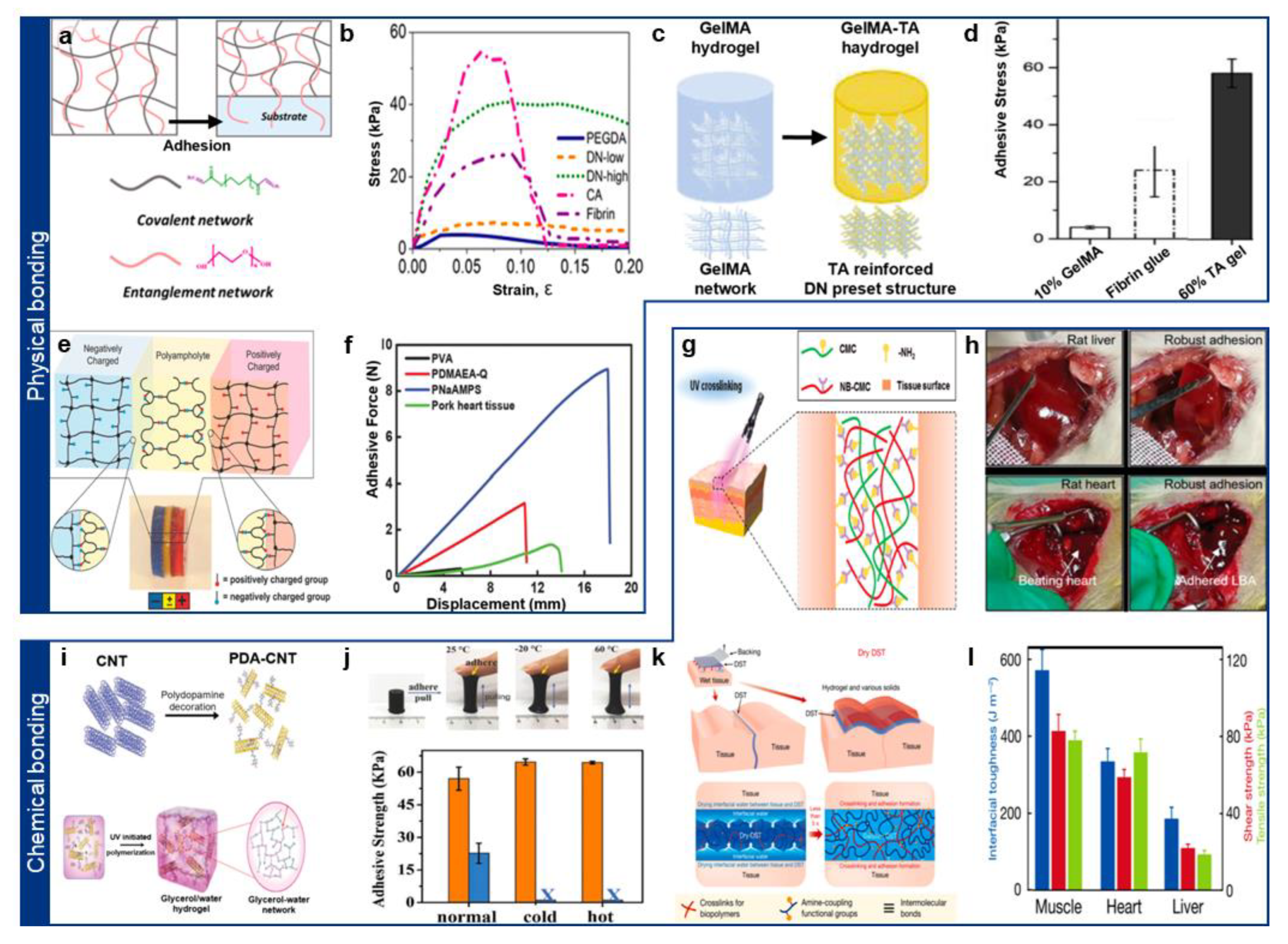
2.2. Chemically Adhesive Hydrogel
3. Conductive Hydrogel
3.1. Ionically Conductive Hydrogel
3.2. Conductive Polymer-Based Hydrogel

3.3. Conductive Hydrogel Nanocomposite
| Properties | Representative Materials | Mechanism of Conductivity Enhancement | Advantages | Limitations | Ref. | |
|---|---|---|---|---|---|---|
| Materials | ||||||
| Metallic Nanomaterials | Silver nanowires | Percolated metallic networks for electronic conduction | High conductivity, facile synthesis | Potential cytotoxicity, poor flexibility at high loadings | [80,81] | |
| Liquid metals | Flowable metallic phases with deformability | Intrinsic softness, good mechanical compliance | Stability issues, oxidation | [82,83] | ||
| MXenes | 2D layered structure allowing both ionic and electronic conduction | High surface area, excellent mixed conductivity | Sensitive to oxidation, dispersion stability | [84,85] | ||
| Carbon-based Nanomaterials | Carbon nanotubes (CNTs) | Percolated conductive networks | High conductivity, mechanical reinforcement | Aggregation, surface functionalization often required | [86,87] | |
| Graphene/Graphene oxide | Planar sheets forming electron pathways | High electrical and mechanical performance | Restacking tendencies, potential biocompatibility concerns | [19,88,89] | ||
| Carbon black | Conductive filler forming percolated paths | Cost-effective, scalable | Lower conductivity than CNTs or graphene | [90,91] | ||
4. Biomedical Applications of Epicardial Patch
4.1. Minimally Invasive Deployment on Epicardial Surfaces
4.2. Epicardial Patches for Epicardiographic Recording

4.3. Epicardial Patches for Epicardial Stimulation
4.4. Epicardial Patches for Drug and Cell Delivery
5. Current Limitations and Prospects
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sunwoo, S.H.; Ha, K.H.; Lee, S.; Lu, N.; Kim, D.H. Wearable and Implantable Soft Bioelectronics: Device Designs and Material Strategies. Annu. Rev. Chem. Biomol. Eng. 2021, 12, 359–391. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.J.; Jeong, H.; Cho, K.W.; Lu, N.; Kim, D.H. Wearable and Implantable Devices for Cardiovascular Healthcare: From Monitoring to Therapy Based on Flexible and Stretchable Electronics. Adv. Funct. Mater. 2019, 29, 1808247. [Google Scholar] [CrossRef]
- Han, S.I.; Sunwoo, S.H.; Park, C.S.; Lee, S.P.; Hyeon, T.; Kim, D.H. Next-Generation Cardiac Interfacing Technologies Using Nanomaterial-Based Soft Bioelectronics. ACS Nano 2024, 18, 12025–12048. [Google Scholar] [CrossRef]
- Feiner, R.; Dvir, T. Tissue–Electronics Interfaces: From Implantable Devices to Engineered Tissues. Nat. Rev. Mater. 2017, 3, 17076. [Google Scholar] [CrossRef]
- Fallegger, F.; Schiavone, G.; Lacour, S.P. Conformable Hybrid Systems for Implantable Bioelectronic Interfaces. Adv. Mater. 2020, 32, 1903904. [Google Scholar] [CrossRef]
- Robotti, F.; Sterner, I.; Bottan, S.; Monné Rodríguez, J.M.; Pellegrini, G.; Schmidt, T.; Falk, V.; Poulikakos, D.; Ferrari, A.; Starck, C. Microengineered Biosynthesized Cellulose as Anti-Fibrotic in Vivo Protection for Cardiac Implantable Electronic Devices. Biomaterials 2020, 229, 119583. [Google Scholar] [CrossRef]
- Sunwoo, S.-H.; Han, S.I.; Park, C.S.; Kim, J.H.; Georgiou, J.S.; Lee, S.-P.; Kim, D.-H.; Hyeon, T. Soft Bioelectronics for the Management of Cardiovascular Diseases. Nat. Rev. Bioeng. 2023, 2, 8–24. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, N.; Xu, J.; Liu, Z.; Zhou, Y.; Yang, Y.; Li, S.; Huang, Y.; Jiang, S. Flexible Electronics for Cardiovascular Healthcare Monitoring. Innovation 2023, 4, 100485. [Google Scholar] [CrossRef]
- Lin, R.; Lei, M.; Ding, S.; Cheng, Q.; Ma, Z.; Wang, L.; Tang, Z.; Zhou, B.; Zhou, Y. Applications of Flexible Electronics Related to Cardiocerebral Vascular System. Mater. Today Bio 2023, 23, 100787. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, K.; Shen, G. Wearable, Implantable, and Interventional Medical Devices Based on Smart Electronic Skins. Adv. Mater. Technol. 2021, 6, 2100107. [Google Scholar] [CrossRef]
- Kim, S.; Shin, Y.; Han, J.; Kim, H.J.; Sunwoo, S.; Kim, S.; Shin, Y.; Han, J.; Kim, H.J.; Sunwoo, S.-H. Introductory Review of Soft Implantable Bioelectronics Using Conductive and Functional Hydrogels and Hydrogel Nanocomposites. Gels 2024, 10, 614. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Hong, Y.J.; Jung, J.; Shin, Y.; Sunwoo, S.H.; Baik, S.; Park, O.K.; Choi, S.H.; Hyeon, T.; Kim, J.H.; et al. Tissue-like Skin-Device Interface for Wearable Bioelectronics by Using Ultrasoft, Mass-Permeable, and Low-Impedance Hydrogels. Sci. Adv. 2021, 7, eabd3716. [Google Scholar] [CrossRef]
- Chang, Z.; Wang, B.; Ren, Q.; Nie, J.; Guo, B.; Lu, Y.; Lu, X.; Zhang, Y.; Ji, D.; Lv, Y.; et al. Fully Implantable Wireless Cardiac Pacing and Sensing System Integrated with Hydrogel Electrodes. Adv. Sci. 2024, 11, 2401982. [Google Scholar] [CrossRef]
- Li, J.; Wang, X. Materials Perspectives for Self-Powered Cardiac Implantable Electronic Devices toward Clinical Translation. Acc. Mater. Res. 2021, 2, 739–750. [Google Scholar] [CrossRef]
- Yuk, H.; Lu, B.; Zhao, X. Hydrogel Bioelectronics. Chem. Soc. Rev. 2019, 48, 1642–1667. [Google Scholar] [CrossRef]
- Lee, J.; Cho, H.R.; Cha, G.D.; Seo, H.; Lee, S.; Park, C.K.; Kim, J.W.; Qiao, S.; Wang, L.; Kang, D.; et al. Flexible, Sticky, and Biodegradable Wireless Device for Drug Delivery to Brain Tumors. Nat. Commun. 2019, 10, 5205. [Google Scholar] [CrossRef]
- Yuen, H.Y.; Bei, H.P.; Zhao, X. Underwater and Wet Adhesion Strategies for Hydrogels in Biomedical Applications. Chem. Eng. J. 2022, 431, 133372. [Google Scholar] [CrossRef]
- Kang, T.; Cha, G.D.; Park, O.K.; Cho, H.R.; Kim, M.; Lee, J.; Kim, D.; Lee, B.; Chu, J.; Koo, S.; et al. Penetrative and Sustained Drug Delivery Using Injectable Hydrogel Nanocomposites for Postsurgical Brain Tumor Treatment. ACS Nano 2023, 17, 5435–5447. [Google Scholar] [CrossRef]
- Deng, J.; Yuk, H.; Wu, J.; Varela, C.E.; Chen, X.; Roche, E.T.; Guo, C.F.; Zhao, X. Electrical Bioadhesive Interface for Bioelectronics. Nat. Mater. 2020, 20, 229–236. [Google Scholar] [CrossRef]
- Cha, G.D.; Lee, W.H.; Sunwoo, S.H.; Kang, D.; Kang, T.; Cho, K.W.; Kim, M.; Park, O.K.; Jung, D.; Lee, J.; et al. Multifunctional Injectable Hydrogel for in Vivo Diagnostic and Therapeutic Applications. ACS Nano 2022, 16, 554–567. [Google Scholar] [CrossRef]
- Bovone, G.; Dudaryeva, O.Y.; Marco-Dufort, B.; Tibbitt, M.W. Engineering Hydrogel Adhesion for Biomedical Applications via Chemical Design of the Junction. ACS Biomater. Sci. Eng. 2021, 7, 4048–4076. [Google Scholar] [CrossRef] [PubMed]
- Nam, M.; Lee, J.W.; Cha, G.D. Biomedical Application of Enzymatically Crosslinked Injectable Hydrogels. Gels 2024, 10, 640. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cong, Y.; Fu, J. Tissue Adhesive Hydrogel Bioelectronics. J. Mater. Chem. B 2021, 9, 4423–4443. [Google Scholar] [CrossRef]
- Chen, K.; Feng, Y.; Zhang, Y.; Yu, L.; Hao, X.; Shao, F.; Dou, Z.; An, C.; Zhuang, Z.; Luo, Y.; et al. Entanglement-Driven Adhesion, Self-Healing, and High Stretchability of Double-Network PEG-Based Hydrogels. ACS Appl. Mater. Interfaces 2019, 11, 36458–36468. [Google Scholar] [CrossRef] [PubMed]
- Cintron-Cruz, J.A.; Freedman, B.R.; Lee, M.; Johnson, C.; Ijaz, H.; Mooney, D.J. Rapid Ultratough Topological Tissue Adhesives. Adv. Mater. 2022, 34, 2205567. [Google Scholar] [CrossRef]
- De Gennes, P.G. Dynamics of Entangled Polymer Solutions. I. The Rouse Model. Macromolecules 1976, 9, 587–593. [Google Scholar] [CrossRef]
- Jo, Y.; Lee, Y.; Heo, J.H.; Son, Y.; Kim, T.Y.; Park, K.; Kim, S.; Kim, S.J.; Jin, Y.; Park, S.; et al. Universal Hydrogel Adhesives with Robust Chain Entanglement for Bridging Soft Electronic Materials. NPJ Flex. Electron. 2024, 8, 39. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Y.; Chen, T.; Bai, L.; Li, H.; Tan, H.; Liu, C.; Qu, X. Chain Entanglement-Driven Tough, Fatigue-Resistant PEG-Based Injectable Hydrogel Adhesive for Joint Skin Wound Healing. Compos. Part B Eng. 2023, 266, 110991. [Google Scholar] [CrossRef]
- Chen, B.; Zhu, D.; Li, Q.; Wang, C.; Cui, J.; Zheng, Z.; Wang, X. Mechanically Reinforced and Injectable Universal Adhesive Based on a PEI-PAA/Alg Dual-Network Hydrogel Designed by Topological Entanglement and Catechol Chemistry. ACS Appl. Mater. Interfaces 2023, 15, 59826–59837. [Google Scholar] [CrossRef]
- Liu, H.; Hu, X.; Li, W.; Zhu, M.; Tian, J.; Li, L.; Luo, B.; Zhou, C.; Lu, L. A Highly-Stretchable and Adhesive Hydrogel for Noninvasive Joint Wound Closure Driven by Hydrogen Bonds. Chem. Eng. J. 2023, 452, 139368. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Y.; Li, J.; You, M.; Yu, Y.; Yang, J.; Qin, G.; Chen, Q. Tough Wet Adhesion of Hydrogen-Bond-Based Hydrogel with On-Demand Debonding and Efficient Hemostasis. ACS Appl. Mater. Interfaces 2022, 14, 36166–36177. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Wang, J.; Jin, Z. Tough, Swelling-Resistant, Self-Healing, and Adhesive Dual-Cross-Linked Hydrogels Based on Polymer-Tannic Acid Multiple Hydrogen Bonds. Macromolecules 2018, 51, 1696–1705. [Google Scholar] [CrossRef]
- Han, L.; Lu, X.; Liu, K.; Wang, K.; Fang, L.; Weng, L.T.; Zhang, H.; Tang, Y.; Ren, F.; Zhao, C.; et al. Mussel-Inspired Adhesive and Tough Hydrogel Based on Nanoclay Confined Dopamine Polymerization. ACS Nano 2017, 11, 2561–2574. [Google Scholar] [CrossRef]
- Liu, B.; Wang, Y.; Miao, Y.; Zhang, X.; Fan, Z.; Singh, G.; Zhang, X.; Xu, K.; Li, B.; Hu, Z.; et al. Hydrogen Bonds Autonomously Powered Gelatin Methacrylate Hydrogels with Super-Elasticity, Self-Heal and Underwater Self-Adhesion for Sutureless Skin and Stomach Surgery and E-Skin. Biomaterials 2018, 171, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.K.; Guo, H.L.; Sun, T.L.; Ihsan, A.-B.; Kurokawa, T.; Takahata, M.; Nonoyama, T.; Nakajima, T.; Gong, J.P. Self-Adjustable Adhesion of Polyampholyte Hydrogels. Adv. Mater. 2015, 27, 7344–7349. [Google Scholar] [CrossRef]
- Ma, Y.; Yao, J.; Liu, Q.; Han, T.; Zhao, J.; Ma, X.; Tong, Y.; Jin, G.; Qu, K.; Li, B.; et al. Liquid Bandage Harvests Robust Adhesive, Hemostatic, and Antibacterial Performances as a First-Aid Tissue Adhesive. Adv. Funct. Mater. 2020, 30, 2001820. [Google Scholar] [CrossRef]
- Han, L.; Liu, K.; Wang, M.; Wang, K.; Fang, L.; Chen, H.; Zhou, J.; Lu, X. Mussel-Inspired Adhesive and Conductive Hydrogel with Long-Lasting Moisture and Extreme Temperature Tolerance. Adv. Funct. Mater. 2018, 28, 1704195. [Google Scholar] [CrossRef]
- Yuk, H.; Varela, C.E.; Nabzdyk, C.S.; Mao, X.; Padera, R.F.; Roche, E.T.; Zhao, X. Dry Double-Sided Tape for Adhesion of Wet Tissues and Devices. Nature 2019, 575, 169–174. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, S.; Ren, X.; Zhang, J.; Lin, Q.; Zhao, Y. Supramolecular Adhesive Hydrogels for Tissue Engineering Applications. Chem. Rev. 2022, 122, 5604–5640. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, R.; Li, S.; Xue, X.; Zhang, Q.; Shi, F.; Cheng, M. Robust Electrostatically Interactive Hydrogel Coatings for Macroscopic Supramolecular Assembly via Rapid Wet Adhesion. ACS Appl. Mater. Interfaces 2023, 15, 21640–21650. [Google Scholar] [CrossRef]
- Tang, S.; Huang, L.; Shi, Z.; He, W. Water-Based Synthesis of Cationic Hydrogel Particles: Effect of the Reaction Parameters and in Vitro Cytotoxicity Study. J. Mater. Chem. B 2015, 3, 2842–2852. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liang, X.; Ma, W.; An, X.; Wu, H.; Zhang, Q.; Jia, X. An Integrated Asymmetric Wet Adhesive Hydrogel with Gradient Charge Distribution Induced by Electrostatic Field. Adv. Funct. Mater. 2024, 34, 2310233. [Google Scholar] [CrossRef]
- Yang, J.; Bai, R.; Chen, B.; Suo, Z. Hydrogel Adhesion: A Supramolecular Synergy of Chemistry, Topology, and Mechanics. Adv. Funct. Mater. 2020, 30, 1901693. [Google Scholar] [CrossRef]
- Ho, M.H.; van Hilst, Q.; Cui, X.; Ramaswamy, Y.; Woodfield, T.; Rnjak-Kovacina, J.; Wise, S.G.; Lim, K.S. From Adhesion to Detachment: Strategies to Design Tissue-Adhesive Hydrogels. Adv. NanoBiomed Res. 2024, 4, 2300090. [Google Scholar] [CrossRef]
- Anderson, E.B.; Long, T.E. Imidazole- and Imidazolium-Containing Polymers for Biology and Material Science Applications. Polymer 2010, 51, 2447–2454. [Google Scholar] [CrossRef]
- Yang, J.; Yu, H.; Wang, L.; Liu, J.; Liu, X.; Hong, Y.; Huang, Y.; Ren, S. Advances in Adhesive Hydrogels for Tissue Engineering. Eur. Polym. J. 2022, 172, 111241. [Google Scholar] [CrossRef]
- Leggat, P.A.; Smith, D.R.; Kedjarune, U. Surgical Applications of Cyanoacrylate Adhesives: A Review of Toxicity. ANZ J. Surg. 2007, 77, 209–213. [Google Scholar] [CrossRef]
- Yu, F.; Cao, X.; Du, J.; Wang, G.; Chen, X. Multifunctional Hydrogel with Good Structure Integrity, Self-Healing, and Tissue-Adhesive Property Formed by Combining Diels-Alder Click Reaction and Acylhydrazone Bond. ACS Appl. Mater. Interfaces 2015, 7, 24023–24031. [Google Scholar] [CrossRef]
- Nam, S.; Mooney, D. Polymeric Tissue Adhesives. Chem. Rev. 2021, 121, 11336–11384. [Google Scholar] [CrossRef]
- Uslu, E.; Rana, V.K.; Guo, Y.; Stampoultzis, T.; Gorostidi, F.; Sandu, K.; Pioletti, D.P. Enhancing Robustness of Adhesive Hydrogels through PEG-NHS Incorporation. ACS Appl. Mater. Interfaces 2023, 15, 50095–50105. [Google Scholar] [CrossRef]
- Kim, M.; Lee, H.; Nam, S.; Kim, D.H.; Cha, G.D. Soft Bioelectronics Using Nanomaterials and Nanostructures for Neuroengineering. Acc. Chem. Res. 2024, 57, 1633–1647. [Google Scholar] [CrossRef]
- Yoo, S.; Kim, M.; Choi, C.; Kim, D.H.; Cha, G.D. Soft Bioelectronics for Neuroengineering: New Horizons in the Treatment of Brain Tumor and Epilepsy. Adv. Healthc. Mater. 2024, 13, 2303563. [Google Scholar] [CrossRef]
- Zhu, T.; Ni, Y.; Biesold, G.M.; Cheng, Y.; Ge, M.; Li, H.; Huang, J.; Lin, Z.; Lai, Y. Recent Advances in Conductive Hydrogels: Classifications, Properties, and Applications. Chem. Soc. Rev. 2023, 52, 473–509. [Google Scholar] [CrossRef]
- Cha, G.D.; Kim, M.; Park, O.K.; Sunwoo, S.H.; Kang, T.; Lee, W.H.; Nam, S.; Hyeon, T.; Choi, S.H.; Kim, D.H. Minimally-Invasive and In-Vivo Hydrogel Patterning Method for In Situ Fabrication of Implantable Hydrogel Devices. Small Methods 2023, 7, 2300032. [Google Scholar] [CrossRef]
- Cha, G.D.; Lee, W.H.; Lim, C.; Choi, M.K.; Kim, D.H. Materials Engineering, Processing, and Device Application of Hydrogel Nanocomposites. Nanoscale 2020, 12, 10456–10473. [Google Scholar] [CrossRef]
- Fu, F.; Wang, J.; Zeng, H.; Yu, J. Functional Conductive Hydrogels for Bioelectronics. ACS Mater. Lett. 2020, 2, 1287–1301. [Google Scholar] [CrossRef]
- Liu, K.; Wei, S.; Song, L.; Liu, H.; Wang, T. Conductive Hydrogels—A Novel Material: Recent Advances and Future Perspectives. J. Agric. Food Chem. 2020, 68, 7269–7280. [Google Scholar] [CrossRef]
- Chong, J.; Sung, C.; Nam, K.S.; Kang, T.; Kim, H.; Lee, H.; Park, H.; Park, S.; Kang, J. Highly Conductive Tissue-like Hydrogel Interface through Template-Directed Assembly. Nat. Commun. 2023, 14, 2206. [Google Scholar] [CrossRef]
- Yang, B.; Yuan, W. Highly Stretchable and Transparent Double-Network Hydrogel Ionic Conductors as Flexible Thermal-Mechanical Dual Sensors and Electroluminescent Devices. ACS Appl. Mater. Interfaces 2019, 11, 16765–16775. [Google Scholar] [CrossRef]
- Wang, S.; Yu, L.; Wang, S.; Zhang, L.; Chen, L.; Xu, X.; Song, Z.; Liu, H.; Chen, C. Strong, Tough, Ionic Conductive, and Freezing-Tolerant All-Natural Hydrogel Enabled by Cellulose-Bentonite Coordination Interactions. Nat. Commun. 2022, 13, 3408. [Google Scholar] [CrossRef]
- Mo, F.; Zhou, P.; Lin, S.; Zhong, J.; Wang, Y. A Review of Conductive Hydrogel-Based Wearable Temperature Sensors. Adv. Healthc. Mater. 2024, 13, 2401503. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, T.; Xie, X.; Song, G.; Ma, X.; Mu, Q.; Huang, Z.; Liu, X.; Sun, C.; Xu, W. Advances and Challenges in Conductive Hydrogels: From Properties to Applications. Eur. Polym. J. 2022, 177, 111454. [Google Scholar] [CrossRef]
- Wang, Z.; Lai, Y.C.; Chiang, Y.T.; Scheiger, J.M.; Li, S.; Dong, Z.; Cai, Q.; Liu, S.; Hsu, S.H.; Chou, C.C.; et al. Tough, Self-Healing, and Conductive Elastomer-Ionic PEGgel. ACS Appl. Mater. Interfaces 2022, 14, 50152–50162. [Google Scholar] [CrossRef]
- Ghorbanizamani, F.; Moulahoum, H.; Guler Celik, E.; Timur, S. Ionic Liquids Enhancement of Hydrogels and Impact on Biosensing Applications. J. Mol. Liq. 2022, 357, 119075. [Google Scholar] [CrossRef]
- Hu, X.; Cheng, Y.; Wei, Z.; Zhang, R.; Zhan, Y.; Xia, H. Enhanced Sensing and Electromagnetic Interference Shielding Performance of Hydrogels by the Incorporation of Ionic Liquids. ACS Appl. Electron. Mater. 2024, 6, 1770–1780. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, S.; Qian, L.; Wei, N.; Nica, V.; Coseri, S.; Han, F. Super Stretchable, Self-Healing, Adhesive Ionic Conductive Hydrogels Based on Tailor-Made Ionic Liquid for High-Performance Strain Sensors. Adv. Funct. Mater. 2022, 32, 2204565. [Google Scholar] [CrossRef]
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive Polymers: Opportunities and Challenges in Biomedical Applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef]
- Tan, P.; Wang, H.; Xiao, F.; Lu, X.; Shang, W.; Deng, X.; Song, H.; Xu, Z.; Cao, J.; Gan, T.; et al. Solution-Processable, Soft, Self-Adhesive, and Conductive Polymer Composites for Soft Electronics. Nat. Commun. 2022, 13, 358. [Google Scholar] [CrossRef]
- Namsheer, K.; Rout, C.S. Conducting Polymers: A Comprehensive Review on Recent Advances in Synthesis, Properties and Applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar] [CrossRef]
- Shi, Y.; Pan, L.; Liu, B.; Wang, Y.; Cui, Y.; Bao, Z.; Yu, G. Nanostructured Conductive Polypyrrole Hydrogels as High-Performance, Flexible Supercapacitor Electrodes. J. Mater. Chem. A 2014, 2, 6086–6091. [Google Scholar] [CrossRef]
- Li, L.; Ai, Z.; Wu, J.; Lin, Z.; Huang, M.; Gao, Y.; Bai, H. A Robust Polyaniline Hydrogel Electrode Enables Superior Rate Capability at Ultrahigh Mass Loadings. Nat. Commun. 2024, 15, 6591. [Google Scholar] [CrossRef]
- Inal, S.; Rivnay, J.; Suiu, A.O.; Malliaras, G.G.; McCulloch, I. Conjugated Polymers in Bioelectronics. Acc. Chem. Res. 2018, 51, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Cheng, Y.; Huang, C.; Chen, R.; Wang, Z.; Wei, J. Self-Healing Conductive Hydrogels Based on Alginate, Gelatin and Polypyrrole Serve as a Repairable Circuit and a Mechanical Sensor. J. Mater. Chem. B 2019, 7, 5704–5712. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Yuk, H.; Lin, S.; Jian, N.; Qu, K.; Xu, J.; Zhao, X. Pure PEDOT:PSS Hydrogels. Nat. Commun. 2019, 10, 1043. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, X.; Tan, R.; Zhang, S.; Zhang, K.; Hu, J. Hierarchically Structured Hydrogel Composites with Ultra-High Conductivity for Soft Electronics. Adv. Funct. Mater. 2024, 34, 2312667. [Google Scholar] [CrossRef]
- Feig, V.R.; Tran, H.; Lee, M.; Bao, Z. Mechanically Tunable Conductive Interpenetrating Network Hydrogels That Mimic the Elastic Moduli of Biological Tissue. Nat. Commun. 2018, 9, 2740. [Google Scholar] [CrossRef]
- Ahn, Y.; Lee, H.; Lee, D.; Lee, Y. Highly Conductive and Flexible Silver Nanowire-Based Microelectrodes on Biocompatible Hydrogel. ACS Appl. Mater. Interfaces 2014, 6, 18401–18407. [Google Scholar] [CrossRef]
- Liu, X.; Miller, A.L.; Park, S.; Waletzki, B.E.; Zhou, Z.; Terzic, A.; Lu, L. Functionalized Carbon Nanotube and Graphene Oxide Embedded Electrically Conductive Hydrogel Synergistically Stimulates Nerve Cell Differentiation. ACS Appl. Mater. Interfaces 2017, 9, 14677–14690. [Google Scholar] [CrossRef]
- Zhao, R.; Xu, X.; Hu, L. Highly Strong, Stretchable, and Conductive Reduced Graphene Oxide Composite Hydrogel-Based Sensors for Monitoring Strain and Pressure. ACS Appl. Polym. Mater. 2021, 3, 5155–5161. [Google Scholar] [CrossRef]
- Kim, J.; Cha, G.D.; Kim, M.; Lee, S.-P.; Sunwoo, S.-H.; Kim, D.-H. Soft Cardiac Patch Using a Bifacial Architecture of Adhesive/Low-Impedance Hydrogel Nanocomposites and Highly Conductive Elastomer Nanocomposites. Adv. NanoBiomed Res. 2025, 5, 2400143. [Google Scholar] [CrossRef]
- Du, Y.; Yan, X.; Chen, S.; Zha, Z.; Wu, W.; Song, Y.; Wu, Y.; Li, K.; Liu, X.; Lu, Y. Silver Nanowire Reinforced Conductive and Injectable Colloidal Gel for Effective Wound Healing Via Electrical Stimulation. Adv. Healthc. Mater. 2024, 13, 2301420. [Google Scholar] [CrossRef]
- Xu, Y.; Rothe, R.; Voigt, D.; Hauser, S.; Cui, M.; Miyagawa, T.; Patino Gaillez, M.; Kurth, T.; Bornhäuser, M.; Pietzsch, J.; et al. Convergent Synthesis of Diversified Reversible Network Leads to Liquid Metal-Containing Conductive Hydrogel Adhesives. Nat. Commun. 2021, 12, 1412. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, H.; Yun, G.; Gong, L.; Chen, Z.; Jin, S.; Du, H.; Jiang, Z.; Li, W. A Laminated Gravity-Driven Liquid Metal-Doped Hydrogel of Unparalleled Toughness and Conductivity. Adv. Funct. Mater. 2024, 34, 2318113. [Google Scholar] [CrossRef]
- Hou, M.; Yu, M.; Liu, W.; Zhang, H.; Wang, Z.; Du, J.; Xu, L.; Li, N.; Xu, J. Mxene Hybrid Conductive Hydrogels with Mechanical Flexibility, Frost-Resistance, Photothermoelectric Conversion Characteristics and Their Multiple Applications in Sensing. Chem. Eng. J. 2024, 483, 149299. [Google Scholar] [CrossRef]
- Liu, H.; Du, C.; Liao, L.; Zhang, H.; Zhou, H.; Zhou, W.; Ren, T.; Sun, Z.; Lu, Y.; Nie, Z.; et al. Approaching Intrinsic Dynamics of MXenes Hybrid Hydrogel for 3D Printed Multimodal Intelligent Devices with Ultrahigh Superelasticity and Temperature Sensitivity. Nat. Commun. 2022, 13, 31051. [Google Scholar] [CrossRef]
- Cui, Z.; Zhou, M.; Greensmith, P.J.; Wang, W.; Hoyland, J.A.; Kinloch, I.A.; Freemont, T.; Saunders, B.R. A Study of Conductive Hydrogel Composites of PH-Responsive Microgels and Carbon Nanotubes. Soft Matter 2016, 12, 4004–4013. [Google Scholar] [CrossRef]
- Wei, J.; Wang, R.; Pan, F.; Fu, Z. Polyvinyl Alcohol/GraphAene Oxide Conductive Hydrogels via the Synergy of Freezing and Salting Out for Strain Sensors. Sensors 2022, 22, 3015. [Google Scholar] [CrossRef]
- Gao, Y.; Dai, C.; Zhang, M.; Zhang, J.; Yin, L.; Li, W.; Zhang, K.; Yang, Y.; Zhao, Y. Biomimetic Silk Fibroin Hydrogel for Enhanced Peripheral Nerve Regeneration: Synergistic Effects of Graphene Oxide and Fibroblast Exosome. Adv. Funct. Mater. 2024, 34, 2314610. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, P.; Maity, P.P.; Mondal, S.; Ghosh, S.; Dhara, S.; Das, N.C. Green Reduced Graphene Oxide Toughened Semi-IPN Monolith Hydrogel as Dual Responsive Drug Release System: Rheological, Physicomechanical, and Electrical Evaluations. J. Phys. Chem. B 2018, 122, 7280–7291. [Google Scholar] [CrossRef]
- Hong, W.; Guo, X.; Zhang, T.; Mu, S.; Wu, F.; Yan, Z.; Zhang, H.; Li, X.; Zhang, A.; Wang, J.; et al. Flexible Strain Sensor Based on Nickel Microparticles/Carbon Black Microspheres/Polydimethylsiloxane Conductive Composites for Human Motion Detection. ACS Appl. Mater. Interfaces 2024, 16, 32702–32712. [Google Scholar] [CrossRef]
- Dey, K.; Sandrini, E.; Gobetti, A.; Ramorino, G.; Lopomo, N.F.; Tonello, S.; Sardini, E.; Sartore, L. Designing Biomimetic Conductive Gelatin-Chitosan–Carbon Black Nanocomposite Hydrogels for Tissue Engineering. Biomimetics 2023, 8, 473. [Google Scholar] [CrossRef]
- Shin, Y.; Lee, H.S.; Hong, Y.J.; Sunwoo, S.H.; Park, O.K.; Choi, S.H.; Kim, D.H.; Lee, S. Low-Impedance Tissue-Device Interface Using Homogeneously Conductive Hydrogels Chemically Bonded to Stretchable Bioelectronics. Sci. Adv. 2024, 10, eadi7724. [Google Scholar] [CrossRef]
- Garcia, J.R.; Campbell, P.F.; Kumar, G.; Langberg, J.J.; Cesar, L.; Deppen, J.N.; Shin, E.Y.; Bhatia, N.K.; Wang, L.; Xu, K.; et al. Minimally Invasive Delivery of Hydrogel-Encapsulated Amiodarone to the Epicardium Reduces Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2018, 11, e006408. [Google Scholar] [CrossRef]
- Lu, W.N.; Lü, S.H.; Wang, H.-B.; Li, D.X.; Duan, C.M.; Liu, Z.Q.; Hao, T.; He, W.J.; Xu, B.; Fu, Q.; et al. Functional Improvement of Infarcted Heart by Co-Injection of Embryonic Stem Cells with Temperature-Responsive Chitosan Hydrogel. Tissue Eng. Part A 2009, 15, 1437–1447. [Google Scholar] [CrossRef]
- Garbern, J.C.; Minami, E.; Stayton, P.S.; Murry, C.E. Delivery of Basic Fibroblast Growth Factor with a PH-Responsive, Injectable Hydrogel to Improve Angiogenesis in Infarcted Myocardium. Biomaterials 2011, 32, 2407–2416. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Ghasemi, A.; Sahandi Zangabad, P.; Rahighi, R.; Moosavi Basri, S.M.; Mirshekari, H.; Amiri, M.; Shafaei Pishabad, Z.; Aslani, A.; Bozorgomid, M.; et al. Smart Micro/Nanoparticles in Stimulus-Responsive Drug/Gene Delivery Systems. Chem. Soc. Rev. 2016, 45, 1457–1501. [Google Scholar] [CrossRef]
- Li, J.; Lv, Y.; Zhu, D.; Mei, X.; Huang, K.; Wang, X.; Li, Z.; Zhang, S.; Hu, S.; Popowski, K.D.; et al. Intrapericardial Hydrogel Injection Generates High Cell Retention and Augments Therapeutic Effects of Mesenchymal Stem Cells in Myocardial Infarction. Chem. Eng. J. 2022, 427, 131581. [Google Scholar] [CrossRef]
- Garcia, J.R.; Campbell, P.F.; Kumar, G.; Langberg, J.J.; Cesar, L.; Wang, L.; García, A.J.; Levit, R.D. A Minimally Invasive, Translational Method to Deliver Hydrogels to the Heart Through the Pericardial Space. JACC Basic Transl. Sci. 2017, 2, 601–609. [Google Scholar] [CrossRef]
- Lin, X.; Liu, Y.; Bai, A.; Cai, H.; Bai, Y.; Jiang, W.; Yang, H.; Wang, X.; Yang, L.; Sun, N.; et al. A Viscoelastic Adhesive Epicardial Patch for Treating Myocardial Infarction. Nat. Biomed. Eng. 2019, 3, 632–643. [Google Scholar] [CrossRef]
- He, Y.; Li, Q.; Chen, P.; Duan, Q.; Zhan, J.; Cai, X.; Wang, L.; Hou, H.; Qiu, X. A Smart Adhesive Janus Hydrogel for Non-Invasive Cardiac Repair and Tissue Adhesion Prevention. Nat. Commun. 2022, 13, 7666. [Google Scholar] [CrossRef]
- Sunwoo, S.H.; Han, S.I.; Kang, H.; Cho, Y.S.; Jung, D.; Lim, C.; Cha, M.-J.; Lee, S.P.; Hyeon, T.; Kim, D.-H. Stretchable Low-Impedance Nanocomposite Comprised of Ag–Au Core–Shell Nanowires and Pt Black for Epicardial Recording and Stimulation. Adv. Mater. Technol. 2020, 5, 1900768. [Google Scholar] [CrossRef]
- Zhou, T.; Yuk, H.; Hu, F.; Wu, J.; Tian, F.; Roh, H.; Shen, Z.; Gu, G.; Xu, J.; Lu, B.; et al. 3D Printable High-Performance Conducting Polymer Hydrogel for All-Hydrogel Bioelectronic Interfaces. Nat. Mater. 2023, 22, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xue, Y.; Chen, X.; Zhang, P.; Shan, L.; Duan, Q.; Xing, J.; Lan, Y.; Lu, B.; Liu, J. 3D Printed Implantable Hydrogel Bioelectronics for Electrophysiological Monitoring and Electrical Modulation. Adv. Funct. Mater. 2024, 34, 2314471. [Google Scholar] [CrossRef]
- Rodriguez-Rivera, G.J.; Post, A.; John, M.; Buchan, S.; Bernard, D.; Razavi, M.; Cosgriff-Hernandez, E. Injectable Hydrogel Electrodes as Conduction Highways to Restore Native Pacing. Nat. Commun. 2024, 15, 64. [Google Scholar] [CrossRef]
- Paul, A.; Hasan, A.; Al Kindi, H.; Gaharwar, A.K.; Rao, V.T.S.; Nikkhah, M.; Shin, S.R.; Krafft, D.; Dokmeci, M.R.; Shum-Tim, D.; et al. Injectable Graphene Oxide/Hydrogel-Based Angiogenic Gene Delivery System for Vasculogenesis and Cardiac Repair. ACS Nano 2014, 8, 8050–8062. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, J.; Hua, W.; Darabi, A.; Song, X.; Song, C.; Zhong, W.; Xing, M.M.Q.; Qiu, X. Mussel-Inspired Conductive Cryogel as Cardiac Tissue Patch to Repair Myocardial Infarction by Migration of Conductive Nanoparticles. Adv. Funct. Mater. 2016, 26, 4293–4305. [Google Scholar] [CrossRef]
- Zhu, D.; Li, Z.; Huang, K.; Caranasos, T.G.; Rossi, J.S.; Cheng, K. Minimally Invasive Delivery of Therapeutic Agents by Hydrogel Injection into the Pericardial Cavity for Cardiac Repair. Nat. Commun. 2021, 12, 1412. [Google Scholar] [CrossRef]
- Singelyn, J.M.; Sundaramurthy, P.; Johnson, T.D.; Schup-Magoffin, P.J.; Hu, D.P.; Faulk, D.M.; Wang, J.; Mayle, K.M.; Bartels, K.; Salvatore, M.; et al. Catheter-Deliverable Hydrogel Derived from Decellularized Ventricular Extracellular Matrix Increases Endogenous Cardiomyocytes and Preserves Cardiac Function Post-Myocardial Infarction. J. Am. Coll. Cardiol. 2012, 59, 751–763. [Google Scholar] [CrossRef]
- Marchiori, M.L.; Lubini, G.; Dalla Nora, G.; Friedrich, R.B.; Fontana, M.C.; Ourique, A.F.; Bastos, M.O.; Rigo, L.A.; Silva, C.B.; Tedesco, S.B.; et al. Hydrogel Containing Dexamethasone-Loaded Nanocapsules for Cutaneous Administration: Preparation, Characterization, and in Vitro Drug Release Study. Drug Dev. Ind. Pharm. 2010, 36, 962–971. [Google Scholar] [CrossRef]
- Silva, E.A.; Mooney, D.J. Spatiotemporal Control of Vascular Endothelial Growth Factor Delivery from Injectable Hydrogels Enhances Angiogenesis. J. Thromb. Haemost. 2007, 5, 590–598. [Google Scholar] [CrossRef]
- Elisseeff, J.; McIntosh, W.; Fu, K.; Blunk, T.; Langer, R. Controlled-Release of IGF-I and TGF-Β1 in a Photopolymerizing Hydrogel for Cartilage Tissue Engineering. J. Orthop. Res. 2001, 19, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Panwar, V.; Mondal, B.; Sharma, A.; Murugesan, P.; Arora, M.; Saini, D.; Mandal, D.; Ghosh, D. A Self-Powered, Anti-Bacterial, Moist-Wound Dressing Made with Electroactive Free-Flowing Hydrogel Particles, Encourage Faster Wound Closure. Chem. Eng. J. 2024, 494, 153063. [Google Scholar] [CrossRef]

| Patch Material | Conductivity (S/cm) | Modulus (kPa) | Stretchability (%) | Signal-to-Noise Ratio (dB) |
|---|---|---|---|---|
| Electronically conductive hydrogel | >10 | 25–100 | 400–610 | >60 |
| Nanocomposite hydrogel | ~20 | 50–150 | ~300 | ~55 |
| Ionic conductive hydrogel | 5–10 | 10–50 | >500 | 40–50 |
| Hydrogel Type | Typical Composition | Primary Application | Controlled Release Mechanism | Key Advantages |
|---|---|---|---|---|
| Drug Delivery Hydrogel | PEG, GelMA, alginate | Anti-inflammatory, angiogenic factor delivery | Diffusion-based, stimuli-responsive release | Precise dosage, sustained release |
| Cell Delivery Hydrogel | Gelatin, hyaluronic acid, fibrin | Stem cell, cardiomyocyte encapsulation | Cell encapsulation, mechanical protection | Enhanced cell viability, tissue integration |
| Binding Strategy | Mechanism | Key Features |
|---|---|---|
| Schiff Base | Aldehyde-amine reaction | Reversible, stable under physiological conditions |
| Boronate Ester | Diol-boronic acid reaction | pH-responsive reversibility |
| Diels–Alder | Diene-dienophile cycloaddition | Temperature-responsive reversibility |
| Host–Guest Interaction | Supramolecular cyclodextrin-adamantane binding | Highly reversible, rapid self-healing |
| Catechol-based | Mussel-inspired dopamine adhesion | Strong adhesion, redox-mediated reversibility |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.H.; Lee, J.W.; Kim, D.; Cha, G.D.; Sunwoo, S.-H. Recent Achievements of Epicardial Patch Electronics Using Adhesive and Conductive Hydrogels. Gels 2025, 11, 530. https://doi.org/10.3390/gels11070530
Lee SH, Lee JW, Kim D, Cha GD, Sunwoo S-H. Recent Achievements of Epicardial Patch Electronics Using Adhesive and Conductive Hydrogels. Gels. 2025; 11(7):530. https://doi.org/10.3390/gels11070530
Chicago/Turabian StyleLee, Su Hyeon, Jong Won Lee, Daehyeon Kim, Gi Doo Cha, and Sung-Hyuk Sunwoo. 2025. "Recent Achievements of Epicardial Patch Electronics Using Adhesive and Conductive Hydrogels" Gels 11, no. 7: 530. https://doi.org/10.3390/gels11070530
APA StyleLee, S. H., Lee, J. W., Kim, D., Cha, G. D., & Sunwoo, S.-H. (2025). Recent Achievements of Epicardial Patch Electronics Using Adhesive and Conductive Hydrogels. Gels, 11(7), 530. https://doi.org/10.3390/gels11070530






