Molecular Dynamics Simulation of the Thermosensitive Gelation Mechanism of Phosphorylcholine Groups-Conjugated Methylcellulose Hydrogel
Abstract
1. Introduction
2. Results and Discussion
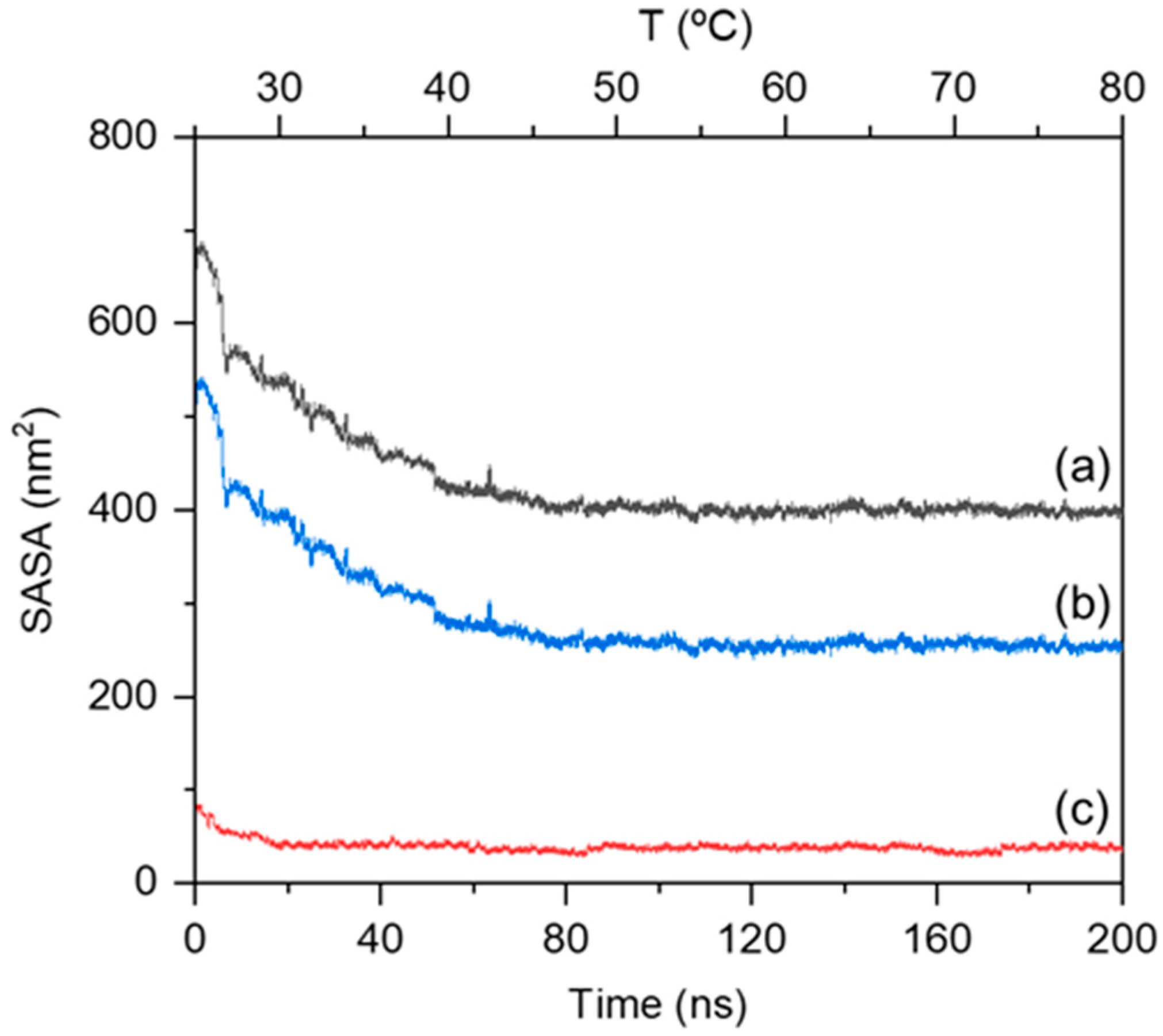
2.1. Thermosensitive Gelation Mechanism of MPC-g-MC Hydrogel
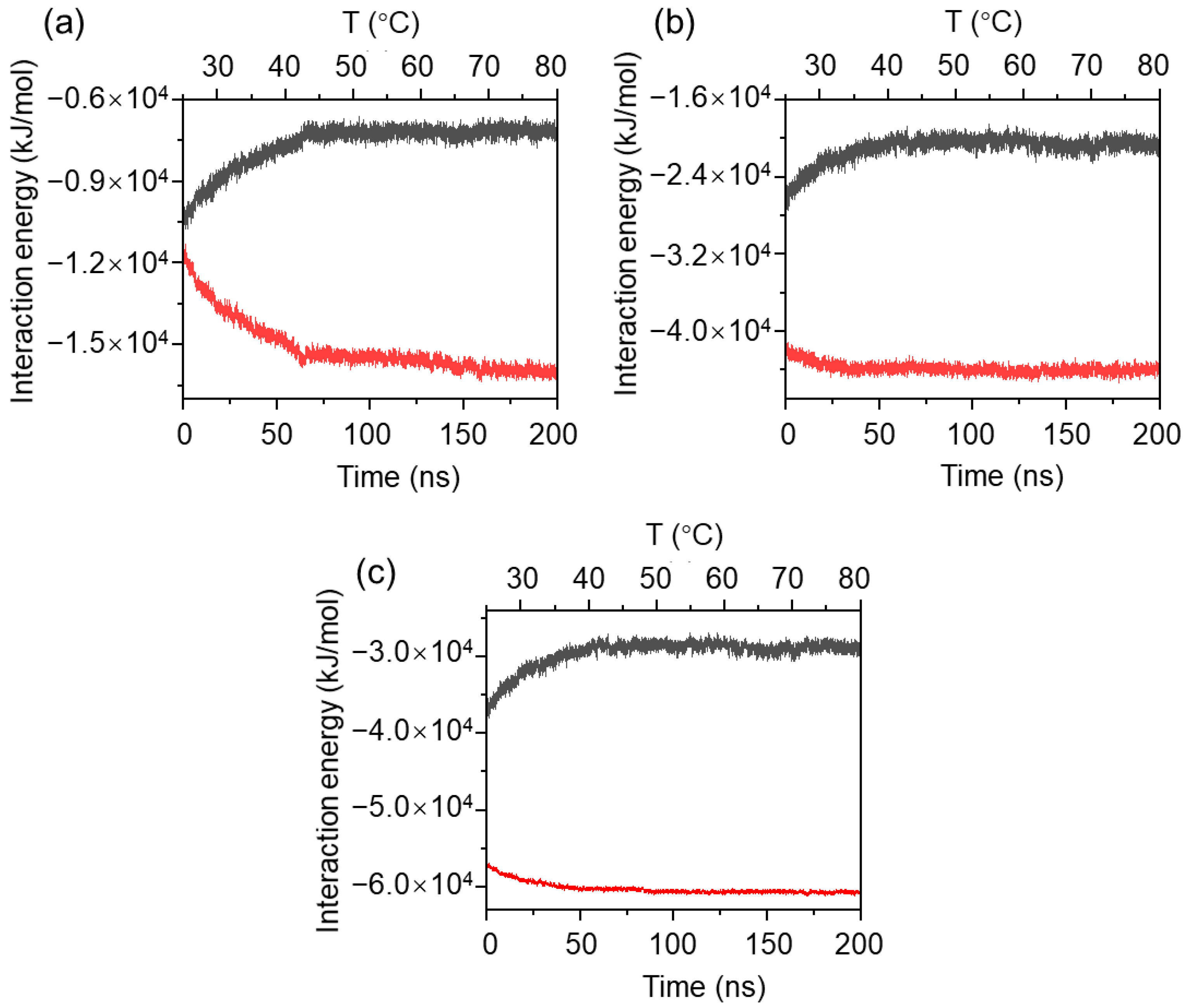
2.2. Aggregation Behavior and Intermolecular Interactions of MPC-g-MC Chains at Constant Temperatures
2.3. Mechanism of Gelation Temperature Modulation by Phosphorylcholine Groups
3. Conclusions
4. Materials and Methods
4.1. Construction of the Model Molecule of MPC-g-MC
4.2. System Setup of MPC-g-MC/H2O Simulation
4.3. MD Simulation
4.4. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MPC | 2-Methacryloyloxyethyl Phosphorylcholine |
| MC | Methylcellulose |
| MPC-g-MC | MPC-Grafted Methylcellulose |
| AGU | Anhydroglucose Unit |
| LCST | Lower Critical Solution Temperature |
| MD | Molecular Dynamics |
| SASA | Solvent Accessible Surface Area |
| RDF | Radial Distribution Function |
| VMD | Visual Molecular Dynamics |
| PME | Particle Mesh Ewald |
| LINCS | Linear Constraint Solver |
| NVT | Constant Number of particles, Volume, and Temperature |
| NPT | Constant Number of particles, Pressure, and Temperature |
| CHARMM | Chemistry at HARvard Macromolecular Mechanics |
| CGenFF | CHARMM General Force Field |
| TIP3P | Transferable Intermolecular Potential with 3 Points |
| DSC | Differential Scanning Calorimetry |
| FTIR | Fourier Transform Infrared Spectroscopy |
| 1H NMR | Proton Nuclear Magnetic Resonance |
| SD Rat | Sprague–Dawley Rat |
| HPC | Hydroxypropyl Cellulose |
| PBC | Periodic Boundary Condition |
References
- Kim, Y.-J.; Matsunaga, Y.T. Thermo-responsive Polymers and Their Application as Smart Biomaterials. J. Mater. Chem. B 2017, 5, 4307–4321. [Google Scholar] [CrossRef]
- Aguilar, M.R.; Elvira, C.; Gallardo, A.; Vazquez, B.; Román, J. Smart Polymers and Their Applications as Biomaterials. Top. Tissue Eng. 2007, 3, 1–27. [Google Scholar]
- Gheysoori, P.; Paydayesh, A.; Jafari, M.; Peidayesh, H. Thermoresponsive Nanocomposite Hydrogels Based on Gelatin/poly (N–isopropylacrylamide) (PNIPAM) for Controlled Drug Delivery. Eur. Polym. J. 2023, 186, 111846. [Google Scholar] [CrossRef]
- Zhang, X.; Aziz, S.; Salahuddin, B.; Zhu, Z. Thermoresponsive Hydrogel Artificial Muscles. Matter 2023, 6, 2735–2775. [Google Scholar] [CrossRef]
- Xu, T.; Hua, Y.; Mei, P.; Zeng, D.; Jiang, S.; Liao, C. Black Phosphorus Thermosensitive Hydrogels Loaded with Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Synergistically Promote Bone Tissue Defect Repair. J. Mater. Chem. B 2023, 11, 4396–4407. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, J.; Ren, K.; Zuo, J.; Ding, J.; Chen, X. Thermosensitive Hydrogels as Scaffolds for Cartilage Tissue Engineering. Biomacromolecules 2019, 20, 1478–1492. [Google Scholar] [CrossRef]
- Sponchioni, M.; Capasso Palmiero, U.; Moscatelli, D. Thermo-responsive polymers: Applications of Smart Materials in Drug Delivery and Tissue Engineering. Mater. Sci. Eng. C 2019, 102, 589–605. [Google Scholar] [CrossRef]
- Jun, I.; Kim, S.J.; Lee, J.-H.; Lee, Y.J.; Shin, Y.M.; Choi, E.; Park, K.M.; Park, J.; Park, K.D.; Shin, H. Transfer Printing of Cell Layers with an Anisotropic Extracellular Matrix Assembly using Cell-Interactive and Thermosensitive Hydrogels. Adv. Funct. Mater. 2012, 22, 4060–4069. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S. Poly(N-isopropylacrylamide)-based Thermo-responsive Surfaces with Controllable Cell Adhesion. Sci. China Chem. 2014, 57, 552–557. [Google Scholar] [CrossRef]
- Bonetti, L.; De Nardo, L.; Farè, S. Crosslinking Strategies in Modulating Methylcellulose Hydrogel Properties. Soft Matter 2023, 19, 7869–7884. [Google Scholar] [CrossRef]
- Bonetti, L.; De Nardo, L.; Farè, S. Thermo-Responsive Methylcellulose Hydrogels: From Design to Applications as Smart Biomaterials. Tissue Eng. Part B 2021, 27, 486–513. [Google Scholar] [CrossRef]
- Niemczyk-Soczynska, B.; Sajkiewicz, P.; Gradys, A. Toward a Better Understanding of the Gelation Mechanism of Methylcellulose via Systematic DSC Studies. Polymers 2022, 14, 1810. [Google Scholar] [CrossRef]
- McAllister, J.W.; Schmidt, P.W.; Dorfman, K.D.; Lodge, T.P.; Bates, F.S. Thermodynamics of Aqueous Methylcellulose Solutions. Macromolecules 2015, 48, 7205–7215. [Google Scholar] [CrossRef]
- Li, L. Thermal Gelation of Methylcellulose in Water: Scaling and Thermoreversibility. Macromolecules 2002, 35, 5990–5998. [Google Scholar] [CrossRef]
- Lam, Y.C.; Joshi, S.C.; Tan, B.K. Thermodynamic Characteristics of Gelation for Methyl-Cellulose Hydrogels. J. Therm. Anal. Calorim. 2007, 87, 475–482. [Google Scholar] [CrossRef]
- Li, L.; Thangamathesvaran, P.M.; Yue, C.Y.; Tam, K.C.; Hu, X.; Lam, Y.C. Gel Network Structure of Methylcellulose in Water. Langmuir 2001, 17, 8062–8068. [Google Scholar] [CrossRef]
- Cochis, A.; Bonetti, L.; Sorrentino, R.; Contessi Negrini, N.; Grassi, F.; Leigheb, M.; Rimondini, L.; Farè, S. 3D Printing of Thermo-Responsive Methylcellulose Hydrogels for Cell-Sheet Engineering. Materials 2018, 11, 579. [Google Scholar] [CrossRef]
- Zheng, T.; Doyle, P.S. Injectable Sustained-Release Hydrogel for High-Concentration Antibody Delivery. RSC Pharm. 2025, 2, 186–196. [Google Scholar] [CrossRef]
- Mahboubian, A.; Vllasaliu, D.; Dorkoosh, F.A.; Stolnik, S. Temperature-Responsive Methylcellulose–Hyaluronic Hydrogel as a 3D Cell Culture Matrix. Biomacromolecules 2020, 21, 4737–4746. [Google Scholar] [CrossRef]
- Liu, Z.; Yao, P. Injectable Thermo-Responsive Hydrogel Composed of Xanthan Gum and Methylcellulose Double Networks with Shear-Thinning Property. Carbohydr. Polym. 2015, 132, 490–498. [Google Scholar] [CrossRef]
- Bonetti, L.; De Nardo, L.; Variola, F.; Farè, S. In-Situ Raman Spectroscopy: An Effective Technique for the Quantification of LCST Transition of Methylcellulose Hydrogels. Mater. Lett. 2020, 274, 128011. [Google Scholar] [CrossRef]
- Contessi, N.; Altomare, L.; Filipponi, A.; Farè, S. Thermo-Responsive Properties of Methylcellulose Hydrogels for Cell Sheet Engineering. Mater. Lett. 2017, 207, 157–160. [Google Scholar] [CrossRef]
- Altomare, L.; Cochis, A.; Carletta, A.; Rimondini, L.; Farè, S. Thermo-Responsive Methylcellulose Hydrogels as Temporary Substrate for Cell Sheet Biofabrication. J. Mater. Sci. Mater. Med. 2016, 27, 95. [Google Scholar] [CrossRef]
- Morozova, S.; Schmidt, P.W.; Bates, F.S.; Lodge, T.P. Effect of Poly(ethylene glycol) Grafting Density on Methylcellulose Fibril Formation. Macromolecules 2018, 51, 9413–9421. [Google Scholar] [CrossRef]
- Sangfai, T.; Tantishaiyakul, V.; Hirun, N.; Li, L. Microphase Separation and Gelation of Methylcellulose in The Presence of Gallic Acid and NaCl as an In Situ Gel-Forming Drug Delivery System. AAPS PharmSciTech 2017, 18, 605–616. [Google Scholar] [CrossRef]
- Xu, Y.; Li, L.; Zheng, P.; Lam, Y.C.; Hu, X. Controllable Gelation of Methylcellulose by a Salt Mixture. Langmuir 2004, 20, 6134–6138. [Google Scholar] [CrossRef]
- Ishihara, K.; Fukazawa, K. 2-Methacryloyloxyethyl Phosphorylcholine Polymers. In Phosphorus-Based Polymers: From Synthesis to Applications; Monge, S., David, G., Eds.; The Royal Society of Chemistry: London, UK, 2014. [Google Scholar]
- Goda, T.; Ishihara, K.; Miyahara, Y. Critical Update on 2-Methacryloyloxyethyl Phosphorylcholine (MPC) Polymer Science. J. Appl. Polym. Sci. 2015, 132, 41766. [Google Scholar] [CrossRef]
- Moro, T.; Takatori, Y.; Kyomoto, M.; Ishihara, K.; Saiga, K.; Nakamura, K.; Kawaguchi, H. Surface Grafting of Biocompatible Phospholipid Polymer MPC Provides Wear Resistance of Tibial Polyethylene Insert in Artificial Knee Joints. Osteoarthr. Cartil. 2010, 18, 1174–1182. [Google Scholar] [CrossRef]
- Amoako, K.; Ukita, R.; Cook, K.E. Antifouling Zwitterionic Polymer Coatings for Blood-Bearing Medical Devices. Langmuir 2025, 41, 2994–3006. [Google Scholar] [CrossRef]
- Zheng, C.; Huang, X.; Ding, K.; Kuang, D.; Yang, L.; Wang, Y.; Zhang, X. Double Bond Crosslinked and Phosphocholinated Biological Heart Valve with Robust Antithrombogenicity, Durability and Anticalcification Property. Compos. Part B Eng. 2023, 250, 110448. [Google Scholar] [CrossRef]
- Wang, Y.; Zhai, W.; Zhang, H.; Cheng, S.; Li, J. Injectable Polyzwitterionic Lubricant for Complete Prevention of Cardiac Adhesion. Macromol. Biosci. 2023, 23, 2200554. [Google Scholar] [CrossRef]
- Shi, X.; Cantu-Crouch, D.; Sharma, V.; Pruitt, J.; Yao, G.; Fukazawa, K.; Wu, J.Y.; Ishihara, K. Surface Characterization of a Silicone Hydrogel Contact Lens Having Bioinspired 2-Methacryloyloxyethyl Phosphorylcholine Polymer Layer in Hydrated State. Colloids Surf. B Biointerfaces 2021, 199, 111539. [Google Scholar] [CrossRef]
- Açarı, İ.K.; Sel, E.; Özcan, İ.; Ateş, B.; Köytepe, S.; Thakur, V.K. Chemistry and Engineering of Brush Type Polymers: Perspective towards Tissue Engineering. Adv. Colloid Interface Sci. 2022, 305, 102694. [Google Scholar] [CrossRef]
- Mirazul Islam, M.; Cėpla, V.; He, C.; Edin, J.; Rakickas, T.; Kobuch, K.; Ruželė, Ž.; Bruce Jackson, W.; Rafat, M.; Lohmann, C.P.; et al. Functional Fabrication of Recombinant Human Collagen–Phosphorylcholine Hydrogels for Regenerative Medicine Applications. Acta Biomater. 2015, 12, 70–80. [Google Scholar] [CrossRef]
- Wen, J.; Zheng, Y.; He, J.; Hu, D.; Huang, Y.; Liu, K.; Zhang, Y.; Wang, T.; Zhou, H.; Wang, K. Injectable Polyphenol Hydrogel Prevents Peritoneal Adhesion Intensified by Intraperitoneal Microbial Infection. Chem. Eng. J. 2025, 513, 163134. [Google Scholar] [CrossRef]
- Wen, J.; Liu, K.; Bu, Y.; Zhang, Y.; Zheng, Y.; He, J.; Huang, Y.; Hu, D.; Wang, K. An Injectable and Antifouling Hydrogel Prevents the Development of abdominal Adhesions by Inhibiting the CCL2/CCR2 Interaction. Biomaterials 2024, 311, 122661. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, W.; Zhang, J.; Peng, X.; Li, G.; Zhang, L.-M.; Yang, L. Thermosensitive Hydrogels Based on Methylcellulose Derivatives for Prevention of Postoperative Adhesion. Cellulose 2020, 27, 1555–1571. [Google Scholar] [CrossRef]
- Deshmukh, S.A.; Sankaranarayanan, S.K.R.S.; Suthar, K.; Mancini, D.C. Role of Solvation Dynamics and Local Ordering of Water in Inducing Conformational Transitions in Poly(N-isopropylacrylamide) Oligomers through the LCST. J. Phys. Chem. B 2012, 116, 2651–2663. [Google Scholar] [CrossRef]
- Ci, J.; Kang, H.; Liu, C.; He, A.; Liu, R. Thermal Sensitivity and Protein Anti-Adsorption of Hydroxypropyl Cellulose-g-Poly(2-(methacryloyloxy) Ethyl phosphorylcholine). Carbohydr. Polym. 2017, 157, 757–765. [Google Scholar] [CrossRef]
- Desbrières, J.; Hirrien, M.; Ross-Murphy, S.B. Thermogelation of methylcellulose: Rheological considerations. Polymer 2000, 41, 2451–2461. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A Web-Based Graphical User Interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Vanommeslaeghe, K.; MacKerell, A.D., Jr. Automation of the CHARMM General Force Field (CGenFF) I: Bond Perception and Atom Typing. J. Chem. Inf. Model. 2012, 52, 3144–3154. [Google Scholar] [CrossRef]
- Páll, S.; Zhmurov, A.; Bauer, P.; Abraham, M.; Lundborg, M.; Gray, A.; Hess, B.; Lindahl, E. Heterogeneous Parallelization and Acceleration of Molecular Dynamics Simulations in GROMACS. J. Chem. Phys. 2020, 153, 134110. [Google Scholar] [CrossRef]
- Best, R.B.; Zhu, X.; Shim, J.; Lopes, P.E.M.; Mittal, J.; Feig, M.; MacKerell, A.D., Jr. Optimization of the Additive CHARMM All-Atom Protein Force Field Targeting Improved Sampling of the Backbone ϕ, ψ and Side-Chain χ1 and χ2 Dihedral Angles. J. Chem. Theory Comput. 2012, 8, 3257–3273. [Google Scholar] [CrossRef]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM General Force field: A Force field for Drug-Like Molecules Compatible with the CHARMM All-Atom Additive Biological Force Fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of Simple Potential Functions for Simulating Liquid Water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- The PyMOL Molecular Graphics System; Version 2.0; Schrödinger, LLC: New York, NY, USA, 2015.
- Kirschner, K.N.; Yongye, A.B.; Tschampel, S.M.; González-Outeiriño, J.; Daniels, C.R.; Foley, B.L.; Woods, R.J. GLYCAM06: A generalizable biomolecular force field. Carbohydrates. J. Comput. Chem. 2008, 29, 622–655. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, H.; Huang, Y.; Yi, J.; Chen, W.; Guan, P.; Guan, S.; Chen, X.; Li, W.; Yang, L. Molecular Dynamics Simulation of the Thermosensitive Gelation Mechanism of Phosphorylcholine Groups-Conjugated Methylcellulose Hydrogel. Gels 2025, 11, 521. https://doi.org/10.3390/gels11070521
Mei H, Huang Y, Yi J, Chen W, Guan P, Guan S, Chen X, Li W, Yang L. Molecular Dynamics Simulation of the Thermosensitive Gelation Mechanism of Phosphorylcholine Groups-Conjugated Methylcellulose Hydrogel. Gels. 2025; 11(7):521. https://doi.org/10.3390/gels11070521
Chicago/Turabian StyleMei, Hongyu, Yaqing Huang, Juzhen Yi, Wencheng Chen, Peng Guan, Shanyue Guan, Xiaohong Chen, Wei Li, and Liqun Yang. 2025. "Molecular Dynamics Simulation of the Thermosensitive Gelation Mechanism of Phosphorylcholine Groups-Conjugated Methylcellulose Hydrogel" Gels 11, no. 7: 521. https://doi.org/10.3390/gels11070521
APA StyleMei, H., Huang, Y., Yi, J., Chen, W., Guan, P., Guan, S., Chen, X., Li, W., & Yang, L. (2025). Molecular Dynamics Simulation of the Thermosensitive Gelation Mechanism of Phosphorylcholine Groups-Conjugated Methylcellulose Hydrogel. Gels, 11(7), 521. https://doi.org/10.3390/gels11070521





