Insights on the Influence of the Drying Method and Surface Wettability on the Final Properties of Silica Aerogels
Abstract
1. Introduction
2. Results and Discussion
2.1. Silica Aerogel Samples and Hydrophobicity
2.2. Effect of Drying on Density and Porosity
2.3. Effect of Drying on the Textural Properties and Porous Structures
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of the Silica Aerogels
4.3. Drying Methods
4.4. Characterization Methods
4.4.1. Bulk Density, Solid Density, and Porosity
4.4.2. Contact Angle
4.4.3. Specific Surface Area (SBET) and Pore Size
4.4.4. Morphology Observation by Scanning Electron Microscopy (SEM)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kistler, S.S. Coherent Expanded Aerogels and Jellies. Nature 1931, 127, 741. [Google Scholar] [CrossRef]
- Kistler, S.S. Coherent expanded aerogels. J. Phys. Chem. 1932, 36, 52–64. [Google Scholar] [CrossRef]
- Aerogel Market Size & Share Report, 2020–2028. 2021. Available online: https://www.grandviewresearch.com/industry-analysis/aerogel-market (accessed on 1 May 2025).
- Hrubesh, L.W.; Tillotson, T.M.; Poco, J.F. Characterization of ultralow-density silica aerogels made from a condensed silica precursor. MRS Proc. 1990, 180, 315. [Google Scholar] [CrossRef]
- Aegerter, M.A.; Leventis, N.; Koebel, M.M. Aerogels Handbook; Springer: Berlin, Germany, 2011. [Google Scholar]
- Woignier, T.; Phalippou, J. Skeletal density of silica aerogels. J. Non-Cryst. Solids 1987, 93, 17–21. [Google Scholar] [CrossRef]
- Ren, J.; Feng, J.; Wang, L.; Chen, G.; Zhou, Z.; Li, Q. High specific surface area hybrid silica aerogel containing POSS. Microporous Mesoporous Mater. 2021, 310, 110456. [Google Scholar] [CrossRef]
- Buzykaev, A.; Danilyuk, A.; Ganzhur, S.; Gorodetskaya, T.; Kravchenko, E.; Onuchin, A.; Vorobiov, A. Aerogels with high optical parameters for Cherenkov counters. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 1996, 379, 465–467. [Google Scholar] [CrossRef]
- Riffat, S.B.; Qiu, G. A review of state-of-the-art aerogel applications in buildings. Int. J. Low-Carbon Technol. 2013, 8, 1–6. [Google Scholar] [CrossRef]
- Huang, Y.; Niu, J.L. Application of super-insulating translucent silica aerogel glazing system on commercial building envelope of humid subtropical climates—Impact on space cooling load. Energy 2015, 83, 316–325. [Google Scholar] [CrossRef]
- YKim, J.; Yoo, S.H.; Lee, H.G.; Won, Y.; Choi, J.; Kang, K. Structural Analysis of silica aerogels for the interlayer dielectric in semiconductor devices. Ceram. Int. 2021, 47, 29722–29729. [Google Scholar] [CrossRef]
- Dunn, B.C.; Cole, P.; Covington, D.; Webster, M.C.; Pugmire, R.J.; Ernst, R.D.; Eyring, E.M.; Shah, N.; Huffman, G.P. Silica aerogel supported catalysts for Fischer-Tropsch synthesis. Appl. Catal. A Gen. 2005, 278, 233–238. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, B.; Hua, Y.; Lu, S.; Nong, Z.; Wang, J. Ultralight MXene/rGO aerogel frames with component and structure controlled electromagnetic wave absorption by direct ink writing. Carbon 2024, 230, 119650. [Google Scholar] [CrossRef]
- Li, S.; Ren, H.; Zhu, J.; Bi, Y.; Xu, Y.; Zhang, L. Facile fabrication of superhydrophobic, mechanically strong multifunctional silica-based aerogels at benign temperatura. J. Non-Cryst. Solids 2017, 473, 59–63. [Google Scholar] [CrossRef]
- Sun, L.; Jiang, Z.; Yuan, B.; Zhi, S.; Zhang, Y. Chemical Engineering Research and Design Ultralight and Superhydrophobic Perfluorooctyltrimethoxysilane Modified Biomass Carbonaceous Aerogel for Oil-Spill Remediation. Chem. Eng. Res. Des. 2021, 174, 71–78. [Google Scholar] [CrossRef]
- Sarawade, P.B.; Kim, J.-K.; Park, J.-K.; Kim, H.-K. Influence of Solvent Exchange on the Physical Properties of Sodium Silicate Based Aerogel Prepared at Ambient Pressure. Aerosol Air Qual. Res. 2006, 6, 93–105. [Google Scholar] [CrossRef]
- Lee, S.; Cha, Y.C.; Hwang, H.J.; Moon, J.W.; Han, I.S. The effect of pH on the physicochemical properties of silica aerogels prepared by an ambient pressure drying method. Mater. Lett. 2007, 61, 3130–3133. [Google Scholar] [CrossRef]
- Nawaz, K.; Schmidt, S.J.; Jacobi, A.M. Effect of catalyst used in the sol-gel process on the microstructure and adsorption/desorption performance of silica aerogels. Int. J. Heat Mass Transf. 2014, 74, 25–34. [Google Scholar] [CrossRef]
- Smitha, S.; Shajesh, P.; Aravind, P.R.; Kumar, S.R.; Pillai, P.K.; Warrier, K.G.K. Effect of aging time and concentration of aging solution on the porosity characteristics of subcritically dried silica aerogels. Microporous Mesoporous Mater. 2006, 91, 286–292. [Google Scholar] [CrossRef]
- Babiarczuk, B.; Lewandowski, D.; Kierzek, K.; Detyna, J.; Jones, W.; Kaleta, J.; Krzak, J. Mechanical properties of silica aerogels controlled by synthesis parameters. J. Non-Cryst. Solids 2023, 606, 122171. [Google Scholar] [CrossRef]
- Linhares, T.; Carneiro, V.H.; Merillas, B.; De Amorim, M.T.P.; Durães, L. Textile waste-reinforced cotton-silica aerogel composites for moisture regulation and thermal/acoustic barrier. J. Sol-Gel Sci. Technol. 2022, 102, 574–588. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, R.; Fang, D. An ultralight silica-modified ZrO2–SiO2 aerogel composite with ultra-low thermal conductivity and enhanced mechanical strength. Scr. Mater. 2018, 143, 113–116. [Google Scholar] [CrossRef]
- Merillas, B.; Lamy-Mendes, A.; Villafañe, F.; Durães, L.; Rodríguez-Pérez, M.Á. Silica-Based Aerogel Composites Reinforced with Reticulated Polyurethane Foams: Thermal and Mechanical Properties. Gels 2022, 8, 392. [Google Scholar] [CrossRef]
- Merillas, B.; Lamy-Mendes, A.; Villafañe, F.; Durães, L.; Rodríguez-Pérez, M.Á. Polyurethane foam scaffold for silica aerogels: Effect of cell size on the mechanical properties and thermal insulation. Mater. Today Chem. 2022, 26, 101257. [Google Scholar] [CrossRef]
- Ye, X.; Chen, Z.; Ai, S.; Hou, B.; Zhang, J.; Zhou, Q.; Wang, F.; Liu, H.; Cui, S. Microstructure characterization and thermal performance of reticulated SiC skeleton reinforced silica aerogel composites. Compos. Part B 2019, 177, 107409. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; Portugal, A. An overview on silica aerogels synthesis and different mechanical reinforcing strategies. J. Non-Cryst. Solids 2014, 385, 55–74. [Google Scholar] [CrossRef]
- Randall, J.P.; Meador, M.A.B.; Jana, S.C. Tailoring mechanical properties of aerogels for aerospace applications. ACS Appl. Mater. Interfaces 2011, 3, 613–626. [Google Scholar] [CrossRef]
- Merillas, B.; García-González, C.A.; Álvarez-Arenas, T.E.G.; Rodríguez-Pérez, M.Á. Towards the Optimization of Polyurethane Aerogel Properties by Densification: Exploring the Structure–Properties Relationship. Small Struct. 2024, 5, 2400120. [Google Scholar] [CrossRef]
- Plappert, S.F.; Nedelec, J.M.; Rennhofer, H.; Lichtenegger, H.C.; Liebner, F.W. Strain Hardening and Pore Size Harmonization by Uniaxial Densification: A Facile Approach Toward Superinsulating Aerogels from Nematic Nanofibrillated 2,3-Dicarboxyl Cellulose. Chem. Mater. 2017, 29, 6630–6641. [Google Scholar] [CrossRef]
- Scherer, G.W. Effect of drying on properties of silica gel. J. Non-Cryst. Solids 1997, 215, 155–168. [Google Scholar] [CrossRef]
- Panda, D.; Sahu, A.K.; Gangawane, K.M. Effect of molar concentration and drying methodologies on monodispersed silica sol for synthesis of silica aerogels with temperature-resistant characteristics. Mater. Sci. Eng. B 2024, 299, 117062. [Google Scholar] [CrossRef]
- Jones, S.M.; Sakamoto, J. Aerogels Handb. In Aerogels Handbook; Springer: New York, NY, USA, 2011; pp. 721–746. [Google Scholar] [CrossRef]
- Bisson, A.; Rigacci, A.; Lecomte, D.; Rodier, E.; Achard, P. Drying of Silica Gels to Obtain Aerogels: Phenomenology and Basic Techniques. Dry. Technol. 2003, 21, 593–628. [Google Scholar] [CrossRef]
- Maleki, H.; Dura, L. Development of Mechanically Strong Ambient Pressure Dried Silica Aerogels with Optimized Properties. J. Phys. Chem. C 2015, 119, 7689–7703. [Google Scholar] [CrossRef]
- Zemke, F.; Scoppola, E.; Simon, U.; Bekheet, M.F.; Wagermaier, W.; Gurlo, A. Springback effect and structural features during the drying of silica aerogels tracked by in-situ synchrotron X-ray scattering. Sci. Rep. 2022, 12, 7537. [Google Scholar] [CrossRef] [PubMed]
- Khedkar, M.V.; Somvanshi, S.B.; Humbe, A.V.; Jadhav, K.M. Surface modified sodium silicate based superhydrophobic silica aerogels prepared via ambient pressure drying process. J. Non-Cryst. Solids 2019, 511, 140–146. [Google Scholar] [CrossRef]
- Khedkar, M.V.; Jadhav, S.A.; Somvanshi, S.B.; Kharat, P.B. Physicochemical properties of ambient pressure dried surface modified silica aerogels: Effect of pH variation. SN Appl. Sci. 2020, 2, 696. [Google Scholar] [CrossRef]
- Nagapriya, S.; Ajith, M.R.; Sreemoolanadhan, H.; Mathew, M.; Sharma, S.C. Hydrophobic silica aerogels by ambient pressure drying. Mater. Sci. Forum 2015, 830–831, 476–479. [Google Scholar] [CrossRef]
- Shewale, P.M.; Rao, A.V.; Rao, A.P. Effect of different trimethyl silylating agents on the hydrophobic and physical properties of silica aerogels. Appl. Surf. Sci. 2008, 254, 6902–6907. [Google Scholar] [CrossRef]
- Duräes, L.; Ochoa, M.; Rocha, N.; Patrício, R.; Duarte, N.; Redondo, V.; Portugal, A. Effect of the drying conditions on the microstructure of silica based xerogels and aerogels. J. Nanosci. Nanotechnol. 2012, 12, 6828–6834. [Google Scholar] [CrossRef] [PubMed]
- Ru, Y.; Guoqiang, L.; Min, L. Analysis of the effect of drying conditions on the structural and surface heterogeneity of silica aerogels and xerogel by using cryogenic nitrogen adsorption characterization. Microporous Mesoporous Mater. 2010, 129, 1–10. [Google Scholar] [CrossRef]
- Simón-Herrero, C.; Caminero-Huertas, S.; Romero, A.; Valverde, J.L.; Sánchez-Silva, L. Effects of freeze-drying conditions on aerogel properties. J. Mater. Sci. 2016, 51, 8977–8985. [Google Scholar] [CrossRef]
- Tajiri, K.; Igarashi, K.; Nishio, T. Effects of supercritical drying media on structure and properties of silica aerogel. J. Non-Cryst. Solids 1995, 186, 83–87. [Google Scholar] [CrossRef]
- Borba, A.; Vareda, J.P.; Duraes, L.; Portugal, A.; Simoes, P.N. Spectroscopic Characterization of Silica Aerogels Prepared Using Several Precursors—Effect on the Formation of Molecular Clusters. New J. Chem. 2017, 41, 6742–6759. [Google Scholar] [CrossRef]
- Kalinin, S.V.; Kheifets, L.I.; Mamchik, A.I.; Ko, A.G.K.; Vertegel, A.A. Influence of the Drying Technique on the Structure of Silica Gels. J. Sol-Gel Sci. Technol. 1999, 35, 31–35. [Google Scholar] [CrossRef]
- Dorcheh, A.S.; Abbasi, M.H. Silica aerogel; synthesis, properties and characterization. J. Mater. Process. Technol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, L.; Li, S.; Liu, X.; Liang, J.; Liu, J.; Duan, X.; Liu, H. Modulating pore microstructure of silica aerogels dried at ambient pressure by adding N-hexane to the solvent. J. Non-Cryst. Solids 2023, 610, 122312. [Google Scholar] [CrossRef]
- Zhang, G.; Li, C.; Wang, Y.; Lin, L.; Ostrikov, K.K. High-Performance Methylsilsesquioxane Aerogels: Hydrolysis Mechanisms and Maximizing Compression Properties. Gels 2023, 9, 720. [Google Scholar] [CrossRef]
- Merillas, B.; Vareda, J.P.; León, J.M.-D.; Rodríguez-Pérez, M.Á.; Durães, L. Thermal Conductivity of Nanoporous Materials: Where Is the Limit? Polymers 2022, 14, 2556. [Google Scholar] [CrossRef]
- Zhu, K.; Gao, F.; Zhao, Z.; Ren, J.; Lasobras, J.; Shen, X.; Cui, S.; Men, M. Ultra-high specific surface area spherical FePOX/SiO2 aerogel with excellent mechanical properties for the highly selective direct oxidation of CH4 to HCHO. J. Alloys Compd. 2024, 971, 172535. [Google Scholar] [CrossRef]
- Mohammad, R.; Soubaihi, A.; Khaled, S.; Ye, F.; Tay, M.; Myint, Z.; Saeed, S.; Dutta, J. Microporous and Mesoporous Materials Synthesis of hierarchically porous silica aerogel supported Palladium catalyst for low-temperature CO oxidation under ignition/extinction conditions. Microporous Mesoporous Mater. 2020, 292, 109758. [Google Scholar] [CrossRef]
- Lamy-Mendes, A.; Malfait, W.J.; Sadeghpour, A.; Girão, A.V.; Silva, R.F.; Durães, L. Influence of 1D and 2D carbon nanostructures in silica-based aerogels. Carbon 2021, 180, 146–162. [Google Scholar] [CrossRef]
- ASTM D1622-08; Standard Test Method for Apparent Density of Rigid Cellular Plastics. ASTM International: West Conshohocken, PA, USA, 2008. [CrossRef]
- ASTM D5946-17; Standard Test Method for Corona-Treated Polymer Films Using Water Contact Angle Measurements. ASTM International: West Conshohocken, PA, USA, 2017. [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]


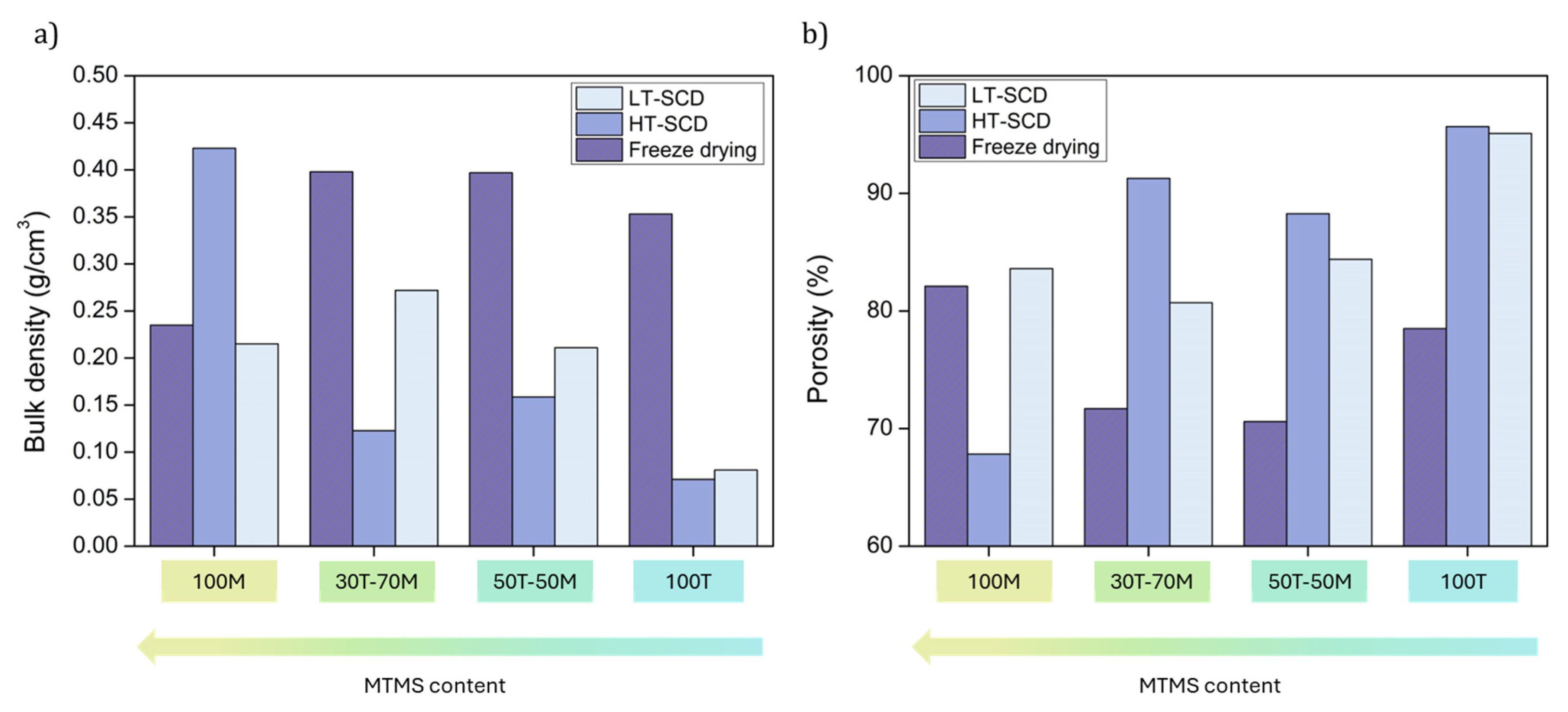
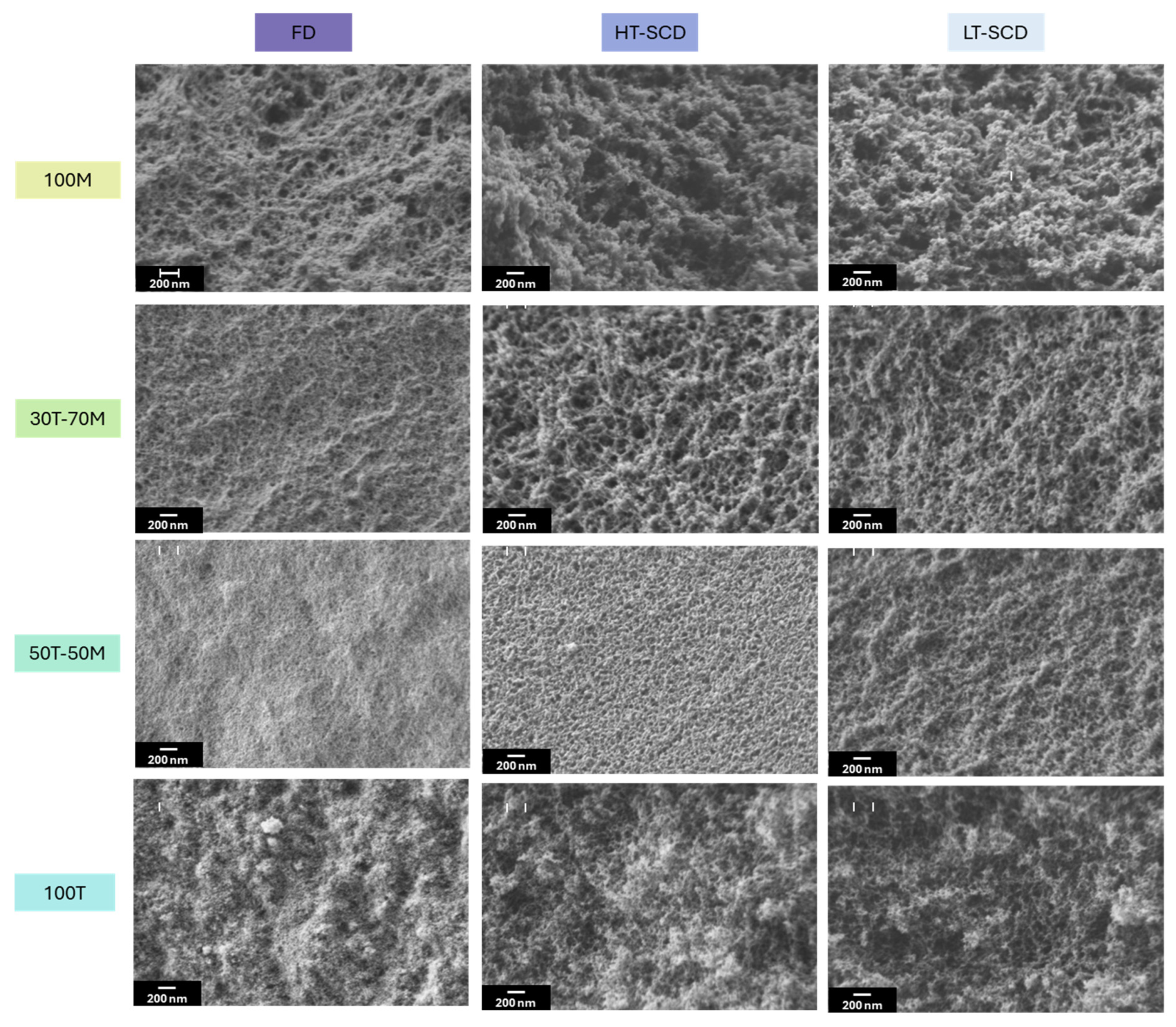
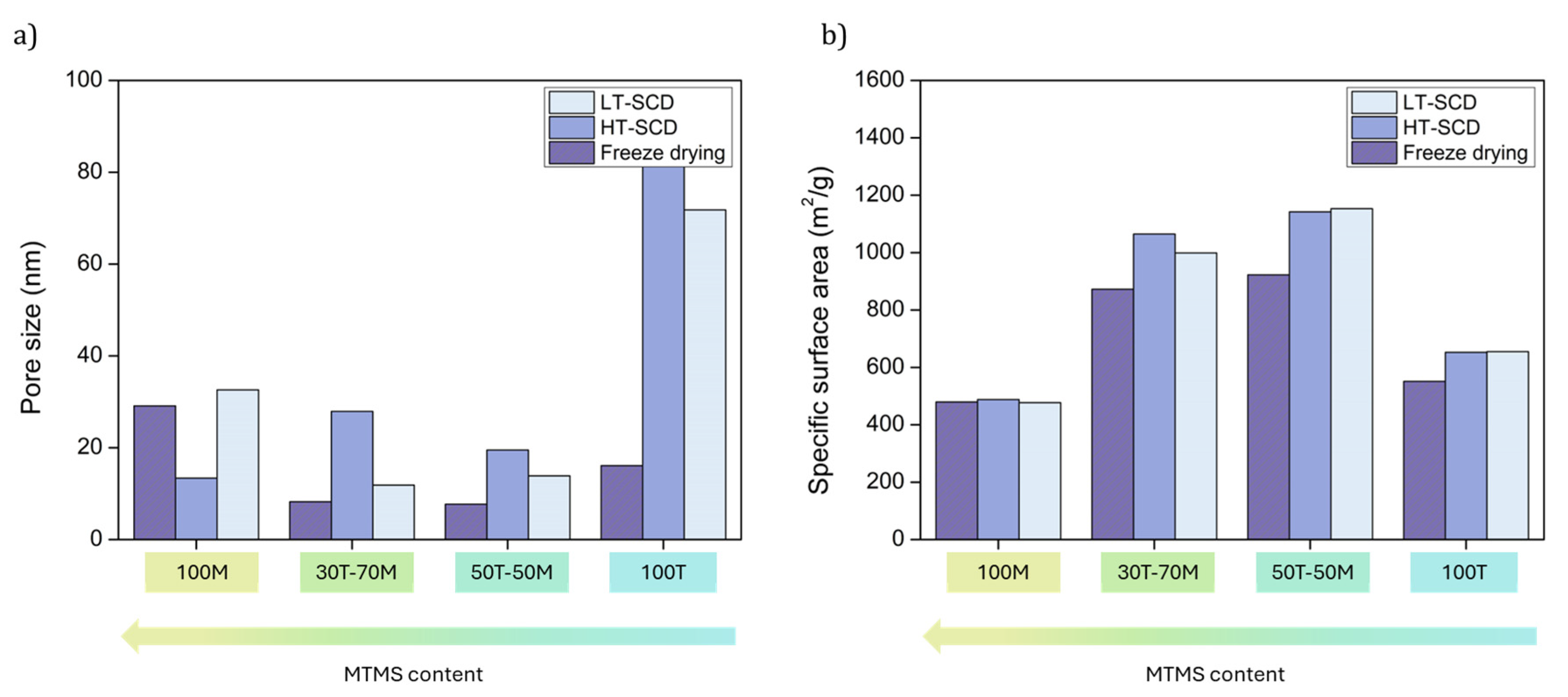

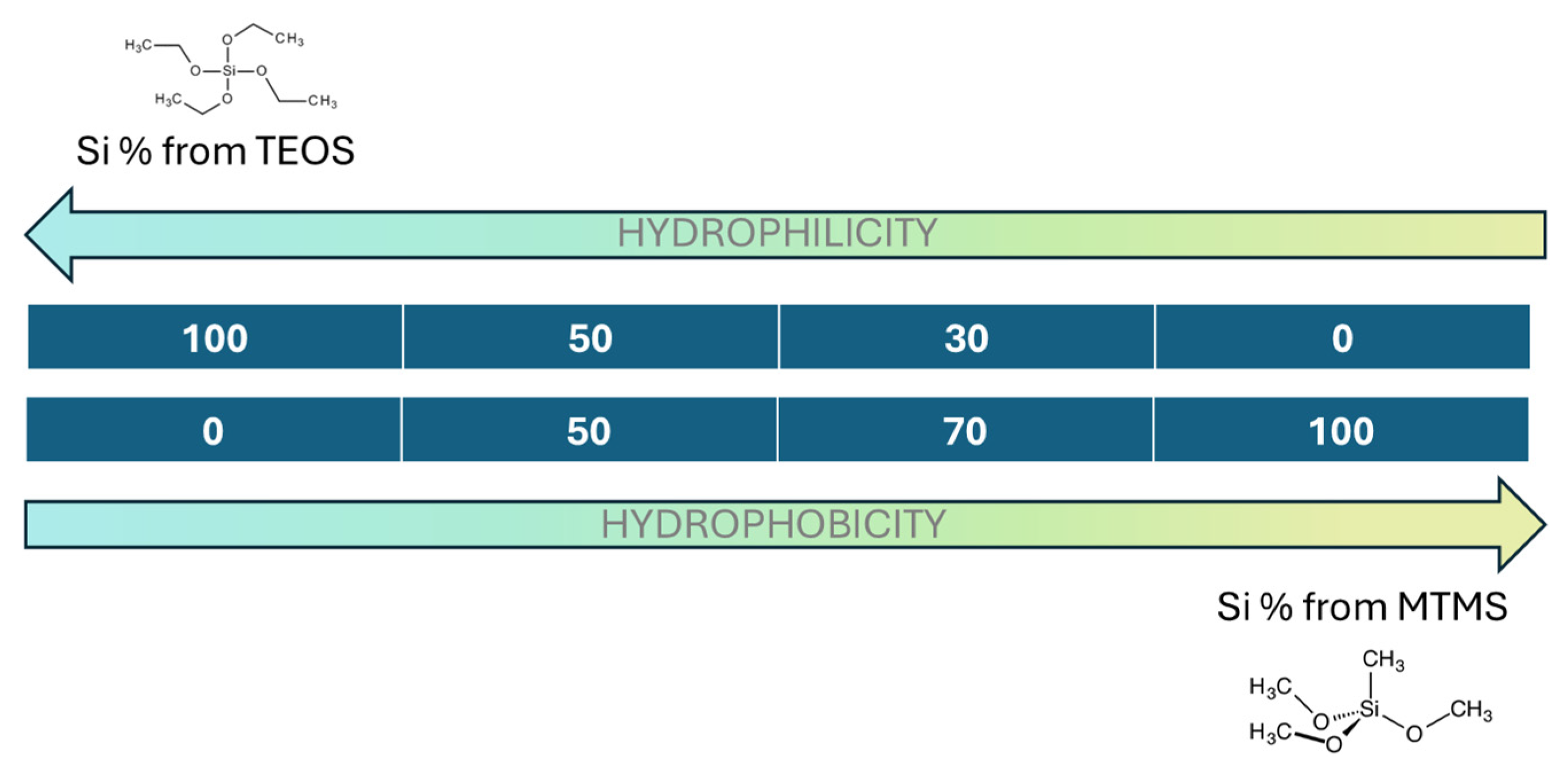
| Sample | Bulk Density (g/cm3) | Porosity (%) | SBET (m2/g) | Mean Pore Size (nm) |
|---|---|---|---|---|
| LT-SCD | ||||
| 100T | 0.081 | 95.1 | 655.1 ± 1.2 | 71.82 |
| 50T-50M | 0.211 | 84.4 | 1153.4 ± 3.4 | 13.9 |
| 30T-70M | 0.272 | 80.7 | 998.7 ± 2.0 | 11.9 |
| 100M | 0.215 | 83.7 | 477.1 ± 3.1 | 32.6 |
| HT-SCD | ||||
| 100T | 0.071 | 95.7 | 652.4 ± 1.7 | 82.6 |
| 50T-50M | 0.159 | 88.3 | 1141.9 ± 3.7 | 19.5 |
| 30T-70M | 0.123 | 91.3 | 1064.6 ± 2.4 | 27.9 |
| 100M | 0.423 | 67.8 | 478.8 ± 2.3 | 13.4 |
| Freeze-drying | ||||
| 100T | 0.353 | 78.5 | 551.5 ± 1.1 | 16.1 |
| 50T-50M | 0.397 | 70.6 | 922.6 ± 3.5 | 7.7 |
| 30T-70M | 0.398 | 71.7 | 872.4 ± 2.6 | 8.3 |
| 100M | 0.235 | 82.1 | 479.8 ± 2.2 | 29.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merillas, B.; Roque, M.I.; Almeida, C.M.R.; Rodríguez-Pérez, M.Á.; Durães, L. Insights on the Influence of the Drying Method and Surface Wettability on the Final Properties of Silica Aerogels. Gels 2025, 11, 511. https://doi.org/10.3390/gels11070511
Merillas B, Roque MI, Almeida CMR, Rodríguez-Pérez MÁ, Durães L. Insights on the Influence of the Drying Method and Surface Wettability on the Final Properties of Silica Aerogels. Gels. 2025; 11(7):511. https://doi.org/10.3390/gels11070511
Chicago/Turabian StyleMerillas, Beatriz, Maria Inês Roque, Cláudio M. R. Almeida, Miguel Ángel Rodríguez-Pérez, and Luisa Durães. 2025. "Insights on the Influence of the Drying Method and Surface Wettability on the Final Properties of Silica Aerogels" Gels 11, no. 7: 511. https://doi.org/10.3390/gels11070511
APA StyleMerillas, B., Roque, M. I., Almeida, C. M. R., Rodríguez-Pérez, M. Á., & Durães, L. (2025). Insights on the Influence of the Drying Method and Surface Wettability on the Final Properties of Silica Aerogels. Gels, 11(7), 511. https://doi.org/10.3390/gels11070511











