The Influence of Concentration and Type of Salts on the Behaviour of Linear Actuators Based on PVA Hydrogel Activated by AC Power
Abstract
1. Introduction
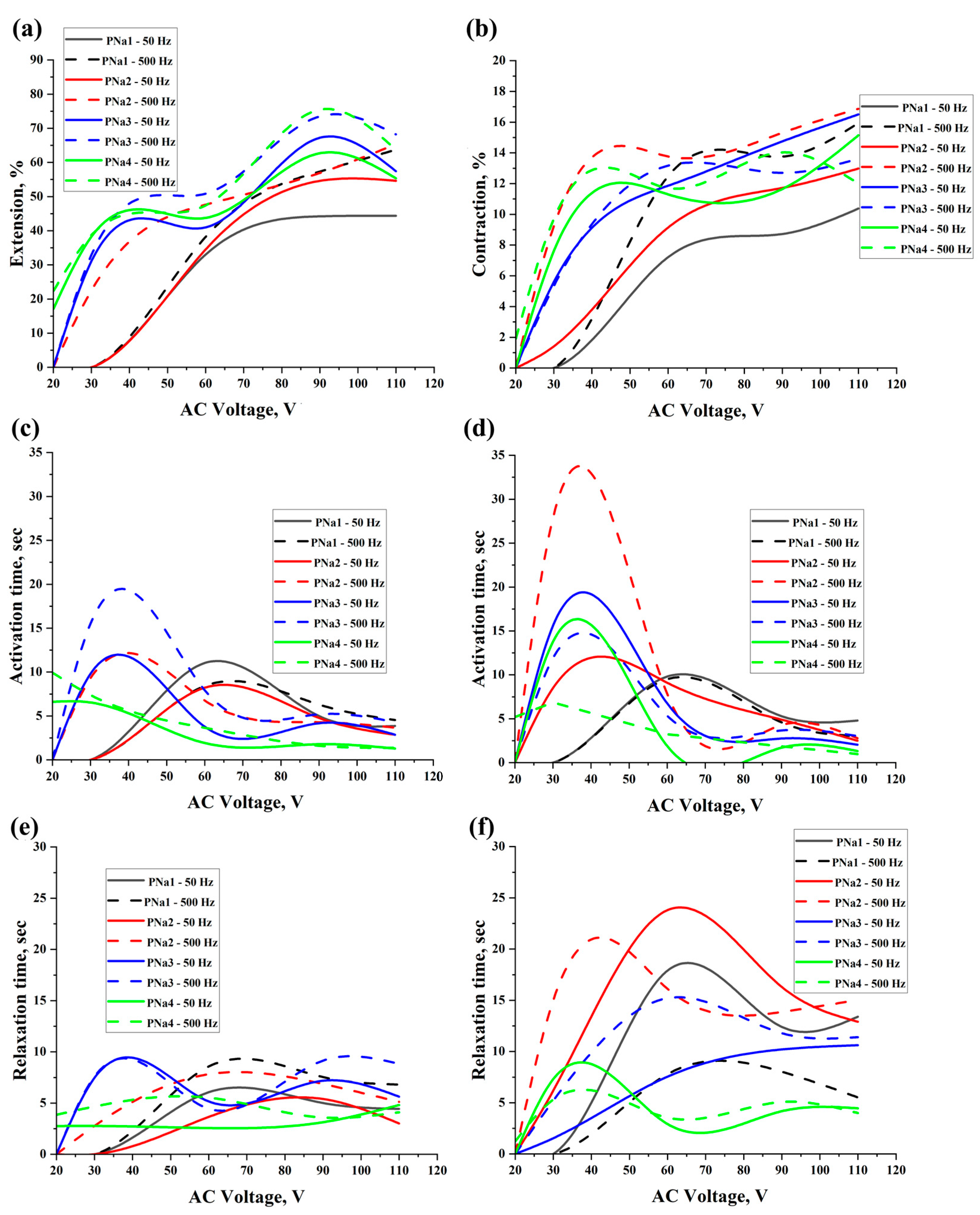
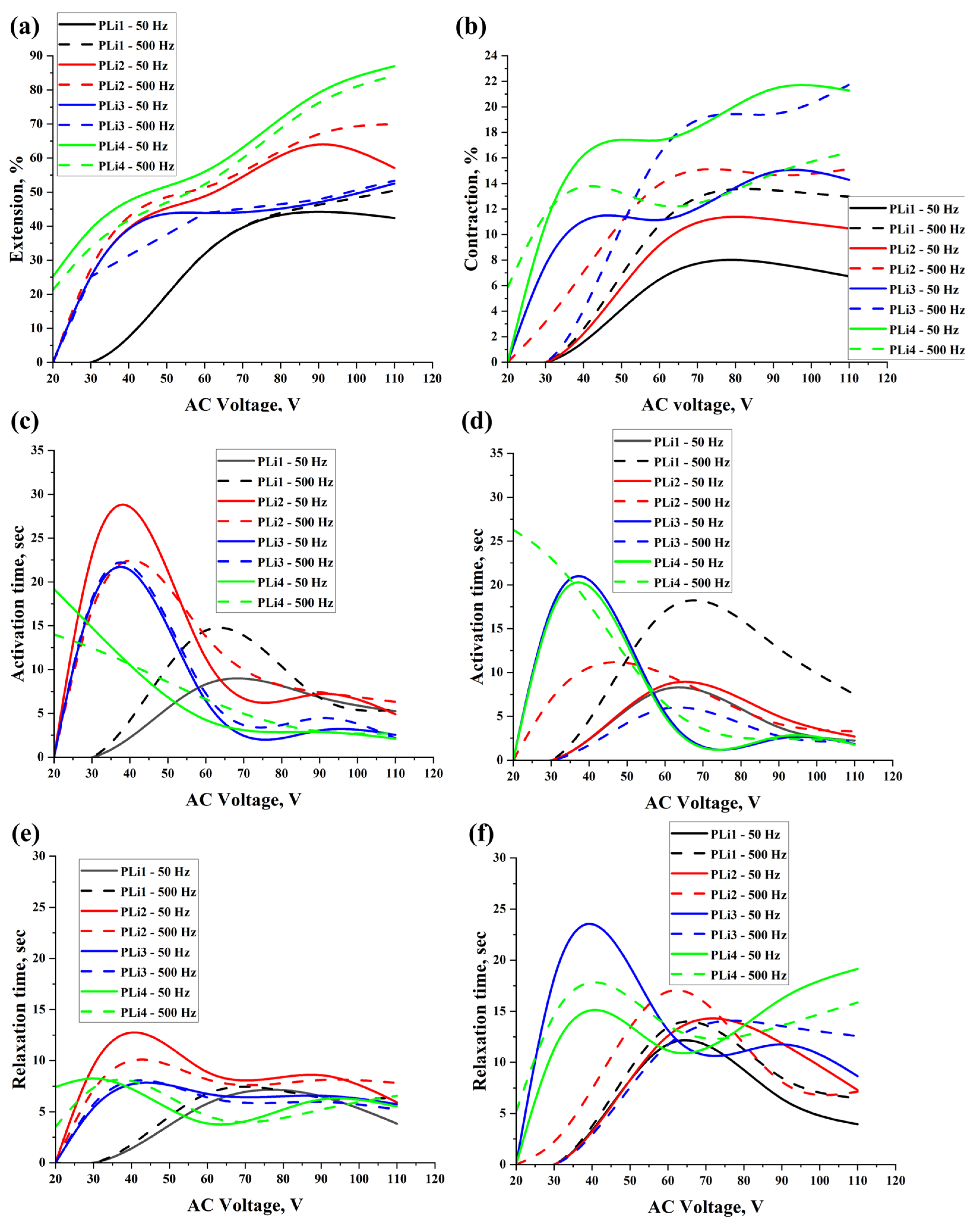
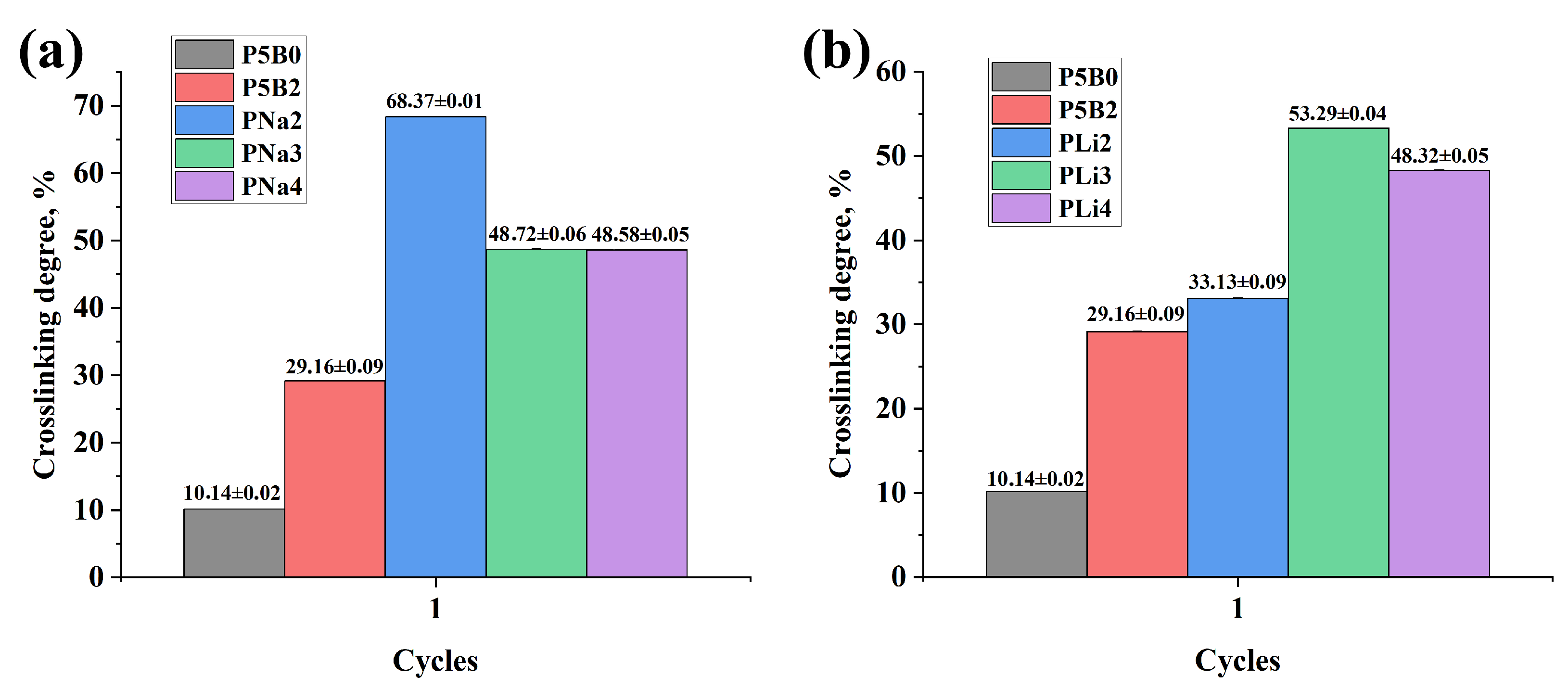
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
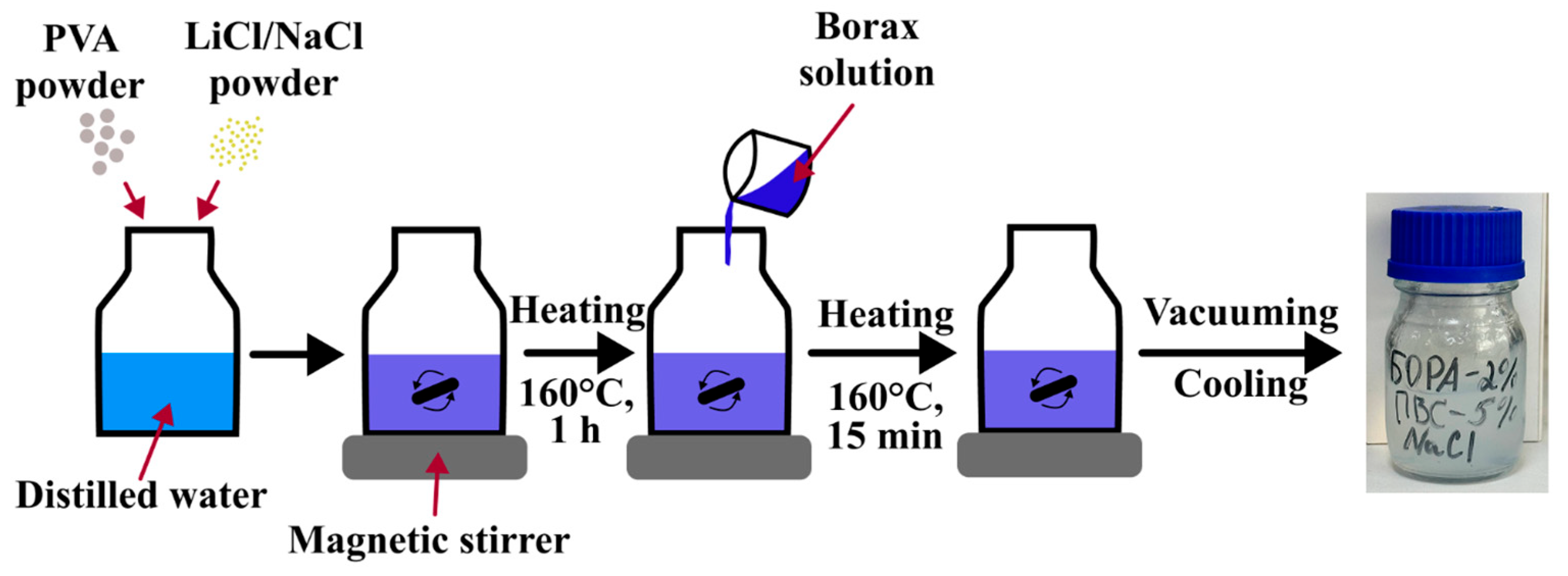
4.2. PVA Hydrogels Preparation
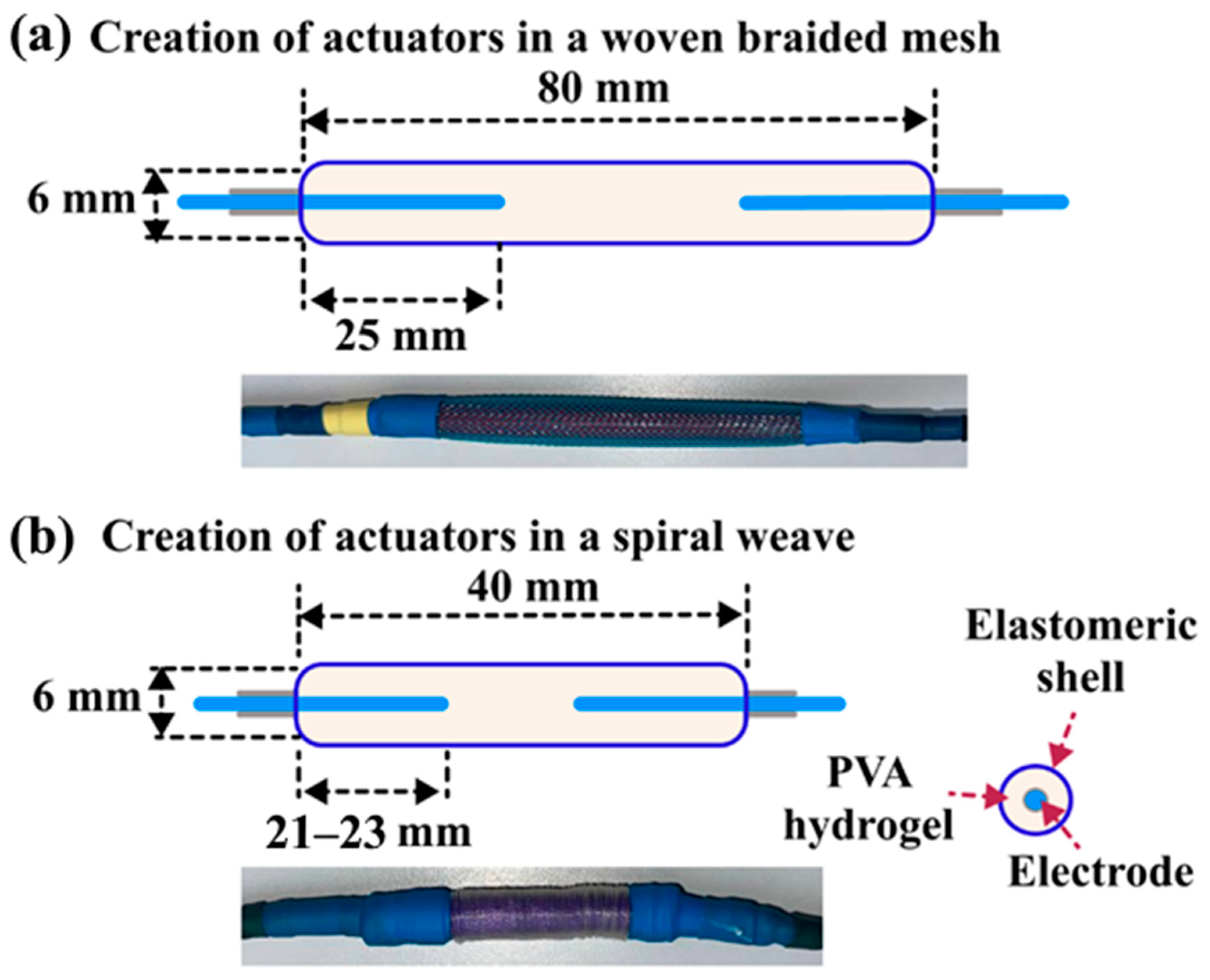
4.3. Hydrogel Actuators Preparation
4.4. Methods
4.4.1. Electrical Conductivity Assessment of Hydrogel Actuators
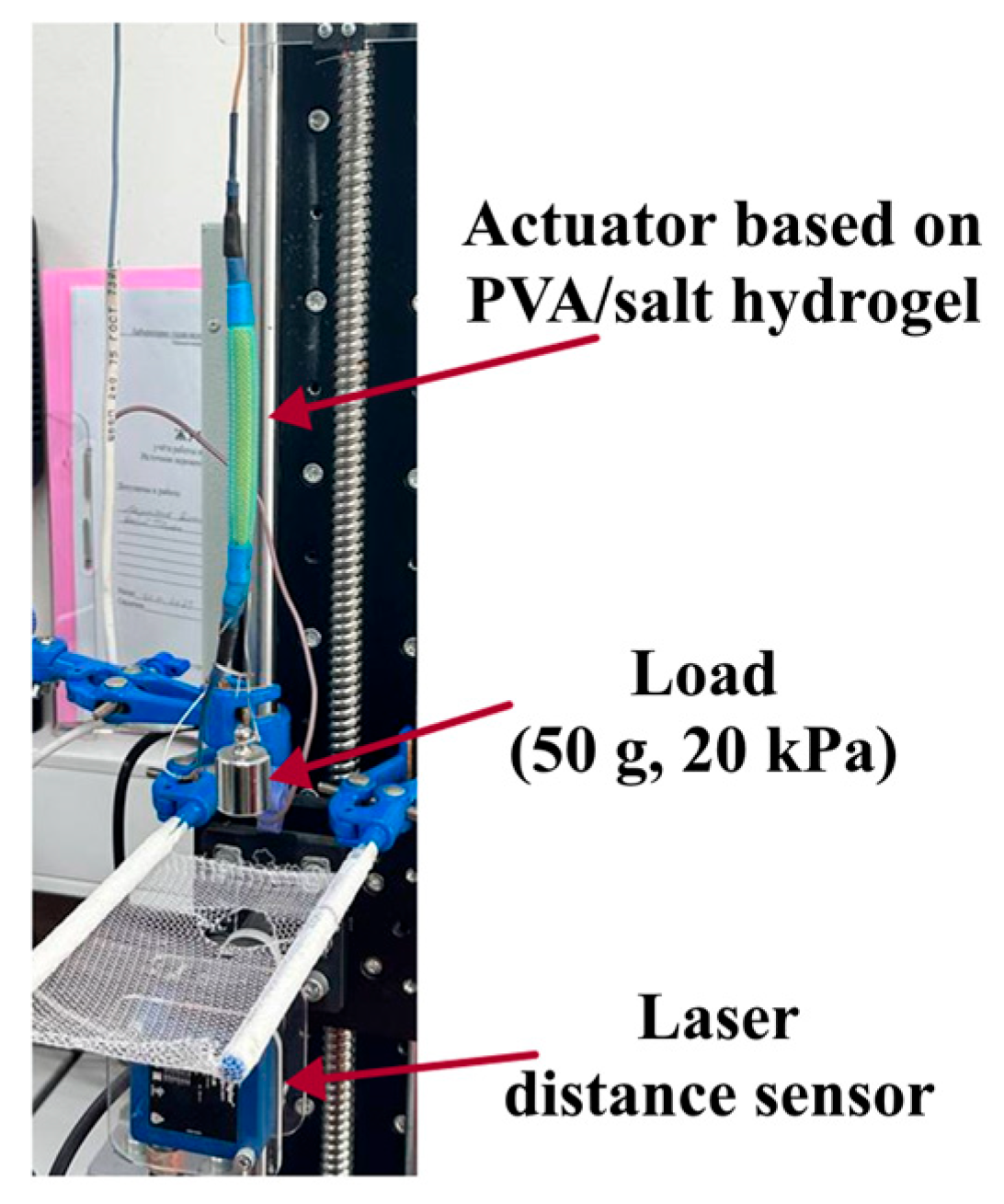
4.4.2. Actuation Tests
4.4.3. Actuation Deformation Assessment
4.4.4. Measurement of Swelling of PVA/Salt Hydrogel
4.4.5. Investigation of Crosslinking Degree of PVA/Salt Hydrogels
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PVA | polyvinyl alcohol |
| LiCl | lithium chloride |
| NaCl | sodium chloride |
| EAPs | electroactive polymers |
| DC | direct current |
| AC | alternating current |
References
- Wei, M.; Gao, Y.; Li, X.; Serpe, M.J. Stimuli-Responsive Polymers and Their Applications. Polym. Chem. 2017, 8, 127–143. [Google Scholar] [CrossRef]
- Ahn, J.; Gu, J.; Choi, J.; Han, C.; Jeong, Y.; Park, J.; Cho, S.; Oh, Y.S.; Jeong, J.H.; Amjadi, M.; et al. A Review of Recent Advances in Electrically Driven Polymer-Based Flexible Actuators: Smart Materials, Structures, and Their Applications. Adv. Mater. Technol. 2022, 7, 2200041. [Google Scholar] [CrossRef]
- Maksimkin, A.V.; Dayyoub, T.; Telyshev, D.V.; Gerasimenko, A.Y. Electroactive Polymer-Based Composites for Artificial Muscle-like Actuators: A Review. Nanomaterials 2022, 12, 2272. [Google Scholar] [CrossRef]
- Dayyoub, T.; Maksimkin, A.V.; Filippova, O.V.; Tcherdyntsev, V.V.; Telyshev, D.V. Shape Memory Polymers as Smart Ma-terials: A Review. Polymers 2022, 14, 3511. [Google Scholar] [CrossRef]
- Behl, M.; Lendlein, A. Shape-memory polymers. Mater. Today 2007, 10, 20–28. [Google Scholar] [CrossRef]
- Ratna, D.; Karger-Kocsis, J. Recent advances in shape memory polymers and composites: A review. J. Mater. Sci. 2008, 43, 254–269. [Google Scholar] [CrossRef]
- Anderson, I.A.; Gisby, T.A.; McKay, T.G.; O’Brien, B.M.; Calius, E. Multi-functional dielectric elastomer artificial muscles for soft and smart machines. J. Appl. Phys. 2012, 112, 041101. [Google Scholar] [CrossRef]
- Bar-Cohen, Y. Electroactive Polymers as Artificial Muscles—Reality, Potential and Challenges, Vol. PM136, 2nd ed.; SPIE Press: Bellingham, WA, USA, 2004; pp. 1–765. ISBN 0-8194–5297-1. [Google Scholar]
- Cardoso, V.F.; Ribeiro, C.; Lanceros-Mendez, S. Metamorphic biomaterials. In Bioinspired Materials for Medical Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 69–99. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, C.; Twigg, S.; Lin, J.-H.; Hatipoglu, G.; Liu, S.; Zhang, Q.M. Ion distribution in ionic electroactive polymer ac-tuators. Electroact. Polym. Actuators Devices 2011, 79762, 776–783. [Google Scholar] [CrossRef]
- Biddiss, E.; Chau, T. Electroactive polymeric sensors in hand prostheses: Bending response of an ionic polymer metal composite. Med. Eng. Phys. 2006, 28, 568–578. [Google Scholar] [CrossRef]
- Adelnia, H.; Ensandoost, R.; Shehzahdi, S.M.; Gavgani, J.N.; Vasafi, E.I.; Ta, H.T. Freeze/thawed polyvinyl alcohol hydro-gels: Present, past and future. Eur. Polym. J. 2022, 164, 110974. [Google Scholar] [CrossRef]
- Chiellini, E.; Corti, A.; D’Antone, S.; Solaro, R. Biodegradation of poly (vinyl alcohol) based materials. Prog. Polym. Sci. 2003, 28, 963–1014. [Google Scholar] [CrossRef]
- Paradossi, G.; Cavalieri, F.; Chiessi, E.; Spagnoli, C.; Cowan, M.K. Poly (vinyl alcohol) as versatile biomaterial for potential biomedical applications. J. Mater. Sci. Mater. Med. 2003, 14, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Dayyoub, T.; Zadorozhnyy, M.; Filippova, K.V.; Iudina, L.D.; Telyshev, D.V.; Zhemchugov, P.V.; Ladokhin, D.G.; Maksimkin, A. Linear Actuators Based on Polyvinyl Alcohol/Lithium Chloride Hydrogels Activated by Low AC-Voltage. J. Compos. Sci. 2024, 8, 323. [Google Scholar] [CrossRef]
- Dayyoub, T.; Maksimkin, A.; Larionov, D.I.; Filippova, O.V.; Telyshev, D.V.; Gerasimenko, A.Y. Preparation of Linear Actuators Based on Polyvinyl Alcohol Hydrogels Activated by AC Voltage. Polymers 2023, 15, 2739. [Google Scholar] [CrossRef] [PubMed]
- Morrow, R.; McKenzie, D.R. The Time-Dependent Development of Electric Double-Layers in Pure Water at Metal Electrodes: The Effect of an Applied Voltage on the Local pH. Proc. R. Soc. A Math. Phys. Eng. Sci. 2012, 468, 18–34. [Google Scholar] [CrossRef]
- Morrow, R.; McKenzie, D.R.; Bilek, M.M.M. The Time-Dependent Development of Electric Double-Layers in Saline Solutions. J. Phys. D Appl. Phys. 2006, 39, 937–943. [Google Scholar] [CrossRef]
- Han, Z.J.; Morrow, R.; Tay, B.K.; McKenzie, D. Time-Dependent Electrical Double Layer with Blocking Electrode. Appl. Phys. Lett. 2009, 94, 043118. [Google Scholar] [CrossRef]
- Hossain, R.; Adamiak, K. Dynamic Properties of the Electric Double Layer in Electrolytes. J. Electrost. 2013, 71, 829–838. [Google Scholar] [CrossRef]
- Hong, F.; Cao, J.; Cheng, P. A parametric study of AC electrothermal flow in microchannels with asymmetrical interdigitated electrodes. Int. Commun. Heat Mass Transf. 2011, 38, 275–279. [Google Scholar] [CrossRef]
- Lian, M.; Wu, J. Microfluidic flow reversal at low frequency by AC electrothermal effect. Microfluid. Nanofluid. 2009, 7, 757–765. [Google Scholar] [CrossRef]
- Zehavi, M.; Boymelgreen, A.; Yossifon, G. Competition between Induced-Charge Electro-Osmosis and Electrothermal Effects at Low Frequencies around a Weakly Polarizable Microchannel Corner. Phys. Rev. Appl. 2016, 5, 044013. [Google Scholar] [CrossRef]
- Wang, H.; Wei, J.; Simon, G.P. Response to Osmotic Pressure versus Swelling Pressure: Comment on “Bifunctional Polymer Hydrogel Layers as Forward Osmosis Draw Agents for Continuous Production of Fresh Water Using Solar Energy”. Environ. Sci. Technol. 2014, 48, 4214–4215. [Google Scholar] [CrossRef]
- Tanveer, S.; Chen, C.C. Thermodynamic analysis of hydrogel swelling in aqueous sodium chloride solutions. J. Mol. Liq. 2022, 348, 118421. [Google Scholar] [CrossRef]
- Maurer, G.; Prausnitz, J.M. Thermodynamics of phase equilibrium for systems containing gels. Fluid Phase Equilibria 1996, 115, 113–133. [Google Scholar] [CrossRef]
- Hüther, A.; Xu, X.; Maurer, G. Swelling of N-isopropyl acrylamide hydrogels in aqueous solutions of sodium chloride. Fluid Phase Equilibria 2006, 240, 186–196. [Google Scholar] [CrossRef]
- Islam, M.R.; Tanveer, S.; Chen, C.-C. Modeling swelling behavior of hydrogels in aqueous organic solvents. Chem. Eng. Sci. 2021, 242, 116744. [Google Scholar] [CrossRef]
- Guo, Y.; Guan, W.; Lei, C.; Lu, H.; Shi, W.; Yu, G. Scalable super hygroscopic polymer films for sustainable moisture harvesting in arid environments. Nat. Commun. 2022, 13, 2761. [Google Scholar] [CrossRef]
- Xu, J.; Li, T.; Yan, T.; Wu, S.; Wu, M.; Chao, J.; Huo, X.; Wang, P.; Wang, R. Ultrahigh solar-driven atmospheric water production enabled by scalable rapid-cycling water harvester with vertically aligned nanocomposite sorbent. Energy Environ. Sci. 2021, 14, 5979. [Google Scholar] [CrossRef]
- Díaz-Marín, C.D.; Zhang, L.; Lu, Z.; Alshrah, M.; Grossman, J.C.; Wang, E.N. Kinetics of Sorption in Hygroscopic Hydrogels. Nano Lett. 2022, 22, 1100. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, B.; Xiang, F.; Zhou, J.; Wang, H.; Suo, Z. Transparent hydrogel with enhanced water retention capacity by introducing highly hydratable salt. Appl. Phys. Lett. 2014, 105, 151903. [Google Scholar] [CrossRef]
- Graeber, G.; Díaz-Marín, C.D.; Gaugler, L.C.; Zhong, Y.; El Fil, B.; Liu, X.; Wang, E.N. Extreme Water Uptake of Hygroscopic Hydrogels through Maximized Swelling-Induced Salt Loading. Adv. Mater. 2023, 36, 2211783. [Google Scholar] [CrossRef]
- Cao, C.; Li, X.; Yin, Y.; Kong, B.; Sun, F.; Liu, Q. Effects of Sodium Chloride on the Physical and Oxidative Stability of Filled Hydrogel Particles Fabricated with Phase Separation Behavior. Foods 2021, 10, 1027. [Google Scholar] [CrossRef]
- Kazimierska-Drobny, K.; Kaczmarek, M. Effect of NaCl and KCl solutions on deformation of PVA hydrogel—Chemo-mechanical coupling. Polimery 2020, 65, 44–50. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Ko, H.-U.; Kim, H.C.; Kim, J.W.; Muthoka, R.M.; Kim, J. Electroactive Hydrogels Made with Polyvinyl Alcohol/Cellulose Nanocrystals. Materials 2018, 11, 1615. [Google Scholar] [CrossRef] [PubMed]
- Valiskó, M.; Boda, D. The effect of concentration- and temperature-dependent dielectric constant on the activity coefficient of NaCl electrolyte solutions. J. Chem. Phys. 2014, 140, 234508. [Google Scholar] [CrossRef]
- Marcus, Y. Electrostriction in Electrolyte Solutions. Chem. Rev. 2011, 111, 2761–2783. [Google Scholar] [CrossRef]
- Anand, G.; Safaripour, S.; Snoeyink, C. Effects of Frequency and Joule Heating on Height Rise between Parallel Electrodes with AC Electric Fields. Langmuir 2022, 38, 1204–1214. [Google Scholar] [CrossRef]
- Das, G.; Hlushak, S.; dos Ramos, M.C.; McCabe, C. Predicting the thermodynamic properties and dielectric behavior of electrolyte solutions using the SAFT-VR+DE equation of state. AIChE J. 2015, 61, 3053–3072. [Google Scholar] [CrossRef]
- Ninjumrat, U.; Chuysinuan, P.; Inprasit, T.; Ummartyotin, S.; Chainok, K.; Pisitsak, P. Fast-Swelling Tamarind Xyloglucan/PVA Hydrogels with Interconnected Macroporous Structures for Biomedical Applications. Polymers 2024, 16, 3457. [Google Scholar] [CrossRef]
- Urushizaki, F.; Yamaguchi, H.; Nakamura, K.; Numajiri, S.; Sugibayashi, K.; Morimoto, Y. Swelling and mechanical properties of poly(vinyl alcohol) hydrogels. Int. J. Pharm. 1990, 58, 135–142. [Google Scholar] [CrossRef]
- Lin, F.; Zhu, Y.; You, Z.; Li, W.; Chen, J.; Zheng, X.; Zheng, G.; Song, Z.; You, X.; Xu, Y. Ultrastrong and Tough Urushiol-Based Ionic Conductive Double Network Hydrogels as Flexible Strain Sensors. Polymers 2023, 15, 3219. [Google Scholar] [CrossRef]
- Hong, Y.; Lin, Z.; Yang, Y.; Jiang, T.; Shang, J.; Luo, Z. Flexible Actuator Based on Conductive PAM Hydrogel Electrodes with Enhanced Water Retention Capacity and Conductivity. Micromachines 2022, 3, 1951. [Google Scholar] [CrossRef]
- Kędzierska, M.; Jamroży, M.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Bańkosz, M.; Gruca, M.; Potemski, P.; Tyliszczak, B. Analysis of the Influence of Both the Average Molecular Weight and the Content of Crosslinking Agent on Physicochemical Properties of PVP-Based Hydrogels Developed as Innovative Dressings. Int. J. Mol. Sci. 2022, 23, 11618. [Google Scholar] [CrossRef]
- Milampure, C.; Jawale, D.; Shingate, S.; Mahala, P.; Upendra Kulshrestha, U.; Ghosh, S.B.; Bandyopadhyay-Ghosh, S. Effect of salt addition towards enhancement of water retention capacity of hydrogel. Mater. Today Proc. 2024; in press. [Google Scholar] [CrossRef]
- Tang, C.; Li, M.; Yao, Y.; Wang, Y.; Zhang, Y.; Wang, G.; Liu, J.; Li, L. High-performance environmental adaptive microsupercapacitors from multifunctional hydrogel via modulating ionic hydration and hydrogen bonds. Energy Storage Mater. 2023, 55, 527–537. [Google Scholar] [CrossRef]
- Li, Z.; Yu, C.; Kumar, H.; He, X.; Lu, Q.; Bai, H.; Kim, K.; Hu, J. The Effect of Crosslinking Degree of Hydrogels on Hydrogel Adhesion. Gels 2022, 8, 682. [Google Scholar] [CrossRef]
- Lambrecht, S.; Schröter, H.; Pohle, H.; Jopp, S. Swelling Behavior of Novel Hydrogels Produced from Glucose-Based Ionic Monomers with Varying Cross-Linkers. ACS Omega 2024, 9, 5418–5428. [Google Scholar] [CrossRef]
- Kazimierska-Drobny, K. PVA hydrogel deformation in response to change in temperature or pH. Bull. Pol. Acad. Sci. Tech. Sci. 2021, 69, e136724. [Google Scholar] [CrossRef]
- Yamamoto, H.; Heyamoto, N.; Matsui, T.; Nurayama, N.; Shibata, J. Volumetric change and surface properties of temperature-sensitive polyvinylalcohol (PVA) hydrogel. Int. J. Thermophys. 2003, 24, 1385–1394. [Google Scholar] [CrossRef]
- Ren, T.; Gan, J.; Zhou, L.; Chen, H. Physically Crosslinked Hydrogels Based on Poly (Vinyl Alcohol) and Fish Gelatin for Wound Dressing Application: Fabrication and Characterization. Polymers 2020, 12, 1729. [Google Scholar] [CrossRef]
- Jalal, N.M.; Ali, Z.A.; Allami, S.A.; Hassan, S.M.; Ali, M.R. Effect of Lithium Chloride Addition on the Electrical Conductivity of Polyvinyl Alcohol Films. Am. J. Eng. Res. (AJER) 2017, 6, 337–343. [Google Scholar] [CrossRef]


| Material | PLi1 | PLi2 | PLi3 | PLi4 | ||||
|---|---|---|---|---|---|---|---|---|
| Frequency, Hz | 50 | 500 | 50 | 500 | 50 | 500 | 50 | 500 |
| Electrical conductivity, S/m | 0.016 ± 0.003 | 0.024 ± 0.002 | 0.049 ± 0.006 | 0.064 ± 0.003 | 0.071 ± 0.002 | 0.092 ± 0.001 | 0.094 ± 0.002 | 0.122 ± 0.002 |
| Material | PNa1 | PNa2 | PNa3 | PNa4 | ||||
|---|---|---|---|---|---|---|---|---|
| Frequency, Hz | 50 | 500 | 50 | 500 | 50 | 500 | 50 | 500 |
| Electrical conductivity, S/m | 0.022 ± 0.004 | 0.025 ± 0.002 | 0.033 ± 0.002 | 0.036 ± 0.003 | 0.088 ± 0.001 | 0.093 ± 0.003 | 0.208 ± 0.004 | 0.222 ± 0.002 |
| Material Code (Activation Voltage) | PNa1 (110 V) | PNa2 (110 V) | PNa3 (90 V) | PNa4 (90 V) | PLi1 (110 V) | PLi2 (110 V) | PLi3 (110 V) | PLi4 (110 V) |
|---|---|---|---|---|---|---|---|---|
| Current, A | 0.031 ± 0.003 | 0.046 ± 0.004 | 0.069 ± 0.004 | 0.055 ± 0.005 | 0.032 ± 0.001 | 0.033 ± 0.002 | 0.027 ± 0.003 | 0.088 ± 0.006 |
| Efficiency, % | 2.02 ± 0. 12 | 1.74 ± 0. 31 | 1.11 ± 0.11 | 4.60 ± 0.21 | 1.32 ± 0.12 | 1.55 ± 0.22 | 4.11 ± 0.33 | 1.60 ± 0.01 |
| Material Code (Activation Voltage) | PNa1 (110 V) | PNa2 (110 V) | PNa3 (110 V) | PNa4 (110 V) | PLi1 (110 V) | PLi2 (110 V) | PLi3 (110 V) | PLi4 (110 V) |
|---|---|---|---|---|---|---|---|---|
| Current, A | 0.031 ± 0.002 | 0.057 ± 0.003 | 0.067 ± 0.003 | 0.075 ± 0.005 | 0.043 ± 0.001 | 0.062 ± 0.003 | 0.069 ± 0.002 | 0.045 ± 0.004 |
| Efficiency, % | 0.81 ± 0.01 | 0.50 ± 0.02 | 0. 31 ± 0.01 | 0.71 ± 0.02 | 0.22 ± 0.01 | 0. 38 ± 0.01 | 0.67 ± 0.03 | 0.83 ± 0.01 |
| Material Code | SD at 35 °C, % | SD at 45 °C, % | SD at 50 °C, % |
|---|---|---|---|
| PNa1 | 16.48 ± 1.79 | 47.88 ± 5.87 | 66.98 ± 1.84 |
| PNa2 | −7.38 ± 0.23 | 22.65 ± 0.44 | 52.75 ± 0.59 |
| PNa3 | 27.80 ± 1.91 | 55.08 ± 1.25 | 58.20 ± 0.49 |
| PNa4 | 26.60 ± 0.98 | 66.18 ± 0.30 | 73.80 ± 2.18 |
| PLi1 | 24.68 ± 2.22 | 55.13 ± 5.81 | 73.80 ± 1.21 |
| PLi2 | −28.10 ± 3.01 | −2.95 ± 0.25 | 20.18 ± 0.86 |
| PLi3 | 14.38 ± 1.42 | 44.63 ± 4.34 | 70.05 ± 2.82 |
| PLi4 | 3.78 ± 0.25 | 32.68 ± 2.46 | 56.28 ± 2.42 |
| P5B2 * | 22.38 ± 1.29 | 61.08 ± 2.78 | 80.20 ± 3.56 |
| Material Code | PNa2 | PNa3 | PNa4 | PLi2 | PLi3 | PLi4 | P5B0 * | P5B2 * |
|---|---|---|---|---|---|---|---|---|
| Crosslinking degree, % | 68.37 ± 0.01 | 48.72 ± 0.06 | 48.58 ± 0.05 | 33.13 ± 0.09 | 53.29 ± 0.04 | 48.32 ± 0.05 | 10.14 ± 0.02 | 29.16 ± 0.09 |
| Material No. | Material Code | LiCl Content, g | LiCl Concentration, mol/L | NaCl Content, g | NaCl Concentration, mol/L |
| 1 | PLi1 | 0.5 | 0.1180 | - | - |
| 2 | PLi2 | 1.5 | 0.3539 | - | - |
| 3 | PLi3 | 2.5 | 0.5898 | - | - |
| 4 | PLi4 | 5.0 | 1.1797 | - | - |
| 5 | PNa1 | - | - | 0.5 | 0.0856 |
| 6 | PNa2 | - | - | 1.5 | 0.2567 |
| 7 | PNa3 | - | - | 2.5 | 0.4278 |
| 8 | PNa4 | - | - | 5.0 | 0.8558 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maksimkin, A.; Zadorozhnyy, M.; Filippova, K.V.; Iudina, L.D.; Telyshev, D.V.; Dayyoub, T. The Influence of Concentration and Type of Salts on the Behaviour of Linear Actuators Based on PVA Hydrogel Activated by AC Power. Gels 2025, 11, 484. https://doi.org/10.3390/gels11070484
Maksimkin A, Zadorozhnyy M, Filippova KV, Iudina LD, Telyshev DV, Dayyoub T. The Influence of Concentration and Type of Salts on the Behaviour of Linear Actuators Based on PVA Hydrogel Activated by AC Power. Gels. 2025; 11(7):484. https://doi.org/10.3390/gels11070484
Chicago/Turabian StyleMaksimkin, Aleksey, Mikhail Zadorozhnyy, Kseniia V. Filippova, Lidiia D. Iudina, Dmitry V. Telyshev, and Tarek Dayyoub. 2025. "The Influence of Concentration and Type of Salts on the Behaviour of Linear Actuators Based on PVA Hydrogel Activated by AC Power" Gels 11, no. 7: 484. https://doi.org/10.3390/gels11070484
APA StyleMaksimkin, A., Zadorozhnyy, M., Filippova, K. V., Iudina, L. D., Telyshev, D. V., & Dayyoub, T. (2025). The Influence of Concentration and Type of Salts on the Behaviour of Linear Actuators Based on PVA Hydrogel Activated by AC Power. Gels, 11(7), 484. https://doi.org/10.3390/gels11070484










