Single- and Multi-Network Hydrogels for Soft Electronics—A Review
Abstract
1. Introduction
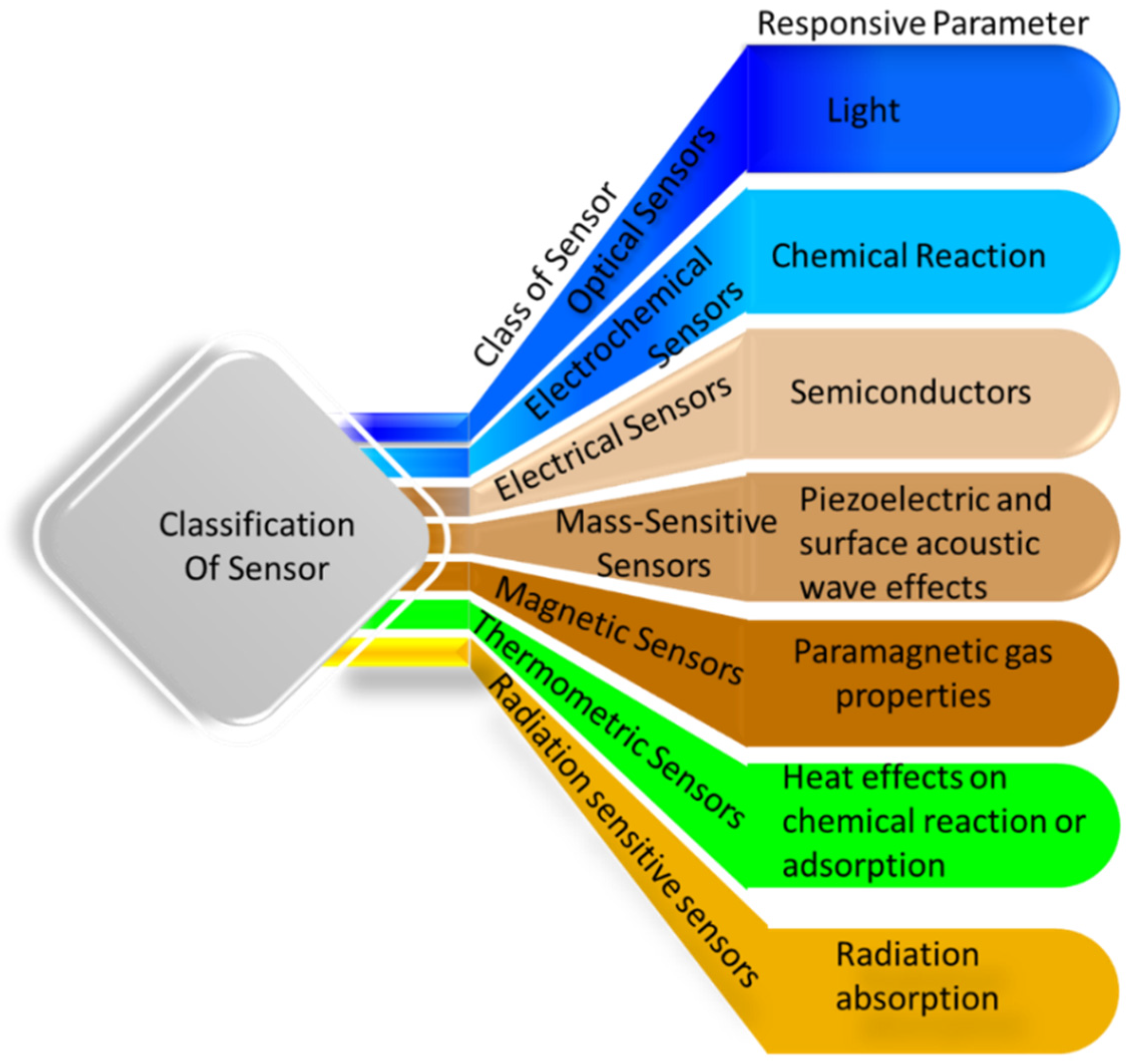
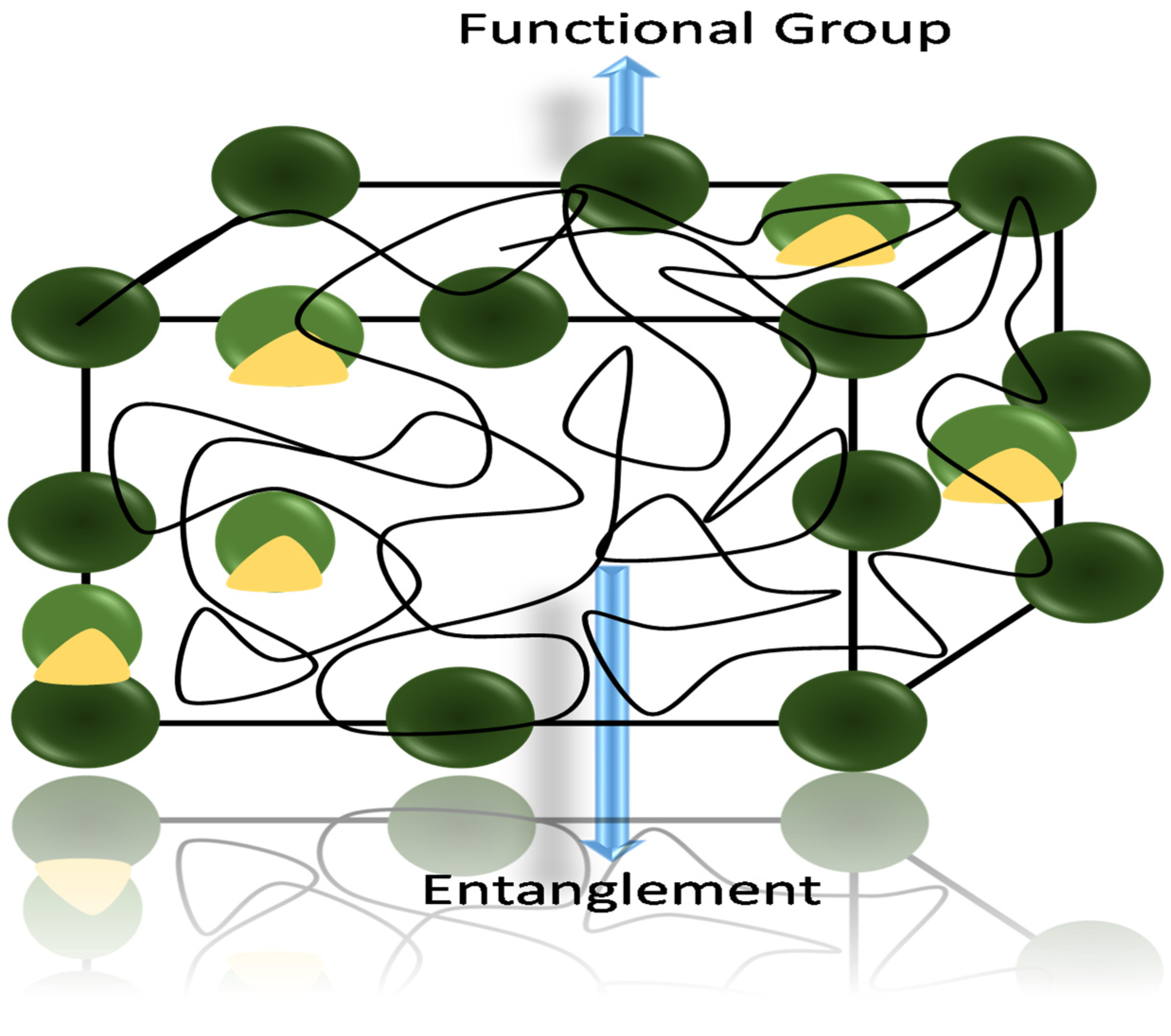
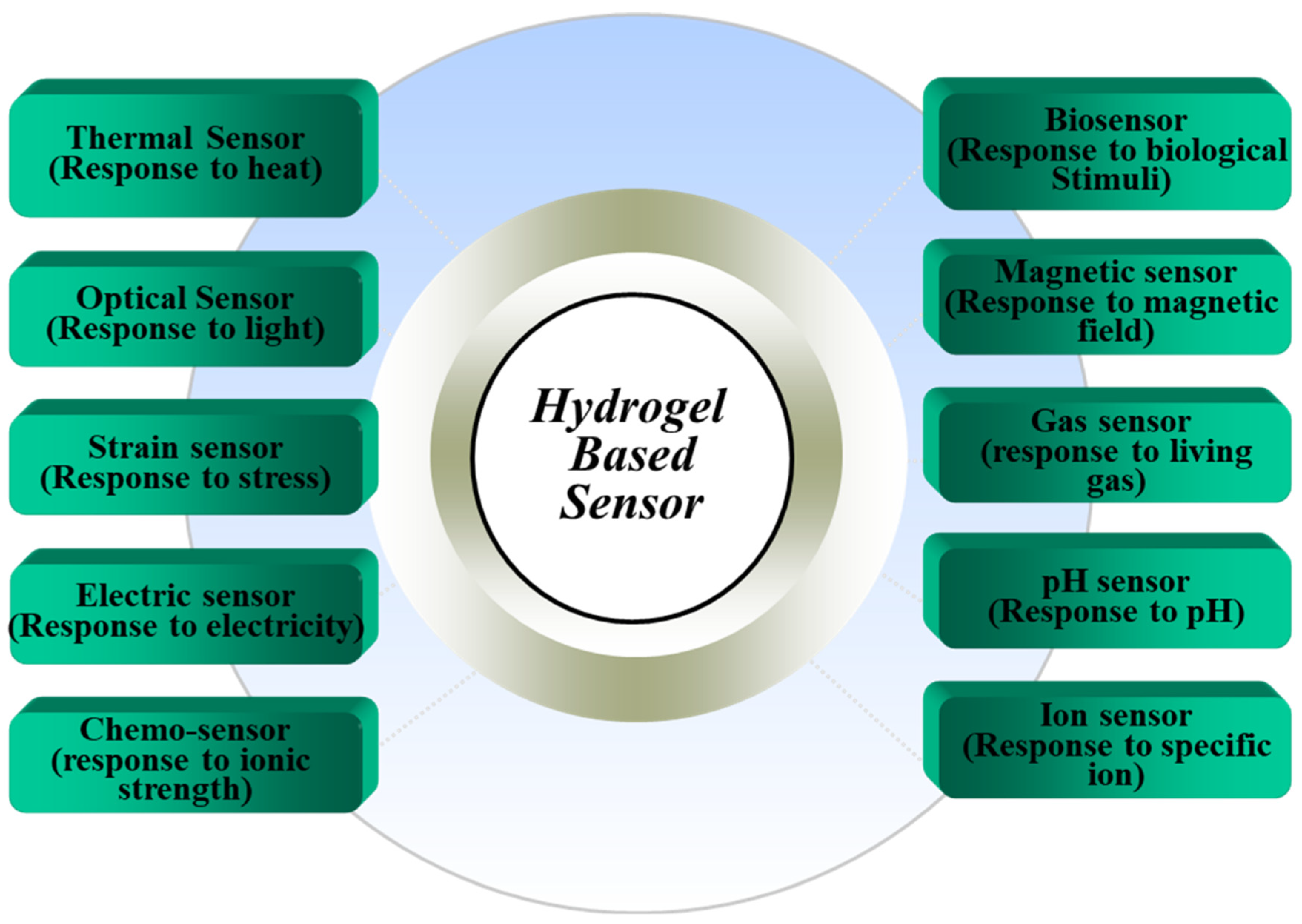
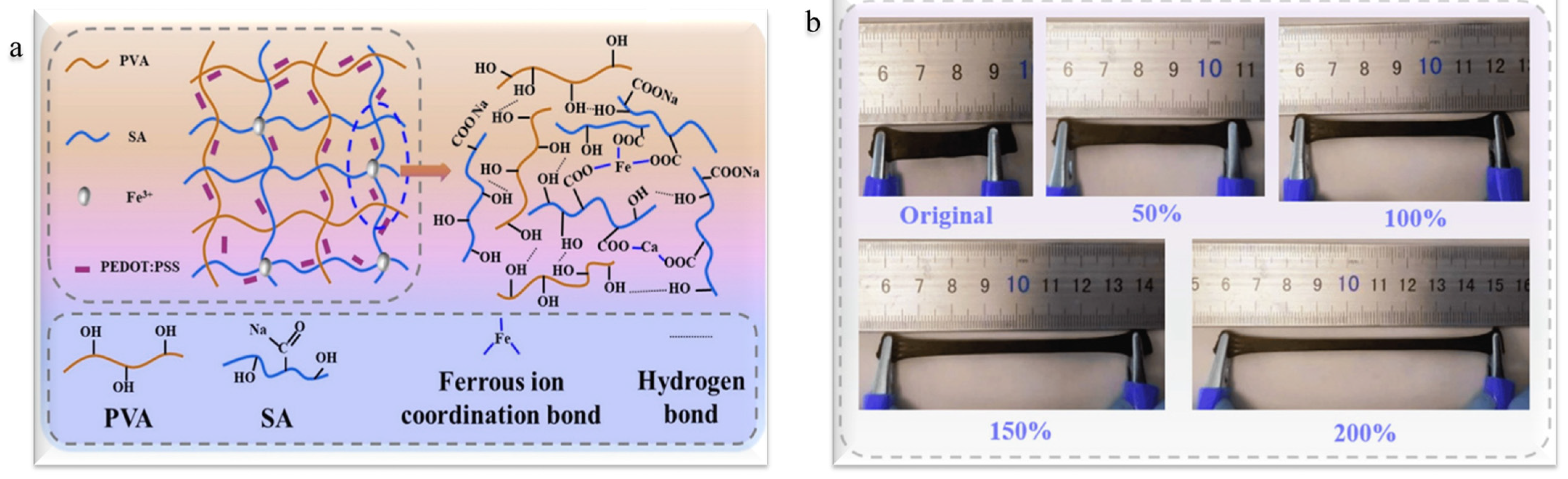
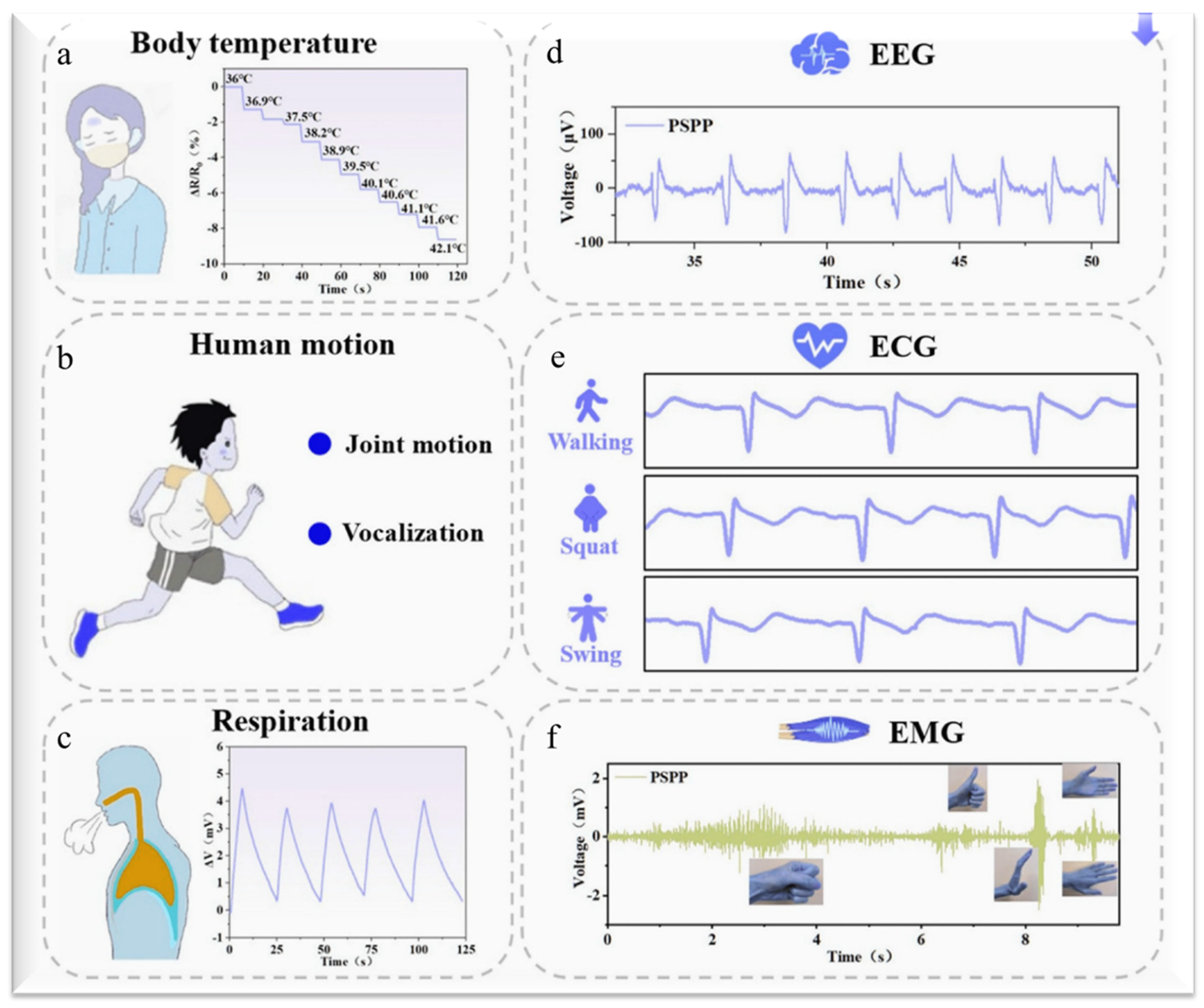
2. Hydrogel-Based Sensors
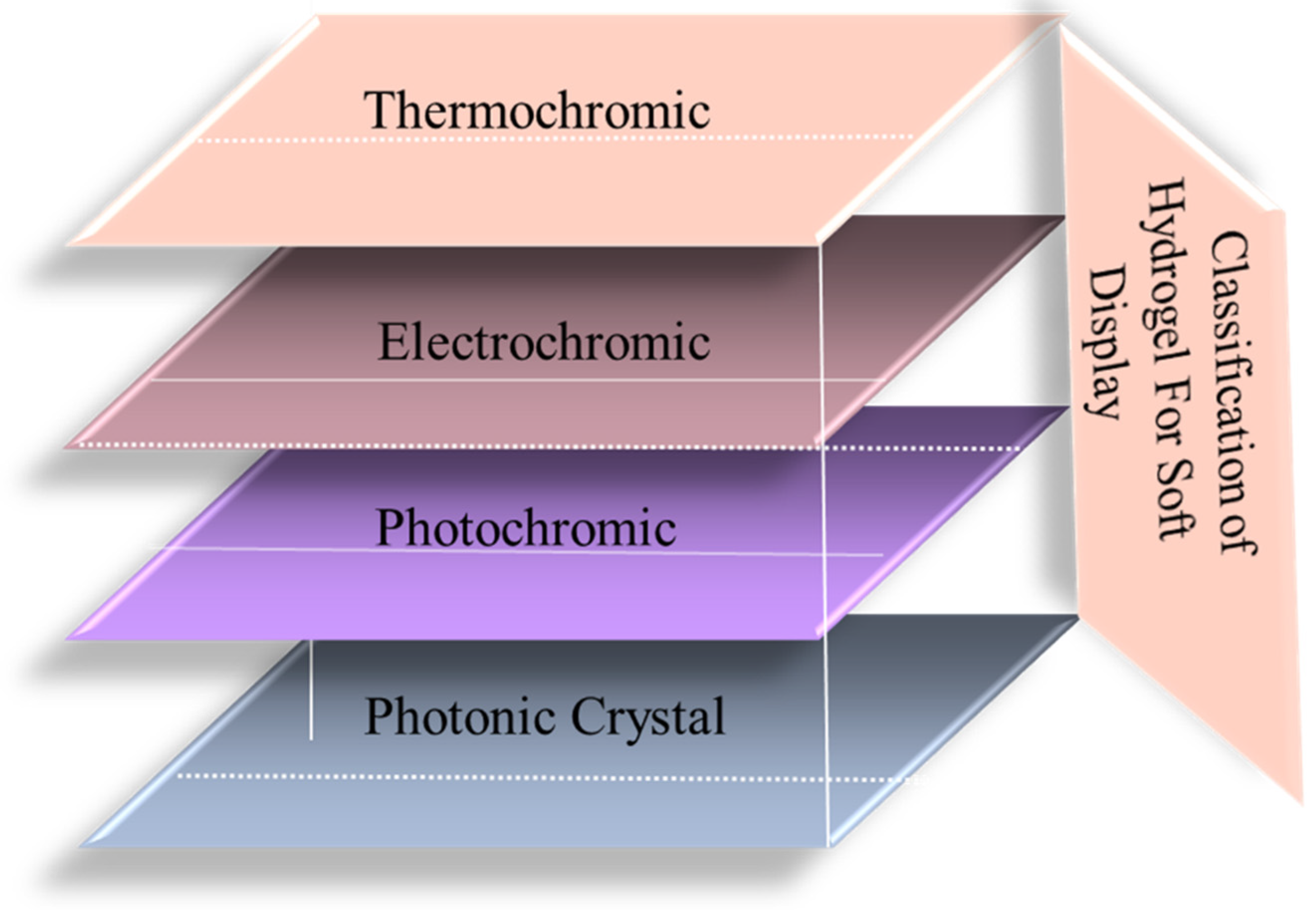
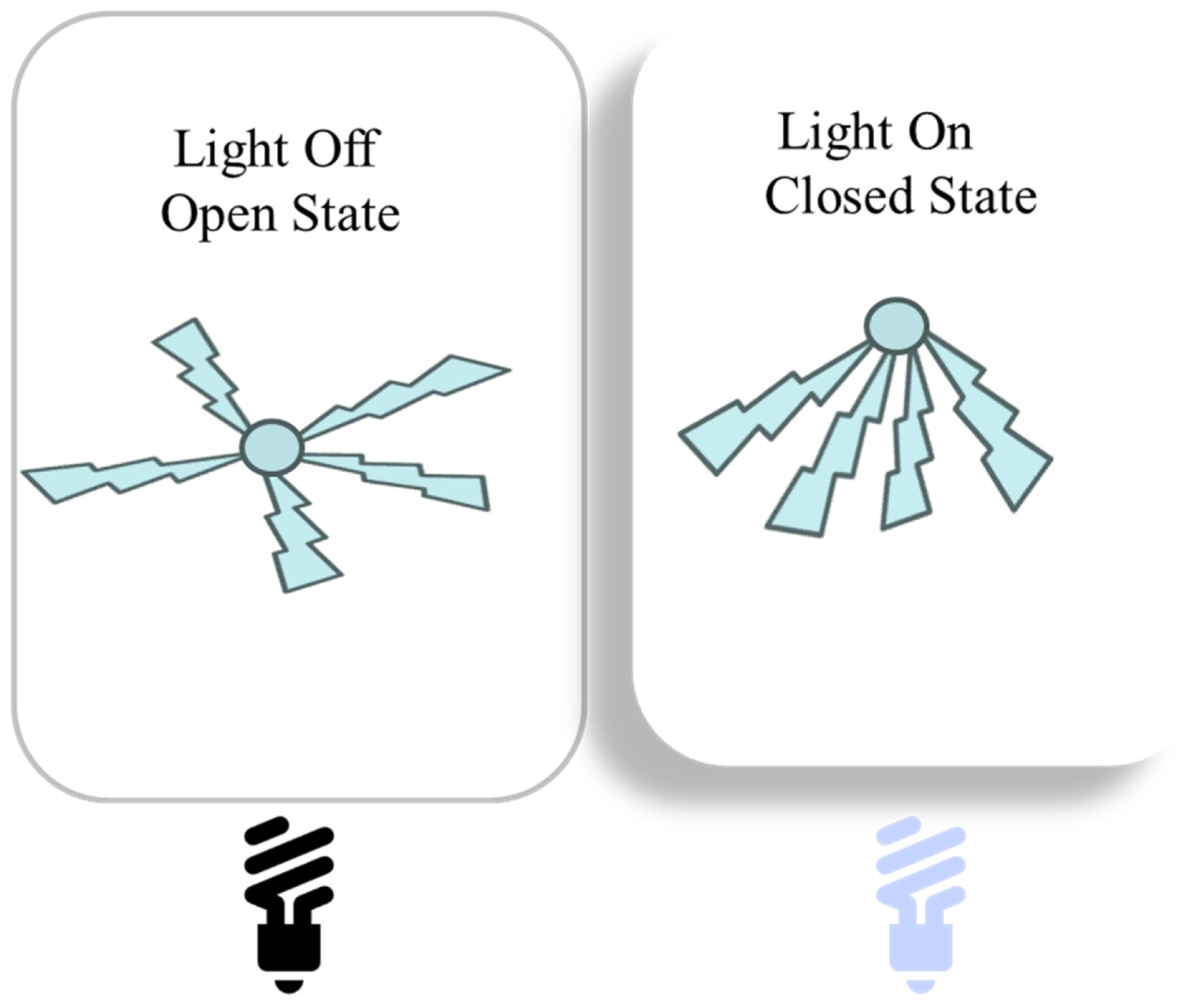
3. Hydrogel-Based Soft Display
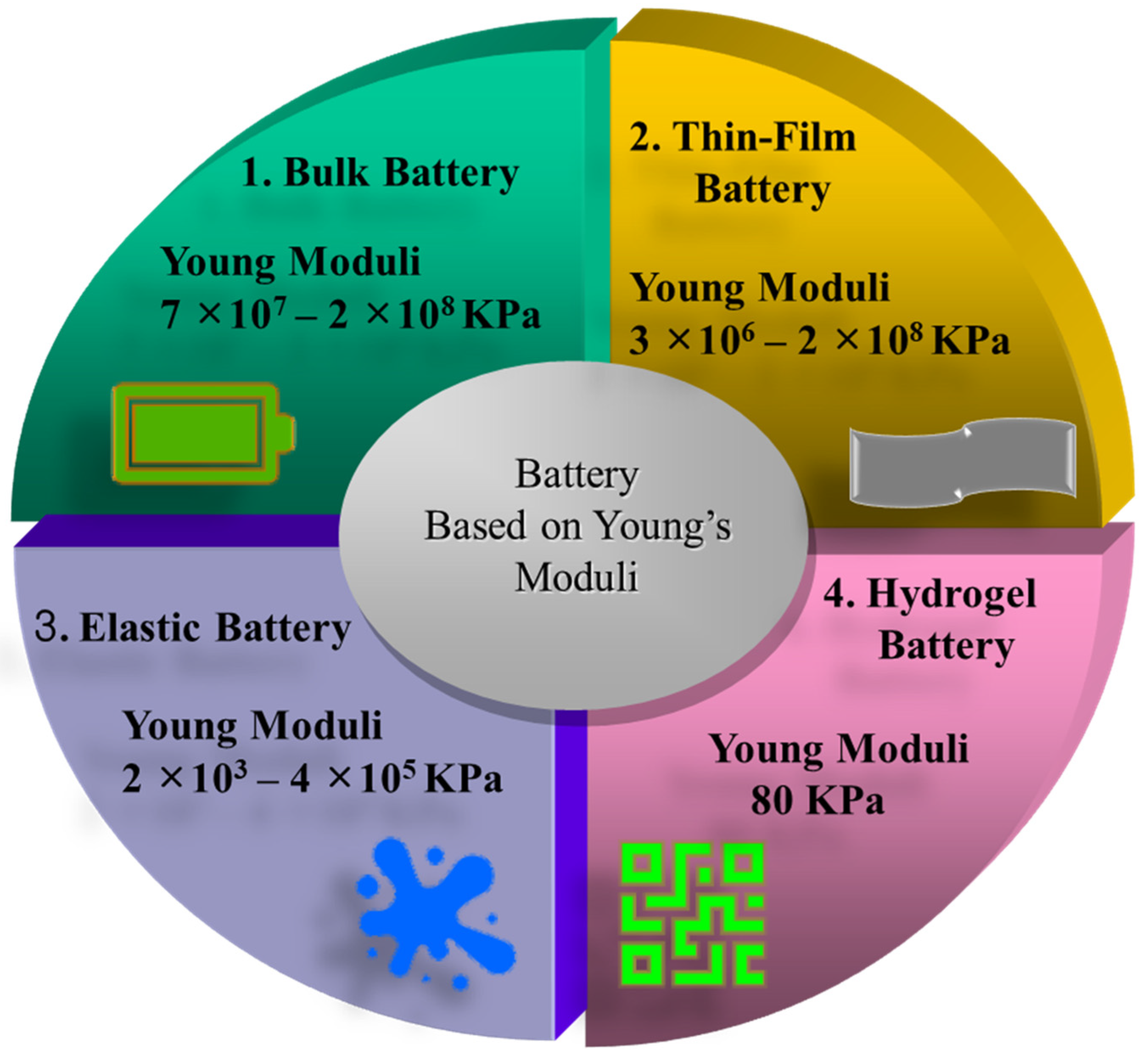
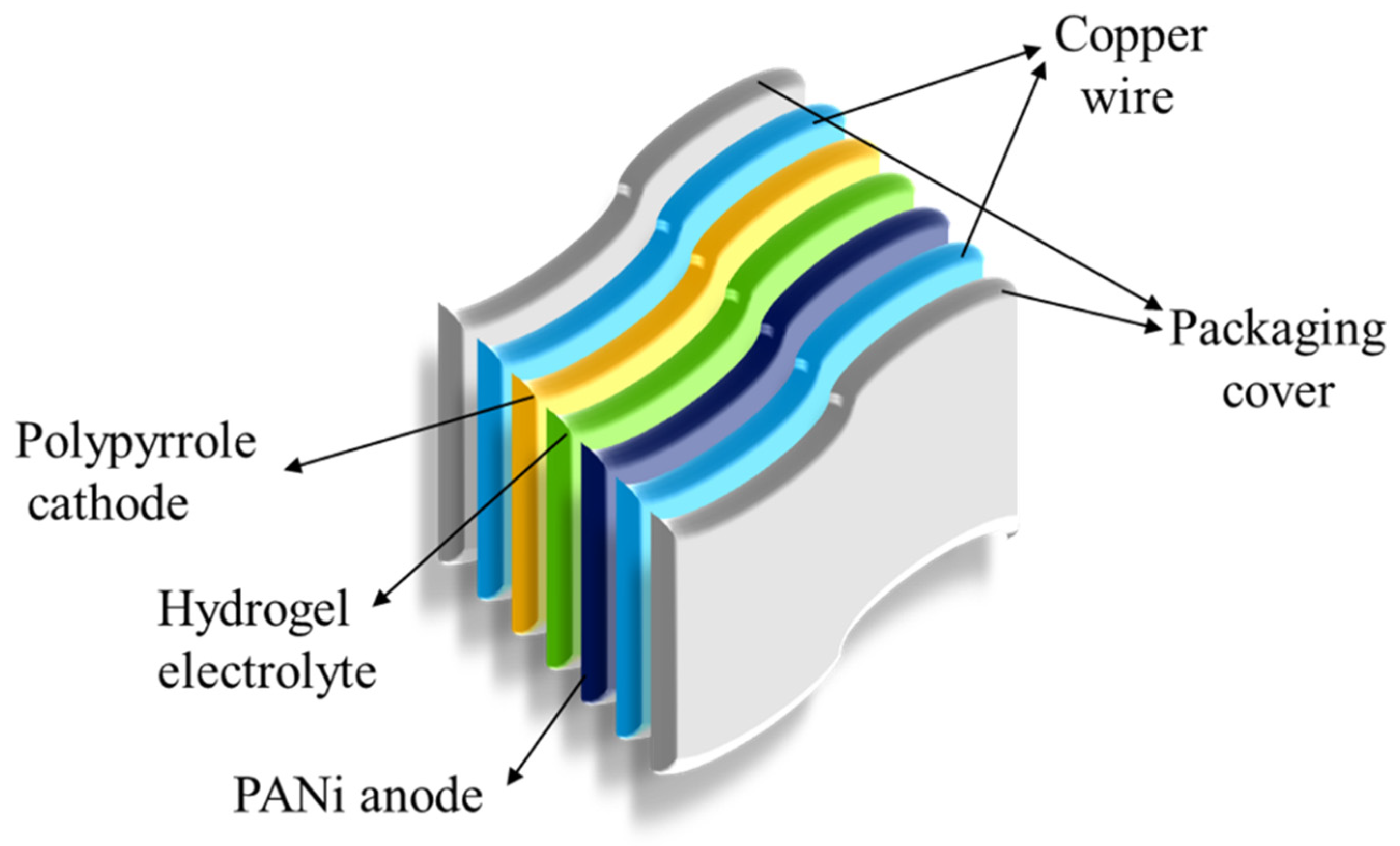
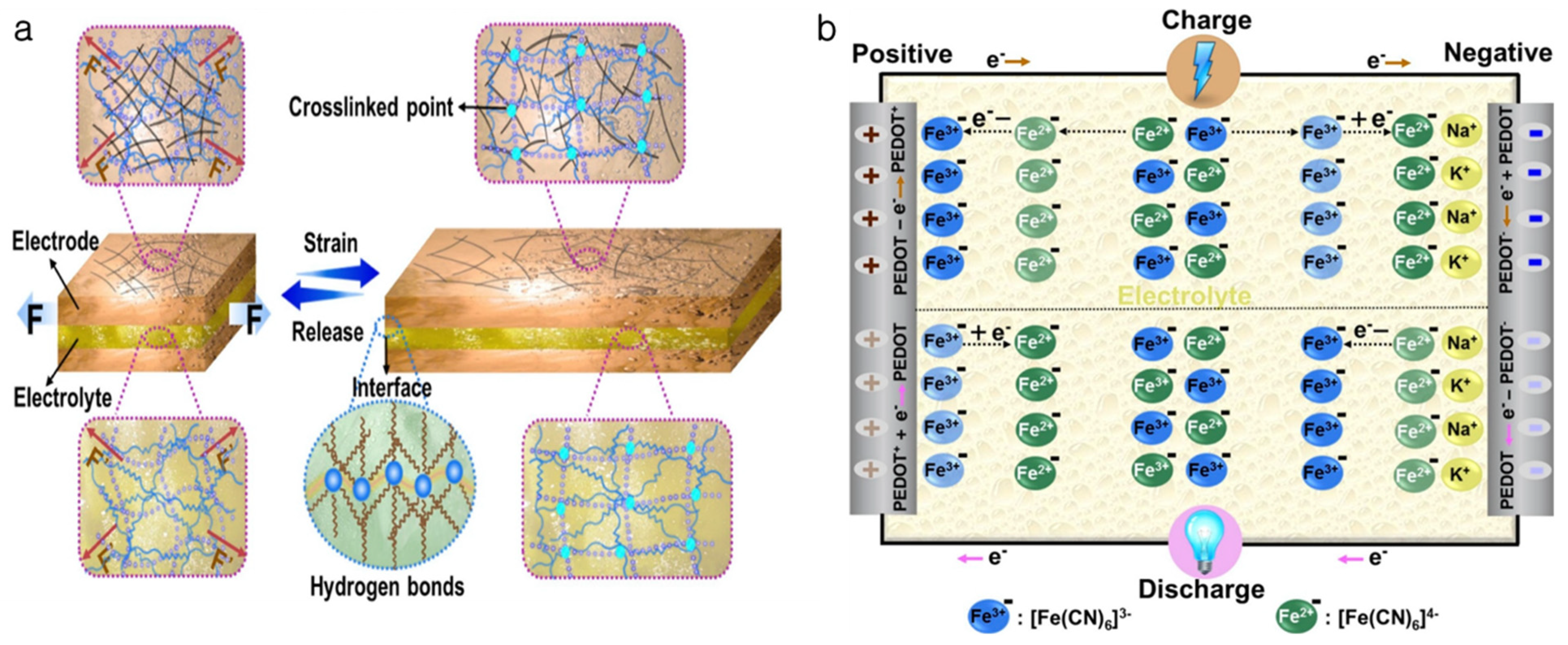
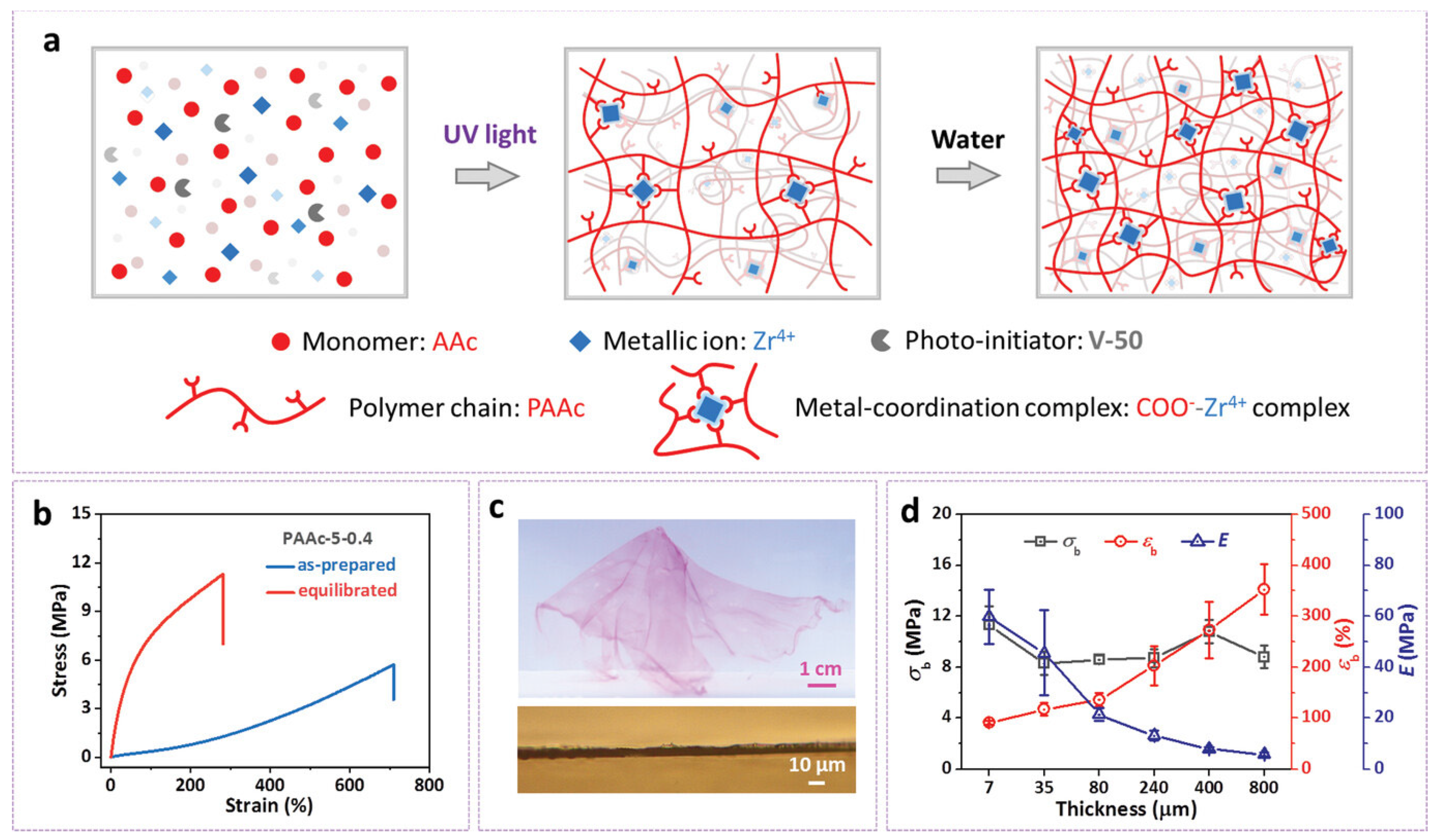
4. Hydrogel-Based Soft Battery and Supercapacitor
5. Hydrogel-Based Soft Circuits
6. Hydrogel-Based Soft Robots
7. Scope of Improvement and Future Prospects
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niu, Y.; Liu, H.; He, R.; Li, Z.; Ren, H.; Gao, B.; Guo, H.; Genin, G.M.; Xu, F. The New Generation of Soft and Wearable Electronics for Health Monitoring in Varying Environment: From Normal to Extreme Conditions. Mater. Today 2020, 41, 219–242. [Google Scholar] [CrossRef]
- Lim, H.R.; Kim, H.S.; Qazi, R.; Kwon, Y.T.; Jeong, J.W.; Yeo, W.H. Advanced Soft Materials, Sensor Integrations, and Applications of Wearable Flexible Hybrid Electronics in Healthcare, Energy, and Environment. Adv. Mater. 2020, 32, e1901924. [Google Scholar] [CrossRef]
- Zhao, Y.; Kim, A.; Wan, G.; Tee, B.C.K. Design and Applications of Stretchable and Self-Healable Conductors for Soft Electronics. Nano Converg. 2019, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Cheng, W. One-Dimensional Nanomaterials for Soft Electronics. Adv. Electron. Mater. 2017, 3, 1600314. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C.; Huang, Z.; Xu, S. Materials and Structures toward Soft Electronics. Adv. Mater. 2018, 30, e1801368. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Ishida, Y. A Design of an Improved Anti-Windup Control Using a Pi Controller Based on a Pole Placement Method. Int. J. Simul. Syst. Sci. Technol. 2016, 17, 1.1–1.7. [Google Scholar] [CrossRef]
- Bhuyan, M.M.; Jophous, M.; Jeong, J.H. Synthesis and Characterization of Gamma Radiation-Induced (3-Acrylamidopropyl) Trimethylammonium Chloride-Acrylic Acid Functional Superabsorbent Hydrogel. Polym. Bull. 2022, 80, 8651–8664. [Google Scholar] [CrossRef]
- Rogovina, L.Z.; Vasil’ev, V.G.; Braudo, E.E. Definition of the Concept of Polymer Gel. Polym. Sci. Ser. C 2008, 50, 85–92. [Google Scholar] [CrossRef]
- Kabiri, K.; Zohuriaan-Mehr, M.J. Superabsorbent Hydrogel Composites. Polym. Adv. Technol. 2003, 14, 438–444. [Google Scholar] [CrossRef]
- Gong, J.P. Why Are Double Network Hydrogels so Tough? Soft Matter 2010, 6, 2583–2590. [Google Scholar] [CrossRef]
- Hasan, N.; Bhuyan, M.M.; Jeong, J.-H. Single/Multi-Network Conductive Hydrogels—A Review. Polymers 2024, 16, 2030. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, D.; Chen, H.; Zhang, Y.; Liu, Y.; Ren, B.; Zheng, J. A Multiscale Polymerization Framework towards Network Structure and Fracture of Double-Network Hydrogels. npj Comput. Mater. 2021, 7, 39. [Google Scholar] [CrossRef]
- Gao, N.; Pan, C. Intelligent Ion Gels: Design, Performance, and Applications. SmartMat 2024, 5, e1215. [Google Scholar] [CrossRef]
- Pinelli, F.; Magagnin, L.; Rossi, F. Progress in Hydrogels for Sensing Applications: A Review. Mater. Today Chem. 2020, 17, 100317. [Google Scholar] [CrossRef]
- Zhang, D.; Jian, J.; Xie, Y.; Gao, S.; Ling, Z.; Lai, C.; Wang, J.; Wang, C.; Chu, F.; Dumont, M.-J. Mimicking Skin Cellulose Hydrogels for Sensor Applications. Chem. Eng. J. 2022, 427, 130921. [Google Scholar] [CrossRef]
- Jo, Y.; Lee, Y.; Heo, J.H.; Son, Y.; Kim, T.Y.; Park, K.; Kim, S.; Kim, S.J.; Jin, Y.; Park, S.; et al. Universal Hydrogel Adhesives with Robust Chain Entanglement for Bridging Soft Electronic Materials. npj Flex. Electron. 2024, 8, 39. [Google Scholar] [CrossRef]
- Wang, J.; Yang, B.; Jiang, Z.; Liu, Y.; Zhou, L.; Liu, Z.; Tang, L. Recent Advances of Conductive Hydrogels for Flexible Electronics. Electron. Mater. 2024, 5, 101–131. [Google Scholar] [CrossRef]
- Khan, B.; Abdullah, S.; Khan, S. Current Progress in Conductive Hydrogels and Their Applications in Wearable Bioelectronics and Therapeutics. Micromachines 2023, 14, 1005. [Google Scholar] [CrossRef]
- Bhuyan, M.M.; Jeong, J.-H. Gels/Hydrogels in Different Devices/Instruments—A Review. Gels 2024, 10, 548. [Google Scholar] [CrossRef]
- Lim, J.H.; Kim, M.J.; Yoon, H.G.; Kim, S.W. Highly Sensitive and Long-Term Stretchable Eutectic Nanogel Conductor with Conducting Interpenetrating Nanogel Networks for Monitoring Human Motions. Compos. Part B Eng. 2022, 247, 110299. [Google Scholar] [CrossRef]
- Gul, O.; Kim, K.; Gu, J.; Choi, J.; Del Orbe Henriquez, D.; Ahn, J.; Park, I. Sensitivity-Controllable Liquid-Metal-Based Pressure Sensor for Wearable Applications. ACS Appl. Electron. Mater. 2021, 3, 4027–4036. [Google Scholar] [CrossRef]
- Liu, C.; Ma, F.; Sun, Q.; Hu, Q.; Tong, W.; Guo, X.; Hu, R.; Liu, P.; Huang, Y.; Hao, X.; et al. Highly Sensitive Flexible Capacitive Pressure Sensor Based on a Multicross-Linked Dual-Network Ionic Hydrogel for Blood Pressure Monitoring Applications. ACS Appl. Mater. Interfaces 2024, 16, 34042–34056. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.P.; Jo, J.W.; Nam, D.; Kim, Y.H.; Park, S.K. High-Performance Metal Oxide TFTs for Flexible Displays: Materials, Fabrication, Architecture, and Applications. Soft Sci. 2025, 5, 1. [Google Scholar] [CrossRef]
- Dutta, T.; Chaturvedi, P.; Llamas-Garro, I.; Velázquez-González, J.S.; Dubey, R.; Mishra, S.K. Smart Materials for Flexible Electronics and Devices: Hydrogel. RSC Adv. 2024, 14, 12984–13004. [Google Scholar] [CrossRef]
- Dai, Y.; Wai, S.; Li, P.; Shan, N.; Cao, Z.; Li, Y.; Wang, Y.; Liu, Y.; Liu, W.; Tang, K.; et al. Soft Hydrogel Semiconductors with Augmented Biointeractive Functions. Science 2024, 386, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Jiang, M.; Zhou, T.; Raijmakers, L.; Vezhlev, E.; Wu, B.; Schülli, T.U.; Danilov, D.L.; Wei, Y.; Eichel, R.A.; et al. Interface Aspects in All-Solid-State Li-Based Batteries Reviewed. Adv. Energy Mater. 2021, 11, 2003939. [Google Scholar] [CrossRef]
- Uyanga, K.A.; Zhao, H.; Bo, X.; Daoud, W.A. Advances and Opportunities of Hydrogels for Metal-Ion Batteries. Energy Storage Mater. 2024, 72, 103707. [Google Scholar] [CrossRef]
- Mehra, P.; Saxena, S.; Bhullar, S. A Comprehensive Analysis of Supercapacitors and Their Equivalent Circuits—A Review. World Electr. Veh. J. 2024, 15, 332. [Google Scholar] [CrossRef]
- Rustamaji, H.; Prakoso, T.; Devianto, H.; Widiatmoko, P.; Nurdin, I. Design, Fabrication, and Testing of Supercapacitor Based on Nanocarbon Composite Material. ASEAN J. Chem. Eng. 2022, 22, 19–32. [Google Scholar] [CrossRef]
- Yin, B.S.; Zhang, S.W.; Ren, Q.Q.; Liu, C.; Ke, K.; Wang, Z.B. Elastic Soft Hydrogel Supercapacitor for Energy Storage. J. Mater. Chem. A 2017, 5, 24942–24950. [Google Scholar] [CrossRef]
- Yin, L.; Cheng, R.; Ding, J.; Jiang, J.; Hou, Y.; Feng, X.; Wen, Y.; He, J. Two-Dimensional Semiconductors and Transistors for Future Integrated Circuits. ACS Nano 2024, 18, 7739–7768. [Google Scholar] [CrossRef]
- Xie, Y.; Ding, C.; Jin, Q.; Zheng, L.; Xu, Y.; Xiao, H.; Cheng, M.; Zhang, Y.; Yang, G.; Li, M.; et al. Organic Transistor-Based Integrated Circuits for Future Smart Life. SmartMat 2024, 5, e1261. [Google Scholar] [CrossRef]
- Hajalilou, A. Liquid Metal–Polymer Hydrogel Composites for Sustainable Electronics: A Review. Molecules 2025, 30, 12–15. [Google Scholar] [CrossRef]
- Rajurkar, A.; Dangra, K.; Deshpande, A.; Gosavi, M.; Phadtare, T.; Gambhire, G. Comparative Finite Element Analysis of a Novel Robot Chassis Using Structural Steel and Aluminium Alloy Materials. Eng. Innov. 2023, 7, 61–73. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Y.; Chen, Y.; Majidi, C.; Iida, F.; Askounis, E.; Pei, Q. Controllable and Reversible Tuning of Material Rigidity for Robot Applications. Mater. Today 2018, 21, 563–576. [Google Scholar] [CrossRef]
- Jiang, J.; Xu, S.; Ma, H.; Li, C.; Huang, Z. Photoresponsive Hydrogel-Based Soft Robot: A Review. Mater. Today Bio 2023, 20, 100657. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; Chen, Y. Light-Driven Soft Microrobots Based on Hydrogels and LCEs: Development and Prospects. RSC Adv. 2024, 14, 14278–14288. [Google Scholar] [CrossRef]
- Buenger, D.; Topuz, F.; Groll, J. Hydrogels in Sensing Applications. Prog. Polym. Sci. 2012, 37, 1678–1719. [Google Scholar] [CrossRef]
- Bhuyan, M.M.; Okabe, H.; Hidaka, Y.; Dafader, N.C.; Rahman, N.; Hara, K. Synthesis of Pectin-N, N-Dimethyl Acrylamide Hydrogel by Gamma Radiation and Application in Drug Delivery (in Vitro). J. Macromol. Sci. Part A Pure Appl. Chem. 2018, 55, 369–376. [Google Scholar] [CrossRef]
- Gawel, K.; Barriet, D.; Sletmoen, M.; Stokke, B.T. Responsive Hydrogels for Label-Free Signal Transduction within Biosensors. Sensors 2010, 10, 4381–4409. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Bajpai, J.; Saini, R.; Gupta, R. Responsive Polymers in Biology and Technology. Polym. Rev. 2011, 51, 53–97. [Google Scholar] [CrossRef]
- Unger, K.; Coclite, A.M. Glucose-Responsive Boronic Acid Hydrogel Thin Films Obtained via Initiated Chemical Vapor Deposition. Biomacromolecules 2022, 23, 4289–4295. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, W.; Veiseh, O.; Appel, E.A.; Xue, K.; Webber, M.J.; Tang, B.C.; Yang, X.W.; Weir, G.C.; Langer, R.; et al. Injectable and Glucose-Responsive Hydrogels Based on Boronic Acid-Glucose Complexation. Langmuir 2016, 32, 8743–8747. [Google Scholar] [CrossRef] [PubMed]
- Guan, P.C.; Qi, Q.J.; Wang, Y.Q.; Lin, J.S.; Zhang, Y.J.; Li, J.F. Development of a 3D Hydrogel SERS Chip for Noninvasive, Real-Time PH and Glucose Monitoring in Sweat. ACS Appl. Mater. Interfaces 2024, 16, 48139–48146. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Su, B.; Wu, H.; Dai, Y.; Chen, T.; Fu, F.; Lin, Z.; Dong, Y. Hydrogel SERS Chip with Strong Localized Surface Plasmon Resonance for Sensitive and Rapid Detection of T-2 Toxin. Talanta 2024, 268, 125329. [Google Scholar] [CrossRef]
- Hilt, J.Z.; Gupta, A.K.; Bashir, R.; Peppas, N.A. Ultrasensitive Biomens Sensors Based on Microcantilevers Patterned with Environmentally Responsive Hydrogels. Biomed. Microdevices 2003, 5, 177–184. [Google Scholar] [CrossRef]
- Feng, L.; Wang, L.; Hu, Z.; Tian, Y.; Xian, Y.; Jin, L. Encapsulation of Horseradish Peroxidase into Hydrogel, and Its Bioelectrochemistry. Microchim. Acta 2009, 164, 49–54. [Google Scholar] [CrossRef]
- Jen, A.C.; Wake, M.C.; Mikos, A.G. Review: Hydrogels for Cell Immobilization. Biotechnol. Bioeng. 1996, 50, 357–364. [Google Scholar] [CrossRef]
- Hong, W.; Hu, X.; Zhao, B.; Zhang, F.; Zhang, D. Tunable Photonic Polyelectrolyte Colorimetric Sensing for Anions, Cations and Zwitterions. Adv. Mater. 2010, 22, 5043–5047. [Google Scholar] [CrossRef]
- Rahmani, P.; Shojaei, A. A Review on the Features, Performance and Potential Applications of Hydrogel-Based Wearable Strain/Pressure Sensors. Adv. Colloid Interface Sci. 2021, 298, 102553. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, J.; Zhao, Y.; Wang, C.; Fan, M.; Li, Y.; Yang, C.; Yang, H. A Low-Cost Hydrogel Electrode for Multifunctional Sensing: Strain, Temperature, and Electrophysiology. Biosensors 2025, 15, 177. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Zhu, Y.; Wang, J.H.; Li, X.N.; Wu, X.G.; Qin, Y.X.; Chen, W.Y. Fe3+, NIR Light and Thermal Responsive Triple Network Composite Hydrogel with Multi-Shape Memory Effect. Compos. Sci. Technol. 2021, 206, 108653. [Google Scholar] [CrossRef]
- Yin, T.; Wu, T.; Zhong, D.; Liu, J.; Liu, X.; Han, Z.; Yu, H.; Qu, S. Soft Display Using Photonic Crystals on Dielectric Elastomers. ACS Appl. Mater. Interfaces 2018, 10, 24758–24766. [Google Scholar] [CrossRef]
- Wu, T.; Yin, T.; Hu, X.; Nian, G.; Qu, S.; Yang, W. A Thermochromic Hydrogel for Camouflage and Soft Display. Adv. Opt. Mater. 2020, 8, 2000031. [Google Scholar] [CrossRef]
- Luo, X.; Wan, R.; Zhang, Z.; Song, M.; Yan, L.; Xu, J.; Yang, H.; Lu, B. 3D-Printed Hydrogel-Based Flexible Electrochromic Device for Wearable Displays. Adv. Sci. 2024, 11, e2404679. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, J.; Zhang, H.; Wei, H.; Zhou, L.; Wang, Z.; Pan, Y.; Su, X.; Zhang, A.; Fu, J. White-Light-Emitting Flexible Display Devices Based on Double Network Hydrogels Crosslinked by YAG:Ce Phosphors. J. Mater. Chem. C 2020, 8, 247–252. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Z.; Yang, S.; Jia, H.; Wei, J. Dual-Mode Multicolor Display Based on Structural and Fluorescent Color CdS Photonic Crystal Hydrogel. Langmuir 2024, 40, 12767–12777. [Google Scholar] [CrossRef]
- Parvini, E.; Hajalilou, A.; Tavakoli, M. A Bright Future of Hydrogels in Flexible Batteries and Supercapacitors Storage Systems: A Review. Int. J. Energy Res. 2022, 46, 13276–13307. [Google Scholar] [CrossRef]
- Ye, T.; Wang, J.; Jiao, Y.; Li, L.; He, E.; Wang, L.; Li, Y.; Yun, Y.; Li, D.; Lu, J.; et al. A Tissue-Like Soft All-Hydrogel Battery. Adv. Mater. 2022, 34, 2105120. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, X.; Lin, Z.; Zhong, X.; Huang, Y.; Duan, X. One-Step Strategy to Graphene/Ni(OH)2 Composite Hydrogels as Advanced Three-Dimensional Supercapacitor Electrode Materials. Nano Res. 2013, 6, 65–76. [Google Scholar] [CrossRef]
- Islam, M.; Ahmed, M.S.; Faizan, M.; Ali, B.; Bhuyan, M.M.; Bari, G.A.K.M.R.; Nam, K. Review on the Polymeric and Chelate Gel Precursor for Li-Ion Battery Cathode Material Synthesis. Gels 2024, 10, 586. [Google Scholar] [CrossRef] [PubMed]
- Paudel, A.; Crum, A.N.; Wang, Y. A Full Metal-Free Flexible Ammonium-Ion Battery with Biodegradable Hydrogel Electrolyte. J. Mater. Chem. A 2024, 12, 11975–11985. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, L.; Wang, W.; Zhang, Q. Soft Materials for Wearable Supercapacitors. Soft Sci. 2021, 1, 5. [Google Scholar] [CrossRef]
- Cheng, T.; Zhang, Y.Z.; Wang, S.; Chen, Y.L.; Gao, S.Y.; Wang, F.; Lai, W.Y.; Huang, W. Conductive Hydrogel-Based Electrodes and Electrolytes for Stretchable and Self-Healable Supercapacitors. Adv. Funct. Mater. 2021, 31, 2101303. [Google Scholar] [CrossRef]
- Zeng, J.; Dong, L.; Sha, W.; Wei, L.; Guo, X. Highly Stretchable, Compressible and Arbitrarily Deformable All-Hydrogel Soft Supercapacitors. Chem. Eng. J. 2020, 383, 123098. [Google Scholar] [CrossRef]
- Jiao, C.; Li, L.; Lu, B.; Wang, Q.; Hong, W.; Chen, X.; Chang, L.; Wang, X.; Wang, Y.; Sun, K.; et al. Giant Electrical Conductivity Difference Enabled Liquid Metal-Hydrogel Hybrid Printed Circuits for Soft Bioelectronics. Chem. Eng. J. 2024, 482, 148951. [Google Scholar] [CrossRef]
- Zhang, C.W.; Hao, X.P.; Zheng, Q.; Wu, Z.L. Hydrogel-Based Multifunctional Soft Electronics with Distributed Sensing Units: A Review. Adv. Sens. Res. 2023, 2, 2200069. [Google Scholar] [CrossRef]
- Ohm, Y.; Pan, C.; Ford, M.J.; Huang, X.; Liao, J.; Majidi, C. An Electrically Conductive Silver–Polyacrylamide–Alginate Hydrogel Composite for Soft Electronics. Nat. Electron. 2021, 4, 185–192. [Google Scholar] [CrossRef]
- Jiao, C.; Liu, J.; Yan, S.; Xu, Z.; Hou, Z.; Xu, W. Hydrogel-Based Soft Bioelectronic Interfaces and Their Applications. J. Mater. Chem. C 2025, 13, 2620–2645. [Google Scholar] [CrossRef]
- Bag, S.P.; Lee, S.; Song, J.; Kim, J. Hydrogel-Gated FETs in Neuromorphic Computing to Mimic Biological Signal: A Review. Biosensors 2024, 14, 150. [Google Scholar] [CrossRef]
- Rong, Q.; Lei, W.; Liu, M. Conductive Hydrogels as Smart Materials for Flexible Electronic Devices. Chem. A Eur. J. 2018, 24, 16930–16943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.W.; Chen, C.; Duan, S.; Yan, Y.; He, P.; He, X. Hydrogel-Based Soft Bioelectronics for Personalized Healthcare. Med-X 2024, 2, 20. [Google Scholar] [CrossRef]
- Jo, Y.J.; Ok, J.; Kim, S.Y.; Kim, T. Stretchable and Soft Organic–Ionic Devices for Body-Integrated Electronic Systems. Adv. Mater. Technol. 2022, 7, 2001273. [Google Scholar] [CrossRef]
- Shi, J.; Liu, N. Making Hydrogels into Circuits. Wearable Electron. 2024, 1, 251–254. [Google Scholar] [CrossRef]
- Li, P.; Sun, W.; Li, J.; Chen, J.P.; Wang, X.; Mei, Z.; Jin, G.; Lei, Y.; Xin, R.; Yang, M.; et al. N-Type Semiconducting Hydrogel. Science 2024, 384, 557–563. [Google Scholar] [CrossRef]
- Han, S.H.; Kim, S., II; Lee, H.-R.; Lim, S.-M.; Yeon, S.Y.; Oh, M.-A.; Lee, S.; Sun, J.-Y.; Joo, Y.-C.; Chung, T.D. Hydrogel-Based Iontronics on a Polydimethylsiloxane Microchip. ACS Appl. Mater. Interfaces 2021, 13, 6606–6614. [Google Scholar] [CrossRef]
- Yu, H.C.; Hao, X.P.; Zhang, C.W.; Zheng, S.Y.; Du, M.; Liang, S.; Wu, Z.L.; Zheng, Q. Engineering Tough Metallosupramolecular Hydrogel Films with Kirigami Structures for Compliant Soft Electronics. Small 2021, 17, 2103836. [Google Scholar] [CrossRef]
- Sotoudeh, M.; Baumgart, S.; Dillenz, M.; Döhn, J.; Forster-Tonigold, K.; Helmbrecht, K.; Stottmeister, D.; Groß, A. Ion Mobility in Crystalline Battery Materials. Adv. Energy Mater. 2024, 14, 2302550. [Google Scholar] [CrossRef]
- Xiang, Z.; Li, Y.; Cheng, X.; Yang, C.; Wang, K.-P.; Zhang, Q.; Wang, L. Lean-Water Hydrogel Electrolyte with Improved Ion Conductivity for Dendrite-Free Zinc-Ion Batteries. Chem. Eng. J. 2024, 490, 151524. [Google Scholar] [CrossRef]
- Yao, B.; Yan, Y.; Cui, Q.; Duan, S.; Wang, C.; Du, Y.; Zhao, Y.; Wu, D.; Wu, S.; Zhu, X.; et al. Ultrastrong, Highly Conductive and Capacitive Hydrogel Electrode for Electron-Ion Transduction. Matter 2022, 5, 4407–4424. [Google Scholar] [CrossRef]
- López-Díaz, A.; Vázquez, A.S.; Vázquez, E. Hydrogels in Soft Robotics: Past, Present, and Future. ACS Nano 2024, 18, 20817–20826. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Du, J.; Mao, Y. Hydrogel-Based Continuum Soft Robots. Gels 2025, 11, 254. [Google Scholar] [CrossRef] [PubMed]
- Selvamuthu, M.G.; Tadakuma, R.; Fujiwara, N.; Yoshida, K.; Takagi, M.; Hoshino, H.; Suzuri, Y.; Furukawa, H. Development of Soft Inchworm Robot with Friction Control of Feet Using Double-Network Gel. Adv. Robot. 2023, 37, 407–422. [Google Scholar] [CrossRef]
- Yin, C.; Wei, F.; Fu, S.; Zhai, Z.; Ge, Z.; Yao, L.; Jiang, M.; Liu, M. Visible Light-Driven Jellyfish-like Miniature Swimming Soft Robot. ACS Appl. Mater. Interfaces 2021, 13, 47147–47154. [Google Scholar] [CrossRef]


| Types of Electronics | Types of Materials | Components | Performance/Feature | References |
|---|---|---|---|---|
| Sensor | Liquid-metal-based pressure sensor | Metals, metal oxides, silicon wafers, glass, ceramics. | Low sensitivity, rigid; stretchability < 1%. | [21] |
| Soft hydrogel-based pressure sensor | Single or multi-layer hydrophilic polymer networks (polyvinyl alcohol, chitosan etc.) | High sensitivity, ultra-stretchable (up to 300–1000% strain. | [22] | |
| Display | Traditional display | Amorphous silicon (a-Si), low-temperature polysilicon (LTPS), or metal oxides | Resistant to environmental stress | [23] |
| Hydrogel-based display | Water-rich polymer networks that provide flexibility, stretchability and self-healing, electronic and optoelectronic functionalities | Can expand over 1500% without losing function, self-healable. | [24,25] | |
| Battery | Traditional battery | Metal ions like lithium-ion, lead-acid, nickel-cadmium etc. | Exhibit better cycle stability, have higher energy density. | [26] |
| Hydrogel-based battery | Polymers, polyaniline anode and polypyrrole cathode, ammonium cations (NH4+), metal ions. | Exhibit better cycle stability and resistance to degradation. | [27] | |
| Supercapacitor | Traditional supercapacitors | Activated carbon, graphene, or carbon nanotubes, | Longer cycle life | [28,29] |
| Hydrogel-based supercapacitors | Polyvinyl alcohol, polypyrrole, carbon nanotube films | Longer cycle life with enhanced stability | [30] | |
| Circuit | Traditional circuits | Silicon-based semiconductors, metallic conductors, organic transistors. | Higher electronic conductivity, more stable | [31,32] |
| Hydrogel | Conductive hydrogels, polymeric networks with ionic or electronic conductors, metal-polymer hydrogel. | Rely on ionic conductivity, require hydration to maintain performance | [33] | |
| Robot | Traditional robots | Steel or aluminum frames, risk of injury on collision | Deformation is resisted by stiff connections. | [34,35] |
| Hydrogel-based robots | Hydrophilic polymer matrices | Can undergo large strains (100–1000%) | [36,37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhuyan, M.M.; Hasan, N.; Jeong, J.-H. Single- and Multi-Network Hydrogels for Soft Electronics—A Review. Gels 2025, 11, 480. https://doi.org/10.3390/gels11070480
Bhuyan MM, Hasan N, Jeong J-H. Single- and Multi-Network Hydrogels for Soft Electronics—A Review. Gels. 2025; 11(7):480. https://doi.org/10.3390/gels11070480
Chicago/Turabian StyleBhuyan, Md Murshed, Nahid Hasan, and Jae-Ho Jeong. 2025. "Single- and Multi-Network Hydrogels for Soft Electronics—A Review" Gels 11, no. 7: 480. https://doi.org/10.3390/gels11070480
APA StyleBhuyan, M. M., Hasan, N., & Jeong, J.-H. (2025). Single- and Multi-Network Hydrogels for Soft Electronics—A Review. Gels, 11(7), 480. https://doi.org/10.3390/gels11070480









