Advances in Gelatin-Based Tissue Engineering Using HRP/H2O2
Abstract
1. Introduction
2. Methodology
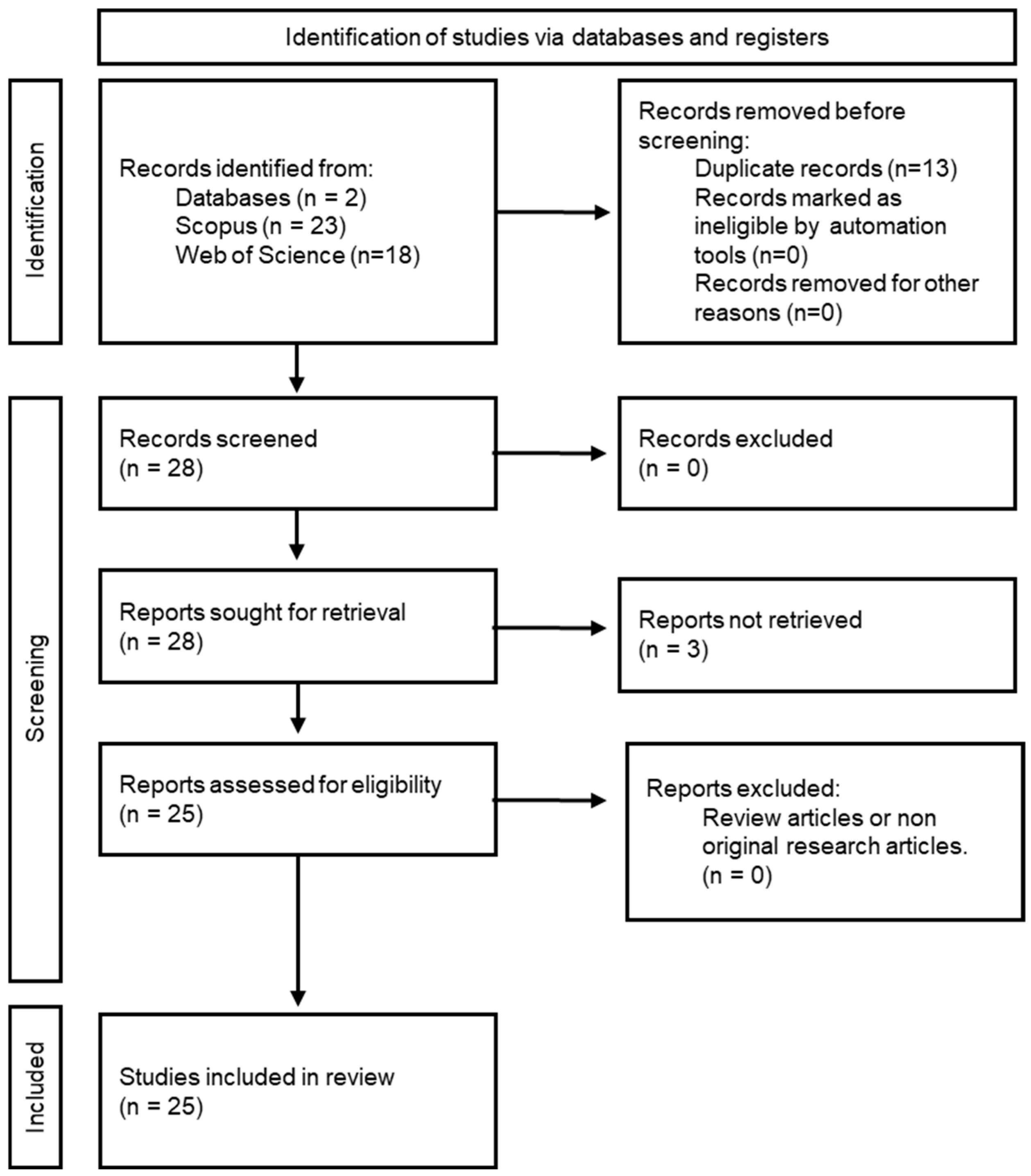
2.1. Study Design and Search Strategy
2.2. Study Selection and Data Extraction
2.3. Quality Assessment
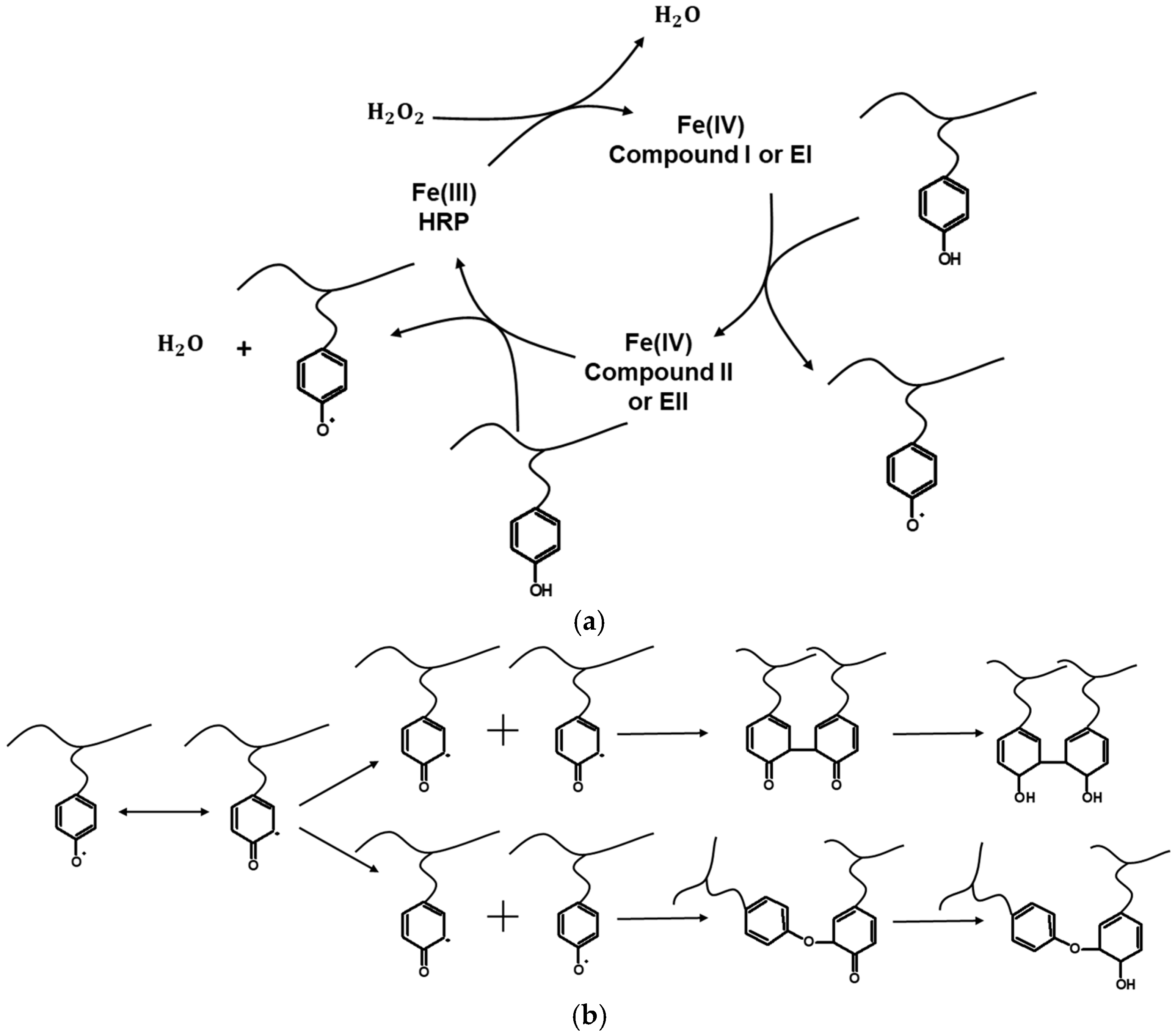
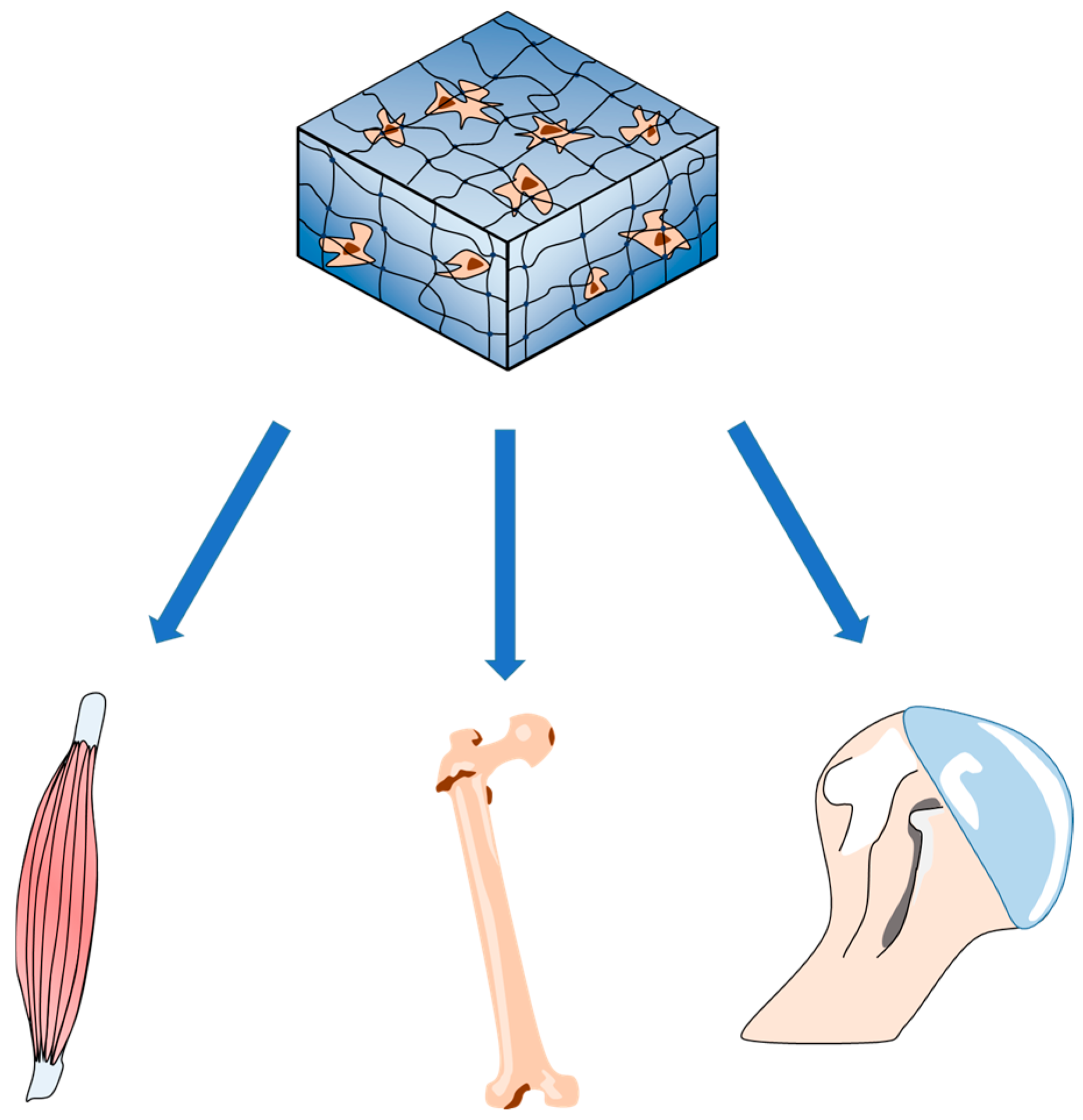
3. Applications of HRP/H2O2-Catalyzed Hydrogels in TE
3.1. Wound Healing and Soft Tissue Repair
3.2. Bone and Cartilage TE
3.3. Muscle Regeneration and Nerve Repair
3.4. Vascular Tissue Engineering
3.5. Gene Delivery and Genome Editing
3.6. Reducing Immunogenicity for In Vivo Applications
3.7. Advances in Fabrication Techniques and Biomaterials
3.8. Limitations of the Review
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TE | Tissue Engineering |
| HRP | Horseradish Peroxidase |
| Tsg | Tilapia skin gelatin |
| Fuc | Fucoidan |
| SD | Sprague-Dawley |
| Aga | Agarose |
| Tyr | Tyramine |
| TA | Tyramine |
| CDTA | crosslink tyramine-functionalized Chondroitine sulfate |
| GTA | tyramine-functionalized gelatin |
| BCP | Biphasic calcium phosphate |
| Gel-AE | Amine-rich highly-branched gelatin |
| HAECs | human aortic endothelial cells |
| VSMCs | Human vascular smooth muscle cells |
| Na-Alg | Sodium alginate |
| EPS | Exopolysaccharide |
| hADS cells | Human adipose-derived stem cells |
| WSCD-HCl | Water-soluble carbodiimide hydrochloride |
| HPA | Hydroxyphenyl propionic acid |
| bMSCs | Bone marrow mesenchymal stem cells |
| Gtn-HPA | Gelatin-Hydroxyphenyl Propionic Acid |
| HPA | Hydroxy-phenyl pro-pionic acid |
| PCNs | polyelectrolyte complex nanoparticles |
| DS | Dextran sulfate |
| CS | Chitosan |
| PDGF | Platelet-derived growth factor |
| SDF | Stromal cell-derived factor |
| HASMCs | Human aortic smooth muscle cells |
| Alg-Ph | Alginate derivative possessing phenolic hydroxyl moieties |
| Gelatin-Ph | Gelatin derivative possessing Ph moieties |
| hMSCs | Human mesenchymal stem cells |
| Tyr.HCl | Tyramine hydrochloride |
| NHS | N-hydroxysuccinimide |
| EDC-HCI | 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride |
| HepG2 | Human hepatoblastoma |
| PDMS | polydimethylsiloxane |
| TG | transglutaminase |
| HUVECs | Human umbilical vein endothelial cells |
| GelMA | methacrylated gelatin |
| GMDA | Dopamine modified methacrylate gelatin |
| MSCs | Mesenchymal stem cells |
| GPT | Gelatin–poly(ethylene glycol)–tyramine |
| mBMSCs | Mouse bone marrow mesenchymal stem cells |
| E-SF/GT | enzymatically cross-linked silk fibroin/gelatin-tyramine |
| GT-DA | gelatin grafted with dopamine |
| CNT-PDA | polydopamine-coated carbon nanotubes |
| GH | Gelatin-hydroxyphenylpropionic acid |
| IPN | interpenetrating polymer network |
| chitosan-PA | chitosan containing phloretic acid |
| PDMS | polydimethylsiloxane |
| GT | gelatin–tyramine |
| SF | silk fibroin |
| gRNA | guide RNA |
| G-TA | gelatin-TA |
| EPS | exopolysaccharide |
References
- Patrick, C.W.; Mikos, A.G.; McIntire, L.V. Prospectus of Tissue Engineering. In Elsevier eBooks; Elsevier: Amsterdam, The Netherlands, 1998; pp. 3–11. [Google Scholar]
- Bianco, P.; Robey, P.G. Stem Cells in Tissue Engineering. Nature 2001, 414, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Karageorgos, F.F.; Alexiou, M.; Tsoulfas, G.; Alexopoulos, A.H. Hydrogel-Based Vascularized Organ Tissue Engineering: A Systematized Review on Abdominal Organs. Gels 2024, 10, 653. [Google Scholar] [CrossRef] [PubMed]
- Lukin, I.; Erezuma, I.; Maeso, L.; Zarate, J.; Desimone, M.F.; Al-Tel, T.H.; Dolatshahi-Pirouz, A.; Orive, G. Progress in Gelatin as Biomaterial for Tissue Engineering. Pharmaceutics 2022, 14, 1177. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, W.; Guo, L.; Yu, Y.; Yuan, Z. Preparation of enzymatically cross-linked sulfated chitosan hydrogel and its potential application in thick tissue engineering. Sci. China Chem. 2013, 56, 1701–1709. [Google Scholar] [CrossRef]
- Jin, R.; Teixeira, L.S.M.; Dijkstra, P.J.; Zhong, Z.; Van Blitterswijk, C.A.; Karperien, M.; Feijen, J. Enzymatically Crosslinked Dextran-Tyramine Hydrogels as Injectable Scaffolds for Cartilage Tissue Engineering. Tissue Eng. Part A 2010, 16, 2429–2440. [Google Scholar] [CrossRef]
- Bi, B.; Liu, H.; Kang, W.; Zhuo, R.; Jiang, X. An Injectable Enzymatically Crosslinked Tyramine-Modified Carboxymethyl Chitin Hydrogel for Biomedical Applications. Colloids Surf. B Biointerfaces 2018, 175, 614–624. [Google Scholar] [CrossRef]
- Lee, F.; Chung, J.E.; Kurisawa, M. An Injectable Enzymatically Crosslinked Hyaluronic Acid–Tyramine Hydrogel System with Independent Tuning of Mechanical Strength and Gelation Rate. Soft Matter 2008, 4, 880. [Google Scholar] [CrossRef]
- Elvitigala, K.C.M.L.; Mubarok, W.; Sakai, S. Tuning the Crosslinking and Degradation of Hyaluronic Acid/Gelatin Hydrogels Using Hydrogen Peroxide for Muscle Cell Sheet Fabrication. Soft Matter 2023, 19, 5880–5887. [Google Scholar] [CrossRef]
- Bae, J.W.; Choi, J.H.; Lee, Y.; Park, K.D. Horseradish Peroxidase-Catalysedin Situ-Forming Hydrogels for Tissue-Engineering Applications. J. Tissue Eng. Regen. Med. 2014, 9, 1225–1232. [Google Scholar] [CrossRef]
- Sakai, S.; Nakahata, M. Horseradish Peroxidase Catalyzed Hydrogelation for Biomedical, Biopharmaceutical, and Biofabrication Applications. Chem. —Asian J. 2017, 12, 3098–3109. [Google Scholar] [CrossRef]
- Karageorgos, F.F.; Kiparissides, C. Prediction of Viscoelastic Properties of Enzymatically Crosslinkable Tyramine–Modified Hyaluronic Acid Solutions Using a Dynamic Monte Carlo Kinetic Approach. Int. J. Mol. Sci. 2021, 22, 7317. [Google Scholar] [CrossRef] [PubMed]
- Karageorgos, F.F.; Vasileiadou, A.C.; Kiparissides, C. Mathematical Modelling of Enzymatically Cross-linked Polymer–Phenol Conjugates Using Deterministic and Stochastic Methods. Can. J. Chem. Eng. 2022, 101, 4871–4885. [Google Scholar] [CrossRef]
- Lopes, G.R.; Pinto, D.C.G.A.; Silva, A.M.S. Horseradish Peroxidase (HRP) as a Tool in Green Chemistry. RSC Adv. 2014, 4, 37244–37265. [Google Scholar] [CrossRef]
- Veitch, N.C. Horseradish Peroxidase: A Modern View of a Classic Enzyme. Phytochemistry 2004, 65, 249–259. [Google Scholar] [CrossRef]
- Azevedo, A.M.; Martins, V.C.; Prazeres, D.M.F.; Vojinović, V.; Cabral, J.M.S.; Fonseca, L.P. Horseradish Peroxidase: A Valuable Tool in Biotechnology. Biotechnol. Annu. Rev. 2003, 9, 199–247. [Google Scholar] [CrossRef] [PubMed]
- Echave, M.C.; Burgo, L.S.; Pedraz, J.L.; Orive, G. Gelatin as Biomaterial for Tissue Engineering. Curr. Pharm. Des. 2017, 23, 3567–3584. [Google Scholar] [CrossRef]
- Sultana, S.; Ali, M.E.; Ahamad, M.N.U. Gelatine, Collagen, and Single Cell Proteins as a Natural and Newly Emerging Food Ingredients. In Elsevier eBooks; Elsevier: Amsterdam, The Netherlands, 2018; pp. 215–239. [Google Scholar]
- Alipal, J.; Pu’ad, N.A.S.M.; Lee, T.C.; Nayan, N.H.M.; Sahari, N.; Basri, H.; Idris, M.I.; Abdullah, H.Z. A Review of Gelatin: Properties, Sources, Process, Applications, and Commercialisation. Mater. Today Proc. 2021, 42, 240–250. [Google Scholar] [CrossRef]
- Hanani, Z.A.N.; Roos, Y.H.; Kerry, J.P. Use and Application of Gelatin as Potential Biodegradable Packaging Materials for Food Products. Int. J. Biol. Macromol. 2014, 71, 94–102. [Google Scholar] [CrossRef]
- Grant, M.J.; Booth, A. A Typology of Reviews: An Analysis of 14 Review Types and Associated Methodologies. Health Inf. Libr. J. 2009, 26, 91–108. [Google Scholar] [CrossRef]
- Lu, Y.; Hu, M.; Huang, Y.; Liao, J.; Zhao, M.; Zhou, Y.; Xia, G.; Zhan, Q. Preparation of Multifunctional Hydrogels with In Situ Dual Network Structure and Promotion of Wound Healing. Biomacromolecules 2024, 25, 4965–4976. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Dang, L.H.; Nguyen, P.; Pham, T.L.-B.; Le, H.K.; Nguyen, M.-T.; Nhi, T.T.Y.; Feng, S.; Chen, J.; Tran, N.Q. Dual Composition Chondroitin Sulfate and Gelatin Biomimetic Hydrogel Based on Tyramine Crosslinking for Tissue Regenerative Medicine. Eur. Polym. J. 2023, 189, 111975. [Google Scholar] [CrossRef]
- Wang, G.; Meng, X.; Wang, P.; Wang, X.; Liu, G.; Wang, D.-A.; Fan, C. A Catechol Bioadhesive for Rapid Hemostasis and Healing of Traumatic Internal Organs and Major Arteries. Biomaterials 2022, 291, 121908. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Jafari, H.; Siminska-Stanny, J.; Okoro, O.V.; Fatimi, A.; Shavandi, A. Anionic Exopolysaccharide from Cryptococcus Laurentii 70766 as an Alternative for Alginate for Biomedical Hydrogels. Int. J. Biol. Macromol. 2022, 212, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Mubarok, W.; Qu, Y.; Sakai, S. Influence of Hydrogen Peroxide-Mediated Cross-Linking and Degradation on Cell-Adhesive Gelatin Hydrogels. ACS Appl. Bio Mater. 2021, 4, 4184–4190. [Google Scholar] [CrossRef]
- Niu, W.; Lim, T.C.; Alshihri, A.; Rajappa, R.; Wang, L.; Kurisawa, M.; Spector, M. Platelet-Derived Growth Factor Stimulated Migration of Bone Marrow Mesenchymal Stem Cells into an Injectable Gelatin-Hydroxyphenyl Propionic Acid Matrix. Biomedicines 2021, 9, 203. [Google Scholar] [CrossRef]
- Wang, L.-S.; Du, C.; Toh, W.S.; Wan, A.C.A.; Gao, S.J.; Kurisawa, M. Modulation of Chondrocyte Functions and Stiffness-Dependent Cartilage Repair Using an Injectable Enzymatically Crosslinked Hydrogel with Tunable Mechanical Properties. Biomaterials 2013, 35, 2207–2217. [Google Scholar] [CrossRef]
- Liu, Y.; Sakai, S.; Taya, M. Production of Endothelial Cell-Enclosing Alginate-Based Hydrogel Fibers with a Cell Adhesive Surface through Simultaneous Cross-Linking by Horseradish Peroxidase-Catalyzed Reaction in a Hydrodynamic Spinning Process. J. Biosci. Bioeng. 2012, 114, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-S.; Du, C.; Chung, J.E.; Kurisawa, M. Enzymatically Cross-Linked Gelatin-Phenol Hydrogels with a Broader Stiffness Range for Osteogenic Differentiation of Human Mesenchymal Stem Cells. Acta Biomater. 2012, 8, 1826–1837. [Google Scholar] [CrossRef]
- Wang, L.-S.; Chung, J.E.; Chan, P.P.-Y.; Kurisawa, M. Injectable Biodegradable Hydrogels with Tunable Mechanical Properties for the Stimulation of Neurogenesic Differentiation of Human Mesenchymal Stem Cells in 3D Culture. Biomaterials 2009, 31, 1148–1157. [Google Scholar] [CrossRef]
- Furuno, K.; Suzuki, K.; Sakai, S. Gelatin-Based Electrospun Nanofibers Cross-Linked Using Horseradish Peroxidase for Plasmid DNA Delivery. Biomolecules 2022, 12, 1638. [Google Scholar] [CrossRef]
- Kotani, T.; Mubarok, W.; Hananouchi, T.; Sakai, S. Horseradish Peroxidase-Mediated Bioprinting via BioINK Gelation by Alternately Extruded Support Material. ACS Biomater. Sci. Eng. 2023, 9, 5804–5812. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Bae, J.W.; Lee, J.W.; Suh, W.; Park, K.D. Enzyme-Catalyzed in Situ Forming Gelatin Hydrogels as Bioactive Wound Dressings: Effects of Fibroblast Delivery on Wound Healing Efficacy. J. Mater. Chem. B 2014, 2, 7712–7718. [Google Scholar] [CrossRef]
- Mubarok, W.; Elvitigala, K.C.M.L.; Sakai, S. Tuning Myogenesis by Controlling Gelatin Hydrogel Properties through Hydrogen Peroxide-Mediated Cross-Linking and Degradation. Gels 2022, 8, 387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fan, Z.; Xu, C.; Fang, S.; Liu, X.; Li, X. Tough Biohydrogels with Interpenetrating Network Structure by Bienzymatic Crosslinking Approach. Eur. Polym. J. 2015, 72, 717–725. [Google Scholar] [CrossRef]
- Nie, K.; Han, S.; Yang, J.; Sun, Q.; Wang, X.; Li, X.; Li, Q. Enzyme-Crosslinked Electrospun Fibrous Gelatin Hydrogel for Potential Soft Tissue Engineering. Polymers 2020, 12, 1977. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-J.; Zhang, Y.-M.; Li, X.-S. Tough Biopolymer IPN Hydrogel Fibers by Bienzymatic Crosslinking Approach. Chin. J. Polym. Sci. 2015, 33, 1741–1749. [Google Scholar] [CrossRef]
- Zhou, F.; Yang, Y.; Zhang, W.; Liu, S.; Shaikh, A.B.; Yang, L.; Lai, Y.; Ouyang, H.; Zhu, W. Bioinspired, Injectable, Tissue-Adhesive and Antibacterial Hydrogel for Multiple Tissue Regeneration by Minimally Invasive Therapy. Appl. Mater. Today 2021, 26, 101290. [Google Scholar] [CrossRef]
- Moghaddam, M.M.; Jooybar, E.; Imani, R.; Ehrbar, M. Development of Injectable Microgel-Based Scaffolds via Enzymatic Cross-Linking of Hyaluronic Acid-Tyramine/Gelatin-Tyramine for Potential Bone Tissue Engineering. Int. J. Biol. Macromol. 2024, 279, 135176. [Google Scholar] [CrossRef]
- Li, L.; Bae, K.H.; Ng, S.; Yamashita, A.; Kurisawa, M. Peroxidase-Immobilized Porous Silica Particles for in Situ Formation of Peroxidase-Free Hydrogels with Attenuated Immune Responses. Acta Biomater. 2018, 81, 103–114. [Google Scholar] [CrossRef]
- Cheng, K.W.; Alhasan, L.; Rezk, A.R.; Al-Abboodi, A.; Doran, P.M.; Yeo, L.Y.; Chan, P.P.Y. Fast Three-Dimensional Micropatterning of PC12 Cells in Rapidly Crosslinked Hydrogel Scaffolds Using Ultrasonic Standing Waves. Biofabrication 2019, 12, 015013. [Google Scholar] [CrossRef]
- Park, K.M.; Lee, Y.; Son, J.Y.; Bae, J.W.; Park, K.D. In Situ SVVYGLR Peptide Conjugation into Injectable Gelatin-Poly(Ethylene Glycol)-Tyramine Hydrogel via Enzyme-Mediated Reaction for Enhancement of Endothelial Cell Activity and Neo-Vascularization. Bioconjugate Chem. 2012, 23, 2042–2050. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, Q.; Pan, H.; Dai, Q.; Feng, Q.; Yu, C.; Zhang, X.; Liang, Z.; Dong, H.; Cao, X. Tubular Silk Fibroin/Gelatin-Tyramine Hydrogel with Controllable Layer Structure and Its Potential Application for Tissue Engineering. ACS Biomater. Sci. Eng. 2020, 6, 6896–6905. [Google Scholar] [CrossRef] [PubMed]
- Hasturk, O.; Jordan, K.E.; Choi, J.; Kaplan, D.L. Enzymatically Crosslinked Silk and Silk-Gelatin Hydrogels with Tunable Gelation Kinetics, Mechanical Properties and Bioactivity for Cell Culture and Encapsulation. Biomaterials 2019, 232, 119720. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhao, X.; Hu, T.; Han, Y.; Guo, B. Mussel-Inspired, Antibacterial, Conductive, Antioxidant, Injectable Composite Hydrogel Wound Dressing to Promote the Regeneration of Infected Skin. J. Colloid Interface Sci. 2019, 556, 514–528. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Karageorgos, F. Development of Computational Methods for the Prediction of Crosslinking Kinetics and Molecular—Viscoelastic Properties of Hydrogels for Biomedical Applications. Ph.D. Thesis, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2023. [Google Scholar] [CrossRef]

| No. | Authors | Type of Study | Cells Used | Animal Model | Method Used for Hydrogel Preparation/Gelation | Material Used for Hydrogel | Application for Tissue Engineering |
|---|---|---|---|---|---|---|---|
| 1 | Lu Y. et al. [22] | In vitro and in vivo | NIH-3T3 mouse embryonic fibroblasts | Sprague-Dawley (SD) rats | - Tyramine modification: Tsg and Fuc were conjugated with tyramine → Tsg-Tyr and FucC-Tyr derivatives - Enzymatic crosslinking: HRP/H2O2 system catalyzed covalent bonding between phenolic groups - Physical crosslinking: Agarose solution was cooled to form a secondary hydrogen-bonded network - Dual-network formation: Combined covalent (Tsg-Tyr/FucC-Tyr) and physical (Aga) networks | - Tilapia skin gelatin (Tsg) - Fucoidan (Fuc)- Agarose (Aga) - Tyramine (Tyr) | Skin tissue engineering (wound healing focus) |
| 2 | Nguyen T.T. [23] | In vitro | Human mesenchymal stem cells | - | - Separate HRP/H2O2 enzymatic crosslinking of tyramine-functionalized Chondroitine sulfate (CDTA) and tyramine-functionalized gelatin (GTA). - In situ formation of GTA-CDTA hybrid hydrogels by mixing solutions containing GTA, CDTA, and HRP with solutions containing GTA, CDTA, and H2O2. - Embedding BCP nanoparticles into the hybrid hydrogel by incorporating them in the polymer solution prior to enzymatic crosslinking. | - Gelatin type A from porcine skin (Bloom 300) - Chondroitine sulfate - Biphasic calcium phosphate (BCP) nanoparticles - Tyramine (TA) | Scaffold-based tissue engineering |
| 3 | Wang G. et al. [24] | In vitro and in vivo | Human vascular smooth muscle cells (VSMCs) and human aortic endothelial cells (HAECs) | Mice | - Synthesis of amine-rich, highly-branched gelatin (Gel-AE) through a nucleophilic substitution reaction. - Functionalization of Gel-AE with catechol groups through a reaction with 3,4-dihydroxyphenylacetic acid → Gel-AE-Ca precursor. - Dual crosslinking to form CAGA: covalent bonds by HRP/H2O2 crosslinking and coordinate bonds through a catechol −Fe3+ reaction. | - Gelatin - 2-chloroethylamine - 3,4-dihydroxyphenylacetic acid -HRP - Hydrogen peroxide (H2O2) - Iron(III) chloride (FeCl3) | Hemostatic bioadhesive for tissue repair |
| 4 | Hamidi M. et al. [25] | In vitro | - 3T3 L fibroblast cell line - Human macrophage - Fibroblast cell lines | - | - Functionalization of EPS and Na-Alg with tyramine hydrochloride. - HRP/H2O2 enzymatic crosslinking of the functionalized products. | - Sodium alginate (Na-Alg) - Exopolysaccharide (EPS) from Cryptococcus laurentii 70766 - Tyramine hydrochloride | Tissue engineering, drug delivery, and wound dressings |
| 5 | Mubarok W. et al. [26] | In vitro | - Human adipose-derived stem cells (hADS cells) - Rat fibroblast 3Y1 cells | - | - Conjugation of 3-(4-hydroxyphenyl)propionic acid with gelatin in DMF-buffered solution using WSCD-HCl → Gelatin-Ph formation. - HRP-catalyzed gelation of aqueous Gelatin-Ph solutions by supplying H2O2 from the gas phase. | - Gelatin from bovine skin (Gelatin Type B) - 3-(4-hydroxyphenyl)propionic acid - Water-soluble carbodiimide hydrochloride (WSCD-HCl) | Tissue engineering |
| 6 | Niu W. et al. [27] | In vitro and in vivo | Bone marrow mesenchymal stem cells (bMSCs) | Adult Spanish goat (for bMSC isolation) | - Gelatin (Gtn) and Hydroxyphenyl Propionic Acid (HPA) conjugation → Gtn-HPA formation. - Preparation of polyelectrolyte complex nanoparticles (PCNs) from dextran sulfate and chitosan. - Encapsulation of PDGF-BB or SDF-1α into the PCNs. - Mixing of protein-encapsulated PCNs or blank PCNs with Gtn-HPA solution, followed by HRP/ H2O2 mediated crosslinking. | -Gelatin - Hydroxyphenyl propionic acid (HPA) - Dextran sulfate (DS) - Chitosan (CS) - Platelet-derived growth factor (PDGF)-BB - Stromal cell-derived factor (SDF)-1α | Tissue engineering/regenerative medicine |
| 7 | Wang L.S. et al. [28] | In vitro and in vivo | Chondrocytes | Rabbit | - Synthesis of Gtn-HPA conjugates. - Formation of hydrogels through HRP/H2O2 mediated crosslinking. - Tunable stiffness achieved through H2O2 and Gtn-HPA concentration modulation. | - Gtn-HPA | - Cartilage tissue engineering - Osteochondral defect repair |
| 8 | Liu Y. et al. [29] | In vitro | - HAECs - Human aortic smooth muscle cells (HASMCs) | - | - Extrusion of an aqueous solution containing Alg-Ph and HRP into a flow of aqueous solution containing H2O2 and Gelatin-Ph. - Simultaneous crosslinking of Alg-Ph to form a hydrogel fiber and immobilization of Gelatin-Ph on the fiber surface via the HRP/H2O2 system. | - Alginate derivative possessing phenolic hydroxyl moieties (Alg-Ph) - Gelatin derivative possessing Ph moieties (Gelatin-Ph) | Tissue Engineering |
| 9 | Fritschen A. et al. [30] | In vitro and in vivo | - Human mesenchymal stem cells (hMSCs) - Human fibroblasts (HFF-1) | Mice | - Synthesis of Gelatin-HPA-Tyr conjugate using a two-step reaction process involving the synthesis of Gtn-HPA conjugate followed by further conjugation of Tyr. - Hydrogel formation through HRP/H2O2 oxidative coupling of phenol moieties. | - Gelatin - 3,4-hydroxyphenyl propionic acid (HPA) - Tyramine hydrochloride (Tyr.HCl) - N-hydroxysuccinimide (NHS) - 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDC-HCI) | Tissue engineering and regenerative medicine |
| 10 | Wang L.S. et al. [31] | In vitro | hMSCs | - | - Synthesis of Gtn-HPA conjugate using carbodiimide/active ester-mediated coupling reaction. - HRP/H2O2 mediated gelation. - Tunable stiffness is achieved by varying the H2O2 concentration. | - Gtn - 3,4-Hydroxyphenylpropionic acid (HPA) - Gtn-HPA | - Neural tissue engineering - Regenerative medicine |
| 11 | Furuno K. et al. [32] | In vitro | Human embryonic kidney-derived HEK293 cells; HEK293 cells constitutively expressing GFP; HEK293 cells possessing a 35 bp deletion in the GFP sequence | - | - Electrospinning of Gelatin, containing phenolic hydroxyl moieties, produces nanofibrils. - Insolubilization of nanofibrils using horseradish peroxidase in the presence of air containing 16 ppm H2O2 for 30 min. - Loading of pDNA onto the nanofibrils through immersion in a solution of Lipofectamine/pDNA complexes. | - Gelatin from porcine skin | -Gene therapy and tissue regeneration |
| 12 | Kotani T. et al. [33] | In vitro | Mouse fibroblasts (10T1/2); Human hepatoblastoma (HepG2) | - | - For Bioprinting: Intermittent extrusion of bioink (containing cells, HRP, and phenolated polymers) and H2O2-containing support material → Improved printing fidelity. - For Scaffolds intended to culture cells: Intermittent deposition of bioink (containing a combination of HA-Ph, gelatin-Ph, and HRP, but no cells) and H2O2-containing support material → The presence of gelatin permits cell attachment and growth on the scaffolds. | - Hyaluronic acid-Ph and Gelatin-Ph | -Scaffolds and cell-laden constructs |
| 13 | Lee Y. et al. [34] | In vitro and in vivo | Human dermal fibroblasts | Nude mice; IRC mice | - In situ GH-hydrogel production (containing 7 × 106 human DFBs/mL) at the exposed wound site in the presence of HRP (0.02 mg/mL) and H2O2 (0.007 wt%). | - Gelatin-hydroxyphenyl propionic acid (GH) | - Wound dressings |
| 14 | Mubarok W. et al. [35] | In vitro | C2C12 cells | - | - Addition of an aqueous solution containing 3.0% w/v Gelatin-Ph and 1 U/mL HRP in PBS to a polydimethylsiloxane (PDMS) mold (diameter: 8 mm, height: 4 mm). - Exposure to air containing H2O2 for 15, 30, 45, and 60 min follows → Hydrogels of varying consistencies. | - Gelatin from bovine | - Skeletal muscle tissue engineering |
| 15 | Zhang Y. et al. [36] | In vitro | L929 cells | - | - Gelatin and chitosan-PA + transglutaminase (TG) and HRP/H2O2 → Formation of IPN. - TG → Amide bonds between glutamine and lysine residues on adjacent gelatin chains - HRP → Crosslinking of phenol groups in chitosan-PA in the presence of H2O2. | - Gelatin and chitosan-PA | - Biocompatible scaffolds for tissue engineering and wound dressings |
| 16 | Nie K. et al. [37] | In vitro and in vivo | Human umbilical vein endothelial cells (HUVECs) | Adult male Sprague Dawley (SD) rats | - Electrospinning of Gelatin–hydroxyphenylpropionic acid (Gel–HPA) → nanofiber formation → enzymatic insolubilization through HRP/H2O2 crosslinking. - Prevention of nanofiber dissolution by an ethanol-water solution (volume ratio of 85:15). | - Gel–HPA | - Soft tissue engineering and regeneration |
| 17 | Liu X. et al. [38] | In vitro | L929 cells | - | - Gelatin and chitosan-PA + TG and HRP/H2O2 → IPN fiber formation under wet spinning conditions. | - Gelatin and chitosan-PA | - Scaffold for tissue engineering |
| 18 | Zhou F. et al. [39] | In vitro and in vivo | L929 cells | Rats; Rabbits | - Production of dopamine-modified methacrylate gelatin (GMDA) → Hydrogelation of GMDA in a two-step process; HRP/H2O2 mediated cross-linking, followed by UV light-induced photo-crosslinking (365 nm). | - GMDA | - Hemostasis, wound closure and healing |
| 19 | Moghaddam M.M. et al. [40] | In vitro | MG-63 cells | - | - Tyramine (TA) is added to hyaluronic acid (HA) and gelatin. Quantification of conjugation via UV–Vis spectroscopy and 1H NMR analysis. HRP/H2O2 crosslinking follows, with concentrations optimized for a 10 s gelation, ensuring spherical microglobules. - Surfactant → Rapid formation of droplets. - Sphere size = inverse to stirring speed, with the appropriate size for cell delivery being 80–100 μm. | - Hyaluronic acid (HA) and Gelatin | - Micro-scaffolds in bone tissue engineering |
| 20 | Li L. et al. [41] | In vitro and in vivo | RAW 264.7 mouse macrophages; HUVECs; Mesenchymal stem cells (MSCs) | immunocompetent C57BL/6J mice | - Covalent bonding of HRP onto porous silica particles (70–140 µm in diameter) via a polyethylene glycol molecule. - Retention of particles within the syringe during H2O2 and hydrogel precursor flow and crosslinking. | - Dextran-tyramine; Gelatin-hydroxyphenyl propionic acid | - Minimizes the immune response |
| 21 | Cheng K.W. et al. [42] | In vitro | PC12 rat pheochromocytoma cells | - | - HRA/H2O2 crosslinking of gelatin-hydroxyphenylpropionic acid. - PC12 cells embedded within the hydrogel precursor. | - Gelatin-hydroxyphenyl propionic acid | - Nerve regeneration |
| 22 | Park K.M. et al. [43] | In vitro and in vivo | HUVECs | mice | - Mixing of Gelatin–poly(ethylene glycol)–tyramine (GPT) with an aqueous solution of an angiogenic peptide in the presence of HRP/H2O2 → Hydrogel embedded with the peptide. | - GPT | - Wound healing |
| 23 | Xu S. et al. [44] | In vitro | Mouse bone marrow mesenchymal stem cells (mBMSCs) | - | - Tubular silk fibroin/gelatin-tyramine (E-SF/GT) hydrogel formation through HRP/H2O2 crosslinking and the thermosensitive properties of gelatin. - Further treatment with methanol → Distinct inner and outer layers of the EM-SF/GT tubular hydrogel. | - Silk fibroin/gelatin-tyramine | - Scaffolds for hollow multilayer tissue engineering, such as blood vessels |
| 24 | Hasturk O. et al. [45] | In vitro and in vivo | Human bone marrow mesenchymal stem cells (hMSCs) | mice | - HRP/H2O2 crosslinking of a mixture containing SF + (SF-TA or G-TA) → opaque hydrogels with quicker gelation times compared to SF alone. | - Silk fibroin (SF) - SF-TA - Gelatin-TA | - Injectable tissue fillings, 3D bioprinting or cell microencapsulation |
| 25 | Liang Y. et al. [46] | In vitro and in vivo | L929 fibroblast cells | Kunming mice, 25–30 g, female | - Chitosan + gelatin grafted with dopamine (GT-DA) + polydopamine-coated carbon nanotubes (CNT-PDA) + HRP/H2O2 → Production of hydrogels with favorable wound healing properties. | - Gelatin-grafted-dopamine (GT-DA) - Polydopamine-coated carbon nanotubes (CNT-PDA) - chitosan | - Wound dressings |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basha, M.; Aburub, A.; Karageorgos, F.F.; Tsoulfas, G.; Alexopoulos, A.H. Advances in Gelatin-Based Tissue Engineering Using HRP/H2O2. Gels 2025, 11, 460. https://doi.org/10.3390/gels11060460
Basha M, Aburub A, Karageorgos FF, Tsoulfas G, Alexopoulos AH. Advances in Gelatin-Based Tissue Engineering Using HRP/H2O2. Gels. 2025; 11(6):460. https://doi.org/10.3390/gels11060460
Chicago/Turabian StyleBasha, Marino, Ahmad Aburub, Filippos F. Karageorgos, Georgios Tsoulfas, and Aleck H. Alexopoulos. 2025. "Advances in Gelatin-Based Tissue Engineering Using HRP/H2O2" Gels 11, no. 6: 460. https://doi.org/10.3390/gels11060460
APA StyleBasha, M., Aburub, A., Karageorgos, F. F., Tsoulfas, G., & Alexopoulos, A. H. (2025). Advances in Gelatin-Based Tissue Engineering Using HRP/H2O2. Gels, 11(6), 460. https://doi.org/10.3390/gels11060460








