Synthesis of Temperature/pH Dual-Responsive Double-Crosslinked Hydrogel on Medical Titanium Alloy Surface
Abstract
1. Introduction
2. Results and Discussion
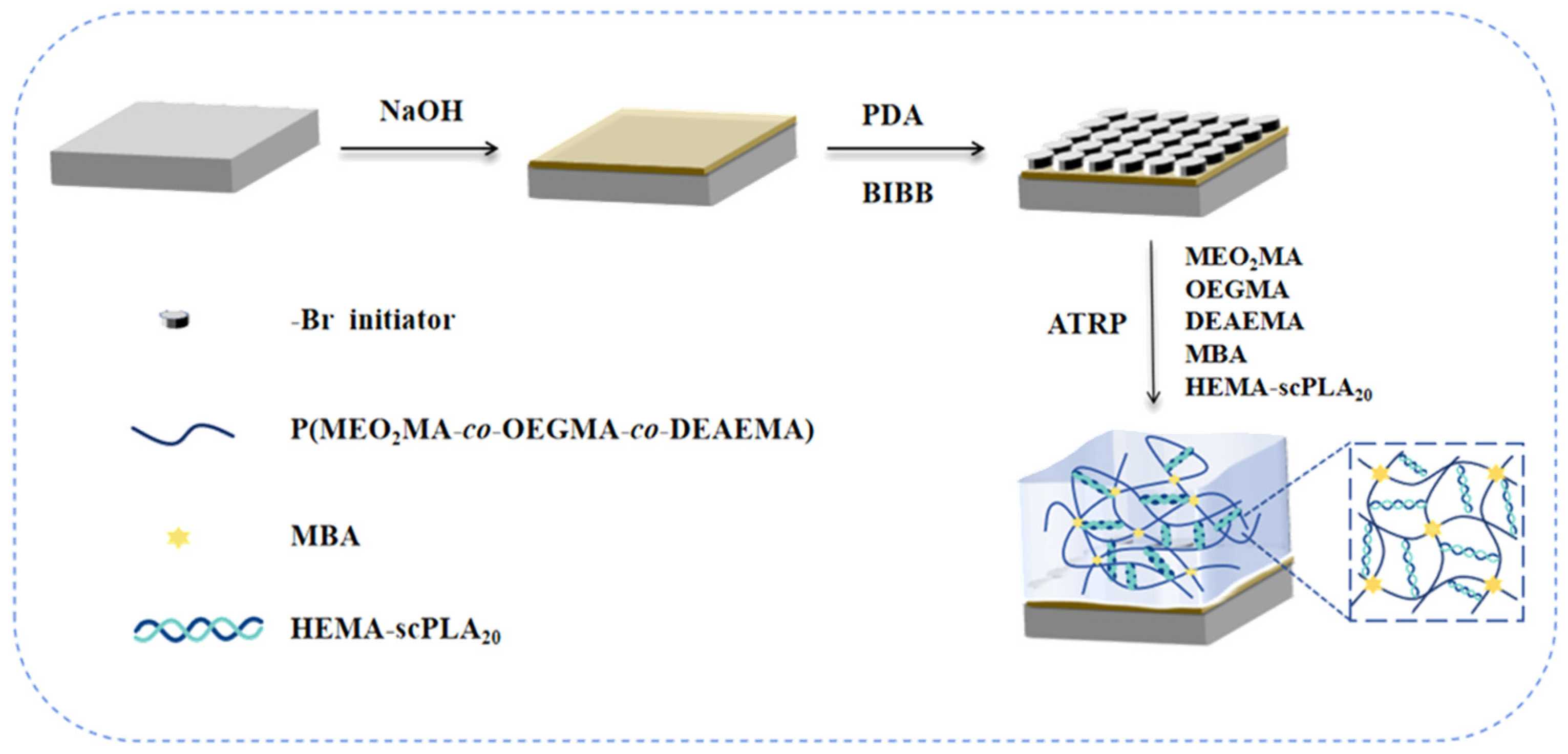
2.1. Synthesis of Hydrogels
2.2. Structural Characterization
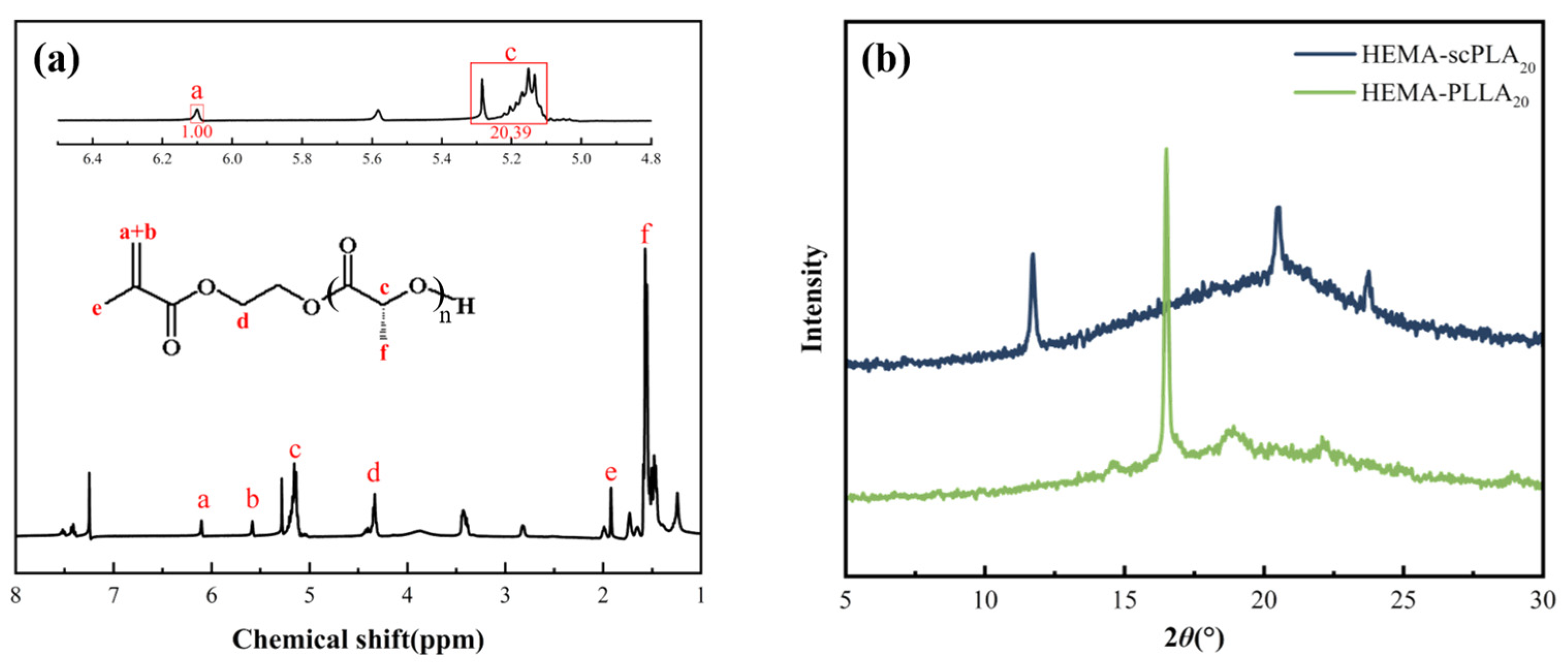
2.2.1. Characterization of HEMA-PLLA20
2.2.2. XRD Characterization of HEMA-scPLA20
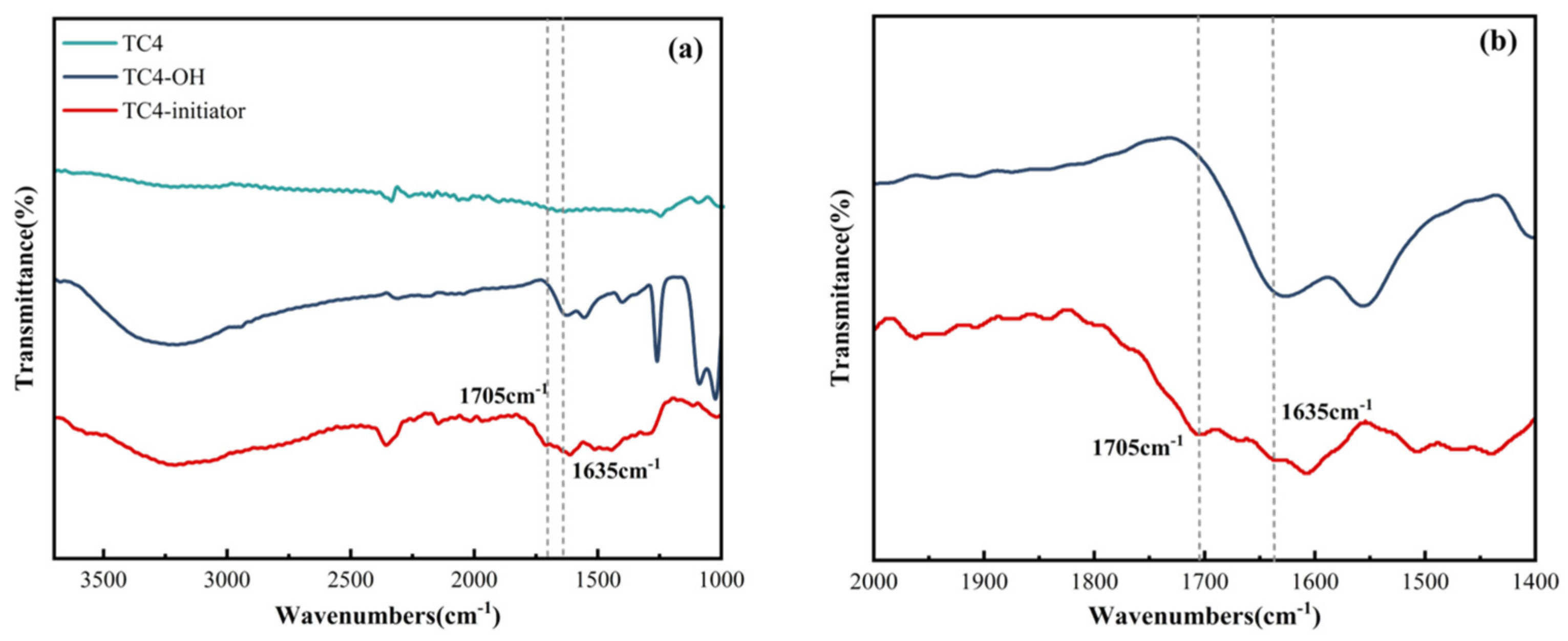
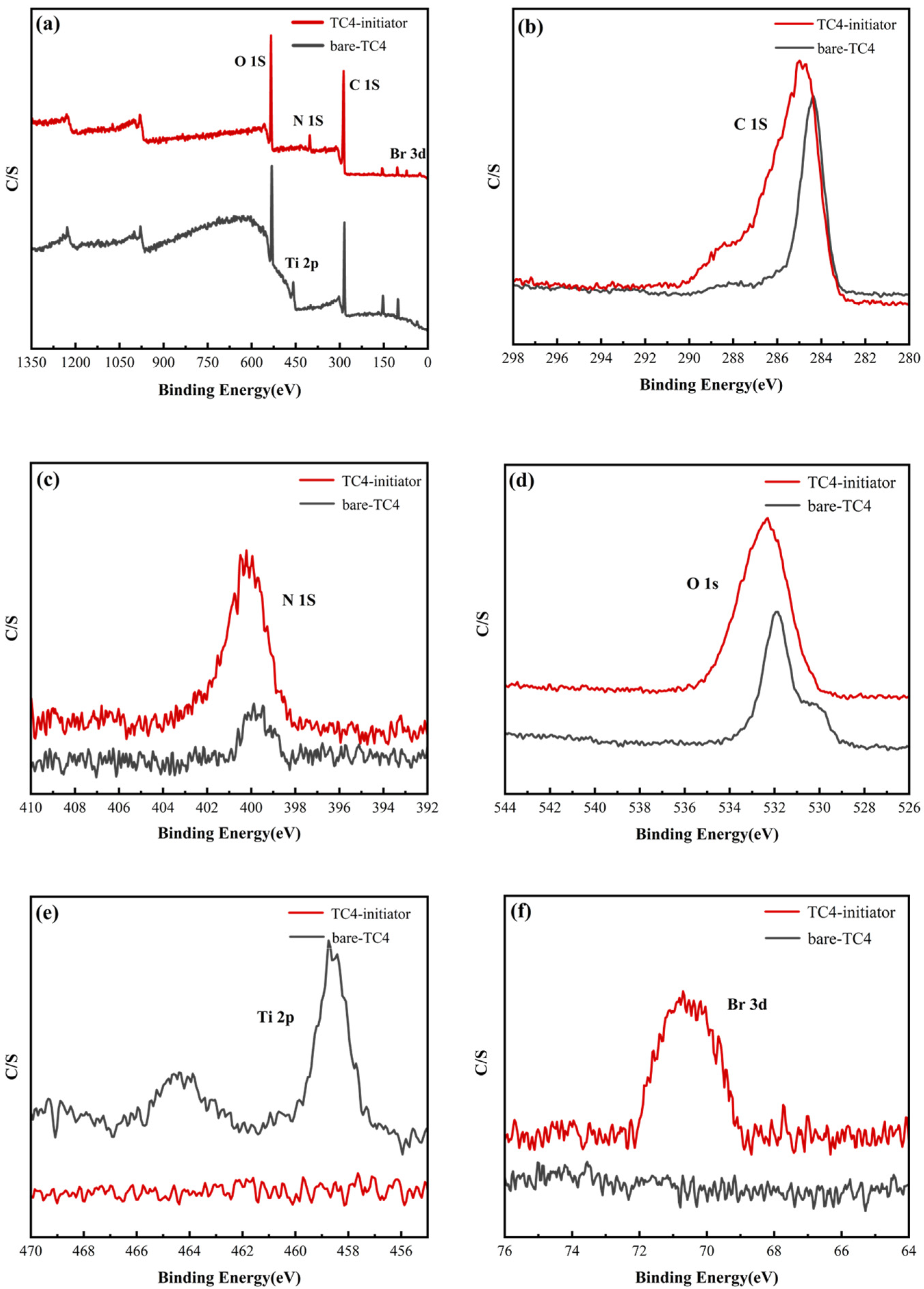
2.2.3. Characterization of the TC4-Initiator
2.2.4. XPS Characterization of TC4-Initiator
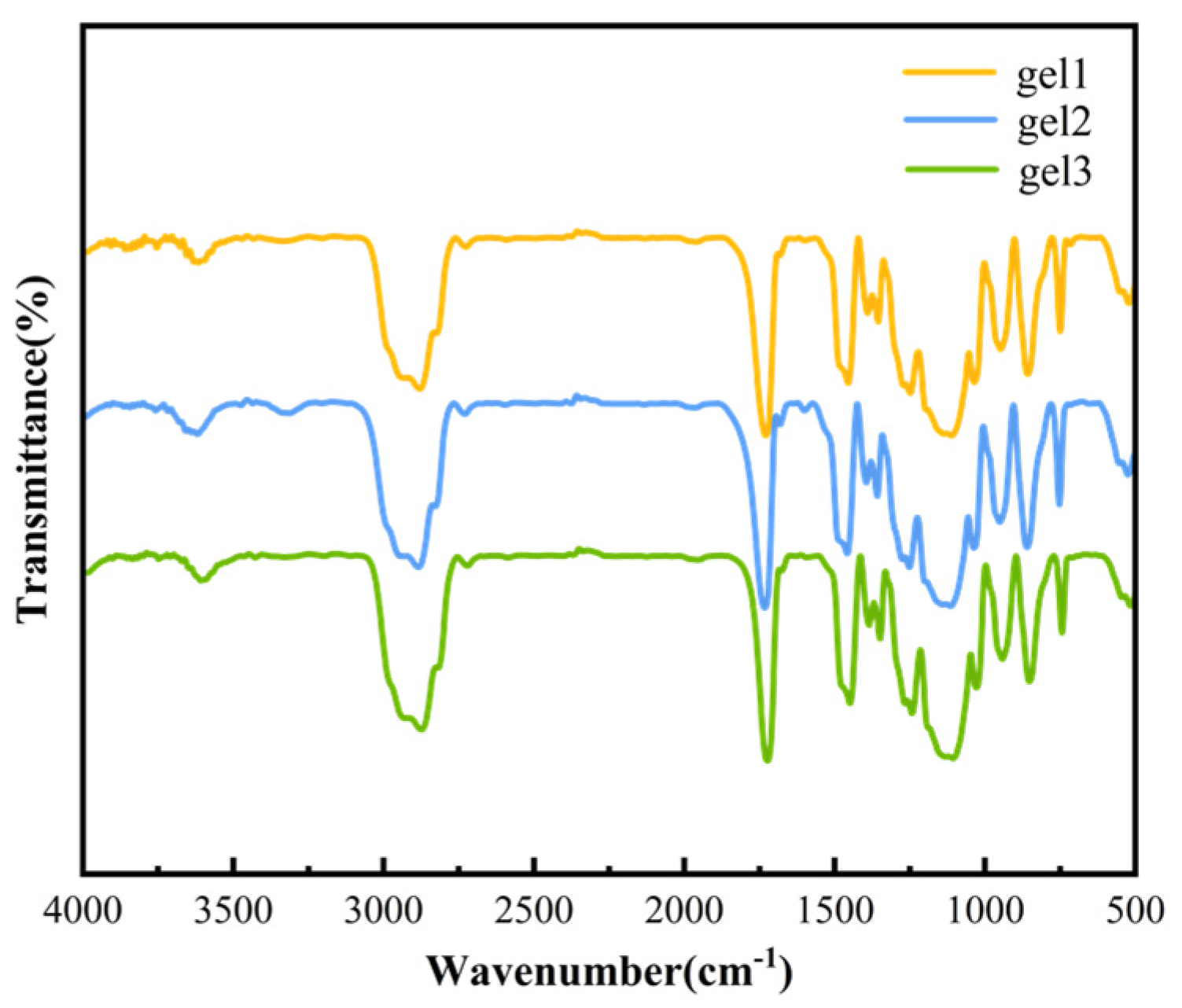
2.2.5. Structural Characterization of Gels
2.3. Morphological Analysis of Gels
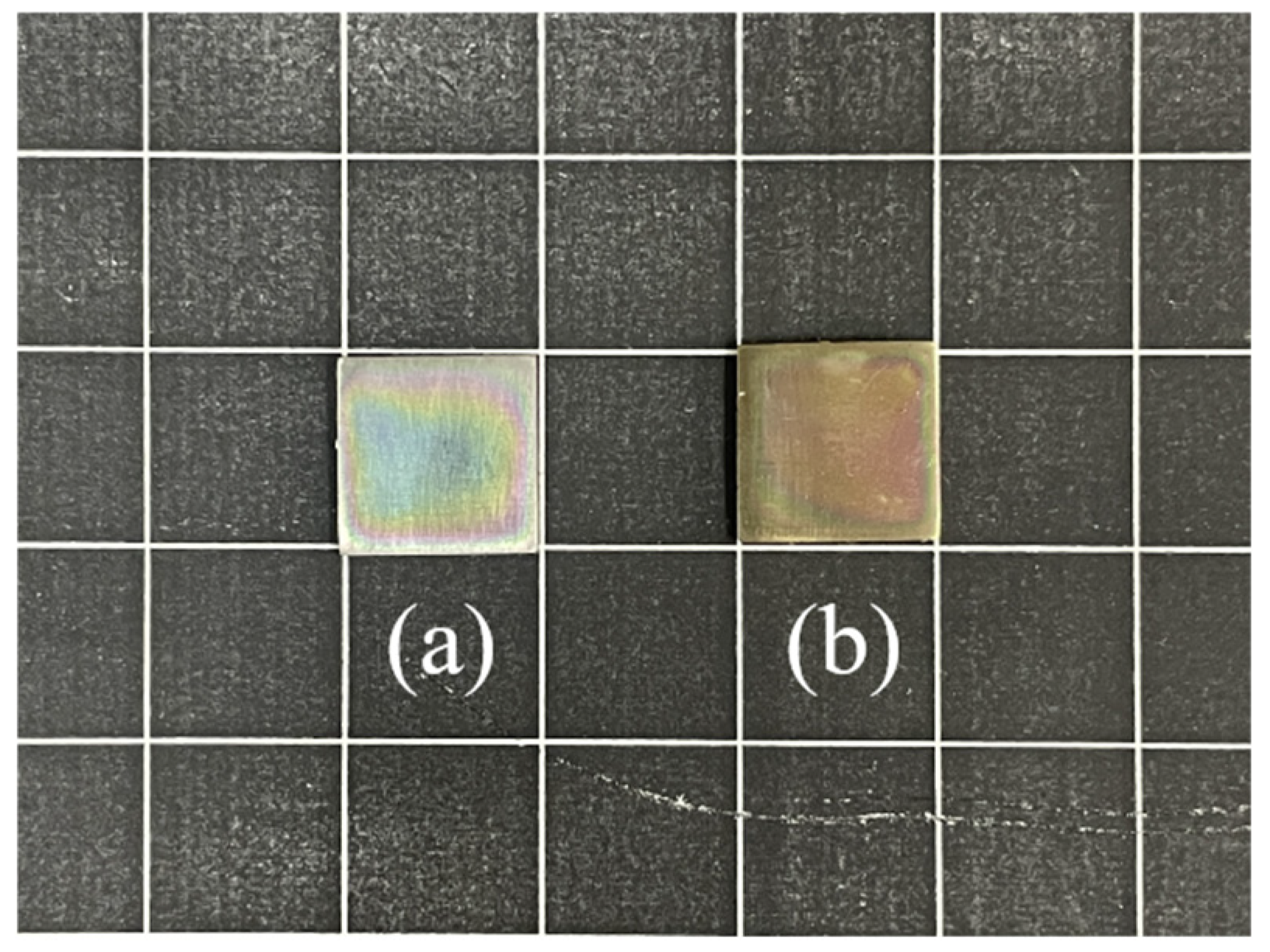
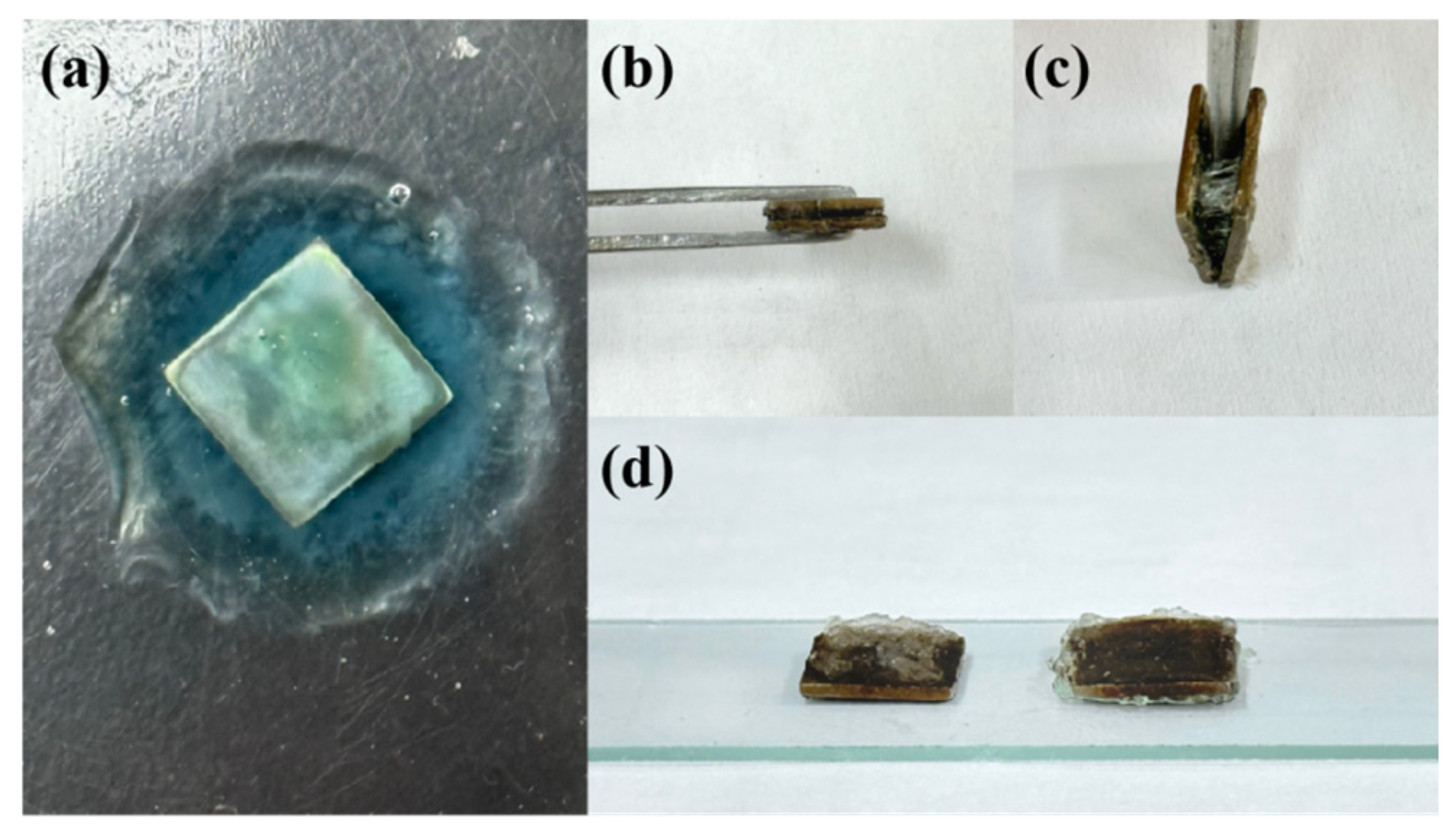
2.4. Bonding Between the Hydrogel and TC4
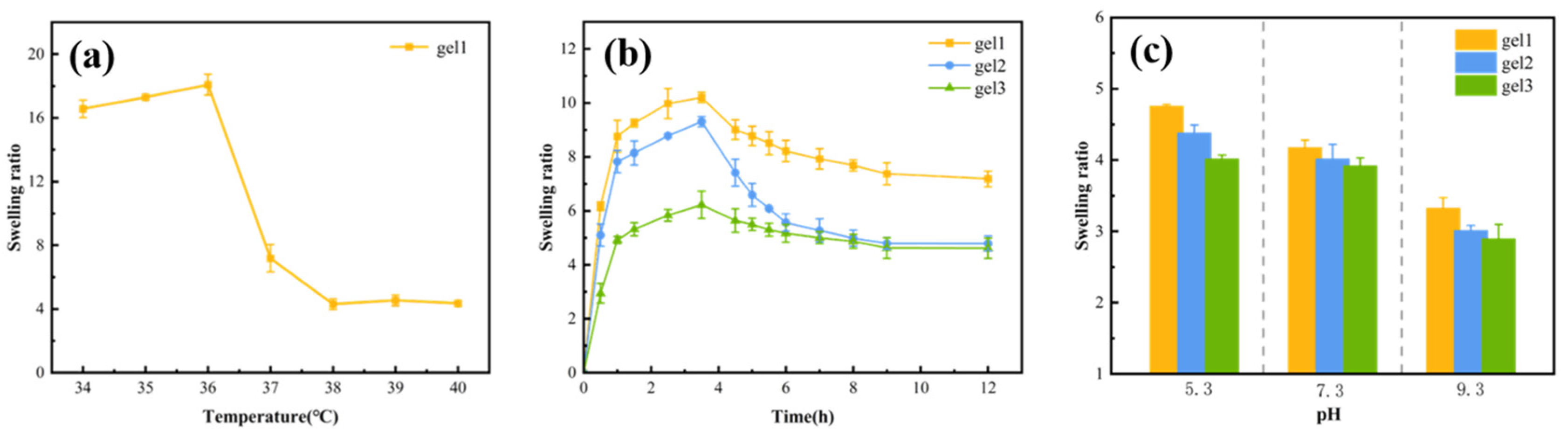
2.5. Temperature Sensitivity of Gels
2.6. pH Sensitivity of Gels
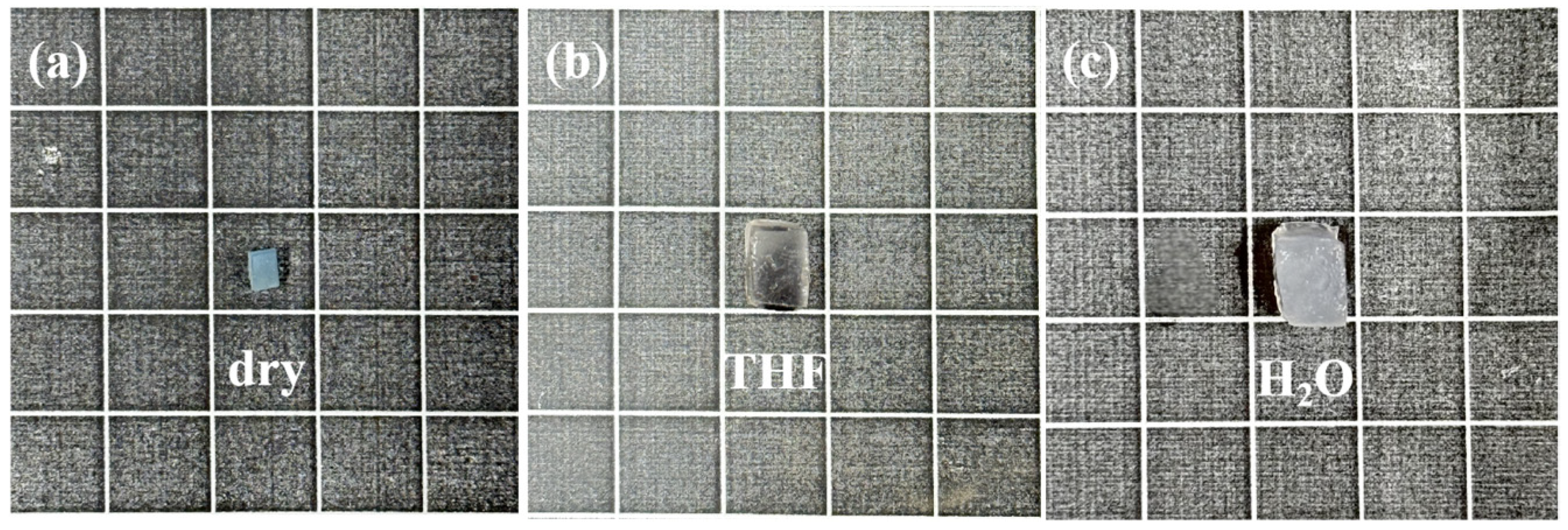
2.7. Amphiphilicity of Gels
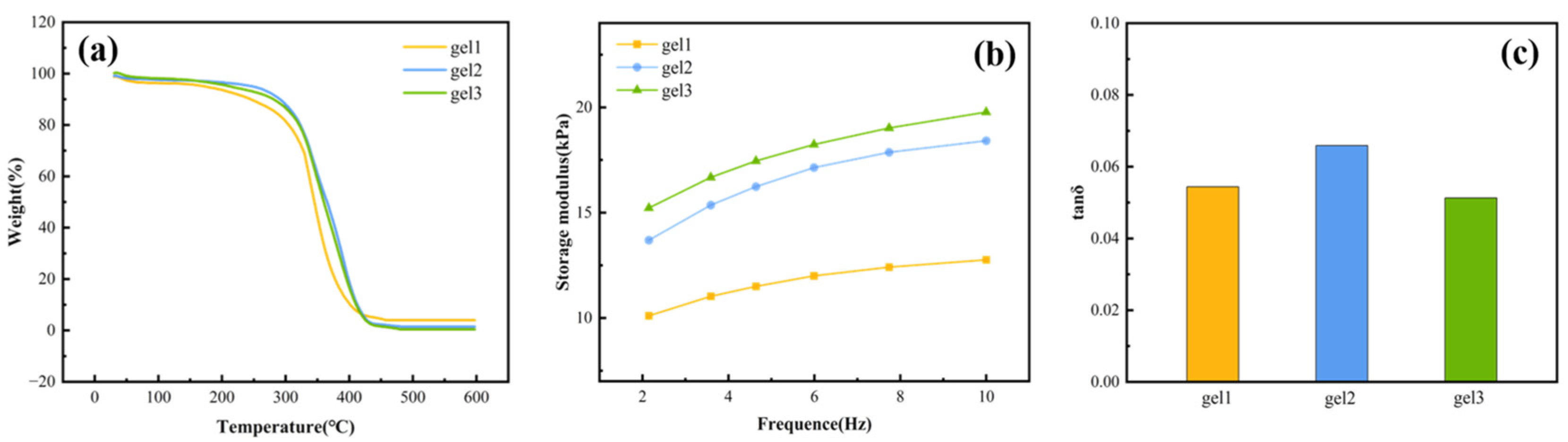
2.8. Thermal Stability of Gels
2.9. Mechanical Properties of Gels
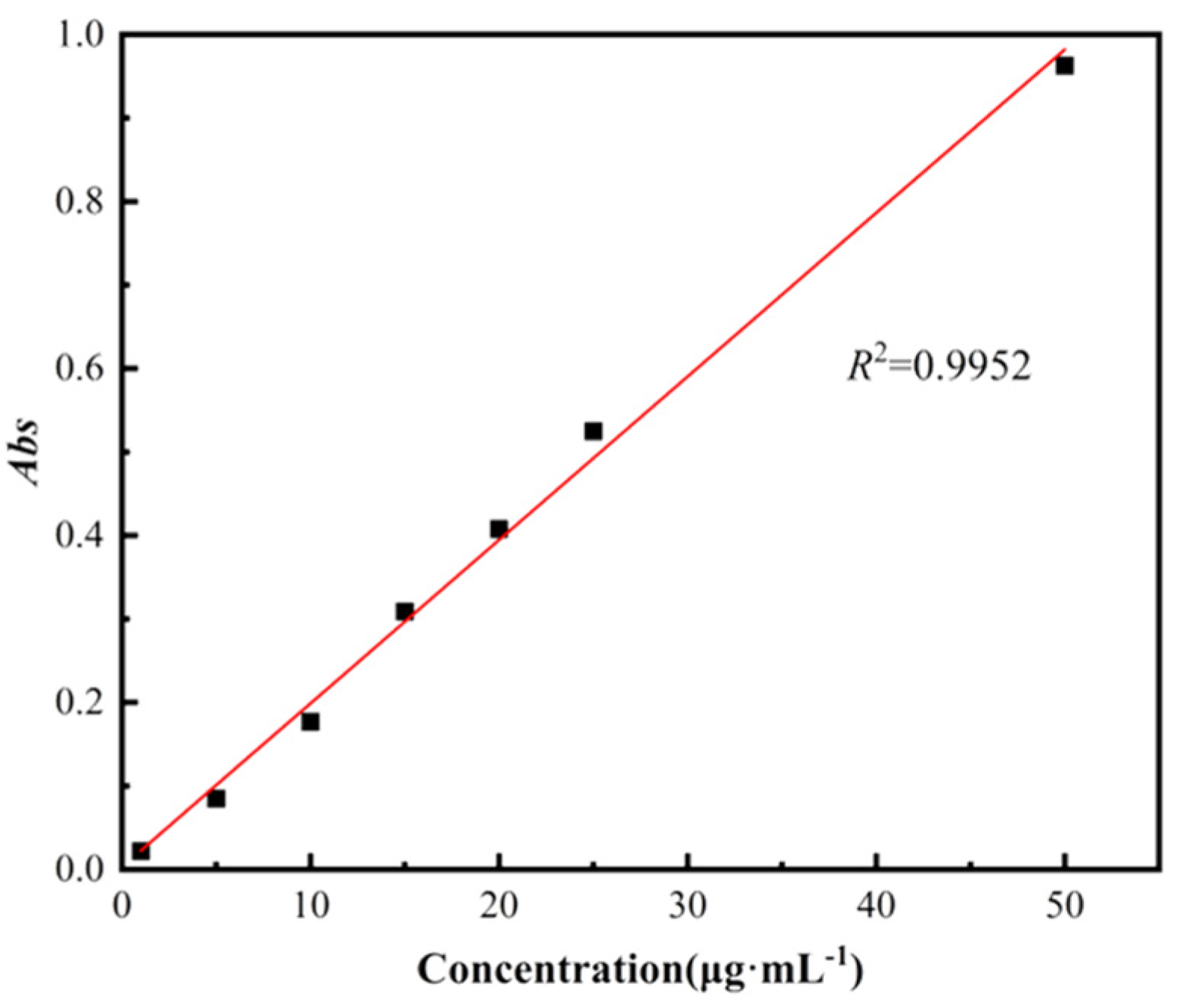
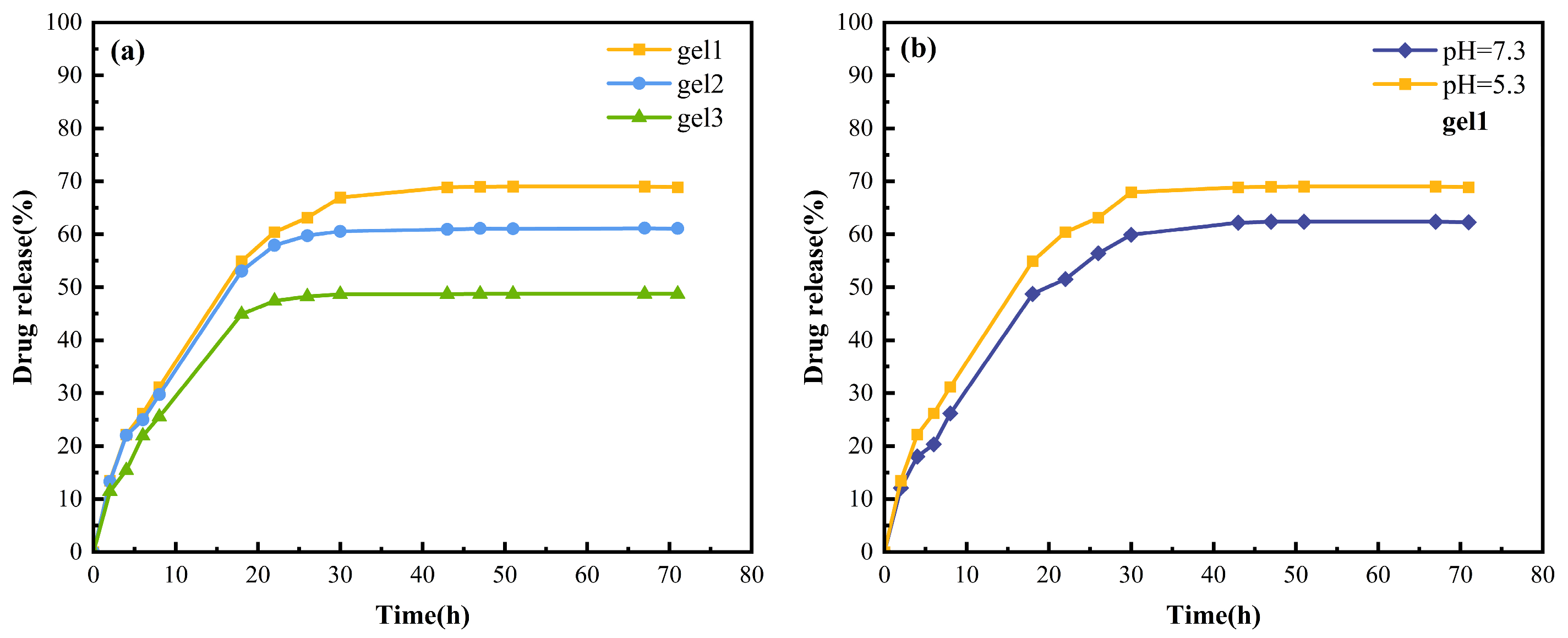
2.10. Sustained Drug Release of Gels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Synthesis of HEMA-PDLA20
4.2.2. Synthesis of HEMA-scPLA20
4.2.3. Synthesis of TC4-Initiator
4.2.4. Synthesis of Gels
4.3. Structural Characterization
4.3.1. Proton Nuclear Magnetic Resonance Spectroscopy (1H NMR)
4.3.2. Fourier-Transform Infrared Spectroscopy (FT-IR)
4.3.3. X-Ray Photoelectron Spectroscopy (XPS)
4.3.4. X-Ray Diffraction (XRD)
4.4. Temperature Sensitivity of Gels
4.5. pH Sensitivity of Gels
4.6. Amphiphilicity of Gels
4.7. Scanning Electron Microscopy (SEM) Analysis of Gels
4.8. Thermal Stability Analysis of Gels
4.9. Mechanical Properties Testing of Gels
4.10. Sustained Drug Release of Gels
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, X.; Ma, J.; Tian, A.; Wang, Y.; Li, Y.; Dong, B.; Tong, X.; Ma, X. Surface modification techniques of titanium and titanium alloys for biomedical orthopaedics applications: A review. Colloids Surf. B Biointerfaces 2023, 227, 113339. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Zhao, X.; Yang, L.; Wang, R.; Qin, G.; Chen, D.; Zhang, E. A novel biomedical titanium alloy with high antibacterial property and low elastic modulus. J. Mater. Sci. Technol. 2021, 81, 13–25. [Google Scholar] [CrossRef]
- He, S.; Zhu, J.; Jing, Y.; Long, S.; Tang, L.; Cheng, L.; Shi, Z. Effect of 3D-Printed Porous Titanium Alloy Pore Structure on Bone Regeneration: A Review. Coatings 2024, 14, 253. [Google Scholar] [CrossRef]
- Hijazi, K.; Dixon, S.; Armstrong, J.; Rizkalla, A. Titanium Alloy Implants with Lattice Structures for Mandibular Reconstruction. Materials 2024, 17, 140. [Google Scholar] [CrossRef]
- Wang, B.; Guo, Y.; Xu, J.; Zeng, F.; Ren, T.; Guo, W. Efficacy of bone defect therapy involving various surface treatments of titanium alloy implants: An in vivo and in vitro study. Sci. Rep. 2023, 13, 20116. [Google Scholar] [CrossRef]
- Wei, P.; Chen, T.; Chen, G.; Liu, H.; Mugaanire, I.; Hou, K.; Zhu, M. Conductive Self-Healing Nanocomposite Hydrogel Skin Sensors with Antifreezing and Thermoresponsive Properties. ACS Appl. Mater. Interfaces 2020, 12, 3068–3079. [Google Scholar] [CrossRef]
- Villegas, M.; Bayat, F.; Kramer, T.; Schwarz, E.; Wilson, D.; Hosseinidoust, Z.; Didar, T. Emerging Strategies to Prevent Bacterial Infections on Titanium-Based Implants. Small 2024, 20, 2404351. [Google Scholar] [CrossRef]
- Yang, J.; Liu, C.; Sun, H.; Liu, Y.; Liu, Z.; Zhang, D.; Zhao, G.; Wang, Q.; Yang, D. The progress in titanium alloys used as biomedical implants: From the view of reactive oxygen species. Front. Bioeng. Biotechnol. 2022, 10, 1092916. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Shi, Y.; Tang, J.; Huang, D.; Yan, M.; Dargusch, M. Surface Modification of Biomedical Ti and Ti Alloys: A Review on Current Advances. Materials 2022, 15, 1749. [Google Scholar] [CrossRef]
- Yu, M.; Wei, Z.; Yang, X.; Xu, Y.; Zhu, W.; Weng, X.; Feng, B. Safety and effectiveness of intraosseous regional prophylactic antibiotics in total knee arthroplasty: A systematic review and meta-analysis. Arch. Orthop. Trauma Surg. 2024, 144, 4233–4245. [Google Scholar] [CrossRef]
- Zhao, X.; Xiong, D.; Liu, Y. Improving surface wettability and lubrication of polyetheretherketone (PEEK) by combining with polyvinyl alcohol (PVA) hydrogel. J. Mech. Behav. Biomed. Mater. 2018, 82, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Javan, A.; Xiong, D. Recent Progress in Bionic Hydrogels for Articular Cartilage: Tribological and Mechanical Characteristics. J. Bionic Eng. 2024, 21, 653–673. [Google Scholar] [CrossRef]
- Deng, Y.; Xu, Y.; Nie, L.; Huang, Y. Crosslinked Polymer Coatings of Poly (Acrylic Acid-co-acrylamide)/Polyethyleneimine (P(AA-co-AAm)/PEI) on Titanium Alloy with Excellent Lubrication Performance for Artificial Joints. Coatings 2024, 14, 28. [Google Scholar] [CrossRef]
- İzmir, M.; Ercan, B. Anodization of titanium alloys for orthopedic applications. Front. Chem. Sci. Eng. 2019, 13, 28–45. [Google Scholar] [CrossRef]
- Wu, B.; Xiong, S.; Guo, Y.; Chen, Y.; Huang, P.; Yang, B. Tooth-colored bioactive titanium alloy prepared with anodic oxidation method for dental implant application. Mater. Lett. 2019, 248, 134–137. [Google Scholar] [CrossRef]
- Katic, J.; Metikos, H.; Babic, R. Nitinol Modified by Calcium Phosphate Coatings Prepared by Sol-Gel Method and Electrodeposition. ECS Meet. Abstr. 2013, 1, 981. [Google Scholar] [CrossRef]
- Ziąbka, M.; Matysiak, K.; Walczak, K.; Gajek, M.; Cholewa, K. Modification of TiAlV Alloys with Hybrid Layers Containing Metallic Nanoparticles Obtained by the Sol–Gel Method: Surface and Structural Properties. Int. J. Mol. Sci. 2022, 23, 2283. [Google Scholar] [CrossRef]
- Cao, L.; Ullah, I.; Li, N.; Niu, S.; Sun, R.; Xia, D.; Yang, R.; Zhang, X. Plasma spray of biofunctional (Mg, Sr)-substituted hydroxyapatite coatings for titanium alloy implants. J. Mater. Sci. Technol. 2019, 35, 719–726. [Google Scholar] [CrossRef]
- Hussain, S.; Shah, Z.; Sabiruddin, K.; Keshri, A. Characterization and tribological behaviour of Indian clam seashell-derived hydroxyapatite coating applied on titanium alloy by plasma spray technique. J. Mech. Behav. Biomed. Mater. 2023, 137, 105550. [Google Scholar] [CrossRef]
- Sun, A.; Ashammakhi, N.; Dokmeci, M. Methacrylate Coatings for Titanium Surfaces to Optimize Biocompatibility. Micromachines 2020, 11, 87. [Google Scholar] [CrossRef]
- Wang, S.; Song, J.; Li, Y.; Zhao, X.; Chen, L.; Li, G.; Wang, L.; Jia, Z.; Ge, X. Grafting antibacterial polymer brushes from titanium surface via polydopamine chemistry and activators regenerated by electron transfer ATRP. React. Funct. Polym. 2019, 140, 48–55. [Google Scholar] [CrossRef]
- Fu, Y.; Wu, Q.; Yang, W.; Wang, J.; Liu, Z.; Shi, h.; Liu, S. Preparation and Properties of Physical Gel on Medical Titanium Alloy Surface. Gels 2023, 9, 558. [Google Scholar] [CrossRef]
- Yang, W.; Wang, J.; Jia, L.; Li, J.; Liu, S. Stereo-Complex and Click-Chemical Bicrosslinked Amphiphilic Network Gels with Temperature/pH Response. Gels 2023, 9, 647. [Google Scholar] [CrossRef] [PubMed]
- Prince, D.; Villamagna, I.; Hopkins, C.; Druyn, J.; Gillies, E. Effect of drug loading on the properties of temperature-responsive polyester–poly(ethylene glycol)–polyester hydrogels. Polym. Int. 2019, 68, 1074–1083. [Google Scholar] [CrossRef]
- Avais, M.; Chattopadhyay, S. Waterborne pH responsive hydrogels: Synthesis, characterization and selective pH responsive behavior around physiological pH. Polymer 2019, 180, 121701. [Google Scholar] [CrossRef]
- Mei, L.; Mei, Q.; Dong, W.; Wu, X. Redox-responsive self-assembled peptide hydrogel for mitochondrial-targeted anticancer drug delivery. Appl. Mater. Today 2024, 41, 102471. [Google Scholar] [CrossRef]
- Yang, H.; Whitby, C.; Travas, S. Dual-network hydrogel capsules for controlled molecular transport via pH and temperature responsiveness. J. Colloid Interface Sci. 2025, 677, 942–951. [Google Scholar] [CrossRef]
- Shi, C.; Yang, F.; Hu, L.; Wang, H.; Wang, Y.; Wang, Z.; Pan, S.; Chen, J. Construction of polysaccharide based physically crosslinked double-network antibacterial hydrogel. Mater. Lett. 2022, 316, 132048. [Google Scholar] [CrossRef]
- Hou, J.; Jiang, Z.; Xiao, Y.; Cheng, R.; Zhao, Y. Room temperature shape self-adjustable tough hydrogel based on multi-physical crosslinking. Chem. Eng. J. 2024, 499, 156144. [Google Scholar] [CrossRef]
- Patacho, I.; Oliveira, A.; Nolasco, P.; Colaco, R.; Serro, A. Chemically crosslinked PVA hydrogels for cartilage substitution. Ann. Med. 2021, 53 (Suppl. S1), S25. [Google Scholar] [CrossRef]
- Barron, V.; Killion, J.; Pilkington, L.; Burke, G.; Geever, L.; Lyons, J.; McCullagh, E.; Higginbotham, C. Development of chemically cross-linked hydrophilic–hydrophobic hydrogels for drug delivery applications. Eur. Polym. J. 2016, 75, 25–35. [Google Scholar] [CrossRef]
- Wu, J.; Xue, W.; Yun, Z.; Liu, Q.; Sun, X. Biomedical applications of stimuli-responsive “smart” interpenetrating polymer network hydrogels. Mater. Today Bio. 2024, 25, 100998. [Google Scholar] [CrossRef] [PubMed]
- El-Husseiny, H.; Mady, E.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications. Mater. Today Bio. 2022, 13, 100186. [Google Scholar] [CrossRef]
- Luo, L.; Nguyen, D.; Huang, C.; Lai, J. Therapeutic hydrogel sheets programmed with multistage drug delivery for effective treatment of corneal abrasion. Chem. Eng. J. 2022, 429, 132409. [Google Scholar] [CrossRef]
- Ding, Z.; Fatollahi, F.; Kwon, I.; Pisturius, P.; Bettinger, C. Polydopamine Nanomembranes as Adhesion Layers for Improved Corrosion Resistance in Low Carbon Steel. Adv. Eng. Mater. 2018, 20, 1800621. [Google Scholar] [CrossRef]
- Kopeć, M.; Spanjers, J.; Scavo, E.; Emens, D.; Duvigneau, J.; Julius, V. Surface-initiated ATRP from polydopamine-modified TiO2 nanoparticles. Eur. Polym. J. 2018, 106, 291–296. [Google Scholar] [CrossRef]
- Dong, L.; Liu, X.; Xiong, Z.; Sheng, D.; Lin, C.; Zhou, Y.; Yang, Y. Preparation of UV-Blocking Poly(vinylidene fluoride) Films through SI-AGET ATRP Using a Colorless Polydopamine Initiator Layer. Ind. Eng. Chem. Res. 2018, 57, 12662–12669. [Google Scholar] [CrossRef]
- Kaseem, M. Poly(Lactic Acid) Composites. Materials 2019, 12, 3586. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, X.; Guo, W.; Zhou, H.; Li, Z.; Ma, Y.; Yan, C.; Dufresne, A. Insights into Stereocomplexation of Poly(lactic acid) Materials: Evolution of Interaction between Enantiomeric Chains and Its Role in Conformational Transformation in Racemic Blends. ACS Appl. Polym. Mater. 2022, 4, 5891–5900. [Google Scholar] [CrossRef]
- Wang, J.; Yang, W.; Li, Y.; Ma, X.; Xie, Y.; Zhou, G.; Liu, S. Dual-Temperature/pH-Sensitive Hydrogels with Excellent Strength and Toughness Crosslinked Using Three Crosslinking Methods. Gels 2024, 10, 480. [Google Scholar] [CrossRef]
- Wellert, S.; Hübner, J.; Boyaciyan, D.; Inanova, O.; VonKlitzing, R.; Soltwedel, O.; Holderer, O. A grazing incidence neutron spin echo study of near surface dynamics in p(MEO2MA-co-OEGMA) copolymer brushes. Colloid Polym. Sci. 2018, 296, 2005–2014. [Google Scholar] [CrossRef]
- Ye, Z.; Hai, P.; Cheng, L.; Hu, J.; Shang, Y.; Liu, H. Molecular understanding of the LCST phase behaviour of P(MEO2MA-b-OEGMA) block copolymers. Mol. Simul. 2021, 47, 299–305. [Google Scholar] [CrossRef]
- Rabiee, H.; Jin, B.; Yun, S.; Dai, S. Gas-responsive cationic microgels for forward osmosis desalination. Chem. Eng. J. 2018, 347, 424–431. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, X.; Han, D.; Zhao, Z.; Lu, W. Preparation of a Novel CO2-Responsive Polymer/Multiwall Carbon Nanotube Composite. Processes 2021, 9, 1638. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Yang, Y.; Lu, T. Swell induced stress in a hydrogel coating. Acta Mech. Sin. 2021, 37, 797–802. [Google Scholar] [CrossRef]
- Matsumoto, K.; Sakikawa, N.; Miyata, T. Thermo-responsive gels that absorb moisture and ooze water. Nat. Commun. 2018, 9, 2315. [Google Scholar] [CrossRef]
- Chen, C.; Yang, J.; Lu, C.; Wu, Y.; Chen, F. Effect of Quercetin on Injury to Indomethacin-Treated Human Embryonic Kidney 293 Cells. Life 2021, 11, 1134. [Google Scholar] [CrossRef]


| Sample | MEO2MA (mmol) | OEGMA (mmol) | DEAEMA (mmol) | HEMA-scPLA20 (g) | MBA (%) |
|---|---|---|---|---|---|
| gel1 | 2.7 | 0.3 | 0.6 | 0.05 | 1.2 |
| gel2 | 2.7 | 0.3 | 0.6 | 0.1 | 1.2 |
| gel3 | 2.7 | 0.3 | 0.6 | 0.2 | 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, J.; Liu, S. Synthesis of Temperature/pH Dual-Responsive Double-Crosslinked Hydrogel on Medical Titanium Alloy Surface. Gels 2025, 11, 443. https://doi.org/10.3390/gels11060443
Li Y, Wang J, Liu S. Synthesis of Temperature/pH Dual-Responsive Double-Crosslinked Hydrogel on Medical Titanium Alloy Surface. Gels. 2025; 11(6):443. https://doi.org/10.3390/gels11060443
Chicago/Turabian StyleLi, Yutong, Jiaqi Wang, and Shouxin Liu. 2025. "Synthesis of Temperature/pH Dual-Responsive Double-Crosslinked Hydrogel on Medical Titanium Alloy Surface" Gels 11, no. 6: 443. https://doi.org/10.3390/gels11060443
APA StyleLi, Y., Wang, J., & Liu, S. (2025). Synthesis of Temperature/pH Dual-Responsive Double-Crosslinked Hydrogel on Medical Titanium Alloy Surface. Gels, 11(6), 443. https://doi.org/10.3390/gels11060443






