Abstract
Bioelectronics for wearable and implantable biomedical devices has attracted significant attention due to its potential for continuous health monitoring, early disease diagnosis, and real-time therapeutic interventions. Among the various materials explored for bioelectronic applications, hydrogels derived from natural biopolymers have emerged as highly promising candidates, owing to their inherent biocompatibility, mechanical compliance akin to biological tissues, and tunable structural properties. This review provides a comprehensive overview of recent advancements in the design and application of protein-based hydrogels, including gelatin, collagen, silk fibroin, and gluten, as well as carbohydrate-based hydrogels such as chitosan, cellulose, alginate, and starch. Particular emphasis is placed on elucidating their intrinsic material characteristics, modification strategies to improve electrical and mechanical performance, and their applicability for bioelectronic interfaces. The review further explores their diverse applications in physiological and biochemical signal sensing, bioelectric signal recording, and electrical stimulation. Finally, current challenges and future perspectives are discussed to guide the ongoing innovation of hydrogel-based systems for next-generation bioelectronic technologies.
1. Introduction
The convergence of artificial intelligence, human–machine interfaces (HMIs), and next-generation healthcare technologies is reshaping the landscape of biomedical innovation [,,,]. This progression is driving the demand for personalized medicine and continuous health monitoring, necessitating the development of intelligent devices capable of reliable operation in dynamic biological environments. A diverse array of wearable and implantable health monitoring devices has been developed to meet this demand, including respiratory, electrophysiological, glucose, and motion monitors. These devices have enabled real-time tracking of vital physiological signals, offering new opportunities for early diagnosis and preventive healthcare [,]. However, many existing systems are hindered by their rigid architectures, limited stretchability, and insufficient long-term biocompatibility, compromising their performance under continuous mechanical deformation and hindering seamless integration with soft biological tissues [,,]. These challenges highlight the pressing need for advanced materials that can conform intimately to the body while maintaining functional stability. As bioelectronic interfaces continue to evolve, the integration of soft, bioinspired materials into electronic systems has emerged as a pivotal strategy for advancing next-generation biomedical technologies [,,].
In response to these material challenges and integration demands, bioelectronics has emerged as a transformative interdisciplinary platform, integrating advances from material science, electronics, and biomedicine [,,,]. However, a key challenge is that interface materials seamlessly integrate with biological tissues without eliciting adverse responses. Although conventional electronic materials often exhibit excellent electrical conductivity, their mechanical mismatch with soft tissues frequently undermines performance and limits long-term biocompatibility [,,]. Hydrogels derived from biomaterials have shown significant promise in addressing these limitations. Their soft mechanical properties facilitate stress-free interfacing with tissues, while their intrinsic ionic conductivity supports efficient transduction of physiological signals [,]. Natural biomaterial-based hydrogels, particularly those derived from proteins and polysaccharides, offer bioactive moieties and versatile functional groups [,,,]. These characteristics enable targeted tuning of electrical, mechanical, and biological properties through chemical crosslinking, conductive network embedding, or topological structuring [,,,,].
This review provides a comprehensive summary of recent advances in hydrogel materials for next-generation bioelectronic devices. As shown in Figure 1, the discussion focuses on two primary categories of natural biopolymers: polysaccharides such as cellulose, chitosan, alginate, and starch, and proteins such as gelatin, collagen, silk fibroin, and gluten. Their intrinsic properties, including biocompatibility, tissue-matching mechanical compliance, and tunable structure, are carefully discussed. On this basis, biomaterial-based hydrogels are analyzed for applications in electrophysiological and biochemical signal monitoring, bioelectric stimulation, and bioelectric recording. Furthermore, key challenges, such as long-term stability, material performance degradation, and seamless integration with electronic components, are discussed. Finally, future perspectives are proposed to guide the ongoing development of multifunctional hydrogel-based platforms for next-generation bioelectronics.
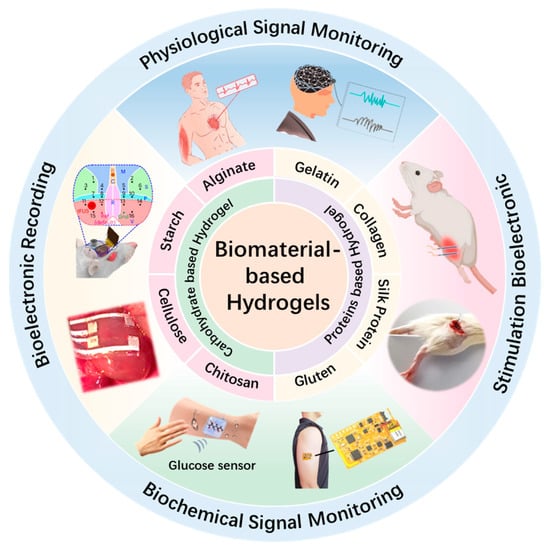
Figure 1.
Recent progress of biomaterial-based hydrogels for wearable and implantable bioelectronics. Physiological Signal Monitoring: reproduced with permission []. Copyright 2023, Elsevier. Reproduced with permission []. Copyright 2023, Wiley. Stimulation Bioelectronic: reproduced with permission []. Copyright 2023, Springer Nature. Reproduced with permission []. Copyright 2025, Elsevier. Biochemical Signal Monitoring: reproduced with permission []. Copyright 2023, Cell Press. Reproduced with permission []. Copyright 2023, Elsevier. Bioelectronic Recording: reproduced with permission []. Copyright 2023, Springer Nature. Reproduced with permission []. Copyright 2024, Springer Nature.
2. Biomaterial-Based Hydrogel
Biomaterial-based hydrogels, an emerging class of intelligent soft materials, offer a promising platform for integrating biofunctionality with tunable physicochemical properties through molecular engineering [,,]. These three-dimensional (3D) networks, composed of natural macromolecules such as proteins and polysaccharides, are typically stabilized via crosslinking strategies, including hydrogen bonding, electrostatic interactions, and covalent linkages [,,]. In representative hydrogel systems, common synthesis routes mainly include chemical crosslinking, physical crosslinking, and enzymatic reactions. Chemical crosslinking, for example, is often achieved by aldehyde–amine reaction, free radical polymerization, or isocyanate reaction to achieve the connection between polymer chains. The crosslinking conditions usually involve the molar ratio of reactants, solution pH, reaction temperature, and reaction time, which directly affect the mechanical properties, gelation rate, and microstructure of hydrogels [,]. This molecular design strategy ensures intrinsic biocompatibility while enabling precise tuning of performance parameters such as conductivity and Young’s modulus through the modulation of polymer architecture and the incorporation of functional groups [,]. Compared to conventional rigid electronic materials such as silicon or metallic components, hydrogels exhibit superior mechanical compatibility with soft biological tissues [,,].
In implantable bioelectronics, the dynamic viscoelasticity of hydrogels alleviates mechanical mismatch at tissue interfaces, enabling over 80% reduction in interfacial stress and strain through optimized energy dissipation mechanisms [,]. Their inherent stretchability and viscoelasticity further support their application in high-fidelity physiological signal monitoring. By integrating conductive nanomaterials, dynamic covalent linkages, and tissue-interactive functional groups, hydrogel-based bioelectronic systems have been engineered with multimodal sensing capabilities and seamless integration with biological tissues [,,].
Despite these advantages, current biomaterial-based hydrogel synthesis strategies still face significant challenges in terms of time efficiency and scalability. Traditional methods such as physical crosslinking, chemical crosslinking, and self-assembly can effectively tailor hydrogel properties on a laboratory scale, but often suffer from long synthesis times, complex reaction conditions, and limited batch-to-batch reproducibility. In addition, achieving consistent performance in large-scale mass production remains a persistent obstacle. However, striking a balance between performance reliability, cost efficiency, and environmental sustainability remains a central challenge in driving hydrogel-based technologies towards practical bioelectronic applications.
2.1. Protein-Based Hydrogels
Hydrogels derived from proteins such as collagen, gelatin, and silk fibroin have drawn considerable attention for use in implantable and wearable bioelectronic devices, owing to their intrinsic biological activity and customizable structural properties [,,]. At the molecular level, protein chains are capable of self-assembly or chemical crosslinking to form 3D networks, in which diverse amino acid sequences create biomimetic microenvironments that resemble native tissues []. Furthermore, functionalization strategies, such as the integration of conductive nanowires, conjugated polymers, and ionic groups, have been employed to precisely modulate the electrical conductivity of protein-based hydrogels []. Additionally, these hydrogels exhibit mechanical compatibility with soft biological tissues [,,,], making them excellent candidates for next-generation wearable and implantable electronics.
2.1.1. Gelatin
Gelatin, a biopolymer naturally produced through the controlled hydrolysis of collagen, retains the characteristic (Gly-X-Y)n triplet sequence in its molecular chains [,]. This characteristic sequence imparts excellent biocompatibility and biomimetic features to gelatin, making it highly suitable as a foundational material for fabricating functional hydrogels. Consequently, gelatin has been widely utilized as a structural platform in the design and development of advanced hydrogel systems [,]. In particular, the pore size distribution and Young’s modulus of gelatin-based 3D networks have been finely tuned through the synergistic control of physical crosslinking and chemical modification. These tunable physicochemical characteristics enable dynamic modulation of hydrogel properties, establishing gelatin-based hydrogels as promising candidates for flexible bioelectronic devices.
However, the intrinsic electrical insulation of native gelatin limits its direct application in conductive bioelectronics. To overcome this limitation, conductive nanomaterials such as MXenes and polypyrrole nanowires have been incorporated to construct biomimetic ionic–electronic dual conduction pathways [,]. Subsequently, several representative studies are summarized to demonstrate recent progress in gelatin-based hydrogels for multifunctional bioelectronic applications. Zhang et al. [] developed a multimodal responsive optical/electrical skin (OE-skin) through a bio-inspired hierarchical design strategy (Figure 2a). The OE-skin comprises a three-layered architecture: The top layer integrates an ionic electrode with an elastic dielectric interface for pressure sensing. The middle layer incorporates a magnetic photonic crystal in a dual-network hydrogel for dynamic color switching. The bottom layer features a carbon nanotube/MXene-based conductive film with microcrack structures for strain sensitivity. This gradient-modulus configuration optimizes mechanical adaptability, presenting a new paradigm for cross-scale signal coupling in intelligent robotics (Figure 2a). In addition, Yu et al. [] synthesized a poly(acrylamide)/gelatin/ammonium sulfate organohydrogel (PGAOH) using a facile one-step fabrication strategy (Figure 2b). In this system, acrylamide (AM) improves gelatin dispersibility in high-salt environments, which significantly reduces gelation time, allows for precise volume control, and promotes the formation of a robust double-network structure that enhances mechanical properties. As shown in Figure 2c, the resulting PGAOH exhibits multiple functional characteristics, including anti-freezing behavior, excellent stability, ionic conductivity, sensitivity, moldability, transparency, and elasticity. These properties have been validated in wireless virtual reality gaming applications. Furthermore, Liu et al. [] developed a thermoresponsive poly(vinyl alcohol)/phytic acid/gelatin (PPG) composite hydrogel based on dynamic hydrogen bonding (Figure 2d). The multivalent hydrogen bond network enables reversible phase transitions within the physiological temperature range (25–37 °C), enabling controllable adhesion behavior. As shown in Figure 2e, PPG hydrogel sensors adhered to finger joints demonstrate stable monitoring of flexion–extension motions over 12 h, indicating substantial potential for sustainable bioelectronic systems.
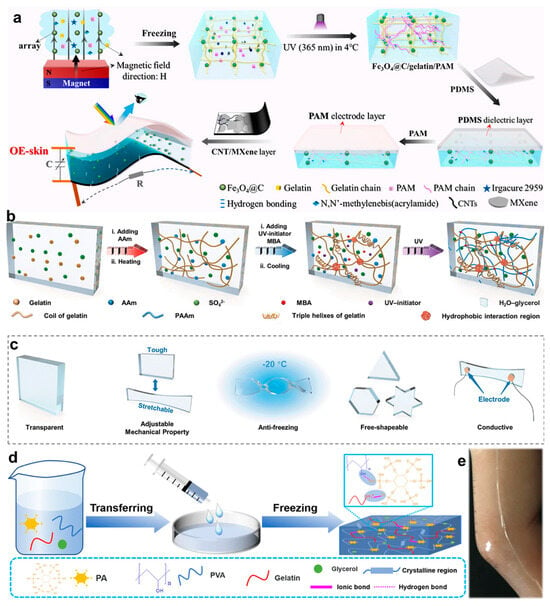
Figure 2.
Fabrication procedures and functional demonstrations of gelatin-based hydrogels. (a) Schematic illustration of the fabrication process for the multilayered chromotropic OE-skin []. (b) Synthetic route of PGAOH. (c) Functional demonstration matrix showcasing the multi-responsive properties of PGAOH []. (d) Schematic representation of the preparation process of PPG hydrogel. (e) Photographs of PPG hydrogel attached to human joints []. All pictures have been adopted with permission.
2.1.2. Collagen
Collagen, a natural structural protein, possesses a unique triple-helical molecular conformation that imparts distinct biofunctional properties and physiological compatibility [,]. In bioelectronic applications, collagen-based hydrogels have garnered significant interest, owing to their adjustable network configurations and exceptional biological properties [,]. These hydrogels are typically constructed through the synergistic combination of physical self-assembly and chemical crosslinking, enabling precise regulation of mechanical and electrical properties.
However, conventional collagen-based hydrogels often suffer from mechanical limitations, restricting their long-term stability under dynamic physiological conditions. To address these challenges, conductive polymers and ionic liquids have been incorporated into collagen matrices, resulting in double-network hydrogel architectures [,]. This section highlights recent advances in strategies for enhancing the mechanical performance of collagen-based hydrogels. Ling et al. [] designed a 3D interpenetrating collagen-based self-healing conductive organohydrogel (CDPAP) by integrating collagen with acrylic acid, dialdehyde carboxymethyl cellulose, and Al3+ ions in a water/propylene glycol cosolvent (Figure 3a). This system, which leverages a dual crosslinking mechanism based on dynamic imine bonds and metal coordination, exhibits high tensile strength, rapid self-healing capability, and robustness under extreme environmental conditions. Compared with conventional collagen hydrogels, the CDPAP hydrogel demonstrates superior mechanical resilience and operational stability. In addition, Li et al. [] introduced a novel biomimetic strategy inspired by the “towel-twisting” dehydration mechanism to achieve controlled fiber alignment. A two-step process combining twist-induced fiber orientation with covalent network reinforcement was established (Figure 3b–d). This method allows for accurate regulation of fiber orientation and facilitates swift covalent crosslinking, which significantly enhances both mechanical strength and toughness while maintaining excellent biocompatibility. Aligned collagen networks fabricated via this strategy exhibited mechanical properties several times greater than those of randomly oriented structures.
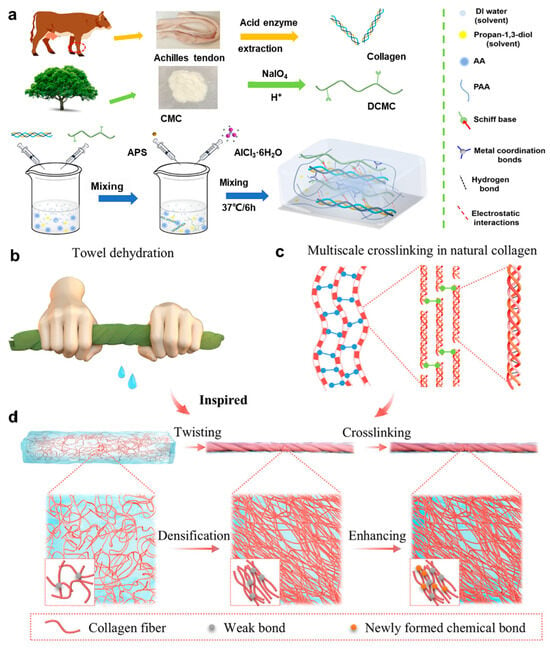
Figure 3.
Structural design and functional demonstration of collagen-based hydrogels. (a) Schematic illustration of the fabrication process for CDPAP hydrogels []. (b) Photographs showing towel-inspired mechanical manipulation, including twisting and squeezing deformations. (c) Hierarchical multiscale crosslinking network in natural collagen fibrils. (d) Bioinspired processing strategy for collagen hydrogels []. All pictures have been adopted with permission.
2.1.3. Silk Fibroin
Silk fibroin has emerged as a promising natural material for bioelectronic applications, forming a 3D β-sheet crystalline network through the self-assembly of its heavy- and light-chain polypeptides, which provides structural stability and tenability [,,]. The high glycine (~45%) and alanine (~30%) content in silkworm silk contributes to molecular flexibility, thereby facilitating β-sheet formation. Consequently, silk fibroin exhibits favorable mechanical strength and can be functionally tuned through chemical modification, physical processing, or molecular engineering strategies [,,]. These hydrogels combine biocompatibility, breathability, and biodegradability, making them ideal as flexible substrates for next-generation implantable electrodes and intelligent wearable sensors.
Recent research has highlighted the versatility of silk fibroin in constructing advanced bioelectronic systems. For example, Mirbakht et al. [] developed a silk fibroin-based electronic skin by modulating its β-sheet crystallization behavior (Figure 4a). Inkjet printing enabled in situ patterning of silk fibroin into micron-scale circuits, enhancing fabrication efficiency and increasing integration density (Figure 4b). At the material level, the system exhibited superior self-adhesiveness, breathability, and stretchability compared with conventional substrates, closely mimicking the mechanical behavior of human skin. At the system level, high-resolution circuits fabricated by inkjet printing are shown in Figure 4c, while robust bonding between the circuit and the flexible substrate, critical for stable biosignal monitoring, is illustrated in Figure 4d. These features enabled reliable, noninvasive monitoring even on challenging skin regions, such as hairy or perspiration-prone areas. Yang et al. [] report a silk protein-based bioelectrode that forms directly on the skin, offering a seamless and conformable interface for improved electrophysiological signal acquisition (Figure 4e). Unlike traditional electrodes, this system adapts well to hairy and irregular skin surfaces, significantly enhancing signal quality with a signal-to-noise ratio (SNR) improvement reaching 38.9%. The fluid–gel transition, triggered by ethanol and sodium alginate, ensures robust skin contact (Figure 4f). In a related study, Wang et al. [] developed a programmable silk fibroin system exhibiting electrical responsiveness and structural reconfigurability. This was achieved via isoelectric point migration driven by low-voltage-induced pH gradients, enabling precise control over the 3D structure. The in situ programming strategy enabled reversible, multimodal deformation in three dimensions, allowing real-time structural reconfiguration in response to electrical stimuli. Therefore, dynamic mechanical matching with biological tissues under diverse application scenarios was realized.
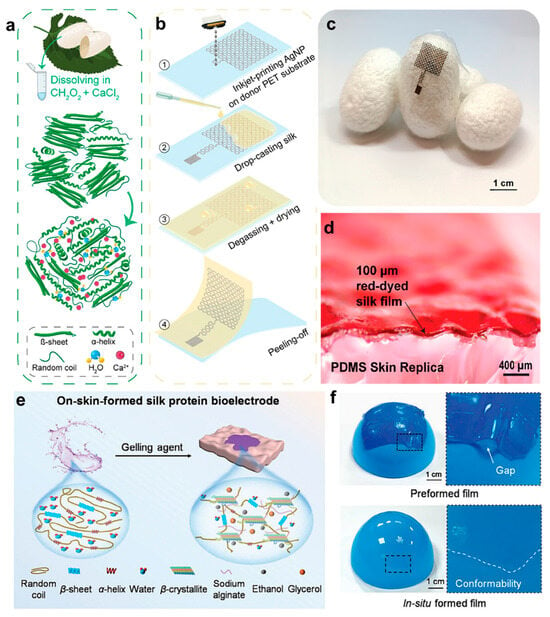
Figure 4.
Fabrication principles and morphological characteristics of silk protein-based hydrogels. (a) Schematic illustration of Ca2+-induced structural transition in silk proteins. (b) Micro-/nanopatterning technique employed in the fabrication of silk-based bioelectronic devices. (c) Optical image demonstrating the direct integration of silk bioelectronics. (d) Cross-sectional SEM image between red-dyed silk and PDMS []. (e) Schematic illustration of the synthesis route of the on-skin-formed silk protein (OSF-SP) bioelectrode and its secondary structure transition in the gelation process. (f) Photographs of a preformed film and an in situ-formed film covering the surface of a ball []. All pictures have been adopted with permission.
2.1.4. Gluten
Gluten is a protein complex naturally extracted from cereals such as wheat and barley, and is abundant in functional groups such as amino and carboxyl moieties. These chains can form 3D dynamic networks via physical or chemical crosslinking, thereby endowing hydrogel systems with tunable mechanical properties [,,]. In flexible bioelectronics, gluten-based hydrogels are considered promising materials for wearable sensors and bio-integrated electrodes due to their tissue-like flexibility, strong interfacial adhesion, and stable ionic conductivity. Chen et al. [] developed a double-network hydrogel composed of polyvinyl alcohol and gluten protein, crosslinked with borax (Figure 5a). The hydrogel demonstrates exceptional mechanical performance, with stretchability up to 1300% and excellent shape moldability (Figure 5b,c). These properties are largely attributed to the incorporation of NaCl, which enhances polymer chain interactions and network dynamics. Additionally, the electronic skin exhibits both biodegradability and biocompatibility, positioning it as a strong candidate for environmentally sustainable wearable electronic applications. Meanwhile, Han et al. [] developed an ionically conductive gluten (i-Gluten) hydrogel by leveraging synergistic coordination between glycerol and ionic species, enabling dynamic reconfiguration of the peptide network (Figure 5d). This strategy imparted self-healing capability and robust interfacial adhesion to the hydrogel (Figure 5e–h). Due to its mechanical compatibility with skin, the material exhibited stable performance across a wide range of physiological activities, from gross limb movements to fine EMG signals, and maintained functionality over 3000 mechanical cycles.
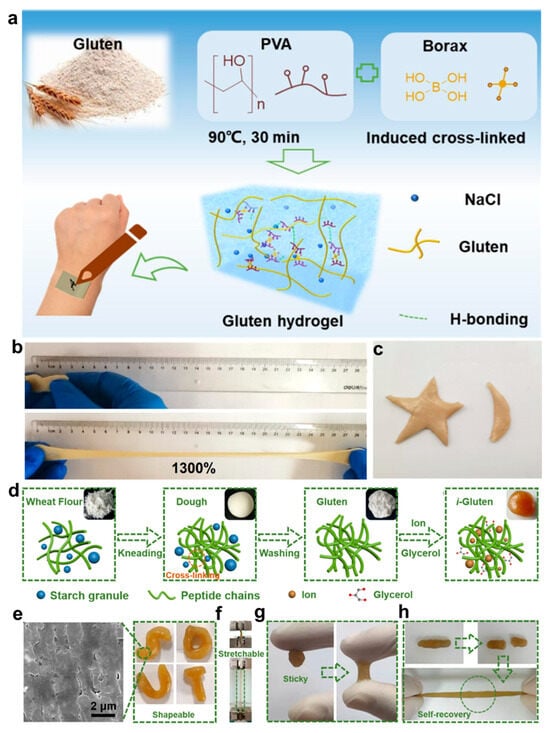
Figure 5.
Fabrication strategy and multifunctional performance of glutenin-based hydrogels. (a) Schematic illustration of the preparation procedure of the gluten-based e-skin. (b) Photographs of stretched gluten hydrogel. (c) Different shapes of gluten hydrogel photographs []. (d) Schematic illustration of the fabrication process of conductive i-Gluten hydrogels. (e) Optical and SEM images displaying the morphology of i-Gluten. (f) Photographs demonstrating the excellent stretchability of i-Gluten. (g) Photographs showing the adhesive capability of i-Gluten on various substrates. (h) Photographs highlighting the self-recovery performance of i-Gluten after mechanical damage []. All pictures have been adopted with permission.
2.2. Polysaccharide-Based Hydrogels
Polysaccharide-based hydrogels, such as those derived from chitosan (CS), hyaluronic acid, and alginate (Alg), have attracted growing interest in the field of wearable bioelectronics, due to their natural abundance, biodegradability, and structurally diverse molecular frameworks [,,]. Although polysaccharide-based materials are often considered to be abundantly sourced and eco-friendly, their cost is not absolutely inexpensive, but rather has some economic advantages over other biomedical polymers or functional synthetic polymers. Enriched with reactive functional groups such as hydroxyl and amino moieties, these hydrogels provide a versatile platform for chemical modification. By grafting conductive monomers or incorporating carbon-based nanomaterials and metal nanoparticles, composite systems with simultaneous ionic and electronic conductivity can be readily engineered [,]. Despite these advantages, achieving sufficient mechanical durability and multifunctionality suitable for dynamic biological environments remains challenging. Rational structural design strategies, such as utilizing hydrogen bonding, ionic crosslinking, or dynamic covalent bonds, can markedly enhance their stretchability and self-healing capabilities [,,]. These mechanical properties are particularly desirable for accommodating complex deformations associated with joint movements and epidermal dynamics. Compared with conventional elastomers, polysaccharide hydrogels exhibit superior adaptability under repetitive strain, facilitating their integration into soft bioelectronic interfaces [,,]. In summary, these characteristics make polysaccharide hydrogels promising candidates for the development of next-generation wearable and implantable bioelectronic devices.
2.2.1. Chitosan
Chitosan is an alkaline polysaccharide of natural origin, containing abundant amino and hydroxyl groups along its molecular chain [,,]. These functional groups enable the formation of a 3D network structure through physical or chemical crosslinking. This structure not only imparts excellent water absorption capacity and mechanical extensibility but also significantly improves ionic conductivity when integrated with conductive components such as polyaniline or carbon nanotubes [,].
However, challenges remain in achieving precise control over the mechanical and electrical properties of chitosan-based hydrogels while maintaining their structural integrity under dynamic conditions. Go et al. [] proposed a dual-network hydrogel system composed of poly(N-hydroxyethylacrylamide-co-acrylamide) and crosslinked chitosan (PHA/x-CS), fabricated via a lithium electrolyte-induced dual crosslinking mechanism (Figure 6a) to overcome this challenge. This system combines dynamic covalent bonding and ion–dipole interactions, enabling refined control over network topology. Compared with conventional single-network hydrogels, this dual-network structure significantly improves mechanical extensibility, shape flexibility at room temperature, and optical transparency (Figure 6b–e). Furthermore, the integration of photopolymerization-assisted shaping technology introduces a novel route for the personalized fabrication of flexible bioelectronic devices. An alternative approach was reported by Jiao et al. [], in which an in situ 3D-printed gelatin–sodium alginate–potassium chloride (GSP)/liquid metal (LM) hybrid hydrogel system was developed. This strategy achieves in situ encapsulation of LM through a calcium ion diffusion-induced phase transition (Figure 6f), thereby eliminating the need for traditional multi-step transfer processes. Compared with conventional fabrication techniques, this method integrates the supporting matrix, conductive pathways, and biological interface functionalities into a single crosslinking step, offering the advantage of rapid curing. Therefore, the hydrogel exhibits mechanical compliance with biological tissues and maintains stable electrochemical interface characteristics (Figure 6g,h), thereby offering essential technological support for the advancement of next-generation fully flexible bioelectronic devices.
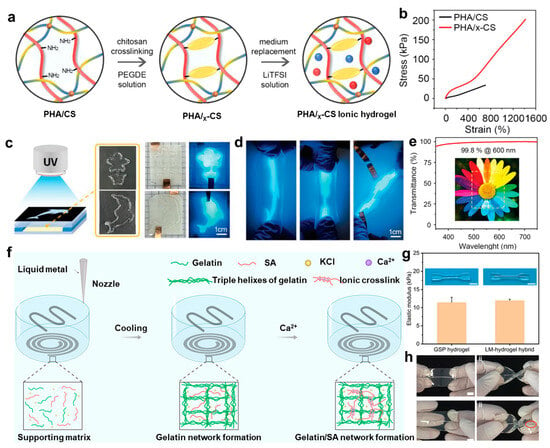
Figure 6.
Fabrication strategies and structural characterization of chitosan-based hydrogels. (a) Schematic illustration of the fabrication process for PHA/x-CS hydrogels. (b) Representative tensile stress–strain curves comparing PHA/CS and PHA/x-CS hydrogels. (c) Schematic of the photomask-assisted patterning approach for PHA/x-CS hydrogels. (d) The mechanical deformation of the patterned hydrogel under stretching. (e) Optical transmittance spectrum of the PHA/x-CS hydrogel []. (f) Schematic of in situ 3D printing of LM-hydrogel hybrids. (g) Elastic modulus comparison between GSP hydrogel and LM-hydrogel hybrid. (h) Twist deformation comparison of LM–hydrogel and metal–hydrogel composites []. All pictures have been adopted with permission.
2.2.2. Cellulose
Cellulose, a natural fiber derived from wood, cotton, hemp plants, agricultural waste, or bacterial synthesis, possesses a backbone composed of D-glucose units linked by β-1,4-glycosidic bonds [,]. Owing to its intrinsic biodegradability, water absorption capability, and biocompatibility, cellulose is widely employed to construct hydrogels through hydrogen bonding and physical or chemical crosslinking with other polymers, forming 3D networks [,]. In addition, molecular modifications, such as oxidative modification and graft copolymerization, have been utilized to enhance its functional versatility [,].
However, balancing electrical conductivity and mechanical durability remains a key challenge in conventional cellulose-based hydrogels [,]. To address this challenge, Wang et al. [] adopted a supramolecular engineering strategy to construct a cellulose/bentonite (BT) nanoconfined system. By forming multidentate coordination bonds between cellulose hydroxyl groups and aluminum oxide octahedra in BT, an intercalated structure was established (Figure 7a), which significantly overcomes the mechanical conductivity tradeoff. The resulting all-natural hydrogel’s (Figure 7b) anti-freezing capability and scalable processing features (Figure 7c) were validated in low-temperature wearable strain sensors, demonstrating substantially enhanced multifunctional performance through nanoscale structure control. In addition, utilizing phenylboronic acid–ionic liquid (PBA-IL) chemistry, Yao et al. [] developed a dynamic semi-interpenetrating network hydrogel. A one-pot method enabled the uniform incorporation of cellulose nanofibrils (CNFs) into the PBA-IL/AM crosslinked matrix (Figure 7d). This system exploits dynamic boronate ester bonds along with electrostatic interactions and hydrogen bonding to achieve ultra-high stretchability (Figure 7e) and a self-healing efficiency of 92%, while maintaining excellent adhesion and optical transparency. Furthermore, Ye et al. [] proposed a mechanical conductivity synergistic optimization strategy based on regulating hydration equilibrium within ionic liquid gels (Figure 7f). Through the competitive interactions between CNF and the IL/H2O system, the dissolution of CNF was effectively inhibited, while maintaining its nanoreinforcing effects. As a result, high-performance ionic gels exhibiting super-stretchability (Figure 7g), covalently crosslinked toughness (Figure 7h), and stable ionic conductivity were successfully developed. Compared with conventional hydrogels, these materials demonstrate substantially enhanced environmental stability under a wide temperature range and low-humidity conditions, thereby overcoming the common limitations of freezing-induced or dehydration-induced performance degradation.
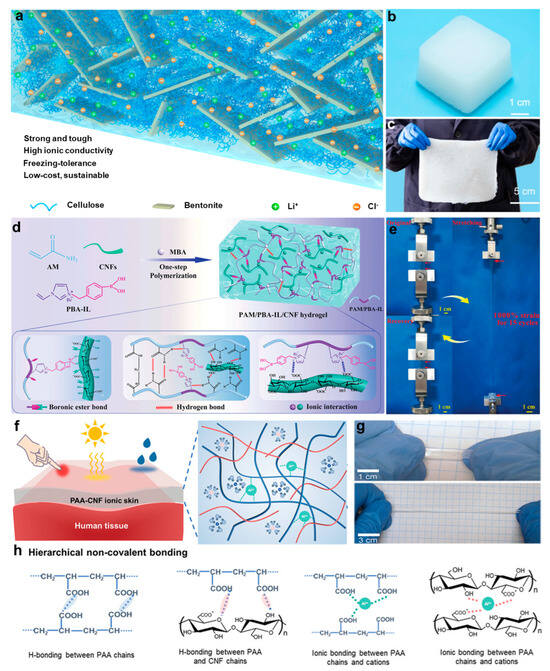
Figure 7.
Preparation strategies and structural characterization of cellulose-based hydrogels. (a) Schematic of hierarchical microstructure in cellulose/BT hydrogels. (b) Photograph of the synthesized ion–CB hydrogel. (c) Optical image demonstrating the scalable fabrication of ion–CB hydrogels []. (d) Schematic of fabrication and dynamic network structure of PAM/PBA-IL/CNF hydrogels. (e) Tensile deformation of PAM/PBA-IL/CNF hydrogels under stress. (f) Design schematic of PAA-CNF-IL-H2O ionogels. (g) Visual of tensile performance of PAA-CNF-IL-H2O ionogels. (h) Molecular schematic of noncovalent bonding in PAA-CNF-IL-H2O ionogels []. All pictures have been adopted with permission.
2.2.3. Alginate
Alginate is a naturally derived polysaccharide extracted from brown algae, featuring linear copolymer chains of β-D-mannuronic acid (M) and α-L-guluronic acid (G) residues joined by 1,4-glycosidic linkages [,]. The M/G ratio plays a pivotal role in determining the pore architecture and swelling capacity of alginate-based hydrogels, both of which are key factors for optimal bioelectronic functionality. These hydrogels can be rapidly fabricated through ionic crosslinking with divalent cations, such as calcium ions (Ca2+) [,]. However, its conventional single-network architecture results in limited mechanical strength and high brittleness, constraining its practical application [,]. To overcome these shortcomings, various strategies have been developed to improve the mechanical robustness and ionic conductivity of alginate-based hydrogels. Specifically, incorporating gelatin, polyvinyl alcohol (PVA), or carbon nanomaterials has enhanced mechanical strength and tuned ionic conductivity within the range of 10−3 to 101 S/m [,,,].
However, a substantial gap remains between the mechanical robustness of these hydrogels and that of native biological tissues, which restricts their broader clinical deployment. Suo et al. [] addressed these limitations by designing an ion–covalent double-network hydrogel that overcomes the brittleness and poor extensibility of traditional systems (Figure 8a–d). This design integrates the crack-bridging effect of a covalent network with the dynamic dissociation–recombination behavior of ionic bonds, enabling hydrogels with 90% water content to achieve over 20 times the elongation at break and a rubber-like fracture energy of 9000 J/m2. The covalent framework provides shape-memory behavior, while the dynamic ionic network reduces notch sensitivity via energy dissipation, jointly contributing to superior mechanical performance. Additionally, Cui et al. [] tackled the dual challenge of enhancing both conductivity and mechanical toughness in ionic hydrogels. They proposed a synergistic mechanism involving both anionic and cationic contributions. A physical double-network hydrogel composed of ion-sensitive polymers was immersed in a tailored salt solution, inducing dual crosslinking and salting-out effects (Figure 8e,f). This treatment dramatically improved the gel’s original fracture energy, ionic conductivity, and tensile strength by factors of 1100, 4.9, and 530, respectively. Consequently, a super-conductive hydrogel was achieved, exhibiting a tensile strength as high as 15 MPa, a toughness of 39 kJ/m2, and an ionic conductivity of 1.5 S/m. Therefore, this approach is broadly applicable across various salt-ion polymer systems and provides a fundamental breakthrough in overcoming the mechanical durability bottleneck of traditional conductive hydrogels, thus laying a foundation for wearable and implantable devices capable of operating under extreme mechanical conditions.
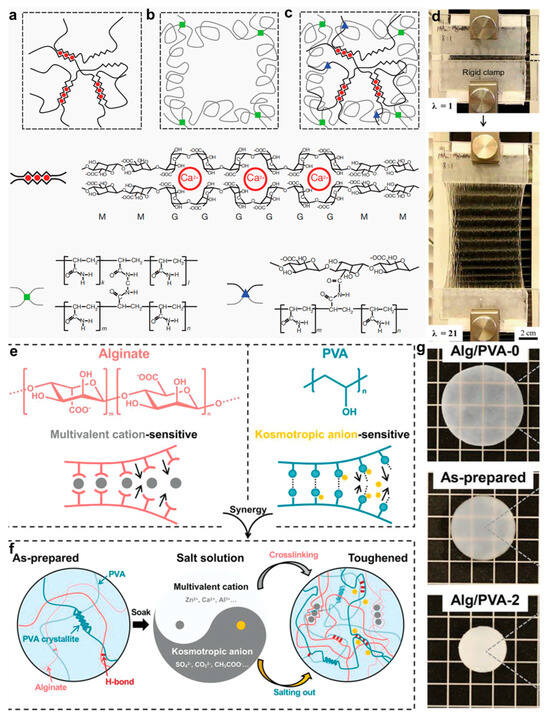
Figure 8.
Structural mechanisms and enhanced mechanical performance of alginate-based hydrogels. (a) Schematic illustration of ionic crosslinking in Alg hydrogels. (b) Covalent crosslinking architecture in PAAm hydrogels formed via free-radical polymerization. (c) Hybrid Alg/PAAm network with ionic and covalent crosslinks. (d) Mechanical performance of the hybrid hydrogel []. (e) Schematic of dual crosslinking in Alg/PVA hydrogels. (f) Reinforcement strategy using ionic crosslinking and salting-out in Alg/PVA hydrogels. (g) Visual comparison of Alg/PVA hydrogels in different treatment states []. All pictures have been adopted with permission.
2.2.4. Starch
Starch-based hydrogels, derived from natural starch, are polymeric 3D network structures formed through physical, chemical, or enzymatic modifications. Their physicochemical behavior is primarily governed by the ratio of amylose to amylopectin and the specific crosslinking techniques employed [,]. Due to the hydroxyl-rich nature of starch molecules, these hydrogels exhibit high hydrophilicity, biocompatibility, and biodegradability.
However, their mechanical properties remain relatively weak [,,]. To improve mechanical strength and self-healing capacity, a number of techniques have been used, including adding cellulose or polyvinyl alcohol and creating dynamic connections. Additionally, the incorporation of conductive materials such as polyaniline and MXene or the formation of ionic networks has been utilized to impart or enhance electrical conductivity [,]. To address the inherent limitations of traditional starch-based hydrogels, Zhao et al. [] constructed a dynamic dual-mode crosslinked hydrogel composed of amylose and polyacrylamide, using a phytic acid-mediated hydrogen bonding strategy (Figure 9a). In this system, phytic acid molecules act as multidentate hydrogen bond donors, forming a reconfigurable physical network with polysaccharide chains, while trace amounts of covalent crosslinking are introduced to enhance structural stability. Here, the material achieves an elongation at break as high as 36,000% and an energy dissipation capacity of 47 MJ·m−3 while maintaining superior mechanical properties compared with conventional hydrogels. The dynamic hydrogen bond network is shown in Figure 9b. Figure 9c presents the material’s optical and environmental stability, including 93% light transmittance, freeze resistance at −20 °C, and seven-day moisture retention. In addition, Su et al. [] proposed a green strategy for constructing a corn starch/Alg dual-network hydrogel through a calcium ion-induced synergistic toughening mechanism (Figure 9d). By combining the rigid segments of high-amylose starch with the flexible groups of Alg and applying gradient calcium ion crosslinking, a dynamic network structure was established via hydrogen bonding and ionic interactions. This design endows the hydrogel with room-temperature self-healing capability and rapid shape recovery driven by its porous structure, as shown in Figure 9e. Compared with conventional starch hydrogels, this system exhibits substantially enhanced mechanical robustness and dynamic responsiveness, offering promising potential for wearable and implantable bioelectronic applications. In order to facilitate a more intuitive understanding of the differences between different biomaterial-based hydrogels in terms of key performance parameters, this paper compiles a comparison of common hydrogels in terms of conductivity, Young’s modulus, and biocompatibility, as shown in Table 1.
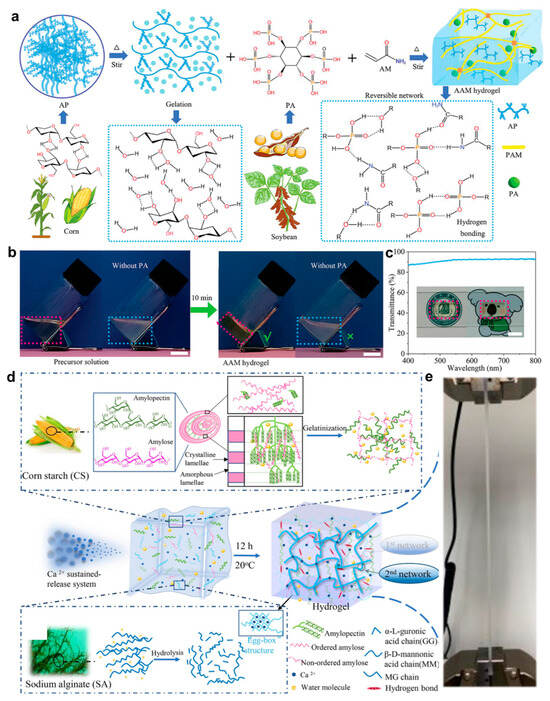
Figure 9.
Preparation strategies and characterization of starch-based hydrogels. (a) Schematic of AAM hydrogel fabrication process. (b) Photographs of AAM hydrogels before and after thermal treatment. (c) Optical transmittance spectra of AAM hydrogels []. (d) Formation mechanism of CS-SA-Ca2+ hydrogel via ionic crosslinking. (e) Tensile deformation behavior of CS-SA-Ca2+ hydrogel []. All pictures have been adopted with permission.

Table 1.
Comparison of the properties of different biomaterial types of conductive hydrogels.
3. Application
With their excellent biocompatibility, tissue-like mechanical compliance, and tunable electrical properties, protein and polysaccharide-based hydrogels have shown great promise for application in next-generation bioelectronic technologies. These materials enable intimate and stable interfaces with biological tissues, making them ideally suited for both wearable and implantable applications. In wearable bioelectronics, hydrogel-based devices can conform to the skin surface for non-invasive, continuous monitoring of physiological and biochemical signals, supporting personalized health management and early disease detection. In implantable bioelectronics, their biodegradability and biological functionality allow for seamless integration into internal tissues, enabling high-fidelity recording and targeted stimulation of biological activity. Recent advancements have propelled hydrogel-based systems into diverse biomedical applications, including electrophysiological signal monitoring and closed-loop therapeutic interventions, illustrating their transformative role in future bioelectronic technologies [,,,]. Although biomaterial-based hydrogels show promising applications in flexible electronics, they still face several key challenges. For example, their mechanical strength is typically lower than that of synthetic polymer hydrogels, limiting their stable use in high-stress environments, and furthermore, the poor physical stability of such hydrogels under humidity and temperature variations may affect their reliability in long-term wear or in vivo environments.
3.1. Wearable Bioelectronics
Recent advances in flexible and wearable bioelectronic devices have driven extensive research efforts toward their application in personalized medicine, HMI, energy harvesting, and health monitoring [,,,,,,]. Due to their superior flexibility, tunable functionality, and biocompatibility, hydrogels derived from biological sources have garnered significant attention [,,].
However, challenges remain in achieving reliable mechanical adaptation and stable electrochemical performance under dynamic bodily movements and complex physiological conditions [,]. To address these challenges, hydrogels derived from natural or synthetic biomaterials have been molecularly designed and engineered, enabling precise regulation of mechanical properties and electrochemical characteristics [,,]. In addition, strategies such as network crosslinking, incorporation of conductive nanomaterials, and surface functionalization have been employed to substantially enhance the ionic conductivity and mechanical resilience, which are critical for bioelectronic interfaces [,]. Therefore, hydrogel systems offer a promising platform for the development of next-generation bioelectronic devices that operate reliably within complex biological environments. In this paper, we not only systematically sort out the core advances in current hydrogel research, but also systematically summarize the cutting-edge directions, such as bioelectronic fusion. Compared with the existing literature, we especially emphasize the potential of hydrogels in cross-cutting applications such as smart medicine and tissue interfaces.
3.1.1. Electrophysiological Signal Monitoring
Physiological electrical signals, such as electrocardiogram (ECG), electroencephalogram (EEG), and electromyogram (EMG), are essential indicators of human physiological activities [,,,]. Accurate monitoring of these signals is critical for disease diagnosis, health management, and HMI [,]. However, conventional rigid electronic devices encounter substantial obstacles in dynamically interfacing with biological tissues, primarily due to mechanical mismatch, high interface impedance, and poor biocompatibility [,,]. These limitations often result in signal attenuation and failure during long-term monitoring. Therefore, alternative materials that can form stable, tissue-adaptable interfaces are highly desired for reliable physiological signal acquisition [,,,]. In addition, dynamic crosslinking networks and ionic conductivity further enhance their potential for continuous bioelectronic monitoring.
Here, recent innovations in hydrogel-based sensing interfaces are highlighted through representative examples. For example, Zhu et al. [] developed a cellulose nanofiber/polyionic liquid composite hydrogel (PAC2V3), which features an ordered honeycomb structure and dynamic ionic bonding synergy. This design enables high stretchability and low hysteresis, characteristics essential for wearable bioelectronics. The hydrogel demonstrated superior performance in dynamic ECG monitoring, accurately capturing heart rate fluctuations during resting, jogging, and jumping (Figure 10a–c). In addition, a wireless transmission system integrated with PAC2V3 facilitated real-time physiological signal interaction, providing an integrated interface solution for wearable health management applications. In parallel, Han et al. [] introduced dopamine-modified polyvinylalcohol/polyvinylpyrrolidone (P-P-PDA) hydrogel electrodes, which were fabricated via an in situ nanoparticle formation strategy induced by peroxide. The resulting hydrogels exhibit a tissue-like modulus and stable adhesion interfaces, which are essential for long-term dynamic monitoring. As shown in Figure 10d, the multi-channel wireless system incorporating P-P-PDA hydrogel electrodes achieves superior anti-interference performance in EEG monitoring, effectively minimizing artifacts induced by blinks, speech, and head movements. Compared with commercial electrodes, the system exhibits a significantly lower signal fluctuation amplitude (Figure 10e), making it suitable for applications such as consciousness assessment and neurological diagnostics. Furthermore, inspired by the hierarchical structure of myelin, Liu et al. [] designed PEDOT-modified sulfonated cellulose hydrogels, incorporating biomimetic conductive channel architectures. This approach imparts the material with exceptional stretchability, strong skin adhesion, self-healing ability, and low electrochemical impedance (Figure 10f–h). The hydrogel demonstrates stable, high-fidelity signal acquisition in facial EMG monitoring and outperformed commercial electrodes in ECG recording.
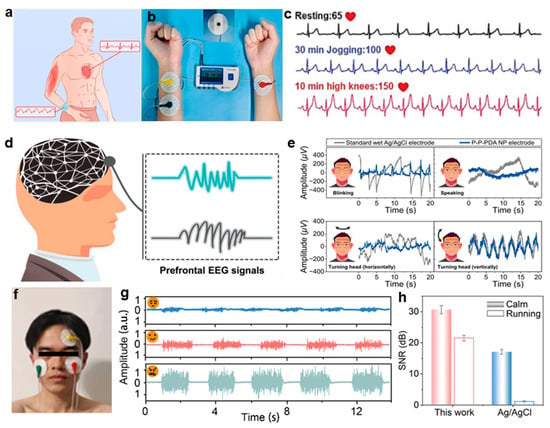
Figure 10.
Applications of biomaterial-based hydrogels in physiological signal monitoring. (a) Schematic of PAC2V3 hydrogel electrodes for ECG monitoring. (b) Diagram of the ECG monitoring mechanism. (c) ECG signals from PAC2V3 electrodes under three physiological states []. (d) Structural layout of the EEG monitoring system employing hydrogel-based components. (e) Comparative analysis of motion artifacts between P-P-PDA nanoparticle electrodes and conventional wet Ag/AgCl electrodes []. (f) Setup for facial EMG recording with hydrogel electrodes. (g) EMG signals for different facial expressions. (h) Comparison of SNR of Ag/AgCl and hydrogel electrodes under different conditions []. All pictures have been adopted with permission.
3.1.2. Biochemical Signal Monitoring
In the field of bioelectronics, the monitoring of biochemical electrical signals serves as a critical bridge between physiological activities in the human body and the functional realization of electronic devices [,,,,]. Hydrogel-based bioelectronic sensors, which can be functionally modified, enable continuous monitoring of target biomarkers such as neurotransmitters, lactate, and glucose [,,]. Hu et al. [] designed a hydrogel-based wearable biosensing platform based on hydrogels for long-term, non-invasive sweat uric acid (UA) monitoring. This platform utilizes a reusable sensing patch combined with a sensor design that mitigates antioxidant accumulation, effectively eliminating environmental interference and ensuring accurate and stable real-time detection (Figure 11a). The UA sensor is constructed on a flexible substrate featuring vertically stacked subcomponents (Figure 11b), minimizing skin contact to the greatest extent. Kanokpaka et al. [] proposed a triboelectric sensor based on self-healing glucose-responsive hydrogels for continuous non-invasive glucose monitoring in sweat. The sensor encapsulates glucose oxidase (GOx) in β-cyclodextrin, embedded in a PVA matrix. The enzymatic conversion of glucose produces gluconic acid and hydrogen peroxide, elevating the local ionic strength, which induces the hydrogel’s swelling and the breaking of mechanochemical bonds, enhancing its conductivity. This conductivity change is converted into detectable signals by a triboelectric nanogenerator system, and as glucose concentration increases, the signal output performance improves (Figure 11c). Qin et al. [] developed a self-healing, stretchable conductive TOCNF/PANI-PVAB hydrogel (CPPH) material based on dynamic crosslinking technology, fabricated using polyaniline with TEMPO-oxidized CNF and a PVA/borax composite. Following encapsulation in polydimethylsiloxane (PDMS), the hydrogel was coupled with ion-selective membranes to fabricate a fully flexible, self-powered sweat sensor capable of selectively detecting Na+, K+, and Ca2+ ions. The sensor system transmits electrical signals from the CPPH hydrogel through a current amplifier to a digital multimeter, which then relays data wirelessly via the smartphone’s Bluetooth module (Figure 11d). To evaluate system functionality, the device was worn by a subject while running, and real-time wireless monitoring of Na+ concentration in sweat was performed (Figure 11e). Figure 11f demonstrates the current signals of Na+, Ca2+, and K+ in sweat 30 min post-exercise, with signal intensity steadily increasing in tandem with the subject’s perspiration.
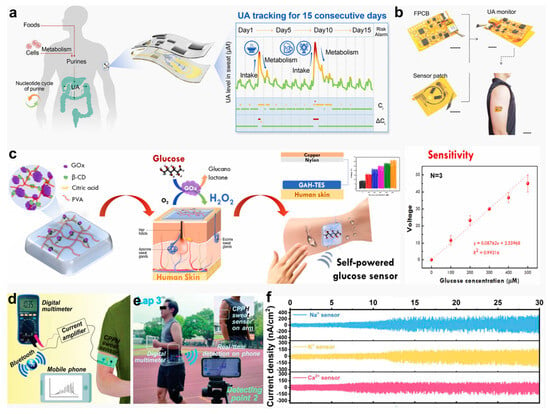
Figure 11.
Hydrogel-based biochemical sensors and their physiological monitoring capabilities. (a) Schematic of a wearable device for UA monitoring. (b) Photographs of the UA sensor patch and its on-skin application []. (c) Diagram of a self-healing, glucose-adaptive hydrogel triboelectric sensor for sweat analysis []. (d) System connectivity of the CPPH sweat sensor with wireless components. (e) Real-time wireless sensing performance during running. (f) Ion detection profiles (Na+, K+, Ca2+) over 30 min of exercise []. All pictures have been adopted with permission.
In addition to this, the application of hydrogels in wound healing sensors and diabetes detection systems has received much attention, demonstrating their great potential in flexible bioelectronics. Such sensors not only help to dynamically assess the state of wound healing, but also enable personalized interventions and adjustment of treatment strategies. In the field of diabetes management, hydrogels have been used to develop non-invasive or minimally invasive glucose detection platforms that enable continuous monitoring of glucose concentration in body fluids through integration with enzymes or photosensitive materials. Such systems are highly flexible and wearable, adapting to be attached to the skin surface for extended periods of time, providing diabetic patients with a more convenient and non-invasive means of blood glucose management.
3.2. Implantable Bioelectronics
Implantable bioelectronic devices are required to achieve long-term compatibility with biological tissues and stable transmission of electrophysiological signals [,]. This requirement imposes stringent demands on the materials used, particularly in terms of biocompatibility, interfacial matching, and controllable degradation behavior [,]. However, conventional electronic materials often suffer from mechanical mismatch and poor degradability, leading to adverse tissue responses and limited device lifespan [,]. To address these challenges, biopolymer-based hydrogels have been developed as promising materials for next-generation implantable devices. These materials mimic the extracellular matrix, offering tissue-like softness, tunable degradation profiles, and low immunogenicity, all of which are essential for seamless tissue integration. Through chemical modification and network design, parameters such as ionic conductivity, Young’s modulus, and degradation kinetics can be precisely controlled, enabling the fabrication of hydrogel systems tailored for specific biomedical applications [,].
Moreover, to achieve stable and flexible coupling between hydrogel systems and conventional electronic components, several interfacial engineering strategies have been developed. These include the incorporation of adhesion-promoting interlayers such as polydopamine and polyimide to strengthen bonding between hydrogels and inorganic surfaces, as well as the construction of dual-network architectures and ionic-conducting hybrid systems to reconcile mechanical compliance with electrical performance. For material applications, flexible conducting polymers such as PEDOT: PSS, liquid metal particle complexes, and surface-functionalized carbon nanomaterials have shown excellent interfacial adaptation and electrical coupling capabilities in several studies. Therefore, these hydrogels offer a substantial advancement over conventional materials in achieving superior biointegration and functional performance.
3.2.1. Bioelectric Recording
Bioelectrical signal recording is a fundamental modality for bidirectional communication between biological systems and electronic devices, typically achieved through invasive or non-invasive electrode interfaces [,,]. Among these, epidermal electrodes have emerged as the most widely utilized in clinical practice due to their non-invasive nature [,,]. For example, large-area epidermal electrodes are routinely employed in EEG, which represents a mainstream method for monitoring cerebral activity. In addition, the acquisition of cardiac and muscular signals, such as ECG and EMG, respectively, also relies on epidermal electrodes, which have become standard tools in clinical diagnostics [,]. However, conventional epidermal electrodes are often limited by insufficient mechanical compliance and poor long-term stability at the bio-interface, particularly under dynamic physiological conditions.
To address these challenges, Lee et al. [] proposed an innovative shape-adaptive cortical adhesive (SMCA) sensor for closed-loop ultrasonic neuromodulation in drug-resistant epilepsy. This system integrates a catechol-conjugated alginate (Alg-CA) hydrogel adhesive, a flexible 16-channel electrode array, and a self-healing polymer substrate, thereby enabling conformal adhesion to curved cortical surfaces and reliable neural signal acquisition in awake epileptic animal models during ultrasonic stimulation (Figure 12a). The SMCA sensor conforms intimately to irregular cortical geometries, forming topographic features of varying depth and demonstrating substantial potential as a brain–device interface (Figure 12b). In addition, Choi et al. [] advanced this field by developing a strain-adaptive fiber-interlocked electronic (SAFIE) patch tailored for implantation applications. Central to this innovation is the synergistic design of a three-layered architecture (Figure 12c). A bottom layer composed of an Alg-CA ionic hydrogel provides tissue adhesion; a middle layer consisting of a gallium-indium LM/polymer nanocomposite ensures electrical conductivity, mechanical durability, and self-healing capacity; and a top layer fabricated via electrospinning forms a PDMS fiber-interlocked network, granting dynamic mechanical adaptability. Guided by an interfacial coupling strategy based on self-assembly, this patch conforms intimately to the cardiac surface, maintaining a stable electrical interface throughout prolonged operation (Figure 12d). Implantation studies revealed that the SAFIE patch could robustly capture ventricular depolarization delays over four weeks, with negligible deterioration in SNR (Figure 12e). Therefore, this decoupled yet integrated design of adhesion, conduction, and deformation offers a scalable architecture for organ-conformal bioelectronic systems. Additionally, Tringides et al. [] further exemplifies the potential of hydrogel-based bioelectronics through the engineering of a flexible surface microelectrode array. In this design, traditional rigid encapsulation and metallic conductors are substituted with viscoelastic materials to achieve superior mechanical compliance with biological tissues such as the heart and cerebral cortex. The innovation lies in the use of carbon nanomaterial-enhanced Alg-CA hydrogels as ionically conductive paths, encapsulated within insulating hydrogel layers. Through precision fabrication processes, the resulting electrode arrays conform seamlessly to intricate organ topographies (Figure 12f) and remain fully compatible with conventional electrophysiological setups (Figure 12g). This approach presents a new paradigm in implantable electronics for both signal acquisition and stimulation.
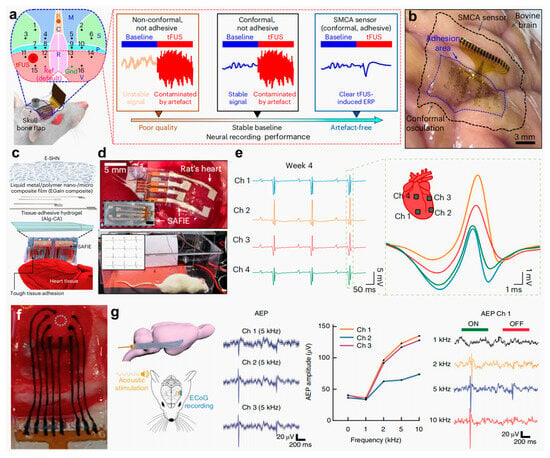
Figure 12.
Biomaterial-based hydrogel electrodes for implantable electrophysiological recording. (a) Schematic of an in vivo material performance testing setup. (b) Images showing ultraconformal adhesion of the SMCA sensor on an ex vivo bovine cortex []. (c) Diagram of a SAFIE system on a rat heart. (d) Photographs of SAFIE deployed on cardiac tissue. (e) Real-time ECG recordings from four channels showing ventricular activity 4 weeks after implantation []. (f) Photograph of a viscoelastic array placed on rat cortical dura. (g) Schematic and data showing flexible array deployment and auditory cortex responses to sound stimulation []. All pictures have been adopted with permission.
3.2.2. Bioelectrical Stimulation
Bioelectrical stimulation is widely employed as a versatile tool in both neuroscience research and clinical therapy [,]. Depending on the degree of invasiveness associated with tissue–electrode interactions, electrical stimulation modalities in biological systems are broadly categorized into invasive and non-invasive approaches. While implantable electrodes establish direct contact with target tissues, they typically entail higher levels of invasiveness. In contrast, epidermal bioelectronic stimulation techniques, such as transcutaneous electrical nerve stimulation, have been widely adopted for clinical pain management [,]. Despite the diversity in electrode configurations, the underlying physical principles governing electrical stimulation exhibit significant commonality across different modalities. However, conventional electrodes suffer from inherent limitations related to mechanical mismatch, limited biocompatibility, and unstable tissue–electrode interfaces.
To address these limitations, Jin et al. [] designed an injectable conductive hydrogel (IT-IC hydrogel) utilizing a PB-mediated multi-crosslinking strategy. The hydrogel network was structurally reinforced through the synergistic combination of irreversible biphenyl crosslinks, gold nanoparticle (AuNP)-mediated coordination bonds, and multiple weak interactions, including hydrogen bonding and metal–π interactions. This composite conductive network substantially enhanced electrical signal transduction, facilitating not only intertissue conductivity but also seamless interfacing between tissues and bioelectronic devices. In addition, the hydrogel was integrated into a closed-loop robotic rehabilitation system to achieve precise neuromodulation and real-time motion feedback. As shown in Figure 13a–c, neural stimulation across a range of frequencies and amplitudes successfully elicited hindlimb muscle contractions without causing detectable tissue damage. Deng et al. [] presented an innovative electrically conductive bioadhesive interface that enables rapid, strong, and reversible adhesion to wet and dynamically moving tissues while simultaneously maintaining electrical conductivity to facilitate bidirectional bioelectronic communication. The e-bioadhesive was evaluated in in vivo rat models, demonstrating excellent biocompatibility, as well as mechanical and electrical stability over time (Figure 13d–f). Notably, in vivo sciatic nerve stimulation using flexible electrodes combined with the bioadhesive interface produced consistent ankle joint motion, highlighting the interface’s potential to enhance tissue–device integration and improve the overall performance of implantable bioelectronic systems (Figure 13g). Furthermore, Jiao et al. [] fabricated an LM–hydrogel composite that leverages the superior wettability and alloying properties of LMs, enabling seamless integration with conventional electronic interfaces while maintaining mechanical compliance (~10 kPa) consistent with soft tissues. This hybrid material supports the direct formation of functional electronic components without requiring additional encapsulation, thereby significantly advancing the application potential of hydrogels in bioelectronics. A schematic of the stimulation setup for the rat tibialis anterior (TA) muscle is shown in Figure 13h, and the corresponding muscle contraction forces under various stimulation intensities are presented in Figure 13i. The threshold voltage required to elicit TA muscle contraction using LM–hydrogel hybrid electrodes was as low as 1.25 V, compared with pure GSP hydrogel electrodes, which failed to generate measurable force even at 3 V. Figure 13j further details the muscle responses across different stimulation frequencies.
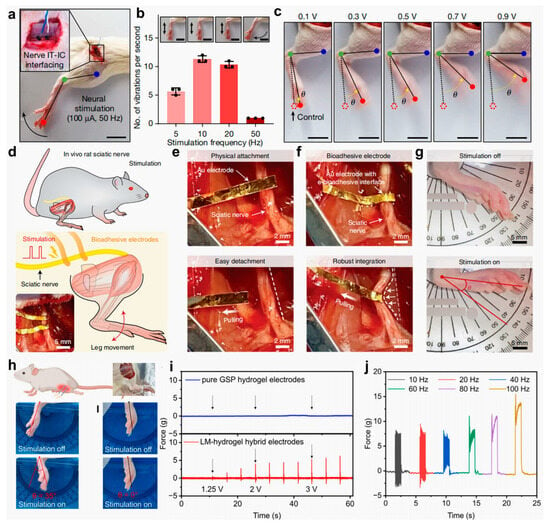
Figure 13.
Schematic illustration and functional characterization of implantable electrical stimulation systems utilizing biomaterial hydrogel electrodes. (a) Photograph of neural stimulation using IT-IC hydrogel interfaces. (b) Correlation between leg vibration and stimulation frequency with motion snapshots. (c) Photographs showing ankle angle changes under different voltages []. (d) Schematic illustrations and an image of the sciatic nerve stimulation setup. (e) Physical attachment of a stimulation electrode on a sciatic nerve without the e-bioadhesive interface. (f) Robust integration of a stimulation electrode on a sciatic nerve with the e-bioadhesive interface. (g) Images of the ankle joint movement in response to electrical stimulation via the e-bioadhesive interface []. (h) Schematic of TA muscle stimulation using LM–hydrogel hybrid electrodes in rats. (i) Comparative images of ankle joint kinematics during TA muscle stimulation, employing LM–hydrogel electrodes versus pure GSP hydrogel controls. (j) Force output measurements under different TA muscle stimulation conditions []. All pictures have been adopted with permission.
4. Conclusions and Future Perspectives
The integration of hydrogels derived from natural biomaterials into bioelectronics is regarded as a promising strategy for advancing next-generation healthcare technologies. In particular, polysaccharide and protein-based hydrogels are considered highly suitable for bioelectronic applications due to their inherent structural tunability and intrinsic biocompatibility. Through functionalization strategies such as conductive polymer blending, nanostructure engineering, and dynamic bond design, these hydrogels have been able to achieve critical performance metrics, including tissue-like mechanical compliance, efficient ionic and electronic conductivity, and strong interfacial adhesion. These features collectively allow seamless interfacing with biological tissues, which is essential for continuous health monitoring, precision diagnostics, and HMI. In wearable electronics and implantable sensors, such hydrogels have been demonstrated to bridge the gap between rigid electronics and soft tissues, thereby mitigating long-standing challenges related to mechanical mismatch and biological contamination.
However, key challenges persist in enhancing conductivity without compromising biocompatibility, ensuring long-term stability under physiological conditions, and achieving seamless integration with electronic components. Firstly, the reliance on conductive additives such as carbon nanotubes and conductive polymers to enhance conductivity has been found to compromise the mechanical integrity and biocompatibility of natural hydrogels. Therefore, future research should prioritize the exploitation of the intrinsic functionalities of proteins and polysaccharides to construct fully biomaterial-derived hydrogels with preserved conductivity and minimally impaired mechanical and biological performance. Secondly, although current hydrogel systems show promise in short-term applications, their long-term operational stability under dynamic physiological conditions, including enzymatic degradation and mechanical fatigue, remains insufficient for chronic implantation scenarios. In addition, challenges persist in the interfacial integration between hydrogels and electronic components, particularly in balancing electrical conductivity and biocompatibility, as well as ensuring stable signal transmission. Overall, the deep integration of natural biomaterial-based hydrogels with bioelectronic systems offers a unique pathway for the development of next-generation biocompatible medical devices. Owing to their structural tunability and inherent biocompatibility, polysaccharide- and protein-derived hydrogels have already demonstrated potential in flexible sensing platforms and soft-tissue interfaces. From a broader perspective, such materials are anticipated to reshape the landscape of medical electronics by converging with flexible machines and electronics, thereby driving forward the realization of personalized medicine and smart bioelectronic technologies.
Author Contributions
Investigation, B.C., Y.Z. and R.Y.; writing—original draft preparation, B.C. and Y.Z.; writing—review and editing, L.L., Y.M., P.Z., C.L., Y.Z., R.Y., Y.F. and Z.H.; supervision, L.L. and Y.M.; project administration, L.L. and Y.M.; funding acquisition, L.L. and Y.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Science and Technology Research and Development Program Joint Fund of Henan (232301420033) and the Scientific and Technological Project in Henan Province (242102231014, 242102231002).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Koo, J.; MacEwan, M.R.; Kang, S.K.; Won, S.M.; Stephen, M.; Gamble, P.; Xie, Z.; Yan, Y.; Chen, Y.Y.; Shin, J.; et al. Wireless bioresorbable electronic system enables sustained nonpharmacological neuroregenerative therapy. Nat. Med. 2018, 24, 1830–1836. [Google Scholar] [CrossRef]
- Tringides, C.M.; Mooney, D.J. Materials for Implantable Surface Electrode Arrays: Current Status and Future Directions. Adv. Mater. 2022, 34, e2107207. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.U.; Shen, Z.; Mugo, S.M.; Wang, H.; Zhang, Q. Implantable hydrogels as pioneering materials for next-generation brain-computer interfaces. Chem. Soc. Rev. 2025, 54, 2832–2880. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.U.; Kim, B.H.; Lee, J.Y.; Lee, J.; Xie, Z.; Ibler, E.M.; Lee, K.; Banks, A.; Jeong, J.Y.; Kim, J.; et al. Binodal, wireless epidermal electronic systems with in-sensor analytics for neonatal intensive care. Science 2019, 363, eaau0780. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, G.; Wang, S.; Zhang, Y.; Jian, Y.; He, L.; Yu, T.; Luo, H.; Kong, D.; Xianyu, Y.; et al. Stretchable graphene–hydrogel interfaces for wearable and implantable bioelectronics. Nat. Electron. 2023, 7, 51. [Google Scholar] [CrossRef]
- Duan, H.; Peng, S.; He, S.; Tang, S.Y.; Goda, K.; Wang, C.H.; Li, M. Wearable Electrochemical Biosensors for Advanced Healthcare Monitoring. Adv. Sci. 2025, 12, e2411433. [Google Scholar] [CrossRef]
- Li, J.; Carlos, C.; Zhou, H.; Sui, J.; Wang, Y.; Silva-Pedraza, Z.; Yang, F.; Dong, Y.; Zhang, Z.; Hacker, T.A.; et al. Stretchable piezoelectric biocrystal thin films. Nat. Commun. 2023, 14, 6562. [Google Scholar] [CrossRef]
- Jiao, F.; Lin, C.; Dong, L.; Mao, X.; Wu, Y.; Dong, F.; Zhang, Z.; Sun, J.; Li, S.; Yang, X.; et al. Silicon Vacancies Diamond/Silk/PVA Hierarchical Physical Unclonable Functions for Multi-Level Encryption. Adv. Sci. 2024, 11, e2308337. [Google Scholar] [CrossRef]
- Hou, Y.; Li, Z.; Wang, Z.; Zhang, X.; Li, Y.; Li, C.; Guo, H.; Yu, H. Programmable and Surface-Conformable Origami Design for Thermoelectric Devices. Adv. Sci. 2024, 11, e2309052. [Google Scholar] [CrossRef]
- Li, Y.; Li, N.; Liu, W.; Prominski, A.; Kang, S.; Dai, Y.; Liu, Y.; Hu, H.; Wai, S.; Dai, S.; et al. Achieving tissue-level softness on stretchable electronics through a generalizable soft interlayer design. Nat. Commun. 2023, 14, 4488. [Google Scholar] [CrossRef]
- Yi, J.; Zou, G.; Huang, J.; Ren, X.; Tian, Q.; Yu, Q.; Wang, P.; Yuan, Y.; Tang, W.; Wang, C.; et al. Water-responsive supercontractile polymer films for bioelectronic interfaces. Nature 2023, 624, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Shi, M.; He, S.; Yao, M.; Sun, H.; Yue, Z.; Qiu, Y.; Liu, B.; Liang, L.; Zhao, Z.; et al. Chronological adhesive cardiac patch for synchronous mechanophysiological monitoring and electrocoupling therapy. Nat. Commun. 2023, 14, 6226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tao, T.H. Skin-Friendly Electronics for Acquiring Human Physiological Signatures. Adv. Mater. 2019, 31, 1905767. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, T.; Zhang, Y.; Qu, G.; Wei, S.; Liu, Z.; Kong, T. Ultrastretchable and Wireless Bioelectronics Based on All-Hydrogel Microfluidics. Adv. Mater. 2019, 31, 1902783. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kum, J.; Kim, S.; Jung, H.; An, S.; Choi, S.J.; Choi, J.H.; Kim, J.; Yu, K.J.; Lee, W.; et al. A shape-morphing cortex-adhesive sensor for closed-loop transcranial ultrasound neurostimulation. Nat. Electron. 2024, 7, 800–814. [Google Scholar] [CrossRef]
- Chowdhury, I.F.; Shawon, M.T.A.; Alam, M.A.; Fatima, S.; Khan, A.A.; Yang, J.; Tang, Z.; Mondal, A.K. Ni2+-Rich Collagen/Lignin Composite Hydrogel: Transforming Industrial Waste Materials into Flexible Electronics. ACS Appl. Polym. Mater. 2024, 6, 15094–15104. [Google Scholar] [CrossRef]
- Cho, K.W.; Sunwoo, S.H.; Hong, Y.J.; Koo, J.H.; Kim, J.H.; Baik, S.; Hyeon, T.; Kim, D.H. Soft Bioelectronics Based on Nanomaterials. Chem. Rev. 2022, 122, 5068–5143. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Yadav, S.; Truong, T.A.; Han, M.; Barton, M.; Leitch, M.; Guzman, P.; Dinh, T.; Ashok, A.; Vu, H.; et al. Integrated, Transparent Silicon Carbide Electronics and Sensors for Radio Frequency Biomedical Therapy. ACS Nano 2022, 16, 10890–10903. [Google Scholar] [CrossRef]
- Fan, H.; Gong, J.P. Bioinspired Underwater Adhesives. Adv. Mater. 2021, 33, e2102983. [Google Scholar] [CrossRef]
- Yuk, H.; Wu, J.; Zhao, X. Hydrogel interfaces for merging humans and machines. Nat. Rev. Mater. 2022, 7, 935–952. [Google Scholar] [CrossRef]
- Chen, C.-K.; Chen, P.-W.; Wang, H.-J.; Yeh, M.-Y. Alkyl Chain Length Effects of Imidazolium Ionic Liquids on Electrical and Mechanical Performances of Polyacrylamide/Alginate-Based Hydrogels. Gels 2021, 7, 164. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Liu, H.; He, R.; Luo, M.; Shu, M.; Xu, F. Environmentally Compatible Wearable Electronics Based on Ionically Conductive Organohydrogels for Health Monitoring with Thermal Compatibility, Anti-Dehydration, and Underwater Adhesion. Small 2021, 17, 2101151. [Google Scholar] [CrossRef]
- Ling, Q.; Fan, X.; Ling, M.; Liu, J.; Zhao, L.; Gu, H. Collagen-Based Organohydrogel Strain Sensor with Self-Healing and Adhesive Properties for Detecting Human Motion. ACS Appl. Mater. Interfaces 2023, 15, 12350–12362. [Google Scholar] [CrossRef]
- Li, S.; Liu, G.; Wen, H.; Liu, G.; Wang, H.; Ye, M.; Yang, Y.; Guo, W.; Liu, Y. A Skin-Like Pressure- and Vibration-Sensitive Tactile Sensor Based on Polyacrylamide/Silk Fibroin Elastomer. Adv. Funct. Mater. 2022, 32, 2111747. [Google Scholar] [CrossRef]
- Li, J.; Sun, Y.; Shi, W.; Li, Y.; Zou, Y.; Zhang, H. Fabrication, characterization, and in vitro digestion of gelatin/gluten oleogels from thermally crosslinked electrospun short fiber aerogel templates. Food. Chem. 2024, 454, 139804. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Park, J.; Choe, G.; Lee, S.; Kang, B.G.; Jun, J.H.; Shin, Y.; Kim, M.C.; Kim, Y.S.; Ahn, Y.; et al. A Conductive and Adhesive Hydrogel Composed of MXene Nanoflakes as a Paintable Cardiac Patch for Infarcted Heart Repair. ACS Nano 2023, 17, 12290–12304. [Google Scholar] [CrossRef]
- Lan, M.; Zhang, J.; Zhou, J.; Gu, H. CQDs-Cross-Linked Conductive Collagen/PAA-Based Nanocomposite Organohydrogel Coupling Flexibility with Multifunctionality for Dual-Modal Sensing of Human Motions. ACS Appl. Mater. Interfaces 2024, 16, 23838–23854. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Shao, C.; Cui, C.; Xu, F.; Lei, J.; Yang, J. Autonomous Self-Healing Silk Fibroin Injectable Hydrogels Formed via Surfactant-Free Hydrophobic Association. ACS Appl. Mater. Interfaces 2019, 12, 1628–1639. [Google Scholar] [CrossRef]
- Ohm, Y.; Pan, C.; Ford, M.J.; Huang, X.; Liao, J.; Majidi, C. An electrically conductive silver–polyacrylamide–alginate hydrogel composite for soft electronics. Nat. Electron. 2021, 4, 185–192. [Google Scholar] [CrossRef]
- Wang, C.; Xia, K.; Zhang, M.; Jian, M.; Zhang, Y. An All-Silk-Derived Dual-Mode E-skin for Simultaneous Temperature–Pressure Detection. ACS Appl. Mater. Interfaces 2017, 9, 39484–39492. [Google Scholar] [CrossRef]
- Zhu, M.; Gong, D.; Ji, Z.; Yang, J.; Wang, M.; Wang, Z.; Tao, S.; Wang, X.; Xu, M. Cellulose-reinforced poly(Ionic Liquids) composite hydrogel for infected wounds therapy and real-time reliable bioelectronic. Chem. Eng. J. 2023, 476, 146816. [Google Scholar] [CrossRef]
- Han, Q.; Zhang, C.; Guo, T.; Tian, Y.; Song, W.; Lei, J.; Li, Q.; Wang, A.; Zhang, M.; Bai, S.; et al. Hydrogel Nanoarchitectonics of a Flexible and Self-Adhesive Electrode for Long-Term Wireless Electroencephalogram Recording and High-Accuracy Sustained Attention Evaluation. Adv. Mater. 2023, 35, 2209606. [Google Scholar] [CrossRef]
- Jin, S.; Choi, H.; Seong, D.; You, C.-L.; Kang, J.-S.; Rho, S.; Lee, W.B.; Son, D.; Shin, M. Injectable tissue prosthesis for instantaneous closed-loop rehabilitation. Nature 2023, 623, 58–65. [Google Scholar] [CrossRef]
- Jiao, C.; Wang, Q.; Li, L.; Chen, W.; Liu, J.; Xu, Y.; Song, L.; Fu, S.; Hu, L. In situ 3D printing of liquid metal-hydrogel hybrid for multifunctional soft bioelectronics and devices. Cell Rep. Phys. Sci. 2023, 4, 101640. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, Y.; Chu, H.; Shi, R.; Li, J.; Xu, G.; Huang, X.; Zhang, B.; Yiu, C.K.; Zhao, G.; et al. Prolonged monitoring and risk management of hyperuricemia using interference-resistant wearable bioelectronics. Device 2025, 3, 100753. [Google Scholar] [CrossRef]
- Kanokpaka, P.; Chang, Y.-H.; Chang, C.-C.; Rinawati, M.; Wang, P.-C.; Chang, L.-Y.; Yeh, M.-H. Enabling glucose adaptive self-healing hydrogel based triboelectric biosensor for tracking a human perspiration. Nano Energy 2023, 112, 108513. [Google Scholar] [CrossRef]
- Choi, H.; Kim, Y.; Kim, S.; Jung, H.; Lee, S.; Kim, K.; Han, H.-S.; Kim, J.Y.; Shin, M.; Son, D. Adhesive bioelectronics for sutureless epicardial interfacing. Nat. Electron. 2023, 6, 779–789. [Google Scholar] [CrossRef]
- Zhang, W.; Lou, Q.; Sun, J. Carbon nanodot-based flexible and self-powered white displays. Nano Res. 2025, 18, 94907117. [Google Scholar] [CrossRef]
- Qureshi, A.T.; Afrin, S.; Asim, S.; Rizwan, M. Imine Crosslinked, Injectable, and Self-Healing Fucoidan Hydrogel with Immunomodulatory Properties. Adv. Healthc. Mater. 2025, 14, e2405260. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, S.; Cao, L.; Lv, Z.; Ren, J.; Shao, Z.; Yao, Y.; Ling, S. Natural Silk Spinning-Inspired Meso-Assembly-Processing Engineering Strategy for Fabricating Soft Tissue-Mimicking Biomaterials. Adv. Funct. Mater. 2022, 32, 2200267. [Google Scholar] [CrossRef]
- Bi, S.; Wang, P.; Hu, S.; Li, S.; Pang, J.; Zhou, Z.; Sun, G.; Huang, L.; Cheng, X.; Xing, S.; et al. Construction of physical-crosslink chitosan/PVA double-network hydrogel with surface mineralization for bone repair. Carbohyd. Polym. 2019, 224, 115176. [Google Scholar] [CrossRef] [PubMed]
- Go, Y.; Park, H.Y.; Zhu, Y.; Yoo, K.; Kwak, J.; Jin, S.H.; Yoon, J. Optically Transparent and Mechanically Robust Ionic Hydrogel Electrodes for Bright Electroluminescent Devices Achieving High Stretchability Over 1400%. Adv. Funct. Mater. 2023, 33, 2215193. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Zhao, X.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, H.; Huang, Y.; Wei, Y.; Chen, J. Naturally sourced hydrogels: Emerging fundamental materials for next-generation healthcare sensing. Chem. Soc. Rev. 2023, 52, 2992–3034. [Google Scholar] [CrossRef]
- Feig, V.R.; Tran, H.; Lee, M.; Bao, Z. Mechanically tunable conductive interpenetrating network hydrogels that mimic the elastic moduli of biological tissue. Nat. Commun. 2018, 9, 2740. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, X.; Chi, X.; Feng, Z.; Yang, C.; Gao, R.; Li, S.; Zhang, C.; Chen, X.; Huang, P.; et al. Biomimetic integration of tough polymer elastomer with conductive hydrogel for highly stretchable, flexible electronic. Nano Energy 2022, 92, 106735. [Google Scholar] [CrossRef]
- Fu, Q.; Tang, J.; Wang, W.; Wang, R. Biocomposite Polyvinyl Alcohol/Ferritin Hydrogels with Enhanced Stretchability and Conductivity for Flexible Strain Sensors. Gels 2025, 11, 59. [Google Scholar] [CrossRef]
- Li, Y.; Tan, S.; Zhang, X.; Li, Z.; Cai, J.; Liu, Y. Design Strategies and Emerging Applications of Conductive Hydrogels in Wearable Sensing. Gels 2025, 11, 258. [Google Scholar] [CrossRef]
- Wang, R.; Jin, B.; Li, J.; Li, J.; Xie, J.; Zhang, P.; Fu, Z. Bio-Inspired Synthesis of Injectable, Self-Healing PAA-Zn-Silk Fibroin-MXene Hydrogel for Multifunctional Wearable Capacitive Strain Sensor. Gels 2025, 11, 377. [Google Scholar] [CrossRef]
- Tringides, C.M.; Vachicouras, N.; de Lázaro, I.; Wang, H.; Trouillet, A.; Seo, B.R.; Elosegui-Artola, A.; Fallegger, F.; Shin, Y.; Casiraghi, C.; et al. Viscoelastic surface electrode arrays to interface with viscoelastic tissues. Nat. Nanotechnol. 2021, 16, 1019–1029. [Google Scholar] [CrossRef]
- Wang, Q.; Ling, S.; Liang, X.; Wang, H.; Lu, H.; Zhang, Y. Self-Healable Multifunctional Electronic Tattoos Based on Silk and Graphene. Adv. Funct. Mater. 2019, 29, 1808695. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, S.; Qian, L.; Wei, N.; Nica, V.; Coseri, S.; Han, F. Super Stretchable, Self-Healing, Adhesive Ionic Conductive Hydrogels Based on Tailor-Made Ionic Liquid for High-Performance Strain Sensors. Adv. Funct. Mater. 2022, 32, 2204565. [Google Scholar] [CrossRef]
- Park, B.; Shin, J.H.; Ok, J.; Park, S.; Jung, W.; Jeong, C.; Choy, S.; Jo, Y.J.; Kim, T.-i. Cuticular pad–inspired selective frequency damper for nearly dynamic noise–free bioelectronics. Science 2022, 376, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Ji, Y.-E.; Wang, T.; Fu, Y.; Li, X.; Li, G.; Zheng, T.; Wu, L.; Han, Q.; et al. Silk-protein-based gradient hydrogels with multimode reprogrammable shape changes for biointegrated devices. Proc. Natl. Acad. Sci. USA 2023, 120, e2305704120. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Z.; Zhou, T.; Yu, N.; Yu, R.; Du, X.; Bai, X.; Miao, Z.; Wu, L.; Pan, S.; et al. An On-Skin-Formed Silk Protein Bioelectrode for Conformable and Robust Electrophysiological Interface. Adv. Funct. Mater. 2024, 34, 2402608. [Google Scholar] [CrossRef]
- Sang, F.; Liu, C.; Yan, J.; Su, J.; Niu, S.; Wang, S.; Zhao, Y.; Dang, Q. Polysaccharide- and protein-based hydrogel dressings that enhance wound healing: A review. Int. J. Biol. Macromol. 2024, 280, 135482. [Google Scholar] [CrossRef]
- Song, X.; Wang, X.; Zhang, J.; Shen, S.; Yin, W.; Ye, G.; Wang, L.; Hou, H.; Qiu, X. A tunable self-healing ionic hydrogel with microscopic homogeneous conductivity as a cardiac patch for myocardial infarction repair. Biomaterials 2021, 273, 120811. [Google Scholar] [CrossRef]
- Qie, H.; Wang, Z.; Ren, J.; Lü, S.; Liu, M. A tough shape memory hydrogel strain sensor based on gelatin grafted polypyrrole. Polymer 2022, 263, 125524. [Google Scholar] [CrossRef]
- Wang, J.; Gao, C.; Hou, P.; Liu, Y.; Zhao, J.; Huo, P. All-bio-based, adhesive and low-temperature resistant hydrogel electrolytes for flexible supercapacitors. Chem. Eng. J. 2023, 455, 140952. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, T.H.; Park, S.; Lee, J.; Chae, U.; Jeong, J.-Y.; Park, S.; Kim, S.; Cho, I.-J.; Jung, Y.; et al. Hybrid neural interfacing devices based on Au wires with nanogranular Au shell and hydrogel layer for anti-inflammatory and bi-directional neural communications. Chem. Eng. J. 2023, 465, 142966. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, J.; Wang, H.; Wang, C.; Gu, Y.; Xu, Y.; Lee, S.; Yokota, T.; Haick, H.; Someya, T.; et al. A 10-micrometer-thick nanomesh-reinforced gas-permeable hydrogel skin sensor for long-term electrophysiological monitoring. Sci. Adv. 2024, 10, eadj5389. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Yuan, W. Adhesive, Stretchable, and Transparent Organohydrogels for Antifreezing, Antidrying, and Sensitive Ionic Skins. ACS Appl. Mater. Interfaces 2021, 13, 1474–1485. [Google Scholar] [CrossRef]
- Yu, D.; Yi, J.; Zhu, S.; Tang, Y.; Huang, Y.; Lin, D.; Lin, Y. Hofmeister Effect-Assisted Facile One-Pot Fabrication of Double Network Organohydrogels with Exceptional Multi-Functions. Adv. Funct. Mater. 2024, 34, 2307566. [Google Scholar] [CrossRef]
- Li, T.; Qi, H.; Zhao, C.; Li, Z.; Zhou, W.; Li, G.; Zhuo, H.; Zhai, W. Robust skin-integrated conductive biogel for high-fidelity detection under mechanical stress. Nat. Commun. 2025, 16, 88. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, H.; Lee, J.-H.; Kim, E.; Chan, K.-Y.; Venkatesan, H.; Shen, X.; Yang, J.; Kim, J.-K. Mechanochromic Optical/Electrical Skin for Ultrasensitive Dual-Signal Sensing. ACS Nano 2023, 17, 5921–5934. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xie, R.; Zhu, J.; Wu, J.; Hui, J.; Zheng, X.; Huo, F.; Fan, D. A temperature responsive adhesive hydrogel for fabrication of flexible electronic sensors. npj Flex. Electron. 2022, 6, 68. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, D.; Macedo, M.H.; Cui, W.; Sarmento, B.; Shen, G. Advanced Collagen-Based Biomaterials for Regenerative Biomedicine. Adv. Funct. Mater. 2018, 29, 1804943. [Google Scholar] [CrossRef]
- Shi, X.; Lan, M.; Liu, J.; Zhou, J.; Gu, H. Highly robust, self-adhesive, self-healing, pH-responsive, cytocompatible and degradable collagen/PVA/tannin-based conductive hydrogel sensor for motion-monitoring. Polymer 2024, 308, 127365. [Google Scholar] [CrossRef]
- Song, X.; Zhang, J.; Shen, S.; Liu, D.; Zhang, J.; Yin, W.; Ye, G.; Wang, L.; Cai, L.; Hou, H.; et al. Cardiac-Adaptive Conductive Hydrogel Patch Enabling Construction of Mechanical–Electrical Anisotropic Microenvironment for Heart Repair. Research 2023, 6, 0161. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Q.; Liang, K.; Gao, L.; Lu, P.; Ding, C.; Dang, Y. Collagen/poly(acrylic acid)/MXene hydrogels with tissue-adhesive, biosensing, and photothermal antibacterial properties. Polym. Eng. Sci. 2023, 63, 3672–3683. [Google Scholar] [CrossRef]
- Roshanbinfar, K.; Schiffer, M.; Carls, E.; Angeloni, M.; Koleśnik-Gray, M.; Schruefer, S.; Schubert, D.W.; Ferrazzi, F.; Krstić, V.; Fleischmann, B.K.; et al. Electrically Conductive Collagen-PEDOT:PSS Hydrogel Prevents Post-Infarct Cardiac Arrhythmia and Supports hiPSC-Cardiomyocyte Function. Adv. Mater. 2024, 36, 2403642. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhou, Z.; Xie, Y.; Cai, W.; Zhu, X.; Jia, Y.; Zhang, Z.; Xu, F.; Huang, G. Engineering strong and tough collagen hydrogels and tissue constructs via twisting and crosslinking. Cell Rep. Phys. Sci. 2025, 6, 102454. [Google Scholar] [CrossRef]
- Mao, Z.; Bi, X.; Yu, C.; Chen, L.; Shen, J.; Huang, Y.; Wu, Z.; Qi, H.; Guan, J.; Shu, X.; et al. Mechanically robust and personalized silk fibroin-magnesium composite scaffolds with water-responsive shape-memory for irregular bone regeneration. Nat. Commun. 2024, 15, 4160. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Feng, Y.; Xue, L.; Cui, M.; Zhang, Q.; Xu, F.; Peng, N.; Jiang, Z.; Gao, D.; Zhang, X. Anisotropic conductive reduced graphene oxide/silk matrices promote post-infarction myocardial function by restoring electrical integrity. Acta Biomater. 2022, 139, 190–203. [Google Scholar] [CrossRef]
- Mirbakht, S.S.; Golparvar, A.; Umar, M.; Kuzubasoglu, B.A.; Irani, F.S.; Yapici, M.K. Highly Self-Adhesive and Biodegradable Silk Bioelectronics for All-In-One Imperceptible Long-Term Electrophysiological Biosignals Monitoring. Adv. Sci. 2025, 12, 2405988. [Google Scholar] [CrossRef]
- Chen, B.; Cao, Y.; Li, Q.; Yan, Z.; Liu, R.; Zhao, Y.; Zhang, X.; Wu, M.; Qin, Y.; Sun, C.; et al. Liquid metal-tailored gluten network for protein-based e-skin. Nat. Commun. 2022, 13, 1206. [Google Scholar] [CrossRef]
- Jaroenthai, N.; Srikhao, N.; Kasemsiri, P.; Okhawilai, M.; Theerakulpisut, S.; Uyama, H.; Chindaprasirt, P. Optimization of rapid self-healing and self-adhesive gluten/guar gum crosslinked gel for strain sensors and electronic devices. Int. J. Biol. Macromol. 2023, 253, 127401. [Google Scholar] [CrossRef]
- Peng, S.; Zhao, Y.; Liang, C.; Wang, H.; Zhou, H.; Yang, L. Conductive Ionically Cross-Linked Gluten/Poly(vinyl alcohol) Composite Organohydrogel with Freezing and Water Resistance for Wearable Sensors. ACS Appl. Polym. Mater. 2023, 5, 9515–9524. [Google Scholar] [CrossRef]
- Xiang, H.; Li, Z.; Bai, Z.; Wu, H.; Liu, G.; Zhou, H.; Liu, H. An E-skin for handwriting input at human-machine interface. Chem. Eng. J. 2025, 505, 158879. [Google Scholar] [CrossRef]
- Han, X.; Lu, W.; Yu, W.; Xu, H.; Bi, S.; Cai, H. Conductive and adhesive gluten ionic skin for eco-friendly strain sensor. J. Mater. Sci. 2020, 56, 3970–3980. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Zuo, M.; Zeng, X.; Tang, X.; Sun, Y.; Lin, L. Stretchable, freezing-tolerant conductive hydrogel for wearable electronics reinforced by cellulose nanocrystals toward multiple hydrogen bonding. Carbohydr. Polym. 2022, 280, 119018. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wei, H.; Cui, S.; Hou, H.; Zhang, Y.; Zhang, Y.; Xu, B.B.; Chu, L.; El-Bahy, Z.M.; Melhi, S.; et al. Polyvinyl alcohol/sodium alginate-based conductive hydrogels with in situ formed bimetallic zeolitic imidazolate frameworks towards soft electronics. Carboh. Polym. 2024, 346, 122633. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhou, T.; Han, L.; Zhu, M.; Cheng, Z.; Li, D.; Ren, F.; Wang, K.; Lu, X. Conductive Cellulose Bio-Nanosheets Assembled Biostable Hydrogel for Reliable Bioelectronics. Adv. Funct. Mater. 2021, 31, 2010465. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, B.; Li, J.; Shen, M.; Liu, H.; Xu, X.; Shang, S. Self-healing, self-adhesive, and stretchable conductive hydrogel for multifunctional sensor prepared by catechol modified nanocellulose stabilized poly(α-thioctic acid). Carbohydr. Polym. 2023, 313, 120813. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Huang, K.; You, X.; Huang, B.; Wu, J.; Gu, Z. Hybrid hydrogels with high strength and biocompatibility for bone regeneration. Int. J. Biol. Macromol. 2017, 104, 1143–1149. [Google Scholar] [CrossRef]
- Li, Y.; Wen, X.; Li, X.; Zahid, M.; Wang, H.; Zhang, J. Design of super stretchability, rapid self-healing, and self-adhesion hydrogel based on starch for wearable strain sensors. Carbohydr. Polym. 2025, 348, 122858. [Google Scholar] [CrossRef]
- Zhou, Q.; Abushammala, H.; Gao, D.; Xu, P.; Niu, D.; Yang, W.; Ma, P. Human soft tissues-like PVA/cellulose hydrogels with multifunctional properties towards flexible electronics applications. Carbohydr. Polym. 2025, 357, 123425. [Google Scholar] [CrossRef]
- Sun, X.; Yao, M.; He, S.; Dong, X.; Liang, L.; Yao, F.; Li, J. Antibacterial and UV-Blocking Bioelectronics Based on Transparent, Adhesive, and Strain-Sensitive Multifunctional Hydrogel. Adv. Mater. Technol. 2021, 7, 2101283. [Google Scholar] [CrossRef]
- Ryplida, B.; Lee, K.D.; In, I.; Park, S.Y. Light-Induced Swelling-Responsive Conductive, Adhesive, and Stretchable Wireless Film Hydrogel as Electronic Artificial Skin. Adv. Funct. Mater. 2019, 29, 1903209. [Google Scholar] [CrossRef]
- Yang, J.M.; Fan, C.-S.; Wang, N.-C.; Chang, Y.-H. Evaluation of membrane preparation method on the performance of alkaline polymer electrolyte: Comparison between poly(vinyl alcohol)/chitosan blended membrane and poly(vinyl alcohol)/chitosan electrospun nanofiber composite membranes. Electrochim. Acta 2018, 266, 332–340. [Google Scholar] [CrossRef]
- Pirahmadi, P.; Kokabi, M.; Alamdarnejad, G. Polyvinyl alcohol/chitosan/carbon nanotubes electroactive shape memory nanocomposite hydrogels. J. Appl. Polym. Sci. 2020, 138, e49995. [Google Scholar] [CrossRef]
- Guo, Z.; Xu, X.; Qiu, J.; Yu, W.; Zhang, S.; Li, J.; Zhu, Y.; Lu, J.; Gao, Q.; Nie, B.; et al. Fishing net-inspired PVA-chitosan-CNT hydrogels with high stretchability, sensitivity, and environmentally stability for textile strain sensors. Int. J. Biol. Macromol. 2024, 282, 137576. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Yao, M.; Zhang, D.; He, Y.; Chang, R.; Ren, Y.; Guan, F. One-Step Synthesis of Multifunctional Chitosan Hydrogel for Full-Thickness Wound Closure and Healing. Adv. Healthc. Mater. 2021, 11, 2101808. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Wang, C.; Jia, C.; Kuang, Y.; Pastel, G.; Chen, C.; Chen, G.; He, S.; Huang, H.; Zhang, J.; et al. Muscle-Inspired Highly Anisotropic, Strong, Ion-Conductive Hydrogels. Adv. Mater. 2018, 30, 1801934. [Google Scholar] [CrossRef]
- Zhu, P.; Wei, Y.; Kuang, Y.; Qian, Y.; Liu, Y.; Jiang, F.; Chen, G. Porous and conductive cellulose nanofiber/carbon nanotube foam as a humidity sensor with high sensitivity. Carbohydr. Polym. 2022, 292, 119684. [Google Scholar] [CrossRef]
- Yang, T.; Xu, C.; Liu, C.; Ye, Y.; Sun, Z.; Wang, B.; Luo, Z. Conductive polymer hydrogels crosslinked by electrostatic interaction with PEDOT:PSS dopant for bioelectronics application. Chem. Eng. J. 2022, 429, 132430. [Google Scholar] [CrossRef]
- Teng, Y.; Zhang, Z.; Cui, Y.; Su, Z.; Godwin, M.; Chung, T.; Zhou, Y.; Leontowich, A.F.G.; Islam, M.S.; Tam, K.C.; et al. High-Sensitivity and Flexible Motion Sensing Enabled by Robust, Self-Healing Wood-Based Anisotropic Hydrogel Composites. Small 2025, 21, 2500944. [Google Scholar] [CrossRef]
- Lei, T.; Pan, J.; Wang, N.; Xia, Z.; Zhang, Q.; Fan, J.; Tao, L.; Shou, W.; Gao, Y. Cold-resistant, highly stretchable ionic conductive hydrogels for intelligent motion recognition in winter sports. Mater. Horiz. 2024, 11, 1234–1250. [Google Scholar] [CrossRef]
- Wang, S.; Yu, L.; Wang, S.; Zhang, L.; Chen, L.; Xu, X.; Song, Z.; Liu, H.; Chen, C. Strong, tough, ionic conductive, and freezing-tolerant all-natural hydrogel enabled by cellulose-bentonite coordination interactions. Nat. Commun. 2022, 13, 3408. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, Y.; Chen, Y.; Han, X.; Jiang, F. Cellulose Nanofibrils Enhanced, Strong, Stretchable, Freezing-Tolerant Ionic Conductive Organohydrogel for Multi-Functional Sensors. Adv. Funct. Mater. 2020, 30, 2010465. [Google Scholar] [CrossRef]
- Ye, Y.; Oguzlu, H.; Zhu, J.; Zhu, P.; Yang, P.; Zhu, Y.; Wan, Z.; Rojas, O.J.; Jiang, F. Ultrastretchable Ionogel with Extreme Environmental Resilience through Controlled Hydration Interactions. Adv. Funct. Mater. 2022, 33, 2209787. [Google Scholar] [CrossRef]
- Sun, Z.; Hu, Y.; Wei, C.; Hao, R.; Hao, C.; Liu, W.; Liu, H.; Huang, M.; He, S.; Yang, M. Transparent, photothermal and stretchable alginate-based hydrogels for remote actuation and human motion sensing. Carbohydr. Polym. 2022, 293, 119727. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Shu, Z.; Zhang, T.; Ji, W.; Chen, J.; Wei, Y. Highly Elastic, Sensitive, Stretchable, and Skin-Inspired Conductive Sodium Alginate/Polyacrylamide/Gallium Composite Hydrogel with Toughness as a Flexible Strain Sensor. Biomacromolecules 2022, 23, 2603–2613. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ren, Z.; Liu, X.; Ling, Q.; Li, Z.; Gu, H. A Multifunctional, Self-Healing, Self-Adhesive, and Conductive Sodium Alginate/Poly(vinyl alcohol) Composite Hydrogel as a Flexible Strain Sensor. ACS Appl. Mater. Interfaces 2021, 13, 11344–11355. [Google Scholar] [CrossRef]
- Wu, P.; Qin, Z.; Dassanayake, R.; Sun, Z.; Cao, M.; Fu, K.; Zhou, Y.; Liu, Y. Antimicrobial MXene-based conductive alginate hydrogels as flexible electronics. Chem. Eng. J. 2023, 455, 140546. [Google Scholar] [CrossRef]
- Cui, W.; Zheng, Y.; Zhu, R.; Mu, Q.; Wang, X.; Wang, Z.; Liu, S.; Li, M.; Ran, R. Strong Tough Conductive Hydrogels via the Synergy of Ion-Induced Cross-Linking and Salting-Out. Adv. Funct.Mater. 2022, 32, 2204823. [Google Scholar] [CrossRef]
- Rosciardi, V.; Baglioni, P. Role of amylose and amylopectin in PVA-starch hybrid cryo-gels networks formation from liquid-liquid phase separation. J. Colloid. Interf. Sci. 2023, 630, 415–425. [Google Scholar] [CrossRef]
- Su, C.; Li, D.; Wang, L.; Wang, Y. Green double crosslinked starch-alginate hydrogel regulated by sustained calcium ion-gluconolactone release for human motion monitoring. Chem. Eng. J. 2023, 455, 140653. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Li, X.; Lu, L.; Cui, B.; Yuan, C.; Guo, L.; Yu, B.; Chai, Q. Starch/polyvinyl alcohol with ionic liquid/graphene oxide enabled highly tough, conductive and freezing-resistance hydrogels for multimodal wearable sensors. Carbohydr. Polym. 2023, 320, 121262. [Google Scholar] [CrossRef]
- Li, J.; Deng, Y.; Fu, H.; Zhang, Y.; Zhang, Y.; Fu, L.; Xu, C.; Lin, B. Multifunctional Starch-Based Sensor with Non-Covalent Network to Achieve “3R” Circulation. Small 2023, 19, e2208116. [Google Scholar] [CrossRef]
- Chen, R.; Wang, L.; Ji, D.; Luo, M.; Zhang, Z.; Zhao, G.; Chang, X.; Zhu, Y. Highly stretchable, conductive, and self-adhesive starch-based hydrogel for high-performance flexible electronic devices. Carbohydr. Polym. 2025, 352, 123220. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Hou, M.; Wang, L.; Zhang, X.; Liu, L. Anti-bacterial, anti-freezing starch/ionic liquid/PVA ion-conductive hydrogel with high performance for multi-stimulation sensitive responsive sensors. Chem. Eng. J. 2023, 477, 147065. [Google Scholar] [CrossRef]
- Zeng, S.; Zhang, J.; Zu, G.; Huang, J. Transparent, flexible, and multifunctional starch-based double-network hydrogels as high-performance wearable electronics. Carbohydr. Polym. 2021, 267, 118198. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, R.; Cheng, D.; Yang, X.; Zhang, H.; Zheng, J.; Hu, R. Extremely Ultrahigh Stretchable Starch-Based Hydrogels with Continuous Hydrogen Bonding. Adv. Funct. Mater. 2024, 35, 2415530. [Google Scholar] [CrossRef]
- Liu, Y.; Wong, T.H.; Huang, X.; Yiu, C.K.; Gao, Y.; Zhao, L.; Zhou, J.; Park, W.; Zhao, Z.; Yao, K.; et al. Skin-integrated, stretchable, transparent triboelectric nanogenerators based on ion-conducting hydrogel for energy harvesting and tactile sensing. Nano Energy 2022, 99, 107442. [Google Scholar] [CrossRef]
- Roshanbinfar, K.; Kolesnik-Gray, M.; Angeloni, M.; Schruefer, S.; Fiedler, M.; Schubert, D.W.; Ferrazzi, F.; Krstic, V.; Engel, F.B. Collagen Hydrogel Containing Polyethylenimine-Gold Nanoparticles for Drug Release and Enhanced Beating Properties of Engineered Cardiac Tissues. Adv. Healthc. Mater. 2023, 12, 2202408. [Google Scholar] [CrossRef]
- Yan, X.; Liu, Z.; Fu, Y.; Chen, X.; Gan, S.; Yang, W.; Chen, S.; Liu, L. Liquid Metal@Silk Fibroin Peptide Particles Initiated Hydrogels with High Toughness, Adhesion, and Conductivity for Portable and Continuous Electrophysiological Monitoring. Adv. Funct. Mater. 2025, 35, 2420240. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, Y.; Huang, S.; Geng, L.; Wu, J.; Mao, G.; Peng, X.; Cheng, Y. Self-assembly Polysaccharide Network Regulated Hydrogel Sensors with Toughness, Anti-freezing, Conductivity and Wide Working Conditions. Chem. Eng. J. 2024, 497, 154409. [Google Scholar] [CrossRef]
- Zhou, Y.; Wan, C.; Yang, Y.; Yang, H.; Wang, S.; Dai, Z.; Ji, K.; Jiang, H.; Chen, X.; Long, Y. Highly Stretchable, Elastic, and Ionic Conductive Hydrogel for Artificial Soft Electronics. Adv. Funct. Mater. 2018, 29, 1806220. [Google Scholar] [CrossRef]
- Ji, D.; Park, J.M.; Oh, M.S.; Nguyen, T.L.; Shin, H.; Kim, J.S.; Kim, D.; Park, H.S.; Kim, J. Superstrong, superstiff, and conductive alginate hydrogels. Nat. Commun. 2022, 13, 3019. [Google Scholar] [CrossRef]
- Lu, J.; Hu, O.; Hou, L.; Ye, D.; Weng, S.; Jiang, X. Highly tough and ionic conductive starch/poly(vinyl alcohol) hydrogels based on a universal soaking strategy. Int. J. Biol. Macromol. 2022, 221, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, M.; Jing, L.; Jia, Q.; Lv, S.; Xu, Z.; Liu, J.; Cai, X. Enhanced neural activity detection with microelectrode arrays modified by drug-loaded calcium alginate/chitosan hydrogel. Biosens. Bioelectron. 2025, 267, 116837. [Google Scholar] [CrossRef]
- Gao, M.; Liu, W.; Chen, K. Piezoresistive Effect: A New Concept for Hearing Aids. Adv. Sci. 2025, 2501227. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wei, T.; Yin, R.T.; Wu, M.; Xu, Y.; Koo, J.; Choi, Y.S.; Xie, Z.; Chen, S.W.; Kandela, I.; et al. Photocurable bioresorbable adhesives as functional interfaces between flexible bioelectronic devices and soft biological tissues. Nat. Mater. 2021, 20, 1559–1570. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Yang, Y.; Liu, Z.; Li, W.; Zhang, Y.; Huang, Y.; Kang, T.; Yu, Y.; Li, N.; Tian, Y.; et al. Injectable ultrasonic sensor for wireless monitoring of intracranial signals. Nature 2024, 630, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Bao, R.; Wang, C.; Dong, L.; Shen, C.; Zhao, K.; Pan, C. CdS nanorods/organic hybrid LED array and the piezo-phototronic effect of the device for pressure mapping. Nanoscale 2016, 8, 8078–8082. [Google Scholar] [CrossRef]
- Feng, T.; Ling, D.; Li, C.; Zheng, W.; Zhang, S.; Li, C.; Emel’yanov, A.; Pozdnyakov, A.S.; Lu, L.; Mao, Y. Stretchable on-skin touchless screen sensor enabled by ionic hydrogel. Nano Res. 2023, 17, 4462–4470. [Google Scholar] [CrossRef]
- Xu, Q.; Chu, N.; Wang, Y.; Wang, H.; Xu, T.; Li, X.; Huang, S.; Li, X.; Luo, Y.; Yang, H.Y.; et al. 3D Printed Low-Tortuosity and Ultra-Thick Hierarchical Porous Electrodes for High-Performance Wearable Quasi-Solid-State Zn-VOH Batteries. Adv. Sci. 2025, 12, 2401660. [Google Scholar] [CrossRef]
- Liu, H.; Qin, J.; Yang, X.; Lv, C.; Huang, W.; Li, F.; Zhang, C.; Wu, Y.; Dong, L.; Shan, C. Highly sensitive humidity sensors based on hexagonal boron nitride nanosheets for contactless sensing. Nano Res. 2023, 16, 10279–10286. [Google Scholar] [CrossRef]
- Ma, F.; Wu, Y.; Dai, S.; Lin, P.; Sun, J.; Dong, L. A soft-contact hybrid electromagnetic–triboelectric nanogenerator for self-powered water splitting towards hydrogen production. Nano Res. 2024, 17, 6567–6574. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, N.; Tang, Y.; Wang, M.; Chao, M.; Liang, E. A paper triboelectric nanogenerator for self-powered electronic systems. Nanoscale 2017, 9, 14499–14505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Qin, C.; Feng, T.; Li, J.; Yang, Z.; Sun, X.; Liang, E.; Mao, Y.; Wang, X. Non-contact cylindrical rotating triboelectric nanogenerator for harvesting kinetic energy from hydraulics. Nano Res. 2020, 13, 1903–1907. [Google Scholar] [CrossRef]
- Li, G.; Chen, N.; Xu, T.; Zhang, Y. Flexible Fe3+-doped gelatin/poly(acrylate-co-acrylamide) conductive hydrogels for biopotential acquisition, salt recognition, and supercapacitors. Sens. Actuators A Phys. 2025, 387, 116425. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Kumar, V.; Jansson, E.; Huttunen, O.H.; Yamamoto, A.; Vikman, M.; Khakalo, A.; Hiltunen, J.; Behfar, M.H. Biodegradable Cellulose Nanocomposite Substrate for Recyclable Flexible Printed Electronics. Adv. Electron. Mater. 2023, 9, 2201094. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Wei, W.; Zhang, Z.; Li, A.A.; Huang, G.; Li, X.; Ge, S.S.; Zhou, L.; Kong, H. High-precision flexible sweat self-collection sensor for mental stress evaluation. npj Flex. Electron. 2024, 8, 47. [Google Scholar] [CrossRef]
- Lu, L.; Hu, G.; Liu, J.; Yang, B. 5G NB-IoT System Integrated with High-Performance Fiber Sensor Inspired by Cirrus and Spider Structures. Adv. Sci. 2024, 11, 2309894. [Google Scholar] [CrossRef]
- Liu, D.; Zhu, P.; Zhang, F.; Li, P.; Huang, W.; Li, C.; Han, N.; Mu, S.; Zhou, H.; Mao, Y. Intrinsically stretchable polymer semiconductor based electronic skin for multiple perceptions of force, temperature, and visible light. Nano Res. 2022, 16, 1196–1204. [Google Scholar] [CrossRef]
- Miao, Y.; Xu, M.; Zhang, L. Electrochemistry-Induced Improvements of Mechanical Strength, Self-Healing, and Interfacial Adhesion of Hydrogels. Adv. Mater. 2021, 33, 2102308. [Google Scholar] [CrossRef]
- Campuzano, S.; Pedrero, M.; Barderas, R.; Pingarrón, J.M. Empowering Electrochemical Biosensing through Nanostructured or Multifunctional Nucleic Acid or Peptide Biomaterials. Adv. Mater. Technol. 2022, 7, 2200310. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, H.; Li, Y.; Wang, J.; Ma, L. A ferrocene-based hydrogel as flexible electrochemical biosensor for oxidative stress detection and antioxidation treatment. Biosens. Bioelectron. 2024, 248, 115997. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Shi, S.; Zheng, Y.; Ye, Z.; Liao, J.; Sun, Q.; Dang, B.; Shen, X. Myelin Sheath-Inspired Hydrogel Electrode for Artificial Skin and Physiological Monitoring. ACS Nano 2024, 18, 27420–27432. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, R.; Wang, L.; Wang, X. Multifunctional flexible graphene oxide/bacterial cellulose composite paper platforms for realtime monitoring sweat and strain in wearable devices. Chem. Eng. J. 2024, 481, 148390. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Q.; Wang, A.; Zhu, Z.; Moses, K.; Wang, T.; Li, P.; Huang, W. Sponge Inspired Flexible, Antibacterial Aerogel Electrode with Long-Term High-Quality Electrophysiological Signal Recording for Human-Machine Interface. Adv. Funct. Mater. 2024, 34, 2309704. [Google Scholar] [CrossRef]
- Jiang, L.; Gan, D.; Xu, C.; Zhang, T.; Gao, M.; Xie, C.; Zhang, D.; Lu, X. Polyphenol-Mediated Multifunctional Human-Machine Interface Hydrogel Electrodes in Bioelectronics. Small. Sci. 2025, 5, 2400362. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yao, K.; Zhang, Q.; Huang, X.; Chen, Z.; Zhou, Y.; Yu, X. Bioelectronics for electrical stimulation: Materials, devices and biomedical applications. Chem. Soc. Rev. 2024, 53, 8632–8712. [Google Scholar] [CrossRef]
- Zhu, P.; Niu, M.; Liang, S.; Yang, W.; Zhang, Y.; Chen, K.; Pan, Z.; Mao, Y. Non-hand-worn, load-free VR hand rehabilitation system assisted by deep learning based on ionic hydrogel. Nano Res. 2025, 18, 94907301. [Google Scholar] [CrossRef]
- Yang, M.; Cheng, Y.; Yue, Y.; Chen, Y.; Gao, H.; Li, L.; Cai, B.; Liu, W.; Wang, Z.; Guo, H.; et al. High-Performance Flexible Pressure Sensor with a Self-Healing Function for Tactile Feedback. Adv. Sci. 2022, 9, 2200507. [Google Scholar] [CrossRef]
- Zhu, P.; Mu, S.; Huang, W.; Sun, Z.; Lin, Y.; Chen, K.; Pan, Z.; Haghighi, M.G.; Sedghi, R.; Wang, J.; et al. Soft multifunctional neurological electronic skin through intrinsically stretchable synaptic transistor. Nano Res. 2024, 17, 6550–6559. [Google Scholar] [CrossRef]
- Roubert Martinez, S.; Le Floch, P.; Liu, J.; Howe, R.D. Pure Conducting Polymer Hydrogels Increase Signal-to-Noise of Cutaneous Electrodes by Lowering Skin Interface Impedance. Adv. Healthc. Mater. 2023, 12, e2202661. [Google Scholar] [CrossRef]
- Li, N.; Wang, X.; Liu, Y.; Li, Y.; Li, J.; Qin, Z.; Jiao, T. Ultrastretchable, Self-Adhesive and conductive MXene nanocomposite hydrogel for body-surface temperature distinguishing and electrophysiological signal monitoring. Chem. Eng. J. 2024, 483, 149303. [Google Scholar] [CrossRef]
- Xia, X.; Liang, Q.; Sun, X.; Yu, D.; Huang, X.; Mugo, S.M.; Chen, W.; Wang, D.; Zhang, Q. Intrinsically Electron Conductive, Antibacterial, and Anti-swelling Hydrogels as Implantable Sensors for Bioelectronics. Adv. Funct. Mater. 2022, 32, 2208024. [Google Scholar] [CrossRef]
- Shen, G.; Zheng, K.; Jiang, C.; Shao, S.; Zhao, N.; Liu, J. A Gelatin-Based Hydrogel Electrode With High Moisturizing Ability for Wearable EEG Recording. IEEE Sens. J. 2023, 23, 25689–25697. [Google Scholar] [CrossRef]
- Liu, R.; Wang, T.; Li, G.; Fan, Z.; Zhou, Q.; Wang, K.; Li, P.; Huang, W. Self-Reinforced Hydrogel-Based Skin-Contactable Flexible Electronics for Multimodal Electrophysiological Signal Monitoring and Emergency Alarming System. Adv. Funct. Mater. 2023, 33, 2214917. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.; Xue, J.; Huang, G.; Zheng, S.; Zhao, K.; Huang, J.; Wang, Y.; Zhang, Y.; Yin, T.; et al. Body Temperature Enhanced Adhesive, Antibacterial, and Recyclable Ionic Hydrogel for Epidermal Electrophysiological Monitoring. Adv. Healthc. Mater. 2022, 11, e2200653. [Google Scholar] [CrossRef]
- Huang, H.; Shen, J.; Wan, S.; Han, L.; Dou, G.; Sun, L. Wet-Adhesive Multifunctional Hydrogel with Anti-swelling and a Skin-Seamless Interface for Underwater Electrophysiological Monitoring and Communication. ACS Appl. Mater. Interfaces 2023, 15, 11549–11562. [Google Scholar] [CrossRef]
- Gong, R.; Dong, Y.; Ge, D.; Miao, Z.; Yu, H.-Y. Wet Spinning Fabrication of Robust and Uniform Intrinsically Conductive Cellulose Nanofibril/Silk Conductive Fibers as Bifunctional Strain/Humidity Sensor in Potential Smart Dressing. Adv. Fiber Mater. 2024, 6, 993–1007. [Google Scholar] [CrossRef]
- Li, Q.; He, C.; Wang, C.; Huang, Y.; Yu, J.; Wang, C.; Li, W.; Zhang, X.; Zhang, F.; Qing, G. Sustainable, Insoluble, and Photonic Cellulose Nanocrystal Patches for Calcium Ion Sensing in Sweat. Small 2023, 19, 2207932. [Google Scholar] [CrossRef]
- Siripongpreda, T.; Somchob, B.; Rodthongkum, N.; Hoven, V.P. Bacterial cellulose-based re-swellable hydrogel: Facile preparation and its potential application as colorimetric sensor of sweat pH and glucose. Carbohydr. Polym. 2021, 256, 117506. [Google Scholar] [CrossRef]
- Zou, J.; Lin, Z.; Yuan, Z.; Zhou, B.; Fu, X.; Ye, D. High-strength, high-toughness regenerated cellulose/graphene oxide nanofluidic membrane with highly oriented and charged nanochannels for wearable sweat-monitoring systems. Chem. Eng. J. 2023, 467, 143485. [Google Scholar] [CrossRef]
- Qin, Y.; Mo, J.; Liu, Y.; Zhang, S.; Wang, J.; Fu, Q.; Wang, S.; Nie, S. Stretchable Triboelectric Self-Powered Sweat Sensor Fabricated from Self-Healing Nanocellulose Hydrogels. Adv. Funct. Mater. 2022, 32, 2201846. [Google Scholar] [CrossRef]
- Liu, X.; Rao, S.; Chen, W.; Felix, K.; Ni, J.; Sahasrabudhe, A.; Lin, S.; Wang, Q.; Liu, Y.; He, Z.; et al. Fatigue-resistant hydrogel optical fibers enable peripheral nerve optogenetics during locomotion. Nat. Meth. 2023, 20, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.L.; Peng, C.W.; Chiu, S.C.; Lu, H.E.; Wu, C.W.; Cheng, T.Y.; Huang, W.C. All Biodisintegratable Hydrogel Biohybrid Neural Interfaces with Synergistic Performances of Microelectrode Array Technologies, Tissue Scaffolding, and Cell Therapy. Adv. Funct. Mater. 2023, 34, 2307365. [Google Scholar] [CrossRef]
- Park, S.; Yuk, H.; Zhao, R.; Yim, Y.S.; Woldeghebriel, E.W.; Kang, J.; Canales, A.; Fink, Y.; Choi, G.B.; Zhao, X.; et al. Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity. Nat. Commun. 2021, 12, 3435. [Google Scholar] [CrossRef]
- Han, I.K.; Song, K.I.; Jung, S.M.; Jo, Y.; Kwon, J.; Chung, T.; Yoo, S.; Jang, J.; Kim, Y.T.; Hwang, D.S.; et al. Electroconductive, Adhesive, Non-Swelling, and Viscoelastic Hydrogels for Bioelectronics. Adv. Mater. 2022, 35, 2203431. [Google Scholar] [CrossRef]
- Zhou, T.; Yuk, H.; Hu, F.; Wu, J.; Tian, F.; Roh, H.; Shen, Z.; Gu, G.; Xu, J.; Lu, B.; et al. 3D printable high-performance conducting polymer hydrogel for all-hydrogel bioelectronic interfaces. Nat. Mater. 2023, 22, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Feig, V.R.; Bao, Z. Conjugated Polymer for Implantable Electronics toward Clinical Application. Adv. Healthc. Mater. 2021, 10, 2001916. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Xue, Y.; Lei, I.M.; Chen, X.; Zhang, P.; Cai, C.; Liang, X.; Lu, Y.; Liu, J. Engineering Electrodes with Robust Conducting Hydrogel Coating for Neural Recording and Modulation. Adv. Mater. 2023, 35, e2209324. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, J.; Chen, X.; Zhang, J.; Chen, G.; Zhang, K.; Lin, J.; Guo, C.; Liu, J. Trigger-Detachable Hydrogel Adhesives for Bioelectronic Interfaces. Adv. Funct. Mater. 2021, 31, 2106446. [Google Scholar] [CrossRef]
- Yuk, H.; Lu, B.; Zhao, X. Hydrogel bioelectronics. Chem. Soc. Rev. 2019, 48, 1642–1667. [Google Scholar] [CrossRef]
- Li, Z.; Lu, J.; Ji, T.; Xue, Y.; Zhao, L.; Zhao, K.; Jia, B.; Wang, B.; Wang, J.; Zhang, S.; et al. Self-Healing Hydrogel Bioelectronics. Adv. Mater. 2024, 36, e2306350. [Google Scholar] [CrossRef]
- Liu, G.; Lv, Z.; Batool, S.; Li, M.Z.; Zhao, P.; Guo, L.; Wang, Y.; Zhou, Y.; Han, S.T. Biocompatible Material-Based Flexible Biosensors: From Materials Design to Wearable/Implantable Devices and Integrated Sensing Systems. Small 2023, 19, e2207879. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Yuk, H.; Wu, J.; Varela, C.E.; Chen, X.; Roche, E.T.; Guo, C.F.; Zhao, X. Electrical bioadhesive interface for bioelectronics. Nat. Mater. 2020, 20, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Zhu, Z.; Xia, P.; Wang, Z.; Zhao, X.; Jiang, X.; Wang, T.; Gao, Q.; Xu, J.; Shan, D.; et al. Tough Gelatin Hydrogel for Tissue Engineering. Adv. Sci. 2023, 10, 2301665. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Yan, Z.; Ni, S.; Yang, H.; Xie, Y.; Wang, X.; Zou, D.; Tao, C.; Jiang, W.; Jiang, J.; et al. Tissue/Organ Adaptable Bioelectronic Silk-Based Implants. Adv. Mater. 2024, 36, 2405892. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).