Alginate Sphere-Based Soft Actuators
Abstract
1. Introduction
2. Fabrication Strategies for Alginate Spheres
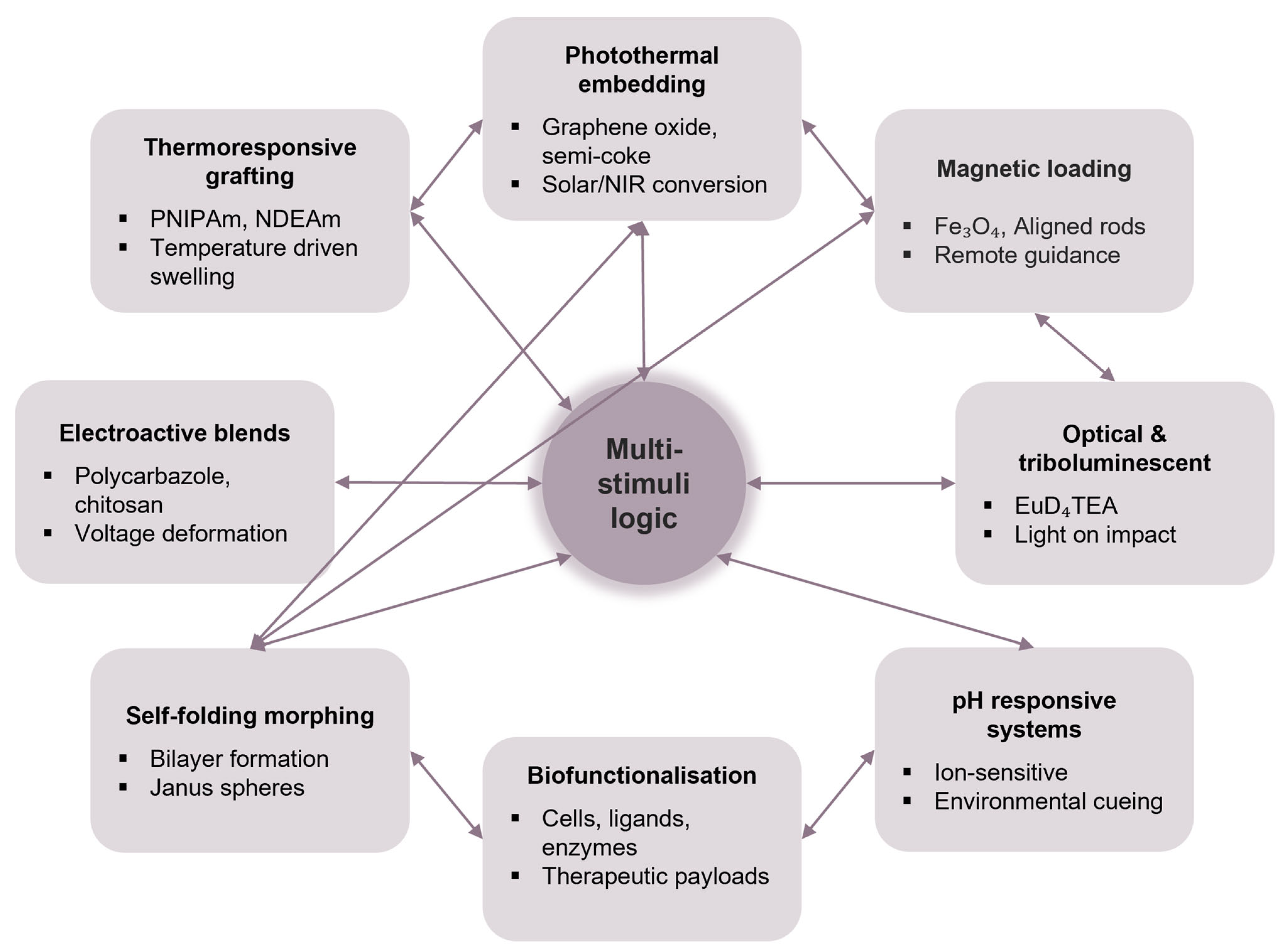
3. Functionalisation Approaches
4. Actuation Mechanisms and Performance
| System/Material | Stimulus | Max Actuation Strain (%) | Blocking Force (N) | Response Time | Actuation Mode | Application Domain | Reversibility/Cyclic Use | Reference |
|---|---|---|---|---|---|---|---|---|
| Alginate-g-P(NIPAm-co-NDEAm)/SC | Temperature, pH, light | ~50% | Not reported | 1–3 min | Volumetric swelling/deswelling | Controlled agrochemical release | High, multi-cycle-tested | [56] |
| Magnetic alginate beads | Magnetic field | ~10% | Not reported | Instantaneous | Magnetic rotation/translation | Targeted drug delivery | High, magnetic field-controlled | [57,107,108] |
| Braided hydrogel muscle | Temperature (cooling from 60 °C) | 7–8% | 5–6 N | Slow (minutes) | Contraction due to swelling | Artificial muscles/soft robotics | Moderate (fatigue observed) | [50] |
| Electroactive alginate–polycarbazole | Electric field (low voltage) | 1–2% | Low (µN-mN) | Seconds | Voltage-induced deformation | Electroactive sensing or actuation | Limited (electrochemical fatigue) | [55] |
| Photothermal GO–alginate bilayers | NIR light | 15–20% | Not reported | Fast (~seconds) | Bending/folding due to heating | Microrobotics, biomedical folding | Good, NIR-cycled | [94] |
| Magnetic alginate micromotors | Magnetic field | ~12% | Not reported | Sub-second rotation | Magnetic propulsion | Remote-controlled microswimmers | Yes, in fluid environment | [103,109] |
| pH-responsive Ca–alginate beads | pH variation (acidic) | 5–10% | Not applicable | 2–5 min | Swelling and gel softening | Environmental remediation | Yes, but pH-limited | [110,111] |
| Triboluminescent EuD4TEA–alginate beads | Mechanical impact | Swelling-dependent | Not applicable | Instantaneous flash | Optical emission due to deformation | Mechanical sensing and diagnostics | No, single flash | [96] |
| Piezoelectric alginate microspheres | Electric field (piezoelectric) | 0.5–1% | Very low | Milliseconds | Electric field-induced deformation | Biosignal-responsive systems | Yes, piezoelectric loop | [112] |
5. Design Logic and Actuation Modelling
5.1. Integration into Functional Architectures
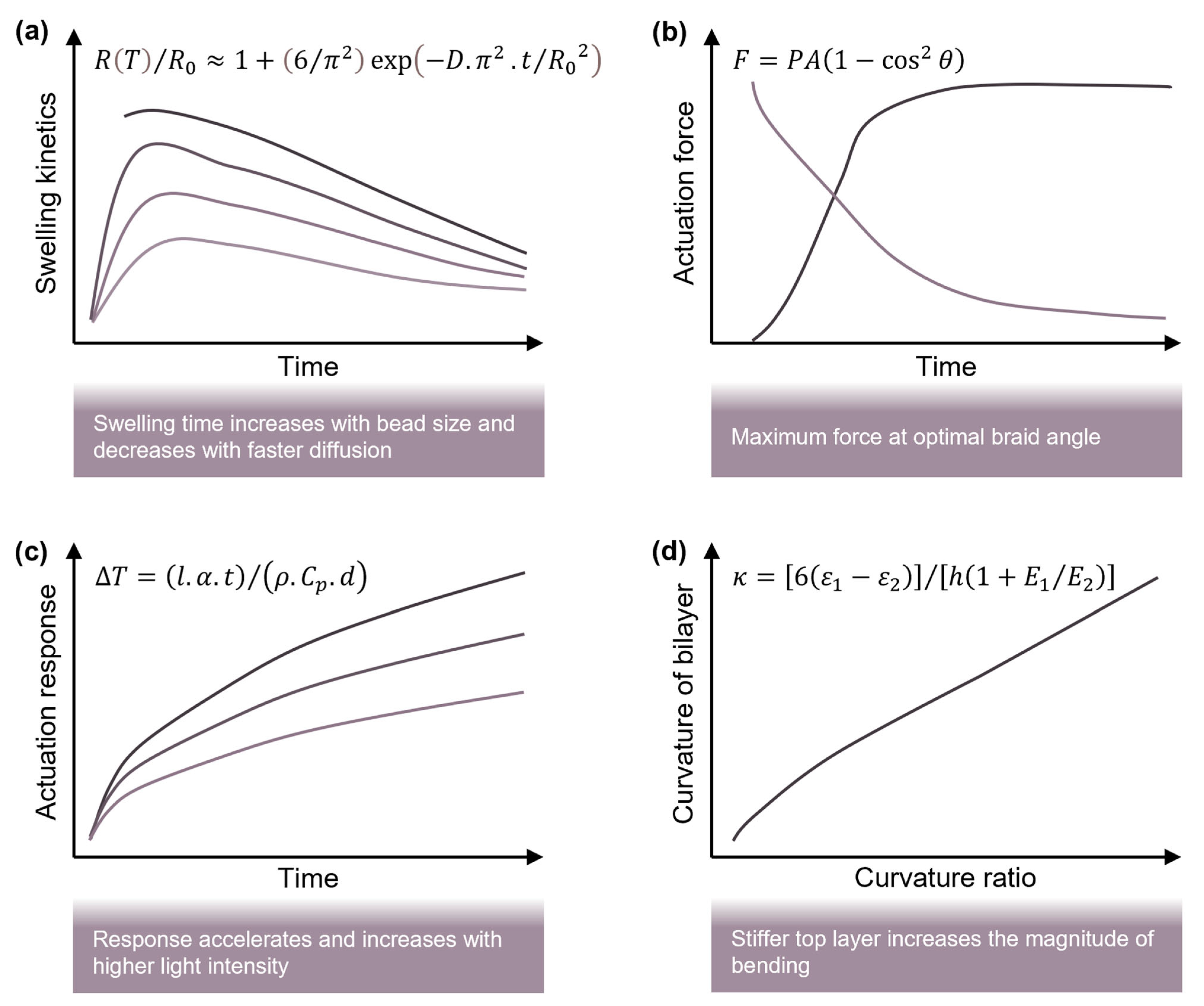
5.2. Fundamental Actuation Models
5.3. Stimuli-Induced Actuation Models
6. Application Demonstrations and Potential
6.1. Microrobotic Locomotion and Artificial Muscles
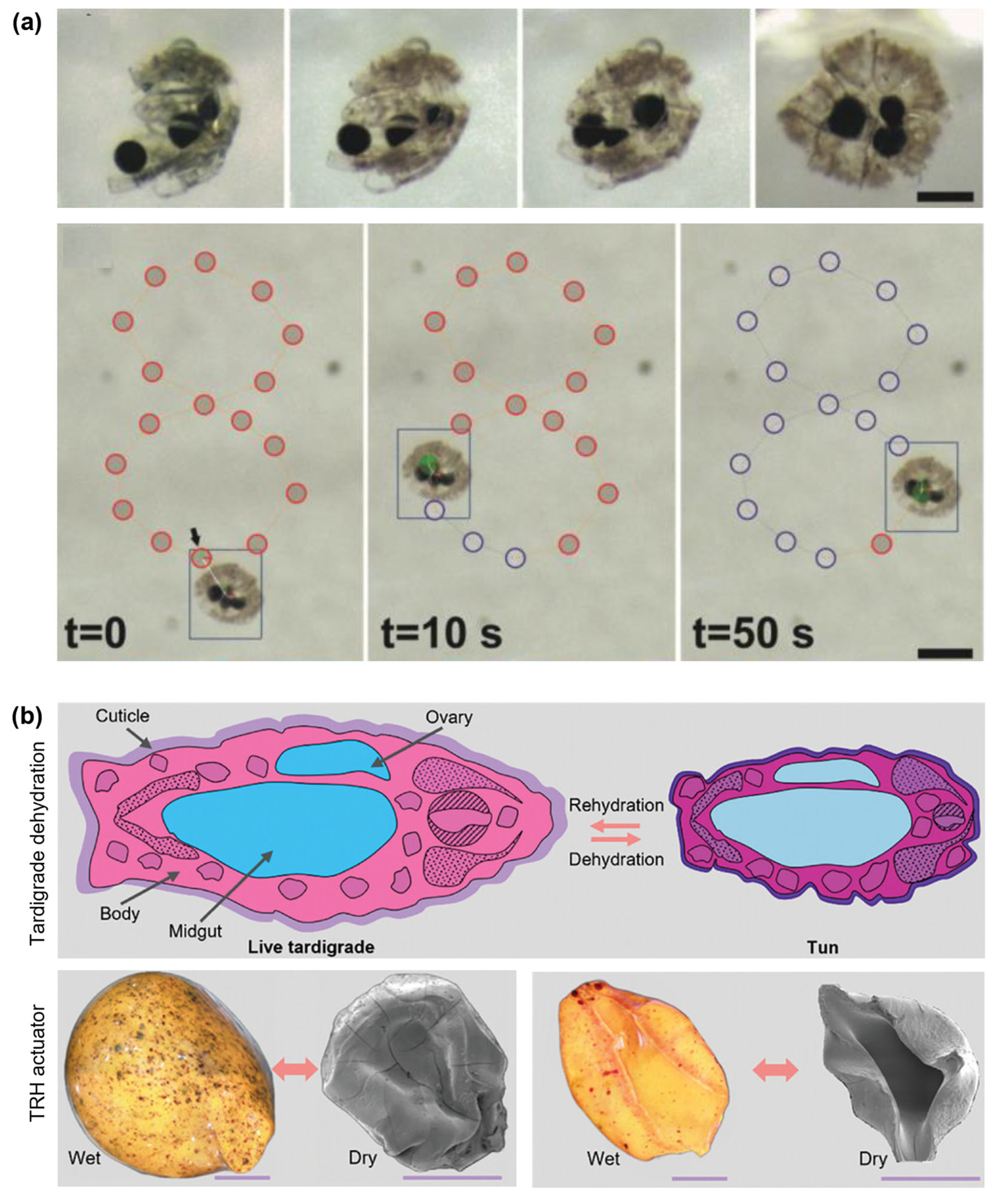
6.2. Shape Morphing and Biomimetic Resilience
6.3. Biomedical Delivery and Regenerative Applications
6.4. Sustainable Actuator Solutions
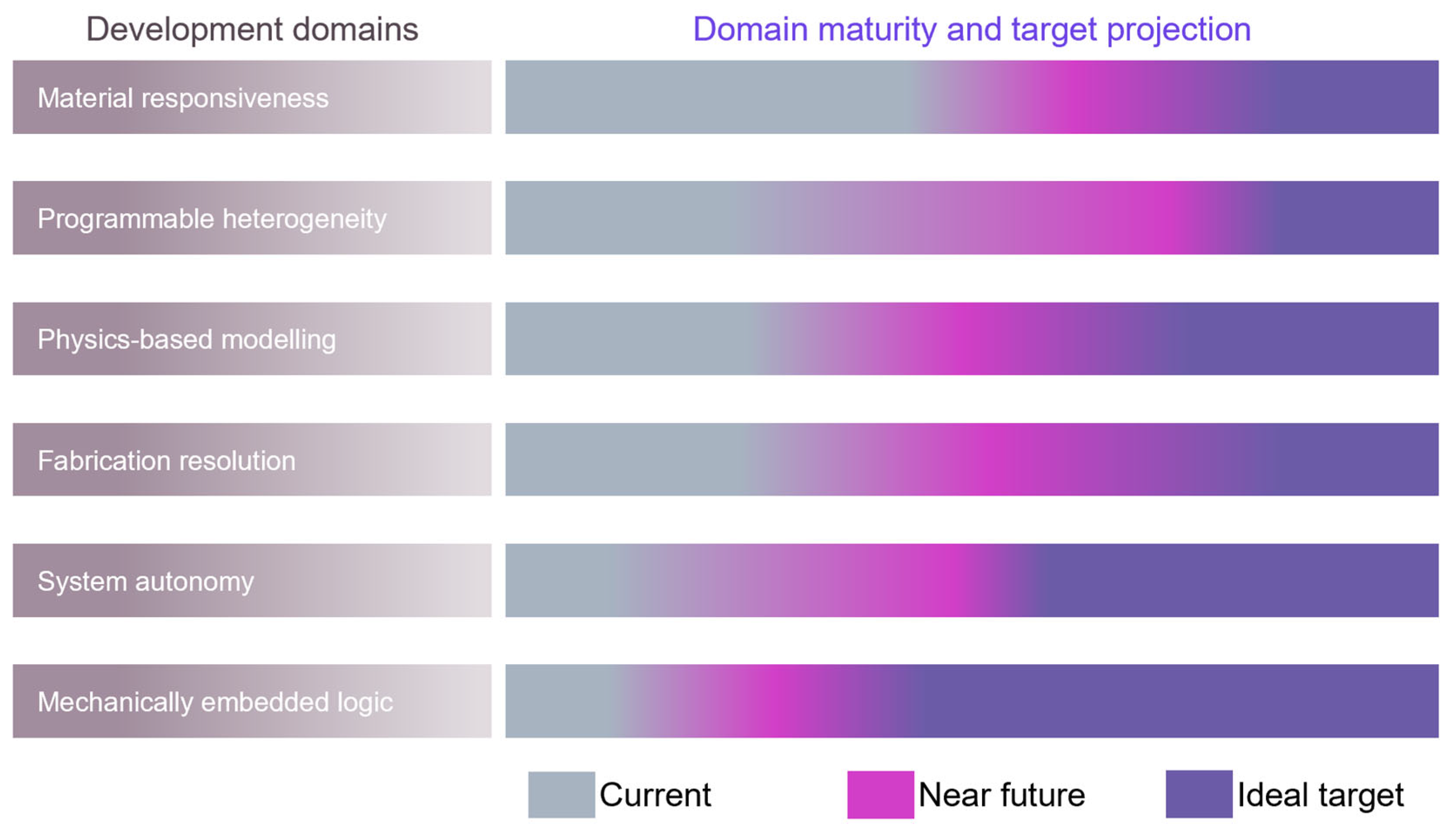
7. Outlook
- ▪ Integrate actuation, sensing and mechanical logic into unified composite hydrogels with reconfigurable and adaptive functionality.
- ▪ Develop real-time, multiphysics modelling platforms that incorporate environmental coupling and deformation-based feedback mechanisms.
- ▪ Scale high-resolution fabrication strategies capable of encoding spatial heterogeneity and internal actuation logic across large bead or scaffold arrays.
- ▪ Incorporate adaptive features such as mechanical memory, self-healing or chemo-mechanical conditioning to improve devices’ resilience and operational lifespan.
- ▪ Transition to using recyclable, biodegradable and bio-derived alginate composites to align with sustainability and circular economy frameworks.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ding, M.; Jing, L.; Yang, H.; Machnicki, C.E.; Fu, X.; Li, K.; Wong, I.Y.; Chen, P.Y. Multifunctional soft machines based on stimuli-responsive hydrogels: From freestanding hydrogels to smart integrated systems. Mater. Today Adv. 2020, 8, 100088. [Google Scholar] [CrossRef]
- Jiao, D.J.; Zhu, Q.L.; Li, C.Y.; Zheng, Q.; Wu, Z.L. Programmable Morphing Hydrogels for Soft Actuators and Robots: From Structure Designs to Active Functions. Accounts Chem. Res. 2022, 55, 1533–1545. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Z.; Liu, F.F.; Abdiryim, T.; Liu, X. Stimuli-responsive hydrogels as promising platforms for soft actuators. Mater. Today Phys. 2024, 40, 101281. [Google Scholar] [CrossRef]
- Ionov, L. Hydrogel-based actuators: Possibilities and limitations. Mater. Today 2014, 17, 494–503. [Google Scholar] [CrossRef]
- Zhang, X.; Aziz, S.; Salahuddin, B.; Zhu, Z. Thermoresponsive hydrogel artificial muscles. Matter 2023, 6, 2735–2775. [Google Scholar] [CrossRef]
- Ionov, L. Biomimetic Hydrogel-Based Actuating Systems. Adv. Funct. Mater. 2013, 23, 4555–4570. [Google Scholar] [CrossRef]
- Zhao, X.H.; Chen, X.Y.; Yuk, H.; Lin, S.T.; Liu, X.Y.; Parada, G. Soft Materials by Design: Unconventional Polymer Networks Give Extreme Properties. Chem. Rev. 2021, 121, 4309–4372. [Google Scholar] [CrossRef]
- Wang, Y. Programmable hydrogels. Biomaterials 2018, 178, 663–680. [Google Scholar] [CrossRef]
- Wang, H.H.; Du, J.L.; Mao, Y. Hydrogel-Based Continuum Soft Robots. Gels 2025, 11, 254. [Google Scholar] [CrossRef]
- Jayakumar, A.; Jose, V.K.; Lee, J.M. Hydrogels for Medical and Environmental Applications. Small Methods 2020, 4, 1900735. [Google Scholar] [CrossRef]
- López-Díaz, A.; Vázquez, A.S.; Vázquez, E. Hydrogels in Soft Robotics: Past, Present, and Future. ACS Nano 2024, 18, 20817–20826. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Tan, J.Y.; Luo, Y.N.; Guo, Y.Q.; Zhou, Y.; Liao, X.Y.; Li, D.X.L.; Lai, X.Y.; Liu, Y. Development of alginate-based hydrogels: Crosslinking strategies and biomedical applications. Int. J. Biol. Macromol. 2023, 239, 124275. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.S.; Xie, Y.J.; He, W. Research progress on chemical modification of alginate: A review. Carbohydr. Polym. 2011, 84, 33–39. [Google Scholar] [CrossRef]
- Aksakal, B.; Kaplan, Z.; Turhan, K. The influence of plasticizer on the mechanical, structural, thermal and strain recovery properties following stress-relaxation process of silk fibroin/sodium alginate biocomposites for biomedical applications. J. Mech. Behav. Biomed. Mater. 2025, 161, 106797. [Google Scholar] [CrossRef]
- Ren, Y.Z.; Wang, Q.; Xu, W.L.; Yang, M.C.; Guo, W.H.; He, S.Q.; Liu, W.T. Alginate-based hydrogels mediated biomedical applications: A review. Int. J. Biol. Macromol. 2024, 279, 135019. [Google Scholar] [CrossRef]
- King, A.H. Brown seaweed extracts (alginates). In Food hydrocolloids; CRC Press: Boca Raton, FL, USA, 2019; pp. 115–188. [Google Scholar]
- Leong, J.Y.; Lam, W.H.; Ho, K.W.; Voo, W.P.; Lee, M.F.X.; Lim, H.P.; Lim, S.L.; Tey, B.T.; Poncelet, D.; Chan, E.S. Advances in fabricating spherical alginate hydrogels with controlled particle designs by ionotropic gelation as encapsulation systems. Particuology 2016, 24, 44–60. [Google Scholar] [CrossRef]
- Tan, W.H.; Takeuchi, S. Monodisperse alginate hydrogel microbeads for cell encapsulation. Adv. Mater. 2007, 19, 2696. [Google Scholar] [CrossRef]
- Song, W.L.; Lima, A.C.; Mano, J.F. Bioinspired methodology to fabricate hydrogel spheres for multi-applications using superhydrophobic substrates. Soft Matter 2010, 6, 5868–5871. [Google Scholar] [CrossRef]
- Sun, R.; Gao, S.; Zhang, K.; Cheng, W.-T.; Hu, G. Recent advances in alginate-based composite gel spheres for removal of heavy metals. Int. J. Biol. Macromol. 2024, 268, 131853. [Google Scholar] [CrossRef]
- Dong, Y.X.; Ramey-Ward, A.N.; Salaita, K. Programmable Mechanically Active Hydrogel-Based Materials. Adv. Mater. 2021, 33, 2006600. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Tang, J.; Hu, Y.; Yang, S.; Xu, F.; Zrínyi, M.; Chen, Y.M. Magnetic hydrogel-based flexible actuators: A comprehensive review on design, properties, and applications. Chem. Eng. J. 2023, 462, 142193. [Google Scholar] [CrossRef]
- Ouyang, Y.; Huang, G.S.; Cui, J.Z.; Zhu, H.; Yan, G.H.; Mei, Y.F. Advances and Challenges of Hydrogel Materials for Robotic and Sensing Applications. Chem. Mater. 2022, 34, 9307–9328. [Google Scholar] [CrossRef]
- Wang, Z.H.; Li, W.J.; Li, C.; Klingner, A.; Pei, Y.T.; Misra, S.; Khalil, I.S.M. Magnetic alginate microrobots with dual-motion patterns through centrifugally driven flow control. Mater. Des. 2024, 246, 113337. [Google Scholar] [CrossRef]
- Chen, X.; Tian, C.Y.; Zhang, H.; Xie, H. Magnetic-actuated hydrogel microrobots with multimodal motion and collective behavior. J. Mater. Chem. B 2024, 12, 7440–7449. [Google Scholar] [CrossRef]
- Li, B.-Y.; Lin, T.-Y.; Lai, Y.-J.; Chiu, T.-H.; Yeh, Y.-C. Engineering Multiresponsive Alginate/PNIPAM/Carbon Nanotube Nanocomposite Hydrogels as On-Demand Drug Delivery Platforms. Small 2025, 21, 2407420. [Google Scholar] [CrossRef]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles-A review of production techniques and physical properties. Crit. Rev. Food Sci. 2017, 57, 1133–1152. [Google Scholar] [CrossRef]
- Wang, Y.T.; Shen, Z.P.; Wang, H.L.; Song, Z.P.; Yu, D.H.; Li, G.D.; Liu, X.N.; Liu, W.X. Progress in Research on Metal Ion Crosslinking Alginate-Based Gels. Gels 2025, 11, 16. [Google Scholar] [CrossRef]
- Cao, L.Q.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y.P. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef]
- Lee, B.B.; Ravindra, P.; Chan, E.S. Size and Shape of Calcium Alginate Beads Produced by Extrusion Dripping. Chem. Eng. Technol. 2013, 36, 1627–1642. [Google Scholar] [CrossRef]
- Mohamed, M.G.A.; Ambhorkar, P.; Samanipour, R.; Yang, A.; Ghafoor, A.; Kim, K. Microfluidics-based fabrication of cell-laden microgels. Biomicrofluidics 2020, 14, 021501. [Google Scholar] [CrossRef]
- Zhang, C.; Grossier, R.; Candoni, N.; Veesler, S. Preparation of alginate hydrogel microparticles by gelation introducing cross-linkers using droplet-based microfluidics: A review of methods. Biomater. Res. 2021, 25, 41. [Google Scholar] [CrossRef] [PubMed]
- Oveysi, M.; Zaker, M.A.; Peregrino, G.; Bazargan, V.; Marengo, M. Droplet-based fabrication of alginate hydrogel microparticles in presence of surfactants. Microfluid. Nanofluid 2023, 27, 45. [Google Scholar] [CrossRef]
- Liu, H.X.; Wang, C.Y.; Gao, Q.X.; Liu, X.X.; Tong, Z. Fabrication of novel core-shell hybrid alginate hydrogel beads. Int. J. Pharm. 2008, 351, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.F.; Ni, C.; Grist, S.M.; Bayly, C.; Cheung, K.C. Alginate core-shell beads for simplified three-dimensional tumor spheroid culture and drug screening. Biomed. Microdevices 2015, 17, 33. [Google Scholar] [CrossRef]
- Bennacef, C.; Desobry-Banon, S.; Probst, L.; Desobry, S. Alginate Core-Shell Capsules Production through Coextrusion Methods: Principles and Technologies. Mar. Drugs 2023, 21, 235. [Google Scholar] [CrossRef]
- Zhu, T.F.; Wan, L.; Li, R.Q.; Zhang, M.; Li, X.L.; Liu, Y.L.; Cai, D.J.; Lu, H.B. Janus structure hydrogels: Recent advances in synthetic strategies, biomedical microstructure and (bio)applications. Biomater. Sci. 2024, 12, 3003–3026. [Google Scholar] [CrossRef]
- Zhao, L.B.; Pan, L.; Zhang, K.; Guo, S.S.; Liu, W.; Wang, Y.; Chen, Y.; Zhao, X.Z.; Chan, H.L.W. Generation of Janus alginate hydrogel particles with magnetic anisotropy for cell encapsulation. Lab A Chip 2009, 9, 2981–2986. [Google Scholar] [CrossRef]
- Hwang, S.; Kwak, B.K.; Lee, J.; Kim, D.S.; Chang, S.T.; Park, J.; Lee, J. Janus hydrogel particles and their aggregation behavior. Macromol. Res. 2012, 20, 899–901. [Google Scholar] [CrossRef]
- Schmidt, B.V.K.J. Multicompartment Hydrogels. Macromol. Rapid Commun. 2022, 43, 2100895. [Google Scholar] [CrossRef]
- Håti, A.G.; Arnfinnsdottir, N.B.; Ostevold, C.; Sletmoen, M.; Etienne, G.; Amstad, E.; Stokke, B.T. Microarrays for the study of compartmentalized microorganisms in alginate microbeads and (W/O/W) double emulsions. RSC Adv. 2016, 6, 114830–114842. [Google Scholar] [CrossRef]
- Ciarleglio, G.; Placido, M.; Toto, E.; Santonicola, M.G. Dual-Responsive Alginate/PNIPAM Microspheres Fabricated by Microemulsion-Based Electrospray. Polymers 2024, 16, 2765. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Su, P.; Zhang, W.; Raston, C.L. Microfluidic Devices in Fabricating Nano or Micromaterials for Biomedical Applications. Adv. Mater. Technol. 2019, 4, 1900488. [Google Scholar] [CrossRef]
- Mazzitelli, S.; Bottaro, E.; Nastruzzi, C. Fabrication of Multifunctional Materials for Cell Transplantation by Microfluidics. RSC Smart Mater. 2017, 24, 566–609. [Google Scholar]
- Hinojosa-Ventura, G.; Acosta-Cuevas, J.M.; Velázquez-Carriles, C.A.; Navarro-López, D.E.; López-Alvarez, M.Á.; Ortega-de la Rosa, N.D.; Silva-Jara, J.M. From Basic to Breakthroughs: The Journey of Microfluidic Devices in Hydrogel Droplet Generation. Gels 2025, 11, 309. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Fu, Y.Q.; Jiang, R.; Yao, J.; Xiao, L.; Zeng, G.M. Optimization of Copper(II) Adsorption onto Novel Magnetic Calcium Alginate/Maghemite Hydrogel Beads Using Response Surface Methodology. Ind. Eng. Chem. Res. 2014, 53, 4059–4066. [Google Scholar] [CrossRef]
- Cholewinski, A.; Yang, F.K.; Zhao, B.X. Underwater Contact Behavior of Alginate and Catechol-Conjugated Alginate Hydrogel Beads. Langmuir 2017, 33, 8353–8361. [Google Scholar] [CrossRef]
- Lekka, M.; Sainz-Serp, D.; Kulik, A.J.; Wandrey, C. Hydrogel microspheres: Influence of chemical composition on surface morphology, local elastic properties, and bulk mechanical characteristics. Langmuir 2004, 20, 9968–9977. [Google Scholar] [CrossRef]
- Salahuddin, B.; Warren, H.; Spinks, G.M. Thermally actuated hydrogel bead based braided artificial muscle. Smart Mater. Struct. 2020, 29, 055042. [Google Scholar] [CrossRef]
- Zhang, H.J.; Zhang, Y.A.; He, L.F.; Yang, B.A.; Zhu, S.J.; Yao, M.H. Thermal-responsive poly(N-isopropyl acrylamide)/sodium alginate hydrogels: Preparation, swelling behaviors, and mechanical properties. Colloid Polym. Sci. 2016, 294, 1959–1967. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.W.; Qu, Q.D.; Pan, S.; Yu, K.Y.; Liu, Y.S. Graphene oxide modified sodium alginate/polyethylene glycol phase change material hydrogel scaffold composite with photothermal temperature control for potential bone tissue regeneration. J. Mater. Res. Technol. 2024, 30, 2446–2457. [Google Scholar] [CrossRef]
- Gan, L.; Li, H.; Chen, L.W.; Xu, L.J.; Liu, J.; Geng, A.B.; Mei, C.T.; Shang, S.M. Graphene oxide incorporated alginate hydrogel beads for the removal of various organic dyes and bisphenol A in water. Colloid Polym. Sci. 2018, 296, 607–615. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Wang, Y.; Li, Q.; Yu, C.; Chu, W.L. Natural Polymer-based Stimuli-responsive Hydrogels. Curr. Med. Chem. 2020, 27, 2631–2657. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, W.; Petcharoen, K.; Paradee, N.; Lerdwijitjarud, W.; Sirivat, A. Electrically responsive materials based on polycarbazole/sodium alginate hydrogel blend for soft and flexible actuator application. Carbohydr. Polym. 2016, 151, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Wang, K.; Bai, B.; Hu, N.; Wang, H. Swelling and glyphosate-controlled release behavior of multi-responsive alginate-gP (NIPAm-co-NDEAm)-based hydrogel. Carbohydr. Polym. 2022, 282, 119113. [Google Scholar] [CrossRef]
- Mair, L.O.; Chowdhury, S.; Paredes-Juarez, G.A.; Guix, M.; Bi, C.; Johnson, B.; English, B.W.; Jafari, S.; Baker-McKee, J.; Watson-Daniels, J. Magnetically aligned nanorods in alginate capsules (MANiACs): Soft matter tumbling robots for manipulation and drug delivery. Micromachines 2019, 10, 230. [Google Scholar] [CrossRef]
- Tirella, A.; Magliaro, C.; Penta, M.; Troncone, M.; Pimentel, R.; Ahluwalia, A. Sphyga: A multiparameter open source tool for fabricating smart and tunable hydrogel microbeads. Biofabrication 2014, 6, 025009. [Google Scholar] [CrossRef]
- Alshehri, A.M.; Wilson, O.C.; Dahal, B.; Philip, J.; Luo, X.L.; Raub, C.B. Magnetic nanoparticle-loaded alginate beads for local micro-actuation of tissue constructs. Colloid Surface B 2017, 159, 945–955. [Google Scholar] [CrossRef]
- Salahuddin, B.; Aziz, S.; Gao, S.; Hossain, M.S.A.; Billah, M.; Zhu, Z.H.; Amiralian, N. Magnetic Hydrogel Composite for Wastewater Treatment. Polymers 2022, 14, 5074. [Google Scholar] [CrossRef]
- Majumdar, S.; Krishnatreya, G.; Gogoi, N.; Thakur, D.; Chowdhury, D. Carbon-Dot-Coated Alginate Beads as a Smart Stimuli-Responsive Drug Delivery System. ACS Appl. Mater. Interfaces 2016, 8, 34179–34184. [Google Scholar] [CrossRef]
- Tiwari, A.; Gajbhiye, V.; Jain, A.; Verma, A.; Shaikh, A.; Salve, R.; Jain, S.K. Hyaluronic acid functionalized liposomes embedded in biodegradable beads for duo drugs delivery to oxaliplatin-resistant colon cancer. J. Drug Deliv. Sci. Technol. 2022, 77, 103891. [Google Scholar] [CrossRef]
- Roquero, D.M.; Katz, E. “Smart” alginate hydrogels in biosensing, bioactuation and biocomputing: State-of-the-art and perspectives. Sens. Actuators Rep. 2022, 4, 100095. [Google Scholar] [CrossRef]
- Lu, L.; Zhao, H.; Lu, Y.; Zhang, Y.; Wang, X.; Fan, C.; Li, Z.; Wu, Z. Design and Control of the Magnetically Actuated Micro/Nanorobot Swarm toward Biomedical Applications. Adv. Healthc. Mater. 2024, 13, 2400414. [Google Scholar] [CrossRef] [PubMed]
- Maity, C.; Das, N. Alginate-Based Smart Materials and Their Application: Recent Advances and Perspectives. Top. Curr. Chem. 2022, 380, 1–67. [Google Scholar] [CrossRef] [PubMed]
- Swamy, B.Y.; Chang, J.H.; Ahn, H.; Lee, W.K.; Chung, I. Thermoresponsive N-vinyl caprolactam grafted sodium alginate hydrogel beads for the controlled release of an anticancer drug. Cellulose 2013, 20, 1261–1273. [Google Scholar] [CrossRef]
- Lencina, M.M.S.; Iatridi, Z.; Villar, M.A.; Tsitsilianis, C. Thermoresponsive hydrogels from alginate-based graft copolymers. Eur. Polym. J. 2014, 61, 33–44. [Google Scholar] [CrossRef]
- Liu, M.; Wen, Y.T.; Song, X.; Zhu, J.L.; Li, J. A smart thermoresponsive adsorption system for efficient copper ion removal based on alginate-poly(-isopropylacrylamide) graft copolymer. Carbohydr. Polym. 2019, 219, 280–289. [Google Scholar] [CrossRef]
- Zhang, J.N.; Cui, Z.F.; Field, R.; Moloney, M.G.; Rimmer, S.; Ye, H. Thermo-responsive microcarriers based on poly(-isopropylacrylamide). Eur. Polym. J. 2015, 67, 346–364. [Google Scholar] [CrossRef]
- Degen, P.; Leick, S.; Siedenbiedel, F.; Rehage, H. Magnetic switchable alginate beads. Colloid Polym. Sci. 2012, 290, 97–106. [Google Scholar] [CrossRef]
- Fu, Y.; Yao, J.J.; Zhao, H.H.; Zhao, G.; Wan, Z.S.; Guo, R.Z. A muscle-like magnetorheological actuator based on bidisperse magnetic particles enhanced flexible alginate-gelatin sponges. Smart Mater. Struct. 2020, 29, 015019. [Google Scholar] [CrossRef]
- Gunatilake, U.B.; Venkatesan, M.; Basabe-Desmonts, L.; Benito-Lopez, F. and u Magnetic Phase Synthesised Magneto-Driven Alginate Beads. J. Colloid Interface Sci. 2022, 610, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, S.; Yao, J.; Wei, K.; Yu, T.; Fang, M.; Jiang, Z. Electrically actuated characteristics and electrochemical mechanism of a flexible biomimetic artificial muscle regulated by the responsive polymer of the calcium alginate gelation. Polym. Test. 2023, 126, 108163. [Google Scholar] [CrossRef]
- Palza, H.; Zapata, P.A.; Angulo-Pineda, C. Electroactive Smart Polymers for Biomedical Applications. Materials 2019, 12, 277. [Google Scholar] [CrossRef]
- Gehlen, D.B. Microgel-based Regenerative Materials and Biofunctionalization. Doctoral Dissertation, RWTH Aachen University, Aachen, Germany, 2021. [Google Scholar]
- Appiah, C.; Arndt, C.; Siemsen, K.; Heitmann, A.; Staubitz, A.; Selhuber-Unkel, C. Living Materials Herald a New Era in Soft Robotics. Adv. Mater. 2019, 31, 1807747. [Google Scholar] [CrossRef] [PubMed]
- Tordi, P.; Tamayo, A.; Jeong, Y.; Bonini, M.; Samorì, P. Multiresponsive Ionic Conductive Alginate/Gelatin Organohydrogels with Tunable Functions. Adv. Funct. Mater. 2024, 34, 2410663. [Google Scholar] [CrossRef]
- Das, A.; Babu, A.; Chakraborty, S.; Van Guyse, J.F.R.; Hoogenboom, R.; Maji, S. Poly(-isopropylacrylamide) and Its Copolymers: A Review on Recent Advances in the Areas of Sensing and Biosensing. Adv. Funct. Mater. 2024, 34, 2402432. [Google Scholar] [CrossRef]
- Hanyková, L.; Stastná, J.; Krakovsky, I. Responsive Acrylamide-Based Hydrogels: Advances in Interpenetrating Polymer Structures. Gels 2024, 10, 414. [Google Scholar] [CrossRef]
- Deng, Z.X.; Yu, R.; Guo, B.L. Stimuli-responsive conductive hydrogels: Design, properties, and applications. Mater. Chem. Front. 2021, 5, 2092–2123. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Chen, Z.; Xu, J.H. Recent advances in the microfluidic generation of shape-controllable hydrogel microparticles and their applications. Green Chem. Eng. 2024, 5, 16–30. [Google Scholar] [CrossRef]
- Shang, J.J.; Le, X.X.; Zhang, J.W.; Chen, T.; Theato, P. Trends in polymeric shape memory hydrogels and hydrogel actuators (vol 10, pg 1036, 2019). Polym. Chem. 2019, 10, 1036–1055. [Google Scholar] [CrossRef]
- Hu, L.; Wan, Y.; Zhang, Q.; Serpe, M.J. Harnessing the Power of Stimuli-Responsive Polymers for Actuation. Adv. Funct. Mater. 2020, 30, 1903471. [Google Scholar] [CrossRef]
- Zhang, X.; Aziz, S.; Zhu, Z.H. Tough and Fast Thermoresponsive Hydrogel Soft Actuators. Adv. Mater. Technol. 2025, 2401920. [Google Scholar] [CrossRef]
- Zhang, X.; Aziz, S.; Salahuddin, B.; Zhu, Z.H. Bioinspired Hydro- and Hydrothermally Responsive Tubular Soft Actuators. ACS Appl. Mater. Interfaces 2024, 16, 59202–59215. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.; Zhang, X.; Naficy, S.; Salahuddin, B.; Jager, E.W.H.; Zhu, Z.H. Plant-Like Tropisms in Artificial Muscles. Adv. Mater. 2023, 35, 2212046. [Google Scholar] [CrossRef]
- Park, J.; Guan, W.X.; Yu, G.H. Smart Hydrogels for Sustainable Agriculture. EcoMat 2025, 7, e70011. [Google Scholar] [CrossRef]
- Mao, S.D.; Johir, M.A.; Onggowarsito, C.; Feng, A.; Nghiem, L.D.; Fu, Q. Recent developments of hydrogel based solar water purification technology. Mater. Adv. 2022, 3, 1322–1340. [Google Scholar] [CrossRef]
- Sroka, K.; Sroka, P. Superabsorbent Hydrogels in the Agriculture and Reclamation of Degraded Areas. Sustainability 2024, 16, 2945. [Google Scholar] [CrossRef]
- Chung, H.J.; Parsons, A.M.; Zheng, L.L. Magnetically Controlled Soft Robotics Utilizing Elastomers and Gels in Actuation: A Review. Adv. Intell. Syst. 2021, 3, 2000186. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Bi, C.H.; Cappelleri, D.J.; Ciuti, G.; Conn, A.T.; Faivre, D.; Habibi, N.; Hosovsky, A.; Iacovacci, V.; Khalil, I.S.M.; et al. Magnetic Actuation Methods in Bio/Soft Robotics. Adv. Funct. Mater. 2021, 31, 2005137. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, G.G.; Medina-Sánchez, M.; Guix, M.; Makarov, D.; Jin, D.Y. Responsive Magnetic Nanocomposites for Intelligent Shape-Morphing Microrobots. ACS Nano 2023, 17, 8899–8917. [Google Scholar] [CrossRef]
- Philippova, O.; Barabanova, A.; Molchanov, V.; Khokhlov, A. Magnetic polymer beads: Recent trends and developments in synthetic design and applications. Eur. Polym. J. 2011, 47, 542–559. [Google Scholar] [CrossRef]
- Fusco, S.; Sakar, M.S.; Kennedy, S.; Peters, C.; Pane, S.; Mooney, D.; Nelson, B.J. Self-folding mobile microrobots for biomedical applications. In Proceedings of the 2014 IEEE International Conference on Robotics and Automation (ICRA), Hong Kong, China, 31 May–7 June 2014; pp. 3777–3782. [Google Scholar]
- Chen, Q.; Wu, S. Stimuli-Responsive Polymers for Tubal Actuators. Chem. Eur. J. 2025, 31, e202403429. [Google Scholar] [CrossRef] [PubMed]
- Incel, A.; Demir, M.M. Triboluminescent composite microspheres consisting of alginate and EuD TEA crystals. Sens. Actuators A Phys. 2018, 269, 556–562. [Google Scholar] [CrossRef]
- Habib, M.; Berthalon, S.; Leclercq, L.; Tourrette, A.; Sharkawi, T.; Blanquer, S. Dual Cross-Linked Stimuli-Responsive Alginate-Based Hydrogels. Biomacromolecules 2024, 25, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Tordi, P.; Ridi, F.; Samori, P.; Bonini, M. Cation-Alginate Complexes and Their Hydrogels: A Powerful Toolkit for the Development of Next-Generation Sustainable Functional Materials. Adv. Funct. Mater. 2025, 35, 2416390. [Google Scholar] [CrossRef]
- Banerjee, H.; Suhail, M.; Ren, H.L. Hydrogel Actuators and Sensors for Biomedical Soft Robots: Brief Overview with Impending Challenges. Biomimetics 2018, 3, 15. [Google Scholar] [CrossRef]
- Bercea, M. Bioinspired Hydrogels as Platforms for Life-Science Applications: Challenges and Opportunities. Polymers 2022, 14, 2365. [Google Scholar] [CrossRef]
- Buenger, D.; Topuz, F.; Groll, J. Hydrogels in sensing applications. Prog. Polym. Sci. 2012, 37, 1678–1719. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Tottori, N.; Nisisako, T. Microfluidic synthesis of highly spherical calcium alginate hydrogels based on external gelation using an emulsion reactant. Sens. Actuators B Chem. 2019, 283, 802–809. [Google Scholar] [CrossRef]
- Li, T.; Yu, S.; Sun, B.; Li, Y.; Wang, X.; Pan, Y.; Song, C.; Ren, Y.; Zhang, Z.; Grattan, K.T.V.; et al. Bioinspired claw-engaged and biolubricated swimming microrobots creating active retention in blood vessels. Sci. Adv. 2023, 9, eadg4501. [Google Scholar] [CrossRef]
- Moe, S.T.; Skjaak-Braek, G.; Elgsaeter, A.; Smidsroed, O. Swelling of covalently crosslinked alginate gels: Influence of ionic solutes and nonpolar solvents. Macromolecules 1993, 26, 3589–3597. [Google Scholar] [CrossRef]
- Lee, K.Y.; Rowley, J.A.; Eiselt, P.; Moy, E.M.; Bouhadir, K.H.; Mooney, D.J. Controlling mechanical and swelling properties of alginate hydrogels independently by cross-linker type and cross-linking density. Macromolecules 2000, 33, 4291–4294. [Google Scholar] [CrossRef]
- Salahuddin, B.B. Hydrogel Based Braided Artificial Muscles; University of Wollongong: Wollongong, NSW, Australia, 2020. [Google Scholar]
- Supramaniam, J.; Adnan, R.; Kaus, N.H.M.; Bushra, R. Magnetic nanocellulose alginate hydrogel beads as potential drug delivery system. Int. J. Biol. Macromol. 2018, 118, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Solanki, S.; Chaudhari, R.; Bahadur, D.; Aslam, M.; Srivastava, R. Multifunctional alginate microspheres for biosensing, drug delivery and magnetic resonance imaging. Acta Biomater. 2011, 7, 3955–3963. [Google Scholar] [CrossRef]
- Li, J.Y.; Li, X.J.; Luo, T.; Wang, R.; Liu, C.C.; Chen, S.X.; Li, D.F.; Yue, J.B.; Cheng, S.H.; Sun, D. Development of a magnetic microrobot for carrying and delivering targeted cells. Sci. Robot. 2018, 3, eaat8829. [Google Scholar] [CrossRef]
- Singh, A.; Kar, A.K.; Singh, D.; Verma, R.; Shraogi, N.; Zehra, A.; Gautam, K.; Anbumani, S.; Ghosh, D.; Patnaik, S. pH-responsive eco-friendly chitosan modified cenosphere/alginate composite hydrogel beads as carrier for controlled release of Imidacloprid towards sustainable pest control. Chem. Eng. J. 2022, 427, 131215. [Google Scholar] [CrossRef]
- Ahmad, A.; Ahmad, I.; Kamal, T.; Asiri, A.M.; Tabassum, S. Sodium Alginate-Based Nanomaterials for Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Li, K.; Sun, J.H.; He, S.P.; Zhou, X.X.; Li, H.Y.; Liu, Y.X. On-demand preparation of calcium alginate microspheres via piezoelectric microfluidics. Sens. Actuators A Phys. 2022, 347, 113925. [Google Scholar] [CrossRef]
- Ding, F.; Zhang, L.; Chen, X.; Yin, W.; Ni, L.; Wang, M. Photothermal nanohybrid hydrogels for biomedical applications. Front. Bioeng. Biotechnol. 2022, 10, 1066617. [Google Scholar] [CrossRef]
- Cui, X.; Ruan, Q.; Zhuo, X.; Xia, X.; Hu, J.; Fu, R.; Li, Y.; Wang, J.; Xu, H. Photothermal Nanomaterials: A Powerful Light-to-Heat Converter. Chem. Rev. 2023, 123, 6891–6952. [Google Scholar] [CrossRef]
- van der Sman, R.G.M. Biopolymer gel swelling analysed with scaling laws and Flory–Rehner theory. Food Hydrocoll. 2015, 48, 94–101. [Google Scholar] [CrossRef]
- Malektaj, H.; Drozdov, A.D.; deClaville Christiansen, J. Mechanical Properties of Alginate Hydrogels Cross-Linked with Multivalent Cations. Polymers 2023, 15, 3012. [Google Scholar] [CrossRef] [PubMed]
- Abroug, N.; Schöbel, L.; Boccaccini, A.R.; Seitz, H. Quantitative Macromolecular Modeling Assay of Biopolymer-Based Hydrogels. Gels 2024, 10, 676. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.I. Kinetics of drug release from hydrogel matrices. J. Control. Release 1985, 2, 277–288. [Google Scholar] [CrossRef]
- Jastram, A.; Lindner, T.; Luebbert, C.; Sadowski, G.; Kragl, U. Swelling and Diffusion in Polymerized Ionic Liquids-Based Hydrogels. Polymers 2021, 13, 1834. [Google Scholar] [CrossRef]
- Bertos, G.A.; Papadopoulos, E.G. Chapter Six—Upper-Limb Prosthetic Devices. In Handbook of Biomechatronics; Segil, J., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 177–240. [Google Scholar]
- Yang, C.; Wang, W.; Yao, C.; Xie, R.; Ju, X.-J.; Liu, Z.; Chu, L.-Y. Hydrogel Walkers with Electro-Driven Motility for Cargo Transport. Sci. Rep. 2015, 5, 13622. [Google Scholar] [CrossRef]
- Shen, S.; Qiu, J.; Huo, D.; Xia, Y. Nanomaterial-Enabled Photothermal Heating and Its Use for Cancer Therapy via Localized Hyperthermia. Small 2024, 20, 2305426. [Google Scholar] [CrossRef]
- Zhuang, J.; Jia, L.; Li, C.; Yang, R.; Wang, J.; Wang, W.-a.; Zhou, H.; Luo, X. Recent advances in photothermal nanomaterials for ophthalmic applications. Beilstein J. Nanotechnol. 2025, 16, 195–215. [Google Scholar] [CrossRef]
- Yang, Y.; Kirmizitas, F.C.; Sokolich, M.; Valencia, A.; Rivas, D.; Karakan, M.Ç.; White, A.E.; Malikopoulos, A.A.; Das, S. Rolling Helical Microrobots for Cell Patterning. In Proceedings of the 2023 International Conference on Manipulation, Automation and Robotics at Small Scales (MARSS), Abu Dhabi, United Arab Emirates, 9–13 October 2023; pp. 1–6. [Google Scholar]
- Zhou, H.; Mayorga-Martinez, C.C.; Pané, S.; Zhang, L.; Pumera, M. Magnetically Driven Micro and Nanorobots. Chem. Rev. 2021, 121, 4999–5041. [Google Scholar] [CrossRef]
- Xu, T.; Yu, J.; Yan, X.; Choi, H.; Zhang, L. Magnetic Actuation Based Motion Control for Microrobots: An Overview. Micromachines 2015, 6, 1346–1364. [Google Scholar] [CrossRef]
- Ahmadinejad, M.; Marshall, J.S. Magnetic nanoparticle interaction with a hydrogel in an oscillating magnetic field. Phys. Fluids 2024, 36, 013104. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, W.; Xie, L.; Liu, Y.; Wang, X.; Wu, X.; Zhou, F.; Hu, H. Experimental study on mechanism of stable drag reduction with hydrogel interface. Tribol. Int. 2023, 178, 108013. [Google Scholar] [CrossRef]
- Zang, J.; Liu, F. Modified Timoshenko formula for bending of ultrathin strained bilayer films. Appl. Phys. Lett. 2008, 92, 021905. [Google Scholar] [CrossRef]
- Kim, J.; Kim, C.; Song, Y.; Jeong, S.-G.; Kim, T.-S.; Lee, C.-S. Reversible self-bending soft hydrogel microstructures with mechanically optimized designs. Chem. Eng. J. 2017, 321, 384–393. [Google Scholar] [CrossRef]
- Scalet, G. Programmable materials: Current trends, challenges, and perspectives. Appl. Mater. Today 2024, 40, 102372. [Google Scholar] [CrossRef]
- Xu, K.; Yuan, G.; Zheng, J.; Zhang, Y.; Wang, J.; Guo, H. Bioinspired microrobots and their biomedical applications. Nanoscale 2024, 16, 20434–20450. [Google Scholar] [CrossRef]
- Li, J.; Esteban-Fernández de Ávila, B.; Gao, W.; Zhang, L.; Wang, J. Micro/nanorobots for biomedicine: Delivery, surgery, sensing, and detoxification. Sci. Robot. 2017, 2, eaam6431. [Google Scholar] [CrossRef]
- Salahuddin, B.; Aziz, S.; Zhang, X.; Feng, D.; Zhu, Z. Biomorph Soft Actuators with Tardigrade-Like Resilience. Adv. Funct. Mater. 2024, 34, 2404962. [Google Scholar] [CrossRef]
- Tian, Y.; Han, W.; Yeung, K.L. Magnetic Microsphere Scaffold-Based Soft Microbots for Targeted Mesenchymal Stem Cell Delivery. Small 2023, 19, 2300430. [Google Scholar] [CrossRef]
- Jeong, C.; Kim, S.; Lee, C.; Cho, S.; Kim, S.-B. Changes in the Physical Properties of Calcium Alginate Gel Beads under a Wide Range of Gelation Temperature Conditions. Foods 2020, 9, 180. [Google Scholar] [CrossRef]
- Schneider, F.; Draheim, J.; Kamberger, R.; Wallrabe, U. Process and material properties of polydimethylsiloxane (PDMS) for Optical MEMS. Sens. Actuators A Phys. 2009, 151, 95–99. [Google Scholar] [CrossRef]
- Zhou, B.; Aouraghe, M.A.; Chen, W.; Jiang, Q.; Xu, F. Highly Responsive Soft Electrothermal Actuator with High-Output Force Based on Polydimethylsiloxane (PDMS)-Coated Carbon Nanotube (CNT) Sponge. Nano Lett. 2023, 23, 6504–6511. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.; Spinks, G.M. Torsional artificial muscles. Mater. Horiz. 2020, 7, 667–693. [Google Scholar] [CrossRef]
- Aziz, S.; Villacorta, B.; Naficy, S.; Salahuddin, B.; Gao, S.; Baigh, T.A.; Sangian, D.; Zhu, Z. A microwave powered polymeric artificial muscle. Appl. Mater. Today 2021, 23, 101021. [Google Scholar] [CrossRef]
- Peng, S.; Cao, X.; Sun, Y.; Chen, L.; Ma, C.; Yang, L.; Zhao, H.; Liu, Q.; Liu, Z.; Ma, C. Polyurethane Shape Memory Polymer/pH-Responsive Hydrogel Hybrid for Bi-Function Synergistic Actuations. Gels 2023, 9, 428. [Google Scholar] [CrossRef]
- Pringpromsuk, S.; Xia, H.; Ni, Q.-Q. Multifunctional stimuli-responsive shape memory polyurethane gels for soft actuators. Sens. Actuators A Phys. 2020, 313, 112207. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanam, U.S.; Jeong, H.T.; Mutlu, R.; Aziz, S. Alginate Sphere-Based Soft Actuators. Gels 2025, 11, 432. https://doi.org/10.3390/gels11060432
Khanam US, Jeong HT, Mutlu R, Aziz S. Alginate Sphere-Based Soft Actuators. Gels. 2025; 11(6):432. https://doi.org/10.3390/gels11060432
Chicago/Turabian StyleKhanam, Umme Salma, Hyeon Teak Jeong, Rahim Mutlu, and Shazed Aziz. 2025. "Alginate Sphere-Based Soft Actuators" Gels 11, no. 6: 432. https://doi.org/10.3390/gels11060432
APA StyleKhanam, U. S., Jeong, H. T., Mutlu, R., & Aziz, S. (2025). Alginate Sphere-Based Soft Actuators. Gels, 11(6), 432. https://doi.org/10.3390/gels11060432











