Multiplexing 3D Natural Scaffolds to Optimize the Repair and Regeneration of Chronic Diabetic Wounds
Abstract
1. Introduction and Background
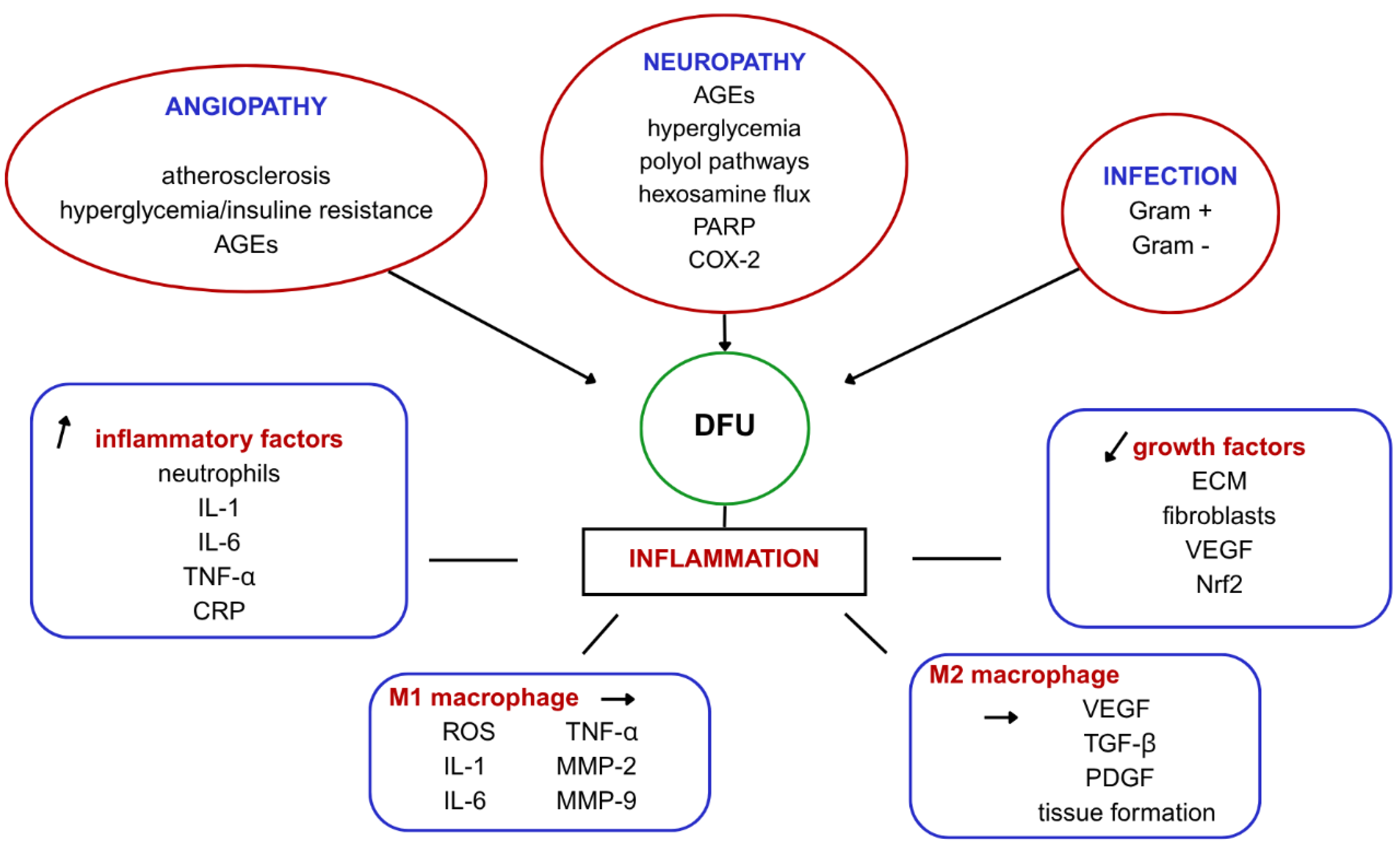
2. Mechanisms Behind Impaired Wound Healing in DFU
3. Conventional Management of DFU
4. Novel Therapeutic Approaches
4.1. Stem Cell Therapy: Advanced Wound Healing
4.2. Exosomes—Exploring a New Frontier in Wound Healing
5. Drug-Loaded Scaffolds: A Breakthrough in Chronic Wound Healing
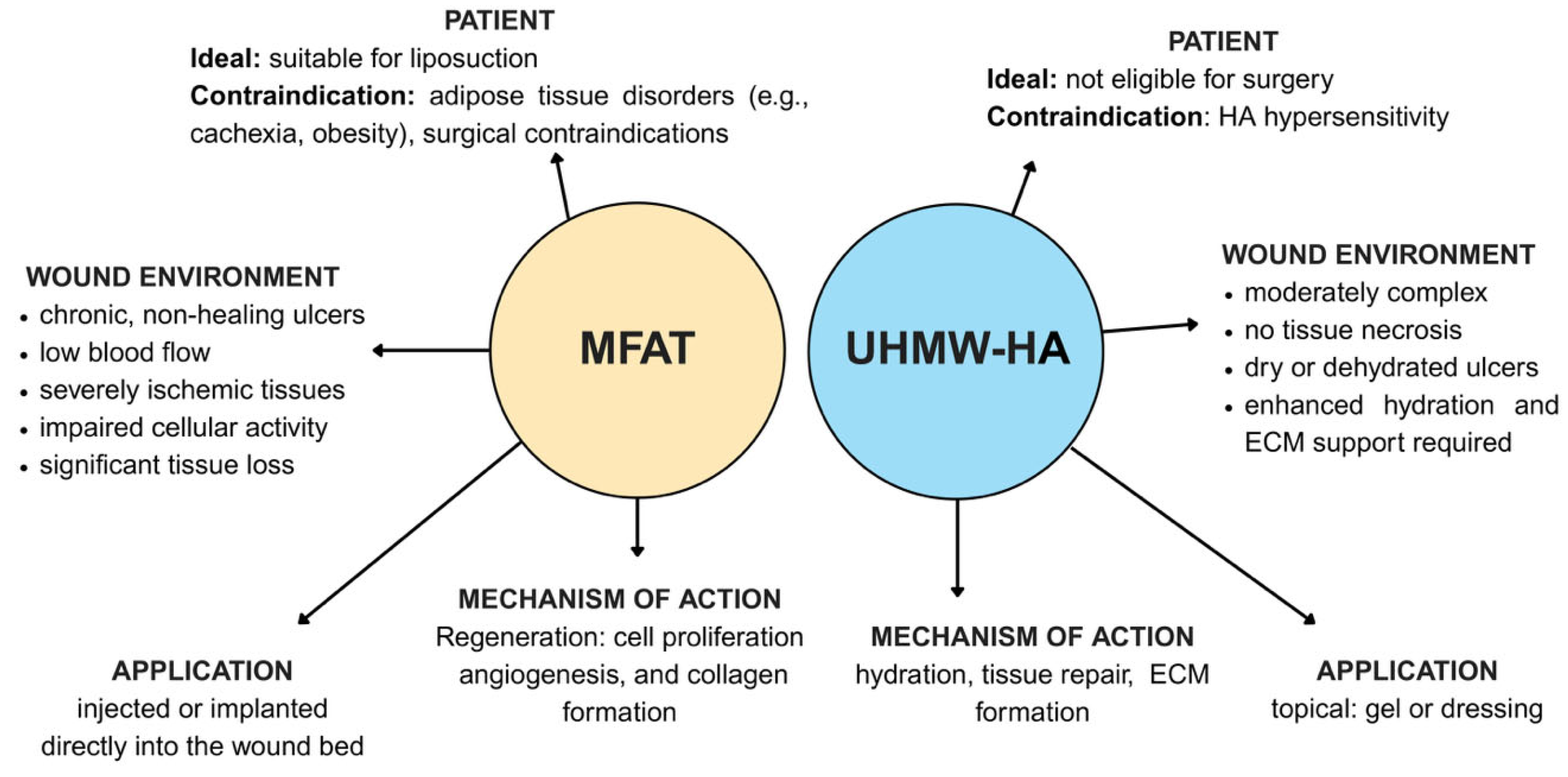
5.1. Microfragmented Adipose Tissue (MFAT) in DFU Healing: A Revolutionary Approach for Regeneration and Repair
5.2. Hydrogels in Chronic Wound Care
5.2.1. Chitosan—Properties, Applications, and Limitations in Diabetic Wounds
5.2.2. Alginate—Therapeutic Use and Challenges in Diabetic Ulcers
5.2.3. Collagen—Use and Limitations in Tissue Repair
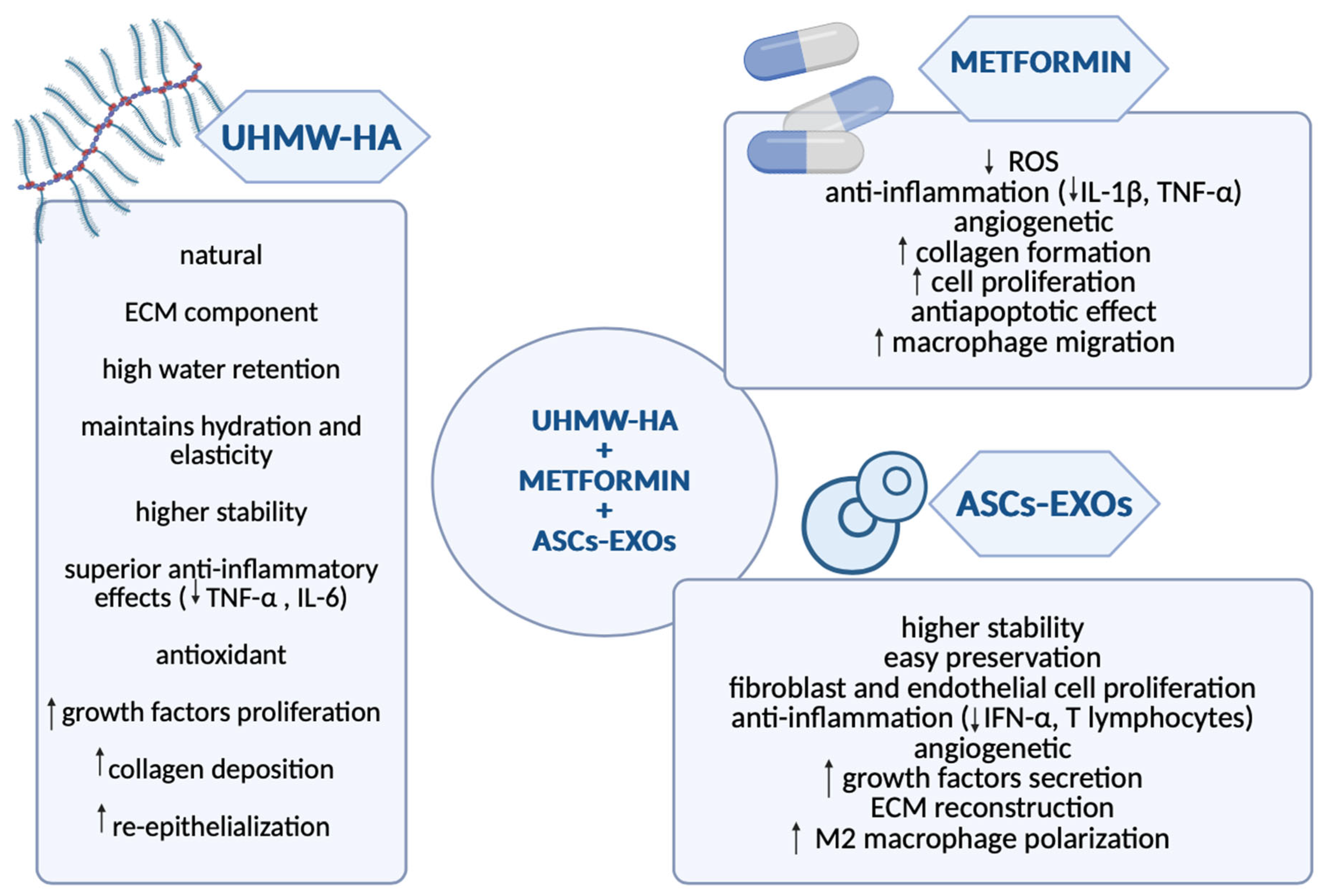
5.3. Hyaluronic Acid: Promoting Rapid Recovery and Efficient Drug Delivery for DFU Healing
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DM | diabetes mellitus |
| DFU | diabetic foot ulcer |
| VSMC | vascular smooth muscle cell |
| AGEs | advanced glycation end products |
| PARP | poly-ADP ribose polymerase |
| COX-2 | cyclooxygenase-2 |
| ECM | extracellular matrix |
| IL-1 | interleukin- 1 |
| IL-6 | interleukin-6 |
| TNF α | tumor necrosis factor alpha |
| CRP | C-reactive protein |
| VEGF | vascular endothelial growth factor |
| Nrf2 | nuclear factor erythroid-2-related factor 2 |
| ROS | reactive oxygen species |
| MMP 2 | metalloproteinase 2 |
| MMP 9 | metalloproteinase 9 |
| TGF β | tumor growth factor beta |
| PDGF | platelet-derived growth factor |
| HBOT | hyperbaric oxygen therapy |
| NPWT | negative pressure wound therapy |
| PRP | platelet-rich plasma |
| ESCs | embryonic stem cells |
| iPSCs | induced pluripotent stem cells |
| MSCs | mesenchymal stem cells |
| BM-MSCs | bone-marrow-derived MSCs |
| ASCs | adipose-derived MSCs |
| SVF | stromal vascular fraction |
| T2DM | type 2 diabetes mellitus |
| FGF-2 | fibroblast growth factor-2 |
| IGF-1 | insulin growth factor-1 |
| HGF | hepatocyte growth factor |
| KGF | keratinocyte growth factor |
| EXOs | exosomes |
| DNA | deoxyribonucleic acid |
| RNA | ribonucleic acid |
| miRNA | micro ribonucleic acid |
| IFN-α | interferon alpha |
| MFAT | micro-fragmented adipose tissue |
| GLP-1 | glucagon-like peptide-1 |
| HA | hyaluronic acid |
| UHMWA-HA | ultra-high molecular weight hyaluronic acid |
| pDA | polydopamine |
| EGF | epidermal growth factor |
| IL-10 | interleukin 10 |
References
- Shaw, J.; Sicree, R.; Zimmet, P. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Tan, T.-W.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers: A Review. JAMA 2023, 330, 62. [Google Scholar] [CrossRef] [PubMed]
- McDermott, K.; Fang, M.; Boulton, A.J.; Selvin, E.; Hicks, C.W. Etiology, Epidemiology, and Disparities in the Burden of Diabetic Foot Ulcers. Diabetes Care 2023, 46, 209–221. [Google Scholar] [CrossRef]
- Raja, J.M.; A Maturana, M.; Kayali, S.; Khouzam, A.; Efeovbokhan, N. Diabetic foot ulcer: A comprehensive review of pathophysiology and management modalities. World J. Clin. Cases 2023, 11, 1684–1693. [Google Scholar] [CrossRef]
- Deng, H.; Li, B.; Shen, Q.; Zhang, C.; Kuang, L.; Chen, R.; Wang, S.; Ma, Z.; Li, G. Mechanisms of diabetic foot ulceration: A review. J. Diabetes 2023, 15, 299–312. [Google Scholar] [CrossRef]
- Sandireddy, R.; Yerra, V.G.; Areti, A.; Komirishetty, P.; Kumar, A. Neuroinflammation and Oxidative Stress in Diabetic Neuropathy: Futuristic Strategies Based on These Targets. Int. J. Endocrinol. 2014, 2014, 674987. [Google Scholar] [CrossRef] [PubMed]
- Pitocco, D.; Spanu, T.; Di Leo, M.; Vitiello, R.; Rizzi, A.; Tartaglione, L.; Fiori, B.; Caputo, S.; Tinelli, G.; Zaccardi, F.; et al. Diabetic foot infections: A comprehensive overview. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 26–37. [Google Scholar] [CrossRef]
- Macdonald, K.E.; Boeckh, S.; Stacey, H.J.; Jones, J.D. The microbiology of diabetic foot infections: A meta-analysis. BMC Infect. Dis. 2021, 21, 770. [Google Scholar] [CrossRef]
- MacLeod, A.S.; Mansbridge, J.N. The Innate Immune System in Acute and Chronic Wounds. Adv. Wound Care 2016, 5, 65–78. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; He, W.; Mu, X.; Wu, X.; Deng, J.; Nie, X. Fibroblasts: Immunomodulatory factors in refractory diabetic wound healing. Front. Immunol. 2022, 13, 918223. [Google Scholar] [CrossRef]
- Burgess, J.L.; Wyant, W.A.; Abujamra, B.A.; Kirsner, R.S.; Jozic, I. Diabetic Wound-Healing Science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef]
- Jayasuriya, R.; Dhamodharan, U.; Karan, A.N.; Anandharaj, A.; Rajesh, K.; Ramkumar, K.M. Role of Nrf2 in MALAT1/ HIF-1α loop on the regulation of angiogenesis in diabetic foot ulcer. Free Radic. Biol. Med. 2020, 156, 168–175. [Google Scholar] [CrossRef]
- Morey, M.; O’Gaora, P.; Pandit, A.; Hélary, C. Hyperglycemia acts in synergy with hypoxia to maintain the pro-inflammatory phenotype of macrophages. PLoS ONE 2019, 14, e0220577. [Google Scholar] [CrossRef]
- Bandyk, D.F. The diabetic foot: Pathophysiology, evaluation, and treatment. Semin. Vasc. Surg. 2018, 31, 43–48. [Google Scholar] [CrossRef]
- Lim, J.Z.M.; Ng, N.S.L.; Thomas, C. Prevention and treatment of diabetic foot ulcers. J. R. Soc. Med. 2017, 110, 104–109. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Khan, J.; Noordin, S. Diabetic foot ulcers: Contemporary assessment and management. J. Pak. Med. Assoc. 2023, 73, 1480–1488. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, W.; Xu, Y.; Liu, D. Efficacy of hyperbaric oxygen therapy for diabetic foot ulcers: An updated systematic review and meta-analysis. Asian J. Surg. 2022, 45, 68–78. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, S.K.; Mudgal, S.K.; Jelly, P.; Thakur, K. Efficacy of hyperbaric oxygen therapy for diabetic foot ulcer, a systematic review and meta-analysis of controlled clinical trials. Sci. Rep. 2021, 11, 2189. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, S.; Da, J.; Wu, W.; Ma, F.; Tang, C.; Li, G.; Zhong, D.; Liao, B. A systematic review and meta-analysis of efficacy and safety of negative pressure wound therapy in the treatment of diabetic foot ulcer. Ann. Palliat. Med. 2021, 10, 10830–10839. [Google Scholar] [CrossRef]
- OuYang, H.; Tang, Y.; Yang, F.; Ren, X.; Yang, J.; Cao, H.; Yin, Y. Platelet-rich plasma for the treatment of diabetic foot ulcer: A systematic review. Front. Endocrinol. 2023, 14, 1256081. [Google Scholar] [CrossRef]
- Gupta, A.; Channaveera, C.; Sethi, S.; Ranga, S.; Anand, V. Efficacy of Intralesional Platelet-Rich Plasma in Diabetic Foot Ulcer. J. Am. Podiatr. Med. Assoc. 2021, 111, 07. [Google Scholar] [CrossRef]
- Canonico, S.; Campitiello, F.; Della, A.; Padovano, V.; Pellino, G. Treatment of Leg Chronic Wounds with Dermal Substitutes and Thin Skin Grafts. In Skin Grafts; Gore, M., Ed.; InTech: Rang-du-Fliers, France, 2013. [Google Scholar] [CrossRef]
- Lee, K.; Choi, J.; Cho, S.; Chung, J.; Moon, E.; Kim, N.; Han, H. Topical embryonic stem cells enhance wound healing in diabetic rats. J. Orthop. Res. 2011, 29, 1554–1562. [Google Scholar] [CrossRef]
- Kanji, S.; Das, H. Advances of Stem Cell Therapeutics in Cutaneous Wound Healing and Regeneration. Mediat. Inflamm. 2017, 2017, 5217967. [Google Scholar] [CrossRef]
- Clayton, Z.E.; Tan, R.P.; Miravet, M.M.; Lennartsson, K.; Cooke, J.P.; Bursill, C.A.; Wise, S.G.; Patel, S. Induced pluripotent stem cell-derived endothelial cells promote angiogenesis and accelerate wound closure in a murine excisional wound healing model. Biosci. Rep. 2018, 38, BSR20180563. [Google Scholar] [CrossRef]
- Ding, D.C.; Shyu, W.C.; Lin, S.Z. Mesenchymal Stem Cells. Cell Transpl. 2011, 20, 5–14. [Google Scholar] [CrossRef]
- Yu, X.; Liu, P.; Li, Z.; Zhang, Z. Function and mechanism of mesenchymal stem cells in the healing of diabetic foot wounds. Front. Endocrinol. 2023, 14, 1099310. [Google Scholar] [CrossRef]
- Farabi, B.; Roster, K.; Hirani, R.; Tepper, K.; Atak, M.F.; Safai, B. The Efficacy of Stem Cells in Wound Healing: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 3006. [Google Scholar] [CrossRef]
- Deptuła, M.; Brzezicka, A.; Skoniecka, A.; Zieliński, J.; Pikuła, M. Adipose-derived stromal cells for nonhealing wounds: Emerging opportunities and challenges. Med. Res. Rev. 2021, 41, 2130–2171. [Google Scholar] [CrossRef]
- Hassanshahi, A.; Hassanshahi, M.; Khabbazi, S.; Hosseini-Khah, Z.; Peymanfar, Y.; Ghalamkari, S.; Su, Y.; Xian, C.J. Adipose-derived stem cells for wound healing. J. Cell. Physiol. 2019, 234, 7903–7914. [Google Scholar] [CrossRef]
- Bellei, B.; Migliano, E.; Tedesco, M.; Caputo, S.; Papaccio, F.; Lopez, G.; Picardo, M. Adipose tissue-derived extracellular fraction characterization: Biological and clinical considerations in regenerative medicine. Stem Cell Res. Ther. 2018, 9, 207. [Google Scholar] [CrossRef]
- Carstens, M.H.; Quintana, F.J.; Calderwood, S.T.; Sevilla, J.P.; Ríos, A.B.; Rivera, C.M.; Calero, D.W.; Zelaya, M.L.; Garcia, N.; Bertram, K.A.; et al. Treatment of Chronic Diabetic Foot Ulcers with Adipose-Derived Stromal Vascular Fraction Cell Injections: Safety and Evidence of Efficacy at 1 Year. Stem Cells Transl. Med. 2021, 10, 1138–1147. [Google Scholar] [CrossRef]
- Bi, H.; Li, H.; Zhang, C.; Mao, Y.; Nie, F.; Xing, Y.; Sha, W.; Wang, X.; Irwin, D.M.; Tan, H. Stromal vascular fraction promotes migration of fibroblasts and angiogenesis through regulation of extracellular matrix in the skin wound healing process. Stem Cell Res. Ther. 2019, 10, 302. [Google Scholar] [CrossRef]
- Brembilla, N.C.; Vuagnat, H.; Boehncke, W.-H.; Krause, K.-H.; Preynat-Seauve, O. Adipose-Derived Stromal Cells for Chronic Wounds: Scientific Evidence and Roadmap Toward Clinical Practice. Stem Cells Transl. Med. 2022, 12, 17–25. [Google Scholar] [CrossRef]
- Vanderstichele, S.; Vranckx, J.J. Anti-fibrotic effect of adipose-derived stem cells on fibrotic scars. World J. Stem Cells 2022, 14, 200–213. [Google Scholar] [CrossRef]
- Han, S.; Kim, H.; Kim, W. The treatment of diabetic foot ulcers with uncultured, processed lipoaspirate cells: A pilot study. Wound Repair Regen. 2010, 18, 342–348. [Google Scholar] [CrossRef]
- Maharlooei, M.K.; Bagheri, M.; Solhjou, Z.; Jahromi, B.M.; Akrami, M.; Rohani, L.; Monabati, A.; Noorafshan, A.; Omrani, G.R. Adipose tissue derived mesenchymal stem cell (AD-MSC) promotes skin wound healing in diabetic rats. Diabetes Res. Clin. Pract. 2011, 93, 228–234. [Google Scholar] [CrossRef]
- Hamada, M.; Iwata, T.; Kato, Y.; Washio, K.; Morikawa, S.; Sakurai, H.; Yamato, M.; Okano, T.; Uchigata, Y. Xenogeneic transplantation of human adipose-derived stem cell sheets accelerate angiogenesis and the healing of skin wounds in a Zucker Diabetic Fatty rat model of obese diabetes. Regen. Ther. 2017, 6, 65–73. [Google Scholar] [CrossRef]
- Marino, G.; Moraci, M.; Armenia, E.; Orabona, C.; Sergio, R.; De Sena, G.; Capuozzo, V.; Barbarisi, M.; Rosso, F.; Giordano, G.; et al. Therapy with autologous adipose-derived regenerative cells for the care of chronic ulcer of lower limbs in patients with peripheral arterial disease. J. Surg. Res. 2013, 185, 36–44. [Google Scholar] [CrossRef]
- Shi, R.; Jin, Y.; Cao, C.; Han, S.; Shao, X.; Meng, L.; Cheng, J.; Zhang, M.; Zheng, J.; Xu, J.; et al. Localization of human adipose-derived stem cells and their effect in repair of diabetic foot ulcers in rats. Stem Cell Res. Ther. 2016, 7, 155. [Google Scholar] [CrossRef]
- Lombardi, F.; Palumbo, P.; Augello, F.R.; Cifone, M.G.; Cinque, B.; Giuliani, M. Secretome of Adipose Tissue-Derived Stem Cells (ASCs) as a Novel Trend in Chronic Non-Healing Wounds: An Overview of Experimental In Vitro and In Vivo Studies and Methodological Variables. Int. J. Mol. Sci. 2019, 20, 3721. [Google Scholar] [CrossRef]
- Mazini, L.; Rochette, L.; Admou, B.; Amal, S.; Malka, G. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int. J. Mol. Sci. 2020, 21, 1306. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Lin, S.; Tan, X.; Zhu, S.; Nie, F.; Zhen, Y.; Gu, L.; Zhang, C.; Wang, B.; Wei, W.; et al. Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Prolif. 2021, 54, e12993. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, B.; Yang, Y.; Jiang, Q.; Li, T.; Gong, J.; Tang, H.; Zhang, Q. Stem cell-derived exosomes: Emerging therapeutic opportunities for wound healing. Stem Cell Res. Ther. 2023, 14, 107. [Google Scholar] [CrossRef]
- Feng, J.; Yao, Y.; Wang, Q.; Han, X.; Deng, X.; Cao, Y.; Chen, X.; Zhou, M.; Zhao, C. Exosomes: Potential key players towards novel therapeutic options in diabetic wounds. Biomed. Pharmacother. 2023, 166, 115297. [Google Scholar] [CrossRef]
- Sharifiaghdam, M.; Shaabani, E.; Faridi-Majidi, R.; De Smedt, S.C.; Braeckmans, K.; Fraire, J.C. Macrophages as a therapeutic target to promote diabetic wound healing. Mol. Ther. 2022, 30, 2891–2908. [Google Scholar] [CrossRef]
- Xia, W.; Liu, Y.; Jiang, X.; Li, M.; Zheng, S.; Zhang, Z.; Huang, X.; Luo, S.; Khoong, Y.; Hou, M.; et al. Lean adipose tissue macrophage derived exosome confers immunoregulation to improve wound healing in diabetes. J. Nanobiotechnol. 2023, 21, 128. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Su, P.; Zhao, F.; Zhang, Q.; Huang, X.; He, C.; Wu, Q.; Wang, Z.; Ma, J.; Wang, Z. Adipose mesenchymal stem cell-derived exosomes promote skin wound healing in diabetic mice by regulating epidermal autophagy. Burn. Trauma 2024, 12, tkae001. [Google Scholar] [CrossRef]
- Li, X.; Xie, X.; Lian, W.; Shi, R.; Han, S.; Zhang, H.; Lu, L.; Li, M. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef]
- Salagean, A.; Nechifor-Boila, A.; Bajwa, N.; Pastorello, Y.; Slevin, M. Micro-Fragmented Adipose Tissue as a Natural Scaffold for Targeted Drug Delivery in Brain Cancer. Int. J. Mol. Sci. 2023, 24, 11530. [Google Scholar] [CrossRef]
- Nava, S.; Sordi, V.; Pascucci, L.; Tremolada, C.; Ciusani, E.; Zeira, O.; Cadei, M.; Soldati, G.; Pessina, A.; Parati, E.; et al. Long-Lasting Anti-Inflammatory Activity of Human Microfragmented Adipose Tissue. Stem Cells Int. 2019, 2019, 5901479. [Google Scholar] [CrossRef]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, B.; Shaw, I.; Lesme, H.; Yong, L.; Khan, N.; Tremolada, C.; Péault, B. Higher Pericyte Content and Secretory Activity of Microfragmented Human Adipose Tissue Compared to Enzymatically Derived Stromal Vascular Fraction. Stem Cells Transl. Med. 2018, 7, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Sabol, R.A.; Bowles, A.C.; Côté, A.; Wise, R.; Pashos, N.; Bunnell, B.A. Therapeutic Potential of Adipose Stem Cells. In Cell Biology and Translational Medicine, Volume 13; Turksen, K., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2018; Volume 1341, pp. 15–25. [Google Scholar] [CrossRef]
- Gečys, D.; Skredėnienė, R.; Gečytė, E.; Kazlauskas, A.; Balnytė, I.; Jekabsone, A. Adipose Tissue-Derived Stem Cell Extracellular Vesicles Suppress Glioblastoma Proliferation, Invasiveness and Angiogenesis. Cells 2023, 12, 1247. [Google Scholar] [CrossRef]
- Pascucci, L.; Coccè, V.; Bonomi, A.; Ami, D.; Ceccarelli, P.; Ciusani, E.; Viganò, L.; Locatelli, A.; Sisto, F.; Doglia, S.M.; et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: A new approach for drug delivery. J. Control. Release 2014, 192, 262–270. [Google Scholar] [CrossRef]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Corrigendum to “Exosomes as drug delivery vehicles for Parkinson’s disease therapy” [Journal of Controlled Release 207, (2015) 18–30]. J. Control. Release 2021, 339, 232–234. [Google Scholar] [CrossRef]
- Fuhrmann, G.; Serio, A.; Mazo, M.; Nair, R.; Stevens, M.M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Control. Release 2015, 205, 35–44. [Google Scholar] [CrossRef]
- Ceresa, C.; Borrone, A.; Fracchia, L.; Rinaldi, M.; Marchetti, A.; Tremolada, C.; Bosetti, M. Lipoaspirate Shows In Vitro Potential for Wound Healing. Pharmaceutics 2022, 14, 447. [Google Scholar] [CrossRef]
- Copeland, R.; Martin, J. Chronic prosthesis-related residual limb ulcer treated with autologous micro-fragmented adipose tissue. Regen. Ther. 2021, 18, 21–23. [Google Scholar] [CrossRef]
- Lonardi, R.; Leone, N.; Gennai, S.; Borsari, G.T.; Covic, T.; Silingardi, R. Autologous micro-fragmented adipose tissue for the treatment of diabetic foot minor amputations: A randomized controlled single-center clinical trial (MiFrAADiF). Stem Cell Res. Ther. 2019, 10, 223. [Google Scholar] [CrossRef]
- Seo, E.; Lim, J.S.; Jun, J.-B.; Choi, W.; Hong, I.-S.; Jun, H.-S. Exendin-4 in combination with adipose-derived stem cells promotes angiogenesis and improves diabetic wound healing. J. Transl. Med. 2017, 15, 35. [Google Scholar] [CrossRef]
- Rajabi, H.; Ahmadi, M.; Aslani, S.; Saberianpour, S.; Rahbarghazi, R. Exendin-4 as a Versatile Therapeutic Agent for the Amelioration of Diabetic Changes. Adv. Pharm. Bull. 2021, 12, 1. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, S.; Liang, K.; Wu, Z.; Yan, X.; Liu, W.; Li, J.; Wu, B.; Du, Y. Exendin-4 gene modification and microscaffold encapsulation promote self-persistence and antidiabetic activity of MSCs. Sci. Adv. 2021, 7, eabi4379. [Google Scholar] [CrossRef]
- del Moral, L.R.; Salazar, A.A.; Stefanov Kiuri, S.; Tong, H.; de Cubas, L.R.; García-Olmo, D.; García-Arranz, M. Phase Ib Open Clinical Trial to Assess the Safety of Autologous Mesenchymal Stem Cells for the Treatment of Nonrevascularizable Critical Lower Limb Ischemia. J. Stem Cell Res. Ther. 2017, 7, 06. [Google Scholar] [CrossRef]
- Soria-Juan, B.; Garcia-Arranz, M.; Jiménez, L.L.; Aparicio, C.; Gonzalez, A.; Fernandez, I.M.; del Moral, L.R.; Grochowicz, L.; Andreu, E.J.; Marin, P.; et al. Efficacy and safety of intramuscular administration of allogeneic adipose tissue derived and expanded mesenchymal stromal cells in diabetic patients with critical limb ischemia with no possibility of revascularization: Study protocol for a randomized controlled double-blind phase II clinical trial (The NOMA Trial). Trials 2021, 22, 595. [Google Scholar] [CrossRef]
- Ma, Y.; Dong, J.; Li, M.; Du, X.; Yan, Z.; Tian, W. An antimicrobial microneedle patch promotes functional healing of infected wounds through controlled release of adipose tissue-derived apoptotic vesicles. J. Nanobiotechnol. 2024, 22, 579. [Google Scholar] [CrossRef]
- Feng, J.; Mineda, K.; Wu, S.-H.; Mashiko, T.; Doi, K.; Kuno, S.; Kinoshita, K.; Kanayama, K.; Asahi, R.; Sunaga, A.; et al. An injectable non-cross-linked hyaluronic-acid gel containing therapeutic spheroids of human adipose-derived stem cells. Sci. Rep. 2017, 7, 1548. [Google Scholar] [CrossRef]
- Armstrong, D.G.D.; Harris, S.G.; Rasor, Z.D.; Zelen, C.M.D.; Kim, J.; Swerdlow, M.; Isaac, A.L.D. Autologous Minimally Manipulated Homologous Adipose Tissue (AMHAT) for Treatment of Nonhealing Diabetic Foot Ulcers. Plast. Reconstr. Surg.—Glob. Open 2022, 10, e4588. [Google Scholar] [CrossRef]
- Aronowitz, J.A.; Lockhart, R.A.; Hakakian, C.S. Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. SpringerPlus 2015, 4, 713. [Google Scholar] [CrossRef]
- Condé-Green, A.; Kotamarti, V.S.; Sherman, L.S.; Keith, J.D.; Lee, E.S.; Granick, M.S.; Rameshwar, P. Shift toward Mechanical Isolation of Adipose-derived Stromal Vascular Fraction: Review of Upcoming Techniques. Plast. Reconstr. Surg.—Glob. Open 2016, 4, e1017. [Google Scholar] [CrossRef]
- Pak, J.; Lee, J.H.; Kartolo, W.A.; Lee, S.H. Cartilage Regeneration in Human with Adipose Tissue-Derived Stem Cells: Current Status in Clinical Implications. BioMed Res. Int. 2016, 2016, 4702674. [Google Scholar] [CrossRef]
- Cianfarani, F.; Toietta, G.; Di Rocco, G.; Cesareo, E.; Zambruno, G.; Odorisio, T. Diabetes impairs adipose tissue–derived stem cell function and efficiency in promoting wound healing. Wound Repair Regen. 2013, 21, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Pomatto, M.; Gai, C.; Negro, F.; Cedrino, M.; Grange, C.; Ceccotti, E.; Togliatto, G.; Collino, F.; Tapparo, M.; Figliolini, F.; et al. Differential Therapeutic Effect of Extracellular Vesicles Derived by Bone Marrow and Adipose Mesenchymal Stem Cells on Wound Healing of Diabetic Ulcers and Correlation to Their Cargoes. Int. J. Mol. Sci. 2021, 22, 3851. [Google Scholar] [CrossRef]
- Fraser, J.K.; Wulur, I.; Alfonso, Z.; Hedrick, M.H. Fat tissue: An underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006, 24, 150–154. [Google Scholar] [CrossRef]
- Arabpour, Z.; Abedi, F.; Salehi, M.; Baharnoori, S.M.; Soleimani, M.; Djalilian, A.R. Hydrogel-Based Skin Regeneration. Int. J. Mol. Sci. 2024, 25, 1982. [Google Scholar] [CrossRef]
- Huang, C.; Dong, L.; Zhao, B.; Lu, Y.; Huang, S.; Yuan, Z.; Luo, G.; Xu, Y.; Qian, W. Anti-inflammatory hydrogel dressings and skin wound healing. Clin. Transl. Med. 2022, 12, e1094. [Google Scholar] [CrossRef]
- Bardill, J.R.; Laughter, M.R.; Stager, M.; Liechty, K.W.; Krebs, M.D.; Zgheib, C. Topical gel-based biomaterials for the treatment of diabetic foot ulcers. Acta Biomater. 2021, 138, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Rajinikanth, B.S.; Rajkumar, D.S.R.; K, K.; Vijayaragavan, V. Chitosan-Based Biomaterial in Wound Healing: A Review. Cureus 2024, 16, e55193. [Google Scholar] [CrossRef]
- Alven, S.; Aderibigbe, B.A. Chitosan and Cellulose-Based Hydrogels for Wound Management. Int. J. Mol. Sci. 2020, 21, 9656. [Google Scholar] [CrossRef] [PubMed]
- Reay, S.L.; Ferreira, A.M.; Hilkens, C.M.U.; Novakovic, K. The Paradoxical Immunomodulatory Effects of Chitosan in Biomedicine. Polymers 2024, 17, 19. [Google Scholar] [CrossRef]
- Escárcega-Galaz, A.A.; De La Cruz-Mercado, J.L.; López-Cervantes, J.; Sánchez-Machado, D.I.; Brito-Zurita, O.R.; Ornelas-Aguirre, J.M. Chitosan treatment for skin ulcers associated with diabetes. Saudi J. Biol. Sci. 2018, 25, 130–135. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [PubMed]
- Anushree, U.; Punj, P.; Vasumathi; Bharati, S. Phosphorylated chitosan accelerates dermal wound healing in diabetic wistar rats. Glycoconj. J. 2023, 40, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Al Mamun, A.; Zaidi, M.B.; Roome, T.; Hasan, A. A calcium peroxide incorporated oxygen releasing chitosan-PVA patch for Diabetic wound healing. Biomed. Pharmacother. 2023, 165, 115156. [Google Scholar] [CrossRef]
- Shang, S.; Zhuang, K.; Chen, J.; Zhang, M.; Jiang, S.; Li, W. A bioactive composite hydrogel dressing that promotes healing of both acute and chronic diabetic skin wounds. Bioact. Mater. 2024, 34, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Victor, R.D.S.; Santos, A.M.D.C.; De Sousa, B.V.; Neves, G.D.A.; Santana, L.N.D.L.; Menezes, R.R. A Review on Chitosan’s Uses as Biomaterial: Tissue Engineering, Drug Delivery Systems and Cancer Treatment. Materials 2020, 13, 4995. [Google Scholar] [CrossRef]
- Ribeiro, M.; Simões, M.; Vitorino, C.; Mascarenhas-Melo, F. Hydrogels in Cutaneous Wound Healing: Insights into Characterization, Properties, Formulation and Therapeutic Potential. Gels 2024, 10, 188. [Google Scholar] [CrossRef]
- Wang, X.; Guan, S.; Zhang, K.; Li, J. Benlysta-Loaded Sodium Alginate Hydrogel and Its Selective Functions in Promoting Skin Cell Growth and Inhibiting Inflammation. ACS Omega 2020, 5, 10395–10400. [Google Scholar] [CrossRef]
- Wang, T.; Gu, Q.; Zhao, J.; Mei, J.; Shao, M.; Pan, Y.; Zhang, J.; Wu, H.; Zhang, Z.; Liu, F. Calcium alginate enhances wound healing by up-regulating the ratio of collagen types I/III in diabetic rats. Int. J. Clin. Exp. Pathol. 2015, 8, 6636–6645. [Google Scholar]
- Yan, Y.; Ren, P.; Wu, Q.; Zhang, T. Precise Design of Alginate Hydrogels Crosslinked with Microgels for Diabetic Wound Healing. Biomolecules 2022, 12, 1582. [Google Scholar] [CrossRef]
- Shah, S.A.; Sohail, M.; Khan, S.A.; Kousar, M. Improved drug delivery and accelerated diabetic wound healing by chondroitin sulfate grafted alginate-based thermoreversible hydrogels. Mater. Sci. Eng. C 2021, 126, 112169. [Google Scholar] [CrossRef]
- Sheng, W.; Song, Q.; Su, X.; Lu, Y.; Bai, Y.; Ji, F.; Zhang, L.; Yang, R.; Fu, X. Sodium alginate/gelatin hydrogels loaded with adipose-derived mesenchymal stem cells promote wound healing in diabetic rats. J. Cosmet. Dermatol. 2023, 22, 1670–1679. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Raines, R.T. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Veves, A.; Sheehan, P.; Pham, H.T. A Randomized, Controlled Trial of Promogran (a Collagen/Oxidized Regenerated Cellulose Dressing) vs Standard Treatment in the Management of Diabetic Foot Ulcers. Arch. Surg. 2002, 137, 822. [Google Scholar] [CrossRef] [PubMed]
- Salagean, A.-A.; Moldovan, C.-A.; Slevin, M. Utilisation of High Molecular Weight and Ultra-High Molecular Weight Hyaluronan in Management of Glioblastoma. Gels 2025, 11, 50. [Google Scholar] [CrossRef]
- Neuman, M.G.; Nanau, R.M.; Oruña-Sanchez, L.; Coto, G. Hyaluronic Acid and Wound Healing. J. Pharm. Pharm. Sci. 2015, 18, 53. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic Acid Hydrogels for Biomedical Applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef]
- Al Bayaty, F.; Abdulla, M.; Abu Hassan, M.I.; Masud, M. Wound healing potential by hyaluronate gel in streptozotocin-induced diabetic rats. Sci. Res. Essays 2010, 5.18, 2756–2760. [Google Scholar]
- De Angelis, B.; D’autilio, M.F.L.M.; Orlandi, F.; Pepe, G.; Garcovich, S.; Scioli, M.G.; Orlandi, A.; Cervelli, V.; Gentile, P. Wound Healing: In Vitro and In Vivo Evaluation of a Bio-Functionalized Scaffold Based on Hyaluronic Acid and Platelet-Rich Plasma in Chronic Ulcers. J. Clin. Med. 2019, 8, 1486. [Google Scholar] [CrossRef]
- Worathumrong, N.; Grimes, A.J. The Effect of o-Salicylate upon pentose phosphate pathway activity in normal and G6PD-deficient red cells. Br. J. Haematol. 1975, 30, 225–231. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Ren, D.-Y.; Feng, Z.-X.; Zhang, L.-Y.; Zhong, Y.-F.; Jin, M.-Y.; Xu, F.-W.; Feng, C.-Y.; Du, Y.-Z.; et al. Mussel-inspired collagen-hyaluronic acid composite scaffold with excellent antioxidant properties and sustained release of a growth factor for enhancing diabetic wound healing. Mater. Today Bio 2022, 15, 100320. [Google Scholar] [CrossRef]
- Yang, H.; Song, L.; Sun, B.; Chu, D.; Yang, L.; Li, M.; Li, H.; Dai, Y.; Yu, Z.; Guo, J. Modulation of macrophages by a paeoniflorin-loaded hyaluronic acid-based hydrogel promotes diabetic wound healing. Mater. Today Bio 2021, 12, 100139. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhao, B.; Li, M.; Wang, H.; Zhu, J.; Li, Q.; Gao, H.; Feng, Q.; Cao, X. Microenvironment responsive nanocomposite hydrogel with NIR photothermal therapy, vascularization and anti-inflammation for diabetic infected wound healing. Bioact. Mater. 2023, 26, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Tombulturk, F.K.; Soydas, T.; Kanigur-Sultuybek, G. Topical metformin accelerates wound healing by promoting collagen synthesis and inhibiting apoptosis in a diabetic wound model. Int. Wound J. 2024, 21, e14345. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tang, W.; Liu, J.; Han, Y.; Yan, Q.; Dong, Y.; Liu, X.; Yang, D.; Ma, G.; Cao, H. A novel sprayable thermosensitive hydrogel coupled with zinc modified metformin promotes the healing of skin wound. Bioact. Mater. 2023, 20, 610–626. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Wang, Y.; Han, F.; Shen, K.; Luo, L.; Li, Y.; Jia, Y.; Zhang, J.; Cai, W.; et al. Exosome/metformin-loaded self-healing conductive hydrogel rescues microvascular dysfunction and promotes chronic diabetic wound healing by inhibiting mitochondrial fission. Bioact. Mater. 2023, 26, 323–336. [Google Scholar] [CrossRef]
- Sharma, M.; Sahu, K.; Singh, S.P.; Jain, B. Wound healing activity of curcumin conjugated to hyaluronic acid: In vitro and in vivo evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1009–1017. [Google Scholar] [CrossRef]
- Hu, B.; Gao, M.; Boakye-Yiadom, K.O.; Ho, W.; Yu, W.; Xu, X.; Zhang, X.-Q. An intrinsically bioactive hydrogel with on-demand drug release behaviors for diabetic wound healing. Bioact. Mater. 2021, 6, 4592–4606. [Google Scholar] [CrossRef]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef]

| Natural Scaffolds | Advantages | Disadvantages |
|---|---|---|
| MFAT | - a natural source of ASCs/SVF - structural support - biocompatible - anti-inflammatory and pro-healing - antibacterial effect - promotes secretion of regenerative factors (e.g., VEGF, TGF-β) - no adverse effects - flexible delivery methods (injection, microneedles, etc.) - enhanced drug delivery | - requires liposuction (minimally invasive procedure) - fragile - need for complex equipment (Lipogems) - may yield insufficient tissue - inflammatory phenotype in diabetics - harder to standardize |
| Chitosan | - biodegradable, biocompatible - mucoadhesive - non-toxic - non-immunogenic - various application forms (hydrogels, sponges, etc. - moisture retention - hemostatic - antibacterial: against Staphylococcus spp., E. coli, Enterococcus spp. - promotes growth of beneficial bacterial - anti-inflammatory: ↓ TNF-α, IL-6, IL-1β; ↑ IL-10, TGF-β1 - promotes collagen formation, angiogenesis, ECM protein deposition - carrier for growth factors, EXOs, and other drugs | - poor solubility at physiological pH - variable quality - weak mechanical strength - rapid degradation - may induce inflammation in some forms (powder, partially deacetylated, etc.) |
| Alginate | - biodegradable, biocompatible - affordable - non-toxic - moisture retention - hydrophilic - supports fibroblast activity - drug delivery - effective in composites | - weak mechanical strength - rapid degradation - lack of bioactivity when used alone - needs to be combined with other agents |
| HA | - ECM mimic - strong hydration - maintains elasticity - high water retention capacity - anti-inflammatory (↑ IL-10, TGF-β1; M2 macrophage polarization) - promotes angiogenesis, re-epithelization, collagen deposition - effective drug and cell delivery scaffold - UHMW-HA: higher stability; superior anti-inflammatory and tissue-protective effects; maintains ECM integrity | - rapid degradation (standard HA) - UHMW-HA is costly and less available - requires reinforcement for structural applications |
| Collagen | - biocompatible - biodegradable - moisture retention - hemostatic - promotes angiogenesis, cell adhesion | - rapid degradation (especially in diabetic wounds) - immunogenicity risk - weak mechanical strength - needs to be combined with other agents |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moldovan, C.-A.-D.; Salagean, A.-A.; Slevin, M. Multiplexing 3D Natural Scaffolds to Optimize the Repair and Regeneration of Chronic Diabetic Wounds. Gels 2025, 11, 430. https://doi.org/10.3390/gels11060430
Moldovan C-A-D, Salagean A-A, Slevin M. Multiplexing 3D Natural Scaffolds to Optimize the Repair and Regeneration of Chronic Diabetic Wounds. Gels. 2025; 11(6):430. https://doi.org/10.3390/gels11060430
Chicago/Turabian StyleMoldovan, Cezara-Anca-Denisa, Alex-Adrian Salagean, and Mark Slevin. 2025. "Multiplexing 3D Natural Scaffolds to Optimize the Repair and Regeneration of Chronic Diabetic Wounds" Gels 11, no. 6: 430. https://doi.org/10.3390/gels11060430
APA StyleMoldovan, C.-A.-D., Salagean, A.-A., & Slevin, M. (2025). Multiplexing 3D Natural Scaffolds to Optimize the Repair and Regeneration of Chronic Diabetic Wounds. Gels, 11(6), 430. https://doi.org/10.3390/gels11060430







