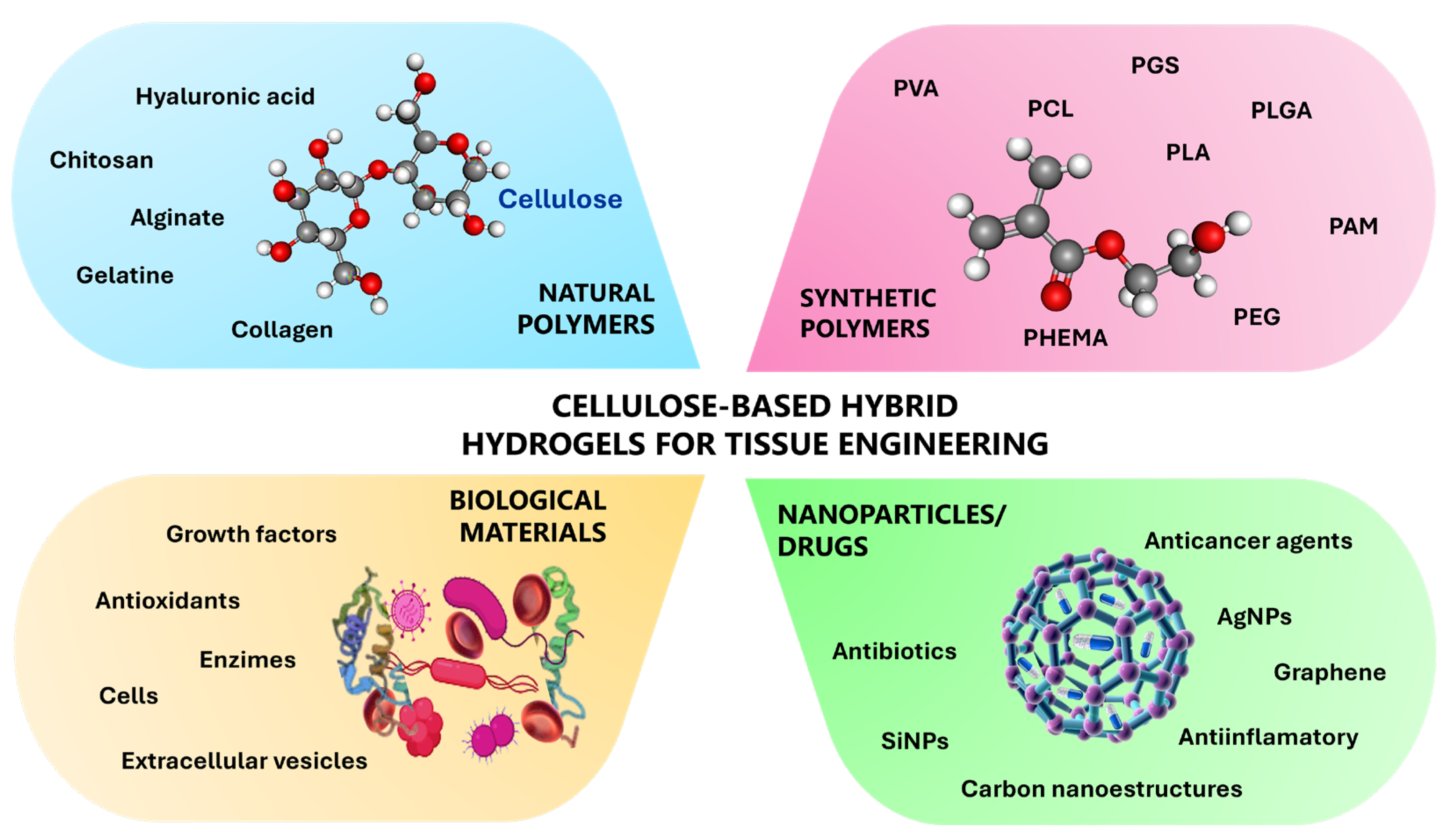
Cellulose-Based Hybrid Hydrogels for Tissue Engineering Applications: A Sustainable Approach
Abstract
1. Introduction
2. Cellulose Sustainable Sources and Cellulose Derivatives
2.1. Cellulose Structure
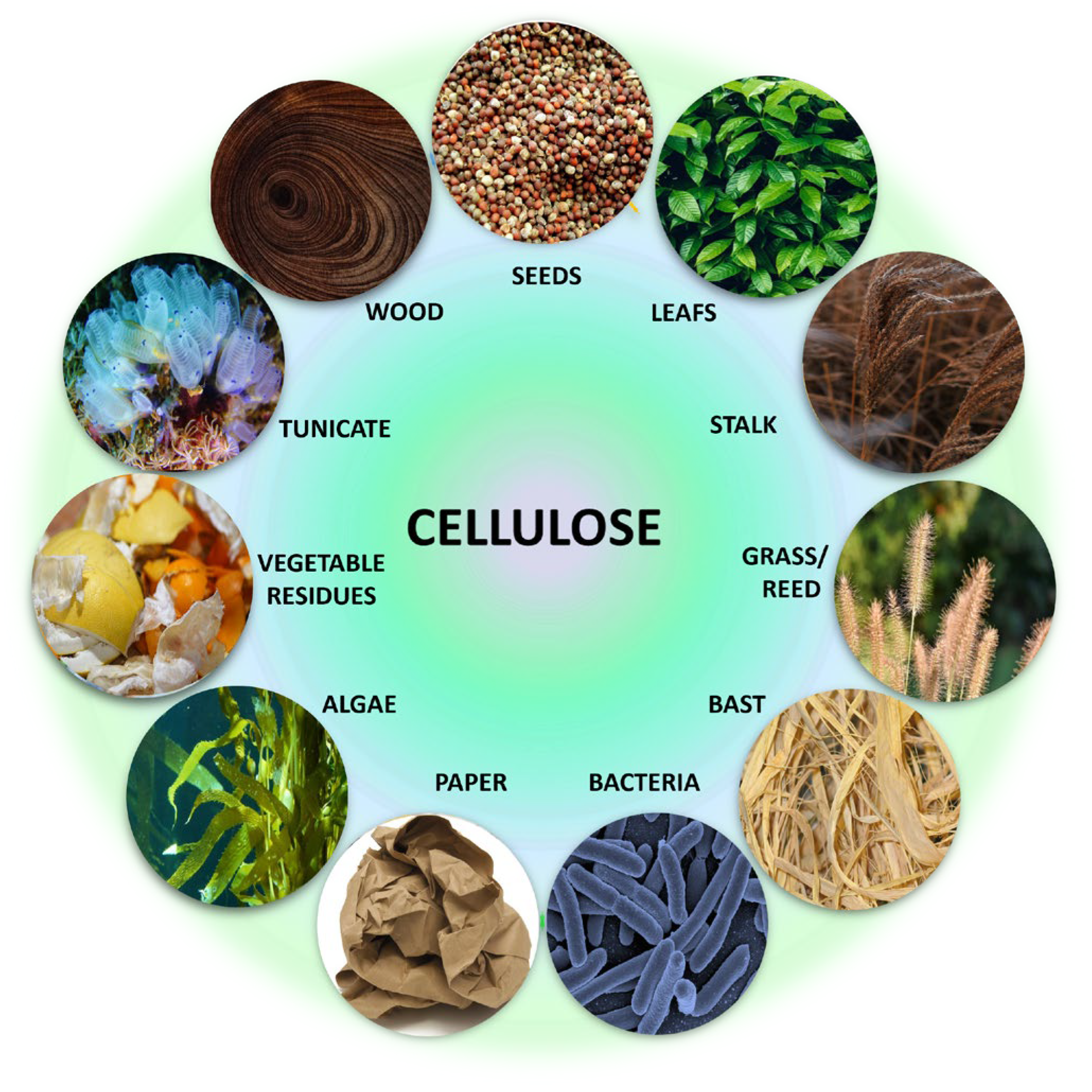
2.2. Cellulose Sources
2.2.1. Plant Cellulose
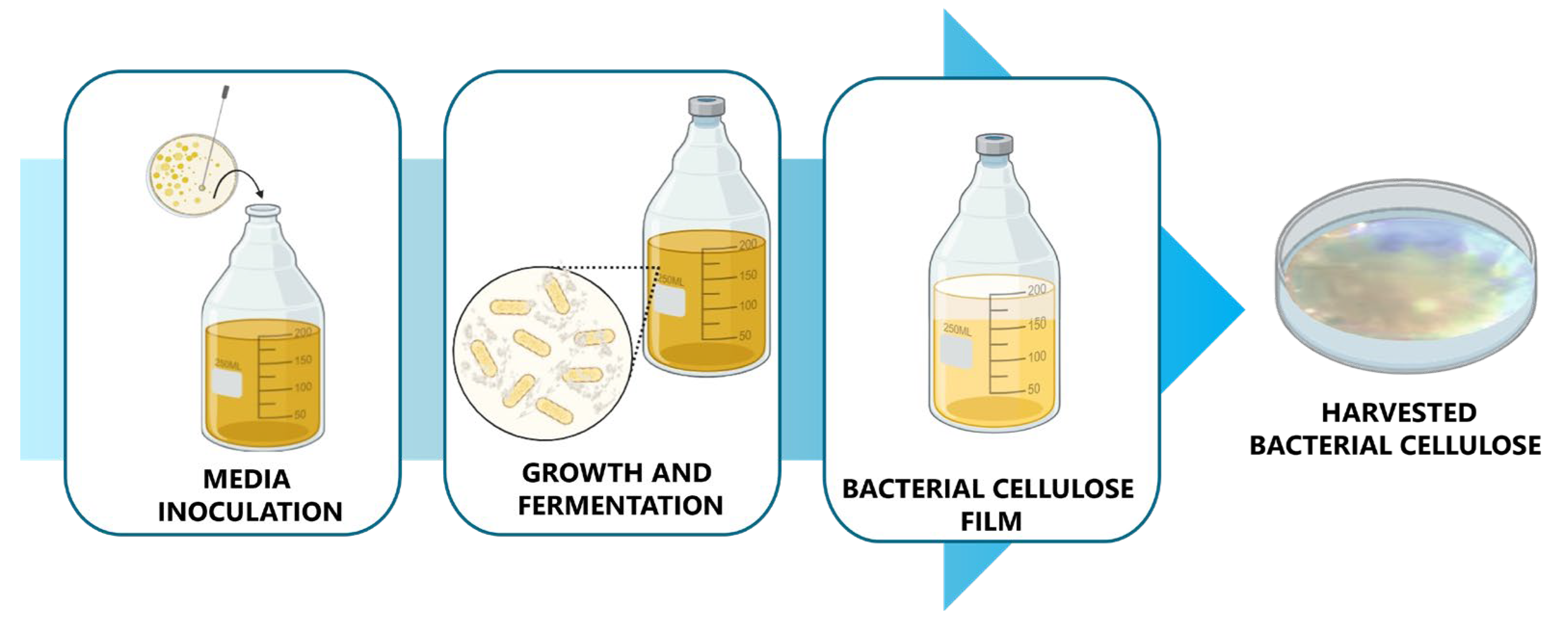
2.2.2. Bacterial Cellulose
2.2.3. Algal Cellulose
2.2.4. Animal Cellulose
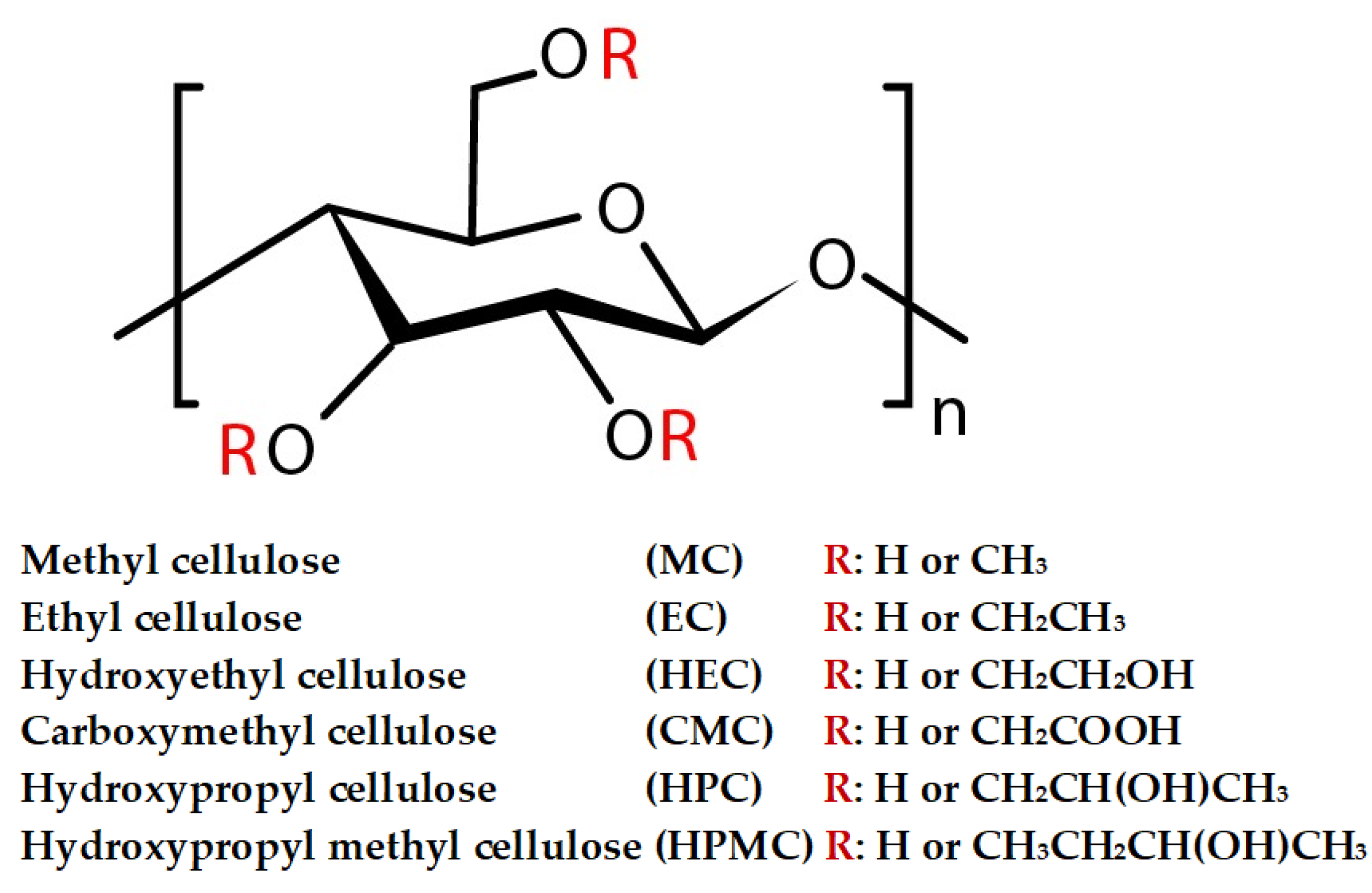
2.2.5. Cellulose Derivatives
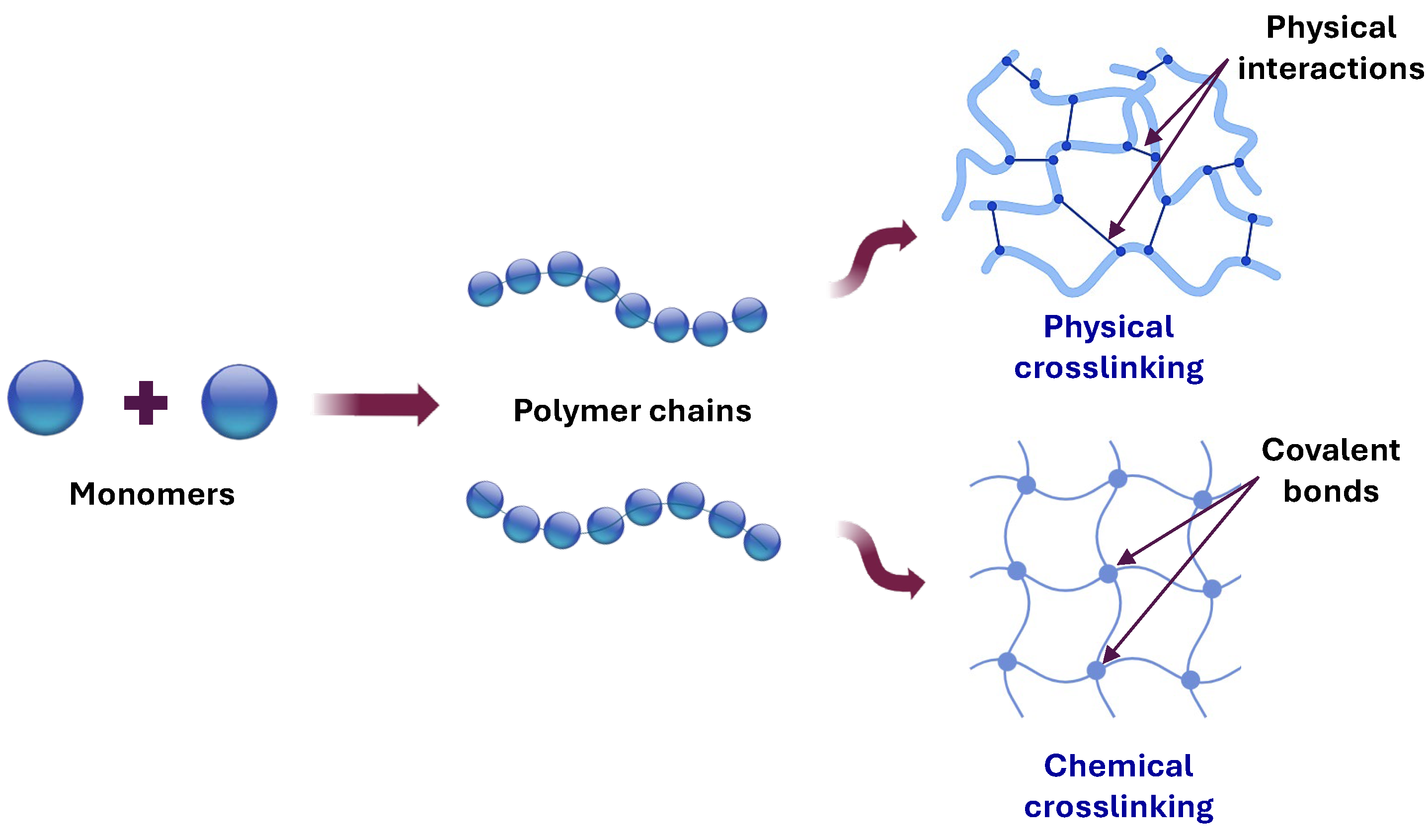
3. Synthesis of Cellulose-Based Hydrogels
3.1. Physically Crosslinked Hydrogels
3.1.1. Crosslinking by Ionic Interactions
3.1.2. Hydrogels Crosslinked by Hydrogen Bonds
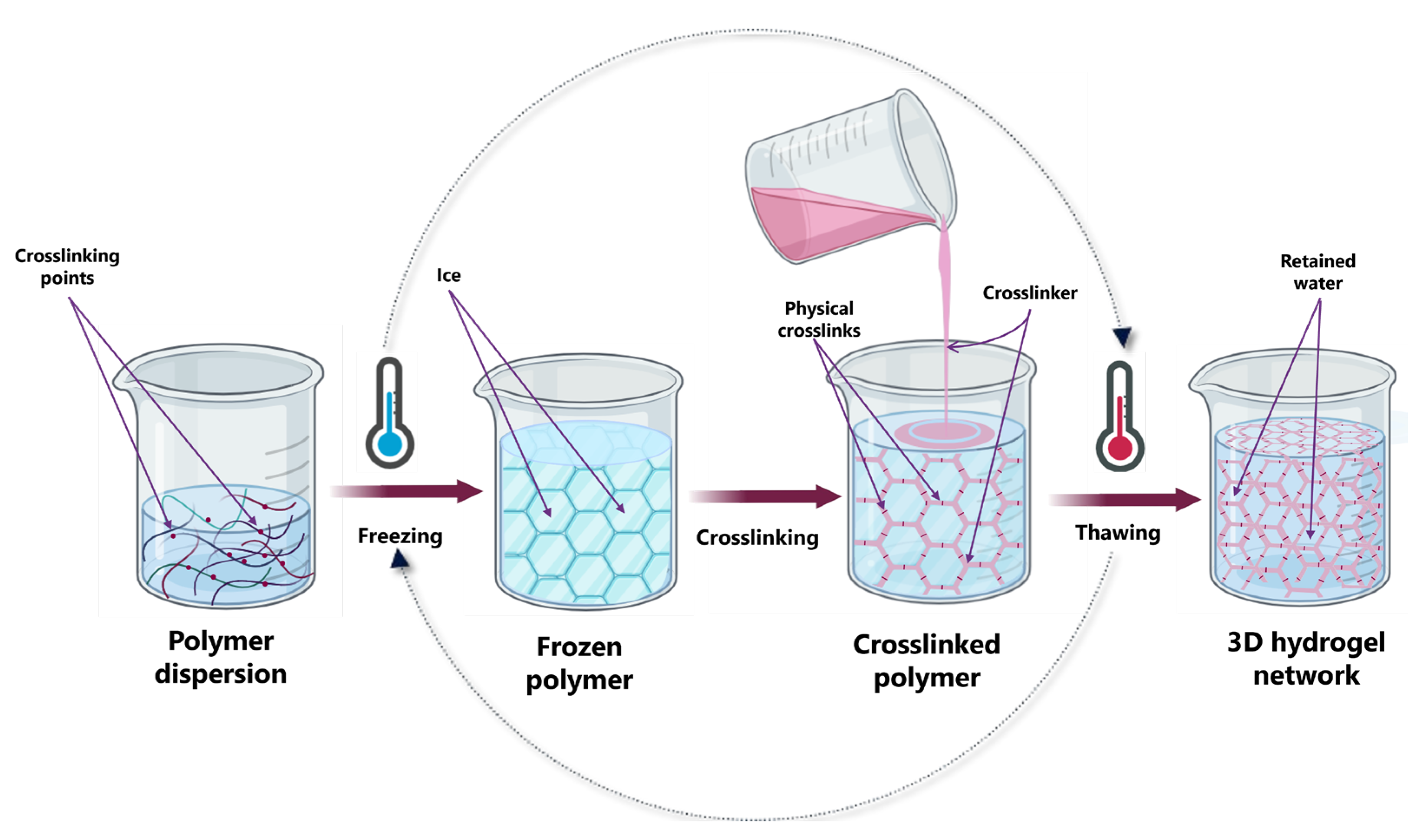
3.1.3. Freeze–Thawing Method
3.1.4. Crosslinking by Host–Guest Interactions
3.2. Chemical Crosslinking
3.2.1. Click Chemistry Reactions
- (a)
- Alkyne–Azide Cycloaddition Reaction: A reaction between azide groups and alkynes leading to the formation of 1,4-disubstituted and 1,5-disubstituted 1,2,3-triazole rings. This process requires elevated temperatures, and when catalyzed by copper ions, it exclusively produces the 1,4-disubstituted isomer. This specific isomer exhibits resistance to oxidation under acidic conditions, is chemically inert to hydrolysis, and has the ability to form hydrogen bonds. This technique has proven useful for synthesizing hydrogels with key advantages, including rapid gelation and high product yields, and the copper-catalyzed version ranks as the most frequently utilized among click reactions. However, copper catalysis is not favorable for applications in tissue engineering due to potential cytotoxicity risks. In this context, Okulmus et al. [104] synthesized multicomponent hydrogels by crosslinking bacterial cellulose (BC), hydroxypropyl methylcellulose (HPMC), and hyaluronic acid (HA) through the Azide–Alkyne Cycloaddition Reaction, catalyzed by copper, at ambient conditions for 24 h. First, hyaluronic acid was prepared using 1-azido-2,3-epoxypropane, and alkyne-terminated cellulose was also ready for the synthesis of the multicomponent hydrogel. The resulting hydrogels proved to be suitable for wound dressing applications. An in vitro cell culturing MTT assay demonstrated that the hydrogels were able to promote cell proliferation, adhesion, and spreading of 3T3 cells.
- (b)
- Strain-Promoted Azide−Alkyne Cycloaddition (SPAAC) Reaction: This reaction is performed at room temperature with no catalyst, between cyclooctyne derivatives and azides, producing aromatic triazoles. Cyclooctyne is the smallest, isolatable, remarkably stable cyclic alkyne whose structure affects the reaction kinetics. Therefore, its reactivity can be improved by introducing electron-withdrawing groups such as fluorine or sp2-hybridized atoms into its ring structure. Additionally, cyclooctane derivatives, including bicyclononynes and difluorinated cyclooctyne (DIFO), can be obtained by fusing cyclopropane units. These non-metal-catalyzed reactions facilitate the development of tailored injectable hydrogels and microstructured gels by carefully controlling factors such as space and time. In a study conducted by Nouri-Felekori et al. [105], azide and alkyne moieties were introduced into the structure of citric acid-modified hydroxyethyl cellulose. Through a strain-promoted azide–alkyne cycloaddition, also known as bioorthogonal click chemistry, a hydrogel was formed. Characterization of the hydrogel showed a porous interconnected microarchitecture adequate for cartilage tissue application. Also, the swelling degree reached about 650%, and the mechanical characteristics of the sample were comparable to those of natural cartilage tissue. In vitro biological assays proved that the hydrogel had significant biocompatibility, chondrogenic ability, and bioorthogonal features.
- (c)
- Thiol–Ene Reaction: Reactions between thiols and various functional groups are common. Common reactions between thiol groups and alkenes are carried out under light exposure or thermal initiators to form thioethers. The reaction is highly selective and can be carried out in water, with a yield close to 100%. This technique allows the alteration of the internal spacing of hydrogels by controlling the time, place, speed, and light exposure of the reaction. Moreover, adverse effects caused by ultraviolet light initiation can be avoided by regulating the wavelength and the dose [106,107,108,109,110].
- (d)
- Diels−Alder (DA) Reaction: The Diels–Alder (DA) reaction is a cycloaddition process that involves an electron-rich diene and an electron-deficient dienophile to create a six-membered ring. This reaction is known for its high selectivity and producing no byproducts; it occurs most rapidly in the presence of water and can be performed with no coupling agent or catalyst. The maleimide–furan reaction, in particular, is extensively utilized for producing hydrogels, which are essential in tissue regeneration and cell encapsulation applications [111,112]. As an illustration, a Diels–Alder reaction was utilized to create hydrogels based on hydroxypropyl methylcellulose. The initial phase consisted of altering hydroxypropylmethylcellulose (HPMC) with a diene compound containing carboxyl groups, which was produced from the synthesis of furfurylamine and succinic anhydride. Following this, dienophile groups were incorporated into HPMC through a coupling reaction with N-maleoyl alanine, employing N,N′-dicyclohexylcarbodiimide and 4-dimethylaminopyridine. Next, the furan- and maleimide-modified HPMC were dissolved in water, leading to gelation at a specified temperature after a certain duration. The duration for gelation was reduced when changes were made to the temperature and concentration of the solution, and the presence of water influenced the kinetics of the Diels–Alder reaction. The swelling characteristics showed that the swelling ratio rose with an increase in temperature [112]. In another study, toluene diisocyanate was employed as a spacer to graft Diels–Alder moieties, such as furyl and protected maleimido moieties, onto cellulose nanocrystals. The reaction time and molar ratio of reactants positively influenced the grafting efficiency. Further characterization confirmed that the grafted moieties and cellulose nanocrystals remained intact after the reaction. However, side reactions were also observed, which impacted the click chemistry reaction on cellulose nanocrystals [113].
3.2.2. Pseudo Click Chemistry Reactions
- (a)
- Schiff Base Reactions: These are condensation reactions that involve the nucleophilic attack on electrophilic carbonyl groups of aldehydes or ketones, forming Schiff bases. Hydrogels have been developed based on imines and their derivatives, such as hydrazones and oximes, which are the products of reactions between aldehydes or ketones (i.e., glutaraldehyde or dialdehydes) with primary amines, hydrazides, and aminooxy groups, respectively. Additionally, benzoic Schiff base linkages, including benzoic imines, hydrazides, and oximes, are generated by connecting benzoic aldehydes with amines, hydrazides, and aminooxy groups, correspondingly. Hydrogels developed in situ maintain stability under physiological conditions. In this context, hydrazones and oximes exhibit greater intrinsic stability than imines, while acylhydrazones present improved hydrolytic stability compared to both hydrazones and oximes [12,114,115,116].
- (b)
- Crosslinking via Michael Addition: The Michael addition reaction forms a C–C bond between a carbanion or other nucleophile (such as amines or thiols) and an α,β-unsaturated carbonyl compound. This reaction is fast at room temperature, has low curing times, involves fewer toxic precursors, and does not require UV radiation, free radicals, or other crosslinking agents [121]. Furthermore, it is characterized by its high regioselectivity, efficiency, low reversibility, and absence of byproducts. There are two main variations: the aza–Michael addition (carbon–nitrogen bond) and the thio–Michael addition (carbon–sulfur bond), both applied in the synthesis of hydrogels, achieving a homogeneous and biocompatible polymer network.
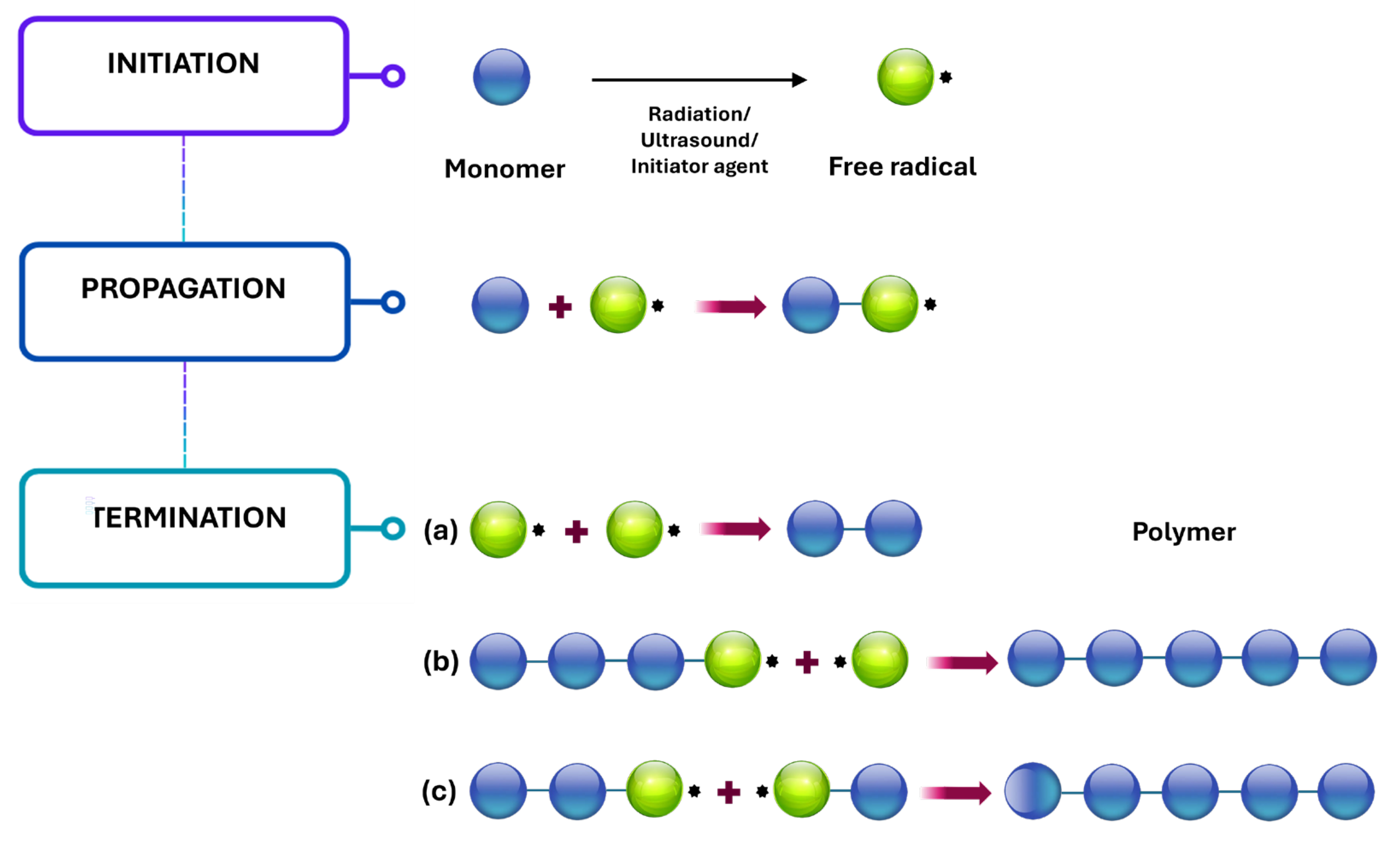
3.3. Free Radical Polymerization
3.4. Thermal Induced Crosslinking
3.5. Photo-Induced Crosslinking
3.6. Radiation-Induced Crosslinking
3.7. Crosslinking via Condensation Polymerization
3.8. Ultrasound-Induced Crosslinking
3.9. Biologic Crosslinking
3.10. Hybrid Hydrogels
4. Cellulose-Based Stimuli-Responsive Hydrogels
4.1. Hydrogels Responsive to Chemical Stimuli
4.2. Ionic Strength and pH Responsive Hydrogels
4.3. Thermal Responsive Hydrogels
4.4. Hydrogels Responsive to Mechanical Stimuli
4.5. Photo Responsive Hydrogels
4.6. Magneto-Electro-Responsive Hydrogels
4.7. Glucose-Sensitive Hydrogels
4.8. Enzyme-Responsive Hydrogels
4.9. Multi-Responsive Hydrogels
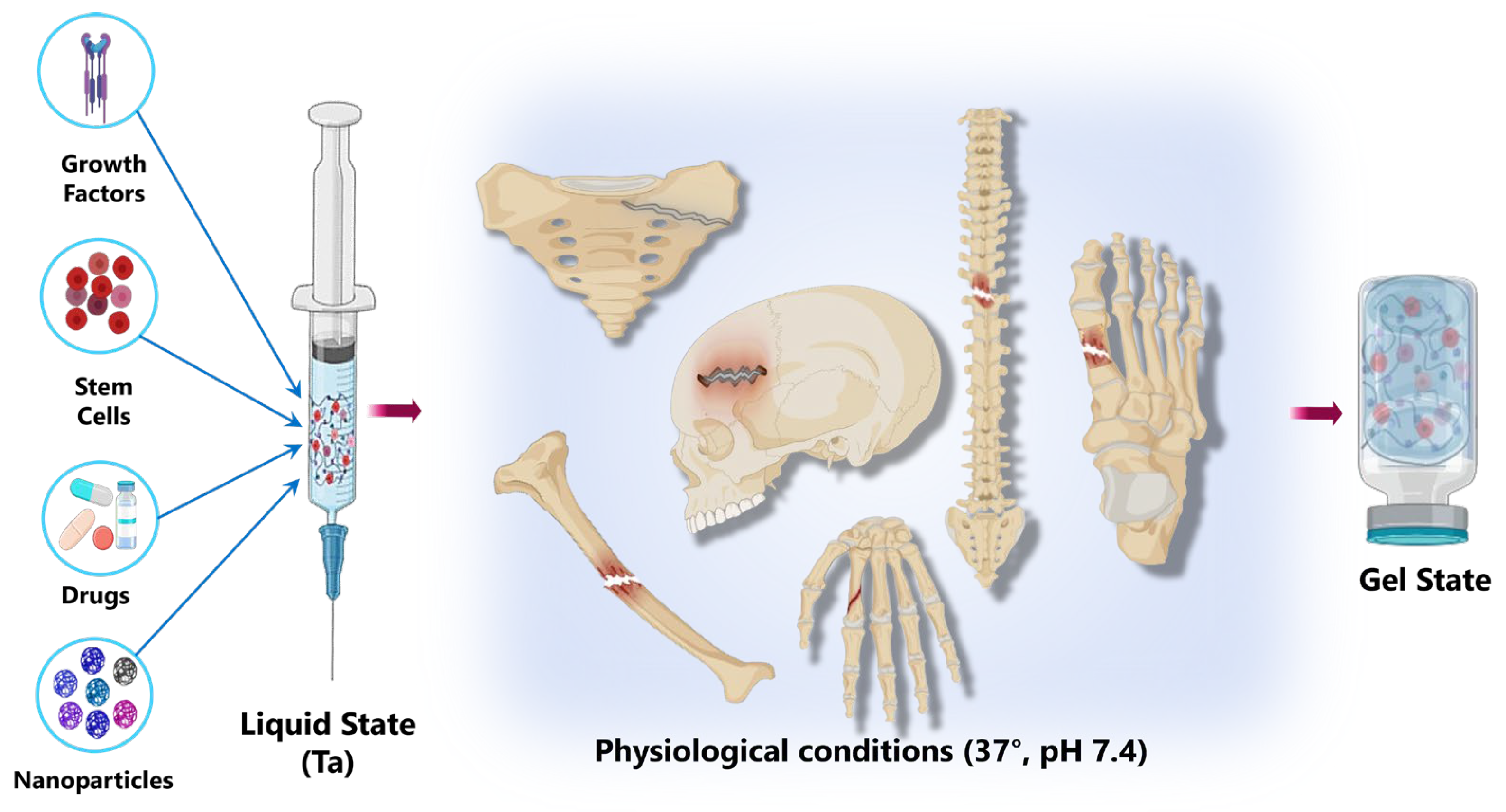
5. Cellulose-Based Hydrogels for Tissue Engineering
5.1. Culture of Pluripotent Stem Cells
5.2. Cartilage Tissue Engineering
5.3. Skin Tissue Engineering
5.4. Bone Tissue Engineering
5.5. Skeletal Muscle Tissue Engineering
5.6. Soft Tissue Engineering
5.7. Nervous System Tissue Engineering
5.8. Cardio and Vascular Tissue Engineering
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mukarakate, C.; Mittal, A.; Ciesielski, P.N.; Budhi, S.; Thompson, L.; Iisa, K.; Nimlos, M.R.; Donohoe, B.S. Influence of Crystal Allomorph and Crystallinity on the Products and Behavior of Cellulose during Fast Pyrolysis. ACS Sustain. Chem. Eng. 2016, 4, 4662–4674. [Google Scholar] [CrossRef]
- Ye, D.; Rongpipi, S.; Kiemle, S.N.; Barnes, W.J.; Chaves, A.M.; Zhu, C.; Norman, V.A.; Liebman-Peláez, A.; Hexemer, A.; Toney, M.F.; et al. Preferred Crystallographic Orientation of Cellulose in Plant Primary Cell Walls. Nat. Commun. 2020, 11, 4720. [Google Scholar] [CrossRef] [PubMed]
- Rongpipi, S.; Ye, D.; Gomez, E.D.; Gomez, E.W. Progress and Opportunities in the Characterization of Cellulose—An Important Regulator of Cell Wall Growth and Mechanics. Front. Plant. Sci. 2019, 9, 410940. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, D.; Ahopelto, J.; Karttunen, A.J. Thermodynamic Properties of Crystalline Cellulose Allomorphs Studied with Dispersion-Corrected Density Functional Methods. Molecules 2022, 27, 6240. [Google Scholar] [CrossRef]
- Lee, C.M.; Mittal, A.; Barnette, A.L.; Kafle, K.; Park, Y.B.; Shin, H.; Johnson, D.K.; Park, S.; Kim, S.H. Cellulose Polymorphism Study with Sum-Frequency-Generation (SFG) Vibration Spectroscopy: Identification of Exocyclic CH2OH Conformation and Chain Orientation. Cellulose 2013, 20, 991–1000. [Google Scholar] [CrossRef]
- Sawada, D.; Nishiyama, Y.; Shah, R.; Forsyth, V.T.; Mossou, E.; O’Neill, H.M.; Wada, M.; Langan, P. Untangling the Threads of Cellulose Mercerization. Nat. Commun. 2022, 13, 6189. [Google Scholar] [CrossRef]
- Wu, Z.; Xu, J.; Gong, J.; Li, J.; Mo, L. Preparation, Characterization and Acetylation of Cellulose Nanocrystal Allomorphs. Cellulose 2018, 25, 4905–4918. [Google Scholar] [CrossRef]
- Wohlert, M.; Benselfelt, T.; Wågberg, L.; Furó, I.; Berglund, L.A.; Wohlert, J. Cellulose and the Role of Hydrogen Bonds: Not in Charge of Everything. Cellulose 2021, 29, 1–23. [Google Scholar] [CrossRef]
- Yatsu, L.Y.; Calamari, T.A.; Benerito, R.R. Conversion of Cellulose I to Stable Cellulose III. Text. Res. J. 1986, 56, 419–424. [Google Scholar] [CrossRef]
- Yui, T.; Okayama, N.; Hayashi, S. Structure Conversions of Cellulose IIII Crystal Models in Solution State: A Molecular Dynamics Study. Cellulose 2010, 17, 679–691. [Google Scholar] [CrossRef]
- Feng, L.; Ling, Z.; Ma, J.; Liu, X.; Jiang, Z. Recrystallization Behavior of Cellulose III from Hydrothermal Treatment: The Dynamic Variations of Polymorphs and Crystallinities. Wood Sci. Technol. 2020, 54, 1605–1616. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, J.; Zhu, S.; Kuang, Y.; Wang, B.; Gao, W. Crystalline Stability of Cellulose III Nanocrystals in the Hydrothermal Treatment and NaOH Solution. Carbohydr. Polym. 2020, 249, 116827. [Google Scholar] [CrossRef] [PubMed]
- Wada, M.; Heux, L.; Sugiyama, J. Polymorphism of Cellulose I Family: Reinvestigation of Cellulose IVI. Biomacromolecules 2004, 5, 1385–1391. [Google Scholar] [CrossRef]
- SriBala, G.; Chennuru, R.; Mahapatra, S.; Vinu, R. Effect of Alkaline Ultrasonic Pretreatment on Crystalline Morphology and Enzymatic Hydrolysis of Cellulose. Cellulose 2016, 23, 1725–1740. [Google Scholar] [CrossRef]
- Li, J.; Lemstra, P.J.; Ma, P. Chapter 7: Can high-performance fibers be(come) bio-based and also biocompostable? Adv. Ind. Eng. Polym. Res. 2022, 5, 117–132. [Google Scholar] [CrossRef]
- Peter, Z. Order in Cellulosics: Historical Review of Crystal Structure Research on Cellulose. Carbohydr. Polym. 2021, 254, 117417. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose Crystallinity Index: Measurement Techniques and Their Impact on Interpreting Cellulase Performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef]
- Montesanti, N.; Lancelon-Pin, C.; Potocki-Veronese, G.; Buléon, A.; Putaux, J.L. A-Amylose Single Crystals: Influence of Amylose Concentration, Crystallization Temperature and Surface Induction on the Crystal Morphology. Cellulose 2023, 30, 8459–8473. [Google Scholar] [CrossRef]
- Eldeeb, A.E.; Salah, S.; Elkasabgy, N.A. Biomaterials for Tissue Engineering Applications and Current Updates in the Field: A Comprehensive Review. AAPS PharmSciTech 2022, 23, 267. [Google Scholar] [CrossRef]
- Biswal, T. Biopolymers for Tissue Engineering Applications: A Review. Mater. Today Proc. 2021, 41, 397–402. [Google Scholar] [CrossRef]
- Janmohammadi, M.; Nazemi, Z.; Salehi, A.O.M.; Seyfoori, A.; John, J.V.; Nourbakhsh, M.S.; Akbari, M. Cellulose-Based Composite Scaffolds for Bone Tissue Engineering and Localized Drug Delivery. Bioact. Mater. 2023, 20, 137–163. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Choi, W.S.; Jeong, J.O.A.; Dupin, D.; Choi, H.; Choi, W.S.; Jeong, J.O. A Review of Advanced Hydrogel Applications for Tissue Engineering and Drug Delivery Systems as Biomaterials. Gels 2024, 10, 693. [Google Scholar] [CrossRef] [PubMed]
- Rezakhani, L.; Gharibshahian, M.; Salehi, M.; Zamani, S.; Abpeikar, Z.; Ghaderzadeh, O.; Alizadeh, M.; Masoudi, A.; Rezaei, N.; Cheraghali, D. Recent advances in hydrogels applications for tissue engineering and clinical trials. Regen. Ther. 2024, 26, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xi, Y.; Weng, Y. Recent Advances in Cellulose-Based Hydrogels for Tissue Engineering Applications. Polymers 2022, 14, 3335. [Google Scholar] [CrossRef]
- Li, S.; Bashline, L.; Lei, L.; Gu, Y. Cellulose Synthesis and Its Regulation. Arab. Book 2014, 12, e0169. [Google Scholar] [CrossRef]
- Salem, K.S.; Kasera, N.K.; Rahman, M.A.; Jameel, H.; Habibi, Y.; Eichhorn, S.J.; French, A.D.; Pal, L.; Lucia, L.A. Comparison and Assessment of Methods for Cellulose Crystallinity Determination. Chem. Soc. Rev. 2023, 52, 6417–6446. [Google Scholar] [CrossRef]
- Melikoğlu, A.Y.; Bilek, S.E.; Cesur, S. Optimum Alkaline Treatment Parameters for the Extraction of Cellulose and Production of Cellulose Nanocrystals from Apple Pomace. Carbohydr. Polym. 2019, 215, 330–337. [Google Scholar] [CrossRef]
- Acharya, S.; Liyanage, S.; Abidi, N.; Parajuli, P.; Rumi, S.S.; Shamshina, J.L. Utilization of Cellulose to Its Full Potential: A Review on Cellulose Dissolution, Regeneration, and Applications. Polymers 2021, 13, 4344. [Google Scholar] [CrossRef]
- Chopra, L. Manikanika Extraction of Cellulosic Fibers from the Natural Resources: A Short Review. Mater. Today Proc. 2022, 48, 1265–1270. [Google Scholar] [CrossRef]
- Husin, M.; Li, A.R.; Ramli, N.; Romli, A.Z.; Hakimi, M.I.; Ilham, Z. Preparation and Characterization of Cellulose and Microcrystalline Cellulose Isolated from Waste Leucaena Leucocephala Seeds. Int. J. Adv. Appl. Sci. 2017, 4, 51–58. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 535734. [Google Scholar] [CrossRef] [PubMed]
- Ansell, M.P.; Mwaikambo, L.Y. The Structure of Cotton and Other Plant Fibres. Handbook Text. Fib. Struct. 2009, 2, 62–94. [Google Scholar] [CrossRef]
- George, J.; Rantschler, J.; Bae, S.-E.; Shahzad, K.; Sohail, M.; Hamid, A. Green Ethanol Production from Cotton Stalk. IOP Conf. Ser. Earth Environ. Sci. 2019, 257, 012025. [Google Scholar] [CrossRef]
- Buzala, K.P.; Kalinowska, H.; Malachowska, E.; Boruszewski, P.; Krajewski, K.; Przybysz, P. The Effect of Lignin Content in Birch and Beech Kraft Cellulosic Pulps on Simple Sugar Yields from the Enzymatic Hydrolysis of Cellulose. Energies 2019, 12, 2952. [Google Scholar] [CrossRef]
- Sebeia, N.; Jabli, M.; Ghith, A.; El Ghoul, Y.; Alminderej, F.M. Populus Tremula, Nerium Oleander and Pergularia Tomentosa Seed Fibers as Sources of Cellulose and Lignin for the Bio-Sorption of Methylene Blue. Int. J. Biol. Macromol. 2019, 121, 655–665. [Google Scholar] [CrossRef]
- Gautam, S.P.; Bundela, P.S.; Pandey, A.K.; Jamaluddin, J.; Awasthi, M.K.; Sarsaiya, S. A Review on Systematic Study of Cellulose. J. Appl. Nat. Sci. 2010, 2, 330–343. [Google Scholar] [CrossRef]
- Madsen, B.; Gamstedt, E.K. Wood versus Plant Fibers: Similarities and Differences in Composite Applications. Adv. Mater. Sci. 2013, 2013, 564346. [Google Scholar] [CrossRef]
- Chand, N.; Fahim, M. Natural Fibers and Their Composites. Tribol. Nat. Fiber Polym. Compos. 2021, 1–59. [Google Scholar] [CrossRef]
- Abualnaja, M.M.; Almotairy, A.R.Z.; Alorabi, A.Q.; Alaysuy, O.; Almahri, A.; Alkhamis, K.; Alrefaee, S.H.; El-Metwaly, N.M. Optimizing Cellulose Nanofiber Composite for Co2+ Ions Recovery from Lithium-Ion Battery Wastes and Removal from Wastewater: A Green Environmental Solution. J. Water Process Eng. 2024, 57, 104621. [Google Scholar] [CrossRef]
- Morán, J.I.; Alvarez, V.A.; Cyras, V.P.; Vázquez, A. Extraction of Cellulose and Preparation of Nanocellulose from Sisal Fibers. Cellulose 2008, 15, 149–159. [Google Scholar] [CrossRef]
- Naim, A.; Tan, C.S.Y.; Liew, F.K. Thermal Properties of Bamboo Cellulose Isolated from Bamboo Culms and Shoots. Bioresources 2022, 17, 4806–4815. [Google Scholar] [CrossRef]
- Ramos, M.; Laveriano, E.; San Sebastián, L.; Perez, M.; Jiménez, A.; Lamuela-Raventos, R.M.; Garrigós, M.C.; Vallverdú-Queralt, A. Rice Straw as a Valuable Source of Cellulose and Polyphenols: Applications in the Food Industry. Trends Food Sci. Technol. 2023, 131, 14–27. [Google Scholar] [CrossRef]
- Nicolas, W.J.; Ghosal, D.; Tocheva, E.I.; Meyerowitz, E.M.; Jensen, G.J. Structure of the Bacterial Cellulose Ribbon and Its Assembly-Guiding Cytoskeleton by Electron Cryotomography. J. Bacteriol. 2021, 203. [Google Scholar] [CrossRef] [PubMed]
- Jamsheera, C.P.; Pradeep, B.V. Production of Bacterial Cellulose from Acetobacter Species and Its Applications—A Review. J Pure Appl. Microbiol. 2021, 15, 544–555. [Google Scholar] [CrossRef]
- Chaiyasat, A.; Jearanai, S.; Moonmangmee, S.; Moonmangmee, D.; Christopher, L.P.; Alam, M.N.; Chaiyasat, P. Novel Green Hydrogel Material Using Bacterial Cellulose. Orient. J. Chem. 2018, 34, 1735–1740. [Google Scholar] [CrossRef]
- Brandão, P.R.; Crespo, M.T.B.; Nascimento, F.X. Phylogenomic and Comparative Analyses Support the Reclassification of Several Komagataeibacter Species as Novel Members of the Novacetimonas Gen. Nov. and Bring New Insights into the Evolution of Cellulose Synthase Genes. Int. J. Syst. Evol. Microbiol. 2022, 72, 005252. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Dutta, B.; Dey, A.; Sarkar, T.; Pati, S.; Edinur, H.A.; Kari, Z.A.; Noor, N.H.M.; Ray, R.R. Bacterial Cellulose: Production, Characterization, and Application as Antimicrobial Agent. Int. J. Mol. Sci. 2021, 22, 12984. [Google Scholar] [CrossRef]
- Buldum, G.; Bismarck, A.; Mantalaris, A. Recombinant Biosynthesis of Bacterial Cellulose in Genetically Modified Escherichia Coli. Bioprocess Biosyst. Eng. 2018, 41, 265–279. [Google Scholar] [CrossRef]
- Jozala, A.F.; de Lencastre-Novaes, L.C.; Lopes, A.M.; de Carvalho Santos-Ebinuma, V.; Mazzola, P.G.; Pessoa-Jr, A.; Grotto, D.; Gerenutti, M.; Chaud, M.V. Bacterial Nanocellulose Production and Application: A 10-Year Overview. Appl. Microbiol. Biotechnol. 2016, 100, 2063–2072. [Google Scholar] [CrossRef]
- Ye, J.; Zheng, S.; Zhang, Z.; Yang, F.; Ma, K.; Feng, Y.; Zheng, J.; Mao, D.; Yang, X. Bacterial Cellulose Production by Acetobacter Xylinum ATCC 23767 Using Tobacco Waste Extract as Culture Medium. Bioresour. Technol. 2019, 274, 518–524. [Google Scholar] [CrossRef]
- Said Azmi, S.N.N.; Mohd Fabli, S.N.N.F.; Faisul Aris, F.A.; Samsu, Z.A.; Mohd Asnawi, A.S.F.; Mohamed Yusof, Y.; Ariffin, H.; Syed Abdullah, S.S. Fresh Oil Palm Frond Juice as a Novel and Alternative Fermentation Medium for Bacterial Cellulose Production. Mater. Today Proc. 2021, 42, 101–106. [Google Scholar] [CrossRef]
- Betlej, I.; Antczak, A.; Szadkowski, J.; Drożdżek, M.; Krajewski, K.; Radomski, A.; Zawadzki, J.; Borysiak, S. Evaluation of the Hydrolysis Efficiency of Bacterial Cellulose Gel Film after the Liquid Hot Water and Steam Explosion Pretreatments. Polymers 2022, 14, 2032. [Google Scholar] [CrossRef] [PubMed]
- Pogorelova, N.; Rogachev, E.; Digel, I.; Chernigova, S.; Nardin, D. Bacterial Cellulose Nanocomposites: Morphology and Mechanical Properties. Materials 2020, 13, 2849. [Google Scholar] [CrossRef]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M. The Future Prospects of Microbial Cellulose in Biomedical Applications. Biomacromolecules 2007, 8, 1–12. [Google Scholar] [CrossRef]
- Zou, C.; Qu, D.; Jiang, H.; Lu, D.; Ma, X.; Zhao, Z.; Xu, Y. Bacterial Cellulose: A Versatile Chiral Host for Circularly Polarized Luminescence. Molecules 2019, 24, 1008. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, R.; Sharma, S.; Shree, N.; Kaur, K. Bacterial Cellulose: An Ecological Alternative as A Biotextile. Biosci. Biotechnol. Res. Asia 2023, 20, 449–463. [Google Scholar] [CrossRef]
- El-Gendi, H.; Taha, T.H.; Ray, J.B.; Saleh, A.K. Recent Advances in Bacterial Cellulose: A Low-Cost Effective Production Media, Optimization Strategies and Applications. Cellulose 2022, 29, 7495–7533. [Google Scholar] [CrossRef]
- Abeer, M.M.; Mohd Amin, M.C.I.; Martin, C. A Review of Bacterial Cellulose-Based Drug Delivery Systems: Their Biochemistry, Current Approaches and Future Prospects. J. Pharm. Pharmacol. 2014, 66, 1047–1061. [Google Scholar] [CrossRef]
- Raven, J.A.; Giordano, M. Algae. Curr. Biol. 2014, 24, R590–R595. [Google Scholar] [CrossRef]
- Baghel, R.S.; Reddy, C.R.K.; Singh, R.P. Seaweed-Based Cellulose: Applications, and Future Perspectives. Carbohydr. Polym. 2021, 267, 118241. [Google Scholar] [CrossRef]
- Zanchetta, E.; Damergi, E.; Patel, B.; Borgmeyer, T.; Pick, H.; Pulgarin, A.; Ludwig, C. Algal Cellulose, Production and Potential Use in Plastics: Challenges and Opportunities. Algal Res. 2021, 56, 102288. [Google Scholar] [CrossRef]
- Jonjaroen, V.; Ummartyotin, S.; Chittapun, S. Algal Cellulose as a Reinforcement in Rigid Polyurethane Foam. Algal Res. 2020, 51, 102057. [Google Scholar] [CrossRef]
- Mihranyan, A. Cellulose from Cladophorales Green Algae: From Environmental Problem to High-Tech Composite Materials. J. Appl. Polym. Sci. 2011, 119, 2449–2460. [Google Scholar] [CrossRef]
- Che, M.; Shan, C.; Huang, R.; Cui, M.; Qi, W.; Klemeš, J.J.; Su, R. A Rapid Removal of Phaeocystis Globosa from Seawater by Peroxymonosulfate Enhanced Cellulose Nanocrystals Coagulation. Ecotoxicol. Environ. Saf. 2023, 262, 115318. [Google Scholar] [CrossRef]
- Drishya, P.K.; Reddy, M.V.; Mohanakrishna, G.; Sarkar, O.; Isha, N.; Rohit, M.V.; Patel, A.; Chang, Y.-C. Advances in Microbial and Plant-Based Biopolymers: Synthesis and applications in Next-Generation materials. Macromol 2025, 5, 21. [Google Scholar] [CrossRef]
- Holland, L.Z. Tunicates. Curr. Biol. 2016, 26, R146–R152. [Google Scholar] [CrossRef]
- Nanglu, K.; Lerosey-Aubril, R.; Weaver, J.C.; Ortega-Hernández, J. A Mid-Cambrian Tunicate and the Deep Origin of the Ascidiacean Body Plan. Nat. Commun. 2023, 14, 3832. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Moser, C.; Lindström, M.E.; Henriksson, G.; Li, J. Cellulose Nanofibers from Softwood, Hardwood, and Tunicate: Preparation-Structure-Film Performance Interrelation. ACS Appl. Mater. Interfaces 2017, 9, 13508–13519. [Google Scholar] [CrossRef]
- Chanthathamrongsiri, N.; Petchsomrit, A.; Leelakanok, N.; Siranonthana, N.; Sirirak, T. The Comparison of the Properties of Nanocellulose Isolated from Colonial and Solitary Marine Tunicates. Heliyon 2021, 7, e07819. [Google Scholar] [CrossRef]
- El Achaby, M.; Kassab, Z.; Aboulkas, A.; Gaillard, C.; Barakat, A. Reuse of Red Algae Waste for the Production of Cellulose Nanocrystals and Its Application in Polymer Nanocomposites. Int. J. Biol. Macromol. 2018, 106, 681–691. [Google Scholar] [CrossRef]
- Valcarcel, J.; Vázquez, J.A.; Varela, U.R.; Reis, R.L.; Novoa-Carballal, R. Isolation and Characterization of Polysaccharides from the Ascidian Styela Clava. Polymers 2022, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Asadnia, M.; Sadat-Shojai, M. Recent perspective of synthesis and modification strategies of cellulose nanocrystals and cellulose nanofibrils and their beneficial impact in scaffold-based tissue engineering: A review. Int. J. Biol. Macromol. 2025, 293, 139409. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Ning, D.; Xu, D.; Cheng, Y.; Mondal, A.K.; Zou, Q.; Zhu, H.; Huang, F. Preparation and Characterization of Super Hydrophobic Aerogels Derived from Tunicate Cellulose Nanocrystals. Carbohydr. Res. 2022, 511, 108488. [Google Scholar] [CrossRef] [PubMed]
- Apelgren, P.; Sämfors, S.; Säljö, K.; Mölne, J.; Gatenholm, P.; Troedsson, C.; Thompson, E.M.; Kölby, L. Biomaterial and Biocompatibility Evaluation of Tunicate Nanocellulose for Tissue Engineering. Adv. Biomater. 2022, 137, 212828. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Y.; Lindström, M.E.; Li, J. Tunicate Cellulose Nanocrystals: Preparation, Neat Films and Nanocomposite Films with Glucomannans. Carbohydr. Polym. 2015, 117, 286–296. [Google Scholar] [CrossRef]
- Xu, D.; Cheng, Y.; Wu, S.; Zou, Q.; Mondal, A.K.; Ning, D.; Huang, F. Study On The Effect of Tunicate Cellulose Nanocrystals in The Preparation of Sodium Alginate-Based Enteric Capsule. Cellulose 2022, 29, 2497–2511. [Google Scholar] [CrossRef]
- Cho, S.Y.; Yu, H.; Choi, J.; Kang, H.; Park, S.; Jang, J.S.; Hong, H.J.; Kim, I.D.; Lee, S.K.; Jeong, H.S.; et al. Continuous Meter-Scale Synthesis of Weavable Tunicate Cellulose/Carbon Nanotube Fibers for High-Performance Wearable Sensors. ACS Nano 2019, 13, 9332–9341. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, Q.; Feng, S.; Zhang, L.; Chang, C. Shear-Aligned Tunicate-Cellulose-Nanocrystal-Reinforced Hydrogels with Mechano-Thermo-Chromic Properties. J. Mater Chem. C. Mater. 2021, 9, 6344–6350. [Google Scholar] [CrossRef]
- Cao, L.; Yuan, D.; Xu, C.; Chen, Y. Biobased, Self-Healable, High Strength Rubber with Tunicate Cellulose Nanocrystals. Nanoscale 2017, 9, 15696–15706. [Google Scholar] [CrossRef]
- Pinto, E.; Aggrey, W.N.; Boakye, P.; Amenuvor, G.; Sokama-Neuyam, Y.A.; Fokuo, M.K.; Karimaie, H.; Sarkodie, K.; Adenutsi, C.D.; Erzuah, S.; et al. Cellulose Processing from Biomass and Its Derivatization into Carboxymethylcellulose: A Review. Sci. Afr. 2022, 15, e01078. [Google Scholar] [CrossRef]
- Heinze, T.; El, O.A.; Koschella, A. Principles of Cellulose Derivatization. In Cellulose Derivatives; Springer Series on Polymer and Composite Materials; Springer, 2018; pp. 259–292. Available online: https://link.springer.com/chapter/10.1007/978-3-319-73168-1_4 (accessed on 19 May 2025). [CrossRef]
- Magalhães, S.; Fernandes, C.; Pedrosa, J.F.S.; Alves, L.; Medronho, B.; Ferreira, P.J.T.; Rasteiro, M. da G. Eco-Friendly Methods for Extraction and Modification of Cellulose: An Overview. Polymers 2023, 15, 3138. [Google Scholar] [CrossRef] [PubMed]
- Nasution, H.; Harahap, H.; Dalimunthe, N.F.; Ginting, M.H.S.; Jaafar, M.; Tan, O.O.H.; Aruan, H.K.; Herfananda, A.L. Hydrogel and Effects of Crosslinking Agent on Cellulose-Based Hydrogels: A Review. Gels 2022, 8, 568. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, L.; Qing, Y.; Yan, N.; Tian, C.; Huang, Y. A Green Route to Prepare Fluorescent and Absorbent Nano-Hybrid Hydrogel for Water Detection. Sci. Rep. 2017, 7, 4380. [Google Scholar] [CrossRef] [PubMed]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New Developments in Medical Applications of Hybrid Hydrogels Containing Natural Polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef]
- Palmese, L.L.; Thapa, R.K.; Sullivan, M.O.; Kiick, K.L. Hybrid Hydrogels for Biomedical Applications. Curr. Opin. Chem. Eng. 2019, 24, 143–157. [Google Scholar] [CrossRef]
- de Siqueira, E.C.; de França, J.A.A.; de Souza, R.F.M.; da Silva Leoterio, D.M.; Cordeiro, J.N.; Doboszewski, B. Recent Advances in the Development of the Physically Crosslinked Hydrogels and Their Biomedical Applications. Res. Soc. Dev. 2023, 12, e18212843073. [Google Scholar] [CrossRef]
- Kopač, T.; Ambrožič, R. Tailored alginate and chitosan hydrogels: Structural control and functional applications via copper-based electrodeposition. Int. J. Biol. Macromol. 2025, 308, 142476. [Google Scholar] [CrossRef]
- Vinchhi, P.; Rawal, S.U.; Patel, M.M. Biodegradable Hydrogels. In Developments in Biomedical Engineering and Bioelectronics, Drug Delivery Devices and Therapeutic Systems; Academic Press, 2021; pp. 395–419. Available online: https://www.sciencedirect.com/science/article/abs/pii/B9780128198384000122?via%3Dihub (accessed on 19 May 2025). [CrossRef]
- Yan, K.; Wan, Y.; Xu, F.; Lu, J.; Yang, C.; Li, X.; Lu, Z.; Wang, X.; Wang, D. Ionic Crosslinking of Alginate/Carboxymethyl Chitosan Fluorescent Hydrogel for Bacterial Detection and Sterilization. Carbohydr. Polym. 2023, 302, 120427. [Google Scholar] [CrossRef]
- Hu, D.; Zeng, M.; Sun, Y.; Yuan, J.; Wei, Y. Cellulose-Based Hydrogels Regulated by Supramolecular Chemistry. SusMat 2021, 1, 266–284. [Google Scholar] [CrossRef]
- Xie, Z.; Hu, B.L.; Li, R.W.; Zhang, Q. Hydrogen Bonding in Self-Healing Elastomers. ACS Omega. 2021, 6, 9319–9333. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Zuo, M.; Zeng, X.; Tang, X.; Sun, Y.; Lin, L. Stretchable, freezing-tolerant conductive hydrogel for wearable electronics reinforced by cellulose nanocrystals toward multiple hydrogen bonding. Carbohydr. Polym. 2022, 280, 119018. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, F.; Lin, X.; Ding, T. Hydrogen-Bonding-Assisted Toughening of Hierarchical Carboxymethyl Cellulose Hydrogels for Biomechanical Sensing. Carbohydr. Polym. 2021, 269, 118252. [Google Scholar] [CrossRef] [PubMed]
- Zainal, S.H.; Mohd, N.H.; Suhaili, N.; Anuar, F.H.; Lazim, A.M.; Othaman, R. Preparation of Cellulose-Based Hydrogel: A Review. J. Mater. Res. Technol. 2021, 10, 935–952. [Google Scholar] [CrossRef]
- Pavandi, N.; Taghavi, E.; Anarjan, N. Preparation of Carboxymethyl Cellulose and Polyvinyl Alcohol (CMC/PVA) Hydrogels Using Freeze−Thaw Processes for Adsorption Of Zn2+ And Cu2+. Cellulose Chem. Technol. 2021, 55, 375–383. [Google Scholar] [CrossRef]
- Yang, H.; Yuan, B.; Zhang, X.; Scherman, O.A. Supramolecular Chemistry at Interfaces: Host-Guest Interactions for Fabricating Multifunctional Biointerfaces. Acc. Chem. Res. 2014, 47, 2106–2115. [Google Scholar] [CrossRef]
- Park, J.; Park, J.; Lee, J.; Lim, C.; Lee, D.W. Size Compatibility and Concentration Dependent Supramolecular Host–Guest Interactions at Interfaces. Nat. Commun. 2022, 13, 112. [Google Scholar] [CrossRef]
- Han, S.; Wang, T.; Yang, L.; Li, B. Building a Bio-Based Hydrogel via Electrostatic and Host-Guest Interactions for Realizing Dual-Controlled Release Mechanism. Int. J. Biol. Macromol. 2017, 105, 377–384. [Google Scholar] [CrossRef]
- Jiang, X.; Zeng, F.; Yang, X.; Jian, C.; Zhang, L.; Yu, A.; Lu, A. Injectable Self-Healing Cellulose Hydrogel Based on Host-Guest Interactions and Acylhydrazone Bonds for Sustained Cancer Therapy. Acta Biomater. 2022, 141, 102–113. [Google Scholar] [CrossRef]
- De Siqueira, E.C.; De França, J.A.A.; De Souza, R.F.M.; Leoterio, D.M.; Cordeiro, J.N.; Doboszewski, B. Mechanisms of the Chemical Crosslinking to Obtain the Hydrogels: Synthesis, Conditions of Crosslinking and Biopharmaceutical Applications. Res. Soc. Dev. 2023, 12, e18312943072. [Google Scholar] [CrossRef]
- Jeong, J.O.; Park, J.S.; Kim, E.J.; Jeong, S.I.; Lee, J.Y.; Lim, Y.M. Preparation of Radiation Cross-Linked Poly(Acrylic Acid) Hydrogel Containing Metronidazole with Enhanced Antibacterial Activity. Int. J. Mol. Sci. 2019, 21, 187. [Google Scholar] [CrossRef]
- Li, X.; Xiong, Y. Application of “Click” Chemistry in Biomedical Hydrogels. ACS Omega 2022, 7, 36918–36928. [Google Scholar] [CrossRef] [PubMed]
- Okulmuş, Ş.T.; Oktay, B.; Kazan, D.; Apohan, N.K. Development of Bacterial Cellulose-Hyaluronic Acid Multicomponent Hydrogels via Click Chemistry for Biomedical Applications. Polym. Sci. Ser. A 2023, 65, 682–691. [Google Scholar] [CrossRef]
- Nouri-Felekori, M.; Nezafati, N.; Moraveji, M.; Hesaraki, S.; Ramezani, T. Bioorthogonal hydroxyethyl cellulose-based scaffold crosslinked via click chemistry for cartilage tissue engineering applications. Int. J. Biol. Macromol. 2021, 183, 2030–2043. [Google Scholar] [CrossRef]
- Atmani, Z.; Steindorfer, T.; Kargl, R.; Kleinschek, K.S.; Heinze, T.; Gericke, M. Allyl-Functionalized Polysaccharides for 3D Printable Hydrogels Through Thiol–Ene Click Chemistry. Polysaccharides 2025, 6, 13. [Google Scholar] [CrossRef]
- Laurano, R.; Boffito, M.; Cassino, C.; Midei, L.; Pappalardo, R.; Chiono, V.; Ciardelli, G. Thiol-Ene Photo-Click Hydrogels with Tunable Mechanical Properties Resulting from the Exposure of Different -Ene Moieties through a Green Chemistry. Materials 2023, 16, 2024. [Google Scholar] [CrossRef]
- Cadamuro, F.; Piazzoni, M.; Gamba, E.; Sonzogni, B.; Previdi, F.; Nicotra, F.; Ferramosca, A.; Russo, L. Artificial Intelligence Tool for Prediction of ECM Mimics Hydrogel Formulations via Click Chemistry. Biomater. Adv. 2025, 175, 214323. [Google Scholar] [CrossRef]
- Hao, K.; Xue, Y.; Li, B.; Liu, H.; Shen, G.; Wu, H.; Yao, Y. Cellulose-keratin polymer based on “thiol-ene” click reaction and ease-to-process performance. Int. J. Biol. Macromol. 2025, 310, 142920. [Google Scholar] [CrossRef]
- Troncoso-Afonso, L.; Henríquez-Banegas, Y.M.; Vinnacombe-Willson, G.A.; Gutierrez, J.; Gallastegui, G.; Liz-Marzán, L.M.; García-Astrain, C. Using Thiol–Ene Click Chemistry to Engineer 3D Printed Plasmonic Hydrogel Scaffolds for SERS Biosensing. Biomater. Sci. 2025, 13, 2936–2950. [Google Scholar] [CrossRef]
- Michniak-Kohn, B.B.; Deol, P.K.; Morozova, S.M. Recent Advances in Hydrogels via Diels–Alder Crosslinking: Design and Applications. Gels 2023, 9, 102. [Google Scholar] [CrossRef]
- Wang, G.F.; Chu, H.J.; Wei, H.L.; Liu, X.Q.; Zhao, Z.X.; Zhu, J. Click Synthesis by Diels-Alder Reaction and Characterisation of Hydroxypropyl Methylcellulose-Based Hydrogels. Chem. Pap. 2014, 68, 1390–1399. [Google Scholar] [CrossRef]
- Carneiro de Oliveira, J.; Rigolet, S.; Marichal, C.; Roucoules, V.; Laborie, M.P. Grafting Diels-Alder moieties on cellulose nanocrystals through carbamation. Carbohydr. Polym. 2020, 250, 116966. [Google Scholar] [CrossRef]
- Khin, M.N.; Easdani, M.; Aziz, T.; Shami, A.; Alharbi, N.K.; Al-Asmari, F.; Lin, L. Schiff’s base crosslinked gelatin-dialdehyde cellulose film with gallic acid for improved water resistance and antimicrobial properties. Food Hydrocoll. 2025, 166, 111331. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Hsu, S. Hui. Hydrogels Based on Schiff Base Linkages for Biomedical Applications. Molecules 2019, 24, 3005. [Google Scholar] [CrossRef]
- Raczuk, E.; Dmochowska, B.; Samaszko-Fiertek, J.; Madaj, J. Different Schiff Bases, Structure, Importance and Classification. Molecules 2022, 27, 787. [Google Scholar] [CrossRef]
- Fabbrizzi, L. Beauty in Chemistry: Making Artistic Molecules with Schiff Bases. J. Org. Chem. 2020, 85, 12212–12226. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Li, X.; Zhang, M.; Xu, C.; Li, W.; Wan, L. Antibacterial Cellulose Nanocrystal-Incorporated Hydrogels with Satisfactory Vascularization for Enhancing Skin Regeneration. Front. Bioeng. Biotechnol. 2022, 10, 876936. [Google Scholar] [CrossRef]
- Sethi, S.; Kaith, B.S.; Kaur, M.; Sharma, N.; Khullar, S. A Hydrogel Based on Dialdehyde Carboxymethyl Cellulose–Gelatin and Its Utilization as a Bio Adsorbent. J. Chem. Sci. 2020, 132, 15. [Google Scholar] [CrossRef]
- Sheng, X.; Li, X.; Li, M.; Zhang, R.; Deng, S.; Yang, W.; Chang, G.; Ye, X. An Injectable Oxidized Carboxymethyl Cellulose/Polyacryloyl Hydrazide Hydrogel via Schiff Base Reaction. Aust. J. Chem. 2017, 71, 74–79. [Google Scholar] [CrossRef]
- Mather, B.D.; Viswanathan, K.; Miller, K.M.; Long, T.E. Michael addition Reactions in Macromolecular Design for Emerging Technologies. Prog. Polym. Sci. 2006, 31, 487–531. [Google Scholar] [CrossRef]
- Kang, H.; Liu, R.; Huang, Y. Cellulose-Based Gels. Macromol. Chem. Phys. 2016, 217, 1322–1334. [Google Scholar] [CrossRef]
- Chen, J.; Ma, X.; Edgar, K.J. A Versatile Method for Preparing Polysaccharide Conjugates via Thiol-Michael Addition. Polymers 2021, 13, 1905. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Medronho, B.; Lindman, B.; Norgren, M. Simple One Pot Preparation of Chemical Hydrogels from Cellulose Dissolved in Cold LiOH/Urea. Polymers 2020, 12, 373. [Google Scholar] [CrossRef] [PubMed]
- Varghese, S.A.; Rangappa, S.M.; Siengchin, S.; Parameswaranpillai, J. Natural polymers and the hydrogels prepared from them. In Hydrogels Based on Natural Polymers; Elsevier, 2020; pp. 17–47. Available online: https://www.sciencedirect.com/science/article/abs/pii/B9780128164211000021?via%3Dihub (accessed on 19 May 2025). [CrossRef]
- Emam, H.E.; Shaheen, T.I. Design of a Dual PH and Temperature Responsive Hydrogel Based on Esterified Cellulose Nanocrystals for Potential Drug Release. Carbohydr. Polym. 2022, 278, 118925. [Google Scholar] [CrossRef]
- Talbi, A.; Merouani, S.; Dehane, A.; Bouchoucha, H.; Abdessemed, A.; Belahmadi, M.S.O. Thermo-Catalytic Persulfate Activation in Tubular Microreactors for Advanced Oxidation of Safranin O: Insights into Process Benefits and Limitations. Processes 2025, 13, 1494. [Google Scholar] [CrossRef]
- Bao, Y.; Ma, J.; Li, N. Synthesis and Swelling Behaviors of Sodium Carboxymethyl Cellulose-g-Poly(AA-Co-AM-Co-AMPS)/MMT Superabsorbent Hydrogel. Carbohydr. Polym. 2011, 84, 76–82. [Google Scholar] [CrossRef]
- Lu, M.; Liu, Y.; Huang, Y.C.; Huang, C.J.; Tsai, W.B. Fabrication of Photo-Crosslinkable Glycol Chitosan Hydrogel as a Tissue Adhesive. Carbohydr. Polym. 2018, 181, 668–674. [Google Scholar] [CrossRef]
- Silva, R.; Rebelo, R.C.; Paula, C.T.B.; Pereira, P.; Fonseca, A.C.; Serra, A.C.; Coelho, J.F.J. All-Cellulose Resin for 3D Printing Hydrogels via Digital Light Processing (DLP). Int. J. Biol. Macromol. 2025, 306, 141389. [Google Scholar] [CrossRef]
- Chen, J.; Edgar, K.J.; Frazier, C.E. Photo-Curable, Double-Crosslinked, in Situ-Forming Hydrogels Based on Oxidized Hydroxypropyl Cellulose. Cellulose 2021, 28, 3903–3915. [Google Scholar] [CrossRef]
- Hu, W.; Chen, Z.; Chen, X.; Feng, K.; Hu, T.; Huang, B.; Tang, J.; Wang, G.; Liu, S.; Yang, G.; et al. Double-Network Cellulose-Based Hybrid Hydrogels with Favourable Biocompatibility and Antibacterial Activity for Wound Healing. Carbohydr. Polym. 2023, 319, 121193. [Google Scholar] [CrossRef]
- Meléndez-Ortiz, H.I.; Varca, G.H.C.; Lugão, A.B.; Bucio, E. Smart Polymers and Coatings Obtained by Ionizing Radiation: Synthesis and Biomedical Applications. Open J. Polym. Chem. 2015, 5, 17–33. [Google Scholar] [CrossRef]
- Ramos-Ballesteros, A.; Pino-Ramos, V.H.; López-Saucedo, F.; Flores-Rojas, G.G.; Bucio, E. γ-Rays and Ions Irradiation. In Surface Modification of Polymers; John Wiley and Sons, 2019; pp. 185–209. Available online: https://onlinelibrary.wiley.com/doi/10.1002/9783527819249.ch7 (accessed on 19 May 2025). [CrossRef]
- Araujo, L.M.G.; Morales, A.R. Compatibilization of Recycled Polypropylene and Recycled Poly (Ethylene Terephthalate) Blends with SEBS-g-MA. Polimeros 2018, 28, 84–91. [Google Scholar] [CrossRef]
- Hiroki, A.; Taguchi, M.; Swiatla-Wojcik, D.; Katsumura, Y.; Wach, R.A. Development of Environmentally Friendly Cellulose Derivative-Based Hydrogels for Contact Lenses Using a Radiation Crosslinking Technique. Appl. Sci. 2021, 11, 9168. [Google Scholar] [CrossRef]
- Blažic, R.; Marušić, K.; Vidović, E. Swelling and Viscoelastic Properties of Cellulose-Based Hydrogels Prepared by Free Radical Polymerization of Dimethylaminoethyl Methacrylate in Cellulose Solution. Gels 2023, 9, 94. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of Hybrid Organic–Inorganic Nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.M.; Mahapatra, C.; Kim, H.W.; Knowles, J.C. Sol–Gel Based Materials for Biomedical Applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef]
- Mehdi, A.; Reyé, C.; Brandès, S.; Guilard, R.; Corriu, R.J.P. Synthesis of Large-Pore Ordered Mesoporous Silicas Containing Aminopropyl Groups. New J. Chem. 2005, 29, 965–968. [Google Scholar] [CrossRef]
- Demitri, C.; Del Sole, R.; Scalera, F.; Sannino, A.; Vasapollo, G.; Maffezzoli, A.; Ambrosio, L.; Nicolais, L. Novel Superabsorbent Cellulose-Based Hydrogels Crosslinked with Citric Acid. J. Appl. Polym. Sci. 2008, 110, 2453–2460. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Wang, Z.; Wang, L. Ultrasound in Cellulose-Based Hydrogel for Biomedical Use: From Extraction to Preparation. Colloids Surf. Biointerfaces 2022, 212, 112368. [Google Scholar] [CrossRef]
- Maestri, C.A.; Abrami, M.; Hazan, S.; Chistè, E.; Golan, Y.; Rohrer, J.; Bernkop-Schnürch, A.; Grassi, M.; Scarpa, M.; Bettotti, P. Role of Sonication Pre-Treatment and Cation Valence in the Sol-Gel Transition of Nano-Cellulose Suspensions. Sci. Rep. 2017, 7, 11129. [Google Scholar] [CrossRef]
- Pan, H.; Qu, Y.; Wang, F.; Zhao, S.; Chen, G. Horseradish peroxidase-catalyzed crosslinking injectable hydrogel for bone repair and regeneration. Colloid Interfac. Sci. 2025, 66, 00828. [Google Scholar] [CrossRef]
- Enping, L.; Binyu, B.; Yifei, Z.; Haitao, L. Transglutaminase-Catalyzed Bottom-Up Synthesis of Polymer Hydrogel. Front. Bioeng. Biotechnol. 2022, 10, 824747. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, S.; Lu, W.; Chen, N.; Zhu, D.; Li, Y. Preparation and Characterization of Enzymatically Cross-Linked Gelatin/Cellulose Nanocrystal Composite Hydrogels. RSC Adv. 2021, 11, 10794–10803. [Google Scholar] [CrossRef]
- Cai, M.H.; Chen, X.Y.; Fu, L.Q.; Du, W.L.; Yang, X.; Mou, X.Z.; Hu, P.Y. Design and Development of Hybrid Hydrogels for Biomedical Applications: Recent Trends in Anticancer Drug Delivery and Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 630943. [Google Scholar] [CrossRef]
- Karchoubi, F.; Ghotli, R.A.; Pahlevani, H.; Salehi, M.B. New insights into nanocomposite hydrogels; a review on recent advances in characteristics and applications. Adv. Ind. Eng. Polym. Res. 2024, 7, 54–78. [Google Scholar] [CrossRef]
- Zou, P.; Yao, J.; Cui, Y.-N.; Zhao, T.; Che, J.; Yang, M.; Li, Z.; Demitri, C.; Riva, L.; Zou, P.; et al. Advances in Cellulose-Based Hydrogels for Biomedical Engineering: A Review Summary. Gels 2022, 8, 364. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Fugolin, A.P.P.; Huynh, B.; Rajasekaran, S.P. Innovations in the Design and Application of Stimuli-Responsive Restorative Dental Polymers. Polymers 2023, 15, 3346. [Google Scholar] [CrossRef]
- Klouda, L.; Mikos, A.G. Thermoresponsive Hydrogels in Biomedical Applications. Eur. J. Pharm. Biopharm. 2008, 68, 34–45. [Google Scholar] [CrossRef]
- Decuzzi, P.; Cook, A.B. Harnessing Endogenous Stimuli for Responsive Materials in Theranostics. ACS Nano 2021, 15, 2068–2098. [Google Scholar] [CrossRef]
- Guild, J.D.; Knox, S.T.; Burholt, S.B.; Hilton, E.M.; Terrill, N.J.; Schroeder, S.L.M.; Warren, N.J. Continuous-Flow Laboratory SAXS for In Situ Determination of the Impact of Hydrophilic Block Length on Spherical Nano-Object Formation during Polymerization-Induced Self-Assembly. Macromolecules 2023, 56, 6426–6435. [Google Scholar] [CrossRef]
- Reyes-Ortega, F. pH-responsive polymers: Properties, synthesis and applications. In Smart Polymers and their Applications; Aguilar, M.R., Román, J.S., Eds.; Woodhead Publishing: Cambridge, UK, 2014; pp. 45–92. ISBN 9780857096951. [Google Scholar] [CrossRef]
- Nesrinne, S.; Djamel, A. Synthesis, Characterization and Rheological Behavior of PH Sensitive Poly(Acrylamide-Co-Acrylic Acid) Hydrogels. Arab. J. Chem. 2017, 10, 539–547. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Arola, S.; Kou, Z.; Rooijakkers, B.J.M.; Velagapudi, R.; Sammalkorpi, M.; Linder, M.B. On the Mechanism for the Highly Sensitive Response of Cellulose Nanofiber Hydrogels to the Presence of Ionic Solutes. Cellulose 2022, 29, 6109–6121. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, M.; Tu, Y.; Lin, S.; Hu, J. Stimuli-Responsive Cellulose-Based Hydrogels. In Cellulose-Based Superabsorbent Hydrogels; Springer: Cham, Switzerland, 2019; pp. 269–308. [Google Scholar] [CrossRef]
- Ding, H.; Tan, P.; Fu, S.; Tian, X.; Zhang, H.; Ma, X.; Gu, Z.; Luo, K. Preparation and Application of PH-Responsive Drug Delivery Systems. J. Control. Release 2022, 348, 206–238. [Google Scholar] [CrossRef]
- Sampath Udeni Gunathilake, T.M.; Ching, Y.C.; Chuah, C.H.; Rahman, N.A.; Liou, N.S. Recent Advances in Celluloses and Their Hybrids for Stimuli-Responsive Drug Delivery. Int. J. Biol. Macromol. 2020, 158, 670–688. [Google Scholar] [CrossRef]
- Seo, J.Y.; Lee, B.; Kang, T.W.; Noh, J.H.; Kim, M.J.; Ji, Y.B.; Ju, H.J.; Min, B.H.; Kim, M.S. Electrostatically Interactive Injectable Hydrogels for Drug Delivery. Tissue Eng. Regen. Med. 2018, 15, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Anwar, N. Gelatin/Carboxymethyl Cellulose Based Stimuli-Responsive Hydrogels for Controlled Delivery of 5-Fluorouracil, Development, in Vitro Characterization, in Vivo Safety and Bioavailability Evaluation. Carbohydr. Polym. 2021, 257, 117617. [Google Scholar] [CrossRef]
- Chang, C.; He, M.; Zhou, J.; Zhang, L. Swelling Behaviors of PH- and Salt-Responsive Cellulose-Based Hydrogels. Macromolecules 2011, 44, 1642–1648. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, X.; Wei, X.; Zhang, J.; Wang, D.; Lu, H.; Jia, P. Ultrastretchable, Tough, Antifreezing, and Conductive Cellulose Hydrogel for Wearable Strain Sensor. ACS Appl. Mater. Interfaces 2020, 12, 53247–53256. [Google Scholar] [CrossRef]
- Vegad, U.; Patel, M.; Khunt, D.; Zupančič, O.; Chauhan, S.; Paudel, A. pH Stimuli-Responsive Hydrogels from Non-Cellulosic Biopolymers for Drug Delivery. Front. Bioeng. Biotechnol. 2023, 11, 1270364. [Google Scholar] [CrossRef]
- Chen, N.; Wang, H.; Ling, C.; Vermerris, W.; Wang, B.; Tong, Z. Cellulose-Based Injectable Hydrogel Composite for PH-Responsive and Controllable Drug Delivery. Carbohydr. Polym. 2019, 225, 115207. [Google Scholar] [CrossRef] [PubMed]
- Pedige, M.P.H.; Asoh, T.A.; Hsu, Y.I.; Uyama, H. Stimuli-Responsive Composite Hydrogels with Three-Dimensional Stability Prepared Using Oxidized Cellulose Nanofibers and Chitosan. Carbohydr. Polym. 2022, 278, 118907. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Hui, P.C.L.; Kan, C.W. Thermoresponsive Hydrogels and Their Biomedical Applications: Special Insight into Their Applications in Textile Based Transdermal Therapy. Polymers 2018, 10, 480. [Google Scholar] [CrossRef]
- Pazhouhnia, Z.; Beheshtizadeh, N.; Lotfibakhshaiesh, N. Encapsulation of Cartilage Cells. Princ. Biomater. Encapsul. 2023, 2, 525–555. [Google Scholar] [CrossRef]
- Duarte-Peña, L.; Fragoso-Medina, A.J.; Bucio, E.; López-Saucedo, F. Improving the Therapeutic Value of Sutures. In Advanced Technologies and Polymer Materials for Surgical Sutures; Woodhead Publishing: Cambridge, UK, 2023; pp. 45–76. [Google Scholar] [CrossRef]
- Yuan, Y.; Raheja, K.; Milbrandt, N.B.; Beilharz, S.; Tene, S.; Oshabaheebwa, S.; Gurkan, U.A.; Cristina, A.; Samia, S.; Karayilan, M. Thermoresponsive Polymers with LCST Transition: Synthesis, Characterization, and Their Impact on Biomedical Frontiers. RSC Appl. Polym. 2023, 1, 158–189. [Google Scholar] [CrossRef]
- Le, M.; Huang, W.; Chen, K.F.; Lin, C.; Cai, L.; Zhang, H.; Jia, Y.G. Upper Critical Solution Temperature Polymeric Drug Carriers. Chem. Eng. J. 2022, 432, 134354. [Google Scholar] [CrossRef]
- Ieong, N.S.; Hasan, M.; Phillips, D.J.; Saaka, Y.; O’Reilly, R.K.; Gibson, M.I. Polymers with Molecular Weight Dependent LCSTs Are Essential for Cooperative Behaviour. Polym. Chem. 2012, 3, 794–799. [Google Scholar] [CrossRef]
- Wei, M.; Gao, Y.; Li, X.; Serpe, M.J. Stimuli-Responsive Polymers and Their Applications. Polym. Chem. 2016, 8, 127–143. [Google Scholar] [CrossRef]
- Pang, J.; Wang, L.; Xu, Y.; Wu, M.; Wang, M.; Liu, Y.; Yu, S.; Li, L. Skin-Inspired Cellulose Conductive Hydrogels with Integrated Self-Healing, Strain, and Thermal Sensitive Performance. Carbohydr. Polym. 2020, 240, 116360. [Google Scholar] [CrossRef]
- Parvari, G.; Rotbaum, Y.; Eichen, Y.; Rittel, D. Impact-Induced Gelation in Aqueous Methylcellulose Solutions. Chem. Commun. 2018, 54, 12578–12581. [Google Scholar] [CrossRef]
- Coughlin, M.L.; Liberman, L.; Ertem, S.P.; Edmund, J.; Bates, F.S.; Lodge, T.P. Methyl Cellulose Solutions and Gels: Fibril Formation and Gelation Properties. Prog. Polym. Sci. 2021, 112, 101324. [Google Scholar] [CrossRef]
- Lyytikäinen, J.; Laukala, T.; Backfolk, K. Temperature-Dependent Interactions between Hydrophobically Modified Ethyl(Hydroxyethyl)Cellulose and Methyl Nanocellulose. Cellulose 2019, 26, 7079–7087. [Google Scholar] [CrossRef]
- Lynen, F.; Ampe, A.; Bandini, E.; Baert, M.; Wicht, K.; Kajtazi, A.; Rahmani, T.; Veenhoven, J.; Spileers, G. Perspectives in Hydrophobic Interaction Temperature-Responsive Liquid Chromatography (TRLC). LC-GC N. Am. 2022, 40, 566–572. [Google Scholar] [CrossRef]
- Wang, T.; Chen, L.; Shen, T.; Wu, D. Preparation and Properties of a Novel Thermo-Sensitive Hydrogel Based on Chitosan/Hydroxypropyl Methylcellulose/Glycerol. Int. J. Biol. Macromol. 2016, 93, 775–782. [Google Scholar] [CrossRef]
- Arai, K.; Shikata, T. Hydration/Dehydration Behavior of Hydroxyethyl Cellulose Ether in Aqueous Solution. Molecules 2020, 25, 4726. [Google Scholar] [CrossRef] [PubMed]
- Dashtimoghadam, E.; Salimi-Kenari, H.; Nasseri, R.; Knudsen, K.D.; Mirzadeh, H.; Nyström, B. Tunable Viscoelastic Features of Aqueous Mixtures of Thermosensitive Ethyl(Hydroxyethyl)Cellulose and Cellulose Nanowhiskers. Colloids Surf. A Physicochem. Eng. Asp. 2020, 590, 124489. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Z.; Wang, J.; Zong, W.; Liu, R. The Interaction Mechanism between Anionic or Cationic Surfactant with HSA by Using Spectroscopy, Calorimetry and Molecular Docking Methods. J. Mol. Liq. 2016, 224, 1008–1015. [Google Scholar] [CrossRef]
- Gregory, K.P.; Elliott, G.R.; Robertson, H.; Kumar, A.; Wanless, E.J.; Webber, G.B.; Craig, V.S.J.; Andersson, G.G.; Page, A.J. Understanding Specific Ion Effects and the Hofmeister Series. Phys. Chem. Chem. Phys. 2022, 24, 12682–12718. [Google Scholar] [CrossRef]
- Li, L.; Wang, Q.; Xu, Y. Thermoreversible Association and Gelation of Methylcellulose in Aqueous Solutions. Nihon Reoroji Gakkaishi 2003, 31, 287–296. [Google Scholar] [CrossRef][Green Version]
- Png, Z.M.; Wang, C.G.; Yeo, J.C.C.; Lee, J.J.C.; Surat’man, N.E.; Tan, Y.L.; Liu, H.; Wang, P.; Tan, B.H.; Xu, J.W.; et al. Stimuli-Responsive Structure–Property Switchable Polymer Materials. Mol. Syst. Des. Eng. 2023, 8, 1097–1129. [Google Scholar] [CrossRef]
- Li, W. Mechanophores in Polymer Mechanochemistry: Insights from Single-Molecule Experiments and Computer Simulations. In Functional Tactile Sensors: Materials, Devices and Integrations; Woodhead Publishing: Cambridge, UK, 2021; pp. 113–139. [Google Scholar] [CrossRef]
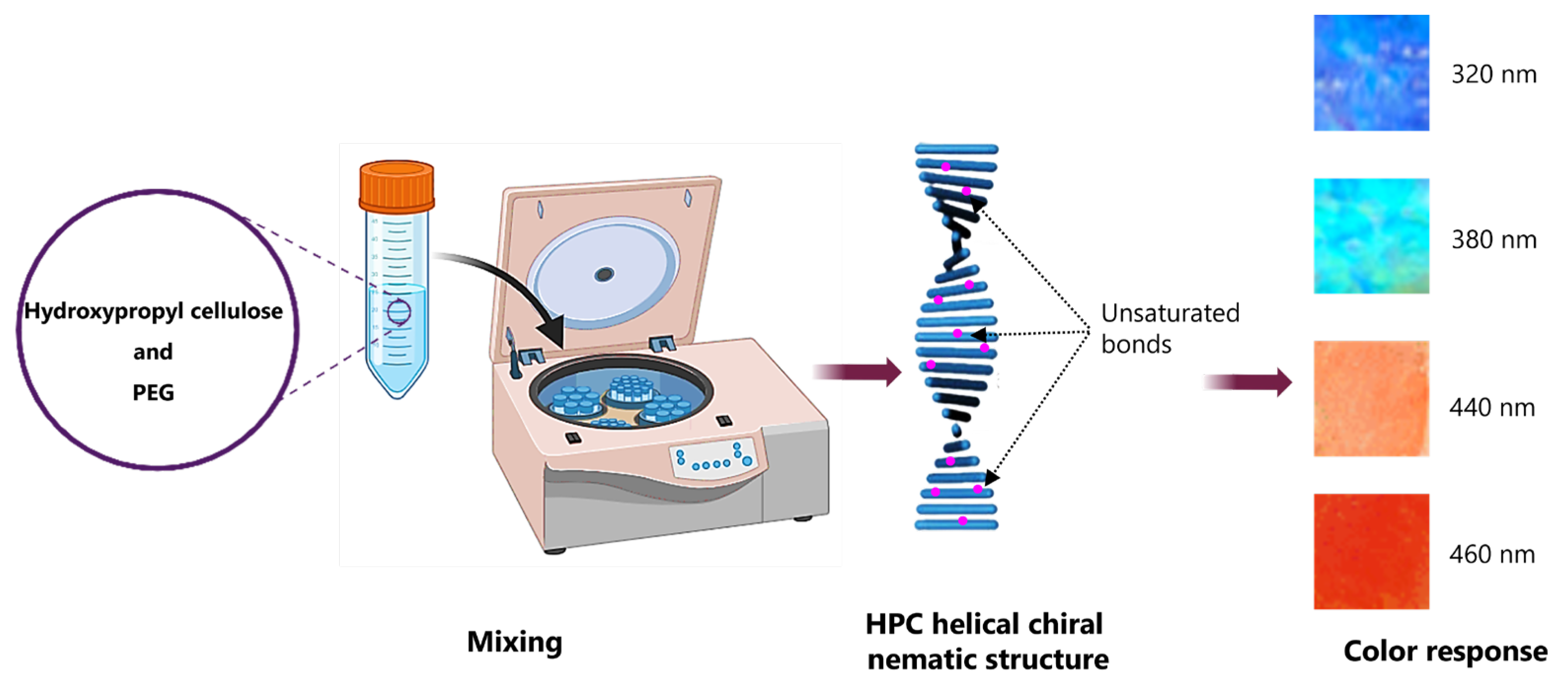
- Barty-King, C.H.; Chan, C.L.C.; Parker, R.M.; Bay, M.M.; Vadrucci, R.; De Volder, M.; Vignolini, S. Mechanochromic, Structurally Colored, and Edible Hydrogels Prepared from Hydroxypropyl Cellulose and Gelatin. Adv. Mater. 2021, 33, e2102112. [Google Scholar] [CrossRef] [PubMed]
- Buaksuntear, K.; Limarun, P.; Suethao, S.; Smitthipong, W. Non-Covalent Interaction on the Self-Healing of Mechanical Properties in Supramolecular Polymers. Int. J. Mol. Sci. 2022, 23, 6902. [Google Scholar] [CrossRef]
- Hussain, I.; Sayed, S.M.; Liu, S.; Oderinde, O.; Kang, M.; Yao, F.; Fu, G. Enhancing the Mechanical Properties and Self-Healing Efficiency of Hydroxyethyl Cellulose-Based Conductive Hydrogels via Supramolecular Interactions. Eur. Polym. J. 2018, 105, 85–94. [Google Scholar] [CrossRef]
- Kang, M.; Oderinde, O.; Han, X.; Fu, G.; Zhang, Z. Development of oxidized hydroxyethyl cellulose-based hydrogel enabling unique mechanical, transparent, and photochromic properties for contact lenses. Int. J. Biol. Macromol. 2021, 183, 1162–1173. [Google Scholar] [CrossRef]
- Sikdar, P.; Uddin, M.M.; Dip, T.M.; Islam, S.; Hoque, M.S.; Dhar, A.K.; Wu, S. Recent Advances in the Synthesis of Smart Hydrogels. Mater. Adv. 2021, 2, 4532–4573. [Google Scholar] [CrossRef]
- Chiang, C.W.; Hsiao, Y.C.; Jheng, P.R.; Chen, C.H.; Manga, Y.B.; Lekha, R.; Chao, K.M.; Ho, Y.C.; Chuang, E.Y. Strontium Ranelate-Laden near-Infrared Photothermal-Inspired Methylcellulose Hydrogel for Arthritis Treatment. Mater. Sci. Eng. C 2021, 123, 111980. [Google Scholar] [CrossRef]
- Shi, Q.; Liu, H.; Tang, D.; Li, Y.; Li, X.J.; Xu, F. Bioactuators Based on Stimulus-Responsive Hydrogels and Their Emerging Biomedical Applications. NPG Asia Mater. 2019, 11, 64. [Google Scholar] [CrossRef]
- Gebeyehu, E.K.; Sui, X.; Adamu, B.F.; Beyene, K.A.; Tadesse, M.G. Cellulosic-Based Conductive Hydrogels for Electro-Active Tissues: A Review Summary. Gels 2022, 8, 140. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.S. Conducting polymers in biosensing: A review. Chem. Phys. Impact. 2024, 8, 100642. [Google Scholar] [CrossRef]
- Danmatam, N.; Pearce, J.T.H.; Pattavarakorn, D. Intelligent self-healable electroactive carboxymethyl cellulose hydrogel containing conductive polythiophene and acid hydrolyzed cellulose. J. Appl. Polym. Sci. 2023, 141, e5475. [Google Scholar] [CrossRef]
- Liu, S.; Luo, X.; Zhou, J. Magnetic Responsive Cellulose Nanocomposites and Their Applications. In Cellulose—Medical, Pharmaceutical and Electronic Applications; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Fragal, E.H.; Fragal, V.H.; Silva, E.P.; Paulino, A.T.; da Silva Filho, E.C.; Mauricio, M.R.; Silva, R.; Rubira, A.F.; Muniz, E.C. Magnetic-Responsive Polysaccharide Hydrogels as Smart Biomaterials: Synthesis, Properties, and Biomedical Applications. Carbohydr. Polym. 2022, 292, 119665. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.I.; Biliuta, G.; Socoliuc, V.; Coseri, S. Affordable Magnetic Hydrogels Prepared from Biocompatible and Biodegradable Sources. Polymers 2021, 13, 1693. [Google Scholar] [CrossRef]
- Mansoor, S.; Kondiah, P.P.D.; Choonara, Y.E. Advanced Hydrogels for the Controlled Delivery of Insulin. Pharmaceutics 2021, 13, 2113. [Google Scholar] [CrossRef]
- Mohanty, A.R.; Ravikumar, A.; Peppas, N.A. Recent Advances in Glucose-Responsive Insulin Delivery Systems: Novel Hydrogels and Future Applications. Regen. Biomater. 2022, 9, rbac056. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, Z.; Wang, Y.; Meng, Z.; Zhao, Z. A Self-Healing Carboxymethyl Chitosan/Oxidized Carboxymethyl Cellulose Hydrogel with Fluorescent Bioprobes for Glucose Detection. Carbohydr. Polym. 2021, 274, 118642. [Google Scholar] [CrossRef] [PubMed]
- Morariu, S. Advances in the Design of Phenylboronic Acid-Based Glucose-Sensitive Hydrogels. Polymers 2023, 15, 582. [Google Scholar] [CrossRef]
- Peng, H.; Ning, X.; Wei, G.; Wang, S.; Dai, G.; Ju, A. The Preparations of Novel Cellulose/Phenylboronic Acid Composite Intelligent Bio-Hydrogel and Its Glucose, pH-Responsive Behaviors. Carbohydr. Polym. 2018, 195, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Yi, J.; Mao, X.; Wu, H.; Zhang, L.M.; Yang, L. Glucose-Sensitive Hydrogels from Covalently Modified Carboxylated Pullulan and Concanavalin A for Smart Controlled Release of Insulin. React. Funct. Polym. 2019, 139, 112–119. [Google Scholar] [CrossRef]
- Reduwan Billah, S.M.; Mondal, M.I.H.; Somoal, S.H.; Nahid Pervez, M.; Haque, M.O. Enzyme-Responsive Hydrogels. In Cellulose-Based Superabsorbent Hydrogels; Springer: Cham, Switzerland, 2018; pp. 1–23. [Google Scholar] [CrossRef]
- Mallikarjun, P.N.; Anusha, S.; Sai Nandini, V.; Rama Rao, B.; Kamala Kumari, P.V.; Srinivasa Rao, Y. Hydrogel: Responsive Structures for Drug Delivery. Int. J. Appl. Pharm. 2021, 13, 65–76. [Google Scholar] [CrossRef]
- Baretta, R.; Gabrielli, V.; Frasconi, M. Nanozyme-Cellulose Hydrogel Composites Enabling Cascade Catalysis for the Colorimetric Detection of Glucose. ACS Appl. Nano Mater. 2022, 5, 13845–13853. [Google Scholar] [CrossRef]
- Xue, H.; Zhu, C.; Wang, Y.; Gu, Q.; Shao, Y.; Jin, A.; Zhang, X.; Lei, L.; Li, Y. Stimulus-Responsive Cellulose Hydrogels in Biomedical Applications and Challenges. Mater. Tday. Biol. 2025, 32, 101814. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhai, Z.; Yao, Y.; Stant, J.C.; Landrum, S.L.; Bortner, M.J.; Frazier, C.E.; Edgar, K.J. Oxidized Hydroxypropyl Cellulose/Carboxymethyl Chitosan Hydrogels Permit PH-Responsive, Targeted Drug Release. Carbohydr. Polym. 2023, 300, 120213. [Google Scholar] [CrossRef] [PubMed]
- Maturavongsadit, P.; Paravyan, G.; Shrivastava, R.; Benhabbour, S.R. Thermo-/pH-responsive chitosan-cellulose nanocrystals based hydrogel with tunable mechanical properties for tissue regeneration applications. Materialia 2020, 12, 100681. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Liu, Y.; Hua, S.; Meng, F.; Ma, Q.; Kong, L.; Pan, S.; Che, Y. Injectable, self-healable and antibacterial multi-responsive tunicate cellulose nanocrystals strengthened supramolecular hydrogels for wound dressings. Int. J. Biol. Macromol. 2023, 240, 124365. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Cellulose-Based Composites as Scaffolds for Tissue Engineering: Recent Advances. Molecules 2022, 27, 8830. [Google Scholar] [CrossRef]
- Jooken, S.; Deschaume, O.; Bartic, C. Nanocomposite Hydrogels as Functional Extracellular Matrices. Gels 2023, 9, 153. [Google Scholar] [CrossRef]
- Namjoo, A.R.; Abrbekoh, F.N.; Saghati, S.; Amini, H.; Saadatlou, M.A.E.; Rahbarghazi, R. Tissue Engineering Modalities in Skeletal Muscles: Focus on Angiogenesis and Immunomodulation Properties. Stem Cell Res. Ther. 2023, 14, 90. [Google Scholar] [CrossRef]
- Phatchayawat, P.P.; Khamkeaw, A.; Yodmuang, S.; Phisalaphong, M. 3D Bacterial Cellulose-Chitosan-Alginate-Gelatin Hydrogel Scaffold for Cartilage Tissue Engineering. Biochem. Eng. J. 2022, 184, 108476. [Google Scholar] [CrossRef]
- Al-Sabah, A.; Burnell, S.E.A.; Simoes, I.N.; Jessop, Z.; Badiei, N.; Blain, E.; Whitaker, I.S. Structural and Mechanical Characterization of Crosslinked and Sterilised Nanocellulose-Based Hydrogels for Cartilage Tissue Engineering. Carbohydr. Polym. 2019, 212, 242–251. [Google Scholar] [CrossRef]
- Hao, J.; Chen, Y.; Zhu, M.; Zhao, Y.; Zhang, K.; Xu, X. Spatial-Temporal Heterogeneity in Large Three-Dimensional Nanofibrillar Cellulose Hydrogel for Human Pluripotent Stem Cell Culture. Gels 2023, 9, 324. [Google Scholar] [CrossRef]
- Wasyłeczko, M.; Sikorska, W.; Chwojnowski, A. Review of Synthetic and Hybrid Scaffolds in Cartilage Tissue Engineering. Membranes 2020, 10, 348. [Google Scholar] [CrossRef]
- Hettinger, A.Z.; Roth, E.M.; Bisantz, A.M. Cognitive Engineering and Health Informatics: Applications and Intersections. J. Biomed. Inform. 2017, 67, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Boyer, C.; Figueiredo, L.; Pace, R.; Lesoeur, J.; Rouillon, T.; Le Visage, C.; Tassin, J.F.; Weiss, P.; Guicheux, J.; Rethore, G. Laponite Nanoparticle-Associated Silated Hydroxypropylmethyl Cellulose as an Injectable Reinforced Interpenetrating Network Hydrogel for Cartilage Tissue Engineering. Acta Biomater. 2018, 65, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Thivya, P.; Akalya, S.; Sinija, V.R. A Comprehensive Review on Cellulose-Based Hydrogel and Its Potential Application in the Food Industry. Appl. Food Res. 2022, 2, 100161. [Google Scholar] [CrossRef]
- Torabizadeh, F.; Talaei-Khozani, T.; Yaghobi, A.; Walker, M.; Mirzaei, E. Enhancing Chondrogenic Differentiation of Mesenchymal Stem Cells through Synergistic Effects of Cellulose Nanocrystals and Plastic Compression in Collagen-Based Hydrogel for Cartilage Formation. Int. J. Biol. Macromol. 2024, 272, 132848. [Google Scholar] [CrossRef] [PubMed]
- Buchtová, N.; D’Orlando, A.; Judeinstein, P.; Chauvet, O.; Weiss, P.; Le Bideau, J. Water Dynamics in Silanized Hydroxypropyl Methylcellulose Based Hydrogels Designed for Tissue Engineering. Carbohydr. Polym. 2018, 202, 404–408. [Google Scholar] [CrossRef]
- Boer, M.; Duchnik, E.; Maleszka, R.; Marchlewicz, M. Structural and Biophysical Characteristics of Human Skin in Maintaining Proper Epidermal Barrier Function. Postep. Dermatol. Alergol. 2016, 33, 1–5. [Google Scholar] [CrossRef]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflam. 2019, 2019, 3706315. [Google Scholar] [CrossRef]
- Bektas, C.; Kimiz, I.; Sendemir, A.; Hasirci, V.; Hasirci, N. A Bilayer Scaffold Prepared from Collagen and Carboxymethyl Cellulose for Skin Tissue Engineering Applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 1764–1784. [Google Scholar] [CrossRef]
- Koivunotko, E.; Koivuniemi, R.; Monola, J.; Harjumäki, R.; Pridgeon, C.S.; Madetoja, M.; Linden, J.; Paasonen, L.; Laitinen, S.; Yliperttula, M. Cellulase-Assisted Platelet-Rich Plasma Release from Nanofibrillated Cellulose Hydrogel Enhances Wound Healing. J. Control. Release 2024, 368, 397–412. [Google Scholar] [CrossRef]
- Palem, R.R.; Kim, B.J.; Baek, I.; Choi, H.; Suneetha, M.; Shimoga, G.; Lee, S.H. In Situ Fabricated ZnO Nanostructures within Carboxymethyl Cellulose-Based Ternary Hydrogels for Wound Healing Applications. Carbohydr. Polym. 2024, 334, 122020. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Pajorova, J.; Bacakova, M.; Skogberg, A.; Kallio, P.; Kolarova, K.; Svorcik, V. Versatile Application of Nanocellulose: From Industry to Skin Tissue Engineering and Wound Healing. Nanomaterials 2019, 9, 164. [Google Scholar] [CrossRef]
- Utoiu, E.; Manoiu, V.S.; Oprita, E.I.; Craciunescu, O. Bacterial Cellulose: A Sustainable Source for Hydrogels and 3D-Printed Scaffolds for Tissue Engineering. Gels 2024, 10, 387. [Google Scholar] [CrossRef]
- Hosseini, S.M.R.; Heydari, P.; Namnabat, M.; Nasr Azadani, R.; Azimi Gharibdousti, F.; Mousavi Rizi, E.; Khosravi, A.; Zarepour, A.; Zarrabi, A. Carboxymethyl Cellulose/Sodium Alginate Hydrogel with Anti-Inflammatory Capabilities for Accelerated Wound Healing; In Vitro and In Vivo Study. Eur. J. Pharmacol. 2024, 976, 176671. [Google Scholar] [CrossRef] [PubMed]
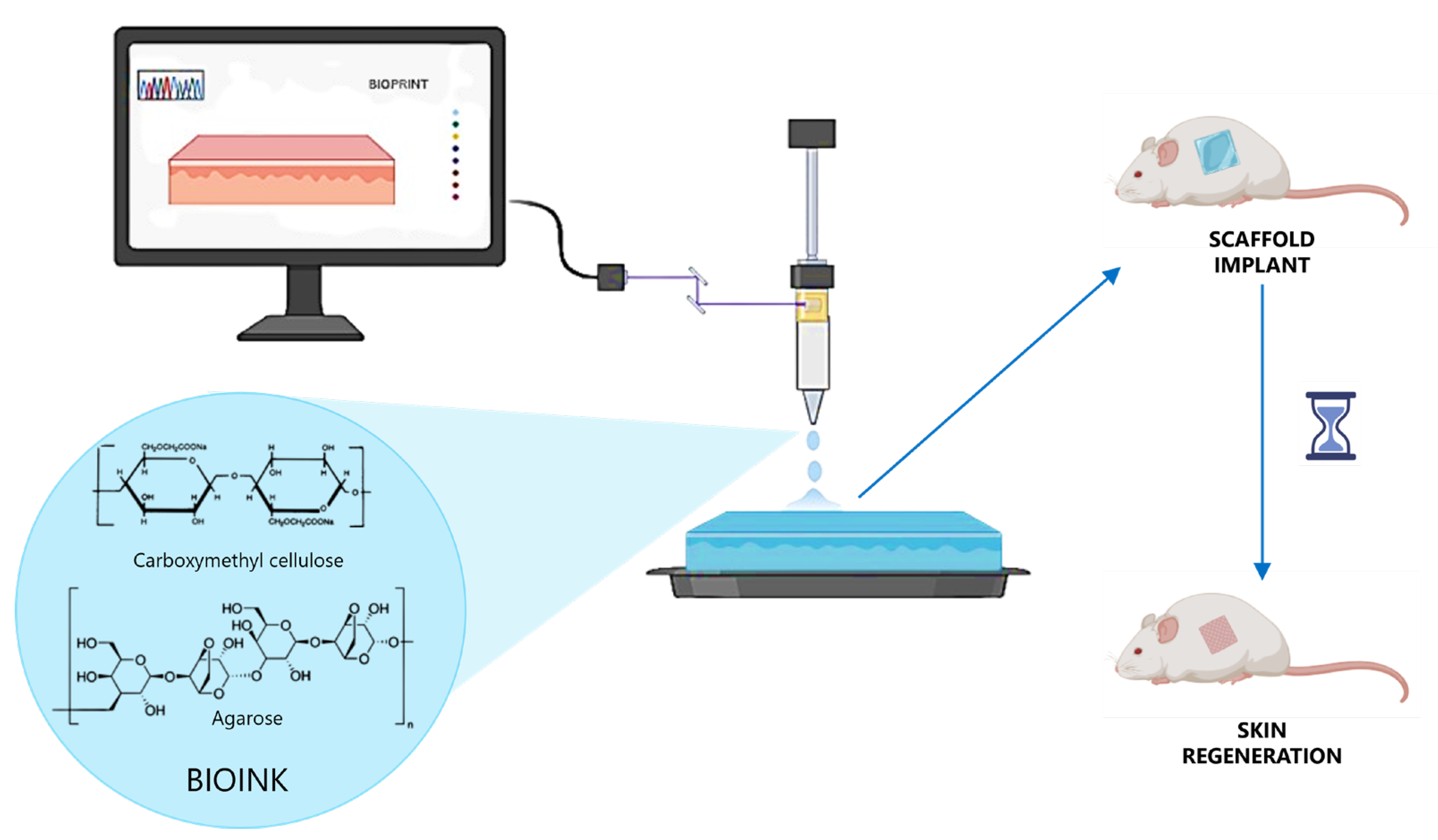
- Budharaju, H.; Bagewadi, S.; Devanathan, P.; Chellappan, D.; Chinnaswamy, P.; Sethuraman, S.; Sundaramurthi, D. Carboxymethyl Cellulose-Agarose Hydrogel in Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Nanofibers: A Novel Tissue Engineered Skin Graft. Int. J. Biol. Macromol. 2024, 264, 130565. [Google Scholar] [CrossRef]
- Damle, M.N.; Chaudhari, L.; Tardalkar, K.; Bhamare, N.; Jagdale, S.; Gaikwad, V.; Chhabra, D.; Kumar, B.; Manuja, A.; Joshi, M.G. A biologically functional bioink based on extracellular matrix derived collagen for 3D printing of skin. Int. J. Biol. Macromol. 2024, 258, 128851. [Google Scholar] [CrossRef]
- Budharaju, H.; Chandrababu, H.; Zennifer, A.; Chellappan, D.; Sethuraman, S.; Sundaramurthi, D. Tuning Thermoresponsive Properties of Carboxymethyl Cellulose (CMC)–Agarose Composite Bioinks to Fabricate Complex 3D Constructs for Regenerative Medicine. Int. J. Biol. Macromol. 2024, 260, 129443. [Google Scholar] [CrossRef]
- Qiu, H.; Wang, J.; Hu, H.; Song, L.; Liu, Z.; Xu, Y.; Liu, S.; Zhu, X.; Wang, H.; Bao, C.; et al. Preparation of an Injectable and Photocurable Carboxymethyl Cellulose/Hydroxyapatite Composite and Its Application in Cranial Regeneration. Carbohydr. Polym. 2024, 333, 121987. [Google Scholar] [CrossRef] [PubMed]
- Suneetha, M.; Kim, H.; Han, S.S. Bone-like Apatite Formation in Biocompatible Phosphate-Crosslinked Bacterial Cellulose-Based Hydrogels for Bone Tissue Engineering Applications. Int. J. Biol. Macromol. 2024, 256, 128364. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Xiao, X.; Li, X.; Chen, C.; Sun, D. Biomimetic Design of Platelet-Rich Plasma Controlled Release Bacterial Cellulose/Hydroxyapatite Composite Hydrogel for Bone Tissue Engineering. Int. J. Biol. Macromol. 2024, 269, 132124. [Google Scholar] [CrossRef]
- Niknafs, B.; Meskaraf-asadabadi, M.; Hamdi, K.; Ghanbari, E. Incorporating bioactive glass nanoparticles in silk fibroin/bacterial nanocellulose composite scaffolds improves their biological and osteogenic properties for bone tissue engineering applications. Int. J. Biol. Macromol. 2024, 266, 131167. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Niu, Y.; Zhang, Y.; Wang, W.; Zhou, J.; Bai, Y.; Ma, G. Metal Ion-Containing Hydrogels: Synthesis, Properties, and Applications in Bone Tissue Engineering. Biomacromolecules 2024, 25, 3217–3248. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Li, Y.M.; Jiang, K.; Wang, K.; Kuzmanović, M.; You, X.H.; Zhang, Y.; Lei, J.; Huang, S.S.; Xu, J.Z. ECM-Inspired Calcium/Zinc Laden Cellulose Scaffold for Enhanced Bone Regeneration. Carbohydr. Polym. 2024, 331, 121823. [Google Scholar] [CrossRef]
- Jiao, H.; Lu, X.; Li, Y.; Zhang, H.; Fu, Y.; Zhong, C.; Wang, Q.; Ullah, M.W.; Liu, H.; Yong, Y.C.; et al. In Situ Biomineralization Reinforcing Anisotropic Nanocellulose Scaffolds for Guiding the Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells. Int. J. Biol. Macromol. 2024, 274, 133515. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, H.; Shi, J.; Luo, H.; Yin, C.; Wan, Y. Hydroxyapatite Gradient Poly (Vinyl Alcohol)/Bacteria Cellulose Bone Scaffold via Buoyancy-Driven Gradient Method. Fibers Polym. 2024, 25, 1951–1963. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, P.; Liang, H.; Zhou, J. Hemostatic Tranexamic Acid-Induced Fast Gelation and Mechanical Reinforcement of Polydimethylacrylamide/Carboxymethyl Chitosan Hydrogel for Hemostasis and Wound Healing. Biomacromolecules 2024, 25, 819–828. [Google Scholar] [CrossRef]
- Peyravian, N.; Milan, P.B.; Kebria, M.M.; Mashayekhan, S.; Ghasemian, M.; Amiri, S.; Hamidi, M.; Shavandi, A.; Moghtadaei, M. Designing and Synthesis of Injectable Hydrogel Based on Carboxymethyl Cellulose/Carboxymethyl Chitosan Containing QK Peptide for Femoral Head Osteonecrosis Healing. Int. J. Biol. Macromol. 2024, 270, 132127. [Google Scholar] [CrossRef]
- Almeida-Pinto, J.; Moura, B.S.; Gaspar, V.M.; Mano, J.F. Advances in Cell-Rich Inks for Biofabricating Living Architectures. Adv. Mater. 2024, 36, 2313776. [Google Scholar] [CrossRef]
- Afra, S.; Koch, M.; Żur-Pińska, J.; Dolatshahi, M.; Bahrami, A.R.; Sayed, J.E.; Moradi, A.; Matin, M.M.; Włodarczyk-Biegun, M.K. Chitosan/Nanohydroxyapatite/Hydroxyethyl-Cellulose-Based Printable Formulations for Local Alendronate Drug Delivery in Osteoporosis Treatment. Carbohydr. Polym. Technol. Appl. 2024, 7, 100418. [Google Scholar] [CrossRef]
- Volpi, M.; Paradiso, A.; Costantini, M.; Świȩszkowski, W. Hydrogel-Based Fiber Biofabrication Techniques for Skeletal Muscle Tissue Engineering. ACS Biomater. Sci. Eng. 2022, 8, 379–405. [Google Scholar] [CrossRef]
- Gahlawat, S.; Oruc, D.; Paul, N.; Ragheb, M.; Patel, S.; Fasasi, O.; Sharma, P.; Shreiber, D.I.; Freeman, J.W. Tissue Engineered 3D Constructs for Volumetric Muscle Loss. Ann. Biomed. Eng. 2024, 52, 2325–2347. [Google Scholar] [CrossRef] [PubMed]
- Rzhepakovsky, I.; Piskov, S.; Avanesyan, S.; Sizonenko, M.; Timchenko, L.; Anfinogenova, O.; Nagdalian, A.; Blinov, A.; Denisova, E.; Kochergin, S.; et al. Composite of Bacterial Cellulose and Gelatin: A Versatile Biocompatible Scaffold for Tissue Engineering. Int. J. Biol. Macromol. 2024, 256, 128369. [Google Scholar] [CrossRef]
- Wang, X.; Wang, M.; Xu, Y.; Yin, J.; Hu, J. A 3D-Printable Gelatin/Alginate/ε-Poly-l-Lysine Hydrogel Scaffold to Enable Porcine Muscle Stem Cells Expansion and Differentiation for Cultured Meat Development. Int. J. Biol. Macromol. 2024, 271, 131980. [Google Scholar] [CrossRef] [PubMed]
- Mastrodimos, M.; Jain, S.; Badv, M.; Shen, J.; Montazerian, H.; Meyer, C.E.; Annabi, N.; Weiss, P.S. Human Skeletal Muscle Myoblast Culture in Aligned Bacterial Nanocellulose and Commercial Matrices. ACS Appl. Mater. Interfaces 2024, 16, 47150–47162. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Cai, J.; Fang, Z.; Wang, H.; Qiu, X.; Liu, W. Anisotropic Muscle-like Conductive Composite Hydrogel Reinforced by Lignin and Cellulose Nanofibrils. ACS Sustain. Chem. Eng. 2022, 10, 12993–13003. [Google Scholar] [CrossRef]
- Zhao, W.; Cao, S.; Cai, H.; Wu, Y.; Pan, Q.; Lin, H.; Fang, J.; He, Y.; Deng, H.; Liu, Z. Chitosan/Silk Fibroin Biomimic Scaffolds Reinforced by Cellulose Acetate Nanofibers for Smooth Muscle Tissue Engineering. Carbohydr. Polym. 2022, 298, 120056. [Google Scholar] [CrossRef]
- Khan, R.; Aslam Khan, M.U.; Stojanović, G.M.; Javed, A.; Haider, S.; Abd Razak, S.I. Fabrication of Bilayer Nanofibrous-Hydrogel Scaffold from Bacterial Cellulose, PVA, and Gelatin as Advanced Dressing for Wound Healing and Soft Tissue Engineering. ACS Omega 2024, 9, 6527–6536. [Google Scholar] [CrossRef]
- Yue, Z.; Wen, F.; Gao, S.; Ang, M.Y.; Pallathadka, P.K.; Liu, L.; Yu, H. Preparation of Three-Dimensional Interconnected Macroporous Cellulosic Hydrogels for Soft Tissue Engineering. Biomaterials 2010, 31, 8141–8152. [Google Scholar] [CrossRef]
- Ollier, R.C.; Webber, M.J. Strain-Stiffening Mechanoresponse in Dynamic-Covalent Cellulose Hydrogels. Biomacromolecules 2024, 25, 4406–4419. [Google Scholar] [CrossRef]
- Mijiritsky, E.; Assaf, H.D.; Kolerman, R.; Mangani, L.; Ivanova, V.; Zlatev, S. Autologous Platelet Concentrates (APCs) for Hard Tissue Regeneration in Oral Implantology, Sinus Floor Elevation, Peri-Implantitis, Socket Preservation, and Medication-Related Osteonecrosis of the Jaw (MRONJ): A Literature Review. Biology 2022, 11, 1254. [Google Scholar] [CrossRef]
- Grandjean, T.; Perumal, N.; Manicam, C.; Matthey, B.; Wu, T.; Thiem, D.G.E.; Stein, S.; Henrich, D.; Kämmerer, P.W.; Al-Nawas, B.; et al. Towards Optimized Tissue Regeneration: A New 3D Printable Bioink of Alginate/Cellulose Hydrogel Loaded with Thrombocyte Concentrate. Front. Bioeng. Biotechnol. 2024, 12, 1363380. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Tu, H.; Chen, J.; Wang, J.; Liu, H.; Zhang, F.; Li, J. Functionalized Hydrogels in Neural Injury Repairing. Front. Neurosci. 2023, 17, 1199299. [Google Scholar] [CrossRef]
- Kurdtabar, M.; Shafiei, F.; Amini, T.; Shahsavari, F.; Rezanejad Amirdehi, A.; Darestanifarahani, M.; Bagherpour, M.; Farhadi, M. Stimuli-Responsive Double Network CMC-Based Hydrogel Nanocomposite with Enhanced Mechanical Properties for Proliferation and Differentiation of PC12. Polym.-Plast. Technol. Mater. 2024, 63, 489–503. [Google Scholar] [CrossRef]
- Najafi, M.; Pourmadadi, M.; Abdous, M.; Rahdar, A.; Pandey, S. Formulation of Double Nanoemulsions Based on PH-Sensitive Carboxymethyl Cellulose /Starch Copper Doped Carbon Quantum Dots for Quercetin Controlled Release. J. Mol. Liq. 2024, 400, 124543. [Google Scholar] [CrossRef]
- Molina, B.G.; Arnau, M.; Sánchez, M.; Alemán, C. Controlled Dopamine Release from Cellulose-Based Conducting Hydrogel. Eur. Polym. J. 2024, 202, 112635. [Google Scholar] [CrossRef]
- Hasanzadeh, E.; Seifalian, A.; Mellati, A.; Saremi, J.; Asadpour, S.; Enderami, S.E.; Nekounam, H.; Mahmoodi, N. Injectable Hydrogels in Central Nervous System: Unique and Novel Platforms for Promoting Extracellular Matrix Remodeling and Tissue Engineering. Mater. Today Bio 2023, 20, 100614. [Google Scholar] [CrossRef]
- Politrón-Zepeda, G.A.; Fletes-Vargas, G.; Rodríguez-Rodríguez, R. Injectable Hydrogels for Nervous Tissue Repair—A Brief Review. Gels 2024, 10, 190. [Google Scholar] [CrossRef]
- Belyaeva, A.A.; Averchuk, A.S.; Rozanova, N.A.; Alexandrova, O.P.; Solomakha, O.A.; Nashchekina, Y.A.; Korzhikov-Vlakh, V.A.; Yurchenko, S.O.; Salmina, A.B.; Korzhikova-Vlakh, E.G.; et al. Thermosensitive Injectable Fibrillar Gels Based on Cellulose Nanocrystals Grafted with Poly(N-Isopropylacrylamide) as Biocompatible Brain Implants. Carbohydr. Polym. 2024, 346, 122596. [Google Scholar] [CrossRef] [PubMed]
- Digma, L.A.; Upadhyayula, P.S.; Martin, J.R.; Ciacci, J.D. Stem Cells and Chronic Spinal Cord Injury: Overview. In Diagnosis and Treatment of Spinal Cord Injury; Academic Press: Cambridge, MA, USA, 2022; pp. 397–409. [Google Scholar] [CrossRef]
- Sun, Z.; Hu, H.; Xu, Y.; Zhang, X.; Zheng, L.; Sun, Z.; Xiao, Y.; Dong, F.; Wei, G.; Zhang, X. Neurophilic Peptide-Reinforced Dual-Fiber-Network Bioactive Hydrogels for Spinal Cord Injury Repair. Chem. Eng. J. 2024, 498, 155301. [Google Scholar] [CrossRef]
- Laureano, A.; Kim, J.; Martinez, E.; Kwan, K.Y. Chromodomain Helicase DNA Binding Protein 4 in Cell Fate Decisions. Hear. Res. 2023, 436, 108813. [Google Scholar] [CrossRef]
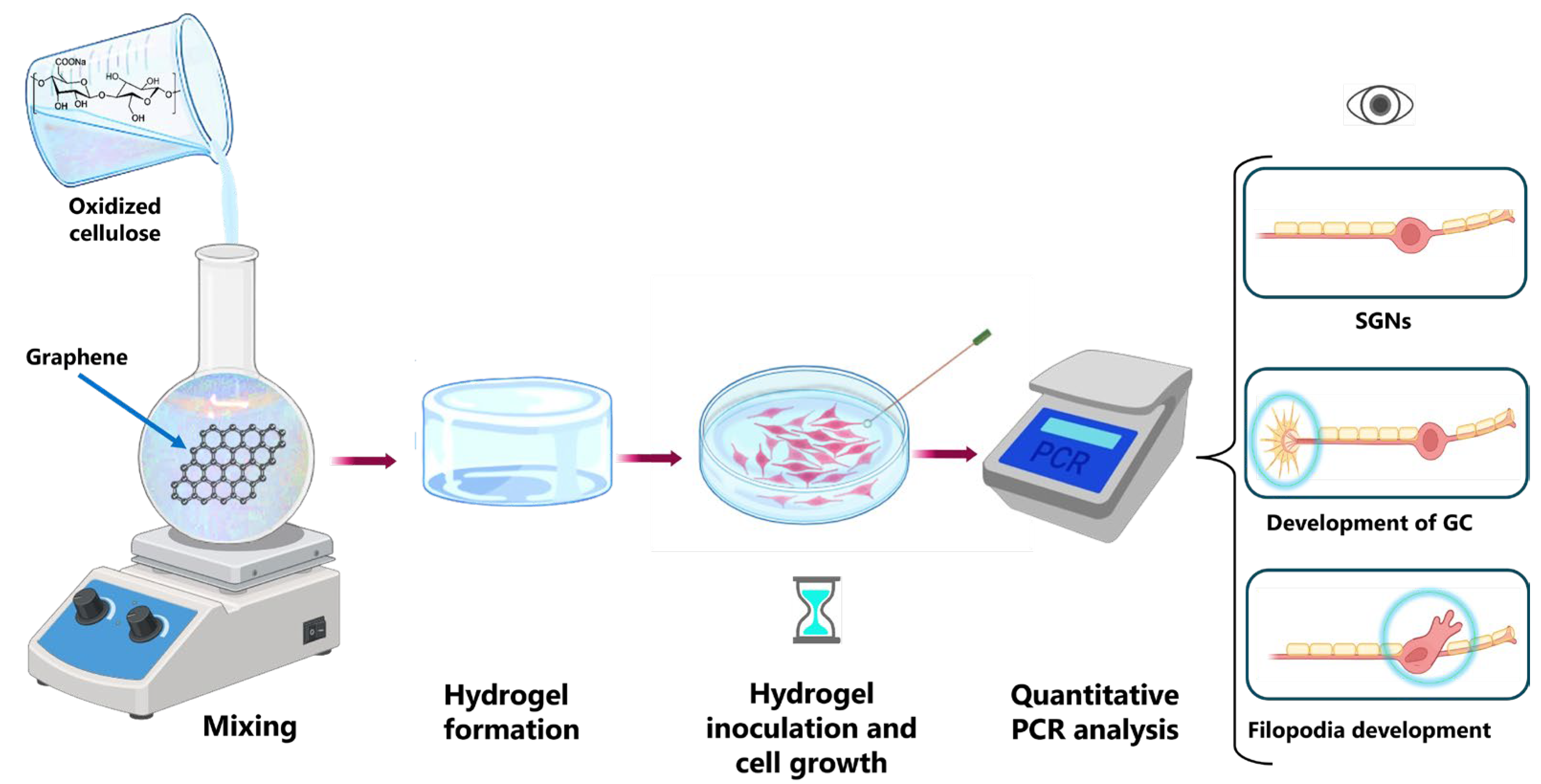
- Shi, L.; Hong, G.; Chen, C.; Li, X.; Zhang, H.; Chai, R.; Sun, D. Growth of Spiral Ganglion Neurons Induced by Graphene Oxide/Oxidized Bacterial Cellulose Composite Hydrogel. Carbohydr. Polym. 2023, 311, 120749. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Fan, Z.; Fang, B.; Zhao, X.; Yao, H.; Cai, G.; Yang, S.; Zhang, G.; Cheng, X.; Feng, Y.; et al. Oriented Cellulose Hydrogel: Directed Tissue Regeneration for Reducing Corneal Leukoplakia and Managing Fungal Corneal Ulcers. Bioact. Mater. 2024, 41, 15–29. [Google Scholar] [CrossRef]
- Vijayaraghavan, R.; Loganathan, S.; Valapa, R.B. 3D Bioprinted Photo Crosslinkable GelMA/Methylcellulose Hydrogel Mimicking Native Corneal Model with Enhanced in Vitro Cytocompatibility and Sustained Keratocyte Phenotype for Stromal Regeneration. Int. J. Biol. Macromol. 2024, 264, 130472. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, Y.; Xu, D.; Zhao, Y. Emerging Technologies for Cardiac Tissue Engineering and Artificial Hearts. Smart Med. 2023, 2, e20220040. [Google Scholar] [CrossRef]
- Alamdari, S.G.; Alibakhshi, A.; de la Guardia, M.; Baradaran, B.; Mohammadzadeh, R.; Amini, M.; Kesharwani, P.; Mokhtarzadeh, A.; Oroojalian, F.; Sahebkar, A. Conductive and Semiconductive Nanocomposite-Based Hydrogels for Cardiac Tissue Engineering. Adv. Heal. Mater. 2022, 11, 2200526. [Google Scholar] [CrossRef]
- Tohidi, H.; Maleki, N.; Simchi, A. Conductive, injectable, and self-healing collagen-hyaluronic acid hydrogels loaded with bacterial cellulose and gold nanoparticles for heart tissue engineering. Int. J. Biol. Macromol. 2024, 280, 135749. [Google Scholar] [CrossRef]
- Chainoglou, E.; Karagkiozaki, V.; Choli-Papadopoulou, T.; Mavromanolis, C.; Laskarakis, A.; Logothetidis, S.; Chainoglou, E.; Karagkiozaki, V.; Choli-Papadopoulou, T.; Mavromanolis, C.; et al. Development of Biofunctionalized Cellulose Acetate Nanoscaffolds for Heart Valve Tissue Engineering. World J. Nano Sci. Eng. 2016, 6, 129–152. [Google Scholar] [CrossRef]
- Ma, N.; Cheung, D.Y.; Butcher, J.T. Incorporating Nanocrystalline Cellulose into a Multifunctional Hydrogel for Heart Valve Tissue Engineering Applications. J. Biomed. Mater. Res. A 2022, 110, 76–91. [Google Scholar] [CrossRef]
- Hua, Y.; He, Z.; Ni, Y.; Sun, L.; Wang, R.; Li, Y.; Li, X.; Jiang, G. Silk fibroin and hydroxypropyl cellulose composite injectable hydrogel-containing extracellular vesicles for myocardial infarction repair. Biomed. Phys. Eng. Express. 2024, 10, 045001. [Google Scholar] [CrossRef]

| Unit | Occurrence | Precursor | Structure | Arrangement | Reference |
|---|---|---|---|---|---|
| Iα | Natural: bacteria, alga, tunicates | Glucose | Triclinic | One chain (42 atoms) per unit, parallel | [1,2,3,4] |
| Iβ | Natural: plants | Glucose | Monoclinic | Two parallel chains per unit (84 atoms) | [1,2,3,4] |
| II | Synthetic: (a) chemical regeneration, (b) caustic mercerization, (c) alkaline salt precipitation, and (d) microbiological cultures | Cellulose I | Monoclinic | Two antiparallel chains per unit | [1,2,3,4] |
| IIII | Synthetic: (a) exposure to amines or ammonia at 140 °C, (b) removal by evaporation | Cellulose Iβ | Monoclinic | One cellulose chain per unit, parallel | [4,8,9,10,11,12] |
| IIIII | Synthetic: (a) exposure to amines or ammonia at 140 °C, (b) removal by evaporation | Cellulose II | Monoclinic | Undefined | [4,8,9,10,11,12] |
| IVI | Natural and synthetic: (a) glycerol heat treatment at 260 °C, (b) super-critical ammonia treatment at 105 °C | Cellulose IIII | Orthorhombic | Two antiparallel chains per unit | [8,13,14,15] |
| IVII | Synthetic: (a) glycerol heat treatment, (b) deacetylation at 150–160 °C | (a) Cellulose IIII and IIIII(b) Cellulose triacetate | Undefined | Two parallel chains per unit | [13,14,15,16,17,18] |
| Source | Cellulose% | Hemicellulose% | Lignin% | Reference |
|---|---|---|---|---|
| Cotton | 82–96.4 | 2–6 | 0–5 | [32] |
| Cotton stalks | 5% | 20% | 21 | [33] |
| Birch | 40.0 | 36 | 20 | [34] |
| Beech | 41 | 33 | 22 | [34] |
| European aspen | 60.0 | 15 | 12 | [35] |
| Nerium oleander | 45 | 15 | 21 | [35] |
| Asclepias | 43.8 | 16 | 8.6 | [35] |
| Flax | 63–71 | 12–21 | 2–3 | [36] |
| Hemp | 63–64 | 12–15 | 3–6 | [37] |
| Jute | 64.4 | 12 | 11.8 | [38] |
| Kenaf | 49.88 | 13.82 | 10.33 | [39] |
| Sisal | 50–74 | 10–14 | 8–11 | [40] |
| Ramie | 68–76 | 13–15 | 0.6–1 | [32] |
| Coir | 32–43 | 10–20 | 43–49 | [36] |
| Bamboo | 73.8 | 12.5 | 10.1 | [41] |
| Rice straw | 28–45 | 12–32 | 5–24 | [42] |
| Response | Transition | ||
|---|---|---|---|
| Size change (swelling–deswelling) |  Hydrogel |  |  |
 Dendrimer hydrogel |  |  | |
 Hyperbranched hydrogel |  |  | |
| Morphology change |  Block copolymer |  |  Micelle |
 |  |  Reverse micelle | |
 Coiled |  |  Globular | |
 Planar |  |  Curved | |
 Stripe |  |  Coiled | |
| Degradation |  Sintered |  |  Degraded |
| Phase transition |  Liquid |  |  Gel |
| pH-Responsive Cationic Polymers | pH-Responsive Anionic Polymers | pH-Cleavable Linkers |
|---|---|---|
| Poly(2-dimethylaminoethyl methacrylate) | Poly(acrylic acid) | Ortho-esters |
| Poly(2-diethylaminoethyl methacrylate) | Poly(2-carboxyethyl acrylate) | Ketals/acetals |
| Poly(2-diisopropylaminoethyl methacrylate) | Poly(2-propylacrylic acid) | Hydrazone |
| Poly(4-vinylpyridine) | Poly(aspartic acid) | Imines |
| Poly(4-(1H-imidazol-1-yl)butyl methacrylate) | Poly(4-vinylbenzoic acid) | Maleic acid |
| Poly(lysine) | Poly(glutamic acid) | Amide derivatives |
| Poly(histidine) (PHis) | Poly(vinylsulfonic acid) | Silyl ethers |
| Poly(ethylenimine) (PEI), | Poly(vinylphenylboronic acid) | Trityl derivatives |
| Poly(β-amino ester) (PbAE). | Poly(vinylphenylboronic acid) | |
| Chitosan | Hyaluronic acid | |
| Dextran | Sodium alginate | |
| Xanthan | ||
| Carragenan | ||
| Chondroitin sulfate | ||
| Fucan and flucoidans | ||
| Mannan and galactomannan |
| LCST Behavior | UCST Behavior |
|---|---|
| Poly (N-isopropylacrylamide) | Poly(N-acryloyl glycinamide) |
| Poly(N-vinyl caprolactam) | Poly(sulfopropyl dimethylammonium propylacrylamide) |
| Poly(N,N-diethylaminoethyl methacrylate) | Poly (2-(N-3-Sulfopropyl-N,N-dimethyl ammonium)ethyl methacrylate) |
| Poly(2-(N-morpholine) ethyl methacrylate) | Polymethacrylamide |
| Poly(oligo(ethylene glycol)methacrylate) | |
| Poly(N,N-diethylacrylamide) | |
| Poly(N,N-dimethylaminoethyl methacry-late, poly(2(diethylamino)ethyl acrylamide)) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Rivas, E.; Desales-Guzmán, L.A.; Pacheco-Sánchez, J.H.; Burillo-Amezcua, S.G. Cellulose-Based Hybrid Hydrogels for Tissue Engineering Applications: A Sustainable Approach. Gels 2025, 11, 438. https://doi.org/10.3390/gels11060438
Vázquez-Rivas E, Desales-Guzmán LA, Pacheco-Sánchez JH, Burillo-Amezcua SG. Cellulose-Based Hybrid Hydrogels for Tissue Engineering Applications: A Sustainable Approach. Gels. 2025; 11(6):438. https://doi.org/10.3390/gels11060438
Chicago/Turabian StyleVázquez-Rivas, Elizabeth, Luis Alberto Desales-Guzmán, Juan Horacio Pacheco-Sánchez, and Sofia Guillermina Burillo-Amezcua. 2025. "Cellulose-Based Hybrid Hydrogels for Tissue Engineering Applications: A Sustainable Approach" Gels 11, no. 6: 438. https://doi.org/10.3390/gels11060438
APA StyleVázquez-Rivas, E., Desales-Guzmán, L. A., Pacheco-Sánchez, J. H., & Burillo-Amezcua, S. G. (2025). Cellulose-Based Hybrid Hydrogels for Tissue Engineering Applications: A Sustainable Approach. Gels, 11(6), 438. https://doi.org/10.3390/gels11060438








