Recyclable and Degradable Poly(vinyl alcohol)/Betaine-Based Deep Eutectic Polymer Dry Gel Plastics with a High Mechanical Strength
Abstract
1. Introduction
2. Results and Discussion
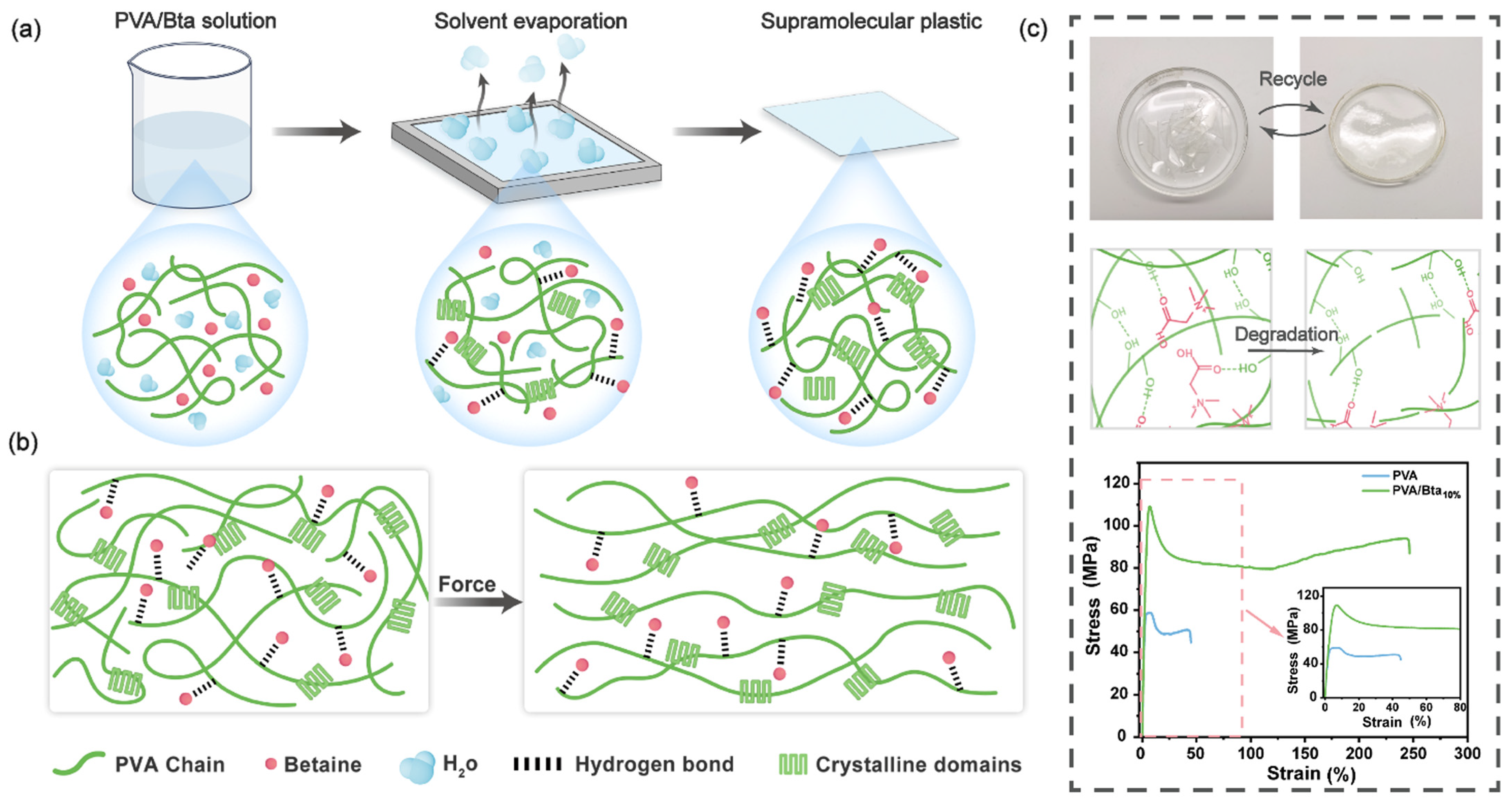
2.1. Mechanical Performance of the PVA/Bta Dry Gel Plastics
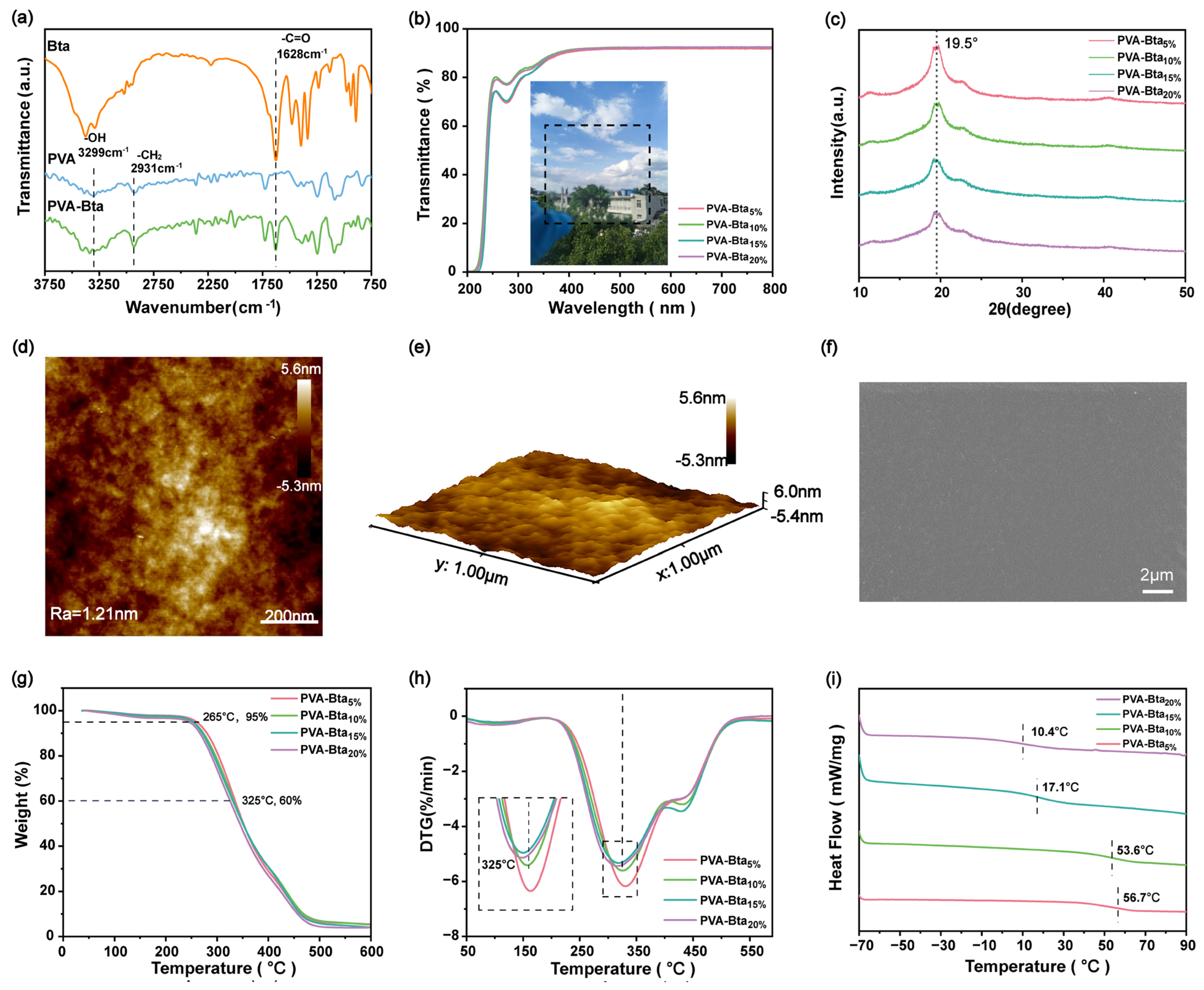
2.2. Synthesis and Characterization of the PVA/Bta Dry Gel Plastics
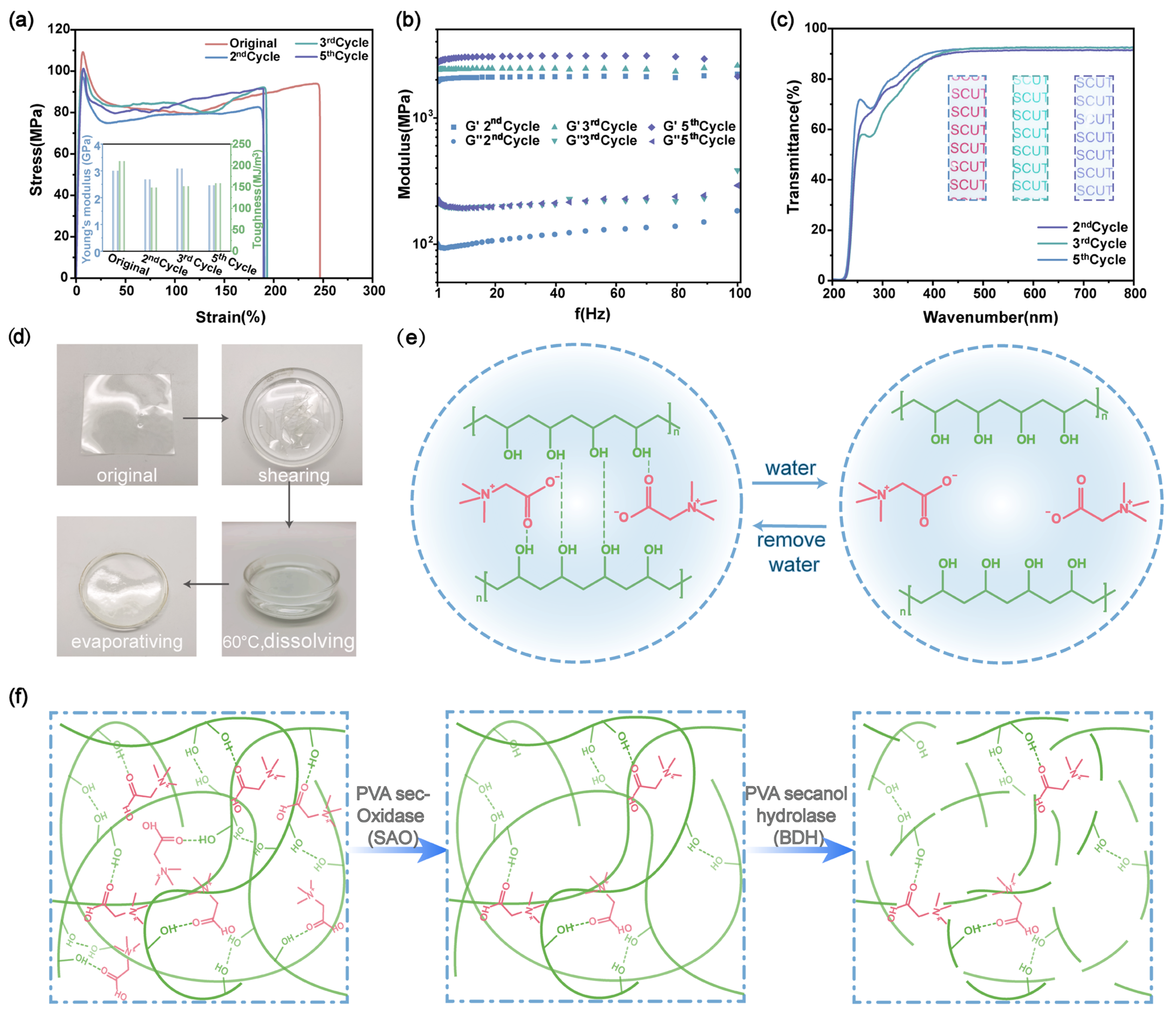
2.3. Recyclability of PVA/Bta Dry Gel Plastics
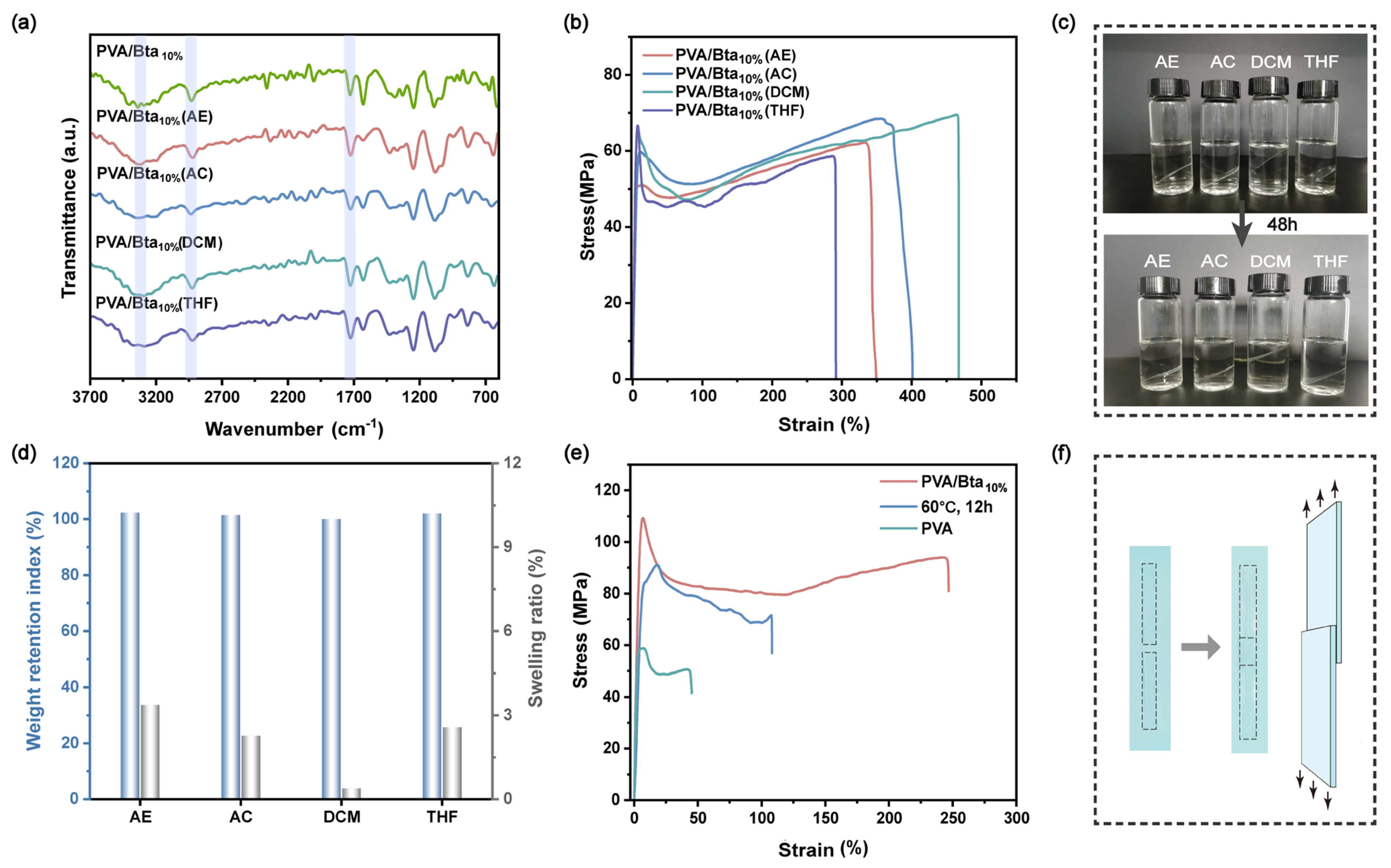
2.4. Solvent Resistance of the PVA/Bta Dry Gel Plastics
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Polymer Preparation
4.3. Characterization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DSC | Differential scanning calorimetry |
| HBA | Hydrogen bond acceptor |
| HBD | Hydrogen bond donor |
| PDES | Polymerizable deep eutectic solvent |
| PALBs | PVA/Bta dry gel plastics |
| AA | Acrylic acid |
| ChCl | Choline chloride |
References
- Fang, X.; Qing, Y.; Lou, Y.; Gao, X.; Wang, H.; Wang, X.; Li, Y.; Qin, Y.; Sun, J. Degradable, Recyclable, Water-Resistant, and Eco-Friendly Poly(vinyl alcohol)-Based Supramolecular Plastics. ACS Mater. Lett. 2022, 4, 1132–1138. [Google Scholar] [CrossRef]
- Coates, G.W.; Getzler, Y.D.Y.L. Chemical recycling to monomer for an ideal, circular polymer economy. Nat. Rev. Mater. 2020, 5, 501–516. [Google Scholar] [CrossRef]
- Jung, H.; Shin, G.; Kwak, H.; Hao, L.T.; Jegal, J.; Kim, H.J.; Jeon, H.; Park, J.; Oh, D.X. Review of polymer technologies for improving the recycling and upcycling efficiency of plastic waste. Chemosphere 2023, 320, 138089. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhao, B.; Zhang, J. Recent development of repairable, malleable and recyclable thermosetting polymers through dynamic transesterification. Polymer 2020, 194, 122392. [Google Scholar] [CrossRef]
- Sanchez, C. Fungal potential for the degradation of petroleum-based polymers: An overview of macro- and microplastics biodegradation. Biotechnol. Adv. 2020, 40, 107501. [Google Scholar] [CrossRef]
- Zhao, X.-L.; Tian, P.-X.; Li, Y.-D.; Zeng, J.-B. Biobased covalent adaptable networks: Towards better sustainability of thermosets. Green Chem. 2022, 24, 4363–4387. [Google Scholar] [CrossRef]
- Luo, J.; Demchuk, Z.; Zhao, X.; Saito, T.; Tian, M.; Sokolov, A.P.; Cao, P.-F. Elastic vitrimers: Beyond thermoplastic and thermoset elastomers. Matter 2022, 5, 1391–1422. [Google Scholar] [CrossRef]
- Naser, A.Z.; Deiab, I.; Darras, B.M. Poly(lactic acid) (PLA) and polyhydroxyalkanoates (PHAs), green alternatives to petroleum-based plastics: A review. RSC Adv. 2021, 11, 17151–17196. [Google Scholar] [CrossRef]
- Atiwesh, G.; Mikhael, A.; Parrish, C.C.; Banoub, J.; Le, T.-A.T. Environmental impact of bioplastic use: A review. Heliyon 2021, 7, e07918. [Google Scholar] [CrossRef]
- Kabir, E.; Kaur, R.; Lee, J.; Kim, K.-H.; Kwon, E.E. Prospects of biopolymer technology as an alternative option for non-degradable plastics and sustainable management of plastic wastes. J. Clean. Prod. 2020, 258, 120536. [Google Scholar] [CrossRef]
- Dang, X.; Du, Y.; Wang, X. Engineering eco-friendly and biodegradable biomass-based multifunctional antibacterial packaging films for sustainable food preservation. Food Chem. 2024, 439, 138119. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Nishide, H.; Tsuchida, E. Oxygen permeability of biodegradable copolycaprolactones. Polym. Adv. Technol. 1999, 10, 282–286. [Google Scholar] [CrossRef]
- Cho, Y.S.; Choi, S.; Lee, S.-H.; Kim, K.K.; Cho, Y.-S. Assessments of polycaprolactone/hydroxyapatite composite scaffold with enhanced biomimetic mineralization by exposure to hydroxyapatite via a 3D-printing system and alkaline erosion. Eur. Polym. J. 2019, 113, 340–348. [Google Scholar] [CrossRef]
- Weidner, E.; Kabasci, S.; Kopitzky, R.; Mörbitz, P. Thermal and Morphological Properties of Poly(L-Lactic Acid)/Poly(D-Lactic Acid)-B-Polycaprolactone Diblock Copolymer Blends. Materials 2020, 13, 2550. [Google Scholar] [CrossRef]
- Hatti-Kaul, R.; Nilsson, L.J.; Zhang, B.; Rehnberg, N.; Lundmark, S. Designing Biobased Recyclable Polymers for Plastics. Trends Biotechnol. 2020, 38, 50–67. [Google Scholar] [CrossRef]
- Fortman, D.J.; Brutman, J.P.; De Hoe, G.X.; Snyder, R.L.; Dichtel, W.R.; Hillmyer, M.A. Approaches to Sustainable and Continually Recyclable Cross-Linked Polymers. ACS Sustain. Chem. Eng. 2018, 6, 11145–11159. [Google Scholar] [CrossRef]
- Hong, M.; Chen, E.Y.X. Chemically recyclable polymers: A circular economy approach to sustainability. Green Chem. 2017, 19, 3692–3706. [Google Scholar] [CrossRef]
- Zhang, L.; Sheng, H.; Liu, R.; Yang, M.; Guo, Y.; Xu, Q.; Hu, L.; Liang, S.; Xie, H. Engineering chitosan into fully bio-sourced, water-soluble and enhanced antibacterial poly(aprotic/protic ionic liquid)s packaging membrane. Int. J. Biol. Macromol. 2023, 230, 123182. [Google Scholar] [CrossRef]
- Tian, G.; Li, L.; Li, Y.; Wang, Q. Water-Soluble Poly(vinyl alcohol)/Biomass Waste Composites: A New Route toward Ecofriendly Materials. ACS Omega 2022, 7, 42515–42523. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Vanda, H.; Dai, Y.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Green solvents from ionic liquids and deep eutectic solvents to natural deep eutectic solvents. Comptes Rendus Chim. 2018, 21, 628–638. [Google Scholar] [CrossRef]
- Li, R.; Fan, T.; Chen, G.; Zhang, K.; Su, B.; Tian, J.; He, M. Autonomous Self-Healing, Antifreezing, and Transparent Conductive Elastomers. Chem. Mater. 2020, 32, 874–881. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, K.; Li, R.; Sang, P.; Hu, H.; He, M. A very mechanically strong and stretchable liquid-free double-network ionic conductor. J. Mater. Chem. A 2021, 9, 23714–23721. [Google Scholar] [CrossRef]
- Dillingham, E.O.; Lawrence, W.H.; Autian, J.; Schmalz, G. Acrylate and methacrylate esters: Relationship of hemolytic activity and in vivo toxicity. J. Biomed. Mater. Res. 1983, 17, 945–957. [Google Scholar] [CrossRef]
- Saillenfait, A.M.; Bonnet, P.; Gallissot, F.; Peltier, A.; Fabriès, J.F. Developmental toxicities of methacrylic acid, ethyl methacrylate, n-butyl methacrylate, and allyl methacrylate in rats following inhalation exposure. Toxicol. Sci. 1999, 50, 136–145. [Google Scholar] [CrossRef]
- Yoshii, E. Cytotoxic effects of acrylates and methacrylates: Relationships of monomer structures and cytotoxicity. J. Biomed. Mater. Res. 1997, 37, 517–524. [Google Scholar] [CrossRef]
- McCarthy, K.L.; Thomas, W.C.; Aardema, M.J.; Seymour, J.L.; Putman, D.L.; Yang, L.L.; Curren, R.D.; Valencia, R. Genetic toxicology of acrylic acid. Food Chem. Toxicol. 1992, 30, 505–515. [Google Scholar] [CrossRef]
- Wu, S.; Cai, C.; Li, F.; Tan, Z.; Dong, S. Deep Eutectic Supramolecular Polymers: Bulk Supramolecular Materials. Angew. Chem.-Int. Ed. 2020, 59, 11871–11875. [Google Scholar] [CrossRef]
- Zheng, L.; Hua, H.; Zhang, Z.; Zhu, Y.; Wang, L.; Li, Y. PVA/ChCl Deep Eutectic Polymer Blends for Transparent Strain Sensors with Antifreeze, Flexible, and Recyclable Properties. ACS Appl. Mater. Interfaces 2022, 14, 49212–49223. [Google Scholar] [CrossRef]
- Lai, J.; Huang, S.; Wu, S.; Li, F.; Dong, S. Adhesion behaviour of bulk supramolecular polymers via pillar[5]arene-based molecular recognition. Chem. Commun. 2021, 57, 13317–13320. [Google Scholar] [CrossRef] [PubMed]
- Duman, O.; Ugurlu, H.; Diker, C.O.; Tunc, S. Fabrication of highly hydrophobic or superhydrophobic electrospun PVA and agar/PVA membrane materials for efficient and selective oil/water separation. J. Environ. Chem. Eng. 2022, 10, 107405. [Google Scholar] [CrossRef]
- Mallakpour, S.; Tabesh, F.; Hussain, C.M. A new trend of using poly(vinyl alcohol) in 3D and 4D printing technologies: Process and applications. Adv. Colloid Interface Sci. 2022, 301, 102605. [Google Scholar] [CrossRef] [PubMed]
- Macarie, L.; Pekar, M.; Simulescu, V.; Plesu, N.; Iliescu, S.; Ilia, G.; Tara-Lunga-Mihali, M. Properties in aqueous solution of homo- and copolymers of vinylphosphonic acid derivatives obtained by UV-curing. Macromol. Res. 2017, 25, 214–221. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Wang, Q.; Ma, J.; Cao, J.; Hu, W.; Wu, Z. Relationship between polymers compatibility and casting solution stability in fabricating PVDF/PVA membranes. J. Membr. Sci. 2017, 537, 263–271. [Google Scholar] [CrossRef]
- Woo, J.H.; Kim, N.H.; Kim, S.I.; Park, O.-K.; Lee, J.H. Effects of the addition of boric acid on the physical properties of MXene/polyvinyl alcohol (PVA) nanocomposite. Compos. Part B Eng. 2020, 199, 108205. [Google Scholar] [CrossRef]
- Sarwar, M.S.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Hussain, A. Preparation and characterization of PVA/nanocellulose/Ag nanocomposite films for antimicrobial food packaging. Carbohydr. Polym. 2018, 184, 453–464. [Google Scholar] [CrossRef]
- Yilmaz Dogan, H.; Altin, Y.; Bedeloğlu, A.Ç. Fabrication and properties of graphene oxide and reduced graphene oxide reinforced Poly(Vinyl alcohol) nanocomposite films for packaging applications. Polym. Polym. Compos. 2022, 30, 09673911221113328. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, J.; Qiu, X.; Zhang, J.; Chang, H.; He, H.; Zhao, L.; Liu, X. Enteromorpha cellulose micro-nanofibrils/poly(vinyl alcohol) based composite films with excellent hydrophilic, mechanical properties and improved thermal stability. Int. J. Biol. Macromol. 2022, 217, 229–242. [Google Scholar] [CrossRef]
- Ismayati, M.; Fatah, N.A.N.; Ernawati, E.E.; Juliandri; Kusumaningrum, W.B.; Lubis, M.A.R.; Fatriasari, W.; Solihat, N.N.; Sari, F.P.; Halim, A.; et al. Antioxidant and UV-blocking activity of PVA/tannin-based bioplastics in food packaging application. Int. J. Biol. Macromol. 2024, 257, 128332. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Sun, J. Degradable Poly(vinyl alcohol)-Based Supramolecular Plastics with High Mechanical Strength in a Watery Environment. Adv. Mater. 2021, 33, 2007371. [Google Scholar] [CrossRef] [PubMed]
- Kawai, F.; Hu, X. Biochemistry of microbial polyvinyl alcohol degradation. Appl. Microbiol. Biotechnol. 2009, 84, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ko, T.-P.; Liu, L.; Li, J.; Huang, C.-H.; Chan, H.-C.; Ren, F.; Jia, D.; Wang, A.H.J.; Guo, R.-T.; et al. Structural Insights into Enzymatic Degradation of Oxidized Polyvinyl Alcohol. Chembiochem 2014, 15, 1882–1886. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, Z.W.; Dong, Y.; Davies, I.J.; Barbhuiya, S. PVA, PVA Blends, and Their Nanocomposites for Biodegradable Packaging Application. Polym.-Plast. Technol. Eng. 2017, 56, 1307–1344. [Google Scholar] [CrossRef]
- Teodorescu, M.; Maria, B.; Morariu, S. Biomaterials of Poly(vinyl alcohol) and Natural Polymers. Polym. Rev. 2018, 58, 247–287. [Google Scholar] [CrossRef]
- Deng, H.; Su, J.; Zhang, W.; Khan, A.; Sani, M.A.; Goksen, G.; Kashyap, P.; Ezati, P.; Rhim, J.-W. A review of starch/polyvinyl alcohol (PVA) blend film: A potential replacement for traditional plastic-based food packaging film. Int. J. Biol. Macromol. 2024, 273, 132926. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Yang, D.; Qiu, X. Biomimetic Supertough and Strong Biodegradable Polymeric Materials with Improved Thermal Properties and Excellent UV-Blocking Performance. Adv. Funct. Mater. 2019, 29, 1806912. [Google Scholar] [CrossRef]
- Yu, Z.; Li, B.; Chu, J.; Zhang, P. Silica in situ enhanced PVA/chitosan biodegradable films for food packages. Carbohydr. Polym. 2018, 184, 214–220. [Google Scholar] [CrossRef]
- Huang, J.; Yang, S.; Zhang, S. Degradation Performance and Biodiversity of an Anaerobic Polyvinyl Alcohol-Degrading Microbial Community. J. Donghua Univ. (Engl. Ed.) 2017, 34, 591–595. [Google Scholar]
- Kucan, K.Z.; Perkovic, M.; Cmrk, K.; Nacinovic, D.; Rogosic, M. Betaine plus (Glycerol or Ethylene Glycol or Propylene Glycol) Deep Eutectic Solvents for Extractive Purification of Gasoline. Chemistryselect 2018, 3, 12582–12590. [Google Scholar] [CrossRef]
- Rogosic, M.; Kristo, A.; Kucan, K.Z. Deep Eutectic Solvents Based on Betaine and Propylene Glycol as Potential Denitrification Agents: A Liquid-Liquid Equilibrium Study. Braz. J. Chem. Eng. 2019, 36, 1703–1716. [Google Scholar] [CrossRef]
- Abranches, D.O.; Silva, L.P.; Martins, M.A.R.; Pinho, S.P.; Coutinho, J.A.P. Understanding the Formation of Deep Eutectic Solvents: Betaine as a Universal Hydrogen Bond Acceptor. Chemsuschem 2020, 13, 4916–4921. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, L.; Liu, Z.; Zheng, S.; Li, Q.; Yan, F. Supramolecular Ionogels Tougher than Metals. Adv. Mater. 2023, 35, e2301383. [Google Scholar] [CrossRef]
- Wu, L.; Kang, Y.; Shi, X.; Yuezhen, B.; Qu, M.; Li, J.; Wu, Z.-S. Natural-Wood-Inspired Ultrastrong Anisotropic Hybrid Hydrogels Targeting Artificial Tendons or Ligaments. ACS Nano 2023, 17, 13522–13532. [Google Scholar] [CrossRef] [PubMed]
- Abdo, S.M.; Youssef, M.; El Nagar, I.; Mohamed, H.E.; El-Kholy, S.A.; Youssef, A.M. Processing and characterization of antimicrobial bioplastic films based on green microalgae Scenedesmus obliquus extract-loaded polyurethane. Int. J. Biol. Macromol. 2024, 257, 128711. [Google Scholar] [CrossRef]
- Plotka-Wasylka, J.; de la Guardia, M.; Andruch, V.; Vilkova, M. Deep eutectic solvents vs ionic liquids: Similarities and differences. Microchem. J. 2020, 159, 105539. [Google Scholar] [CrossRef]
- El Achkar, T.; Greige-Gerges, H.; Fourmentin, S. Basics and properties of deep eutectic solvents: A review. Environ. Chem. Lett. 2021, 19, 3397–3408. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, J.; Yang, Y.; Wang, Q.; Zhang, X.; Hu, H.; Zhu, J. Bio-based poly(butylene diglycolate-co-furandicarboxylate) copolyesters with balanced mechanical, barrier and biodegradable properties: A prospective substitute for PBAT. Polym. Degrad. Stab. 2022, 202, 110010. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, G.; Du, J.; Pei, X.; Du, P.; Zhou, L. Effects of several polymeric materials on the improvement of the sandy soil under rainfall simulation. J. Environ. Manag. 2023, 345, 118847. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Bregado, J.L.; Secchi, A.R.; Tavares, F.W. A density functional theory study on interactions in water-bridged dimeric complexes of lignin. Phys. Chem. Chem. Phys. 2024, 26, 9234–9252. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, Q. Independent gradient model based on Hirshfeld partition: A new method for visual study of interactions in chemical systems. J. Comput. Chem. 2022, 43, 539–555. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Jia, Y.; Cai, L.; Wang, X.; He, M.; Chen, G. Recyclable and Degradable Poly(vinyl alcohol)/Betaine-Based Deep Eutectic Polymer Dry Gel Plastics with a High Mechanical Strength. Gels 2025, 11, 421. https://doi.org/10.3390/gels11060421
Zhao H, Jia Y, Cai L, Wang X, He M, Chen G. Recyclable and Degradable Poly(vinyl alcohol)/Betaine-Based Deep Eutectic Polymer Dry Gel Plastics with a High Mechanical Strength. Gels. 2025; 11(6):421. https://doi.org/10.3390/gels11060421
Chicago/Turabian StyleZhao, Hanyu, Ying Jia, Ling Cai, Xiaochun Wang, Minghui He, and Guangxue Chen. 2025. "Recyclable and Degradable Poly(vinyl alcohol)/Betaine-Based Deep Eutectic Polymer Dry Gel Plastics with a High Mechanical Strength" Gels 11, no. 6: 421. https://doi.org/10.3390/gels11060421
APA StyleZhao, H., Jia, Y., Cai, L., Wang, X., He, M., & Chen, G. (2025). Recyclable and Degradable Poly(vinyl alcohol)/Betaine-Based Deep Eutectic Polymer Dry Gel Plastics with a High Mechanical Strength. Gels, 11(6), 421. https://doi.org/10.3390/gels11060421






