Study on the Feasibility of Self-Assembling Peptides as a Three-Dimensional Culture Tool for Drug Screening of Colorectal Adenocarcinoma Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Physicochemical Properties of SCIOBIO III
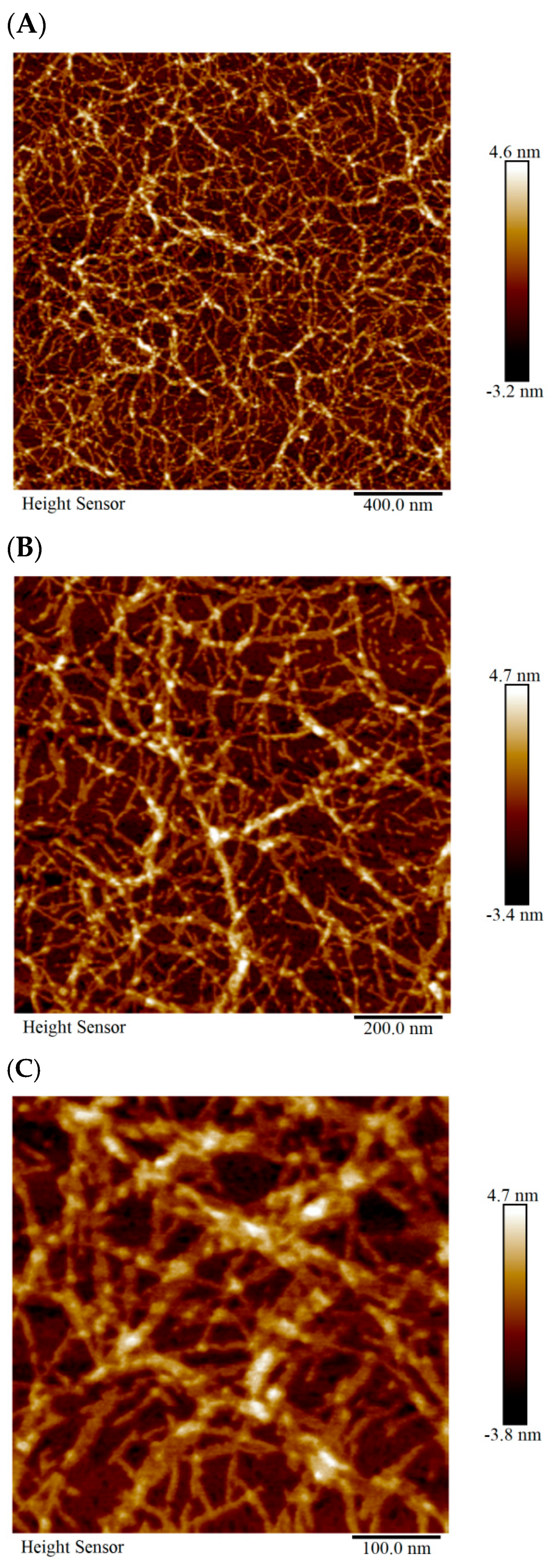
2.1.1. AFM Detection of the Microstructure of SCIOBIO III
2.1.2. Cryogenic-Scanning Electron Microscope (Cryo-SEM) Detection of the Microstructure of SCIOBIO III
2.1.3. Congo Red/Aniline Blue Detection of the Macroscopic Morphology of SCIOBIO III
2.2. SCIOBIO III for 3D Culture of Colorectal Adenocarcinoma Cells
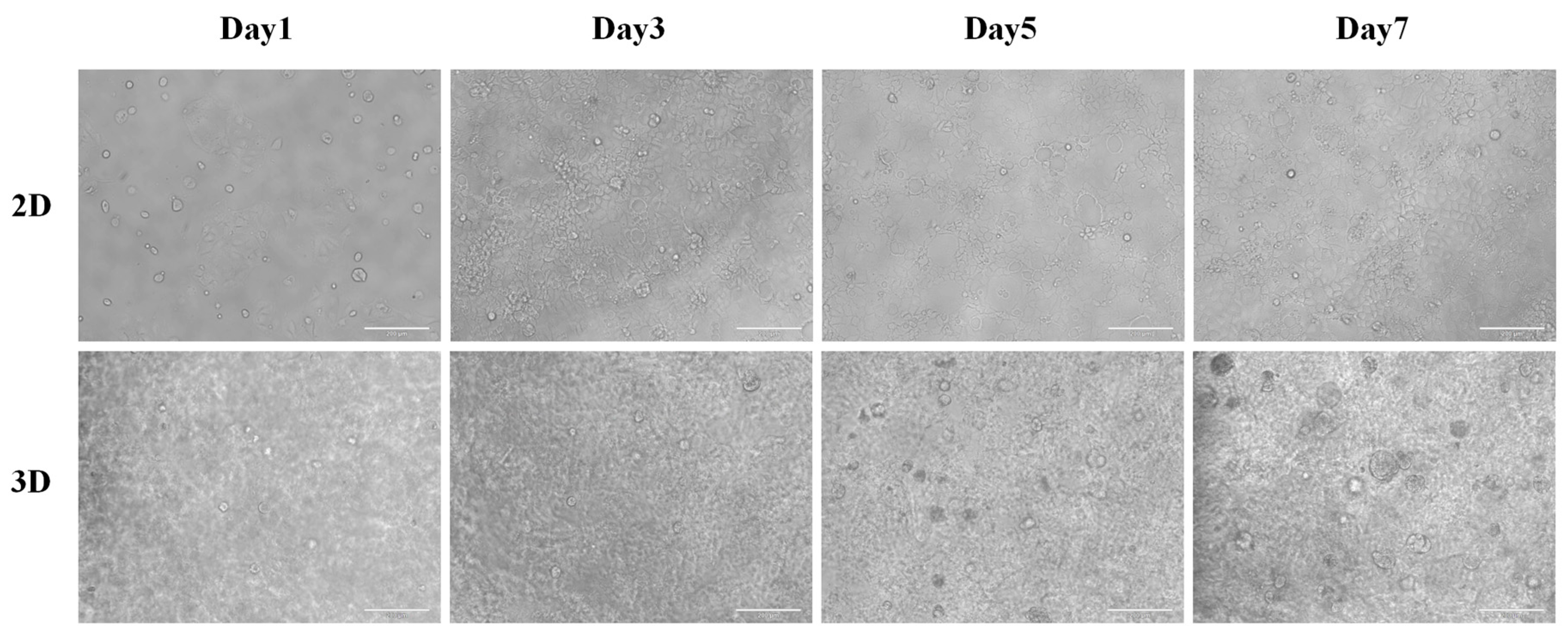
2.2.1. Three-Dimensional Culture of CACO-2 in SCIOBIO III
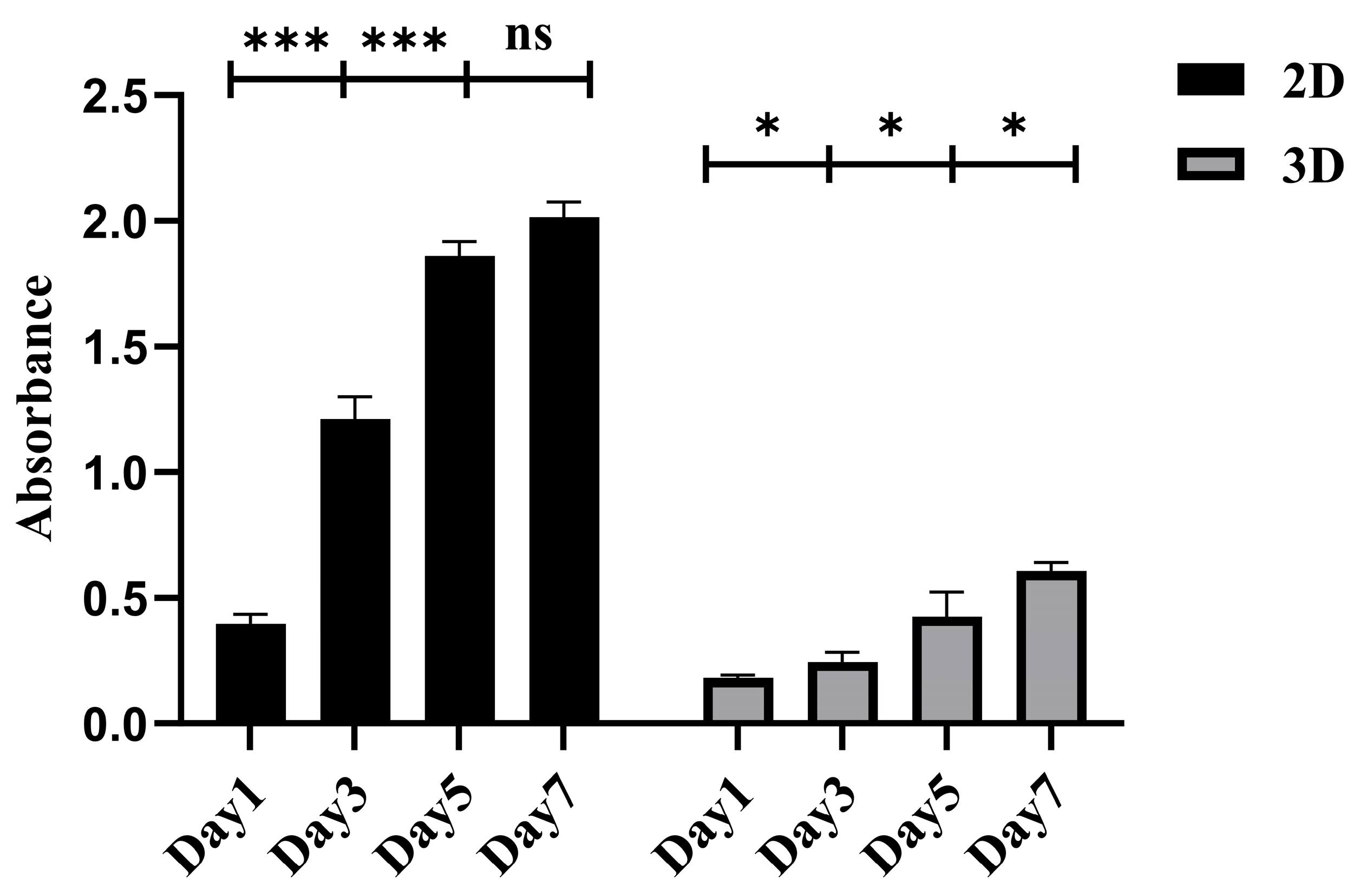
2.2.2. The Proliferative Activity of CACO-2 Cultured in 3D with SCIOBIO III
2.2.3. CACO-2 Staining of Live/Dead Cells in SCIOBIO III
2.3. Screening of Anticancer Drugs Based on SCIOBIO III CACO-2 Cell 3D Culture
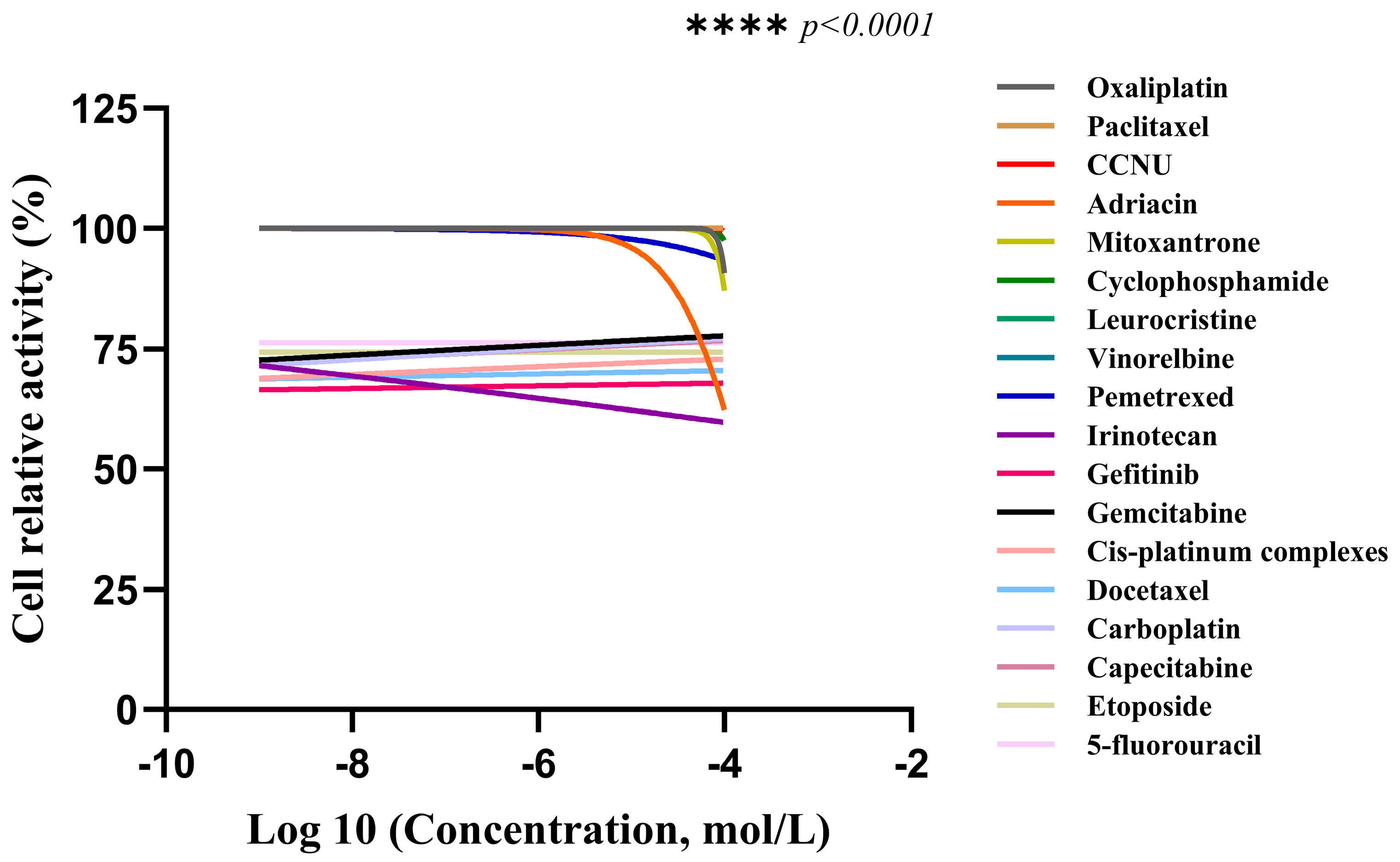
2.3.1. Drug Sensitivity Detection of CACO-2 Cells Cultured in 2D
2.3.2. Drug Sensitivity Detection of CACO-2 Cells Cultured in 3D
2.4. Discussion
3. Conclusions
4. Materials and Methods
4.1. SCIOBIO III Preparation
4.2. AFM
4.3. Cryo-SEM
4.4. Congo Red/Aniline Blue Staining
4.5. Two-Dimensional Culture of CACO-2 Cells
4.6. Three-Dimensional Culture of CACO-2 Cells
4.7. CCK-8 Detection
4.8. Live/Dead Cell Staining
4.9. Drug Sensitivity Detection of CACO-2 Cells in 2D/3D Culture
4.9.1. Drug Sensitivity Detection of CACO-2 Cells in 2D Cultures
4.9.2. Drug Sensitivity Detection of CACO-2 Cells in 3D Cultures
4.10. Data Analysis
5. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roerink, S.F.; Sasaki, N.; Lee-Six, H.; Young, M.D.; Alexandrov, L.B.; Behjati, S.; Mitchell, T.J.; Grossmann, S.; Lightfoot, H.; Egan, D.A.; et al. Intra-tumour diversification in colorectal cancer at the single-cell level. Nature 2018, 556, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Tang, Y.; Pan, W.; Yan, B.; Hao, Y.; Zeng, Y.; Chen, Z.; Lan, J.; Zhao, S.; Deng, C.; et al. Patient-Derived Tumor Organoids Can Predict the Progression-Free Survival of Patients with Stage IV Colorectal Cancer After Surgery. Dis. Colon Rectum 2023, 66, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Sabouni, E.; Nejad, M.M.; Mojtabavi, S.; Khoshduz, S.; Mojtabavi, M.; Nadafzadeh, N.; Nikpanjeh, N.; Mirzaei, S.; Hashemi, M.; Aref, A.R.; et al. Unraveling the function of epithelial-mesenchymal transition (EMT) in colorectal cancer: Metastasis, therapy response, and revisiting molecular pathways. Biomed. Pharmacother. 2023, 160, 114395. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Han, C.; Wan, G.; Huang, X.; Ivan, C.; Jiang, D.; Rodriguez-Aguayo, C.; Lopez-Berestein, G.; Rao, P.H.; et al. TP53 loss creates therapeutic vulnerability in colorectal cancer. Nature 2015, 520, 697–701. [Google Scholar] [CrossRef]
- Al-Shamma, S.A.; Zaher, D.M.; Hersi, F.; Abu Jayab, N.N.; Omar, H.A. Targeting aldehyde dehydrogenase enzymes in combination with chemotherapy and immunotherapy: An approach to tackle resistance in cancer cells. Life Sci. 2023, 320, 121541. [Google Scholar] [CrossRef]
- Short, S.P.; Costacurta, P.W.; Williams, C.S. Using 3D Organoid Cultures to Model Intestinal Physiology and Colorectal Cancer. Curr. Color. Cancer Rep. 2017, 13, 183–191. [Google Scholar] [CrossRef]
- Roper, J.; Tammela, T.; Cetinbas, N.M.; Akkad, A.; Roghanian, A.; Rickelt, S.; Almeqdadi, M.; Wu, K.; Oberli, M.A.; Sánchez-Rivera, F.; et al. Corrigendum: In vivo genome editing and organoid transplantation models of colorectal cancer and metastasis. Nat. Biotechnol. 2017, 35, 1211. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yan, C.; Hu, Y.; Mu, L.; Huang, K.; Li, Q.; Li, X.; Tao, D.; Qin, J. Sphere-forming assay vs. organoid culture: Determining long-term stemness and the chemoresistant capacity of primary colorectal cancer cells. Int. J. Oncol. 2019, 54, 893–904. [Google Scholar] [CrossRef]
- Hao, M.; Cao, Z.; Wang, Z.; Xin, J.; Kong, B.; Xu, J.; Zhang, L.; Chen, P. Patient-Derived Organoid Model in the Prediction of Chemotherapeutic Drug Response in Colorectal Cancer. ACS Biomater. Sci. Eng. 2022, 8, 3515–3525. [Google Scholar] [CrossRef]
- Blatchley, M.R.; Günay, K.A.; Yavitt, F.M.; Hawat, E.M.; Dempsey, P.J.; Anseth, K.S. In Situ Super-Resolution Imaging of Organoids and Extracellular Matrix Interactions via Phototransfer by Allyl Sulfide Exchange-Expansion Microscopy (PhASE-ExM). Adv. Mater. 2022, 34, e2109252. [Google Scholar] [CrossRef]
- Geevimaan, K.; Guo, J.Y.; Shen, C.N.; Jiang, J.K.; Fann, C.S.J.; Hwang, M.J.; Shui, J.W.; Lin, H.T.; Wang, M.J.; Shih, H.C.; et al. Patient-Derived Organoid Serves as a Platform for Personalized Chemotherapy in Advanced Colorectal Cancer Patients. Front. Oncol. 2022, 12, 883437. [Google Scholar] [CrossRef] [PubMed]
- Osuna de la Peña, D.; Trabulo, S.M.D.; Collin, E.; Liu, Y.; Sharma, S.; Tatari, M.; Behrens, D.; Erkan, M.; Lawlor, R.T.; Scarpa, A.; et al. Bioengineered 3D models of human pancreatic cancer recapitulate in vivo tumour biology. Nat. Commun. 2021, 12, 5623. [Google Scholar] [CrossRef] [PubMed]
- Broguiere, N.; Isenmann, L.; Hirt, C.; Ringel, T.; Placzek, S.; Cavalli, E.; Ringnalda, F.; Villiger, L.; Züllig, R.; Lehmann, R.; et al. Growth of Epithelial Organoids in a Defined Hydrogel. Adv. Mater. 2018, 30, e1801621. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, Z.; Zhao, M.; Liu, G.; Wu, J. Advances of hydrogel dressings in diabetic wounds. Biomater. Sci. 2021, 9, 1530–1546. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Lin, W.; Yuan, Z.; Wu, J.; Qian, H.; Xu, L.; Chen, S. Development of ionic strength/pH/enzyme triple-responsive zwitterionic hydrogel of the mixed l-glutamic acid and l-lysine polypeptide for site-specific drug delivery. J. Mater. Chem. B 2017, 5, 935–943. [Google Scholar] [CrossRef]
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef]
- Gelain, F.; Luo, Z.; Rioult, M.; Zhang, S. Self-assembling peptide scaffolds in the clinic. NPJ Regen. Med. 2021, 6, 9. [Google Scholar] [CrossRef]
- Lou, P.; Liu, S.; Wang, Y.; Pan, C.; Xu, X.; Zhao, M.; Liao, G.; Yang, G.; Yuan, Y.; Li, L.; et al. Injectable self-assembling peptide nanofiber hydrogel as a bioactive 3D platform to promote chronic wound tissue regeneration. Acta Biomater. 2021, 135, 100–112. [Google Scholar] [CrossRef]
- Loo, Y.; Zhang, S.; Hauser, C.A. From short peptides to nanofibers to macromolecular assemblies in biomedicine. Biotechnol. Adv. 2012, 30, 593–603. [Google Scholar] [CrossRef]
- Wang, T.W.; Chang, K.C.; Chen, L.H.; Liao, S.Y.; Yeh, C.W.; Chuang, Y.J. Effects of an injectable functionalized self-assembling nanopeptide hydrogel on angiogenesis and neurogenesis for regeneration of the central nervous system. Nanoscale 2017, 9, 16281–16292. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.Y.; Wu, H.C.; Huang, M.Y.; Chang, W.H.; Lee, C.H.; Wang, T.W. Self-assembling functionalized nanopeptides for immediate hemostasis and accelerative liver tissue regeneration. Nanoscale 2013, 5, 2734–2744. [Google Scholar] [CrossRef]
- Gelain, F.; Bottai, D.; Vescovi, A.; Zhang, S. Designer self-assembling peptide nanofiber scaffolds for adult mouse neural stem cell 3-dimensional cultures. PLoS ONE 2006, 1, e119. [Google Scholar] [CrossRef]
- Meng, H.; Chen, L.; Ye, Z.; Wang, S.; Zhao, X. The effect of a self-assembling peptide nanofiber scaffold (peptide) when used as a wound dressing for the treatment of deep second degree burns in rats. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 89, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Gelain, F.; Luo, Z.; Zhang, S. Self-Assembling Peptide EAK16 and RADA16 Nanofiber Scaffold Hydrogel. Chem. Rev. 2020, 120, 13434–13460. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, M.; Zhou, Y.; Li, L.; Wang, C.; Yuan, Y.; Li, L.; Liao, G.; Bresette, W.; Chen, Y.; et al. A self-assembling peptide hydrogel-based drug co-delivery platform to improve tissue repair after ischemia-reperfusion injury. Acta Biomater. 2020, 103, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Bernal, P.N.; Bouwmeester, M.; Madrid-Wolff, J.; Falandt, M.; Florczak, S.; Rodriguez, N.G.; Li, Y.; Größbacher, G.; Samsom, R.A.; van Wolferen, M.; et al. Volumetric Bioprinting of Organoids and Optically Tuned Hydrogels to Build Liver-Like Metabolic Biofactories. Adv. Mater. 2022, 34, e2110054. [Google Scholar] [CrossRef]
- Yu, W.; Lu, C.; Wang, G.; Liang, Z.; Jiang, Z.; Liu, Y.; Yan, J. Pharmacological Mechanism of Pingxiao Formula against Colorectal Cancer. Evid.-Based Complement. Altern. Med. eCAM 2022, 2022, 7884740. [Google Scholar] [CrossRef]
- Nethi, S.K.; Das, S.; Patra, C.R.; Mukherjee, S. Recent advances in inorganic nanomaterials for wound-healing applications. Biomater. Sci. 2019, 7, 2652–2674. [Google Scholar] [CrossRef]
- Hauser, C.A.; Maurer-Stroh, S.; Martins, I.C. Amyloid-based nanosensors and nanodevices. Chem. Soc. Rev. 2014, 43, 5326–5345. [Google Scholar] [CrossRef]
- Mohajeri, M.; Behnam, B.; Sahebkar, A. Biomedical applications of carbon nanomaterials: Drug and gene delivery potentials. J. Cell. Physiol. 2018, 234, 298–319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gelain, F.; Zhao, X. Designer self-assembling peptide nanofiber scaffolds for 3D tissue cell cultures. Semin. Cancer Biol. 2005, 15, 413–420. [Google Scholar] [CrossRef]
- Ba, Z.; Chen, Z.; Huang, Y.; Feng, D.; Zhao, Q.; Zhu, J.; Wu, D. Nanoporous diopside modulates biocompatibility, degradability and osteogenesis of bioactive scaffolds of gliadin-based composites for new bone formation. Int. J. Nanomed. 2018, 13, 3883–3896. [Google Scholar] [CrossRef]
- Di Martino, P. Extracellular polymeric substances, a key element in understanding biofilm phenotype. AIMS Microbiol. 2018, 4, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Serfozo, Z.; Elekes, K. Chemical properties of the extracellular matrix of the snail nervous system: A comprehensive study using a combination of histochemical techniques. Micron 2010, 41, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S. Emerging biological materials through molecular self-assembly. Biotechnol. Adv. 2002, 20, 321–339. [Google Scholar] [CrossRef]
- Davis, M.E.; Motion, J.P.; Narmoneva, D.A.; Takahashi, T.; Hakuno, D.; Kamm, R.D.; Zhang, S.; Lee, R.T. Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells. Circulation 2005, 111, 442–450. [Google Scholar] [CrossRef]
- Kisiday, J.; Jin, M.; Kurz, B.; Hung, H.; Semino, C.; Zhang, S.; Grodzinsky, A.J. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: Implications for cartilage tissue repair. Proc. Natl. Acad. Sci. USA 2002, 99, 9996–10001. [Google Scholar] [CrossRef]
- Wen, Y.; Roudebush, S.L.; Buckholtz, G.A.; Goehring, T.R.; Giannoukakis, N.; Gawalt, E.S.; Meng, W.S. Coassembly of amphiphilic peptide EAK16-II with histidinylated analogues and implications for functionalization of β-sheet fibrils in vivo. Biomaterials 2014, 35, 5196–5205. [Google Scholar] [CrossRef]
- Nikdouz, A.; Orso, F. Emerging roles of 3D-culture systems in tackling tumor drug resistance. Cancer Drug Resist. 2023, 6, 788–804. [Google Scholar] [CrossRef]
- Imamura, Y.; Mukohara, T.; Shimono, Y.; Funakoshi, Y.; Chayahara, N.; Toyoda, M.; Kiyota, N.; Takao, S.; Kono, S.; Nakatsura, T.; et al. Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Oncol. Rep. 2015, 33, 1837–1843. [Google Scholar] [CrossRef] [PubMed]



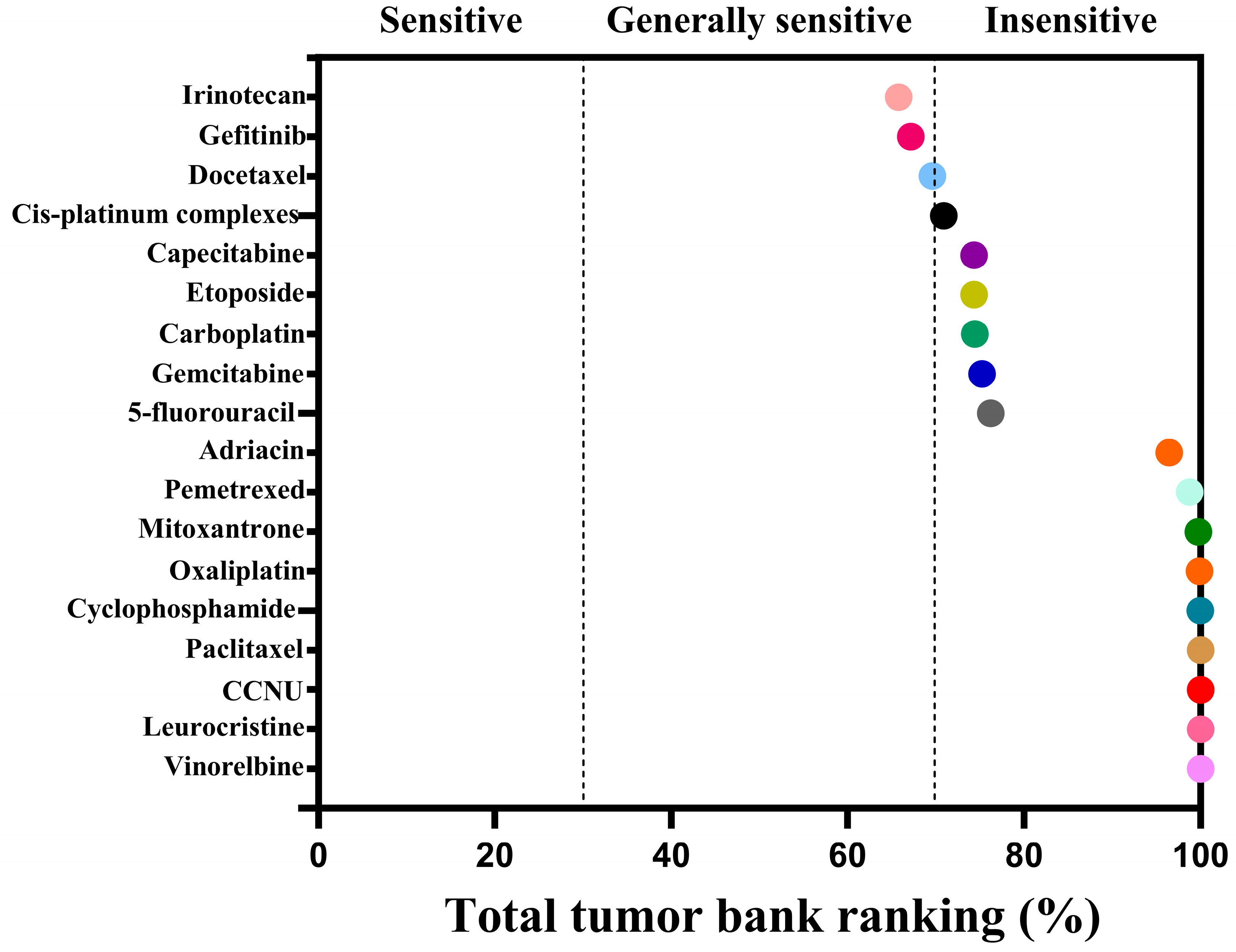
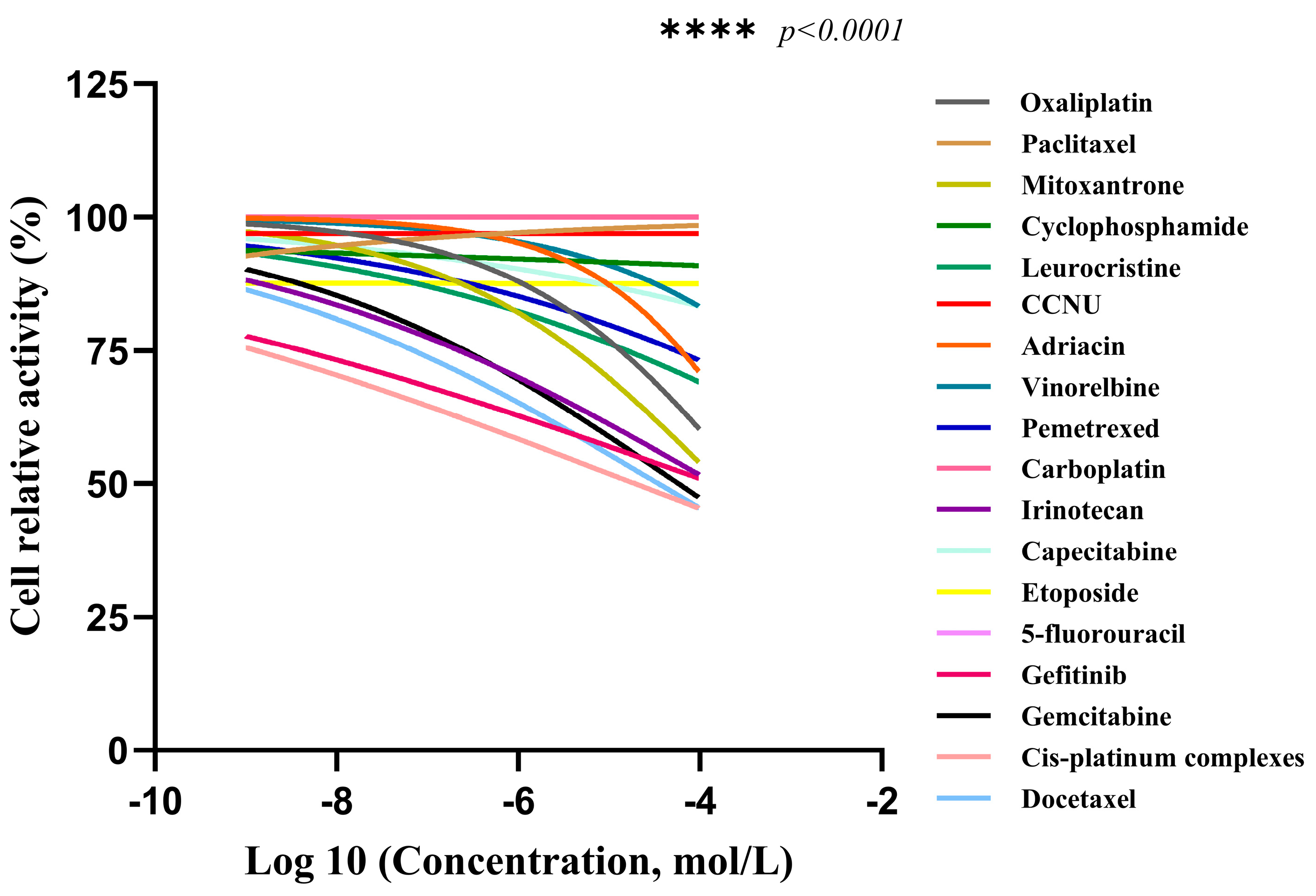
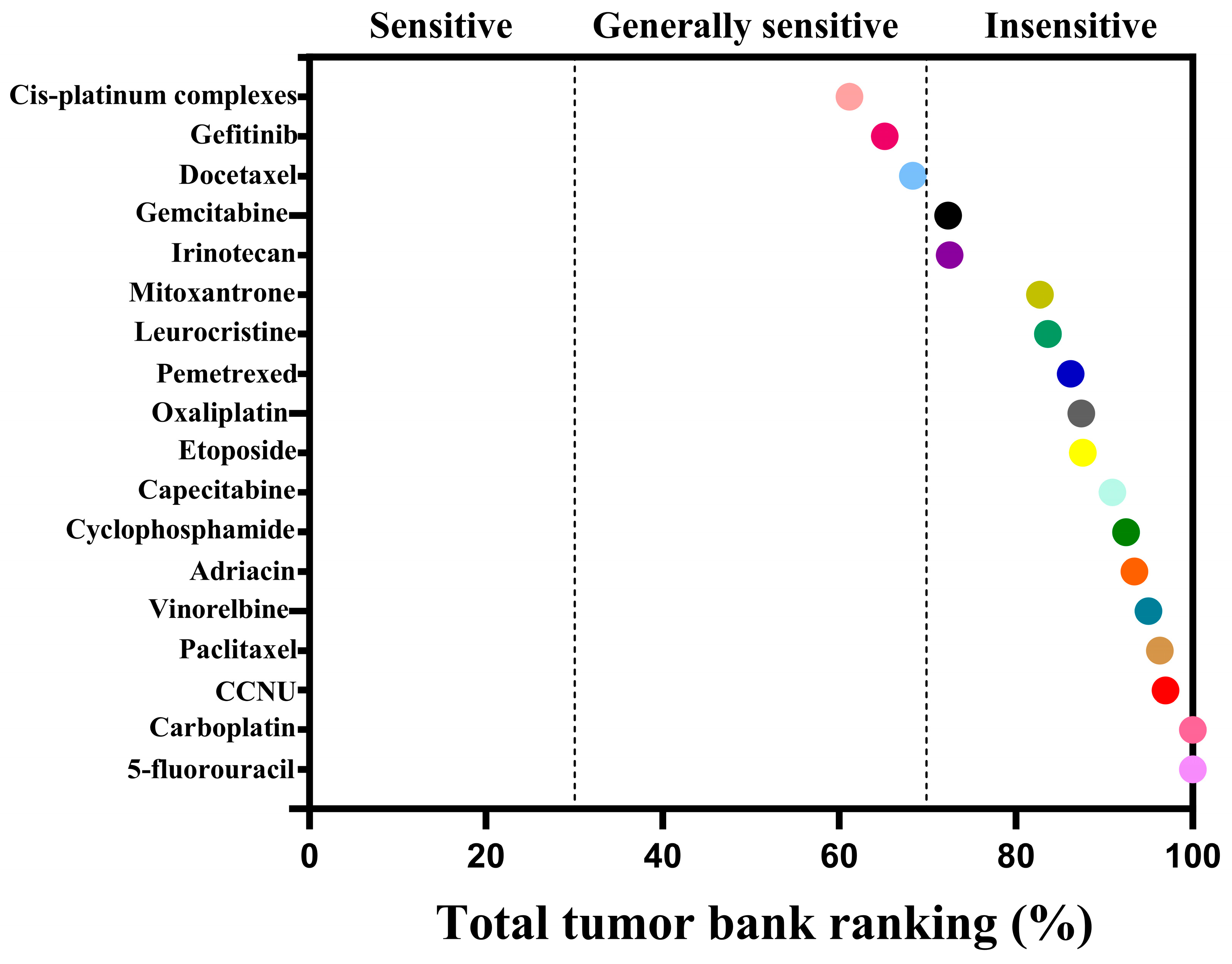
| Drug Classification | Name of the Drug | AUC | Sensibility |
|---|---|---|---|
| Chemotherapy drugs | Irinotecan | 65.78 | Generally sensitive |
| Gefitinib | 67.14 | Generally sensitive | |
| Docetaxel | 69.60 | Generally sensitive | |
| Cis-platinum Complexes | 70.88 | insensitivity | |
| Capecitabine | 74.32 | insensitivity | |
| Etoposide | 74.34 | insensitivity | |
| Carboplatin | 74.42 | insensitivity | |
| Gemcitabine | 75.22 | insensitivity | |
| 5-fluorouracil | 76.24 | insensitivity | |
| Adriacin | 96.46 | insensitivity | |
| Pemetrexed | 98.78 | insensitivity | |
| Mitoxantrone | 99.76 | insensitivity | |
| Oxaliplatin | 99.90 | insensitivity | |
| Cyclophosphamide | 99.96 | insensitivity | |
| Paclitaxel | 100.00 | insensitivity | |
| CCNU | 100.00 | insensitivity | |
| Leurocristine | 100.00 | insensitivity | |
| Vinorelbine | 100.00 | insensitivity |
| Drug Classification | Name of the Drug | AUC | Sensibility |
|---|---|---|---|
| Chemotherapy drugs | Cis-platinum Complexes | 61.16 | Generally sensitive |
| Gefitinib | 65.14 | Generally sensitive | |
| Docetaxel | 68.34 | Generally sensitive | |
| Gemcitabine | 72.30 | insensitivity | |
| Irinotecan | 72.50 | insensitivity | |
| Mitoxantrone | 82.70 | insensitivity | |
| Leurocristine | 83.60 | insensitivity | |
| Pemetrexed | 86.18 | insensitivity | |
| Oxaliplatin | 87.40 | insensitivity | |
| Etoposide | 87.58 | insensitivity | |
| Capecitabine | 90.92 | insensitivity | |
| Cyclophosphamide | 92.44 | insensitivity | |
| Adriacin | 93.42 | insensitivity | |
| Vinorelbine | 95.00 | insensitivity | |
| Paclitaxel | 96.28 | insensitivity | |
| CCNU | 96.92 | insensitivity | |
| Carboplatin | 100.00 | insensitivity | |
| 5-fluorouracil | 100.00 | insensitivity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Su, D.; Zhao, J.; Luo, Z.; Lin, X. Study on the Feasibility of Self-Assembling Peptides as a Three-Dimensional Culture Tool for Drug Screening of Colorectal Adenocarcinoma Cells. Gels 2025, 11, 394. https://doi.org/10.3390/gels11060394
Gao Y, Su D, Zhao J, Luo Z, Lin X. Study on the Feasibility of Self-Assembling Peptides as a Three-Dimensional Culture Tool for Drug Screening of Colorectal Adenocarcinoma Cells. Gels. 2025; 11(6):394. https://doi.org/10.3390/gels11060394
Chicago/Turabian StyleGao, Yu, Di Su, Jiawei Zhao, Zhongli Luo, and Xuemei Lin. 2025. "Study on the Feasibility of Self-Assembling Peptides as a Three-Dimensional Culture Tool for Drug Screening of Colorectal Adenocarcinoma Cells" Gels 11, no. 6: 394. https://doi.org/10.3390/gels11060394
APA StyleGao, Y., Su, D., Zhao, J., Luo, Z., & Lin, X. (2025). Study on the Feasibility of Self-Assembling Peptides as a Three-Dimensional Culture Tool for Drug Screening of Colorectal Adenocarcinoma Cells. Gels, 11(6), 394. https://doi.org/10.3390/gels11060394






