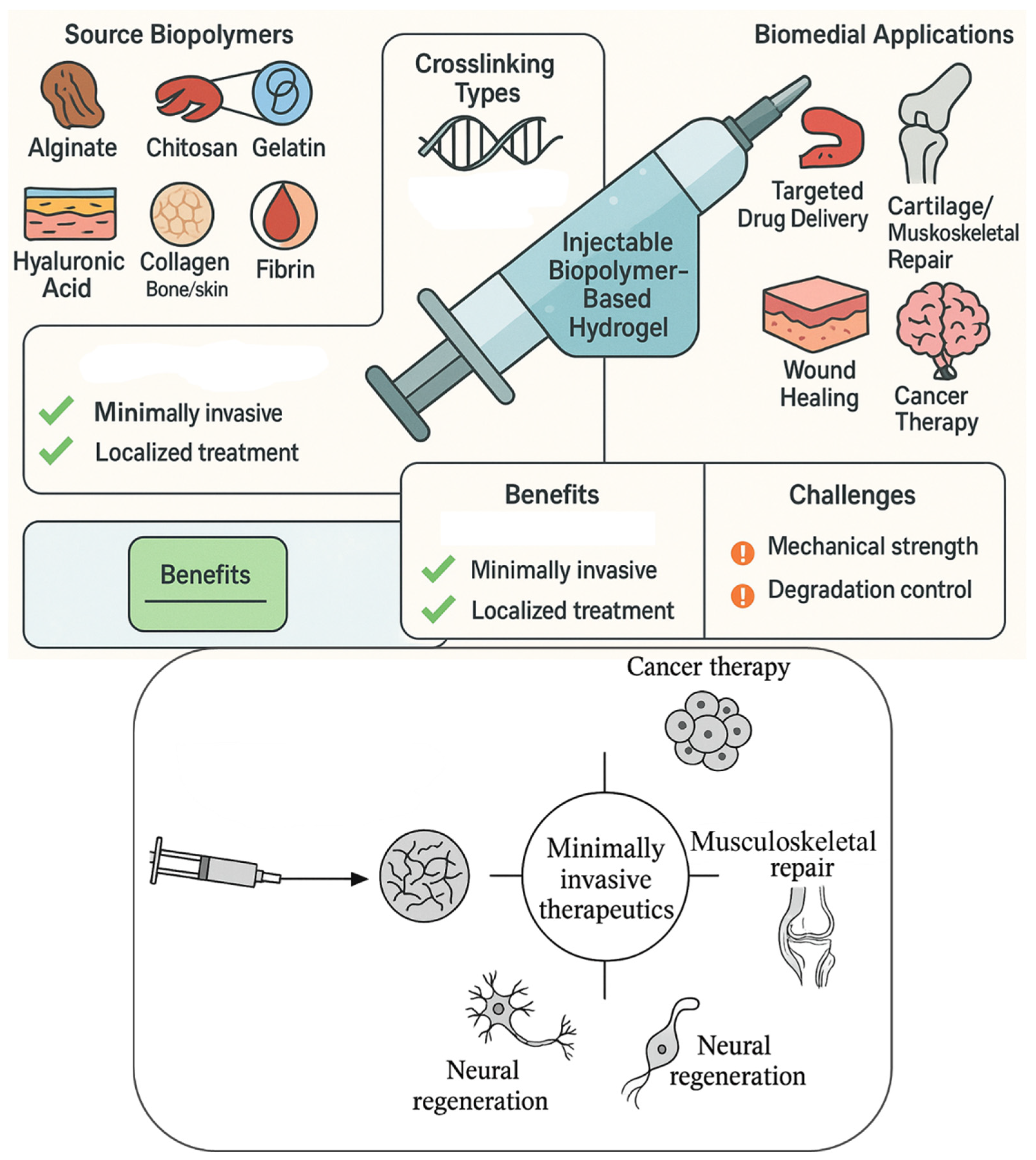
Injectable Biopolymer-Based Hydrogels: A Next-Generation Platform for Minimally Invasive Therapeutics
Abstract
1. Introduction
1.1. Background and Significance of Injectable Hydrogels
1.2. Motivation for Using Biopolymer-Based Gels in Medicine
1.3. Objectives and Scope of the Review
- The physicochemical properties of natural biopolymers and their transformation into injectable hydrogel systems.
- Various crosslinking mechanisms—both physical (e.g., ionic, thermal, or hydrogen bonding) and chemical (e.g., enzymatic or photo-crosslinking) that facilitate in situ gelation under physiological conditions.
- Biofunctional characteristics of these hydrogels, including responsiveness to environmental stimuli, drug-loading capacity, degradation kinetics, and support for cellular activities.
- Biomedical applications spanning targeted drug delivery, tissue engineering, neural regeneration, cancer therapy, and musculoskeletal repair.
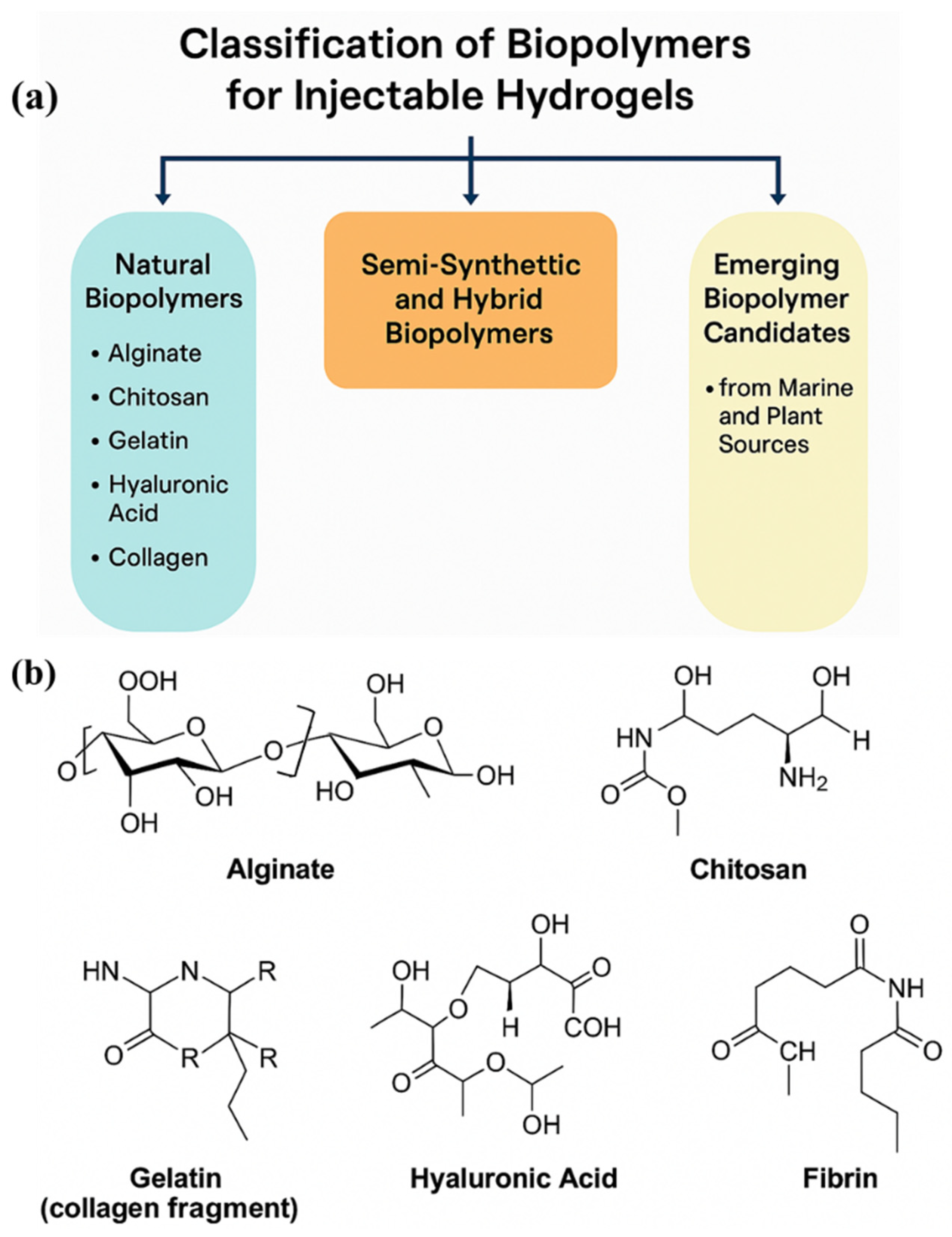
2. Classification of Biopolymers for Injectable Hydrogels
2.1. Natural Biopolymers
2.2. Semi-Synthetic and Hybrid Biopolymers
2.3. Emerging Biopolymer Candidates from Marine and Plant Sources
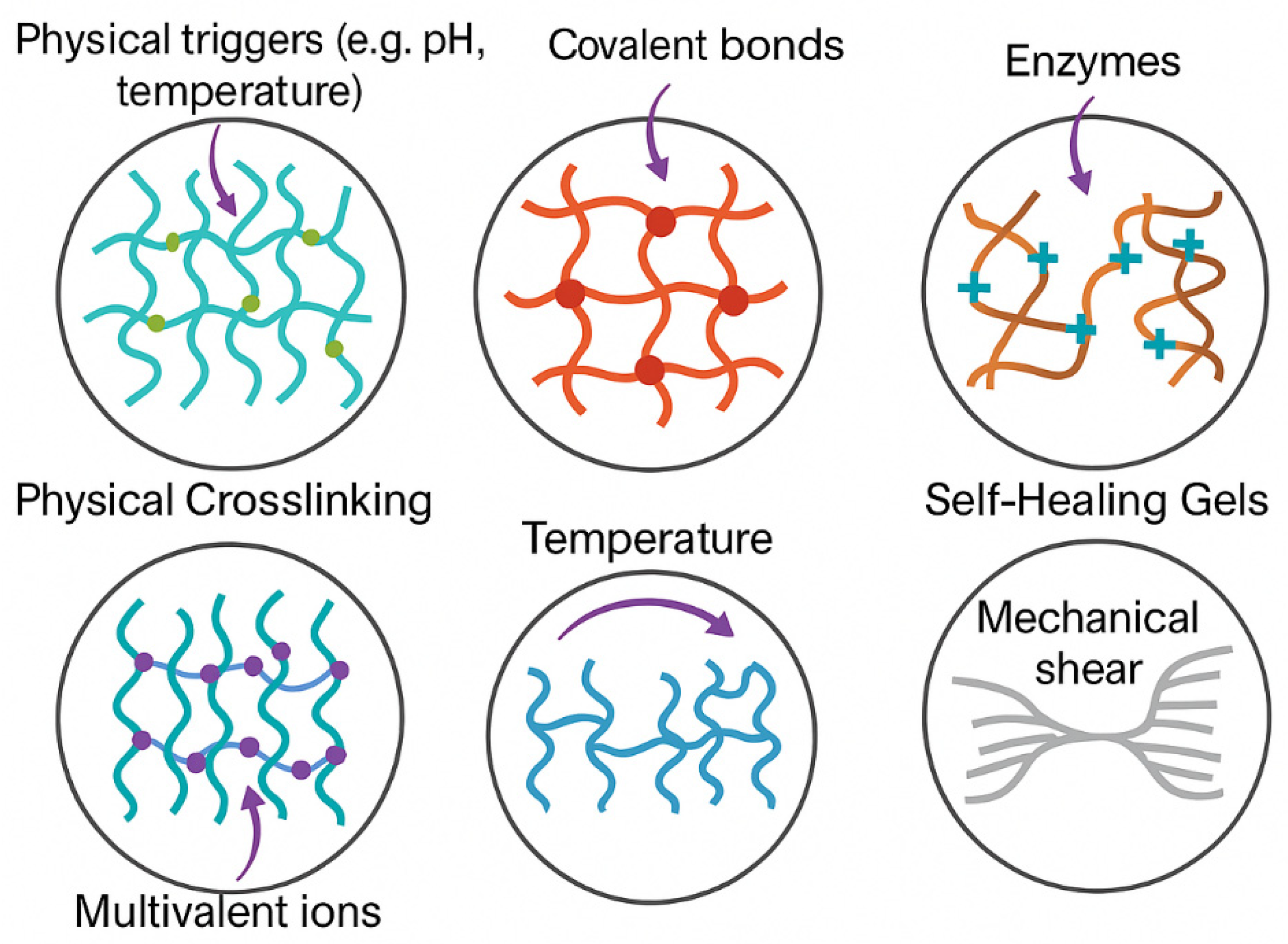
3. Gelation Mechanisms and Injectable Formulations
3.1. Physical Crosslinking
3.2. Chemical Crosslinking
- Photo-crosslinking, using photoinitiators (e.g., Irgacure 2959 or LAP) under UV or visible light exposure, is widely applied to polymers, like gelatin methacryloyl (GelMA) and methacrylated hyaluronic acid. This strategy enables spatial and temporal control over gelation, particularly in 3D bioprinting and microfluidic hydrogel systems [82].
- Click chemistry, such as thiol–ene, azide–alkyne, and Diels–Alder reactions, offers high efficiency, bioorthogonality, and minimal toxicity. Hydrogels formed via strain-promoted azide–alkyne cycloaddition (SPAAC) or Michael addition enable encapsulation of cells and drugs under mild conditions [83].
- Schiff base chemistry, often used in aldehyde-modified polysaccharides (e.g., oxidized alginate or HA) and amine-containing polymers (e.g., chitosan), facilitates rapid in situ gelation via imine bond formation, useful in wound dressing or localized therapy [84].
3.3. Enzymatic and Ionic Gelation
- Alginate–Ca2+ hydrogels, with rapid gelation under divalent ion exposure and tunable porosity based on the G/M ratio.
- Chitosan–TPP (tripolyphosphate) systems, where positively charged chitosan interacts with negatively charged polyphosphates to form injectable gels for vaccine delivery or mucosal therapy [90].
3.4. In Situ Gelation and Thermo-Responsive Systems
3.5. Self-Healing and Shear-Thinning Properties
- Hydrogen bonds;
- Ionic interactions;
- Host–guest inclusion complexes;
- Dynamic covalent bonds (e.g., imine, disulfide, boronate ester bonds) [107].
- Chitosan/β-GP hydrogels;
- Gelatin and methylcellulose-based formulations;
- Peptide- or protein-based supramolecular hydrogels [110].
4. Design Strategies for Biomedical Applications
4.1. Rheological and Mechanical Property Optimization
4.2. Biodegradability and Biocompatibility Considerations
4.3. Incorporation of Bioactive Molecules
4.4. Controlled and Sustained Drug Release Behavior
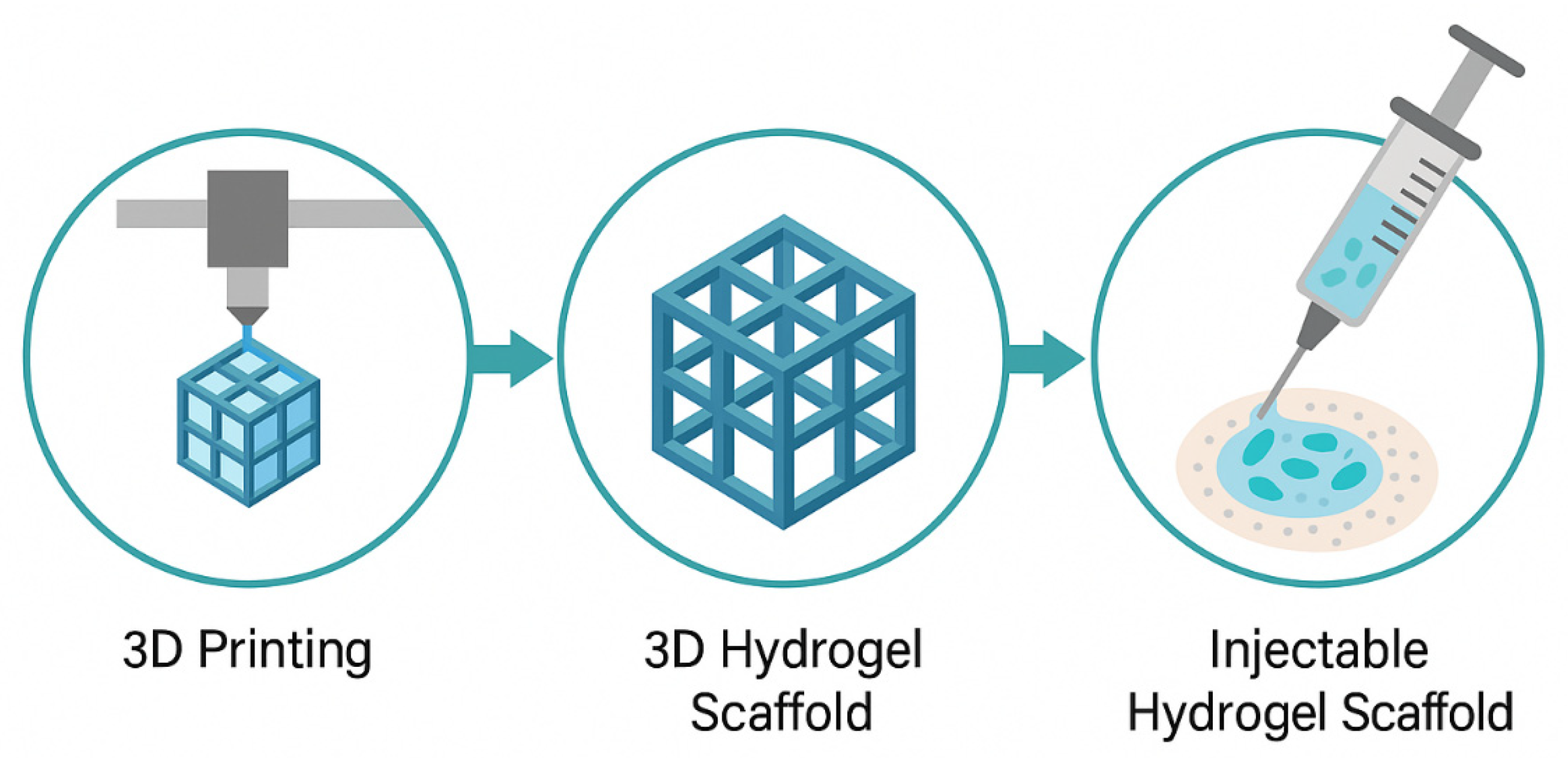
4.5. 3D Structuring and Injectable Scaffolds
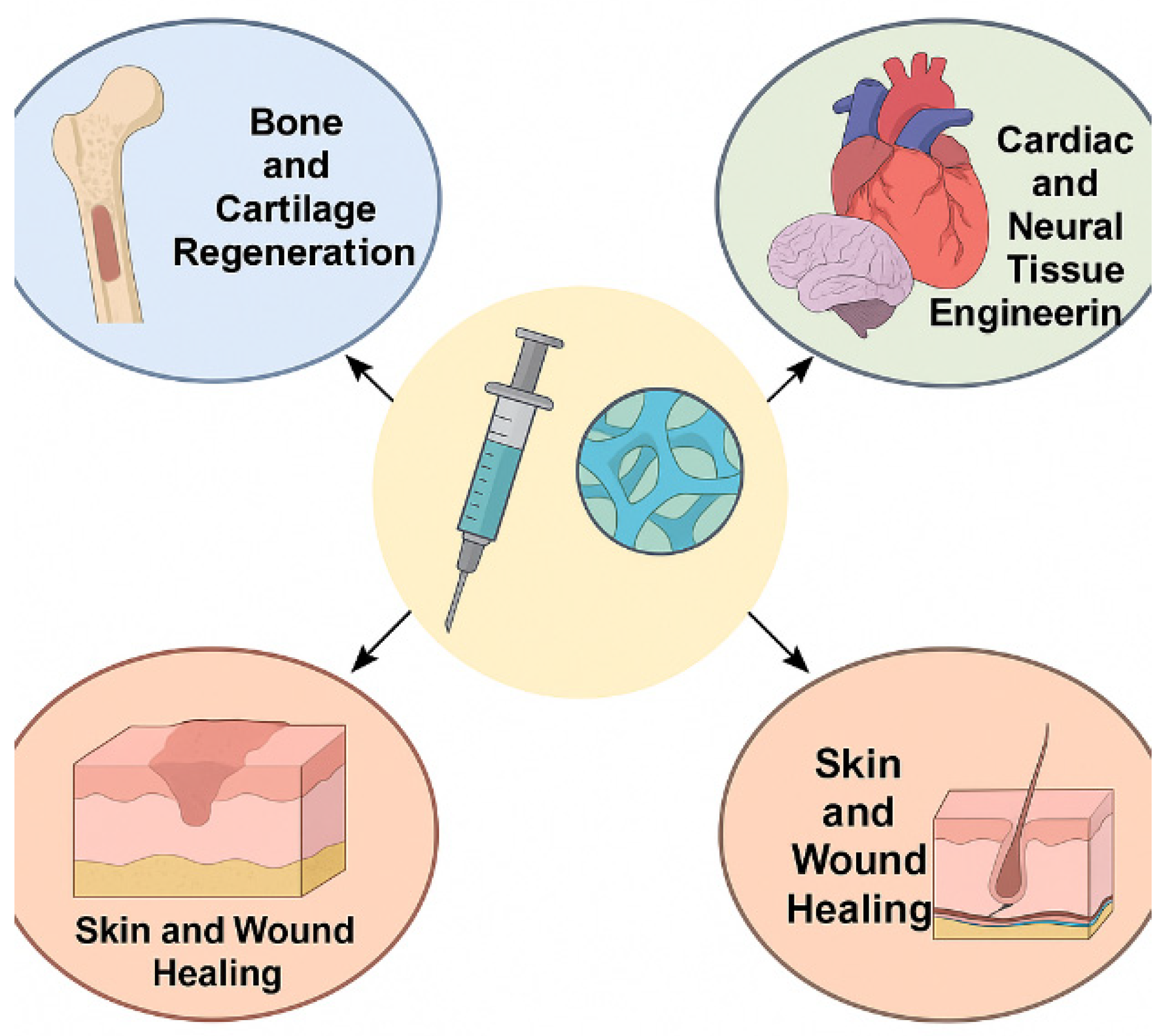
5. Biomedical Applications of Injectable Biopolymer-Based Hydrogels
5.1. Targeted Drug Delivery
5.1.1. Cancer Therapeutics
5.1.2. Anti-Inflammatory and Antimicrobial Delivery
5.2. Tissue Engineering and Regenerative Medicine
5.2.1. Bone and Cartilage Regeneration
5.2.2. Cardiac and Neural Tissue Engineering
5.2.3. Skin and Wound Healing
5.3. Injectable Platforms for Gene and Cell Delivery
5.4. Use in Minimally Invasive Surgery and Localized Therapy
6. Challenges and Limitations
6.1. Mechanical Fragility and Structural Instability
6.2. Sterilization and Storage Constraints
6.3. Batch-to-Batch Variation in Biopolymers
6.4. Immunogenicity and Regulatory Barriers
7. Future Perspectives and Emerging Trends
7.1. Bioinspired and Smart Responsive Hydrogels
7.2. Integration with Nanotechnology and 3D Bioprinting
7.3. Personalized Medicine and Patient-Specific Gels
7.4. Clinical Translation and Commercialization Potential
8. Conclusions
8.1. Summary of Key Findings
8.2. Final Remarks on the Promise of Injectable Biopolymer Hydrogels
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, J.; Yu, L.; Ding, J. PEG-Based Thermosensitive and Biodegradable Hydrogels. Acta Biomater. 2021, 128, 42–59. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Pablos, J.L.; Lozano, D.; Manzano, M.; Vallet-Regí, M. Regenerative Medicine: Hydrogels and Mesoporous Silica Nanoparticles. Mater. Today Bio 2024, 29, 101342. [Google Scholar] [CrossRef]
- Parvin, N.; Kumar, V.; Joo, S.W.; Mandal, T.K. Cutting-Edge Hydrogel Technologies in Tissue Engineering and Biosensing: An Updated Review. Materials 2024, 17, 4792. [Google Scholar] [CrossRef]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-Based Hydrogels Applied in Drug Delivery: An Overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef]
- Mandal, T.K.; Parvin, N.; Joo, S.W. PH-Responsive Biocompatible Fluorescent Core-Shell Nanogel for Intracellular Imaging and Control Drug Release. Part. Part. Syst. Charact. 2021, 38, 2100110. [Google Scholar] [CrossRef]
- Barajaa, M.A.; Ghosh, D.; Laurencin, C.T. Decellularized Extracellular Matrix-Derived Hydrogels: A Powerful Class of Biomaterials for Skeletal Muscle Regenerative Engineering Applications. Regen. Eng. Transl. Med. 2025, 11, 39–63. [Google Scholar] [CrossRef]
- Wu, J.; Xue, W.; Yun, Z.; Liu, Q.; Sun, X. Biomedical Applications of Stimuli-Responsive “Smart” Interpenetrating Polymer Network Hydrogels. Mater. Today Bio 2024, 25, 100998. [Google Scholar] [CrossRef]
- Almawash, S.; Osman, S.K.; Mustafa, G.; El Hamd, M.A. Current and Future Prospective of Injectable Hydrogels—Design Challenges and Limitations. Pharmaceuticals 2022, 15, 371. [Google Scholar] [CrossRef]
- Mehvari, F.; Ramezanzade, V.; An, J.; Kim, J.; Dinari, M.; Seung Kim, J. Biopolymer-Based Hydrogels for Biomedical Applications: Bioactivity and Wound Healing Properties. Coord. Chem. Rev. 2024, 518, 216093. [Google Scholar] [CrossRef]
- Mitura, S.; Sionkowska, A.; Jaiswal, A. Biopolymers for Hydrogels in Cosmetics: Review. J. Mater. Sci. Mater. Med. 2020, 31, 50. [Google Scholar] [CrossRef]
- Hong, F.; Qiu, P.; Wang, Y.; Ren, P.; Liu, J.; Zhao, J.; Gou, D. Chitosan-Based Hydrogels: From Preparation to Applications, a Review. Food Chem. X 2024, 21, 101095. [Google Scholar] [CrossRef]
- Diller, R.B.; Tabor, A.J. The Role of the Extracellular Matrix (ECM) in Wound Healing: A Review. Biomimetics 2022, 7, 87. [Google Scholar] [CrossRef]
- Li, S.; Molina, I.; Martinez, M.B.; Vert, M. Hydrolytic and Enzymatic Degradations of Physically Crosslinked Hydrogels Prepared from PLA/PEO/PLA Triblock Copolymers. J. Mater. Sci. Mater. Med. 2002, 13, 81–86. [Google Scholar] [CrossRef]
- Santhamoorthy, M.; Kim, S.-C. A Review of the Development of Biopolymer Hydrogel-Based Scaffold Materials for Drug Delivery and Tissue Engineering Applications. Gels 2025, 11, 178. [Google Scholar] [CrossRef]
- Klouda, L.; Mikos, A.G. Thermoresponsive Hydrogels in Biomedical Applications. Eur. J. Pharm. Biopharm. 2008, 68, 34–45. [Google Scholar] [CrossRef]
- Brovold, M.; Almeida, J.I.; Pla-Palacín, I.; Sainz-Arnal, P.; Sánchez-Romero, N.; Rivas, J.J.; Almeida, H.; Dachary, P.R.; Serrano-Aulló, T.; Soker, S.; et al. Naturally-Derived Biomaterials for Tissue Engineering Applications. Adv. Exp. Med. Biol. 2018, 1077, 421–449. [Google Scholar]
- Hua, C.; Qiu, L. Polymersomes for Therapeutic Protein and Peptide Delivery: Towards Better Loading Properties. Int. J. Nanomed. 2024, 19, 2317–2340. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, X.; Wu, Y.; Gao, J. Locally Injectable Hydrogels for Tumor Immunotherapy. Gels 2021, 7, 224. [Google Scholar] [CrossRef]
- Wang, X.; Sun, X.; Gan, D.; Soubrier, M.; Chiang, H.-Y.; Yan, L.; Li, Y.; Li, J.; Yu, S.; Xia, Y.; et al. Bioadhesive and Conductive Hydrogel-Integrated Brain-Machine Interfaces for Conformal and Immune-Evasive Contact with Brain Tissue. Matter 2022, 5, 1204–1223. [Google Scholar] [CrossRef]
- Haq, F.; Kiran, M.; Khan, I.A.; Mehmood, S.; Aziz, T.; Haroon, M. Exploring the Pathways to Sustainability: A Comprehensive Review of Biodegradable Plastics in the Circular Economy. Mater. Today Sustain. 2025, 29, 101067. [Google Scholar] [CrossRef]
- Aguero, L.; Alpdagtas, S.; Ilhan, E.; Zaldivar-Silva, D.; Gunduz, O. Functional Role of Crosslinking in Alginate Scaffold for Drug Delivery and Tissue Engineering: A Review. Eur. Polym. J. 2021, 160, 110807. [Google Scholar] [CrossRef]
- Iqbal, Y.; Amin, F.; Usman, Y.; Farrukh Sarfraz, M. Alginate-Based Hydrogels with Inorganic Nanomaterials: A Promising Approach for Wound Healing and Bone Tissue Regeneration. Eur. Polym. J. 2024, 212, 113057. [Google Scholar] [CrossRef]
- Farjaminejad, S.; Farjaminejad, R.; Hasani, M.; Garcia-Godoy, F.; Abdouss, M.; Marya, A.; Harsoputranto, A.; Jamilian, A. Advances and Challenges in Polymer-Based Scaffolds for Bone Tissue Engineering: A Path Towards Personalized Regenerative Medicine. Polymers 2024, 16, 3303. [Google Scholar] [CrossRef]
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: A Potential Biopolymer in Drug Delivery and Biomedical Applications. Pharmaceutics 2023, 15, 1313. [Google Scholar] [CrossRef]
- Haider, A.; Khan, S.; Iqbal, D.N.; Shrahili, M.; Haider, S.; Mohammad, K.; Mohammad, A.; Rizwan, M.; Kanwal, Q.; Mustafa, G. Advances in Chitosan-Based Drug Delivery Systems: A Comprehensive Review for Therapeutic Applications. Eur. Polym. J. 2024, 210, 112983. [Google Scholar] [CrossRef]
- Shafi, S.; Sidiq, S.; Chat, O.A.; Kumar, G.; Dar, A.A.; Bhat, P.A. Surfactant–Rescued Gelation: Stabilizing P123-Chitosan Hydrogels in Blood-Isotonic Aqueous Solutions for Controlled Drug Delivery. Int. J. Biol. Macromol. 2025, 310, 143166. [Google Scholar] [CrossRef]
- Contessi Negrini, N.; Angelova Volponi, A.; Sharpe, P.T.; Celiz, A.D. Tunable Cross-Linking and Adhesion of Gelatin Hydrogels via Bioorthogonal Click Chemistry. ACS Biomater. Sci. Eng. 2021, 7, 4330–4346. [Google Scholar] [CrossRef]
- Sisso, A.M.; Boit, M.O.; DeForest, C.A. Self-healing Injectable Gelatin Hydrogels for Localized Therapeutic Cell Delivery. J. Biomed. Mater. Res. Part. A 2020, 108, 1112–1121. [Google Scholar] [CrossRef]
- Zhou, L.; Fan, L.; Zhang, F.-M.; Jiang, Y.; Cai, M.; Dai, C.; Luo, Y.-A.; Tu, L.-J.; Zhou, Z.-N.; Li, X.-J.; et al. Hybrid Gelatin/Oxidized Chondroitin Sulfate Hydrogels Incorporating Bioactive Glass Nanoparticles with Enhanced Mechanical Properties, Mineralization, and Osteogenic Differentiation. Bioact. Mater. 2021, 6, 890–904. [Google Scholar] [CrossRef]
- Lierova, A.; Kasparova, J.; Filipova, A.; Cizkova, J.; Pekarova, L.; Korecka, L.; Mannova, N.; Bilkova, Z.; Sinkorova, Z. Hyaluronic Acid: Known for Almost a Century, but Still in Vogue. Pharmaceutics 2022, 14, 838. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, Y.; Xu, Y.; Wang, J.; Yu, Y. Modification and Crosslinking Strategies for Hyaluronic Acid-based Hydrogel Biomaterials. Smart Med. 2023, 2, e20230029. [Google Scholar] [CrossRef]
- Petit, N.; Chang, Y.J.; Lobianco, F.A.; Hodgkinson, T.; Browne, S. Hyaluronic Acid as a Versatile Building Block for the Development of Biofunctional Hydrogels: In Vitro Models and Preclinical Innovations. Mater. Today Bio 2025, 31, 101596. [Google Scholar] [CrossRef]
- Faivre, J.; Pigweh, A.I.; Iehl, J.; Maffert, P.; Goekjian, P.; Bourdon, F. Crosslinking Hyaluronic Acid Soft-Tissue Fillers: Current Status and Perspectives from an Industrial Point of View. Expert. Rev. Med. Devices 2021, 18, 1175–1187. [Google Scholar] [CrossRef]
- Amirrah, I.N.; Lokanathan, Y.; Zulkiflee, I.; Wee, M.F.M.R.; Motta, A.; Fauzi, M.B. A Comprehensive Review on Collagen Type I Development of Biomaterials for Tissue Engineering: From Biosynthesis to Bioscaffold. Biomedicines 2022, 10, 2307. [Google Scholar] [CrossRef]
- Ullah, S.; Zainol, I. Fabrication and Applications of Biofunctional Collagen Biomaterials in Tissue Engineering. Int. J. Biol. Macromol. 2025, 298, 139952. [Google Scholar] [CrossRef]
- Xu, Q.; Torres, J.E.; Hakim, M.; Babiak, P.M.; Pal, P.; Battistoni, C.M.; Nguyen, M.; Panitch, A.; Solorio, L.; Liu, J.C. Collagen- and Hyaluronic Acid-Based Hydrogels and Their Biomedical Applications. Mater. Sci. Eng. R. Rep. 2021, 146, 100641. [Google Scholar] [CrossRef]
- Mortier, C.; Costa, D.C.S.; Oliveira, M.B.; Haugen, H.J.; Lyngstadaas, S.P.; Blaker, J.J.; Mano, J.F. Advanced Hydrogels Based on Natural Macromolecules: Chemical Routes to Achieve Mechanical Versatility. Mater. Today Chem. 2022, 26, 101222. [Google Scholar] [CrossRef]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the Potential of Hydrogels for Advanced Therapeutic Applications: Current Achievements and Future Directions. Signal Transduct. Target. Ther. 2024, 9, 166. [Google Scholar] [CrossRef]
- Kurian, A.G.; Singh, R.K.; Patel, K.D.; Lee, J.-H.; Kim, H.-W. Multifunctional GelMA Platforms with Nanomaterials for Advanced Tissue Therapeutics. Bioact. Mater. 2022, 8, 267–295. [Google Scholar] [CrossRef]
- Wang, W.; Shi, D.; Zhang, Y.; Li, W.; Li, F.; Feng, H.; Ma, L.; Yang, C.; Peng, Z.; Song, G.; et al. An Injectable Hydrogel Based on Hyaluronic Acid Prepared by Schiff Base for Long-Term Controlled Drug Release. Int. J. Biol. Macromol. 2023, 245, 125341. [Google Scholar] [CrossRef]
- Wang, R.; Cheng, C.; Wang, H.; Wang, D. Swollen Hydrogel Nanotechnology: Advanced Applications of the Rudimentary Swelling Properties of Hydrogels. ChemPhysMater 2024, 3, 357–375. [Google Scholar] [CrossRef]
- Rana, M.M.; De la Hoz Siegler, H. Evolution of Hybrid Hydrogels: Next-Generation Biomaterials for Drug Delivery and Tissue Engineering. Gels 2024, 10, 216. [Google Scholar] [CrossRef]
- Yin, B.; Gosecka, M.; Bodaghi, M.; Crespy, D.; Youssef, G.; Dodda, J.M.; Wong, S.H.D.; Imran, A.B.; Gosecki, M.; Jobdeedamrong, A.; et al. Engineering Multifunctional Dynamic Hydrogel for Biomedical and Tissue Regenerative Applications. Chem. Eng. J. 2024, 487, 150403. [Google Scholar] [CrossRef]
- Jia, Z.; Zeng, H.; Ye, X.; Dai, M.; Tang, C.; Liu, L. Hydrogel-Based Treatments for Spinal Cord Injuries. Heliyon 2023, 9, e19933. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Ma, Y.; Wang, M.; Pan, G. Nano-Crosslinked Dynamic Hydrogels for Biomedical Applications. Mater. Today Bio 2023, 20, 100640. [Google Scholar] [CrossRef]
- Rahmanian-Devin, P.; Baradaran Rahimi, V.; Askari, V.R. Thermosensitive Chitosan-β-Glycerophosphate Hydrogels as Targeted Drug Delivery Systems: An Overview on Preparation and Their Applications. Adv. Pharmacol. Pharm. Sci. 2021, 2021, 1–17. [Google Scholar] [CrossRef]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/Stimuli-Responsive Hydrogels: Cutting-Edge Platforms for Tissue Engineering and Other Biomedical Applications. Mater. Today Bio 2022, 13, 100186. [Google Scholar] [CrossRef]
- Zorrón, M.; Cabrera, A.L.; Sharma, R.; Radhakrishnan, J.; Abbaszadeh, S.; Shahbazi, M.; Tafreshi, O.A.; Karamikamkar, S.; Maleki, H. Emerging 2D Nanomaterials-Integrated Hydrogels: Advancements in Designing Theragenerative Materials for Bone Regeneration and Disease Therapy. Adv. Sci. 2024, 11, e2403204. [Google Scholar] [CrossRef]
- Wu, K.Y.; Qian, S.Y.; Faucher, A.; Tran, S.D. Advancements in Hydrogels for Corneal Healing and Tissue Engineering. Gels 2024, 10, 662. [Google Scholar] [CrossRef]
- Tsanaktsidou, E.; Kammona, O.; Kiparissides, C. Recent Developments in Hyaluronic Acid-Based Hydrogels for Cartilage Tissue Engineering Applications. Polymers 2022, 14, 839. [Google Scholar] [CrossRef]
- Yu, C.; Xu, J.; Heidari, G.; Jiang, H.; Shi, Y.; Wu, A.; Makvandi, P.; Neisiany, R.E.; Zare, E.N.; Shao, M.; et al. Injectable Hydrogels Based on Biopolymers for the Treatment of Ocular Diseases. Int. J. Biol. Macromol. 2024, 269, 132086. [Google Scholar] [CrossRef]
- Haggag, Y.; Abd Elrahman, A.; Ulber, R.; Zayed, A. Fucoidan in Pharmaceutical Formulations: A Comprehensive Review for Smart Drug Delivery Systems. Mar. Drugs 2023, 21, 112. [Google Scholar] [CrossRef]
- Chelu, M.; Calderon Moreno, J.M.; Musuc, A.M.; Popa, M. Natural Regenerative Hydrogels for Wound Healing. Gels 2024, 10, 547. [Google Scholar] [CrossRef]
- Joy, R.; Vigneshkumar, P.N.; John, F.; George, J. Hydrogels Based on Carrageenan. In Plant and Algal Hydrogels for Drug Delivery and Regenerative Medicine; Woodhead Publishing: Cambridge, UK, 2021; pp. 293–325. [Google Scholar]
- Barakat, K.M.; Ismail, M.M.; Abou El Hassayeb, H.E.; El Sersy, N.A.; Elshobary, M.E. Chemical Characterization and Biological Activities of Ulvan Extracted from Ulva fasciata (Chlorophyta). Rend. Lincei. Sci. Fis. Nat. 2022, 33, 829–841. [Google Scholar] [CrossRef]
- Hamed, R.; Magamseh, K.H.; Al-Shalabi, E.; Hammad, A.; Abu-Sini, M.; Abulebdah, D.H.; Tarawneh, O.; Sunoqrot, S. Green Hydrogels Prepared from Pectin Extracted from Orange Peels as a Potential Carrier for Dermal Delivery Systems. ACS Omega 2025, 10, 17182–17200. [Google Scholar] [CrossRef]
- Nasution, H.; Harahap, H.; Dalimunthe, N.F.; Ginting, M.H.S.; Jaafar, M.; Tan, O.O.H.; Aruan, H.K.; Herfananda, A.L. Hydrogel and Effects of Crosslinking Agent on Cellulose-Based Hydrogels: A Review. Gels 2022, 8, 568. [Google Scholar] [CrossRef]
- Lee, D.; Park, J.; Hwang, K.; Chun, S.-J.; Kim, J.H.; Lee, T.-J.; Lee, B.-T.; Cho, H.-J.; Kim, B.-J.; Wu, Q.; et al. Poly(Vinyl Alcohol) Hydrogels Reinforced with Cellulose Nanocrystals for Sustained Delivery of Salicylic Acid. ACS Appl. Nano Mater. 2024, 7, 3918–3930. [Google Scholar] [CrossRef]
- Wu, F.; Misra, M.; Mohanty, A.K. Challenges and New Opportunities on Barrier Performance of Biodegradable Polymers for Sustainable Packaging. Prog. Polym. Sci. 2021, 117, 101395. [Google Scholar] [CrossRef]
- Kim, Y.; Hu, Y.; Jeong, J.; Jung, S. Injectable, Self-Healable and Adhesive Hydrogels Using Oxidized Succinoglycan/Chitosan for PH-Responsive Drug Delivery. Carbohydr. Polym. 2022, 284, 119195. [Google Scholar] [CrossRef]
- Lu, H.-T.; Chang, W.-T.; Tsai, M.-L.; Chen, C.-H.; Chen, W.-Y.; Mi, F.-L. Development of Injectable Fucoidan and Biological Macromolecules Hybrid Hydrogels for Intra-Articular Delivery of Platelet-Rich Plasma. Mar. Drugs 2019, 17, 236. [Google Scholar] [CrossRef]
- Tennakoon, P.; Chandika, P.; Yi, M.; Jung, W.-K. Marine-Derived Biopolymers as Potential Bioplastics, an Eco-Friendly Alternative. iScience 2023, 26, 106404. [Google Scholar] [CrossRef]
- Lee, Y.B.; Kyun, M.-L.; Lee, Y.J.; Shim, H.-E.; Huh, K.M.; Kang, S.-W. Cyclodextrins as Multifunctional Tools for Advanced Biomaterials in Tissue Repair and Regeneration. Bioact. Mater. 2025, 49, 627–651. [Google Scholar] [CrossRef]
- Meng, H.; Su, J.; Shen, Q.; Hu, W.; Li, P.; Guo, K.; Liu, X.; Ma, K.; Zhong, W.; Chen, S.; et al. A Smart MMP-9-responsive Hydrogel Releasing M2 Macrophage-derived Exosomes for Diabetic Wound Healing. Adv. Healthc. Mater. 2025, 14. [Google Scholar] [CrossRef]
- Bupphathong, S.; Quiroz, C.; Huang, W.; Chung, P.-F.; Tao, H.-Y.; Lin, C.-H. Gelatin Methacrylate Hydrogel for Tissue Engineering Applications—A Review on Material Modifications. Pharmaceuticals 2022, 15, 171. [Google Scholar] [CrossRef]
- Zhang, M.; Ye, Q.; Zhu, Z.; Shi, S.; Xu, C.; Xie, R.; Li, Y. Hyaluronic Acid-Based Dynamic Hydrogels for Cartilage Repair and Regeneration. Gels 2024, 10, 703. [Google Scholar] [CrossRef]
- Kavand, A.; Noverraz, F.; Gerber-Lemaire, S. Recent Advances in Alginate-Based Hydrogels for Cell Transplantation Applications. Pharmaceutics 2024, 16, 469. [Google Scholar] [CrossRef]
- Sacco, P.; Furlani, F.; De Marzo, G.; Marsich, E.; Paoletti, S.; Donati, I. Concepts for Developing Physical Gels of Chitosan and of Chitosan Derivatives. Gels 2018, 4, 67. [Google Scholar] [CrossRef]
- Neamtu, B.; Barbu, A.; Negrea, M.O.; Berghea-Neamțu, C.Ș.; Popescu, D.; Zăhan, M.; Mireșan, V. Carrageenan-Based Compounds as Wound Healing Materials. Int. J. Mol. Sci. 2022, 23, 9117. [Google Scholar] [CrossRef]
- Beaumont, M.; Tran, R.; Vera, G.; Niedrist, D.; Rousset, A.; Pierre, R.; Shastri, V.P.; Forget, A. Hydrogel-Forming Algae Polysaccharides: From Seaweed to Biomedical Applications. Biomacromolecules 2021, 22, 1027–1052. [Google Scholar] [CrossRef]
- Sepe, F.; Valentino, A.; Marcolongo, L.; Petillo, O.; Calarco, A.; Margarucci, S.; Peluso, G.; Conte, R. Polysaccharide Hydrogels as Delivery Platforms for Natural Bioactive Molecules: From Tissue Regeneration to Infection Control. Gels 2025, 11, 198. [Google Scholar] [CrossRef]
- Tudoroiu, E.-E.; Dinu-Pîrvu, C.-E.; Albu Kaya, M.G.; Popa, L.; Anuța, V.; Prisada, R.M.; Ghica, M.V. An Overview of Cellulose Derivatives-Based Dressings for Wound-Healing Management. Pharmaceuticals 2021, 14, 1215. [Google Scholar] [CrossRef]
- Koshenaj, K.; Ferrari, G. A Comprehensive Review on Starch-Based Hydrogels: From Tradition to Innovation, Opportunities, and Drawbacks. Polymers 2024, 16, 1991. [Google Scholar] [CrossRef]
- Hong, Y.; Lin, Z.; Yang, Y.; Jiang, T.; Shang, J.; Luo, Z. Biocompatible Conductive Hydrogels: Applications in the Field of Biomedicine. Int. J. Mol. Sci. 2022, 23, 4578. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Ansari, M.J.; Rajendran, R.R.; Mohanto, S.; Agarwal, U.; Panda, K.; Dhotre, K.; Manne, R.; Deepak, A.; Zafar, A.; Yasir, M.; et al. Poly(N-Isopropylacrylamide)-Based Hydrogels for Biomedical Applications: A Review of the State-of-the-Art. Gels 2022, 8, 454. [Google Scholar] [CrossRef]
- Liu, L.; Gao, Q.; Lu, X.; Zhou, H. In Situ Forming Hydrogels Based on Chitosan for Drug Delivery and Tissue Regeneration. Asian J. Pharm. Sci. 2016, 11, 673–683. [Google Scholar] [CrossRef]
- Huang, L.; Guo, Z.; Yang, X.; Zhang, Y.; Liang, Y.; Chen, X.; Qiu, X.; Chen, X. Advancements in GelMA Bioactive Hydrogels: Strategies for Infection Control and Bone Tissue Regeneration. Theranostics 2025, 15, 460–493. [Google Scholar] [CrossRef]
- Fernandes-Cunha, G.M.; Jeong, S.H.; Logan, C.M.; Le, P.; Mundy, D.; Chen, F.; Chen, K.M.; Kim, M.; Lee, G.-H.; Na, K.-S.; et al. Supramolecular Host-Guest Hyaluronic Acid Hydrogels Enhance Corneal Wound Healing through Dynamic Spatiotemporal Effects. Ocul. Surf. 2022, 23, 148–161. [Google Scholar] [CrossRef]
- Nanda, D.; Behera, D.; Pattnaik, S.S.; Behera, A.K. Advances in Natural Polymer-Based Hydrogels: Synthesis, Applications, and Future Directions in Biomedical and Environmental Fields. Discov. Polym. 2025, 2, 6. [Google Scholar] [CrossRef]
- Salisbury, E.; Rawlings, T.M.; Efstathiou, S.; Tryfonos, M.; Makwana, K.; Fitzgerald, H.C.; Gargett, C.E.; Cameron, N.R.; Haddleton, D.M.; Brosens, J.J.; et al. Photo-Cross-Linked Gelatin Methacryloyl Hydrogels Enable the Growth of Primary Human Endometrial Stromal Cells and Epithelial Gland Organoids. ACS Appl. Mater. Interfaces 2024, 16, 39140–39152. [Google Scholar] [CrossRef]
- Hui, E.; Sumey, J.L.; Caliari, S.R. Click-Functionalized Hydrogel Design for Mechanobiology Investigations. Mol. Syst. Des. Eng. 2021, 6, 670–707. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, S.; Lin, R.; Cui, S.; Jing, X.; Coseri, S. Injectable Multifunctional Carboxymethyl Chitosan/Hyaluronic Acid Hydrogel for Drug Delivery Systems. Int. J. Biol. Macromol. 2023, 249, 125801. [Google Scholar] [CrossRef]
- Mohseni, M.; Zobeiry, N.; Fernlund, G. Process-Induced Matrix Defects: Post-Gelation. Compos. Part. A Appl. Sci. Manuf. 2020, 137, 106007. [Google Scholar] [CrossRef]
- Rumon, M.M.H. Advances in Cellulose-Based Hydrogels: Tunable Swelling Dynamics and Their Versatile Real-Time Applications. RSC Adv. 2025, 15, 11688–11729. [Google Scholar] [CrossRef]
- Gomez-Florit, M.; Pardo, A.; Domingues, R.M.A.; Graça, A.L.; Babo, P.S.; Reis, R.L.; Gomes, M.E. Natural-Based Hydrogels for Tissue Engineering Applications. Molecules 2020, 25, 5858. [Google Scholar] [CrossRef]
- Pan, H.; Qu, Y.; Wang, F.; Zhao, S.; Chen, G. Horseradish Peroxidase-Catalyzed Crosslinking Injectable Hydrogel for Bone Repair and Regeneration. Colloid Interface Sci. Commun. 2025, 66, 100828. [Google Scholar] [CrossRef]
- He, L.; Meng, F.; Chen, R.; Qin, J.; Sun, M.; Fan, Z.; Du, J. Precise Regulations at the Subcellular Level through Intracellular Polymerization, Assembly, and Transformation. JACS Au 2024, 4, 4162–4186. [Google Scholar] [CrossRef]
- Pérez-Pacheco, Y.; Tylkowski, B.; García-Valls, R. Chitosan Micro/Nanocapsules in Action: Linking Design, Production, and Therapeutic Application. Molecules 2025, 30, 252. [Google Scholar] [CrossRef]
- Nam, M.; Lee, J.W.; Cha, G.D. Biomedical Application of Enzymatically Crosslinked Injectable Hydrogels. Gels 2024, 10, 640. [Google Scholar] [CrossRef]
- Kolawole, O.M.; Cook, M.T. In Situ Gelling Drug Delivery Systems for Topical Drug Delivery. Eur. J. Pharm. Biopharm. 2023, 184, 36–49. [Google Scholar] [CrossRef]
- Boonlai, W.; Tantishaiyakul, V.; Hirun, N.; Sangfai, T.; Suknuntha, K. Thermosensitive Poloxamer 407/Poly(Acrylic Acid) Hydrogels with Potential Application as Injectable Drug Delivery System. AAPS PharmSciTech 2018, 19, 2103–2117. [Google Scholar] [CrossRef]
- Irmukhametova, G.S.; Kazybayeva, D.S.; Mun, G.A.; Khutoryanskiy, V.V. Synthesis and Chemistry of Hydrogels. In Hydrogels in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2025; pp. 1–37. [Google Scholar]
- Bodratti, A.; Alexandridis, P. Formulation of Poloxamers for Drug Delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef]
- Conzatti, G.; Nadal, C.; Berthelot, J.; Vachoud, L.; Labour, M.-N.; Tourrette, A.; Belamie, E. Chitosan-PNIPAM Thermogel Associated with Hydrogel Microspheres as a Smart Formulation for MSC Injection. ACS Appl. Bio Mater. 2024, 7, 3033–3040. [Google Scholar] [CrossRef]
- Jahanbekam, S.; Asare-Addo, K.; Alipour, S.; Nokhodchi, A. Smart Hydrogels and the Promise of Multi-Responsive in-Situ Systems. J. Drug Deliv. Sci. Technol. 2025, 107, 106758. [Google Scholar] [CrossRef]
- Li, J.; Jia, X.; Yin, L. Hydrogel: Diversity of Structures and Applications in Food Science. Food Rev. Int. 2021, 37, 313–372. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, X.; Liu, J.; Zhang, H.; Fu, D. Advances in the Application of Natural/Synthetic Hybrid Hydrogels in Tissue Engineering and Delivery Systems: A Comprehensive Review. Int. J. Pharm. 2025, 672, 125323. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Wang, S.; Chen, X.; Jiang, Y.; Su, J. Fabrication of Physical and Chemical Crosslinked Hydrogels for Bone Tissue Engineering. Bioact. Mater. 2022, 12, 327–339. [Google Scholar] [CrossRef]
- Alavarse, A.C.; Frachini, E.C.G.; da Silva, R.L.C.G.; Lima, V.H.; Shavandi, A.; Petri, D.F.S. Crosslinkers for Polysaccharides and Proteins: Synthesis Conditions, Mechanisms, and Crosslinking Efficiency, a Review. Int. J. Biol. Macromol. 2022, 202, 558–596. [Google Scholar] [CrossRef]
- Hasturk, O.; Jordan, K.E.; Choi, J.; Kaplan, D.L. Enzymatically Crosslinked Silk and Silk-Gelatin Hydrogels with Tunable Gelation Kinetics, Mechanical Properties and Bioactivity for Cell Culture and Encapsulation. Biomaterials 2020, 232, 119720. [Google Scholar] [CrossRef]
- Massana Roquero, D.; Othman, A.; Melman, A.; Katz, E. Iron(iii)-Cross-Linked Alginate Hydrogels: A Critical Review. Mater. Adv. 2022, 3, 1849–1873. [Google Scholar] [CrossRef]
- Pardeshi, S.; Damiri, F.; Zehravi, M.; Joshi, R.; Kapare, H.; Prajapati, M.K.; Munot, N.; Berrada, M.; Giram, P.S.; Rojekar, S.; et al. Functional Thermoresponsive Hydrogel Molecule to Material Design for Biomedical Applications. Polymers 2022, 14, 3126. [Google Scholar] [CrossRef]
- Duceac, I.A.; Coseri, S. Chitosan Schiff-Base Hydrogels—A Critical Perspective Review. Gels 2022, 8, 779. [Google Scholar] [CrossRef]
- Bertsch, P.; Diba, M.; Mooney, D.J.; Leeuwenburgh, S.C.G. Self-Healing Injectable Hydrogels for Tissue Regeneration. Chem. Rev. 2023, 123, 834–873. [Google Scholar] [CrossRef]
- Park, J.; Kim, H.; Ham, J.; Lee, W.; Koh, W.-G. Cell-Adhesive Double Network Self-Healing Hydrogel Capable of Cell and Drug Encapsulation: New Platform to Construct Biomimetic Environment with Bottom-up Approach. Carbohydr. Polym. 2024, 338, 122204. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.J.; Yang, Y.; Chen, Y.; Zhu, X.; You, X. Biopolymer-Based Self-Healing Hydrogels: A Short Review. Giant 2023, 16, 100188. [Google Scholar] [CrossRef]
- Choi, H.; Choi, W.-S.; Jeong, J.-O. A Review of Advanced Hydrogel Applications for Tissue Engineering and Drug Delivery Systems as Biomaterials. Gels 2024, 10, 693. [Google Scholar] [CrossRef]
- Londhe, P.V.; Londhe, M.V.; Salunkhe, A.B.; Laha, S.S.; Mefford, O.T.; Thorat, N.D.; Khot, V.M. Magnetic Hydrogel (MagGel): An Evolutionary Pedestal for Anticancer Therapy. Coord. Chem. Rev. 2025, 522, 216228. [Google Scholar] [CrossRef]
- Lu, X.; Dai, S.; Huang, B.; Li, S.; Wang, P.; Zhao, Z.; Li, X.; Li, N.; Wen, J.; Sun, Y.; et al. Exosomes Loaded a Smart Bilayer-Hydrogel Scaffold with ROS-Scavenging and Macrophage-Reprogramming Properties for Repairing Cartilage Defect. Bioact. Mater. 2024, 38, 137–153. [Google Scholar] [CrossRef]
- Karvinen, J.; Kellomäki, M. Characterization of Self-Healing Hydrogels for Biomedical Applications. Eur. Polym. J. 2022, 181, 111641. [Google Scholar] [CrossRef]
- Protsak, I.S.; Morozov, Y.M. Fundamentals and Advances in Stimuli-Responsive Hydrogels and Their Applications: A Review. Gels 2025, 11, 30. [Google Scholar] [CrossRef]
- Bezold, M.G.; Dollinger, B.R.; DeJulius, C.R.; Keech, M.C.; Hanna, A.R.; Kittel, A.R.; Yu, F.; Gupta, M.K.; D’Arcy, R.; Brunger, J.M.; et al. Shear-Thinning Hydrogel for Allograft Cell Transplantation and Externally Controlled Transgene Expression. Biomaterials 2025, 314, 122812. [Google Scholar] [CrossRef]
- Hussain, S.; Maktedar, S.S. Structural, Functional and Mechanical Performance of Advanced Graphene-Based Composite Hydrogels. Results Chem. 2023, 6, 101029. [Google Scholar] [CrossRef]
- Stowers, R.S. Advances in Extracellular Matrix-Mimetic Hydrogels to Guide Stem Cell Fate. Cells Tissues Organs 2022, 211, 703–720. [Google Scholar] [CrossRef]
- Garcia-Garcia, A.; Muñana-González, S.; Lanceros-Mendez, S.; Ruiz-Rubio, L.; Alvarez, L.P.; Vilas-Vilela, J.L. Biodegradable Natural Hydrogels for Tissue Engineering, Controlled Release, and Soil Remediation. Polymers 2024, 16, 2599. [Google Scholar] [CrossRef]
- Pramanik, S.; Kharche, S.; More, N.; Ranglani, D.; Singh, G.; Kapusetti, G. Natural Biopolymers for Bone Tissue Engineering: A Brief Review. Eng. Regen. 2023, 4, 193–204. [Google Scholar] [CrossRef]
- Thai, V.L.; Ramos-Rodriguez, D.H.; Mesfin, M.; Leach, J.K. Hydrogel Degradation Promotes Angiogenic and Regenerative Potential of Cell Spheroids for Wound Healing. Mater. Today Bio 2023, 22, 100769. [Google Scholar] [CrossRef]
- Øvrebø, Ø.; Perale, G.; Wojciechowski, J.P.; Echalier, C.; Jeffers, J.R.T.; Stevens, M.M.; Haugen, H.J.; Rossi, F. Design and Clinical Application of Injectable Hydrogels for Musculoskeletal Therapy. Bioeng. Transl. Med. 2022, 7, e10295. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, X.; Wang, Y.; Chen, W.; Zhu, Z.; Wang, S. Hydrogels and Hydrogel-Based Drug Delivery Systems for Promoting Refractory Wound Healing: Applications and Prospects. Int. J. Biol. Macromol. 2025, 285, 138098. [Google Scholar] [CrossRef]
- Arias-Betancur, A.; Badilla-Wenzel, N.; Astete-Sanhueza, Á.; Farfán-Beltrán, N.; Dias, F.J. Carrier Systems for Bone Morphogenetic Proteins: An Overview of Biomaterials Used for Dentoalveolar and Maxillofacial Bone Regeneration. Jpn. Dent. Sci. Rev. 2022, 58, 316–327. [Google Scholar] [CrossRef]
- Davis-Hall, D.; Nguyen, V.; D’Ovidio, T.J.; Tsai, E.; Bilousova, G.; Magin, C.M. Peptide-Functionalized Hydrogels Modulate Integrin Expression and Stemness in Adult Human Epidermal Keratinocytes. Adv. Biosyst. 2019, 3, e1900022. [Google Scholar] [CrossRef]
- Omidian, H.; Wilson, R.; Dey Chowdhury, S. Injectable Biomimetic Gels for Biomedical Applications. Biomimetics 2024, 9, 418. [Google Scholar] [CrossRef]
- Chabria, Y.; Duffy, G.; Lowery, A.; Dwyer, R. Hydrogels: 3D Drug Delivery Systems for Nanoparticles and Extracellular Vesicles. Biomedicines 2021, 9, 1694. [Google Scholar] [CrossRef]
- Ghosh, S.; Kumar, N.; Chattopadhyay, S. Electrically Conductive “SMART” Hydrogels for on-Demand Drug Delivery. Asian J. Pharm. Sci. 2025, 20, 101007. [Google Scholar] [CrossRef]
- Chen, H.; Xu, J.; Sun, J.; Jiang, Y.; Zheng, W.; Hu, W.; Qian, H. Recent Advances on Thermosensitive Hydrogels-Mediated Precision Therapy. Asian J. Pharm. Sci. 2024, 19, 100911. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef]
- Visan, A.I.; Negut, I. Development and Applications of PLGA Hydrogels for Sustained Delivery of Therapeutic Agents. Gels 2024, 10, 497. [Google Scholar] [CrossRef]
- Volpi, M.; Paradiso, A.; Costantini, M.; Świȩszkowski, W. Hydrogel-Based Fiber Biofabrication Techniques for Skeletal Muscle Tissue Engineering. ACS Biomater. Sci. Eng. 2022, 8, 379–405. [Google Scholar] [CrossRef]
- He, Z.; He, S. Two-Photon Polymerization of Hydrogel Cellular Scaffolds. Opt. Commun. 2025, 574, 131161. [Google Scholar] [CrossRef]
- Li, P.; Zhang, M.; Chen, Z.; Tian, B.; Kang, X. Tissue-Engineered Injectable Gelatin–Methacryloyl Hydrogel-Based Adjunctive Therapy for Intervertebral Disc Degeneration. ACS Omega 2023, 8, 13509–13518. [Google Scholar] [CrossRef]
- Satchanska, G.; Davidova, S.; Petrov, P.D. Natural and Synthetic Polymers for Biomedical and Environmental Applications. Polymers 2024, 16, 1159. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Mohan, M.; Rajeev, M.R. Modified Chitosan-Hyaluronic Acid Based Hydrogel for the PH-Responsive Co-Delivery of Cisplatin and Doxorubicin. Int. J. Biol. Macromol. 2022, 201, 378–388. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, T.; Li, J.; Yan, H.; Lyu, L.; Yu, Y.; Yang, G.; Zhang, T.; Zhou, Y.; Wang, X.; et al. Matrix Metalloproteinase-Responsive Hydrogel with On-Demand Release of Phosphatidylserine Promotes Bone Regeneration Through Immunomodulation. Adv. Sci. 2024, 11, e2306924. [Google Scholar] [CrossRef]
- Bernhard, S.; Tibbitt, M.W. Supramolecular Engineering of Hydrogels for Drug Delivery. Adv. Drug Deliv. Rev. 2021, 171, 240–256. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, F.; Long, L.; Kong, Q.; Luo, R.; Wang, Y. Dual-Responsive Injectable Hydrogels Encapsulating Drug-Loaded Micelles for on-Demand Antimicrobial Activity and Accelerated Wound Healing. J. Control. Release 2020, 324, 204–217. [Google Scholar] [CrossRef]
- Mikhail, A.S.; Morhard, R.; Mauda-Havakuk, M.; Kassin, M.; Arrichiello, A.; Wood, B.J. Hydrogel Drug Delivery Systems for Minimally Invasive Local Immunotherapy of Cancer. Adv. Drug Deliv. Rev. 2023, 202, 115083. [Google Scholar] [CrossRef]
- Huang, G.; Huang, H. Application of Hyaluronic Acid as Carriers in Drug Delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef]
- Ahsan, A.; Farooq, M.A.; Parveen, A. Thermosensitive Chitosan-Based Injectable Hydrogel as an Efficient Anticancer Drug Carrier. ACS Omega 2020, 5, 20450–20460. [Google Scholar] [CrossRef]
- Liu, C.; Liao, Y.; Liu, L.; Xie, L.; Liu, J.; Zhang, Y.; Li, Y. Application of Injectable Hydrogels in Cancer Immunotherapy. Front. Bioeng. Biotechnol. 2023, 11, 1121887. [Google Scholar] [CrossRef]
- Binaymotlagh, R.; Hajareh Haghighi, F.; Chronopoulou, L.; Palocci, C. Liposome–Hydrogel Composites for Controlled Drug Delivery Applications. Gels 2024, 10, 284. [Google Scholar] [CrossRef]
- Zhong, Z.; Gan, L.; Feng, Z.; Wang, W.; Pan, X.; Wu, C.; Huang, Y. Hydrogel Local Drug Delivery Systems for Postsurgical Management of Tumors: Status Quo and Perspectives. Mater. Today Bio 2024, 29, 101308. [Google Scholar] [CrossRef]
- Shadab, A.; Farokhi, S.; Fakouri, A.; Mohagheghzadeh, N.; Noroozi, A.; Razavi, Z.S.; Karimi Rouzbahani, A.; Zalpoor, H.; Mahjoor, M. Hydrogel-Based Nanoparticles: Revolutionizing Brain Tumor Treatment and Paving the Way for Future Innovations. Eur. J. Med. Res. 2025, 30, 71. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, L.; Xu, Y.; Du, W.; Cai, X.; Wang, F.; Ling, Y.; Chen, H.; Wang, Z.; Hu, B.; et al. A PH and Magnetic Dual-Response Hydrogel for Synergistic Chemo-Magnetic Hyperthermia Tumor Therapy. RSC Adv. 2018, 8, 9812–9821. [Google Scholar] [CrossRef]
- Zhao, B.; Li, L.; Cui, K.; Wang, C.-L.; Wang, A.-L.; Sun, Z.-Q.; Zhang, B.; Zhou, W.-Y.; Niu, Z.-X.; Tian, H.; et al. Mechanisms of TRAIL and Gemcitabine Induction of Pancreatic Cancer Cell Apoptosis. Asian Pac. J. Cancer Prev. 2011, 12, 2675–2678. [Google Scholar]
- Olteanu, G.; Neacșu, S.M.; Joița, F.A.; Musuc, A.M.; Lupu, E.C.; Ioniță-Mîndrican, C.-B.; Lupuliasa, D.; Mititelu, M. Advancements in Regenerative Hydrogels in Skin Wound Treatment: A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 3849. [Google Scholar] [CrossRef]
- Yu, S.; Sun, H.; Li, Y.; Wei, S.; Xu, J.; Liu, J. Hydrogels as Promising Platforms for Engineered Living Bacteria-Mediated Therapeutic Systems. Mater. Today Bio 2022, 16, 100435. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Gong, Y.; Yan, X.; Wang, L.; Zheng, W.; Ai, H.; Zhao, Y. Recent Advances in Nanoantibiotics against Multidrug-Resistant Bacteria. Nanoscale Adv. 2023, 5, 6278–6317. [Google Scholar] [CrossRef]
- Leyva-Jiménez, F.J.; Oliver-Simancas, R.; Castangia, I.; Rodríguez-García, A.M.; Alañón, M.E. Comprehensive Review of Natural Based Hydrogels as an Upcoming Trend for Food Packing. Food Hydrocoll. 2023, 135, 108124. [Google Scholar] [CrossRef]
- Walvekar, P.; Lulinski, P.; Kumar, P.; Aminabhavi, T.M.; Choonara, Y.E. A Review of Hyaluronic Acid-Based Therapeutics for the Treatment and Management of Arthritis. Int. J. Biol. Macromol. 2024, 264, 130645. [Google Scholar] [CrossRef]
- Duarte, J.; Mascarenhas-Melo, F.; Pires, P.C.; Veiga, F.; Paiva-Santos, A.C. Multifunctional Hydrogels-Based Therapies for Chronic Diabetic Wound Healing. Eur. Polym. J. 2024, 211, 113026. [Google Scholar] [CrossRef]
- Sathiya, K.; Ganesamoorthi, S.; Mohan, S.; Shanmugavadivu, A.; Selvamurugan, N. Natural Polymers-Based Surface Engineering of Bone Scaffolds—A Review. Int. J. Biol. Macromol. 2024, 282, 136840. [Google Scholar] [CrossRef]
- Huang, S.; Wang, Z.; Sun, X.; Li, K. Bone Morphogenetic Protein 7-Loaded Gelatin Methacrylate/Oxidized Sodium Alginate/Nano-Hydroxyapatite Composite Hydrogel for Bone Tissue Engineering. Int. J. Nanomed. 2024, 19, 6359–6376. [Google Scholar] [CrossRef]
- Shi, W.; Jiang, Y.; Wu, T.; Zhang, Y.; Li, T. Advancements in Drug-Loaded Hydrogel Systems for Bone Defect Repair. Regen. Ther. 2024, 25, 174–185. [Google Scholar] [CrossRef]
- Hashemi-Afzal, F.; Fallahi, H.; Bagheri, F.; Collins, M.N.; Eslaminejad, M.B.; Seitz, H. Advancements in Hydrogel Design for Articular Cartilage Regeneration: A Comprehensive Review. Bioact. Mater. 2025, 43, 1–31. [Google Scholar] [CrossRef]
- Lin, T.-H.; Wang, H.-C.; Tseng, Y.-L.; Yeh, M.-L. A Bioactive Composite Scaffold Enhances Osteochondral Repair by Using Thermosensitive Chitosan Hydrogel and Endothelial Lineage Cell-Derived Chondrogenic Cell. Mater. Today Bio 2024, 28, 101174. [Google Scholar] [CrossRef]
- Wu, J.; Chen, Q.; Deng, C.; Xu, B.; Zhang, Z.; Yang, Y.; Lu, T. Exquisite Design of Injectable Hydrogels in Cartilage Repair. Theranostics 2020, 10, 9843–9864. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Q.; Tian, Z.; Yao, Q.; Zhang, M. Recent Advances in 3D Bioprinted Cartilage-Mimicking Constructs for Applications in Tissue Engineering. Mater. Today Bio 2023, 23, 100870. [Google Scholar] [CrossRef]
- Niu, X.; Li, N.; Du, Z.; Li, X. Integrated Gradient Tissue-Engineered Osteochondral Scaffolds: Challenges, Current Efforts and Future Perspectives. Bioact. Mater. 2023, 20, 574–597. [Google Scholar] [CrossRef]
- Patel, R.; Patel, D. Injectable Hydrogels in Cardiovascular Tissue Engineering. Polymers 2024, 16, 1878. [Google Scholar] [CrossRef]
- Munarin, F.; Kabelac, C.; Coulombe, K.L.K. Heparin-modified Alginate Microspheres Enhance Neovessel Formation in hiPSC-derived Endothelial Cells and Heterocellular in Vitro Models by Controlled Release of vascular Endothelial Growth Factor. J. Biomed. Mater. Res. Part A 2021, 109, 1726–1736. [Google Scholar] [CrossRef]
- Hoeeg, C.; Dolatshahi-Pirouz, A.; Follin, B. Injectable Hydrogels for Improving Cardiac Cell Therapy—In Vivo Evidence and Translational Challenges. Gels 2021, 7, 7. [Google Scholar] [CrossRef]
- Zare, I.; Mirshafiei, M.; Kheilnezhad, B.; Far, B.F.; Hassanpour, M.; Pishbin, E.; Eftekhar Vaghefi, S.S.; Yazdian, F.; Rashedi, H.; Hasan, A.; et al. Hydrogel-Integrated Graphene Superstructures for Tissue Engineering: From Periodontal to Neural Regeneration. Carbon 2024, 223, 118970. [Google Scholar] [CrossRef]
- Hlavac, N.; Kasper, M.; Schmidt, C.E. Progress toward Finding the Perfect Match: Hydrogels for Treatment of Central Nervous System Injury. Mater. Today Adv. 2020, 6, 100039. [Google Scholar] [CrossRef]
- Jiang, S.; Geng, R.; Wang, R.; Li, X.; Bao, X. The Potential of Hydrogels as a Niche for Promoting Neurogenesis and Regulating Neuroinflammation in Ischemic Stroke. Mater. Des. 2023, 229, 111916. [Google Scholar] [CrossRef]
- Bellotti, E.; Schilling, A.L.; Little, S.R.; Decuzzi, P. Injectable Thermoresponsive Hydrogels as Drug Delivery System for the Treatment of Central Nervous System Disorders: A Review. J. Control. Release 2021, 329, 16–35. [Google Scholar] [CrossRef]
- Bu, W.; Wu, Y.; Ghaemmaghami, A.M.; Sun, H.; Mata, A. Rational Design of Hydrogels for Immunomodulation. Regen. Biomater. 2022, 9, rbac009. [Google Scholar] [CrossRef]
- Che, X.; Zhao, T.; Hu, J.; Yang, K.; Ma, N.; Li, A.; Sun, Q.; Ding, C.; Ding, Q. Application of Chitosan-Based Hydrogel in Promoting Wound Healing: A Review. Polymers 2024, 16, 344. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Li, Y.; Yang, Y.; Jin, M.; Lin, X.; Zhuang, Z.; Guo, K.; Zhang, T.; Tan, W. Application of Collagen-Based Hydrogel in Skin Wound Healing. Gels 2023, 9, 185. [Google Scholar] [CrossRef]
- Cao, H.; Wang, J.; Hao, Z.; Zhao, D. Gelatin-Based Biomaterials and Gelatin as an Additive for Chronic Wound Repair. Front. Pharmacol. 2024, 15, 1398939. [Google Scholar] [CrossRef]
- Dong, Y.; Cui, M.; Qu, J.; Wang, X.; Kwon, S.H.; Barrera, J.; Elvassore, N.; Gurtner, G.C. Conformable Hyaluronic Acid Hydrogel Delivers Adipose-Derived Stem Cells and Promotes Regeneration of Burn Injury. Acta Biomater. 2020, 108, 56–66. [Google Scholar] [CrossRef]
- Diniz, F.; Maia, R.; de Andrade, L.R.; Andrade, L.; Vinicius Chaud, M.; da Silva, C.; Corrêa, C.; de Albuquerque Junior, R.; Pereira da Costa, L.; Shin, S.; et al. Silver Nanoparticles-Composing Alginate/Gelatine Hydrogel Improves Wound Healing In Vivo. Nanomaterials 2020, 10, 390. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, B.M. Current Advances in Stimuli-Responsive Hydrogels as Smart Drug Delivery Carriers. Gels 2023, 9, 838. [Google Scholar] [CrossRef]
- Karvinen, J.; Kellomäki, M. 3D-Bioprinting of Self-Healing Hydrogels. Eur. Polym. J. 2024, 209, 112864. [Google Scholar] [CrossRef]
- Yadav, P.; Singh, S.; Jaiswal, S.; Kumar, R. Synthetic and Natural Polymer Hydrogels: A Review of 3D Spheroids and Drug Delivery. Int. J. Biol. Macromol. 2024, 280, 136126. [Google Scholar] [CrossRef]
- García-Couce, J.; Tomás, M.; Fuentes, G.; Que, I.; Almirall, A.; Cruz, L.J. Chitosan/Pluronic F127 Thermosensitive Hydrogel as an Injectable Dexamethasone Delivery Carrier. Gels 2022, 8, 44. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Xu, X.; Chen, C.; Akiyama, K.; Snead, M.L.; Shi, S. Dental Mesenchymal Stem Cells Encapsulated in an Alginate Hydrogel Co-Delivery Microencapsulation System for Cartilage Regeneration. Acta Biomater. 2013, 9, 9343–9350. [Google Scholar] [CrossRef]
- Xia, J.; Gao, X.; Yao, J.; Fei, Y.; Song, D.; Gu, Z.; Zheng, G.; Gu, Y.; Tu, C. Injectable Brain Extracellular Matrix Hydrogels Enhance Neuronal Migration and Functional Recovery After Intracerebral Hemorrhage. Biomater. Res. 2025, 29, 0192. [Google Scholar] [CrossRef]
- Fan, M.-H.; Pi, J.-K.; Zou, C.-Y.; Jiang, Y.-L.; Li, Q.-J.; Zhang, X.-Z.; Xing, F.; Nie, R.; Han, C.; Xie, H.-Q. Hydrogel-Exosome System in Tissue Engineering: A Promising Therapeutic Strategy. Bioact. Mater. 2024, 38, 1–30. [Google Scholar] [CrossRef]
- Su, C.; Lin, D.; Huang, X.; Feng, J.; Jin, A.; Wang, F.; Lv, Q.; Lei, L.; Pan, W. Developing Hydrogels for Gene Therapy and Tissue Engineering. J. Nanobiotechnol. 2024, 22, 182. [Google Scholar] [CrossRef]
- He, L.; Chen, X. Cardiomyocyte Induction and Regeneration for Myocardial Infarction Treatment: Cell Sources and Administration Strategies. Adv. Healthc. Mater. 2020, 9, e2001175. [Google Scholar] [CrossRef]
- Distler, T.; Lauria, I.; Detsch, R.; Sauter, C.M.; Bendt, F.; Kapr, J.; Rütten, S.; Boccaccini, A.R.; Fritsche, E. Neuronal Differentiation from Induced Pluripotent Stem Cell-Derived Neurospheres by the Application of Oxidized Alginate-Gelatin-Laminin Hydrogels. Biomedicines 2021, 9, 261. [Google Scholar] [CrossRef]
- Fan, P.; Zeng, Y.; Zaldivar-Silva, D.; Agüero, L.; Wang, S. Chitosan-Based Hemostatic Hydrogels: The Concept, Mechanism, Application, and Prospects. Molecules 2023, 28, 1473. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, L.; Cheng, H.; Zhu, J.; Li, X.; Ye, S.; Li, X. Hydrogel-Based Dressings Designed to Facilitate Wound Healing. Mater. Adv. 2024, 5, 1364–1394. [Google Scholar] [CrossRef]
- Dethe, M.R.; A, P.; Ahmed, H.; Agrawal, M.; Roy, U.; Alexander, A. PCL-PEG Copolymer Based Injectable Thermosensitive Hydrogels. J. Control. Release 2022, 343, 217–236. [Google Scholar] [CrossRef]
- Ghandforoushan, P.; Alehosseini, M.; Golafshan, N.; Castilho, M.; Dolatshahi-Pirouz, A.; Hanaee, J.; Davaran, S.; Orive, G. Injectable Hydrogels for Cartilage and Bone Tissue Regeneration: A Review. Int. J. Biol. Macromol. 2023, 246, 125674. [Google Scholar] [CrossRef]
- Lv, Q.; Zhou, D.; He, Y.; Xu, T.; Qiu, X.; Zeng, J. Engineering Functional Electroconductive Hydrogels for Targeted Therapy in Myocardial Infarction Repair. Bioact. Mater. 2025, 49, 172–192. [Google Scholar] [CrossRef]
- Dong, Y.C.; Bouché, M.; Uman, S.; Burdick, J.A.; Cormode, D.P. Detecting and Monitoring Hydrogels with Medical Imaging. ACS Biomater. Sci. Eng. 2021, 7, 4027–4047. [Google Scholar] [CrossRef]
- Yu, J.; Liu, Y.; Zhang, Y.; Ran, R.; Kong, Z.; Zhao, D.; Liu, M.; Zhao, W.; Cui, Y.; Hua, Y.; et al. Smart Nanogels for Cancer Treatment from the Perspective of Functional Groups. Front. Bioeng. Biotechnol. 2024, 11, 1329311. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, Z.; Zhang, J.; Yang, Y.; Huang, C.; Zhang, W.; Ding, D.; Liu, G.; Cheng, N. Multifunctional Chitosan/Alginate Hydrogel Incorporated with Bioactive Glass Nanocomposites Enabling Photothermal and Nitric Oxide Release Activities for Bacteria-Infected Wound Healing. Int. J. Biol. Macromol. 2023, 232, 123445. [Google Scholar] [CrossRef]
- Lee, S.S.; Du, X.; Kim, I.; Ferguson, S.J. Scaffolds for Bone-Tissue Engineering. Matter 2022, 5, 2722–2759. [Google Scholar] [CrossRef]
- Xu, Q.; Xiao, Z.; Yang, Q.; Yu, T.; Deng, X.; Chen, N.; Huang, Y.; Wang, L.; Guo, J.; Wang, J. Hydrogel-Based Cardiac Repair and Regeneration Function in the Treatment of Myocardial Infarction. Mater. Today Bio 2024, 25, 100978. [Google Scholar] [CrossRef]
- Taisescu, O.; Dinescu, V.C.; Rotaru-Zavaleanu, A.D.; Gresita, A.; Hadjiargyrou, M. Hydrogels for Peripheral Nerve Repair: Emerging Materials and Therapeutic Applications. Gels 2025, 11, 126. [Google Scholar] [CrossRef]
- Duong, T.K.N.; Truong, T.T.; Phan, T.N.L.; Nguyen, T.X.; Doan, V.H.M.; Vo, T.T.; Choi, J.; Pal, U.; Dhar, P.; Lee, B.; et al. Hydrogel-Based Smart Materials for Wound Healing and Sensing. Aggregate 2025. [Google Scholar] [CrossRef]
- Zhong, R.; Talebian, S.; Mendes, B.B.; Wallace, G.; Langer, R.; Conde, J.; Shi, J. Hydrogels for RNA Delivery. Nat. Mater. 2023, 22, 818–831. [Google Scholar] [CrossRef]
- Farasati Far, B.; Safaei, M.; Nahavandi, R.; Gholami, A.; Naimi-Jamal, M.R.; Tamang, S.; Ahn, J.E.; Ramezani Farani, M.; Huh, Y.S. Hydrogel Encapsulation Techniques and Its Clinical Applications in Drug Delivery and Regenerative Medicine: A Systematic Review. ACS Omega 2024, 9, 29139–29158. [Google Scholar] [CrossRef]
- Tang, Z.; Deng, L.; Zhang, J.; Jiang, T.; Xiang, H.; Chen, Y.; Liu, H.; Cai, Z.; Cui, W.; Xiong, Y. Intelligent Hydrogel-Assisted Hepatocellular Carcinoma Therapy. Research 2024, 7, 0477. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, Y.; Zhang, J.; Liang, H.; Chen, X.; Tan, H. Natural Polymer-Based Hydrogels: From Polymer to Biomedical Applications. Pharmaceutics 2023, 15, 2514. [Google Scholar] [CrossRef]
- Grosskopf, A.K.; Mann, J.L.; Baillet, J.; Lopez Hernandez, H.; Autzen, A.A.A.; Yu, A.C.; Appel, E.A. Extreme Extensibility in Physically Cross-Linked Nanocomposite Hydrogels Leveraging Dynamic Polymer–Nanoparticle Interactions. Macromolecules 2022, 55, 7498–7511. [Google Scholar] [CrossRef]
- Han, X.; Wu, Y.; Shan, Y.; Zhang, X.; Liao, J. Effect of Micro-/Nanoparticle Hybrid Hydrogel Platform on the Treatment of Articular Cartilage-Related Diseases. Gels 2021, 7, 155. [Google Scholar] [CrossRef]
- Irfan, M.; Liu, J.; Du, X.; Chen, S.; Xiao, J.J. Hydrogen Bond-reinforced Double-network Hydrogels with Enhanced Mechanical Strength: Preparation, Characterization and Swelling Behavior. J. Polym. Sci. 2024, 62, 3426–3438. [Google Scholar] [CrossRef]
- Xu, F.; Dawson, C.; Lamb, M.; Mueller, E.; Stefanek, E.; Akbari, M.; Hoare, T. Hydrogels for Tissue Engineering: Addressing Key Design Needs Toward Clinical Translation. Front. Bioeng. Biotechnol. 2022, 10, 849831. [Google Scholar] [CrossRef]
- Galante, R.; Pinto, T.J.A.; Colaço, R.; Serro, A.P. Sterilization of Hydrogels for Biomedical Applications: A Review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2472–2492. [Google Scholar] [CrossRef]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current Hydrogel Advances in Physicochemical and Biological Response-Driven Biomedical Application Diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef]
- Bento, C.S.A.; Gaspar, M.C.; Coimbra, P.; de Sousa, H.C.; Braga, M.E.M. A Review of Conventional and Emerging Technologies for Hydrogels Sterilization. Int. J. Pharm. 2023, 634, 122671. [Google Scholar] [CrossRef]
- Odziomek, K.; Drabczyk, A.K.; Kościelniak, P.; Konieczny, P.; Barczewski, M.; Bialik-Wąs, K. The Role of Freeze-Drying as a Multifunctional Process in Improving the Properties of Hydrogels for Medical Use. Pharmaceuticals 2024, 17, 1512. [Google Scholar] [CrossRef]
- Kang, N.-W.; Yoon, S.-Y.; Kim, S.; Yu, N.-Y.; Park, J.-H.; Lee, J.-Y.; Cho, H.-J.; Kim, D.-D. Subcutaneously Injectable Hyaluronic Acid Hydrogel for Sustained Release of Donepezil with Reduced Initial Burst Release: Effect of Hybridization of Microstructured Lipid Carriers and Albumin. Pharmaceutics 2021, 13, 864. [Google Scholar] [CrossRef]
- Cascone, S.; Lamberti, G. Hydrogel-Based Commercial Products for Biomedical Applications: A Review. Int. J. Pharm. 2020, 573, 118803. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Tian, X.; Wen, Y.; Zhang, Z.; Zhu, J.; Song, X.; Phan, T.T.; Li, J. Recent Advances in Smart Hydrogels Derived from Polysaccharides and Their Applications for Wound Dressing and Healing. Biomaterials 2025, 318, 123134. [Google Scholar] [CrossRef]
- Sarrigiannidis, S.O.; Rey, J.M.; Dobre, O.; González-García, C.; Dalby, M.J.; Salmeron-Sanchez, M. A Tough Act to Follow: Collagen Hydrogel Modifications to Improve Mechanical and Growth Factor Loading Capabilities. Mater. Today Bio 2021, 10, 100098. [Google Scholar] [CrossRef]
- Yu, G.; Niu, C.; Liu, J.; Wu, J.; Jin, Z.; Wang, Y.; Zhao, K. Preparation and Properties of Self-Cross-Linking Hydrogels Based on Chitosan Derivatives and Oxidized Sodium Alginate. ACS Omega 2023, 8, 19752–19766. [Google Scholar] [CrossRef]
- Edo, G.I.; Ndudi, W.; Ali, A.M.; Yousif, E.; Jikah, A.N.; Isoje, E.F.; Igbuku, U.A.; Mafe, A.N.; Opiti, R.A.; Madueke, C.J.; et al. Biopolymers: An Inclusive Review. Hybrid Adv. 2025, 9, 100418. [Google Scholar] [CrossRef]
- Tamo, A.K. Nanocellulose-Based Hydrogels as Versatile Materials with Interesting Functional Properties for Tissue Engineering Applications. J. Mater. Chem. B 2024, 12, 7692–7759. [Google Scholar] [CrossRef]
- Patil, V.A.; Masters, K.S. Engineered Collagen Matrices. Bioengineering 2020, 7, 163. [Google Scholar] [CrossRef]
- Perez-Puyana, V.; Jiménez-Rosado, M.; Romero, A.; Guerrero, A. Crosslinking of Hybrid Scaffolds Produced from Collagen and Chitosan. Int. J. Biol. Macromol. 2019, 139, 262–269. [Google Scholar] [CrossRef]
- Guarino, V.; Caputo, T.; Altobelli, R.; Ambrosio, L. Degradation Properties and Metabolic Activity of Alginate and Chitosan Polyelectrolytes for Drug Delivery and Tissue Engineering Applications. AIMS Mater. Sci. 2015, 2, 497–502. [Google Scholar] [CrossRef]
- Karami, P.; Stampoultzis, T.; Guo, Y.; Pioletti, D.P. A Guide to Preclinical Evaluation of Hydrogel-Based Devices for Treatment of Cartilage Lesions. Acta Biomater. 2023, 158, 12–31. [Google Scholar] [CrossRef]
- Rumon, M.M.H.; Rahman, M.S.; Akib, A.A.; Sohag, M.S.; Rakib, M.R.A.; Khan, M.A.R.; Yesmin, F.; Shakil, M.S.; Rahman Khan, M.M. Progress in Hydrogel Toughening: Addressing Structural and Crosslinking Challenges for Biomedical Applications. Discov. Mater. 2025, 5, 5. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, J.S.; Yang, D.H.; Nah, H.; Min, S.J.; Lee, S.Y.; Yoo, J.H.; Chun, H.J.; Moon, H.-J.; Hong, Y.K.; et al. Development of a Temperature-Responsive Hydrogel Incorporating PVA into NIPAAm for Controllable Drug Release in Skin Regeneration. ACS Omega 2023, 8, 44076–44085. [Google Scholar] [CrossRef]
- Solanki, R.; Bhatia, D. Stimulus-Responsive Hydrogels for Targeted Cancer Therapy. Gels 2024, 10, 440. [Google Scholar] [CrossRef]
- He, Q.; Chen, J.; Yan, J.; Cai, S.; Xiong, H.; Liu, Y.; Peng, D.; Mo, M.; Liu, Z. Tumor Microenvironment Responsive Drug Delivery Systems. Asian J. Pharm. Sci. 2020, 15, 416–448. [Google Scholar] [CrossRef]
- Condò, I.; Giannitelli, S.M.; Lo Presti, D.; Cortese, B.; Ursini, O. Overview of Dynamic Bond Based Hydrogels for Reversible Adhesion Processes. Gels 2024, 10, 442. [Google Scholar] [CrossRef]
- Jo, Y.K.; Kim, H.J.; Jeong, Y.; Joo, K., II; Cha, H.J. Biomimetic Surface Engineering of Biomaterials by Using Recombinant Mussel Adhesive Proteins. Adv. Mater. Interfaces 2018, 5, 1800068. [Google Scholar] [CrossRef]
- Min, J.H.; Patel, M.; Koh, W.-G. Incorporation of Conductive Materials into Hydrogels for Tissue Engineering Applications. Polymers 2018, 10, 1078. [Google Scholar] [CrossRef]
- Xue, L.; Sun, J. Magnetic Hydrogels with Ordered Structure for Biomedical Applications. Front. Chem. 2022, 10, 1040492. [Google Scholar] [CrossRef]
- Iyer, K.S.; Bao, L.; Zhai, J.; Jayachandran, A.; Luwor, R.; Li, J.J.; Li, H. Microgel-Based Bioink for Extrusion-Based 3D Bioprinting and Its Applications in Tissue Engineering. Bioact. Mater. 2025, 48, 273–293. [Google Scholar] [CrossRef]
- Yoon, J.; Han, H.; Jang, J. Nanomaterials-Incorporated Hydrogels for 3D Bioprinting Technology. Nano Converg. 2023, 10, 52. [Google Scholar] [CrossRef]
- Aronsson, C.; Jury, M.; Naeimipour, S.; Boroojeni, F.R.; Christoffersson, J.; Lifwergren, P.; Mandenius, C.-F.; Selegård, R.; Aili, D. Dynamic Peptide-Folding Mediated Biofunctionalization and Modulation of Hydrogels for 4D Bioprinting. Biofabrication 2020, 12, 035031. [Google Scholar] [CrossRef]
- Zöller, K.; To, D.; Bernkop-Schnürch, A. Biomedical Applications of Functional Hydrogels: Innovative Developments, Relevant Clinical Trials and Advanced Products. Biomaterials 2025, 312, 122718. [Google Scholar] [CrossRef]
- Gan, Z.; Qin, X.; Liu, H.; Liu, J.; Qin, J. Recent Advances in Defined Hydrogels in Organoid Research. Bioact. Mater. 2023, 28, 386–401. [Google Scholar] [CrossRef]
- Wong, J.F.; Mohan, M.D.; Young, E.W.K.; Simmons, C.A. Integrated Electrochemical Measurement of Endothelial Permeability in a 3D Hydrogel-Based Microfluidic Vascular Model. Biosens. Bioelectron. 2020, 147, 111757. [Google Scholar] [CrossRef]
- Negut, I.; Bita, B. Exploring the Potential of Artificial Intelligence for Hydrogel Development—A Short Review. Gels 2023, 9, 845. [Google Scholar] [CrossRef]
- Raeisi, A.; Farjadian, F. Commercial Hydrogel Product for Drug Delivery Based on Route of Administration. Front. Chem. 2024, 12, 1336717. [Google Scholar] [CrossRef]
- Delgado-Pujol, E.J.; Martínez, G.; Casado-Jurado, D.; Vázquez, J.; León-Barberena, J.; Rodríguez-Lucena, D.; Torres, Y.; Alcudia, A.; Begines, B. Hydrogels and Nanogels: Pioneering the Future of Advanced Drug Delivery Systems. Pharmaceutics 2025, 17, 215. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Z.; Li, G.; Cai, Z.; Wu, J.; Wang, L.; Deng, L.; Cai, M.; Cui, W. Injectable Microfluidic Hydrogel Microspheres for Cell and Drug Delivery. Adv. Funct. Mater. 2021, 31, 2103339. [Google Scholar] [CrossRef]
- Wang, S.; Ma, Y.; Wang, F.; Lu, C.; Liu, Y.; Zhang, S.; Ma, S.; Wang, L. Development of Cellulose-Based Self-Healing Hydrogel Smart Packaging for Fish Preservation and Freshness Indication. Carbohydr. Polym. 2025, 348, 122806. [Google Scholar] [CrossRef]

| Biopolymer | Source | Key Properties | Biomedical Applications | Limitations |
|---|---|---|---|---|
| Alginate | Brown seaweed | Ionic crosslinking, biocompatible, fast gelation | Wound healing, drug delivery, cartilage regeneration | Poor cell adhesion, mechanical weakness |
| Chitosan | Crustacean shells | Antibacterial, mucoadhesive, biodegradable | Skin repair, nerve regeneration, hemostasis | Insoluble at physiological pH |
| Gelatin | Denatured collagen | Thermoresponsive, promotes cell adhesion and migration | Tissue scaffolding, drug release, cell encapsulation | Weak mechanical properties, batch variability |
| Hyaluronic Acid | Connective tissue | ECM mimic, promotes angiogenesis, hydrophilic | Wound healing, osteoarthritis treatment, cosmetic fillers | Rapid degradation, low mechanical strength |
| Collagen | Animal tissue | Native ECM protein, supports cell growth and differentiation | Bone regeneration, skin tissue engineering | Immunogenicity (depending on source), gelation control required |
| Fibrin | Blood plasma protein | Fast gelation, supports angiogenesis and hemostasis | Wound sealants, cardiovascular applications | Rapid degradation, weak long-term mechanical support |
| Silk Fibroin | Silkworm cocoons | High mechanical strength, tunable degradation | Bone tissue, nerve repair, drug carriers | Slow gelation, requires pre-processing |
| Biopolymer Type | Source | Key Functional Properties | Common Applications | Advantages | Limitations | References |
|---|---|---|---|---|---|---|
| Gelatin Methacryloyl (GelMA) | Animal-derived (modified) | Photopolymerizable; cell adhesion sites; enzymatic degradation | Tissue engineering, wound healing | ECM mimicry; tunable stiffness; photo-crosslinkable | UV exposure risks; needs photoinitiators | [66] |
| HA-derivatives (e.g., HA-CHO) | Animal-derived (modified) | Injectable via Schiff base reactions; supports cell migration | Cartilage repair, drug delivery | Biocompatible; modifiable for in situ gelling | Rapid degradation; variable gelation | [67] |
| Alginate–PEG Hybrid | Marine + synthetic blend | Ionic/covalent crosslinking; improved mechanical strength | Bone tissue scaffolds, 3D bioprinting | Customizable rheology; dual crosslinking | Bioinert unless modified | [68] |
| Chitosan–β-GP Hybrid | Marine-derived hybrid | Thermo-responsive (sol–gel transition at ~37 °C) | Injectable scaffolds, CNS regeneration | Easy injection; physiological gelation | Low long-term mechanical strength | [69] |
| Carrageenan | Red algae (marine) | Thermoreversible gelation; sulfate groups for bioactivity | Mucosal drug delivery, wound dressing | Natural gelling; anti-inflammatory | Weak mechanical properties; low cell adhesion | [70] |
| Fucoidan | Brown algae (marine) | Anticoagulant, anti-inflammatory; ionic crosslinking | Cancer therapy, angiogenesis scaffolds | Bioactive; supports vascularization | Extraction variability; limited mechanical resilience | [71] |
| Pectin | Citrus/apple waste (plant) | Ionically crosslinkable with Ca2+; pH-sensitive | Colon-specific delivery, wound dressing | Biodegradable; widely available | Poor mechanical strength; fast degradation | [72] |
| CMC/HPMC | Cellulose derivative (plant) | Viscosity control; injectable as shear-thinning fluid | Ophthalmics, dermal fillers | Renewable; modifiable functional groups | Limited bioactivity; often inert | [73] |
| Oxidized Starch Derivatives | Corn/potato starch (plant) | Injectable; forms gels with polymers like PVA | Drug delivery, tissue adhesives | Eco-friendly; abundant source | Needs chemical functionalization for gelling | [74] |
| Gelation Mechanism | Trigger | Key Biopolymers | Advantages | Limitations | References |
|---|---|---|---|---|---|
| Physical Crosslinking | Temperature, pH, ionic strength | Gelatin, chitosan, alginate | Mild conditions, reversible, injectable, biocompatible | Poor mechanical strength, uncontrollable degradation | [100] |
| Chemical Crosslinking | Covalent bonding (photo, redox, click) | GelMA, HA-DA, PEG-aldehyde systems | Strong and stable gels, tunable mechanical properties | Toxic initiators, complex synthesis | [101] |
| Enzymatic Gelation | HRP/H2O2, tyrosinase, transglutaminase | Gelatin–phenol, HA–tyramine, fibrin | Biocompatible, site-specific, in situ gelation | Costly enzymes, sensitivity to enzymatic activity | [102] |
| Ionic Gelation | Multivalent ions (e.g., Ca2+, Fe3+) | Alginate, chitosan, carrageenan | Fast gelation, low toxicity, good for cell encapsulation | Weak mechanical integrity, ion leaching | [103] |
| Thermo-Responsive Systems | Body temperature (~37 °C) | Chitosan/β–GP, Pluronic F127, PNIPAAm hybrids | Injectable, sol-to-gel transition in vivo | Poor mechanical strength, unstable at elevated temperatures | [104] |
| Self-Healing Gels | Dynamic bonds (Schiff base, host–guest) | Oxidized alginate, HA–borate, gelatin–PEG hydrazone | Durable, injectable, regenerable network | Slow recovery in some cases, trade-off with strength | [105] |
| Shear-Thinning Gels | Mechanical shear | Chitosan blends, collagen, peptide hydrogels | Easily injectable, shape-conforming, ideal for bioprinting | Rapid breakdown under shear, recovery kinetics variability | [106] |
| Design Strategy | Description | Advantages | Challenges | Applications |
|---|---|---|---|---|
| Rheological and Mechanical Property Optimization | Optimizing viscosity, elasticity, and mechanical strength for ease of injection and functional support in tissue. | Improved injectability, tailored mechanical properties for specific tissues, enhanced post-injection integrity. | Balancing viscosity for injectability vs. mechanical strength, maintaining shear-thinning properties without compromising stability. | Cartilage repair, bone regeneration, neural scaffolds, tissue engineering. |
| Biodegradability and Biocompatibility | Ensuring the hydrogel degrades at a rate matching tissue healing and does not provoke an immune response. | Biocompatibility with minimal adverse reactions, controlled degradation for tissue integration. | Achieving controlled degradation without impeding healing; potential for long-term systemic effects if not properly designed. | Soft tissue regeneration, wound healing, in vivo drug delivery. |
| Incorporation of Bioactive Molecules | Loading the hydrogel with growth factors, peptides, or drugs to accelerate healing and promote cell behavior. | Enhanced tissue regeneration, targeted therapeutic effects, localized delivery of active compounds. | Maintaining bioactivity during encapsulation and release, potential for rapid clearance of bioactive agents. | Cancer therapy, bone and cartilage regeneration, stem cell therapies, gene therapy. |
| Controlled and Sustained Drug Release | Developing hydrogels that release drugs over extended periods with controlled kinetics. | Prolonged therapeutic effects, reduction in drug dosing frequency, reduced side effects. | Ensuring consistent release profile, preventing burst release, overcoming biological barriers to effective drug release. | Cancer therapy, chronic disease treatment, antimicrobial treatments, pain management. |
| 3D Structuring and Injectable Scaffolds | Creating 3D structures within hydrogels to mimic natural tissues and promote cell organization, migration, and regeneration. | Mimics natural tissue architecture, supports cell growth and vascularization, adaptable to irregular defects. | Complexity of 3D patterning, maintaining cell viability, optimizing scaffold degradation for long-term healing. | Cartilage repair, wound healing, skin regeneration, organ tissue engineering, personalized medicine. |
| Application Area | Hydrogel Type/Composition | Therapeutic Target | Delivery Mechanism | Advantages | Representative References |
|---|---|---|---|---|---|
| Cancer Therapeutics | HA-based, PEGylated chitosan, alginate–gelatin composites | Tumor-specific drug delivery (e.g., DOX, PTX) | Localized, pH/temperature-responsive in situ gelation | Reduced systemic toxicity, improved bioavailability | [190] |
| Anti-inflammatory and Antimicrobial | Chitosan–silver nanocomposites, alginate–AMP hydrogels | Inflammation, bacterial infections (e.g., wound beds) | Controlled release of anti-inflammatory or antimicrobial agents | Dual functionality: tissue regeneration and microbial suppression | [191] |
| Bone and Cartilage Regeneration | Collagen–hydroxyapatite, GelMA, alginate–BMP composites | Bone defects, cartilage repair | Injectable scaffold with osteoinductive molecules/cells | Supports osteogenesis/chondrogenesis, promotes matrix mineralization | [192] |
| Cardiac Tissue Engineering | HA–collagen, alginate–fibrin hybrid hydrogels | Myocardial infarction repair | Cell-laden or drug-loaded injectables post-MI | Enhances vascularization, promotes cardiomyocyte survival | [193] |
| Neural Tissue Engineering | Chitosan–GelMA, PEG–HA hybrid systems | Neural regeneration post-stroke or trauma | Injectable neuro-supportive matrix with growth factors | Reduces glial scarring, supports axonal growth and neural integration | [194] |
| Skin and Wound Healing | Gelatin/HA, chitosan–aloe vera, collagen–silver NP hydrogels | Chronic wounds, burns, diabetic ulcers | In situ gel with anti-microbial, anti-inflammatory agents | Promotes granulation, moisture retention, antimicrobial effects | [195] |
| Gene Delivery | PEI-functionalized gelatin, chitosan-DNA, HA-based nanoparticles | siRNA, plasmid DNA, miRNA delivery | Encapsulation or complexation within hydrogels | Protects genetic cargo, localized and sustained transfection | [196] |
| Cell Delivery (Stem/Progenitor) | GelMA, collagen–PEGDA, alginate–MSC encapsulation | Stem cell therapy for regenerative medicine | Injectable matrices for cell viability and localization | Enhances cell retention, survival, and paracrine signaling | [197] |
| Minimally Invasive Surgery | Thermosensitive chitosan, Pluronic-based composites | Postoperative drug release, tissue repair | In situ gelation in body temperature-responsive manner | Easy administration, adaptable to irregular defect geometries | [143] |
| Localized Therapy | Alginate–GelMA with targeted ligands | Site-specific chemotherapy or anti-inflammatories | Ligand-guided hydrogel deposition | Improves localization, lowers off-target effects | [198] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parvin, N.; Joo, S.W.; Mandal, T.K. Injectable Biopolymer-Based Hydrogels: A Next-Generation Platform for Minimally Invasive Therapeutics. Gels 2025, 11, 383. https://doi.org/10.3390/gels11060383
Parvin N, Joo SW, Mandal TK. Injectable Biopolymer-Based Hydrogels: A Next-Generation Platform for Minimally Invasive Therapeutics. Gels. 2025; 11(6):383. https://doi.org/10.3390/gels11060383
Chicago/Turabian StyleParvin, Nargish, Sang Woo Joo, and Tapas Kumar Mandal. 2025. "Injectable Biopolymer-Based Hydrogels: A Next-Generation Platform for Minimally Invasive Therapeutics" Gels 11, no. 6: 383. https://doi.org/10.3390/gels11060383
APA StyleParvin, N., Joo, S. W., & Mandal, T. K. (2025). Injectable Biopolymer-Based Hydrogels: A Next-Generation Platform for Minimally Invasive Therapeutics. Gels, 11(6), 383. https://doi.org/10.3390/gels11060383








