Dynamic Boronate Ester Based Hydrogel with Enhanced Mechanical Properties and Multi-Stimuli-Triggered Release for Tissue Repair and Antioxidant Therapy
Abstract
1. Introduction
2. Results and Discussion
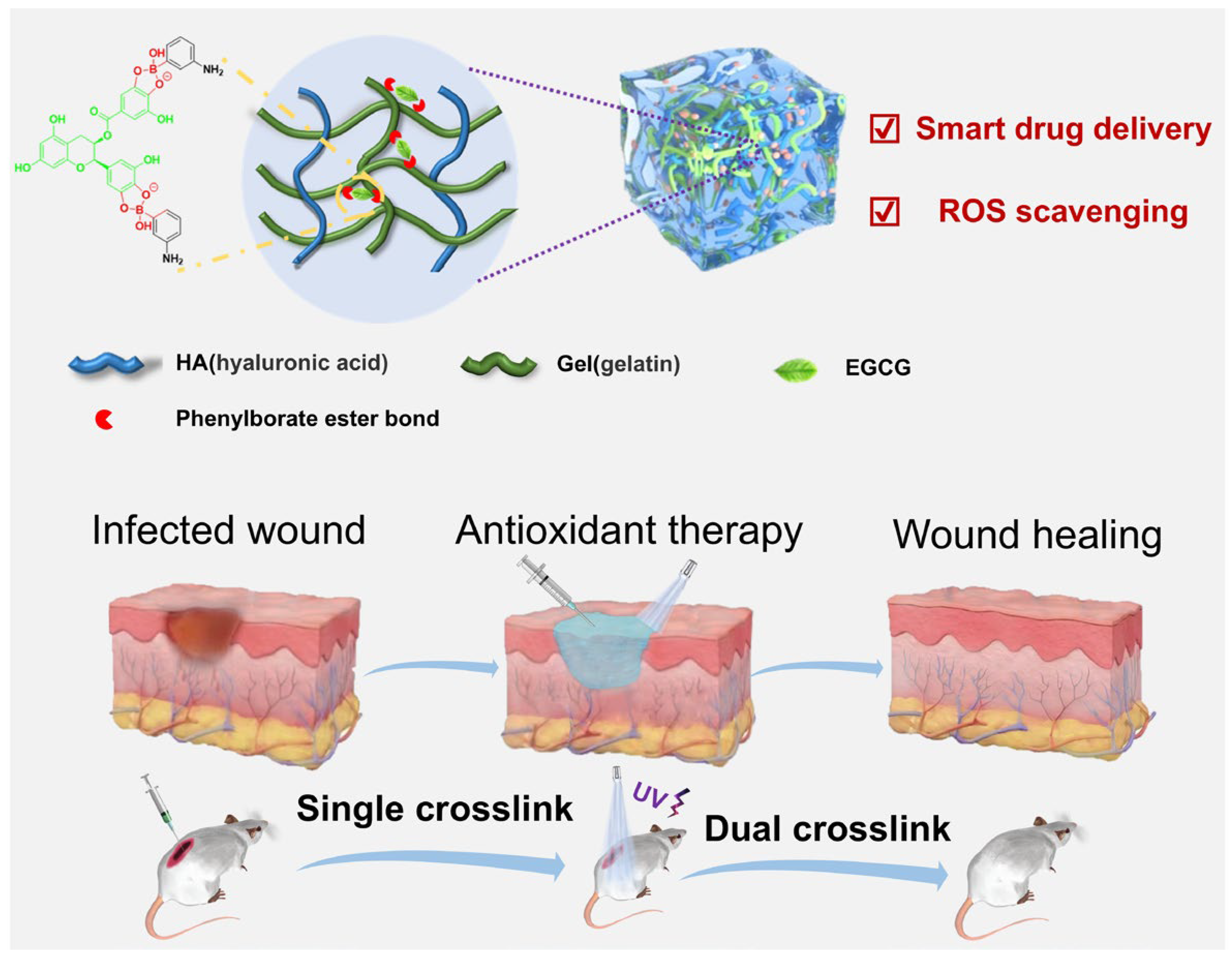
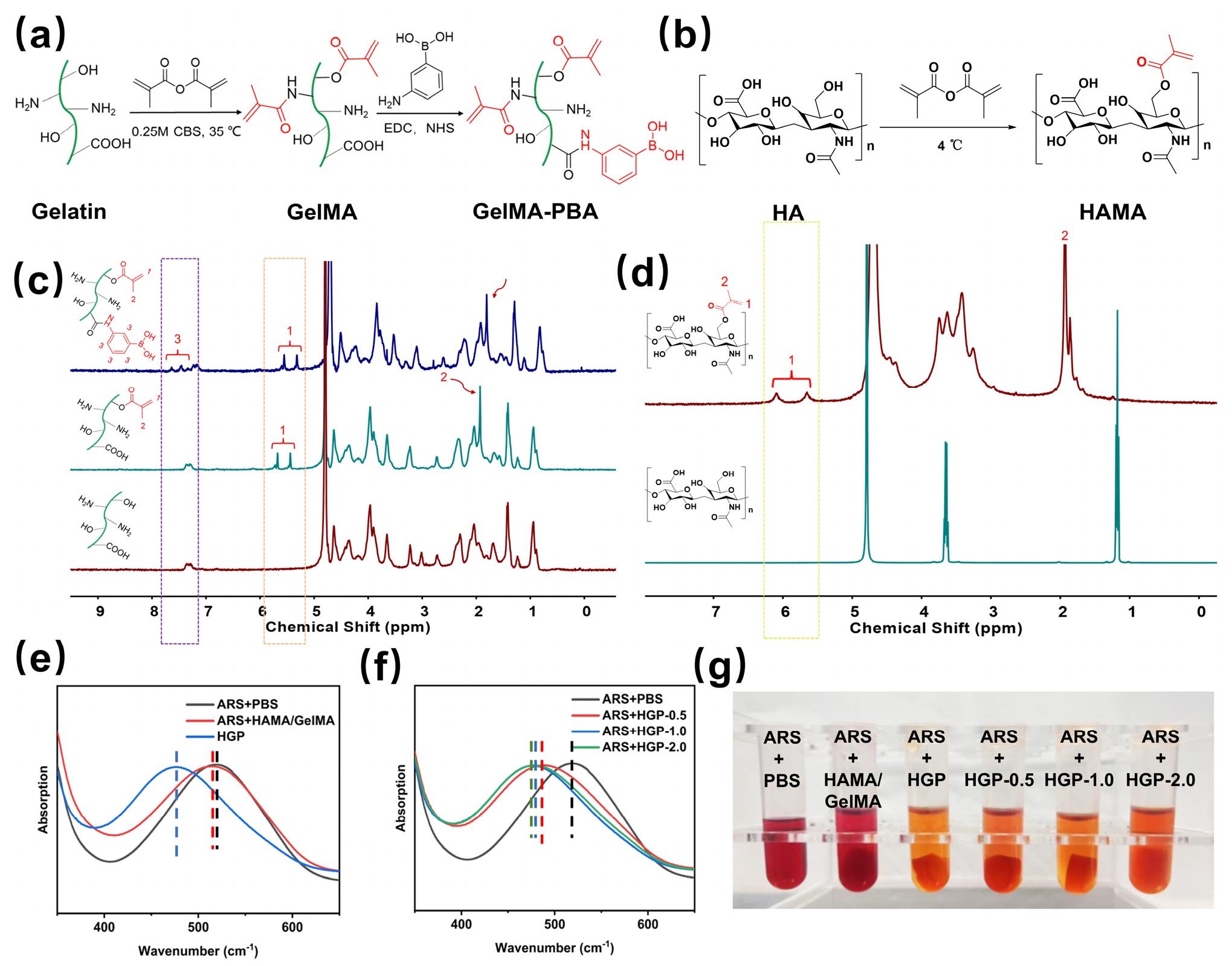
2.1. Design and Synthesis of the Hydrogels
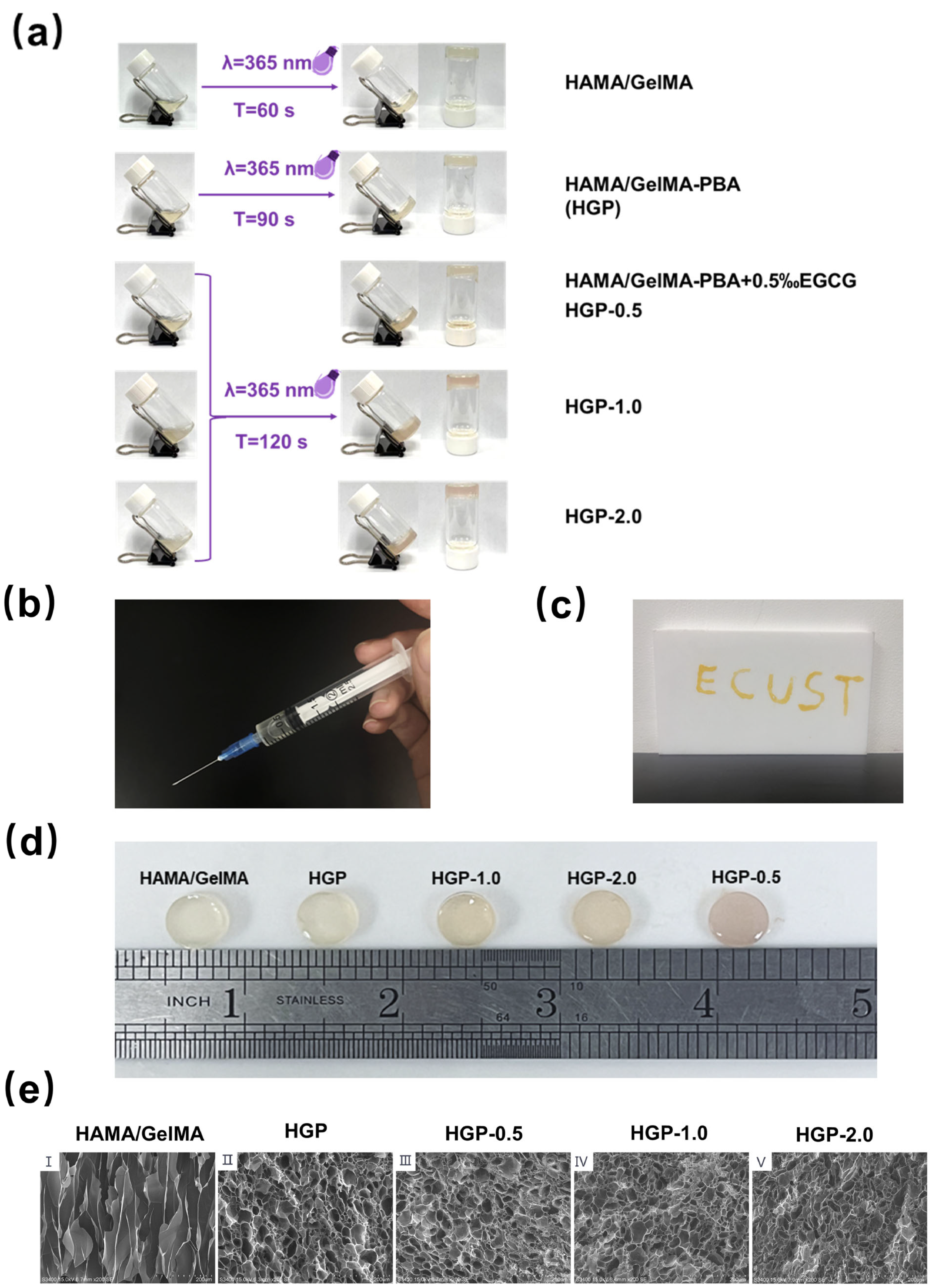
2.2. Morphology of Hydrogels
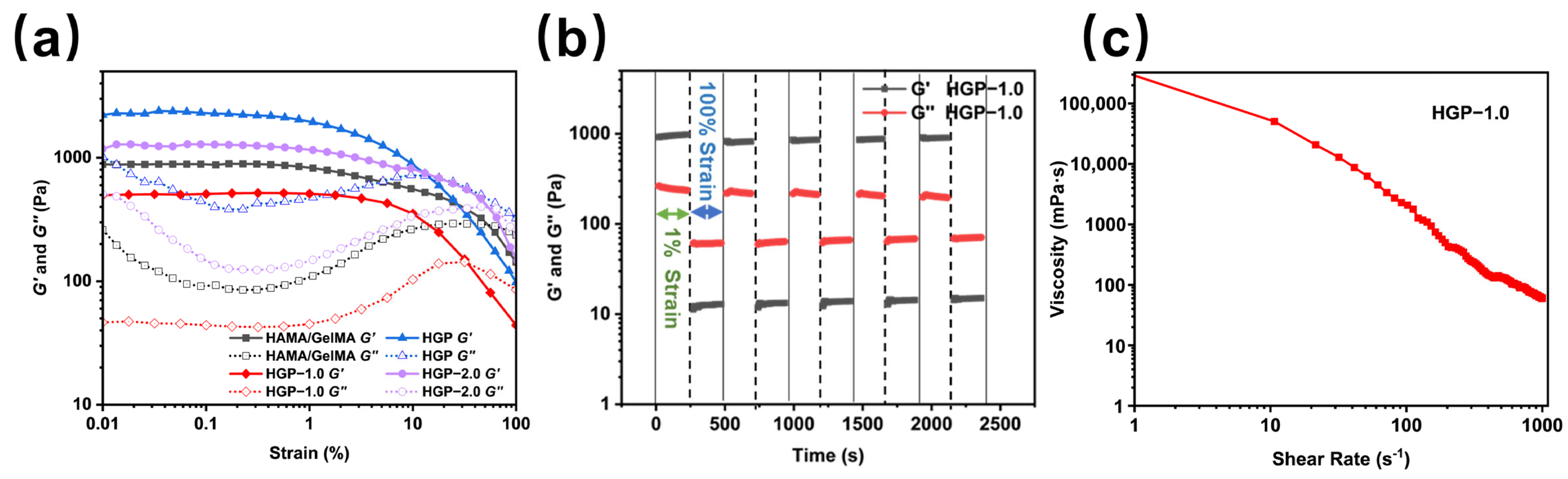
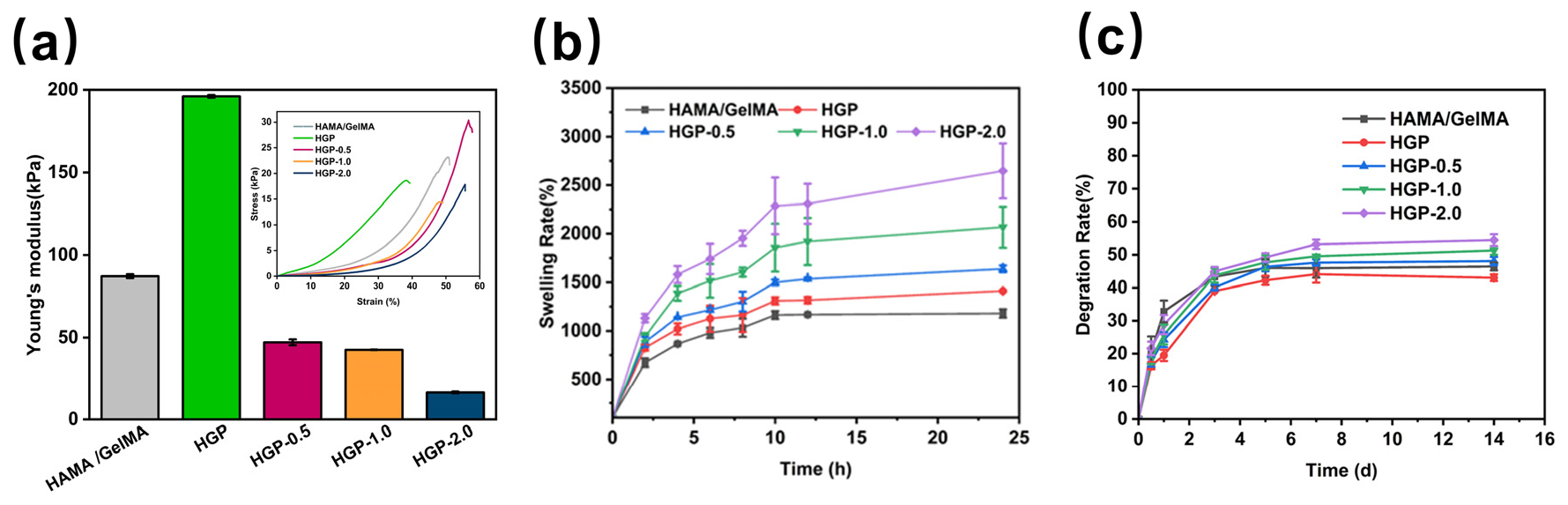
2.3. Rheological and Mechanical Properties of Hydrogels
2.4. Swelling and Degradation Behavior of Hydrogels
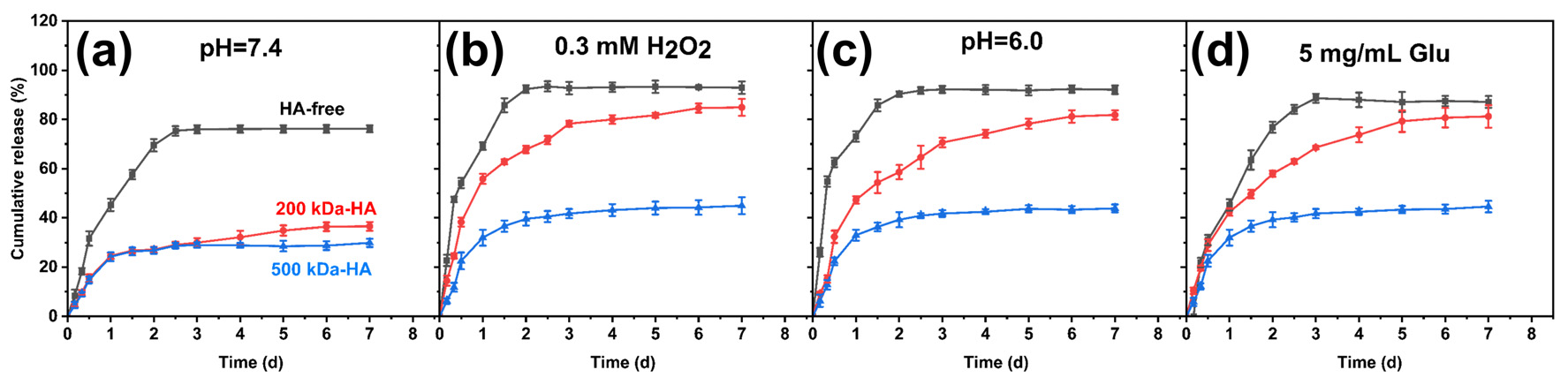
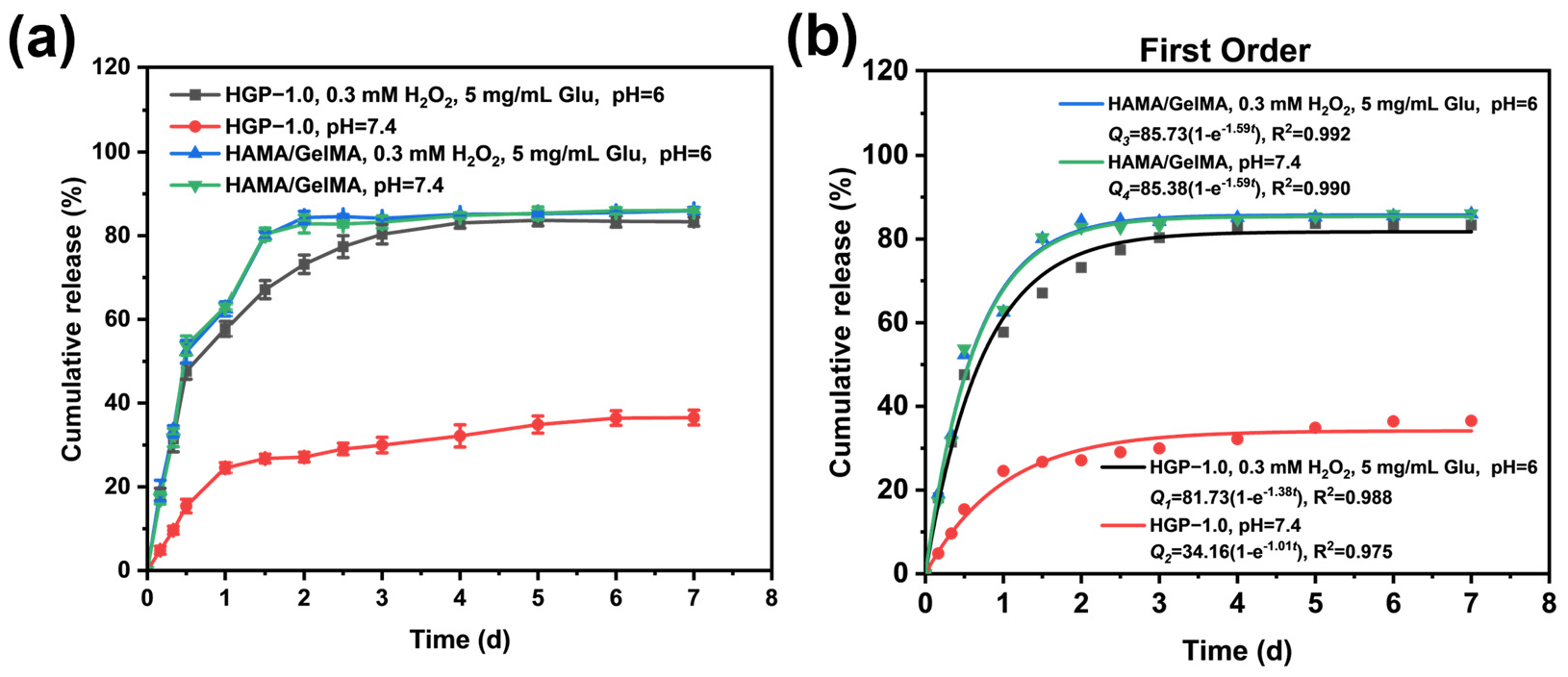
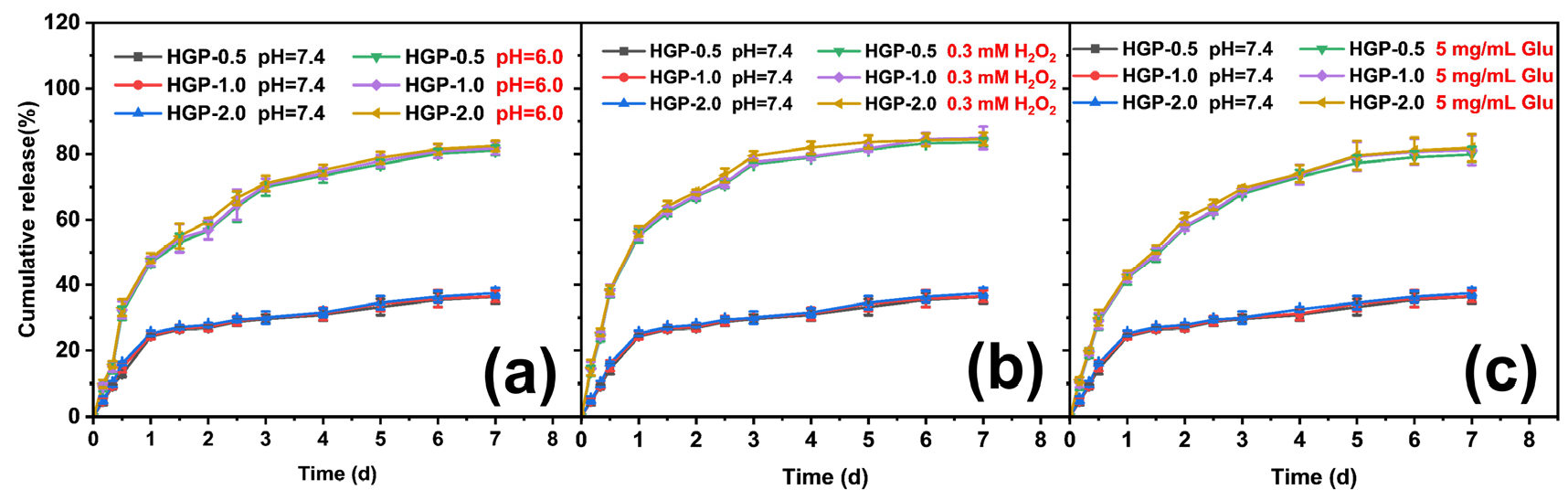
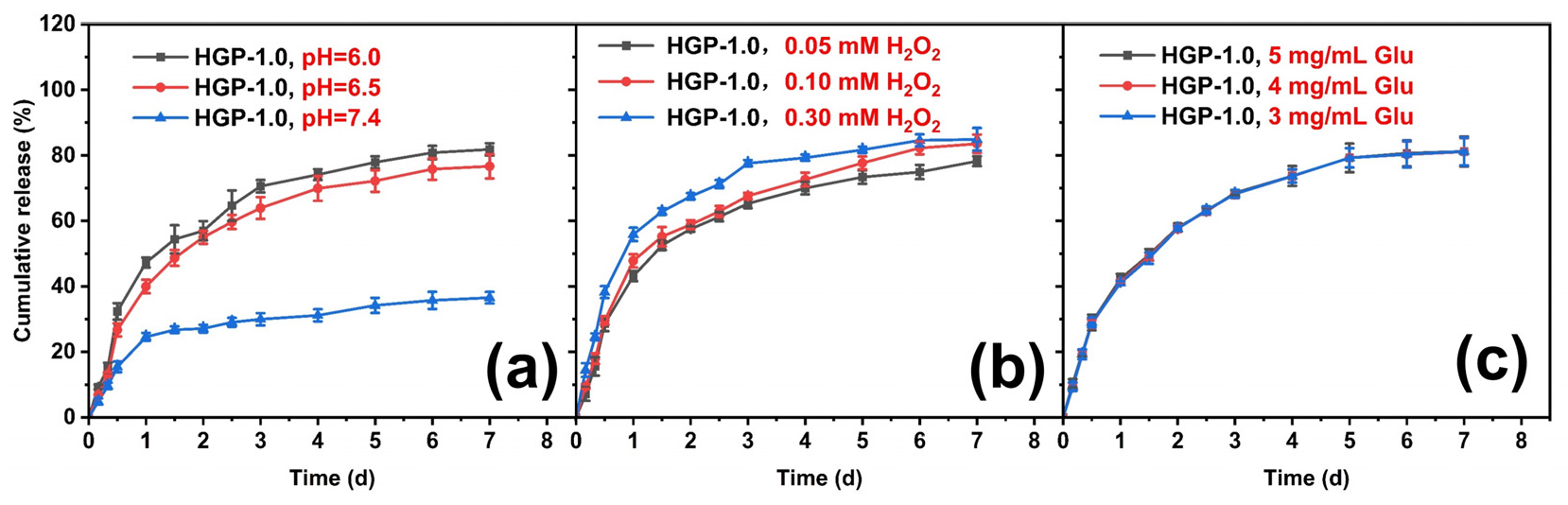
2.5. In Vitro Drug Release Behavior of Drug-Loaded Hydrogels
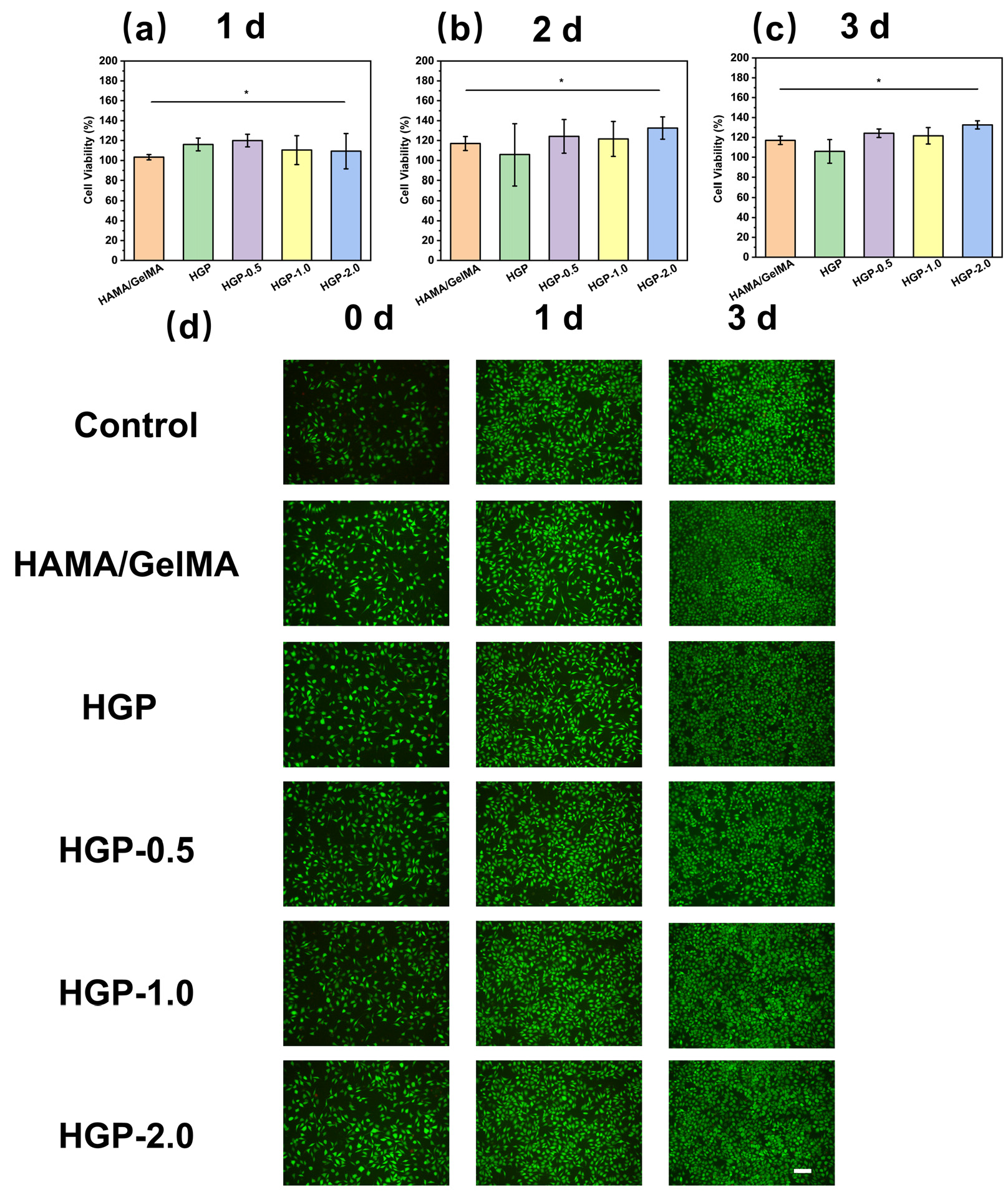
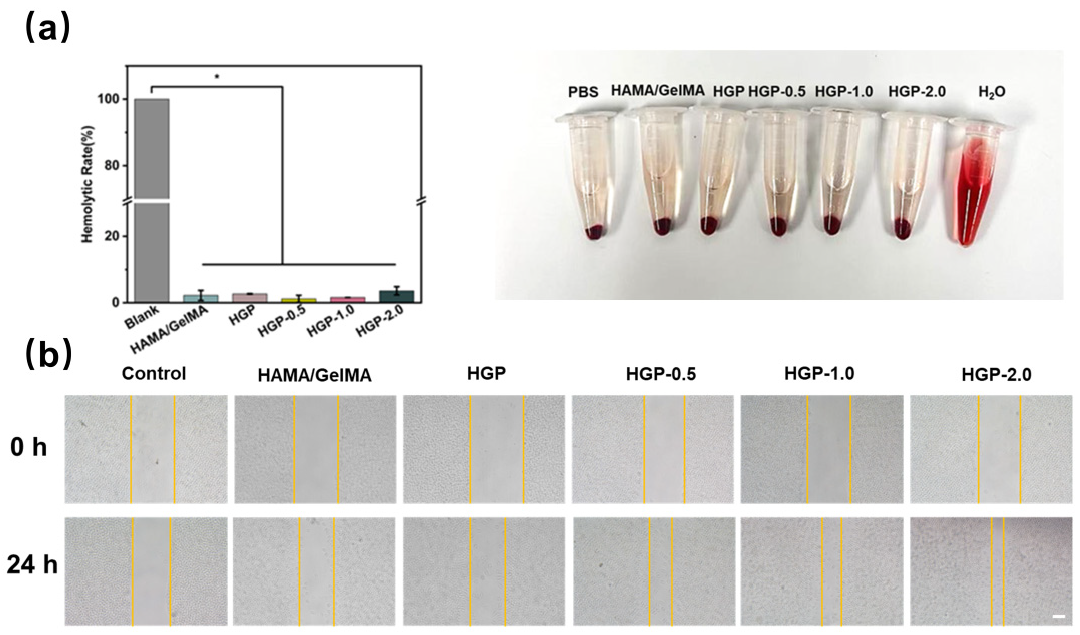
2.6. Biocompatibility of Hydrogels
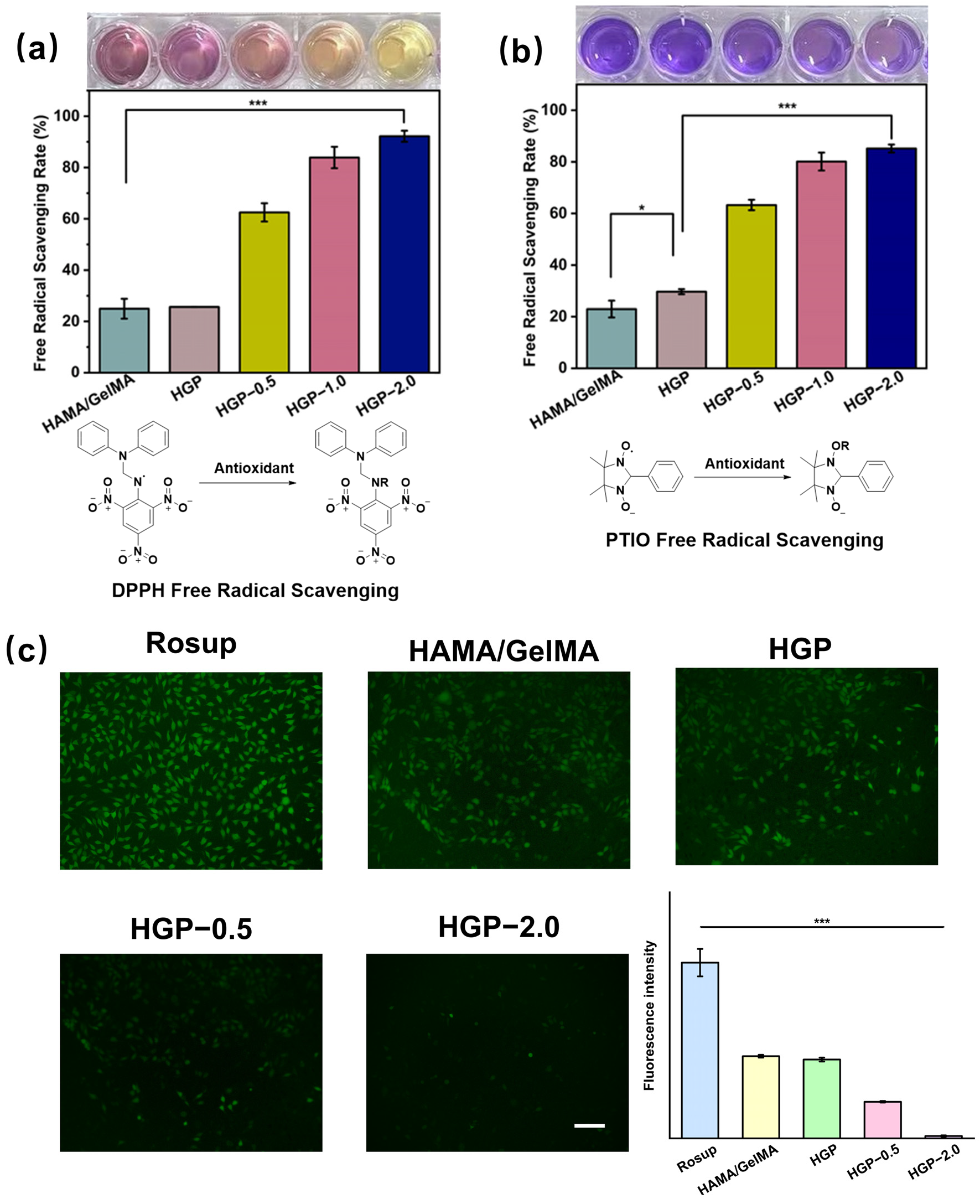
2.7. Antioxidant Efficiency of Hydrogels
3. Conclusions
4. Materials and Methods
4.1. Reagents and Materials
4.2. Synthesis and Characterization of Methacrylated Hyaluronic Acid (HAMA)
4.3. Synthesis and Characterization of Phenylmethacrylated Gelatin (GelMA-PBA)
4.4. Preparation of HAMA/GelMA and HGP Hydrogels
4.5. Physical Properties of HAMA/GelMA and HGP Hydrogels
4.6. Mechanical Properties of Hydrogels
4.7. Rheological Properties of Hydrogels
4.8. In Vitro Drug Release Behavior of Hydrogels
4.9. Cell Culture
4.10. Cell Compatibility and Hemolysis Tests
4.11. In Vitro Scratch Healing Assay
4.12. Free Radical Scavenging Ability of Hydrogels
4.13. Intracellular Antioxidant Ability Assay
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HA | Hyaluronic acid |
| PBA | Phenylboronic acid |
| MA | Methacrylic anhydride |
| ARS | Alizarin Red S |
| EGCG | Epigallocatechin gallate |
| EDC | 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide |
| NHS | N-Hydroxy succinimide |
| DPPH | 1,1-Diphenyl-2-picrylhydrazyl radical 2,2-Diphenyl-1-(2,4,6-trinitrophenyl) hydrazyl |
| PTIO | 2-Phenyl-4,4,5,5-tetramethylimidazoline-3-oxide-1-oxyl |
| DS | Degree of substitution |
| WVTR | Water vapor transmission rate |
| OD | Optical density |
| Cytc | Cytochrome c |
| SR | Swelling rate |
| DR | Degradation rate |
References
- Halliwell, B. Free Radicals and Antioxidants: Updating a Personal View. Nutr. Rev. 2012, 70, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Abdal Dayem, A.; Yan, E.; Do, M.; Kim, Y.; Lee, Y.; Cho, S.-G.; Kim, D.-H. Engineering Extracellular Vesicles for ROS Scavenging and Tissue Regeneration. Nano Converg. 2024, 11, 24. [Google Scholar] [CrossRef]
- He, X.; Xue, J.; Shi, L.; Kong, Y.; Zhan, Q.; Sun, Y.; Zhang, Q.; Ramakrishna, S.; Dai, Y. Recent Antioxidative Nanomaterials toward Wound Dressing and Disease Treatment via ROS Scavenging. Mater. Today Nano 2022, 17, 100149. [Google Scholar] [CrossRef]
- Liu, Z.; Xiao, T.S. Partners with a Killer: Metabolic Signaling Promotes Inflammatory Cell Death. Cell 2021, 184, 4374–4376. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Maruhashi, T.; Sakamoto, W.; Yogo, T. Organic–Inorganic Hybrid Hollow Nanoparticles Suppress Oxidative Stress and Repair Damaged Tissues for Treatment of Hepatic Fibrosis. Adv. Funct. Mater. 2018, 28, 1706332. [Google Scholar] [CrossRef]
- Ozawa, Y. Oxidative Stress in the Light-Exposed Retina and Its Implication in Age-Related Macular Degeneration. Redox Biol. 2020, 37, 101779. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, P.; Ding, Y.; Xie, X.; Fu, J.; Zhao, R.; Xiao, Y.; Lukic, M.J.; Li, B.; Wang, W.; et al. Bioactives from Biomass: Treasure for Future Potent Antimicrobial Applications. Chem. Eng. J. 2024, 499, 155669. [Google Scholar] [CrossRef]
- Mistry, S.; Roy, R.; Jha, A.K.; Pandit, N.; Das, S.; Burman, S.; Joy, M. Treatment of Long Bone Infection by a Biodegradable Bone Cement Releasing Antibiotics in Human. J. Control. Release 2022, 346, 180–192. [Google Scholar] [CrossRef]
- Oda, Y.; Takahashi, C.; Harada, S.; Nakamura, S.; Sun, D.; Kiso, K.; Urata, Y.; Miyachi, H.; Fujiyoshi, Y.; Honigmann, A.; et al. Discovery of Anti-Inflammatory Physiological Peptides That Promote Tissue Repair by Reinforcing Epithelial Barrier Formation. Sci. Adv. 2021, 7, eabj6895. [Google Scholar] [CrossRef]
- Wan, R.; Hussain, A.; Behfar, A.; Moran, S.L.; Zhao, C. The Therapeutic Potential of Exosomes in Soft Tissue Repair and Regeneration. Int. J. Mol. Sci. 2022, 23, 3869. [Google Scholar] [CrossRef]
- Gresham, R.C.H.; Bahney, C.S.; Leach, J.K. Growth Factor Delivery Using Extracellular Matrix-Mimicking Substrates for Musculoskeletal Tissue Engineering and Repair. Bioact. Mater. 2021, 6, 1945–1956. [Google Scholar] [CrossRef] [PubMed]
- Lord, M.; Whitelock, J.; Turnbull, J.E. Better Growth-Factor Binding Aids Tissue Repair. Nat. Biomed. Eng. 2020, 4, 368–369. [Google Scholar] [CrossRef] [PubMed]
- Kurt Yilmaz, N.; Schiffer, C.A. Introduction: Drug Resistance. Chem. Rev. 2021, 121, 3235–3237. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cao, G.; Wang, Z.; Liu, D.; Ren, L.; Ma, D. The Recent Progress of Bone Regeneration Materials Containing EGCG. J. Mater. Chem. B 2024, 12, 9835–9844. [Google Scholar] [CrossRef]
- Zawani, M.; Fauzi, M. Epigallocatechin Gallate: The Emerging Wound Healing Potential of Multifunctional Biomaterials for Future Precision Medicine Treatment Strategies. Polymers 2021, 13, 3656. [Google Scholar] [CrossRef]
- Huang, H.-T.; Cheng, T.-L.; Yang, C.-D.; Chang, C.-F.; Ho, C.-J.; Chuang, S.-C.; Li, J.-Y.; Huang, S.-H.; Lin, Y.-S.; Shen, H.-Y.; et al. Intra-Articular Injection of (−)-Epigallocatechin 3-Gallate (EGCG) Ameliorates Cartilage Degeneration in Guinea Pigs with Spontaneous Osteoarthritis. Antioxidants 2021, 10, 178. [Google Scholar] [CrossRef]
- Kim, Y.; Baek, Y.; Jeong, E.; Lee, H.G. Development of Gelatinized-Core Liposomes for the Oral Delivery of EGCG with Improved Stability, Release Property, and Cellular Antioxidant Activity. Colloids Surf. B Biointerfaces 2024, 234, 113723. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Liu, N.; Feng, D.; Wu, W.; Gu, K.; Wu, A.; Li, C.; Wang, X. Multi-Mechanism Antitumor/Antibacterial Effects of Cu-EGCG Self-Assembling Nanocomposite in Tumor Nanotherapy and Drug-Resistant Bacterial Wound Infections. J. Colloid Interface Sci. 2024, 671, 751–769. [Google Scholar] [CrossRef]
- Talib, W.H.; Awajan, D.; Alqudah, A.; Alsawwaf, R.; Althunibat, R.; Abu AlRoos, M.; Al Safadi, A.; Abu Asab, S.; Hadi, R.W.; Al Kury, L.T. Targeting Cancer Hallmarks with Epigallocatechin Gallate (EGCG): Mechanistic Basis and Therapeutic Targets. Molecules 2024, 29, 1373. [Google Scholar] [CrossRef]
- Yin, X.; Huang, S.; Xu, S.; Chang, L.; Zhao, X.; Chen, Z.; Mei, X.; Gao, X. Preparation of Pro-Angiogenic, Antibacterial and EGCG-Modified ZnO Quantum Dots for Treating Bacterial Infected Wound of Diabetic Rats. Biomater. Adv. 2022, 133, 112638. [Google Scholar] [CrossRef]
- Kar, A.K.; Singh, A.; Dhiman, N.; Purohit, M.P.; Jagdale, P.; Kamthan, M.; Singh, D.; Kumar, M.; Ghosh, D.; Patnaik, S. Polymer-Assisted In Situ Synthesis of Silver Nanoparticles with Epigallocatechin Gallate (EGCG) Impregnated Wound Patch Potentiate Controlled Inflammatory Responses for Brisk Wound Healing. Int. J. Nanomed. 2019, 14, 9837–9854. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; You, Y.; Yang, Y.; Liu, X.; Yang, L.; Li, Y.; Zhang, C.; Yang, H.; Sha, Z.; Ma, Y.; et al. Multifunctional EGCG@ZIF-8 Nanoplatform with Photodynamic Therapy/Chemodynamic Therapy Antibacterial Properties Promotes Infected Wound Healing. ACS Appl. Mater. Interfaces 2024, 16, 50238–50250. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.A.; Pinheiro, M.; Granja, A.; Farabegoli, F.; Reis, S.; Attar, R.; Sabitaliyevich, U.Y.; Xu, B.; Ahmad, A. EGCG Mediated Targeting of Deregulated Signaling Pathways and Non-Coding RNAs in Different Cancers: Focus on JAK/STAT, Wnt/β-Catenin, TGF/SMAD, NOTCH, SHH/GLI, and TRAIL Mediated Signaling Pathways. Cancers 2020, 12, 951. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ding, C.; Qin, M.; Li, J. Hydrogel-Based Biosensors for Bacterial Infections. Small 2024, 20, 2306960. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Liu, J.; Wang, C.; Ouyang, Z.; Kuang, J.; Pang, Q.; Fan, R. Sex-Specific Oxidative Damage Effects Induced by BPA and Its Analogs on Primary Hippocampal Neurons Attenuated by EGCG. Chemosphere 2021, 264, 128450. [Google Scholar] [CrossRef]
- Chen, X.; Liu, B.; Tong, R.; Ding, S.; Wu, J.; Lei, Q.; Fang, W. Improved Stability and Targeted Cytotoxicity of Epigallocatechin-3-Gallate Palmitate for Anticancer Therapy. Langmuir 2021, 37, 969–977. [Google Scholar] [CrossRef]
- Dai, W.; Ruan, C.; Zhang, Y.; Wang, J.; Han, J.; Shao, Z.; Sun, Y.; Liang, J. Bioavailability Enhancement of EGCG by Structural Modification and Nano-Delivery: A Review. J. Funct. Foods 2020, 65, 103732. [Google Scholar] [CrossRef]
- Terriac, L.; Helesbeux, J.-J.; Maugars, Y.; Guicheux, J.; Tibbitt, M.W.; Delplace, V. Boronate Ester Hydrogels for Biomedical Applications: Challenges and Opportunities. Chem. Mater. 2024, 36, 6674–6695. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Stojanović, G.M.; Abdullah, M.F.B.; Dolatshahi-Pirouz, A.; Marei, H.E.; Ashammakhi, N.; Hasan, A. Fundamental Properties of Smart Hydrogels for Tissue Engineering Applications: A Review. Int. J. Biol. Macromol. 2024, 254, 127882. [Google Scholar] [CrossRef]
- Nasra, S.; Patel, M.; Shukla, H.; Bhatt, M.; Kumar, A. Functional Hydrogel-Based Wound Dressings: A Review on Biocompatibility and Therapeutic Efficacy. Life Sci. 2023, 334, 122232. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cai, L.; Fan, L.; Wang, L.; Bian, F.; Sun, W.; Zhao, Y. Electrical Microneedles for Wound Treatment. Adv. Sci. 2024, e2409519. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, J.; Wang, X.; Meng, Y.; Feng, X.; Liu, G.; Wang, D.-A.; Fan, C. Porous Materials with Water-Triggered Instant Transformation to Robust Hydrogel Adhesives for Rapid Hemostasis of High-Pressure Hemorrhages. Adv. Funct. Mater. 2024, 35, 2419456. [Google Scholar] [CrossRef]
- Catanzano, O.; Soriente, A.; La Gatta, A.; Cammarota, M.; Ricci, G.; Fasolino, I.; Schiraldi, C.; Ambrosio, L.; Malinconico, M.; Laurienzo, P.; et al. Macroporous Alginate Foams Crosslinked with Strontium for Bone Tissue Engineering. Carbohydr. Polym. 2018, 202, 72–83. [Google Scholar] [CrossRef]
- Wang, L.; Mei, Z.; Jin, G.; Liu, H.; Lv, S.; Fu, R.; Li, M.; Yao, C. In Situ Sustained Release Hydrogel System Delivering GLUT1 Inhibitor and Chemo-Drug for Cancer Post-Surgical Treatment. Bioact. Mater. 2024, 36, 541–550. [Google Scholar] [CrossRef]
- Pang, S.; Wu, R.; Lv, W.; Zou, J.; Li, Y.; Li, Y.; Zhang, P.; Ma, X.; Wang, Y.; Liu, S. Use of a pH-Responsive Imatinib Mesylate Sustained-Release Hydrogel for the Treatment of Tendon Adhesion by Inhibiting PDGFRβ/CLDN1 Pathway. Bioact. Mater. 2024, 38, 124–136. [Google Scholar] [CrossRef]
- Liang, X.; Ding, L.; Ma, J.; Li, J.; Cao, L.; Liu, H.; Teng, M.; Li, Z.; Peng, Y.; Chen, H.; et al. Enhanced Mechanical Strength and Sustained Drug Release in Carrier-Free Silver-Coordinated Anthraquinone Natural Antibacterial Anti-Inflammatory Hydrogel for Infectious Wound Healing. Adv. Healthc. Mater. 2024, 13, 2400841. [Google Scholar] [CrossRef]
- Steverink, J.G.; Van Tol, F.R.; Oosterman, B.J.; Vermonden, T.; Verlaan, J.-J.; Malda, J.; Piluso, S. Robust Gelatin Hydrogels for Local Sustained Release of Bupivacaine Following Spinal Surgery. Acta Biomater. 2022, 146, 145–158. [Google Scholar] [CrossRef]
- Cho, S.; Hwang, S.Y.; Oh, D.X.; Park, J. Recent Progress in Self-Healing Polymers and Hydrogels Based on Reversible Dynamic B–O Bonds: Boronic/Boronate Esters, Borax, and Benzoxaborole. J. Mater. Chem. A 2021, 9, 14630–14655. [Google Scholar] [CrossRef]
- Narkar, A.R.; Lee, B.P. Incorporation of Anionic Monomer to Tune the Reversible Catechol–Boronate Complex for pH-Responsive, Reversible Adhesion. Langmuir 2018, 34, 9410–9417. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, Y.; Gong, Y.; Shi, X.; Xiao, D.; Ren, L.; Dai, X.; Zeng, Z.; Zhao, C. A ROS-Responsive and Scavenging Hydrogel for Postoperative Abdominal Adhesion Prevention. Acta Biomater. 2024, 184, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, W.; Xiao, J.; Yang, R.; Feng, L.; Xu, H.; Xu, L.; Xing, Y. ROS-Responsive Injectable Hydrogels Loaded with Exosomes Carrying miR-4500 Reverse Liver Fibrosis. Biomaterials 2025, 314, 122887. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Nouseen, S.; Saroj, S.; Shegane, M.; Majumder, P.; Puri, A.; Rakshit, T.; Manna, D.; Pal, S. Repurposing Pinacol Esters of Boronic Acids for Tuning Viscoelastic Properties of Glucose-Responsive Polymer Hydrogels: Effects on Insulin Release Kinetics. J. Mater. Chem. B 2022, 10, 7591–7599. [Google Scholar] [CrossRef]
- Maity, B.; Moorthy, H.; Govindaraju, T. Glucose-Responsive Self-Regulated Injectable Silk Fibroin Hydrogel for Controlled Insulin Delivery. ACS Appl. Mater. Interfaces 2023, 15, 49953–49963. [Google Scholar] [CrossRef]
- Chen, F.; Qin, J.; Wu, P.; Gao, W.; Sun, G. Glucose-Responsive Antioxidant Hydrogel Accelerates Diabetic Wound Healing. Adv. Healthc. Mater. 2023, 12, 2300074. [Google Scholar] [CrossRef] [PubMed]
- Wahlsten, A.; Stracuzzi, A.; Lüchtefeld, I.; Restivo, G.; Lindenblatt, N.; Giampietro, C.; Ehret, A.E.; Mazza, E. Multiscale Mechanical Analysis of the Elastic Modulus of Skin. Acta Biomater. 2023, 170, 155–168. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, G.; Li, Q.; Wu, J. A Novel Hydrogel with Glucose-Responsive Hyperglycemia Regulation and Antioxidant Activity for Enhanced Diabetic Wound Repair. Nano Res. 2022, 15, 5305–5315. [Google Scholar] [CrossRef]
- Tang, H.; Chu, W.; Xiong, J.; Wu, H.; Cheng, L.; Cheng, L.; Luo, J.; Yin, H.; Li, J.; Li, J.; et al. Seeking Cells, Targeting Bacteria: A Cascade-Targeting Bacteria-Responsive Nanosystem for Combating Intracellular Bacterial Infections. Small 2024, 20, 2311967. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, Y.; Ji, Z.; Feng, J.; Shu, L.; Xiao, S.; Huang, Z. Wound Microenvironment Sensing and Self-Adjusting Hydrogel with Glucose, ROS, and MMP-9 Responsiveness for Improving Microcirculation of Diabetes Foot Ulcers. Chem. Eng. J. 2025, 505, 159537. [Google Scholar] [CrossRef]
- Mohsin, M.E.A.; Siddiqa, A.J.; Mousa, S.; Shrivastava, N.K. Design, Characterization, and Release Kinetics of a Hybrid Hydrogel Drug Delivery System for Sustained Hormone Therapy. Polymers 2025, 17, 999. [Google Scholar] [CrossRef]
- Sahu, K.M.; Biswal, A.; Manisha, U.; Swain, S.K. Synthesis and Drug Release Kinetics of Ciprofloxacin from Polyacrylamide/Dextran/Carbon Quantum Dots (PAM/Dex/CQD) Hydrogels. Int. J. Biol. Macromol. 2024, 269, 132132. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, G.; Liu, P.; Hu, Y.; Chen, Y.; Fang, Y.; Sun, G.; Huang, H.; Wu, J. Hyaluronic Acid-Based Glucose-Responsive Antioxidant Hydrogel Platform for Enhanced Diabetic Wound Repair. Acta Biomater. 2022, 147, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zeng, W.; Xu, P.; Fu, X.; Yu, X.; Chen, L.; Leng, F.; Yu, C.; Yang, Z. Glucose-Responsive Multifunctional Metal–Organic Drug-Loaded Hydrogel for Diabetic Wound Healing. Acta Biomater. 2022, 140, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yamanaka, J.; Sato, S.; Miyama, I.; Yonese, M. Characteristics of Complexes Composed of Sodium Hyaluronate and Bovine Serum Albumin. Chem. Pharm. Bull. 2000, 48, 779–783. [Google Scholar] [CrossRef]

| Name | HAMA (mg/mL) | GelMA (mg/mL) | GelMA-PBA (mg/mL) | EGCG (mg/mL) |
|---|---|---|---|---|
| HAMA/GelMA | 10 | 100 | 0 | 0 |
| HGP | 10 | 0 | 100 | 0 |
| HGP-0.5 | 10 | 0 | 100 | 0.5 |
| HGP-1.0 | 10 | 0 | 100 | 1 |
| HGP-2.0 | 10 | 0 | 100 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Li, G.; An, Z.; Wang, S.; Xu, S.; Liu, H. Dynamic Boronate Ester Based Hydrogel with Enhanced Mechanical Properties and Multi-Stimuli-Triggered Release for Tissue Repair and Antioxidant Therapy. Gels 2025, 11, 370. https://doi.org/10.3390/gels11050370
Liu F, Li G, An Z, Wang S, Xu S, Liu H. Dynamic Boronate Ester Based Hydrogel with Enhanced Mechanical Properties and Multi-Stimuli-Triggered Release for Tissue Repair and Antioxidant Therapy. Gels. 2025; 11(5):370. https://doi.org/10.3390/gels11050370
Chicago/Turabian StyleLiu, Fangyi, Gaoyang Li, Zhenhui An, Sijia Wang, Shouhong Xu, and Honglai Liu. 2025. "Dynamic Boronate Ester Based Hydrogel with Enhanced Mechanical Properties and Multi-Stimuli-Triggered Release for Tissue Repair and Antioxidant Therapy" Gels 11, no. 5: 370. https://doi.org/10.3390/gels11050370
APA StyleLiu, F., Li, G., An, Z., Wang, S., Xu, S., & Liu, H. (2025). Dynamic Boronate Ester Based Hydrogel with Enhanced Mechanical Properties and Multi-Stimuli-Triggered Release for Tissue Repair and Antioxidant Therapy. Gels, 11(5), 370. https://doi.org/10.3390/gels11050370






