Research and Development of a High-Temperature-Resistant, Gel-Breaking Chemical Gel Plugging Agent and Evaluation of Its Physicochemical Properties
Abstract
1. Introduction
2. Construction of Chemical Gel Plugging Agent System
2.1. Selection of Polymer Type and Concentration
2.1.1. Molecular Weight and Hydrolysis Degree of Different Types of Polymers
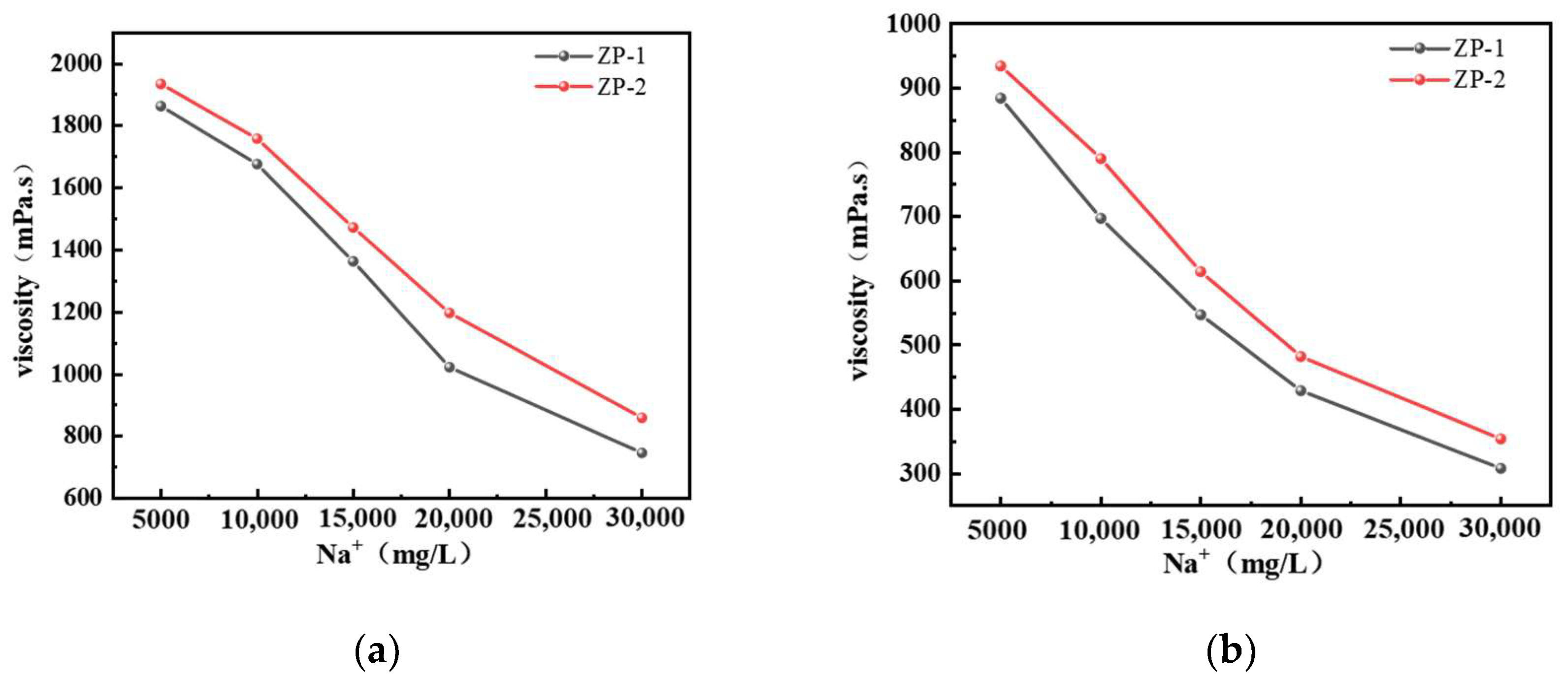
2.1.2. Salt Resistance of Different Types of Polymer Solutions
2.1.3. TemperatureResistance of Different Polymer Solutions
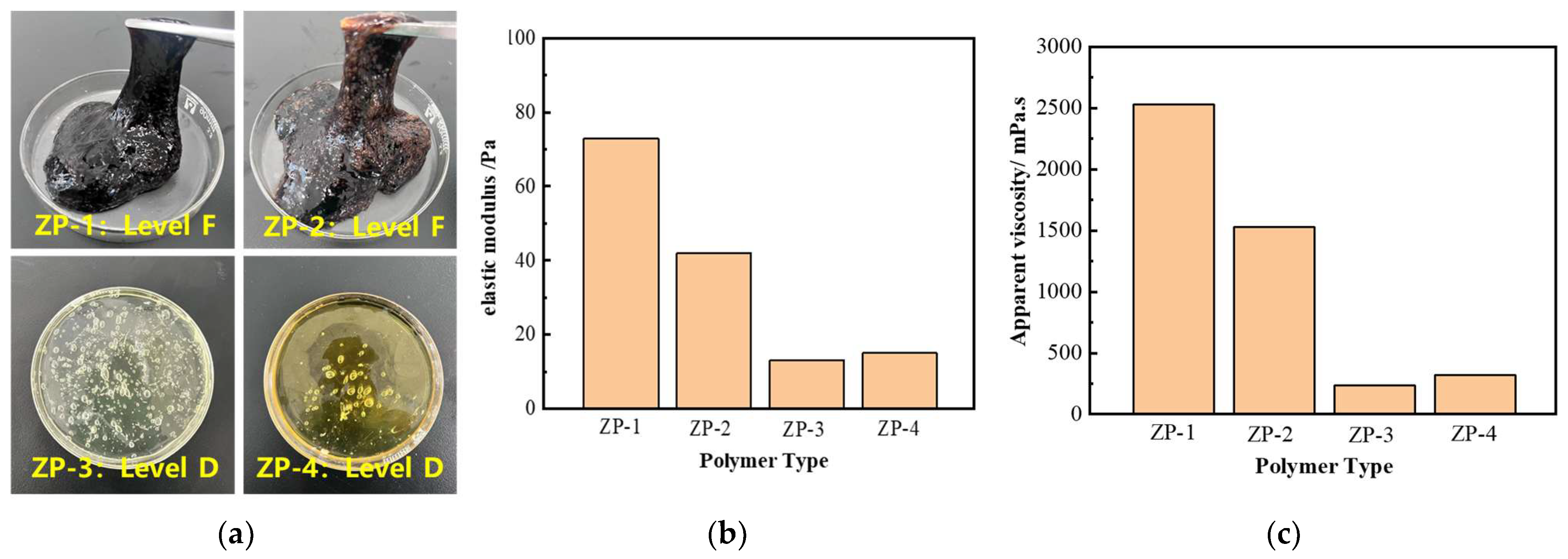
2.1.4. The Effect of Polymer Blends on Viscosity
2.1.5. Optimization of Polymer Concentration and Type for Gel Composition
2.2. Crosslinking Agent Type and Concentration Optimization
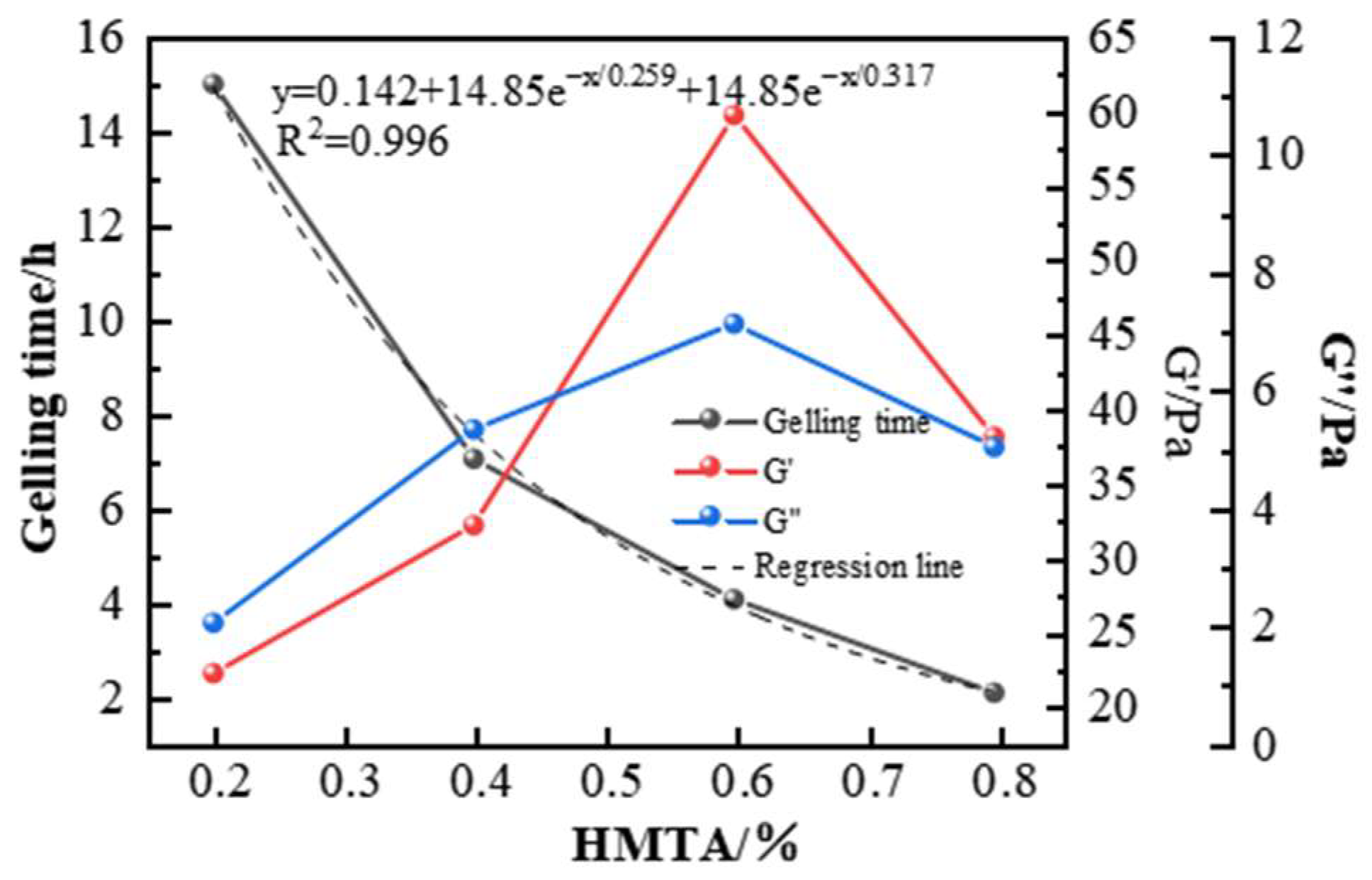
2.2.1. Selection of Aldehyde Crosslinking Agents
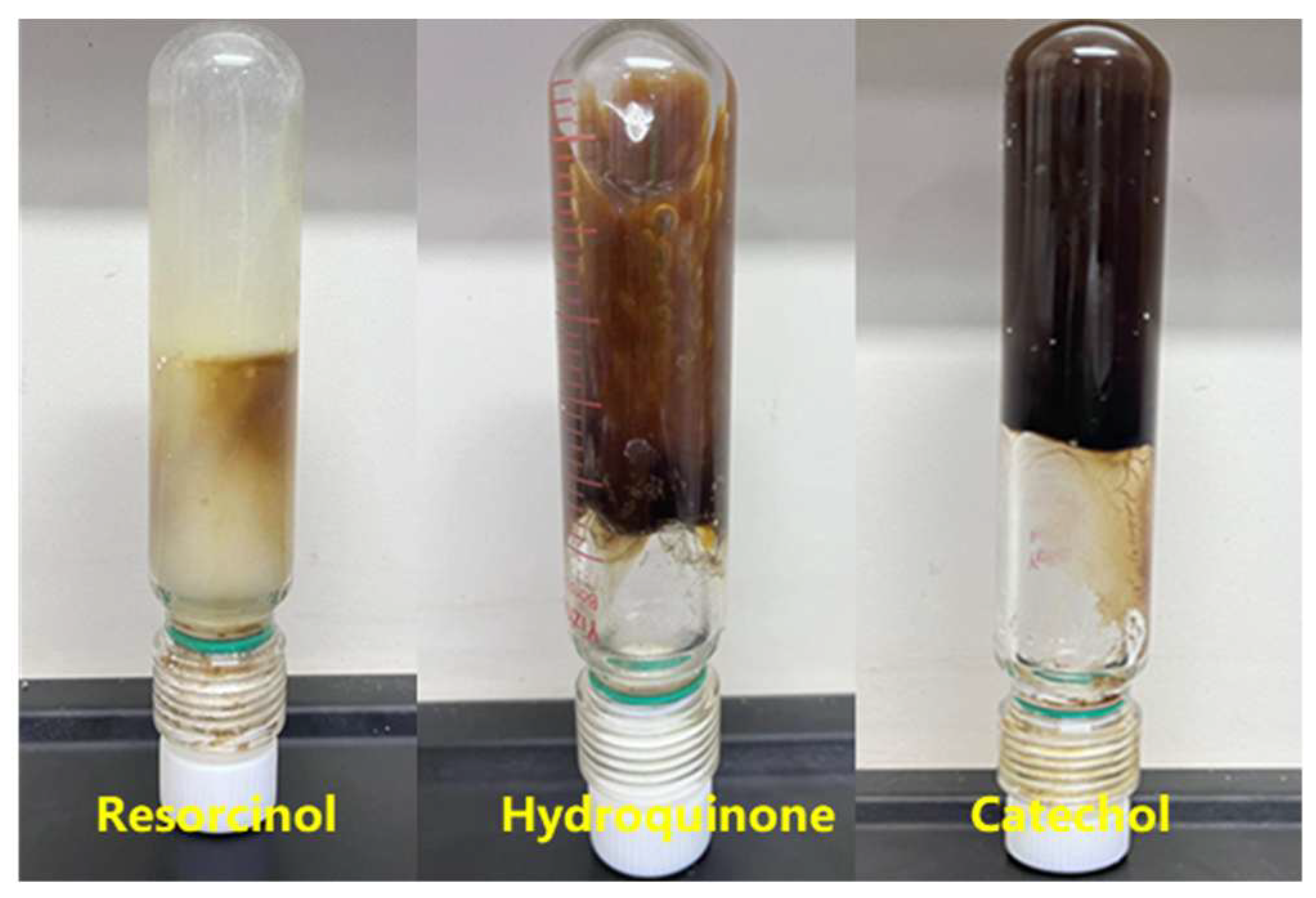
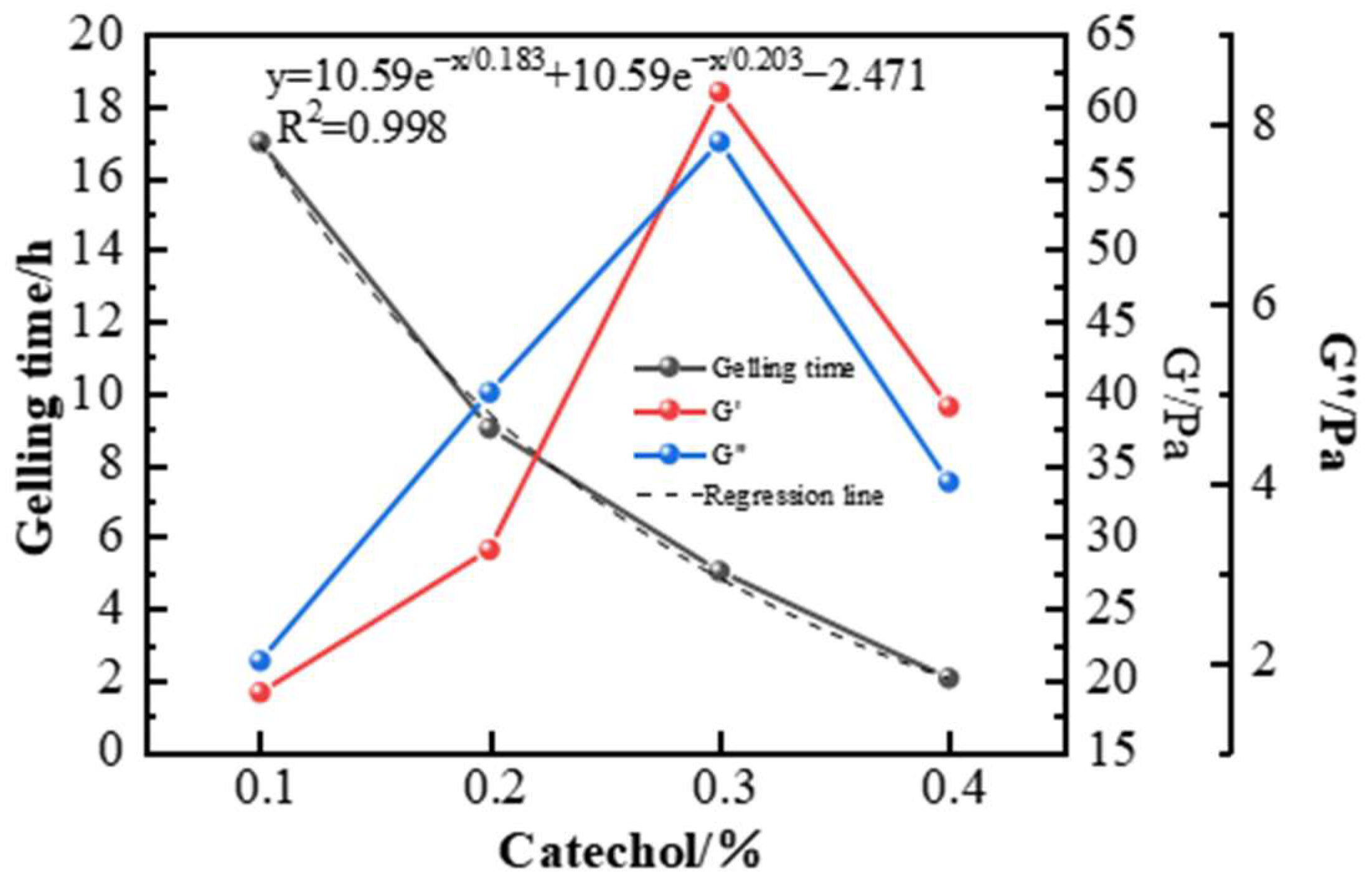
2.2.2. Phenolic Crosslinking Agent Selection
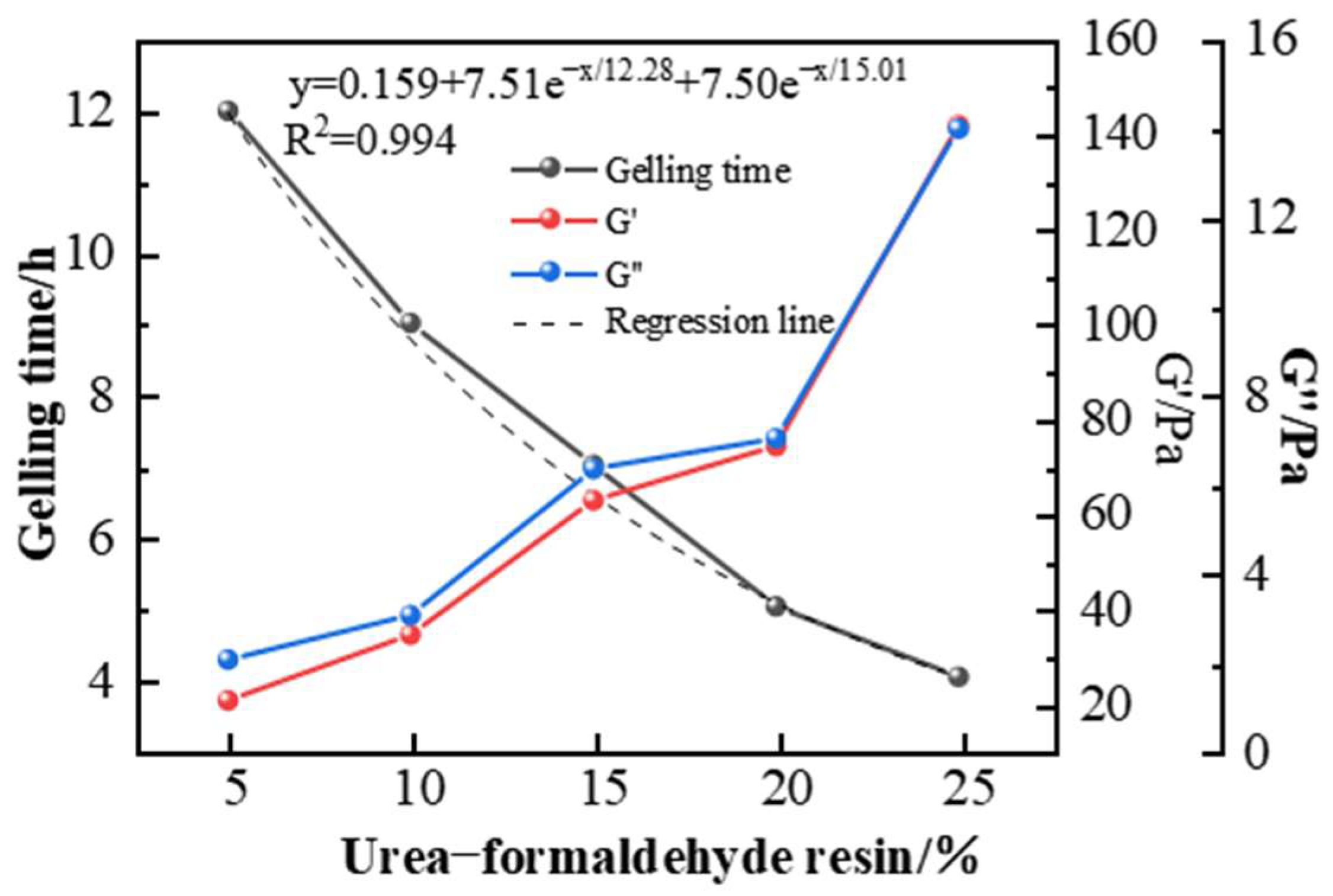
2.3. Selection of Resin Curing Agent Type and Concentration
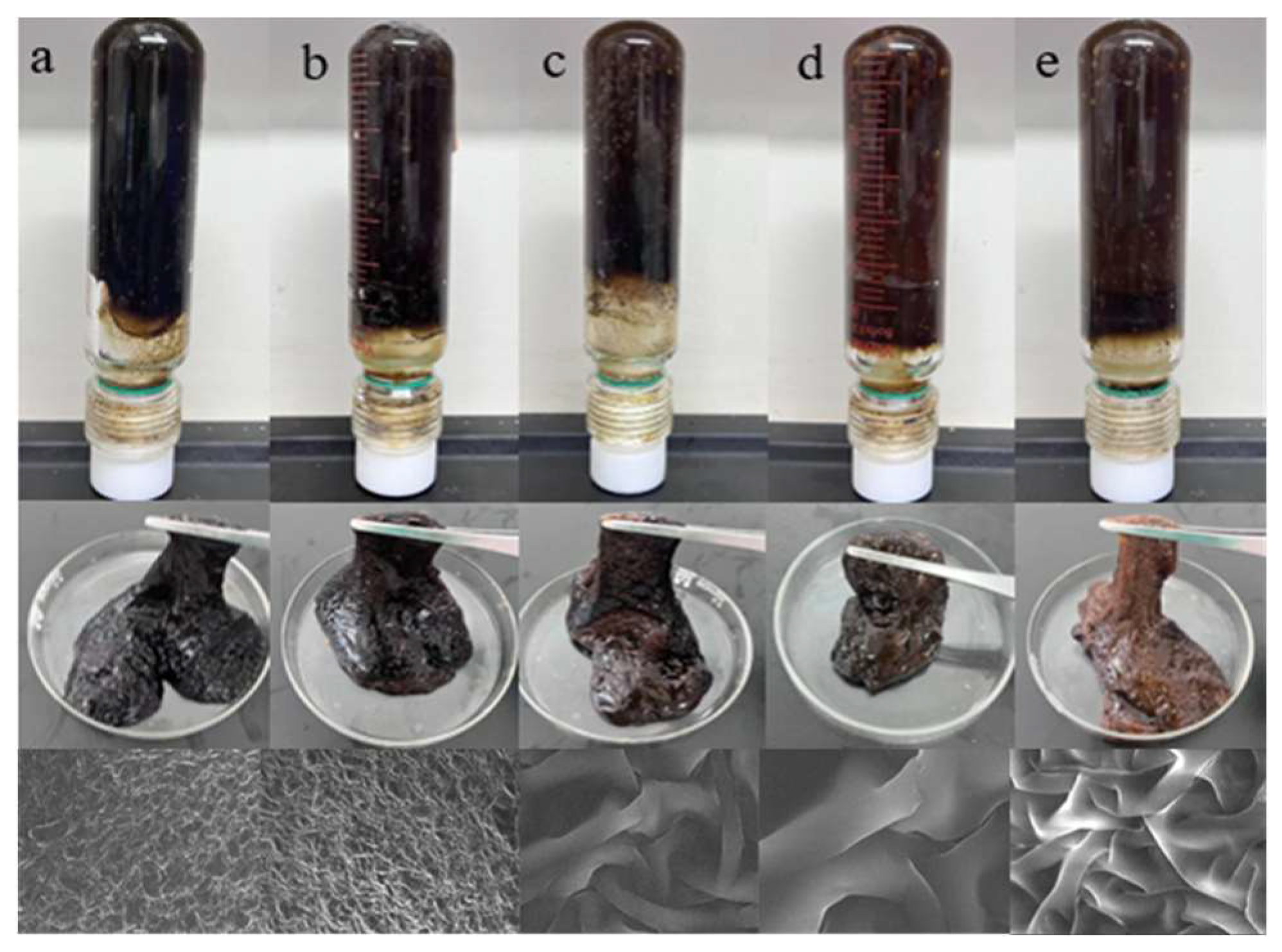
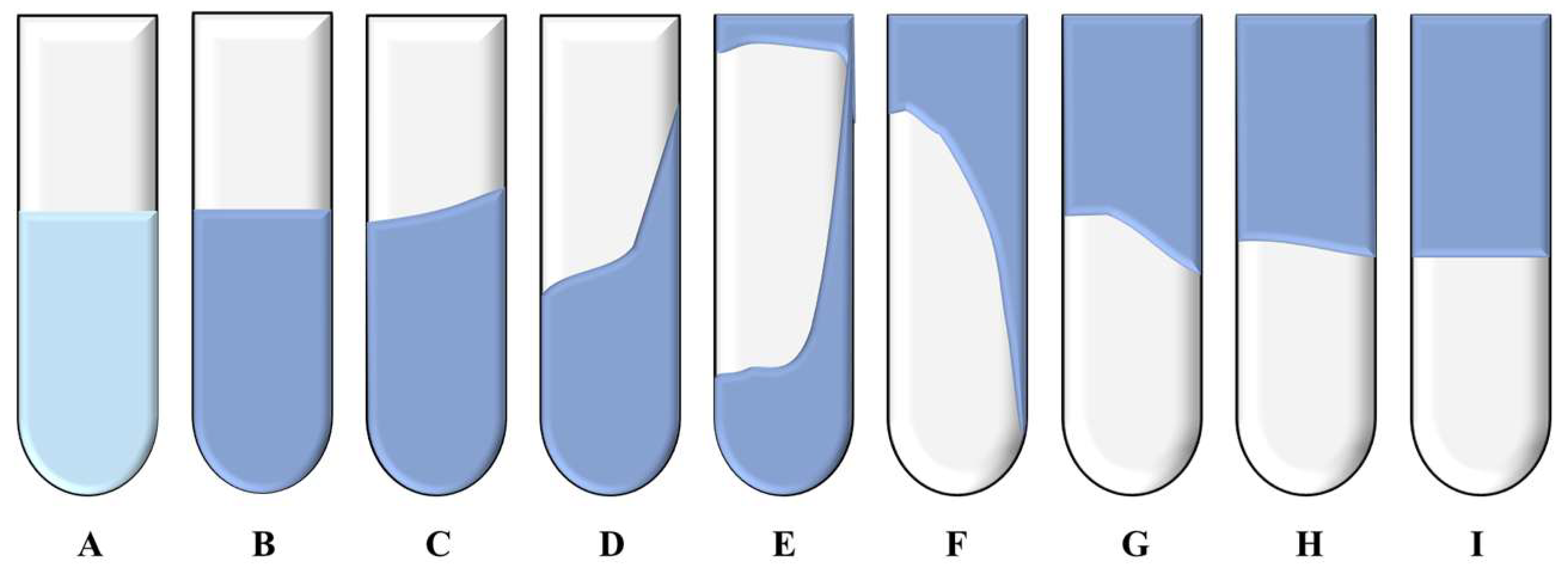
2.4. Gel Plugging System Construction and Characterization
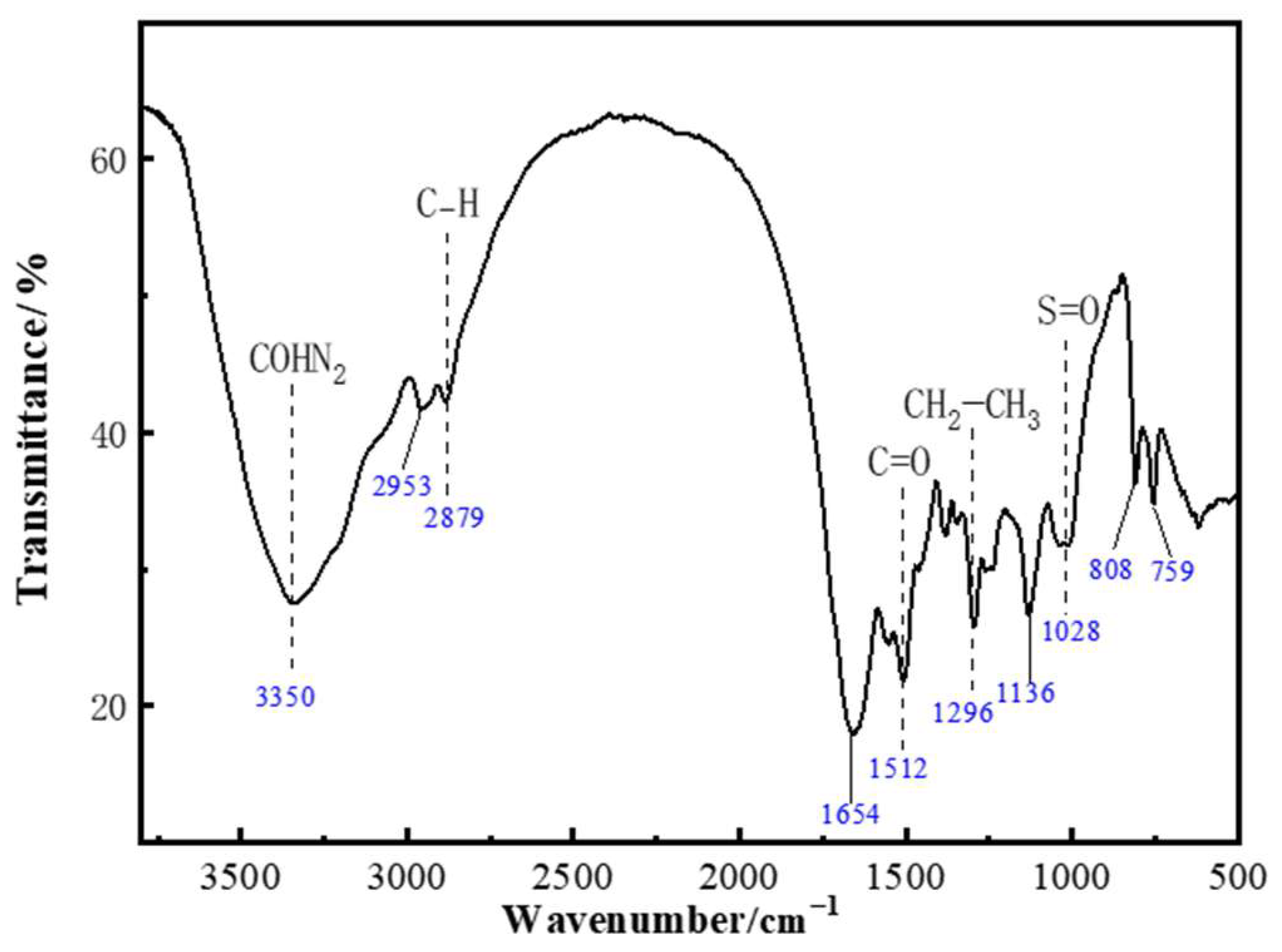
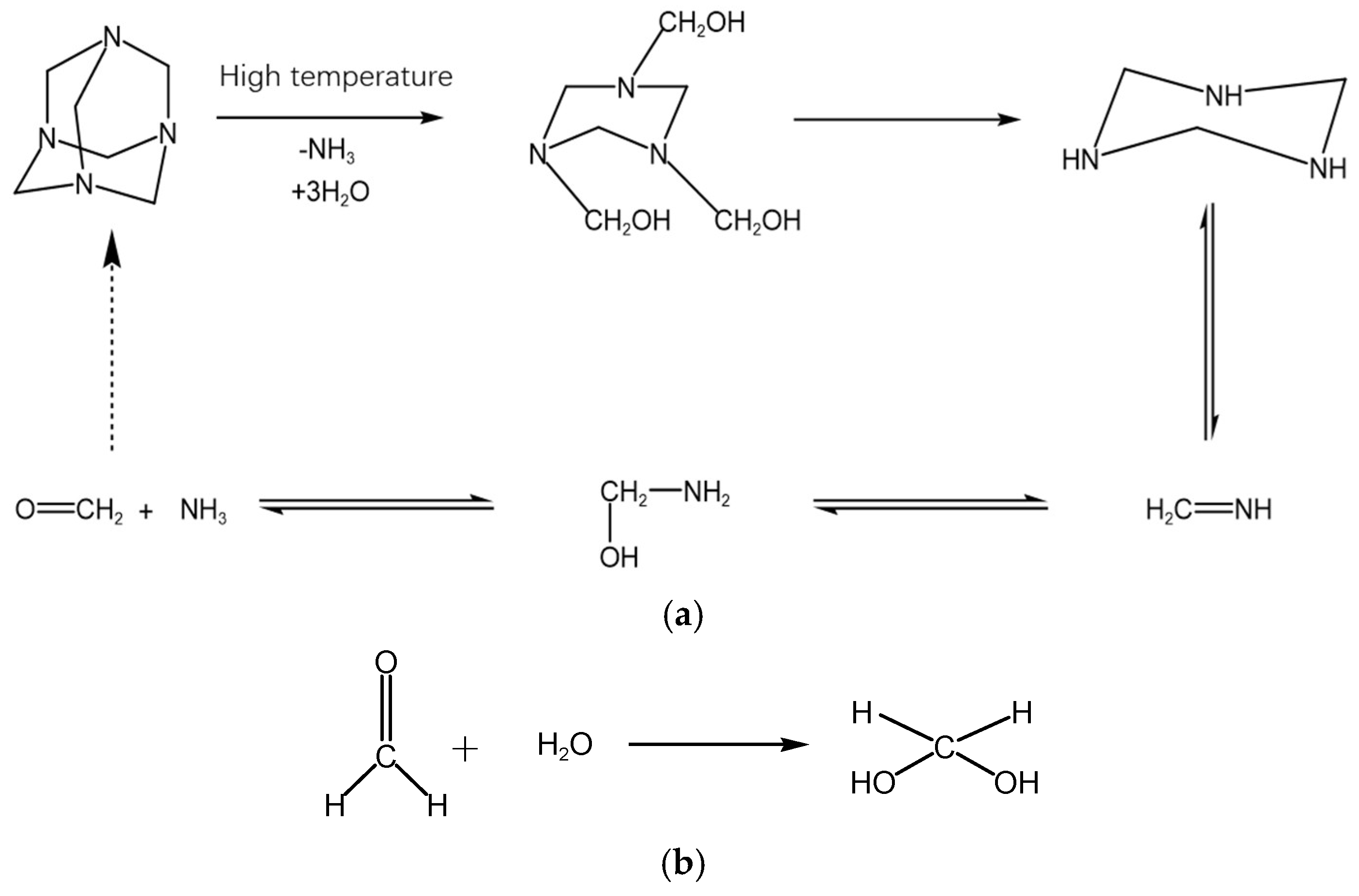
2.5. Analysis of Polymer Gel-Forming Mechanism
3. Physicochemical Properties of the Chemical Gel Plugging System
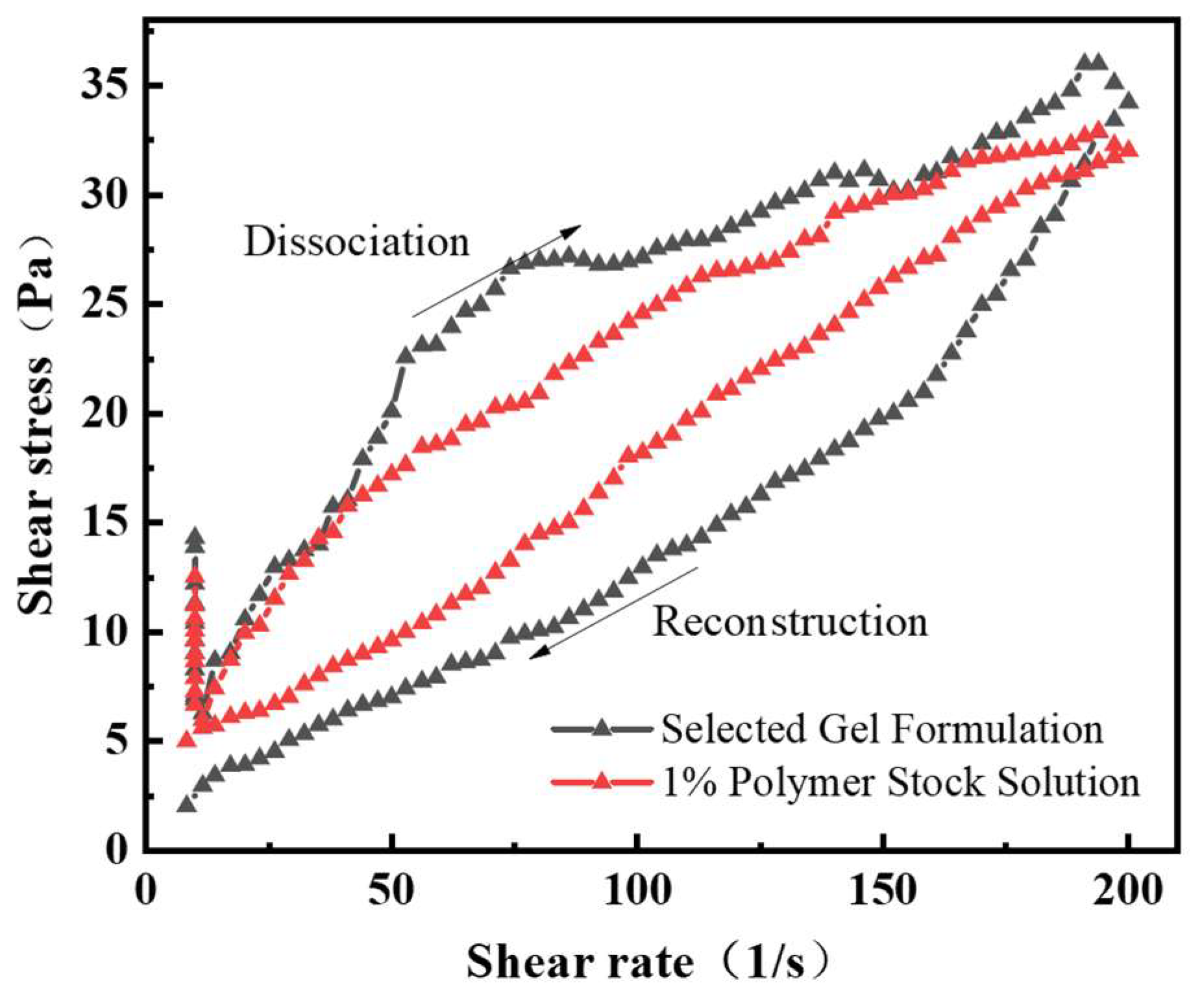
3.1. Shear Resistance of Gel Plugging System Solution
3.2. Thixotropic Properties of the Gel Plugging System Solution
3.3. High-Temperature Gelling Performance of the Gel Plugging System
3.4. High-Temperature Stability of Gel Plugging Systems
3.5. Salt Resistance of Gel Plugging Systems
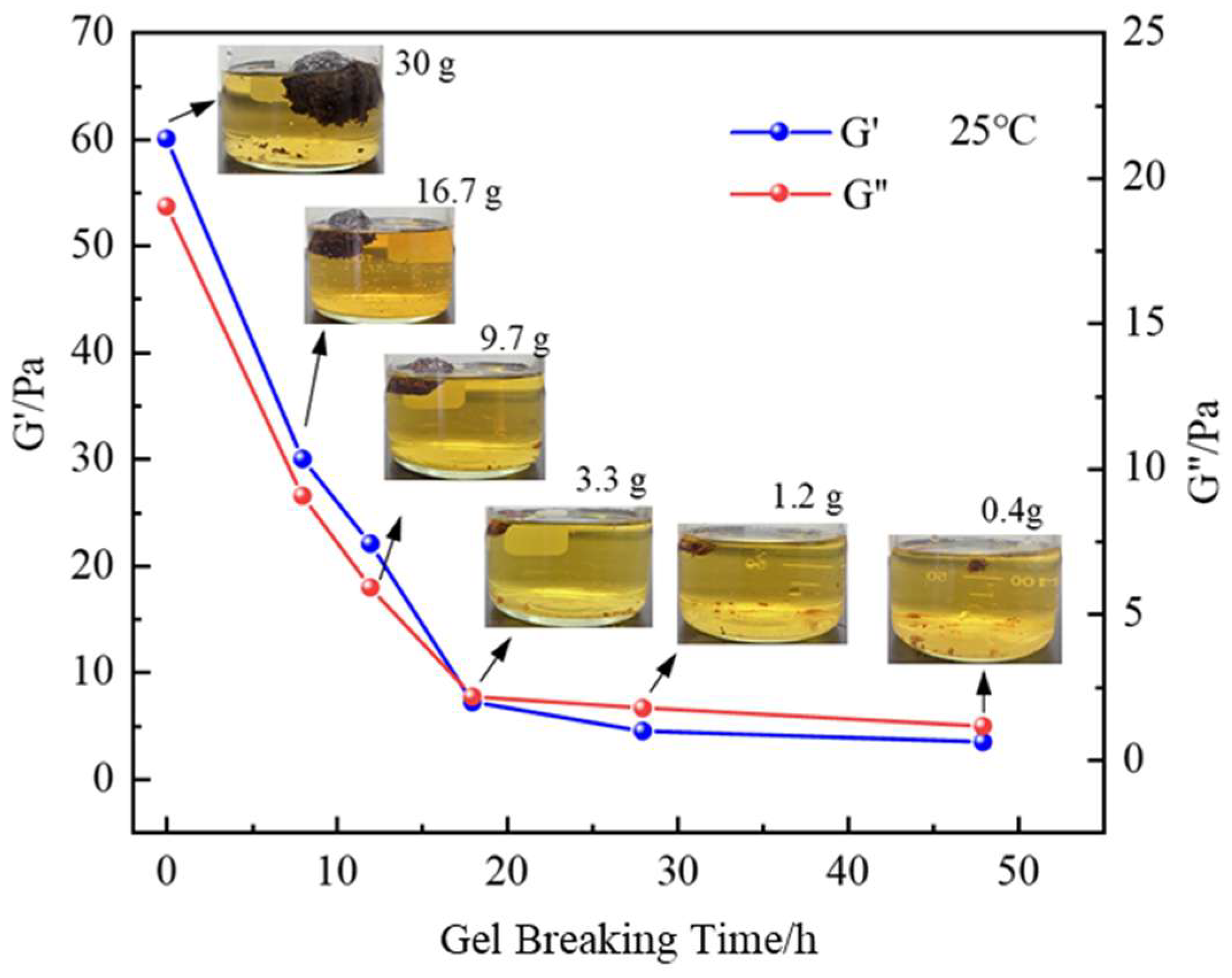
3.6. Gel Degradation Performance of Gel Plugging Systems
4. Conclusions
- (1)
- By optimizing key additives such as high-temperature-resistant crosslinking polymers and crosslinking agents, and determining the optimal types and ratios of the main agent and crosslinking agent, the formulation for a chemical gel plugging agent system resistant to temperatures up to 150 °C was developed: 0.5% ZP-1 + 0.5% ZP-2 + 0.6% HMTA + 0.3% phenol + 25% resin curing agent.
- (2)
- The apparent viscosity of the chemical gel plugging agent solution shows a significant lag between the breakdown and reconstruction of the solution during the shear rate cycles, with a large lag area, indicating the excellent thixotropic behavior of the system. The system is formed into a gel at 150 °C, the storage modulus is 125 Pa, the apparent viscosity is about 7500 mPa·s, and the viscosity retention rate of the gel system is more than 82% after aging at 150 °C for 9 days, which shows excellent high-temperature stability performance.
- (3)
- For an in-depth investigation of the influence mechanism of multivalent cations (Na+, Ca2+) on the gel network, the concentration gradient experiment of Na+ and Ca2+ was constructed. The composite modulus level of 96 Pa versus 19 Pa was still maintained under the condition of high Na+ concentration of 30,000 mg/L, and the viscosity retention was kept at the level of 91%. With the increase of Ca2+ concentration, the gel formation time of the system was shortened from 9 h to 3 h, and the apparent viscosity decreased to 6515 mPa-s, with a viscosity retention of 82%. This indicates that the preferred gel system has excellent salt resistance.
- (4)
- For the gel system at room temperature and 150 °C high-temperature conditions, with the use of a concentration of 15% ammonium persulfate-soaked gel block (fixed solution 100 mL and gel block 30 g) for 12 h, the gel had a breakage rate of more than 70%, and a breakage rate of 99.8% after 24 h. High-temperature conditions result in faster breakage of the gel and breakthrough of the chemical plugging agent of the bottleneck.
5. Experimental Materials and Methods
5.1. Experimental Materials
5.2. Experimental Methods
5.2.1. Preparation of Polymer Solution
5.2.2. Evaluation of Gelling Time and Gelling Effect of Polymer Gel System
5.2.3. Strength Testing of Polymer Gel System
5.2.4. High-Temperature Stability of Polymer Gel System
5.2.5. Microstructure Analysis of Polymer Gel System
5.2.6. Infrared Spectroscopy Characterization of Polymer Gel System
5.2.7. Thermogravimetric Analysis of Polymer Gel System
5.2.8. Pollution Resistance Evaluation of Polymer Gel System
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seright, R. Gel Placement in Fractured Systems. SPE Prod. Facil. 1995, 10, 241–248. [Google Scholar] [CrossRef]
- Luo, X.; Wei, B.; Gao, K.; Jing, B.; Huang, B.-L.; Guo, P.; Yin, H.; Feng, Y.-J.; Zhang, X. Gas Channeling Control with an In-Situ Smart Surfactant Gel during Water-Alternating-CO2 Enhanced Oil Recovery. Pet. Sci. 2023, 10, 2835–2851. [Google Scholar] [CrossRef]
- Du, Q.; Zhao, D.; Wu, J.; Hou, J.; Wei, Z.; Shi, L.; Zhou, K.; Zheng, H.; Zhang, J.; Liu, Y. Early Warning Methods of Chemical Agent Channeling in Polymer–Surfactant Flooding Reservoirs. Energy Sci. Eng. 2024, 12, 2180–2197. [Google Scholar] [CrossRef]
- Wang, L.; Yan, Y.; Han, X. Microscopic Plugging Adjustment Mechanism in a New Heterogeneous Combined Flooding System. SSRN Electron. J. 2022, 8, 15350–15364. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Q.; Trivedi, J.; Li, Y.; Liu, J.; Liu, Z.; Liu, S. Investigation on Enhanced Oil Recovery and CO2 Storage Efficiency of Temperature-Resistant CO2 Foam Flooding. Fuel 2024, 364, 130870. [Google Scholar] [CrossRef]
- Goudarzi, A.M.; Zhang, H.; Varavei, A.; Taksaudom, P.; Hu, Y.; Delshad, M.; Bai, B.; Sepehrnoori, K. A Laboratory and Simulation Study of Preformed Particle Gels for Water Conformance Control. Fuel 2015, 140, 502–513. [Google Scholar] [CrossRef]
- Bai, B.; Li, L.; Liu, Y.; Liu, H.; Wang, Z.; You, C.-M. Preformed Particle Gel for Conformance Control: Factors Affecting Its Properties and Applications. SPE Reserv. Eval. Eng. 2007, 10, 415–422. [Google Scholar] [CrossRef]
- Farajzadeh, R.; Andrianov, A.; Krastev, R.; Hirasaki, G.J.; Rossen, W.R. Foam-Oil Interaction in Porous Media: Implications for Foam Assisted Enhanced Oil Recovery. Adv. Colloid Interface Sci. 2012, 183–184, 1–13. [Google Scholar] [CrossRef]
- Song, X.; Li, G.; Huang, Z.; Shi, Y.; Wang, G.; Song, G.; Xu, F. Review of High-Temperature Geothermal Drilling and Exploitation Technologies. Gondwana Res. 2022, 122, 315–330. [Google Scholar] [CrossRef]
- El-Karsani, K.S.M.; Al-Muntasheri, G.A.; Sultan, A.S.; Hussein, I.A. Gelling of a Water-Shutoff Gel at High Pressure and High Temperature: Rheological Investigation. SPE J. 2015, 20, 1103–1112. [Google Scholar] [CrossRef]
- Juárez, J.L.; Rodriguez, M.R.; Montes, J.; Trujillo, F.D.; Monzòn, J.; Dupuis, G.; Gaillard, N. Conformance Gel Design for High Temperature Reservoirs. In Proceedings of the SPE Euro Featured 82nd EAGE Conference and Exhibition, Amsterdam, The Netherlands, 1–3 December 2020. SPE-200640-MS. [Google Scholar]
- Chen, L.F.; Zhang, G.C.; Ge, J.J.; Jiang, P.; Zhu, X.; Lin, Y.; Han, S. A Novel Thermal-Resistance and Salt-Tolerance Gel with Low-Concentration Crosslinkers for Water Shutoff in Tahe Oilfield. In Proceedings of the SPE Asia Pacific Unconventional Resources Conference and Exhibition, Brisbane, Australia, 9–11 November 2015. SPE-176848-MS. [Google Scholar]
- Wang, Q.; Cai, J.; Wang, J.; Zhou, C.; Wen, X.; Zhang, J.; Mao, H. Development and Application of the Anti-High-Temperature Delayed Crosslinking Polymer as a Gel Plugging Additive for Drilling Fluid. Gels 2024, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Yu, B.; Zhu, C. Conformance Improvement for Ultra-High-Temperature Reservoir: A Comparative Study between Hydrostable and Conventional Preformed Particle Gel. In Proceedings of the Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, UAE, 12–15 November 2018. SPE-192738-MS. [Google Scholar]
- Zhu, D.; Hou, J.; Wei, Q.; Chen, Y.; Peng, K. Development of a High-Temperature Resistant Polymer Gel System for Conformance Control in Jidong Oilfield. In Proceedings of the SPE/IATMI Asia Pacific Oil and Gas Conference and Exhibition, Jakarta, Indonesia, 17–19 October 2017. SPE-186235-MS. [Google Scholar]
- Miao, G.; Zhang, H.; Yang, Y.; Qu, J.; Ma, X.; Zheng, J.; Liu, X. Synthesis and Performance Evaluation of Crosslinker for Seawater-Based Fracturing Fluid. J. Appl. Polym. Sci. 2023, 140, e53372. [Google Scholar] [CrossRef]
- Wang, G.; Shen, H.; Gao, Y.; Xiang, Y.; Yang, Z.; Lv, Q.; Dong, Z.; Lin, M. Study on Plugging, Migration, and EOR Capability of Dual Crosslinked Terpolymer Microspheres. J. Mol. Liq. 2025, 424, 127101. [Google Scholar] [CrossRef]
- Sokhanvarian, K.; Nasr-El-Din, H.A.; Harper, T.L. Effect of Ligand Type Attached to Zirconium-Based Crosslinkers and the Effect of a New Dual Crosslinker on the Properties of Crosslinked Carboxymethylhydroxypropylguar. SPE J. 2019, 24, 1741–1756. [Google Scholar] [CrossRef]
- Niu, C.; Fan, S.; Chen, X.; He, Z.; Dai, L.; Wen, Z.; Li, M. Preparation and Performance Evaluation of a Supramolecular Polymer Gel-Based Temporary Plugging agent for Heavy Oil Reservoir. Gels 2024, 10, 536. [Google Scholar] [CrossRef]
- El-Karsani, K.S.M.; Al-Muntasheri, G.A.; Sultan, A.S.; Hussein, I.A. Gelling Kinetics of PAM/PEI System. J. Therm. Anal. Calorim. 2014, 116, 1409–1415. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, J.; Gu, Y.; Zhao, Q.; Yang, R.; Yang, Y. Polyacrylamide Gel Formed by Cr(III) and Phenolic Resin for Water Control in High-Temperature Reservoirs. J. Pet. Sci. Eng. 2020, 194, 107423. [Google Scholar] [CrossRef]
- Yang, H.; Jiang, H.; Xu, Z.; Zhang, X.; Wang, T.; Liu, H.; Ma, X.; Zhu, J.; Zhang, X.; Kang, W. Development and Evaluation of Organic/Metal Ion Double Crosslinking Polymer Gel for Anti-CO2 Gas Channeling in High Temperature and Low Permeability Reservoirs. Pet. Sci. 2024, 22, 724–738. [Google Scholar] [CrossRef]
- Shafiei, M.; Kazemzadeh, Y.; Shirazy, G.M.; Riazi, M. Evaluating the Role of Salts on Emulsion Properties during Water-Based Enhanced Oil Recovery: Ion Type, Concentration, and Water Content. J. Mol. Liq. 2022, 364, 120028. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, Z.; Zhang, J.; Li, Q.; Zhang, Q. Free Radical Induced Degradation of High Molecular Weight Partial Hydrolysis Polyacrylamide (HPAM) in a Ferrous Iron Containing System. Polym. Bull. 2022, 79, 9397–9406. [Google Scholar] [CrossRef]
- Zhang, Q.; Mao, J.; Yang, X.; Lin, C.; Zhang, H.; Xu, T.; Wang, Q. Synthesis of a Hydrophobic Association Polymer with an Inner Salt Structure for Fracture Fluid with Ultra-High-Salinity Water. Colloids Surf. A Physicochem. Eng. Asp. 2021, 636, 128062. [Google Scholar] [CrossRef]
- Zhang, L.; Pu, C.; Sang, H.; Zhao, Q. Mechanism Study of the Crosslinking Reaction of Hydrolyzed Polyacrylamide/Ac3Cr in Formation Water. Energy Fuels 2015, 29, 4701–4710. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, C.; Zhang, H.; Zhang, X.; Wen, X.; Bai, J.; Mao, H. Preparation of Low-Molecular-Weight Polyacrylamide as the Delayed Crosslinking Plugging agent for Drilling Fluid. Gels 2024, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhang, X.; He, L.; Zhao, G.; Dai, C. Experimental Research of Hydroquinone (HQ)/Hexamethylene Tetramine (HMTA) Gel for Water Plugging Treatments in High-Temperature and High-Salinity Reservoirs. J. Appl. Polym. Sci. 2017, 134, 44359. [Google Scholar] [CrossRef]
- Ren, J.M.; McKenzie, T.G.; Fu, Q.; Wong, E.H.H.; Xu, J.; An, Z.; Shanmugam, S.; Davis, T.P.; Boyer, C.; Qiao, G.G. Star Polymers. Chem. Rev. 2016, 116, 6743–6836. [Google Scholar] [CrossRef]
- Nie, X.; Luo, P.; Wang, P.; Zhang, X.; Yang, L. Rheology of a New Gel Used for Severe Lost Circulation Control. In Proceedings of the International Oil and Gas Conference and Exhibition in China, Beijing, China, 8–10 June 2010; pp. 2882–2891. [Google Scholar]
- Corredor, L.M.; Husein, M.M.; Maini, B.B. Impact of PAM-Grafted Nanoparticles on the Performance of Hydrolyzed Polyacrylamide Solutions for Heavy Oil Recovery at Different Salinities. Ind. Eng. Chem. Res. 2019, 58, 9888–9899. [Google Scholar] [CrossRef]
- Behdadfar, M.H.; Sheng, J.J.; Esmaeilnezhad, E. Designing Effective Enhanced Oil Recovery Fluid: Combination of Graphene Oxide, D118 SuperPusher, and Chuback Surfactant. Gels 2023, 390, 123081. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, C.; Sun, J.; Shang, X.; Lv, K.; Zhu, Y.; Wang, F. High Temperature Resistant Polymer Gel as Lost Circulation Material for Fractured Formation during Drilling. Colloids Surf. A Physicochem. Eng. Asp. 2022, 637, 128244. [Google Scholar] [CrossRef]
- Du, X.; Shahzadi, K.; Wei, L. Multifunctional Conductive Composite Hydrogel Facilitated by High-Functionality Crosslinker Strategy for Strain Sensing Applications. Polym. Bull. 2025, 82, 245–262. [Google Scholar] [CrossRef]
- Lv, K.; Zhang, G.; Bai, Y.; Yang, J. Preparation of Encapsulated Breakers for Polymer Gels and Evaluation of Their Properties. Gels 2023, 9, 387. [Google Scholar] [CrossRef]





















| Polymer ID | Polymer Molecular Weight (Daltons) | Polymer Hydrolysis Degree (%) | Monomer Composition |
|---|---|---|---|
| ZP-1 | 700~900 | 10~15 | AM/AMPS/NVP |
| ZP-2 | 800~1000 | 15~20 | AM/AMPS |
| ZP-3 | 400~600 | 20–25 | AM |
| ZP-4 | 500~700 | 20–25 | AM |
| Polymer Type | Blend Ratio | Phenol Concentration (%) | Aldehyde Concentration (%) | Gelling Time (h) | Gelling Strength |
|---|---|---|---|---|---|
| ZP-1/ZP-2 | 1:2 | 0.2 | 0.4 | 14 | E |
| 2:1 | 0.2 | 0.4 | 10 | F | |
| 1:1 | 0.2 | 0.4 | 8 | H |
| Aldehyde Crosslinker and Concentration (%) | Phenolic Concentration (%) | Gelling Time (h) | Gelling Effect and Thermal Stability | |
|---|---|---|---|---|
| Formaldehyde | 0.6 | 0.3 | 1 | The gelling strength reaches G, followed by 5 h of high-temperature degradation. |
| Paraformaldehyde | 0.6 | 0.3 | Not gelled | / |
| HMTA | 0.6 | 0.3 | 6 | Gelling strength H, with dehydration less than 10% after 7 days. |
| Phenolic Crosslinking Agent and Concentration (%) | Phenolic Concentration (%) | Gelling Time (h) | Gelling Effect and Thermal Stability | |
|---|---|---|---|---|
| Resorcinol | 0.3 | 0.6 | 8 | Gel strength reached D; 12 h high-temperature degradation. |
| Hydroquinone | 0.3 | 0.6 | 6 | Gel strength G; dehydration < 10% after 7 days. |
| Catechol | 0.3 | 0.6 | 6 | Gel strength H; dehydration < 10% after 7 days. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, J.; Sun, J.; Feng, X.; Pan, L.; Bai, Y.; Yang, J. Research and Development of a High-Temperature-Resistant, Gel-Breaking Chemical Gel Plugging Agent and Evaluation of Its Physicochemical Properties. Gels 2025, 11, 350. https://doi.org/10.3390/gels11050350
Fang J, Sun J, Feng X, Pan L, Bai Y, Yang J. Research and Development of a High-Temperature-Resistant, Gel-Breaking Chemical Gel Plugging Agent and Evaluation of Its Physicochemical Properties. Gels. 2025; 11(5):350. https://doi.org/10.3390/gels11050350
Chicago/Turabian StyleFang, Junwei, Jinsheng Sun, Xingen Feng, Lijuan Pan, Yingrui Bai, and Jingbin Yang. 2025. "Research and Development of a High-Temperature-Resistant, Gel-Breaking Chemical Gel Plugging Agent and Evaluation of Its Physicochemical Properties" Gels 11, no. 5: 350. https://doi.org/10.3390/gels11050350
APA StyleFang, J., Sun, J., Feng, X., Pan, L., Bai, Y., & Yang, J. (2025). Research and Development of a High-Temperature-Resistant, Gel-Breaking Chemical Gel Plugging Agent and Evaluation of Its Physicochemical Properties. Gels, 11(5), 350. https://doi.org/10.3390/gels11050350








