Preparation of a Novel Perilla Essential Oil/Grape Seed Extract–Chitosan/Gelatin Composite Edible Gel Film and Its Application in the Preservation of Grass Carp
Abstract
1. Introduction
2. Results and Discussion
2.1. Results of Single Factor
2.1.1. Effect of GSE Addition on Properties of PE/GSE–Gelatin/Chitosan Composite Edible Gel Films
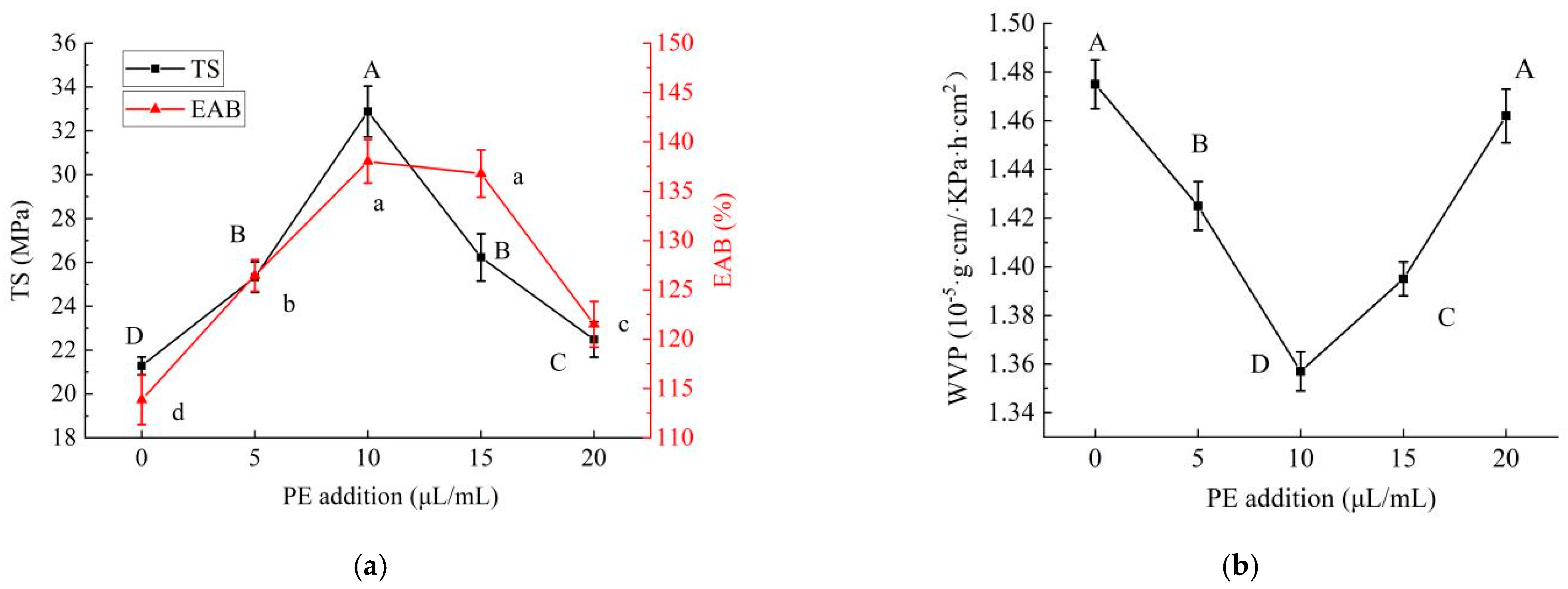
2.1.2. Effects of PE Addition on Properties of PE/GSE–Gelatin/Chitosan Composite Edible Gel Films
2.1.3. Effects of Gelatin Addition on Properties of PE/GSE–Gelatin/Chitosan Composite Edible Gel Films
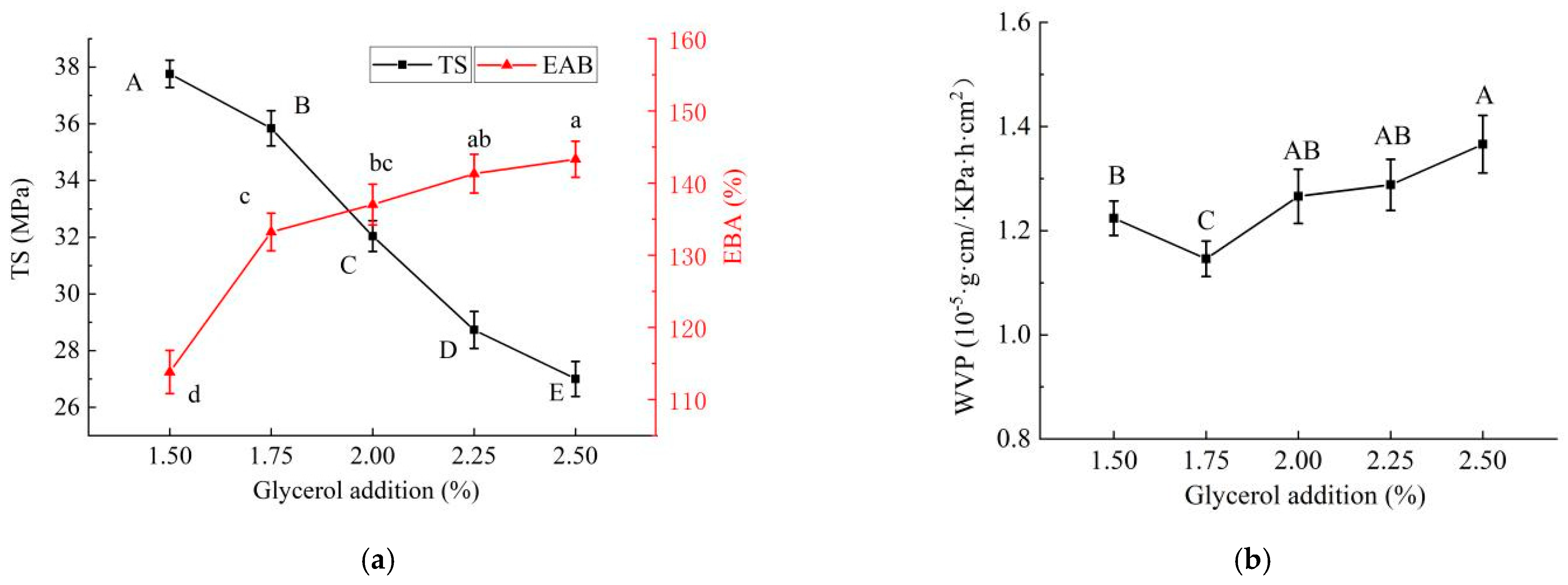
2.1.4. Effect of Glycerol Addition on Properties of PE/GSE–Gelatin/Chitosan Composite Edible Gel Films
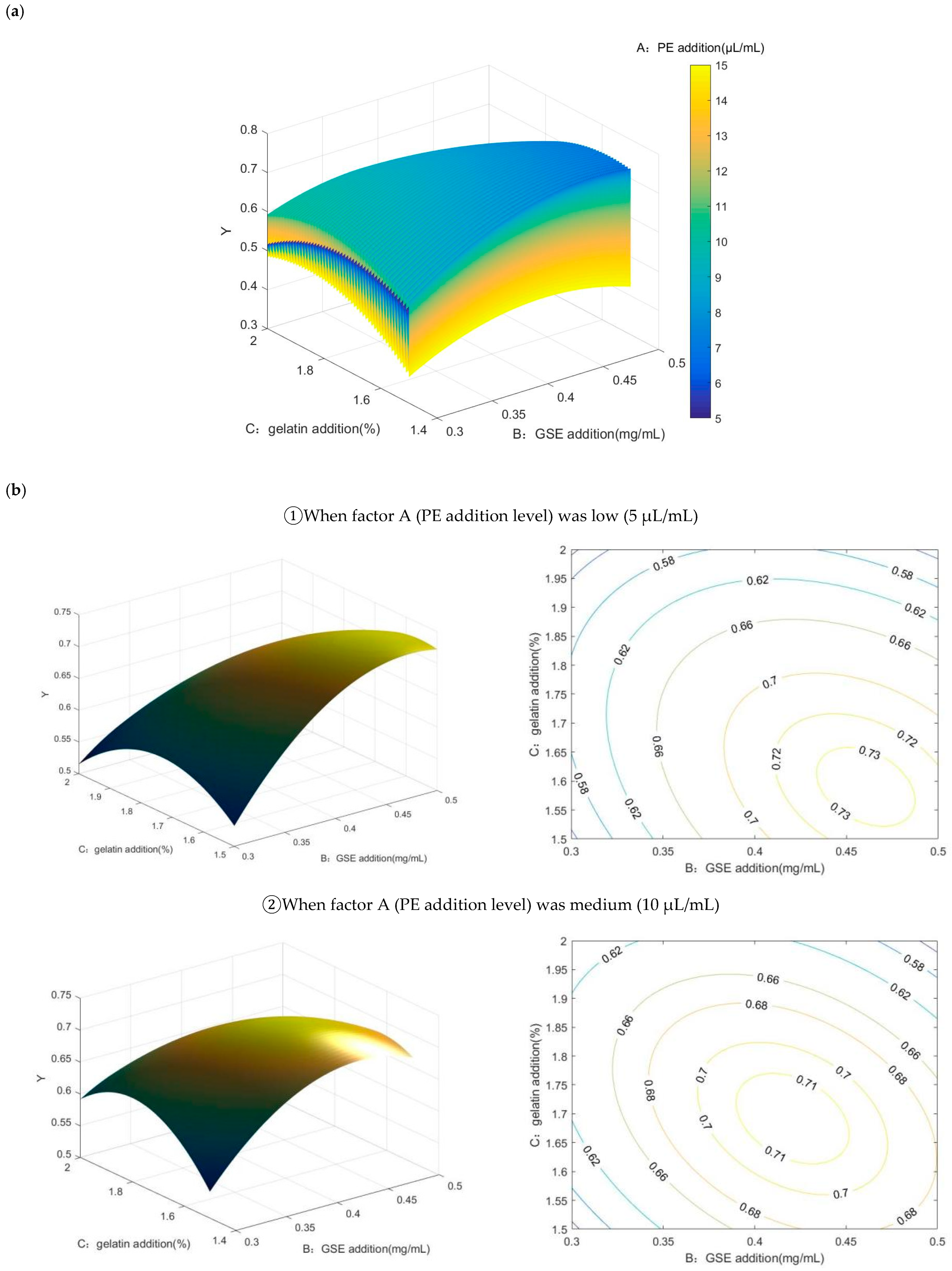
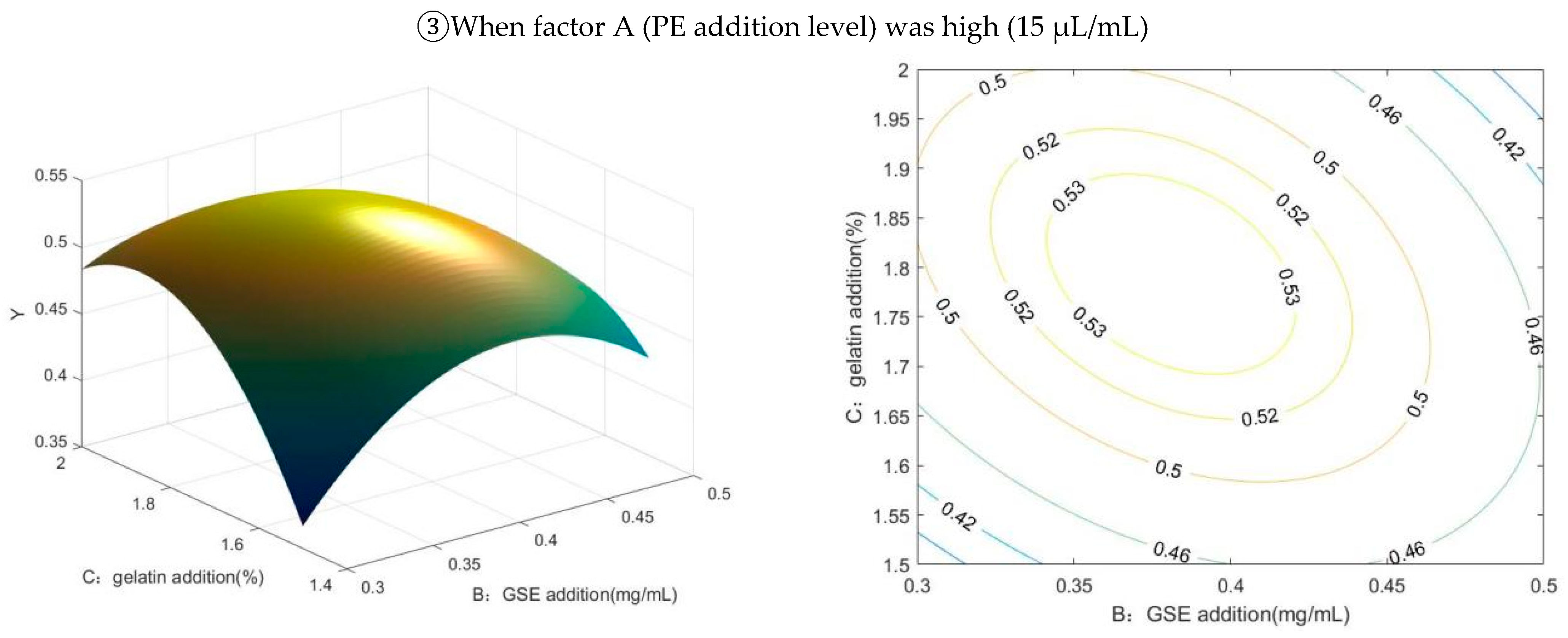
2.2. Results of Box–Behnken Design and Matlab Analysis
2.2.1. Model Building
2.2.2. The Results of Response Surface Analysis and the Significance Test
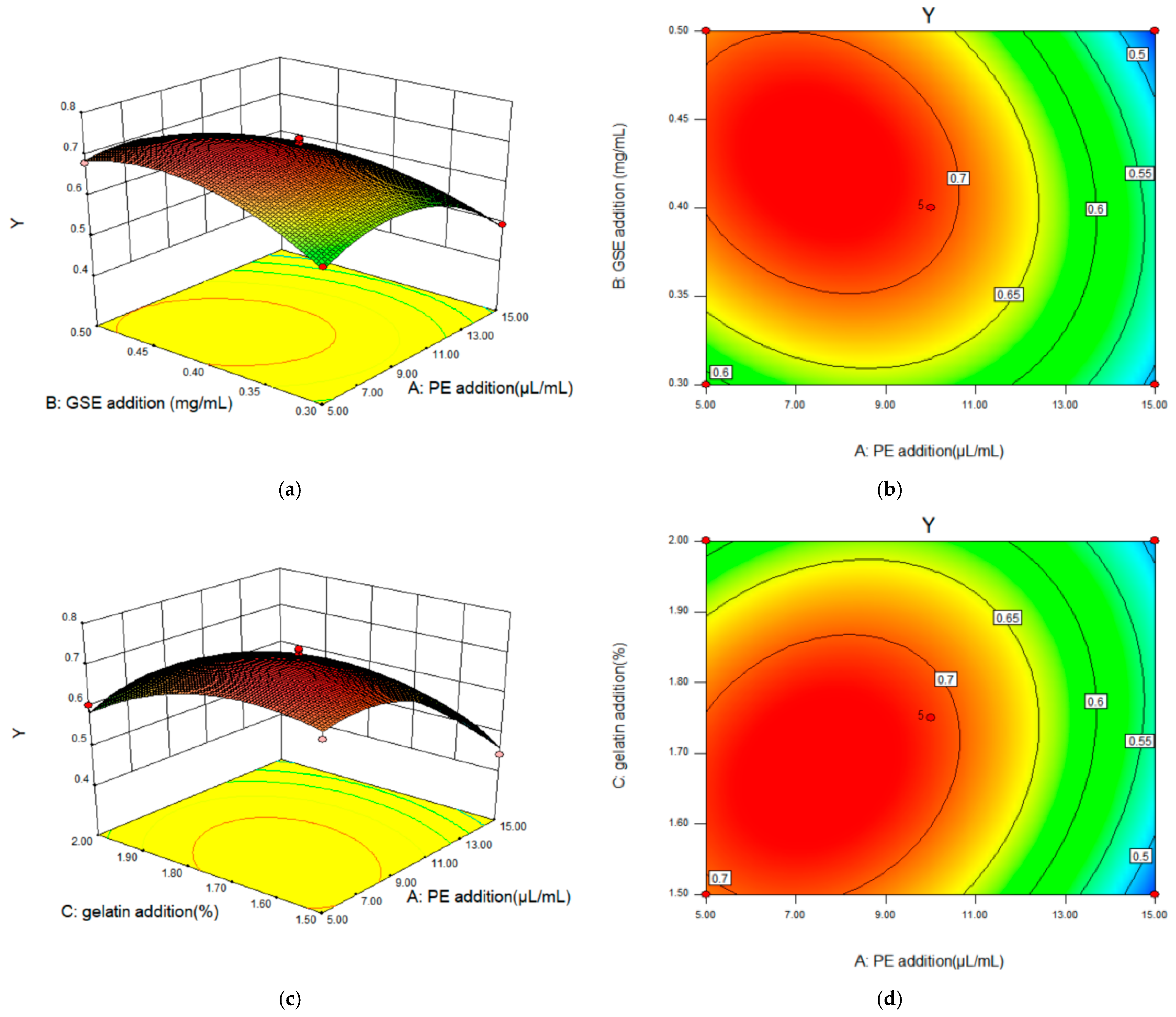
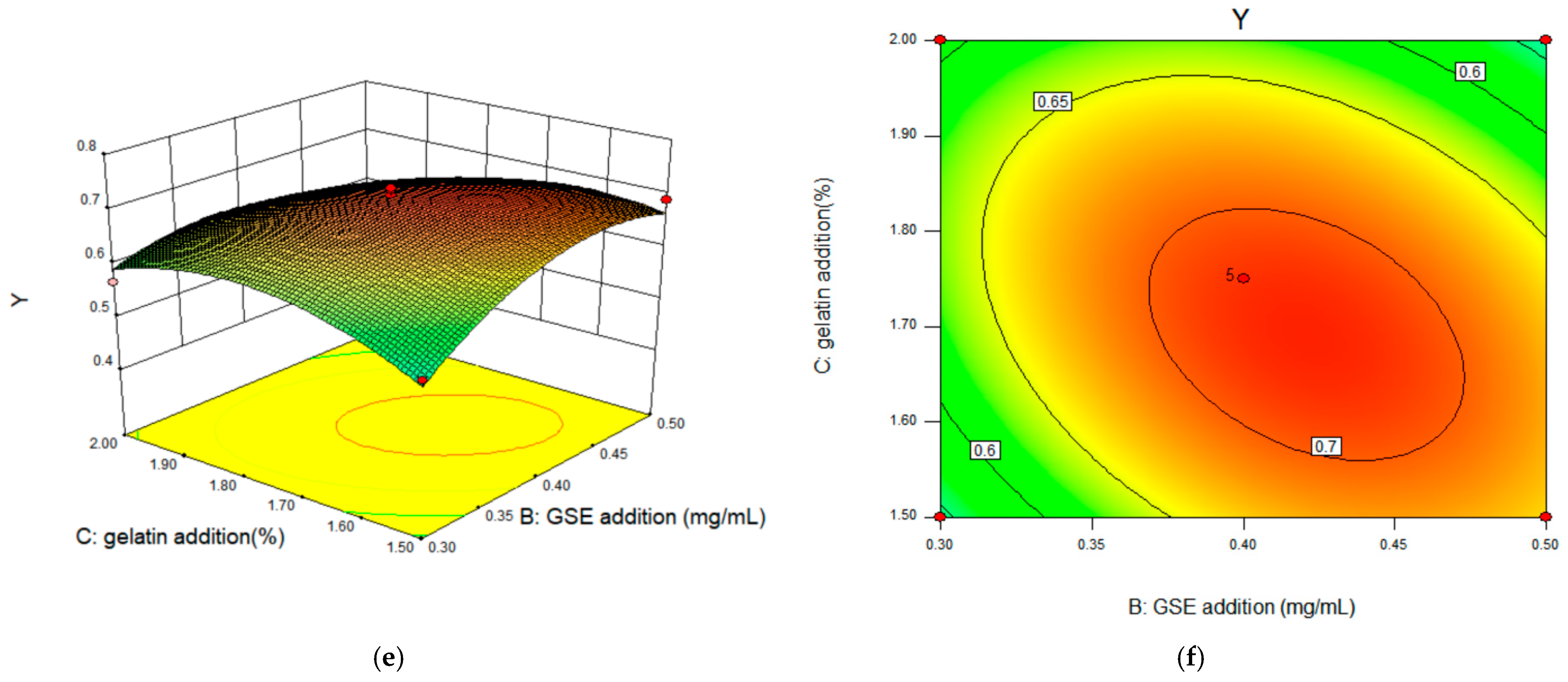
2.2.3. Response Surface Interaction and Optimization Results Analysis
2.2.4. Matlab Analysis
2.2.5. Verification Test
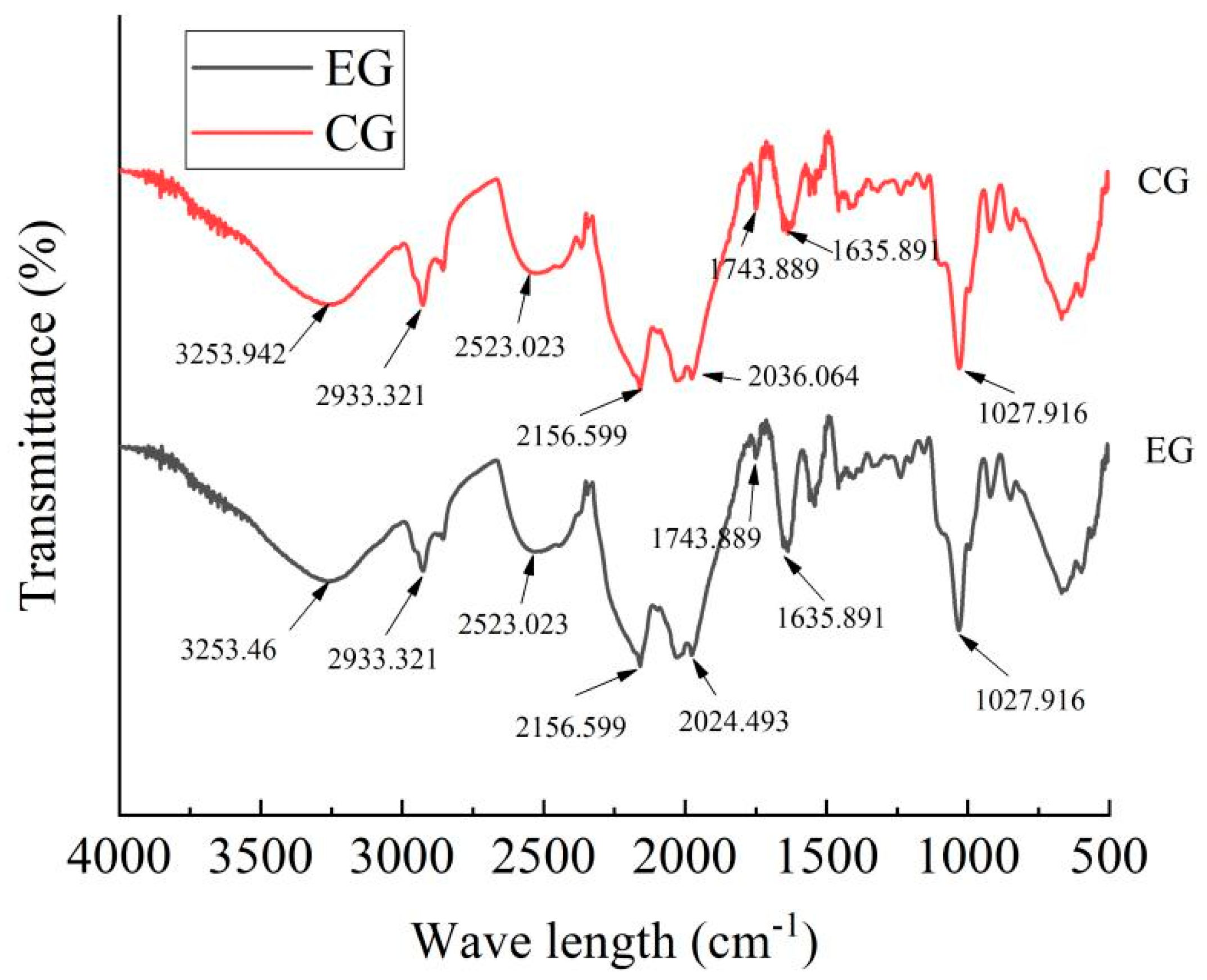
2.3. Results of Fourier-Transform Infrared Spectrometer (FTIR)
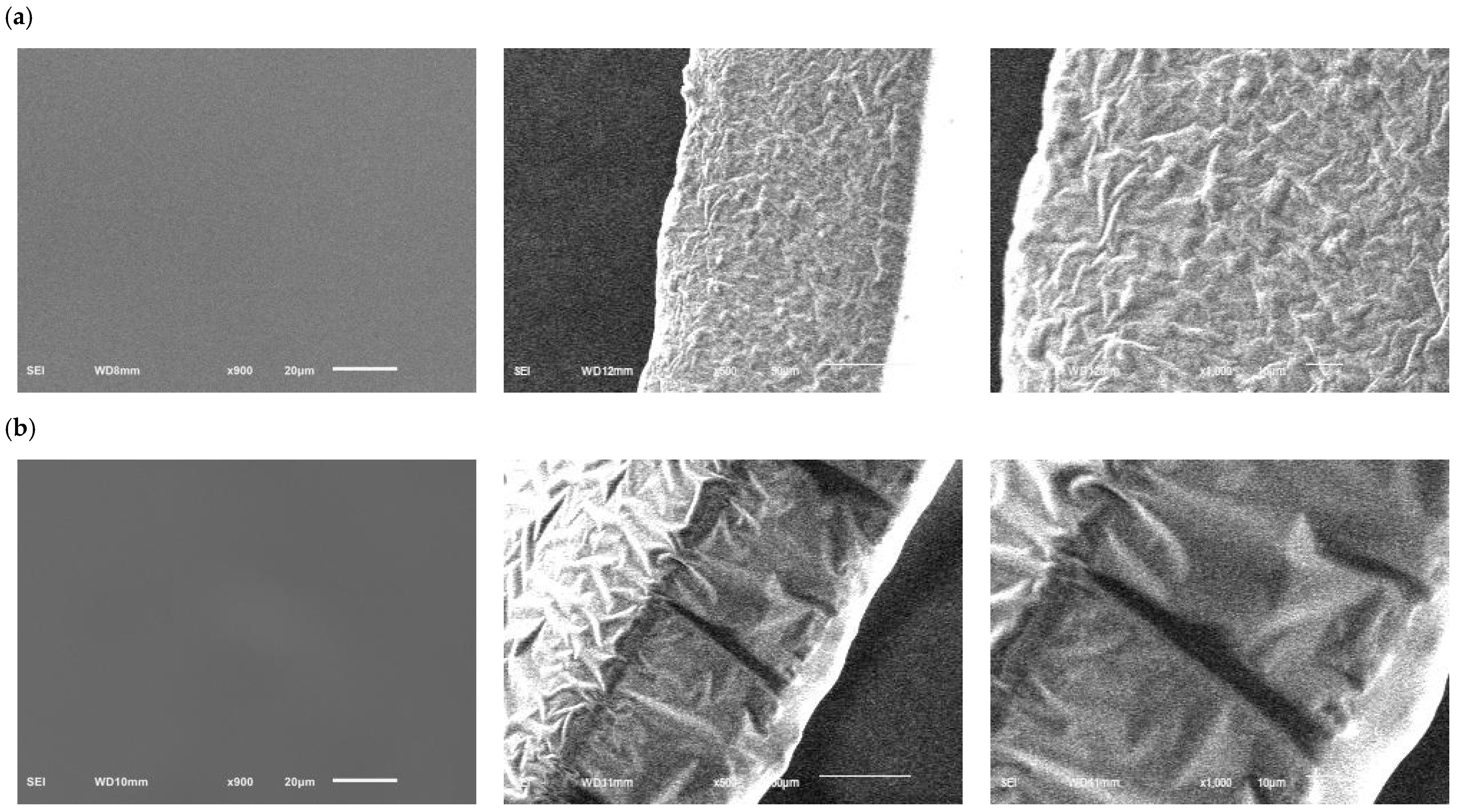
2.4. Results of Scanning Electron Microscope (SEM)
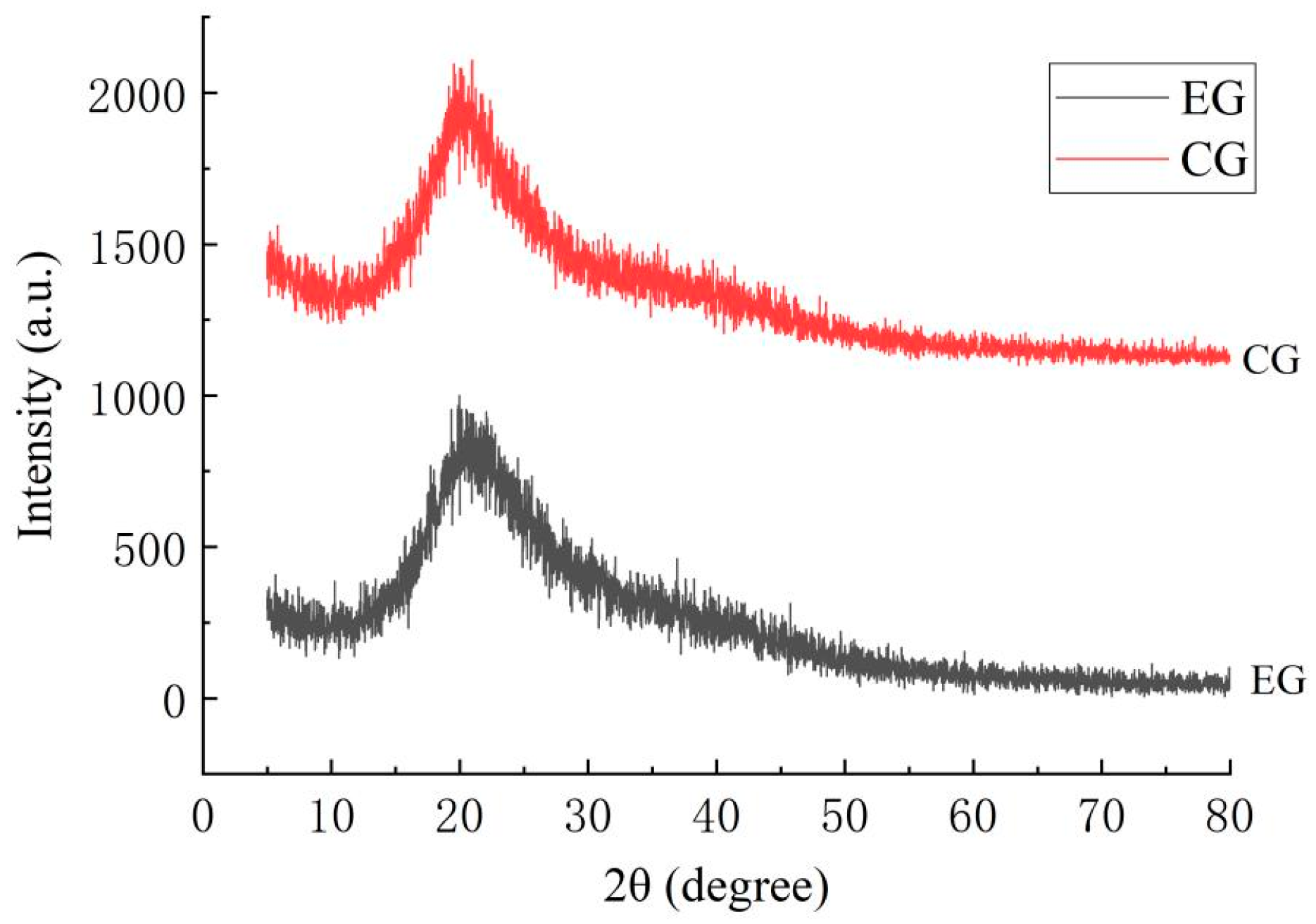
2.5. Results of X-Ray Diffraction (XRD)
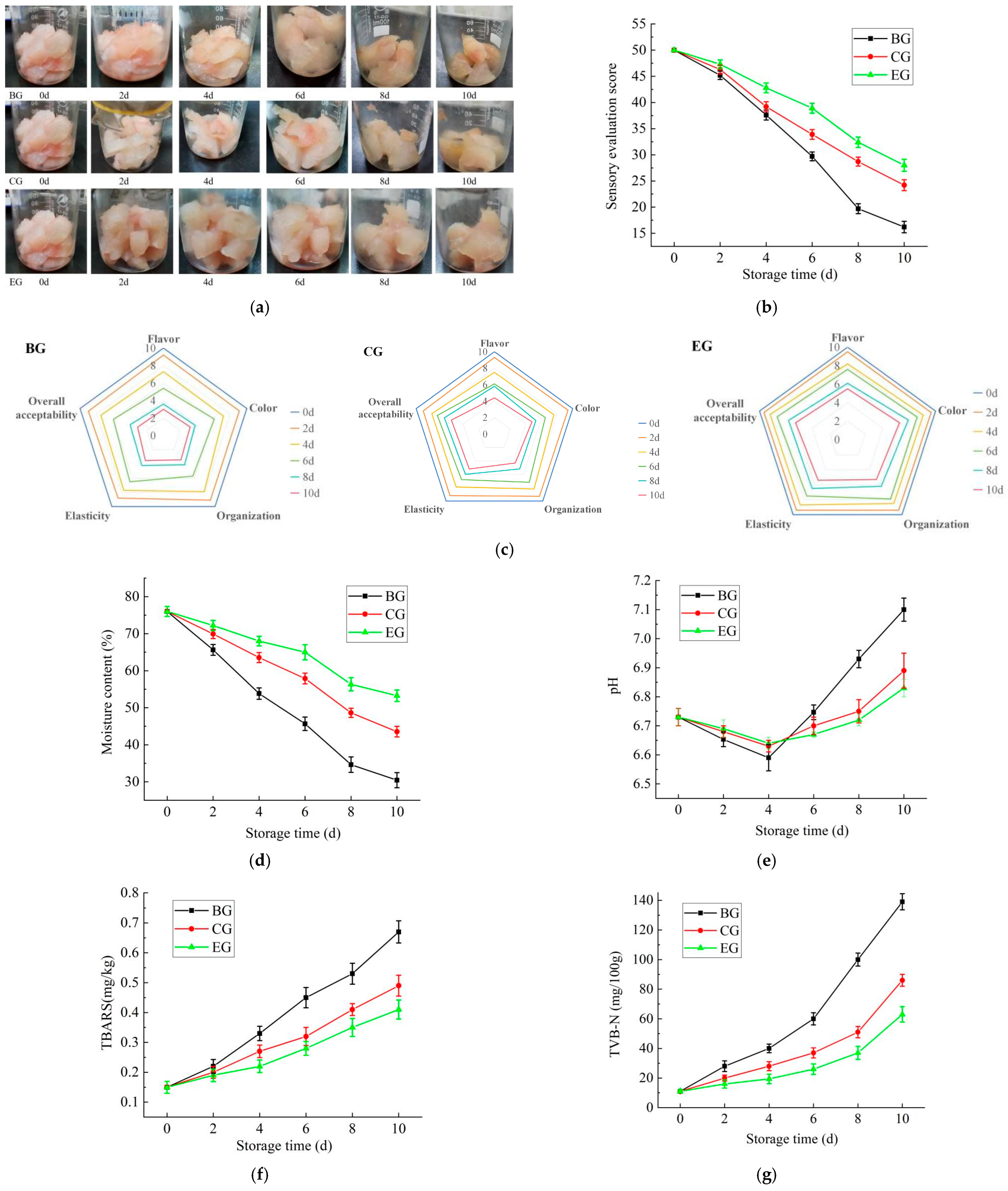
2.6. The Results of the Preservation Experiment
2.6.1. The Change in Sensory Score During Storage
2.6.2. The Change in Moisture Content During Storage
2.6.3. The Change in pH Value During Storage
2.6.4. The Change in Thiobarbituric Acid Reactive Substances (TBARS) During Storage
2.6.5. The Change in Total Volatile Base Nitrogen (TVB-N) During Storage
2.6.6. Correlation Analysis of Indicator Changes
3. Conclusions
4. Materials and Methods
4.1. Material
4.2. Preparation of PE/GSE–Chitosan/Gelatin Composite Edible Gel Films
4.3. Process Optimization of the PE/GSE–Chitosan/Gelatin Composite Edible Gel Films
4.3.1. Single-Factor Experiment
4.3.2. The Box–Behnken Test and Matlab Analysis
4.4. Characterization of PE/GSE–Chitosan/Gelatin Composite Edible Gel Films
4.4.1. Thickness Test
4.4.2. The Tests of TS and EAB
4.4.3. The Test of WVP
4.4.4. Comprehensive Evaluation of Fuzzy Mathematics
4.4.5. X-Ray Diffraction (XRD) Analysis
4.4.6. FTIR Analysis
4.4.7. SEM Analysis
4.5. Effect of PE/GSE–Chitosan/Gelatin Composite Edible Gel Films on Physicochemical Properties of Grass Carp During Storage
4.5.1. Preservation Experiment
4.5.2. Measurement of Sensory Scores
4.5.3. Measurement of Moisture Content
4.5.4. Measurement of pH
4.5.5. Measurement of TBARS
4.5.6. Measurement of TVB-N
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McClements, D.J. Food hydrocolloids: Application as functional ingredients to control lipid digestion and bioavailability. Food Hydrocoll. 2021, 111, 106404. [Google Scholar] [CrossRef]
- Hidayat, M.I.; Hardiansyah, A.; Khoiriah, K.; Yulianti, E.; Wardhani, R.A.K.; Fahrialdi, F.; Yusuf, M.R.I. Composite films based on chitosan incorporating molybdenum disulfide nanosheets and zinc oxide nanoparticles with potential antibacterial application. Food Chem. 2025, 477, 143480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Argenziano, R.; Konate, A.; Shi, X.; Salazar, S.A.; Cerruti, P.; Panzella, L.; Terrasson, V.; Guénin, E. Preparation of chitosan/lignin nanoparticles-based nanocomposite films with high-performance and improved physicochemical properties for food packaging applications. Int. J. Biol. Macromol. 2025, 293, 139079. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, F.; Yong, H.; Chen, D.; Tang, C.; Kan, J.; Liu, J. Recent advances in chitosan-based active and intelligent packaging films incorporated with flavonoids. Food Chem. X 2025, 25, 102200. [Google Scholar] [CrossRef]
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H.W.; Pulvirenti, A. Recent advances on chitosan-based films for sustainable food packaging applications. Food Packag. Shelf Life 2020, 26, 100551. [Google Scholar] [CrossRef]
- Sharma, R.; Nath, P.C.; Das, P.; Rustagi, S.; Sharma, M.; Sridhar, N.; Hazarika, T.K.; Rana, P.; Nayak, P.K.; Sridhar, K. Essential oil-nanoemulsion based edible coating: Innovative sustainable preservation method for fresh/fresh-cut fruits and vegetables. Food Chem. 2024, 460, 140545. [Google Scholar] [CrossRef]
- Karnwal, A.; Kumar, G.; Singh, R.; Selvaraj, M.; Malik, T.; Al Tawaha, A.R.M. Natural biopolymers in edible coatings: Applications in food preservation. Food Chem. X 2025, 25, 102171. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, K.; Zhu, W.; Wang, Y.; Su, C.; Yi, F. Chemical compositions and bioactivities of essential oil from perilla leaf (Perillae Folium) obtained by ultrasonic-assisted hydro-distillation with natural deep eutectic solvents. Food Chem. 2022, 375, 131834. [Google Scholar] [CrossRef]
- Sharma, N.; Kaur, H.; Kaur, G.; Singh, A.; Sharma, S. Appraisal of cutting-edge techniques for prolonging fresh berries shelf life: Innovations in essential oil nanoemulsion-based edible coatings. Sci. Hortic. 2024, 337, 113564. [Google Scholar] [CrossRef]
- Cozmuta, A.M.; Peter, A.; Nicula, C.; Jastrzębska, A.; Jakubczak, M.; Purbayanto, M.A.K.; Bunea, A.; Bora, F.; Uivarasan, A.; Szakács, Z.; et al. The impact of visible light component bands on polyphenols from red grape seed extract powder encapsulated in alginate–whey protein matrix. Food Chem. X 2024, 23, 101758. [Google Scholar] [CrossRef]
- Ghoshal, G.; Singh, J. Study of coating effectiveness of grape fruit seed extract incorporated chitosan/cornstarch based active packaging film on grapes. Food Chem. Adv. 2024, 4, 100651. [Google Scholar] [CrossRef]
- Zhao, X.; Tian, R.; Zhou, J.; Liu, Y. Multifunctional chitosan/grape seed extract/silver nanoparticle composite for food packaging application. Int. J. Biol. Macromol. 2022, 207, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Zhu, Y.; Zhuang, Y.; Tan, K.B.; Chen, J. Effects of grape seed extract on the properties of pullulan polysaccharide/xanthan gum active films for apple preservation. Int. J. Biol. Macromol. 2023, 241, 124617. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadeh, S.; Hettiarachchy, N.; Luthra, K.; Chen, J.; Seo, H.-S.; Atungulu, G.G.; Ubeyitogullari, A. Effects of polyphenol-rich grape seed and green tea extracts on the physicochemical properties of 3D-printed edible soy protein films. Food Packag. Shelf Life 2023, 40, 101184. [Google Scholar] [CrossRef]
- Eranda, D.H.U.; Chaijan, M.; Panpipat, W.; Karnjanapratum, S.; Cerqueira, M.A.; Castro-Muñoz, R. Gelatin-chitosan interactions in edible films and coatings doped with plant extracts for biopreservation of fresh tuna fish products: A review. Int. J. Biol. Macromol. 2024, 280, 135661. [Google Scholar] [CrossRef]
- Ma, Y.; Cao, Y.; Zhang, L.; Yu, Q. Preservation of chilled beef using active films based on bacterial cellulose and polyvinyl alcohol with the incorporation of perilla essential oil Pickering emulsion. Int. J. Biol. Macromol. 2024, 271, 132118. [Google Scholar] [CrossRef]
- Cao, L.; Liu, J.; Meng, Y.; Hou, M.; Li, J.; Song, Y.; Wang, Y.; Song, H.; Zhang, R.; Liang, R.; et al. A tear-free and edible dehydrated vegetables packaging film with enhanced mechanical and barrier properties from soluble soybean polysaccharide blending carboxylated nanocellulose. Int. J. Biol. Macromol. 2024, 264, 130707. [Google Scholar] [CrossRef]
- Garg, R.; Rana, H.; Singh, N.; Goswami, S. Guargum/nanocellulose based novel crosslinked antimicrobial film with enhanced barrier and mechanical properties for food packaging. J. Environ. Chem. Eng. 2023, 11, 109254. [Google Scholar] [CrossRef]
- Fang, Z.; Yang, Y.; Lin, S.; Xu, L.; Chen, S.; Lv, W.; Wang, N.; Dong, S.; Lin, C.; Xie, Y.; et al. Development and antimicrobial activity of composite edible films of chitosan and nisin incorporated with perilla essential oil-glycerol monolaurate emulsions. Food Chem. 2025, 462, 141006. [Google Scholar] [CrossRef]
- Ding, W.; Guo, S.; Wang, K.; Pang, X.; Asres, B.S.; Ding, Z. Aminated graphene oxide reinforced gelatin-chitosan composite films toward biopackaging: Preparation and properties. Int. J. Biol. Macromol. 2025, 284, 138104. [Google Scholar] [CrossRef]
- Hong, S.J.; Riahi, Z.; Shin, G.H.; Kim, J.T. Development of innovative active packaging films using gelatin/pullulan-based composites incorporated with cinnamon essential oil-loaded metal-organic frameworks for meat preservation. Int. J. Biol. Macromol. 2024, 267, 131606. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.S.; Ludueña, L.N.; Flores, S.K. Combined effect of oregano essential oil and glycerol on physicochemical properties of antimicrobial films based on chitosan and acetylated cassava starch. Food Hydrocoll. 2024, 156, 110259. [Google Scholar] [CrossRef]
- Chillo, S.; Flores, S.; Mastromatteo, M.; Conte, A.; Gerschenson, L.; Del Nobile, M.A. Influence of glycerol and chitosan on tapioca starch-based edible film properties. J. Food Eng. 2008, 88, 159–168. [Google Scholar] [CrossRef]
- Laskar, A.A.; Ahmed, M.; Vo, D.-V.N.; Abdullah, A.; Shahadat, M.; Mahmoud, M.H.; Khan, W.; Yusuf, M. Mathematical modeling and regression analysis using MATLAB for optimization of microwave drying efficiency of banana. Therm. Sci. Eng. Prog. 2023, 46, 102157. [Google Scholar] [CrossRef]
- Lin, D. Application of mathematical modeling based on MATLAB in experimental data analysis. Procedia Comput. Sci. 2024, 247, 86–92. [Google Scholar] [CrossRef]
- Xue, S.; Li, C.; Xiong, Z. Preparation of complex polysaccharide gels with Zanthoxylum bungeanum essential oil and their application in fish preservation. Gels 2024, 10, 533. [Google Scholar] [CrossRef]
- Liu, W.W.; Ma, L.; Zhang, Y.H.; Wang, H.X. Development and application of gelatin-grape skin red pigment-based edible ink. J. Food Sci. Biotechnol. 2022, 48, 121–127. (In Chinese) [Google Scholar]
- Gao, C.; Xu, Y.; Xu, X.; Chen, Y.; Meng, L.; Tang, X. Effects of different essential oil nanoemulsions co-stabilized by two emulsifiers on the structure and properties of chitosan active films. Food Packag. Shelf Life 2024, 46, 101404. [Google Scholar] [CrossRef]
- Jahed, E.; Khaledabad, M.A.; Ahmed, S.A.; Almasi, H.; Hassanzadeh, H. Application of chitosan-based edible films reinforced with nanofibers to improve the shelf life of rapeseed oil: Release kinetics of essential oils and biodegradability. Int. J. Biol. Macromol. 2025, 307, 141832. [Google Scholar] [CrossRef]
- Liu, J.; Cao, A.; Liu, Y.; Zheng, X.; Tang, K. Development and characterization of soluble soybean polysaccharide/pullulan blend films enriched with essential oils. Int. J. Biol. Macromol. 2025, 309, 143092. [Google Scholar] [CrossRef]
- Liu, M.; He, C.; Chen, W.; Li, Y.; Yang, N.; Chen, X.; Xue, J.; Wang, X.; Lu, A.; Xu, Z.; et al. Carboxymethyl chitosan/peach gum polysaccharide packaging film incorporating Citrus sinensis essential oil effectively enhances the quality preservation of strawberries. Food Packag. Shelf Life 2024, 46, 101409. [Google Scholar] [CrossRef]
- Zhao, R.; Guan, W.; Zhou, X.; Lao, M.; Cai, L. The physiochemical and preservation properties of anthocyanidin/chitosan nanocomposite-based edible films containing cinnamon-perilla essential oil Pickering nanoemulsions. LWT 2022, 153, 112506. [Google Scholar] [CrossRef]
- Gao, Y.; Shi, H.; Xiong, Q.; Wu, R.; Hu, Y.; Liu, R. A novel strategy for inhibiting AGEs in fried fish cakes: Grape seed extract surimi slurry coating. Food Control 2023, 154, 109948. [Google Scholar] [CrossRef]
- Pu, Y.; Chen, L.; Jiang, W. Antimicrobial guar gum films optimized with Pickering emulsions of zein-gum arabic nanoparticle-stabilized composite essential oil for food preservation. Int. J. Biol. Macromol. 2024, 278, 134911. [Google Scholar] [CrossRef]
- Kitsiou, M.; Purk, L.; Ioannou, C.; Wantock, T.; Sandison, G.; Harle, T.; Gutierrez-Merino, J.; Klymenko, O.V.; Velliou, E. On the evaluation of the antimicrobial effect of grape seed extract and cold atmospheric plasma on the dynamics of Listeria monocytogenes in novel multiphase 3D viscoelastic models. Int. J. Food Microbiol. 2023, 406, 110395. [Google Scholar] [CrossRef]
- Xiang, H.; Liao, J.; Tang, Y.; Wen, P.; Wang, H. Development of cinnamon essential oil-loaded core-shell nano film for the preservation of chilled flesh foods. Food Packag. Shelf Life 2024, 45, 101310. [Google Scholar] [CrossRef]
- Teymoorian, M.; Moghimi, R.; Hosseinzadeh, R.; Zandi, F.; Lakouraj, M.M. Fabrication the emulsion-based edible film containing Dracocephalum kotschyi Boiss essential oil using chitosan–gelatin composite for grape preservation. Carbohydr. Polym. Technol. Appl. 2024, 7, 100444. [Google Scholar] [CrossRef]
- Zhang, X.; Ismail, B.B.; Cheng, H.; Jin, T.Z.; Qian, M.; Arabi, S.A.; Liu, D.; Guo, M. Emerging chitosan-essential oil films and coatings for food preservation—A review of advances and applications. Carbohydr. Polym. 2021, 273, 118616. [Google Scholar] [CrossRef]
- Xue, S.; Huang, Q. Preparation of novel flaxseed oil/beeswax oleogel systems and its application in the improvement of sodium alginate films. Gels 2024, 10, 78. [Google Scholar] [CrossRef]
- Xue, S.; Chen, S.; Liu, B.; Liu, J. Effects of red vinasse on physicochemical qualities of blue round scad (Decapterus maruadsi) during storage, and shelf life prediction. Foods 2024, 13, 3654. [Google Scholar] [CrossRef]



| No. | A: PE Addition (μL/mL) | B: GSE Addition (mg/mL) | C: Gelatin Addition (%) | TS (MPa) | EAB (%) | WVP (10−5 g cm/(KPa h kPa·cm2)) |
|---|---|---|---|---|---|---|
| 1 | −1 (5) | 0 (0.4) | 1 (2) | 35.23 | 188.90 | 1.889 |
| 2 | −1 | −1 (0.3) | 0 (1.75) | 37.05 | 128.17 | 1.199 |
| 3 | −1 | 0 | −1 (1.5) | 36.4 | 144.27 | 1.143 |
| 4 | −1 | 1 (0.5) | 0 | 46.02 | 147.13 | 1.284 |
| 5 | 0 (10) | 1 | 1 | 22.36 | 193.47 | 2.018 |
| 6 | 0 | 1 | −1 | 44.33 | 148.90 | 1.256 |
| 7 | 0 | −1 | 1 | 37.05 | 162.97 | 1.702 |
| 8 | 0 | −1 | −1 | 21.97 | 127.03 | 1.113 |
| 9 | 0 | 0 | 0 | 25.09 | 168.27 | 1.179 |
| 10 | 0 | 0 | 0 | 24.44 | 170.73 | 1.179 |
| 11 | 0 | 0 | 0 | 26.26 | 162.70 | 1.179 |
| 12 | 0 | 0 | 0 | 23.79 | 172.53 | 1.179 |
| 13 | 0 | 0 | 0 | 24.57 | 166.07 | 1.179 |
| 14 | 1 (15) | 1 | 0 | 39.26 | 141.27 | 1.817 |
| 15 | 1 | −1 | 0 | 58.63 | 108.33 | 1.517 |
| 16 | 1 | 0 | −1 | 15.73 | 140.27 | 1.529 |
| 17 | 1 | 0 | 1 | 63.18 | 182.27 | 2.454 |
| M | A | B | C | r1n | r2n | r3n | Y |
|---|---|---|---|---|---|---|---|
| 1 | −1 | 0 | 1 | 0.411 | 0.946 | 0.421 | 0.603 |
| 2 | −1 | −1 | 0 | 0.449 | 0.233 | 0.936 | 0.593 |
| 3 | −1 | 0 | −1 | 0.436 | 0.422 | 0.978 | 0.675 |
| 4 | −1 | 1 | 0 | 0.638 | 0.456 | 0.872 | 0.68 |
| 5 | 0 | 1 | 1 | 0.140 | 1 | 0.325 | 0.524 |
| 6 | 0 | 1 | −1 | 0.603 | 0.477 | 0.893 | 0.689 |
| 7 | 0 | −1 | 1 | 0.449 | 0.642 | 0.561 | 0.567 |
| 8 | 0 | −1 | −1 | 0.136 | 0.22 | 1.000 | 0.553 |
| 9 | 0 | 0 | 0 | 0.197 | 0.704 | 0.951 | 0.712 |
| 10 | 0 | 0 | 0 | 0.184 | 0.733 | 0.951 | 0.721 |
| 11 | 0 | 0 | 0 | 0.222 | 0.639 | 0.951 | 0.696 |
| 12 | 0 | 0 | 0 | 0.170 | 0.754 | 0.951 | 0.726 |
| 13 | 0 | 0 | 0 | 0.186 | 0.678 | 0.951 | 0.702 |
| 14 | 1 | 1 | 0 | 0.496 | 0.387 | 0.475 | 0.448 |
| 15 | 1 | −1 | 0 | 0.904 | 0 | 0.699 | 0.495 |
| 16 | 1 | 0 | −1 | 0.000 | 0.375 | 0.699 | 0.442 |
| 17 | 1 | 0 | 1 | 1.000 | 0.868 | 0.000 | 0.504 |
| Source | Sum of Squares | df | Mean Square | F Value | p Value | Significance |
|---|---|---|---|---|---|---|
| Model | 0.1556 | 9 | 0.0173 | 34.90 | < 0.0001 | ** |
| A | 0.0548 | 1 | 0.0548 | 110.57 | < 0.0001 | ** |
| B | 0.0032 | 1 | 0.0032 | 6.54 | 0.0377 | * |
| C | 0.0022 | 1 | 0.0022 | 4.46 | 0.0725 | |
| AB | 0.0045 | 1 | 0.0045 | 9.06 | 0.0197 | * |
| AC | 0.0045 | 1 | 0.0045 | 9.06 | 0.0197 | * |
| BC | 0.0080 | 1 | 0.0080 | 16.17 | 0.0051 | ** |
| A2 | 0.0359 | 1 | 0.0359 | 72.44 | < 0.0001 | ** |
| B2 | 0.0168 | 11 | 0.0168 | 33.81 | 0.0007 | * |
| C2 | 0.0178 | 1 | 0.0178 | 35.99 | 0.0005 | ** |
| Residual | 0.0035 | 7 | 0.0005 | |||
| Lack of fit | 0.0028 | 3 | 0.0009 | 5.99 | 0.0582 | |
| Pure error | 0.0006 | 4 | 0.0002 | |||
| Cor total | 0.1591 | 16 |
| Sensory Score | Moisture Content | pH | TBARS | TVB-N | |
|---|---|---|---|---|---|
| BG | −0.9937 | −0.9935 | 0.7995 | 0.9964 | 0.9716 |
| CG | −0.9974 | −0.9975 | 0.6503 | 0.9950 | 0.9472 |
| EG | −0.9935 | −0.9884 | 0.5027 | 0.9901 | 0.9250 |
| Factor | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| The addition of PE (μL/mL) | 5 | 10 | 15 |
| The addition of GSE (mg/mL) | 0.3 | 0.4 | 0.5 |
| The addition of gelatin (%) | 1.5 | 1.75 | 2 |
| The addition of glycerol (%) | 1 | 1.25 | 1.5 |
| Score | Odor | Color | Tissue | Elasticity |
|---|---|---|---|---|
| 9–10 | Fish smell was rich; no odor | White | Close section; clear texture | Rapid recovery after compression |
| 7–8 | Fish smell; no odor | Yellowish white | Partially close and clear | Slow recovery after pressing |
| 5–6 | Fish smell was light; slight odor | Yellowish | Not tight, but not loose | Recovery after compression is slow |
| 3–4 | Fishy and unpleasant smell | Grayish yellow | Soft section; partially loose | It is difficult to recover after pressing |
| 1–2 | Very fishy and smelly | gray | Oar-shaped and loose | It will not recover after compression |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, S.; Xu, R.; Liu, J. Preparation of a Novel Perilla Essential Oil/Grape Seed Extract–Chitosan/Gelatin Composite Edible Gel Film and Its Application in the Preservation of Grass Carp. Gels 2025, 11, 321. https://doi.org/10.3390/gels11050321
Xue S, Xu R, Liu J. Preparation of a Novel Perilla Essential Oil/Grape Seed Extract–Chitosan/Gelatin Composite Edible Gel Film and Its Application in the Preservation of Grass Carp. Gels. 2025; 11(5):321. https://doi.org/10.3390/gels11050321
Chicago/Turabian StyleXue, Shan, Rui Xu, and Jia Liu. 2025. "Preparation of a Novel Perilla Essential Oil/Grape Seed Extract–Chitosan/Gelatin Composite Edible Gel Film and Its Application in the Preservation of Grass Carp" Gels 11, no. 5: 321. https://doi.org/10.3390/gels11050321
APA StyleXue, S., Xu, R., & Liu, J. (2025). Preparation of a Novel Perilla Essential Oil/Grape Seed Extract–Chitosan/Gelatin Composite Edible Gel Film and Its Application in the Preservation of Grass Carp. Gels, 11(5), 321. https://doi.org/10.3390/gels11050321







