Lanthanum Recovery from Aqueous Solutions by Adsorption onto Silica Xerogel with Iron Oxide and Zinc Oxide
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of SFZ
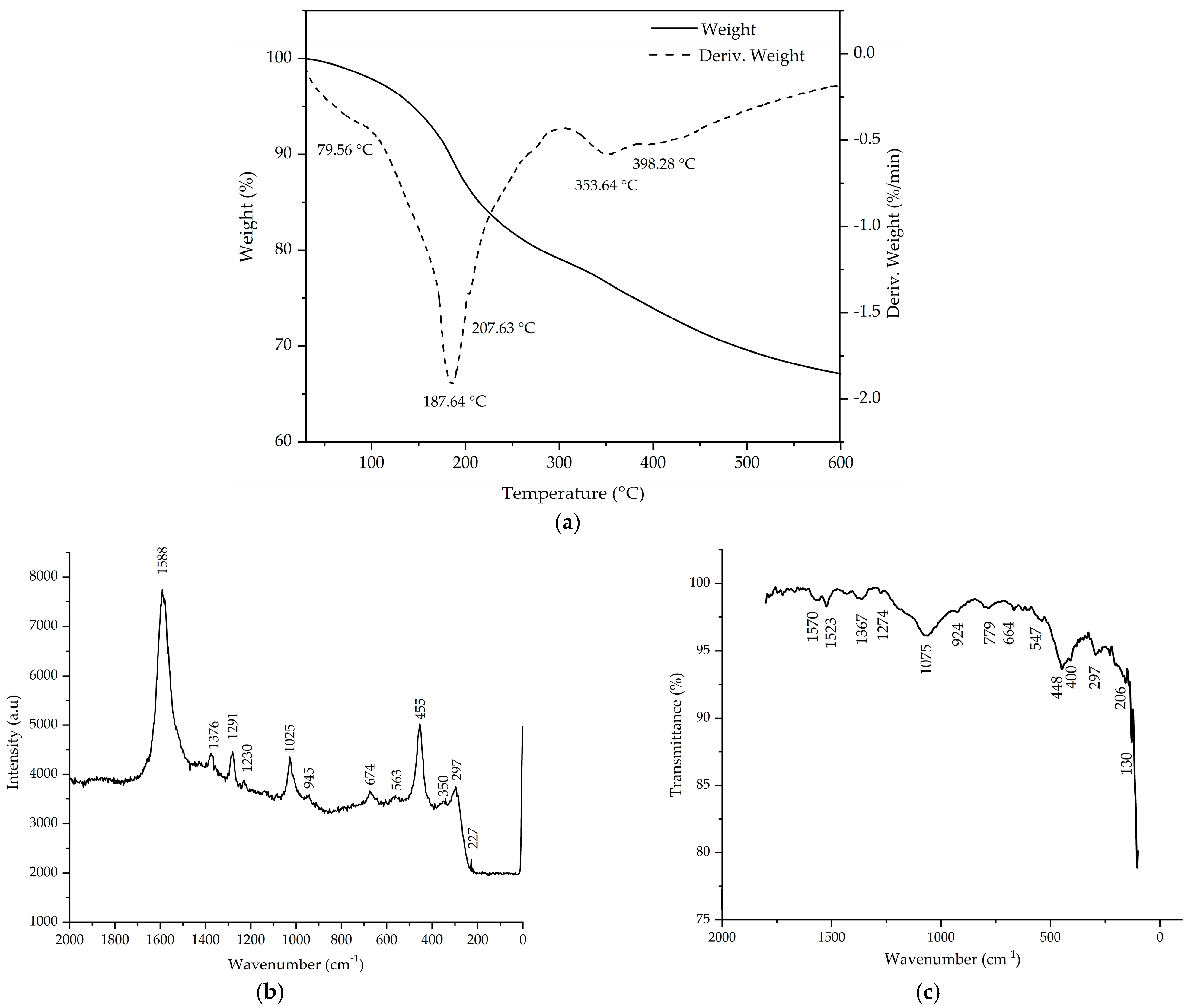
2.1.1. Thermogravimetry and Differential Thermogravimetry (TG–DTG), Raman and Fourier Transform Infrared Spectroscopy (FT–IR) Methods of Investigation
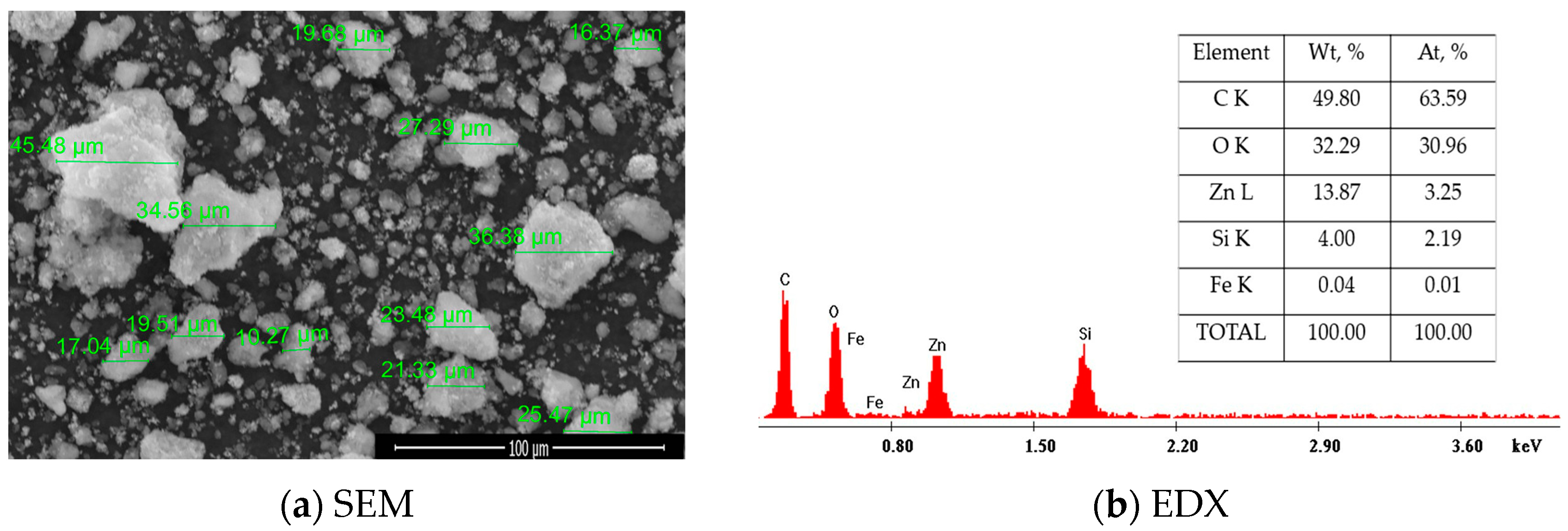
2.1.2. Scanning Electron Microscopy Analysis (SEM) Coupled with Energy-Dispersive X-Ray Spectroscopy (EDX)
2.1.3. Atomic Force Microscopy (AFM)
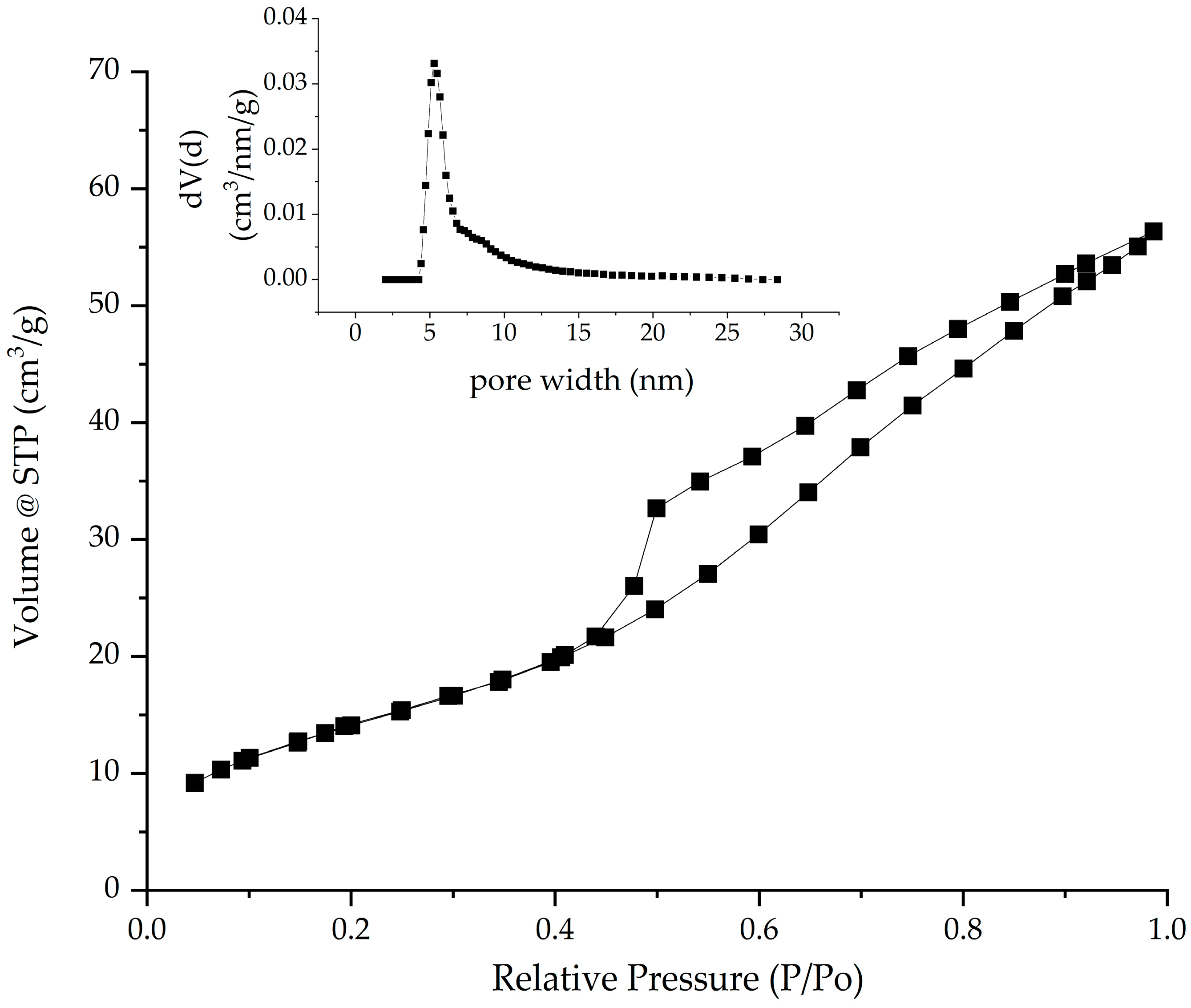
2.1.4. Specific Surface Area Evaluated by BET (Brunauer–Emmett–Teller) Method
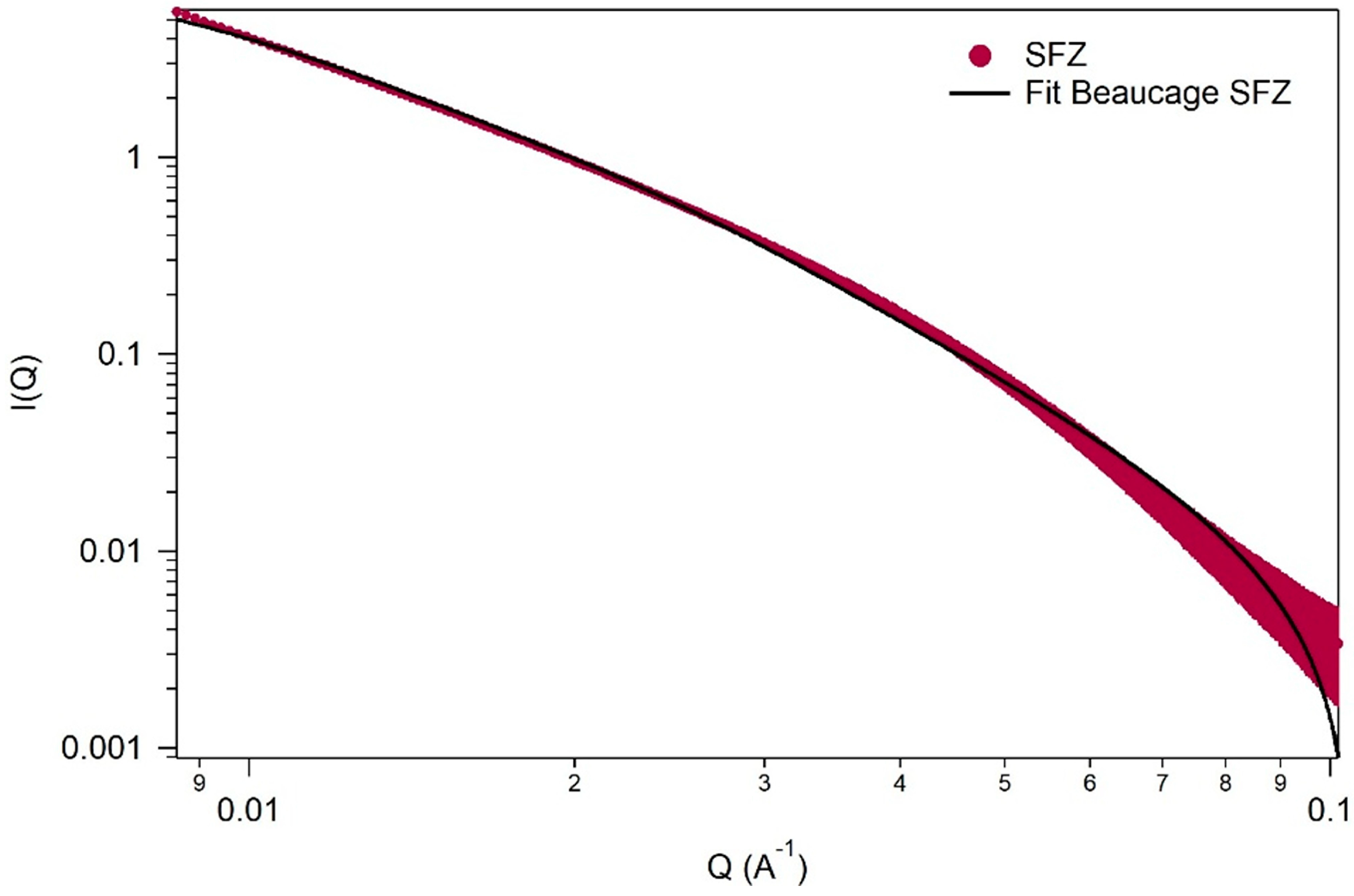
2.1.5. Small-Angle X-Ray Scattering (SAXS) Measurements
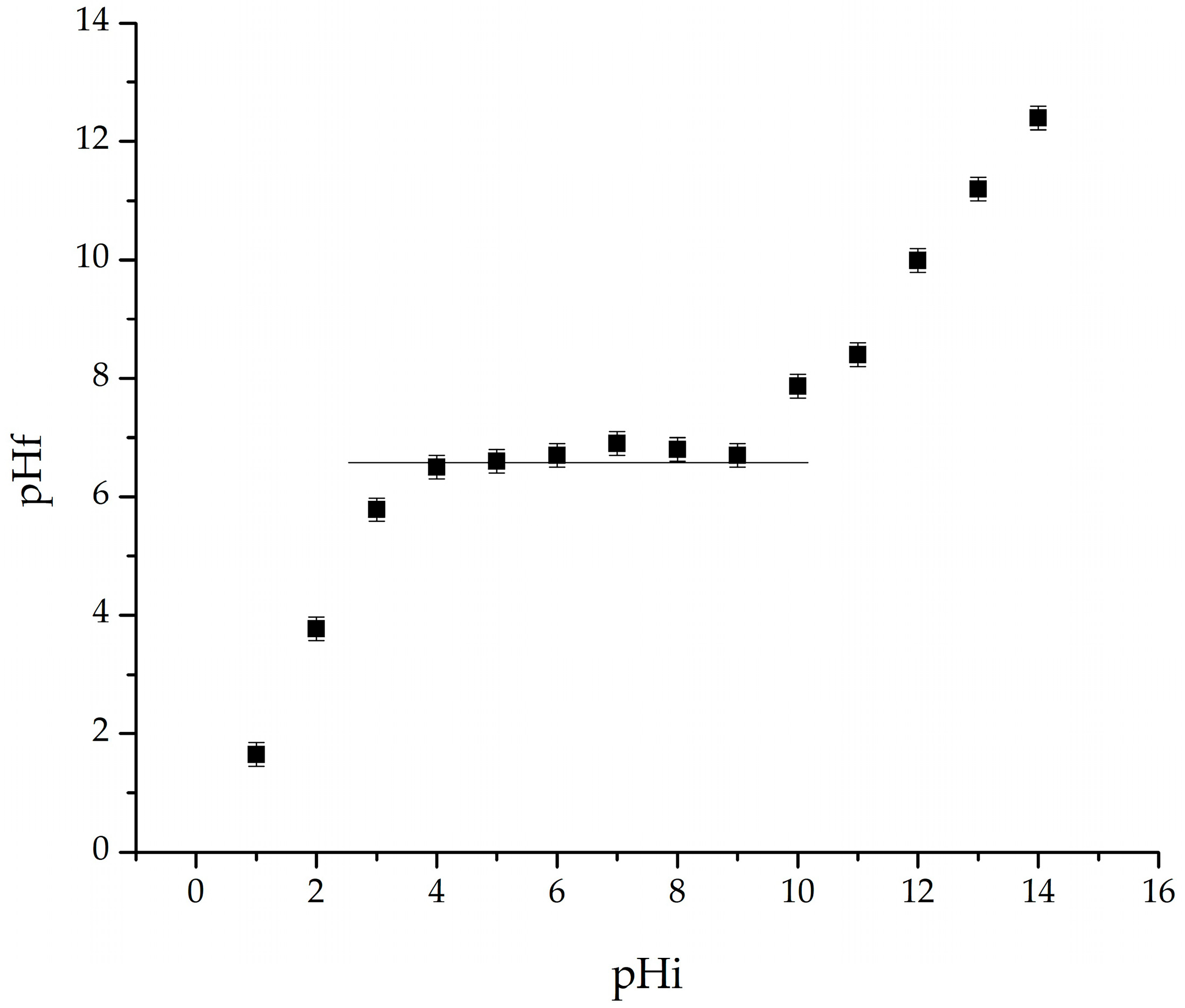
2.1.6. Determination of Point of Zero Charge (pHpZc) for Adsorbent Particle
2.2. Adsorption Studies
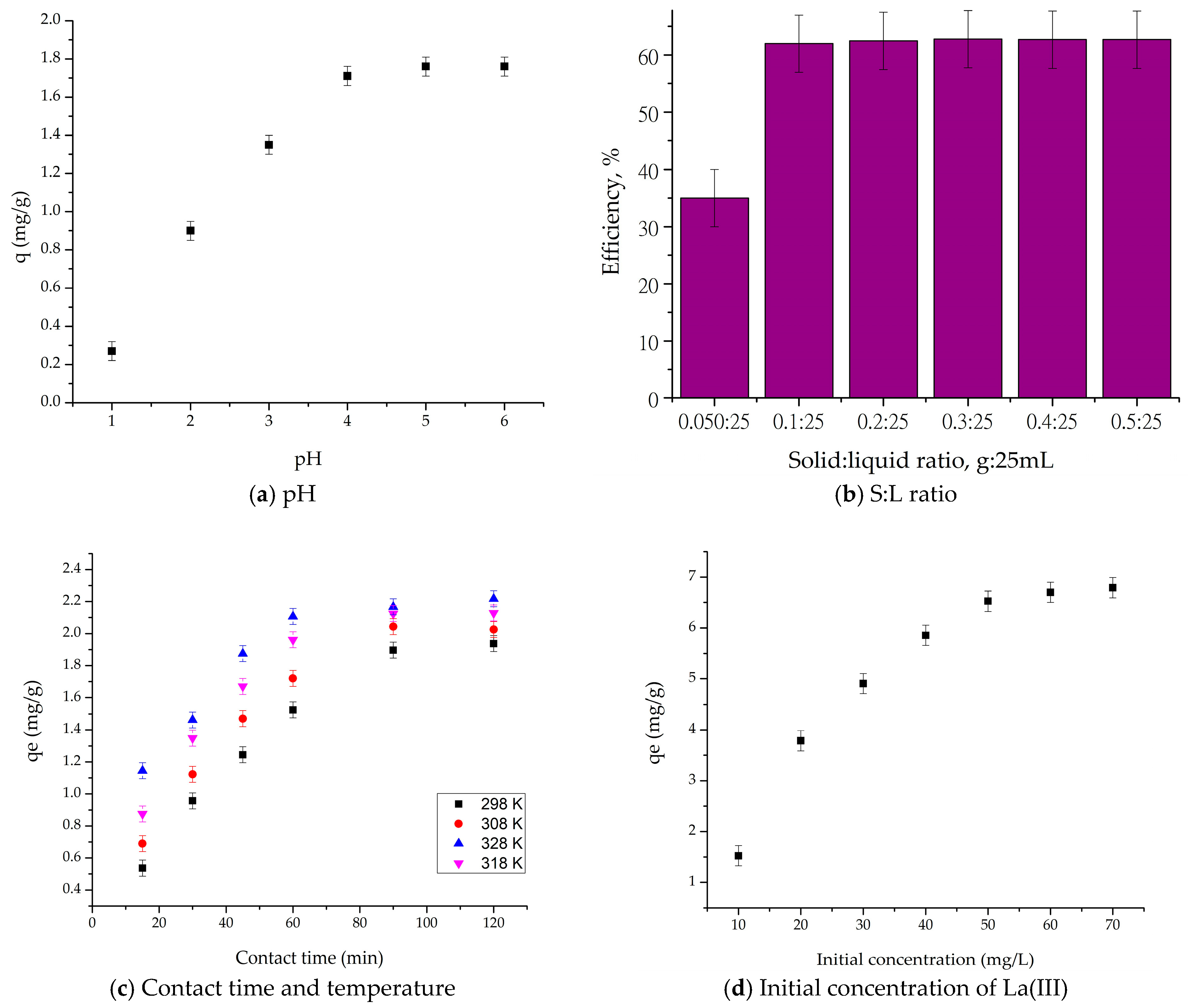
2.2.1. Adsorption Parameter Optimization for La(III) Recovery
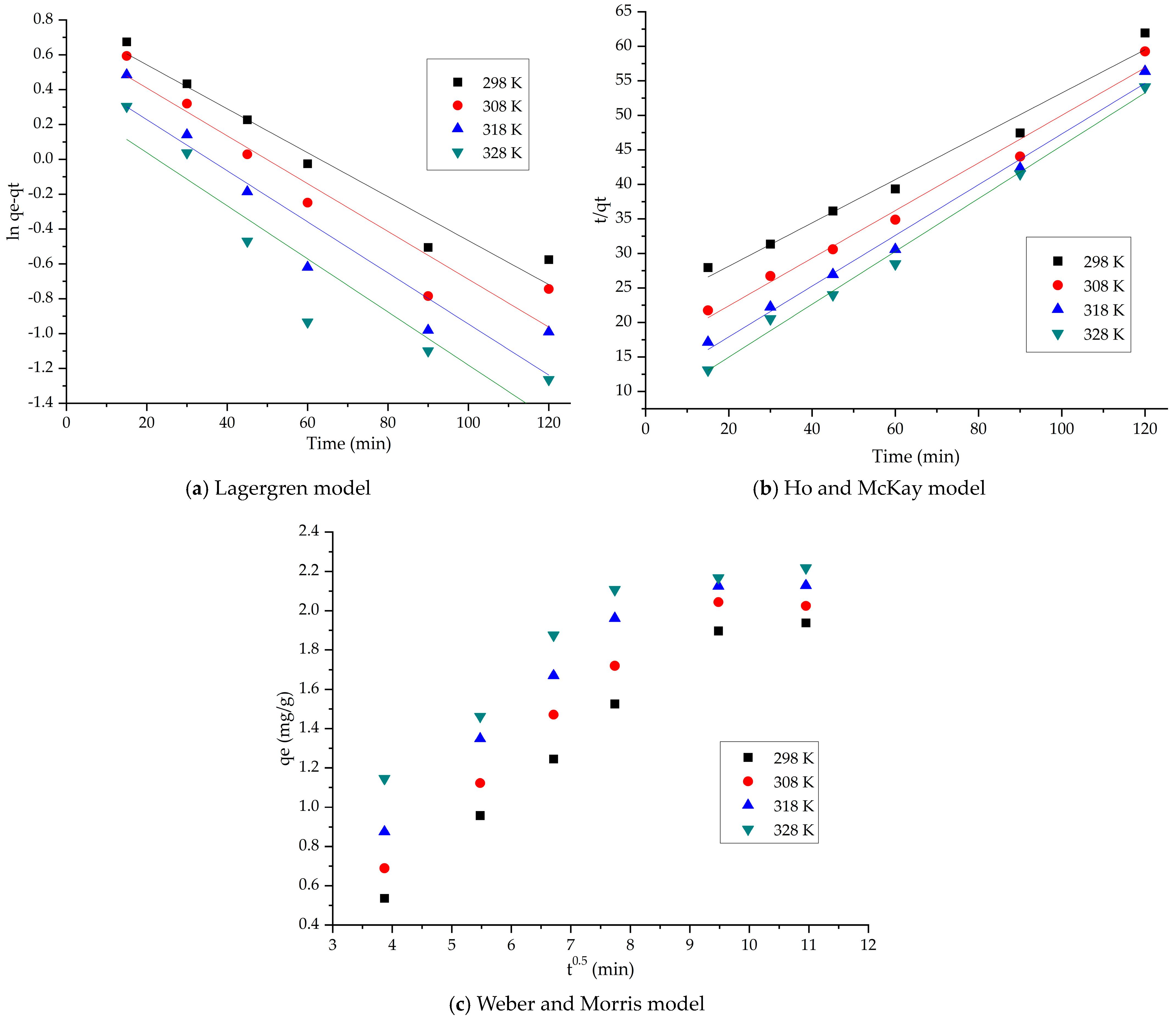
2.2.2. Kinetic Studies
2.2.3. Thermodynamic Studies
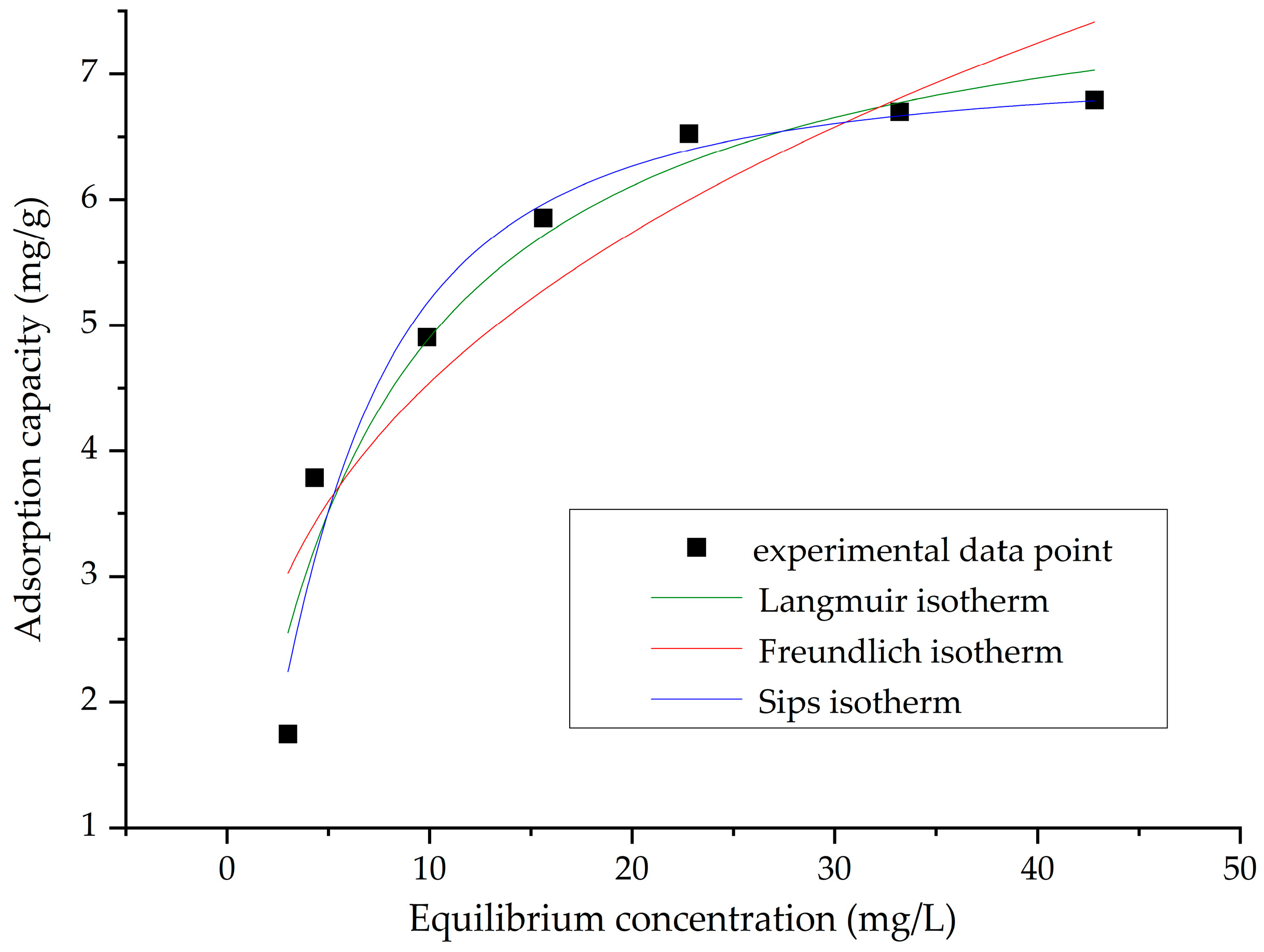
2.2.4. Equilibrium Studies
2.3. Regeneration Degree of Adsorbent
3. Conclusions
4. Materials and Methods
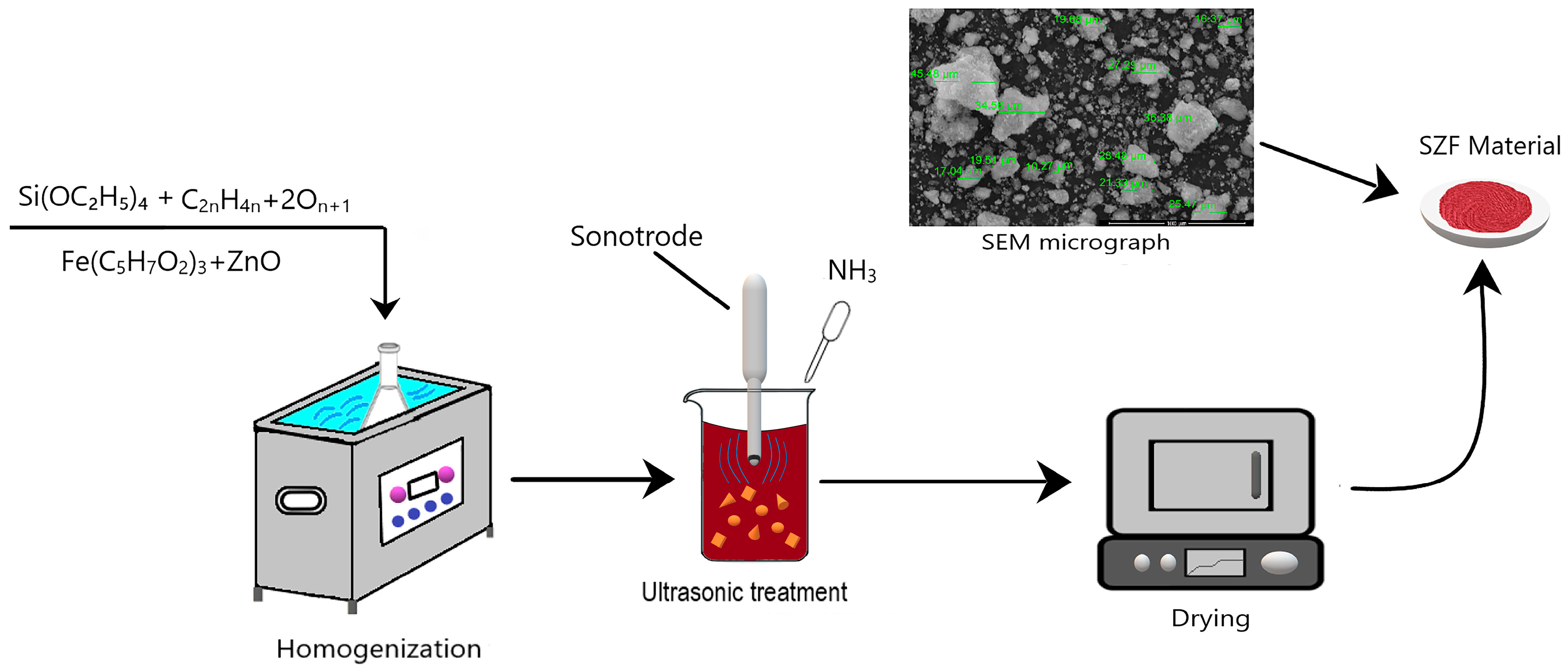
4.1. Synthesis of SFZ
4.2. Characterization of SFZ
- —scattered intensity;
- —gyration radius;
- —scattering variable;
- —radius of gyration of the mass fractal object;
- —Porod constant;
- —2Gi/Rg.i.
4.3. Adsorption Studies
4.3.1. Solid/Liquid (S/L) Ratio Effect
- —La(III) initial concentration (mg/L)
- —La(III) residual concentration (mg/L)
4.3.2. pH Effect
- —adsorption capacity (mg/g)
- —La(III) initial concentration (mg/L)
- —La(III) residual concentration (mg/L)
- —solution volume (L)
- —SFZ material mass (g)
4.3.3. Contact Time and Temperature Effect
4.3.4. Initial Concentration Effect
4.4. Modeling of Sorption Isotherms, Kinetics and Thermodynamics
4.4.1. Adsorption Isotherms
- —equilibrium adsorption capacity (mg/g)
- —equilibrium concentration (mg/L)
- —Langmuir maximum adsorption capacity (mg/g)
- —Langmuir constant.
- —maximum adsorption capacity (mg/g)
- —equilibrium concentration (mg/L)
- and —characteristic constants, which can be associated with the relative adsorption capacity of the adsorbent and the adsorption intensity.
- —Sips maximum adsorption capacity (mg/g)
- —constant related to adsorbent adsorption capacity
- —heterogeneity factor.
4.4.2. Adsorption Kinetics
- —equilibrium adsorption capacity (mg/g)
- —adsorption capacity at a specific time t (mg/g)
- pseudo-first-order speed constant (min−1)
- —contact time (min)
- —equilibrium absorption capacity (mg/g)
- —adsorption capacity at a specific time t (mg/g)
- —pseudo-second-order speed constant (g/mg·min)
- —contact time (min)
- —adsorption capacity at t time (mg/g)
- —speed constant for intraparticle diffusion (mg/g·min1/2)
- —constant correlated with the thickness of the liquid film surrounding the adsorbent particles.
- —rate constant (g/min·mg)
- —Arrhenius constant (g·min/mg)
- —activation energy (kJ/mol)
- —absolute temperature (K)
- —ideal gas constant (8.314 J/mol·K)
4.4.3. Thermodynamics of the Adsorption Process
- —free Gibbs energy standard variation (J/mol)
- —enthalpy standard variation (J/mol)
- —entropy standard variation (J/mol·K)
- —absolute temperature (K)
- —constant of equilibrium
- —standard variation of entropy (J/mol·K)
- —standard variation of enthalpy (kJ/mol)
- —absolute temperature (K)
- —ideal gas constant (8.314 J/mol·K)
4.5. Regeneration Experiments
4.5.1. Regeneration Ratio
- —La(III) equilibrium concentration (mg/L)
- —La(III) residual concentration (mg/L).
4.5.2. Adsorption–Desorption Studies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, J. Pathways for greening the supply of rare earth elements in China. Nat. Sustain. 2018, 1, 598–605. [Google Scholar] [CrossRef]
- Cockell, C.S.; Santomartino, R.; Finster, K.; Waajen, A.C.; Eades, L.J.; Moeller, R.; Rettberg, P.; Fuchs, F.M.; Van Houdt, R.; Leys, N.; et al. Space station biomining experiment demonstrates rare earth element extraction in microgravity and Mars gravity. Nat. Commun. 2020, 11, 5523. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Jowitt, S.; Mudd, G.; Haque, N. A detailed assessment of global rare earth element resources: Opportunities and challenges. Econ. Geol. 2015, 110, 1925–1952. [Google Scholar] [CrossRef]
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Unal Yesiller, S.; Eroğlu, A.E.; Shahwan, T. Removal of aqueous rare earth elements (REEs) using nano-iron based materials. J. Ind. Eng. Chem. 2013, 19, 898–907. [Google Scholar] [CrossRef]
- Oruji, S.; Khoshbin, R.; Karimzadeh, R. Combination of precipitation and ultrasound irradiation methods for preparation of lanthanum-modified Y zeolite nano-catalysts used in catalytic cracking of bulky hydrocarbons. Mater. Chem. Phys. 2019, 230, 131–144. [Google Scholar] [CrossRef]
- Dhanuskar, S.; Naik, S.N.; Pant, K.K. Catalytic cracking and deoxygenation of cottonseed oil to yield light olefins over lanthanum-impregnated zeolite catalysts. Sustain. Energy Fuels 2025, 9, 868–878. [Google Scholar] [CrossRef]
- Jain, P.; Tibrewal, A.; Krishnakumar, V.; Barathraj, R.K. Design, analysis and fabrication of lanthanum strontium manganite catalytic converter. IOP Conf. Ser. Mater. Sci. Eng. 2020, 912, 042051. [Google Scholar] [CrossRef]
- Furfori, S.; Bensaid, S.; Russo, N.; Fino, D. Towards practical application of lanthanum ferrite catalysts for NO reduction with H2. Chem. Eng. J. 2009, 154, 348–354. [Google Scholar] [CrossRef]
- Sicius, H. Rare earth metals: Lanthanides and third subgroup. In Handbook of the Chemical Elements; Sicius, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2024; pp. 875–998. [Google Scholar]
- Alshehri, S.A.; Almotawa, R.M.; Sadeq, M.S.; Alzahrani, J.A.; Mahmoud, A.E.-R.; Amin, H.Y. Unlocking the secrets of colored glass: Structural-optical behaviors and non-linear optical properties of La3+ and Fe3+ ions-doped borates. Opt. Mater. 2024, 157, 116008. [Google Scholar] [CrossRef]
- Mrabet, C.; Boukhachem, A.; Amlouk, M.; Manoubi, T. Improvement of the optoelectronic properties of tin oxide transparent conductive thin films through lanthanum doping. J. Alloys Compd. 2016, 666, 392–405. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Rammah, Y.S.; Laariedh, F.; Abouhaswa, A.S.; Badeche, T.B. Lead borate glasses doped by lanthanum: Synthesis, physical, optical, and gamma photon shielding properties. J. Non-Cryst. Solids 2020, 527, 119731. [Google Scholar] [CrossRef]
- Jeevarajan, J.A.; Lamb, J. Chapter 21-Battery safety. In Safety Design for Space Systems, 2nd ed.; Sgobba, T., Musgrave, G.E., Johnson, G., Kezirian, M.T., Eds.; Butterworth-Heinemann: Oxford, UK, 2023; pp. 825–878. [Google Scholar]
- Na, C.K.; Park, H.J. Defluoridation from aqueous solution by lanthanum hydroxide. J. Hazard. Mater. 2010, 183, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, C.; Zhang, L.; Lu, X.; Liu, Y.; Li, X.; Wang, Y.; Wang, S. Arsenic oxidation and removal from water via core–shell MnO2@La(OH)3 nanocomposite adsorption. Int. J. Environ. Res. Public Health 2022, 19, 10649. [Google Scholar] [CrossRef]
- Biddau, R.; Bensimon, M.; Cidu, R.; Parriaux, A. Rare earth elements in groundwater from different alpine aquifers. Geochemistry 2009, 69, 327–339. [Google Scholar] [CrossRef]
- Kulkarni, P.; Chellam, S.; Fraser, M.P. Lanthanum and lanthanides in atmospheric fine particles and their apportionment to refinery and petrochemical operations in Houston, TX. Atmos. Environ. 2006, 40, 508–520. [Google Scholar] [CrossRef]
- Guan, W.; Zhang, J.; Liu, Q. Quantitative evaluation of anthropogenic sources and health risks of rare earth elements in airborne particulate matter. Sci. Total Environ. 2024, 945, 173960. [Google Scholar] [CrossRef]
- Arienzo, M.; Ferrara, L.; Trifuoggi, M.; Toscanesi, M. Advances in the fate of rare earth elements, REE, in transitional environments: Coasts and estuaries. Water 2022, 14, 401. [Google Scholar] [CrossRef]
- Neira, P.; Romero-Freire, A.; Basallote, M.D.; Qiu, H.; Cobelo-García, A.; Cánovas, C.R. Review of the concentration, bioaccumulation, and effects of lanthanides in marine systems. Front. Mar. Sci. 2022, 9, 920405. [Google Scholar] [CrossRef]
- Herrmann, H.; Nolde, J.; Berger, S.; Heise, S. Aquatic ecotoxicity of lanthanum—A review and an attempt to derive water and sediment quality criteria. Ecotoxicol. Environ. Saf. 2016, 124, 213–238. [Google Scholar] [CrossRef]
- Fraile, P.; Cacharro, L.M.; Garcia-Cosmes, P.; Rosado, C.; Tabernero, J.M. Encephalopathy caused by lanthanum carbonate. Nephrol. Dial. Transplant. Plus 2011, 4, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Rim, K.T.; Koo, K.H.; Park, J.S. Toxicological evaluations of rare earths and their health impacts to workers: A literature review. Saf. Health Work. 2013, 4, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Kampmann, J.; Hansen, N.P.; Ørsted Schultz, A.N.; Brandt, A.H.; Brandt, F. Lanthanum carbonate opacities-a systematic review. Diagnostics 2022, 12, 464. [Google Scholar] [CrossRef]
- Betha, R.; Selvam, V.; Blake, D.R.; Balasubramanian, R. Emission characteristics of ultrafine particles and volatile organic compounds in a commercial printing center. J. Air Waste Manag. Assoc. 2011, 61, 1093–1101. [Google Scholar] [CrossRef][Green Version]
- Dufresne, A.; Krier, G.; Muller, J.F.; Case, B.W.; Perrault, G. Lanthanide particles in the lung of a printer. Sci. Total environment 1994, 151, 249–252. [Google Scholar] [CrossRef]
- Hernández-Campos, M.; Polo, A.M.S.; Sánchez-Polo, M.; Rivera-Utrilla, J.; Berber-Mendoza, M.S.; Andrade-Espinosa, G.; López-Ramón, M.V. Lanthanum-doped silica xerogels for the removal of fluorides from waters. J. Environ. Manag. 2018, 213, 549–554. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Esmaeilzadeh, A. Hybrid silica aerogel nanocomposite adsorbents designed for Cd(II) removal from aqueous solution. Water Environ. Res. 2019, 91, 1624–1637. [Google Scholar] [CrossRef]
- Vareda, J.P.; Durães, L. Efficient adsorption of multiple heavy metals with tailored silica aerogel-like materials. Environmental Technology 2019, 40, 529–541. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, L.; Zhao, Z.; Peng, S.; Yu, C.; Zhang, X.; Zong, Y.; Wu, D. Enhanced phosphate removal by nano-lanthanum hydroxide embedded silica aerogel composites: Superior performance and insights into specific adsorption mechanism. Sep. Purif. Technol. 2022, 285, 120365. [Google Scholar] [CrossRef]
- Akti, F.; Balci, S. Silica xerogel and iron doped silica xerogel synthesis in presence of drying control chemical additives. Mater. Chem. Phys. 2023, 297, 127347. [Google Scholar] [CrossRef]
- Parashar, M.; Shukla, V.K.; Singh, R. Metal oxides nanoparticles via sol–gel method: A review on synthesis, characterization and applications. J. Mater. Sci. Mater. Electron. 2020, 31, 3729–3749. [Google Scholar] [CrossRef]
- Raileanu, M.; Crisan, M.; Petrache, C.; Crisan, D.; Zaharescu, M. Fe2O3-SiO2 nanocomposites obtained by different sol-gel routes. J. Optoelectron. Adv. Mater. 2003, 5, 693–698. [Google Scholar]
- Jitianu, A.; Raileanu, M.; Crisan, M.; Predoi, D.; Jitianu, M.; Stanciu, L.; Zaharescu, M. Fe3O4–SiO2 nanocomposites obtained via alkoxide and colloidal route. J. Sol-Gel Sci. Technol. 2006, 40, 317–323. [Google Scholar] [CrossRef]
- Kumar, R.V.; Koltypin, Y.; Cohen, Y.; Cohen, Y.; Aurbach, D.; Palchik, O.; Felner, I.; Gedanken, A. Preparation of amorphous magnetite nanoparticles embedded in polyvinyl alcohol using ultrasound radiation. J. Mater. Chem. 2000, 10, 1125–1129. [Google Scholar] [CrossRef]
- Ianasi, C.; Costisor, O.; Putz, A.-M.; Lazau, R.; Negrea, A.; Niznansky, D.; Sacarescu, L.; Savii, C. Low temperature superparamagnetic nanocomposites obtained by Fe(acac)3-SiO2-PVA hybrid xerogel thermolysis. Process. Appl. Ceram. 2016, 10, 265–275. [Google Scholar] [CrossRef]
- Pal, B.; Sharon, M. Preparation of iron oxide thin film by metal organic deposition from Fe(III)-acetylacetonate: A study of photocatalytic properties. Thin Solid Film. 2000, 379, 83–88. [Google Scholar] [CrossRef]
- Ismail, H.M. A thermoanalytic study of metal acetylacetonates. J. Anal. Appl. Pyrolysis 1991, 21, 315–326. [Google Scholar] [CrossRef]
- Putz, A.-M.; Ianăși, C.; Dudás, Z.; Coricovac, D.; Watz, C.; Len, A.; Almásy, L.; Sacarescu, L.; Dehelean, C. SiO2-PVA-Fe(acac)3 hybrid based superparamagnetic nanocomposites for nanomedicine: Morpho-textural evaluation and in vitro cytotoxicity assay. Molecules 2020, 25, 653. [Google Scholar] [CrossRef]
- Panczer, G.; De Ligny, D.; Mendoza, C.; Gaft, M.; Seydoux-Guillaume, A.-M.; Wang, X. Raman and fluorescence. Raman Spectrosc. Appl. Earth Sci. Cult. Herit. 2012, 12, 61–82. [Google Scholar] [CrossRef]
- Cai, J.; Chen, S.; Ji, M.; Hu, J.; Ma, Y.; Qi, L. Organic additive-free synthesis of mesocrystalline hematite nanoplates via two-dimensional oriented attachment. CrystEngComm 2014, 16, 1553–1559. [Google Scholar] [CrossRef]
- Mladin, G.; Ciopec, M.; Negrea, A.; Duteanu, N.; Negrea, P.; Ianasi, P.; Ianași, C. Silica-iron oxide nanocomposite enhanced with porogen agent used for arsenic removal. Materials 2022, 15, 5366. [Google Scholar] [CrossRef] [PubMed]
- Ianăşi, C.; Ianăşi, P.; Negrea, A.; Ciopec, M.; Ivankov, O.I.; Kuklin, A.I.; Almásy, L.; Putz, A.-M. Effects of catalysts on structural and adsorptive properties of iron oxide-silica nanocomposites. Korean J. Chem. Eng. 2021, 38, 292–305. [Google Scholar] [CrossRef]
- Matusoiu, F.; Negrea, A.; Nemes, N.S.; Ianasi, C.; Ciopec, M.; Negrea, P.; Duteanu, N.; Ianasi, P.; Duda-Seiman, D.; Muntean, D. Antimicrobial perspectives of active SiO2FexOy/ZnO composites. Pharmaceutics 2022, 14, 2063. [Google Scholar] [CrossRef]
- Lenza, R.F.; Vasconcelos, W.L. Structural evolution of silica sols modified with formamide. Mater. Res. 2001, 4, 175–179. [Google Scholar] [CrossRef]
- Motoc, S.; Ianasi, C.; Anamaria, B.; Delcioiu, C.; Sacarescu, L.; Lacrămă, A.-M.; Manea, F. Humic acid removal from water by sorption and photocatalysis under Vis irradiation using Fe2O3/silica nanocomposite. Environ. Eng. Manag. J. 2021, 20, 335–345. [Google Scholar] [CrossRef]
- Ivetic, T.; Dimitrievska, M.; Gúth, I.; Dacanin, L.; Lukic-Petrovic, S. Structural and optical properties of europium-doped zinc oxide nanopowders prepared by mechanochemical and combustion reaction methods. J. Res. Phys. 2012, 36, 43. [Google Scholar] [CrossRef]
- Gandomi, F.; Vakili, M.; Darugar, V.; Takjoo, R.; Tayyari, S. Optimized molecular geometry, vibrational analysis, and Fe-O bond strength of Tris(α-cyanoacetylacetonate)iron(III):An experimental and theoretical study. J. Mol. Struct. 2021, 1248, 131444. [Google Scholar] [CrossRef]
- Geisler, T.; Dohmen, L.; Lenting, C.; Fritzsche, M.B.K. Real-time in situ observations of reaction and transport phenomena during silicate glass corrosion by fluid-cell Raman spectroscopy. Nat. Mater. 2019, 18, 342–348. [Google Scholar] [CrossRef]
- Santillán, J.M.J.; Muñetón Arboleda, D.; Coral, D.F.; Fernández van Raap, M.B.; Muraca, D.; Schinca, D.C.; Scaffardi, L.B. Optical and magnetic properties of Fe nanoparticles fabricated by femtosecond laser ablation in organic and inorganic solvents. ChemPhysChem 2017, 18, 1192–1209. [Google Scholar] [CrossRef]
- Uma, K.; Chen, S.W.; Arjun, N.; Pan, G.T.; Yang, T.C. The production of an efficient visible light photocatalyst for CO oxidation through the surface plasmonic effect of Ag nanoparticles on SiO2@α-Fe2O3 nanocomposites. RSC Adv. 2018, 8, 12547–12555. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Bhatt, R.; Katare, R. Atomic force microscopy enabled roughness analysis of nanostructured poly (diaminonaphthalene) doped poly (vinyl alcohol) conducting polymer thin films. Micron 2016, 90, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Maksumov, A.; Vidu, R.; Palazoglu, A.; Stroeve, P. Enhanced feature analysis using wavelets for scanning probe microscopy images of surfaces. J. Colloid Interface Sci. 2004, 272, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Bazaka, K.; Jacob, M.V.; Truong, V.K.; Crawford, R.J.; Ivanova, E.P. The effect of polyterpenol thin film surfaces on bacterial viability and adhesion. Polymers 2011, 3, 388–404. [Google Scholar] [CrossRef]
- Sedlaček, M.; Gregorčič, P.; Podgornik, B. Use of the roughness parameters Ssk and Sku to control friction—A method for designing surface texturing. Tribol. Trans. 2017, 60, 260–266. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Beaucage, G. Approximations leading to a unified exponential/power-law approach to small-angle scattering. J. Appl. Crystallogr. 1995, 28, 717–728. [Google Scholar] [CrossRef]
- Beaucage, G. Small-angle scattering from polymeric mass fractals of arbitrary mass-fractal dimension. J. Appl. Crystallogr. 1996, 29, 134–146. [Google Scholar] [CrossRef]
- Savii, C.; Almásy, L.; Ionescu, C.; Székely, N.K.; Enache, C.; Popovici, M.I.; Sora, I.; Nicoara, D.; Savii, G.; Resiga, D.; et al. Mesoporous silica matrices derived from sol-gel process assisted by low power ultrasonic activation. Proc. Appl. Cer. 2009, 3, 59–64. [Google Scholar] [CrossRef]
- Anitas, E. Small-angle scattering from mass and surface fractals. In Complexity in Biological and Physical Systems—Bifurcations, Solitons and Fractals; López-Ruiz, R., Ed.; IntechOpen: Rijeka, Yugoslavia, 2017. [Google Scholar]
- Kosmulski, M. The pH dependent surface charging and points of zero charge. Adv. Colloid Interface Sci. 2020, 275, 102064. [Google Scholar] [CrossRef]
- Al-Maliky, E.A.; Gzar, H.A.; Al-Azawy, M.G. Determination of point of zero charge (PZC) of concrete particles adsorbents. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1184, 012004. [Google Scholar] [CrossRef]
- He, Z.; Ren, B.; Hursthouse, A.; Wang, Z. Efficient removal of Cd(II) using SiO2-Mg(OH)2 nanocomposites derived from sepiolite. Int. J. Environ. Res. Public Health 2020, 17, 2223. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, E.; Carmenica Doina, J.; Trofin, A.; Fortuna, M.; Ungureanu, O.; Ariton, A.; Trinca, L.C.; Brezuleanu, S.; Popa, V. Use of Sarkanda Grass lignin as a possible adsorbent for As (III) from aqueous solutions—Kinetic and equilibrium studies. Cellul. Chem. Technol. 2022, 56, 681–689. [Google Scholar] [CrossRef]
- Zhang, S.; Ning, S.; Liu, H.; Zhou, J.; Wang, S.; Zhang, W.; Wang, X.; Wei, Y. Highly-efficient separation and recovery of ruthenium from electroplating wastewater by a mesoporous silica-polymer based adsorbent. Microporous Mesoporous Mater. 2020, 303, 110293. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, F.; Cheng, W.; Wang, J.; Ma, J. Adsorption equilibrium and kinetics of the removal of ammoniacal nitrogen by zeolite X/activated carbon composite synthesized from elutrilithe. J. Chem. 2017, 2017, 1936829. [Google Scholar] [CrossRef]
- He, Q.; Chen, X.; Gong, S.; Huang, L.; Xiao, Y. Adsorption behaviors and mechanisms of lanthanum ions and exchanger cations at halloysite-solution interface: Perspectives from electrical double layer model and spectral analyses. J. Mol. Liq. 2024, 411, 125711. [Google Scholar] [CrossRef]
- El-Sofany, E.A. Removal of lanthanum and gadolinium from nitrate medium using Aliquat-336 impregnated onto Amberlite XAD-4. J. Hazard. Mater. 2008, 153, 948–954. [Google Scholar] [CrossRef]
- Sepehrian, H.; Cheraghali, R.; Rezaei, P.; Abdi, H. Adsorption behavior of lanthanum on modified mesoporous aluminosilicates. Int. J. Ind. Chem. 2011, 2, 235–241. [Google Scholar]
- Varshini, C.; Das, N. Relevant approach to assess the performance of biowaste materials for the recovery of Lanthanum (III) from aqueous medium. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 88–94. [Google Scholar]
- Chaibou Yacouba, A.-R.; Oral, A.E.; Sert, S.; Kaptanoglu, I.G.; Natatou, I.; Yusan, S.; Aytas, S. Removal of lanthanum and cerium from aqueous solution using chitosan-functionalized magnetite-pectin. Discov. Water 2024, 4, 1. [Google Scholar] [CrossRef]
- Granados-Correa, F.; Vilchis-Granados, J.; Jiménez-Reyes, M.; Quiroz-Granados, L.A. Adsorption behaviour of La(III) and Eu(III) ions from aqueous solutions by hydroxyapatite: Kinetic, isotherm, and thermodynamic studies. J. Chem. 2013, 2013, 751696. [Google Scholar] [CrossRef]
- Iftekhar, S.; Srivastava, V.; Casas, A.; Sillanpää, M. Synthesis of novel GA-g-PAM/SiO2 nanocomposite for the recovery of rare earth elements (REE) ions from aqueous solution. J. Clean. Prod. 2018, 170, 251–259. [Google Scholar] [CrossRef]
- Patnaik, P. Handbook of Inorganic Chemicals; McGraw-Hill: New York, NY, USA, 2003; Volume 529. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Über die adsorption in lösungen. Z. Für Phys. Chemie 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Sips, R. On the structure of a catalyst surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. K. Sven. Vetenskapsakademiens Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.-S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef]
- Weber, W.J., Jr.; Morris, J.C. Equilibria and capacities for adsorption on carbon. J. Sanit. Eng. Div. 1964, 90, 79–108. [Google Scholar] [CrossRef]
- Atkins, P.W.; De Paula, J.; Keeler, J. Atkins’ Physical Chemistry; Oxford University Press: Oxford, UK, 2023. [Google Scholar]
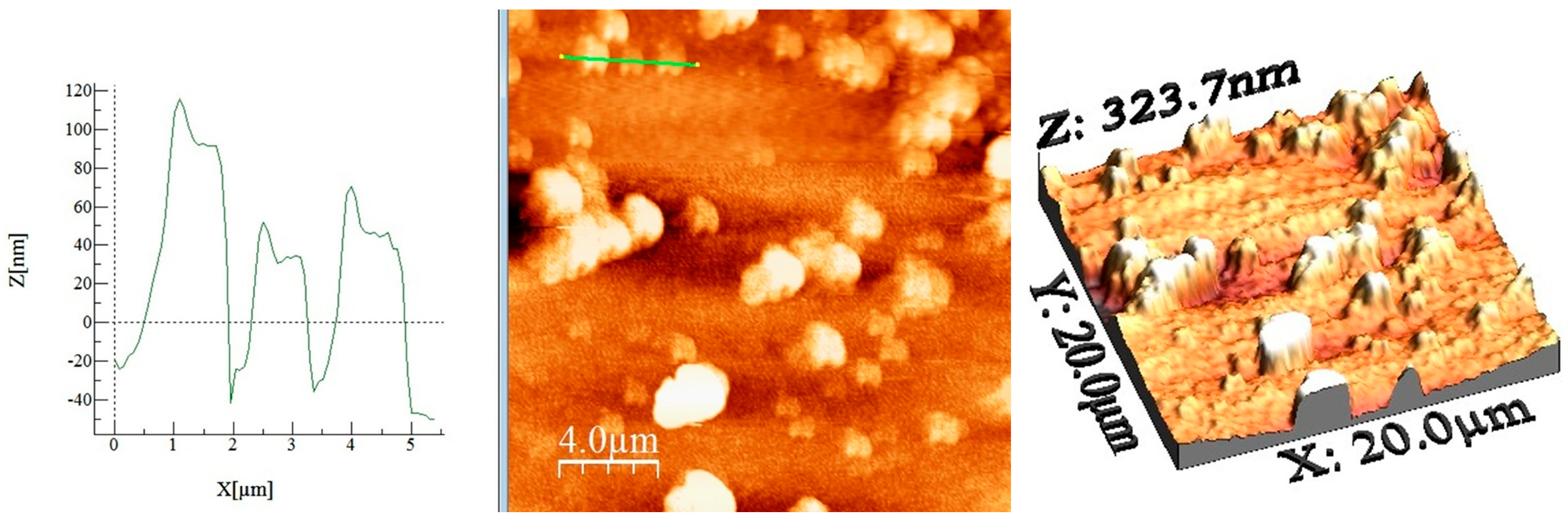


| Sample Name | Ironed Area (µm2) | Sa (nm) | Sq (µm) | Sp (nm) | Sv (nm) | Sy (nm) | Sku | Ssk |
|---|---|---|---|---|---|---|---|---|
| SFZ | 422.156 | 39.5616 | 53.9842 | 176.330 | −147.405 | 323.735 | 4.0171 | 0.7808 |
| Lagergren (Pseudo-First-Order) Model | ||||
|---|---|---|---|---|
| Temperature (K) | qe,exp (mg/g) | k1 (min−1) | qe,calc (mg/g) | R2 |
| 298 | 1.89 | 0.0102 | 2.05 | 0.9554 |
| 308 | 2.04 | 0.0131 | 1.96 | 0.9165 |
| 318 | 2.12 | 0.0147 | 1.68 | 0.8904 |
| 328 | 2.16 | 0.0152 | 1.40 | 0.8721 |
| Ho and McKay (Pseudo-Second-Order) Model | ||||
| Temperature (K) | qe,exp (mg/g) | k2 (g/mg∙min) | qe,calc (mg/g) | R2 |
| 298 | 1.89 | 0.46 | 3.18 | 0.9784 |
| 308 | 2.04 | 0.54 | 2.90 | 0.9830 |
| 318 | 2.12 | 0.70 | 2.72 | 0.9901 |
| 328 | 2.16 | 0.93 | 2.61 | 0.9935 |
| Weber and Morris (IPD) Model | ||||
| Temperature (K) | Kdiff (mg/g·min1/2) | C | R2 | |
| 298 | 0.156 | 0.151 | 0.9548 | |
| 308 | 0.181 | 0.349 | 0.9168 | |
| 318 | 0.198 | 0.452 | 0.8673 | |
| 328 | 0.207 | 0.676 | 0.8365 | |
| ΔH° (kJ/mol) | ΔS° (J/mol∙K) | ΔG° (kJ/mol) | R2 | |||
|---|---|---|---|---|---|---|
| 21.76 | 71.06 | 298 K | 308 K | 318 K | 328 K | 0.9839 |
| −21.1 | −21.8 | −22.5 | −23.2 | |||
| Langmuir Isotherm | |||
|---|---|---|---|
| qm,exp (mg/g) | KL (L/mg) | qL (mg/g) | R2 |
| 6.7 | 0.153 | 8.1 | 0.9374 |
| Freundlich Isotherm | |||
| KF (mg/g) | 1/nF | R2 | |
| 2.09 | 0.33 | 0.8325 | |
| Sips Isotherm | |||
| KS | qS (mg/g) | 1/nS | R2 |
| 0.09 | 7.07 | 0.4 | 0.9451 |
| Materials | Adsorption Capacities, mg/g | References |
|---|---|---|
| Halloysite | 5.65 | [68] |
| Aliquat-336 impregnated onto Amberlite XAD-4 | 3.29 | [69] |
| Nanoporous aluminosilicates | 1.25 | [70] |
| Neem sawdust | 2.3 | [71] |
| Magnetite-pectin-chitosan | 8.17 | [72] |
| Hydroxyapatite | 0.25 | [73] |
| GA-g-PAM/SiO2 | 7.9 | [74] |
| SFZ | 6.7 | This paper |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bălescu, I.; Ciopec, M.; Negrea, A.; Nemeş, N.S.; Ianăşi, C.; Verdes, O.; Suba, M.; Svera, P.; Pascu, B.; Negrea, P.; et al. Lanthanum Recovery from Aqueous Solutions by Adsorption onto Silica Xerogel with Iron Oxide and Zinc Oxide. Gels 2025, 11, 314. https://doi.org/10.3390/gels11050314
Bălescu I, Ciopec M, Negrea A, Nemeş NS, Ianăşi C, Verdes O, Suba M, Svera P, Pascu B, Negrea P, et al. Lanthanum Recovery from Aqueous Solutions by Adsorption onto Silica Xerogel with Iron Oxide and Zinc Oxide. Gels. 2025; 11(5):314. https://doi.org/10.3390/gels11050314
Chicago/Turabian StyleBălescu, Ionuţ, Mihaela Ciopec, Adina Negrea, Nicoleta Sorina Nemeş, Cătălin Ianăşi, Orsina Verdes, Mariana Suba, Paula Svera, Bogdan Pascu, Petru Negrea, and et al. 2025. "Lanthanum Recovery from Aqueous Solutions by Adsorption onto Silica Xerogel with Iron Oxide and Zinc Oxide" Gels 11, no. 5: 314. https://doi.org/10.3390/gels11050314
APA StyleBălescu, I., Ciopec, M., Negrea, A., Nemeş, N. S., Ianăşi, C., Verdes, O., Suba, M., Svera, P., Pascu, B., Negrea, P., & Buzatu, A. R. (2025). Lanthanum Recovery from Aqueous Solutions by Adsorption onto Silica Xerogel with Iron Oxide and Zinc Oxide. Gels, 11(5), 314. https://doi.org/10.3390/gels11050314












