Advancements and Prospects of pH-Responsive Hydrogels in Biomedicine
Abstract
1. Introduction
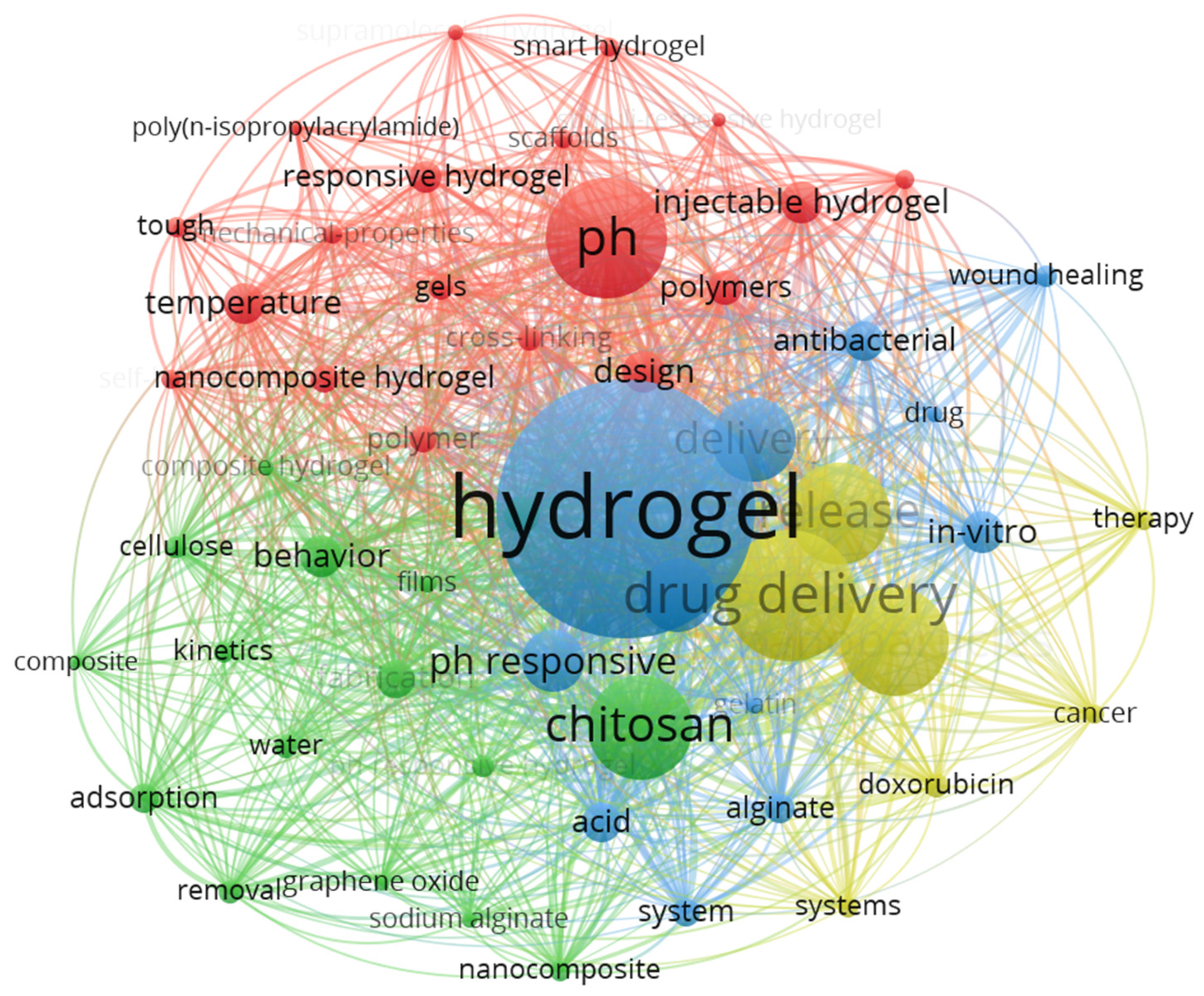
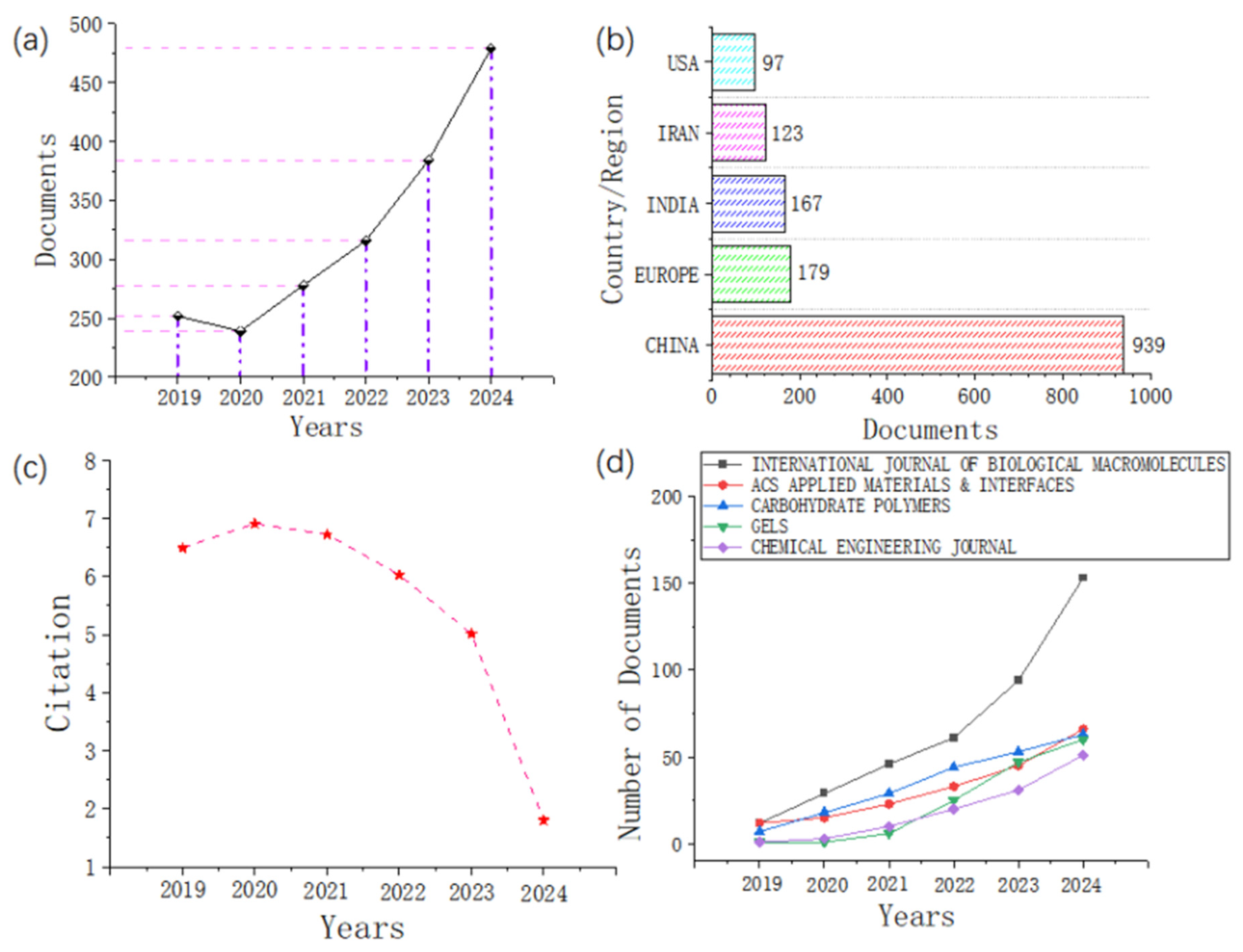
2. Bibliometric Analysis
2.1. Software Overview
2.2. Data Screening
2.3. Statistical Analysis
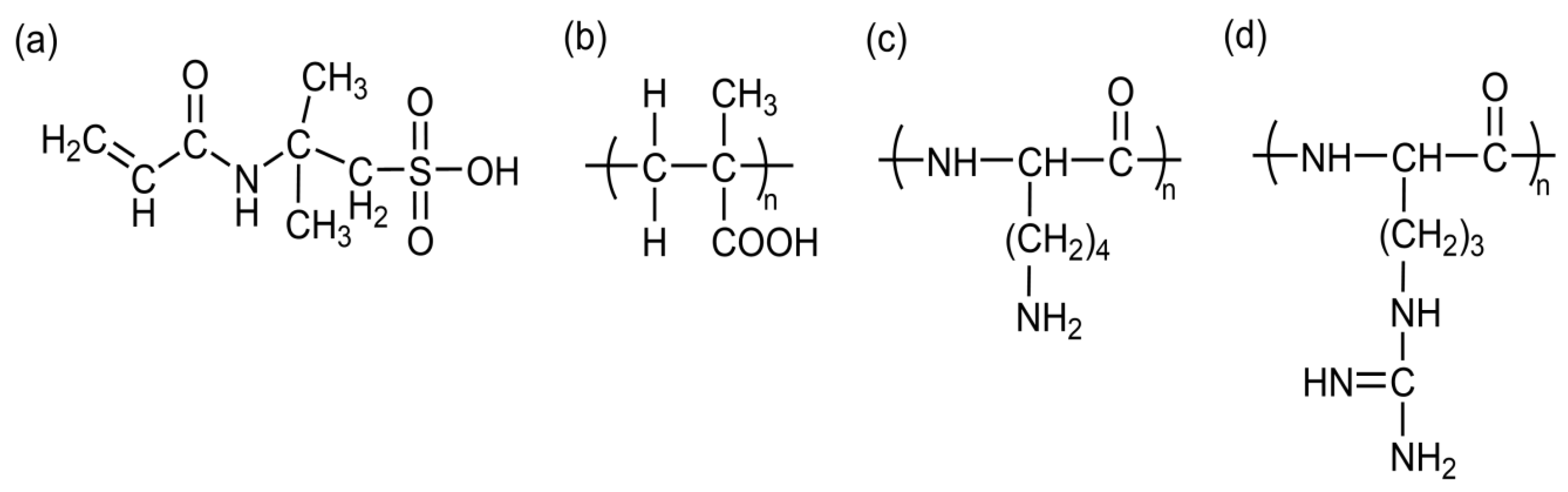
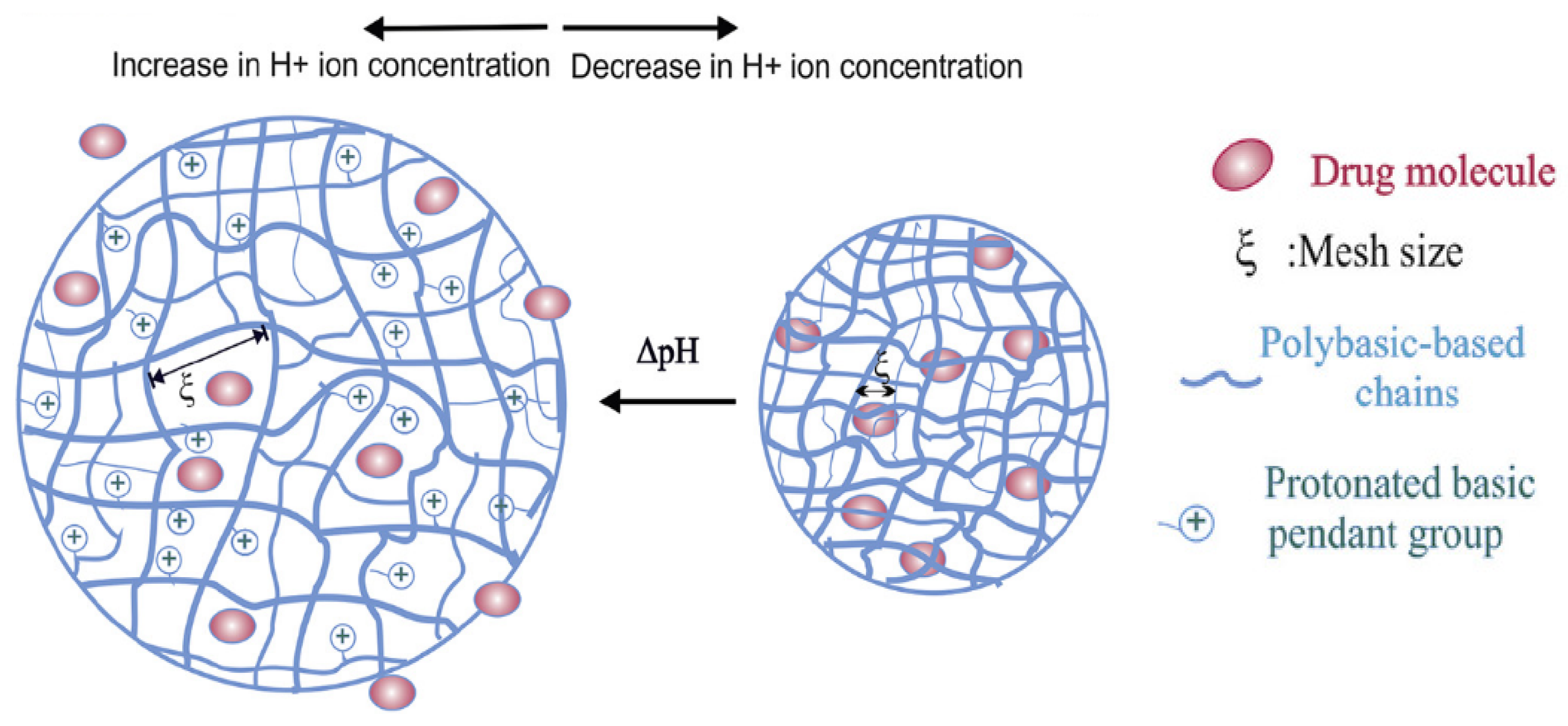
3. Principles of pH-Responsive Hydrogels
4. pH-Sensitive Multistimuli-Responsive Hydrogels
4.1. Dual-Responsive Hydrogels
4.1.1. pH/Temperature Dual-Responsive Hydrogels
4.1.2. pH/Redox Dual-Responsive Hydrogels
4.1.3. pH/Enzyme Dual-Responsive Hydrogels
4.1.4. pH/Glucose Dual-Responsive Hydrogels
4.2. Triple-Responsive Hydrogels
5. Applications of pH-Responsive Hydrogels
5.1. Innovations in Drug Delivery Systems
5.2. Tissue Engineering and Regenerative Medicine
5.3. Diagnostics and Biosensing
6. Advanced Materials and Technological Innovation
6.1. Multifunctional Integrated Hydrogels
6.2. Biomimetic Design
6.3. Microfluidic Technology
7. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAEM | Acetoacetoxyethyl methacrylate |
| AAM | Acrylamide |
| ADR | Anthracycline |
| ARX | Arabinoxylan |
| BACy | N,N-bis(acryloyl) cystamine |
| BBR | Berberine |
| BC | Bacterial cellulose |
| BPCH-B | Bamboo cellulose and in situ crosslinked carboxylated β-cyclodextrin |
| BSA | Bovine serum albumin |
| CA | L-arginine-modified chitosan |
| CCM | Cancer cell membrane |
| CDE | L-cystine dimethyl ester dihydrochloride |
| CHX | Chlorhexidine;methacrylated pectin |
| CLC | Cholesteric liquid crystal |
| CLCsolid | Cholesteric liquid crystal solid |
| CNPs | Cell membrane-coated nanoparticles |
| CS | Chitosan |
| DFUs | Diabetic foot ulcers |
| DMPA | N-[3-(dimethylamino)propyl]methacrylamide |
| DOX | Doxorubicin |
| DTT | Dithiothreitol |
| FA | Folic acid |
| GelMA | Gelatin methacryloyl |
| GO | Graphene oxide |
| GP | Glycerophosphate |
| GS | Gentamicin sulfate |
| GSH | Glutathione |
| HA | Hyaluronic acid |
| HA-CHO | Hyaluronic acid aldehyde |
| HP-β-CD | hydroxypropyl-β-cyclodextrin |
| IL | Interleukin |
| IPN | Interpenetrating polymer network |
| Kana | Kanamycin |
| Lox | lactate oxidase |
| LZM | Lysozyme |
| MAG | Magnolol |
| Met | Metformin |
| MMA | Methyl methacrylate |
| MOF | Metal–organic framework |
| MSCO | composite nanozyme |
| MSN@PDA | Polydopamine-modified mesoporous silica nanoparticles |
| NIPAAM | N-isopropylacrylamide |
| OCS | Oxidized chondroitin sulfate |
| ODP | phenylboronic acid modified oxidized dextran |
| OSU | Oxidized sucrose crosslinker |
| PAA | Polyacrylic acid |
| PB | Poly(acrylamide-co-dimethylaminopropylacrylamide-co-methacrylamidophenylboronic acid) |
| PDEA | Poly(2-diethylaminoethyl acrylate) |
| PED | Pirfenidone |
| PEG | Polyethylene glycol |
| PeMA | Methacrylated pectin |
| PLP | Poly(l-lysine isophthalamide) |
| PMAA | Poly(methacrylic acid) |
| PNIAAm | Poly(N-isopropylacrylamide) |
| PPE | polyethylene glycol-polycaprolactone-poly-β-aminoester triblock copolymer micelles |
| PPEL | PPE micelles loaded with Lox |
| PTX | Paclitaxel |
| PVA | Polyvinyl alcohol |
| ROS | Reactive oxygen species |
| SEBS | Poly(styrene-b-(ethylene-co-butylene)-b-styrene) |
| SGF | Simulated gastric fluid |
| SIF | Simulated intestinal fluid |
| SiNPs | Silica nanoparticles |
| TEOS | Tetraethyl orthosilicate |
| TH | Tetracycline hydrochloride |
| VCL | N-vinylcaprolactam |
| VIm | Vinylimidazole |
| ZIF−8 | Zeolitic imidazolate framework−8 |
References
- Ribeiro, M.; Simões, M.; Vitorino, C.; Mascarenhas-Melo, F. Hydrogels in Cutaneous Wound Healing: Insights into Characterization, Properties, Formulation and Therapeutic Potential. Gels 2024, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X.; Tao, S.; Wang, Q.; Ma, P.Q.; Li, Z.B.; Wu, Y.L.; Li, D.W. Research advances in smart responsive-hydrogel dressings with potential clinical diabetic wound healing properties. Mil. Med. Res. 2023, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.D.; Ye, J.; Yang, Y.C.; Huang, Y.Y.; Xiao, M.T. Self-healing polysaccharide-based injectable hydrogels with antibacterial activity for wound healing. Carbohydr. Polym. 2022, 275, 118770. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef]
- Wan, Y.; Lin, Y.; Tan, X.; Gong, L.; Lei, F.; Wang, C.; Sun, X.; Du, X.; Zhang, Z.; Jiang, J.; et al. Injectable Hydrogel To Deliver Bone Mesenchymal Stem Cells Preloaded with Azithromycin To Promote Spinal Cord Repair. ACS Nano 2024, 18, 8934–8951. [Google Scholar] [CrossRef]
- Hou, X.; Huang, B.; Zhou, L.; Liu, S.; Kong, J.; He, C. An Amphiphilic Entangled Network Design Toward Ultratough Hydrogels. Adv. Mater. 2023, 35, e2301532. [Google Scholar] [CrossRef]
- Li, J.; Chen, G.; Xu, X.; Abdou, P.; Jiang, Q.; Shi, D.; Gu, Z. Advances of injectable hydrogel-based scaffolds for cartilage regeneration. Regen. Biomater. 2019, 6, 129–140. [Google Scholar] [CrossRef]
- Zhao, L.; Qi, X.; Cai, T.; Fan, Z.; Wang, H.; Du, X. Gelatin hydrogel/contact lens composites as rutin delivery systems for promoting corneal wound healing. Drug Deliv. 2021, 28, 1951–1961. [Google Scholar] [CrossRef]
- Jin, X.; Wei, C.-X.; Wu, C.-W.; Zhang, W. Customized Hydrogel for Sustained Release of Highly Water-Soluble Drugs. ACS Omega 2022, 7, 8493–8497. [Google Scholar] [CrossRef]
- Tian, B.; Liu, J. Smart stimuli-responsive chitosan hydrogel for drug delivery: A review. Int. J. Biol. Macromol. 2023, 235, 123902. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Y.; Li, Q.; Yu, C.; Chu, W. Natural Polymer-based Stimuli-responsive Hydrogels. Curr. Med. Chem. 2020, 27, 2631–2657. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Liu, Y.; Pan, Y.; Li, Y.; Xu, L.; Zhong, Y.; Wang, W.; Zuo, J.; Yu, H.; Lv, Z.; et al. Long-term induction of endogenous BMPs growth factor from antibacterial dual network hydrogels for fast large bone defect repair. J. Colloid. Interface Sci. 2022, 607, 1500–1515. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, M.; van Nostrum, C.F.; Hennink, W.E.; Rijkers, D.T.; Liskamp, R.M. Synthesis and characterization of enzymatically biodegradable PEG and peptide-based hydrogels prepared by click chemistry. Biomacromolecules 2010, 11, 1608–1614. [Google Scholar] [CrossRef]
- Yang, Q.Z.; Liu, J.; Jiang, X.L. Application of Click Chemistry in Biomedical Polymers. Progress. Chem. 2010, 22, 2377–2387. [Google Scholar]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Hajebi, S.; Rabiee, N.; Bagherzadeh, M.; Ahmadi, S.; Rabiee, M.; Roghani-Mamaqani, H.; Tahriri, M.; Tayebi, L.; Hamblin, M.R. Stimulus-responsive polymeric nanogels as smart drug delivery systems. Acta Biomater. 2019, 92, 1–18. [Google Scholar] [CrossRef]
- Pérez-Herrero, E.; Fernández-Medarde, A. The reversed intra- and extracellular pH in tumors as a unified strategy to chemotherapeutic delivery using targeted nanocarriers. Acta Pharm. Sin. B 2021, 11, 2243–2264. [Google Scholar] [CrossRef]
- Druzhkova, I.; Lukina, M.; Dudenkova, V.; Ignatova, N.; Snopova, L.; Gavrina, A.; Shimolina, L.; Belousov, V.; Zagaynova, E.; Shirmanova, M. Tracing of intracellular pH in cancer cells in response to Taxol treatment. Cell Cycle 2021, 20, 1540–1551. [Google Scholar] [CrossRef]
- Lin, L.; Shen, L.; Zhang, J.; Xu, Y.; Fang, Z.; Müller-Buschbaum, P.; Zhong, Q. Ionic Hydrogels Based Wearable Sensors to Monitor the Solar Radiation Dose for Vitamin D Production and Sunburn Prevention. ACS Appl. Mater. Interfaces 2021, 13, 45995–46002. [Google Scholar] [CrossRef]
- Morya, V.; Shukla, A.K.; Ghoroi, C.; Bhatia, D. pH-Responsive and Reversible A-Motif-Based DNA Hydrogel: Synthesis and Biosensing Application. Chembiochem 2023, 24, e202300067. [Google Scholar] [CrossRef] [PubMed]
- Haidari, H.; Vasilev, K.; Cowin, A.J.; Kopecki, Z. Bacteria-Activated Dual pH- and Temperature-Responsive Hydrogel for Targeted Elimination of Infection and Improved Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 51744–51762. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A.; He, J.; Bochani, S.; Nosrati, V.; Shahbazi, M.A.; Guo, B. Multifunctional Photoactive Hydrogels for Wound Healing Acceleration. ACS Nano 2021, 15, 18895–18930. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Huang, J.; Li, Y.; Lv, X.; Zhou, H.; Wang, H.; Xu, Y.; Wang, C.; Wang, J.; Liu, Z. ROS-scavenging hydrogel to promote healing of bacteria infected diabetic wounds. Biomaterials 2020, 258, 120286. [Google Scholar] [CrossRef]
- Liang, Y.; Li, M.; Yang, Y.; Qiao, L.; Xu, H.; Guo, B. pH/Glucose Dual Responsive Metformin Release Hydrogel Dressings with Adhesion and Self-Healing via Dual-Dynamic Bonding for Athletic Diabetic Foot Wound Healing. ACS Nano 2022, 16, 3194–3207. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chi-Leung Hui, P. Review of Stimuli-Responsive Polymers in Drug Delivery and Textile Application. Molecules 2019, 24, 2547. [Google Scholar] [CrossRef]
- Jia, X.; Dou, Z.; Zhang, Y.; Li, F.; Xing, B.; Hu, Z.; Li, X.; Liu, Z.; Yang, W.; Liu, Z. Smart Responsive and Controlled-Release Hydrogels for Chronic Wound Treatment. Pharmaceutics 2023, 15, 2735. [Google Scholar] [CrossRef]
- Ding, H.T.; Tan, P.; Fu, S.Q.; Tian, X.H.; Zhang, H.; Ma, X.L.; Gu, Z.W.; Luo, K. Preparation and application of pH-responsive drug delivery systems. J. Control. Release 2022, 348, 206–238. [Google Scholar] [CrossRef]
- Hendi, A.; Umair Hassan, M.; Elsherif, M.; Alqattan, B.; Park, S.; Yetisen, A.K.; Butt, H. Healthcare Applications of pH-Sensitive Hydrogel-Based Devices: A Review. Int. J. Nanomedicine 2020, 15, 3887–3901. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Xu, J.; Zhao, R.; Xiong, C.; Habu, J.; Wang, Y.; Luo, X. Global publication trends and research hotspots of curcumin application in tumor: A 20-year bibliometric approach. Front. Oncol. 2022, 12, 1033683. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, Y.; Zheng, H.; Zhang, L.; Zhao, H.; Sang, X.; Xu, Y.; Lu, X. Evolutionary patterns and research frontiers in neoadjuvant immunotherapy: A bibliometric analysis. Int. J. Surg. 2023, 109, 2774–2783. [Google Scholar] [CrossRef] [PubMed]
- Arruda, H.; Silva, E.R.; Lessa, M.; Proença, D., Jr.; Bartholo, R. VOSviewer and Bibliometrix. J. Med. Libr. Assoc. 2022, 110, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Xu, H.; Lai, Y.; Sun, X.; Zhu, Z.; Zang, H.; Wu, Y. A VOSviewer-Based Bibliometric Analysis of Prescription Refills. Front. Med. 2022, 9, 856420. [Google Scholar] [CrossRef] [PubMed]
- Campra, M.; Riva, P.; Oricchio, G.; Brescia, V. Bibliometrix analysis of medical tourism. Health Serv. Manag. Res. 2022, 35, 172–188. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Che, T.; Zheng, Y.; Nan, X.; Wu, Z. Nanomaterials for diabetic wound healing: Visualization and bibliometric analysis from 2011 to 2021. Front. Endocrinol. 2023, 14, 1124027. [Google Scholar] [CrossRef]
- Tanaka, T.; Sun, S.T.; Nishio, I.; Swislow, G.; Shah, A. Phase Transitions in Ionic Gels. Ferroelectrics 1980, 30, 97. [Google Scholar] [CrossRef]
- Díez-Peña, E.; Quijada-Garrido, I.; Barrales-Rienda, J.M. On the water swelling behaviour of poly(N-isopropylacrylamide) [P(N-iPAAm)], poly(methacrylic acid) [P(MAA)], their random copolymers and sequential interpenetrating polymer networks (IPNs). Polymer 2002, 43, 4341–4348. [Google Scholar] [CrossRef]
- Murali Mohan, Y.; Keshava Murthy, P.S.; Mohana Raju, K. Synthesis, characterization and effect of reaction parameters on swelling properties of acrylamide–sodium methacrylate superabsorbent copolymers. React. Funct. Polym. 2005, 63, 11–26. [Google Scholar] [CrossRef]
- Edirisinghe, D.I.U.; D’Souza, A.; Ramezani, M.; Carroll, R.J.; Chicón, Q.; Muenzel, C.L.; Soule, J.; Monroe, M.B.B.; Patteson, A.E.; Makhlynets, O.V. Antibacterial and Cytocompatible pH-Responsive Peptide Hydrogel. Molecules 2023, 28, 4390. [Google Scholar] [CrossRef]
- Ding, C.; Tian, M.; Feng, R.; Dang, Y.; Zhang, M. Novel Self-Healing Hydrogel with Injectable, pH-Responsive, Strain-Sensitive, Promoting Wound-Healing, and Hemostatic Properties Based on Collagen and Chitosan. ACS Biomater. Sci. Eng. 2020, 6, 3855–3867. [Google Scholar] [CrossRef]
- Qureshi, M.A.; Khatoon, F. In Vitro Study of Temperature and pH-Responsive Gentamycin Sulphate-Loaded Chitosan-Based Hydrogel Films for Wound Dressing Applications. Polym.-Plast. Technol. Eng. 2015, 54, 573–580. [Google Scholar] [CrossRef]
- Shao, W.; Liu, H.; Wu, J.M.; Wang, S.X.; Liu, X.F.; Huang, M.; Xu, P. Preparation, antibacterial activity and pH-responsive release behavior of silver sulfadiazine loaded bacterial cellulose for wound dressing applications. J. Taiwan Inst. Chem. Eng. 2016, 63, 404–410. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Han, R.; Long, Z.; Si, P.; Zhang, D. Dual-Cross-Linked PEI/PVA Hydrogel for pH-Responsive Drug Delivery. Biomacromolecules 2023, 24, 5364–5370. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.A.; Iqbal, I.; Ansari, M.N.M.; Razak, S.I.A.; Raza, M.A.; Sajjad, A.; Jabeen, F.; Riduan Mohamad, M.; Jusoh, N. Development of Antibacterial, Degradable and pH-Responsive Chitosan/Guar Gum/Polyvinyl Alcohol Blended Hydrogels for Wound Dressing. Molecules 2021, 26, 5937. [Google Scholar] [CrossRef] [PubMed]
- Reshmi, C.R.; Suja, P.S.; Manaf, O.; Sanu, P.P.; Sujith, A. Nanochitosan enriched poly ε-caprolactone electrospun wound dressing membranes: A fine tuning of physicochemical properties, hemocompatibility and curcumin release profile. Int. J. Biol. Macromol. 2018, 108, 1261–1272. [Google Scholar]
- Bazban-Shotorbani, S.; Hasani-Sadrabadi, M.M.; Karkhaneh, A.; Serpooshan, V.; Jacob, K.I.; Moshaverinia, A.; Mahmoudi, M. Revisiting structure-property relationship of pH-responsive polymers for drug delivery applications. J. Control Release 2017, 253, 46–63. [Google Scholar] [CrossRef]
- Al-Arjan, W.S.; Khan, M.U.A.; Almutairi, H.H.; Alharbi, S.M.; Razak, S.I.A. pH-Responsive PVA/BC-f-GO Dressing Materials for Burn and Chronic Wound Healing with Curcumin Release Kinetics. Polymers 2022, 14, 1949. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, W.; Li, G.; Ma, Z.; Zhu, M.; Gao, Q.; Xu, K.; Liu, X.; Lu, W.; Zhang, W.; et al. A Factor-Free Hydrogel with ROS Scavenging and Responsive Degradation for Enhanced Diabetic Bone Healing. Small 2024, 20, e2306389. [Google Scholar] [CrossRef]
- Zhou, D.; Li, S.; Fei, Z.; Zhou, P.; Zhao, Y.; Zhi, L.; Li, C.; Peng, X.; Liu, X.; Zhao, C. Glucose and pH Dual-Responsive Polymersomes with Multilevel Self-Regulation of Blood Glucose for Insulin Delivery. Biomacromolecules 2021, 22, 3971–3979. [Google Scholar] [CrossRef]
- Lin, Z.; Ding, J.; Chen, X.; He, C. pH- and Temperature-responsive Hydrogels Based on Tertiary Amine-modified Polypeptides for Stimuli-responsive Drug Delivery. Chem. Asian J. 2023, 18, e202300021. [Google Scholar] [CrossRef]
- Gu, J.; Zhao, G.; Yu, J.; Xu, P.; Yan, J.; Jin, Z.; Chen, S.; Wang, Y.; Zhang, L.W.; Wang, Y. Injectable pH-responsive hydrogel for combinatorial chemoimmunotherapy tailored to the tumor microenvironment. J. Nanobiotechnol. 2022, 20, 372. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, R.; Yang, S.; Li, S.; Gao, Z. Design and application of stimuli-responsive DNA hydrogels: A review. Mater. Today Bio 2022, 16, 100430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lin, W.; Yang, L.; Zhang, A.; Zhang, Y.; Liu, J.; Liu, J. Injectable and pH-responsive self-assembled peptide hydrogel for promoted tumor cell uptake and enhanced cancer chemotherapy. Biomater. Sci. 2022, 10, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Zhang, Z.; Chen, X.; He, C. Stimuli-Responsive Hydrogel Adhesives for Wound Closure and Tissue Regeneration. Macromol. Biosci. 2024, 24, e2300379. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Long, L.; Hu, C.; Kong, Q.; Wang, Y. A spatiotemporal release platform based on pH/ROS stimuli-responsive hydrogel in wound repairing. J. Control Release 2022, 341, 147–165. [Google Scholar] [CrossRef]
- Chen, F.; Qin, J.; Wu, P.; Gao, W.; Sun, G. Glucose-Responsive Antioxidant Hydrogel Accelerates Diabetic Wound Healing. Adv. Healthc. Mater. 2023, 12, e2300074. [Google Scholar] [CrossRef]
- Fathi, M.; Alami-Milani, M.; Geranmayeh, M.H.; Barar, J.; Erfan-Niya, H.; Omidi, Y. Dual thermo-and pH-sensitive injectable hydrogels of chitosan/(poly(N-isopropylacrylamide-co-itaconic acid)) for doxorubicin delivery in breast cancer. Int. J. Biol. Macromol. 2019, 128, 957–964. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, X.; Tang, X.; Peng, L.; Zhang, H.; Zhang, S.; Hu, Q.; Li, J. A dual pH- and temperature-responsive hydrogel produced in situ crosslinking of cyclodextrin-cellulose for wound healing. Int. J. Biol. Macromol. 2023, 253, 126693. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Pérez-Felpete, N.; Fernández-Fernández, C.; Donapetry-García, C.; Pazos-García, C. Liver glucose metabolism in humans. Biosci. Rep. 2016, 36, e00416. [Google Scholar] [CrossRef]
- Liu, J.; Bai, X.; Zhang, M.; Wu, S.; Xiao, J.; Zeng, X.; Li, Y.; Zhang, Z. Energy metabolism: A new target for gastric cancer treatment. Clin. Transl. Oncol. 2024, 26, 338–351. [Google Scholar] [CrossRef]
- Pascual, G.; Majem, B.; Benitah, S.A. Targeting lipid metabolism in cancer metastasis. Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189051. [Google Scholar] [CrossRef]
- Schwärzler, J.; Mayr, L.; Vich Vila, A.; Grabherr, F.; Niederreiter, L.; Philipp, M.; Grander, C.; Meyer, M.; Jukic, A.; Tröger, S.; et al. PUFA-Induced Metabolic Enteritis as a Fuel for Crohn’s Disease. Gastroenterology 2022, 162, 1690–1704. [Google Scholar] [CrossRef]
- Yao, L.; Zhu, X.; Shan, Y.; Zhang, L.; Yao, J.; Xiong, H. Recent Progress in Anti-Tumor Nanodrugs Based on Tumor Microenvironment Redox Regulation. Small 2024, 20, e2310018. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lu, G.; Chen, M.; Wang, P.; Li, Z.; Han, X.; Liang, J.; Sun, Y.; Fan, Y.; Zhang, X. Redox and pH dual-responsive injectable hyaluronan hydrogels with shape-recovery and self-healing properties for protein and cell delivery. Carbohydr. Polym. 2020, 250, 116979. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Z.; Zhang, G.; Ren, J.; Yu, L.; Liu, X.; Yang, Y.; Ravindran, A.; Wong, C.; Chen, R. A pH/redox-dual responsive, nanoemulsion-embedded hydrogel for efficient oral delivery and controlled intestinal release of magnesium ions. J. Mater. Chem. B 2021, 9, 1888–1895. [Google Scholar] [CrossRef]
- Hu, C.; Long, L.; Cao, J.; Zhang, S.; Wang, Y. Dual-crosslinked mussel-inspired smart hydrogels with enhanced antibacterial and angiogenic properties for chronic infected diabetic wound treatment via pH-responsive quick cargo release. Chem. Eng. J. 2021, 411, 128564. [Google Scholar] [CrossRef]
- Rabeh, M.E.; Vora, L.K.; Moore, J.V.; Bayan, M.F.; McCoy, C.P.; Wylie, M.P. Dual stimuli-responsive delivery system for self-regulated colon-targeted delivery of poorly water-soluble drugs. Biomater. Adv. 2024, 157, 213735. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.T.; Wang, J.X.; Huang, S.F.; Li, M.; Chen, J.Y.; Pei, D.D.; Tang, Z.; Guo, B.L. Bacteria-responsive programmed self-activating antibacterial hydrogel to remodel regeneration microenvironment for infected wound healing. Natl. Sci. Rev. 2024, 11, nwae044. [Google Scholar] [CrossRef] [PubMed]
- Keni, R.; Begum, F.; Gourishetti, K.; Viswanatha, G.L.; Nayak, P.G.; Nandakumar, K.; Shenoy, R.R. Diabetic wound healing approaches: An update. J. Basic. Clin. Physiol. Pharmacol. 2023, 34, 137–150. [Google Scholar] [CrossRef]
- Zhao, L.; Niu, L.; Liang, H.; Tan, H.; Liu, C.; Zhu, F. pH and Glucose Dual-Responsive Injectable Hydrogels with Insulin and Fibroblasts as Bioactive Dressings for Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 37563–37574. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, Z.; Zhang, M.; He, J.; Li, S.; Sun, X.; Ni, P. A hybrid hydrogel constructed using drug loaded mesoporous silica and multiple response copolymer as an intelligent dressing for wound healing of diabetic foot ulcers. J. Mater. Chem. B 2023, 11, 4922–4933. [Google Scholar] [CrossRef] [PubMed]
- Dai, F.; Zhang, J.; Chen, F.; Chen, X.; Lee, C.J.; Liang, H.; Zhao, L.; Tan, H. A Multi-Responsive Hydrogel Combined With Mild Heat Stimulation Promotes Diabetic Wound Healing by Regulating Inflammatory and Enhancing Angiogenesis. Adv. Sci. 2024, 11, e2408783. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zheng, X.; Yi, K.; Du, X.; Wang, C.; Cui, P.; Jiang, P.; Ni, X.; Qiu, L.; Wang, J. Temperature-Ion-pH Triple Responsive Gellan Gum as In Situ Hydrogel for Long-Acting Cancer Treatment. Gels 2022, 8, 508. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wu, Q.; Chen, L.; Sun, Y.; Chen, L.; Zhang, C.; Li, S.; Ma, C.; Jiang, S. Remotely Controlled Light/Electric/Magnetic Multiresponsive Hydrogel for Fast Actuations. ACS Appl. Mater. Interfaces 2023, 15, 10030–10043. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Eckert, F.; Boyko, V.; Pich, A. Temperature-, pH-, and magnetic-field-sensitive hybrid microgels. Small 2007, 3, 650–657. [Google Scholar] [CrossRef]
- Jin, S.; Wan, J.; Meng, L.; Huang, X.; Guo, J.; Liu, L.; Wang, C. Biodegradation and Toxicity of Protease/Redox/pH Stimuli-Responsive PEGlated PMAA Nanohydrogels for Targeting Drug delivery. ACS Appl. Mater. Interfaces 2015, 7, 19843–19852. [Google Scholar] [CrossRef]
- Xue, C.; Xu, X.; Zhang, L.; Liu, Y.; Liu, S.; Liu, Z.; Wu, M.; Shuai, Q. Self-healing/pH-responsive/inherently antibacterial polysaccharide-based hydrogel for a photothermal strengthened wound dressing. Colloids Surf. B Biointerfaces 2022, 218, 112738. [Google Scholar] [CrossRef]
- Das, I.J.; Bal, T. pH factors in chronic wound and pH-responsive polysaccharide-based hydrogel dressings. Int. J. Biol. Macromol. 2024, 279, 135118. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, H.; Chen, H.; Ling, Y.; Xi, Z.; Lv, M.; Chen, J. pH-responsive nanocomposite hydrogel for simultaneous prevention of postoperative adhesion and tumor recurrence. Acta Biomater. 2023, 158, 228–238. [Google Scholar] [CrossRef]
- Bian, Z.; Dai, C.; Chu, F.; Hu, A.; Xue, L.; Xu, Q.; Feng, Y.; Zhou, B. pH biosensors based on hydrogel optical fiber. Appl. Opt. 2023, 62, 8272–8278. [Google Scholar] [CrossRef]
- Qin, C.; Qi, Z.; Pan, S.; Xia, P.; Kong, W.; Sun, B.; Du, H.; Zhang, R.; Zhu, L.; Zhou, D.; et al. Advances in Conductive Hydrogel for Spinal Cord Injury Repair and Regeneration. Int. J. Nanomedicine 2023, 18, 7305–7333. [Google Scholar] [CrossRef]
- Zheng, Z.; Yang, X.; Zhang, Y.; Zu, W.; Wen, M.; Liu, T.; Zhou, C.; Li, L. An injectable and pH-responsive hyaluronic acid hydrogel as metformin carrier for prevention of breast cancer recurrence. Carbohydr. Polym. 2023, 304, 120493. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Tamayol, A.; Uquillas, J.A.; Akbari, M.; Bertassoni, L.E.; Cha, C.; Camci-Unal, G.; Dokmeci, M.R.; Peppas, N.A.; Khademhosseini, A. 25th anniversary article: Rational design and applications of hydrogels in regenerative medicine. Adv. Mater. 2014, 26, 85–123. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, L.A.; Daily, A.M.; Horava, S.D.; Peppas, N.A. Therapeutic applications of hydrogels in oral drug delivery. Expert. Opin. Drug Deliv. 2014, 11, 901–915. [Google Scholar] [CrossRef]
- Du, M.; Jin, J.; Zhou, F.; Chen, J.; Jiang, W. Dual drug-loaded hydrogels with pH-responsive and antibacterial activity for skin wound dressing. Colloids Surf. B Biointerfaces 2023, 222, 113063. [Google Scholar] [CrossRef]
- Pan, F.; Giovannini, G.; Zhang, S.; Altenried, S.; Zuber, F.; Chen, Q.; Boesel, L.F.; Ren, Q. pH-responsive silica nanoparticles for the treatment of skin wound infections. Acta Biomater. 2022, 145, 172–184. [Google Scholar] [CrossRef]
- Bostancı, N.S.; Büyüksungur, S.; Hasirci, N.; Tezcaner, A. pH responsive release of curcumin from photocrosslinked pectin/gelatin hydrogel wound dressings. Biomater. Adv. 2022, 134, 112717. [Google Scholar] [CrossRef]
- Wang, M.; Huang, H.; Huang, C.; Liu, S.; Peng, X. pH-responsive magnolol nanocapsule-embedded magnolol-grafted-chitosan hydrochloride hydrogels for promoting wound healing. Carbohydr. Polym. 2022, 292, 119643. [Google Scholar] [CrossRef]
- Zu, Y.; Wang, Y.; Yao, H.; Yan, L.; Yin, W.; Gu, Z. A Copper Peroxide Fenton Nanoagent-Hydrogel as an In Situ pH-Responsive Wound Dressing for Effectively Trapping and Eliminating Bacteria. ACS Appl. Bio. Mater. 2022, 5, 1779–1793. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Haider, S.; Raza, M.A.; Shah, S.A.; Razak, S.I.A.; Kadir, M.R.A.; Subhan, F.; Haider, A. Smart and pH-sensitive rGO/Arabinoxylan/chitosan composite for wound dressing: In-vitro drug delivery, antibacterial activity, and biological activities. Int. J. Biol. Macromol. 2021, 192, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, L.; Li, Y.; Huang, Z.; Luo, S.; He, Y.; Han, H.; Raza, F.; Wu, J.; Ge, L. Injectable pH and redox dual responsive hydrogels based on self-assembled peptides for anti-tumor drug delivery. Biomater. Sci. 2020, 8, 5415–5426. [Google Scholar] [CrossRef] [PubMed]
- Baliga, S.; Muglikar, S.; Kale, R. Salivary pH: A diagnostic biomarker. J. Indian. Soc. Periodontol. 2013, 17, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Phan, V.H.G.; Mathiyalagan, R.; Nguyen, M.T.; Tran, T.T.; Murugesan, M.; Ho, T.N.; Huong, H.; Yang, D.C.; Li, Y.; Thambi, T. Ionically cross-linked alginate-chitosan core-shell hydrogel beads for oral delivery of insulin. Int. J. Biol. Macromol. 2022, 222, 262–271. [Google Scholar] [CrossRef]
- Xu, Q.; Huang, W.; Jiang, L.; Lei, Z.; Li, X.; Deng, H. KGM and PMAA based pH-sensitive interpenetrating polymer network hydrogel for controlled drug release. Carbohydr. Polym. 2013, 97, 565–570. [Google Scholar] [CrossRef]
- Duan, D.; Liu, H.; Xu, M.; Chen, M.; Han, Y.; Shi, Y.; Liu, Z. Size-Controlled Synthesis of Drug-Loaded Zeolitic Imidazolate Framework in Aqueous Solution and Size Effect on Their Cancer Theranostics in Vivo. ACS Appl. Mater. Interfaces 2018, 10, 42165–42174. [Google Scholar] [CrossRef]
- Xiong, M.; Chen, Y.; Hu, H.J.; Cheng, H.; Li, W.X.; Tang, S.; Hu, X.; Lan, L.M.; Zhang, H.; Jiang, G.B. Multifunctional pH-responsive hydrogel dressings based on carboxymethyl chitosan: Synthesis, characterization fostering the wound healing. Carbohydr. Polym. 2024, 341, 122348. [Google Scholar] [CrossRef]
- Li, P.; Li, Y.; Fu, R.; Duan, Z.; Zhu, C.; Fan, D. NIR- and pH-responsive injectable nanocomposite alginate-graft-dopamine hydrogel for melanoma suppression and wound repair. Carbohydr. Polym. 2023, 314, 120899. [Google Scholar] [CrossRef]
- Asadi, M.; Salehi, Z.; Akrami, M.; Hosseinpour, M.; Jockenhövel, S.; Ghazanfari, S. 3D printed pH-responsive tablets containing N-acetylglucosamine-loaded methylcellulose hydrogel for colon drug delivery applications. Int. J. Pharm. 2023, 645, 123366. [Google Scholar] [CrossRef]
- Yi, S.A.; Zhang, Y.; Rathnam, C.; Pongkulapa, T.; Lee, K.B. Bioengineering Approaches for the Advanced Organoid Research. Adv. Mater. 2021, 33, e2007949. [Google Scholar] [CrossRef]
- Park, H.; Lee, Y.; Kim, J.; Sim, J.Y.; Na, Y.; Yoon, C. 3D printed swelling-driven shape-morphing pH-responsive hydrogel gripper. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2023, 2023, 1–4. [Google Scholar] [PubMed]
- Montheil, T.; Maumus, M.; Valot, L.; Lebrun, A.; Martinez, J.; Amblard, M.; Noël, D.; Mehdi, A.; Subra, G. Inorganic Sol–Gel Polymerization for Hydrogel Bioprinting. ACS Omega 2020, 5, 2640–2647. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Shie, M.Y.; Lee, A.K.; Chou, Y.T.; Chiang, C.; Lin, C.P. 3D-Printed Ginsenoside Rb1-Loaded Mesoporous Calcium Silicate/Calcium Sulfate Scaffolds for Inflammation Inhibition and Bone Regeneration. Biomedicines 2021, 9, 907. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.H.; Chen, C.Y.; Chang, C.Y.; Chen, Y.W.; Lin, C.P. The synergistic effects of quercetin-containing 3D-printed mesoporous calcium silicate/calcium sulfate/poly-ε-caprolactone scaffolds for the promotion of osteogenesis in mesenchymal stem cells. J. Formos. Med. Assoc. 2021, 120, 1627–1634. [Google Scholar] [CrossRef]
- Liu, Z.; Xin, W.; Ji, J.; Xu, J.; Zheng, L.; Qu, X.; Yue, B. 3D-Printed Hydrogels in Orthopedics: Developments, Limitations, and Perspectives. Front. Bioeng. Biotechnol. 2022, 10, 845342. [Google Scholar] [CrossRef]
- Su, H.; Li, Q.; Li, D.; Li, H.; Feng, Q.; Cao, X.; Dong, H. A versatile strategy to construct free-standing multi-furcated vessels and a complicated vascular network in heterogeneous porous scaffolds via combination of 3D printing and stimuli-responsive hydrogels. Mater. Horiz. 2022, 9, 2393–2407. [Google Scholar] [CrossRef]
- Schneider, L.A.; Korber, A.; Grabbe, S.; Dissemond, J. Influence of pH on wound-healing: A new perspective for wound-therapy? Arch. Dermatol. Res. 2007, 298, 413–420. [Google Scholar] [CrossRef]
- Mokrý, M.; Gál, P.; Vidinský, B.; Kusnír, J.; Dubayová, K.; Mozes, S.; Sabo, J. In vivo monitoring the changes of interstitial pH and FAD/NADH ratio by fluorescence spectroscopy in healing skin wounds. Photochem. Photobiol. 2006, 82, 793–797. [Google Scholar] [CrossRef]
- Pasche, S.; Angeloni, S.; Ischer, R.; Liley, M.; Lupranoe, J.; Voirin, G. Wearable Biosensors for Monitoring Wound Healing. Biomed. Appl. Smart Mater. 2008, 57, 80–87. [Google Scholar]
- Tsegay, F.; Hisham, M.; Elsherif, M.; Schiffer, A.; Butt, H. 3D Printing of pH Indicator Auxetic Hydrogel Skin Wound Dressing. Molecules 2023, 28, 1339. [Google Scholar] [CrossRef]
- Xiao, J.Y.; Zhou, Z.Z.; Zhong, G.; Xu, T.L.; Zhang, X.J. Self-Sterilizing Microneedle Sensing Patches for Machine Learning-Enabled Wound pH Visual Monitoring. Adv. Funct. Mater. 2024, 34, 2315067. [Google Scholar] [CrossRef]
- Kim, Y.J.; Park, S.Y. Optical Multisensor Array with Functionalized Photonic Droplets by an Interpenetrating Polymer Network for Human Blood Analysis. ACS Appl. Mater. Interfaces 2020, 12, 47342–47354. [Google Scholar] [CrossRef] [PubMed]
- Refardt, J.; Winzeler, B.; Christ-Crain, M. Diabetes Insipidus: An Update. Endocrinol. Metab. Clin. N. Am. 2020, 49, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, L.; Yang, S.; Han, J.; Zheng, Y.; Chen, Z.; Shi, X.; Yang, J. Glucose and pH dual-responsive hydrogels with antibacterial, reactive oxygen species scavenging, and angiogenesis properties for promoting the healing of infected diabetic foot ulcers. Acta Biomater. 2024, 190, 205–218. [Google Scholar] [CrossRef]
- Pan, G.Y.; Li, M.; Mu, L.; Huang, Y.; Liang, Y.P.; Guo, B.L. Photothermal/Photodynamic Synergistic Antibacterial Hydrogel Dressing with pH/Glucose Dual Responsive Pirfenidone Release for Diabetic Foot Ulcers. Adv. Funct. Mater. 2024, 35, 2416205. [Google Scholar] [CrossRef]
- Min, H.; Wang, J.; Qi, Y.; Zhang, Y.; Han, X.; Xu, Y.; Xu, J.; Li, Y.; Chen, L.; Cheng, K.; et al. Biomimetic Metal-Organic Framework Nanoparticles for Cooperative Combination of Antiangiogenesis and Photodynamic Therapy for Enhanced Efficacy. Adv. Mater. 2019, 31, e1808200. [Google Scholar] [CrossRef]
- Wu, Y.; Chang, X.; Yang, G.; Chen, L.; Wu, Q.; Gao, J.; Tian, R.; Mu, W.; Gooding, J.J.; Chen, X.; et al. A Physiologically Responsive Nanocomposite Hydrogel for Treatment of Head and Neck Squamous Cell Carcinoma via Proteolysis-Targeting Chimeras Enhanced Immunotherapy. Adv. Mater. 2023, 35, e2210787. [Google Scholar] [CrossRef]
- Nie, D.; Dai, Z.; Li, J.; Yang, Y.; Xi, Z.; Wang, J.; Zhang, W.; Qian, K.; Guo, S.; Zhu, C.; et al. Cancer-Cell-Membrane-Coated Nanoparticles with a Yolk-Shell Structure Augment Cancer Chemotherapy. Nano Lett. 2020, 20, 936–946. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, S.; Xu, R.; Tang, Y.; Xia, X. Cancer cell membrane-coated nanoparticles: A promising anti-tumor bionic platform. RSC Adv. 2024, 14, 10608–10637. [Google Scholar] [CrossRef]
- Shang, L.; Jiang, X.; Yang, T.; Xu, H.; Xie, Q.; Hu, M.; Yang, C.; Kong, L.; Zhang, Z. Enhancing cancer chemo-immunotherapy by biomimetic nanogel with tumor targeting capacity and rapid drug-releasing in tumor microenvironment. Acta Pharm. Sin. B 2022, 12, 2550–2567. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Chen, W.; Zheng, X.; Ma, L. Preparation of pH-sensitive alginate-based hydrogel by microfluidic technology for intestinal targeting drug delivery. Int. J. Biol. Macromol. 2024, 254, 127649. [Google Scholar] [CrossRef] [PubMed]
- Abourehab, M.A.S.; Rajendran, R.R.; Singh, A.; Pramanik, S.; Shrivastav, P.; Ansari, M.J.; Manne, R.; Amaral, L.S.; Deepak, A. Alginate as a Promising Biopolymer in Drug Delivery and Wound Healing: A Review of the State-of-the-Art. Int. J. Mol. Sci. 2022, 23, 9035. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wu, Q.; Huang, X.; Zhao, X.; Chen, X.; Chen, Y.; Weitz, D.A.; Song, Y. Synthesis of nanomedicine hydrogel microcapsules by droplet microfluidic process and their pH and temperature dependent release. RSC Adv. 2021, 11, 37814–37823. [Google Scholar] [CrossRef]

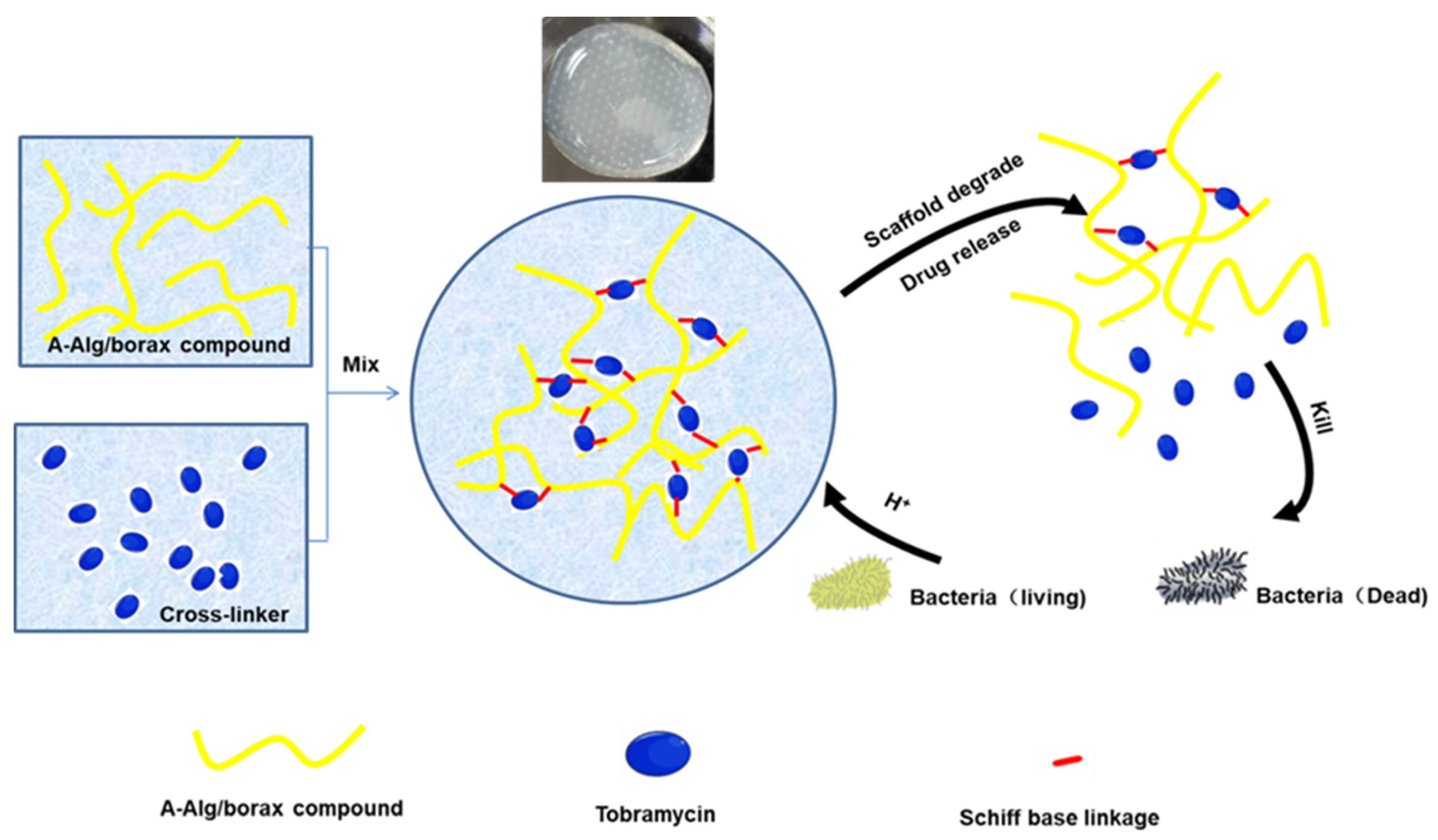


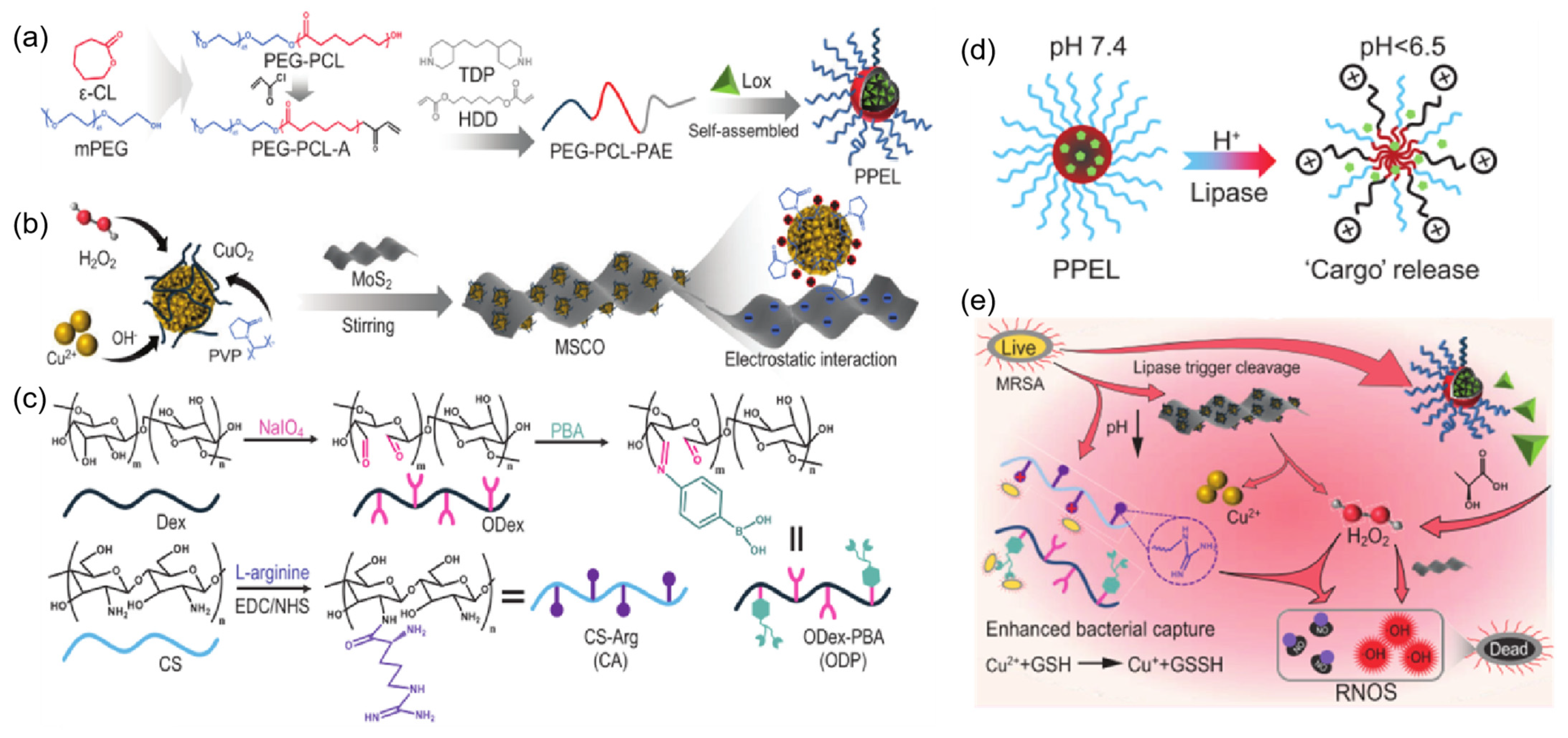
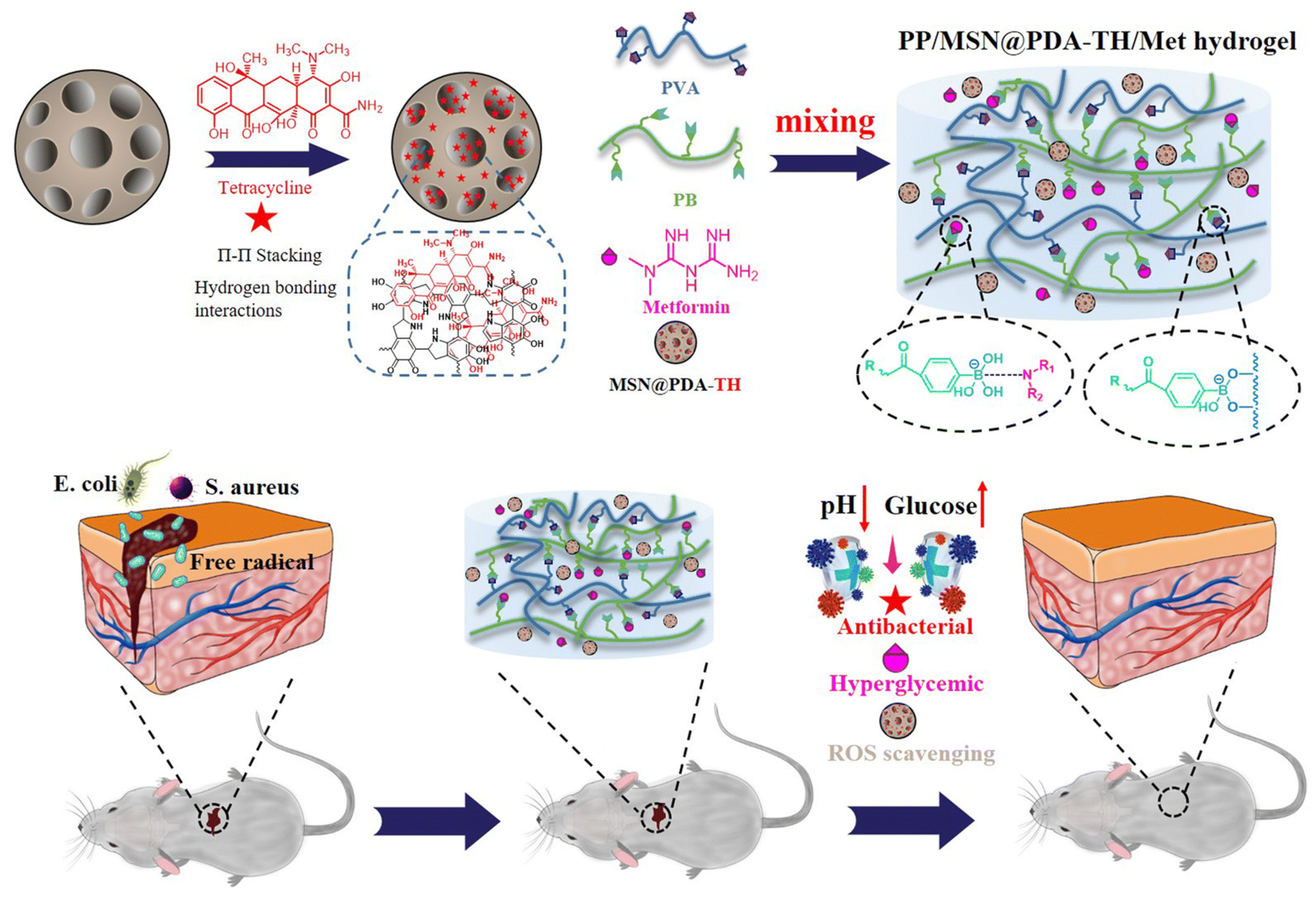
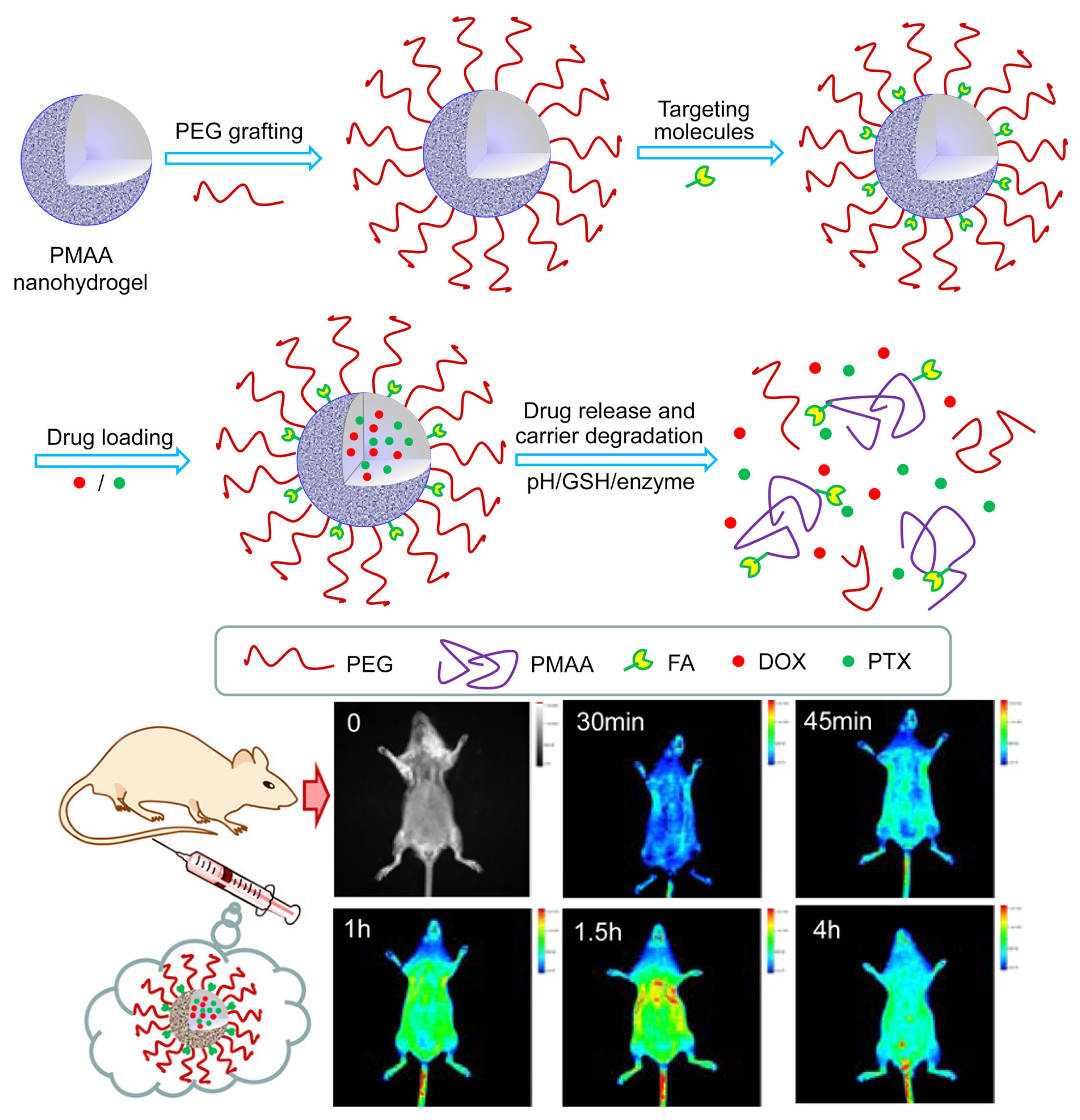
| Composition | Application | Response Conditions | Mechanism of pH Response | Ref. |
|---|---|---|---|---|
| GelMA, HA-CHO, SEBS | Release of antibiotic: GS and LZM | pH = 5.0 | The cleaved Schiff base bonds and the electrostatic interactions | [86] |
| SiNPs, alginates | Release of antibiotic: CHX | pH > 8.0 | Microporous SiNPs is a simple diffusion of the loaded compounds or a degradation of the silica matrix | [87] |
| GelMA, PeMA | Release of natural antibacterial substance: curcumin | pH = 7.4 | Carboxylic acid groups on both PeMA and GelMA and pH-dependent solubility of curcumin | [88] |
| CS, OSU | Release of natural antibacterial substance: MAG | pH = 6.2 | pH-responsive swelling behavior | [89] |
| NIPAAM, AAM, DMPA, methylene-N,N-bis(acrylamide) | Antibiotics (Kana) are used within the hydrogel to kill bacteria through Bacterial trap behavior and Fenton reaction | pH = 5.5 | The responsive released Cu2+ in the weak acidic environment can catalyze the decomposition of self-supplied H2O2 into •OH. | [90] |
| ARX, CS, rGO, TEOS | Delivery of antibiotic: silver-sulphadiazine | pH = 7.4 | The protonation of the alcoholic and carboxylic acid functional groups of ARX and CS | [91] |
| CS, PNIAAm-co-IA, GP | Release antitumor drugs: DOX | pH = 5.5, 40 °C | Result in preventing the proper interaction of functional groups of CS in the acidic pH | [57] |
| Bamboo parenchymal cellulose, carboxylated-β-cyclodextrin | Release antimicrobials: BBR | pH = 7.6, 40 °C | The entanglements between the modified cyclodextrin-grafted cellulose chains are gradually broken | [58] |
| Self-assembled peptide, NaCl | Release antitumor drugs: PTX | pH = 5.8, GSH | The three-dimensional network is damaged under the conditions of slight acidity | [92] |
| PLP, CDE | Deliver Mg2+ | pH = 6.8, DTT | Protonation/deprotonation of carboxyl (-COOH) groups and cleavage of disulfide bonds | [65] |
| PPE, CS, ODP, MSCO | Release Lox to break down lactic acid produced by bacteria, achieving an antibacterial effect. | pH=5.5, lipase | The cleaved Schiff base bonds in the acidic pH | [68] |
| Met, PVA, mesoporous silica nanoparticles (MSN@PDA), PB | Release hypoglycemic drugs: Met Release antimicrobials: TH | pH = 5.0, glucose | The phenyl borate group is unstable at acidic pH | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, K.; Xu, K. Advancements and Prospects of pH-Responsive Hydrogels in Biomedicine. Gels 2025, 11, 293. https://doi.org/10.3390/gels11040293
Gao K, Xu K. Advancements and Prospects of pH-Responsive Hydrogels in Biomedicine. Gels. 2025; 11(4):293. https://doi.org/10.3390/gels11040293
Chicago/Turabian StyleGao, Ke, and Ke Xu. 2025. "Advancements and Prospects of pH-Responsive Hydrogels in Biomedicine" Gels 11, no. 4: 293. https://doi.org/10.3390/gels11040293
APA StyleGao, K., & Xu, K. (2025). Advancements and Prospects of pH-Responsive Hydrogels in Biomedicine. Gels, 11(4), 293. https://doi.org/10.3390/gels11040293






