Microstructure of Sea Cucumber Parastichopus tremulus Peptide Hydrogels and Bioactivity in Caco-2 Cell Culture Model
Abstract
1. Introduction
2. Results and Discussion
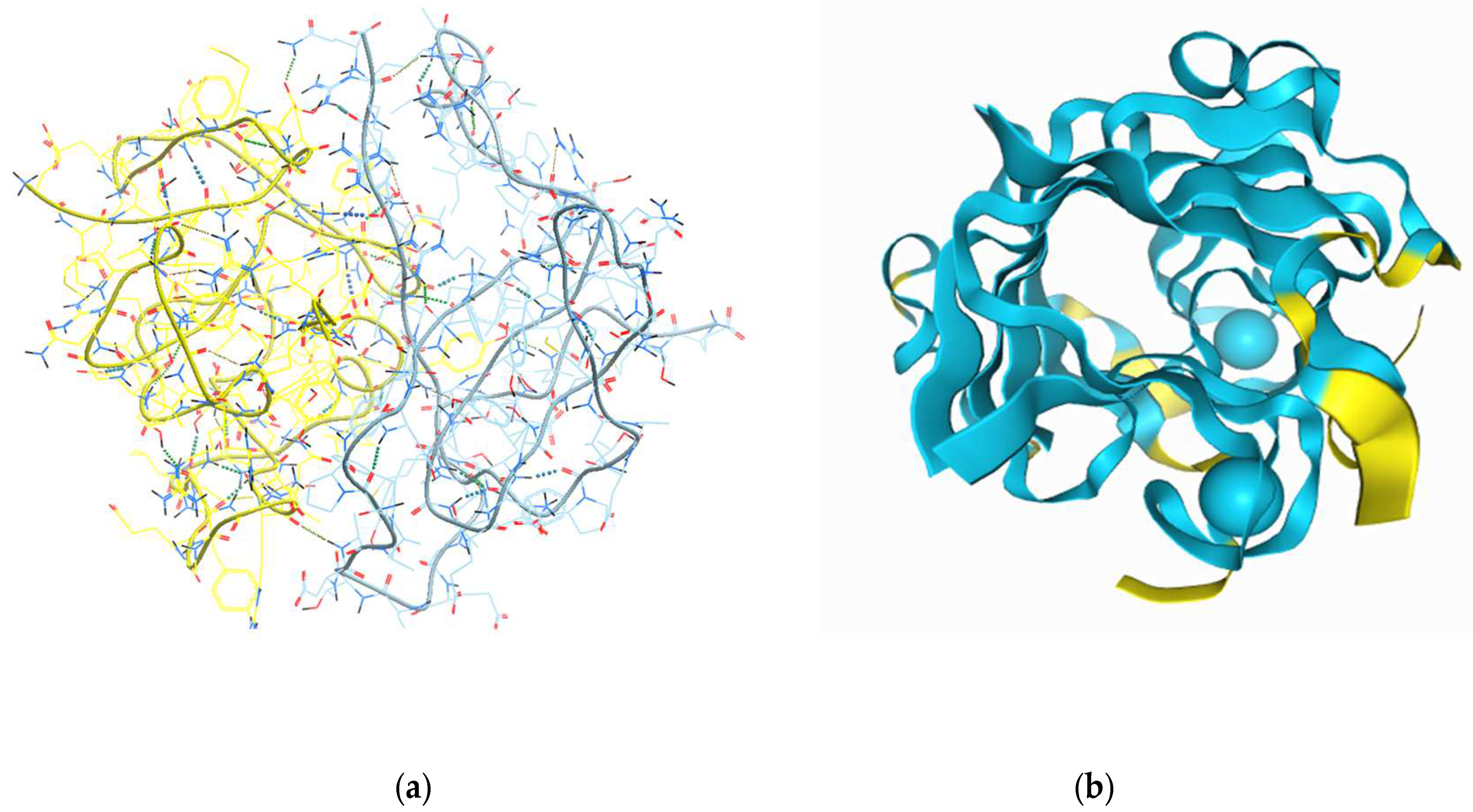
2.1. In Silico Modelling of the Expected 3D Structure of the Peptide Gel in Presence of Chelators
2.2. Experimental Solubility and Minimum Gelation Conditions
2.3. Structural Characterization
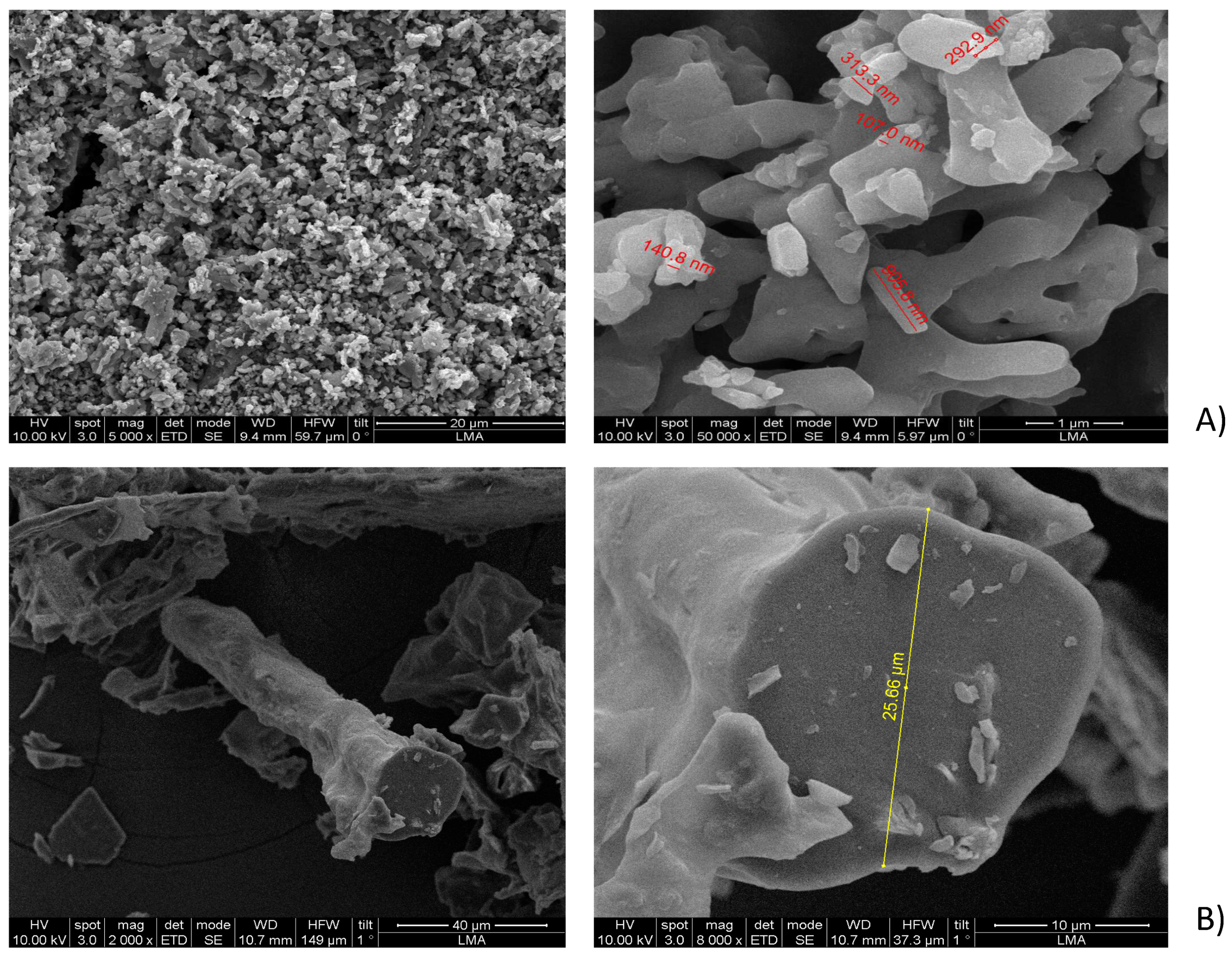
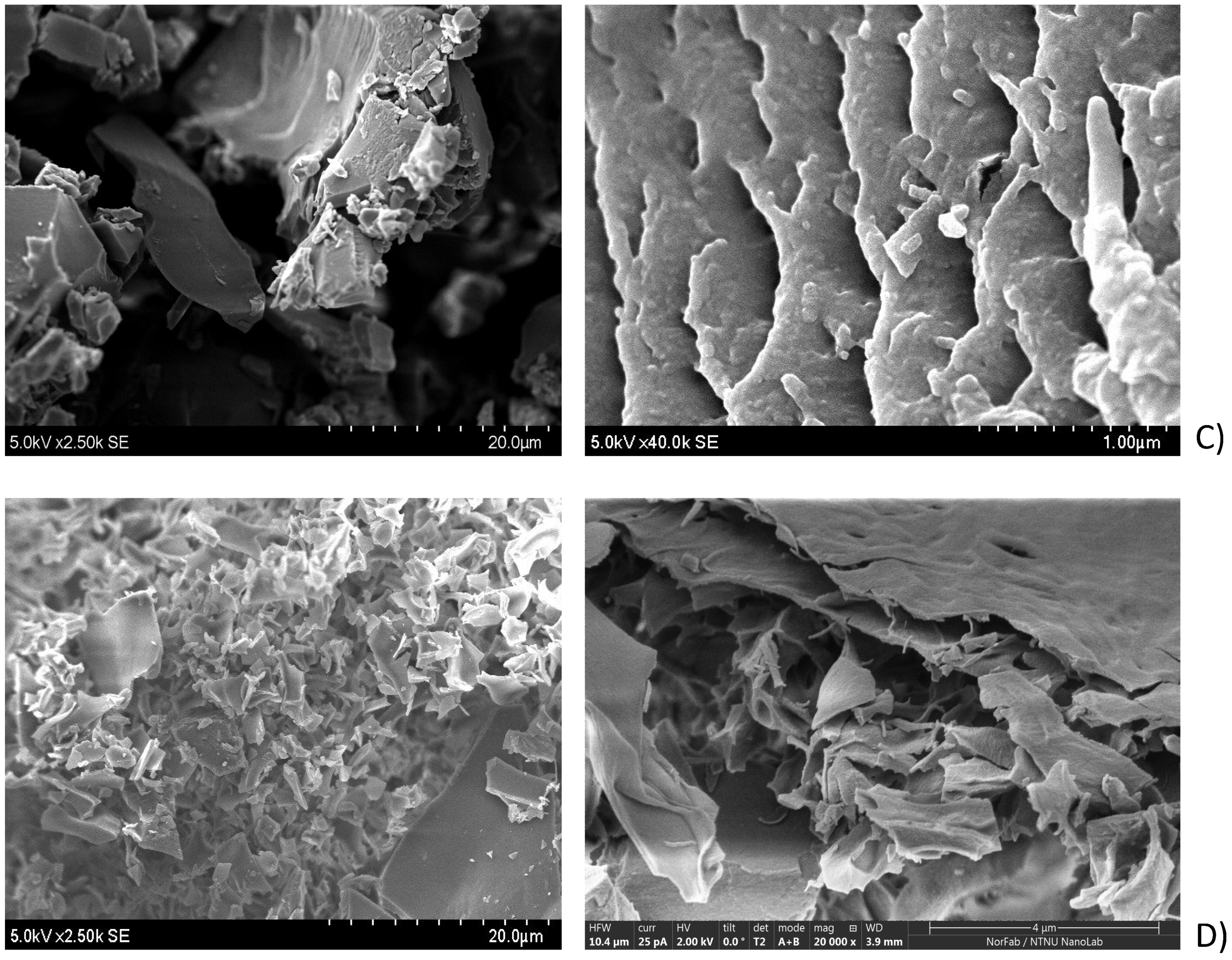
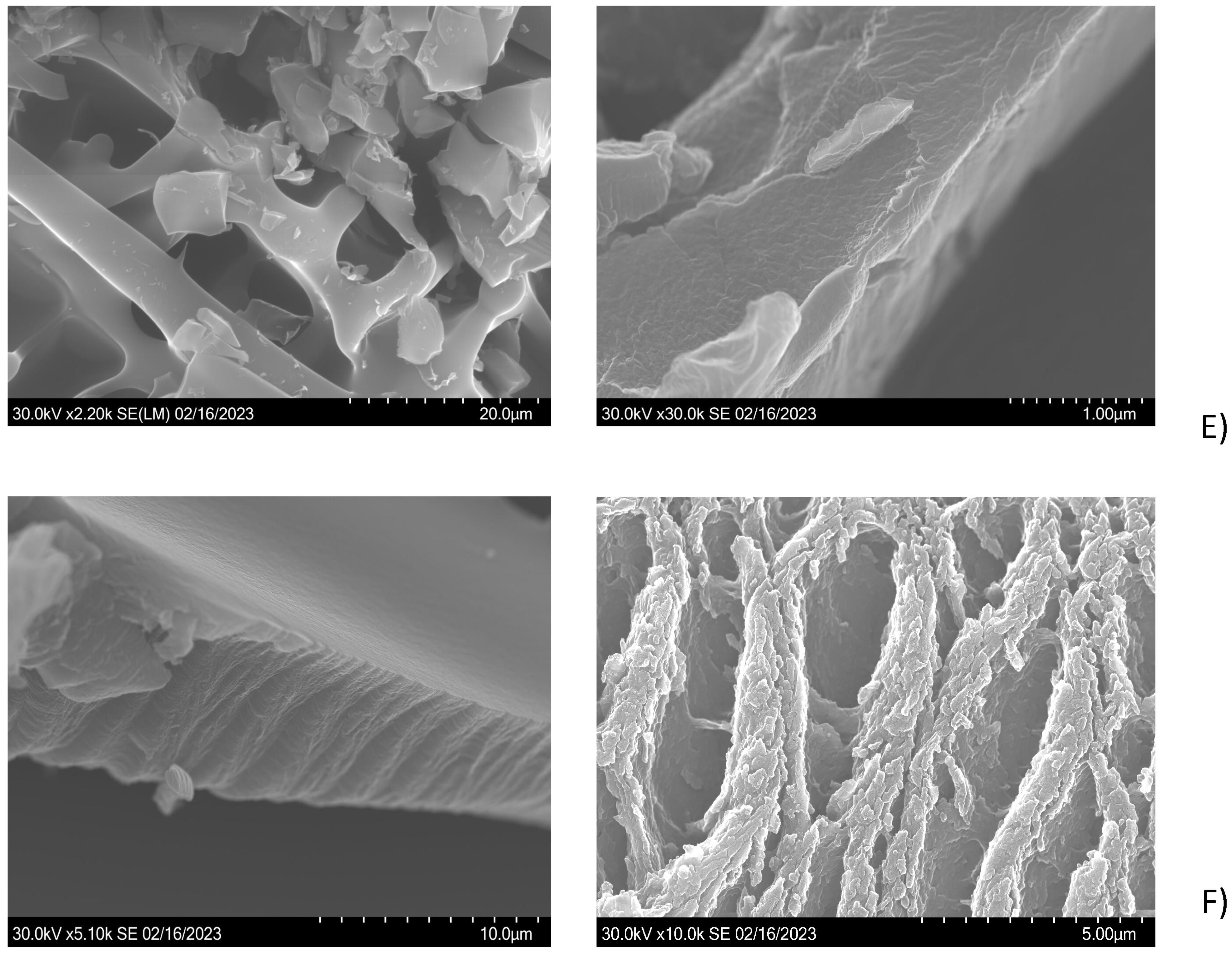
2.3.1. SEM Analysis
2.3.2. UV Spectroscopic Analysis
2.4. Experimentally Determined Bioactivities
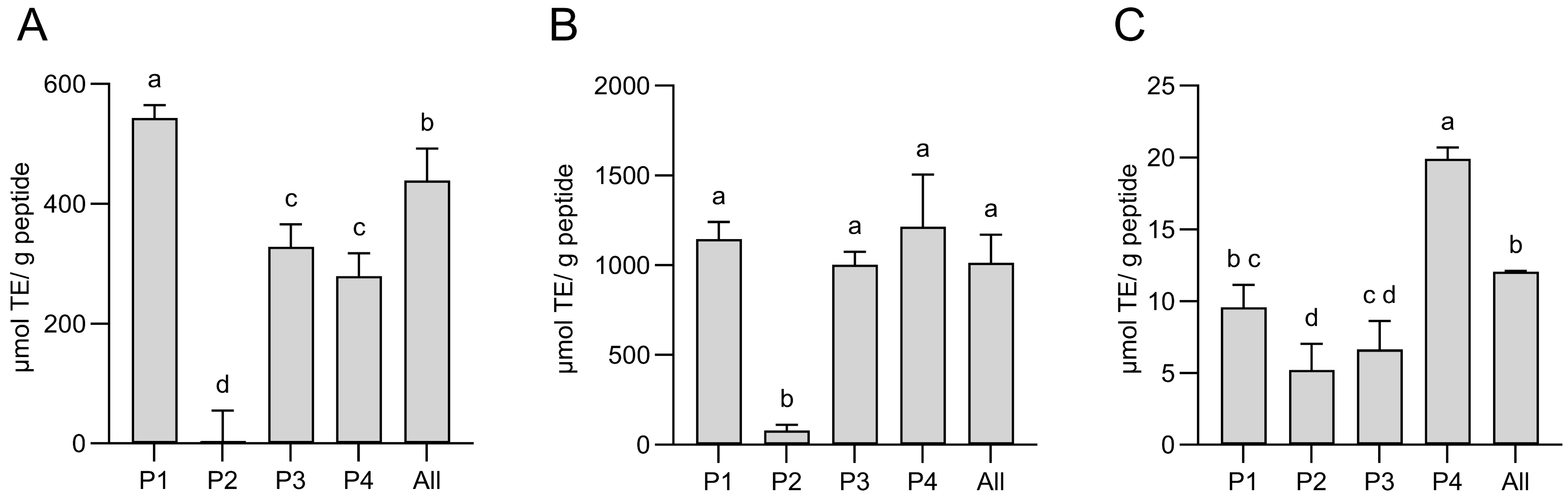
2.4.1. Measurement of the Antioxidant Capacity
2.4.2. Measurement of the Angiotensin-I Converting Enzyme Inhibitory Capacity
2.4.3. Oxidative Stress Protection in Caco-2 Cell Culture Model
3. Conclusions
4. Materials and Methods
4.1. Peptide Synthesis
4.2. Molecular Structure Modelling of the Peptide Complexes
4.3. Establishing Minimal Gelation Conditions
4.4. Structural Characterization of Sea Cucumber Peptide Gels
4.4.1. Scanning Electron Microscopy Analyses
4.4.2. Ultraviolet–Visible (UV) Absorption Spectrum of the Gel
4.5. Experimental Bioactivity Confirmation
4.5.1. Antioxidant Capacity
- ORAC
- ABTS
- DPPH
4.5.2. Angiotensin-I Converting Enzyme Inhibitory Activity Measurement
4.5.3. Oxidative Stress Protection Capacity Testing in a Caco-2 Cell Culture Model
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Candia Carnevali, M.D.; Sugni, M.; Bonasoro, F.; Wilkie, I.C. Mutable Collagenous Tissue: A Concept Generator for Biomimetic Materials and Devices. Mar. Drugs 2024, 22, 37. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.; Kozlovskaya, E.; Rutckova, T.; Styshova, O.; Makhankov, V.; Vakhrushev, A.; Hushpulian, D.; Gazaryan, I.; Son, O.; Tekutyeva, L. Matrikines of Sea Cucumbers: Structure, Biological Activity and Mechanisms of Action. Int. J. Mol. Sci. 2024, 10, 12068. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, M.; Chang, Y.; Xue, C.; Li, Z. Investigation of structural proteins in sea cucumber (Apostichopus japonicus) body wall. Sci. Rep. 2020, 10, 18744. [Google Scholar] [CrossRef]
- Saallah, S.; Roslan, J.; Julius, F.S.; Saallah, S.; Mohamad Razali, U.H.; Pindi, W.; Sulaiman, M.R.; Pa’ee, K.F.; Mustapa Kamal, S.M. Comparative Study of The Yield and Physicochemical Properties of Collagen from Sea Cucumber (Holothuria scabra), Obtained through Dialysis and the Ultrafiltration Membrane. Molecules 2021, 26, 2564. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.-J.; Sun, L.-C.; Cao, K.-Y.; Chen, Y.-L.; Zhang, L.-J.; Liu, G.-M.; Jin, T.; Cao, M.-J. Type I collagen from sea cucumber (Stichopus japonicus) and the role of matrix metalloproteinase-2 in autolysis. Food Biosci. 2021, 41, 100959. [Google Scholar] [CrossRef]
- Bonneel, M.; Hennebert, E.; Aranko, A.S.; Hwang, D.S.; Lefevre, M.; Pommier, V.; Wattiez, R.; Delroisse, J.; Flammang, P. Molecular mechanisms mediating stiffening in the mechanically adaptable connective tissues of sea cucumbers. Matrix Biol. 2022, 108, 39–54. [Google Scholar] [CrossRef]
- Panggabean, J.A.; Adiguna, S.P.; Hardhiyuna, M.; Rahmawati, S.I.; Sadi, N.H.; Yoga, G.P.; Nafisyah, E.; Bayu, A.; Putra, M.Y. Cutting Edge Aquatic-Based Collagens in Tissue Engineering. Mar. Drugs 2023, 21, 87. [Google Scholar] [CrossRef]
- Carvalho, D.N.; Gonçalves, C.; Sousa, R.O.; Reis, R.L.; Oliveira, J.M.; Silva, T.H. Extraction and Purification of Biopolymers from Marine Origin Sources Envisaging Their Use for Biotechnological Applications. Mar. Biotechnol. 2024, 26, 1079–1119. [Google Scholar] [CrossRef]
- Chen, Z.; Du, C.; Liu, S.; Liu, J.; Yang, Y.; Dong, L.; Zhao, W.; Huang, W.; Lei, Y. Progress in biomaterials inspired by the extracellular matrix. Giant 2024, 19, 100323. [Google Scholar] [CrossRef]
- Li, Y.; Liu, S.; Zhang, J.; Wang, Y.; Lu, H.; Zhang, Y.; Song, G.; Niu, F.; Shen, Y.; Midgley, A.C.; et al. Elastic porous microspheres/extracellular matrix hydrogel injectable composites releasing dual bio-factors enable tissue regeneration. Nat. Commun. 2024, 15, 1377. [Google Scholar] [CrossRef]
- Man, J.; Abd El-Aty, A.M.; Wang, Z.; Tan, M. Recent advances in sea cucumber peptide: Production, bioactive properties, and prospects. Food Front. 2023, 4, 131–163. [Google Scholar] [CrossRef]
- Xu, Z.; Han, S.; Guan, S.; Zhang, R.; Chen, H.; Zhang, L.; Han, L.; Tan, Z.; Du, M.; Li, T. Preparation, design, identification and application of self-assembly peptides from seafood: A review. Food Chem. X 2024, 23, 101557. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Lin, S.; Han, W.; Jiang, P.; Zhu, B.; Sun, N. Calcium delivery system assembled by a nanostructured peptide derived from the sea cucumber ovum. J. Agric. Food Chem. 2019, 67, 12283–12292. [Google Scholar] [PubMed]
- Nie, C.; Zou, Y.; Liao, S.; Gao, Q.; Li, Q. Peptides as carriers of active ingredients. Rev. Article. Curr. Res. Food Sci. 2023, 14, 100592. [Google Scholar] [CrossRef]
- Vu, D.T.; Kletthagen, M.C.; Elvevoll, E.O.; Falch, E.; Jensen, I.-J. Simulated Digestion of Red Sea Cucumber (Parastichopus tremulus): A Study of Protein Quality and Antioxidant Activity. Appl. Sci. 2024, 14, 3267. [Google Scholar] [CrossRef]
- Mildenberger, J.; Remm, M.; Atanassova, M. Self-assembly potential of bioactive peptides from Norwegian sea cucumber Parastichopus tremulus for development of functional hydrogels. LWT 2021, 148, 111678. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Senadheera, T.R.L.; Hossain, A.; Dave, D.; Shahidi, F. Antioxidant and ACE-Inhibitory Activity of Protein Hydrolysates Produced from Atlantic Sea Cucumber (Cucumaria frondosa). Molecules 2023, 28, 5263. [Google Scholar] [CrossRef]
- Lamiable, A.; Thévenet, P.; Rey, J.; Vavrusa, M.; Derreumaux, P.; Tufféry, P. PEP-FOLD3: Faster de novo structure prediction for linear peptides in solution and in complex. Nucleic Acids Res. 2016, 44, W449–W454. [Google Scholar] [CrossRef]
- Wargasetia, T.L.; Ratnawati, H.; Widodo, N.; Widyananda, M.H. Bioinformatics Study of Sea Cucumber Peptides as Antibreast Cancer Through Inhibiting the Activity of Overexpressed Protein (EGFR, PI3K, AKT1, and CDK4). Cancer Inform. 2021, 20, 1–11. [Google Scholar] [CrossRef]
- Wee, J.; Wei, G.W. Evaluation of AlphaFold 3’s Protein–Protein Complexes for Predicting Binding Free Energy Changes upon Mutation. J. Chem. Inf. Model. 2024, 64, 6676–6683. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zhang, Y.; Li, Y.; Xu, B.; Cao, Z.; Liu, W. Sea Cucumber-Inspired Autolytic Hydrogels Exhibiting Tunable High Mechanical Performances, Repairability, and Reusability. ACS Appl. Mater. Interfaces 2016, 8, 8956–8966. [Google Scholar] [CrossRef] [PubMed]
- Prior, C.; Davies, O.R.; Bruce, D.; Pohl, E. Obtaining Tertiary Protein Structures by the ab Initio Interpretation of Small Angle X-ray Scattering Data. J. Chem. Theory Comput. 2020, 16, 1985–2001. [Google Scholar] [CrossRef]
- Sun, N.; Cui, P.; Lin, S.; Yu, C.; Tang, Y.; Wei, Y.; Wu, H. Characterization of sea cucumber (Stichopus japonicus) ovum hydrolysates: Calcium chelation, solubility and absorption into intestinal epithelial cells. J. Sci. Food Agric. 2017, 97, 4604–4611. [Google Scholar] [CrossRef]
- Pace, C.N.; Fu, H.; Lee Fryar, K.; Landua, J.; Trevino, S.R.; Schell, D.; Thurlkill, R.L.; Imura, S.; Scholtz, J.M.; Gajiwala, K.; et al. Contribution of hydrogen bonds to protein stability. Protein Sci. 2014, 23, 652–661. [Google Scholar] [CrossRef]
- Rahman, A.M. Collagen of extracellular matrix from marine invertebrates and its medical applications. Rev. Mar. Drugs 2019, 17, 118. [Google Scholar] [CrossRef]
- Delroisse, J.; Van Wayneberghe, K.; Flammang, P.; Gillan, D.; Gerbaux, P.; Opina, N.; Todinanahary, G.G.B.; Eeckhaut, I. Epidemiology of a SKin Ulceration Disease (SKUD) in the sea cucumber Holothuria scabra with a review on the SKUDs in Holothuroidea (Echinodermata). Sci. Rep. 2020, 10, 22150. [Google Scholar] [CrossRef]
- Byrne, M. Chapter 19—Autotomy, evisceration, and regeneration in dendrochirotid holothuroids. In The World of Sea Cucumbers; Mercier, A., Hamel, J.-F., Suhrbier, A.D., Pearce, C.M., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 309–327. ISBN 9780323953771. [Google Scholar] [CrossRef]
- Byrne, M. Morphological, Physiological and Mechanical Features of the Mutable Collagenous Tissues Associated with Autotomy and Evisceration in Dendrochirotid Holothuroids. Mar. Drugs. 2023, 21, 134. [Google Scholar] [CrossRef]
- Bonneel, M.; Hennebert, E.; Byrne, M.; Flammang, P. Chapter 36—Mutable collagenous tissues in sea cucumbers. In The World of Sea Cucumbers; Mercier, A., Hamel, J.-F., Suhrbier, A.D., Pearce, C.M., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 573–584. [Google Scholar] [CrossRef]
- Ansharullah, A.; Tamarin, A.; Patadjai, B.; Asranuddin. Powder production of sea cucumber (Holothuria scabra): Effect of processing methods on the antioxidant activities and physico-chemical characteristics. IOP Conf. Ser. Earth Environ. Sci. 2020, 443, 012024. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Liu, Y.-X.; Zhou, D.-Y.; Liu, X.-Y.; Dong, X.-P.; Li, D.-M.; Shahidi, F. The role of matrix metalloprotease (MMP) to the autolysis of sea cucumber (Stichopus japonicus). J. Sci. Food Agric. 2019, 99, 5752–5759. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Barbaglio, A.; Benedetto, C.D.; Ribeiro, C.C.; Wilkie, I.C.; Carnevali, M.D.C.; Barbosa, M.A. New Insights into Mutable Collagenous Tissue: Correlations between the Microstructure and Mechanical State of a Sea-Urchin Ligament. PLoS ONE 2011, 6, e24822. [Google Scholar] [CrossRef]
- Ferrario, C.; Leggio, L.; Leone, R.; Di Benedetto, C.; Guidetti, L.; Coccè, V.; Ascagni, M.; Bonasoro, F.; La Porta, C.A.M.; Candia Carnevali, M.D.; et al. Marine-derived collagen biomaterials from echinoderm connective tissues. Mar. Environ. Res. 2017, 128, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Lavrinenko, I.A.; Holyavka, M.G.; Chernov, V.E.; Artyukhov, V.G. Second derivative analysis of synthesized spectra for resolution and identification of overlapped absorption bands of amino acid residues in proteins: Bromelain and ficin spectra in the 240–320 nm range. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 27, 117722. [Google Scholar] [CrossRef]
- Lin, X.; Yao, M.; Lu, J.H.; Wang, Y.; Yin, X.; Liang, M.; Yuan, E.; Ren, J. Identification of novel oligopeptides from the simulated digestion of sea cucumber (Stichopus japonicus) to alleviate Aβ aggregation progression. J. Funct. Foods 2019, 60, 103412. [Google Scholar] [CrossRef]
- Purohit, K.; Reddy, N.; Sunna, A. Exploring the Potential of Bioactive Peptides: From Natural Sources to Therapeutics. Int. J. Mol. Sci. 2024, 25, 1391. [Google Scholar] [CrossRef]
- Dewi, A.S.; Patantis, G.; Fawzya, Y.N.; Irianto, H.E.; Sa’diah, S. Angiotensin-Converting Enzyme (ACE) Inhibitory Activities of Protein Hydrolysates from Indonesian Sea Cucumbers. Int. J. Pept. Res. Ther. 2020, 26, 2485–2493. [Google Scholar] [CrossRef]
- Zhong, C.; Sun, L.C.; Yan, L.J.; Lin, Y.C.; Liu, G.M.; Cao, M.J. Production, optimisation and characterisation of angiotensin converting enzyme inhibitory peptides from sea cucumber (Stichopus japonicus) gonad. Food Funct. 2018, 9, 594–603. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, B.; Liu, Z.; Dong, S.; Zhao, X.; Zeng, M. Antihypertensive effect and purification of an ACE inhibitory peptide from sea cucumber gelatin hydrolysate. Process Biochem. 2007, 42, 1586–1591. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Evaluation of The Antioxidant Capacity of Food Products: Methods, Applications and Limitations. Processes 2022, 10, 2031. [Google Scholar] [CrossRef]
- Navajas-Porras, B.; Pérez-Burillo, S.; Valverde-Moya, Á.; Hinojosa-Nogueira, D.; Pastoriza, S.; Rufián-Henares, J.Á. Effect of Cooking Methods on the Antioxidant Capacity of Foods of Animal Origin Submitted to In Vitro Digestion-Fermentation. Antioxidants 2021, 10, 445. [Google Scholar] [CrossRef]
- Ng, Z.X.; Rosman, N.F. In vitro digestion and domestic cooking improved the total antioxidant activity and carbohydrate-digestive enzymes inhibitory potential of selected edible mushrooms. J. Food Sci. Technol. 2019, 56, 865–877. [Google Scholar] [CrossRef]
- Mirzaei, M.; Mirdamadi, S.; Safavi, M. Antioxidant activity and protective effects of Saccharomyces cerevisiae peptide fractions against H2O2-induced oxidative stress in Caco-2 cells. Food Meas. 2019, 13, 2654–2662. [Google Scholar] [CrossRef]
- Seok, C.; Baek, M.; Steinegger, M.; Park, H.; Lee, G.R.; Won, J. Accurate protein structure prediction: What comes next? Biodesign 2021, 9, 47–50. [Google Scholar] [CrossRef]
- Pozzati, G.; Kundrotas, P.; Elofsson, A. Scoring of protein–protein docking models utilizing predicted interface residues. Proteins 2022, 90, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Lear, S.; Cobb, S.L. Pep-Calc.com: A set of web utilities for the calculation of peptide and peptoid properties and automatic mass spectral peak assignment. J Comput. Aided Mol. Des. 2016, 30, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Su, X.; Shan, L.; Liu, Y.; Zhang, P.; Jia, Y. Preparation and tribocorrosion performance of CrCN coatings in artificial seawater on different substrates with different bias voltages. Ceram. Int. 2019, 45, 9901–9911. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; Dezonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Held, P.; Performing Oxygen Radical Absorbance Capacity Assays with SynergyTMHT. Application Note. Bio, 9. 2006. Available online: http://www.biotek.com/resources/docs/ORAC_Assay_Application_Note.pdf (accessed on 30 March 2025).
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Medicine 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Sandoval-Gallardo, J.M.; Osuna-Ruiz, I.; Martínez-Montaño, E.; Hernández, C.; Hurtado-Oliva, M.Á.; Valdez-Ortiz, Á.; Rios-Herrera, G.D.; Salazar-Leyva, J.A.; Ramírez-Pérez, J.S. Influence of enzymatic hydrolysis conditions on biochemical and antioxidant properties of pacific thread herring (Ophistonema libertate) hydrolysates. CyTA J. Food 2020, 18, 392–400. [Google Scholar] [CrossRef]
- Ween, O.; Stangeland, J.K.; Fylling, T.S.; Aas, G.H. Nutritional and functional properties of fishmeal produced from fresh by-products of cod (Gadus morhua L.) and saithe (Pollachius virens). Heliyon 2017, 3, e00343. [Google Scholar] [CrossRef]



| Assigned Peptide Code | Sequence | MW [g/mol] and Reconstitution Advice | Purity (HPLC) [%] | Net Charge at pH 7 | Isoelectric Point |
|---|---|---|---|---|---|
| Peptide 1 (P1) | Ac—EMLWLSDGSMGFAEDTDAAFLPGDTIFGRI | 3305.64; ACN:H2O (1:3) | 87.68 | −5.99 | 2.60 |
| Peptide 2 (P2) | Ac—RAGQPITAFLVRD | 1485.68; ACN:H2O (1:4) | 91.20 | 0.00 | 8.01 |
| Peptide 3 (P3) | Ac—SRPSDPASAVAGEDYTGISRN | 2192.25; ACN:H2O (1:5) | 85.74 | −2.00 | 3.64 |
| Peptide 4 (P4) | Ac—QNGEYGCVADTPNLLYAFKILDYRQ | 2934.23; ACN:H2O (1:3) | 88.81 | −2.04 | 3.64 |
| Calculated net charge of the peptide mixture at pH 7: | −10.03 | 3.88 | |||
| Peptide | Buffer | Solubility/ Cross Linking/Additive | Mixing/Vortexing (1 min) * | Peptide Concentration [mM] |
|---|---|---|---|---|
| 1 | Ca2+ containing (artificial sea water), pH 7.7 | DMSO | + | 1; 15; 30 |
| 2 | Ca2+ containing (artificial sea water), pH 7.7 | DMSO | ++ | 1; 15; 30 |
| 3 | Ca2+ containing (artificial sea water), pH 7.7 | ------ | + | 1; 15; 30 |
| 4 | Ca2+ containing (artificial sea water), pH 7.7 | DMSO | + | 1; 15; 30 |
| 1 + 2 + 3 + 4 (1:1 ratio) | Ca2+ containing (artificial sea water), pH 7.7 | -------- | + | 1; 15; 30 |
| 1 | 75 mM sodium phosphate buffer, pH 7.4 | ------ | + | 1.5; 7.5; 15 |
| 2 | 75 mM sodium phosphate buffer, pH 7.4 | DMSO | ++ | 1.5; 7.5; 15 |
| 3 | 75 mM sodium phosphate buffer, pH 7.4 | ------ | + | 1.5; 7.5; 15 |
| 4 | 75 mM sodium phosphate buffer, pH 7.4 | ------ | ++ | 1.5; 7.5; 15 |
| 1 + 2 + 3 + 4 (1:1:1:1) | 75 mM sodium phosphate buffer, pH 7.4 | DMSO | + | 1.5; 15; 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atanassova, M.R.; Mildenberger, J.; Hansen, M.D.; Tamm, T. Microstructure of Sea Cucumber Parastichopus tremulus Peptide Hydrogels and Bioactivity in Caco-2 Cell Culture Model. Gels 2025, 11, 280. https://doi.org/10.3390/gels11040280
Atanassova MR, Mildenberger J, Hansen MD, Tamm T. Microstructure of Sea Cucumber Parastichopus tremulus Peptide Hydrogels and Bioactivity in Caco-2 Cell Culture Model. Gels. 2025; 11(4):280. https://doi.org/10.3390/gels11040280
Chicago/Turabian StyleAtanassova, Miroslava Rossenova, Jennifer Mildenberger, Marianne Doré Hansen, and Tarmo Tamm. 2025. "Microstructure of Sea Cucumber Parastichopus tremulus Peptide Hydrogels and Bioactivity in Caco-2 Cell Culture Model" Gels 11, no. 4: 280. https://doi.org/10.3390/gels11040280
APA StyleAtanassova, M. R., Mildenberger, J., Hansen, M. D., & Tamm, T. (2025). Microstructure of Sea Cucumber Parastichopus tremulus Peptide Hydrogels and Bioactivity in Caco-2 Cell Culture Model. Gels, 11(4), 280. https://doi.org/10.3390/gels11040280









