Histological Evaluation of Chemo Mechanical Caries Removal with a Babaco-Based Formulation Gel
Abstract
1. Introduction
2. Results and Discussion
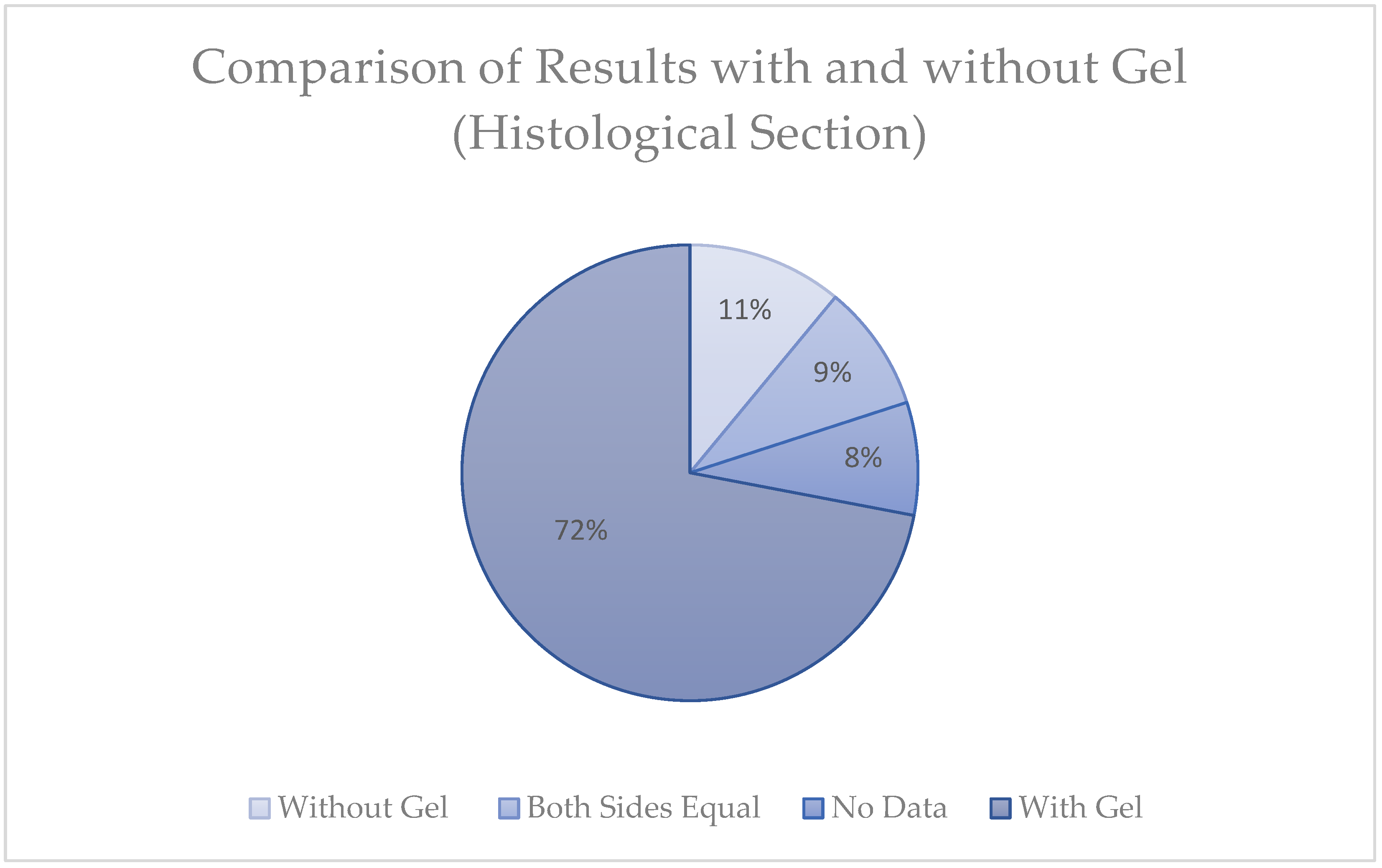
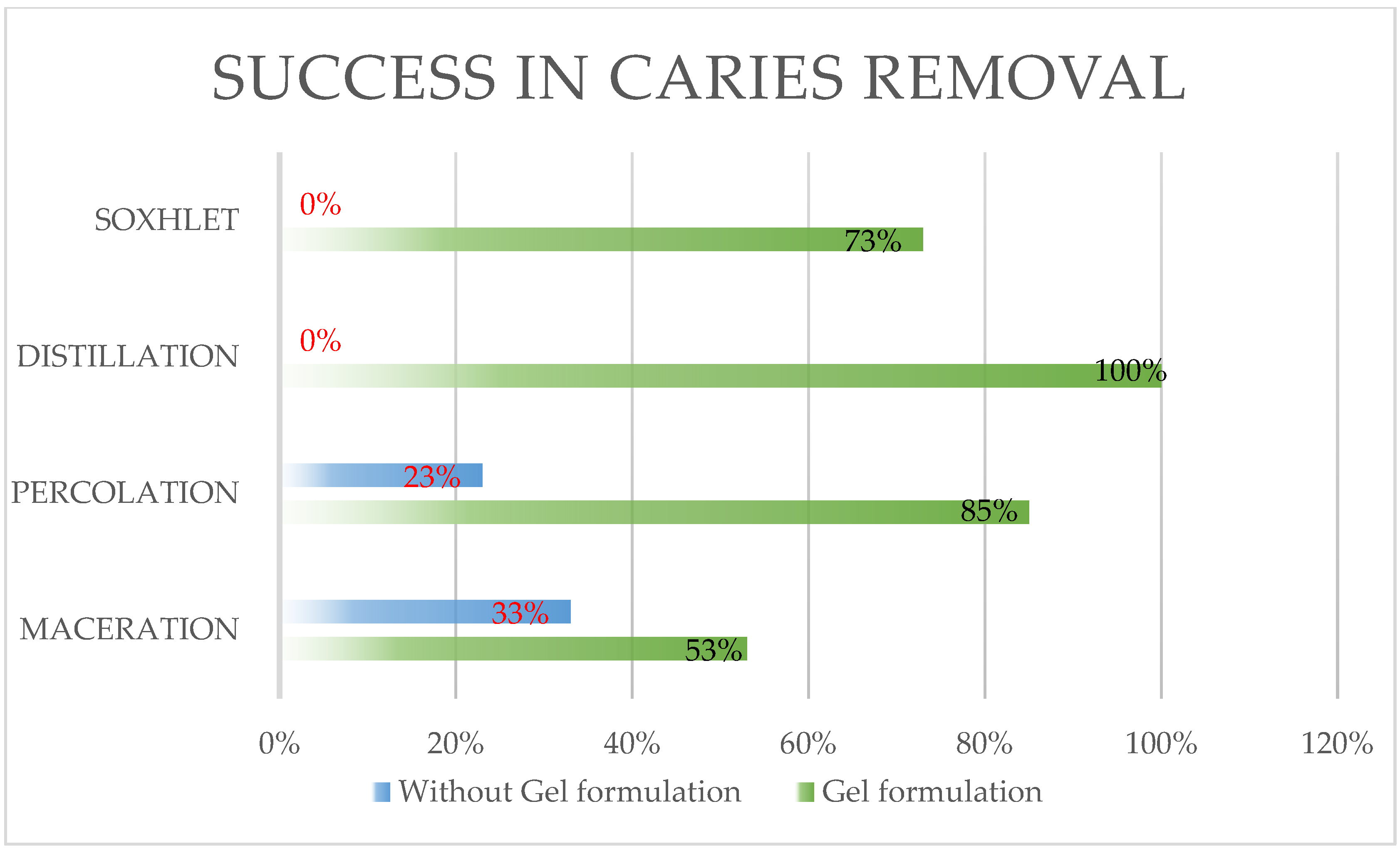
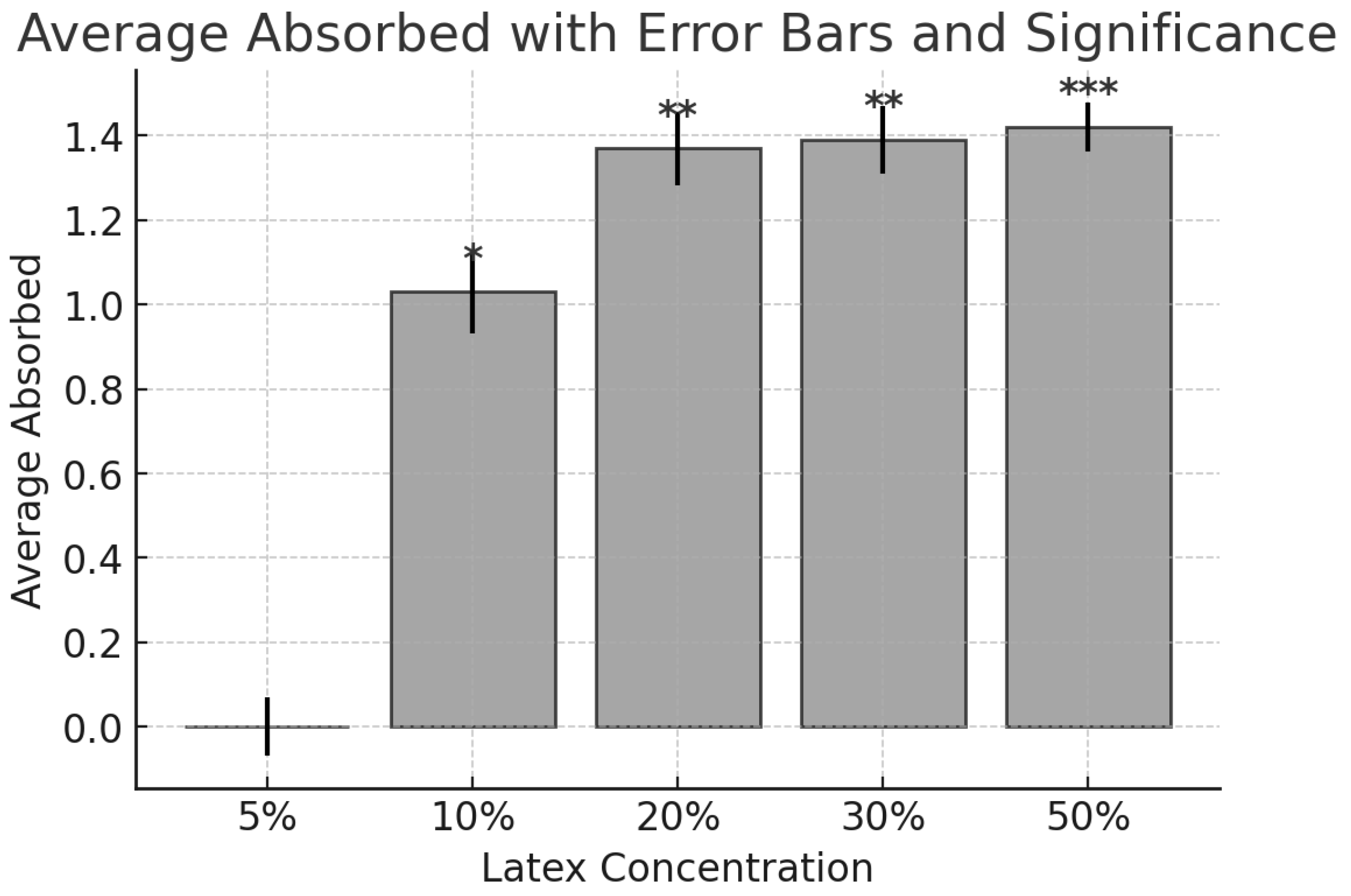
2.1. Results
2.1.1. Extraction Methods
2.1.2. Statistical Analysis
2.2. Discussion
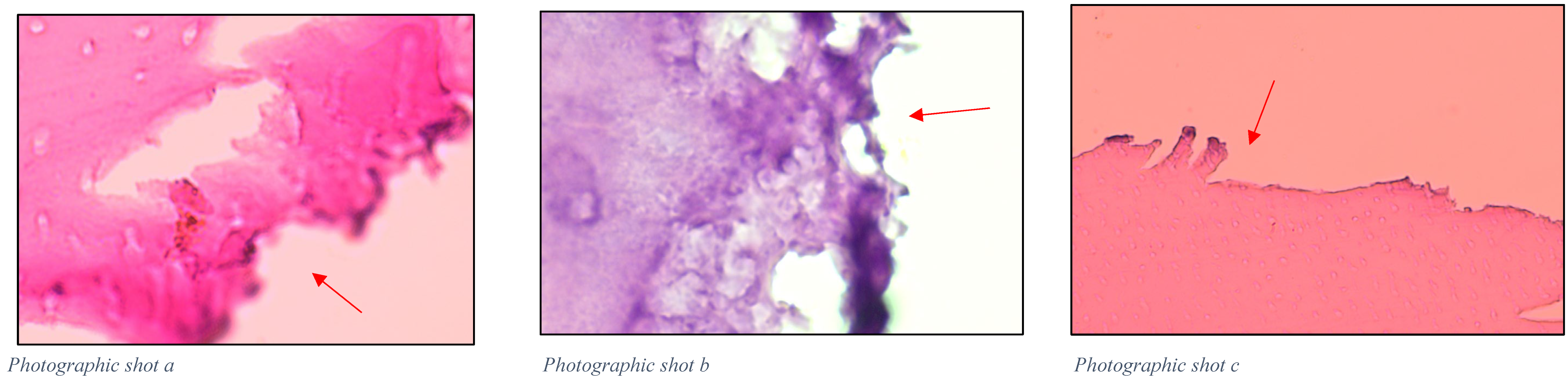
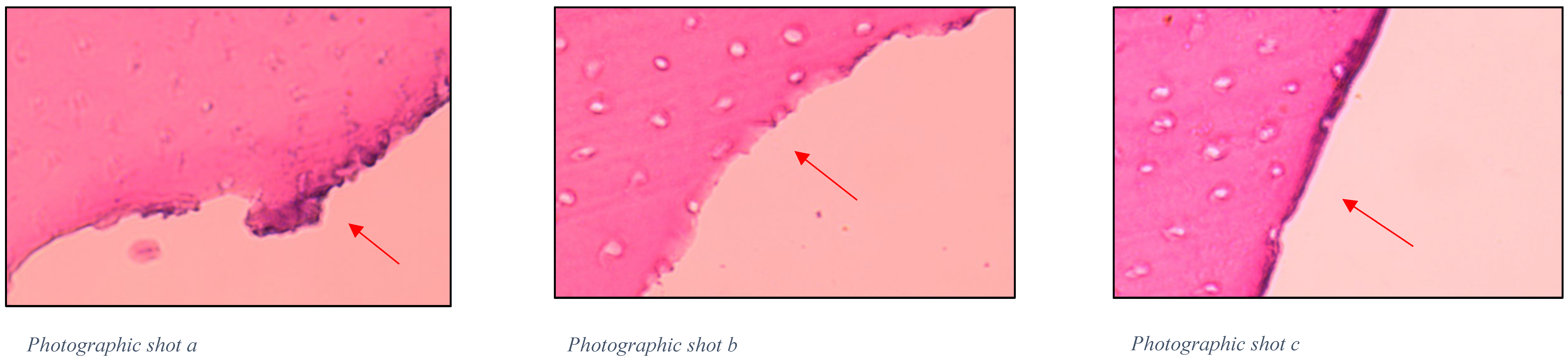
2.2.1. Efficacy of Chemomechanical Caries Removal
2.2.2. Extraction Methods and Their Impact on Efficacy
2.2.3. Comparative Efficacy with Conventional Curettage
2.2.4. Patient-Centered Outcomes
2.2.5. Limitations and Future Directions
2.2.6. Clinical Applicability
2.2.7. Critical Analysis of Potential Limitations
3. Conclusions
4. Materials and Methods
4.1. Procedure for Obtaining Raw Material
- Raw Material Collection: Fresh babaco (Carica pentagona) was harvested directly from the plant provided by the Catholic University of Cuenca, Cuenca, Azuay, Ecuador.
- Sample Processing: The collected babaco was thoroughly washed, dried, and disinfected using antiseptic alcohol. Laminar sections were carefully prepared and placed in pre-sterilized aluminum trays to isolate the second layer of babaco tissue.
- Dehydration: The prepared babaco sections were transferred to an incubator and dehydrated at 60 °C for 48 h.
- Storage: After dehydration, the material was ground into a fine powder using a mortar and stored in airtight plastic tubes at room temperature, ensuring protection from moisture.
4.2. Chemical Methods for Obtaining Papain
4.2.1. Distillation
- Historical Context: Distillation, a technique pioneered by Egyptian alchemists, employs specialized devices to vaporize and separate volatile substances. Over time, it has become a cornerstone process in the food, cosmetics, and chemical industries due to its ability to purify liquid mixtures.
- Principle: The effectiveness of distillation relies on the differences in boiling points of the components in a mixture. A significant difference in boiling points facilitates the efficient separation of components, yielding a higher degree of purity in the final product [41].
- Two clamps are placed on the universal support. The first clamp holds the asbestos mesh on which the pot with water up to half is placed, and the second clamp holds the measuring flask.
- The clamp that will support the coolant is placed on the second universal support, which is joined to the measuring flask using glass tubes and rubber stoppers.
- The lighter is connected to the gas cylinder with the valve and hose. The burner containing the measuring flask is then placed under the pot.
- The reagents are placed in the measuring flask: 200 mL of alcohol at 96° and 20 g of the second layer of crushed babaco. After this, a rubber stopper with a thermometer is placed to control the liquid output.
- The burner is turned on, and the water flow for the coolant is opened.
- A beaker is placed at the end of the coolant to obtain the result of the distillation in a water bath; since it reached a boiling point of 78.3° C, 150 mL of alcohol evaporated, and the distillation ended.
- The solution in the measuring flask is filtered using a funnel and filter paper placed inside it.
- The result of the enzymatic complex is 40 mL and is placed in sterile syringes [42].
4.2.2. Soxhlet
- The 20 g sample of babaco is placed on filter paper inside the cartridge, and 200 mL of solvent (96% alcohol) is placed in the flask.
- The Soxhlet apparatus is assembled without forgetting the water inlet and outlet through hoses.
- The heater is turned on, and the process begins. The heater waits for it to complete its automatic cycle but constantly monitors the procedure.
- Once thirty turns have been completed, and the sample is exhausted, the heater is turned off, and the sample is left to rest until it has thoroughly cooled.
- The solvent must be removed to obtain the enzymatic complex, and the distillation method is used.
- The clamp that will support the coolant is placed on the second universal support. This clamp is attached to the measuring flask using glass tubes and rubber stoppers.
- The burner is connected to the gas cylinder using the valve and hose, after which the burner is placed under the pot containing the measuring flask.
- The reagents are placed in the measuring flask: 175 mL of alcohol plus the enzymatic complex resulting from the process. After that, a rubber stopper with a thermometer is placed to control the liquid output.
- The burner is turned on, and the water flow of the coolant is opened.
- Place a beaker at the end of the condenser to obtain the result of the water bath distillation. When the water bath reaches a boiling point of 78.3 °C, the alcohol evaporates, and the distillation ends [44].
4.2.3. Maceration
- The pulverized babaco is weighed on the laboratory scale, resulting in 34.25 g. This weight is important to determine the amount of soluble needed in the maceration process.
- The crushed raw material is poured into an amber jar, and a double quantity of soluble (96° alcohol) is added to it, for a total of 68.5 mL.
- The amber jar is then covered and wrapped in aluminum foil to prevent light from passing through, and it is left to rest in a cool, dark, and dry place for seven days.
- After the days of rest, the product is filtered using a funnel with filter paper.
- Once the product is filtered, 38 mL of a solution consisting of alcohol and an enzymatic complex rich in Carica pentagona is obtained.
- Subsequently, the distillation process is carried out in a water bath to separate the alcohol in the solution obtained.
- Once the distillation process is finished, 15 mL of alcohol and 21 mL of enzyme complex are obtained, which are placed in sterile syringes to preserve them before verifying their effectiveness [45].
4.2.4. Percolation
- The percolator equipment is prepared, and a layer of sterile cotton and two paper filters are placed.
- The percentage of active ingredients is then weighed on the scale.
- 25.05 g of the active ingredient and 14.8 mL of 96% alcohol are added until all the raw material is covered.
- Two paper filters are placed to cover the active ingredient, and marbles are placed to apply force.
- It is completely covered with aluminum foil.
- It is left to rest for 3 days.
- On the fourth day, an amber glass is placed on the tip of the bottle, and force is applied to cause the active ingredient to fall.
- The active ingredient is obtained and placed in a completely sterile syringe [48].
4.3. Formulation Components
- 10% hydroalcoholic extract of Carica pentagona
- Natrusol: 0.075%
- Methylparaben: 0.05%
- Mint
- Glycerin q.s.p.
- Distilled water q.s.p.
4.4. Preparation and Evaluation of the Papain-Based Gel
- Organoleptic Evaluation: The physical properties of the gel, such as appearance, color, odor, and texture, were assessed. A sample of the gel was examined under adequate lighting against a white background to identify any irregularities. Consistency was evaluated by observing the gel’s flow behavior using a syringe.
- pH Determination: The pH of the final formulation was measured using a pH meter and found to be approximately 7, indicating neutrality.
- Microbiological Tests: Microorganism counting and sterility tests were conducted to confirm the absence of microbiological contaminants and to verify the effectiveness of the preservatives over the product’s shelf life.
- Dental Tissue Adhesion Test: This test evaluated the gel’s ability to adhere to dental tissue for a sufficient duration to exert its therapeutic effect. The test was performed on extracted human teeth, where the gel was applied, and its adhesion time was measured.
4.5. Pilot Test
- Tube 1: 5 drops of milk + 5 drops of latex
- Tube 2: 5 drops of milk + 5 drops of latex + 95% ethanol
- Tube 3: 5 drops of milk + 5 drops of 95% ethanol (negative control)
4.6. Macroscopic Determination of the Effectiveness in Caries Removal
4.7. Standardized Gel Application and Experimental Protocol
4.8. Histological Evaluation of Chemomechanical Removal
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ART | Atraumatic Restorative Treatment |
| CMCR | Chemomechanical Caries Removal |
References
- Santos, T.M.L.; Bresciani, E.; de Matos, F.S.; Camargo, S.E.A.; Hidalgo, A.P.T.; Rivera, L.M.L.; de Bernardino, Í.M.; Paranhos, L.R. Comparison between conventional and chemomechanical approaches for the removal of carious dentin: An in vitro study. Sci. Rep. 2020, 10, 8127. [Google Scholar] [CrossRef]
- Maashi, M.S.; Elkhodary, H.M.; Alamoudi, N.M.; Bamashmous, N.O. Chemomechanical caries removal methods: A literature review. Saudi. Dent. J. 2023, 35, 233–243. [Google Scholar] [CrossRef]
- Nair, M.; Rao, A.; Kukkila, J.; Natarajan, S.; Baranya Srikrishna, S. A comparative evaluation of micro shear bond strength and microleakage between the resin-modified glass ionomer cement and residual dentin following excavation of carious dentin using Carie CareTM and conventional caries removal in primary teeth: An in vitro study. F1000Research 2023, 12, 332. [Google Scholar] [CrossRef] [PubMed]
- Silva, Z.S., Jr.; Huang, Y.Y.; de Freitas, L.F.; França, C.M.; Botta, S.B.; Ana, P.A.; Mesquita-Ferrari, R.A.; Fernandes, K.P.S.; Deana, A.; Leal, C.R.L.; et al. Papain gel containing methylene blue for simultaneous caries removal and antimicrobial photoinactivation against Streptococcus mutans biofilms. Sci. Rep. 2016, 6, 33270. [Google Scholar] [CrossRef]
- Yun, J.; Shim, Y.S.; Park, S.Y.; An, S.Y. New treatment method for pain and reduction of local anesthesia use in deep caries. J. Dent. Anesthesia Pain Med. 2018, 18, 277–285. [Google Scholar] [CrossRef]
- Bratu, D.C.; Nikolajevic-Stoican, N.; Popa, G.; Pop, S.I.; Dragoș, B.; Luca, M.M. A Bibliometric Analysis (2010–2020) of the Dental Scientific Literature on Chemomechanical Methods of Caries Removal Using Carisolv and BRIX3000. Medicina 2022, 58, 788. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Patil, S.D.S.; Kush, A.; Madhu, K. SEM Analysis of Residual Dentin Surface in Primary Teeth Using Different Chemomechanical Caries Removal Agents. J. Clin. Pediatr. Dent. 2017, 41, 289–293. [Google Scholar] [CrossRef]
- Alkhouli, M.M.; Al Nesser, S.F.; Bshara, N.G.; AlMidani, A.N.; Comisi, J.C. Comparing the efficacies of two chemomechanical caries removal agents (2.25% sodium hypochlorite gel and brix 3000), in caries removal and patient cooperation: A randomized controlled clinical trial. J. Dent. 2020, 93, 103280. [Google Scholar] [CrossRef]
- Cornejo-Franco, J.F.; Medina-Salguero, A.; Flores, F.; Chica, E.; Grinstead, S.; Mollov, D.; Quito-Avila, D.F. Exploring the virome of Vasconcellea x heilbornii: The first step towards a sustainable production program for babaco in Ecuador. Eur. J. Plant Pathol. 2020, 157, 961–968. [Google Scholar] [CrossRef]
- Villavicencio-Caparó, E.; Cuenca-Leon, K.; Pachecho-Quito, E.; Rios-Jimenez, J. Elaboración de un removedor de caries en base a babaco. Investig. Clin. 2022, 63, 480–488. Available online: https://www.researchgate.net/publication/378902529_Elaboracion_de_un_removedor_de_caries_en_base_a_Babaco (accessed on 2 February 2025).
- Jadán, M.G.; Dorca-Fornell, C. Universidad de las Fuerzas Armadas-ESPE.; Av. General Rumiñahui s/n, Sangolquí- Ecuador, P.O.Box:171-5-231B. Propagation methods in Babaco plants (Vasconcella x helbornii). Trop. Plant Res. 2019, 6, 37–45. [Google Scholar] [CrossRef]
- Mosquera-Yuqui, F.; Flores, F.J.; Moncayo, E.A.; Garzón-Proaño, B.A.; Méndez, M.A.; Guevara, F.E.; Quito-Avila, D.F.; Viera, W.; Cornejo-Franco, J.F.; Izquierdo, A.R.; et al. Phylodynamics and Coat Protein Analysis of Babaco Mosaic Virus in Ecuador. Plants 2022, 11, 1646. [Google Scholar] [CrossRef] [PubMed]
- Hamama, H.H.H.; Yiu, C.K.Y.; Burrow, M.F.; King, N.M. Systematic Review and Meta-Analysis of Randomized Clinical Trials on Chemomechanical Caries Removal. Oper. Dent. 2015, 40, E167–E178. [Google Scholar] [CrossRef]
- Maru, V.P.; Shakuntala, B.S.; Nagarathna, C. Caries Removal by Chemomechanical (CarisolvTM) vs. Rotary Drill: A Systematic Review. Open Dent. J. 2015, 9, 462–472. [Google Scholar] [CrossRef]
- Lai, G.; Capi, C.L.; Cocco, F.; Cagetti, M.G.; Lingström, P.; Almhöjd, U.; Campus, G. Comparison of Carisolv system vs traditional rotating instruments for caries removal in the primary dentition: A systematic review and meta-analysis. Acta Odont. Scandinavica 2015, 73, 569–580. [Google Scholar] [CrossRef]
- Li, R.; Zhao, Y.; Ye, L. How to make the choice of the carious removal methods, Carisolv or traditional drilling? A meta-analysis. J. Oral Rehabil. 2014, 41, 432–442. [Google Scholar] [CrossRef]
- da Silva, L.B.; Magno, M.B.; Fonseca-Gonçalves, A.; Pintor, A.V.B. ART with or without the aid of chemomechanical agents: A systematic review. Clin. Oral Investig. 2024, 28, 1–19. [Google Scholar] [CrossRef]
- Senthilkumar, V.; Ramesh, S. Systematic review on alternative methods for caries removal in permanent teeth. J. Conserv. Dent. 2020, 23, 2–9. [Google Scholar] [CrossRef]
- de Ferreira, L.A.Q.; Diniz, I.M.A.; da Peixoto, R.T.R.C.; Gomes, N.A.; de Caneschi, C.S.; Spineli, L.M.; Martins, C.C. Efficacy of antiseptics and chemomechanical methods for dentin caries lesions: A systematic review with GRADE approach. Front. Oral Health 2023, 4, 1110634. [Google Scholar] [CrossRef]
- Mehrotra, D.; Kodical, S.R.; Naik, S.S. Comparison of smart burs and chemomechanical caries removal systems in primary molars—A systematic review and meta-analysis. J. Indian Soc. Pedod. Prev. Dent. 2024, 42, 257. [Google Scholar] [CrossRef]
- Rossoni, N.B.; Cavalheiro, C.P.; Casagrande, L.; Lenzi, T.L. Influence of the chemomechanical and mechanical carious tissue removal on the risk of restorative failure: A systematic review and meta-analysis. Clin. Oral Investig. 2022, 26, 6457–6467. [Google Scholar] [CrossRef] [PubMed]
- Schwendicke, F.; Paris, S.; Tu, Y.K. Effects of using different criteria for caries removal: A systematic review and network meta-analysis. J. Dent. 2015, 43, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Acosta, R. Estudio de la Variación de la Actividad Enzimática Proteolítica del Látex del Babaco (Vasconcellea heilbornii cv babaco) en Función de la Edad del Fruto”. Bachelor’s Thesis, Escuela Politecnica Nacional, Quito, Ecuador, 2011. [Google Scholar]
- Reyes, F. Determination of Glycosidades in Babaco Purification and Characterization of a-Mannodidase from Babaco Latex; Escuela Politecnica Nacional: Quito, Ecuador, 2007; Volume 7. [Google Scholar]
- Cuenca-León, K.; Pacheco-Quito, E.M.; Granda-Granda, Y.; Vélez-León, E.; Zarzuelo-Castañeda, A. Phytotherapy: A Solution to Decrease Antifungal Resistance in the Dental Field. Biomolecules 2022, 12, 789. [Google Scholar] [CrossRef] [PubMed]
- Cuenca-León, K.; Lima-Illescas, M.; Pacheco-Quito, E.; Vélez-León, E.; Zarzuelo-Castañeda, A. Effectiveness of Lemon Verbena (Cymbopogon citratus) in Oral Candidiasis: A Systematic Review. Clin. Cosmet. Investig. Dent. 2024, 16, 295–305. [Google Scholar]
- Guedes, F.R.; Bonvicini, J.F.S.; de Souza, G.L.; da Silva, W.H.T.; Moura, C.C.G.; Paranhos, L.R.; Turrioni, A. Cytotoxicity and dentin composition alterations promoted by different chemomechanical caries removal agents: A preliminary study. J. Clin. Exp. Dent. 2021, 13, e826–e834. [Google Scholar] [CrossRef]
- Silva, Z.S., Jr.; França, C.M.; Araújo Prates, R.; Botta, S.B.; Ferrari, R.A.M.; Ana, P.A.; Pavani, C.; Fernandes, K.P.S.; de da Silva, D.F.T.; Hamblin, M.R.; et al. The effects of photodynamic therapy with blue light and papain-based gel associated with Urucum, on collagen and fibroblasts: A spectroscopic and cytotoxicity analysis. Lasers Med. Sci. 2020, 35, 767–775. [Google Scholar] [CrossRef]
- AlHumaid, J. Efficacy and Efficiency of Papacarie versus Conventional Method in Caries Removal in Primary Teeth: An SEM Study. Saudi J. Med. Med. Sci. 2020, 8, 41–45. [Google Scholar] [CrossRef]
- Sharma, N.; Sisodia, S.; Jain, A.; Bhargava, T.; Kumar, P.; Rana, K.S. Evaluation of the Efficacy of Recent Caries Removal Techniques: An In Vitro Study. Cureus 2023, 15, e34432. [Google Scholar] [CrossRef]
- Saber, A.M.; Altoukhi, D.H.; Horaib, M.F.; El-Housseiny, A.A.; Alamoudi, N.M.; Sabbagh, H.J. Consequences of early extraction of compromised first permanent molar: A systematic review. BMC Oral Health 2018, 18, 59. [Google Scholar] [CrossRef]
- Cardoso, M.; Coelho, A.; Lima, R.; Amaro, I.; Paula, A.; Marto, C.M.; Sousa, J.; Spagnuolo, G.; Ferreira, M.M.; Carrilho, E. Efficacy and Patient’s Acceptance of Alternative Methods for Caries Removal-a Systematic Review. J. Clin. Med. 2020, 9, 3407. [Google Scholar] [CrossRef]
- Sierra, L.A.V.; Guette, S.S.C. Ansiedad, miedo y comportamiento en odontopediatria utilizando Brix 3000 y método rotatorio para remoción de caries. Duazary: Rev. Int. Cien. Salud. 2019, 16, 383–394. [Google Scholar]
- Souza, T.F.; Martins, M.L.; Magno, M.B.; Vicente-Gomila, J.M.; Fonseca-Gonçalves, A.; Maia, L.C. Worldwide research trends on using chemical-mechanical caries removal products over the years: A critical review. Eur. Arch. Paediatr. Dent. 2022, 23, 869–883. [Google Scholar] [CrossRef]
- Bottega, F.; Bussadori, S.K.; Battisti, I.D.E.; Vieira, E.P.; Pompeo, T.S.; Winkelmann, E.R. Costs and benefits of Papacarie in pediatric dentistry: A randomized clinical trial. Sci. Rep. 2018, 8, 17908. [Google Scholar] [CrossRef]
- Nair, S.; R Nadig, R.; S Pai, V.; Gowda, Y. Effect of a Papain-based Chemomechanical Agent on Structure of Dentin and Bond Strength: An Study. Int. J. Clin. Pediatr. Dent. 2018, 11, 161–166. [Google Scholar] [CrossRef]
- Ghanem, A.Y.; Talaat, D.M.; Essawy, M.M.; Bakry, N. The effectiveness of Carie-CareTM; chemomechanical caries removal technique in primary teeth: Randomized controlled clinical trial. BMC Oral Health 2023, 23, 882. [Google Scholar] [CrossRef]
- Deng, Y.; Feng, G.; Hu, B.; Kuang, Y.; Song, J. Effects of Papacarie on children with dental caries in primary teeth: A systematic review and meta-analysis. Int. J. Paediatr. Dent. 2018, 28, 361–372. [Google Scholar] [CrossRef]
- Soni, H.K.; Sharma, A.; Sood, P.B. A comparative clinical study of various methods of caries removal in children. Eur. Arch. Paediatr. Dent. 2015, 16, 19–26. [Google Scholar] [CrossRef]
- Valiente, A. Historia de la Destilación. de Facultad. Educ. Química 2018, 7, 76. Available online: https://www.revistavirtualpro.com/biblioteca/historia-de-la-destilacion (accessed on 23 March 2025).
- Tesfaye, M.; Gonfa, Y.; Tadesse, G.; Temesgen, T.; Periyasamy, S. Green synthesis of silver nanoparticles using Vernonia amygdalina plant extract and its antimicrobial activities. Heliyon 2023, 9, e17356. [Google Scholar] [CrossRef]
- Mulet-Hing, M. Automatización de la destilación de alcohol de la UEB destilería de la ronera Santiago de Cuba. Tecnol. Química 2013, 33, 1–9. Available online: https://www.redalyc.org/articulo.oa?id=445543778001 (accessed on 23 March 2025).
- Viera, O.; Morales, S. Comparación de los métodos de extracción para hidrocarburos aromáticos policíclicos en sedimentos marinos empleando soxhlet y baño ultrasónico. Rev. Cent. Azúcar 2020, 47, 14–23. Available online: http://scielo.sld.cu/pdf/caz/v47n3/2223-4861-caz-47-03-14.pdf (accessed on 23 March 2025).
- Wirth, T. Organic synthesis in flow for medicinal chemistry. Bioorg. Med. Chem. 2017, 25, 6179. [Google Scholar] [CrossRef] [PubMed]
- Azwanida, N.N. A review on the extraction methods use in medicinal plants, principle, strength, and limitation. Med. Aromat. Plants 2015, 4, 196. [Google Scholar]
- Velickovic, V.; Durovic, S.; Radojkovic, M.; Cvetanovic, A.; Svarc, J.; Vujic, J.; Trifunović, S.; Mašković, P.Z. Application of conventional and non-conventional extraction approaches for extraction of Erica carnea L.: Chemical profile and biological activity of obtainde extracts. J. Supercrit. Fluids 2017, 128, 331–337. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. International Natural Product Sciences Taskforce, Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Pandey, A.; Tripathi, S. Concept of standardization, extraction, and pre phytochemical screening strategies for herbal drug. J. Pharmacogn. Phytochem. 2014, 2, 115–119. [Google Scholar]
| Success in Caries Removal | |||||
|---|---|---|---|---|---|
| Gel Formulation | Without Gel Formulation | ||||
| N | Success Rate (%) | N | Success Rate (%) | Sig. | |
| MACERATION n = 15 | 8 | 53% | 5 | 33% | 0.3008 * |
| PERCOLATION n = 13 | 11 | 85% | 3 | 23% | 0.013 ** |
| DISTILLATION n = 15 | 15 | 100% | 0 | 0% | N.A. |
| SOXHLET n = 15 | 11 | 73% | 0 | 0% | N.A. |
| TOTAL n = 58 | 45 | 78% | 8 | 14% | <0.01 * |
| Chi-square * | Fisher ** | ||||
| Post Hoc Comparison | ||||||
|---|---|---|---|---|---|---|
| Gel Formulation | Total | Post Hoc Group | ||||
| Success | Failure | |||||
| Extraction Method | Distillation | Count | 15 | 0 | 15 | A |
| Expected | 11.6 | 3.4 | 15.0 | |||
| Residual | 2.4 | −2.4 | ||||
| Maceration | Count | 8 | 7 | 15 | C | |
| Expected | 11.6 | 3.4 | 15.0 | |||
| Residual | −2.6 | 2.6 | ||||
| Percolation | Count | 11 | 2 | 13 | B | |
| Expected | 10.1 | 2.9 | 13.0 | |||
| Residual | 0.7 | −0.7 | ||||
| Soxhlet | Count | 11 | 4 | 15 | B | |
| Expected | 11.6 | 3.4 | 15.0 | |||
| Residual | −0.5 | 0.5 | ||||
| Total | Count | 45 | 13 | 58 | ||
| Expected | 45.0 | 13.0 | 58.0 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pariona-Minaya, M.d.C.; Berrezueta-Pérez, M.; Cabezas-Bernhardt, G.; Villavicencio-Caparo, E. Histological Evaluation of Chemo Mechanical Caries Removal with a Babaco-Based Formulation Gel. Gels 2025, 11, 257. https://doi.org/10.3390/gels11040257
Pariona-Minaya MdC, Berrezueta-Pérez M, Cabezas-Bernhardt G, Villavicencio-Caparo E. Histological Evaluation of Chemo Mechanical Caries Removal with a Babaco-Based Formulation Gel. Gels. 2025; 11(4):257. https://doi.org/10.3390/gels11040257
Chicago/Turabian StylePariona-Minaya, María del Carmen, Melissa Berrezueta-Pérez, Gerson Cabezas-Bernhardt, and Ebingen Villavicencio-Caparo. 2025. "Histological Evaluation of Chemo Mechanical Caries Removal with a Babaco-Based Formulation Gel" Gels 11, no. 4: 257. https://doi.org/10.3390/gels11040257
APA StylePariona-Minaya, M. d. C., Berrezueta-Pérez, M., Cabezas-Bernhardt, G., & Villavicencio-Caparo, E. (2025). Histological Evaluation of Chemo Mechanical Caries Removal with a Babaco-Based Formulation Gel. Gels, 11(4), 257. https://doi.org/10.3390/gels11040257






