Hydrophobic Silica Aerogel with Higher Flame Retardancy, Thermal Radiation Shielding, and High-Temperature Insulation Properties Through Introduction of TiO2
Abstract
1. Introduction
2. Results and Discussion
2.1. Microstructure and Pore Structure
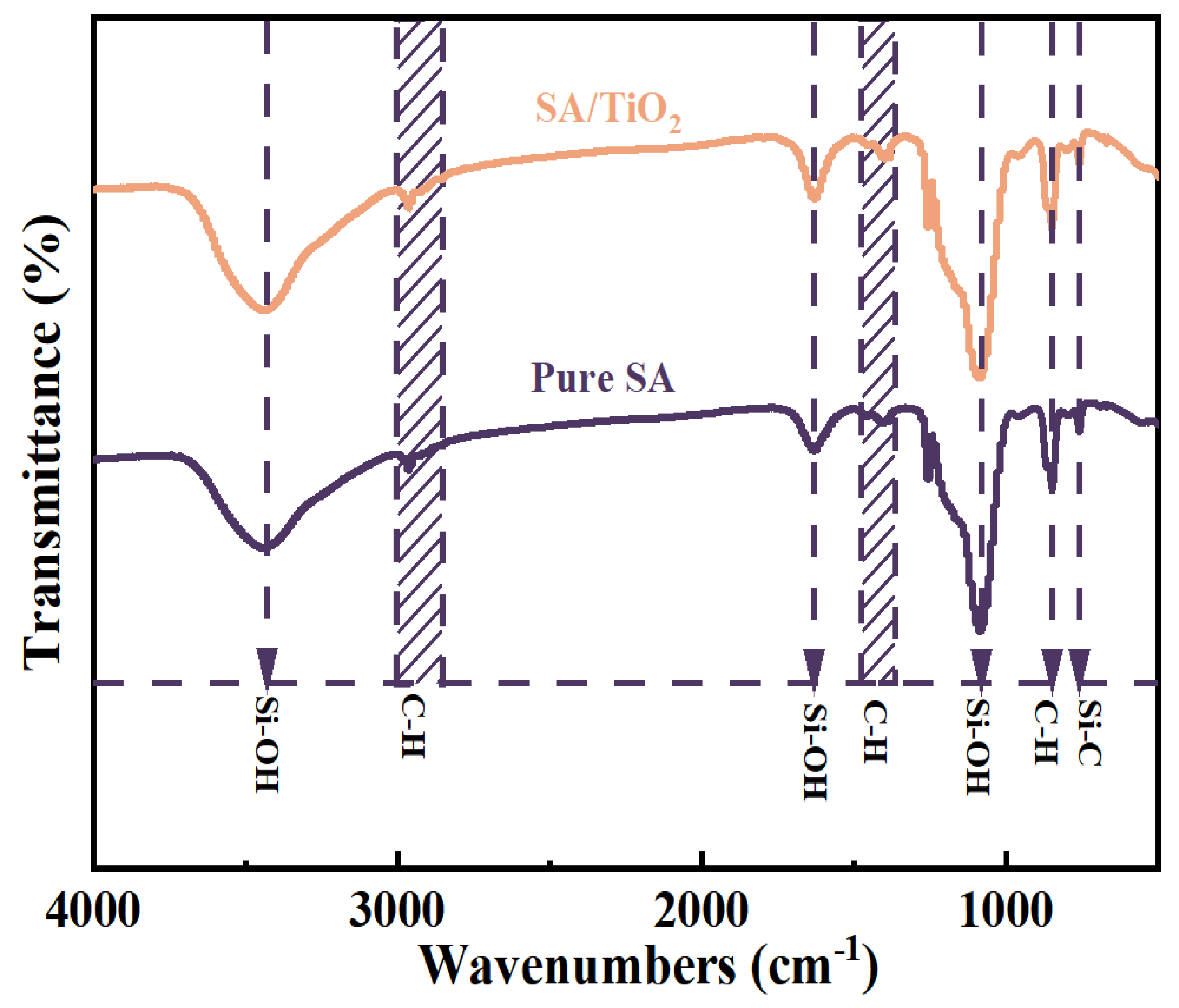
2.2. Basic Physicochemical Properties
2.3. Conbustion Behaviors
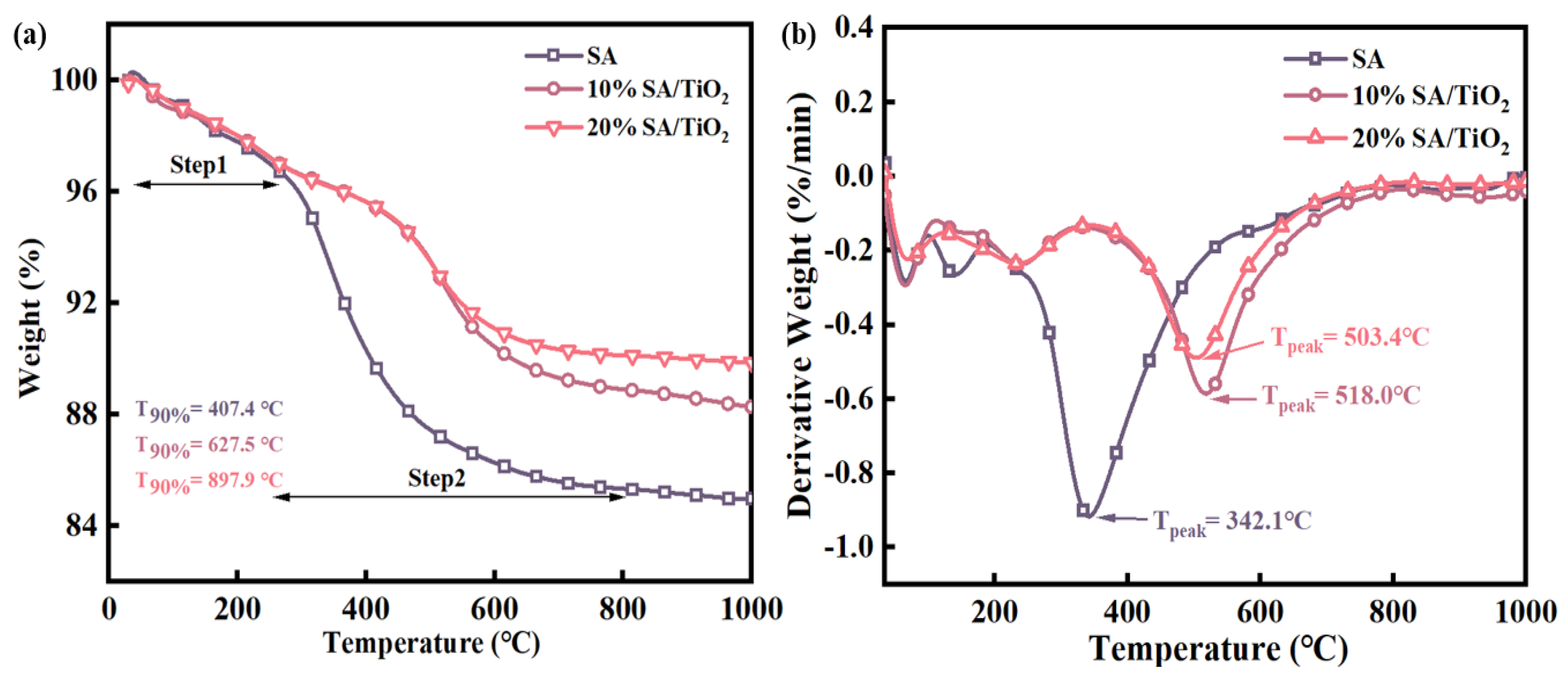
2.3.1. Thermal Analysis
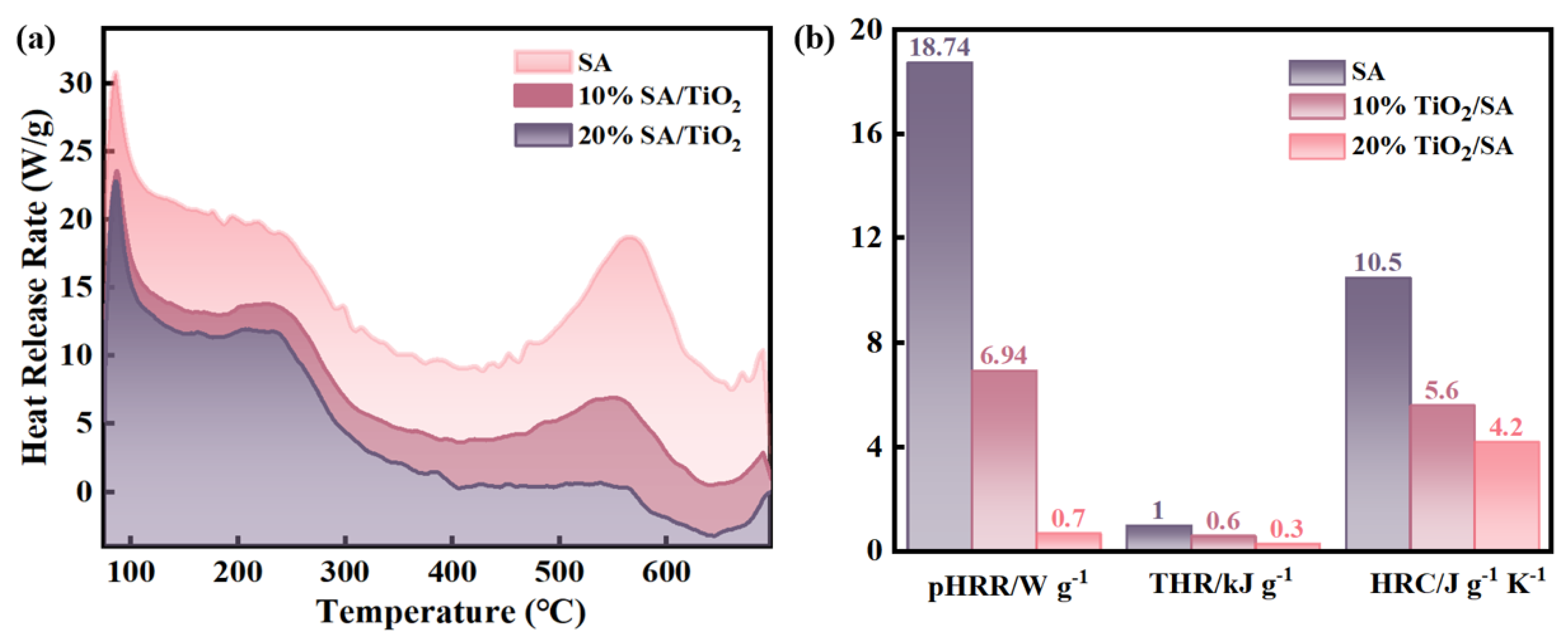
2.3.2. Flame-Retardant Properties
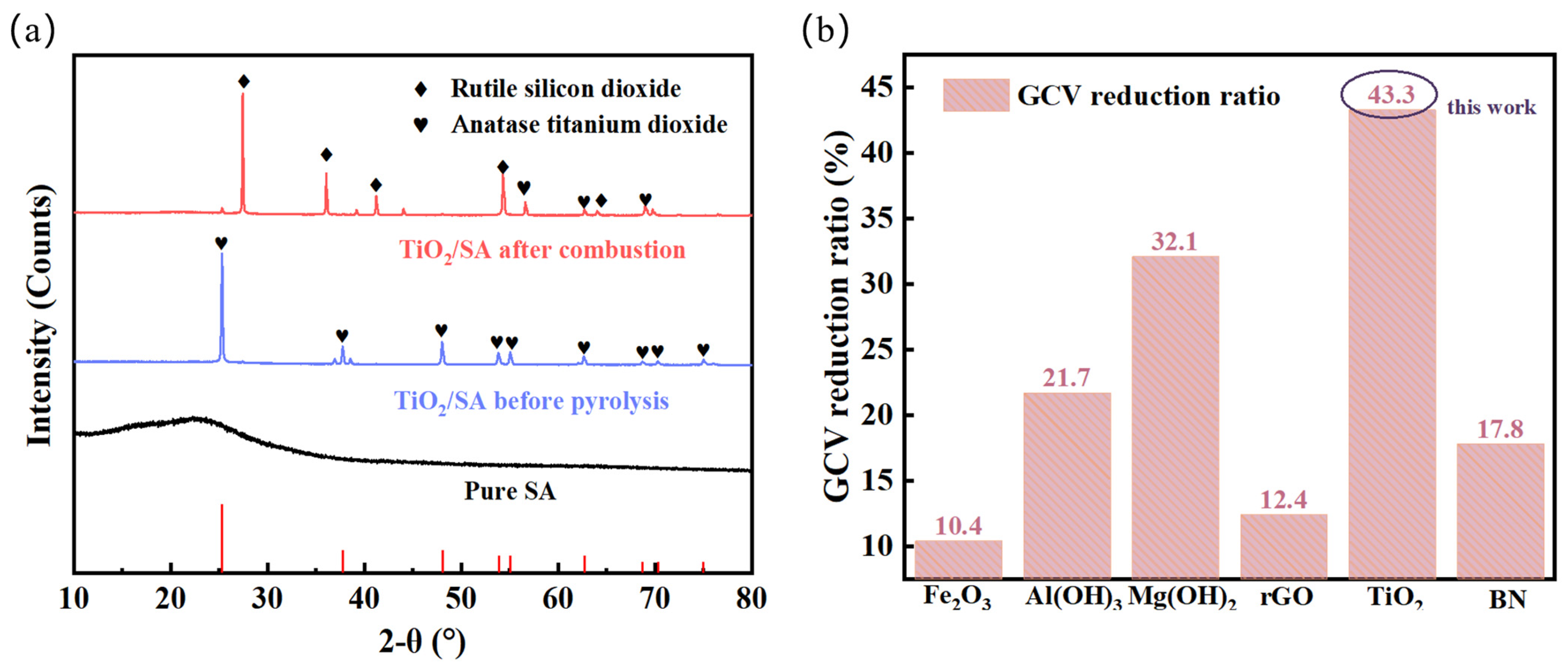
2.3.3. Composition of Pyrolysis Products
2.4. SA/TiO2 Aerogel Composites
2.4.1. Density and Hydrophobicity
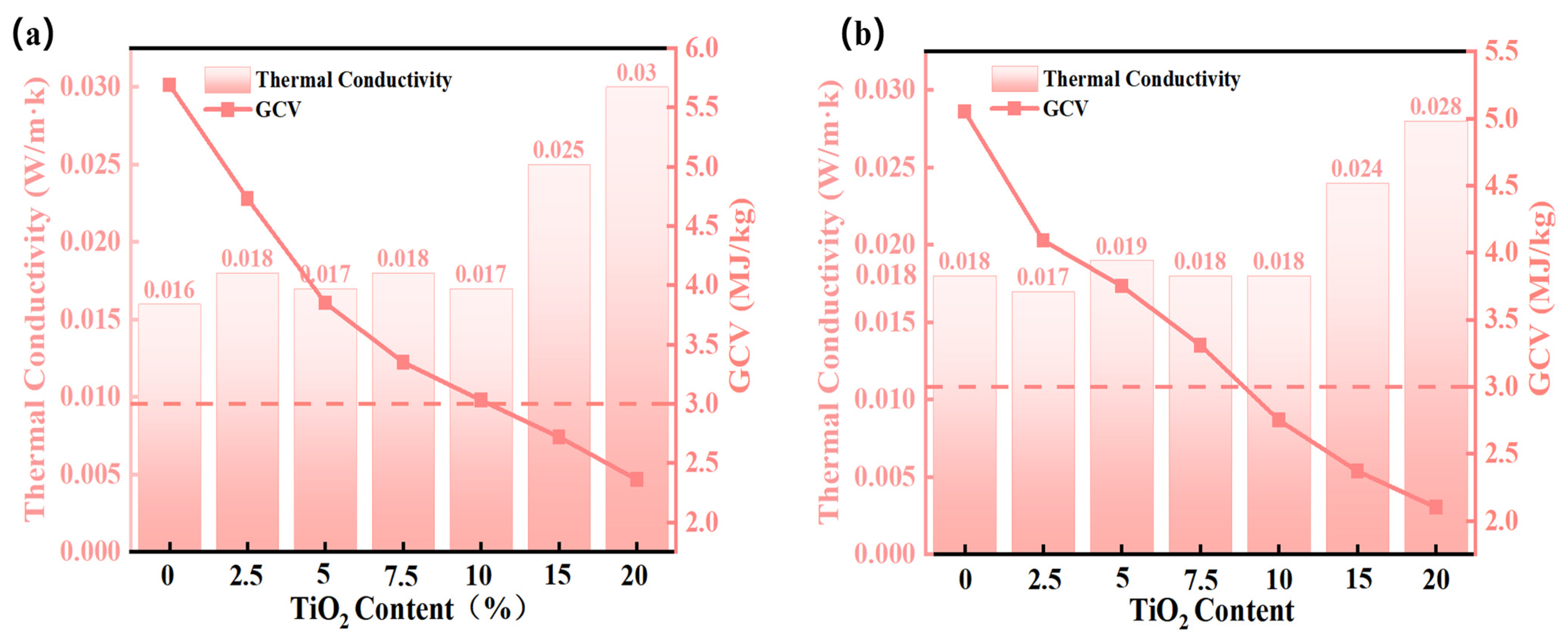
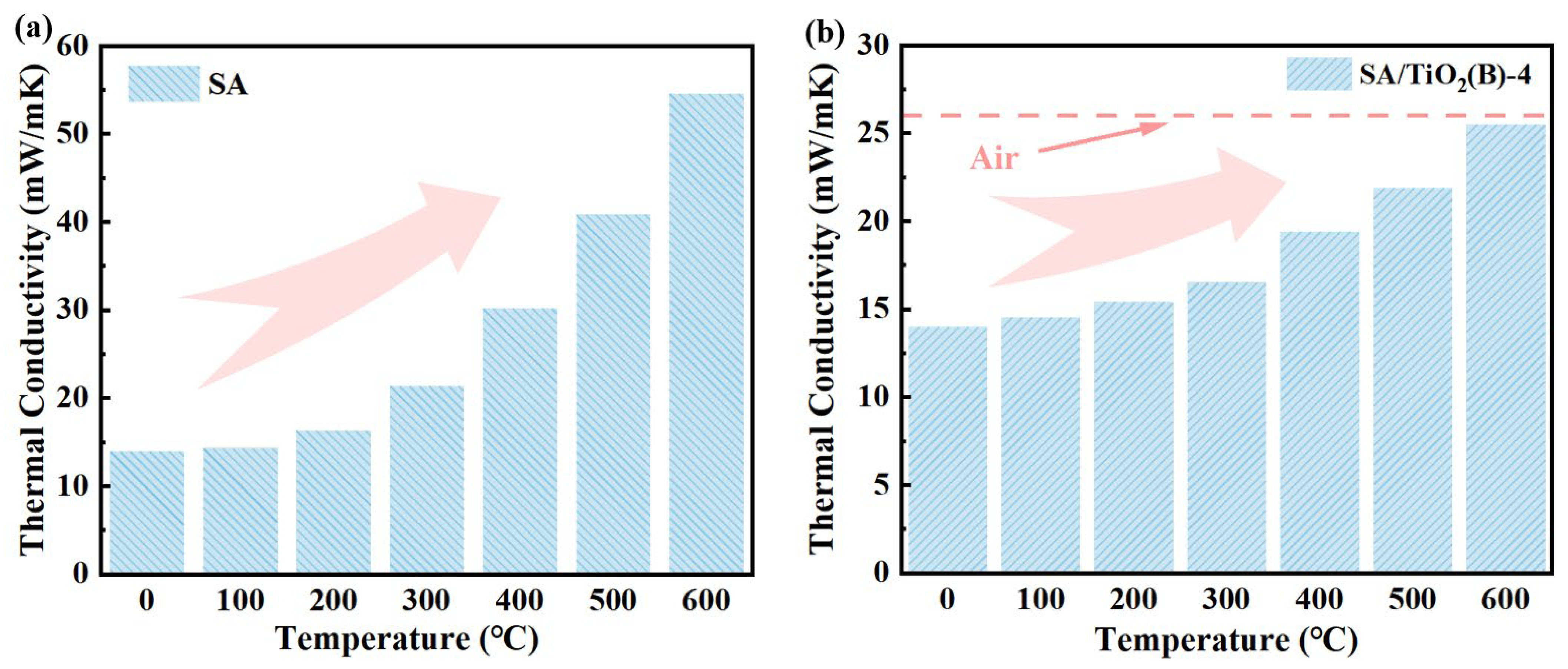
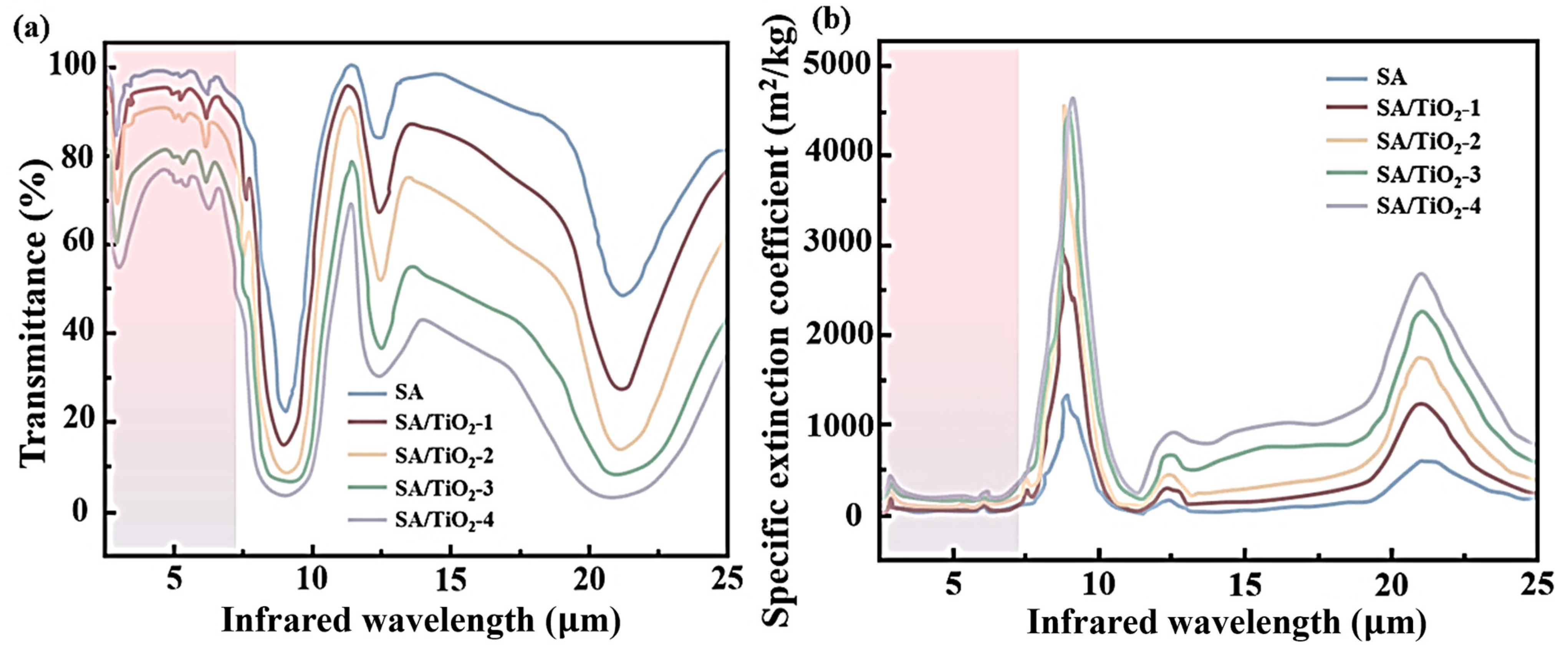
2.4.2. GCV and Thermal Insulation Performance
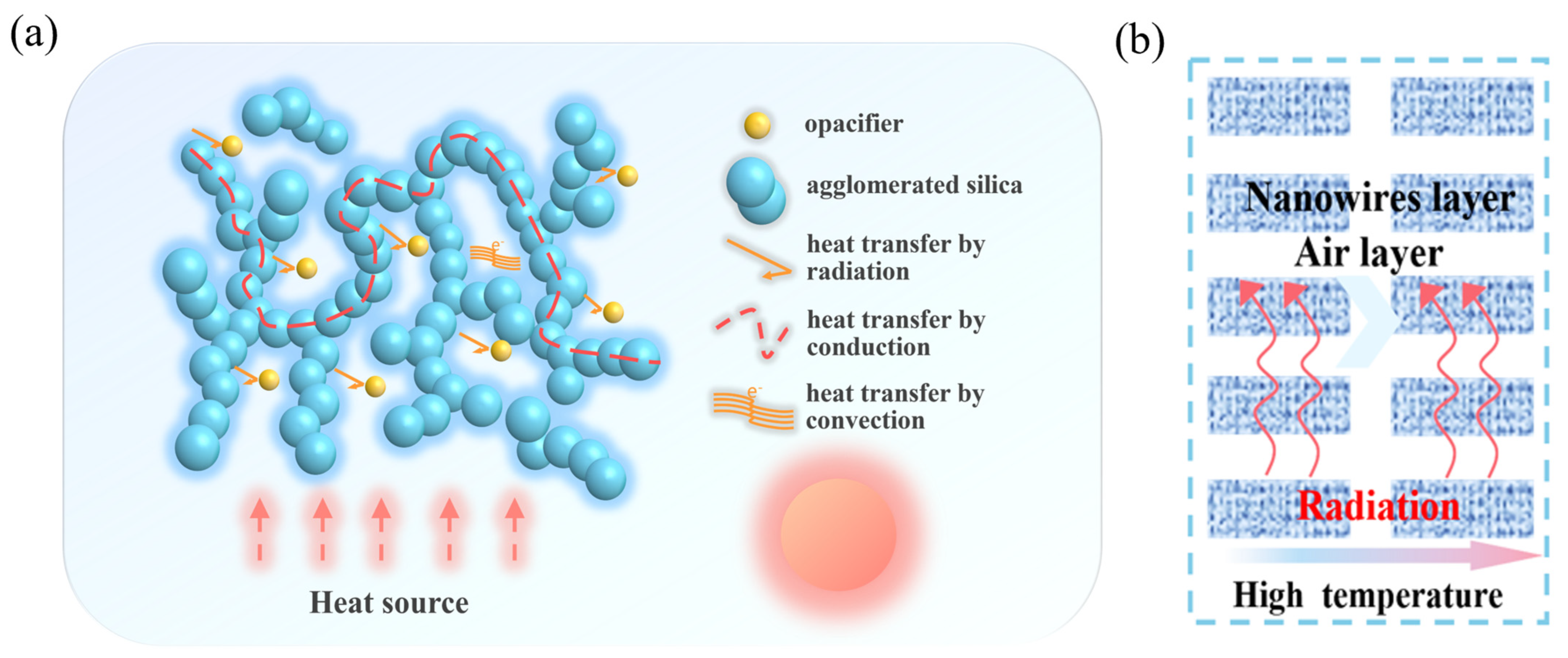
2.4.3. Flame-Retardant Mechanism
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of SA/TiO2 Aerogel Powders
4.3. Preparation of SA/TiO2 Aerogel Composites
4.4. Methods for Characterization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hüsing, N.; Schubert, U. Aerogels—Airy Materials: Chemistry, Structure, and Properties. Angew. Chem. Int. Ed. 1998, 37, 22–45. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, X.; Gong, L.; Liu, Q.; Li, S. Enhanced Flame Retardancy of Hydrophobic Silica Aerogels by Using Sodium Silicate as Precursor and Phosphoric Acid as Catalyst. J. Non-Cryst. Solids 2018, 481, 267–275. [Google Scholar] [CrossRef]
- Wang, L.; Shen, A.; Li, Z.; Wang, C.; Liu, M.; Guo, Y. SiO2 Aerogel Modified Aggregates: Preparation, Heat Resistance and Improvement Mechanism. Constr. Build. Mater. 2024, 449, 138332. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Tudorache, D.-I.; Bocioagă, M.; Mihaiescu, D.E.; Hadibarata, T.; Grumezescu, A.M. An Updated Overview of Silica Aerogel-Based Nanomaterials. Nanomaterials 2024, 14, 469. [Google Scholar] [CrossRef]
- Linhares, T.; Pessoa De Amorim, M.T.; Durães, L. Silica Aerogel Composites with Embedded Fibres: A Review on Their Preparation, Properties and Applications. J. Mater. Chem. A 2019, 7, 22768–22802. [Google Scholar] [CrossRef]
- Tai, M.H.; Kumar, P.S. Harnessing the Power of Silica Aerogels for Applications in Energy and Water Sustainability. J. Mater. Chem. A 2024, 12, 18879–18900. [Google Scholar] [CrossRef]
- Feng, L.; Cai, M.; Fu, Y.; Ma, Q.; Sun, B.; Waterhouse, G.I.N. Short Jute Fiber-Reinforced Silica Aerogel with Excellent Mechanical Properties. J. Mater. Sci. 2024, 59, 19892–19903. [Google Scholar] [CrossRef]
- Rao, A.P.; Rao, A.V.; Pajonk, G.M. Hydrophobic and Physical Properties of the Ambient Pressure Dried Silica Aerogels with Sodium Silicate Precursor Using Various Surface Modification Agents. Appl. Surf. Sci. 2007, 253, 6032–6040. [Google Scholar] [CrossRef]
- Rao, A.V.; Pajonk, G.M.; Bhagat, S.D.; Barboux, P. Comparative Studies on the Surface Chemical Modification of Silica Aerogels Based on Various Organosilane Compounds of the Type RnSiX4−n. J. Non-Cryst. Solids 2004, 350, 216–223. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, X.; He, S.; Shi, X.; Yang, H. Characteristics of Ambient-Pressure-Dried Aerogels Synthesized via Different Surface Modification Methods. J. Sol-Gel. Sci. Technol. 2015, 76, 138–149. [Google Scholar] [CrossRef]
- Halim, Z.A.A.; Yajid, M.A.M.; Hamdan, H. Effects of Solvent Exchange Period and Heat Treatment on Physical and Chemical Properties of Rice Husk Derived Silica Aerogels. Silicon 2021, 13, 251–257. [Google Scholar] [CrossRef]
- Guo, J.; Fu, S.; Deng, Y.; Xu, X.; Laima, S.; Liu, D.; Zhang, P.; Zhou, J.; Zhao, H.; Yu, H.; et al. Hypocrystalline Ceramic Aerogels for Thermal Insulation at Extreme Conditions. Nature 2022, 606, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Jiang, Y.; Feng, J.; Feng, J.; Yue, C. Infrared-Opacified Al2O3–SiO2 Aerogel Composites Reinforced by SiC-Coated Mullite Fibers for Thermal Insulations. Ceram. Int. 2015, 41, 437–442. [Google Scholar] [CrossRef]
- Lun, Z.; Gong, L.; Zhang, Z.; Deng, Y.; Zhou, Y.; Pan, Y.; Cheng, X. Improvement of the Thermal Insulation Performance of Silica Aerogel by Proper Heat Treatment: Microporous Structures Changes and Pyrolysis Mechanism. Gels 2022, 8, 141. [Google Scholar] [CrossRef]
- Baiker, A.; Dollenmeier, P.; Glinski, M.; Reller, A. Selective Catalytic Reduction of Nitric Oxide with Ammonia. Appl. Catal. 1987, 35, 365–380. [Google Scholar] [CrossRef]
- Xi, S.; Wang, Y.; Zhang, X.; Cao, K.; Su, J.; Shen, J.; Wang, X. Fire-Resistant Polyimide-Silica Aerogel Composite Aerogels with Low Shrinkage, Low Density and High Hydrophobicity for Aerospace Applications. Polym. Test. 2023, 129, 108259. [Google Scholar] [CrossRef]
- Zhang, P.; Zhao, S.; Li, K.; Zhang, Z.; Yang, F.; Li, X.; Song, Y.; Gan, Z.; Yang, Z. Large-Scale Production of Elastic SiC/SiO2 Nanofibrous Composite Aerogels with a Labyrinth Structure for High-Temperature Insulation, Fire Prevention, and Noise Absorption. Chem. Eng. J. 2025, 505, 159166. [Google Scholar] [CrossRef]
- Han, Y.; Wu, Y.; Zhang, H.; Huang, S.; Wu, S.; Liang, Z. A Three-Dimensional Network Modifier (Dimethyldiethoxysilane) Makes ZrO2-SiO2 Aerogel with Excellent Thermal Insulation Performance and High-Temperature Stability. Colloids Surf. A Physicochem. Eng. Asp. 2023, 671, 131716. [Google Scholar] [CrossRef]
- Li, Z.; Hu, M.; Shen, K.; Liu, Q.; Li, M.; Chen, Z.; Cheng, X.; Wu, X. Tuning Thermal Stability and Fire Hazards of Hydrophobic Silica Aerogels via Doping Reduced Graphene Oxide. J. Non-Cryst. Solids 2024, 625, 122747. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Huber, L.; Wu, X.; Huang, S.; Zhang, Y.; Huang, R.; Liu, Q. Reducing the Thermal Hazard of Hydrophobic Silica Aerogels by Using Dimethyldichlorosilane as Modifier. J. Sol-Gel. Sci. Technol. 2020, 93, 111–122. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, L.; Deng, X.; Deng, Y.; Wu, X.; Shi, L.; Li, M.; Liu, Q.; Cheng, X.; Li, Z. Improving the Flame Retardance of Hydrophobic Silica Aerogels through a Facile Post-Doping of Magnesium Hydroxide. Adv. Powder Technol. 2021, 32, 1891–1901. [Google Scholar] [CrossRef]
- Sun, M.; Li, Z.; Zhang, Y.; Wu, X.; Shi, L.; Liu, Q.; Li, M. Assessment on Thermal Safety of Aluminum Hydroxide Doping Hydrophobic Silica Aerogels. J. Nanopart. Res. 2022, 24, 87. [Google Scholar] [CrossRef]
- Sun, M.; Wang, Y.; Wang, X.; Liu, Q.; Li, M.; Shulga, Y.M.; Li, Z. In-Situ Synthesis of Layered Double Hydroxide/Silica Aerogel Composite and Its Thermal Safety Characteristics. Gels 2022, 8, 581. [Google Scholar] [CrossRef]
- Yang, S.; Strobach, E.; Bierman, D.; Zhao, L.; Bhatia, B.; Wang, E.N. Effect of Al2O3 ALD Coating on Thermal Stability of Silica Aerogel. J. Porous Mater. 2022, 29, 193–200. [Google Scholar] [CrossRef]
- Park, Y.S.; Choi, J.; Kim, B.S.; Baeck, S.-H.; Shim, S.E.; Qian, Y. Synergistic Effects of P and Si on the Flame Retardancy in a Polymethylsilsesquioxane Aerogel Prepared under Ambient Pressure Drying. J. Therm. Anal Calorim. 2023, 148, 7623–7632. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, S.; Koebel, M.M.; Malfait, W.J. Silica Aerogels with Tailored Chemical Functionality. Mater. Des. 2020, 193, 108833. [Google Scholar] [CrossRef]
- Nie, L.; Li, S.; Cao, M.; Han, N.; Chen, Y. A Brief Review of Preparation and Applications of Monolithic Aerogels in Atmospheric Environmental Purification. J. Environ. Sci. 2025, 149, 209–220. [Google Scholar] [CrossRef]
- Ren, J.; Zhao, Z.; Kong, Y.; Zhu, K.; Jiang, W.; Yuan, M.; Tang, J.; Shen, X. General Approach to the Synthesis of Metal Hybrid Carbon/Titania Aerogel for the Oxygen Reduction Reaction. Energy Fuels 2024, 38, 8262–8276. [Google Scholar] [CrossRef]
- Rojas, F.; Kornhauser, I.; Felipe, C.; Esparza, J.M.; Cordero, S.; Domínguez, A.; Riccardo, J.L. Capillary Condensation in Heterogeneous Mesoporous Networks Consisting of Variable Connectivity and Pore-Size Correlation. Phys. Chem. Chem. Phys. 2002, 4, 2346–2355. [Google Scholar] [CrossRef]
- He, S.; Huang, D.; Bi, H.; Li, Z.; Yang, H.; Cheng, X. Synthesis and Characterization of Silica Aerogels Dried under Ambient Pressure Bed on Water Glass. J. Non-Cryst. Solids 2015, 410, 58–64. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, L.; Yang, M.; Chen, Z.; Song, K.; Wang, Y.; Erisen, D.E.; Xie, J.; Wu, Q.; Kou, Z. Electrospinning of SiO2-Based Composites Embedded TiO2 Nanoparticles with Ultra-Strong Suppression of Radiative Heat Transfer. J. Alloys Compd. 2023, 957, 170331. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Mehmood, M.A.; Taqvi, S.T.H.; Elkamel, A.; Liu, C.-G.; Xu, J.; Rahimuddin, S.A.; Gull, M. Pyrolysis, Kinetics Analysis, Thermodynamics Parameters and Reaction Mechanism of Typha Latifolia to Evaluate Its Bioenergy Potential. Bioresour. Technol. 2017, 245, 491–501. [Google Scholar] [CrossRef]
- Yang, Z.L.; Walvekar, R.; Wong, W.P.; Sharma, R.K.; Dharaskar, S.; Khalid, M. Advances in Phase Change Materials, Heat Transfer Enhancement Techniques, and Their Applications in Thermal Energy Storage: A Comprehensive Review. J. Energy Storage 2024, 87, 111329. [Google Scholar] [CrossRef]
- Fan, C.; Lu, J.; Duan, C.; Wu, C.; Lin, J.; Qiu, R.; Zhang, Z.; Yang, J.; Zhou, B.; Du, A. Effect of Titanium Dioxide Particles on the Thermal Stability of Silica Aerogels. Nanomaterials 2024, 14, 1304. [Google Scholar] [CrossRef]
- Yan, M.; Cheng, X.; Gong, L.; Lun, Z.; He, P.; Shi, L.; Liu, C.; Pan, Y. Growth Mechanism and Structure Regulation of Super-Elastic SiC Aerogels for Thermal Insulation and Electromagnetic Wave Absorption. Chem. Eng. J. 2023, 475, 146417. [Google Scholar] [CrossRef]
- Li, X.K.; Liu, L.; Zhang, Y.X.; Shen, S.D.; Ge, S.; Ling, L.C. Synthesis of Nanometre Silicon Carbide Whiskers from Binary Carbonaceous Silica Aerogels. Carbon 2001, 39, 159–165. [Google Scholar] [CrossRef]
- Al-Oweini, R.; El-Rassy, H. Synthesis and Characterization by FTIR Spectroscopy of Silica Aerogels Prepared Using Several Si(OR)4 and R′′Si(OR′)3 Precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
- Gurav, J.L.; Rao, A.V.; Rao, A.P.; Nadargi, D.Y.; Bhagat, S.D. Physical Properties of Sodium Silicate Based Silica Aerogels Prepared by Single Step Sol–Gel Process Dried at Ambient Pressure. J. Alloys Compd. 2009, 476, 397–402. [Google Scholar] [CrossRef]
- Li, Z.; Huang, S.; Shi, L.; Li, Z.; Liu, Q.; Li, M. Reducing the Flammability of Hydrophobic Silica Aerogels by Doping with Hydroxides. J. Hazard. Mater. 2019, 373, 536–546. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Z.; Shi, L.; Li, Z.; Luo, Y.; Liu, Q.; Huang, R. Methyltrichlorosilane Modified Hydrophobic Silica Aerogels and Their Kinetic and Thermodynamic Behaviors: Graphical Abstract. J. Sol-Gel. Sci. Technol. 2019, 89, 448–457. [Google Scholar] [CrossRef]
- Matin, S.S.; Chelgani, S.C. Estimation of Coal Gross Calorific Value Based on Various Analyses by Random Forest Method. Fuel 2016, 177, 274–278. [Google Scholar] [CrossRef]
- Bangi, U.K.H.; Park, C.-S.; Baek, S.; Park, H.-H. Improvement in Optical and Physical Properties of TEOS Based Aerogels Using Acetonitrile via Ambient Pressure Drying. Ceram. Int. 2012, 38, 6883–6888. [Google Scholar] [CrossRef]
- Mahadik, D.B.; Lee, Y.K.; Chavan, N.K.; Mahadik, S.A.; Park, H.-H. Monolithic and Shrinkage-Free Hydrophobic Silica Aerogels via New Rapid Supercritical Extraction Process. J. Supercrit. Fluids 2016, 107, 84–91. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, X.; Shi, L.; He, S.; Gong, L.; Li, C.; Zhang, H. Flammability and Oxidation Kinetics of Hydrophobic Silica Aerogels. J. Hazard. Mater. 2016, 320, 350–358. [Google Scholar] [CrossRef]
- Lyon, R.E.; Walters, R.N.; Stoliarov, S.I. Screening Flame Retardants for Plastics Using Microscale Combustion Calorimetry. Polym. Eng. Sci 2007, 47, 1501–1510. [Google Scholar] [CrossRef]
- Sonnier, R.; Otazaghine, B.; Vagner, C.; Bier, F.; Six, J.-L.; Durand, A.; Vahabi, H. Exploring the Contribution of Two Phosphorus-Based Groups to Polymer Flammability via Pyrolysis–Combustion Flow Calorimetry. Materials 2019, 12, 2961. [Google Scholar] [CrossRef]
- Huang, D.; Guo, C.; Zhang, M.; Shi, L. Characteristics of Nanoporous Silica Aerogel under High Temperature from 950 °C to 1200 °C. Mater. Des. 2017, 129, 82–90. [Google Scholar] [CrossRef]
- GB 8624-2012; Classification for Burning Behavior of Building Materials and Products. China Standards Press: Beijing, China, 2012. (In Chinese)
- Singh, H.; Geisler, M.; Menzel, F. Experimental Investigations into Thermal Transport Phenomena in Vacuum Insulation Panels (VIPs) Using Fumed Silica Cores. Energy Build. 2015, 107, 76–83. [Google Scholar] [CrossRef]
- Pan, Y.; Jin, X.; Wang, H.; Huang, H.; Wu, C.; Yan, X.; Hong, C.; Zhang, X. Nano-TiO2 Coated Needle Carbon Fiber Reinforced Phenolic Aerogel Composite with Low Density, Excellent Heat-Insulating and Infrared Radiation Shielding Performance. J. Mater. Sci. Technol. 2023, 152, 181–189. [Google Scholar] [CrossRef]
- ISO 1716:2002; Reaction to Fire Tests for Building Products—Determination of the Heat of Combustion. ISO: Geneva, Switzerland, 2002.

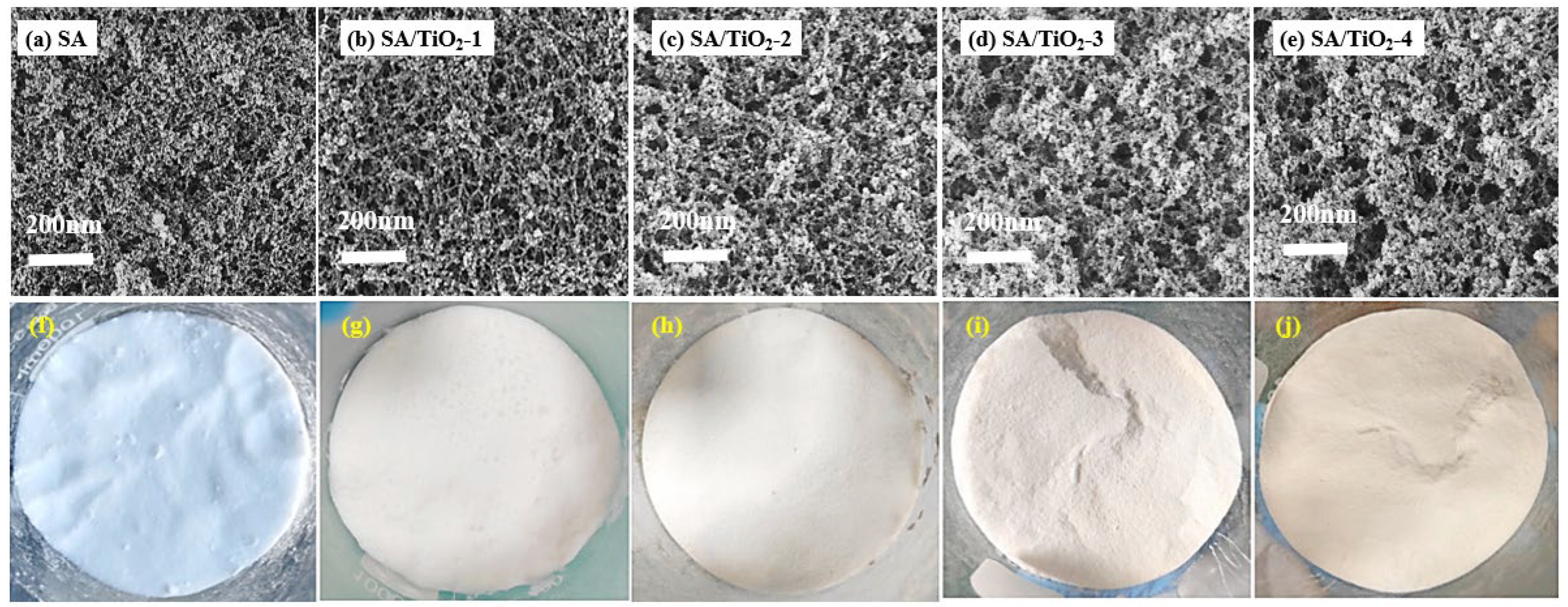
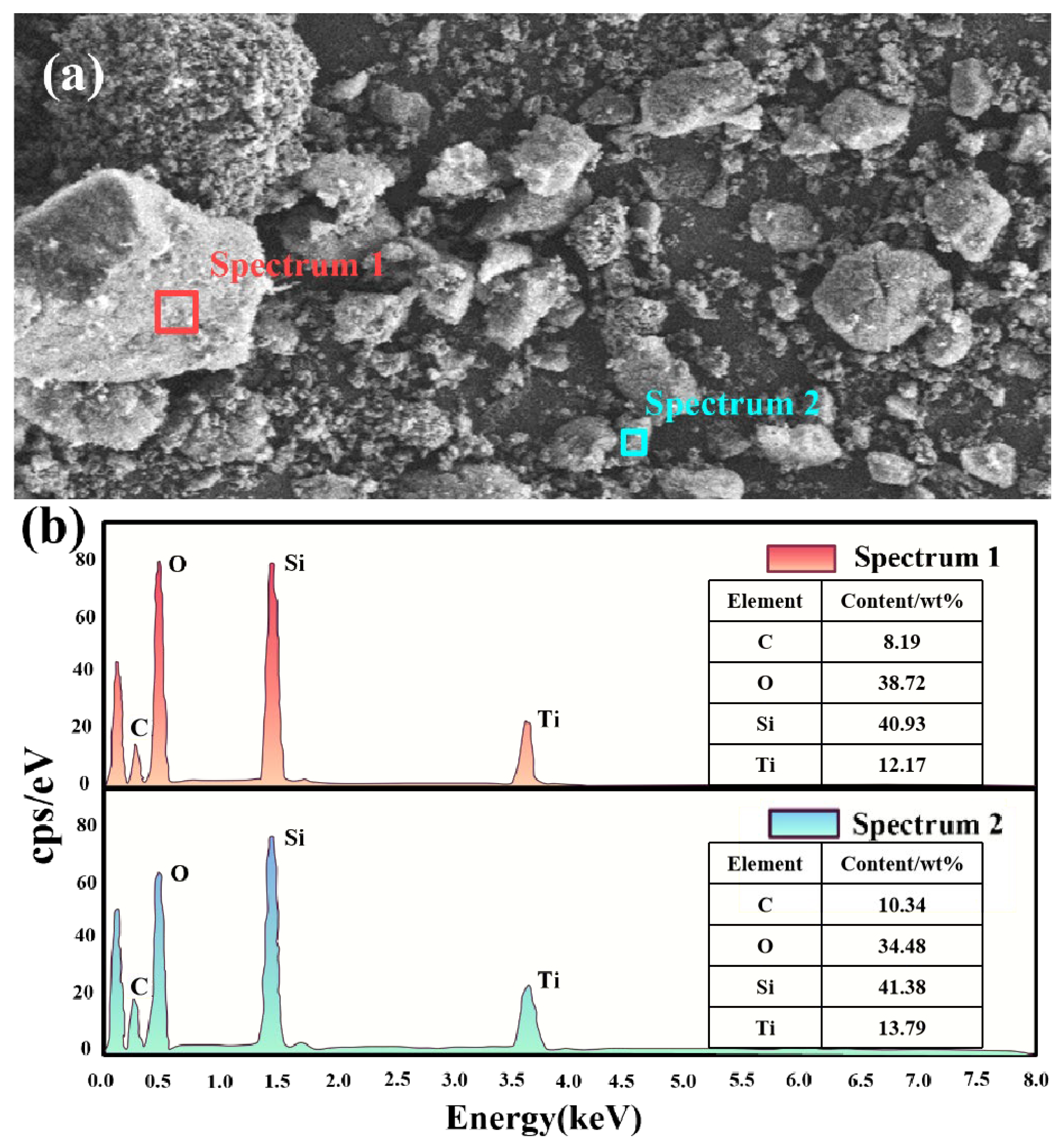
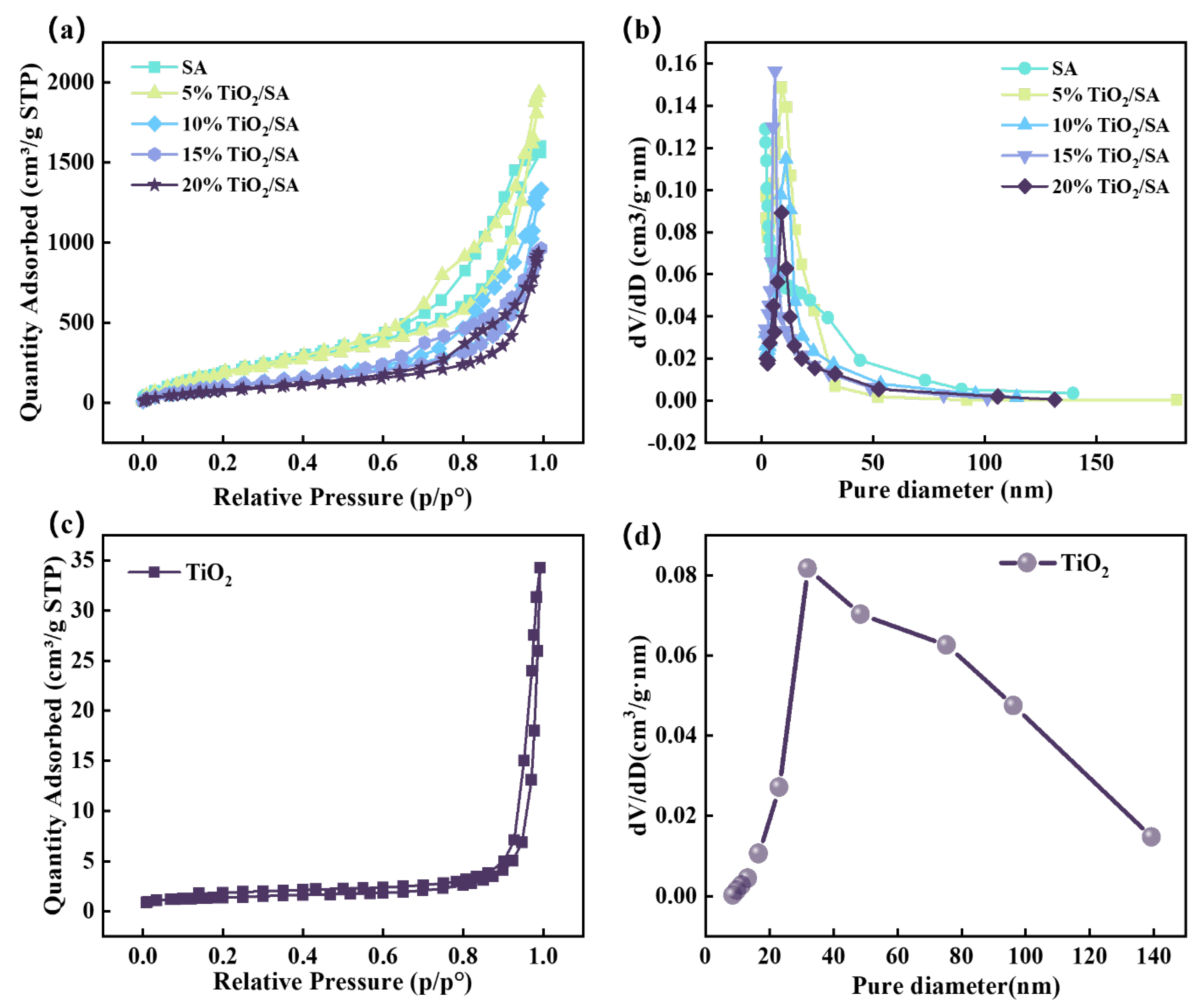
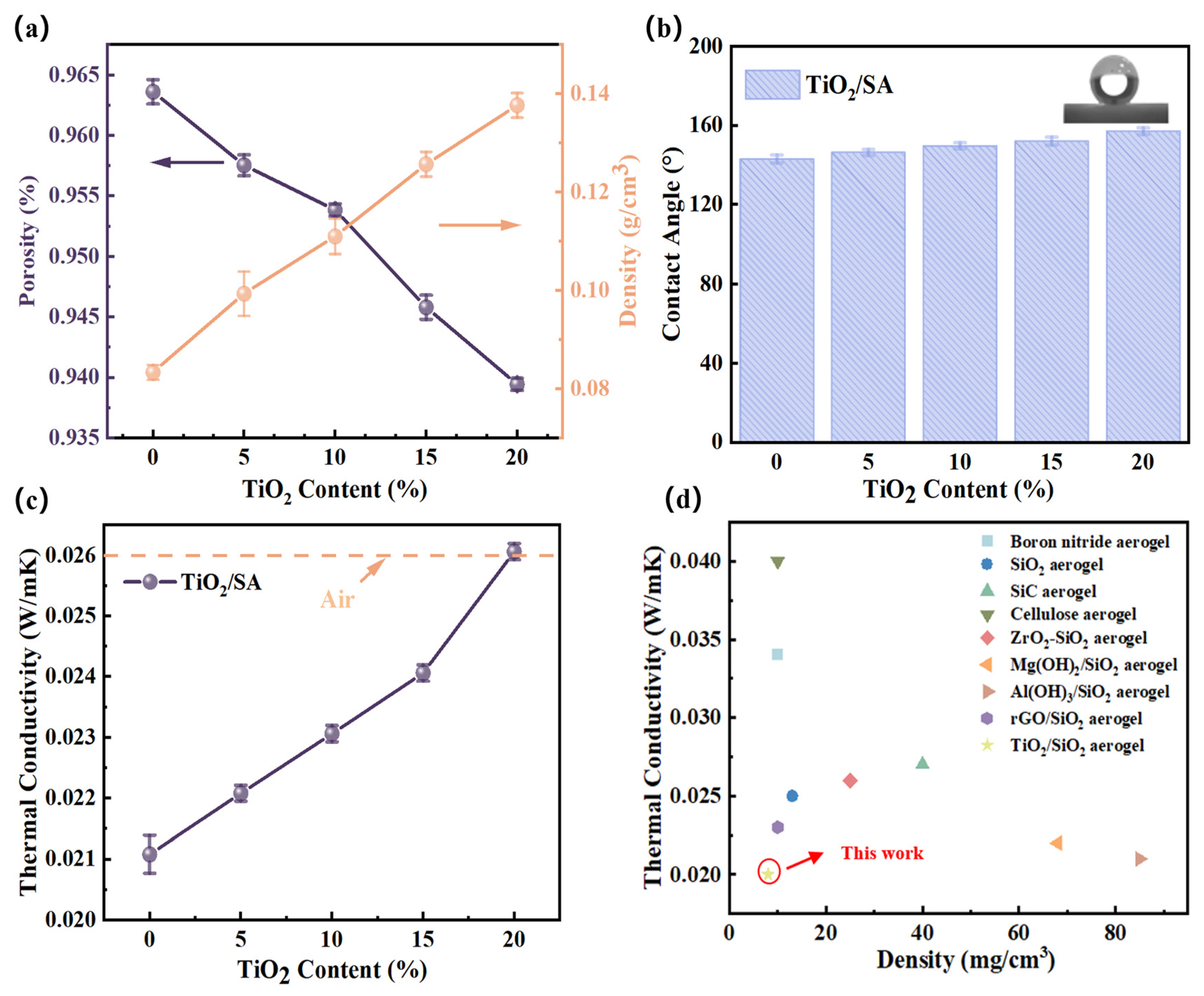


| Samples | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| Pure SA | 682.89 | 0.11 | 9.38 |
| 5% TiO2/SA | 658.28 | 0.14 | 10.82 |
| 10% TiO2/SA | 370.57 | 0.14 | 13.22 |
| 15% TiO2/SA | 361.59 | 0.24 | 13.29 |
| 20% TiO2/SA | 275.46 | 0.25 | 13.34 |
| TiO2 | 5 | 0.03 | 42.44 |
| Concentration of TiO2 (%) | Thermal Conductivity (W/mK) | Infrared Transmittance (%, at 3 μm) | Specific Extinction Coefficient (m2/kg, at 3 μm) |
|---|---|---|---|
| 2.5 | 0.017 | 92 | 98 |
| 5 | 0.019 | 88 | 142 |
| 7.5 | 0.018 | 76 | 189 |
| 10 | 0.018 | 68 | 282 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Pan, Y.; He, S.; Gong, L.; Zhang, Z.; Cheng, X.; Zhang, H. Hydrophobic Silica Aerogel with Higher Flame Retardancy, Thermal Radiation Shielding, and High-Temperature Insulation Properties Through Introduction of TiO2. Gels 2025, 11, 249. https://doi.org/10.3390/gels11040249
Sun H, Pan Y, He S, Gong L, Zhang Z, Cheng X, Zhang H. Hydrophobic Silica Aerogel with Higher Flame Retardancy, Thermal Radiation Shielding, and High-Temperature Insulation Properties Through Introduction of TiO2. Gels. 2025; 11(4):249. https://doi.org/10.3390/gels11040249
Chicago/Turabian StyleSun, Huiying, Yuelei Pan, Song He, Lunlun Gong, Zhongxin Zhang, Xudong Cheng, and Heping Zhang. 2025. "Hydrophobic Silica Aerogel with Higher Flame Retardancy, Thermal Radiation Shielding, and High-Temperature Insulation Properties Through Introduction of TiO2" Gels 11, no. 4: 249. https://doi.org/10.3390/gels11040249
APA StyleSun, H., Pan, Y., He, S., Gong, L., Zhang, Z., Cheng, X., & Zhang, H. (2025). Hydrophobic Silica Aerogel with Higher Flame Retardancy, Thermal Radiation Shielding, and High-Temperature Insulation Properties Through Introduction of TiO2. Gels, 11(4), 249. https://doi.org/10.3390/gels11040249









