New Trends in Preparation and Use of Hydrogels for Water Treatment
Abstract
1. Introduction
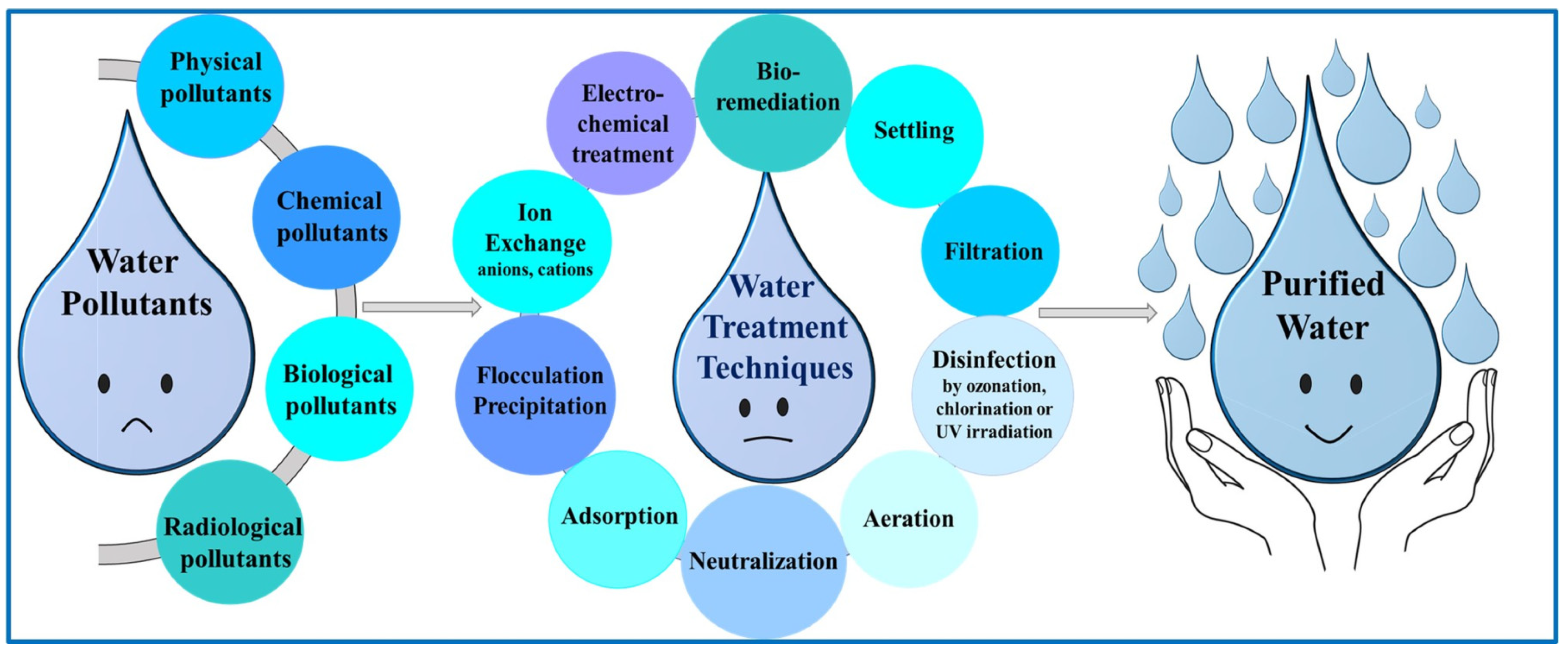
1.1. Water Pollution
1.2. Water Pollutants
1.3. Water Treatments
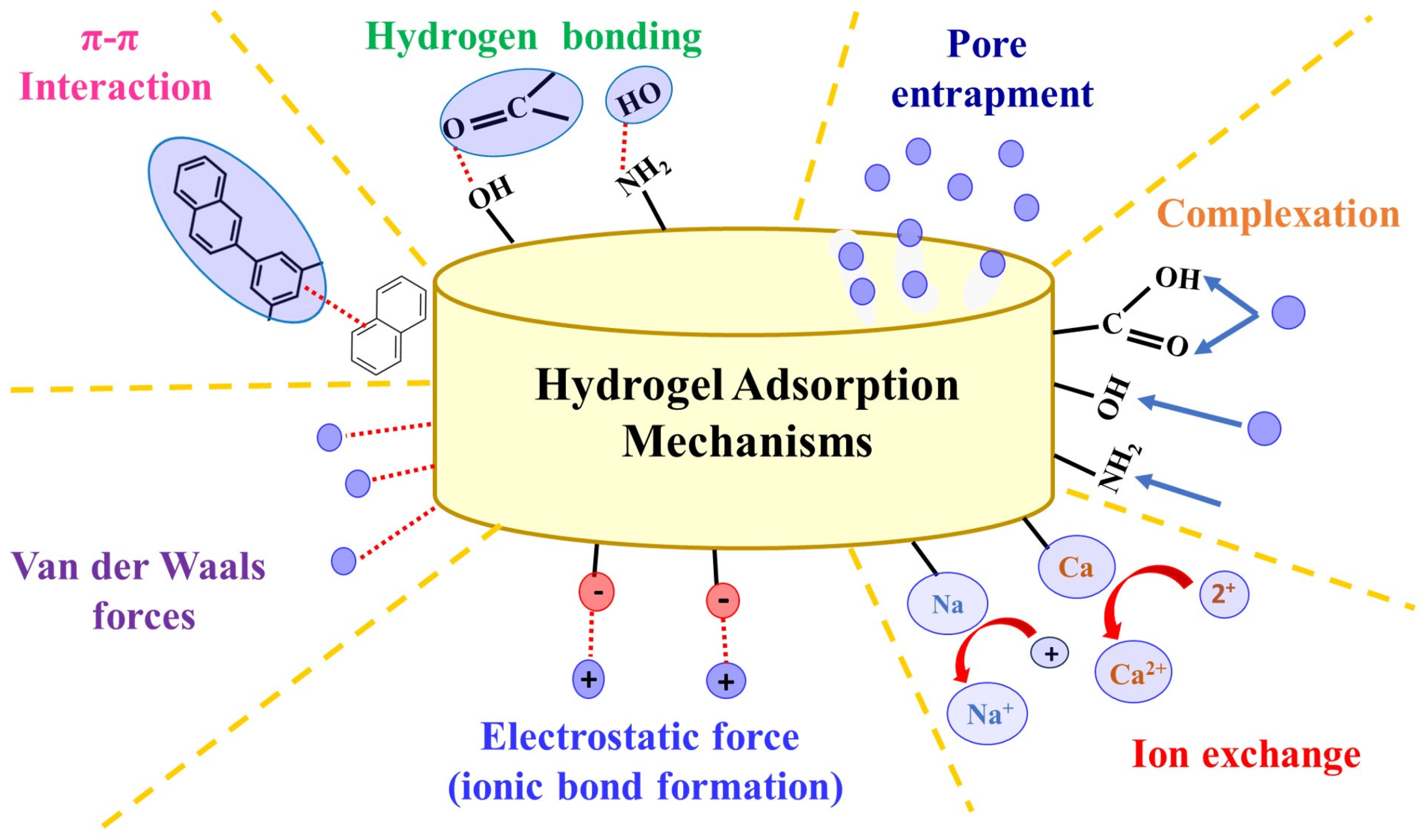
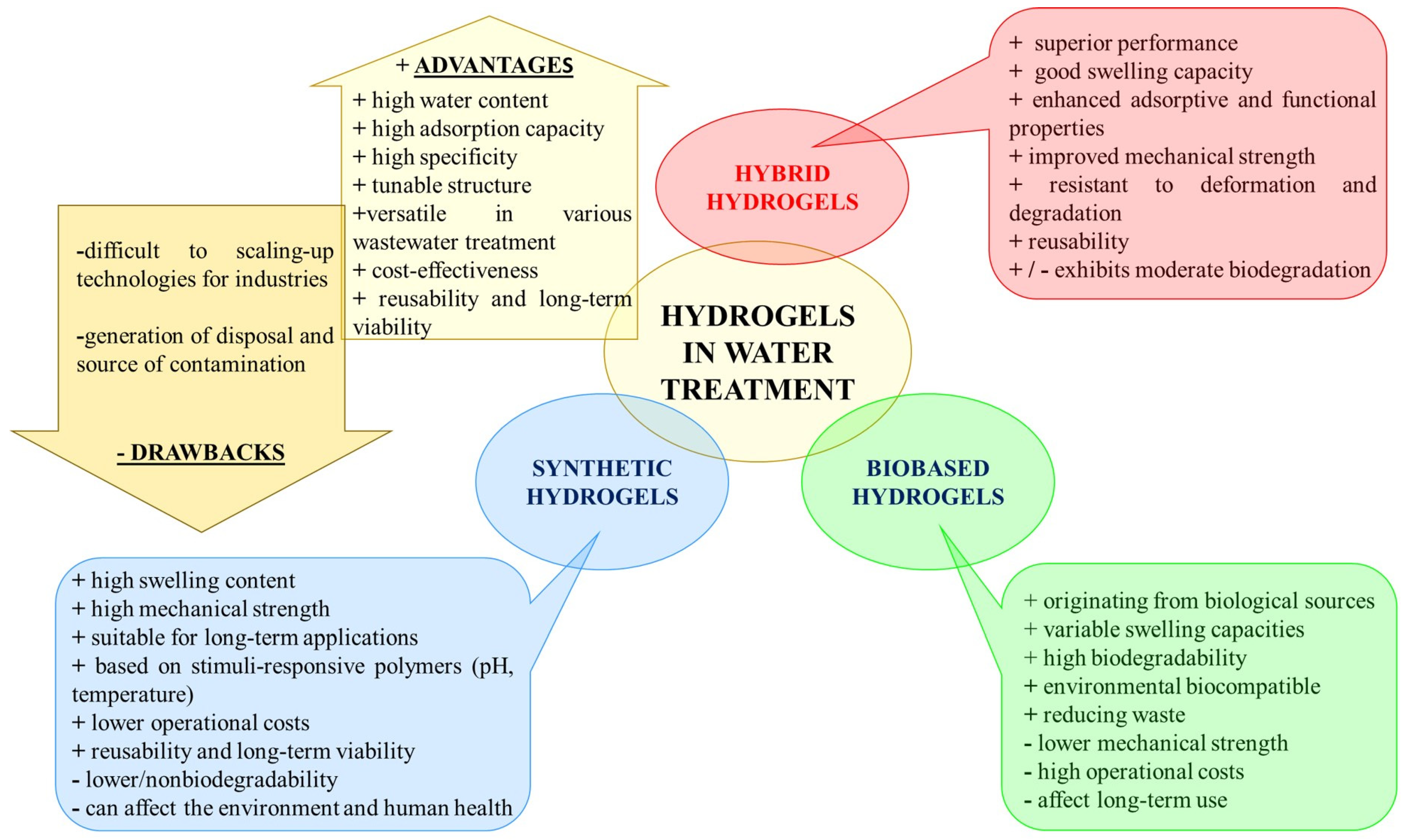
1.4. Hydrogels in Water Treatment
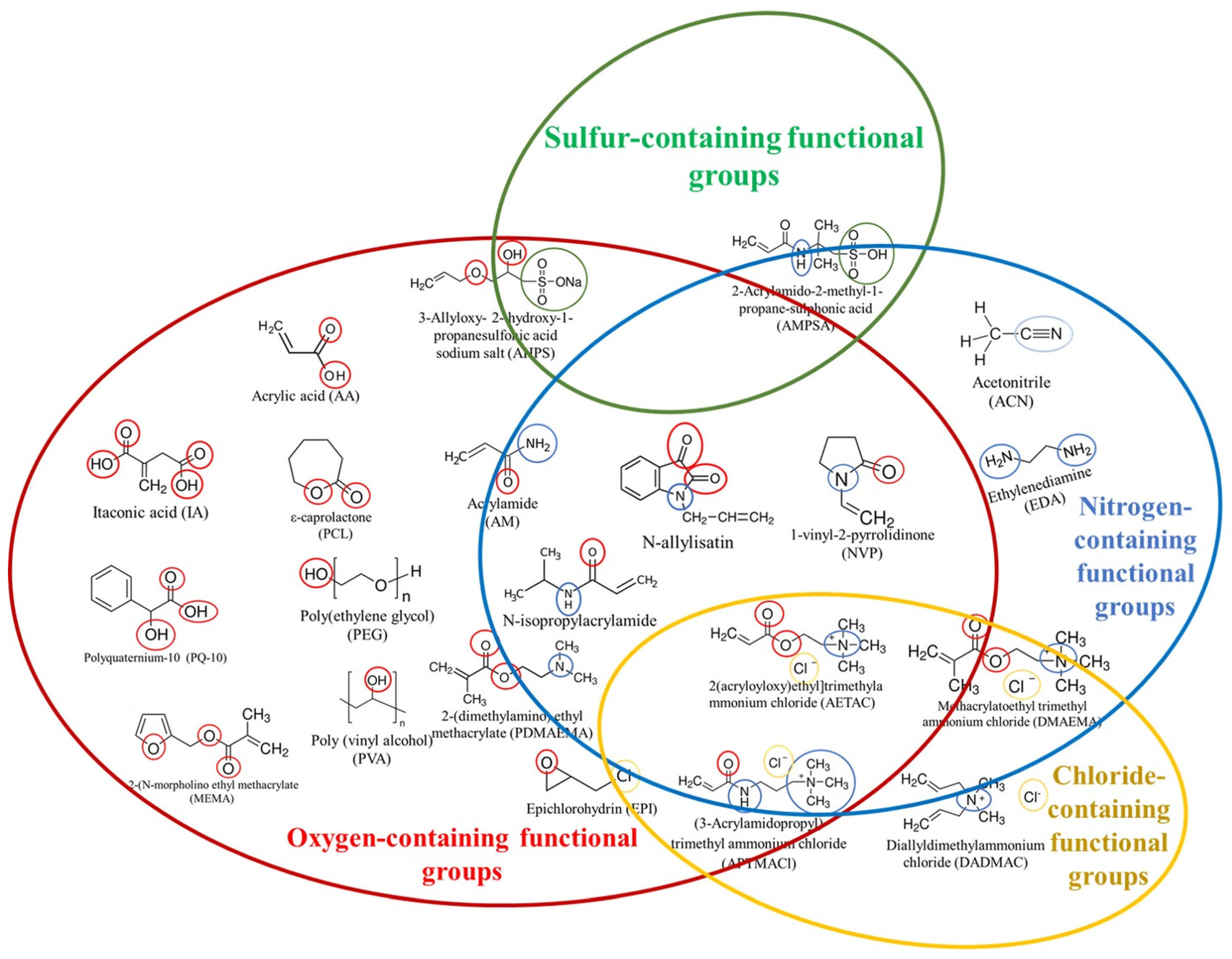
2. Synthetic Polymer-Based Hydrogels for Water Treatments
2.1. Retention of Organic Dyes Using Synthetic Polymer-Based Hydrogels
2.1.1. Polyacrylate Hydrogels and Their Polymer Blends for Efficient Organic Dye Retention
2.1.2. Sulfonic Acid Hydrogels Based on Synthetic Polymers for Efficient Organic Dye Retention
2.1.3. Quaternary Ammonium Hydrogels for Efficient Organic Dye Retention
2.1.4. Nanocomposite Hydrogels Based on Synthetic Polymers for Efficient Organic Dye Retention
2.2. Synthetic Polymer-Based Hydrogels for Heavy Metals Retention
3. Bio-Based Hydrogels Engineered to Capture Water Pollutants
3.1. Polysaccharides-Based Hydrogels for Heavy Metals’ Retention
3.1.1. Cellulose-Based Hydrogels for Heavy Metals’ Retention
3.1.2. Chitosan-Based Hydrogels for Heavy Metals’ Retention
3.1.3. Alginate-Based Hydrogels for Heavy Metals’ Retention
3.2. Polysaccharides-Based Hydrogels for Dyes’ Removal
3.2.1. Cellulose-Based Hydrogels for Dyes’ Removal
3.2.2. Chitosan-Based Hydrogels for Dyes’ Removal
3.2.3. Alginate-Based Hydrogels for Dyes’ Removal
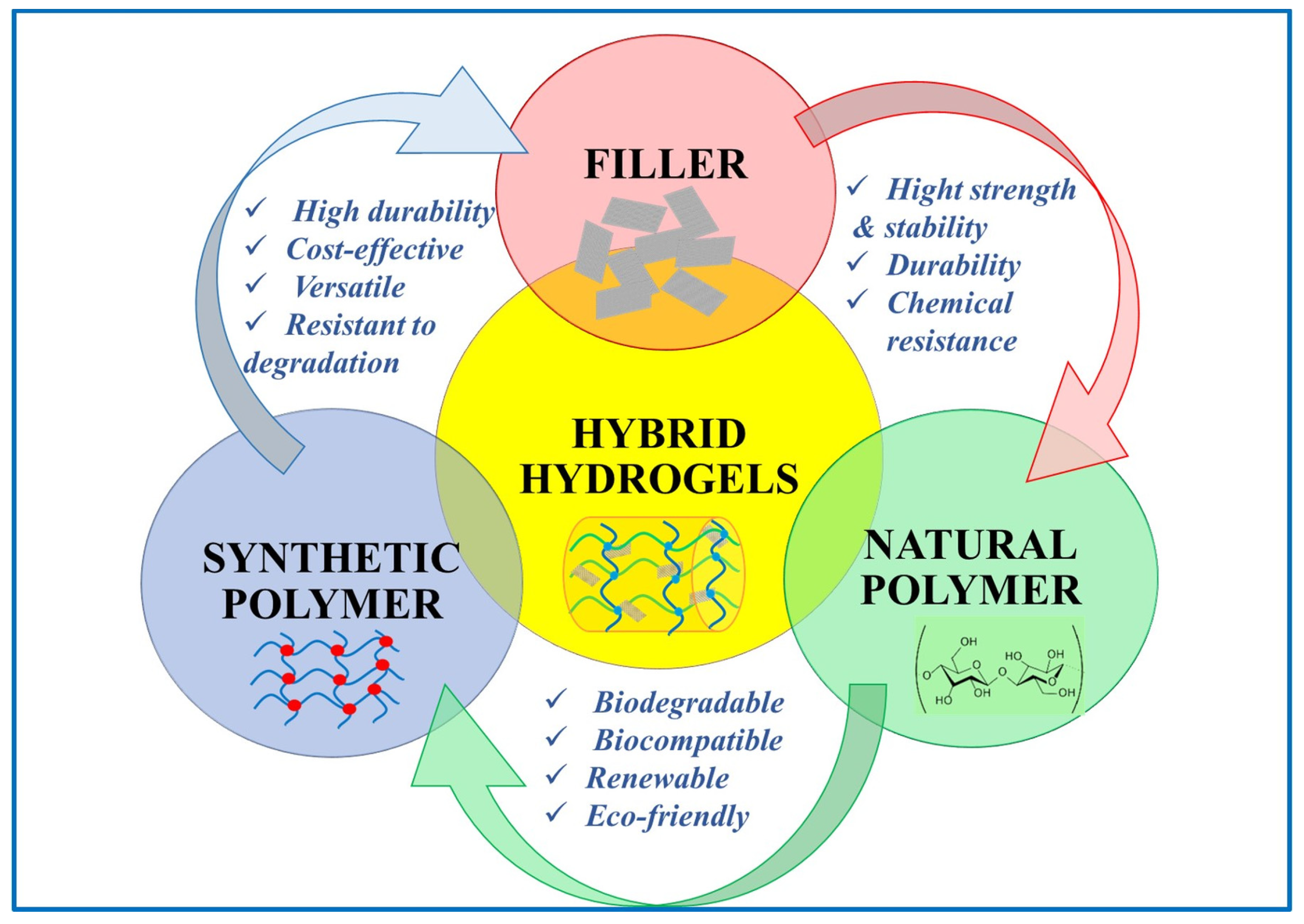
4. Hybrid Hydrogels for Water Treatment
4.1. Hybrid Hydrogels for Dye Removal
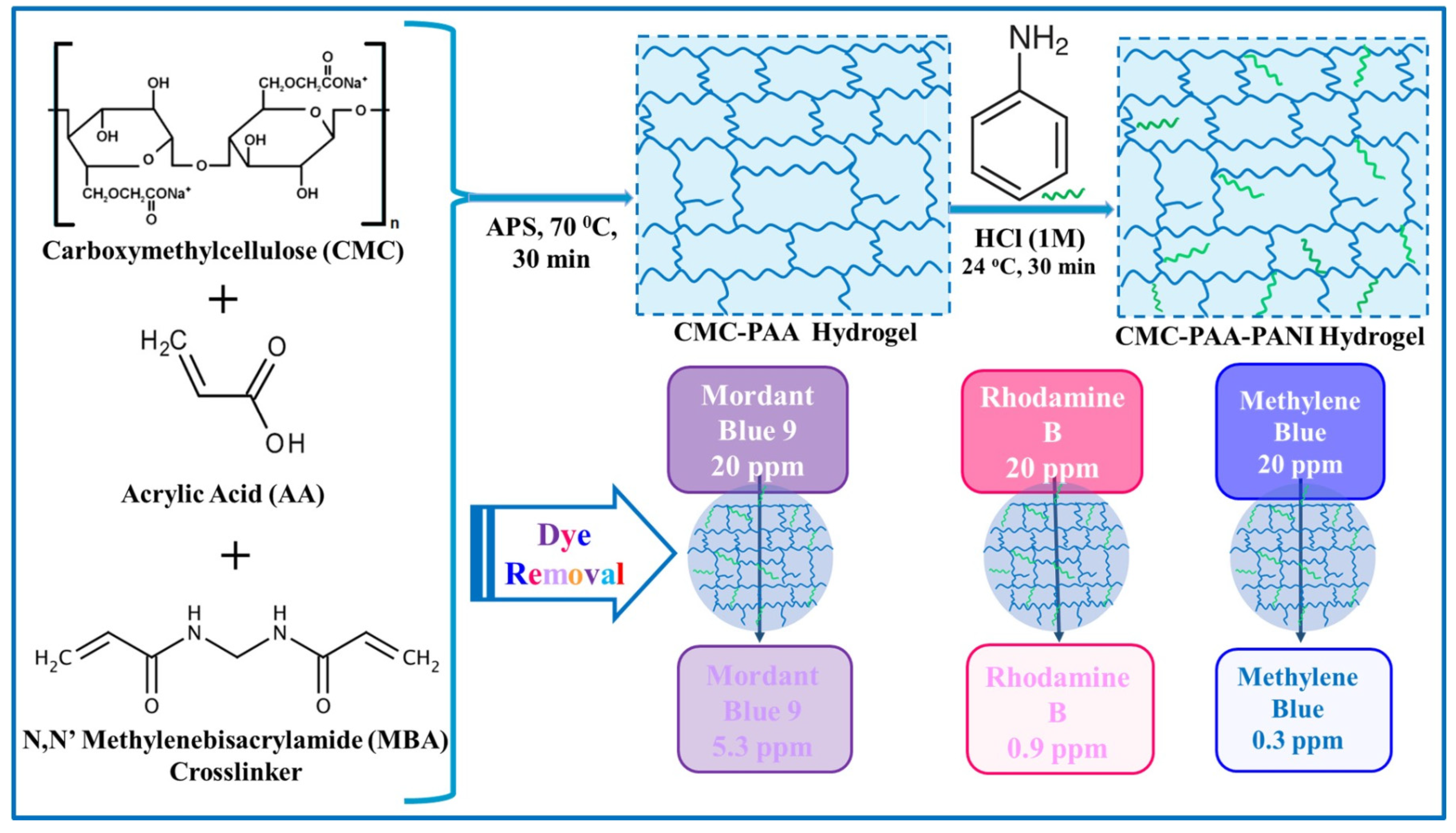
4.1.1. Hybrid Hydrogels Based on Polysaccharides for Dye Removal
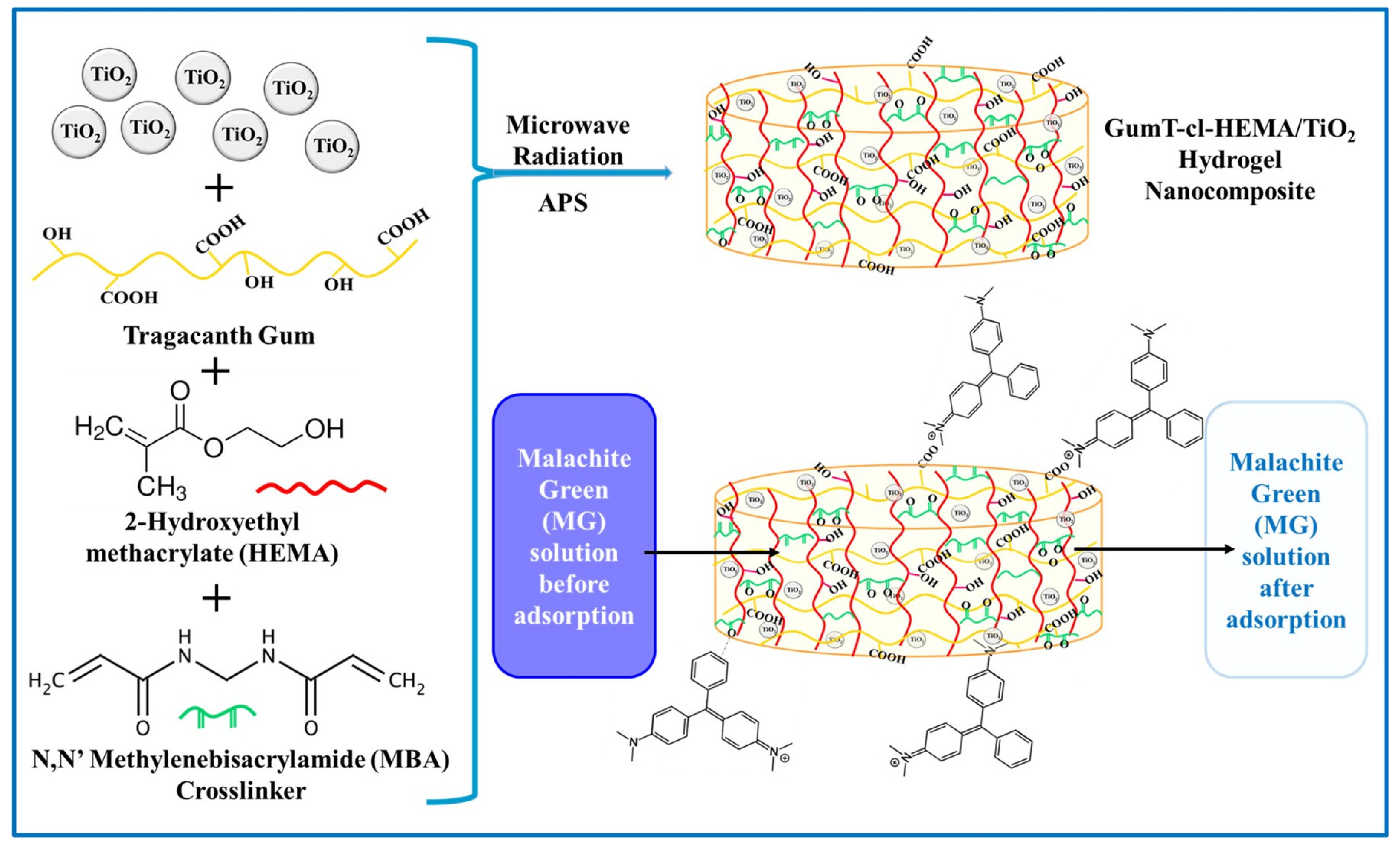
4.1.2. Hybrid Hydrogels Based on Gum Polymers for Dye’ Removal
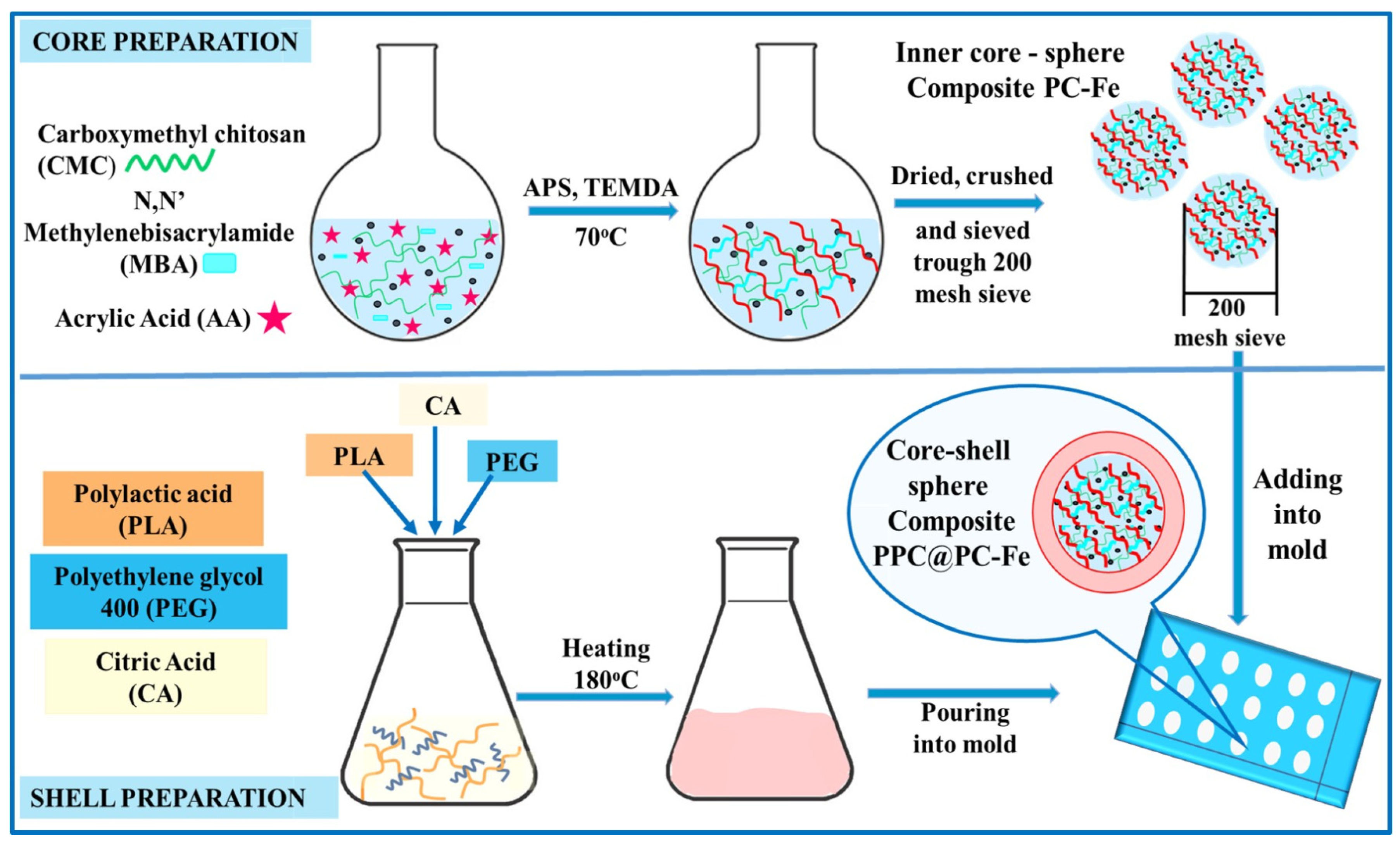
4.1.3. Hybrid Hydrogels Based on Other Kinds of Polymers for Dye’ Removal
4.2. Hybrid Hydrogels for Heavy Metals’ Retention
4.2.1. Hybrid Hydrogels Based on Polysaccharides for Heavy Metals’ Removal
4.2.2. Hybrid Hydrogels Based on Gum Polymers for Heavy Metals’ Removal
4.2.3. Hybrid Hydrogels Based on Other Kinds of Polymers for Heavy Metals’ Removal
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | acrylic acid | KG | Karaya gum |
| AB 113 | Acid Blue 113 | KPS | Potassium persulphate |
| AC | activated carbon | Lap | Laponite |
| AETAC | 2-(acryloyloxy)ethyl trimethylammonium chloride | LS | Lignosulfonate |
| AHPS | 3-Allyloxy-2-hydroxy-1-propane sulfonic acid sodium salt | MA | Maleic acid |
| AIBN | azobisisobuteronitrile | MAA | Methacrylic acid |
| AIPH | 2,2′-azobis [2-(2-imidazolin-2-yl)propane] dihydrochloride | MAAn | Methacrylic anhydride |
| AM | acrylamide | MAC | [2-(Methacryloxy) ethyl] trimethyl ammonium chloride |
| AMPSA | 2-acrylamide-2-methylpropane sulfonic acid | MADA | Methacrylamide dopamine |
| ANi | aniline | MB | Methylene Blue |
| AO | Auramine O | MBA | N,N-methylenebisacrylamide |
| AO7 | Acid Orange 7 | MEMA | 2-(N-morpholino ethyl methacrylate) |
| APS | Ammonium persulfate | MEMA@GO | 2-(N-morpholino ethyl) methacrylate/graphene oxide |
| APTMACl | (3-acrylamidopropyl) trimethyl ammonium chloride | MG | Malachite Green |
| APTES | aminopropyltrimethoxysilane | MMA | Methyl methacrylate |
| AR 18 | Acid Red 18 | MMT | Montmorillonite |
| AR | Alizarin Red | MO | Methyl orange |
| ARS | Alizarin red S | MOFs | Metal–organic frameworks |
| AY36 | Acid Yellow 36 | MPS | 2-Acrylamido-2-methyl-1-propanesulfonic acid |
| BCB | Brilliant Cresyl Blue | MV | Methyl Violet |
| BIO | Biochar | MWCNTs | Multi-walled carbon nanotubes |
| BIO-Ox | Oxidized biochar | NA | N-allylisatin |
| BG | Brilliant Green | NIPAM | N-isopropyl acrylamide |
| BMB | Bromophenol Blue | NMBA | N,N-methylenebisacrylamide |
| BPO | Benzoyl peroxide | NVP | 1-vinyl-2-pyrrolidinone |
| BzO2 | Benzoyl peroxide | OG | Orange G |
| BY28 | Basic yellow 28 | O II | Orange II sodium Salt |
| CA | Carminic acid | PAA | Polyacrylic acid |
| CAN | Ceric Ammonium Nitrate | PCL | ε-caprolactone |
| CCA | Calconcarboxylic Acid | PDMAEMA | 2-(dimethylamino) ethyl methacrylate |
| CCB | Coomassie brilliant blue | P(DMC-co-AM) | Poly (methacrylatoethyl trimethyl ammonium chloride-co-acrylamide) |
| CG | Chrysoidine G | P(DMAEMA) | Poly(2-(dimethylamino) ethyl methacrylate) |
| CMC | Carboxymethyl cellulose | PEG1500 | Poly(ethylene glycol)1500 |
| CMS | Carboxymethyl starch | PEGDA | Polyethylene glycol diacrylate |
| CNS | Calcium hydroxide nano-spherulites | PEGDMA | Poly (ethylene glycol) dimetacrylate |
| CNT | Carbon nanotubes | PEI | Polyethyleneimine |
| cPAA | Crosslinked PAA | Ph-In | 2-hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone |
| CR | Congo Red | PHPAm | Partially hydrolyzed polyacrylamide |
| Cur | Curcuma longa rhizome | PLA | Polylactic acid |
| CV | Crystal Violet | PQ-10 | Polyquaternium-10 |
| DADMAC | Diallyl dimethylammonium chloride | PTE | Pentaerythritol Tetraallyl Ether |
| Darocur117 | 3 2-hydroxyl-2-methylpropiophenone | PVA | Polyvinyl alcohol |
| DB 15 | Direct Blue 15 | PVP | Polyvinylpyrrolidone |
| DB 78 | Direct Blue 78 | RBBR | Remazol Brilliant Blue R |
| DMAEMA | 2-(Dimethylamino)ethyl methacrylate | RBP-5 | Reactive black 5 |
| DMC | methacrylatoethyl trimethyl ammonium chloride | RhB | Rhodamine B |
| 2DMMT | Two-dimensional montmorillonite nanosheets | RPGS | Palygorskite clay |
| DR23 | Direct Red 23 | SA | Sodium alginate |
| DY | Direct yellow | SH | Sodium humate |
| EB | Eosin B | SO | Safranin O |
| EBT | Eriochrome Black T | ST | Safranin T |
| EDA | Ethylenediamine | SY | Sunset Yellow |
| EG | Ethylene glycol | TB | Trypan Blue |
| EGDMA | N,N- ethylene glycol dimethacrylate | TEMED | N,N,N′,N′-tetramethylethylenediamine |
| EMS | Electrolytic manganese slag | TEOS | Tetraethyl orthosilicate |
| EPC | Epichlorohydrin | TMPTA | 1,1,1-trimethylolpropane trimethacrylate |
| FBL2 | Food Blue 2 | TiO2 | Titanium dioxide |
| FeCl2·4H2O | Ferrous chloride tetrahydrate | VBC | 4 vinylbenzyl chloride |
| FeCl3·6H2O | Ferric chloride hexahydrate | KG | Karaya gum |
| FR17 | Food Red 17 | KPS | Potassium persulphate |
| FS | Fluorescein sodium salt | Lap | Laponite |
| GA | Glutaraldehyde | LS | Lignosulfonate |
| Gg | Gum ghatti | MA | Maleic acid |
| GO | Graphene oxide | MAA | Methacrylic acid |
| GOX | lyoxal | MAAn | Methacrylic anhydride |
| GumT | Tragacanth gum | MAC | [2-(Methacryloxy) ethyl] trimethyl ammonium chloride |
| HHMP | 2-hydroxy-4′-(2 hydroxyethoxy)-2-methyl-propiophenone | MADA | Methacrylamide dopamine |
| HEC | Hydroxyl ethyl cellulose | WT | Waste textiles |
| HEMA 2 | Hydroxyethyl methacrylate | XG | Xantam gum |
| IA | Itaconic acid | γ-MPS | γ-methacryloxypropyltrimethoxysilane |
| IA-g-poly (AA-co-Ani) | Poly (acrylic acid-co-aniline) | ZnO | Zinc oxide |
| ILA-1VIM | Ionic liquid vinylimidazole | ZSM-5 | Zeolite |
| IC | Indigo Carmine | VG1 | Vat Green 1 |
| KCuHCF | Potassium copper hexacyanoferrate | VOCs | Volatile organic compounds |
| V50 | 2′-azobis(2-amidinopropane dihydrochloride) | ||
| [Vim]Br2 | Bis1-vinylimidazole ethyl bromide |
References
- Available online: https://www.eea.europa.eu/en/analysis/indicators/industrial-pollutant-releases-to-water (accessed on 15 November 2024).
- Shmeis, R.M.A. Chapter Four: Nanotechnology in wastewater treatment. Compr. Anal. Chem. 2022, 99, 105–134. [Google Scholar] [CrossRef]
- Available online: https://www.epa.gov/ccl/types-drinking-water-contaminants (accessed on 15 November 2024).
- Available online: https://www.toppr.com/guides/chemistry/environmental-chemistry/water-pollutants/ (accessed on 15 November 2024).
- Ortiz-Prado, E.; Simbana-Rivera, K.; Cevallos, G.; Gomez-Barreno, L.; Cevallos, D.; Lister, A.; Fernandez-Naranjo, R.; Rios-Touma, B.; Vasconez-Gonzalez, J.; Izquierdo-Condoy, J.S. Waterborne diseases and ethnic-related disparities: A 10 years nationwide mortality and burden of disease analysis from Ecuador. Public Health Front. 2022, 10, 1029375. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.D.; Jackson, K.; Peters, H.; Herrera Lima, S. Lack of Safe Drinking Water for Lake Chapala Basin Communities in Mexico Inhibits Progress toward Sustainable Development Goals 3 and 6. Int. J. Environ. Res. Public Health 2020, 17, 8328. [Google Scholar] [CrossRef] [PubMed]
- Wampler, P.J. Karst aquifers and water resource contamination in Haiti. Hydrogeol. J. 2022, 30, 1453–1467. [Google Scholar] [CrossRef]
- Warren-Vega, W.M.; Campos-Rodríguez, A.; Zárate-Guzmán, A.I.; Romero-Cano, L.A. A Current Review of Water Pollutants in American Continent: Trends and Perspectives in Detection, Health Risks, and Treatment Technologies. Int. J. Environ. Res. Public Health 2023, 20, 4499. [Google Scholar] [CrossRef]
- Shmeis, R.M.A. Water Chemistry and Microbiology. Compr. Anal. Chem. 2018, 81, 1–56. [Google Scholar] [CrossRef]
- Saravanan, A.; Senthil, K.P.; Jeevanantham, S.; Karishma, S.; Tajsabreen, B.; Yaashikaa, P.R.; Reshma, B. Effective water/wastewater treatment methodologies for toxic pollutants removal: Processes and applications towards sustainable development. Chemosphere 2021, 280, 130595. [Google Scholar] [CrossRef]
- Available online: https://chinawaterrisk.org/glossary-measurement/drinking-water-preparation (accessed on 19 November 2024).
- Available online: https://www.britannica.com/topic/water-purification/Other-purification-steps (accessed on 19 November 2024).
- Available online: https://www.dombor.com/different-methods-of-wastewater-treatment/ (accessed on 19 November 2024).
- Dutta, D.; Arya, S.; Kumar, S. Industrial wastewater treatment: Current trends, bottlenecks, and best practices. Chemosphere 2021, 285, 131245. [Google Scholar] [CrossRef]
- Kong, Z.; Li, L.; Xue, Y.; Yang, M.; Li, Y.-Y. Challenges and prospects for the anaerobic treatment of chemical-industrial organic wastewater: A review. J. Clean. Prod. 2019, 231, 913–927. [Google Scholar] [CrossRef]
- Deng, Y.; Xi, J.; Meng, L.; Lou, Y.; Seidi, F.; Wu, W.; Xiao, H. Stimuli-Responsive nanocellulose Hydrogels: An overview. Eur. Polym. J. 2022, 180, 111591. [Google Scholar] [CrossRef]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable cellulose-based hydrogels: Design and applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Maleki, B.; Kargar, P.G.; Ashrafi, S.S.; Ghani, M. Perspective Chapter: Introduction to Hydrogels—Definition, Classifications, Applications and Methods of Preparation. In Ionic Liquids—Recent Advances; Bhowmik, P.K., Ed.; InTech Europe: Rijeka, Croatia, 2016; pp. 7–9. [Google Scholar]
- Zaharia, A.; Radu, A.-L.; Iancu, S.; Florea, A.-M.; Sandu, T.; Minca, I.; Fruth-Oprisan, V.; Teodorescu, M.; Sarbu, A.L.; Iordache, T.-V. Bacterialcellulose-poly(acrylic acid-co-N,N′-methylene-bis-acrylamide) interpenetrated networks for the controlled release of fertilizers. RSC Adv. 2018, 32, 17635–17644. [Google Scholar] [CrossRef]
- Neblea, I.E.; Gavrila, A.-M.; Iordache, T.V.; Zaharia, A.; Stanescu, P.O.; Radu, I.C.; Burlacu, S.G.; Neagu, G.; Chiriac, A.L.; Sarbu, A. Interpenetrating networks of bacterial cellulose and poly (ethylene glycol) diacrylate as potential cephalexin carriers in wound therapy. J. Polym. Res. 2022, 29, 406. [Google Scholar] [CrossRef]
- Neblea, I.E.; Chiriac, A.-L.; Zaharia, A.; Sarbu, A.; Teodorescu, M.; Miron, A.; Paruch, L.; Paruch, A.M.; Olaru, A.G.; Iordache, T.-V. Introducing Semi-Interpenetrating Networks of Chitosan and Ammonium-Quaternary Polymers for the Effective Removal of Waterborne Pathogens from Wastewaters. Polymers 2023, 15, 1091. [Google Scholar] [CrossRef]
- Cursaru, B.; Radu, A.-L.; Perrin, F.-X.; Sarbu, A.; Teodorescu, M.; Gavrila, A.-M.; Damian, C.-M.; Sandu, T.; Iordache, T.-V.; Zaharia, A. Poly(ethylene glycol) Composite Hydrogels with Natural Zeolite as Filler for Controlled Delivery Applications. Macromol. Res. 2020, 28, 211–220. [Google Scholar] [CrossRef]
- Spatarelu, C.P.; Chiriac, A.-L.; Cursaru, B.; Iordache, T.-V.; Gavrila, A.-M.; Cojocaru, C.-T.; Botez, R.-E.; Trica, B.; Sarbu, A.; Teodorescu, M.; et al. Composite Nanogels Based on Zeolite-Poly(ethylene glycol) Diacrylate for Controlled Drug Delivery. Nanomaterials 2020, 10, 195. [Google Scholar] [CrossRef]
- Dumitru, M.-V.; Neagu, A.-L.; Miron, A.; Roque, M.I.; Durães, L.M.R.; Gavrila, A.-M.; Sarbu, A.; Iovu, H.; Chiriac, A.-L.; Iordache, T.-V. Retention of ciprofloxacin and carbamazepine from aqueous solutions using chitosan-based cryostructured composites. Polymers 2024, 16, 639. [Google Scholar] [CrossRef]
- Akhtar, M.F.; Hanif, M.; Ranjha, N.M. Methods of synthesis of hydrogels. A review. Saudi Pharm. J. 2016, 24, 554–559. [Google Scholar] [CrossRef]
- Gulrez, S.K.H.; Al-Assaf, S.; Phillips, G.O. Hydrogels: Chapter 5 Methods of Preparation, Characterisation and Applications. In Progress in Molecular and Environmental Bioengineering—From Analysis and Modeling to Technology Applications; Carpi, A., Ed.; InTech Europe: Rijeka, Croatia, 2011; pp. 118–138. [Google Scholar]
- Bahram, M.; Mohseni, N.; Moghtader, M. Chapter 2: An Introduction to Hydrogels and Some Recent Applications. In Emerging Concepts in Analysis and Applications of Hydrogels; Majee, S.B., Ed.; InTech Europe: Rijeka, Croatia, 2016; pp. 12–15. [Google Scholar] [CrossRef]
- Chamkouri, H.; Chamkouri, M. A Review of Hydrogels, Their Properties and Applications in Medicine. Am. J. Biomed. Sci. Res. 2021, 11, 485–493. [Google Scholar] [CrossRef]
- Ahearne, M.; Yang, Y.; Liu, K.-K. Chapter 12 Mechanical Characterisation of Hydrogels for Tissue Engineering Applications. In Topics in Tissue Engineering; Ashammakhi, N., Reis, R., Chiellini, F., Eds.; Oulu University: Oulu, Finland, 2008; Volume 4. [Google Scholar]
- Santo, V.E.; Prieto, S.; Testera, A.M.; Arias, F.J.; Alonso, M.; Mano, J.F.; Rodríguez-Cabello, J.C. Temperature-responsive bioactive hydrogels based on a multifunctional recombinant elastin-like polymer. Biomater. Biomech. Eng. 2015, 2, 47–59. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Sultana, M.; Rownok, M.H.; Sabrin, M.; Rahaman, M.; Alam, S.M.N. A review on experimental chemically modified activated carbon to enhance dye and heavy metals adsorption. Clean. Eng. Technol. 2022, 6, 100382. [Google Scholar] [CrossRef]
- Mei, Y.; Zhuang, S.; Wang, J. Adsorption of heavy metals by biochar in aqueous solution: A review. Sci. Total Environ. 2025, 968, 178898. [Google Scholar] [CrossRef] [PubMed]
- Rudžioni, Ž.; Adhikary, S.K.; Manhanga, F.C.; Ashish, D.K.; Ivanauskas, R.; Stelmokaitis, G.; Navickas, A.A. Natural zeolite powder in cementitious composites and its application as heavy metal absorbents. J. Build. Eng. 2021, 43, 103085. [Google Scholar] [CrossRef]
- Lin, G.; Zeng, B.; Li, J.; Wang, Z.; Wang, S.; Hu, T.; Zhang, L. A systematic review of metal organic frameworks materials for heavy metal removal: Synthesis, applications and mechanism. Chem. Eng. J. 2023, 460, 141710. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Roy, P.; Bonilla-Petriciolet, A.; Badawi, M.; Ganachari, S.V.; Shetti, N.P.; Aminabhavi, T.M. Polymeric hydrogels-based materials for wastewater treatment. Chemosphere 2023, 331, 138743. [Google Scholar] [CrossRef]
- Gao, M.; Sun, M.; Guo, X.; Li, F.; Bi, J.; Liu, J.; Wang, S.; Zhao, Y. Removal of ciprofloxacin by PAA-PAM hydrogel: Adsorption performance and mechanism studies. J. Water Process Eng. 2025, 71, 107361. [Google Scholar] [CrossRef]
- Foudazi, R.; Zowada, R.; Manas-Zloczower, I.; Feke, D.L. Porous Hydrogels: Present Challenges and Future Opportunities. Langmuir 2023, 39, 2092–2111. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, S.; Luo, Y. Adsorption mechanisms of hydrogels for heavy metal and organic dyes removal: A short review. J. Agric. Food Res. 2023, 12, 100552. [Google Scholar] [CrossRef]
- Khan, M.; Lo, I.M.C. A holistic review of hydrogel applications in the adsorptive removal of aqueous pollutants: Recent progress, challenges, and perspectives. Water Res. 2016, 106, 259–271. [Google Scholar] [CrossRef]
- Seera, S.D.K.; Kundu, D.; Gami, P.; Naik, P.K.; Banerjee, T. Synthesis and characterization of xylan-gelatin cross-linked reusable hydrogel for the adsorption of methylene blue. Carbohydr. Polym. 2021, 256, 117520. [Google Scholar] [CrossRef] [PubMed]
- Rando, G.; Scalone, E.; Sfameni, S.; Plutino, M.R. Functional Bio-Based Polymeric Hydrogels for Wastewater Treatment: From Remediation to Sensing Applications. Gels 2024, 10, 498. [Google Scholar] [CrossRef] [PubMed]
- Alcalde-Garcia, F.; Prasher, S.; Kaliaguine, S.; Tavares, J.R.; Dumont, M.-J. Desorption Strategies and Reusability of Biopolymeric Adsorbentsand Semisynthetic Derivatives in Hydrogel and HydrogelComposites Used in Adsorption Processes. ACS Eng. Au 2023, 3, 364–545. [Google Scholar] [CrossRef]
- Maroulas, K.N.; Efthymiopoulos, P.; Iliadou, V.; Zamboulis, A.; Bikiaris, N.D.; Bakalis, E.; Kyzas, G.Z. Interfacial interactions in aqueous systems of poly(ε-caprolactone)-co-poly (2-hydroxyethyl methacrylate) hydrogel with dye molecules in synthetic wastewaters. J. Ind. Eng. Chem. 2025, 142, 309–320. [Google Scholar] [CrossRef]
- Sanchez, L.M.; Ollier, R.P.; Alvarez, V.A. Sorption behavior of polyvinyl alcohol/bentonite hydrogels for dyes removal. J. Polym. Res. 2019, 26, 142. [Google Scholar] [CrossRef]
- Lamkhao, S.; Tandorn, S.; Rujijanagul, G.; Randorn, C. A practical approach using a novel porous photocatalyst/hydrogel composite for wastewater treatment. Mater. Today Sustain. 2023, 23, 100482. [Google Scholar] [CrossRef]
- Rahmatpour, A.; Soleimani, P.; Mirkani, A. Eco-friendly poly(vinyl alcohol)/partially hydrolyzed polyacrylamide graphene oxide semi-IPN nanocomposite hydrogel as a reusable and efficient adsorbent of cationic dye methylene blue from water. React. Funct. Polym. 2022, 175, 105290. [Google Scholar] [CrossRef]
- Zhou, G.; Luo, J.; Liu, C.; Chu, L.; Ma, J.; Tang, Y.; Zeng, Z.; Luo, S. A highly efficient polyampholyte hydrogel sorbent based fixed-bed process for heavy metal removal in actual industrial effluent. Water Res. 2016, 89, 151–160. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, P.; Yang, K.; Wang, Q.; Feng, W.; Tang, Y. PAA/TiO2@C composite hydrogels with hierarchical pore structures as high efficiency adsorbents for heavy metal ions and organic dyes removal. Desalination 2023, 558, 116620. [Google Scholar] [CrossRef]
- Adjuik, T.A.; Nokes, S.E.; Montross, M.D. Biodegradability of bio-based and synthetic hydrogels as sustainable soil amendments: A review. J. Appl. Polym. Sci. 2023, 140, 12. [Google Scholar] [CrossRef]
- Pattnaik, A.; Ghosh, P.; Poonia, A.K. An overview on advancements in hydrogels for effective wastewater treatment. J. Mol. Liq. 2025, 424, 127120. [Google Scholar] [CrossRef]
- Wichterle, O.; Lìm, D. Hydrophilic Gels for Biological Use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Kumar, M.; Bhujbal, S.K.; Kohli, K.; Prajapati, R.; Sharma, B.K.; Sawarkar, A.D.; Abhishek, K.; Bolan, S.; Ghosh, P.; Kirkham, M.B.; et al. A review on value-addition to plastic waste towards achieving a circular economy. Sci. Total Environ. 2024, 921, 171106. [Google Scholar] [CrossRef]
- Feldman, D.; Barbalata, A. Synthetic Polymers: Technology, Properties, Applications; Chapman & Hall: London, UK, 1996. [Google Scholar]
- Hossain, M.S.; Hossain, M.M.; Khatun, M.K.; Hossain, K.R. Hydrogel-based superadsorbents for efficient removal of heavy metals in industrial wastewater treatment and environmental conservation. Environ. Func. Mater. 2023, 2, 142–158. [Google Scholar] [CrossRef]
- Teodorescu, M.; Lungu, A.; Stanescu, P.O.; Neamtu, C. Preparation and Properties of Novel Slow-Release NPK Agrochemical Formulations Based on Poly(acrylic acid) Hydrogels and Liquid Fertilizers. Ind. Eng. Chem. Res. 2009, 48, 6527–6534. [Google Scholar] [CrossRef]
- Panic, V.V.; Jovanovic, J.D.; Spasojevic, J.P.; Savic, S.I.; Markovic, M.D.; Radulovic, A.M.; Adnadjevic, B.K. Structure-property correlations for composite hydrogels based on poly(methacrylic acid) and high concentrations of LTA zeolite. Chem. Eng. Sci. 2024, 292, 119981. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Lu, Z.; Miao, R.; Zhang, N. Thermal and light-driven soft actuators based on a conductive polypyrrole nanofibers integrated poly(N-isopropylacrylamide) hydrogel with intelligent response. J. Colloid Interface Sci. 2024, 675, 336–346. [Google Scholar] [CrossRef]
- Hanyková, L.; Št’astná, J.; Krakovský, I. Responsive Acrylamide-Based Hydrogels: Advances in Interpenetrating Polymer Structures. Gels 2024, 10, 414. [Google Scholar] [CrossRef]
- Isaac, A.H.; Phillips, S.Y.R.; Ruben, E.; Estes, M.; Rajavel, V.; Baig, T.; Paleti, C.; Landsgaard, K.; Lee, R.H.; Guda, T.; et al. Impact of PEG sensitization on the efficacy of PEG hydrogel-mediated tissue engineering. Nat. Commun. 2024, 15, 3283. [Google Scholar] [CrossRef]
- Negru, I.; Teodorescu, M.; Stănescu, P.O.; Drăghici, C.; Lungu, A.; Sârbu, A. Thermogelation properties of ABA triblock copolymers of poly (ethylene glycol)(B) and copolyacrylates of oligo (ethylene glycol)s (A) in aqueous solution. Soft Mater. 2013, 11, 149–156. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, J.; He, Y.; Zhao, C.; An, M.; Li, L. Porous polyvinyl alcohol/polyacrylamide hydrogels loaded with HTO lithium-ion sieves for highly rapid and efficient Li+ extraction. Desalination 2024, 580, 117587. [Google Scholar] [CrossRef]
- Sekizkardes, B.; Su, E.; Okay, O. Mechanically Strong Superabsorbent Terpolymer Hydrogels Based on AMPS via Hydrogen-Bonding Interactions. ACS Appl. Polym. Mater. 2023, 5, 2043–2050. [Google Scholar] [CrossRef]
- Xue, X.; He, Y.; Jia, R.; Qian, H.; Lin, Y.; Xu, X.; Shi, J.; Chang, S.; Cui, A. pH-Responsive itaconic acid-based villous-like hydrogels for water-plugging materials. J. Appl. Polym. Sci. 2024, 141, e55440. [Google Scholar] [CrossRef]
- Madduma-Bandarage, U.S.K.; Madihally, S.V. Synthetic hydrogels: Synthesis, novel trends, and applications. J. Appl. Polym. Sci. 2021, 138, 50376. [Google Scholar] [CrossRef]
- Henderson, L. Global perceptions of plastic pollution: The contours and limits of debate. Camb. Prism. Plast. 2023, 1, e21. [Google Scholar] [CrossRef]
- Pakdel, P.M.; Peighambardoust, S.J. A review on acrylic based hydrogels and their applications in wastewater treatment. J. Environ. Manag. 2018, 217, 123–143. [Google Scholar] [CrossRef]
- Yu, Y.; Peng, R.; Yang, C.; Tang, Y. Eco-friendly and cost-effective superabsorbent sodium polyacrylate composites for environmental remediation. J. Mater. Sci. 2015, 50, 5799–5808. [Google Scholar] [CrossRef]
- Alkhaldi, H.; Alharthi, S.; Alharthi, S.; AlGhamdi, H.A.; AlZahrani, Y.M.; Mahmoud, S.A.; Amin, L.G.; AlShaalan, N.H.; Boraie, W.E.; Attia, M.S.; et al. Sustainable polymeric adsorbents for adsorption based water remediation and pathogen deactivation: A review. RSC Adv. 2024, 14, 33143. [Google Scholar] [CrossRef]
- Lin, J.; Ye, W.; Xie, M.; Seo, D.H.; Luo, J.; Wan, Y.; Van der Bruggen, B. Environmental impacts and remediation of dye-containing wastewater. Nat. Rev. Earth Environ. 2023, 4, 785–803. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef]
- Slama, H.B.; ChenariBouket, A.; Pourhassan, Z.; Alenezi, F.N.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golinska, P.; Belbahri, L. Diversity of Synthetic Dyes from Textile Industries, Discharge Impacts and Treatment Methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Azam, K.; Shezad, N.; Shafiq, I.; Akhter, P.; Akhtar, F.; Jamil, F.; Shafique, S.; Park, Y.-K.; Hussain, M. A review on activated carbon modifications for the treatment of wastewater containing anionic dyes. Chemosphere 2022, 306, 135566. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Ji, L.; Guo, J.; Ge, S.; Lu, W.; Chen, Y.; Cai, L.; Wang, Y.; Song, W. An abundant porous biochar material derived from wakame (Undaria pinnatifida) with high adsorption performance for three organic dyes. Bioresour. Technol. 2020, 318, 124082. [Google Scholar] [CrossRef]
- Shabil, S.M.; Anwar, H.; Musthafa, F.N.; Al-Lohedan, H.; Alfarwati, S.; Rajabathar, J.R.; Khalid, A.J.; Cabibihan, J.J.; Karnan, M.; Kumar, S.K. Photocatalytic degradation of organic dyes using reduced graphene oxide (rGO). Sci Rep. 2024, 14, 3608. [Google Scholar] [CrossRef] [PubMed]
- Gadore, V.; Mishra, S.R.; Yadav, N.; Yadav, G.; Ahmaruzzaman, M. Advances in zeolite-based materials for dye removal: Current trends and future prospects. Inorg. Chem. Commun. 2024, 166, 112606. [Google Scholar] [CrossRef]
- Uddin, M.J.; Ampiaw, R.E.; Lee, W. Adsorptive removal of dyes from wastewater using a metal-organic framework: A review. Chemosphere 2021, 284, 131314. [Google Scholar] [CrossRef]
- Zango, Z.U.; Binzowaimil, A.M.; Aldaghri, O.A.; Eisa, M.H.; Garba, A.; Ahmed, N.M.; Lim, J.W.; Ng, H.-S.; Daud, H.; Jumbri, K.; et al. Applications of covalent organic frameworks for the elimination of dyes from wastewater: A state-of-the-arts review. Chemosphere 2023, 343, 140223. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Shunmugam, R. Polymer based gels and their applications in remediation of dyes from textile effluents. J. Macromol. Sci. Part A Pure Appl. Chem. 2020, 57, 906–926. [Google Scholar] [CrossRef]
- Ahmadian, M.; Jaymand, M. Interpenetrating polymer network hydrogels for removal of synthetic dyes: A comprehensive review. Coord. Chem. Rev. 2023, 486, 215152. [Google Scholar] [CrossRef]
- Dey, A.; Bera, R.; Chakrabarty, D. Synthesis of poly(ethylene glycol) di-itaconate and investigation of its influence on acrylamide based hydrogels meant for water treatment. Polymer 2017, 116, 178–190. [Google Scholar] [CrossRef]
- Banerjee, P.; Dinda, P.; Kar, M.; Uchman, M.; Mandal, T.K. Ionic Liquid Cross-Linked High-Absorbent Polymer Hydrogels: Kinetics of Swelling and Dye Adsorption. Langmuir 2023, 39, 9757–9772. [Google Scholar] [CrossRef] [PubMed]
- Mahida, V.P.; Patel, M.P. Removal of some most hazardous cationic dyes using novel poly (NIPAAm/AA/N-allylisatin) nanohydrogel. Arab. J. Chem. 2016, 9, 430–442. [Google Scholar] [CrossRef]
- Üzüm, Ö.B.; Çetin, G.; Kundakcı, S.; Karadağ, E. Swelling and dye adsorption properties of polyelectrolyte semi-IPNs including of acrylamide/(3-acrylamidopropyl)trimethyl ammonium chloride/poly(ethylene glycol). Sep. Sci. Technol. 2020, 55, 18. [Google Scholar] [CrossRef]
- Dalalibera, A.; Vilela, P.B.; Vieira, T.; Becegato, V.A.; Paulino, A.T. Removal and selective separation of synthetic dyes from water using a polyacrylic acid-based hydrogel: Characterization, isotherm, kinetic, and thermodynamic data. J. Environ. Chem. Eng. 2020, 8, 104465. [Google Scholar] [CrossRef]
- Mekewi, M.A.; Madkour, T.M.; Darwish, A.S.; Hashish, Y.M. Does poly(acrylic acid-co-acrylamide) hydrogel be the pluperfect choiceness in treatment of dyeing wastewater? From simple opolymer to gigantic aqua-waste remover. J. Ind. Eng. Chem. 2015, 30, 359–371. [Google Scholar] [CrossRef]
- Sakthivel, M.; Franklin, D.S.; Guhanathan, S. pH-sensitive Itaconic acid based polymeric hydrogels for dye removal applications. Ecotoxicol. Environ. Saf. 2016, 134, 427–432. [Google Scholar] [CrossRef]
- Thakur, S.; Chaudhary, J.; Thakur, A.; Gunduz, O.; Alsanie, W.F.; Makatsoris, C.; Thakur, V.K. Highly efficient poly(acrylic acid-co-aniline) grafted itaconic acid hydrogel: Application in water retention and adsorption of rhodamine B dye for a sustainable environment. Chemosphere 2002, 303, 134917. [Google Scholar] [CrossRef]
- Mohammadzadeh, F.; Haddadi-Asl, V.; Salami-Kalajahi, M. pH-sensitive multi-arm star polyampholytes: A novel approach for simultaneous adsorption of anionic and cationic dyes. J. Mol. Liq. 2024, 395, 123863. [Google Scholar] [CrossRef]
- Salahshoori, I.; Wang, Q.; Nobre, M.A.L.; Mohammadi, A.H.; Dawi, E.A.; Khonakdar, H.A. Molecular simulation-based insights into dye pollutant adsorption: A perspective review. Adv. Colloid Interface Sci. 2024, 333, 103281. [Google Scholar] [CrossRef]
- Ibrahim, A.G.; Hai, F.A.; El-Wahab, H.A.; Aboelanin, H. Methylene blue removal using a novel hydrogel containing 3-Allyloxy-2-hydroxy-1-propanesulfonic acid sodium salt. Adv. Polym. Technol. 2018, 37, 2699–3899. [Google Scholar] [CrossRef]
- Rehman, T.U.; Shah, L.A.; Khan, M.; Irfan, M.; Khattak, N.S. Zwitterionic superabsorbent polymer hydrogels for efficient and selective removal of organic dyes. RSC Adv. 2019, 9, 18565. [Google Scholar] [CrossRef] [PubMed]
- Shoueira, K.R.; Sarhana, A.A.; Attab, A.M.; Akla, M.A. Macrogel and nanogel networks based on crosslinked poly (vinyl alcohol) for adsorption of methylene blue from aqua system. Environ. Nanotechnol. Monit. Manag. 2016, 5, 62–73. [Google Scholar] [CrossRef]
- Du, J.; Dong, Z.; Yang, X.; Zhao, L. Facile fabrication of polymeric quaternary ammonium salt hydrogel by radiation for dyes removal from aqueous solution. Radiat. Phys. Chem. 2021, 188, 109670. [Google Scholar] [CrossRef]
- Tang, Z.; Guo, H.; Xu, J.; Li, Z.; Sun, G. Cationic poly(diallyldimethylammonium chloride) based hydrogel for effective anionic dyes adsorption from aqueous solution. React. Funct. Polym. 2022, 174, 105239. [Google Scholar] [CrossRef]
- Onder, A.; Ilgin, P.; Ozay, H.; Ozay, O. Removal of dye from aqueous medium with pH-sensitive poly [(2-(acryloyloxy)ethyl]trimethylammonium chloride-co-1-vinyl-2-pyrrolidone] cationic hydrogel. J. Environ. Chem. Eng. 2020, 8, 104436. [Google Scholar] [CrossRef]
- Tang, Z.; Hu, X.; Ding, H.; Li, Z.; Liang, R.; Sun, G. Villi-like poly(acrylic acid) based hydrogel adsorbent with fast and highly efficient methylene blue removing ability. J. Colloid Interface Sci. 2021, 594, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Işıkver, Y.; Saraydın, D.; Karakuş, N. An Experimental and Computational Evaluation of the Interaction Between Intelligent Ampholyte Acrylamide/Acrylic Acid/2-(Acryloyloxy)ethyl Trimethylammonium Chloride Hydrogel and Dyes. J. Polym. Environ. 2024, 32, 441–459. [Google Scholar] [CrossRef]
- Li, D.; Li, Q.; Bai, N.; Dong, H.; Mao, D. One-Step Synthesis of Cationic Hydrogel for Efficient Dye Adsorption and Its Second Use for Emulsified Oil Separation. ACS Sustain. Chem. Eng. 2017, 5, 5598–5607. [Google Scholar] [CrossRef]
- Liu, X.; Zhoua, Z.; Yina, J.; Hea, C.; Zhaoa, W.; Zhao, C. Fast and environmental-friendly approach towards uniform hydrogel particles with ultrahigh and selective removal of anionic dyes. J. Environ. Chem. Eng. 2020, 8, 104352. [Google Scholar] [CrossRef]
- Zhao, S.; An, X.; An, W.; Hu, J.; Wu, P.; Cui, W. Efficient adsorption of anionic and cationic dyes by PVA/PAA/GO composite hydrogel with three-dimensional porous double network structure. Mater. Chem. Phys. 2024, 313, 128716. [Google Scholar] [CrossRef]
- Mosaffa, E.; Patel, R.I.; Purohit, A.M.; Basak, B.B.; Banerjee, A. Efficient Decontamination of Cationic Dyes from Synthetic Textile Wastewater Using Poly(acrylic acid) Composite Containing Amino Functionalized Biochar: A Mechanism Kinetic and Isotherm Study. J. Polym. Environ. 2023, 31, 2486–2503. [Google Scholar] [CrossRef]
- Wen, T.; Huang, B.; Zhou, L. Facile Fabrication of Magnetic Poly(Vinyl Alcohol)/Activated Carbon Composite Gel for Adsorptive Removal of Dyes. J. Compos. Sci. 2022, 6, 55. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, G.; Wang, X.; Liu, J.; Li, D.; Wu, C.; Zhang, W. Composite hydrogel membrane with high mechanical strength for treatment of dye pollutant. Sep. Purif. Technol. 2021, 275, 119154. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.; Shi, X.; Nawar, S.; Werner, J.G.; Huang, G.; Ye, M.; Weitz, D.A.; Solovev, A.A.; Mei, Y. Hydrogel microcapsules with photocatalytic nanoparticles for removal of organic pollutants. Environ. Sci. Nano 2020, 7, 656–664. [Google Scholar] [CrossRef]
- Kangwansupamonkon, W.; Klaikaew, N.; Kiatkamjornwong, S. Green synthesis of titanium dioxide/acrylamide-based hydrogel composite, self-degradation and environmental applications. Eur. Polym. J. 2018, 107, 118–131. [Google Scholar] [CrossRef]
- Gao, B.; Yu, H.; Wen, J.; Zeng, H.; Liang, T.; Zuo, F.; Cheng, C. Super-adsorbent poly(acrylic acid)/laponite hydrogel with ultrahigh mechanical property for adsorption of methylene blue. J. Environ. Chem. Eng. 2021, 9, 106346. [Google Scholar] [CrossRef]
- Safarzadeh, H.; Peighambardoust, S.J.; Mousavi, S.H.; Foroutan, R.; Mohammadi, R.; Peighambardoust, S.H. Adsorption ability evaluation of the poly(methacrylic acid-co-acrylamide)/cloisite 30B nanocomposite hydrogel as a new adsorbent for cationic dye removal. Environ. Res. 2022, 212, 113349. [Google Scholar] [CrossRef]
- Jiang, R.N.; Chen, Y.A.; Liu, Y.; Liu, H. Multi-functional nanocomposite hydrogels based on assembled catechin-modified TiO2 nanoparticle crosslinker for efficient dye removal from wastewater. Mater. Commun. 2023, 37, 107216. [Google Scholar] [CrossRef]
- Tasdelen, B.; Cifci, D.I.; Meric, S. Preparation of N-isopropylacrylamide/itaconic acid/Pumice highly swollen composite hydrogels to explore their removal capacity of methylene blue. Colloids Surf. A Physicochem. Eng. Asp. 2017, 519, 245–253. [Google Scholar] [CrossRef]
- Panic, V.V.; Velickovic, S.J. Removal of model cationic dye by adsorption onto poly(methacrylic acid)/zeolite hydrogel composites: Kinetics, equilibrium study and image analysis. Sep. Purif. Technol. 2014, 122, 384–394. [Google Scholar] [CrossRef]
- Taktak, F.; Gokçe, S. Ultrasound-assisted synthesis of biodegradable graphene oxide-based hydrogel nanocomposites for highly efficient and cost-effective removal of methyl orange from aqueous media. J. Water Process Eng. 2024, 58, 104837. [Google Scholar] [CrossRef]
- Mosaffa, E.; Patel, R.I.; Banerjee, A.; Basak, B.B.; Oroujzadeh, M. Comprehensive analysis of cationic dye removal from synthetic and industrial wastewater using a semi-natural curcumin grafted biochar/poly acrylic acid composite hydrogel. RSC Adv. 2024, 14, 7745–7762. [Google Scholar] [CrossRef]
- Yang, C.-X.; Lei, L.; Zhou, P.-X.; Zhang, Z.; Lei, Z.-Q. Preparation and characterization of poly(AA co PVP)/PGS composite and its application for methylene blue adsorption. J. Colloid Interface Sci. 2015, 443, 97–104. [Google Scholar] [CrossRef]
- Kongsen, P.; Amornpitoksuk, P.; Chantarak, S. Development of multifunctional hydrogel composite based on poly(vinyl alcohol-g-acrylamide) for removal and photocatalytic degradation of organic dyes. React. Funct. Polym. 2022, 172, 105207. [Google Scholar] [CrossRef]
- Ortega, J.A.C.; Hernández-Montelong, J.; Hernández-Montelongo, R.; Mendoza, A.G.A. Effective Removal of Cu2+ Ions from Aqueous Media Using Poly(acrylamide-co-itaconic acid) Hydrogels in a Semi-Continuous Process. Gels 2023, 9, 702. [Google Scholar] [CrossRef] [PubMed]
- Diacon, A.; Albota, F.; Mocanu, A.; Brincoveanu, O.; Podaru, A.I.; Rotariu, T.; Ahmad, A.A.; Rusen, E.; Toader, G. Dual-Responsive Hydrogels for Mercury Ion Detection and Removal from Wastewater. Gels 2024, 10, 113. [Google Scholar] [CrossRef]
- Chen, J.; Liao, C.; Guo, X.X.; Hou, S.-C.; He, W.-D. PAAO cryogels from amidoximatedP(acrylic acid-co-acrylonitrile) for the adsorption of lead ion. Eur. Polym. J. 2022, 171, 111192. [Google Scholar] [CrossRef]
- Kafetzi, M.; Borchert, K.B.L.; Steinbach, C.; Schwarz, D.; Pispas, S.; Schwarz, S. Thermoresponsive PNIPAM-b-PAA block copolymers as “smart” adsorbents of Cu(II) for water restore treatments. Colloids Surf. A Physicochem. Eng. Asp. 2021, 614, 126049. [Google Scholar] [CrossRef]
- Lv, Q.; Hu, X.; Zhang, X.; Huang, L.; Liu, Z.; Sun, G. Highly efficient removal of trace metal ions by using poly(acrylic acid) hydrogel adsorbent. Mater. Des. 2019, 181, 107934. [Google Scholar] [CrossRef]
- Wanga, X.; Hou, H.; Li, Y.; Wang, Y.; Hao, C.; Ge, C. A novel semi-IPN hydrogel: Preparation, swelling properties and adsorption studies of Co (II). J. Ind. Eng. Chem. 2016, 41, 82–90. [Google Scholar] [CrossRef]
- Sun, J.; Jin, Z.; Wang, J.; Wang, H.; Zhang, Q.; Gao, H.; Jin, Z.; Zhang, J.; Wang, Z. Application of Ionic Liquid Crosslinked Hydrogel for Removing Heavy Metal Ions from Water: Different Concentration Ranges with Different Adsorption Mechanisms. Polymers 2023, 15, 2784. [Google Scholar] [CrossRef] [PubMed]
- Maksoud, I.K.A.; Bassioni, G.; Nady, N.; Younis, S.A.; Ghobashy, M.M.; Abdel-Mottaleb, M.S.A. Radiation synthesis and chemical modifications of p(AAm-co-AAc) hydrogel for improving their adsorptive removal of metal ions from polluted water. Sci. Rep. 2023, 13, 21879. [Google Scholar] [CrossRef]
- Kaya, S.Ç.Y.; Gönder, Z.B.; Gürdaa, G.; Vergili, I. Removal of Cu(II), Ni(II) and Zn(II) from aqueous solutions using poly(2-acrylamido-2-methyl-1-propanesulfonic acid) gel: Sorption kinetics and characterization. Desalin. Water Treat. 2018, 133, 348–358. [Google Scholar] [CrossRef]
- Çavuş, S.; Yasara, G.; Kaya, Y.; Gönder, Z.B.; Gürdag, G.; Vergili, S. Synthesis and characterization of gel beads based on ethyleneglycoldimethacrylate and 2-acrylamido-2-methyl-1-propane sulfonic acid: Removal of Fe(II), Cu(II), Zn(II), and Ni(II) from metal finishing wastewater. Process Saf. Environ. Prot. 2016, 103, 227–236. [Google Scholar] [CrossRef]
- Hui, B.; Ye, L. Structure of polyvinyl alcohol-g-acrylic acid-2-acrylamido-2-methyl-1-propanesulfonic acid hydrogel and adsorption mechanism for advanced Pb(II) removal. J. Ind. Eng. Chem. 2016, 35, 309–317. [Google Scholar] [CrossRef]
- Shoueir, K.R.; Akl, M.A.; Sarhan, A.A.; Atta, A.M. New core@shell nanogel based 2-acrylamido-2-methyl-1-propane sulfonic acid for preconcentration of Pb(II) from various water samples. Appl. Water Sci. 2017, 7, 3729–3740. [Google Scholar] [CrossRef]
- Yang, X.; Debeli, D.K.; Shan, G.; Pan, P. Selective adsorption and high recovery of La3+ using graphene oxide/poly (N-isopropyl acrylamide-maleic acid) cryogel. Chem. Eng. J. 2020, 379, 122335. [Google Scholar] [CrossRef]
- Bakr, A.-S.A.; Al-Shafey, H.I.; Arafa, E.I.; El Naggar, A.M.A. Synthesis and Characterization of Polymerized Acrylamide Coupled with Acrylamido-2-Methyl-1-Propane Sulfonic Acid-Montmorillonite Structure as a Novel Nanocomposite for Cd (II) Removal from Aqueous Solutions. J. Chem. Eng. Data 2020, 65, 4079–4091. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Ai, Z.; Li, M.; Li, H.; Peng, W.; Zhao, Y.; Song, S. Adsorption toward Pb(II) occurring on three-dimensional reticular-structured montmorillonite hydrogel surface. Appl. Clay Sci. 2021, 210, 106153. [Google Scholar] [CrossRef]
- Gao, B.; Yu, H.-R.; Zhang, H.-Y.; Liang, T.; Cheng, C.-J. High-density immobilization of potassium copper hexacyanoferrate in poly (acrylic acid)/laponite hydrogel for enhanced Cs+ removal. J. Environ. Chem. Eng. 2022, 10, 107979. [Google Scholar] [CrossRef]
- Mohammadinezhada, A.; Marandia, G.B.; Farsadroohb, M.; Javadian, H. Synthesis of poly(acrylamide-co-itaconic acid)/MWCNTs superabsorbent hydrogel nanocomposite by ultrasound-assisted technique: Swelling behavior and Pb (II) adsorption capacity. Ultrason. Sonochem. 2018, 49, 1–12. [Google Scholar] [CrossRef]
- Ge, H.; Wang, J. Ear-like poly (acrylic acid)-activated carbon nanocomposite: A highly efficient adsorbent for removal of Cd(II) from aqueous solutions. Chemosphere 2017, 169, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Souda, P.; Sreejith, L. Magnetic hydrogel for better adsorption of heavy metals from aqueous solutions. J. Environ. Chem. Eng. 2015, 3, 1882–1891. [Google Scholar] [CrossRef]
- Chen, J.; Ahmad, A.L.; Lim, J.K.; Ooi, B.S. Adsorption-desorption characteristic of thermo-magneto-responsive poly(Nisopropylacrylamide)-co-acrylic acid composite hydrogel towards chromium (III) ions. J. Water Process Eng. 2019, 32, 100957. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Wu, Y.; Liu, C.; Liu, X. Preparation of Graphene Oxide Hydrogels and Their Adsorption Applications toward Various Heavy Metal Ions in Aqueous Media. Appl. Sci. 2023, 13, 11948. [Google Scholar] [CrossRef]
- Ma, M.; Ke, X.; Wang, T.; Li, J.; Ye, H. A novel double-network hydrogel made from electrolytic manganese slag and polyacrylic acid-polyacrylamide for removal of heavy metals in wastewater. J. Hazard. Mater. 2024, 462, 132722. [Google Scholar] [CrossRef]
- Jamnongkan, T.; Kantarot, K.; Niemtang, K.; Pansila, P.P.; Wattanakornsiri, A. Kinetics and mechanism of adsorptive removal of copper from aqueous solution with poly(vinyl alcohol) hydrogel. Trans. Nonferrous Met. Soc. China 2014, 24, 3386–3393. [Google Scholar] [CrossRef]
- Zhou, T.; Xia, F.; Deng, Y.; Zhao, Y. Removal of Pb(II) from aqueous solutions using waste textiles/poly(acrylic acid) composite synthesized by radical polymerization technique. J. Environ. Sci. 2018, 67, 368–377. [Google Scholar] [CrossRef]
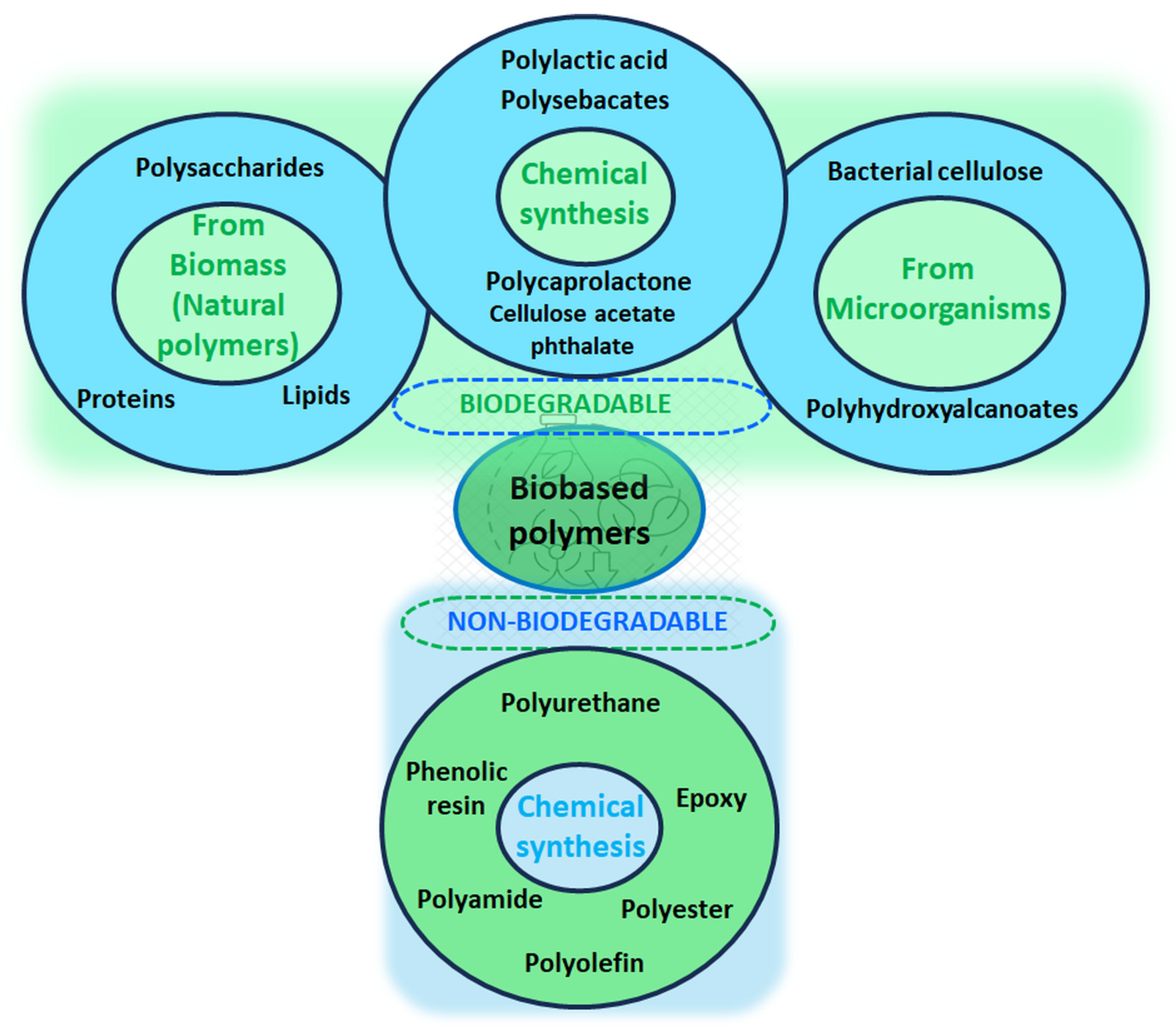
- Muñoz-Bonilla, A.; Echeverria, C.; Sonseca, Á.; Arrieta, M.P.; Fernández-García, M. Bio-Based Polymers with Antimicrobial Properties towards Sustainable Development. Materials 2019, 12, 641. [Google Scholar] [CrossRef]
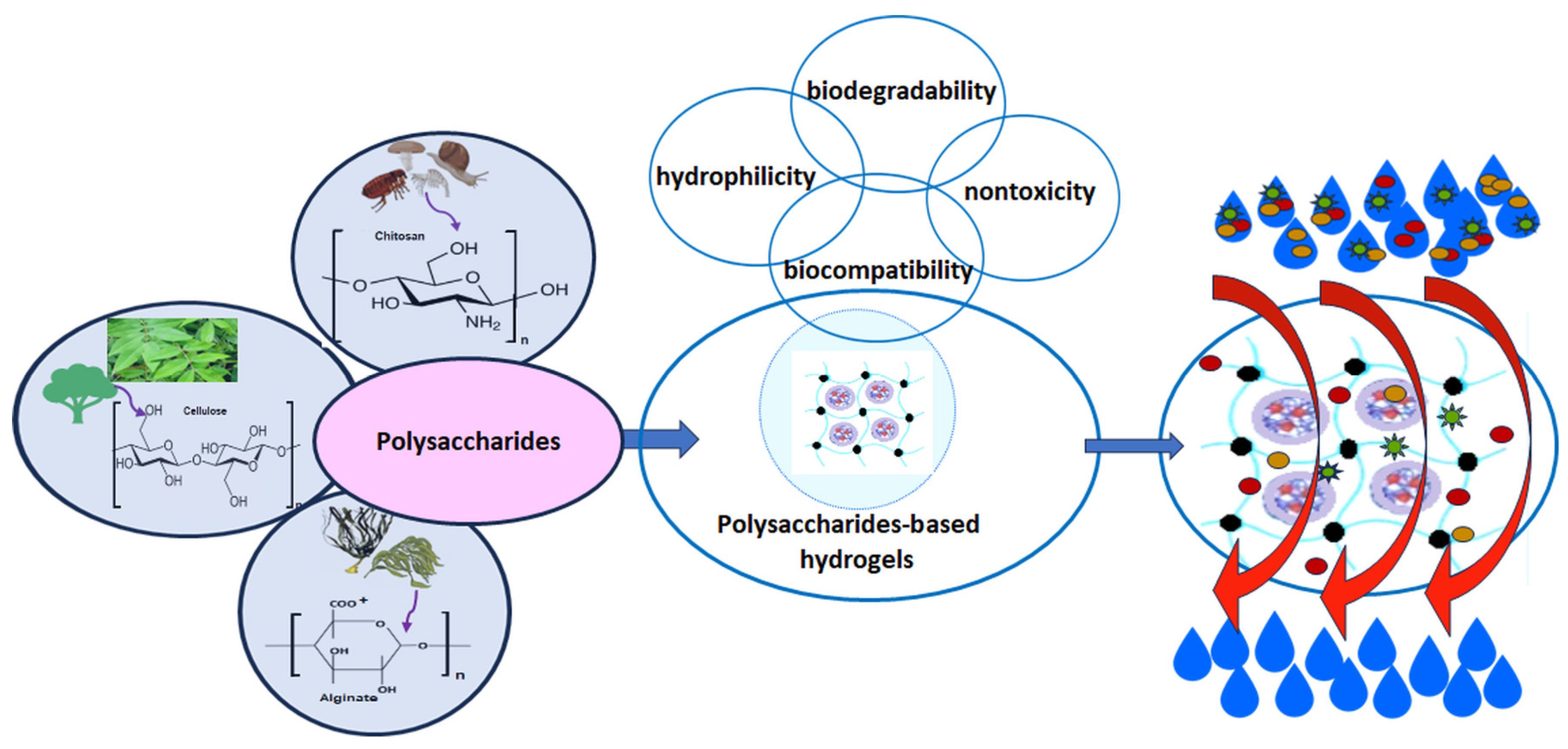
- Benalaya, I.; Alves, G.; Lopes, J.; Silva, L.R. A Review of Natural Polysaccharides: Sources, Characteristics, Properties, Food, and Pharmaceutical Applications. Int. J. Mol. Sci. 2024, 25, 1322. [Google Scholar] [CrossRef]
- Tudu, M.; Samanta, A. Natural polysaccharides: Chemical properties and application in pharmaceutical formulations. Eur. Polym. J. 2023, 184, 111801. [Google Scholar] [CrossRef]
- Qi, X.; Tong, X.; Pan, W.; Zeng, Q.; You, S.; Shen, J. Recent advances in polysaccharide-based adsorbents for wastewater treatment. J. Clean. Prod. 2021, 315, 128221. [Google Scholar] [CrossRef]
- Cywar, R.M.; Rorrer, N.A.; Hoyt, C.B.; Beckham, G.T.; Chen, E.Y.-X. Bio-based polymers with performance-advantaged properties. Nat. Rev. Mater. 2022, 7, 83–103. [Google Scholar] [CrossRef]
- Qureshi, M.A.; Nishat, N.; Jadoun, S.; Ansari, M.Z. Polysaccharide based superabsorbent hydrogels and their methods of synthesis: A review. Carbohydr. Polym. Technol. Appl. 2020, 1, 100014. [Google Scholar] [CrossRef]
- Berradi, A.; Aziz, F.; El Achaby, M.; Ouazzani, N.; Mandi, L. A Comprehensive Review of Polysaccharide-Based Hydrogels as Promising Biomaterials. Polymers 2023, 15, 2908. [Google Scholar] [CrossRef]
- Alsaka, L.; Alsaka, L.; Altaee, A.; Zaidi, S.J.; Zhou, J.; Kazwini, T. A Review of Hydrogel Application in Wastewater Purification. Separations 2025, 12, 51. [Google Scholar] [CrossRef]
- El Halah, A.; López-Carrasquero, F.; Contreras, J. Applications of hydrogels in the adsorption of metallic ions. Cienc. Ing. 2018, 39, 57–69. [Google Scholar]
- Zhou, G.; Luo, J.; Liu, C.; Chu, L.; Crittenden, J. Efficient heavy metal removal from industrial melting effluent using fixed-bed process based on porous hydrogel adsorbents. Water Res. 2018, 131, 246–254. [Google Scholar] [CrossRef]
- Blachnio, M.; Zienkiewicz-Strzalka, M. Evaluation of the Dye Extraction Using Designed Hydrogels for Further Applications towards Water Treatment. Gels 2024, 10, 159. [Google Scholar] [CrossRef]
- Qi, X.; Wu, L.; Su, T.; Zhang, J.; Dong, W. Polysaccharide-based cationic hydrogels for dye adsorption. Colloids Surf. B Biointerfaces 2018, 170, 364–372. [Google Scholar] [CrossRef]
- Utzeri, G.; Verissimo, L.; Ribeiro, A.C.F.; Valente, A.J.M. Pesticides and Their Environment and Health Impact: An Approach to Remediation Using Hydrogels. In Optical and Molecular Physics; Apple Academic Press: Palm Bay, FL, USA, 2021; pp. 447–472. [Google Scholar]
- Akter, M.; Bhattacharjee, M.; Dhar, A.K.; Rahman, F.B.A.; Haque, S.; Rashid, T.U.; Kabir, S.M.F. Cellulose-Based Hydrogels for Wastewater Treatment: A Concise Review. Gels 2021, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Sjahro, N.; Yunus, R.; Abdullah, L.C.; Rashid, S.A.; Asis, A.J.; Akhlisah, Z.N. Recent advances in the application of cellulose derivatives for removal of contaminants from aquatic environments. Cellulose 2021, 28, 7521–7557. [Google Scholar] [CrossRef]
- Chen, C.; Xi, Y.; Weng, Y. Recent advances in cellulose-based hydrogels for tissue engineering applications. Polymers 2022, 14, 3335. [Google Scholar] [CrossRef] [PubMed]
- Topare, N.S.; Wadgaonkar, V.S. A review on application of low-cost adsorbents for heavy metals removal from wastewater. Mater. Today Proc. 2023, 77, 8–18. [Google Scholar] [CrossRef]
- Zainal, S.H.; Mohd, N.H.; Suhaili, N.; Anuar, F.H.; Lazim, A.M.; Othaman, R. Preparation of cellulose-based hydrogel: A review. J. Mater. Res. Technol. 2021, 10, 935–952. [Google Scholar] [CrossRef]
- Yang, L.; Bao, L.; Dong, T.; Xie, H.; Wang, X.; Wang, H.; Wu, J.; Hao, C. Adsorption properties of cellulose/guar gum/biochar composite hydrogel for Cu2+, Co2+ and methylene blue. Ind. Crops Prod. 2024, 210, 118179. [Google Scholar] [CrossRef]
- Lunardi, V.B.; Santoso, S.P.; Angkawijaya, A.E.; Cheng, K.-C.; Tran-Nguyen, P.L.; Go, A.W.; Nakamura, Y.; Lin, S.-P.; Hsu, H.-Y.; Yuliana, M.; et al. Synthesis of cellulose hydrogel by utilizing agricultural waste and zeolite for adsorption of copper metal ions. Ind. Crops Prod. 2024, 210, 118179. [Google Scholar] [CrossRef]
- Yang, S.-C.; Liao, Y.; Karthikeyan, K.G.; Pan, X.J. Mesoporous cellulose-chitosan composite hydrogel fabricated via the co-dissolution-regeneration process as biosorbent of heavy metals. Environ. Pollut. 2021, 286, 117324. [Google Scholar] [CrossRef]
- Rahman, A.S.A.; Khalil, N.A.; Hossain, M.S.; Yahaya, A.N.A.; Zulkifli, M. A composite hydrogel beads bio-sorbent for removal of copper: Effect of pH of copper solution. Mater. Today 2023, 74, 485–488. [Google Scholar] [CrossRef]
- Hisham, F.; Maziati Akmal, M.H.; Ahmad, F.; Ahmad, K.; Samat, N. Biopolymer chitosan: Potential sources, extraction methods, and emerging applications. Ain Shams Eng. J. 2024, 15, 102424. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, H.E.; Hassan, S.Y.; Mostafa, M.A.; El Sadek, M.M. Synthesis and Characterization of Glutamic-Chitosan Hydrogel for Copper and Nickel Removal from Wastewater. Molecules 2016, 21, 684. [Google Scholar] [CrossRef] [PubMed]
- Zidan, T.A.; Abdelhamid, A.E.; Zaki, E.G. N-Aminorhodanine modified chitosan hydrogel for antibacterial and copper ions removal from aqueous solutions. Int. J. Biol. Macromol. 2020, 158, 32–42. [Google Scholar] [CrossRef]
- Lone, S.; Ho Yoon, D.; Leea, H.; Cheong, I.W. Gelatin-chitosan hydrogel particles for efficient removal of Hg(II) from wastewater. Environ. Sci. Water Res. Technol. 2019, 5, 83. [Google Scholar] [CrossRef]
- Facchi, D.P.; Cazetta, A.L.; Canesin, E.A.; Almeida, V.C.; Bonafé, E.G.; Kipper, M.J.; Martins, A.F. New magnetic chitosan/alginate/Fe3O4@SiO2 hydrogel composites applied for removal of Pb(II) ions from aqueous systems. J. Chem. Eng. 2018, 337, 595–608. [Google Scholar] [CrossRef]
- Zhang, H.; Omer, A.M.; Hu, Z.; Yang, L.-Y.; Ji, C.; Ouyang, X.-K. Fabrication of magnetic bentonite/carboxymethyl chitosan/sodium alginate hydrogel beads for Cu (II) adsorption. Int. J. Biol. Macromol. 2019, 135, 490–500. [Google Scholar] [CrossRef]
- Yang, J.; Li, M.; Wang, Y.; Wu, H.; Ji, N.; Dai, L.; Li, Y.; Xiong, L.; Shi, R.; Sun, Q. High-Strength Physically Multi-Cross-Linked Chitosan Hydrogels and Aerogels for Removing Heavy-Metal Ions. J. Agric. Food Chem. 2019, 67, 13648–13657. [Google Scholar] [CrossRef]
- Khalil, N.A.; Syafiqah, A.; Rahman, A.; Huraira, A.M.A.; Janurin, S.N.D.F.; Fizal, A.N.S.; Ahmad, N.; Zulkifli, M.; Hossain, M.S.; Yahaya, A.N.A. Magnetic chitosan hydrogel beads as adsorbent for copper removal from aqueous solution. Mater. Today 2023, 74, 499–503. [Google Scholar] [CrossRef]
- Miron, A.; Iordache, T.-V.; Valente, A.J.M.; Durães, L.M.R.; Sarbu, A.; Ivan, G.R.; Zaharia, A.; Sandu, T.; Iovu, H.; Chiriac, A.-L. Chitosan-Based Beads Incorporating Inorganic–Organic Composites for Copper Ion Retention in Aqueous Solutions. Int. J. Mol. Sci. 2024, 25, 2411. [Google Scholar] [CrossRef]
- Arafa, E.G.; Mahmoud, R.; Gadelhak, Y.; Gawad, O.F.A. Design, preparation, and performance of different adsorbents based on carboxymethyl chitosan/sodium alginate hydrogel beads for selective adsorption of Cadmium (II) and Chromium (III) metal ions. Int. J. Biol. Macromol. 2024, 273, 132809. [Google Scholar] [CrossRef]
- Tang, S.; Yang, J.; Lin, L.; Peng, K.; Chen, Y.; Jin, S.; Yao, W. Construction of physically crosslinked chitosan/sodium alginate/calciumion double-network hydrogel and its application to heavy metal ions removal. J. Chem. Eng. 2020, 393, 124728. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Ho Yoon, D.; Joo, J.; Cheong, I.W. Spherical Chitosan/Gelatin Hydrogel Particles for Removal of Multiple Heavy Metal Ions from Wastewater. Ind. Eng. Chem. Res. 2019, 58, 9900–9907. [Google Scholar] [CrossRef]
- Meetam, P.; Phonlakan, K.; Nijpanich, S.; Budsombat, S. Chitosan-grafted hydrogels for heavy metal ion adsorption and catalytic reduction of nitroaromatic pollutants and dyes. Int. J. Biol. Macromol. 2024, 255, 128261. [Google Scholar] [CrossRef]
- Bi, S.C.; Wang, P.J.; Hu, S.H.; Li, S.K.; Pang, J.H.; Zhou, Z.Z.; Sun, G.; Huang, L.; Cheng, X.; Xing, S.; et al. Construction of physical-crosslink chitosan/PVA double-network hydrogel with surface mineralization for bone repair. Carbohydr. Polym. 2019, 224, 115176. [Google Scholar] [CrossRef]
- Tao, H.C.; Li, S.; Zhang, L.J.; Chen, Y.Z.; Deng, L.P. Magnetic chitosan/sodium alginate gel bead as a novel composite adsorbent for Cu(II) removal from aqueous solution. Environ. Geochem. Health 2019, 41, 297–308. [Google Scholar] [CrossRef]
- Bojorges, H.; López-Rubio, A.; Martínez-Abad, A.; Fabra, M.J. Overview of alginate extraction processes: Impact on alginate molecular structure and techno-functional properties. Trends Food Sci. 2023, 140, 104142. [Google Scholar] [CrossRef]
- Ren, H.; Gao, Z.; Wu, D.; Jiang, J.; Sun, Y.; Luo, C. Efficient Pb(II) removal using sodium alginate-carboxymethyl cellulose gel beads: Preparation, characterization, and adsorption mechanism. Carbohydr. Polym. 2016, 137, 402–409. [Google Scholar] [CrossRef]
- Benettayeb, A.; Ghosh, S.; Usman, M.; Seihoub, F.Z.; Sohoo, I.; Chia, C.H.; Sillanpää, M. Some Well-Known Alginate and Chitosan Modifications Used in Adsorption: A Review. Water 2022, 14, 1353. [Google Scholar] [CrossRef]
- Gao, X.; Guo, C.; Hao, J.; Zhao, Z.; Long, H.; Li, M. Adsorption of heavy metal ions by sodium alginate-based adsorbent-a review and new perspectives. Int. J. Biol. Macromol. 2020, 164, 4423–4434. [Google Scholar] [CrossRef]
- Shehzad, H.; Ahmed, E.; Sharif, A.; Farooqi, Z.H.; Din, M.I.; Begum, R.; Nawaz, I. Modified alginate-chitosan-TiO2 composites for adsorptive removal of Ni(II) ions from aqueous medium. Int. J. Biol. Macromol. 2022, 194, 117–127. [Google Scholar] [CrossRef]
- Song, Q.; Ouyang, B.; Lin, Y.; Wang, C. Cross-linked sodium alginate-carboxymethyl chitosan hydrogel beads for adsorption of Ni(II) ions. Desalin. Water Treat. 2023, 286, 183–191. [Google Scholar] [CrossRef]
- Yu, C.; Wang, M.; Dong, X.; Shi, Z.; Zhang, X.; Lin, Q. Removal of Cu(II) from aqueous solution using Fe3O4–alginate modified biochar microspheres. RSC Adv. 2017, 7, 53135–53144. [Google Scholar] [CrossRef]
- Yu, C.; Li, H.; Ma, H.; Zhang, L.; Li, Y.; Lin, Q. Characteristics and mechanism of Cu(II) adsorption on prepared calcium alginate/carboxymethyl cellulose@MnFe2O4. Polym. Bull. 2022, 79, 1201–1216. [Google Scholar] [CrossRef]
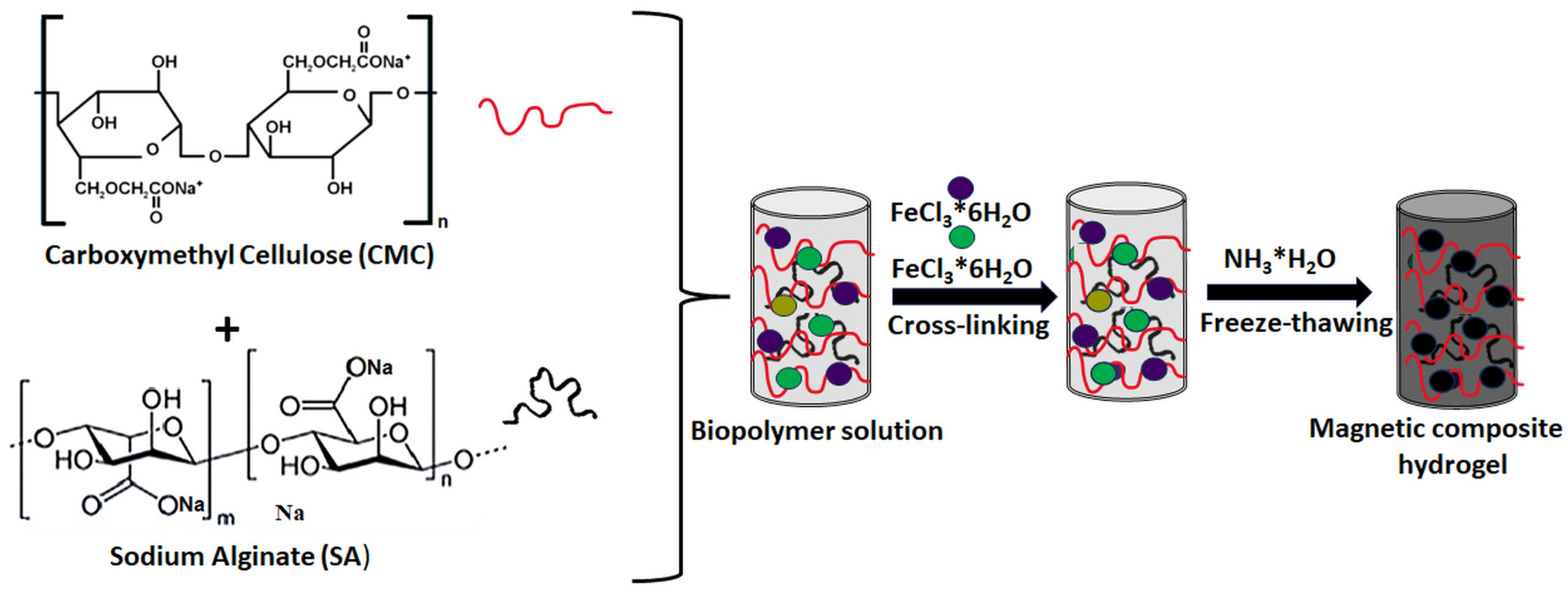
- Wu, S.; Guo, J.; Wang, Y.; Huang, C.; Hu, Y. Facile preparation of magnetic sodium alginate/carboxymethyl cellulose composite hydrogel for removal of heavy metal ions from aqueous solution. J. Mater. Sci. 2021, 56, 13096–13107. [Google Scholar] [CrossRef]
- Zhou, S.; Xie, Y.; Zhu, F.; Gao, Y.; Liu, Y.; Tang, Z.; Duan, Y. Amidoxime modified chitosan/graphene oxide composite for efficient adsorption of U(VI) from aqueous solutions. J. Environ. Chem. Eng. 2021, 9, 106363. [Google Scholar] [CrossRef]
- Ji, D.-B.; Yang, J.-L.; Wang, T.-Y.; Li, X.-R.; Li, G.-H.; Bai, Z.-H.; Yuan, D.-D.; Zhao, X.-F.; Wu, H.-J. Nano-ZnO enhanced amidoxime-functionalized sodium alginate composite hydrogel microspheres for uranium capture from wastewater. Sep. Purif. Technol. 2024, 338, 126568. [Google Scholar] [CrossRef]
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Raj, S.; Singh, H.; Bhattacharya, J. Treatment of textile industry wastewater based on coagulation-flocculation aided sedimentation followed by adsorption: Process studies in an industrial ecology concept. Sci. Total Environ. 2023, 857, 159464. [Google Scholar] [CrossRef]
- Li, M.; Wang, Z.; Li, B. Adsorption behaviour of congo red by cellulose/chitosan hydrogel beads regenerated from ionic liquid. Desalin. Water Treat. 2016, 57, 16970–16980. [Google Scholar] [CrossRef]
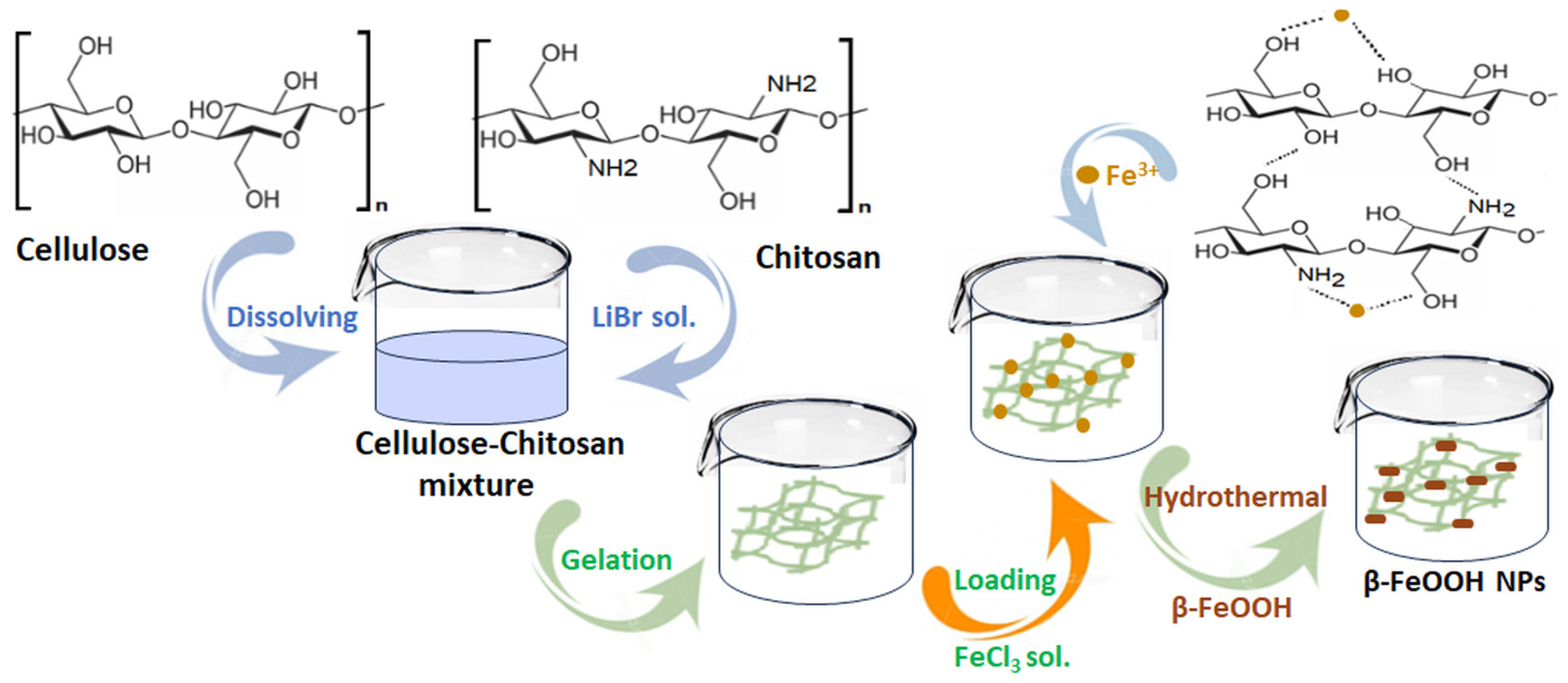
- Yang, X.; Ci, Y.; Zhu, P.; Chen, T.; Li, F.; Tang, Y. Preparation and characterization of cellulose-chitosan/β-FeOOH composite hydrogels for adsorption and photocatalytic degradation of methyl orange. Int. J. Biol. Macromol. 2024, 274, 133201. [Google Scholar] [CrossRef]
- Wang, W.; Hu, J.; Zhang, R.; Yan, C.; Cui, L.; Zhu, J. A pH-responsive carboxymethyl cellulose/chitosan hydrogelfor adsorption and desorption of anionic and cationic dyes. Cellulose 2021, 28, 897–909. [Google Scholar] [CrossRef]
- Meas, A.; Wi, E.; Chang, M.; Hwang, H.S. Carboxylmethyl cellulose produced from wood sawdust for improving properties of sodium alginate hydrogel in dye adsorption. Sep. Purif. Technol. 2024, 341, 126906. [Google Scholar] [CrossRef]
- Mohammed, N.; Grishkewich, N.; Berry, R.M.; Tam, K.C. Cellulose nanocrystal-alginate hydrogel beads as novel adsorbents for organic dyes in aqueous solutions. Cellulose 2015, 22, 3725–3738. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Q.; Zhu, S.; Yu, M. Green and facile fabrication of nano-ZnO coated cellulose/starch/activated carbon hydrogel for enhanced dyes adsorption and antibacterial activity. Mater. Today Commun. 2022, 33, 104355. [Google Scholar] [CrossRef]
- Ngwabebhoh, F.A.; Gazi, M.; Oladipo, A.A. Adsorptive removal of multi-azo dye from aqueous phase using a semi-IPN superabsorbent chitosan-starch hydrogel. Chem. Eng. Res. Des. 2016, 112, 274–288. [Google Scholar] [CrossRef]
- Cui, L.; Xiong, Z.; Guo, Y.; Liu, Y.; Zhao, J.; Zhang, C.; Zhu, P. Fabrication of interpenetrating polymer network chitosan/gelatin porous materials and study on dye adsorption properties. Carbohydr. Polym. 2015, 132, 330–337. [Google Scholar] [CrossRef]
- Peng, Q.; Liu, M.; Zheng, J.; Zhou, C. Adsorption of dyes in aqueous solutions by chitosan-halloysite nanotubes composite hydrogel beads. Micropor. Mesopor. Mater. 2015, 201, 190–201. [Google Scholar] [CrossRef]
- Cınar, S.; Kaynarb, Ü.H.; Aydemir, T.; Kaynar, S.C.; Ayvacıklı, M. An efficient removal of RB5 from aqueous solution by adsorption onto nano-ZnO/Chitosan composite beads. Int. J. Biol. Macromol. 2017, 96, 459–465. [Google Scholar] [CrossRef]
- Gonçalves, J.O.; Santos, J.P.; Rios, E.C.; Crispim, M.M.; Dotto, G.L.; Pinto, L.A.A. Development of chitosan based hybrid hydrogels for dyes removal fromaqueous binary system. J. Mol. Liq. 2017, 225, 265–270. [Google Scholar] [CrossRef]
- Murcia-Salvador, A.; Rodríguez-López, M.I.; Pellicer, J.A.; Gómez-Morte, T.; Aunón-Calles, D.; Yánez-Gascón, M.J.; Cerón-Carrasco, J.P.; Gil-Izquierdo, Á.; Núnez-Delicado, E.; Gabaldón, J.A. Development of Chitosan Polysaccharide-Based Magnetic Gel for Direct Red 83:1 Removal from Water. Gels 2024, 10, 496. [Google Scholar] [CrossRef]
- Ohemeng-Boahen, G.; Sewu, D.D.; Tran, H.N.; Woo, S.H. Enhanced adsorption of congo red from aqueous solution using chitosan/hematite nanocomposite hydrogel capsule fabricated via anionic surfactant gelation. Colloids Surf. A. 2021, 625, 126911. [Google Scholar] [CrossRef]
- Han, D.; Zhao, H.; Gao, L.; Qin, Z.; Ma, J.; Han, Y.; Jiao, T. Preparation of carboxymethyl chitosan/phytic acid composite hydrogels for rapid dye adsorption in wastewater treatment. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127355. [Google Scholar] [CrossRef]
- Far, B.F.; Naimi-Jamal, M.R.; Jahanbakhshi, M.; Khalafvandi, S.A.; Alian, M.; Jahromi, D.R. Decontamination of Congo red dye from aqueous solution using nanoclay/chitosan-graft-gelatin nanocomposite hydrogel. J. Mol. Liq. 2024, 395, 123839. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Bai, X.; Li, X.; Zhang, G.; Zou, J.; Fei, P.; Lai, W. Double network self-healing hydrogels based on carboxyethyl chitosan/oxidized sodium alginate/Ca2+: Preparation, characterization and application in dye adsorption. Int. J. Biol. Macromol. 2024, 264, 130564. [Google Scholar] [CrossRef]
- Mittal, H.; Alili, A.A.; Morajka, P.P.; Alhassan, S.M. GO crosslinked hydrogel nanocomposites of chitosan/carboxymethyl cellulose—A versatile adsorbent for the treatment of dyes contaminated wastewater. Int. J. Biol. Macromol. 2021, 167, 1248–1261. [Google Scholar] [CrossRef]
- Tu, H.; Yu, Y.; Chen, J.; Shi, X.; Zhou, J.; Deng, H.; Dua, Y. Highly cost-effective and high-strength hydrogels as dye adsorbents from natural polymers: Chitosan and cellulose. Polym. Chem. 2017, 8, 2913. [Google Scholar] [CrossRef]
- Wang, C.; Feng, X.; Shang, S.; Liu, H.; Song, Z.; Zhang, H. Lignin/sodium alginate hydrogel for efficient removal of methylene blue. Int. J. Biol. Macromol. 2023, 237, 124200. [Google Scholar] [CrossRef]
- Belhouchat, N.; Zaghouane-Boudiaf, H.; Viseras, C. Removal of anionic and cationic dyes from aqueous solution with activated organo-bentonite/sodium alginate encapsulated beads. Appl. Clay Sci. 2017, 135, 9–15. [Google Scholar] [CrossRef]
- Gan, L.; Li, H.; Chen, L.; Xu, L.; Liu, J.; Geng, A.; Mei, C.; Shang, S. Graphene oxide incorporated alginate hydrogel beads for the removal of various organic dyes and bisphenol A in water. Colloid Polym. Sci. 2018, 296, 607–615. [Google Scholar] [CrossRef]
- Mittal, H.; Maity, A.; Ray, S.S. Effective removal of cationic dyes from aqueous solution using gum ghatti-based biodegradable hydrogel. Int. J. Biol. Macromol. 2015, 79, 8–20. [Google Scholar] [CrossRef]
- Zhang, P.; Raza, S.; Cheng, Y.; Claudine, U.; Hayat, A.; Bashir, T.; Ali, T.; Ghasali, E.; Orooji, Y. Fabrication of maleic anhydride-acrylamide copolymer based sodium alginate hydrogel for elimination of metal ions and dyes contaminants from polluted water. Int. J. Biol. Macromol. 2024, 261, 129146. [Google Scholar] [CrossRef] [PubMed]
- Malatji, N.; Makhado, E.; Modibane, K.D.; Ramohlola, K.E.; Maponya, T.C.; Monama, G.R.; Hato, M.J. Removal of methylene blue from wastewater using hydrogel nanocomposites. Nanomater. Nanotechnol. 2021, 11, 1–27. [Google Scholar] [CrossRef]
- Turkmen, D.; Bakhshpour, M.; Akgonullu, S.; Asir, S.; Denizli, A. Heavy metal ions removal from wastewater using cryogels: A review. Front. Sustain. 2022, 3, 765592. [Google Scholar] [CrossRef]
- Yu, C.; Wang, F.; Zhang, C.; Fu, S.; Lucia, L.A. The synthesis and adsorption dynamics of lignin-based hydrogel for remediation of cationic dye-contaminated effluent. React. Funct. Polym. 2016, 106, 137–142. [Google Scholar] [CrossRef]
- Krishnappa, P.B.; Badalamoole, V. Karaya gum-graft-poly(2-(dimethylamino)ethyl methacrylate) gel: An efficient adsorbent for removal of ionic dyes from water. Int. J. Biol. Macromol. 2019, 122, 997–1007. [Google Scholar] [CrossRef]
- Bagheri, N.; Lakouraj, M.M.; Hasantabar, V.; Mohseni, M. Biodegradable macro-porous CMC-polyaniline hydrogel: Synthesis, characterization and study of antimicrobial elimination and sorption capacity of dyes from waste water. J. Hazard. Mater. 2021, 403, 123631. [Google Scholar] [CrossRef]
- Li, F.; Miao, G.; Gao, Z.; Xu, T.; Zhu, X.; Miao, X.; Song, Y.; Ren, G.; Li, X. A versatile hydrogel platform for oil/water separation, dye adsorption, and wastewater purification. Cellulose 2022, 29, 4427–4438. [Google Scholar] [CrossRef]
- Huang, Q.; Zhou, Y.; Fu, Z.; Zhu, J. Preparation of an injectable hydrogel reinforced by graphene oxide and its application in dye wastewater treatment. J. Mater. Sci. 2023, 58, 3117–3133. [Google Scholar] [CrossRef]
- Zhang, F.; Guo, Y.; Wu, X.; Gao, C.; An, Q.; Tian, Z.; Sun, R. Preparation of a novel lactose-lignin hydrogel catalyst with self-reduction capacity for nitrogeneous wastewater treatment. Front. Chem. Sci. Eng. 2024, 18, 99. [Google Scholar] [CrossRef]
- Zamani-Babgohari, F.; Irannejad, A.; Pour, M.K.; Khayati, G.R. Synthesis of carboxymethyl starch co(polyacrylamide/polyacrylic acid) hydrogel for removing methylene blue dye from aqueous solution. Int. J. Biol. Macromol. 2024, 269, 132053. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Gulcan, H.O.; Saber-Samandari, S.; Gazi, M. Efficient removal of anionic cationic dyes from an aqueous solution using pullulan-graft-polyacrylamide porous hydrogel. Water Air Soil Pollut. 2014, 225, 2177. [Google Scholar] [CrossRef]
- Qian, H.; Wang, J.; Yan, L. Synthesis of lignin-poly(N-methylaniline)-reduced graphene oxide hydrogel for organic dye and lead ions removal. J. Bioresour. Bioprod. 2020, 5, 204–210. [Google Scholar] [CrossRef]
- Gong, G.; Zhang, F.; Cheng, Z.; Zhou, L. Facile fabrication of magnetic carboxymethyl starch/poly(vinyl alcohol) composite gel for methylene blue removal. Int. J. Biol. Macromol. 2015, 81, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.; Seiffert, S. Composite hydrogels based on calcium alginate and polyethyleneimine for wastewater treatment. J. Polym. Sci. 2023, 61, 2203–2222. [Google Scholar] [CrossRef]
- Qi, X.; Wei, W.; Su, T.; Zhang, J.; Dong, W. Fabrication of a new polysaccharide-based adsorbent for water purification. Carbohydr. Polym. 2018, 195, 368–377. [Google Scholar] [CrossRef]
- Toledo, P.V.O.; Limeira, D.P.C.; Siqueira, N.C.; Petri, D.F.S. Carboxymethyl cellulose/poly(acrylic acid) interpenetrating polymer network hydrogels as multifunctional adsorbents. Cellulose 2019, 26, 597–615. [Google Scholar] [CrossRef]
- Rahman, O.; Halim, A.; Deb, A.; Ahmed, S.; Rahman, W.; Dafader, N.C.; Alam, S.M.N.; Khandaker, S.; Alam, J. Modification of superabsorbent hydrogels for industrial wastewater treatment. Adv. Polym. Technol. 2022, 2022, 8405230. [Google Scholar] [CrossRef]
- Getya, D.; Lucas, A.; Gitsov, I. Composite hydrogels based on poly(ethylene glycol) and cellulose macromonomers as fortified materials for environmental cleanup and clean water safeguarding. Int. J. Mol. Sci. 2023, 24, 7558. [Google Scholar] [CrossRef]
- Liu, Z.; Li, D.; Dai, H.; Huang, H. Enhanced properties of tea residue cellulose hydrogels by addition of graphene oxide. J. Mol. Liq. 2017, 244, 110–116. [Google Scholar] [CrossRef]
- Tao, E.; Yang, S.; Hao, X. Graphene oxide-montmorillonite/sodium alginate aerogel beads for selective adsorption of methylene blue in wastewater. J. Alloys Compd. 2020, 832, 154833. [Google Scholar] [CrossRef]
- Mittal, H.; Kumar, V.; Alhassan, S.M.; Ray, S.S. Modification of gum ghatti via grafting with acrylamide and analysis of its flocculation, adsorption, and biodegradation properties. Int. J. Biol. Macromol. 2018, 114, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.; Ghasali, E.; Hayat, A.; Zhang, P.; Orooji, Y.; Lin, H. Sodium alginate hydrogel-encapsulated trans-anethole based polymer: Synthesis and applications as an eradicator of metals and dyes from wastewater. Int. J. Biol. Macromol. 2024, 254, 127153. [Google Scholar] [CrossRef] [PubMed]
- Mittal, H.; Maity, A.; Ray, S.S. Gum karaya based hydrogel nanocomposites for the effective removal of cationic dyes from aqueous solutions. Appl. Surf. Sci. 2016, 364, 917–930. [Google Scholar] [CrossRef]
- Bardajee, G.R.; Mahmoodian, H.; Boraghi, S.A.; Elmizadeh, H.; Ziarani, N.B.; Rezanejad, Z.; Tyagi, I.; Gaur, R.; Javadian, H. Nanoporous hydrogel adsorbent based on salep: Swelling behavior and methyl orange adsorption capacity. Environ. Res. 2023, 225, 115571. [Google Scholar] [CrossRef]
- Goddeti, S.R.M.; Bhaumik, M.; Maity, A.; Ray, S.S. Removal of Congo red from aqueous solution by adsorption using gum ghatti and acrylamide graft copolymer coated with zero valent iron. Int. J. Biol. Macromol. 2020, 149, 21–30. [Google Scholar] [CrossRef]
- Tang, Y.; Zeng, Y.; Hu, T.; Zhou, Q.; Peng, Y. Preparation of lignin sulfonate-based mesoporous materials for adsorbing malachite green from aqueous solution. J. Environ. Chem. Eng. 2016, 4, 2900–2910. [Google Scholar] [CrossRef]
- Aljeboree, A.M.; Hasan, I.T.; Al-Warthan, A.; Alkaim, A.F. Preparation of sodium alginate-based SA-g-poly(ITA-co-VBS)/RC hydrogel nanocomposites: And their application towards dye adsorption. Arab. J. Chem. 2024, 17, 105589. [Google Scholar] [CrossRef]
- Sharma, B.; Thakur, S.; Mamba, G.; Prateek; Gupta, R.K.; Gupta, V.K.; Thakur, V.K. Titania modified gum tragacanth based hydrogel nanocomposite for water remediation. J. Environ. Chem. Eng. 2021, 9, 104608. [Google Scholar] [CrossRef]
- Salzano de Luna, M.; Castaldo, R.; Altobelli, R.; Gioiella, L.; Filippone, G.; Gentile, G.; Ambrogi, V. Chitosan hydrogels embedding hyper-crosslinked polymer particles as reusable broad-spectrum adsorbents for dye removal. Carbohydr. Polym. 2017, 177, 347–354. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Zarkhaneh, S.I.; Foroughi, M.; Foroutan, R.; Azimi, H.; Ramavandi, B. Effectiveness of polyacrylamide-g-gelatin/ACL/Mg-Fe LDH composite hydrogel as an eliminator of crystal violet dye. Environ. Res. 2024, 258, 119428. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, H.; Wang, Y.; Chen, X.; Zhao, C.; An, Y.; Tang, X. A novel carboxyl-rich chitosan-based polymer and its application for clay flocculation and cationic dye removal. Sci. Total Environ. 2018, 640–641, 107–115. [Google Scholar] [CrossRef]
- Wu, L.; Shi, M.; Guo, R.; Dong, W. Development of a novel pullulan/polydopamine composite hydrogel adsorbent for dye removal. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129632. [Google Scholar] [CrossRef]
- Mohamed, R.R.; Abu Elella, M.H.; Sabaa, M.W.; Saad, G.R. Synthesis of an efficient adsorbent hydrogel based on biodegradable polymers for removing crystal violet dye from aqueous solution. Cellulose 2018, 25, 6513–6529. [Google Scholar] [CrossRef]
- Ullah, B.; Alam, S.; Shah, L.A.; Zahoor, M.; Umar, M.N.; Ullah, R.; Ali, E.A. Synthesis, characterization and adsorption studies of hydroxyl ethyl cellulose grafted polyacrylic acid hydrogels; using basic yellow-28 as model dye. Desalination Water Treat. 2024, 318, 100360. [Google Scholar] [CrossRef]
- Mandal, B.; Kumar, R. Removal of safranine T and brilliant cresyl blue dyes from water by carboxy methyl cellulose incorporated acrylic hydrogels: Isotherms, kinetics and thermodynamic study. J. Taiwan Inst. Chem. Eng. 2016, 60, 313–327. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. Npj Clean Water. 2021, 4, 36. [Google Scholar] [CrossRef]
- Ying, Z.; Yu, C.; Jian, Z.; Zongrui, T.; Shaoghua, J. Preparation of SA-g-(PAA-co-PDMC) polyampholytic superabsorbent polymer and its application to the anionic dye adsorption removal from effluents. Sep. Purif. Technol. 2017, 188, 329–340. [Google Scholar] [CrossRef]
- Kono, H.; Ogasawara, K.; Kusumoto, R.; Oshima, K.; Hashimoto, H.; Shimizu, Y. Cationic cellulose hydrogels cross-linked by poly(ethylene glycol): Preparation, molecular dynamic, and adsorption of anionic dyes. Carbohydr. Polym. 2016, 152, 170–180. [Google Scholar] [CrossRef]
- Skoric, M.L.; Terzic, I.; Milosavljevic, N.; Radetic, M.; Saponjic, Z.; Radoicic, M.; Kalagasidis Krusic, M. Chitosan-based microparticles for immobilization of TiO2 nanoparticles and their application for degradation of textile dyes. Eur. Polym. J. 2016, 82, 57–70. [Google Scholar] [CrossRef]
- Becalli Vilela, P.; Dalalibera, A.; Costa Duminelli, E.; Becegato, V.A.; Paulino, A.T. Adsorption and removal of chromium (VI) contained in aqueous solutions using a chitosan-based hydrogel. Environ. Sci. Pollut. Res. 2019, 26, 28481–28489. [Google Scholar] [CrossRef]
- Nowruzi, R.; Heydari, M.; Javanbakht, V. Synthesis of a chitosan/polyvinyl alcohol/activate carbon biocomposite for removal of hexavalent chromium from aqueous solution. Int. J. Biol. Macromol. 2020, 147, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zou, K.; Yan, L.; Liu, J.; Liu, B.; Qing, T.P.; Feng, B. A biomass resource strategy for alginate-polyvinyl alcohol double network hydrogels and their adsorption to heavy metals. Sep. Purif. Technol. 2022, 301, 122050. [Google Scholar] [CrossRef]
- Haque, O.; Mondal, I.H. Synthesis and characterization of cellulose-based eco-friendly hydrogels. Rajshahi Univ. J. Sci. Eng. 2016, 44, 45–53. [Google Scholar] [CrossRef]
- Darban, Z.; Shahabuddin, S.; Gaur, R.; Ahmad, I.; Sridewi, N. Hydrogel-based adsorbent material for the effective removal of heavy metals from wastewater: A comprehensive review. Gels 2022, 8, 263. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, L.; Tan, B.; Li, J.; Gao, X.; He, Y.; Du, X.; Zhang, W.; Wang, W. Adsorption behavior of heavy metal ions from aqueous solution onto composite dextran-chitosan macromolecule resin adsorbent. Int. J. Biol. Macromol. 2019, 141, 738–746. [Google Scholar] [CrossRef]
- Jamaluddin, S.S.; Ghazi, R.M.; Yusoff, N.R.M.; Yusoff, S.F.M. Adsorption of Cu2+ ions using rubber-based hydrogel. BIO Web Conf. 2023, 73, 05003. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, Y.; Wu, Y.; Li, S.; Yin, H.; Wang, J. α-ketoglutaric acid modified chitosan/polyacrylamide semi-interpenetrating polymer network for removal of heavy metal ions. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127262. [Google Scholar] [CrossRef]
- Zheng, Y.; Hua, S.; Wang, A. Adsorption behavior of Cu2+ from from aqueous solutions onto starch-g-poly(acrylic acid)/sodium humate hydrogels. Desalination 2010, 263, 170–175. [Google Scholar] [CrossRef]
- Apopei, D.F.; Dinu, M.V.; Trochimczuk, A.W.; Drăgan, E.S. Sorption isotherms of heavy metal ions onto semi-interpenetrating polymer network hydrogels based on polyacrylamide and anionically modified potato starch. Ind. Eng. Chem. Res. 2012, 51, 10462–10471. [Google Scholar] [CrossRef]
- Dolgormaa, A.; Lv, C.-J.; Li, Y.; Yang, J.; Yang, J.-X.; Chen, P.; Wang, H.-P.; Huang, J. Adsorption of Cu (II) and Zn (II) ions from aqueous solution by Gel/PVA-modified super-paramagnetic iron oxide nanoparticles. Molecules 2018, 23, 2982. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, L.; Fu, S.; Zheng, L.; Zhan, H. Adsorption behavior of Cd2+, Pb2+, and Ni2+ from aqueous solutions on cellulose-based hydrogels. Bioresources 2012, 7, 2752–2765. [Google Scholar] [CrossRef]
- Jafarigol, E.; Ghotli, R.A.; Hajipour, A.; Pahlevani, H.; Salehi, M.B. Tough dual-network GAMAAX hydrogel for efficient removal of cadmium and nickel ions in wastewater treatment applications. J. Ind. Eng. Chem. 2021, 94, 352–360. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, G.; Chu, L.; Liu, Y.; Liu, C.; Luo, S.; Wei, Y. Efficient removal of heavy metal ions with an EDTA functionalized chitosan/polyacrylamide double network hydrogel. ACS Sustain. Chem. Eng. 2017, 5, 843–851. [Google Scholar] [CrossRef]
- Astrini, N.; Anah, L.; Haryadi, H.R. Adsorption of heavy metal ion from aqueous solution by using cellulose based hydrogel composite. Macromol. Symp. 2015, 353, 191–197. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, Y.; Wang, F.; Wang, A. Fabrication of magnetic macroporous chitosan-g-poly(acrylic acid) hydrogel for removal of Cd2+ and Pb2+. Int. J. Biol. Macromol. 2016, 93, 483–492. [Google Scholar] [CrossRef]
- Huang, S.; Liu, X.; Hu, Q.; Wei, T.; Wang, J.; Chen, H.; Wu, C. Temperature-driven metalloprotein-based hybrid hydrogels for selective and reversible removal of cadmium (II) from water. ACS Appl. Mater. Interfaces 2020, 12, 2991–2998. [Google Scholar] [CrossRef]
- Hu, T.; Liu, B.N.; Bu, H.; Hu, H.J.; Zhu, Q.S.; Tang, S.; Li, Y.; Wang, J.; Jiang, G.B. Self-separating core-shell spheres with a carboxymethyl chitosan/acrylic acid/Fe3O4 composite core for soil Cd removal. Carbohydr. Polym. 2024, 343, 122428. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, C.; Chu, L.; Tang, Y.; Luo, S. Rapid and efficient treatment of wastewater with high-concentration heavy metals using a new type of hydrogel-based adsorption process. Bioresour. Technol. 2016, 219, 451–457. [Google Scholar] [CrossRef]
- Khademi, F.; Salehi, M.B.; Mortaheb, H.R.; Nozaeim, A.A.; Ahmadi, S.H. Design and fabrication of co([CHITOSAN-AMPS-AA]/PEI-MBA) nanocomposite hydrogel as an effective solution for removing tin and platinum ions in wastewater treatment applications: Selective recovery of platinum. J. Environ. Polym. Degrad. 2024, 32, 6011–6028. [Google Scholar] [CrossRef]
- El-saied, H.A.A.; Motawea, E.A.T. Optimization and adsorption behavior of nanostructured NiFe2O4/poly AMPS grafted biopolymer. J. Polym. Environ. 2020, 28, 2335–2351. [Google Scholar] [CrossRef]
- Weerasundara, L.; Gabriele, B.; Figoli, A.; Ok, Y.-S.; Bundschuh, J. Hydrogels: Novel materials for contaminant removal in water-A review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 1970–2014. [Google Scholar] [CrossRef]
- Sivagangi Reddy, N.; Krishna Rao, K.S.V. Polymeric Hydrogels: Recent Advances in Toxic Metal Ion Removal and Anticancer Drug Delivery Applications. Int. J. Adv. Chem. Sci. 2016, 4, 214–234. [Google Scholar]
- Zhang, M.; Yu, X.; Zhu, M.; Xiang, A.; Bai, Y.; Zhou, H. Adsorptive behaviors and mechanisms for removing three organic pollutants from aqueous solutions by polyvinyl alcohol/porous carbon composite hydrogels. J. Environ. Chem. Eng. 2023, 11, 111095. [Google Scholar] [CrossRef]
- Lotfi, M.; Bahram, M.; Moghadam, P.N. The study of the removal of penconazole fungicide from surface water using carboxymethyl tragacanth-based hydrogel grafted with poly(acrylic acid-co-acrylamide). Sci. Rep. 2023, 13, 13569. [Google Scholar] [CrossRef]
- Li, I.-C.; Chen, Y.-H.; Chen, Y.-C. Adsorption properties of ammonium nitrogen from aqueous solutions using sodium humate/poly(sodium acrylate) hydrogel. J. Taiwan Inst. Chem. Eng. 2024, 161, 105516. [Google Scholar] [CrossRef]
- Wang, H.; Chen, X.; Gong, C.; Bu, Y.; Wu, T.; Yan, H.; Lin, Q. Intelligent response organo-montmorillonite/Fe3+-alginate/poly(N-isopropylacrylamide) interpenetrating network composite hydrogels for controlled release of water-insoluble pesticides. Appl. Clay Sci. 2024, 251, 107302. [Google Scholar] [CrossRef]
- Kunori, M.; Tokuyama, H. Development of a polyethyleneimine/poly(N-isopropylacrylamide) semi-IPN hydrogel for use in the temperature-swing adsorption and selective desorption of hydrophobic organic compounds. J. Taiwan Inst. Chem. Eng. 2024, 156, 105331. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Y.; Zhong, L.; Feng, Q.; Dong, Z.; Xu, Z. Dialdehyde modified and cationic aerogel for efficient microplastics adsorption from environmental waters. Int. J. Biol. Macromol. 2024, 256, 128326. [Google Scholar] [CrossRef]
- Madhuranthakam, C.M.R.; Alsubaei, A.; Elkamel, A. Performance of polyacrylamide and poly(acrylamide/sodium acrylate) hydrogel-coated mesh for separation of oil/water mixture. J. Water Process Eng. 2018, 26, 62–71. [Google Scholar] [CrossRef]
- Drinking Water Adsorbents Market Size, Share & Trends Analysis Report by Product (Activated Carbon, Zeolite, Alumina, Clay, Manganese Oxide, Cellulose), by Region, and Segment Forecasts. 2019–2025. Available online: https://www.grandviewresearch.com/industry-analysis/drinking-water-adsorbents-market (accessed on 12 February 2025).
- Ighalo, J.O.; Omoarukhe, F.O.; Ojukwu, V.E.; Iwuozor, K.O.; Igwegbe, C.A. Cost of adsorbent preparation and usage in wastewater treatment: A review. Clean. Chem. Eng. 2022, 3, 100042. [Google Scholar] [CrossRef]
- Hydrogel Market Size, Share, Competitive Landscape and Trend Analysis Report, by Raw Material Type, by Composition, by Form, by Product, by Application: Global Opportunity Analysis and Industry Forecast. 2024–2033. Available online: https://www.alliedmarketresearch.com/hydrogel-market (accessed on 12 February 2025).
- Urban Wastewater. Available online: https://environment.ec.europa.eu/topics/water/urban-wastewater_en (accessed on 12 February 2025).
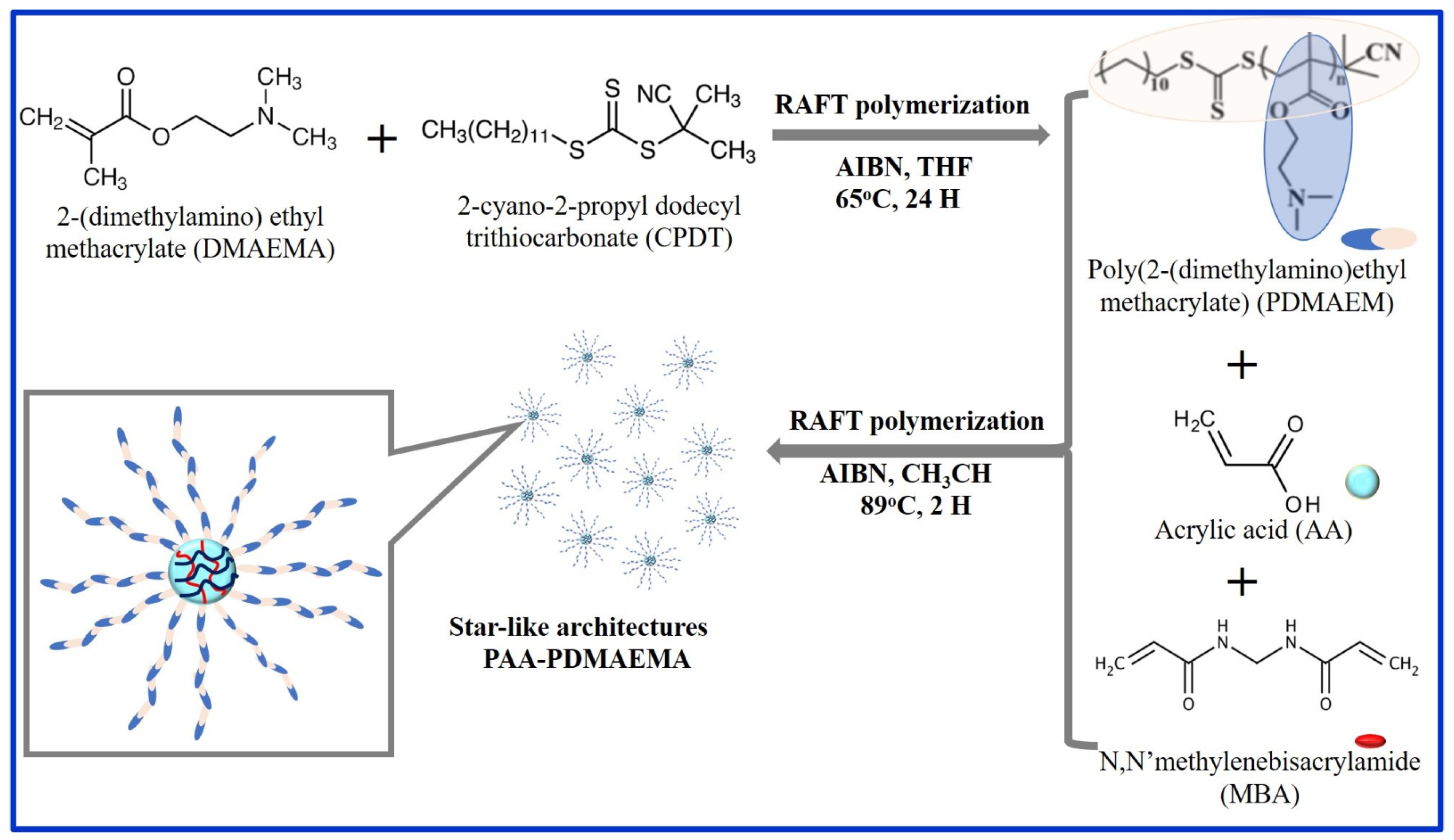
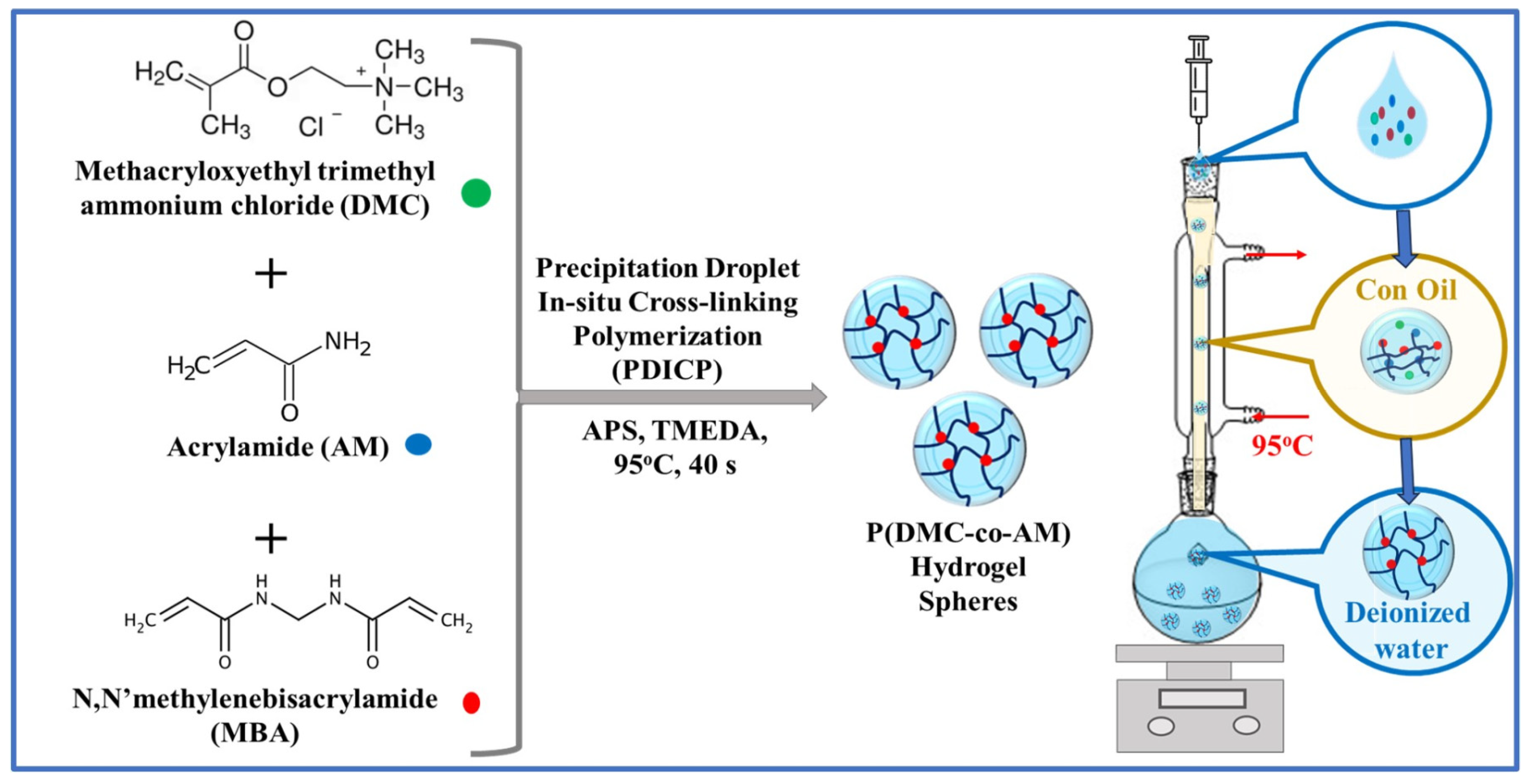

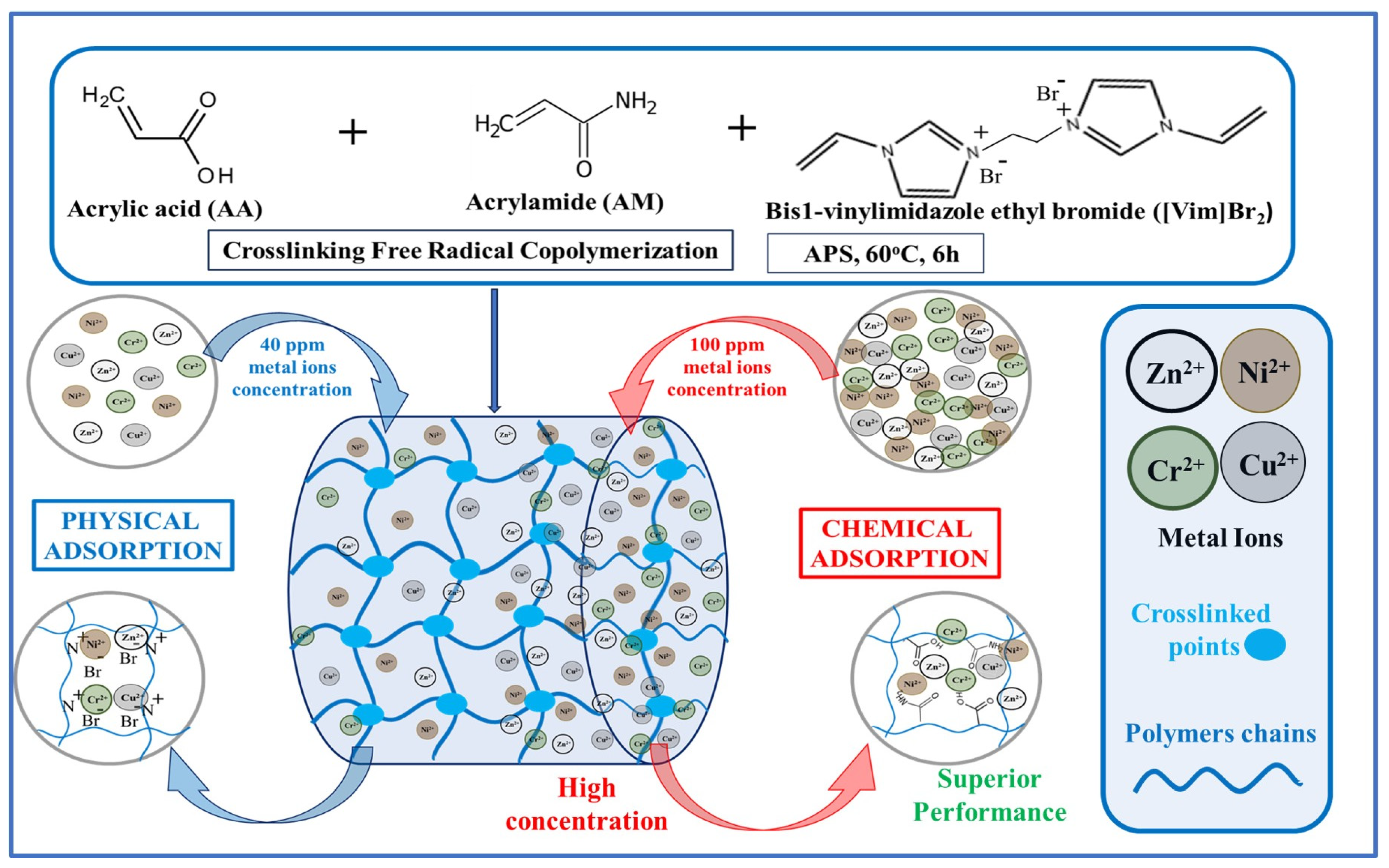
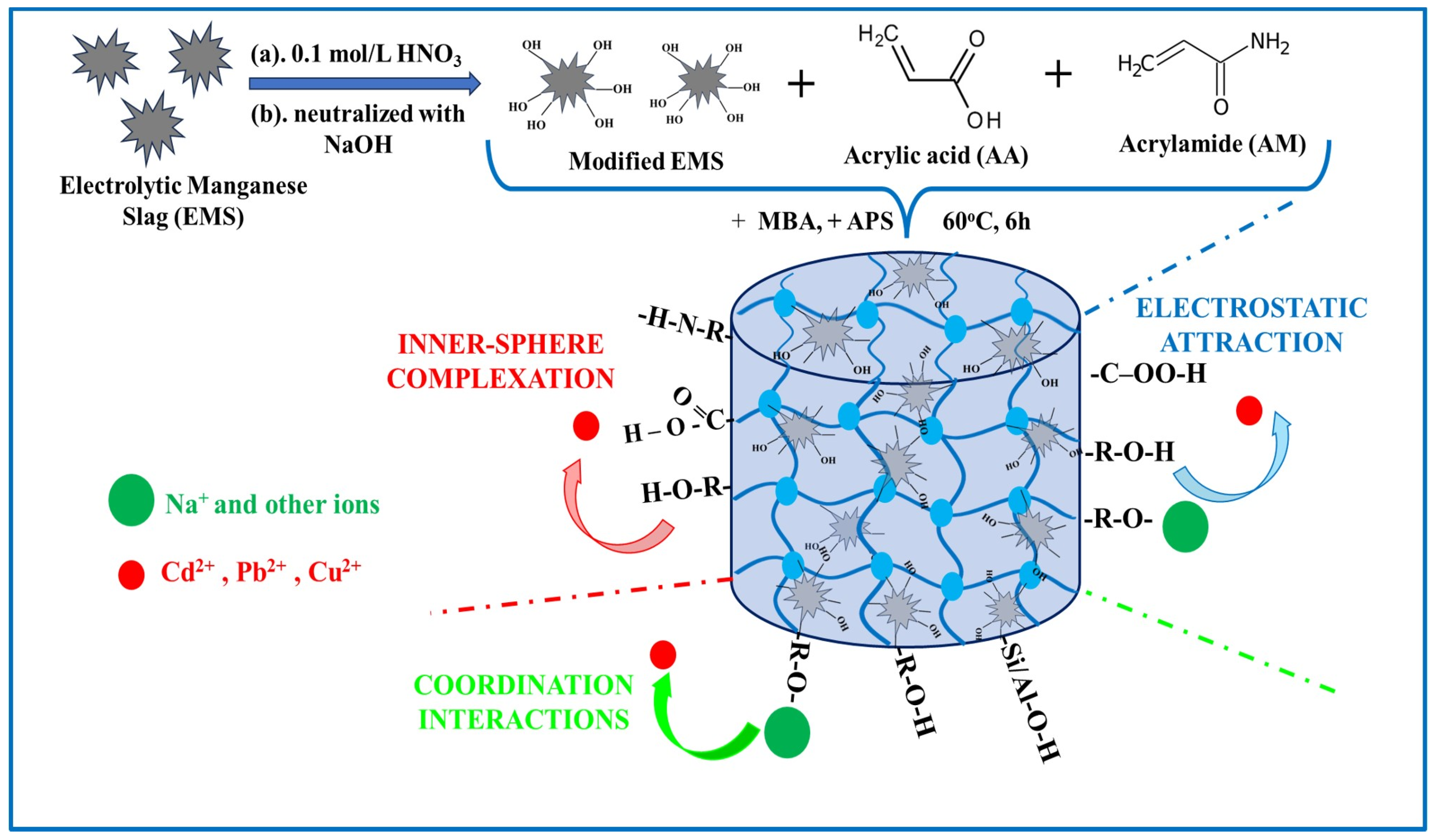
| Dyes | Hydrogel-Based Materials | Preparation Method | Performance | Regeneration and Reusability | Ref. |
|---|---|---|---|---|---|
| MB cationic dye RBBR anionic dye | - Monomers: PCL and HEMA - Initiator: AIBN | - by radical copolymerization | - maximum swelling equilibrium (%)—929% - removal efficiency (%)—95% cationic dyes (MB) and 40% anionic dyes (RBBR) - adsorption capacity (Qmax, mg/g)—521.39 mg/g for MB (24 h, ci = 100 mg/L, pH = 12) and 197.84 mg/g for RBBR (24 h, c0 = 100 mg/L, pH = 2) - best-fit kinetic—pseudo-second-order kinetic model (R2 = 0.992) - best fit isotherm—Langmuir isotherm (R2 = 0.999) - negative ΔG0 values; positive ΔH0 and ΔS0 values indicate that MB and RBBR adsorption spontaneous and thermodynamically favorable | - desorption eluent: HCl and NaOH solutions - 10th regeneration run - 10th reusable run with 80% removal efficiency | [44] |
| MV cationic dye | - Monomers: AM, IA - Polymer: PEG1500 - Crosslinker: NMBA - Initiator: KPS | - by free-radical crosslinking copolymerization | - maximum swelling equilibrium (%)—4500% - removal efficiency (%):— - adsorption capacity (Qmax, mg/g)—28 mg/L*gm - best fit kinetic:— - best fit isotherm:— - thermodynamic:— | - | [81] |
| EB, ARS anionic dyes CV cationic dye | - Monomers: AA and HEMA - Crosslinker: ILA: 1VIM and 4VBC; - ILB: 1VIM and 4VBC and DMAEMA our NMBA - Initiator: APS andTEMED | - by redox polymerization technique using a redox initiator | - maximum swelling equilibrium (%)—929% - removal efficiency (%)—99.8% for anionic dyes (EB) and 72.4% for cationic dyes (CV) - adsorption capacity (Qmax, mg/g)—119 mg/g (48 h, c0 = 250 mg/L, pH = 7) - best-fit kinetic—pseudo-second-order kinetic model (R2 ≥ 0.997) - best-fit isotherm—Langmuir isotherm (R2 = 0.999) - thermodynamic equilibrium constants:— | - desorption eluent: HCl and NaOH solutions - 3rd regeneration run - 3rd reusable run with >80% removal efficiency | [82] |
| MB, AO and CG cationic dyes | - Monomers: AA, NIPAAM, N-allylisatin - Crosslinker: EGDMA - Initiator: AIBN | - by inverse microemulsion polymerization, free-radical polymerization | - maximum swelling equilibrium (%)—36,790% - removal efficiency (%)—98.24% (MB), 87.42% (AO), and 70.12% (CG) - adsorption capacity (Qmax, mg/g)—584.78 mg/g (MB), 347.17 mg/g (AO), and 212.69 mg/g (CG) (48 h, c0 = 250 mg/L, natural pH value of dye solutions) - best-fit kinetic:— - best-fit isotherm—Langmuir and Freundlich isotherm - (R2 > 1), the kinetic and equilibrium adsorption isotherms failed to explain the adsorption process due to the extremely small size - thermodynamic:— | - desorption eluent HCl our NaOH, washed with bidistilled water -2nd regeneration cycles with percent desorption efficiency 100 (MB), 92 (AO), and 86 (CG) (%); -2nd reusable cycles | [83] |
| CCA anionic dye | - Monomers: AM, APTMACl - Crosslinker: PEGDMA - Initiator system: APS, TEMED | - by free-radical crosslinking copolymerization | - maximum swelling equilibrium (%)—1198% - removal efficiency (%)—99% - adsorption capacity (Qmax, mg/g)—79.42 mg/g (48 h, c0 = 200 mg/L, pH = 7) - best-fit kinetic:— - best-fit isotherm—Langmuir (R2 = 0.9952)- thermodynamic: — | - | [84] |
| MB cationic dye MO anionic dye | - Monomers: AA - Crosslinker: NMBA - Initiator: APS | - by free-radical polymerization | - maximum swelling equilibrium:— - removal efficiency (%)—99.99% for MB and 17.5% for MO - adsorption capacity (Qmax, mg/g)—454.45 mg/g for MB (58 h, c0 = 1500 mg/L, pH = 8) - best-fit kinetic—pseudo-first-order model (R2 = 0.9946) for MB - best-fit isotherm—Redlich–Peterson and Sips isotherm models (R2 = 0.998) for MB - thermodynamic equilibrium constants—ΔG0 = −3.7 kJ/mol; ΔH0 = 65.6 kJ/mol, ΔS0 = 221.4 J/K*mol for MB, thermodynamically spontaneous, favorable, and endothermic | - desorption eluent: inorganic acid solutions - 5th regeneration run - 5th reusable run with >87% removal efficiency | [85] |
| MB cationic dye | - Monomers: AA and AM - Crosslinker: NMBA - Initiator:APS | - by free-radical polymerization | - removal efficiency (%)—90% - adsorption capacity (Qmax, mg/g)—1315 mg/g (12 h, c0 = 50 mg/L, pH = 8) - best-fit kinetic—pseudo-second-order model (R2 = 0.99) - best-fit isotherm—Dubinin–Radushkevich model (R2 = 0.99) - thermodynamic equilibrium constants: ΔG0 = −40 kJ/mol; ΔH0 = −51 kJ/mol, ΔS0 = −51 kJ/K*mol; adsorption with an endothermic chemical integration | - desorption eluent: NaNO3, washed with bidistilled water and dried - 20th regeneration run - 21st reusable run | [86] |
| MB cationic dye | - Monomers: IA, EG, AA - Crosslinker: NMBA - Initiator: APS | - by free-radical polymerization | - maximum swelling equilibrium (%)—600% - removal efficiency (%)—83% - adsorption capacity (Qmax, mg/g)—1270 mg/g - best-fit kinetic:— - best-fit isotherm:— - thermodynamic equilibrium constants:— | - | [87] |
| RhB cationic dye | - Monomers: IA, AA, and ANi - Crosslinker: NMBA - Initiator: KPS | - by free-radical crosslinking copolymerization | - maximum swelling equilibrium (%)—1755.3% - removal efficiency (%)—87.9% - adsorption capacity (Qmax, mg/g)—925.92 mg/g for MB (60 min, c0 = 50 mg/L, pH = 7) - best-fit kinetic—pseudo-first-order model (R2 = 0.92) for MB - best-fit isotherm—Freundlich isotherms (R2 = 0.996) - thermodynamic equilibrium constants:— | - desorption eluent: Cl and NaOH solutions - 4th regeneration run - 4th reusable run with >85% removal efficiency | [88] |
| MB cationic dye MO anionic dye | - Monomers: AA and ACN - Homopolymer: PDMAEMA - Crosslinker: NMBA - Initiator: AIBN | - by combining distillation precipitation polymerization and RAFT polymerization | - maximum swelling equilibrium:— - removal efficiency (%)—76.6% for MO and 48.3% for MB - adsorption capacity (Qmax, mg/g)—54.4 mg/g for MO (c0 = 12 mg/L, pH = 2) and 35.9 mg/g for MB (ci = 12 mg/L, pH = 10) - best-fit kinetic—pseudo-second-order kinetic model (R2 =0.99) - best-fit isotherm—Freundlich model isotherm (R2 = 0.999) | - | [89] |
| MB cationic dye | - Monomers: AM, AA, and AHPS - Crosslinker: PTE - Initiator: BzO2 | - by free-radical polymerization | - maximum swelling equilibrium (%)—7071%- removal efficiency (%):— - adsorption capacity (Qmax, mg/g)—79.42 mg/g (8 h, c0 = 100 mg/L, pH = 7) - best-fit kinetic— pseudo-second-order kinetic model (R2 = 0.999). - best-fit isotherm— Freundlich isotherm (R2 =0.957) - thermodynamic:— | - | [90] |
| CV cationic dye CR anionic dye | - Monomers: APTMACl and AMPSA - Crosslinker: PEGDMA - Initiator system: APS and TEMED | - by free-radical crosslinking copolymerization | - maximum swelling equilibrium (%)—3715% - removal efficiency (%)—98% for CV and 86% for CR - adsorption capacity (Qmax, mg/g)—13.6 mg/g for CV (14 h, c0 = 50 mg/L, pH = 9) and 9.07 mg/g for CR (14 h, c0 = 50 mg/L, pH = 5) - best-fit kinetic—pseudo-second-order kinetics model (R2 = 0.9930) - best-fit isotherm—Freundlich isotherms (R2 = 0.98) - thermodynamic equilibrium constants— ΔG0 = −5.869 and −0.54 kJ/mol; ΔH0 = 63.93 and 66.09 kJ/mol, ΔS0 = 0.23 and 0.22 J/K*mol for CV and CR, respectively | - with acetone via the solvent extraction method - 5th regeneration run - 5th reusable run with >75% removal efficiency | [91] |
| MB cationic dye RBBR anionic dye | - Monomers: PCL and HEMA - Initiator: AIBN | - by radical copolymerization | - maximum swelling equilibrium (%)—929% - removal efficiency (%)—95% cationic dyes (MB) and 40% anionic dyes (RBBR) - adsorption capacity (Qmax, mg/g)—521.39 mg/g for MB (100 min, c0 = 50 mg/L, pH = 6) and 197.84 mg/g for RBBR (100 min, c0 = 50 mg/L, pH = 6) - best-fit kinetic— pseudo-second-order kinetic model (R2 = 0.992) - best-fit isotherm—Langmuir isotherm (R2 = 0.999) - negative ΔG0 values; positive ΔH0 and ΔS0 values indicate that MB and RBBR adsorption is spontaneous and thermodynamically favorable | - desorption eluent: HCl and NaOH solutions - 10th regeneration run - 10th reusable run with 80% removal efficiency | [92] |
| MO CR CCB anionic dye | - Monomers: PQ-10 - Crosslinker: PEGDMA | - by electron beam irradiation crosslinking technique | - maximum swelling equilibrium (%)—704%- removal efficiency (%):— - adsorption capacity (Qmax, mg/g)—628.93% for MO; 427.35% for CR; and 628.93% for CBR (24 h, c0 = 25 mg/L, pH = 8) - best-fit kinetic—kinetic pseudo-second-order model (R2 = 0.992) - best-fit isotherm—Langmuir isotherm (R2 = 0.999) | - desorption eluent: HCl solutions - 5th regeneration run - 5th reusable run with 86% removal efficiency | [93] |
| MB cationic dye MO, CR, EBT anionic dye | - Monomers: AA and DADMAC - Crosslinker: CNS - Initiator: APS, TEMED | - by free-radical crosslinking copolymerization | - maximum swelling equilibrium (%)—704% - removal efficiency (%):— - adsorption capacity (Qmax, mg/g)—409% for MO, 862% for CR, 1139% for EBT, and 722 mg/g for MB (135 min, c0 = 500 mg/L, pH = 7) - best-fit kinetic—kinetic pseudo-first-order model (R2 = 0.965) - best-fit isotherm—Sips isotherm (R2 = 0.992) | - | [94] |
| MO, AR anionic dye | - Monomers: AETAC, NVP - Crosslinker: NMBA - Initiator: APS, TEMED | - by free-radical crosslinking copolymerization | - maximum swelling equilibrium (%)—28.12 g/g - removal efficiency (%)—74% for CA, 77% for CV, and 62% for MV - adsorption capacity (Qmax, mg/g)—905.60 mg/g for MO; 843.58 mg/g for AR (24 h, c0 = 2000 mg/L, pH ≤ 5) - best-fit kinetic—kinetic intraparticle diffusion model (R2 = 0.99) - best-fit isotherm—Freundlich isotherm (R2 = 0.99) -negative ΔG0 values; positive ΔH0 and ΔS0 values indicate the adsorption is spontaneous and thermodynamically favorable and is physical | [95] | |
| MB cationic dye | - Monomers: AA, MAC - Crosslinker: CNS - Initiator: APS, TEMED | - by free-radical crosslinking copolymerization | - maximum swelling equilibrium—3800 g/g - removal efficiency (%)—98% - adsorption capacity (Qmax, mg/g)—2249 mg/g (1 h, c0 = 400 mg/L, pH = 7) - best-fit kinetic—kinetic pseudo-second-order model (R2 = 0.99) - best-fit isotherm—Langmuir isotherm (R2 = 0.998) | - | [96] |
| CV, MV, CA anionic dye | - Monomers: AM, AA, AETAC - Crosslinker: EDMA - Initiator: APS, TEMED | - by free-radical crosslinking copolymerization | - maximum swelling equilibrium—28.12 g/g - removal efficiency (%)—97% for MO and 98.6% for AR - adsorption capacity (Qmax, mg/g)—2.445 mg/g for CA; 0.156 mg/g for CV; and 0.115 mg/g for MV (24 h, c0 = 10 mg/L, natural pH of the dye solution) - best-fit kinetic:— - best-fit isotherm— Langmuir model for CA, Saraydın model isotherm for CV and MV | - | [97] |
| AR1, AY36, DR23, AO7, and DB15 anionic dyes | - Monomers: EDA, EPC - Crosslinker: NMBA - Initiator: APS | - by one-step cross-linked copolymerization | - maximum swelling equilibrium:— - removal efficiency (%)—99.1%, 99.8%, 98.0%, 98.8%, and 100% for DR23, AR1, AO7, AY36, and DB15, respectively - adsorption capacity (Qmax, mg/g)—1541.64 mg/g (DR23) (24 h, c0 = 10 mg/L, pH = 2–12) - best-fit kinetic: pseudo-second-order model—(R2 = 0.9991) - best-fit isotherm: Langmuir isotherm—(R2 =0.9995) - thermodynamic:— | - desorption experiments were washed twice with distilled water | [98] |
| MO anionic dye | - Monomers: DMC, AM - Crosslinker: NMBA - Initiator: APS | - by precipitated droplets’ in situ crosslinking polymerization | - maximum swelling equilibrium:— - removal efficiency (%)—94% - adsorption capacity (Qmax, mg/g)—992.63 mg/g for OG, 1388.55 mg/g for AR, and 744.17 mg/g for MO (24 h, c0 = 10 µmol/L, pH = 3–9) - best-fit kinetic: pseudo-second-order model—(R2 = 0.99) - best-fit isotherm: Langmuir isotherm—(R2 =0.99) - thermodynamic:— | - desorption eluent: HCl solutions - 5th regeneration run - 5th reusable run with 80% removal efficiency | [99] |
| Dyes | Composite Hydrogel-Based Materials | Preparation Method | Performance | Regeneration and Reusability | Ref. |
|---|---|---|---|---|---|
| MB, cationic dye | - Homopolymer: PVA - Filler/Crosslinker: Bentonite Initiator:— | - by an innocuous physical crosslinking method | - maximum swelling equilibrium:— - removal efficiency (%)—93% - adsorption capacity (Qmax, mg/g)—27 mg/g (15 h, c0 = 670 mg/L) - best-fit kinetic— pseudo-second-order model -(R2 = 0.999) - best-fit isotherm— Freundlich isotherm model (R2 = 0.993) | - desorption eluent: HCl solutions - 5th regeneration run - 5th reusable run with 80% removal efficiency for MB | [45] |
| MB, cationic dye MO anionic dye | - Monomer: AM - Filler: ZnO - Crosslinker: NMBA - Initiator: ZnO photocatalytic initiator | - self-initiated photopolymerization | - maximum swelling equilibrium—1.46 g/g - adsorption capacity (Qmax, mg/g)—0.9 mg/g for MB (11 h, c0 = 5 mg/L, pH = 6), 0.025 mg/g for MO (11 h, ci = 5 mg/L, pH = 2) - best-fit kinetic—pseudo-second-order model (R2 = 0.99) - best-fit isotherm—Langmuir isotherm model (R2 > 0.998) | - desorption eluent: NaOH solutions - 15th regeneration run - 15th reusable run with 81% and 75% removal efficiency for MB and MO, respectively | [46] |
| MB, cationic dye | - Homopolymer: PVA, PHPAm - Filler: GO - Crosslinker: Chromium III acetate | - a one-stage approach via the internal ionic gelation | - removal efficiency (%)—99% - adsorption capacity (Qmax, mg/g)—714.8 mg/g for MB (3 h, c0 = 200 mg/L, pH = 7) - best-fit kinetic— pseudo-second-order model (R2 = 0.997) - best-fit isotherm—Langmuir isotherm model (R2 = 0.994) - negative ΔG0 values; positive ΔH0 and ΔS0 values; indicate the adsorption was a spontaneous, endothermic, and physical adsorption process. | - desorption eluent: HCl solutions - 5th regeneration run - 5th reusable run with 70% removal efficiency | [47] |
| MG anionic dye CR cationic dye | - Monomers: AA, - Homopolymer: PVA - Filler: GO - Crosslinker: NMBA - Initiator: APS | - by free-radical crosslinking copolymerization | - maximum swelling equilibrium:— - removal efficiency (%)—93% for MG and 55% for CR - adsorption capacity (Qmax, mg/g)—1458 mg/g for MG and 826 mg/g for CR (30 min, c0 = 100 mg/L, pH = 3–5) - best-fit kinetic—pseudo-second-order model (R2 = 0.999) - best-fit isotherm—Freundlich isotherm (R2 = 0.984) | - desorption eluent: HCl and NaOH solutions - 5th regeneration run - 5th reusable run with 74% and 43% removal efficiency for MG and CR, respectively | [101] |
| MB, CV and SO cationic dye | - Monomer: AA - Filler: Melamine-coated BIO-Ox - Crosslinker: NMBA - Initiator: AIBN | - by in situ free-radical polymerization | - maximum swelling equilibrium(%)—60% - removal efficiency (%)—97% for CV, 99% for SO, 95% for MB - adsorption capacity (Qmax, mg/g)—463 mg/g for CV, 711 mg/g for SO, and 638 mg/g for MB (2 h, c0 = 200 mg/L, pH = 10 for MB, pH = 9 for CV, and pH = 7 for SO) - best-fit kinetic—pseudo-second-order model (R2 = 0.999) - best-fit isotherm—Langmuir isotherm model (R2 > 0.998) | - desorption eluent: NaOH solutions - 7th regeneration run - 7th reusable run with 71 removal efficiency for MG | [102] |
| MB, cationic dye MO anionic dye | - Homopolymer: PVA - Filler/Crosslinker: AC, FeCl3, FeSO4*7H2O | - a one-pot approach | - maximum swelling equilibrium:— - removal efficiency (%)—99% - adsorption capacity (Qmax,mg/g)—174 mg/g for MB, 147 mg/g for MO (5 h, c0 = 5 m, pH = 11) - best-fit kinetic—pseudo-second-order model (R2 = 0.999) - best-fit isotherm—Langmuir isotherm model (R2 > 0.992) | - desorption eluent: ethanol - 5th regeneration run - 5th reusables run with 72% removal efficiency for MB. | [103] |
| MB, cationic dye | - Homopolymer: PVA - Fillers: TiO2 and Na2CO3 - Crosslinker: NMBA - Initiator: KPS | -by freeze–thaw cycle method | - maximum swelling equilibrium:— - removal efficiency (%)—99.85% - adsorption capacity (Qmax, mg/g)—318.47 mg/g 303.83 mg/g (12 h, c0 = 100 mg/L) - best-fit kinetic—pseudo-second-order model (R2 = 0.999) - best-fit isotherm—Langmuir isotherm model (R2 = 0.999) | - | [104] |
| MB, cationic dye | - Monomer: MAAn - Fillers: TiO2, ZnO - Crosslinker: EGDMA - Initiator: Darocur1173 | - by water-in-oil-in-water double emulsion | - maximum swelling equilibrium:— - removal efficiency (%)—100% (24 h, c0 = 5 mg/L, pH = 3–5) - adsorption capacity (Qmax, mg/g):— - best-fit kinetic:— - best-fit isotherm:— | - | [105] |
| MB, cationic dye CR anionic dye | - Monomer: AM, AA, IA - Filler: TiO2 - Crosslinker: NMBA - Initiator: TiO2 | - by photopolymerization | - maximum swelling equilibrium(%)—473 g/g - removal efficiency (%)—90% for MB; 91% for CR - adsorption capacity (Qmax, mg/g)—4.86 for MB, 2.22 mg/g for CR (24 h, c0 = 50 mg/L) - best-fit kinetic—pseudo-second-order model (R2 = 0.999) - best-fit isotherm—Freundlich isotherm model (R2 = 0.999) | - | [106] |
| MB, cationic dye | - Monomer: AA - Filler: Lap - Crosslinker: NMBA - Initiator: KPS | - by in situ free-radical polymerization | - maximum swelling equilibrium(%)—1360 g/g - removal efficiency (%)—98% - adsorption capacity (Qmax, mg/g)—3846 mg/g (12 h, c0 = 1000 mg/L, pH = 7) - best-fit kinetic—pseudo-second-order model (R2 = 0.999) - best-fit isotherm—Langmuir isotherm model (R2 = 0.999) - negative ΔG0 values; positive ΔH0 and ΔS0 values; indicate the adsorption was a spontaneous, endothermic, and physical adsorption process | - desorption eluent: HCl and NaOH solutions - 6th regeneration run - 6th reusable run with 93% removal efficiency for MB | [107] |
| MB, cationic dye | - Monomer: MAA, AM - Filler: Cloisite 30B - Crosslinker: NMBA - Initiator: KPS | - by in situ free-radical polymerization | - maximum swelling equilibrium(%)—2800% - removal efficiency (%)—98.6% - adsorption capacity (Qmax, mg/g)—32.83 mg/gMB (1 h, c0 = 1500 mg/L, pH = 8) - best-fit kinetic—pseudo-first-order model (R2 = 0.98) - best-fit isotherm—Freundlich isotherm model (R2 = 0.97) - negative ΔG0, ΔH0,and ΔS0 values; indicate the adsorption was a spontaneous and exothermic process | - | [108] |
| MB, cationic dye | - Monomer: AA, MAC - Filler/Crosslinker: CNS - Initiator: APS, TEMED | - by in situ free-radical copolymerization | - maximum swelling equilibrium(%)—3800 g/g - removal efficiency (%)—98% - adsorption capacity (Qmax, mg/g)—2249 mg/g (1 h, c0 = 1500 mg/L, pH = 8) - best-fit kinetic—pseudo-second-order model (R2 = 0.99) - best-fit isotherm—Langmuir isotherm model (R2 = 0.99) | - | [109] |
| MB, cationic dye | - Monomer: NIPAM, IA - Filler: Pumice (mineral) -Crosslinker:— - Initiator:— | - by γ-radiation copolymerization | - maximum swelling equilibrium(%)—5000% - removal efficiency (%)—30% - adsorption capacity (Qmax, mg/g)—22.52 mg/24 h, c0 = 150 mg/L, pH = 7 - best-fit kinetic: - - best-fit isotherm—Langmuir isotherm model (R2 = 0.993) | - | [110] |
| BY28 cationic dye | - Monomer: MAA - Filler: ZSM-5 - Crosslinker: NMBA - Initiator: AIPH | - by free-radical polymerization | - maximum swelling equilibrium:— - removal efficiency (%):— - adsorption capacity (Qmax, mg/g)—180 mg/g48 h, c0 = 50 mg/L, pH = 6.8 - best-fit kinetic—pseudo-first-order model (R2 = 0.99) - best-fit isotherm—Freundlich isotherm model (R2 = 0.98) | - | [111] |
| MO anionic dye | - Monomers: MEMA - Filler: GO - Crosslinker: NMBA - Initiator: APS | - by in situ free-radical polymerization | - maximum swelling equilibrium:— - removal efficiency (%)—94% - adsorption capacity (Qmax, mg/g)—243.63 mg/g (8.5 h, c0 = 2500 mg/L, pH = 2) - best-fit kinetic—pseudo-second-order model—(R2 = 0.984) - best-fit isotherm—Langmuir isotherm—(R2 = 0.99) - negative ΔG0 values; positive ΔH0 and ΔS0 values indicate the adsorption is spontaneous and endothermic, driven by entropy | - desorption eluent: HCl and NaOH solutions - 8th regeneration run - 8th reusable run with 95% removal efficiency | [112] |
| MG, RhB cationic dye | - Monomer: AA - Filler: Curcumin-modified BIO - Crosslinker: NMBA - Initiator: AIBN | - by in situ free-radical polymerization | - maximum swelling equilibrium(%)—60% - removal efficiency (%)—98.75% for MG and 98.50% for RhB - adsorption capacity (Qmax, mg/g)—521 mg/g for MG (8.5 h, c0 = 1200 mg/L, pH = 7) and 742 mg/g for RhB (8.5 h, ci = 1200 mg/L, pH = 6) - best-fit kinetic—pseudo-second-order model (R2 = 0.998) and Elovich (R2 = 0.995) - best-fit isotherm—Koble–Corrigan and Langmuir isotherm models (R2 > 0.997) | - desorption eluent: HCl and NaOH solutions - 7th regeneration run - 7th reusables run with 80% and 72% removal efficiency for MG and RhB, respectively | [113] |
| MB, cationic dye | - Monomer: AA, PVP - Filler: RPGS - Crosslinker: NMBA - Initiator: KPS | - by radical polymerization | - maximum swelling equilibrium:— - removal efficiency (%):— - adsorption capacity (Qmax, mg/g)—1815 mg/g (2 h, c0 = 200 mg/L, pH = 7) - best-fit kinetic—pseudo-second-order model (R2 = 0.999) - best-fit isotherm—Langmuir isotherm model (R2 = 0.995) - negative ΔG0, ΔH0, and ΔS0 values; indicate the adsorption was a spontaneous and exothermic process | - | [114] |
| MB, CV cationic dye CR anionic dye | - Monomer: AM - Homopolymer: PVA - Filler: SiO2-coated ZnO or TiO2 particles - Crosslinker: NMBA - Initiator: CAN | - by free-radical crosslinking copolymerization | - maximum swelling equilibrium(%)—6400% - removal efficiency (%)—90% (MB, CV, and CR) - adsorption capacity (Qmax, mg/g)—703 mg/g for MB, 863 mg/g for CV, and 174 mg/g for CR (24 h, c0 = 4000 mg/L, pH = 4–10) - best-fit kinetic—pseudo-second-order model (R2 = 0.999) - best-fit isotherm—Freundlich isotherm model (R2 = 0.992) | - desorption eluent: HCl solutions - 5th regeneration run - 5th reusable run with 23% for MB, 30% for CV, and 13% for CR; removal efficiency | [115] |
| Heavy Metals | Hydrogel/Composite Hydrogel-Based Materials | Preparation Method | Performance | Regeneration and Reusability | Ref. |
|---|---|---|---|---|---|
| Cu2+ | - Monomer: AM, IA - Crosslinker: NMBA - Initiator: KPS, TEMEDA | - by radical polymerization | - maximum swelling equilibrium(%)—64% - removal efficiency (%)—98% - adsorption capacity (Qmax, mg/g)—336 mg Cu/g (tc = 48 h, c0 = 1750 mg/L) | - desorption eluent: NaOH solution - 6th regeneration run - 6th reusable run with 83% removal efficiency | [116] |
| Hg2+ | - Monomer: NVP, IA - Homopolymer: PVA - Crosslinker: NMBA - Initiator: Pn-In | - by photopolymerization | - maximum swelling equilibrium: 57%- removal efficiency (%):— - adsorption capacity (Qmax, mg/g)—56 mg Hg/g (tc = 48 h, c0 = 1000 mg/L) | - | [117] |
| Pb2+ | - Monomer: AA, AN, amidoxime group - Crosslinker: NMBA - Initiator: HHMP | - by cryo-polymerization | - maximum swelling equilibrium(%)—40% - removal efficiency (%)—99.8% - adsorption capacity (Qmax, mg/g)—450 mg Pb/g and 650 mg Pb/g after regeneration (tc = 24 h, c0 = 100 mg/L) - best-fit kinetic—pseudo-second-order kinetic (R2 = 0.999) | - desorption eluent: alkali solution - 7th regeneration run - 7th reusable run with 90% removal efficiency | [118] |
| Cu2+ | - Monomer: AA, NIPAM, - Crosslinker: NMBA - Initiator: AIBN | - by RAFT polymerization | - removal efficiency (%)—69.8% - adsorption capacity (Qmax, mg/g)—435 mg Cu/g (c0 = 84 ppm) | - | [119] |
| Cu2+, Cd2+, Cr6+, Fe3+, Mn2+, Ni2+, Zn2+, Ce3+ and Ag+ | - Monomer: AA, - Crosslinker: CNS - Initiator: APS, TEMEDA | - by free-radical polymerization | - maximum swelling equilibrium(%)—8130% - removal efficiency (%)—values for Cu2+, Cd2+, Mn2+, Ni2+, Zn2+, and Ce3+ exceeding 50% - adsorption capacity (Qmax, mg/g)—132.9 mg Cu/g (c0 = 0.16 mg/L), 132.9 mg Cd/g (c0 = 0.56 mg/L), 58.1 mg Cr/g (c0 = 0.52 mg/L), 12.4 mg Fe/g (c0 = 0.56 mg/L), 120.4 mg Mn/g (c0 = 0.55 mg/L), 128.8 mg Ni/g (c0 = 0.59 mg/L), 157.8 mg Zn/g (c0 = 0.65 mg/L), 203.5 mg Ce/g (c0 = 0.7 mg/L), and 203.5 mg Ag/g (c0 = 1.08 mg/L, tc = 4 day) - best-fit kinetic—pseudo-second-order kinetic (R2 = 0.98) - best-fit isotherm—Freundlich isotherm (R2 = 0.95) | - desorption eluent: alkali solution - 10th regeneration run - 10th reusable run with 70% removal efficiency | [120] |
| Co2+ | - Monomer: AA, AM - Homopolymer: PVA - Crosslinker: NMBA - Initiator: KPS | - by free-radical polymerization | - maximum swelling equilibrium(%)—1243% - adsorption capacity (Qmax, mg/g)—184 mg Co/g (tc = 10 h, c0 = 250 mg/L) - best-fit kinetic—pseudo-second-order kinetic (R2 = 0.99) - best-fit isotherm—Freundlich isotherm (R2 = 0.99) - the negative values of ΔGo and positive values of ΔHo and ΔSo indicating the spontaneous and endothermic nature of the adsorption | - | [121] |
| Ni2+, Cu2+, Zn2+, and Cr3+ | - Monomer: AM, AA - Crosslinker: [Vim]Br2 - Initiator: APS | - by crosslinking free-radical polymerization | - maximum swelling equilibrium(%)—40% - removal efficiency (%)—values all exceeding 90% -adsorption capacity (Qmax, mg/g)—38.5 mg Ni/gxerogel (tc = 3 h, c0 = 40 mg/L) and 89 mg Ni/g (tc = 10 h, c0 = 100 mg/L) - best-fit kinetic—pseudo-second-order kinetic (R2 = 0.998) - best-fit isotherm—Langmuir model (R2 = 0.995) at higher concentrations (100 ppm), and Freundlich isotherm at low concentrations (40 ppm) | - | [122] |
| Cu2+ Ba2+ and Sr2+ | - Monomer: AM, AA - Crosslinker: NMBA - Initiator: Pn-In | - by crosslinking free-radical polymerization using the gamma irradiation technique | - maximum swelling equilibrium(%)—400% - adsorption capacity (Qmax,, mg/g)—36.4 mg Ba/g,(tc = 24 h, c0 = 100 mg/L), 27.31 mg Sr/g (tc = 24 h, c0 = 100 mg/L), 13.7 mg Cu/g, (tc = 24 h, c0 = 50 mg/L) - best-fit kinetic—pseudo-first-order kinetic - best-fit isotherm—Freundlich model (R2 = 0.90–0.992) -the thermodynamic parameters (ΔGo,ΔHo andΔSo) indicated that the adsorption of ions exhibit both exothermic and endothermic characteristics | - | [123] |
| Cu2+, Ni2+ and Zn2+ | - Monomer: AMPS - Crosslinker: NMBA - Initiator: APS, TEMEDA | - by free-radical polymerization | - removal efficiency (%)—all values exceeding 95% - adsorption capacity (Qmax, mg/g)—36 mg Zn/g, 33.1 mg Ni/g, 32.9 mg Cu/g (tc = 3 h, c0 = 100 mg/L) - best-fit kinetic—pseudo-second-order kinetic (R2 = 0.999) | - | [124] |
| Fe3+, Cu2+, Zn2+, and Ni2+ | - Monomer: AMPSA - Crosslinker: EGDMA - Initiator: BPO, TMEDA | - by suspension polymerization | - removal efficiency (%) –97.7% Fe, 46.3% Ni, 42.6% Cu, 29.5% Zn - adsorption capacity (Qmax, mg/g)—60.9 mg Fe/g (tc = 2 h, c0 = 616 mg/L), 3.07 mg Cu/g (tc = 2 h, c0 = 72 mg/L), 1.62 mg Zn/g (tc = 2 h, c0 = 55 mg/L), 0.74 mg Ni/g (tc = 2 h, c0 = 16 mg/L) - best-fit kinetic—pseudo-second-order kinetic (R2 = 0.99) | - | [125] |
| Pb2+ | - Monomer: AMPS, AA - Homopolymer: PVA - Crosslinker: EGDMA - Initiator: KPS, TMED | - by free-radical polymerization | - maximum swelling equilibrium(%)—8130% - removal efficiency (%)—90.2% - adsorption capacity (Qmax, mg/g)—200.7 mg Pb/g (tc = 8 h, c0 = 300 mg/L) - best-fit kinetic—pseudo-second-order kinetic (R2 = 0.996) - best-fit isotherm—Langmuir isotherm (R2 = 0.99) -the negative values of ΔGo and positive values of ΔHo and ΔSo indicating the spontaneous and endothermic nature of the adsorption | - | [126] |
| Pb2+ | - Monomer: NIPAM, AMPSA - Homopolymer: PVA - Crosslinker: NMBA - Initiator: APS | - by a surfactant-free emulsion polymerization | - maximum swelling equilibrium(%)—8130% - removal efficiency (%)—99% - adsorption capacity (Qmax, mg/g)—510.2 mgPb/g (tc = 4 h, c0 = 800 mg/L) - best-fit kinetic—pseudo-second-order kinetic (R2 = 0.99) - best-fit isotherm—Langmuir isotherm (R2 = 0.99) -the negative values of ΔGo and, ΔHo indicate the spontaneous and exothermic nature of the adsorption | - desorption eluent: alkali solution - 4th regeneration run - 4th reusable run with recovery efficiency ranging from 95.7–104.4 for Pb2+ ions | [127] |
| La3+ | - Monomer: NIPAM, MA - Filler: GO - Crosslinker: NMBA - Initiator: KPS, TEMED | - by free-radical cryopolymerization | - maximum swelling equilibrium(%)—28.76 g/g - removal efficiency (%)—99% - adsorption capacity (Qmax, mg/g)—33.1 mg La/g (tc = 12 h, c0 = 208.37 mg/L) - best-fit kinetic—pseudo-second-order kinetic (R2 = 0.98) - best-fit isotherm—Langmuir isotherm (R2 = 0.99) | - desorption eluent: HCl solution - 4th regeneration run - 4th reusable run with 94% recovery efficiency | [128] |
| Pb2+ and Cd2+ | - Monomer: AA, MMA - Filler: GO - Crosslinker: NMBA - Initiator: APS, TEMED | - by free-radical crosslinking copolymerization | - removal efficiency (%)—98.5% - adsorption capacity (Qmax, mg/g)—2.225 mgPb/g and 4.175 mg Cd/g (tc = 2 h, c0 = 40 mg/L) - best-fit kinetic—pseudo-second-order kinetic (R2 = 0.99) - best-fit isotherm—Langmuir isotherm (R2 = 0.999) -the negative values of ΔGo and positive values of ΔHo and ΔSo indicating the spontaneous and endothermic nature of the adsorption | - desorption eluent: 0.1 M HCl solution - 5th regeneration run - 5th reusable run with 91% and 87% recovery efficiency for Pb2+ and Cd2+, respectively | [48] |
| Cd2+ | - Monomer: AM, AMPSA - Filler: MMT - Crosslinker: NMBA - Initiator: KPS, TEMED | - by free-radical polymerization | - maximum swelling equilibrium(%)—513.3% - removal efficiency (%)—99% - adsorption capacity (Qmax, mg/g)—301.5 mg Cd/g (tc = ~2 h, c0 = 500 mg/L) - best-fit kinetic—pseudo-second-order kinetic (R2 = 0.98) - best-fit isotherm—Langmuir isotherm (R2 = 0.99) | - desorption eluent: 1.0 M HNO3 - 5th regeneration run - 5th reusable run with 99% recovery efficiency | [129] |
| Pb2+ | - Monomer: AA, AM - Filler: Lap-KCuHCF - Crosslinker: NMBA - Initiator: KPS | - by free-radical crosslinking copolymerization | - removal efficiency (%)—99% - adsorption capacity (Qmax, mg/g)—125 mg Pb/g (tc = 1 h, c0 = 500 mg/L) - best-fit kinetic—pseudo-second-order kinetic (R2 = 0.99) - best-fit isotherm—Langmuir isotherm (R2 = 0.99) | - desorption eluent: 1.0 M HNO3 - 5th regeneration run - 5th reusable run with 70% recovery efficiency | [130] |
| Cs+ | - Monomer: AA, - Filler: Lap-KCuHCF - Crosslinker: NMBA - Initiator: V50 | - by free-radical crosslinking copolymerization | - removal efficiency (%)—90% - adsorption capacity (Qmax, mg/g)—146.22 mg Cs/g (tc = ~2 h, c0 = 625 mg/L) - best-fit kinetic—pseudo-second-order kinetic (R2 = 0.99) - best-fit isotherm—Langmuir isotherm (R2 = 0.99) | - desorption eluent: 1.0 M HNO3 - 5th regeneration run - 5th reusable run with 80% recovery efficiency | [131] |
| Pb2+ | - Monomer: AM, IA - Filler: MWCNTs - Crosslinker: NMBA - Initiator: APS | - by graft copolymerization | - maximum swelling equilibrium(%)—355% - removal efficiency (%)—99% - adsorption capacity (Qmax,, mg/g)—101.01 mg Pb/g (tc = 1.5 h, c0 = 175 mg/L) - best-fit kinetic—pseudo-second-order kinetic (R2 = 0.99) - best-fit isotherm—Langmuir isotherm (R2 = 0.98) | - | [132] |
| Cd2+ | - Monomer: AA - Filler: AC - Crosslinker: NMBA - Initiator: KPS | - by free-radical crosslinking polymerization | - removal efficiency (%)—98.5% - adsorption capacity (Qmax, mg/g)—473.2 mg Cd/g (tc = 15 min, c0 = 1124 mg/L) - best-fit kinetic—pseudo-second-order kinetic (R2 = 0.999) - best-fit isotherm—Langmuir isotherm (R2 = 0.999) - the negative values of ΔGo and positive values of ΔHo and ΔSo indicating the spontaneous and endothermic nature of the adsorption | - | [133] |
| Zn2+ and Cd2+ | - Monomer: AMPSA, MA - Filler: Fe3O4 - Crosslinker: NMBA - Initiator: APS, TEMED | - by free-radical crosslinking copolymerization | - maximum swelling equilibrium(%)—5000% - adsorption capacity (Qmax, mg/g)—289.12 mg Zn/g and 385.2 mg Cd/g (tc = 2 h, c0 = 1000 mg/L) - best-fit kinetic—pseudo-second-order kinetic (R2 = 0.999) - best-fit isotherm—Freundlich isotherm (R2 = 0.99) - thermodynamic data showed the adsorption to be spontaneous and exothermic | - | [134] |
| Cr3+ | - Monomer: AA, NIPAM - Homopolymer: PVP - Filler: FeCl3·6H2O, FeCl2·4H2O, TEOS - Crosslinker: NMBA - Initiator: APS | - by free-radical crosslinking copolymerization | - adsorption capacity (Qmax, mg/g)—243.90 mg Cr/g (tc = 24 h, c0 = 100 mg/L) - best-fit kinetic—pseudo-second-order kinetic (R2 = 0.995) - best-fit isotherm—Freundlich isotherm (R2 = 0.954) | - | [135] |
| Cu2+, Pb2+, Zn2+, and Cd2+ | - Monomer: AA, AM - Filler: GO - Crosslinker: NMBA - Initiator: KPS | - by free-radical polymerization | - adsorption capacity (Qmax,mg/g)—1268 mg Cu/g, 2026 mg Pb/g, 704 mg Zn/g, and 632 mg Cd/g (tc = 1 h, c0 = 2000 mg/L) - best-fit kinetic—pseudo-second-order kinetic (R2 = 0.99) - best-fit isotherm—Langmuir isotherm (R2 = 0.85) | - desorption eluent: HCl solution - 3rd regeneration run - 3rd reusable run with 88% recovery efficiency | [136] |
| Pb2+, Cd2+ and Cu2+ | - Monomer: AA, AM - Filler: EMS - Crosslinker: NMBA - Initiator: APS | - by free-radical polymerization | - removal efficiency (%)—62% Pb, 75% Cd, and 78% Cu - adsorption capacity (Qmax, mg/g)—135.5 mg Pb/g (tc = 1 h, c0 = 207.2 mg/L), 134 mg Cd/g (tc = 1 h, c0 = 112.41 mg/L), and 54 mg Cu/g (tc = 1 h, c0 = 63.55 mg/L) - best-fit kinetic—pseudo-second-order kinetic (R2 = 0.99) - best-fit isotherm—Langmuir isotherm (R2 = 0.85) - the negative values of ΔGo and positive values of ΔHo and ΔSo indicating spontaneous and endothermic nature of the adsorption | - desorption eluent: EDTA-2Na liquors and NaOH - 3rd regeneration run - 3rd reusable run with 70% recovery efficiency | [137] |
| Cu2+ | - Homopolymer: PVA - Crosslinker: GA | - by chemical crosslinking reaction | - maximum swelling equilibrium(%)—250% - removal efficiency (%)—90% - adsorption capacity (Qmax, mg/g)—184 mg Co/g (tc = 2 h, c0 = 50 mg/L) - best-fit kinetic—pseudo-second-order kinetic (R2 = 0.999) | - | [138] |
| Pb2+ and Cd2+ | - Monomer: AA, MADA - Filler: Ti3C2 - Crosslinker: NMBA - Initiator: APS | - by copolymerization | - adsorption capacity (Qmax, mg/g)—609 mg Pb/g and 250 mg Cd/g (tc = 2 h, c0 = 50 mg/L) - best-fit isotherm—Langmuir isotherm (R2 = 0.99) | - desorption eluent: HCl solution - 5th regeneration run - 5th reusable run with 95% recovery efficiency | [49] |
| Pb2+ | - Monomer: AA - Filler: WT - Initiator: CAN | - by radical polymerization and microwave and/or UV irradiation | - maximum swelling equilibrium(%)—28.76 g/g - removal efficiency (%)—95% - adsorption capacity (Qmax, mg/g)—35.7 mg Pb/g (tc = 2 h, c0 = 200 mg/L) - best-fit kinetic—pseudo-first-order kinetic (R2 = 0.998) - best-fit isotherm—Langmuir isotherm (R2 = 0.992) | - desorption eluent: dilute nitric acid solution - 3rd regeneration run - 3rd reusable run with 91% recovery efficiency | [139] |
| Heavy Metals | Chitosan-Based Hydrogel | Preparation Method | Performance | Regeneration and Reusability | Ref. |
|---|---|---|---|---|---|
| Cu2+, Ni2+ | Glutamic-chitosan hydrogels | Crosslinking | - maximum swelling equilibrium:— - removal efficiency (%)—95.17% for Cu2+ and 48–95% for Ni2+ - adsorption capacity (Qmax, mg/g)—83.33 mg Cu/g and 103.4 mg Ni/g - best-fit kinetic—pseudo-second-order model (R2 = 0.988) - best-fit isotherm—Langmuir isotherm—(R2 = 0.999) | - | [164] |
| Cu2+ | N-Aminorhodanine-modified chitosan hydrogel beads | Crosslinking | - maximum swelling equilibrium(%)—362%- removal efficiency (%):— - adsorption capacity (Qmax, mg/g)—62.5 mg Cu/g - best-fit kinetic—pseudo-second-order model (R2 = 0.9976) - best-fit isotherm—Freundlich isotherm (R2 = 0.967) | - desorption effluent: mixture of hydrochloric acid (HCl) and Ethylene diamine tetra-acetic acid sodium salt (EDTA) - 6th regeneration run - 6th reusable run with 23% removal efficiency | [165] |
| Pb2+, Hg2+, Cd2+, Cr3+ | Gelatine–chitosan hydrogel particles | Crosslinking | - maximum swelling equilibrium(%)—7000–8000% - removal efficiency (%)—12% for Pb2+, 97% for Hg2+, 2% for Cd2+, and 24% for Cr3+ - adsorption capacity (Qmax, mg/g):— - best-fit kinetic:— - best-fit isotherm:— | - | [166] |
| Pb2+ | Chitosan/alginate/Fe3O4@SiO2 hydrogel composites | By adjusting the ionic strength of chitosan solutions | - maximum swelling equilibrium:— - removal efficiency (%)—99% - adsorption capacity (Qmax, mg/g)—234.77 mg Pb/g - best-fit kinetic—Elovich model (R2 = 0.99) - best-fit isotherm—Langmuir isotherm | - desorption effluent: nitric acid (HNO3) solution - 3rd regeneration run - 3rd reusable run with 18% removal efficiency | [167] |
| Cu2+ | Magnetic bentonite/carboxymethyl chitosan/sodium alginate hydrogel beads | Crosslinking | - maximum swelling equilibrium:— - removal efficiency (%)—93% - adsorption capacity (Qmax, mg/g)—56.79 mg Cu/g - best-fit kinetic—pseudo-second-order model (R2 = 0.999) - best-fit isotherm—Langmuir isotherm (R2 = 0.989) | - desorption effluent: HCl solution - 4th regeneration run - 4th reusable run with 80% removal efficiency | [168] |
| Pb2+, Cd2+, Cu2+ | Gallic acid-modified carboxymethyl chitosan/iron ions hydrogels | Multi-crosslinking | - maximum swelling equilibrium—135% - removal efficiency (%):— - adsorption capacity (Qmax, mg/g)—97.15 mg Pb/g, 99.75 mg Cd/g, and 98.50 mg Cu/g - best-fit kinetic—pseudo-second-order model (R2 = 1) - best-fit isotherm—Langmuir isotherm (R2 = 0.977) | - desorption effluent: HCl solution - 10th regeneration run - 10th reusable run with 80% removal efficiency | [169] |
| Cu2+ | Magnetic chitosan–alginate–magnetite hydrogel beads | Crosslinking | - maximum swelling equilibrium:— - removal efficiency (%)—56.51% - adsorption capacity (Qmax, mg/g):— - best-fit kinetic:— - best-fit isotherm:— | - | [170] |
| Cu2+ | Chitosan-Based Beads Incorporating Inorganic–Organic Composites | Crosslinking | - maximum swelling equilibrium:— - removal efficiency (%)—29–52% - adsorption capacity (Qmax, mg/g)—17 mg Cu/g - best-fit kinetic—pseudo-second-order model (R2 = 0.898–0.989) - best-fit isotherm:— | - | [171] |
| Cd2+, Cr3+ | O-Carboxymethyl chitosan/sodium alginate/zeolite hydrogel beads (ACZ) and O-Carboxymethyl chitosan/sodium alginate/Zn–Fe-layered double-hydroxides hydrogel beads (ACL) | Crosslinking | - maximum swelling equilibrium(%)—200%, 263%, and 272% for AC, ACZ, and ACL - removal efficiency (%)—86.3% (ACZ) and 88.9% (ACL) for Cd2+, 85.8% (ACZ) and 40.6% (ACL) for Cr3+ - adsorption capacity (Qmax, mg/g):— - best-fit kinetic—Avrami model (R2 = 0.99) - best-fit isotherm—Langmuir isotherm (R2 = 0.99) | - desorption effluent: HCl solution - 5th regeneration run - 5th reusable run with an adsorption capacity of 45 mg/g | [172] |
| Pb2+, Cu2+, Cd2+ | Chitosan/sodium alginate/calcium ion hydrogel | Physically crosslinking | - maximum swelling equilibrium—11,000% - removal efficiency (%):— - adsorption capacity (Qmax, mg/g)—176.50 mg Pb/g, 70.83 mg Cu/g, and 81.25 mg Cd/g - best-fit kinetic—pseudo-first-order model (R2 = 0.994) and pseudo-first-order model (R2 = 0.994) - best-fit isotherm—Langmuir–Freundlich isotherm (R2 =0.99) | - | [173] |
| Pb2+, Cd2+, Cr3+, Hg2+ | Chitosan–gelatin hydrogel particles | Inverse suspension | - maximum swelling equilibrium(%)—850% for oven-dried hydrogels and 6000% for freeze-dried hydrogels - removal efficiency (%)—73–94%- adsorption capacity (Qmax, mg/g):— - best-fit kinetic:— - best-fit isotherm:— | - | [174] |
| Dyes | Polysaccharides Hydrogel Adsorbents | Preparation Method | Performance | Regeneration and Reusability | Ref. |
|---|---|---|---|---|---|
| CR | Cellulose–chitosan hydrogel beads | Extruding and blending regeneration | - maximum swelling equilibrium (%):— - removal efficiency (%)—89.6% - adsorption capacity (Qmax, mg/g)—40 mg CR/g - best-fit kinetic—pseudo-second-order model (R2 = 0.99) - best-fit isotherm—Langmuir isotherm (R2 = 0.97) | - | [191] |
| MO | Cellulose–chitosan/β-FeOOH composite hydrogels | Crosslinking | - maximum swelling equilibrium:— - removal efficiency (%)—60% - adsorption capacity (Qmax, mg/g):— - best-fit kinetic—pseudo-second-order model (R2 = 0.99) - best-fit isotherm—Langmuir isotherm (R2 = 0.998) | - desorption eluent: deionized water and ethanol - 5th regeneration run - 5th reusable run with 80.81% removal efficiency | [192] |
| AOII MB | Carboxymethyl cellulose–chitosan hydrogels | Crosslinking | - maximum swelling equilibrium (%)—7000% at pH 2 and 2000–3000% at pH 7 - removal efficiency (%)—90% for AOII and 95% for MB - adsorption capacity (Qmax, mg/g)—100 mg AOII/g and 110 mg MB/g - best-fit kinetic:— - best-fit isotherm:— | - desorption eluent:— - 3rd regeneration run - 3rd reusable run with 18% removal efficiency | [193] |
| CV TB | Carboxylmethyl cellulose/sodium alginate microgel spheres | Crosslinking | - maximum swelling equilibrium:— - removal efficiency (%)—96.84% for CV and 95.41% for TB - adsorption capacity (Qmax, mg/g)—160.87 mg CV/g and 141.48 mg TB/g - best-fit kinetic—pseudo-second-order model (R2 = 0.99) - best-fit isotherm—Sips isotherm (R2 = 0.977 for CV and R2 = 0.951 for TB) | - desorption eluent:— - 5th regeneration run - 5th reusable run with 96.84% for CV and 95.41% for TB removal efficiency | [194] |
| MB | Cellulose nanocrystal–alginate hydrogel beads | Crosslinking | - maximum swelling equilibrium (%)— - removal efficiency (%)—97% - adsorption capacity (Qmax, mg/g)—256.41 mg MB/g - best-fit kinetic: pseudo-second-order model—(R2 = 0.999) - best-fit isotherm: Langmuir isotherm—(R2 = 0.998) | - desorption eluent: -HCl and ethanol (EtOH) mixture - 5th regeneration run - 5th reusable run with 97% removal efficiency | [195] |
| MB MO | Sugar Beet Pulp Cellulose/Starch/Activated Carbon-ZnO hydrogels | Crosslinking and ultrasonic cavitation | - maximum swelling equilibrium (%):— - removal efficiency (%)—91.22% for MB and 90.44% for MO - adsorption capacity (Qmax, mg/g)—142.70 mg MB/g and 72.63 mg MO/g - best-fit kinetic—pseudo-second-order model (R2 = 0.99) - best-fit isotherm—Langmuir isotherm (R2 = 0.99 for MB and R2 = 0.997 for MO) | - desorption eluent: -EtOH and distilled water mixture - 5th regeneration run - 5th reusable run with 91.22% for MB and 90.44% for MO removal efficiency | [196] |
| DR80 | Chitosan–starch hydrogels | Crosslinking | - maximum swelling equilibrium (%)—1500% - removal efficiency (%)—84.2% - adsorption capacity (Qmax, mg/g)—330.86 mg DR80/g - best-fit kinetic: pseudo-second-order model—(R2 = 0.99) - best-fit isotherm: Freundlich isotherm—(R2 = 0.99) | - desorption eluent: NaOH and HCl solution - 4th regeneration run - 4th reusable run with 75% removal efficiency | [197] |
| AOII | Chitosan–gelatin hydrogels | Crosslinking | - maximum swelling equilibrium (%):— - removal efficiency (%) - - adsorption capacity (Qmax, mg/g)—573 mg AOII/g - best-fit kinetic—pseudo-second-order model (R2 = 0.986) - best-fit isotherm:— | - desorption eluent: solutions with a fixed ionic strength - 7th regeneration run - 7th reusable run with 534 mg AOII/G adsorption capacity | [198] |
| MB MG | Chitosan–halloysite nanotube composite hydrogel beads | Dropping and pH precipitation | - maximum swelling equilibrium (%):— - removal efficiency (%)—95.2% for MB and 97.5% for MG - adsorption capacity (Qmax, mg/g)—270.27 mg MB/g and 303.03 mg MG/g - best-fit kinetic—pseudo-second-order model (R2 = 0.999) - best-fit isotherm—Langmuir (R2 = 0.998) and Freundlich isotherm (R2 = 0.994) | - desorption eluent: NaOH aqueous solution and acetone - 2nd regeneration run - 2nd reusable run with 93% removal efficiency for MB | [199] |
| RB-5 | Nano-ZnO–Chitosan composite beads | Crosslinking (by ionic gelation) | - maximum swelling equilibrium (%):— - removal efficiency (%)—76% - adsorption capacity (Qmax, mg/g)—189.44 mg RB-5/g - best-fit kinetic:— - best-fit isotherm—Langmuir isotherm (R2 = 0.99) | - | [200] |
| FBL2 FR17 | Activated carbon-based chitosan hydrogels | Crosslinking | - maximum swelling equilibrium (%):— - removal efficiency (%)—70% for FBL2 and 60% for FR17 - adsorption capacity (Qmax, mg/g)—155.1 mg FBL2/g and 133.9 mg FR17/g - best-fit kinetic—Avrami (R2 = 0.99) - best-fit isotherm:— | - desorption eluent: sodium chloride (NaCl) and NaOH solution - 5th regeneration run - 5th reusable run with 70% removal efficiency for FBL2 and 60% for FR17 | [201] |
| Direct Red 83:1 | Chitosan-Fe magnetic gels | Crosslinking | - maximum swelling equilibrium (%):— - removal efficiency (%):— - adsorption capacity (Qmax, mg/g)—17.46 mg Direct Red 83:1/g - best-fit kinetic—pseudo-second-order model (R2 = 0.999) - best-fit isotherm—Temkin isotherm (R2 = 0.946) | - | [202] |
| CR | Chitosan–hematite nanocomposite hydrogel capsules | Crosslinking (by anionic surfactant gelation) | - maximum swelling equilibrium (%):— - removal efficiency (%):— - adsorption capacity (Qmax, mg/g)—4705.6 mg CR/g - best-fit kinetic—pseudo-second-order model (R2 = 0.962) - best-fit isotherm—Langmuir isotherm (R2 = 0.90), Freundlich (R2 = 0.973) and Redlich–Peterson (R2 = 0.973) | - | [203] |
| MO CR | Carboxymethyl chitosan–phytic acid composite hydrogels | In situ polymerization | - maximum swelling equilibrium (%)—1277% - removal efficiency (%)—88.1% for MO and 89.7% for CR - adsorption capacity (Qmax, mg/g)—13.62 mg MO/g and 8.49 mg CR/g - best-fit kinetic—pseudo-first-order model (R2 = 0.998) - best-fit isotherm:— | - desorption eluent:— - 5th regeneration run - 5th reusable run with 88.1% removal efficiency for MO and 89.7% for CR | [204] |
| CR | Bentonite–chitosan-graft–gelatin nanocomposite hydrogels | Crosslinking | - maximum swelling equilibrium (%)—490.18% - removal efficiency (%)—93.85% - adsorption capacity (Qmax, mg/g)—453.87 mg CR/g - best-fit kinetic—pseudo-first-order model (R2 = 0.965) - best-fit isotherm—Langmuir isotherm (R2 = 0.974) | - desorption eluent: -NaOH and HCl solutions - 5th regeneration run - 5th reusable run with 88% removal efficiency | [205] |
| MB CR | Carboxyethyl chitosan/oxidized sodium alginate/Ca2+ | Crosslinking (freeze–thaw technique) | - maximum swelling equilibrium (%)—11–21% - removal efficiency (%):— - adsorption capacity (Qmax, mg/g)—254.41 mg MB/g and 185.43 mg CR/g - best-fit kinetic—pseudo-second-order model (R2 = 0.999) - best-fit isotherm—Langmuir isotherm (R2 = 0.934) for MB and (R2 = 0.968) for CR | - | [206] |
| MB MO | Chitosan–carboxymethyl cellulose–Graphene oxide nanocomposites hydrogels | Crosslinking | - maximum swelling equilibrium (%):— - removal efficiency (%)—99% for MB and 82% for MO - adsorption capacity (Qmax, mg/g)—655.98 mg MB/g and 404.52 mg MO/g - best-fit kinetic—pseudo-second-order model (R2 = 0.999) - best-fit isotherm—Langmuir isotherm (R2 = 0.999) | - desorption eluent: HCl and NaOH solutions - 20th regeneration run - 20th reusable run with 93% removal efficiency for MB and 90% for MO | [207] |
| CR | Chitosan–rectorite–cellulose composite hydrogels | Crosslinking | - maximum swelling equilibrium (%)—72% - removal efficiency (%):— - adsorption capacity (Qmax, mg/g)—57.44 mg CR/g - best-fit kinetic—seudo-second-order model (R2 = 0.999) - best-fit isotherm—Freundlich isotherm (R2 = 0.994) | - | [208] |
| MO MB | Lignin–sodium alginate hydrogels | Crosslinking (ion exchange gelling process) | - maximum swelling equilibrium (%)—500% - removal efficiency (%)—91.15% - adsorption capacity (Qmax, mg/g)—388.81 mg MB/g - best-fit kinetic—pseudo-second-order model (R2 = 0.999) - best-fit isotherm—Freundlich isotherm (R2 = 0.984) | - desorption eluent: -HCl solution and EtOH - 5th regeneration run - 5th reusable run with 87.64% removal efficiency | [209] |
| MB MO | Activated organo-bentonite–sodium alginate beads | Crosslinking (ion exchange gelling process) | - maximum swelling equilibrium (%):— - removal efficiency (%):— - adsorption capacity (Qmax, mg/g)—1309 mg MB/g and 141 mg MO/g - best-fit kinetic—pseudo-second-order model (R2 = 0.99) - best-fit isotherm—Langmuir isotherm (R2 = 0.99) | - | [210] |
| MB RhB VG1 MO | Graphene oxide-incorporated alginate hydrogel beads | Crosslinking (ion exchange gelling process) | - maximum swelling equilibrium (%)—35% - removal efficiency (%):— - adsorption capacity (Qmax, mg/g)—240 mg MB/g, 250 mg RhB/g, 120 mg VG1/g, and 110 mg MO/g - best-fit kinetic—pseudo-second-order model-(R2 = —) - best-fit isotherm—Langmuir isotherm (R2 = 0.99) | - | [211] |
| Dyes | Hybrid Hydrogel Precursors | Preparation Method | Performance | Regeneration and Reusability | Ref. |
|---|---|---|---|---|---|
| MB, Mordant blue 9, RB | Natural component: CMC Synthetic component: PAA, PANI Filler:— | Radical polymerization and crosslinking | - Removal efficiency (%): 97.5 (MB); 96 (RB), 74 (Mordant blue 9) - Adsorption capacity (Qmax, mg/g): f or MB: 12.2 (Langmuir) and 1.65 (Freundlich) for Mordant Blue 9: 12.20 (Langmuir) and 1.65 (Freundlich for RB: 8.79 (Langmuir) and 2.80 (Freundlich) - Best-fit isotherm: Langmuir for MB and RB and Freundlich for Mordant blue 9 | The hydrogel is 91.7% biodegraded in soil | [218] |
| MB | Natural component: Aldehyde-modified CMC Synthetic component: Hydrazine-modified PNIPAm Filler: GO | Freeze-drying and crosslinking | - Removal efficiency (%): 90.4 for highly concentrated MB solution (500 mg/L) and 60.4 for 200 mg/L MB solution - Adsorption capacity (Qmax, mg/g): 601.7 for highly concentrated MB solution (500 mg/L) and 1622.1 for 200 mg/L MB solution - Best-fit kinetic: pseudo-second-order - Best-fit isotherm: Langmuir | 76.3% reusability after 5 cycles | [220] |
| MB | Natural component: CMC Synthetic component: cPAA Filler:— | IPN | - Adsorption capacity (Qmax, mg/g): 613 for MB. This hydrogel can also act on Cu, but with a lower adsorption capacity of 250 mg/g. - Best-fit kinetic: pseudo-second-order - Best-fit isotherm: Langmuir | After 5 adsorption–desorption cycles, the hydrogel retained 90% of its original removal capacity | [228] |
| MB, CV, BPB | Natural component: Cellulose macromonomer Synthetic component: PEG Filler:— | Williamson etherification with 4-vinyl benzylchloride and preparation of cellu-mers | - Adsorption capacity (Qmax, mg/g): 5.3 for BPB; 8.5 for MB and 67.0 for CV Best-fit kinetic:— Best-fit isotherm:— | [230] | |
| MB | Natural component: Cellulose Synthetic component:— Filler: GO | Heating–cooling–washing process | Maximum swelling equilibrium:— - Removal efficiency (%): 92.7 for TCH-GO10, 72.3 for TCH-GO5, 40.8 for TCH-GO2, 24.3 for TCH-GO1, 14.2 for TCH-GO0.5, and 9.1 for TCH - Adsorption capacity (Qmax, mg/g): 46.4 for TCH-GO10, 36.2 for TCH-GO5, 20.4 for TCH-GO2, 12.1 for TCH-GO1, 7.1 for TCH-GO0.5, and 4.5 for TCH - Best-fit kinetic: pseudo-second-ordeBest-fit isotherm:— | [231] | |
| Basic yellow 28 | Natural component: HEC Synthetic component: PAA Filler:— | In situ free-radical graft copolymerization | - Maximum swelling equilibrium: >400% - Removal efficiency (%): 98% - Adsorption capacity (Qmax, mg/g): 176.3 - Best-fit kinetic: pseudo-second-order - Best-fit isotherm: Langmuir | [246] | |
| ST, BCB | Natural component: CMC Synthetic component: (1) AA/MBA, (2) sodium acrylate/MBA, and (3) comonomers AA/HEMA/MBA Filler:— | Free-radical polymerization | - Maximum swelling equilibrium:— - Removal efficiency (%): the best results were recorded for SPACMC system (94.5% for St and 98.9% for BCB) - Adsorption capacity (Qmax, mg/g): 206.55 (PAACMC/ST); 209.33 (PAACMC/BCB); 186.65 (SPACMC/ST); 192.09 (SPACMC/BCB); 207.49 (CPCMC/ST); 196.24 (CPCMC/BCBest-fit kinetic:— Best-fit isotherm:— | After 5 consecutive cycles, the hydrogels maintained good mechanical properties under pH changes and thus a good recyclability | [247] |
| AR13, AB92, and AR112 | Natural component: Quaternized cellulose Synthetic component: PEGDE Filler:— | PEGDE played a crosslinker role for the quaternized cellulose | - Maximum swelling equilibrium: 5.43 ± 0.21 (CCG1); 5.74 ± 0.22 (CCG2); 12.5 ± 0.22 - Removal efficiency (%): -Adsorption capacity (Qmax, mg/g): 249 (AR13/CCG1); 286 (AR13/CCG2); 430 (AR13/CCG3); 269 (AB92/CCG1); 314 (AB92/CCG2); 447 (AB92/CCG3); 170 (AR112/CCG1); 209 (AR112/CCG2); 322 (AR112/CCG3) - Best-fit kinetic: pseudo-second-order - Best-fit isotherm: Langmuir | Recycling is possible as these materials can withstand changes in pH and temperature | [250] |
| MB, CR | Natural component: SA Synthetic component: maleic anhydride–acrylamide copolymer Filler:— | Radical polymerization and crosslinking | - Adsorption capacity (Qmax, mg/g): 653 (MB), 685 (CR), 738 (Cr), and 754 (Cu) Best-fit kinetic:— Best-fit isotherm:— - This system is also suitable for heavy metals’ (Cr, Cu) removal | The material may be pretty well recycled, using for this purpose three cleaning agents: hydrochloric acid, ethanol, and deionized water | [213] |
| MB, CR | Natural component: Alg Synthetic component:— Filler:— | Calcium alginate spheres were prepared by approaching a microfluidic method | - Better removal performance recorded for MB (99%) instead of only 94 % for CR Best-fit kinetic:— Best-fit isotherm:— | [226] | |
| MB, RB, MO | Natural component: SA Synthetic component:— Filler: GO- MMT | Crosslinking and freeze-drying | - Maximum swelling equilibrium:— - Removal efficiency (%): depending on MMT amount, the MB removal efficiency ranges from 79.02% to 96.77% - Adsorption capacity (Qmax, mg/g): 150.66 - Best-fit kinetic: pseudo-second-order - Best-fit isotherm: Freundlich | A decrease in MB removal rate from 98.2 to 85.4% was found when increasing the reuse time, which proves repeated uses are enabled | [232] |
| RB | Natural component: SA Synthetic component: MA Filler: Trans-anethole | Interpenetrating network process | - Adsorption capacity (Qmax, mg/g): 745 (RB), 734.9 (Pb), and 722 (Cd) - Best-fit kinetic: pseudo-second-order - Best-fit isotherm: Langmuir - This system is also suitable for heavy metals’ (Pb, Cd) removal | The adsorbed pollutant may be desorbed by soaking in water and ethanol | [234] |
| MG | Natural component: SA Synthetic component:—Poly(itaconic acid-co sodium 4—vinyl benzene sulfonate) Filler: Ricinus communis | Free-radical graft copolymerization | - Removal efficiency (%): 96.33% (pH = 7) - Adsorption capacity (Qmax, mg/g): 1339.83 (pseudo-second-order) and 1247.36 (pseudo-first-order) - Best-fit kinetic: pseudo-second-order - Best-fit isotherm: Langmuir | Regeneration and reuse studies revealed that four continuous adsorption–desorption cycles can be performed | [239] |
| FY3 | Natural component: SA Synthetic component: (PAA-co-PDMC) Filler:— | Free-radical graft copolymerization | - Maximum swelling equilibrium: 267.84 g/g - Removal efficiency (%): 98.23% - Adsorption capacity (Qmax, mg/g): 654.87 - Best-fit kinetic: pseudo-second-order - Best-fit isotherm: Langmuir and Dubinin–Radushkevich | The removal efficiency after the 5th cycle was 90.23%, which is a good result | [249] |
| MG, MB, FS, RB, CV, O II, SY, MO | Natural component: CS Synthetic component: PVA Filler: carbon black | Freeze–thawing | - Removal efficiency (%): 98% Adsorption capacity (Qmax, mg/g):— Best-fit kinetic:— Best-fit isotherm:— | - | [219] |
| CV | Natural component: CS Synthetic component: P(AM-IA) Filler:— | UV-initiated grafted copolymerization | -Removal efficiency (%):— Adsorption capacity (Qmax, mg/g): 167.3 Best-fit kinetic:— Best-fit isotherm:— | - | [244] |
| IC; rhodamine 6G; SY | Natural component: CS Synthetic component: Poly(vinylbenzyl chloride-co-p-divinyl benzene)(poly(VBC-DVB)) Filler:- | Hypercrosslinked polymer (HCP) based on poly(VBC-DVB) was prepared by bulk polymerization, followed by hyper-crosslinking, followed by modification with CS by approaching phase inversion | Removal efficiency (%):— Adsorption capacity (Qmax, mg/g):— Best-fit kinetic:— Best-fit isotherm:— | Regeneration possibility was determined by TGA. No significant weight loss was found after exposure to high temperatures, with it being considered that the material can be regenerated | [241] |
| AO 7, AR 18, AB 113 RY 17, RB 5, DB 78 | Natural component: CS Synthetic component: Poly(methacrylic acid) Filler: TiO2 | Inverse suspension polymerization | - | After three cycles of illumination removal, the material maintained more than 95% efficiency | [251] |
| MB | Natural component: salecan Synthetic component: P(AM-IA) Filler:— | Graft copolymerization | - Adsorption capacity (Qmax, mg/g): 107.1 - Best-fit kinetic: pseudo-second-order - Best-fit isotherm: Freundlich | - | [227] |
| MB, RB | Natural component: pullulan Synthetic component: polyacrylamide Filler:— | Graft radical co-polymerization | - Maximum swelling equilibrium: 2063% after 24 h - Adsorption capacity (Qmax, mg/g): 2 (RB) and 2.5 (MB) - Best-fit kinetic: pseudo-second-order - Best-fit isotherm: Langmuir | The adsorbent can be regenerated | [223] |
| CV | Natural component: pullulan Synthetic component: PDA Filler:— | Semi-IPN polymerization | - Adsorption capacity (Qmax, mg/g): 107 - Best-fit kinetic: pseudo-second-order - Best-fit isotherm: Langmuir | HCl was used for desorption, whereas Na OH was used for regeneration. A high adsorption capacity (100 mg/g) was maintained after four cycles of adsorption experiments | [244] |
| MO | Natural component: salep Synthetic component: Poly(3-sulfopropyl acrylate-co- AA-co- AM) Filler:— | Grafted copolymerization | - Maximum swelling equilibrium - Removal efficiency (%) - Adsorption capacity (Qmax, mg/g): 400 - Best-fit kinetic: pseudo-second-order - Best-fit isotherm: intra-particle diffusion | Five cycles of adsorption and reconditioning by drying at 40 °C | [236] |
| MB | Natural component: Carboxymethyl starch Synthetic component: Acrylic acid- acrylamide Filler:— | Graft polymerization and crosslinking | - Removal efficiency (%): 95 - Adsorption capacity (Qmax, mg/g): 1700 - Best-fit kinetic: pseudo-second-order - Best-fit isotherm: Langmuir | Four adsorption–desorption cycles may be carried out, even though the efficiency decreased from 90% (in the 1st cycle) to 50% (in the last cycle) | [222] |
| MB | Natural component: carboxymethyl starch sodium Synthetic component: PVA Filler: magnetite | The polymers were dissolved in water and magnetite was in situ prepared | - Adsorption capacity (Qmax, mg/g): 23.53 - Best-fit kinetic: pseudo-second-order - Best-fit isotherm: Freundlich | Good removal efficiency even after 8 cycles | [225] |
| MB | Natural component: Potato starch Synthetic component: PAA Filler:— | Gamma radiation-initiated copolymerization | - Adsorption capacity (Qmax, mg/g): for MB, 5.93 for potato starch/AA/gamma radiation/KOH and 10.13 for potato starch/AA/gamma radiation; for Cr, 38.77 for potato starch/AA/gamma radiation/KOH and 33.31 for potato starch/AA/gamma radiation | - | [229] |
| MB, MV | Natural component: Gg Synthetic component: P(AAm-co-MAA) Filler:— | Microwave-assisted free-radical graft copolymerization | - Removal efficiency (%): 98 (MB) and 95 (MV) - Adsorption capacity (Qmax, mg/g): 694.4 (MB, 25 °C), 724.63 (MB, 35 °C), and 746.26 (MB, 45 °C); 543.478 (MV, 25 °C), 609.756 (MV, 35 °C), and 645.161 (MV, 45 °C) - Best-fit kinetic: pseudo-second-order - Best-fit isotherm: Langmuir | The adsorbent was fully degraded in 50 days in soil compost | [212] |
| CR, BG, RB, MO | Natural component: Gg Synthetic component: polyacrylamide Filler:— | Microwave-assisted grafting | - Maximum swelling equilibrium(%): 2117% Removal efficiency (%):— - Adsorption capacity (Qmax, mg/g): 179.09 (CR), 523.62 (BG), 421.60 (RB), and 173.69 (M)Best-fit kinetic:— - Best-fit isotherm: Langmuir | The soil-burial composting method showed that the adsorbent was degraded by 93% within 60 days | [233] |
| CR | Natural component: Gg Synthetic component: PAAm Filler: ZVI | Radical copolymerization | - Adsorption capacity (Qmax, mg/g): 153.8 (25 °C), 200 (35 °C), and 250 (45 °C) - Best-fit kinetic: pseudo-second-order - Best-fit isotherm: Langmuir | The adsorbent may be involved in up to three cycles | [237] |
| MB, IC | Natural component: KG Synthetic component: poly(2-(dimethylamino)ethyl methacrylate) Filler:— | Microwave-assisted graft copolymerization | - Adsorption capacity (Qmax, mg/g): 89.28 (MB) and 101.42 (IC) - Best-fit kinetic: pseudo-second-order - Best-fit isotherm: Freundlich | - | [217] |
| MG, RB | Natural component: KG Synthetic component: PAA Filler: SiC | In situ graft copolymerization | - Removal efficiency (%): 91% (MG) and 86% (RB) - Adsorption capacity (Qmax, mg/g): 757.57 (MG) and 497.51 (RB) - Best-fit kinetic: pseudo-second-order - Best-fit isotherm: Langmuir | The adsorbent may be successfully involved within three adsorption–desorption cycles | [235] |
| CV | Natural component: XG Synthetic component: Poly(N-vinyl imidazole) Filler:— | - Adsorption capacity (Qmax, mg/g): 453 - Best-fit kinetic: pseudo-first-order and intraparticle diffusion - Best-fit isotherm: Langmuir | Four adsorption–desorption cycles | [245] | |
| MG | Natural component: GumT Synthetic component: HEMA Filler: TiO2 | The copolymer was synthesized in its crosslinked form by microwave-assisted green polymerization technique | - Maximum swelling equilibrium(%): 396.9% - Removal efficiency (%): 99.3 - Adsorption capacity (Qmax, mg/g): 78.24 (GumT-cl-HEMA, 25 °C), 88.57 (GumT-cl-HEMA, 35 °C), 85.25 (GumT-cl-HEMA, 45 °C), 83.82 (GumT-cl-HEMA/TiO2, 25 °C), 103.09 (GumT-cl-HEMA/TiO2, 35 °C), 92.08 (GumT-cl-HEMA/TiO2, 45 °C) -Best-fit kinetic: pseudo-second-order - Best-fit isotherm: Langmuir | For GumT-cl-HEMA/TiO2, the adsorption dropped from 99.3% (1st cycle) to 94.8 (3rd cycle). For GumT-cl-HEMA/TiO2, the adsorption dropped from 91.3% (1st cycle) to 84.2 (3rd cycle) | [240] |
| MB | Natural component: Lignosulfonate (LS) Synthetic component: Poly(acrylic acid-r-acrylamide) Filler:— | Radical polymerization | - Adsorption capacity (Qmax, mg/g): 2013 - Best-fit kinetic: pseudo-second-order - Best-fit isotherm: Freundlich | - | [216] |
| MB | Natural component: lignin Synthetic component: Poly(N-methyl aniline) Filler: GO | Polymerization of N-methylaniline in the presence of lignin in an aqueous solution | Maximum swelling equilibrium:—Removal efficiency (%):— - Adsorption capacity (Qmax, mg/g): 201.7 (MB) and 753.5 (Pb) - Best-fit kinetic: pseudo-first-order Best-fit isotherm:— - The adsorbent can also be used for heavy metals (Pb) | Five adsorption–desorption cycles, using ethanol as desorbent | [224] |
| MO, MB, RB, p-nitrophenol | Natural component: carboxylated lactose/sodium lignosulfonate Synthetic component: PAA Filler: AgNO3 | Self-assembly and in situ reduction | - Removal efficiency (%): conversion rate was above 80% after 540 min Adsorption capacity (Qmax, mg/g):— - Best-fit kinetic: quasi-first-order Best-fit isotherm:— | Even after 8 cycles, an excellent performance was maintained (99.7%) | [221] |
| MG | Natural component: LS Synthetic component: Poly(acrylic acid-r-acrylamide) Filler:— | Radical grafting copolymerization | Maximum swelling equilibrium:— Removal efficiency (%):— - Adsorption capacity (Qmax, mg/g): 107.066 (15 °C); 120.919 -(25 °C); and 150.376 (35 °C) - Best-fit kinetic: pseudo-second-order - Best-fit isotherm: Langmuir | - | [238] |
| CV | Natural component: gelatin Synthetic component: PAAm Filler: ACL/Mg-Fe LDH | Grafted copolymerization | Maximum swelling equilibrium:— - Removal efficiency (%): 92.81 (PAM-g-gelatin), 95.71 (PAM-g-gelatin/ACL), and 98.25 (PAM-g-gelatin/ACL/Mg-Fe LDH) - Adsorption capacity (Qmax, mg/g): 35.45 (PAM-g-gelatin), 39.865 (PAM-g-gelatin/ACL), and 44.952 (PAM-g-gelatin/ACL/Mg-Fe LDH) - Best-fit kinetic: pseudo-second-order - Best-fit isotherm: Langmuir | - | [242] |
| Heavy Metals | Hybrid Hydrogel Precursors | Preparation Method | Performance | Regeneration and Reusability | Ref. |
|---|---|---|---|---|---|
| Ni, Cd, Pb | Natural component: cellulose Synthetic component: AA Filler:— | Graft copolymerization | Maximum swelling equilibrium:— -Removal efficiency (%) - Adsorption capacity (Qmax, mg/g): 825.7 (Pb2+), 562.7 (Cd2+), and 380.1 (Ni2+) - Best-fit kinetic: pseudo-second-order - Best-fit isotherm: Langmuir | Desorption was achieved using HNO3 0.1 M. After 3 cycles, a 15% efficiency loss was recorded | [263] |
| Pb, Zn | Natural component: CMC Synthetic component: PAA Filler: MMT | Free-radical and solution polymerization | Maximum swelling equilibrium:— Removal efficiency (%) - Adsorption capacity (Qmax, mg/g): 286.67 (Zn2+), and 146.19 (Pb2+) Best-fit kinetic:— Best-fit isotherm:— | Two cycles can be approached upon regeneration in HNO3 (0.1 M) | [266] |
| Cr, Pb, Cd, Cu | Natural component: Alg or Alg-CS Synthetic component: PVA Filler:— | - | Maximum swelling equilibrium:— Removal efficiency (%):— - Adsorption capacity (Qmax, mg/g): 139.37 (PVA-Alg, Pb), 86.14 (PVA-Alg-CS, Cr) - Best-fit kinetic: pseudo-second-order - Best-fit isotherm: Langmuir | 5 adsorption cycles which retained their efficiency (over 85%) | [264] |
| Cu | Natural component: Alg Synthetic component: Poly(2-acrylamido-2-methyl-1-propane sulfonic acid) Filler: NiFe2O4 | - | Maximum swelling equilibrium:— - Removal efficiency (%): 83 (Cu) and 98.32 (MB) - Adsorption capacity (Qmax, mg/g): 22.81 (Cu) and 275.6 (MB) - Best-fit kinetic: pseudo-second-order - Best-fit isotherm: Freundlich - MB can be also adsorbed using this system, in which case, both Langmuir and Freundlich fit | - | [272] |
| Cr | Natural component: CS Synthetic component: PAA Filler:— | Radical polymerization | Maximum swelling equilibrium:— - Removal efficiency (%): 94.72 - Adsorption capacity (Qmax, mg/g): 73.14 (Langmuir) and 93.03 (Sips) - Best-fit kinetic: pseudo-nth-order - Best-fit isotherm: Redlich Peterson | - | [252] |
| Cr | Natural component: CS Synthetic component: PVA Filler: activated carbon | Maximum swelling equilibrium:— Removal efficiency (%):— - Adsorption capacity (Qmax, mg/g): 109.89 - Best-fit kinetic: pseudo-second-order - Best-fit isotherm: Langmuir | - | [253] | |
| Cu, Pb, Cd, Ni | Natural component: dextran- CS Synthetic component: PAA Filler:— | Ultrasonic heating | Maximum swelling equilibrium:— Removal efficiency (%):— - Adsorption capacity (Qmax, mg/g): 395 (Pb), 342 (Cu), 269 (Cd), 232 (Co), and 184 (Ni) - Best-fit kinetic: pseudo-second-order - Best-fit isotherm: Langmuir | NaOH was used for desorption | [257] |
| Cu, Pb, Zn | Natural component: ketoglutaric acid grafting CS Synthetic component: Polyacrylamide crosslinked with MBA Filler:— | Semi-IPN | Maximum swelling equilibrium:— - Removal efficiency (%) was determined for a mixture consisting of five heavy metals, the following values being determined: 56 (Cu), 53 (Pb), and 38 (Zn) - Adsorption capacity (Qmax, mg/g): 72.39 (Cu2+), 61.41 (Pb2+), and 51.89 (Zn2+) - Best-fit kinetic: pseudo-second-order - Best-fit isotherm: Langmuir | Even after 5 cycles, the adsorption capacity was above 90% | [259] |
| Cu, Pb, Cd | Natural component: CS Synthetic component: polyacrylamide Filler:— | AAm was radically polymerized within a CS solution | Maximum swelling equilibrium:— Removal efficiency (%) - Adsorption capacity (Qmax, mg/g): 86.00 (Cd), 99.44 (Cu), and 138.41 (Pb) - Best-fit kinetic: pseudo-second-order - Best-fit isotherm: Langmuir | 5 cycles | [265] |
| Pb, Cd | Natural component: CS Synthetic component: PAA Filler: Fe3O4 | Pickering emulsion | Maximum swelling equilibrium:— Removal efficiency (%):— - Adsorption capacity (Qmax, mg/g): 695.22 (Pb) and 308.84 (Cd) - Best-fit kinetic: pseudo-second-order - Best-fit isotherm: Langmuir | A high adsorption capacity was maintained after 5 cycles | [267] |
| Cd | Natural component: Carboxymethyl CS Synthetic component: PAA Filler: Fe3O4 | Core–shell | Maximum swelling equilibrium:— Removal efficiency (%) - Adsorption capacity (Qmax, mg/g): 155.10 Best-fit kinetic:— - Best-fit isotherm: Langmuir | - | [269] |
| Sn, Pt | Natural component: CS Synthetic component: AMPS, AA, and PEI Filler: GO; silica | Free-radical copolymerization | Maximum swelling equilibrium:— Removal efficiency (%):— - Adsorption capacity (Qmax, mg/g): 263.16 (Pt) and 118.88 mg/g (Sn) - Best-fit kinetic: pseudo-first-order - Best-fit isotherm: Langmuir | After three adsorption–desorption cycles, only a slight decrease (less than 10%) in activity occurred | [271] |
| Cu | Natural component: starch Synthetic component: PAA Filler: Sodium humate (SH) | Radical graft copolymerization | Maximum swelling equilibrium:— Removal efficiency (%):— - Adsorption capacity (Qmax, mg/g): 2.80 (0%SH); 2.83 (5%); 2.75 (10%); 2.73 (15%); 2.63 (10%) - Best-fit kinetic: pseudo-second-order - Best-fit isotherm: Langmuir | Regeneration can be carried out using NaOH and 4 cycles may be carried out | [260] |
| Cu, Cd, Ni, Zn | Natural component: potato starch Synthetic component: PAAm Filler:— | Redox-initiated graft copolymerization | Maximum swelling equilibrium:— Removal efficiency (%):— - Adsorption capacity (Qmax, mg/g): 40.72 (Cu2+), 19.27 (Cd2+), 9.31 (Ni2+), 7.48 (Zn2+) Best-fit kinetic:— - Best-fit isotherm: Sips | Desorption with 0.1 M HCl | [261] |
| Cd | Natural component: amino-functionalized starch Synthetic component: PAA Filler:— | A double-network hydrogel with adsorbent features was elaborated | Maximum swelling equilibrium:— - Removal efficiency (%):— - Adsorption capacity (Qmax, mg/g): 256.4 Best-fit kinetic:— - Best-fit isotherm: Langmuir | Desorption with 0.1 M NaOH | [270] |
| Cr | Natural component: cotton Synthetic component: Poly(acrylamide–acrylic acid) Filler:— | Free-radical graft copolymerization | - Maximum swelling equilibrium: 50 mg/g corresponded to the optimum cotton concentration of 120 wt% Removal efficiency (%):— - Adsorption capacity (Qmax, mg/g): 353 (Cr) and 255 (Cu) | - | [255] |
| Ni, Cd | Natural component: XG Synthetic component: Poly(acrylamide-co-acrylic acid) Filler: GO | Free-radical polymerization | Maximum swelling equilibrium:— Removal efficiency (%):— - Adsorption capacity (Qmax, mg/g): 312.15 (Cd2+, single-ion affinity), 185.0 (Ni2+, single-ion affinity), 259.25 (Cd2+, dual-ion affinity), 80.75 (Ni2+, dual-ion affinity) - Best-fit kinetic: pseudo-second-order - Best-fit isotherm: Langmuir | 4 adsorption–desorption cycles with adsorption capacity decreased by less than 20% | [264] |
| Cu | Natural component: natural rubber Synthetic component: AA Filler:— | Radical copolymerization | Maximum swelling equilibrium:— - Removal efficiency (%): 72.19 Adsorption capacity (Qmax, mg/g):— Best-fit kinetic:— Best-fit isotherm:— | - | [258] |
| Cu, Zn | Natural component: gelatin Synthetic component: PVA Filler: SPIONs | Maximum swelling equilibrium:— Removal efficiency (%):— - Adsorption capacity (Qmax, mg/g): 47.594 (Cu, SPIONs-gelatin) and 40.559 (Zn, SPIONs-gelatin); 56.051 (Cu, SPIONs-gelatin-PVA) and 40.865 (Zn, SPIONs-gelatin-PVA) | - | [262] | |
| Cd | Natural component: CadRP Synthetic component: PNIPAm Filler: SPIONs | Maximum swelling equilibrium:— Removal efficiency (%):— Adsorption capacity (Qmax, mg/g):— Best-fit kinetic:— Best-fit isotherm:— | The adsorbent could capture and release Cd reversibly, which allows for at least 5 cycles | [268] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandu, T.; Chiriac, A.-L.; Zaharia, A.; Iordache, T.-V.; Sarbu, A. New Trends in Preparation and Use of Hydrogels for Water Treatment. Gels 2025, 11, 238. https://doi.org/10.3390/gels11040238
Sandu T, Chiriac A-L, Zaharia A, Iordache T-V, Sarbu A. New Trends in Preparation and Use of Hydrogels for Water Treatment. Gels. 2025; 11(4):238. https://doi.org/10.3390/gels11040238
Chicago/Turabian StyleSandu, Teodor, Anita-Laura Chiriac, Anamaria Zaharia, Tanta-Verona Iordache, and Andrei Sarbu. 2025. "New Trends in Preparation and Use of Hydrogels for Water Treatment" Gels 11, no. 4: 238. https://doi.org/10.3390/gels11040238
APA StyleSandu, T., Chiriac, A.-L., Zaharia, A., Iordache, T.-V., & Sarbu, A. (2025). New Trends in Preparation and Use of Hydrogels for Water Treatment. Gels, 11(4), 238. https://doi.org/10.3390/gels11040238











