A Poly-γ-Glutamic Acid/ε-Polylysine Hydrogel: Synthesis, Characterization, and Its Role in Accelerated Wound Healing
Abstract
1. Introduction
2. Results and Discussion
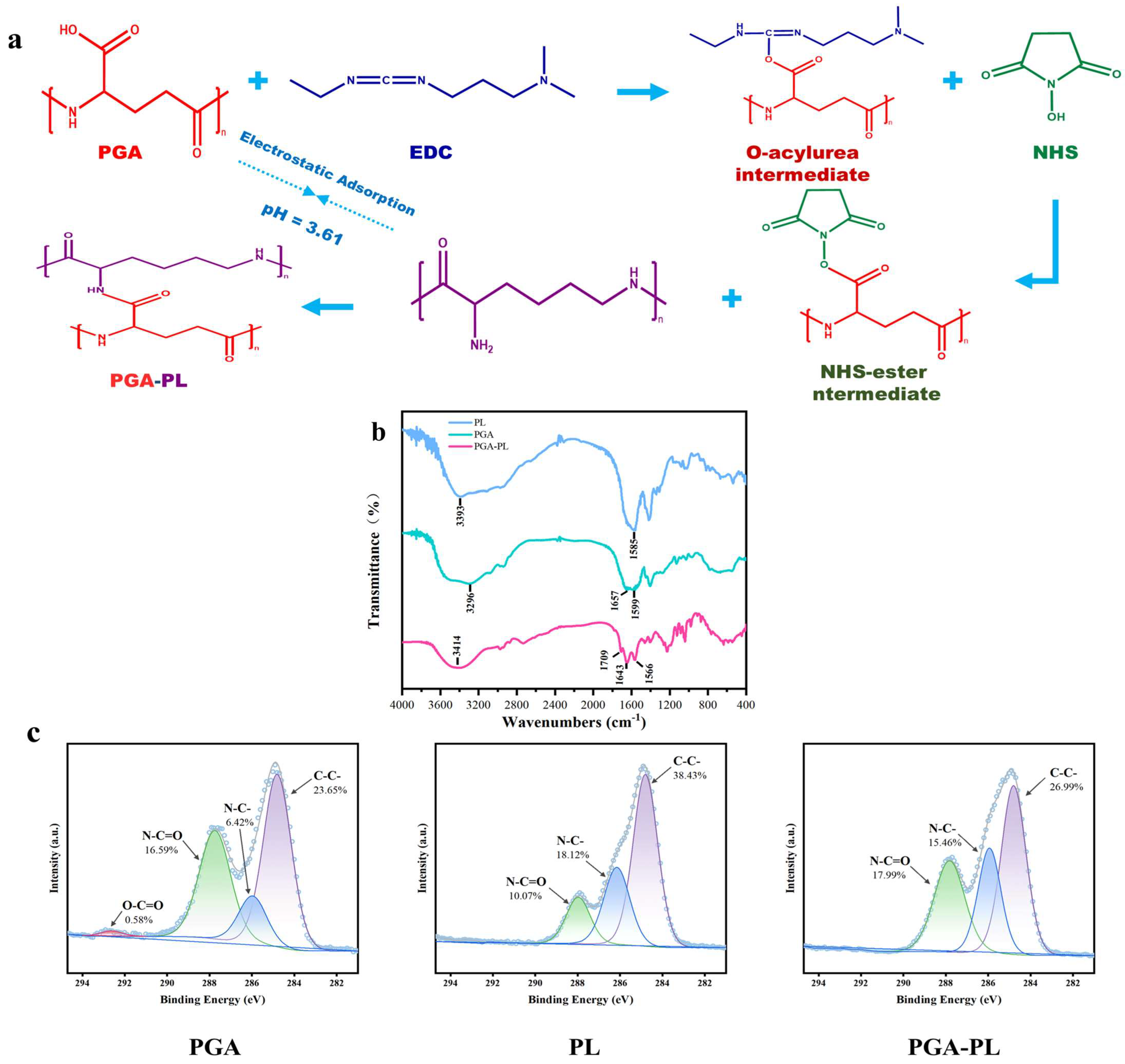
2.1. Synthesis of Hydrogels
2.2. Physicochemical Characterization of Hydrogels
2.2.1. Chemical Structure Analysis of Hydrogels
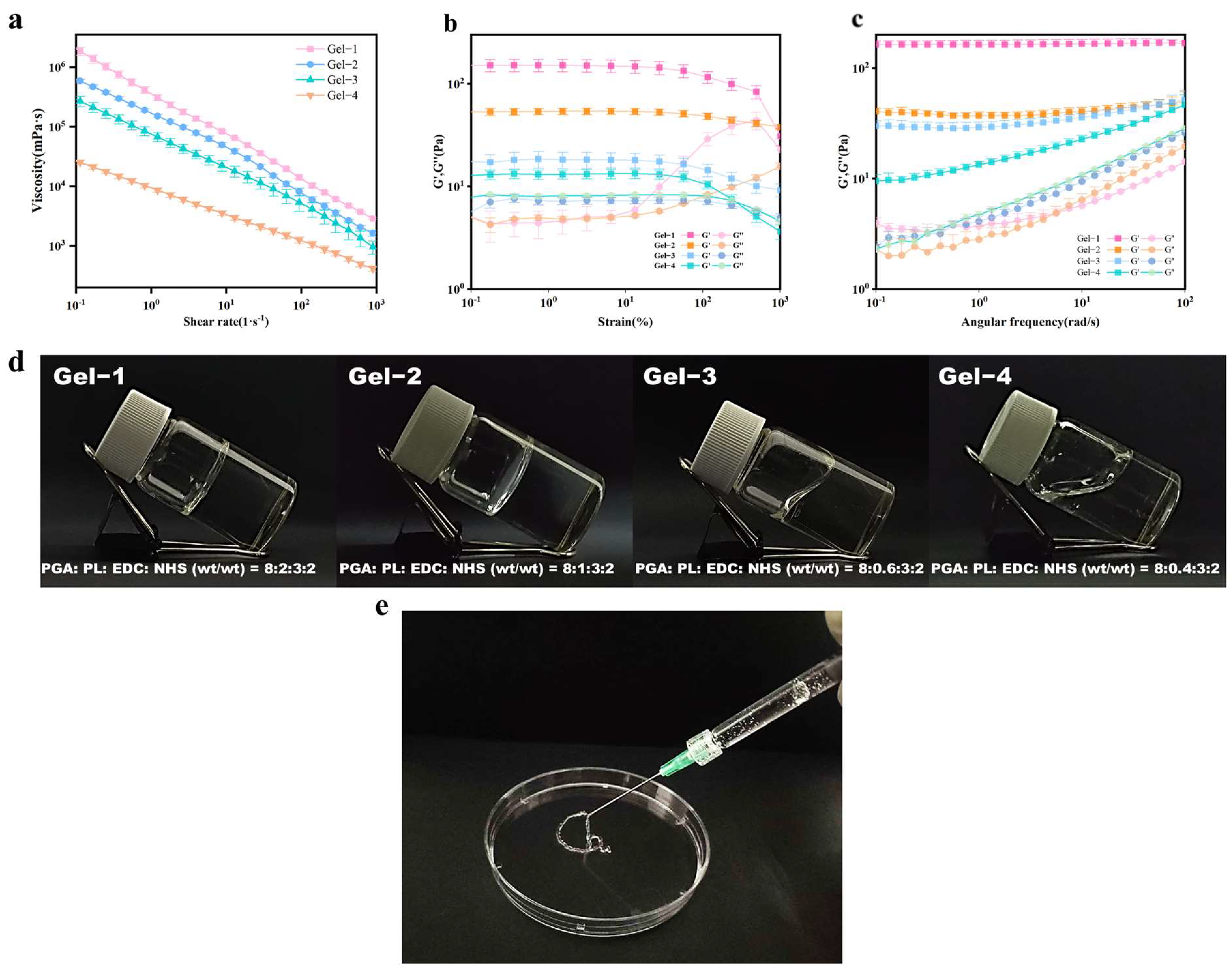
2.2.2. Rheological Analysis of Hydrogels
2.2.3. Swelling Ratio of Hydrogels
2.2.4. In Vitro Degradation of Hydrogels
2.3. In Vitro Biocompatibility of Hydrogels
2.4. In Vivo Biocompatibility of Hydrogels
2.5. In Vivo Wound-Healing Activity
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Hydrogels
4.3. Fourier-Transform Infrared Spectroscopy (FTIR) Characterization
4.4. X-Ray Photoelectron Spectroscopy (XPS) Test
4.5. Rheological Analysis
4.6. Swelling Ratio Test
4.7. In Vitro Degradation of Hydrogels
4.8. Hemolysis Ratios
4.9. In Vitro Biocompatibility of Hydrogels
4.10. In Vivo Degradation of Hydrogels
4.11. In Vivo Wound Healing
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Almadani, Y.H.; Vorstenbosch, J.; Davison, P.G.; Murphy, A.M. Wound Healing: A Comprehensive Review. Semin. Plast. Surg. 2021, 35, 141–144. [Google Scholar]
- Lux, C.N. Wound healing in animals: A review of physiology and clinical evaluation. Vet. Dermatol. 2021, 33, 91-e27. [Google Scholar] [CrossRef]
- Abazari, M.; Ghaffari, A.; Rashidzadeh, H.; Badeleh, S.M.; Maleki, Y. A Systematic Review on Classification, Identification, and Healing Process of Burn Wound Healing. Int. J. Low. Extrem. Wounds 2020, 21, 1534734620924857. [Google Scholar]
- Oliveira, A.; Simões, S.; Ascenso, A.; Reis, C.P. Therapeutic advances in wound healing. J. Dermatol. Treat. 2020, 33, 1–21. [Google Scholar]
- Freedman, B.R.; Hwang, C.; Talbot, S.; Hibler, B.; Matoori, S.; Mooney, D.J. Breakthrough treatments for accelerated wound healing. Sci. Adv. 2023, 9, eade7007. [Google Scholar]
- Wang, X.; Qi, J.; Zhang, W.; Pu, Y.; Yang, R.; Wang, P.; Liu, S.; Tan, X.; Chi, B. 3D-printed antioxidant antibacterial carboxymethyl cellulose/ε-polylysine hydrogel promoted skin wound repair. Int. J. Biol. Macromol. 2021, 187, 91–104. [Google Scholar] [PubMed]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the potential of hydrogels for advanced therapeutic applications: Current achievements and future directions. Signal Transduct. Target. Ther. 2024, 9, 166. [Google Scholar]
- Mahmood, A.; Patel, D.; Hickson, B.; DesRochers, J.; Hu, X. Recent Progress in Biopolymer-Based Hydrogel Materials for Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 1415. [Google Scholar] [CrossRef]
- Yang, R.; Liu, X.; Ren, Y.; Xue, W.; Liu, S.; Wang, P.; Zhao, M.; Xu, H.; Chi, B. Injectable adaptive self-healing hyaluronic acid/poly (γ-glutamic acid) hydrogel for cutaneous wound healing. Acta Biomater. 2021, 127, 102–115. [Google Scholar] [CrossRef]
- Mayandi, V.; Choong, A.C.W.; Dhand, C.; Lim, F.P.; Aung, T.T.; Sriram, H.; Dwivedi, N.; Periayah, M.H.; Sridhar, S.; Fazil, M.H.U.T.; et al. Multifunctional Antimicrobial Nanofiber Dressings Containing ε-Polylysine for the Eradication of Bacterial Bioburden and Promotion of Wound Healing in Critically Colonized Wounds. ACS Appl. Mater. Interfaces 2020, 12, 15989–16005. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [PubMed]
- Liu, S.; Liu, X.; Ren, Y.; Wang, P.H.; Pu, Y.; Yang, R.; Wang, X.; Tan, X.Y.; Ye, Z.; Maurizot, V.; et al. Mussel-Inspired Dual-Cross-linking Hyaluronic Acid/ε-Polylysine Hydrogel with Self-Healing and Antibacterial Properties for Wound Healing. Acs Appl. Mater. Interfaces 2020, 12, 27876–27888. [Google Scholar] [PubMed]
- Li, Y.; Qiu, Y.; Hou, H.; Zhang, G.; Hao, H.; Bi, J. The Preparation and Properties of Amino-Carboxymethyl Chitosan-Based Antibacterial Hydrogel Loaded with ε-Polylysine. Foods 2023, 12, 3807. [Google Scholar] [CrossRef]
- Valachová, K.; El Meligy, M.A.; Šoltés, L. Hyaluronic acid and chitosan-based electrospun wound dressings: Problems and solutions. Int. J. Biol. Macromol. 2022, 206, 74–91. [Google Scholar] [CrossRef]
- Rodrigues, J.P.; Silva, J.R.d.C.; Ferreira, B.A.; Veloso, L.I.; Quirino, L.S.; Rosa, R.R.; Barbosa, M.C.; Rodrigues, C.M.; Gaspari, P.B.F.; Beletti, M.E.; et al. Development of collagenous scaffolds for wound healing: Characterization and in vivo analysis. J. Mater. Sci. Mater. Med. 2024, 35, 12. [Google Scholar]
- Wang, P.; Zhang, W.; Yang, R.; Liu, S.; Ren, Y.; Liu, X.; Tan, X.; Chi, B. Biomimetic poly(γ-glutamic acid) hydrogels based on iron (III) ligand coordination for cartilage tissue engineering. Int. J. Biol. Macromol. 2021, 167, 1508–1516. [Google Scholar] [CrossRef]
- Aunina, K.; Ramata-Stunda, A.; Kovrlija, I.; Tracuma, E.; Merijs-Meri, R.; Nikolajeva, V.; Loca, D. Exploring the Interplay of Antimicrobial Properties and Cellular Response in Physically Crosslinked Hyaluronic Acid/ε-Polylysine Hydrogels. Polymers 2023, 15, 1915. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, J.; Mao, X.; Tang, S. A γ-PGA/KGM-based injectable hydrogel as immunoactive and antibacterial wound dressing for skin wound repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 129, 112374. [Google Scholar]
- Park, S.B.; Sung, M.H.; Uyama, H.; Han, D.K. Poly(glutamic acid): Production, composites, and medical applications of the next-generation biopolymer. Prog. Polym. Sci. 2021, 113, 101341. [Google Scholar]
- Murakami, S.; Aoki, N. Bio-Based Hydrogels Prepared by Cross-Linking of Microbial Poly(γ-glutamic acid) with Various Saccharides. Biomacromolecules 2006, 7, 2122–2127. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Han, Y.; Zheng, X.; Xue, B.; Zhang, X.; Mahmut, Z.; Wang, Y.; Dong, B.; Zhang, C.; Gao, D.; et al. Synthesis of Poly-γ-Glutamic Acid and Its Application in Biomedical Materials. Materials 2023, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, W.; Zhang, Y.; Tan, D.; Cui, M.; Wang, S.; Shi, W. Multifunctional Hydrogels Based on γ-Polyglutamic Acid/Polyethyleneimine for Hemostasis and Wound Healing. Biomater. Res. 2024, 28, 0063. [Google Scholar]
- Wang, G.; Liu, Q.; Yang, Y.M.; Lee, S.U.; Han, J.S.; Jang, K.J.; Kang, D.Y.; Sp, N.; Li, Z.; Tong, T. Applications and Functions of γ-Poly-Glutamic Acid and its Derivatives in Medicine. Curr. Pharm. Biotechnol. 2021, 22, 1404–1411. [Google Scholar] [PubMed]
- Xu, T.; Yang, R.; Ma, X.; Chen, W.; Liu, S.; Liu, X.; Cai, X.; Xu, H.; Chi, B. Bionic Poly(γ-Glutamic Acid) Electrospun Fibrous Scaffolds for Preventing Hypertrophic Scars. Adv. Healthc. Mater. 2019, 8, 1900123. [Google Scholar]
- Zarrintaj, P.; Ghorbani, S.; Barani, M.; Chauhan, N.P.S.; Yazdi, M.K.; Saeb, M.R.; Ramsey, J.D.; Hamblin, M.R.; Mozafari, M.; Mostafavi, E. Polylysine for skin regeneration: A review of recent advances and future perspectives. Bioeng. Transl. Med. 2021, 7, e10261. [Google Scholar]
- Xu, Q.; Dai, X.; Yang, L.; Liu, X.; Li, Y.; Gao, F. ε-Polylysine-Based Macromolecules with Catalase-Like Activity to Accelerate Wound Healing by Clearing Bacteria and Attenuating Inflammatory Response. ACS Biomater. Sci. Eng. 2022, 8, 5018–5026. [Google Scholar]
- Zarrintaj, P.; Yazdi, M.K.; Azarfam, M.Y.; Zare, M.; Ramsey, J.D.; Seidi, F.; Saeb, M.R.; Ramakrishna, S.; Mozafari, M. Injectable Cell-Laden Hydrogels for Tissue Engineering: Recent Advances and Future Opportunities. Tissue Eng. Part A 2021, 27, 821–843. [Google Scholar]
- Wei, M.; Hsu, Y.-I.; Asoh, T.-A.; Sung, M.-H.; Uyama, H. Injectable poly(γ-glutamic acid)-based biodegradable hydrogels with tunable gelation rate and mechanical strength. J. Mater. Chem. B 2021, 9, 3584–3594. [Google Scholar]
- Zhao, C.; Zhuang, X.; He, P.; Xiao, C.; He, C.; Sun, J.; Chen, X.; Jing, X. Synthesis of biodegradable thermo- and pH-responsive hydrogels for controlled drug release. Polymer 2009, 50, 4308–4316. [Google Scholar] [CrossRef]
- Zhu, Q.; Hong, Y.; Huang, Y.; Zhang, Y.; Xie, C.; Liang, R.; Li, C.; Zhang, T.; Wu, H.; Ye, J.; et al. Polyglutamic Acid-Based Elastic and Tough Adhesive Patch Promotes Tissue Regeneration through In Situ Macrophage Modulation. Adv. Sci. 2022, 9, 2106115. [Google Scholar] [CrossRef]
- Manohar, S.M.; A Padgaonkar, A.; Jalota-Badhwar, A.; Sonawane, V.; Rathos, M.J.; Kumar, S.; Joshi, K.S. A novel inhibitor of hypoxia-inducible factor-1α P3155 also modulates PI3K pathway and inhibits growth of prostate cancer cells. BMC Cancer 2011, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yao, L.; Yang, J.; Wang, Z.; Du, G. PI3K/Akt and HIF 1 signaling pathway in hypoxia ischemia (Review). Mol. Med. Rep. 2018, 18, 3547–3554. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, W.; Tang, C.; Wang, C.; Liu, C.; Chen, Q.; Yang, K.; Gu, Y.; Lei, P.; Xu, H.; et al. Antidiabetic effects and mechanism of γ-polyglutamic acid on type II diabetes mice. Int. J. Biol. Macromol. 2024, 261 Pt 1, 129809. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Luo, M.; Chen, M.; Wang, M.; Qu, X.; Lei, B. Bioactive Poly(octanediol-citrate-polyglycol) Accelerates Skin Regeneration through M2 Polarization Immunomodulating and Early Angiogenesis. Adv. Healthc. Mater. 2022, 11, 2101931. [Google Scholar] [CrossRef]
- Ahn, H.; Kang, S.G.; Yoon, S.I.; Kim, P.H.; Kim, D.; Lee, G.S. Poly-gamma-glutamic acid from Bacillus subtilis upregulates pro-inflammatory cytokines while inhibiting NLRP3, NLRC4 and AIM2 inflammasome activation. Cell. Mol. Immunol. 2018, 15, 111–119. [Google Scholar] [CrossRef]
- Lee, K.; Hwang, S.; Paik, D.J.; Kim, W.K.; Kim, J.M.; Youn, J. Bacillus-derived poly-γ-glutamic acid reciprocally regulates the differentiation of T helper 17 and regulatory T cells and attenuates experimental autoimmune encephalomyelitis. Clin. Exp. Immunol. 2012, 170, 66–76. [Google Scholar] [CrossRef][Green Version]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Huang, Y.; Wang, Y.; Han, Q. A Poly-γ-Glutamic Acid/ε-Polylysine Hydrogel: Synthesis, Characterization, and Its Role in Accelerated Wound Healing. Gels 2025, 11, 226. https://doi.org/10.3390/gels11040226
Li J, Huang Y, Wang Y, Han Q. A Poly-γ-Glutamic Acid/ε-Polylysine Hydrogel: Synthesis, Characterization, and Its Role in Accelerated Wound Healing. Gels. 2025; 11(4):226. https://doi.org/10.3390/gels11040226
Chicago/Turabian StyleLi, Jiaqi, Yuanli Huang, Yalu Wang, and Qianqian Han. 2025. "A Poly-γ-Glutamic Acid/ε-Polylysine Hydrogel: Synthesis, Characterization, and Its Role in Accelerated Wound Healing" Gels 11, no. 4: 226. https://doi.org/10.3390/gels11040226
APA StyleLi, J., Huang, Y., Wang, Y., & Han, Q. (2025). A Poly-γ-Glutamic Acid/ε-Polylysine Hydrogel: Synthesis, Characterization, and Its Role in Accelerated Wound Healing. Gels, 11(4), 226. https://doi.org/10.3390/gels11040226







