Preparation and Heavy Metal Adsorption Performance of 2-Aminopyridine-Modified Sodium Alginate/Polyacrylic Acid Hydrogel
Abstract
1. Introduction
2. Results and Discussion
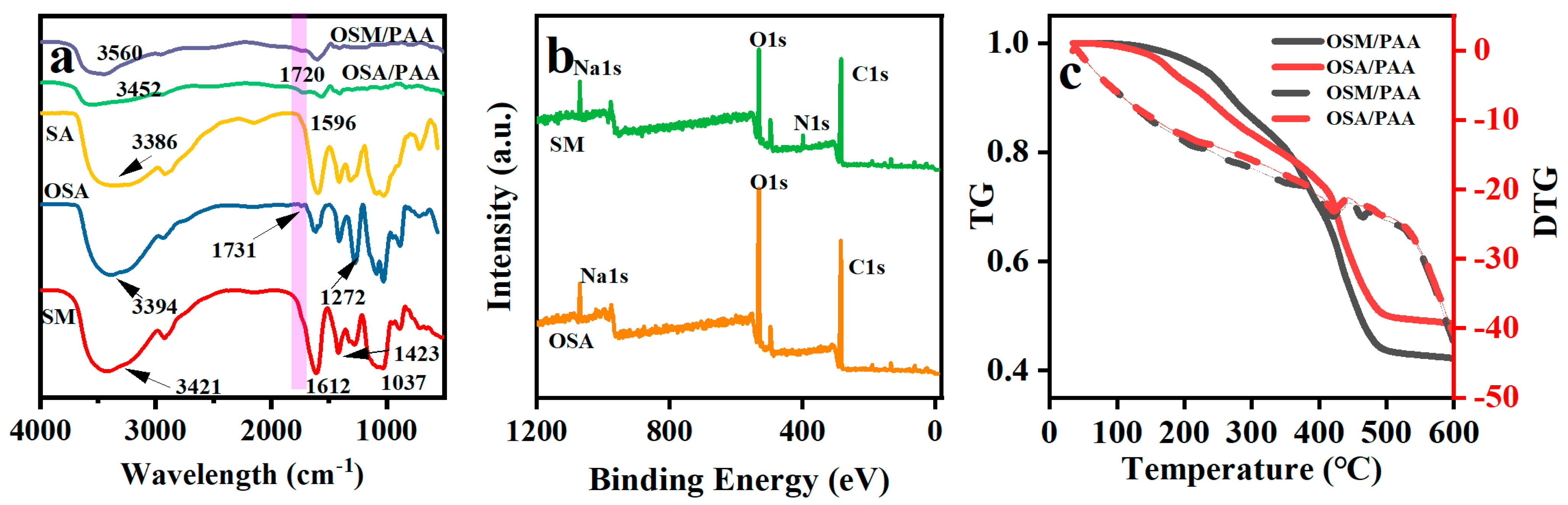
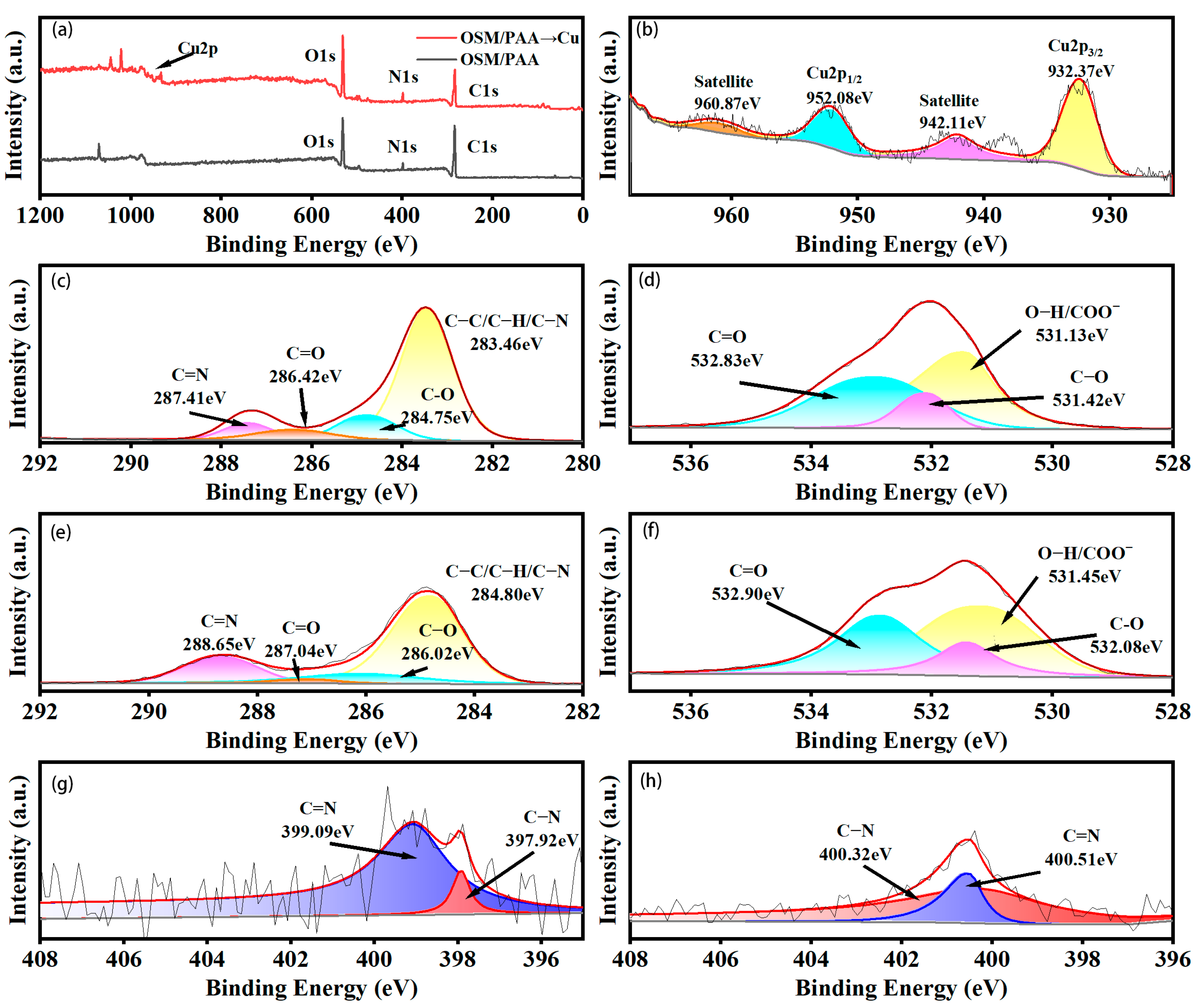
2.1. FT-IR and XPS Analysis
2.2. TGA
2.3. SEM
2.4. Synthesis Conditions and Properties of OSM/PAA Hydrogels
2.4.1. Selection of the Optimal Synthesis Conditions
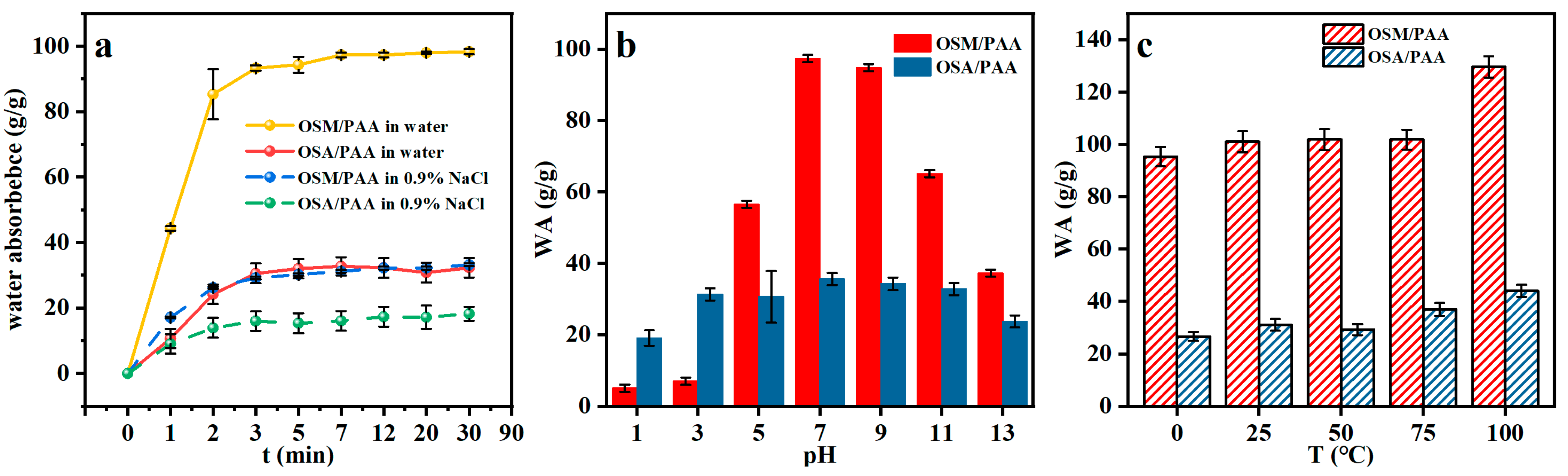
2.4.2. Swelling Behavior of OSM/PAA Hydrogels
2.4.3. Comparison of OSA/PAA and OSM/PAA Adsorption Capacity
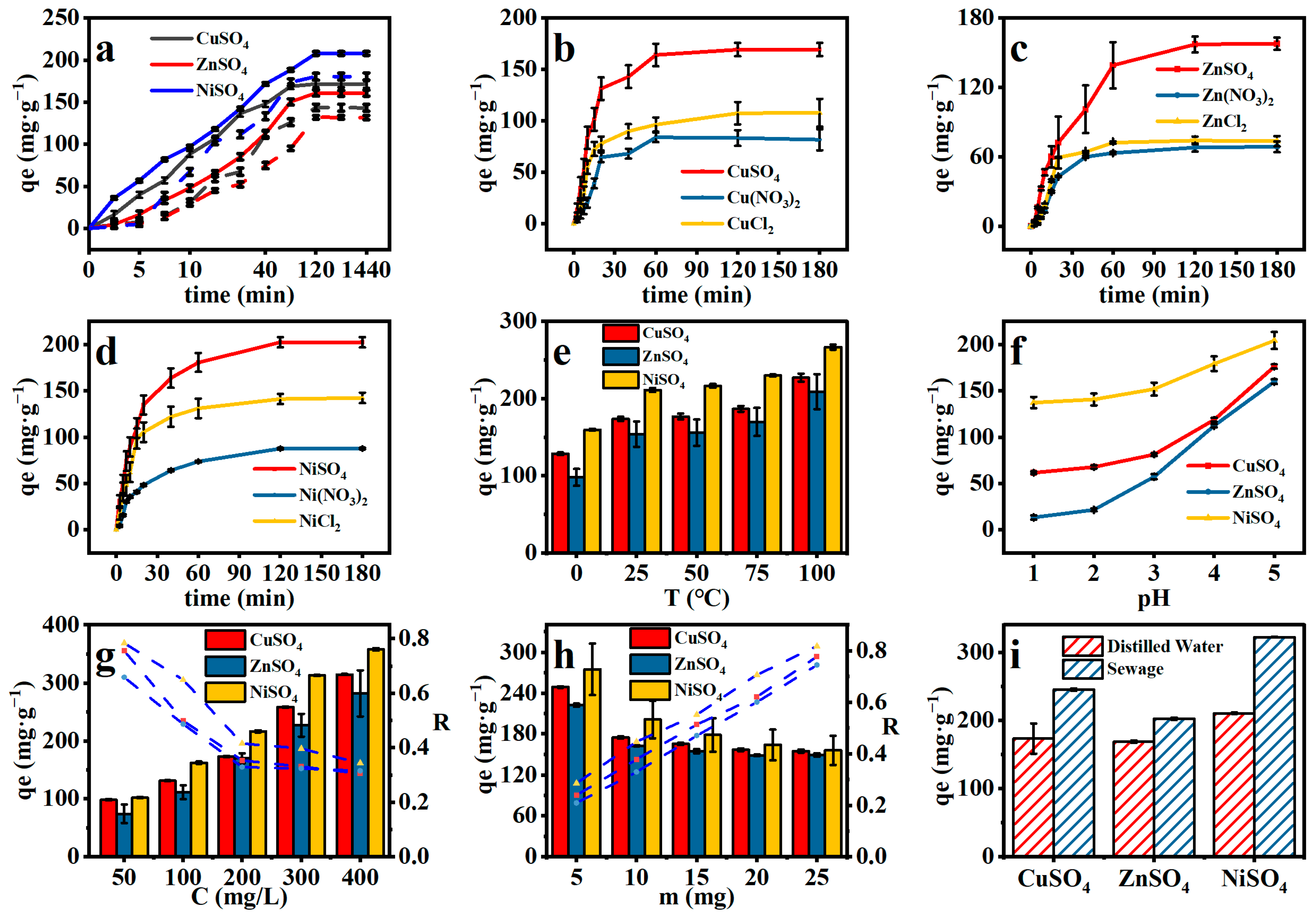
2.4.4. Effect of Anions on Adsorption
2.4.5. Effect of pH and Temperature on Adsorption
2.4.6. Effect of Metal Concentration and Dosage on Adsorption
2.4.7. Realistic Wastewater Simulation Experiments
2.4.8. Competitive Adsorption
2.4.9. Effect of Temperature on Adsorption
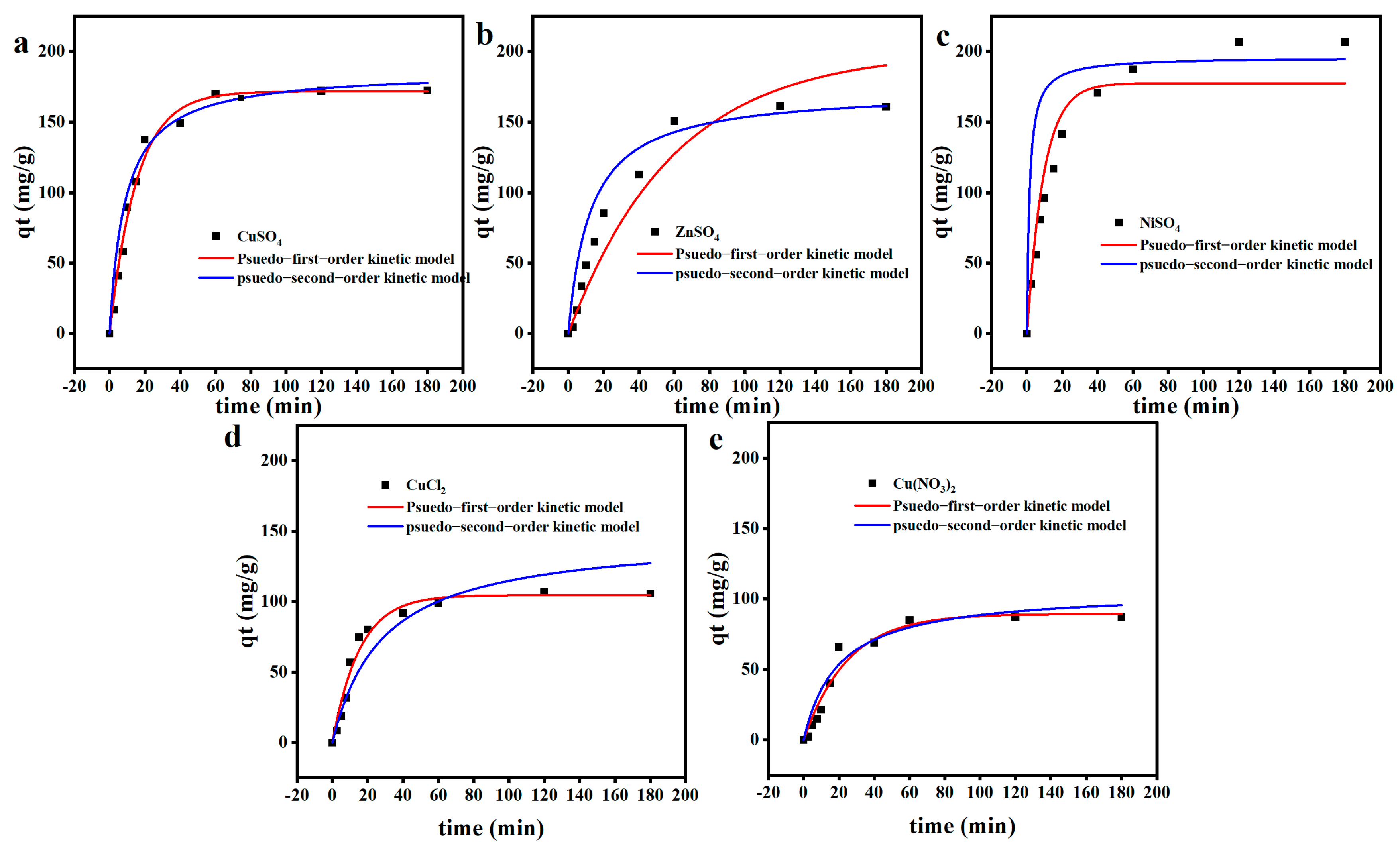
2.5. Adsorption Kinetics
2.6. Adsorption Isotherms
2.7. Repeatability Testing of Hydrogels
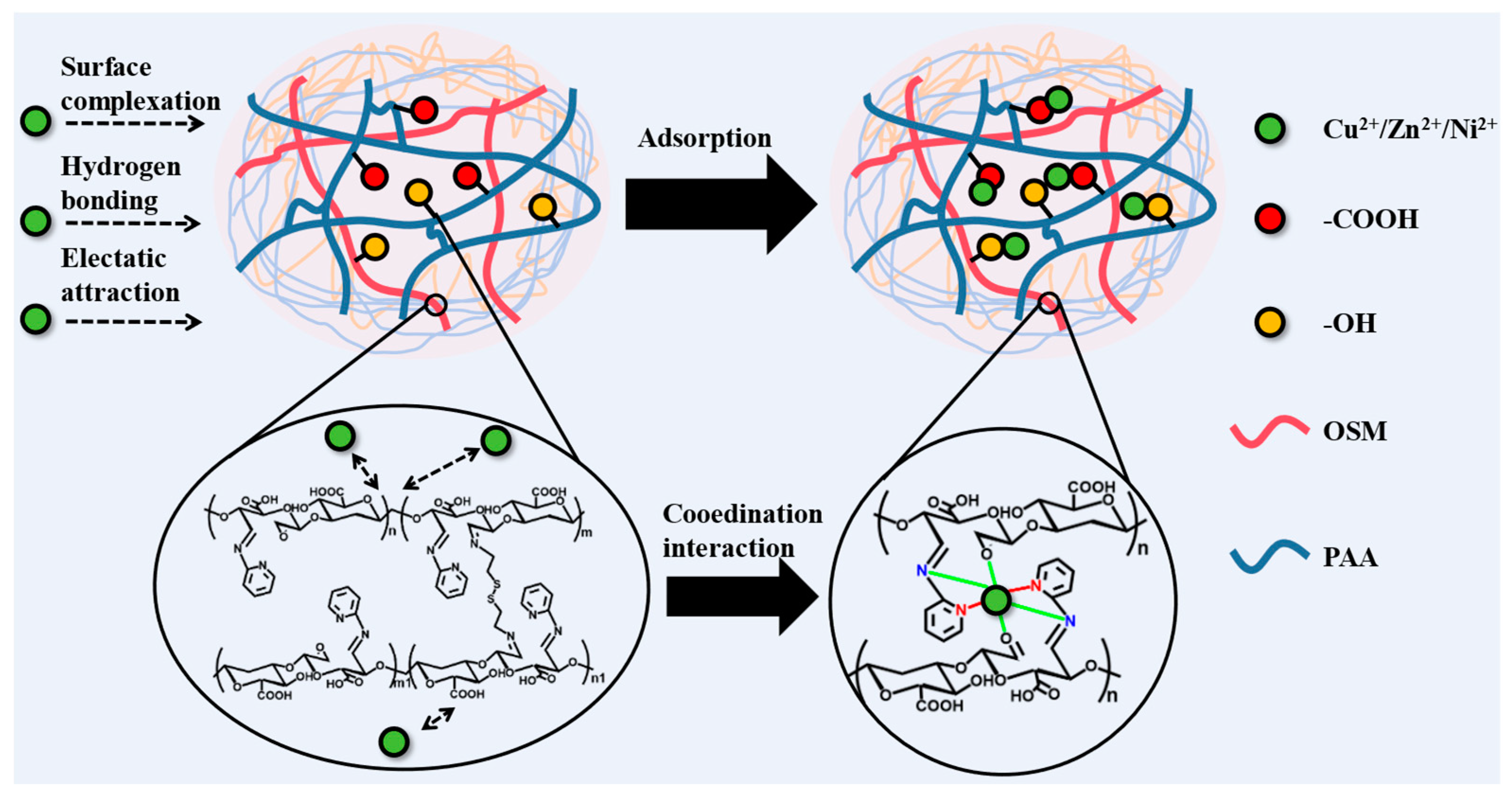
2.8. Adsorption Mechanism of OSM/PAA Hydrogels
3. Conclusions
4. Experimental Section
4.1. Materials
4.2. Oxidation of Sodium Alginate
4.3. Synthesis of Sodium 2-Aminopyridine Alginate (OSM)
4.4. Synthesis of OSM/PAA and OSA/PAA Hydrogels
4.5. Characterization
4.6. Determination of Heavy Metal Adsorption Capacity
4.7. Methods for Determining Adsorption Kinetics and Isotherms
4.8. Recyclability Testing of Hydrogels
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bao, T.; Wang, P.; Hu, B.; Jin, Q.; Zheng, T.; Li, D. Adsorption and distribution of heavy metals in aquatic environments: The role of colloids and effects of environmental factors. J. Hazard. Mater. 2024, 474, 134725. [Google Scholar]
- Waqas, W.; Yuan, Y.; Ali, S.; Zhang, M.; Shafiq, M.; Ali, W.; Chen, Y.; Xiang, Z.; Chen, R.; Ikhwanuddin, M.; et al. Toxic effects of heavy metals on crustaceans and associated health risks in humans: A review. Environ. Chem. Lett. 2024, 22, 1391–1411. [Google Scholar] [CrossRef]
- Yan, J.; Qu, Z.; Li, F.; Li, H. Heavy metals in the water environment of Yangtze River Economic Belt: Status, fuzzy environmental risk assessment and management. Urban Clim. 2021, 40, 100981. [Google Scholar]
- Yi, Z.; Jia, J.; Yang, J.L.; Yu, J.; Tan, S.; Li, J.; Liu, X.; Sang, Z.; Yin, L.; Liu, H.; et al. Zinc Affinity and Hydrogen Evolution Trade-Off for Homogenous Zn Deposition in Reversible Zn Ion Batteries. Small 2024, 20, e2405300. [Google Scholar]
- Sarker, A.; Masud, M.A.A.; Deepo, D.M.; Das, K.; Nandi, R.; Ansary, M.W.R.; Islam, A.R.M.T.; Islam, T. Biological and green remediation of heavy metal contaminated water and soils: A state-of-the-art review. Chemosphere 2023, 332, 138861. [Google Scholar] [PubMed]
- Izydorczyk, G.; Mikula, K.; Skrzypczak, D.; Moustakas, K.; Witek-Krowiak, A.; Chojnacka, K. Potential environmental pollution from copper metallurgy and methods of management. Environ. Res. 2021, 197, 111050. [Google Scholar]
- Lidman, J.; Jonsson, M.; Berglund, Å.M.M. The effect of lead (Pb) and zinc (Zn) contamination on aquatic insect community composition and metamorphosis. Sci. Total Environ. 2020, 734, 139406. [Google Scholar]
- Zhang, P.; Hu, R.; Zhu, L.; Wang, P.; Yin, D.; Zhang, L. Distributions and contamination assessment of heavy metals in the surface sediments of western Laizhou Bay: Implications for the sources and influencing factors. Mar. Pollut. Bull. 2017, 119, 429–438. [Google Scholar]
- Mehnaz, M.; Jolly, Y.N.; Alam, A.K.M.R.; Kabir, J.; Akter, S.; Mamun, K.M.; Rahman, A.; Islam, M.M. Prediction of Hazardous Effect of Heavy Metals of Point-Source Wastewater on Fish (Anabas cobojius) and Human Health. Biol. Trace Elem. Res. 2022, 201, 3031–3049. [Google Scholar] [CrossRef]
- Zaynab, M.; Al-Yahyai, R.; Ameen, A.; Sharif, Y.; Ali, L.; Fatima, M.; Khan, K.A.; Li, S. Health and environmental effects of heavy metals. J. King Saud Univ. Sci. 2022, 34, 101653. [Google Scholar]
- Abdeldayem, R. A preliminary study of heavy metals pollution risk in water. Appl. Water Sci. 2019, 10, 1. [Google Scholar]
- Chen, Q.; Yao, Y.; Li, X.; Lu, J.; Zhou, J.; Huang, Z. Comparison of heavy metal removals from aqueous solutions by chemical precipitation and characteristics of precipitates. J. Water Process Eng. 2018, 26, 289–300. [Google Scholar]
- Fu, Z.-J.; Jiang, S.-K.; Chao, X.-Y.; Zhang, C.-X.; Shi, Q.; Wang, Z.-Y.; Liu, M.-L.; Sun, S.-P. Removing miscellaneous heavy metals by all-in-one ion exchange-nanofiltration membrane. Water Res. 2022, 222, 118888. [Google Scholar] [PubMed]
- Sudilovskiy, P.S.; Kagramanov, G.G.; Trushin, A.M.; Kolesnikov, V.A. Use of membranes for heavy metal cationic wastewater treatment: Flotation and membrane filtration. Clean Technol. Environ. Policy 2007, 9, 189–198. [Google Scholar]
- Dhokpande, S.R.; Deshmukh, S.M.; Khandekar, A.; Sankhe, A. A review outlook on methods for removal of heavy metal ions from wastewater. Sep. Purif. Technol. 2024, 350, 127868. [Google Scholar]
- Qiu, B.; Tao, X.; Wang, H.; Li, W.; Ding, X.; Chu, H. Biochar as a low-cost adsorbent for aqueous heavy metal removal: A review. J. Anal. Appl. Pyrolysis 2021, 155, 105081. [Google Scholar]
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological trends in heavy metals removal from industrial wastewater: A review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar]
- Qu, M.; Xiong, J.; Zhou, J.; Wang, L.; Hu, T.; Liu, F.; Zhang, Q. Modified water treatment residual serves as an adsorbent for the removal of heavy metals from water: A review. J. Ind. Eng. Chem. 2024. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Zhou, R.; Qiao, J.; Liu, J.; Cai, R.; Liu, J.; Rong, J.; Chen, Y. Porous sodium alginate/cellulose nanofiber composite hydrogel microspheres for heavy metal removal in wastewater. Int. J. Biol. Macromol. 2024, 278, 135000. [Google Scholar]
- Wang, L.; Dai, X.; Man, Z.; Li, J.; Jiang, Y.; Liu, D.; Xiao, H.; Shah, S. Dynamics and Treatability of Heavy Metals in Pig Farm Effluent Wastewater by Using UiO-66 and UiO-66-NH2 Nanomaterials as Adsorbents. Water Air Soil Pollut. 2021, 232, 294. [Google Scholar]
- Alsamman, M.T.; Sánchez, J. Recent advances on hydrogels based on chitosan and alginate for the adsorption of dyes and metal ions from water. Arab. J. Chem. 2021, 14, 103455. [Google Scholar] [CrossRef]
- Godiya, C.B.; Martins Ruotolo, L.A.; Cai, W. Functional biobased hydrogels for the removal of aqueous hazardous pollutants: Current status, challenges, and future perspectives. J. Mater. Chem. A 2020, 8, 21585–21612. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, G.; Tan, Q.; Gao, M.; Chen, G.; Huang, X.; Xu, X.; Li, L.; Wang, J.; Zhang, Y.; et al. Polysaccharide-based biopolymer hydrogels for heavy metal detection and adsorption. J. Adv. Res. 2023, 44, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Hu, Y.H. Design, synthesis, and performance of adsorbents for heavy metal removal from wastewater: A review. J. Mater. Chem. A 2022, 10, 1047–1085. [Google Scholar] [CrossRef]
- Cai, R.; Chen, Y.; Hu, J.; Xiong, J.; Lu, J.; Liu, J.; Tan, X.; Liu, W.; Zhou, Y.; Chen, Y. A self-supported sodium alginate composite hydrogel membrane and its performance in filtering heavy metal ions. Carbohydr. Polym. 2023, 300, 120278. [Google Scholar] [CrossRef]
- Kushwaha, J.; Singh, R. Cellulose hydrogel and its derivatives: A review of application in heavy metal adsorption. Inorg. Chem. Commun. 2023, 152, 110721. [Google Scholar] [CrossRef]
- Al-Hazmi, H.E.; Łuczak, J.; Habibzadeh, S.; Hasanin, M.S.; Mohammadi, A.; Esmaeili, A.; Kim, S.-J.; Khodadadi Yazdi, M.; Rabiee, N.; Badawi, M.; et al. Polysaccharide nanocomposites in wastewater treatment: A review. Chemosphere 2024, 347, 140578. [Google Scholar] [CrossRef]
- Sajjadi, M.; Ahmadpoor, F.; Nasrollahzadeh, M.; Ghafuri, H. Lignin-derived (nano)materials for environmental pollution remediation: Current challenges and future perspectives. Int. J. Biol. Macromol. 2021, 178, 394–423. [Google Scholar] [CrossRef]
- Musarurwa, H.; Tavengwa, N.T. Application of carboxymethyl polysaccharides as bio-sorbents for the sequestration of heavy metals in aquatic environments. Carbohydr. Polym. 2020, 237, 116142. [Google Scholar] [CrossRef]
- Kayan, G.Ö.; Kayan, A. Composite of Natural Polymers and Their Adsorbent Properties on the Dyes and Heavy Metal Ions. J. Polym. Environ. 2021, 29, 3477–3496. [Google Scholar] [CrossRef]
- Gao, X.; Guo, C.; Hao, J.; Zhao, Z.; Long, H.; Li, M. Adsorption of heavy metal ions by sodium alginate based adsorbent-a review and new perspectives. Int. J. Biol. Macromol. 2020, 164, 4423–4434. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, F.; Xu, K.; Che, Y.; Qi, M.; Song, C. Modified magnetic chitosan materials for heavy metal adsorption: A review. RSC Adv. 2023, 13, 6713–6736. [Google Scholar] [PubMed]
- Mahdavi, M.; Mahmoudi, N.; Rezaie Anaran, F.; Simchi, A. Electrospinning of Nanodiamond-Modified Polysaccharide Nanofibers with Physico-Mechanical Properties Close to Natural Skins. Mar. Drugs 2016, 14, 128. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Shi, X.; Wu, S.; Liu, X.; Deng, H.; Du, Y.; Li, H. Flexible Polysaccharide Hydrogel with pH-Regulated Recovery of Self-Healing and Mechanical Properties. Macromol. Mater. Eng. 2017, 302, 1700221. [Google Scholar]
- Haddad, C.; Edirisinghe, E.A.K.D.; Brown, H.M.; Ostrowski, A.D. Photoreactivity and Enhanced Mechanical Properties and Water Stability in Polysaccharide-Based Films Using Vanadium Ion Coordination. ACS Appl. Polym. Mater. 2022, 4, 859–867. [Google Scholar]
- Dodda, J.M.; Azar, M.G.; Bělský, P.; Šlouf, M.; Brož, A.; Bačáková, L.; Kadlec, J.; Remiš, T. Biocompatible hydrogels based on chitosan, cellulose/starch, PVA and PEDOT:PSS with high flexibility and high mechanical strength. Cellulose 2022, 29, 6697–6717. [Google Scholar]
- Yang, S.C.; Liao, Y.; Karthikeyan, K.G.; Pan, X.J. Mesoporous cellulose-chitosan composite hydrogel fabricated via the co-dissolution-regeneration process as biosorbent of heavy metals. Environ. Pollut. 2021, 286, 117324. [Google Scholar]
- Alraddadi, H.M.; Fagieh, T.M.; Bakhsh, E.M.; Akhtar, K.; Khan, S.B.; Khan, S.A.; Bahaidarah, E.A.; Homdi, T.A. Adsorptive removal of heavy metals and organic dyes by sodium alginate/coffee waste composite hydrogel. Int. J. Biol. Macromol. 2023, 247, 125708. [Google Scholar]
- Dong, K.; Jiang, Y.; Zhang, Y.; Qin, Z.; Mo, L. Tannic acid-assisted fabrication of antibacterial sodium alginate-based gel beads for the multifunctional adsorption of heavy metal ions and dyes. Int. J. Biol. Macromol. 2023, 252, 126249. [Google Scholar]
- Zhang, P.; Zou, K.; Yuan, L.; Liu, J.; Liu, B.; Qing, T.-P.; Feng, B. A biomass resource strategy for alginate-polyvinyl alcohol double network hydrogels and their adsorption to heavy metals. Sep. Purif. Technol. 2022, 301, 112050. [Google Scholar]
- Pérez-Cid, B.; Calvar, S.; Moldes, A.B.; Manuel Cruz, J. Effective Removal of Cyanide and Heavy Metals from an Industrial Electroplating Stream Using Calcium Alginate Hydrogels. Molecules 2020, 25, 5183. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Wang, K.; Bai, B. A critical review of sodium alginate-based composites in water treatment. Carbohydr. Polym. 2024, 331, 121850. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Xu, X.; Ouyang, X.-K.; Jin, M.-C. Adsorption of Pb(II) from Aqueous Solutions Using Nanocrystalline Cellulose/Sodium Alginate/K-Carrageenan Composite Hydrogel Beads. J. Polym. Environ. 2021, 30, 1995–2006. [Google Scholar] [CrossRef]
- Zheng, C.; Wu, Q.; Sun, K.; Xu, B.; Sun, Y.; Zheng, H. Insight into the impact of environmental factors on heavy metal adsorption by sodium alginate hydrogel: Inspiration on applicable scenarios. Environ. Res. 2024, 262, 119878. [Google Scholar] [CrossRef]
- Liang, L.; Liu, T.; Ouyang, Q.; Li, S.; Li, C. Solid phase synthesis of oxidized sodium alginate-tobramycin conjugate and its application for infected wound healing. Carbohydr. Polym. 2022, 295, 119843. [Google Scholar] [CrossRef]
- Guo, F.; Liu, Y.; Chen, S.; Lin, Y.; Yue, Y. A Schiff base hydrogel dressing loading extracts from Periplaneta Americana for diabetic wound healing. Int. J. Biol. Macromol. 2023, 230, 123256. [Google Scholar] [CrossRef]
- Gomez, C.G.; Rinaudo, M.; Villar, M.A. Oxidation of sodium alginate and characterization of the oxidized derivatives. Carbohydr. Polym. 2007, 67, 296–304. [Google Scholar] [CrossRef]
- Kristiansen, K.A.; Tomren, H.B.; Christensen, B.E. Periodate oxidized alginates: Depolymerization kinetics. Carbohydr. Polym. 2011, 86, 1595–1601. [Google Scholar] [CrossRef]
- Shi, T.; Xie, Z.; Zhu, Z.; Shi, W.; Liu, Y.; Liu, M. Highly efficient and selective adsorption of heavy metal ions by hydrazide-modified sodium alginate. Carbohydr. Polym. 2022, 276, 118797. [Google Scholar] [CrossRef]
- Nematidil, N.; Sadeghi, M.; Nezami, S.; Sadeghi, H. Synthesis and characterization of Schiff-base based chitosan-g-glutaraldehyde/NaMMTNPs-APTES for removal Pb2+ and Hg2+ ions. Carbohydr. Polym. 2019, 222, 114971. [Google Scholar] [CrossRef]
- Li, J.; Xu, Z.; Wu, W.; Jing, Y.; Dai, H.; Fang, G. Nanocellulose/Poly(2-(dimethylamino)ethyl methacrylate)Interpenetrating polymer network hydrogels for removal of Pb(II) and Cu(II) ions. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 474–480. [Google Scholar]
- Zhao, B.; Jiang, H.; Lin, Z.; Xu, S.; Xie, J.; Zhang, A. Preparation of acrylamide/acrylic acid cellulose hydrogels for the adsorption of heavy metal ions. Carbohydr. Polym. 2019, 224, 115022. [Google Scholar] [PubMed]
- Bain, E.D.; Long, T.R.; Beyer, F.L.; Savage, A.M.; Dadmun, M.D.; Martin, H.; Lenhart, J.L.; Mrozek, R.A. Tough, Rapidly Swelling Thermoplastic Elastomer Hydrogels for Hemorrhage Control. Macromolecules 2018, 51, 4705–4717. [Google Scholar]
- Sura, N.; Okabe, H.; Omondi, B.A.; Mufundirwa, A.; Hidaka, Y.; Hara, K. Study on the influence of inductive groups on the performance of carboxylate-based hydrogel polymer network. Polym. Test. 2019, 80, 106117. [Google Scholar]
- Weißpflog, J.; Gündel, A.; Vehlow, D.; Steinbach, C.; Müller, M.; Boldt, R.; Schwarz, S.; Schwarz, D. Solubility and Selectivity Effects of the Anion on the Adsorption of Different Heavy Metal Ions onto Chitosan. Molecules 2020, 25, 2482. [Google Scholar] [CrossRef]
- Millar, G.J.; Couperthwaite, S.J.; Wellner, D.B.; Macfarlane, D.C.; Dalzell, S.A. Removal of fluoride ions from solution by chelating resin with imino-diacetate functionality. J. Water Process Eng. 2017, 20, 113–122. [Google Scholar]
- Yang, L.; Bao, L.; Dong, T.; Xie, H.; Wang, X.; Wang, H.; Wu, J.; Hao, C. Adsorption properties of cellulose/guar gum/biochar composite hydrogel for Cu2+, Co2+ and methylene blue. Int. J. Biol. Macromol. 2023, 242, 125021. [Google Scholar]
- Kong, W.; Li, Q.; Li, X.; Su, Y.; Yue, Q.; Zhou, W.; Gao, B. Removal of copper ions from aqueous solutions by adsorption onto wheat straw cellulose-based polymeric composites. J. Appl. Polym. Sci. 2018, 135, 46680. [Google Scholar]
- Zheng, Z.; Ali, A.; Su, J.; Zhang, S.; Fan, Y.; Sun, Y. Self-immobilized biochar fungal pellet combined with bacterial strain H29 enhanced the removal performance of cadmium and nitrate. Bioresour. Technol. 2021, 341, 125803. [Google Scholar]
- Mo, Z.; Tai, D.; Zhang, H.; Xiao, Y. Advances in the removal of heavy metals by UIO-66-based metal-organic framework. New Chem. Mater. 2023, 51, 33–41. [Google Scholar]
- Zhao, Z.; Huang, Y.; Wu, Y.; Li, S.; Yin, H.; Wang, J. α-ketoglutaric acid modified chitosan/polyacrylamide semi-interpenetrating polymer network hydrogel for removal of heavy metal ions. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127262. [Google Scholar] [CrossRef]
- Zhang, M.; Song, L.; Jiang, H.; Li, S.; Shao, Y.; Yang, J.; Li, J. Biomass based hydrogel as an adsorbent for the fast removal of heavy metal ions from aqueous solutions. J. Mater. Chem. A 2017, 5, 3434–3446. [Google Scholar] [CrossRef]
- Du, M.; Cao, Y.; Luo, X.; Yang, W.; Lin, W.; Wang, Y.; Tang, W.; Li, Z. Shapeable sodium alginate aerogel beads incorporated with L-cysteine-modified defective UiO-67 for heavy metal ions removal. Chem. Eng. J. 2023, 475, 146289. [Google Scholar] [CrossRef]
- Jiang, M.; Simayi, R.; Sawut, A.; Wang, J.; Wu, T.; Gong, X. Modified β-Cyclodextrin hydrogel for selective adsorption and desorption for cationic dyes. Colloids Surf. A Physicochem. Eng. Asp. 2023, 661, 130912. [Google Scholar] [CrossRef]
- Kopinke, F.-D.; Georgi, A.; Goss, K.-U. Comment on “Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solution: A critical review, published by Tran et al. [Water Research 120, 2017, 88–116]”. Water Res. 2018, 129, 520–521. [Google Scholar] [CrossRef]
- Robshaw, T.; Tukra, S.; Hammond, D.B.; Leggett, G.J.; Ogden, M.D. Highly efficient fluoride extraction from simulant leachate of spent potlining via La-loaded chelating resin. An equilibrium study. J. Hazard. Mater. 2019, 361, 200–209. [Google Scholar] [CrossRef]
- Yang, F.; Sun, S.; Chen, X.; Chang, Y.; Zha, F.; Lei, Z. Mg–Al layered double hydroxides modified clay adsorbents for efficient removal of Pb2+, Cu2+ and Ni2+ from water. Appl. Clay Sci. 2016, 123, 134–140. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Y.-F.; Zeng, J.; Zhang, Y.; Zhang, Z.-B.; Zhang, Z.-J.; Ma, S.; Tang, C.-M.; Xu, J.-Q. Chitosan/polyethyleneimine magnetic hydrogels for adsorption of heavy metal ions. Iran. Polym. J. 2022, 31, 1273–1282. [Google Scholar]
- Lin, X.; Jin, J.; Guo, X.; Jia, X. All-carboxymethyl cellulose sponges for removal of heavy metal ions. Cellulose 2021, 28, 3113–3122. [Google Scholar] [CrossRef]
- Yue, H.; Shang, Z.; Xu, P.; Feng, D.; Li, X. Preparation of EDTA modified chitooligosaccharide/sodium alginate/Ca2+ physical double network hydrogel by using of high-salinity oilfield produced water for adsorption of Zn2+, Ni2+ and Mn2+. Sep. Purif. Technol. 2022, 280, 119767. [Google Scholar]
- Aljar, M.A.A.; Rashdan, S.; Almutawah, A.; El-Fattah, A.A. Synthesis and Characterization of Biodegradable Poly(vinyl alcohol)-Chitosan/Cellulose Hydrogel Beads for Efficient Removal of Pb(II), Cd(II), Zn(II), and Co(II) from Water. Gels 2023, 9, 328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shen, B.; Zheng, C.; Huang, Y.; Liang, Y.; Fei, P.; Chen, J.; Lai, W. Chitosan/oxidized sodium alginate/Ca2+ hydrogels: Synthesis, characterization and adsorption properties. Food Hydrocoll. 2024, 156, 110368. [Google Scholar]
- Wang, H.; Chen, X.; Wen, Y.; Li, D.; Sun, X.; Liu, Z.; Yan, H.; Lin, Q. A Study on the Correlation between the Oxidation Degree of Oxidized Sodium Alginate on Its Degradability and Gelation. Polymers 2022, 14, 1679. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Pei, M.; Wan, T.; Yang, H.; Gu, S.; Tao, Y.; Liu, X.; Zhou, Y.; Xu, W.; Xiao, P. Self-healing hyaluronic acid hydrogels based on dynamic Schiff base linkages as biomaterials. Carbohydr. Polym. 2020, 250, 116922. [Google Scholar]
- Sharma, S.; Sharma, G.; Kumar, A.; AlGarni, T.S.; Naushad, M.; Alothman, Z.A.; Stadler, F.J. Adsorption of cationic dyes onto carrageenan and itaconic acid-based superabsorbent hydrogel: Synthesis, characterization and isotherm analysis. J. Hazard. Mater. 2022, 421, 126729. [Google Scholar]
- Gong, X.; Sawut, A.; Simayi, R.; Wang, Z.; Feng, Y. Preparation of modified humic acid/TiO2/P(AA-co-AM) nanocomposite hydrogels with enhanced dye adsorption and photocatalysis. Soft Matter 2024, 20, 2937–2954. [Google Scholar]
- Pashaei-Fakhri, S.; Peighambardoust, S.J.; Foroutan, R.; Arsalani, N.; Ramavandi, B. Crystal violet dye sorption over acrylamide/graphene oxide bonded sodium alginate nanocomposite hydrogel. Chemosphere 2021, 270, 129419. [Google Scholar]



| Thermodynamic Parameter | Temperature (K) | |||
|---|---|---|---|---|
| 298.15 | 323.15 | 348.15 | ||
| Cu2+ | lnKc | 0.3188253 | 0.320327 | 0.409905 |
| ΔG (kJ·mol−1) | −0.79031 | −0.86061 | −1.18648 | |
| ΔS (J·mol−1K−1) | 7.71 | |||
| ΔH (kJ·mol−1) | 1.54682 | |||
| Zn2+ | lnKc | 0.2045785 | 0.203116 | 0.350324 |
| ΔG (kJ·mol−1) | −0.507113 | −0.54571 | −1.01402 | |
| ΔS (J·mol−1K−1) | 9.77 | |||
| ΔH (kJ·mol−1) | 2.472096 | |||
| Ni2+ | lnKc | 0.5941949 | 0.636596 | 0.772223 |
| ΔG (kJ·mol−1) | −1.472902 | −1.71032 | −2.23522 | |
| ΔS (J·mol−1K−1) | 15.03 | |||
| ΔH (kJ·mol−1) | 3.05538 | |||
| ΔG°= −RTlnKc | ||||
| Pseudo First-Order Dynamics | Pseudo-Secondary Dynamics | |||||
|---|---|---|---|---|---|---|
| K1 | q1 | R12 | K2 | q2 | R22 | |
| CuSO4 | 171.684 | 0.0652 | 0.9877 | 186.498 | 0.00061 | 0.9981 |
| Cu(NO3)2 | 89.4957 | 0.0403 | 0.9563 | 106.073 | 0.00048 | 0.9949 |
| CuCl2 | 104.495 | 0.0653 | 0.9705 | 146.996 | 0.00024 | 0.9815 |
| ZnSO4 | 200.002 | 0.0168 | 0.9113 | 172.662 | 0.00046 | 0.9940 |
| NiSO4 | 177.528 | 0.1065 | 0.9269 | 196.153 | 0.00363 | 0.9852 |
| Isotherm Model | Isotherm Parameters | Cu(II) | Zn(II) | Ni(II) |
|---|---|---|---|---|
| Langmuir | qm (mg/g) | 367.64 | 398.4 | 409.83 |
| KL (L/mg) | 0.0124 | 0.0088 | 0.0175 | |
| R2 | 0.81286 | 0.75149 | 0.90659 | |
| Freundlich | 1/n | 0.3602 | 0.4684 | 0.3829 |
| Kf (L/mg) | 36.03 | 20.08 | 40.417 | |
| R2 | 0.88065 | 0.93286 | 0.96404 | |
| Temkin | bT (KJ/mol) | 2.404 | 2.028 | 1.836 |
| AT (L/g) | 15.95 | 7.945 | 2.776 | |
| R2 | 0.74953 | 0.78991 | 0.87864 | |
| Dubinin–Radushkevich | qs (mg/g) | 225.04 | 228.04 | 281.25 |
| β (mol2/J2) | 4.40854 × 10−8 | 7.40329 × 10−8 | 5.12641 × 10−8 | |
| R2 | 0.51875 | 0.65944 | 0.75081 |
| Absorbent | Qt (mg/g) | Refer | ||
|---|---|---|---|---|
| Cu(II) | Zn(II) | Ni(II) | ||
| Pal/CLDH | 55.8 | 23.9 | [67] | |
| PEI-modified chitosan | 128.96 | 105.98 | [68] | |
| Cellulose-chitosan | 94.3 | [37] | ||
| KCTS/PAM | 72.39 | 51.89 | [61] | |
| CMC sponge | 6.8 | 7.2 | [69] | |
| ECSDNH | 160.19 | 72.67 | [70] | |
| PVA-CS/CE | 26.74 | [71] | ||
| OSM/PAA | 172.93 | 162.84 | 208.52 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.; Sawut, A.; Simayi, R. Preparation and Heavy Metal Adsorption Performance of 2-Aminopyridine-Modified Sodium Alginate/Polyacrylic Acid Hydrogel. Gels 2025, 11, 224. https://doi.org/10.3390/gels11040224
Wu T, Sawut A, Simayi R. Preparation and Heavy Metal Adsorption Performance of 2-Aminopyridine-Modified Sodium Alginate/Polyacrylic Acid Hydrogel. Gels. 2025; 11(4):224. https://doi.org/10.3390/gels11040224
Chicago/Turabian StyleWu, Tingxiang, Amatjan Sawut, and Rena Simayi. 2025. "Preparation and Heavy Metal Adsorption Performance of 2-Aminopyridine-Modified Sodium Alginate/Polyacrylic Acid Hydrogel" Gels 11, no. 4: 224. https://doi.org/10.3390/gels11040224
APA StyleWu, T., Sawut, A., & Simayi, R. (2025). Preparation and Heavy Metal Adsorption Performance of 2-Aminopyridine-Modified Sodium Alginate/Polyacrylic Acid Hydrogel. Gels, 11(4), 224. https://doi.org/10.3390/gels11040224









