Hydrogel-Based Biomaterials: A Patent Landscape on Innovation Trends and Patterns
Abstract
1. Introduction
| Type | Sub-Type | Hydrogel | Pros | Cons | Ref. |
|---|---|---|---|---|---|
| Natural polymers | Proteins | Collagen | Excellent biocompatibility; promotes cell adhesion and tissue regeneration; biodegradable. | Low mechanical strength; rapid degradation; risk of immune response (animal-derived). | [28,29] |
| Gelatin | High biocompatibility; promotes cell growth; biodegradable and cost-effective. | Poor mechanical properties; unstable at physiological temperatures. | [30,31] | ||
| Polysaccharides | Alginate | Non-toxic and biocompatible; gelation under mild conditions; ideal for wound healing and drug delivery. | Limited cell adhesion without modification; poor mechanical strength. | [32,33] | |
| Chitosan | Antimicrobial properties; supports wound healing; biodegradable. | Limited solubility at physiological pH; requires chemical modification for enhanced properties. | [34,35] | ||
| Synthetic polymers | Polyethers | Polyethylene glycol (PEG) | Tunable mechanical properties; non-toxic and non-immunogenic; long-term stability. | Lacks inherent biological signals for cell adhesion; non-biodegradable without modification. | [36,37] |
| Poly(lactic-co-glycolic acid) (PLGA) | Biodegradable and biocompatible; tunable degradation rate; FDA-approved for medical applications. | Potential acidic by-products during degradation; requires precise control for uniform degradation. | [38,39] | ||
| Acrylic-based | Polyacrylamide | High mechanical strength non-biodegradable, providing stable structure over time adjustable swelling properties. | Non-biodegradable; toxicity concerns for long-term use. | [40] | |
| Vinyl-based | Polyvinyl alcohol (PVA) | High water content; biocompatible and stable; good mechanical properties for load-bearing applications. | Requires crosslinking for stability; non-degradable in biological environments. | [41,42] |
2. Methodology
2.1. Databases
2.2. Data Collection
2.3. Patent Families
3. Patent Analysis
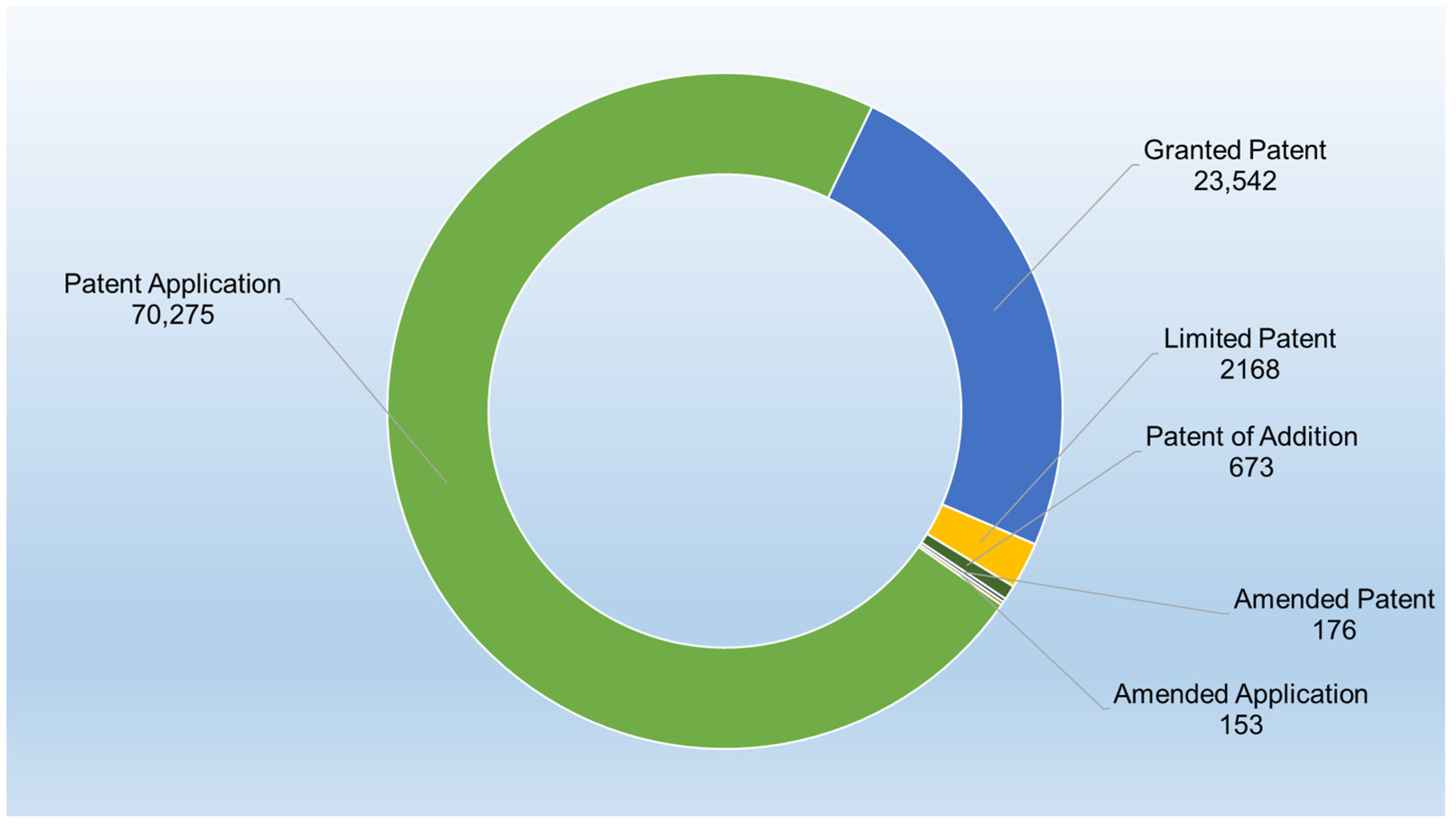
3.1. Patent Documents
3.2. Patent Classification
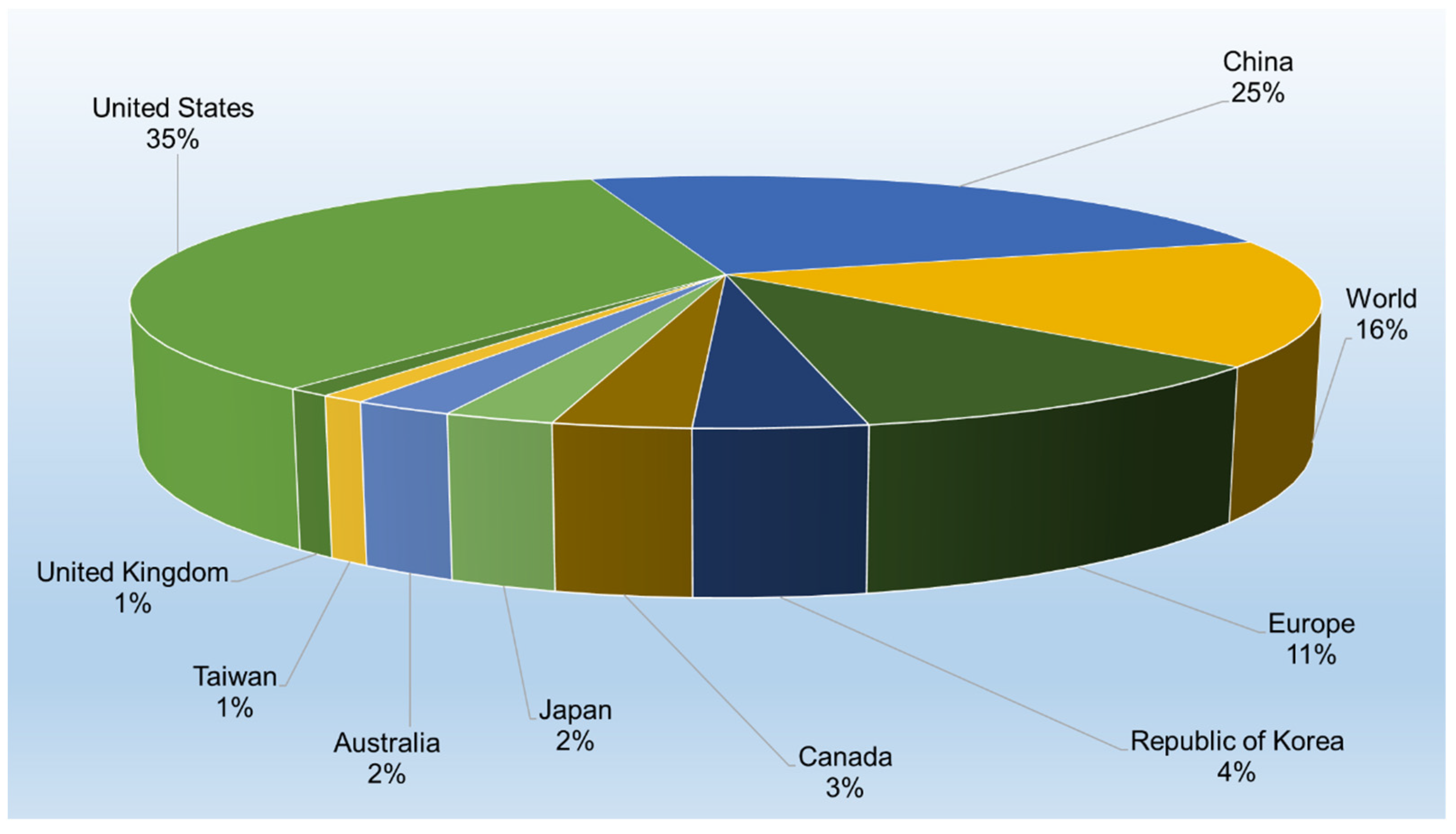
3.3. Jurisdictions
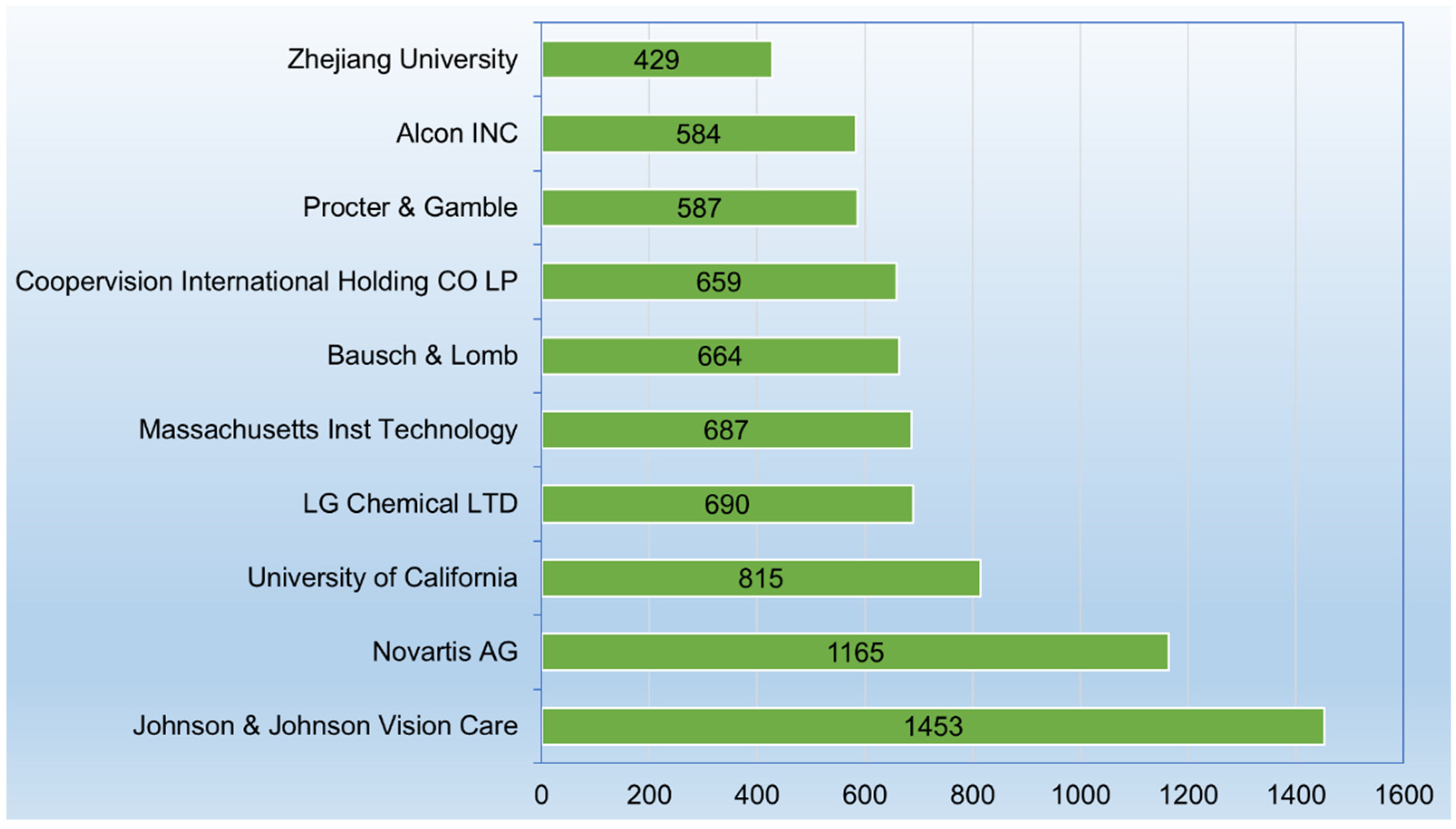
3.4. Applicants
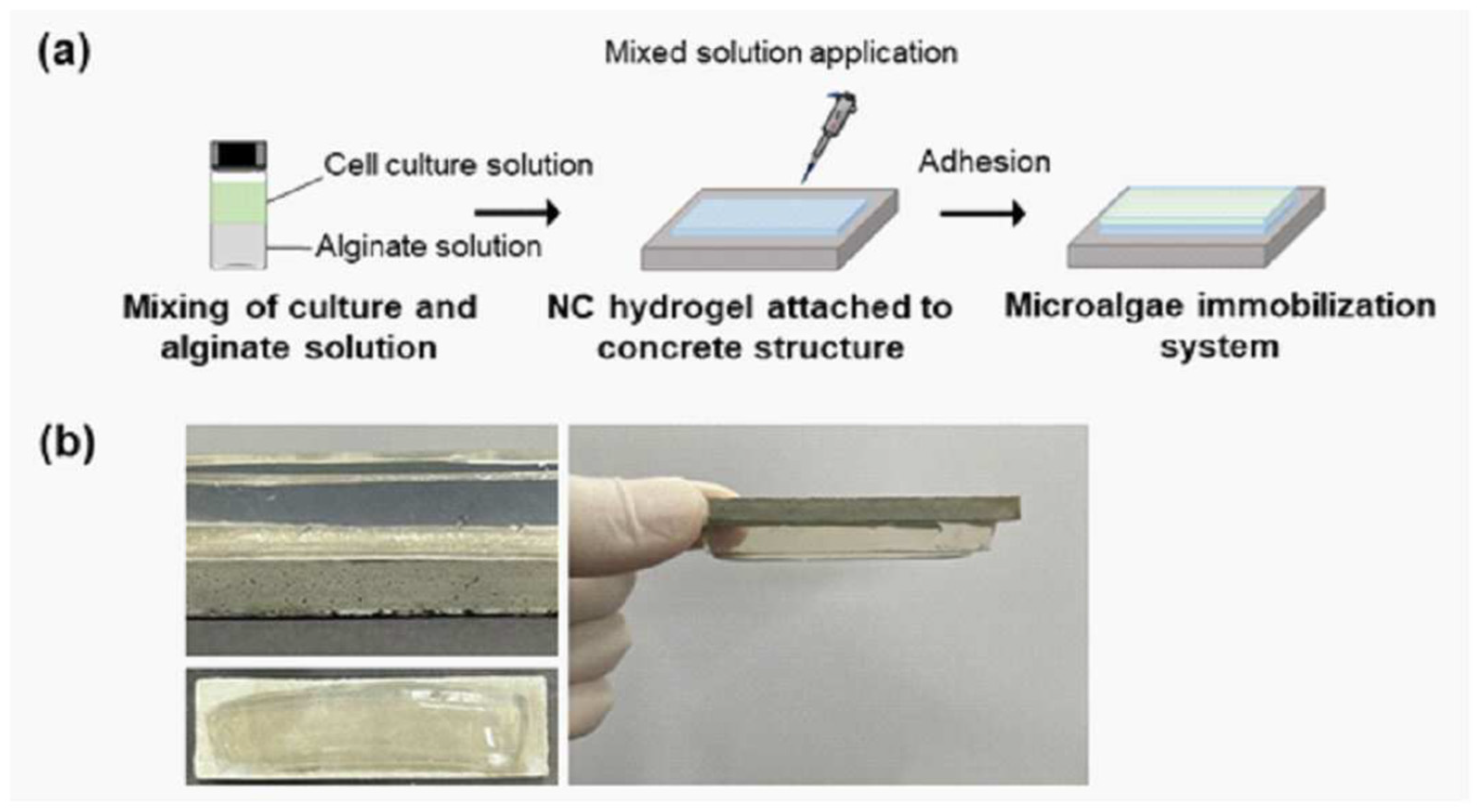
4. Recent Patents on Hydrogel-Based Biomaterials
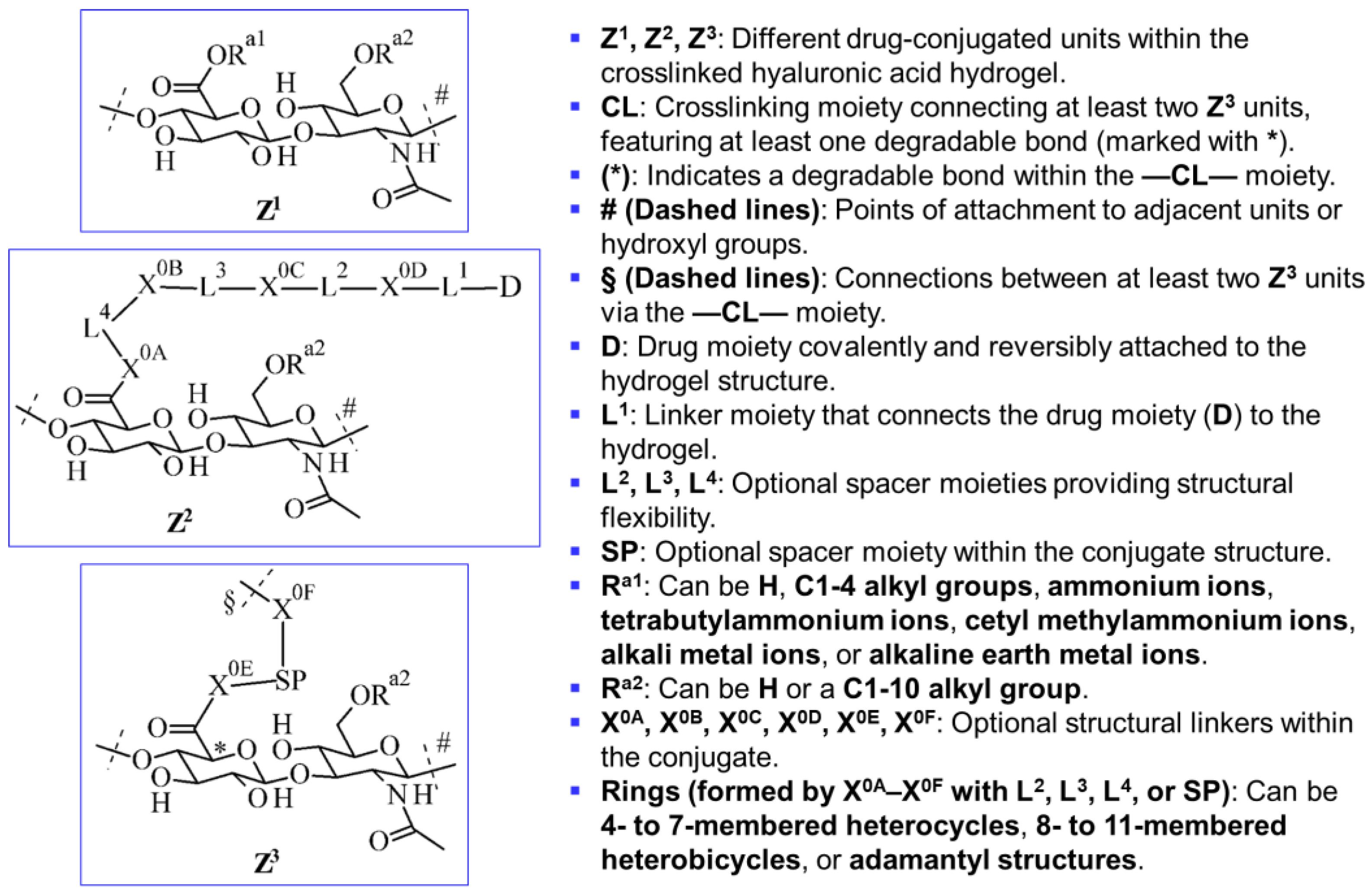
- Degradable crosslinked components (—CL—), ensuring controlled breakdown.
- Spacer moieties (—SP—, L-moieties, X-linkages), enhancing molecular flexibility.
- Drug moieties (—D), covalently attached through –L1– for controlled release.
5. Limitations and Future Research Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Cap | Capping |
| DMA | N,N-dimethylacrylamide |
| ECM | Extracellular matrix |
| EGDMA | Ethylene glycol di(meth)acrylate |
| EPO | European Patent Office |
| Ex | Extended patent family |
| FDA | U.S. Food and Drug Administration |
| HEMA | 2-hydroxyethyl methacrylate |
| IPC | International Patent Classification |
| mRNA | Messenger ribonucleic acid |
| NC | Nanocomposite |
| NVP | N-vinylpyrrolidone |
| PCT | Patent Cooperation Treaty |
| PEG | Polyethylene glycol |
| PEG | Polyethylene glycol |
| PEGMal | PEG-based crosslinker with maleimide functional groups |
| PEO | Poly(ethylene oxide) |
| PLGA | Poly(lactic-co-glycolic acid) |
| Pluronics | (PPO–PEO–PPO) triblock copolymers |
| PPO | Poly(propylene oxide) |
| PTX-3 | Pentraxin 3 |
| PVA | Polyvinyl alcohol |
| S | Simple patent family |
| TSG-6 | Tumor necrosis factor-stimulated gene 6 |
| TSP-1 | Thrombospondin-1 |
| WIPO | World Intellectual Property Organization |
References
- Advincula, R.C.; Dizon, J.R.C.; Caldona, E.B.; Viers, R.A.; Siacor, F.D.C.; Maalihan, R.D.; Espera, A.H. On the progress of 3D-printed hydrogels for tissue engineering. MRS Commun. 2021, 11, 539–553. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging Role of Hydrogels in Drug Delivery Systems, Tissue Engineering and Wound Management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef] [PubMed]
- Revete, A.; Aparicio, A.; Cisterna, B.A.; Revete, J.; Luis, L.; Ibarra, E.; Segura González, E.A.; Molino, J.; Reginensi, D. Advancements in the Use of Hydrogels for Regenerative Medicine: Properties and Biomedical Applications. Int. J. Biomater. 2022, 2022, 3606765. [Google Scholar] [CrossRef]
- Damiri, F.; Fatimi, A.; Liu, Y.; Musuc, A.M.; Fajardo, A.R.; Gowda, B.H.J.; Vora, L.K.; Shavandi, A.; Okoro, O.V. Recent advances in 3D bioprinted polysaccharide hydrogels for biomedical applications: A comprehensive review. Carbohydr. Polym. 2025, 348, 122845. [Google Scholar] [CrossRef] [PubMed]
- Sikdar, P.; Uddin, M.M.; Dip, T.M.; Islam, S.; Hoque, M.S.; Dhar, A.K.; Wu, S. Recent advances in the synthesis of smart hydrogels. Mater. Adv. 2021, 2, 4532–4573. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Damiri, F.; Fatimi, A.; Santos, A.C.P.; Varma, R.S.; Berrada, M. Smart stimuli-responsive polysaccharide nanohydrogels for drug delivery: A review. J. Mater. Chem. B 2023, 11, 10538–10565. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef] [PubMed]
- Hachimi Alaoui, C.; Réthoré, G.; Weiss, P.; Fatimi, A. Sustainable Biomass Lignin-Based Hydrogels: A Review on Properties, Formulation, and Biomedical Applications. Int. J. Mol. Sci. 2023, 24, 13493. [Google Scholar] [CrossRef]
- Xia, Z.; Guo, B.; Wu, D.; Yang, F.; Ding, Y. Advances of natural hydrogel-based vascularization strategies for soft tissue repair. Front. Mater. 2024, 11, 1446035. [Google Scholar] [CrossRef]
- León-Campos, M.I.; Claudio-Rizo, J.A.; Cobos-Puc, L.E.; Cabrera-Munguia, D.A. Cell Differentiation on Hydrogels and its Application in Regenerative Medicine. Front. Stem Cell Regen. Med. Res. 2024, 11, 24–60. [Google Scholar] [CrossRef]
- Kaur, H.; Gogoi, B.; Sharma, I.; Das, D.K.; Azad, M.A.; Pramanik, D.D.; Pramanik, A. Hydrogels as a Potential Biomaterial for Multimodal Therapeutic Applications. Mol. Pharm. 2024, 21, 4827–4848. [Google Scholar] [CrossRef]
- Fatimi, A.; Okoro, O.V.; Podstawczyk, D.; Siminska-Stanny, J.; Shavandi, A. Natural hydrogel-based bio-Inks for 3D bioprinting in tissue engineering: A review. Gels 2022, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Hong, G.; Yuan, X.; Kong, W.; Xia, P.; Li, Y.; Chen, Y.; Liu, N.; He, J.; Shi, J.; et al. 3D Printing of Tough Hydrogel Scaffolds with Functional Surface Structures for Tissue Regeneration. Nano-Micro Lett. 2024, 17, 27. [Google Scholar] [CrossRef]
- Fatimi, A. Hydrogel-based bioinks for three-dimensional bioprinting: Patent analysis. Mater. Proc. 2021, 7, 3. [Google Scholar] [CrossRef]
- Raina, N.; Pahwa, R.; Bhattacharya, J.; Paul, A.K.; Nissapatorn, V.; de Lourdes Pereira, M.; Oliveira, S.M.R.; Dolma, K.G.; Rahmatullah, M.; Wilairatana, P.; et al. Drug Delivery Strategies and Biomedical Significance of Hydrogels: Translational Considerations. Pharmaceutics 2022, 14, 574. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.Y.; Qian, S.Y.; Faucher, A.; Tran, S.D. Advancements in Hydrogels for Corneal Healing and Tissue Engineering. Gels 2024, 10, 662. [Google Scholar] [CrossRef]
- Hachimi Alaoui, C.; Fatimi, A. A 20-year Patent Review and Innovation Trends on Hydrogel-based Coatings Used for Medical Device Biofabrication. J. Biomater. Sci. Polym. Ed. 2023, 34, 1255–1273. [Google Scholar] [CrossRef]
- Fatimi, A.; Damiri, F.; Berrada, M.; Musuc, A.M. Patent Overview of Innovative Hyaluronic Acid-Based Hydrogel Biosensors. Biosensors 2024, 14, 567. [Google Scholar] [CrossRef]
- Choi, Y.; Koh, H.Y.; Han, J.Y.; Seo, S. Synthesis of Hydrogel-Based Microgels and Nanogels Toward Therapeutic and Biomedical Applications. Appl. Sci. 2025, 15, 1368. [Google Scholar] [CrossRef]
- Fatimi, A. Exploring the Patent Landscape and Innovation of Hydrogel-based Bioinks Used for 3D Bioprinting. Recent Adv. Drug Deliv. Formul. 2022, 16, 145–163. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.-H.; Lee, J.-M.; Park, S.-Y.; Kim, M.-H.; Kim, S.-B.; Oh, T.-H. PVA Hydrogels Supplemented with PLA Mesh for Tissue Regeneration Scaffold. Gels 2024, 10, 364. [Google Scholar] [CrossRef]
- Priya, A.S.; Premanand, R.; Ragupathi, I.; Bhaviripudi, V.R.; Aepuru, R.; Kannan, K.; Shanmugaraj, K. Comprehensive Review of Hydrogel Synthesis, Characterization, and Emerging Applications. J. Compos. Sci. 2024, 8, 457. [Google Scholar] [CrossRef]
- Li, C.-S.; Xu, Y.; Li, J.; Qin, S.-H.; Huang, S.-W.; Chen, X.-M.; Luo, Y.; Gao, C.-T.; Xiao, J.-H. Ultramodern natural and synthetic polymer hydrogel scaffolds for articular cartilage repair and regeneration. Biomed. Eng. Online 2025, 24, 13. [Google Scholar] [CrossRef]
- Zhang, C.W.; Si, M.; Chen, C.; He, P.; Fei, Z.; Xu, N.; He, X. Hierarchical Engineering for Biopolymer-based Hydrogels with Tailored Property and Functionality. Adv. Mater. 2025, 2414897. [Google Scholar] [CrossRef]
- Saadan, R.; Hachimi Alaoui, C.; Quraishi, K.S.; Afridi, F.; Chigr, M.; Fatimi, A. Recent Progress in Hydrogel-Based Bioinks for 3D Bioprinting: A Patent Landscape Analysis and Technology Updates. J. Res. Updates Polym. Sci. 2024, 13, 130–146. [Google Scholar] [CrossRef]
- Ilić-Stojanović, S.; Nikolić, L.; Cakić, S. A Review of Patents and Innovative Biopolymer-Based Hydrogels. Gels 2023, 9, 556. [Google Scholar] [CrossRef]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Yang, X.; Ahmad, K.; Yang, T.; Fan, Y.; Zhao, F.; Jiang, S.; Chen, P.; Hou, H. Collagen-based hydrogel sol-gel phase transition mechanism and their applications. Adv. Colloid Interface Sci. 2025, 340, 103456. [Google Scholar] [CrossRef]
- Mushtaq, F.; Raza, Z.A.; Batool, S.R.; Zahid, M.; Onder, O.C.; Rafique, A.; Nazeer, M.A. Preparation, properties, and applications of gelatin-based hydrogels (GHs) in the environmental, technological, and biomedical sectors. Int. J. Biol. Macromol. 2022, 218, 601–633. [Google Scholar] [CrossRef]
- Choi, D.J.; Kho, Y.; Park, S.J.; Kim, Y.-J.; Chung, S.; Kim, C.-H. Effect of cross-linking on the dimensional stability and biocompatibility of a tailored 3D-bioprinted gelatin scaffold. Int. J. Biol. Macromol. 2019, 135, 659–667. [Google Scholar] [CrossRef]
- Farshidfar, N.; Iravani, S.; Varma, R.S. Alginate-Based Biomaterials in Tissue Engineering and Regenerative Medicine. Mar. Drugs 2023, 21, 189. [Google Scholar] [CrossRef]
- Xie, Y.; Li, G.; Wu, J.; Zhu, J.; Cai, X.; Zhao, P.; Zhang, D.; Zhong, Y. Injectable self-healing alginate/PEG hydrogels cross-linked via thiol-Michael addition bonds for hemostasis and wound healing. Carbohydr. Polym. 2025, 348, 122864. [Google Scholar] [CrossRef]
- Gholap, A.D.; Rojekar, S.; Kapare, H.S.; Vishwakarma, N.; Raikwar, S.; Garkal, A.; Mehta, T.A.; Jadhav, H.; Prajapati, M.K.; Annapure, U. Chitosan scaffolds: Expanding horizons in biomedical applications. Carbohydr. Polym. 2024, 323, 121394. [Google Scholar] [CrossRef] [PubMed]
- Rahimnejad, M.; Adoungotchodo, A.; Demarquette, N.R.; Lerouge, S. FRESH bioprinting of biodegradable chitosan thermosensitive hydrogels. Bioprinting 2022, 27, e00209. [Google Scholar] [CrossRef]
- Wancura, M.; Nkansah, A.; Robinson, A.; Toubbeh, S.; Talanker, M.; Jones, S.; Cosgriff-Hernandez, E. PEG-Based Hydrogel Coatings: Design Tools for Biomedical Applications. Ann. Biomed. Eng. 2024, 52, 1804–1815. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Hubbell, K.; Schilling, A.F.; Dai, G.; Cui, X.l. Bioprinting cartilage tissue from mesenchymal stem cells and PEG hydrogel. In 3D Cell Culture: Methods and Protocols; Koledova, Z., Ed.; Springer: New York, NY, USA, 2017; pp. 391–398. [Google Scholar]
- Rocha, C.V.; Gonçalves, V.; da Silva, M.C.; Bañobre-López, M.; Gallo, J. PLGA-Based Composites for Various Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 2034. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Fuoco, T.; Yassin, M.A.; Mustafa, K.; Finne-Wistrand, A. Printability and Critical Insight into Polymer Properties during Direct-Extrusion Based 3D Printing of Medical Grade Polylactide and Copolyesters. Biomacromolecules 2020, 21, 388–396. [Google Scholar] [CrossRef]
- Subramani, R.; Izquierdo-Alvarez, A.; Bhattacharya, P.; Meerts, M.; Moldenaers, P.; Ramon, H.; Van Oosterwyck, H. The Influence of Swelling on Elastic Properties of Polyacrylamide Hydrogels. Front. Mater. 2020, 7, 00212. [Google Scholar] [CrossRef]
- Wang, M.; Bai, J.; Shao, K.; Tang, W.; Zhao, X.; Lin, D.; Huang, S.; Chen, C.; Ding, Z.; Ye, J. Poly(vinyl alcohol) Hydrogels: The Old and New Functional Materials. Int. J. Polym. Sci. 2021, 2021, 2225426. [Google Scholar] [CrossRef]
- Liu, G.; Li, S.; Deng, B.; Huo, L.; Bai, H.; Jiang, S.; Zhou, Y.; Pei, Z.; Kimna, C.; Zhao, Y.; et al. Phase-separated anisotropic PVA hydrogel loaded with tetramethylpyrazine for spinal cord injury repair. Chem. Eng. J. 2025, 506, 159944. [Google Scholar] [CrossRef]
- Cambia Institute. The Lens Patent Data Set. Available online: www.lens.org (accessed on 15 September 2024).
- World Intellectual Property Organization. The International Patent Classification Portal. Available online: https://ipcpub.wipo.int/ (accessed on 15 September 2024).
- Google. Google Patents Research Data. Available online: https://patents.google.com (accessed on 15 September 2024).
- Randolph, L.T.; Long, J.A. Super-Absorbent, Reduced-Pressure System. Europe Patent Application No. EP2594298A1, 22 May 2013. [Google Scholar]
- Say, J.; Tomasco, M.F.; Heller, A.; Gal, Y.; Aria, B.; Heller, E.; Plante, P.J.; Vreeke, M.S.; Friedman, K.A.; Colman, F.C. Analyte Monitoring Device and Methods of Use. U.S. Patent US9326714B2, 3 May 2016. [Google Scholar]
- Serro, A.P.; Silva, D.C.; Fernandes, A.I. Hydrogel-Based Novel Biomaterials: Achievements and Prospects. Gels 2024, 10, 436. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.H.; Kim, W.H. A Hybrid Hydrogel for Propagation of Aquatic Organism and a Method for Manufacturing the Same. Republic of Korea Patent KR102707093B1, 12 September 2024. [Google Scholar]
- Stark, S.; Laufer, B.; Knappe, T.; Voigt, T.; Bisek, N. Degradable Hyaluronic Acid Hydrogels. Australia Patent AU2019348440B2, 12 September 2024. [Google Scholar]
- Chen, Y.-L.; Huang, S.-C.; Ou, H.-W. High Oxygen Permeability Silicone Hydrogel Composition, Contact Lens Made from High Oxygen Permeability Silicone Hydrogel Composition and Manufacturing Method Thereof. Europe Patent EP4269458B1, 11 September 2024. [Google Scholar]
- Le Gac, S.; Leijten Jeroen, C.H.; Paggi, C.A.; Venzac, B. Microfluidic Device for Mechanically Stimulating a Material. Europe Patent EP3870365B1, 11 September 2024. [Google Scholar]
- Heartlein, M.; Derosa, F.; Dias, A. Quantitative Assessment for Capping Efficiency of Messenger RNA. Europe Patent EP3495505B1, 11 September 2024. [Google Scholar]
- Na, J.; Imagawa, D.; Tucci, F.; Beaton, G.; Ravula, S. Chemoembolization Agents. Europe Patent EP3630078B1, 11 September 2024. [Google Scholar]
- Terrell, J.L.; Jahnke, J.P. Biofabrication of Advanced Functional Materials Using Bacterial Cellulose Scaffolds. U.S. Patent US12084701B2, 10 September 2024. [Google Scholar]
- Rangwala, H.S.; Patterson, W.R.; Hewitt, T. Filamentary Devices for Treatment of Vascular Defects. U.S. Patent US12082819B2, 10 September 2024. [Google Scholar]
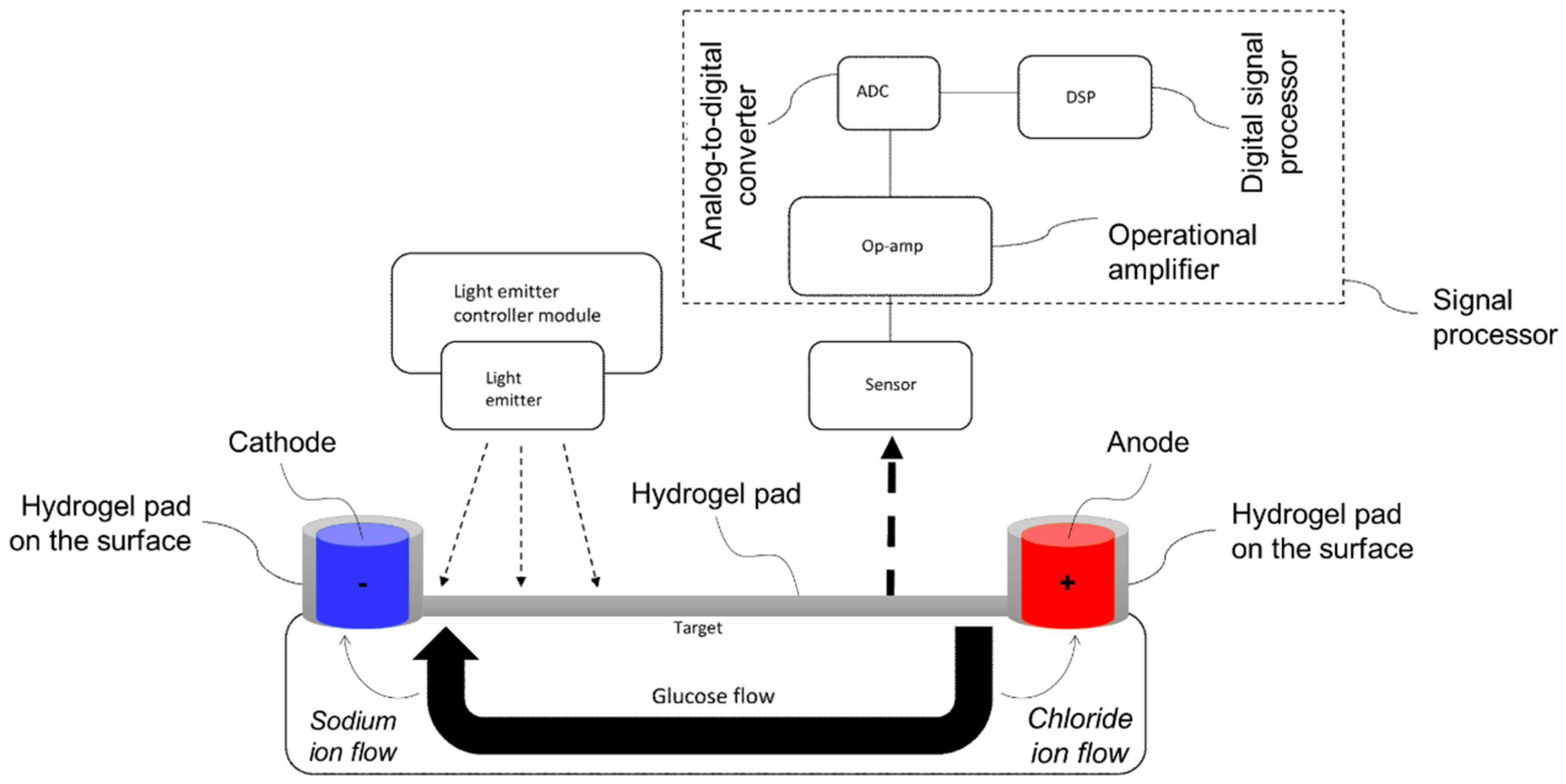
- Zhou, L.; Russell, R.M.; Schultz, P.; Patel, A.M.; Chen, C.; Malekmadani, R.; To, L.; Kow, H.-Y.S.; Raghavendhar, G. Miniaturized Noninvasive Glucose Sensor and Continuous Glucose Monitoring System. U.S. US12082910B2, 10 September 2024. [Google Scholar]
- Murphy, S.V.; Skardal, A.; Atala, A. Amniotic Membrane Powder and Its Use in Wound Healing and Tissue Engineering Constructs. U.S. Patent US12083245B2, 10 September 2024. [Google Scholar]
| Search Query | Patent Documents | Simple Families |
|---|---|---|
| Title: (hydrogel) | 34,858 | 19,506 |
| Abstract: (hydrogel) | 54,797 | 35,832 |
| Claims: (hydrogel) | 50,958 | 24,367 |
| Title: (hydrogel) OR Abstract: (hydrogel) OR Claims: (hydrogel) | 96,987 | 52,120 |
| IPC Code | Definition | Description | Patent Documents |
|---|---|---|---|
| C08J3/075 | Macromolecular gels formed using processes in aqueous media | Encompasses patents dealing with the preparation and processing of macromolecular gels (such as hydrogels) using aqueous solutions, with emphasis on innovations in gel formation methods. | 8865 |
| A61L27/52 | Hydrogels or hydrocolloids used in prostheses or for coating prostheses | Focuses on innovations in hydrogels or hydrocolloids for medical devices and prosthetic coatings, emphasizing biocompatibility and desirable properties for medical applications. | 8109 |
| A61K9/00 | Medicinal preparations characterized by their special physical form | Focuses on pharmaceutical formulations where the physical form (e.g., tablets, gels, capsules, patches, etc.) is crucial for optimizing medicine delivery, absorption, or administration. | 6789 |
| A61K9/06 | Ointments and bases for ointments, including apparatus for making them | Focuses on ointments as a type of medicinal preparation, including formulation bases and the equipment or methods used for producing topical drug delivery forms. | 5561 |
| G02B1/04 | Optical elements made of organic materials, such as plastics | Covers optical elements (e.g., lenses, prisms, etc.) made from organic materials like plastic, particularly important for contact lenses and lightweight, durable optical devices. | 3942 |
| G02C7/04 | Contact lenses for the eyes | Focuses on contact lenses characterized by their material or design for vision correction or therapeutic purposes. | 3924 |
| A61K47/36 | Medicinal preparations involving polysaccharides or their derivatives | Highlights innovations in drug delivery systems, wound care products, or tissue engineering using polysaccharides like gums, alginate, hyaluronic acid, or chitosan. | 3865 |
| C08J3/24 | Crosslinking of macromolecules | Refers to crosslinking macromolecules, focusing on mechanical aspects and crosslinking agents to enhance material strength, elasticity, and thermal stability, commonly used in hydrogels and vulcanization. | 3639 |
| A61L27/54 | Biologically active materials used in medical or prosthetic applications | Covers materials designed for biological interaction in regenerative medicine, wound healing, or medical devices, often incorporating hydrogels or biocompatible polymers for therapeutic substance delivery. | 3575 |
| A61L26/00 | Chemical aspects or materials used for liquid bandages | Focuses on innovations related to the chemical composition and application of liquid bandages for wound care and protection. | 2943 |
| Title | Patent N° | Publication | Family * | Applicants | Ref. |
|---|---|---|---|---|---|
| A hybrid hydrogel for propagation of an aquatic organism and a method for manufacturing the same | KR102707093B1 | 12 September 2024 | 2S./2Ex. | Hannam University Industry-Academia Cooperation | [49] |
| Degradable hyaluronic acid hydrogels | AU2019348440B2 | 12 September 2024 | 12S./12Ex. | Ascendis Pharma As. | [50] |
| High-oxygen-permeability silicone hydrogel composition, contact lens made from high-oxygen-permeability silicone hydrogel composition and manufacturing method thereof | EP4269458B1 | 11 September 2024 | 6S./6Ex. | Innova Vision Inc. | [51] |
| Microfluidic device for mechanically stimulating a material | EP3870365B1 | 11 September 2024 | 5S./5Ex. | University of Twente | [52] |
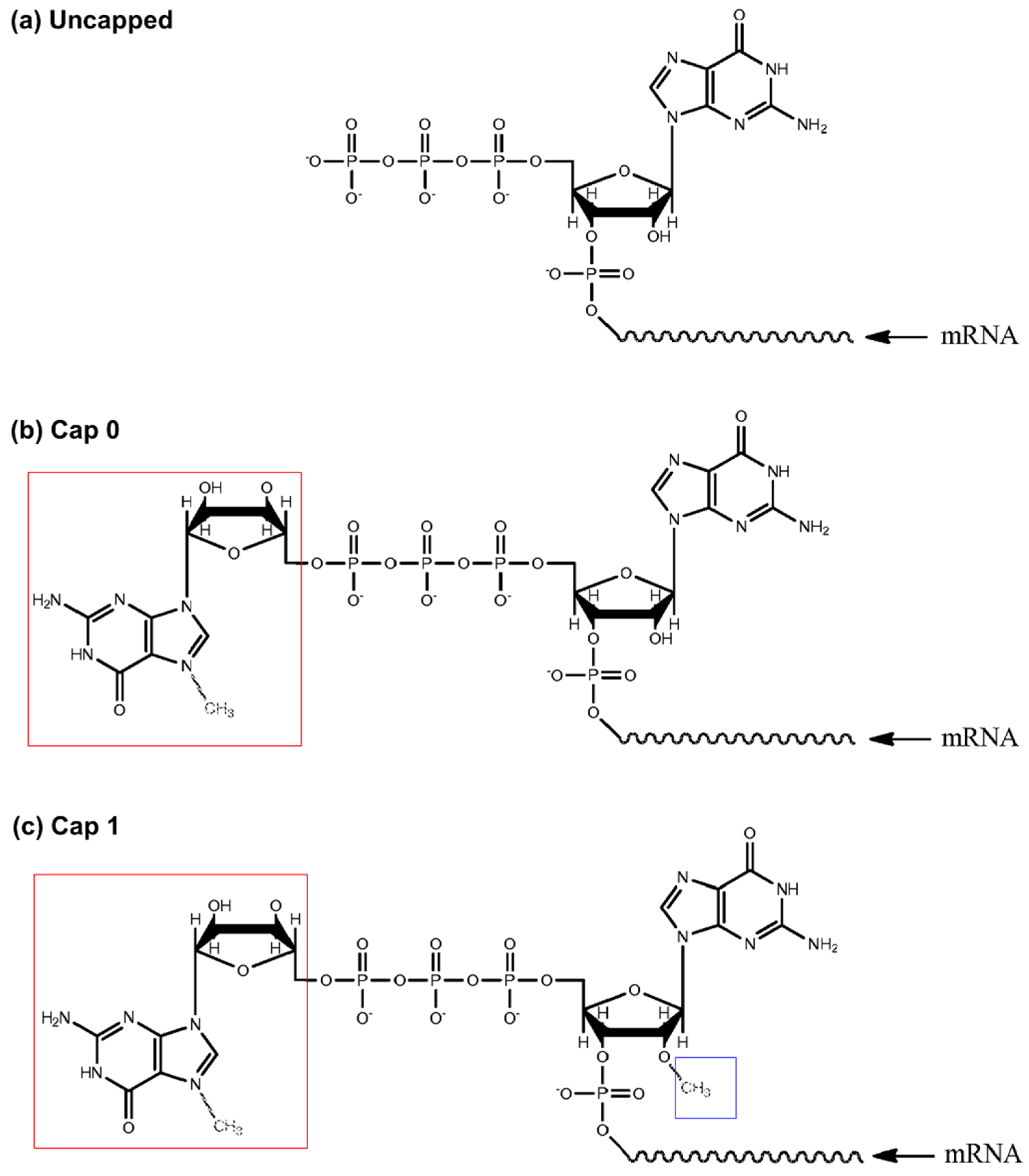
| Quantitative assessment for cap efficiency of messenger RNA | EP3495505B1 | 11 September 2024 | 21S./21Ex. | Translate Bio Inc. | [53] |
| Chemoembolization agents | EP3630078B1 | 11 September 2024 | 20S./20Ex. | Bruin Biosciences Inc.; University of California | [54] |
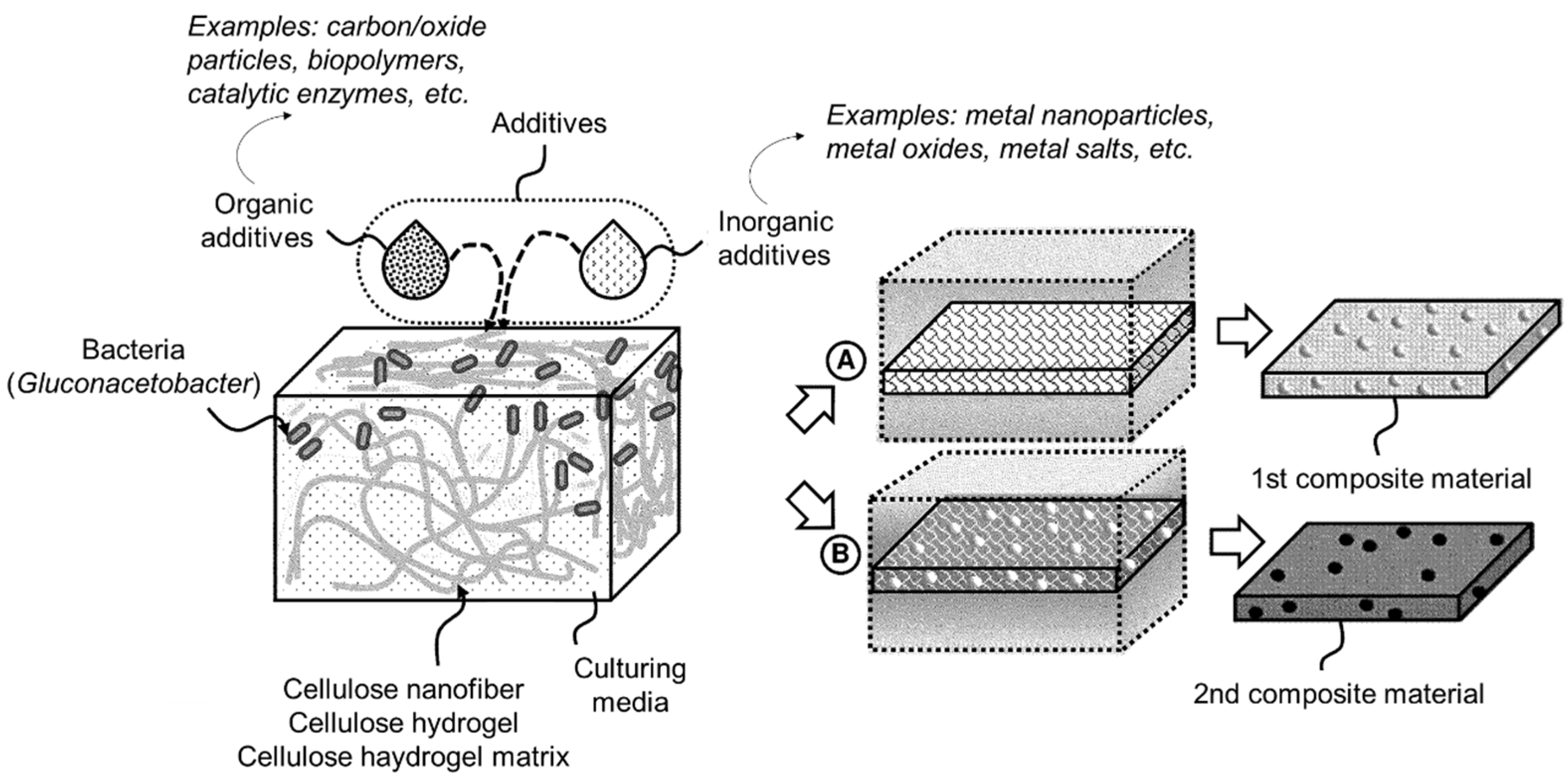
| Biofabrication of advanced functional materials using bacterial cellulose scaffolds | US12084701B2 | 10 September 2024 | 3S./3Ex. | US Army; US Army DEVCOM Army Research Laboratory | [55] |
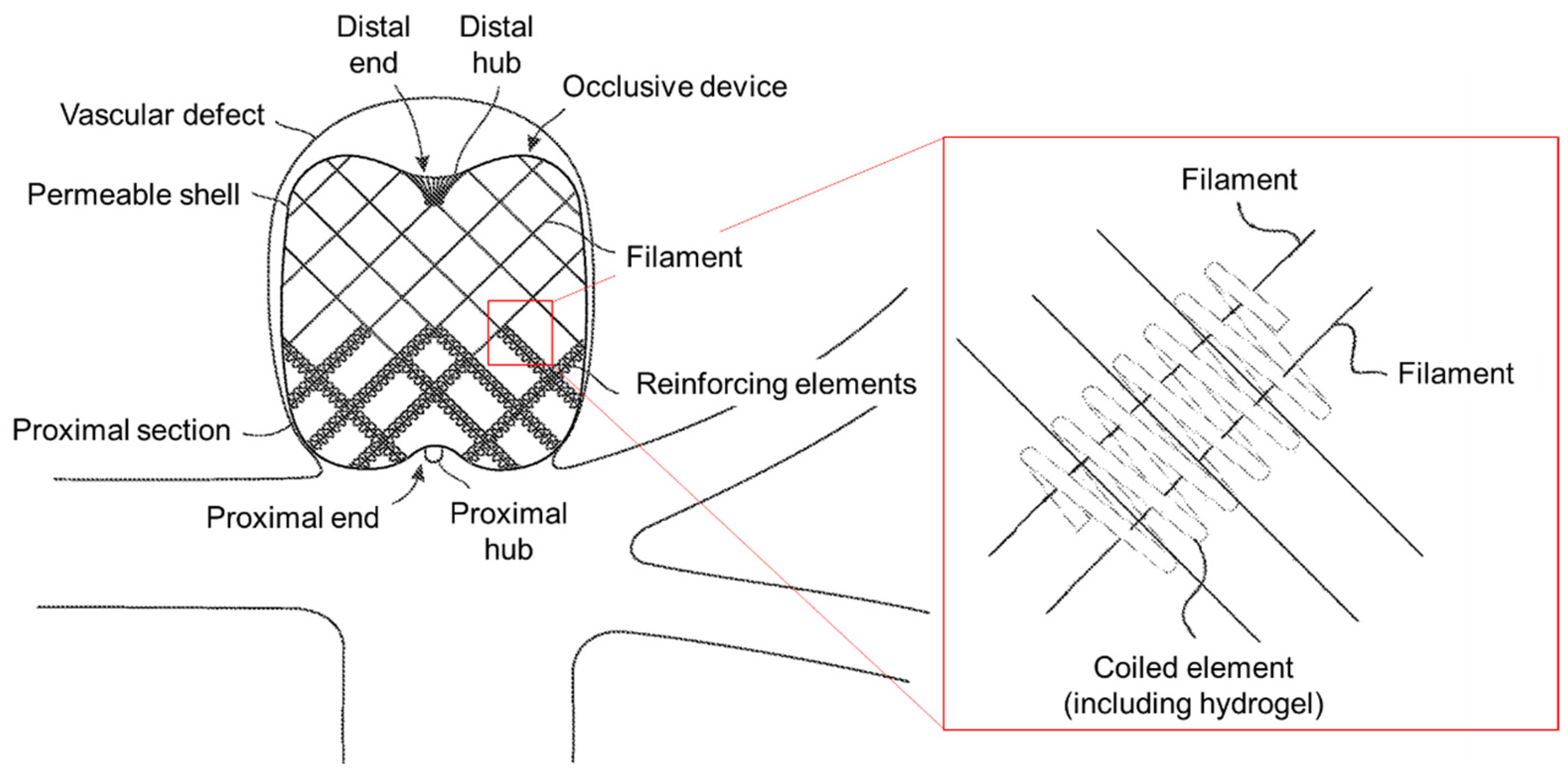
| Filamentary devices for treatment of vascular defects | US12082819B2 | 10 September 2024 | 11S./11Ex. | MicroVention Inc. | [56] |
| Miniaturized noninvasive glucose sensor and continuous glucose-monitoring system | US12082910B2 | 10 September 2024 | 2S./9Ex. | Medtronic MiniMed Inc. | [57] |
| Amniotic membrane powder and its use in wound healing and tissue-engineering constructs | US12083245B2 | 10 September 2024 | 23S./23Ex. | University Wake Forest Health Sciences | [58] |
| Compositions and Physical Properties | Formulation | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Silicone hydrogel compositions | 1st silicone polymer | 15 | 20 | 15 | 20 | 10 | 5 |
| 2nd silicone polymer | 30 | 30 | 30 | 30 | 30 | 30 | |
| 3rd silicone polymer | 0 | 0 | 0 | 0 | 5 | 5 | |
| 1st hydrophilic monomer: HEMA 1 | 35 | 30 | 15 | 5 | 15 | 5 | |
| 2nd hydrophilic monomer: DMA 2 | 0 | 0 | 10 | 15 | 10 | 20 | |
| 3rd hydrophilic monomer: NVP 3 | 0 | 0 | 10 | 15 | 10 | 15 | |
| Crosslinking agent: EGDMA 4 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | |
| Photoinitiator: Irgacure 819 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Solvent: n-hexanol | 18.2 | 18.2 | 18.2 | 18.2 | 18.2 | 18.2 | |
| Physical properties | Water content (%) | 30.2 | 26.6 | 36.1 | 33.5 | 38.3 | 42.3 |
| Tensile modulus (MPa) | 1.1 | 1.5 | 0.9 | 1.2 | 0.8 | 0.6 | |
| Oxygen permeability (barrer) | 90 | 105 | 93 | 115 | 90 | 84 | |
| Claim | Total Protein Content (mg/g) | Elastin Content (mg/g) | Collagen Content (mg/g) | Glycosaminoglycans Content (mg/g) | TSP-1 1 Content (μg/g) | PTX-3 2 Content (μg/g) | TSG-6 3 Content (ng/g) | Crosslinked Hydrogel Matrix |
|---|---|---|---|---|---|---|---|---|
| 1 | 30–500 | — | — | — | — | — | — | Hyaluronic acid crosslinked to gelatin via PEG 4, maleimide-thiol |
| 2 | 50–250 | — | — | — | — | — | — | Same as Claim 1 |
| 3 | 30–500 | 4–100 | — | — | — | — | — | Same as Claim 1 |
| 4 | 30–500 | — | 10–800 | — | — | — | — | Same as Claim 1 |
| 5 | 30–500 | — | — | 0.1–5 | — | — | — | Same as Claim 1 |
| 6 | 30–500 | — | — | — | 30–1000 | — | — | Same as Claim 1 |
| 7 | 30–500 | — | — | — | — | 0.1–50 | — | Same as Claim 1 |
| 8 | 30–500 | — | — | — | — | — | < 1.5 | Same as Claim 1 |
| 9 | 30–500 | — | — | — | — | — | — | PEGMal 5 |
| 10 | 30–500 | — | — | — | — | — | — | Methacrylate, PEO 6, PPO 7, Pluronics 8 |
| 11 | 30–500 | — | 10–600 | — | — | — | — | Same as Claim 1 |
| 12 | — | 4–100 | — | — | — | — | — | Same as Claim 1 |
| 13 | — | 5–60 | — | — | — | — | — | Same as Claim 1 |
| 14 | — | 4–100 | 10–800 | — | — | — | — | Same as Claim 1 |
| 15 | — | 4–100 | — | 0.1–5 | — | — | — | Same as Claim 1 |
| 16 | — | 4–100 | — | — | 30–1000 | — | — | Same as Claim 1 |
| 17 | — | 4–100 | — | — | — | 0.1–50 | — | Same as Claim 1 |
| 18 | — | 4–100 | — | — | — | — | <1.5 | Same as Claim 1 |
| 19 | — | 4–100 | 10–600 | — | — | — | — | Same as Claim 1 |
| 20 | 30–500 | 4–100 | 10–800 | 0.1–5 | — | — | — | Same as Claim 1 |
| 21 | 30–500 | 4–100 | 10–800 | 0.1–5 | 30–1000 | — | — | Same as Claim 1 |
| 22 | 30–500 | 4–100 | 10–800 | 0.1–5 | — | 0.1–50 | — | Same as Claim 1 |
| 23 | 30–500 | 4–100 | 10–800 | 0.1–5 | — | — | <1.5 | Same as Claim 1 |
| 24 | 30–500 | 4–100 | 10–800 | 0.1–5 | 30–1000 | 0.1–50 | <1.5 | Same as Claim 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatimi, A.; Damiri, F.; El Arrach, N.; Hemdani, H.; Musuc, A.M.; Berrada, M. Hydrogel-Based Biomaterials: A Patent Landscape on Innovation Trends and Patterns. Gels 2025, 11, 216. https://doi.org/10.3390/gels11030216
Fatimi A, Damiri F, El Arrach N, Hemdani H, Musuc AM, Berrada M. Hydrogel-Based Biomaterials: A Patent Landscape on Innovation Trends and Patterns. Gels. 2025; 11(3):216. https://doi.org/10.3390/gels11030216
Chicago/Turabian StyleFatimi, Ahmed, Fouad Damiri, Nada El Arrach, Houria Hemdani, Adina Magdalena Musuc, and Mohammed Berrada. 2025. "Hydrogel-Based Biomaterials: A Patent Landscape on Innovation Trends and Patterns" Gels 11, no. 3: 216. https://doi.org/10.3390/gels11030216
APA StyleFatimi, A., Damiri, F., El Arrach, N., Hemdani, H., Musuc, A. M., & Berrada, M. (2025). Hydrogel-Based Biomaterials: A Patent Landscape on Innovation Trends and Patterns. Gels, 11(3), 216. https://doi.org/10.3390/gels11030216









