The Effects of Encapsulating Bioactive Irish Honey into Pluronic-Based Thermoresponsive Hydrogels and Potential Application in Soft Tissue Regeneration
Abstract
1. Introduction
2. Results and Discussion
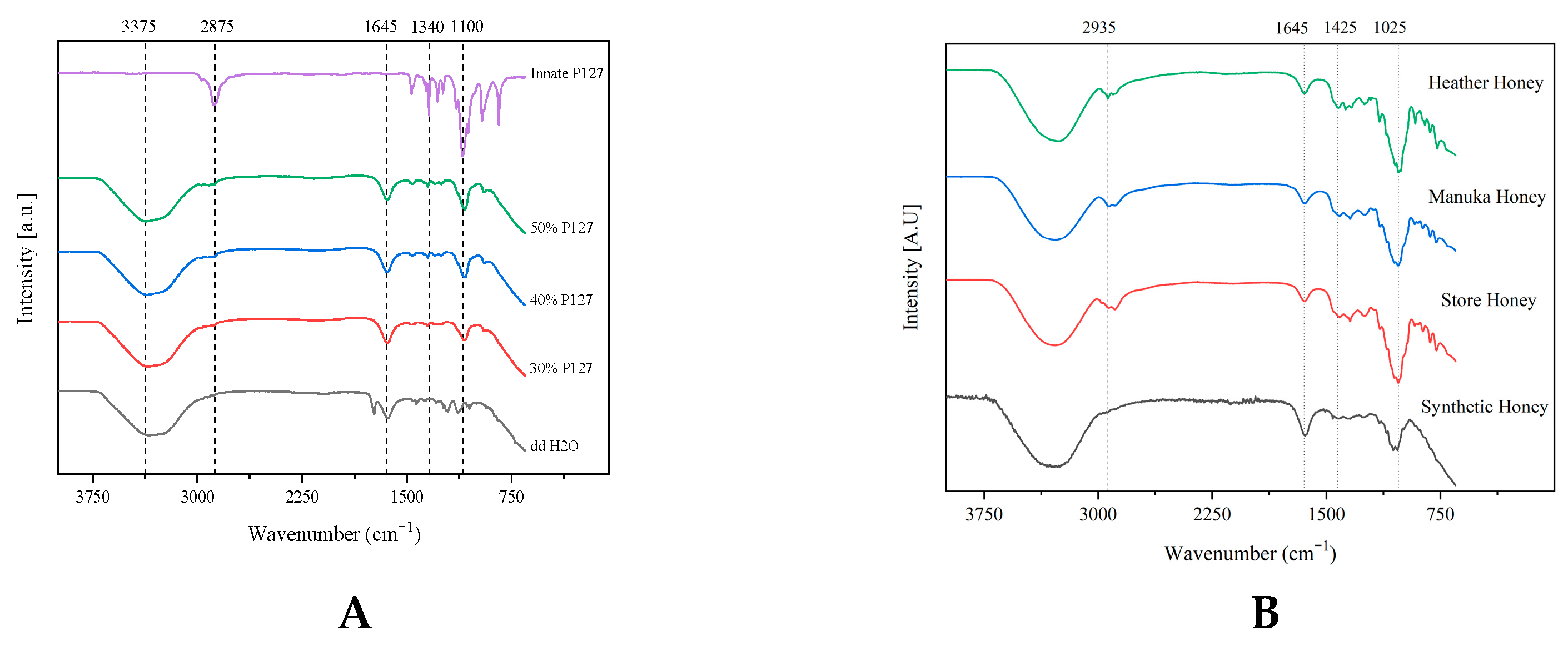
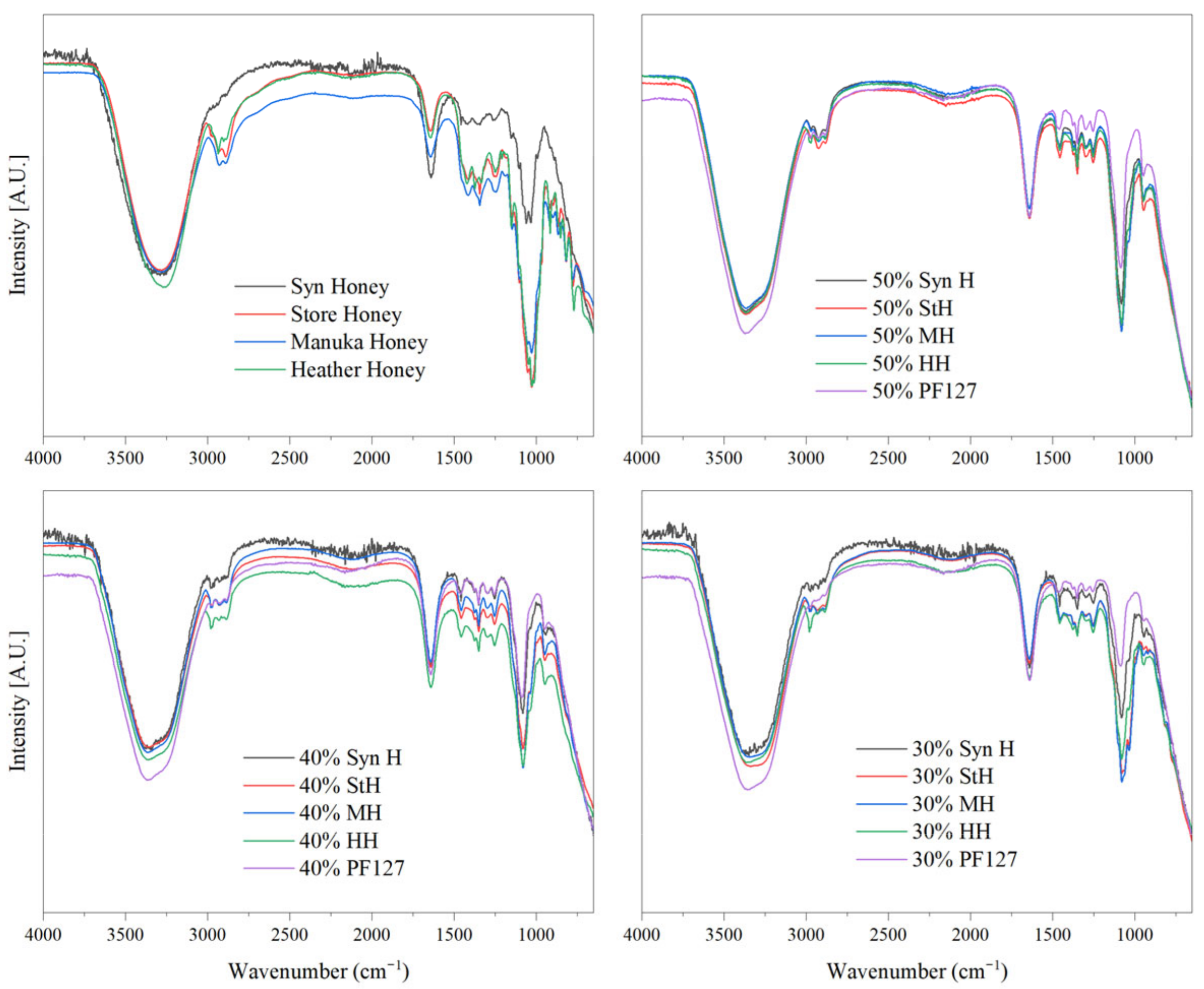
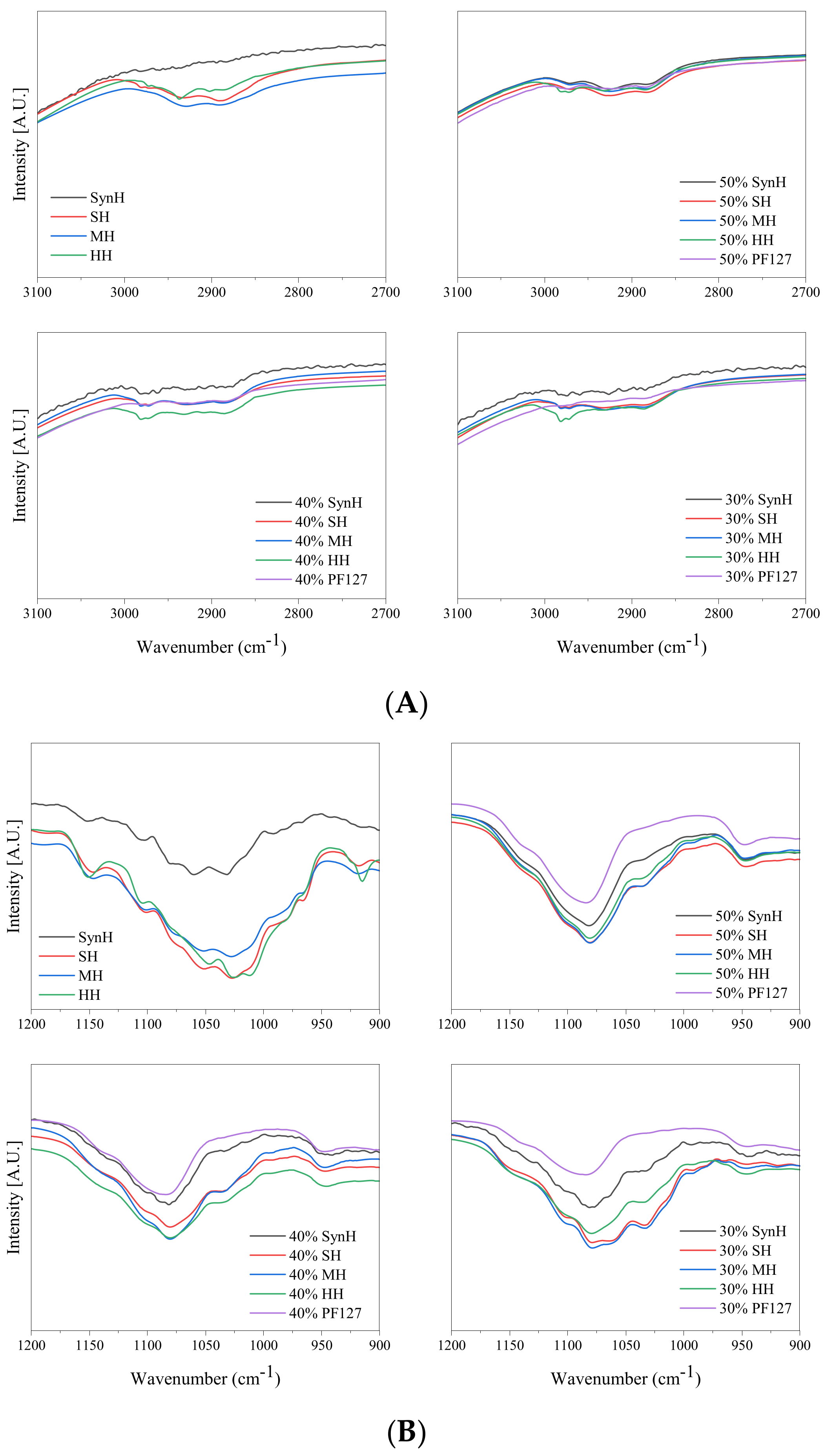
2.1. Attenuated Total Reflectance Fourier-Transform Infrared Spectroscopy (ATR-FTIR)
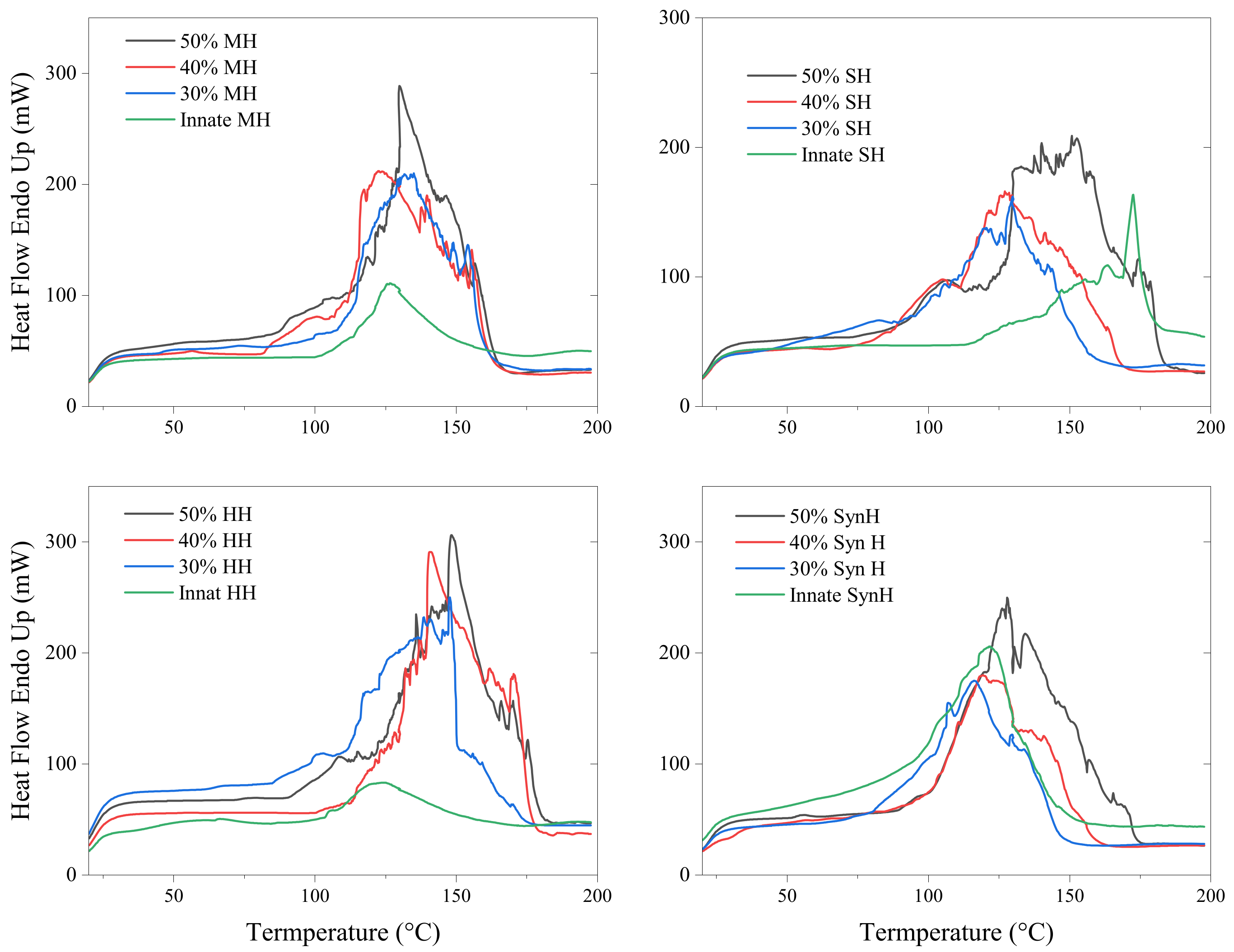
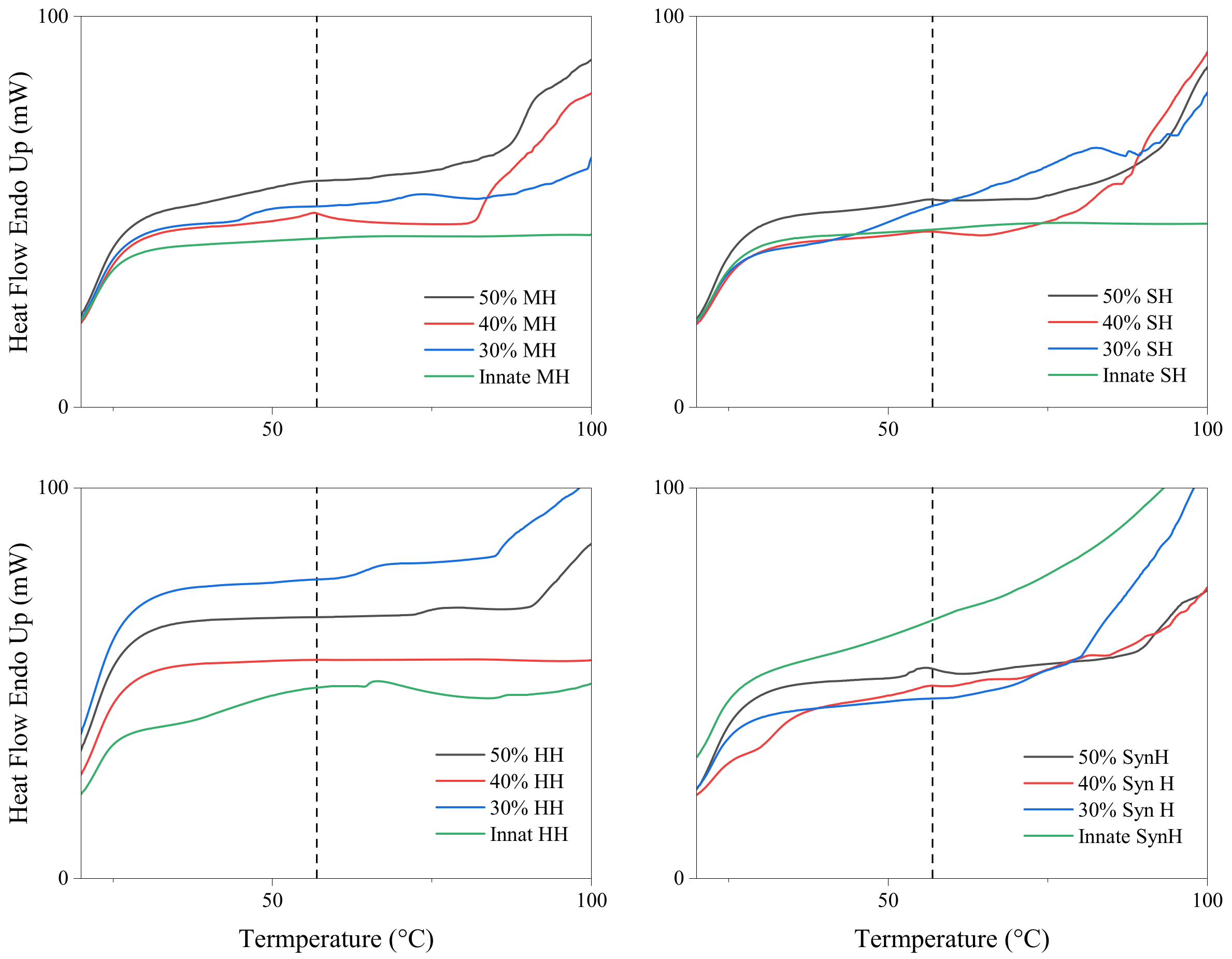
2.2. Differential Scanning Calorimetry (DSC)
2.3. Rheological Evaluation
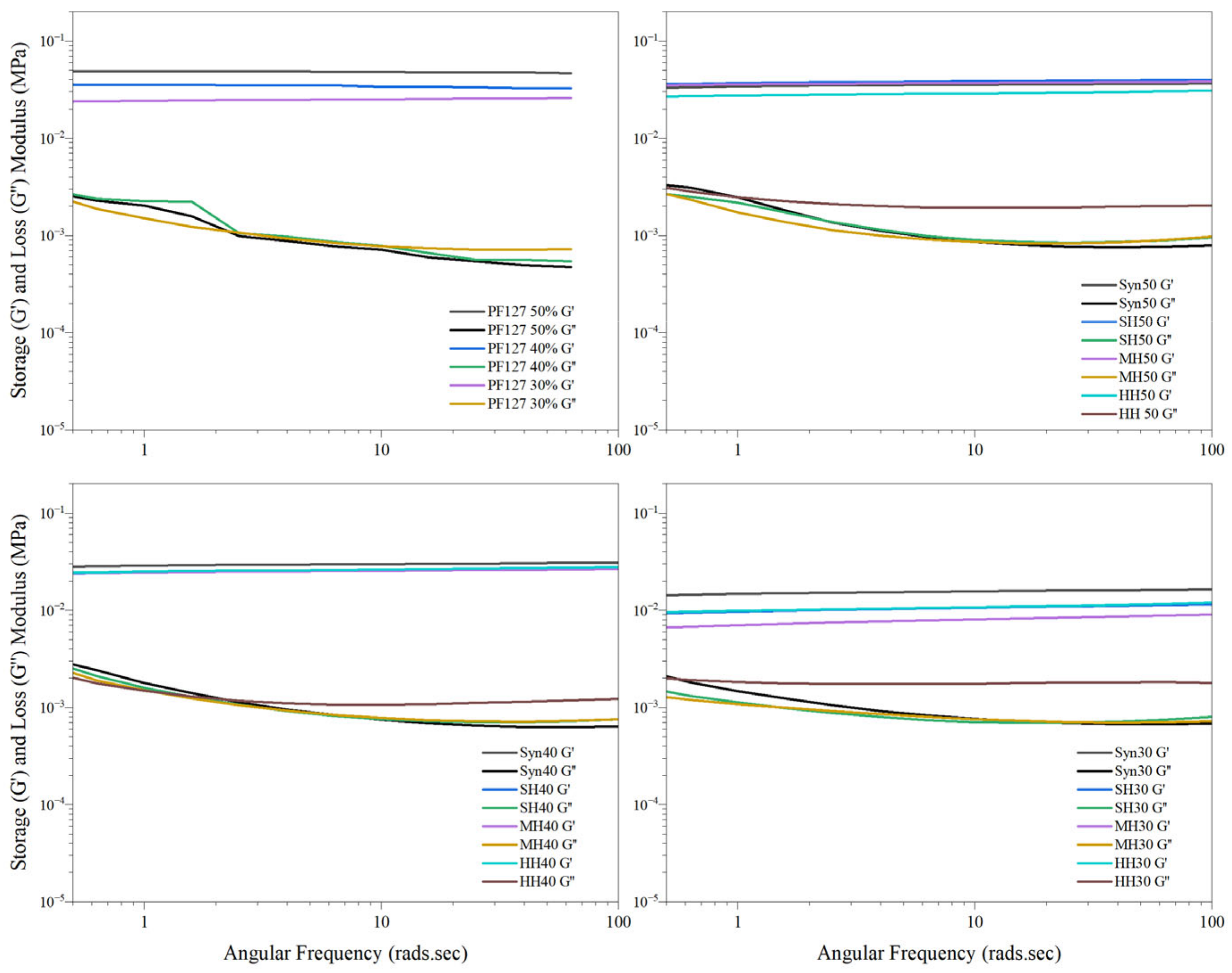
2.3.1. Amplitude Sweep
2.3.2. Frequency Sweep
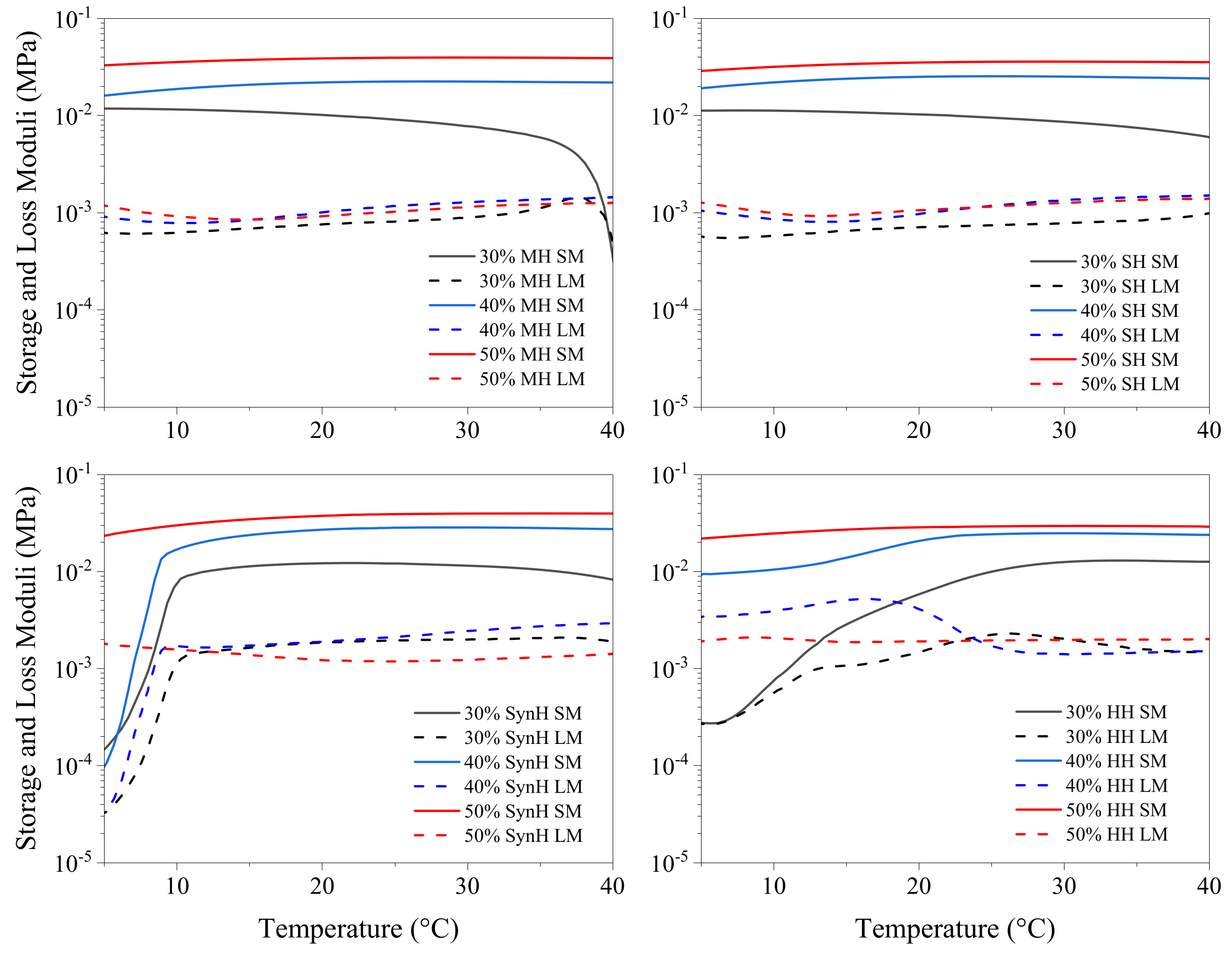
2.3.3. Temperature Sweep
2.4. Dynamic Light Scattering (DLS)
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methodologies
4.2.1. Hydrogel Preparation and API Integration
4.2.2. Preparation of Bioactive Hydrogel
4.2.3. Attenuated Total Reflectance Fourier-Transform Infrared Spectroscopy
4.2.4. Differential Scanning Calorimetry
4.2.5. Rheological Evaluation
Frequency Sweep
Temperature Sweep
4.2.6. Dynamic Light Scattering
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fitzpatrick, D.P.; Kealey, C.; Brady, D.; Goodman, M.; Gately, N. Treatments for the Amelioration of Persistent Factors in Complex Anal Fistula. Biotechnol. Lett. 2021, 44, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Özcan Bülbül, E.; Okur, M.E.; Üstündağ Okur, N.; Siafaka, P.I. Chapter 2—Traditional and Advanced Wound Dressings: Physical Characterization and Desirable Properties for Wound Healing. In Natural Polymers in Wound Healing and Repair; Sah, M.K., Kasoju, N., Mano, J.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 19–50. ISBN 978-0-323-90514-5. [Google Scholar]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound Dressings: Current Advances and Future Directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef] [PubMed]
- Herianto Ritonga, S.; Masraini Daulay, N. Effectiveness of Using Sialang Honey on Wound Bed Preparation in Diabetic Foot Ulcer. Enfermeria Clin. 2019, 29 (Suppl. S1), 88–90. [Google Scholar] [CrossRef]
- Wahdini, S.I.; Seswandhana, M.R.; Vityadewi, N.; Ramli, R.N.; Gabriela, G.C.; Dachlan, I. The Use of Indonesian Randu Honey for Chronic Wounds in a Patient with Uncontrolled Type 2 Diabetes Mellitus: A Case Report. Int. J. Surg. Case Rep. 2022, 95, 107140. [Google Scholar] [CrossRef]
- Sankar, J.; Lalitha, A.V.; Rameshkumar, R.; Mahadevan, S.; Kabra, S.K.; Lodha, R. Use of Honey Versus Standard Care for Hospital-Acquired Pressure Injury in Critically Ill Children: A Multicenter Randomized Controlled Trial. Pediatr. Crit. Care Med. J. Soc. Crit. Care Med. World Fed. Pediatr. Intensive Crit. Care Soc. 2020, 22, e349–e362. [Google Scholar] [CrossRef]
- Bucekova, M.; Buriova, M.; Pekarik, L.; Majtan, V.; Majtan, J. Phytochemicals-Mediated Production of Hydrogen Peroxide Is Crucial for High Antibacterial Activity of Honeydew Honey. Sci. Rep. 2018, 8, 9061. [Google Scholar] [CrossRef]
- Alevia, M.; Rasines, S.; Cantero, L.; Sancho, M.T.; Fernández-Muiño, M.A.; Osés, S.M. Chemical Extraction and Gastrointestinal Digestion of Honey: Influence on Its Antioxidant, Antimicrobial and Anti-Inflammatory Activities. Foods 2021, 10, 1412. [Google Scholar] [CrossRef]
- Lin, T.; Huang, L.; Cheng, N.; Wang, Y.; Ning, Z.; Huang, S.; Wu, Y.; Chen, T.; Su, S.; Lin, Y. The in Vitro and in Vivo Antibacterial Activities of Uniflorous Honey from a Medicinal Plant, Scrophularia Ningpoensis Hemsl., and Characterization of Its Chemical Profile with UPLC-MS/MS. J. Ethnopharmacol. 2022, 296, 115499. [Google Scholar] [CrossRef]
- Sakač, M.; Jovanov, P.; Marić, A.; Četojević-Simin, D.; Novaković, A.; Plavšić, D.; Škrobot, D.; Kovač, R. Antioxidative, Antibacterial and Antiproliferative Properties of Honey Types from the Western Balkans. Antioxidants 2022, 11, 1120. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martínez Flórez, S.; Agudo Toyos, P.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Mol. J. Synth. Chem. Nat. Prod. Chem. 2018, 23, 2322. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Gasparrini, M.; Forbes-Hernández, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Manna, P.P.; Battino, M.; Giampieri, F. Protective Effects of Manuka Honey on LPS-Treated RAW 264.7 Macrophages. Part 1: Enhancement of Cellular Viability, Regulation of Cellular Apoptosis and Improvement of Mitochondrial Functionality. Food Chem. Toxicol. 2018, 121, 203–213. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Giampieri, F.; Cordero, M.; Gasparrini, M.; Forbes-Hernández, T.Y.; Mazzoni, L.; Afrin, S.; Beltrán-Ayala, P.; González-Paramás, A.M.; Santos-Buelga, C.; et al. Activation of AMPK/Nrf2 Signalling by Manuka Honey Protects Human Dermal Fibroblasts against Oxidative Damage by Improving Antioxidant Response and Mitochondrial Function Promoting Wound Healing. J. Funct. Foods 2016, 25, 38–49. [Google Scholar] [CrossRef]
- Schuh, C.M.A.P.; Aguayo, S.; Zavala, G.; Khoury, M. Exosome-like Vesicles in Apis Mellifera Bee Pollen, Honey and Royal Jelly Contribute to Their Antibacterial and pro-Regenerative Activity. J. Exp. Biol. 2019, 222, jeb208702. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Lee, B.H.; Wei, K. 5-Hydroxymethylfurfural Mitigates Lipopolysaccharide-Stimulated Inflammation via Suppression of MAPK, NF-κB and mTOR Activation in RAW 264.7 Cells. Molecules 2019, 24, 275. [Google Scholar] [CrossRef]
- Gannabathula, S.; Skinner, M.A.; Rosendale, D.; Greenwood, J.M.; Mutukumira, A.N.; Steinhorn, G.; Stephens, J.; Krissansen, G.W.; Schlothauer, R.C. Arabinogalactan Proteins Contribute to the Immunostimulatory Properties of New Zealand Honeys. Immunopharmacol. Immunotoxicol. 2012, 34, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Bucekova, M.; Sojka, M.; Valachova, I.; Martinotti, S.; Ranzato, E.; Szep, Z.; Majtan, V.; Klaudiny, J.; Majtan, J. Bee-Derived Antibacterial Peptide, Defensin-1, Promotes Wound Re-Epithelialisation in Vitro and in Vivo. Sci. Rep. 2017, 7, 7340. [Google Scholar] [CrossRef]
- Majtan, J.; Kumar, P.; Majtan, T.; Walls, A.F.; Klaudiny, J. Effect of Honey and Its Major Royal Jelly Protein 1 on Cytokine and MMP-9 mRNA Transcripts in Human Keratinocytes. Exp. Dermatol. 2010, 19, 73–79. [Google Scholar] [CrossRef]
- Lukanc, B.; Potokar, T.; Erjavec, V. Complete Skin Regeneration with Medical Honey after Skin Loss on the Entire Circumference of a Leg in a Cat. J. Tissue Viability 2020, 29, 148–152. [Google Scholar] [CrossRef]
- Ramzi, M.; Kashaninejad, M.; Salehi, F.; Sadeghi Mahoonak, A.; Razavi, M. Rheological and Physicochemical Properties of Honeys As a Function of Temperature, Concentration and Moisture Content. J. Food Biosci. Technol. 2017, 7. [Google Scholar]
- Bosco, J. Principles of Wound Care & Bandaging Techniques. Todays Vet. Pract. 2012.
- Majkut, M.; Kwiecińska-Piróg, J.; Wszelaczyńska, E.; Pobereżny, J.; Gospodarek-Komkowska, E.; Wojtacki, K.; Barczak, T.; Majkut Michałand Kwiecińska-Piróg, J.; Wszelaczyńska, E.; Pobereżny, J.; et al. Antimicrobial Activity of Heat-Treated Polish Honeys. Food Chem. 2021, 343, 128561. [Google Scholar] [CrossRef]
- Al-Waili, N.S. Investigating the Antimicrobial Activity of Natural Honey and Its Effects on the Pathogenic Bacterial Infections of Surgical Wounds and Conjunctiva. J. Med. Food 2004, 7, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.W.; Azman, A.-S.; Tan, J.W. Incorporation of Natural and Synthetic Polymers into Honey Hydrogel for Wound Healing: A Review. Health Sci. Rep. 2024, 7, e2251. [Google Scholar] [CrossRef] [PubMed]
- Nezhad-Mokhtari, P.; Javanbakht, S.; Asadi, N.; Ghorbani, M.; Milani, M.; Hanifehpour, Y.; Gholizadeh, P.; Akbarzadeh, A. Recent Advances in Honey-Based Hydrogels for Wound Healing Applications: Towards Natural Therapeutics. J. Drug Deliv. Sci. Technol. 2021, 66, 102789. [Google Scholar] [CrossRef]
- Hixon, K.R.; Klein, R.C.; Eberlin, C.T.; Linder, H.R.; Ona, W.J.; Gonzalez, H.; Sell, S.A. A Critical Review and Perspective of Honey in Tissue Engineering and Clinical Wound Healing. Adv. Wound Care 2019, 8, 403–415. [Google Scholar] [CrossRef]
- Park, J.-S.; An, S.-J.; Jeong, S.-I.; Gwon, H.-J.; Lim, Y.-M.; Nho, Y.-C. Chestnut Honey Impregnated Carboxymethyl Cellulose Hydrogel for Diabetic Ulcer Healing. Polymers 2017, 9, 248. [Google Scholar] [CrossRef]
- Momin, M.; Kurhade, S.; Khanekar, P.; Mhatre, S. Novel Biodegradable Hydrogel Sponge Containing Curcumin and Honey for Wound Healing. J. Wound Care 2016, 25, 364–372. [Google Scholar] [CrossRef]
- El-Kased, R.F.; Amer, R.I.; Attia, D.; Elmazar, M.M. Honey-Based Hydrogel: In Vitro and Comparative In Vivo Evaluation for Burn Wound Healing. Sci. Rep. 2017, 7, 9692. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Rajput, M.; Barui, A.; Chatterjee, S.S.; Pal, N.K.; Chatterjee, J.; Mukherjee, R. Dual Cross-Linked Honey Coupled 3D Antimicrobial Alginate Hydrogels for Cutaneous Wound Healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111218. [Google Scholar] [CrossRef]
- Stojkovska, J.; Petrovic, P.; Jancic, I.; Milenkovic, M.T.; Obradovic, B. Novel Nano-Composite Hydrogels with Honey Effective against Multi-Resistant Clinical Strains of Acinetobacter Baumannii and Pseudomonas Aeruginosa. Appl. Microbiol. Biotechnol. 2019, 103, 8529–8543. [Google Scholar] [CrossRef]
- Mohd-Aspar, M.A.; Edros, R.; Hamzah, N.A. Optimisation of Topical Antibacterial Preparation from Malaysian Kelulut Honey by Using Xanthan Gum as Polymeric Agent. Trop. Biomed. 2021, 38, 226–238. [Google Scholar] [CrossRef]
- Abraham, S.A.; Yashavanth, G.; Deveswaran, R.; Bharath, S.; Azamathulla, M.; Shanmuganathan, S. Honey Based Hydrogel as Delivery System for Wound Healing. Mater. Today Proc. 2022, 49, 1709–1718. [Google Scholar] [CrossRef]
- Chopra, H.; Bibi, S.; Kumar, S.; Khan, M.S.; Kumar, P.; Singh, I. Preparation and Evaluation of Chitosan/PVA Based Hydrogel Films Loaded with Honey for Wound Healing Application. Gels 2022, 8, 111. [Google Scholar] [CrossRef]
- Sindi, A.M.; Rizg, W.Y.; Khan, M.K.; Alkhalidi, H.M.; Alharbi, W.S.; Sabei, F.Y.; Alfayez, E.; Alkharobi, H.; Korayem, M.; Majrashi, M.; et al. Tailoring and Optimization of a Honey-Based Nanoemulgel Loaded with an Itraconazole-Thyme Oil Nanoemulsion for Oral Candidiasis. Drug Deliv. 2023, 30, 2173337. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Ramsey, J.D.; Samadi, A.; Atoufi, Z.; Yazdi, M.K.; Ganjali, M.R.; Amirabad, L.M.; Zangene, E.; Farokhi, M.; Formela, K.; et al. Poloxamer: A Versatile Tri-Block Copolymer for Biomedical Applications. Acta Biomater. 2020, 110, 37–67. [Google Scholar] [CrossRef]
- Diniz, I.M.A.; Chen, C.; Xu, X.; Ansari, S.; Zadeh, H.H.; Marques, M.M.; Shi, S.; Moshaverinia, A. Pluronic F-127 Hydrogel as a Promising Scaffold for Encapsulation of Dental-Derived Mesenchymal Stem Cells. J. Mater. Sci. Mater. Med. 2015, 26, 153. [Google Scholar] [CrossRef]
- Pitto-Barry, A.; Barry, N.P.E. Pluronic® Block-Copolymers in Medicine: From Chemical and Biological Versatility to Rationalisation and Clinical Advances. Polym. Chem. 2014, 5, 3291–3297. [Google Scholar] [CrossRef]
- Percival, S.L.; Chen, R.; Mayer, D.; Salisbury, A.-M. Mode of Action of Poloxamer-Based Surfactants in Wound Care and Efficacy on Biofilms. Int. Wound J. 2018, 15, 749–755. [Google Scholar] [CrossRef]
- Rajan, A.S.; Sheen, K.M.; Sadhasivam, B.; Gonsalves, D.F.; Nachimuthu, S. Carrageenan Hydrogel Loaded with Pluronic®F68/Curcumin: An Efficient Dressing Material for Chronic Wounds. In AIP Conference Proceedings; AIP Publishing: Seoul, South Korea, 2020; p. 020011. [Google Scholar]
- Ahmad Nayik, G.; Nanda, V.; Zohra, B.; Dar, B.N.; Javed Ansari, M.; Al Obaid, S.; Bobis, O. Response Surface Approach to Optimize Temperature, pH and Time on Antioxidant Properties of Wild Bush (Plectranthus rugosus) Honey from High Altitude Region (Kashmir Valley) of India. Saudi J. Biol. Sci. 2022, 29, 767–773. [Google Scholar] [CrossRef]
- Guttentag, A.; Krishnakumar, K.; Cokcetin, N.; Harry, E.; Carter, D. Factors Affecting the Production and Measurement of Hydrogen Peroxide in Honey Samples. Access Microbiol. 2021, 3, 198. [Google Scholar] [CrossRef] [PubMed]
- Bridelli, M.G. Fourier Transform Infrared Spectroscopy in the Study of Hydrated Biological Macromolecules; IntechOpen: London, UK, 2017; ISBN 978-953-51-2894-6. [Google Scholar]
- Anjos, O.; Guiné, R.P.F.; Santos, A.J.A.; Paula, V.B.; Pereira, H.; Estevinho, L.M. Evaluation of FT-Raman and FTIR-ATR Spectroscopy for the Quality Evaluation of Lavandula Spp. Honey. Open Agric. 2021, 6, 47–56. [Google Scholar] [CrossRef]
- Tomić, S.L.; Vuković, J.S.; Babić Radić, M.M.; Filipović, V.V.; Živanović, D.P.; Nikolić, M.M.; Nikodinovic-Runic, J. Manuka Honey/2-Hydroxyethyl Methacrylate/Gelatin Hybrid Hydrogel Scaffolds for Potential Tissue Regeneration. Polymers 2023, 15, 589. [Google Scholar] [CrossRef]
- Sahlan, M.; Karwita, S.; Gozan, M.; Hermansyah, H.; Yohda, M.; Yoo, Y.J.; Pratami, D.K. Identification and Classification of Honey’s Authenticity by Attenuated Total Reflectance Fourier-Transform Infrared Spectroscopy and Chemometric Method. Vet. World 2019, 12, 1304–1310. [Google Scholar] [CrossRef]
- Seisonen, S.; Kivima, E.; Vene, K. Characterisation of the Aroma Profiles of Different Honeys and Corresponding Flowers Using Solid-Phase Microextraction and Gas Chromatography-Mass Spectrometry/Olfactometry. Food Chem. 2015, 169, 34–40. [Google Scholar] [CrossRef]
- Arango-Ospina, M.; Lasch, K.; Weidinger, J.; Boccaccini, A.R. Manuka Honey and Zein Coatings Impart Bioactive Glass Bone Tissue Scaffolds Antibacterial Properties and Superior Mechanical Properties. Front. Mater. 2021, 7, 610889. [Google Scholar] [CrossRef]
- Nguyen, H.T.L.; Kasapis, S.; Mantri, N. Physicochemical Properties and Effects of Honeys on Key Biomarkers of Oxidative Stress and Cholesterol Homeostasis in HepG2 Cells. Nutrients 2021, 13, 151. [Google Scholar] [CrossRef]
- Sahoo, S.; Chakraborti, C.; Behera, P.; Mishra, S. FTIR and Raman Spectroscopic Investigations of a Norfloxacin/Carbopol934 Polymeric Suspension. J. Young Pharm. JYP 2012, 4, 138–145. [Google Scholar] [CrossRef]
- Swarna; Pattanayek, S.K.; Ghosh, A.K. Role of Hydrogen Bond Interactions in Water–Polyol Medium in the Thickening Behavior of Cornstarch Suspensions. Colloid Polym. Sci. 2017, 295, 1117–1129. [Google Scholar] [CrossRef]
- Cordella, C.; Antinelli, J.-F.; Aurieres, C.; Faucon, J.-P.; Cabrol-Bass, D.; Sbirrazzuoli, N. Use of Differential Scanning Calorimetry (DSC) as a New Technique for Detection of Adulteration in Honeys. 1. Study of Adulteration Effect on Honey Thermal Behavior. J. Agric. Food Chem. 2002, 50, 203–208. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, X.-K.; Xue, X.-T.; Wu, D.-Y. Hydrogel Sheets of Chitosan, Honey and Gelatin as Burn Wound Dressings. Carbohydr. Polym. 2012, 88, 75–83. [Google Scholar] [CrossRef]
- Lang, K.; Sánchez-Leija, R.J.; Gross, R.A.; Linhardt, R.J. Review on the Impact of Polyols on the Properties of Bio-Based Polyesters. Polymers 2020, 12, 2969. [Google Scholar] [CrossRef]
- Mahbub, S.; Akter, S.; Luthfunnessa; Akter, P.; Anamul Hoque, M.; Abdul Rub, M.; Kumar, D.; Alghamdi, Y.G.; Asiri, A.M.; Džudžević-Čančar, H. Effects of Temperature and Polyols on the Ciprofloxacin Hydrochloride-Mediated Micellization of Sodium Dodecyl Sulfate. RSC Adv. 2020, 10, 14531–14541. [Google Scholar] [CrossRef]
- Kumar, P.; Mohan, C.; KanamSrinivasan Uma Shankar, M.; Gulati, M. Physiochemical Characterization and Release Rate Studies of Solid Dispersions of Ketoconazole with Pluronic F127 and PVP K-30. Iran. J. Pharm. Res. IJPR 2011, 10, 685–694. [Google Scholar]
- Mendonsa, N.S.; Murthy, S.N.; Hashemnejad, S.M.; Kundu, S.; Zhang, F.; Repka, M.A. Development of Poloxamer Gel Formulations via Hot-Melt Extrusion Technology. Int. J. Pharm. 2018, 537, 122–131. [Google Scholar] [CrossRef]
- Cordella, C.; Faucon, J.-P.; Cabrol-Bass, D.; Sbirrazzuoli, N. Application of DSC as a Tool for Honey Floral Species Characterization and Adulteration Detection. J. Therm. Anal. Calorim. 2003, 71, 279–290. [Google Scholar] [CrossRef]
- ISO 6721-10 (2015); Plastics—Determination of Dynamic Mechanical Properties—Part 10: Complex Shear Viscosity Using a Parallel-Plate Oscillatory Rheometer. International Organization for Standardization International Organization for Standardization: Geneva, Switzerland, 2015.
- DIN EN 14770:2023-09; Bitumen and Bituminous Binders—Determination of Complex Shear Modulus and Phase Angle—Dynamic Shear Rheometer (DSR). Deutsches Institut für Normung DIN Media GmbH: Berlin, Germany, 2023. [CrossRef]
- Shriky, B.; Kelly, A.; Isreb, M.; Babenko, M.; Mahmoudi, N.; Rogers, S.; Shebanova, O.; Snow, T.; Gough, T. Pluronic F127 Thermosensitive Injectable Smart Hydrogels for Controlled Drug Delivery System Development. J. Colloid Interface Sci. 2020, 565, 119–130. [Google Scholar] [CrossRef]
- Anopchenko, A.; Psurek, T.; VanderHart, D.; Douglas, J.F.; Obrzut, J. Dielectric Study of the Antiplasticization of Trehalose by Glycerol. Phys. Rev. E 2006, 74, 031501. [Google Scholar] [CrossRef]
- Cuomo, F.; Cofelice, M.; Lopez, F. Rheological Characterization of Hydrogels from Alginate-Based Nanodispersion. Polymers 2019, 11, 259. [Google Scholar] [CrossRef] [PubMed]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between Structure and Rheology of Hydrogels for Various Applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef] [PubMed]
- Sun Han Chang, R.; Lee, J.C.-W.; Pedron, S.; Harley, B.A.C.; Rogers, S.A. Rheological Analysis of the Gelation Kinetics of an Enzyme Crosslinked PEG Hydrogel. Biomacromolecules 2019, 20, 2198–2206. [Google Scholar] [CrossRef]
- Afonso, M.J.; Magalhães, M.; Fernandes, L.; Castro, M.; Ramalhosa, E.C.D. Temperature Effect on Rheological Behavior of Portuguese Honeys. Pol. J. Food Nutr. Sci. 2018, 68, 217–222. [Google Scholar] [CrossRef]
- Faustino, C.; Pinheiro, L. Analytical Rheology of Honey: A State-of-the-Art Review. Foods 2021, 10, 1709. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Díaz, D.; Navaza, J.M.; Quintáns-Riveiro, L.C. Effect of Temperature on the Viscosity of Honey. Int. J. Food Prop. 2009, 12, 396–404. [Google Scholar] [CrossRef]
- Yoo, B. Effect of Temperature on Dynamic Rheology of Korean Honeys. J. Food Eng. 2004, 65, 459–463. [Google Scholar] [CrossRef]
- Osuna, M.B.; Michaluk, A.; Romero, A.M.; Judis, M.A.; Bertola, N.C. Plasticizing Effect of Apis Mellifera Honey on Whey Protein Isolate Films. Biopolymers 2022, 113, e23519. [Google Scholar] [CrossRef]
- Chung, C.K.; García-Couce, J.; Campos, Y.; Kralisch, D.; Bierau, K.; Chan, A.; Ossendorp, F.; Cruz, L.J. Doxorubicin Loaded Poloxamer Thermosensitive Hydrogels: Chemical, Pharmacological and Biological Evaluation. Molecules 2020, 25, 2219. [Google Scholar] [CrossRef]
- Schmolka, I.R. Artificial Skin I. Preparation and Properties of Pluronic F-127 Gels for Treatment of Burns. J. Biomed. Mater. Res. 1972, 6, 571–582. [Google Scholar] [CrossRef]
- Beard, M.C.; Cobb, L.H.; Grant, C.S.; Varadarajan, A.; Henry, T.; Swanson, E.A.; Kundu, S.; Priddy, L.B. Autoclaving of Poloxamer 407 Hydrogel and Its Use as a Drug Delivery Vehicle. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 338–347. [Google Scholar] [CrossRef]
- Burak, J.; Grela, K.P.; Pluta, J.; Karolewicz, B.; Marciniak, D.M. Impact of Sterilisation Conditions on the Rheological Properties of Thermoresponsive Pluronic F-127-Based Gels for the Ophthalmic Use. Acta Pol. Pharm.-Drug Res. 2018, 75, 471–481. [Google Scholar] [CrossRef]
- Rafael, D.; Andrade, F.; Martinez-Trucharte, F.; Basas, J.; Seras-Franzoso, J.; Palau, M.; Gomis, X.; Pérez-Burgos, M.; Blanco, A.; López-Fernández, A.; et al. Sterilization Procedure for Temperature-Sensitive Hydrogels Loaded with Silver Nanoparticles for Clinical Applications. Nanomaterials 2019, 9, 380. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, D.P.; Kealey, C.; Brady, D.; Gately, N. Adapted Sterilisation for the Production of Thermoresponsive Hydrogels for Downstream Wound Healing Applications. Polym. Test. 2024, 132, 108379. [Google Scholar] [CrossRef]


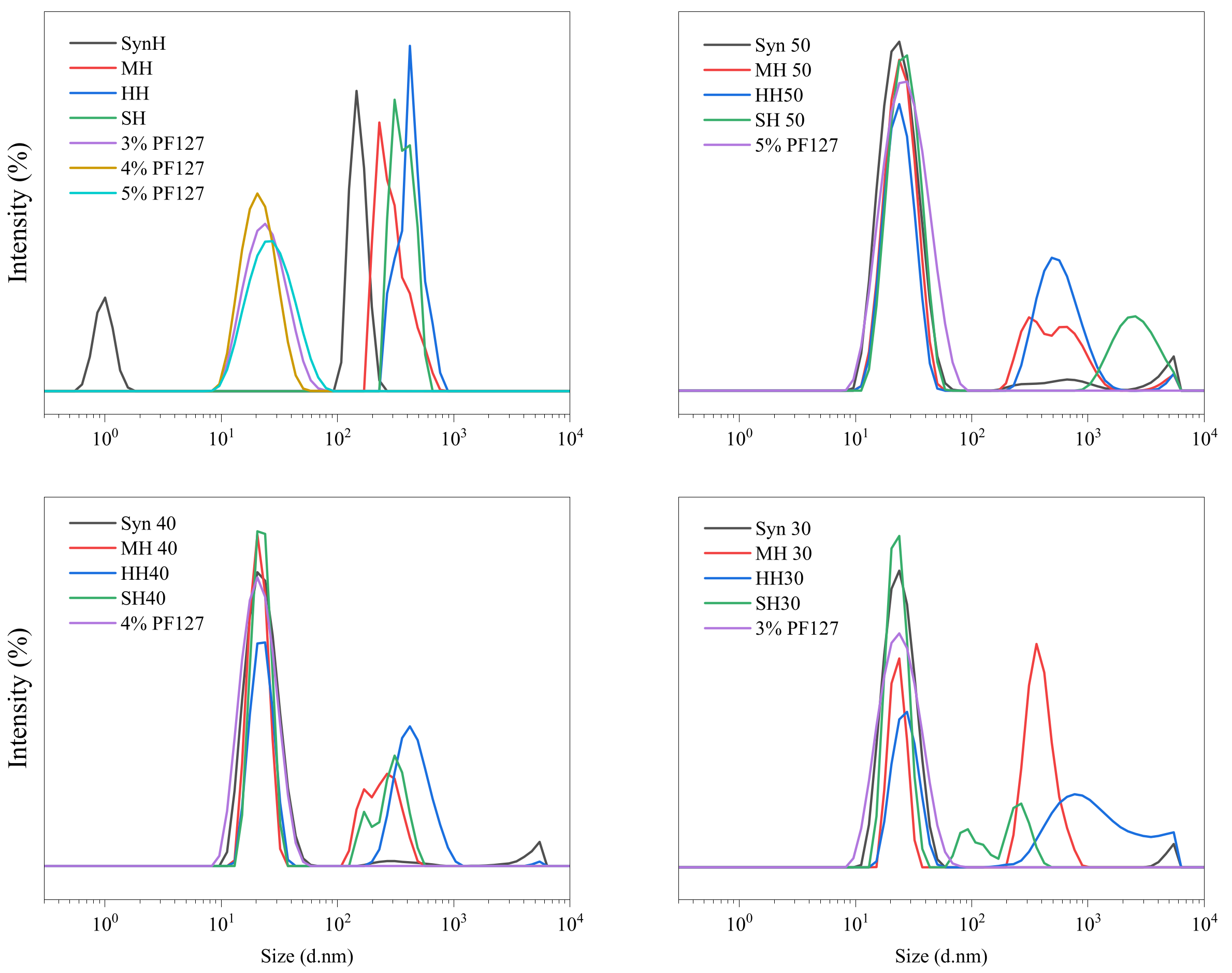
| Z-Average (nm) | Polydispersity Index (PI) | Peak 1 Mean (nm) | Peak 2 Mean (nm) | |
|---|---|---|---|---|
| 50% PF127 | 24.5 ± 1.2 | 0.161 ± 0.015 | 28.98 ± 1.4 | N/A |
| 40% PF127 | 18.4 ± 5.5 | 0.428 ± 0.151 | 30.4 ± 22.5 | N/A |
| 30% PF127 | 22.5 ± 1.5 | 0.135 ± 0.025 | 25.8 ± 2.1 | N/A |
| 50% MH | 59.98 ± 36.2 | 0.437 ± 0.122 | 25.4 ± 2.2 | 523.1 ± 207.5 |
| 40% MH | 152.6 ± 57.1 | 0.398 ± 0.078 | 21.2 ± 1.1 | 245.3 ± 74.8 |
| 30% MH | 159.8 ± 29.9 | 0.631 ± 0.131 | 397.05 ± 92.8 | 23.4 ± 1.4 |
| 50% HH | 40.4 ± 3.6 | 0.609 ± 0.068 | 24.6 ± 0.96 | 590.1 ± 91.2 |
| 40% HH | 52.7 ± 21.5 | 0.702 ± 0.186 | 136.9 ± 256.3 | 344.5 ± 189.3 |
| 30% HH | 84.1 ± 13.4 | 0.8 ± 0.066 | 478.5 ± 619.0 | 791.5 ± 939.4 |
| 50% SynH | 25.1 ± 1.1 | 0.284 ± 0.013 | 24.9 ± 1.4 | 3242.5 ± 2311.7 |
| 40% SynH | 27.1 ± 3.3 | 0.253 ± 0.06 | 23.4 ± 1.6 | 1973.3 ± 2253.1 |
| 30% SynH | 25.5 ± 0.9 | 0.242 ± 0.021 | 24.7 ± 1.2 | 5031.6 ± 125.5 |
| PF127 (g) | Innate Honey (g) | Final [PF127] (%(w/w)) | Final [Honey] (%(w/w)) | |
|---|---|---|---|---|
| 50% Bioactive PF127 | 16.67 * | 3.443 | 50 | 17.215 |
| 40% Bioactive PF127 | 16 ** | 4 | 40 | 20 |
| 30% Bioactive PF127 | 12 ** | 8 | 30 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fitzpatrick, D.P.; Browne, E.; Kealey, C.; Brady, D.; Kavanagh, S.; Devery, S.; Gately, N. The Effects of Encapsulating Bioactive Irish Honey into Pluronic-Based Thermoresponsive Hydrogels and Potential Application in Soft Tissue Regeneration. Gels 2025, 11, 215. https://doi.org/10.3390/gels11030215
Fitzpatrick DP, Browne E, Kealey C, Brady D, Kavanagh S, Devery S, Gately N. The Effects of Encapsulating Bioactive Irish Honey into Pluronic-Based Thermoresponsive Hydrogels and Potential Application in Soft Tissue Regeneration. Gels. 2025; 11(3):215. https://doi.org/10.3390/gels11030215
Chicago/Turabian StyleFitzpatrick, Daniel P., Emma Browne, Carmel Kealey, Damien Brady, Siobhan Kavanagh, Sinead Devery, and Noel Gately. 2025. "The Effects of Encapsulating Bioactive Irish Honey into Pluronic-Based Thermoresponsive Hydrogels and Potential Application in Soft Tissue Regeneration" Gels 11, no. 3: 215. https://doi.org/10.3390/gels11030215
APA StyleFitzpatrick, D. P., Browne, E., Kealey, C., Brady, D., Kavanagh, S., Devery, S., & Gately, N. (2025). The Effects of Encapsulating Bioactive Irish Honey into Pluronic-Based Thermoresponsive Hydrogels and Potential Application in Soft Tissue Regeneration. Gels, 11(3), 215. https://doi.org/10.3390/gels11030215






