Tyrosinase-Catalyzed Soy Protein and Tannic Acid Interaction: Effects on Structural and Rheological Properties of Complexes
Abstract
1. Introduction
2. Results and Discussion
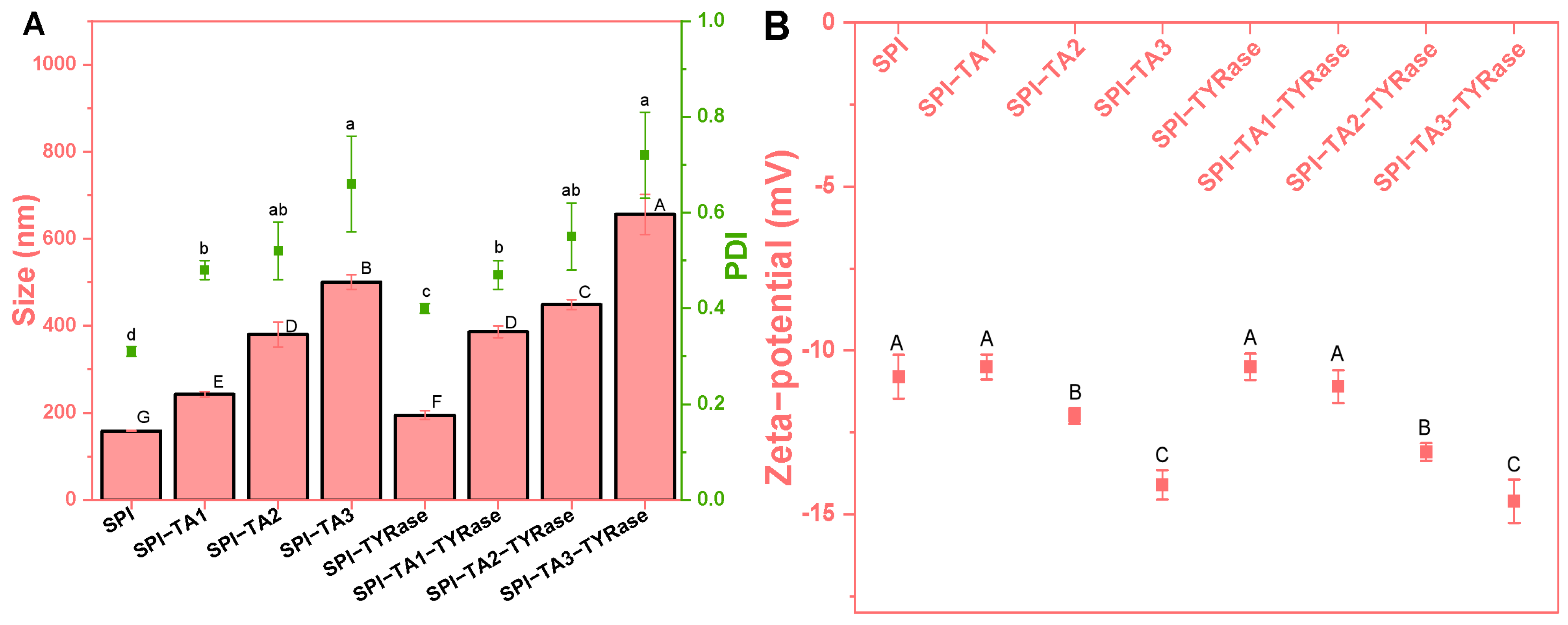
2.1. The Particle Size and Zeta-Potential
2.2. Intrinsic Fluorescence Spectroscopy Analysis
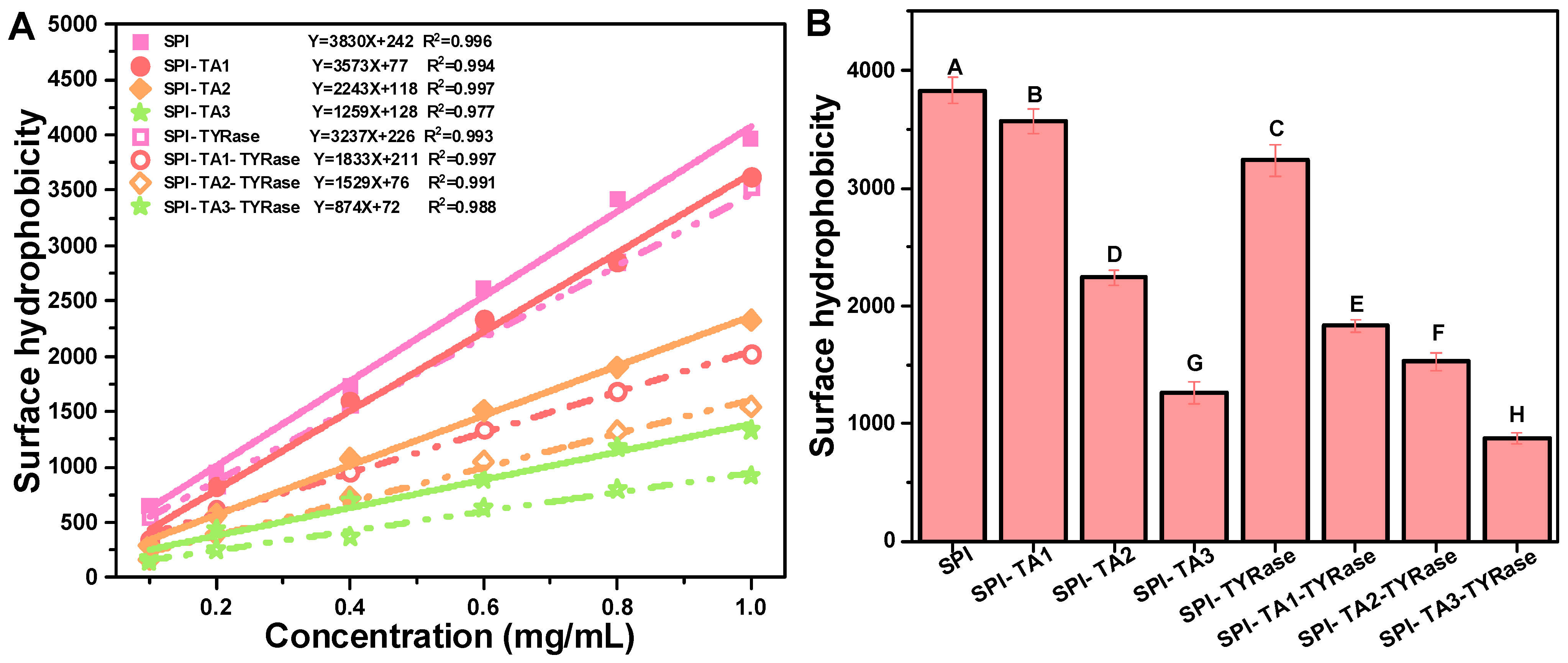
2.3. Surface Hydrophobicity Analysis
2.4. Fourier Transform Infrared (FTIR) Analysis
2.5. Rheological Properties
2.5.1. Apparent Viscosity
2.5.2. Viscoelastic Properties
2.6. Protein Microstructure
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Sample Preparation
4.3. Particle Size and Zeta-Potential Measurement
4.4. Intrinsic Fluorescence Spectroscopy Measurement
4.5. Surface Hydrophobicity Measurement
4.6. Fourier Transform Infrared (FTIR) Measurement
4.7. Rheological Properties Measurement
4.8. Microstructure Observation
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SPI | Soy protein isolate |
| TA | Tannic acid |
| TYRase | Tyrosinase |
| PDI | Polydispersity index |
| G′ | Storage modulus |
| G″ | Loss modulus |
References
- Paglarini, C.; Martini, S.; Pollonio, M. Using emulsion gels made with sonicated soy protein isolate dispersions to replace fat in frankfurters. LWT 2019, 99, 453–459. [Google Scholar] [CrossRef]
- Chen, L.; Chen, J.; Ren, J.; Zhao, M. Modifications of soy protein isolates using combined extrusion pre-treatment and controlled enzymatic hydrolysis for improved emulsifying properties. Food Hydrocoll. 2011, 25, 887–897. [Google Scholar] [CrossRef]
- You, Y.; Yang, L.; Chen, H.; Xiong, L.; Yang, F. Effects of (−)-Epigallocatechin-3-gallate on the functional and structural properties of soybean protein isolate. J. Agric. Food Chem. 2021, 69, 2306–2315. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Xu, J.; Zhang, S.; Li, Y. Effects of flexibility and surface hydrophobicity on emulsifying properties: Ultrasound-treated soybean protein isolate. LWT 2021, 142, 110881. [Google Scholar] [CrossRef]
- Shao, Y.; Tang, C. Characteristics and oxidative stability of soy protein-stabilized oil-in-water emulsions: Influence of ionic strength and heat pretreatment. Food Hydrocoll. 2014, 37, 149–158. [Google Scholar] [CrossRef]
- Shi, R.; Mu, Z.; Hu, J.; Jiang, Z.; Hou, J. Non-thermal techniques as an approach to modify the structure of milk proteins and improve their functionalities: A review of novel preparation. Crit. Rev. Food Sci. Nutr. 2023, 65, 1–29. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P.; Lin, H.; Fei, X.; Zhang, G.; Hu, X. Covalent binding of β-Lactoglobulin and rosmarinic acid by alkaline and free-radical treatment: Improved thermal stability and antioxidant capability. Food Biosci. 2024, 61, 104574. [Google Scholar] [CrossRef]
- Pan, X.; Fang, Y.; Wang, L.; Shi, Y.; Xie, M.; Xia, J.; Pei, F.; Li, P.; Xiong, W.; Shen, X.; et al. Covalent interaction between rice protein hydrolysates and chlorogenic acid: Improving the stability of oil-in-water emulsions. J. Agric. Food Chem. 2019, 67, 4023–4030. [Google Scholar] [CrossRef]
- Ali, M.; Keppler, J.; Coenye, T.; Schwarz, K. Covalent whey protein-rosmarinic acid interactions: A comparison of alkaline and enzymatic modifications on physicochemical, antioxidative, and antibacterial properties. J. Food Sci. 2018, 83, 2092–2100. [Google Scholar] [CrossRef]
- Wang, T.; Wang, N.; Yu, Y.; Yu, D.; Xu, S.; Wang, L. Study of soybean protein isolate-tannic acid non-covalent complexes by multi-spectroscopic analysis, molecular docking, and interfacial adsorption kinetics. Food Hydrocoll. 2023, 137, 108330. [Google Scholar] [CrossRef]
- Pham, L.; Wang, B.; Zisu, B.; Adhikari, B. Covalent modification of flaxseed protein isolate by phenolic compounds and the structure and functional properties of the adducts. Food Chem. 2019, 293, 463–471. [Google Scholar] [CrossRef]
- Guo, Y.; Bao, Y.; Sun, K.; Chang, C.; Liu, W. Effects of covalent interactions and gel characteristics on soy protein-tannic acid conjugates prepared under alkaline conditions. Food Hydrocoll. 2021, 112, 106293. [Google Scholar] [CrossRef]
- Pang, X.; Yang, Y.; Bian, X.; Wang, B.; Ren, L.; Liu, L.; Yu, D.; Yang, J.; Guo, J.; Wang, L. Hemp (Cannabis sativa L.) seed protein-EGCG conjugates: Covalent bonding and functional research. Foods 2021, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Peng, X.; Cheng, Y.; Zhu, Y.; Huang, Y.; Zhang, S.; Qi, B. Effects of catechin types found in tea polyphenols on the structural and functional properties of soybean protein isolate-catechin covalent complexes. LWT 2023, 173, 114336. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Liang, X.; McClements, D.; Liu, X.; Liu, F. Applications of oxidases in modification of food molecules and colloidal systems: Laccase, peroxidase and tyrosinase. Trends Food Sci. Technol. 2020, 103, 78–93. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, S.; Liu, H.; Gu, Y.; Zhu, J. Design and characterization of low salt myofibrillar protein-sugar beet pectin double-crosslinked gels pretreated by ultrasound and konjac glucomannan: Conformational and gelling properties. Food Hydrocoll. 2023, 141, 108717. [Google Scholar] [CrossRef]
- Jiang, Z.; Yuan, X.; Yao, K.; Li, X.; Zhang, X.; Mu, Z.; Jiang, L.; Hou, J. Laccase-aided modification: Effects on structure, gel properties and antioxidant activities of α-lactalbumin. LWT 2017, 80, 355–363. [Google Scholar] [CrossRef]
- Yi, J.; Chen, X.; Wen, Z.; Fan, Y. Improving the functionality of pea protein with laccase-catalyzed crosslinking mediated by chlorogenic acid. Food Chem. 2024, 433, 137344. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lv, P.; Zhang, L.; Yang, S.; Gao, Y. Structural and functional characterization of laccase-induced β-Lactoglobulin-ferulic acid-chitosan ternary conjugates. J. Agric. Food Chem. 2019, 67, 12054–12060. [Google Scholar] [CrossRef]
- Fan, Y.; Li, G.; Yi, J.; Huang, H. Structural characteristics, emulsifying and foaming properties of laccase-crosslinked bovine α-lactalbumin mediated by caffeic acid. Food Hydrocoll. 2022, 133, 107948. [Google Scholar] [CrossRef]
- Chen, C.; Yang, H.; Yang, X.; Ma, Q. Tannic acid: A crosslinker leading to versatile functional polymeric networks: A review. RSC Adv. 2022, 12, 7689–7711. [Google Scholar] [CrossRef]
- Li, R.; Dai, T.; Tan, Y.; Fu, G.; Wan, Y.; Liu, C.; McClements, D. Fabrication of pea protein-tannic acid complexes: Impact on formation, stability, and digestion of flaxseed oil emulsions. Food Chem. 2020, 310, 125828. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Huang, X.; Jiang, C.; Wang, L.; Du, X.; Ma, C.; Wang, H. Effects of tannic acid on the structure and proteolytic digestion of bovine lactoferrin. Food Hydrocoll. 2021, 117, 106666. [Google Scholar] [CrossRef]
- Li, R.; Dai, T.; Zhou, W.; Fu, G.; Wan, Y.; McClements, D.; Li, J. Impact of pH, ferrous ions, and tannic acid on lipid oxidation in plant-based emulsions containing saponin-coated flaxseed oil droplets. Food Res. Int. 2020, 136, 109618. [Google Scholar] [CrossRef]
- Deng, Z.; Wang, S.; Pei, Y.; Zhou, B.; Li, J.; Hou, X.; Li, B.; Liang, H. Tuning of molecular interactions between zein and tannic acid to modify sunflower sporopollenin exine capsules: Enhanced stability and targeted delivery of bioactive macromolecules. AACS Appl. Bio Mater. 2021, 4, 2686–2695. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chen, Y.; Hu, Y.; Han, Y.; Xu, J.; Zhao, Y.; Chen, X.; Li, B. Tuning the molecular interactions between gliadin and tannic acid to prepare pickering stabilizers with improved emulsifying properties. Food Hydrocoll. 2020, 111, 106179. [Google Scholar] [CrossRef]
- Boachie, R.; Okagu, O.; Abioye, R.; Hüttmann, N.; Oliviero, T.; Capuano, E.; Fogliano, V.; Udenigwe, C. Lentil protein and tannic acid interaction limits in vitro peptic hydrolysis and alters peptidomic profiles of the proteins. J. Agric. Food Chem. 2022, 70, 6519–6529. [Google Scholar] [CrossRef]
- Kong, W.; Du, Q.; Qu, Y.; Shao, C.; Chen, C.; Sun, J.; Mao, C.; Tang, R.; Gu, X. Tannic acid induces dentin biomineralization by crosslinking and surface modification. RSC Adv. 2022, 12, 3454–3464. [Google Scholar] [CrossRef]
- Wu, K.; Wu, Z.; Kang, Y.; Su, C.; Yi, F. Hydrogen bond-driven assembly of coral-like soy protein isolate-tannic acid microcomplex for encapsulation of limonene. J. Sci. Food Agric. 2022, 103, 185–194. [Google Scholar] [CrossRef]
- Liao, Y.; Sun, Y.; Peng, X.; Qi, B.; Li, Y. Effects of tannic acid on the physical stability, interfacial properties, and protein/lipid co-oxidation characteristics of oil body emulsions. Food Hydrocoll. 2023, 135, 108230. [Google Scholar] [CrossRef]
- Balange, A.; Benjakul, S. Effect of oxidised tannic acid on the gel properties of mackerel (Rastrelliger kanagurta) mince and surimi prepared by different washing processes. Food Hydrocoll. 2009, 23, 1693–1701. [Google Scholar] [CrossRef]
- Intarasirisawat, R.; Benjakul, S.; Visessanguan, W. Stability of emulsion containing skipjack roe protein hydrolysate modified by oxidised tannic acid. Food Hydrocoll. 2014, 41, 146–155. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, S.; Chen, Q.; Liu, Q.; Kong, B. Antioxidant activities and emulsifying properties of porcine plasma protein hydrolysates modified by oxidized tannic acid and oxidized chlorogenic acid. Process Biochem. 2019, 79, 105–113. [Google Scholar] [CrossRef]
- Vate, N.; Benjakul, S. Combined effect of squid ink tyrosinase and tannic acid on heat induced aggregation of natural actomyosin from sardine. Food Hydrocoll. 2016, 56, 62–70. [Google Scholar] [CrossRef]
- Vate, N.; Benjakul, S. Effect of the mixtures of squid ink tyrosinase and tannic acid on properties of sardine surimi gel. J. Food Sci. Technol. 2016, 53, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Vernhet, A.; Carrillo, S.; Poncet-Legrand, C. Condensed tannin changes induced by autoxidation: Effect of the initial degree of polymerization and concentration. J. Agric. Food Chem. 2014, 62, 7833–7842. [Google Scholar] [CrossRef]
- Jia, Y.; Yan, X.; Li, X.; Zhang, S.; Huang, Y.; Zhang, D.; Li, Y.; Qi, B. Soy protein-phlorizin conjugate prepared by tyrosinase catalysis: Identification of covalent binding sites and alterations in protein structure and functionality. Food Chem. 2023, 404, 134610. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ma, C.; McClements, D.; Gao, Y. A comparative study of covalent and non-covalent interactions between zein and polyphenols in ethanol-water solution. Food Hydrocoll. 2017, 63, 625–634. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Zhang, T.; McClements, D.; Liu, X.; Wu, X.; Liu, F. Enzymatic and nonenzymatic conjugates of lactoferrin and (−)-epigallocatechin gallate: Formation, structure, functionality, and allergenicity. J. Agric. Food Chem. 2021, 69, 6291–6302. [Google Scholar] [CrossRef]
- Wang, H.; You, S.; Wang, W.; Zeng, Y.; Su, R.; Qi, W.; Wang, K.; He, Z. Laccase-catalyzed soy protein and gallic acid complexation: Effects on conformational structures and antioxidant activity. Food Chem. 2022, 375, 131865. [Google Scholar] [CrossRef]
- Gu, M.; Shi, J.; Zhang, B.; Wang, X.; Wang, X. Covalent modification of soy protein isolates by hydroxytyrosol: Effects on structural and functional properties of adducts. LWT 2024, 198, 116041. [Google Scholar] [CrossRef]
- Tong, P.; Xu, X.; Liu, K.; Chen, H.; Gao, J. Denatured pre-treatment assisted polyphenol oxidase-catalyzed cross-linking: Effects on the cross-linking potential, structure, allergenicity and functional properties of OVA. Food Funct. 2021, 12, 10083–10096. [Google Scholar] [CrossRef]
- Dai, S.; Lian, Z.; Qi, W.; Chen, Y.; Tong, X.; Tian, T.; Lyu, B.; Wang, M.; Wang, H.; Jiang, L. Non-covalent interaction of soy protein isolate and catechin: Mechanism and effects on protein conformation. Food Chem. 2022, 384, 132507. [Google Scholar] [CrossRef] [PubMed]
- Zhan, F.; Yang, J.; Li, J.; Wang, Y.; Li, B. Characteristics of the interaction mechanism between tannic acid and sodium caseinate using multispectroscopic and thermodynamics methods. Food Hydrocoll. 2018, 75, 81–87. [Google Scholar] [CrossRef]
- Mo, J.; Wang, F.; Xu, Z.; Feng, C.; Fang, Y.; Tang, X.; Shen, X. Characterization and performance of soybean protein modified by tyrosinase. Int. J. Adhes. 2019, 92, 111–118. [Google Scholar] [CrossRef]
- Lantto, R.; Puolanne, E.; Kruus, K.; Buchert, J.; Autio, K. Tyrosinase-aided protein cross-linking: Effects on gel formation of chicken breast myofibrils and texture and water-holding of chicken breast meat homogenate gels. J. Agric. Food Chem. 2007, 55, 1248–1255. [Google Scholar] [CrossRef]
- Glusac, J.; Davidesko-Vardi, I.; Isaschar-Ovdat, S.; Kukavica, B.; Fishman, A. Gel-like emulsions stabilized by tyrosinase-crosslinked potato and zein proteins. Food Hydrocoll. 2018, 82, 53–63. [Google Scholar] [CrossRef]
- Qayum, A.; Hussain, M.; Li, M.; Li, J.; Shi, R.; Li, T.; Anwar, A.; Ahmed, Z.; Hou, J.; Jiang, Z. Gelling, microstructure and water-holding properties of alpha-lactalbumin emulsion gel: Impact of combined ultrasound pretreatment and laccase cross-linking. Food Hydrocoll. 2021, 110, 106122. [Google Scholar] [CrossRef]
- Isaschar-Ovdat, S.; Davidovich-Pinhas, M.; Fishman, A. Modulating the gel properties of soy glycinin by crosslinking with tyrosinase. Food Res. Int. 2016, 87, 42–49. [Google Scholar] [CrossRef]
- Yuan, X.; Li, X.; Zhang, X.; Mu, Z.; Gao, Z.; Jiang, L.; Jiang, Z. Effect of ultrasound on structure and functional properties of laccase-catalyzed α-lactalbumin. J. Food Eng. 2018, 223, 116–123. [Google Scholar] [CrossRef]
- Islam, S.; Moinuddin; Mir, A.R.; Arfat, M.Y.; Alam, K.; Ali, A. Studies on glycoxidatively modified human IgG: Implications in immuno-pathology of type 2 diabetes mellitus. Int. J. Biol. Macromol. 2017, 104, 19–29. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, Y.; Yuan, L.; Zhou, W.; Yang, J. Tyrosinase-Catalyzed Soy Protein and Tannic Acid Interaction: Effects on Structural and Rheological Properties of Complexes. Gels 2025, 11, 195. https://doi.org/10.3390/gels11030195
Pei Y, Yuan L, Zhou W, Yang J. Tyrosinase-Catalyzed Soy Protein and Tannic Acid Interaction: Effects on Structural and Rheological Properties of Complexes. Gels. 2025; 11(3):195. https://doi.org/10.3390/gels11030195
Chicago/Turabian StylePei, Yaqiong, Lei Yuan, Wenjing Zhou, and Jun Yang. 2025. "Tyrosinase-Catalyzed Soy Protein and Tannic Acid Interaction: Effects on Structural and Rheological Properties of Complexes" Gels 11, no. 3: 195. https://doi.org/10.3390/gels11030195
APA StylePei, Y., Yuan, L., Zhou, W., & Yang, J. (2025). Tyrosinase-Catalyzed Soy Protein and Tannic Acid Interaction: Effects on Structural and Rheological Properties of Complexes. Gels, 11(3), 195. https://doi.org/10.3390/gels11030195




