Hydrogels and Microgels: Driving Revolutionary Innovations in Targeted Drug Delivery, Strengthening Infection Management, and Advancing Tissue Repair and Regeneration
Abstract
1. Introduction
1.1. Natural and Synthetic Hydrogels
1.2. Mechanical Characterization of the Hydrogels
1.3. Effect of Hydrogel Stiffness on Stem Cell Behavior
2. Synthesis of Hydrogel
2.1. Physically Cross-Linked Hydrogels
2.2. Chemically Cross-Linked Hydrogels
3. Biomedical Applications of Hydrogels
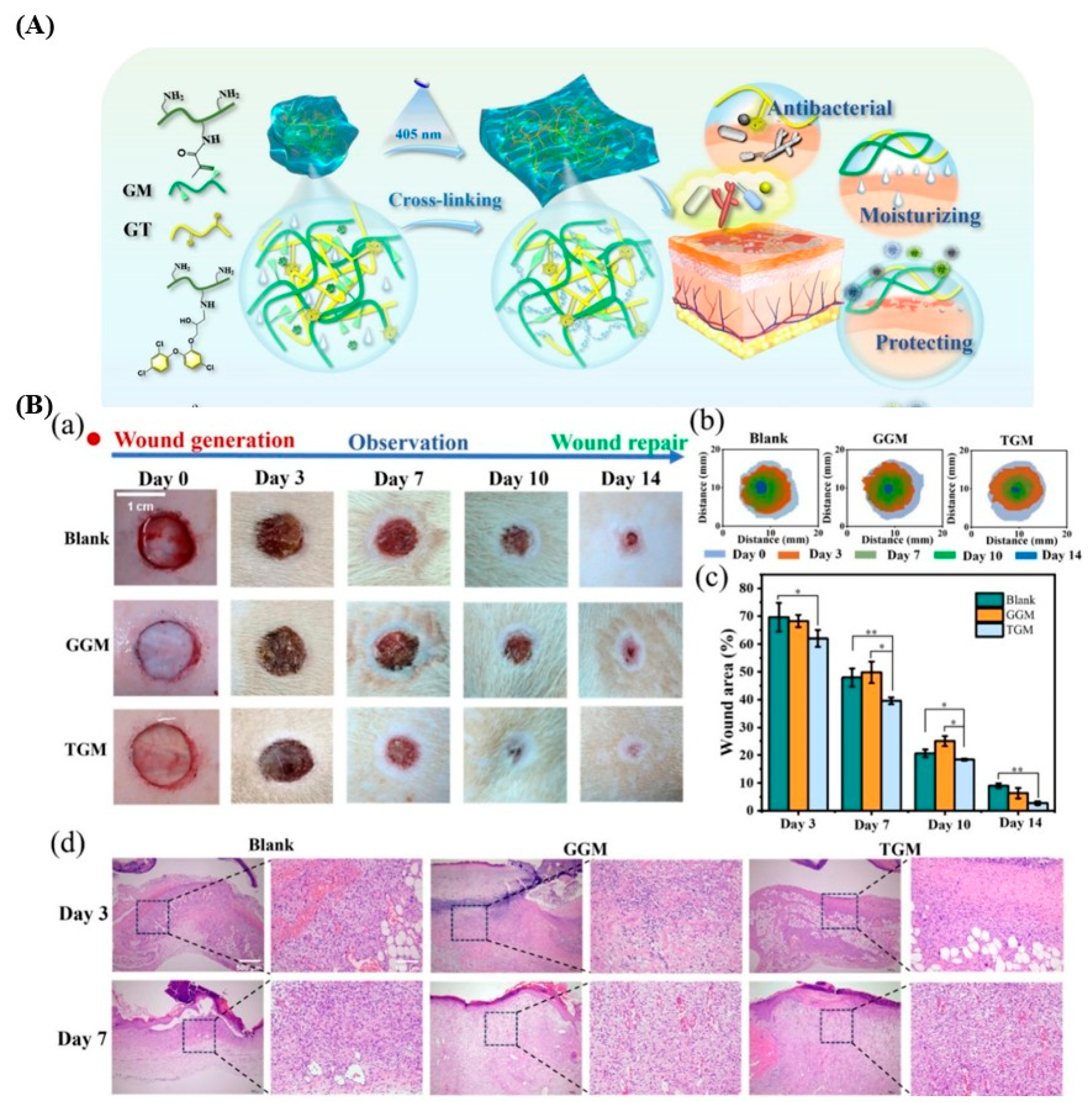
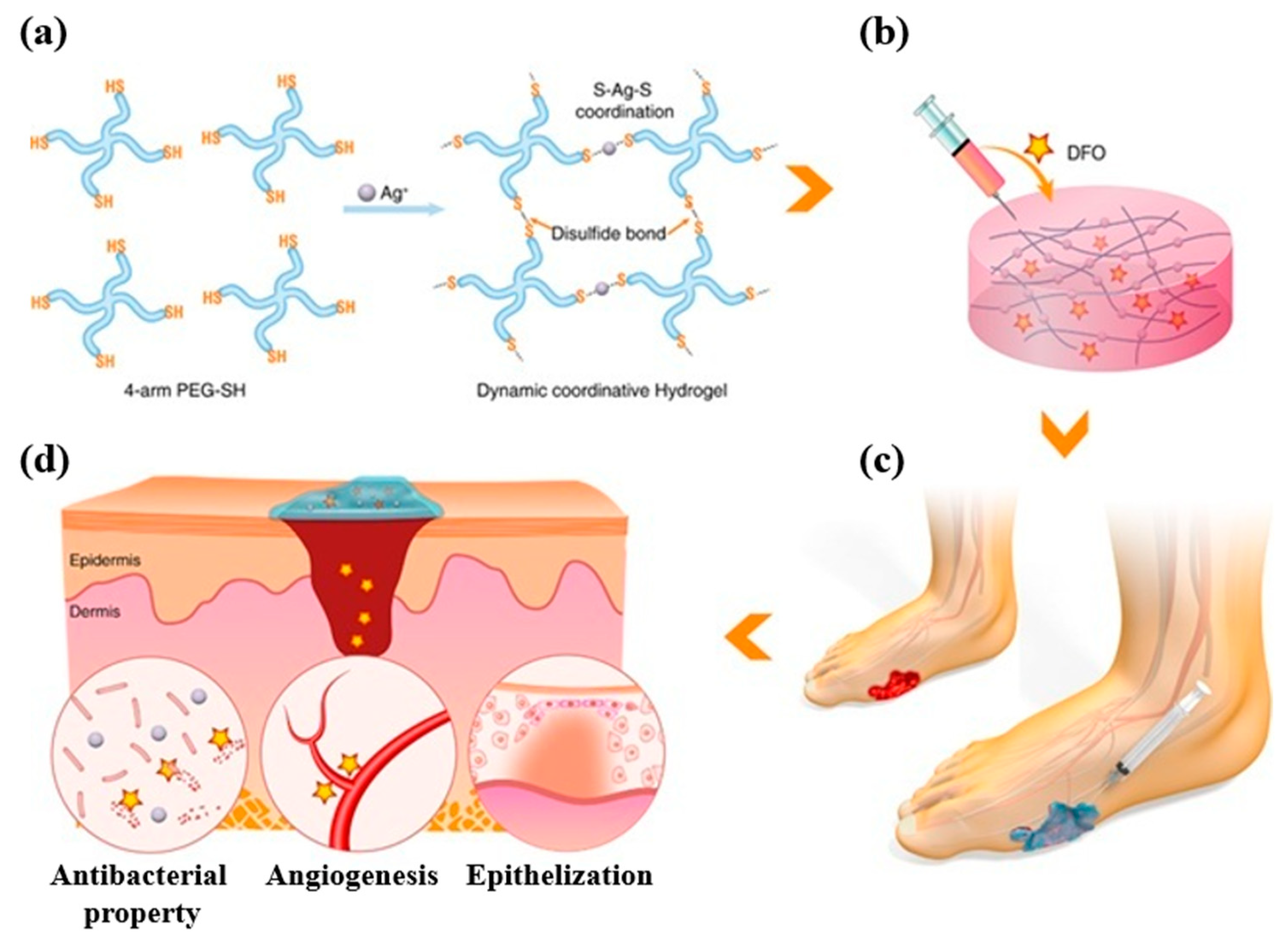
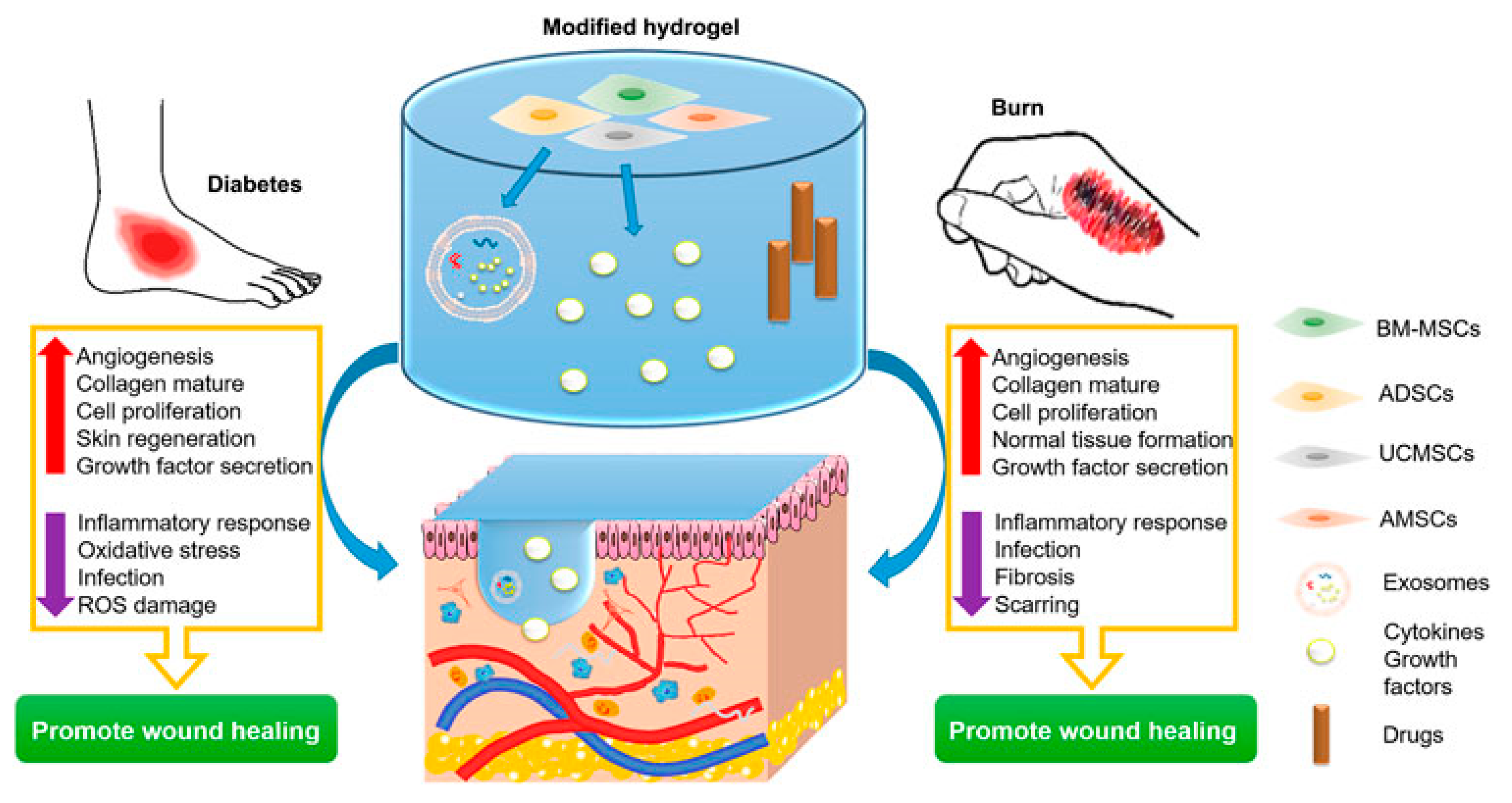
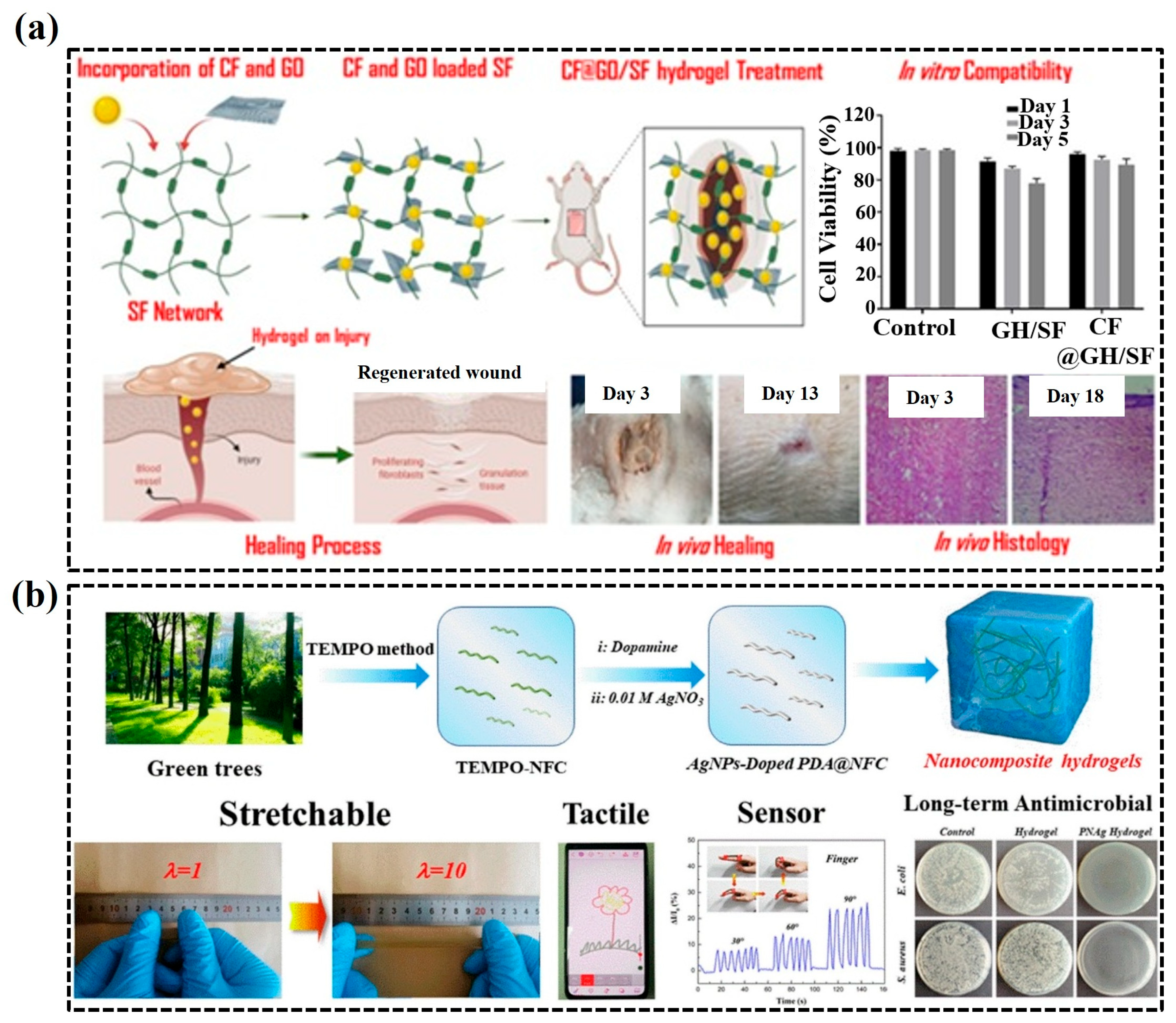
3.1. Wound Dressings
Dressing for Burn Wounds
3.2. Delivery of Therapeutic Agents
3.2.1. Antibacterial Application
3.2.2. Anti-Inflammatory Action
3.3. Hydrogel for Cancer Treatment
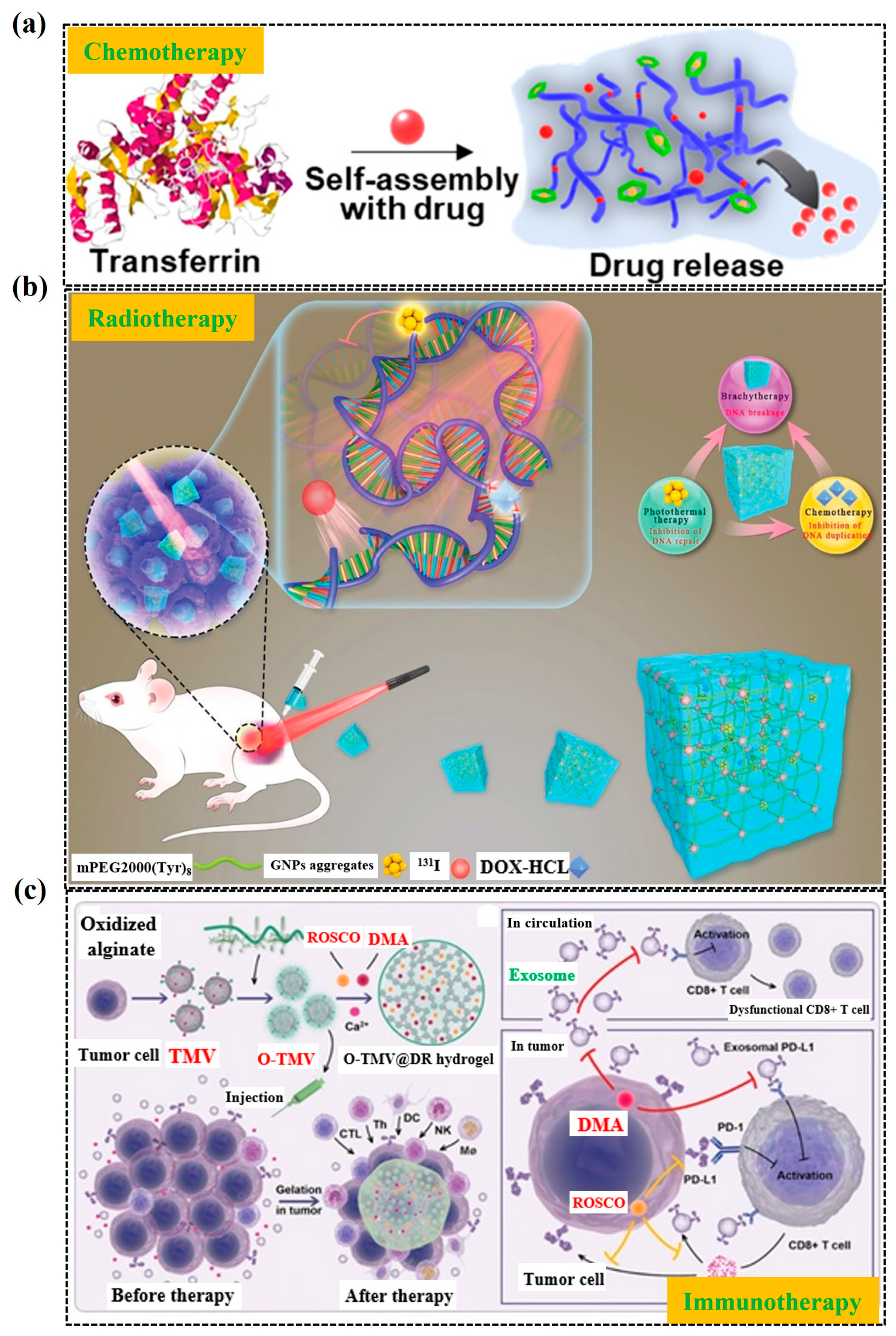
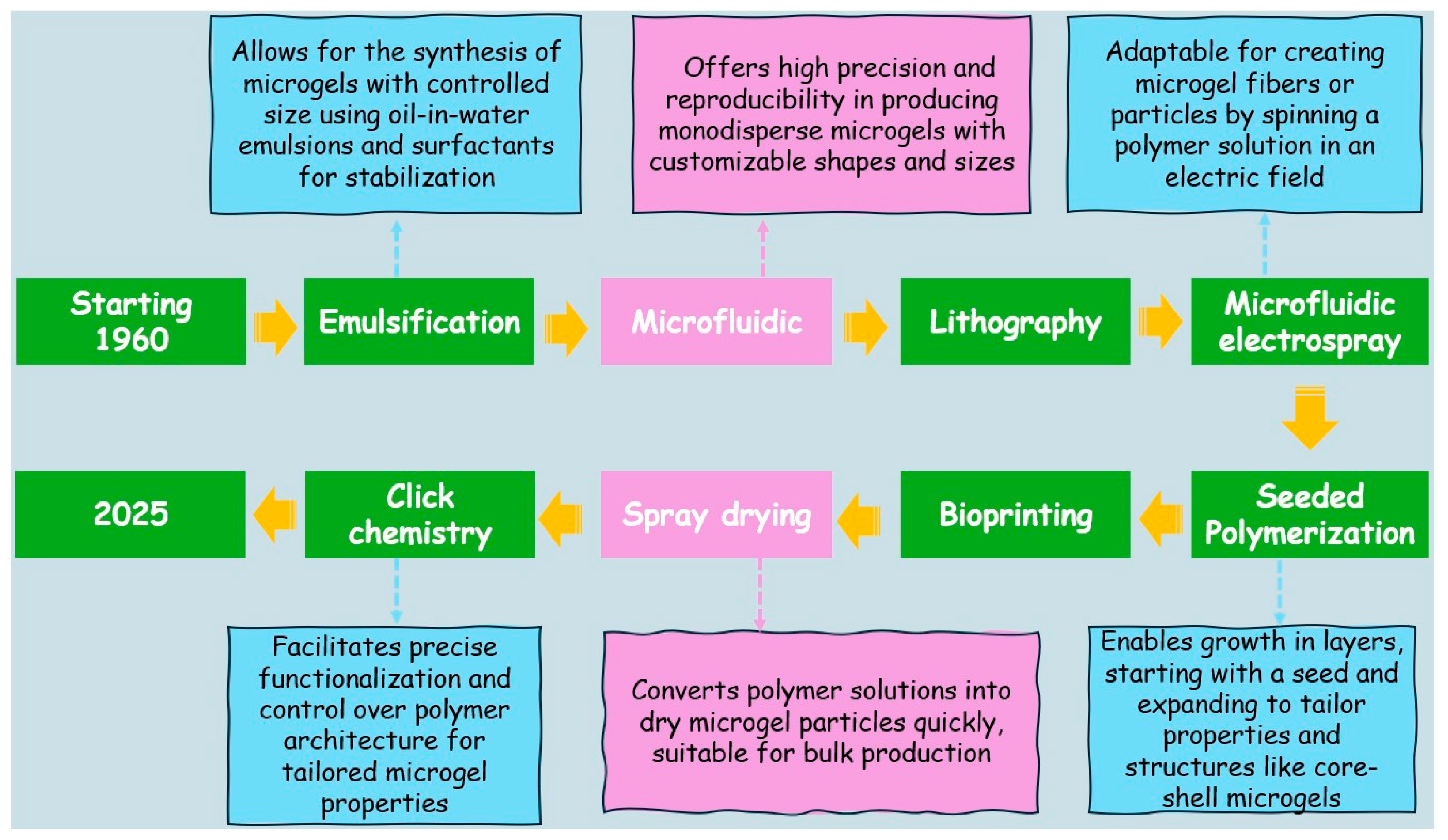
4. Microgels
Fabrication of Microgels
5. Application of Microgels
5.1. Delivery of Therapeutic Agents/Drug Delivery
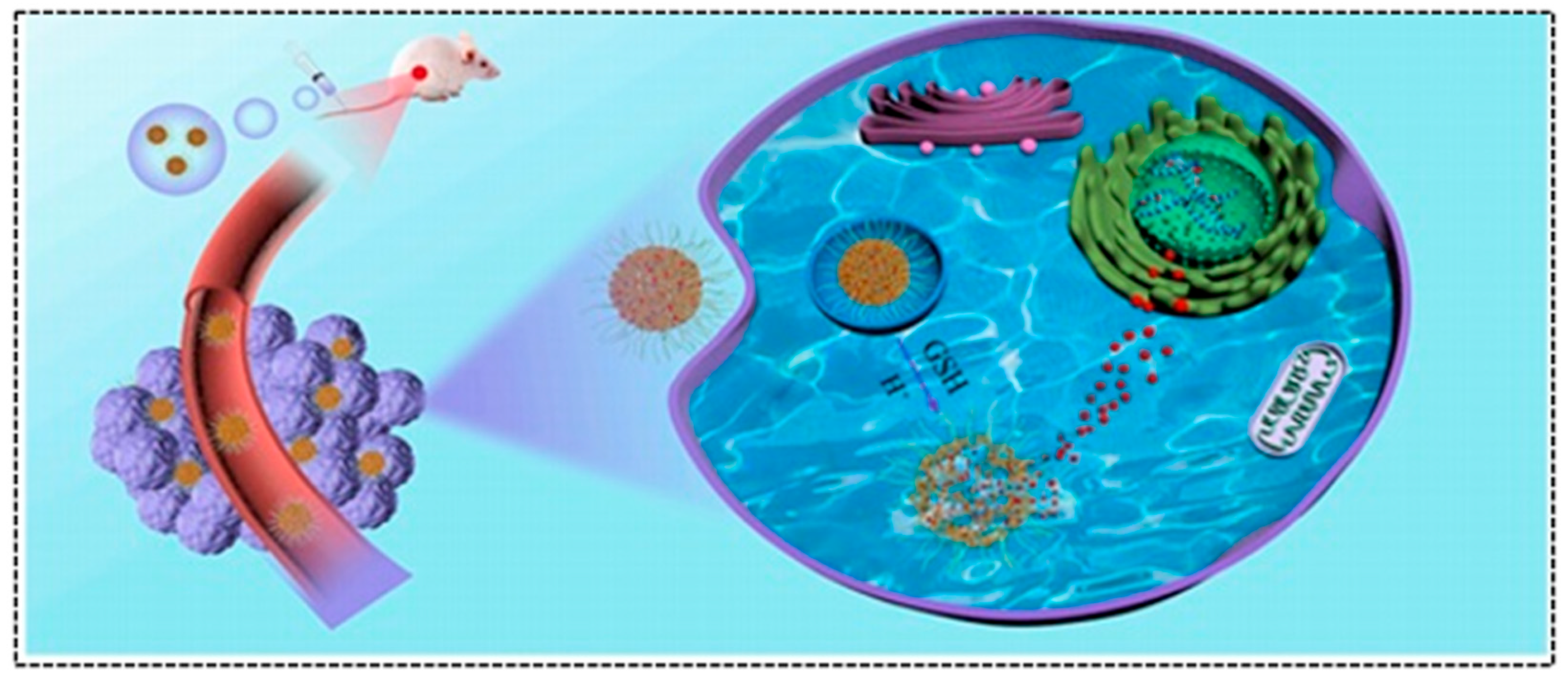
5.2. Cancer Treatment
5.3. Microgels-Based Wound Dressings
Injectable Hydrogels for Wound Treatment
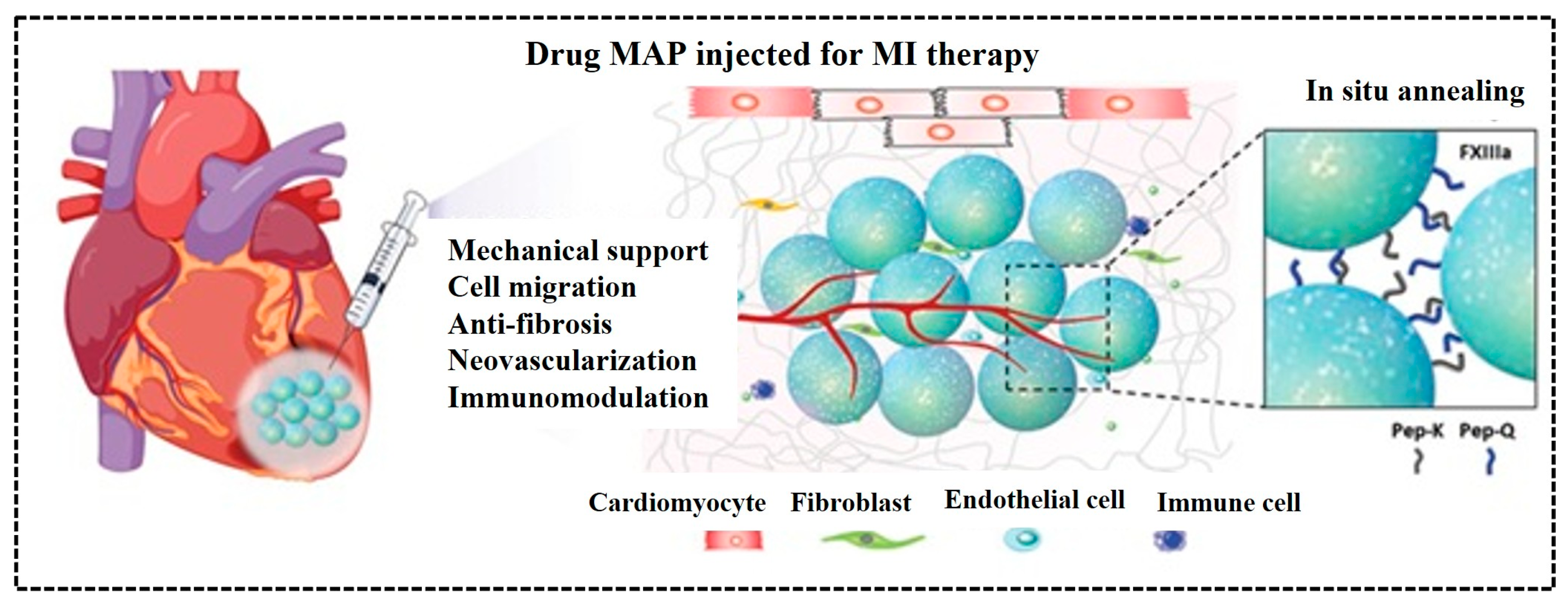
5.4. Microgels-Based Scaffolds for Tissue
6. Conclusions and Future Directions
- Explore the development of multifunctional hydrogels and microgels with integrated diagnostic and therapeutic features, which could include imaging agents or sensors for real-time monitoring of treatment progression.
- Utilize emerging technologies, such as 3D bioprinting, to create complex tissue scaffolds that mimic native tissue architecture, thereby enhancing strategies in regenerative medicine.
- Optimize fabrication techniques to improve the uniformity and reproducibility of hydrogel and microgel production, employing advanced methods such as microfluidics and electrospinning for precisely engineered systems.
- Investigate the interactions between hydrogels/microgels and biological systems, focusing on understanding immune responses and degradation pathways to ensure their safety and efficacy in clinical applications.
- Continue interdisciplinary research to develop next-generation therapeutics, ultimately improving patient outcomes and advancing the field of regenerative medicine.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Croitoriu, A.; Nita, L.E.; Chiriac, A.P.; Rusu, A.G.; Bercea, M. New physical hydrogels based on co-assembling of fmoc–amino acids. Gels 2021, 7, 208. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.; Sharma, A.; Singh, A.; Han, S.S.; Sood, A. Strategy and Advancement in Hybrid Hydrogel and Their Applications: Recent Progress and Trends. Adv. Eng. Mater. 2024, 26, 2400944. [Google Scholar] [CrossRef]
- Sahu, K.; Chakma, S. Recent trends on hydrogel development and sustainable applications: A bibliometric analysis and concise review. Polym. Bull. 2024, 81, 7687–7711. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, C.; Yu, H.; Wang, L.; Ni, Z.; Liu, X.; Shen, D.; Yang, J.; Shi, K.; Wang, H. Tough adhesion enhancing strategies for injectable hydrogel adhesives in biomedical applications. Adv. Colloid Interface Sci. 2023, 319, 102982. [Google Scholar] [CrossRef]
- Ding, Y.-W.; Wang, Z.Y.; Ren, Z.W.; Zhang, X.W.; Wei, D.X. Advances in modified hyaluronic acid-based hydrogels for skin wound healing. Biomater. Sci. 2022, 10, 3393–3409. [Google Scholar] [CrossRef]
- Xu, Q.; Hu, X.; Wang, Y. Alternatives to conventional antibiotic therapy: Potential therapeutic strategies of combating antimicrobial-resistance and biofilm-related infections. Mol. Biotechnol. 2021, 63, 1103–1124. [Google Scholar] [CrossRef]
- Ding, Y.W.; Zhang, X.W.; Mi, C.H.; Qi, X.Y.; Zhou, J.; Wei, D.X. Recent advances in hyaluronic acid-based hydrogels for 3D bioprinting in tissue engineering applications. Smart Mater. Med. 2023, 4, 59–68. [Google Scholar] [CrossRef]
- Shafiei, M.; Ansari, M.N.M.; Razak, S.I.A.; Khan, M.U.A. A comprehensive review on the applications of exosomes and liposomes in regenerative medicine and tissue engineering. Polymers 2021, 13, 2529. [Google Scholar] [CrossRef]
- Nazir, S.; Khan, M.U.A.; Al-Arjan, W.S.; Abd Razak, S.I.; Javed, A.; Kadir, M.R.A. Nanocomposite hydrogels for melanoma skin cancer care and treatment: In-vitro drug delivery, drug release kinetics and anti-cancer activities. Arab. J. Chem. 2021, 14, 103120. [Google Scholar] [CrossRef]
- Khan, R.; Aslam Khan, M.U.; Stojanović, G.M.; Javed, A.; Haider, S.; Abd Razak, S.I. Fabrication of bilayer nanofibrous-hydrogel scaffold from bacterial cellulose, PVA, and gelatin as advanced dressing for wound healing and soft tissue engineering. ACS Omega 2024, 9, 6527–6536. [Google Scholar] [CrossRef]
- Seidi, F.; Zhao, W.; Xiao, H.; Jin, Y.; Saeb, M.R.; Zhao, C. Radical polymerization as a versatile tool for surface grafting of thin hydrogel films. Polym. Chem. 2020, 11, 4355–4381. [Google Scholar] [CrossRef]
- Karg, M.; Pich, A.; Hellweg, T.; Hoare, T.; Lyon, L.A.; Crassous, J.J.; Suzuki, D.; Gumerov, R.A.; Schneider, S.; Potemkin, I.I.; et al. Nanogels and Microgels: From Model Colloids to Applications, Recent Developments, and Future Trends. Langmuir ACS J. Surf. Colloids 2019, 35, 6231–6255. [Google Scholar] [CrossRef]
- Devi, L.; Gaba, P. Hydrogel: An Updated Primer. J. Crit. Rev. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, T.; Chen, S.; Wang, X.; He, J.; Luo, Y. Construction and characterization of chitosan/poly(acrylamide-[2-(methacryloyloxy)ethyl]trimethylammonium chloride) double-network hydrogel with enhanced antibacterial activity. Adv. Compos. Hybrid Mater. 2023, 6, 192. [Google Scholar] [CrossRef]
- Deng, P.; Yao, L.; Chen, J.; Tang, Z.; Zhou, J. Chitosan-based hydrogels with injectable, self-healing and antibacterial properties for wound healing. Carbohydr. Polym. 2022, 276, 118718. [Google Scholar] [CrossRef]
- Sahiner, M.; Yilmaz, A.S.; Demirci, S.; Sahiner, N. Physically and Chemically Crosslinked, Tannic Acid Embedded LinearPEI-Based Hydrogels and Cryogels with Natural Antibacterial and Antioxidant Properties. Biomedicines 2023, 11, 706. [Google Scholar] [CrossRef]
- Wang, Z.; Ye, Q.; Yu, S.; Akhavan, B. Poly Ethylene Glycol (PEG)-Based Hydrogels for Drug Delivery in Cancer Therapy: A Comprehensive Review. Adv. Healthc. Mater. 2023, 12, 2300105. [Google Scholar] [CrossRef]
- Tan, S.J.; Fang, J.Y.; Yang, Z.; Nimni, M.E.; Han, B. The synergetic effect of hydrogel stiffness and growth factor on osteogenic differentiation. Biomaterials 2014, 35, 5294–5306. [Google Scholar] [CrossRef]
- Nair, S.; Remya, N.S.; Remya, S.; Nair, P.D. A biodegradable in situ injectable hydrogel based on chitosan and oxidized hyaluronic acid for tissue engineering applications. Carbohydr. Polym. 2011, 85, 838–844. [Google Scholar] [CrossRef]
- Abalymov, A.; Parakhonskiy, B.; Skirtach, A. Polymer- and HybridBased Biomaterials for Interstitial, Connective, Vascular, Nerve, Visceral and Musculoskeletal Tissue Engineering. Polymers 2020, 12, 620. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D. Creating Complex Polyacrylamide Hydrogel Structures Using 3D Printing with Applications to Mechanobiology. Macromol. Biosci. 2020, 20, 2000082. [Google Scholar] [CrossRef]
- Hogrebe, N.J.; Reinhardt, J.W.; Tram, N.K.; Debski, A.C.; Agarwal, G.; Reilly, M.A.; Gooch, K.J. Independent Control of Matrix Adhesiveness and Stiffness within a 3D Self-Assembling Peptide Hydrogel. Acta Biomater. 2018, 70, 110–119. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Li, J.; Yang, S.; Zhang, Y.; Yao, B.; Song, W.; Fu, X.; Huang, S. Stiffness-mediated Mesenchymal Stem Cell Fate Decision in 3D-Bioprinted Hydrogels. Burn. Trauma 2020, 8, tkaa029. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Zöller, K.; To, D.; Bernkop-Schnürch, A. Biomedical applications of functional hydrogels: Innovative developments, relevant clinical trials and advanced products. Biomaterials 2025, 312, 122718. [Google Scholar] [CrossRef]
- Tang, K.; Wang, J.; Pei, X.; Zhu, Z.; Liu, J.; Wan, Q.; Zhang, X. Flexible coatings based on hydrogel to enhance the biointerface of biomedical implants. Adv. Mather Colloid Interface Sci. 2025, 335, 103358. [Google Scholar] [CrossRef]
- Goding, J.; Vallejo-Giraldo, C.; Syed, O.; Green, R. Considerations for hydrogel applications to neural bioelectronics. J. Mater. Chem. B 2019, 7, 1625–1636. [Google Scholar] [CrossRef]
- Mather, M.L.; Tomlins, P.E. Hydrogels in regenerative medicine: Towards understanding structure–function relationships. Regen. Med. 2010, 5, 809–821. [Google Scholar] [CrossRef]
- Subramaniam, V.; Shetty, A.M.; Chisolm, S.J.; Lansberry, T.R.; Balachandra, A.; Morley, C.D.; Angelini, T.E. Biopolymer networks packed with microgels combine strain stiffening and shape programmability. Giant 2024, 19, 100297. [Google Scholar] [CrossRef]
- Burchard, W. Macrogels, Microgels and Reversible Gels-What is the Difference? Polym. Bull. 2007, 58, 3–14. [Google Scholar] [CrossRef]
- Oh, J.K.; Drumright, R.; Siegwart, D.J.; Matyjaszewski, K. The development of microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2008, 33, 448–477. [Google Scholar] [CrossRef]
- Thorne, J.B.; Vine, G.J.; Snowden, M.J. Microgel applications and commercial considerations. Colloid. Polym. Sci. 2011, 289, 625. [Google Scholar] [CrossRef]
- Dai, Z.; Ngai, T. Microgel particles: The structure-property relationships and their biomedical applications. J. Polym. Sci. Part. Polym. Chem. 2013, 51, 2995–3003. [Google Scholar] [CrossRef]
- Yang, Y.; Xiao, Y.; Wu, X.; Deng, J.; Wei, R.; Liu, A.; Chai, H.; Wang, R. Microgel-Crosslinked Thermo-Responsive Hydrogel Actuators with High Mechanical Properties and Rapid Response. Macromol. Rapid Commun. 2024, 45, 2300643. [Google Scholar] [CrossRef]
- Rivest, C.; Morrison, D.; Ni, B.; Rubin, J.; Yadav, V.; Mahdavi, A.; Karp, J.; Khademhosseini, A. Microscale hydrogels for medicine and biology: Synthesis, characteristics and applications. J. Mech. Mater. Struct. 2007, 2, 1103–1119. [Google Scholar] [CrossRef]
- Xuan, L.; Hou, Y.; Liang, L.; Wu, J.; Fan, K.; Lian, L.; Qiu, J.; Miao, Y.; Ravanbakhsh, H.; Xu, M.; et al. Microgels for Cell Delivery in Tissue Engineering and Regenerative Medicine. Nano-Micro Lett. 2024, 16, 218. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-linking in Hydrogels—A Review. Am. J. Polym. 2014, 4, 25–31. [Google Scholar]
- Nahar, Y.; Horne, J.; Truong, V.; Bissember, A.C.; Thickett, S.C. Preparation of thermoresponsive hydrogels: Via polymerizable deep eutectic monomer solvents. Polym. Chem. 2021, 12, 254–264. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Islam, M.; Hasan, M.K.; Nam, K.-W. A Comprehensive Review of Radiation-Induced Hydrogels: Synthesis, Properties, and Multidimensional Applications. Gels 2024, 10, 381. [Google Scholar] [CrossRef]
- Liu, T.; Jiao, C.; Peng, X.; Chen, Y.N.; Chen, Y.; He, C.; Liu, R.; Wang, H. Super-strong and tough poly(vinyl alcohol)/poly(acrylic acid) hydrogels reinforced by hydrogen bonding. J. Mater. Chem. B 2018, 6, 8105–8114. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhong, H.J.; Ding, H.; Yu, B.; Ma, X.; Liu, X.; Chong, C.M.; He, J. Polyvinyl Alcohol (PVA)-Based Hydrogels: Recent Progress in Fabrication, Properties, and Multifunctional Applications. Polymers 2024, 16, 2755. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Rao, T.; Ma, X.; Du, P.; Liu, Y.; He, X. Facile preparation of physically crosslinked hydrogel based on the glassy state with high strength. New J. Chem. 2024, 48, 1696–1704. [Google Scholar] [CrossRef]
- Akhtar, M.F.; Hanif, M.; Ranjha, N.M. Methods of synthesis of hydrogels … A review. Saudi Pharm. J. 2016, 24, 554–559. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpur, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental concepts of hydrogels: Synthesis, properties, and their applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Jeong, J.O.; Park, J.S.; Kim, E.J.; Jeong, S.I.; Lee, J.Y.; Lim, Y.M. Preparation of radiation cross-linked poly(Acrylic acid) hydrogel containing metronidazole with enhanced antibacterial activity. Int. J. Mol. Sci. 2020, 21, 187. [Google Scholar] [CrossRef]
- Li, X.; Xiong, Y. Application of “Click” Chemistry in Biomedical Hydrogels. ACS Omega 2022, 7, 36918–36928. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Ma, P.X.; Guo, B.; Du, Y.; Han, X. pH-responsive injectable hydrogels with mucosal adhesiveness based on chitosan-grafted-dihydrocaffeic acid and oxidized pullulan for localized drug delivery. J. Colloid Interface Sci. 2019, 536, 224–234. [Google Scholar] [CrossRef]
- Tallet, L.; Gribova, V.; Ploux, L.; Vrana, N.E.; Lavalle, P. New Smart Antimicrobial Hydrogels, Nanomaterials, and Coatings: Earlier Action, More Specific, Better Dosing? Adv. Healthc. Mater. 2021, 10, 2001199. [Google Scholar] [CrossRef]
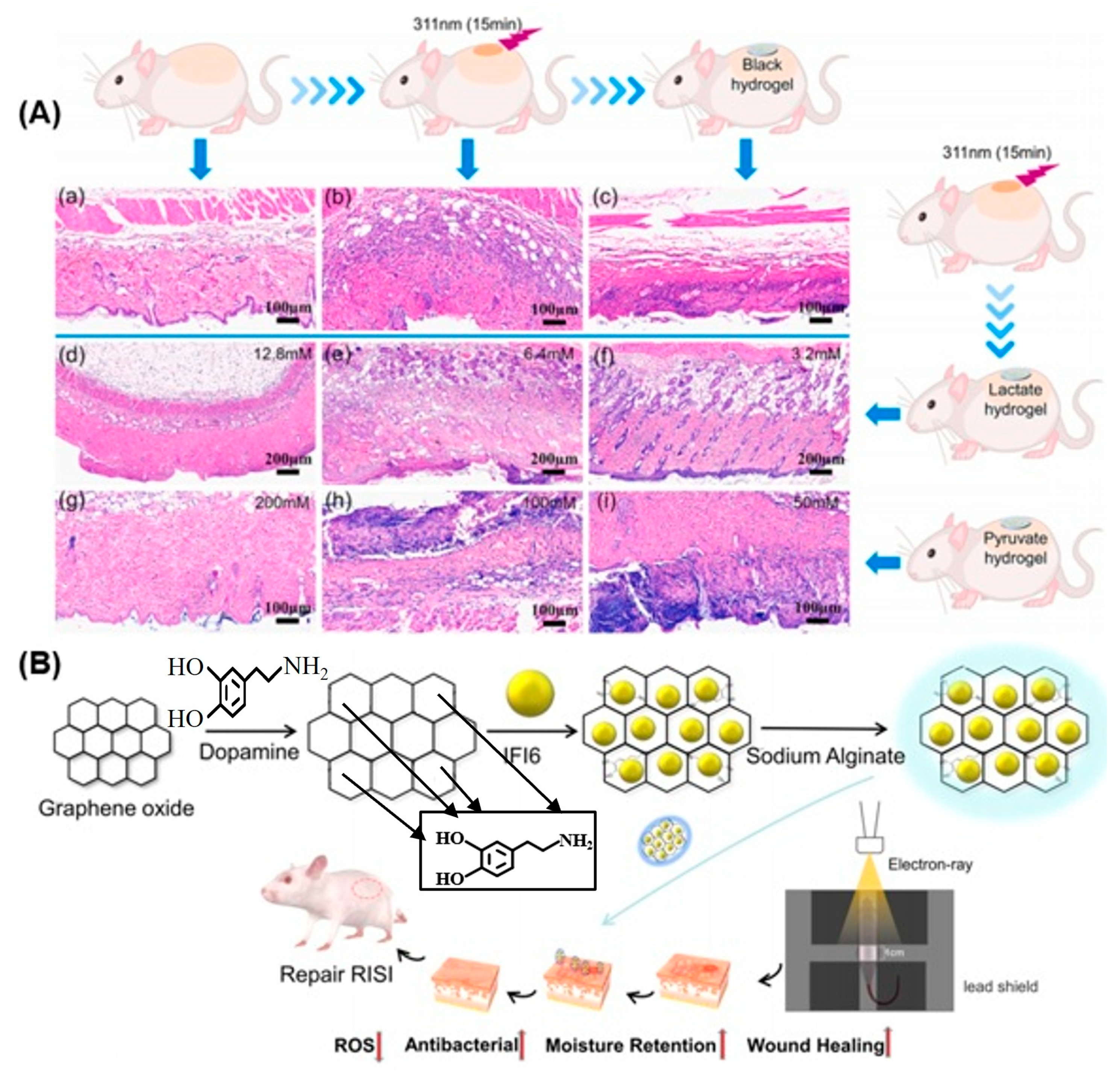
- Liu, M.; Yu, W.; Fang, Y.; Zhou, H.; Liang, Y.; Huang, C.; Liu, H.; Zhao, G. Pyruvate and lactate-based hydrogel film inhibits UV radiation-induced skin inflammation and oxidative stress. Int. J. Pharm. 2023, 634, 122697. [Google Scholar] [CrossRef]
- Hao, J.; Sun, M.; Li, D.; Zhang, T.; Li, J.; Zhou, D. An IFI6-based hydrogel promotes the healing of radiation-induced skin injury through regulation of HSF1 activity. J. Nanobiotechnol. 2022, 20, 288. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Li, H.; Guo, J.; Wang, D.; Zhang, J.; Liu, J. Bio-Inspired Antioxidant Heparin-Mimetic Peptide Hydrogel for Radiation Induced Skin Injury Repair. Adv. Healthc. Mater. 2023, 12, 2203387. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Cui, H.; Yang, C.; Li, L.; Xu, F.; Gao, J. Hydrogels for the treatment of radiation-induced skin and mucosa damages: An up-to-date overview. Front. Mater. 2022, 9, 1018815. [Google Scholar] [CrossRef]
- Wang, M.; Yang, F.; Luo, H.; Jiang, Y.; Zhuang, K.; Tan, L. Photocuring and Gelatin-Based Antibacterial Hydrogel for Skin Care. Biomacromolecules 2023, 24, 4218–4228. [Google Scholar] [CrossRef]
- Liu, W.; Yang, C.; Gao, R.; Zhang, C.; Ou-Yang, W.; Feng, Z.; Zhang, C.; Pan, X.; Huang, P.; Kong, D.; et al. Polymer Composite Sponges with Inherent Antibacterial, Hemostatic, Inflammation-Modulating and Proregenerative Performances for Methicillin-Resistant Staphylococcus aureus-Infected Wound Healing. Adv. Healthc. Mater. 2021, 10, 2101247. [Google Scholar] [CrossRef]
- Cao, J.; Wang, P.; Liu, Y.; Zhu, C.; Fan, D. Double crosslinked HLC-CCS hydrogel tissue engineering scaffold for skin wound healing. Int. J. Biol. Macromol. 2020, 155, 625–635. [Google Scholar] [CrossRef]
- Liu, W.C.; Wang, H.Y.; Lee, T.H.; Chung, R.J. Gamma-poly glutamate/gelatin composite hydrogels crosslinked by proanthocyanidins for wound healing. Mater. Sci. Eng. C 2019, 101, 630–639. [Google Scholar] [CrossRef]
- Qi, L.; Zhang, C.; Wang, B.; Yin, J.; Yan, S. Progress in Hydrogels for Skin Wound Repair. Macromol. Biosci. 2022, 22, e2100475. [Google Scholar] [CrossRef]
- Shen, C.; Li, Y.; Meng, Q. Adhesive polyethylene glycol-based hydrogel patch for tissue repair. Colloids Surf. B Biointerfaces 2022, 218, 112751. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, R.; Zhao, X.; Zhang, Y.; Tam, A.; Yan, Y.; Shen, H.; Zhang, Y.S.; Qi, J.; Feng, Y.; et al. An injectable self-healing coordinative hydrogel with antibacterial and angiogenic properties for diabetic skin wound repair. NPG Asia Mater. 2019, 11, 3. [Google Scholar] [CrossRef]
- Surowiecka, A.; Strużyna, J.; Winiarska, A.; Korzeniowski, T. Hydrogels in Burn Wound Management-A Review. Gels 2022, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Burd, A. Evaluating the use of hydrogel sheet dressings in comprehensive burn wound care. Ostomy Wound Manag. 2007, 53, 52–62. [Google Scholar]
- Goertz, O.; Abels, C.; Knie, U.; May, T.; Hirsch, T.; Daigeler, A.; Steinau, H.-U.; Langer, S. Clinical Safety and Efficacy of a Novel Thermoreversible Polyhexanide-Preserved Wound Covering Gel. Eur. Surg. Res. 2010, 44, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Ou, K.-L.; Tzeng, Y.-S.; Chiao, H.-Y.; Chiu, H.-T.; Chen, C.-Y.; Chu, T.-S.; Huang, D.-W.; Hsu, K.-F.; Chang, C.-K.; Wang, C.-H.; et al. Clinical Performance of Hydrogel-based Dressing in Facial Burn Wounds. Ann. Plast. Surg. 2021, 86, S18–S22. [Google Scholar] [CrossRef]
- Markeson, D.; Pleat, J.M.; Sharpe, J.R.; Harris, A.L.; Seifalian, A.M.; Watt, S.M. Scarring, stem cells, scaffolds and skin repair. J. Tissue Eng. Regen. Med. 2015, 9, 649–668. [Google Scholar] [CrossRef]
- Li, Q.; Wang, D.; Jiang, Z.; Li, R.; Xue, T.; Lin, C.; Deng, Y.; Jin, Y.; Sun, B. Advances of hydrogel combined with stem cells in promoting chronic wound healing. Front. Chem. 2022, 10, 1038839. [Google Scholar] [CrossRef]
- Aminov, R.I. A brief history of the antibiotic era: Lessons learned and challenges for the future. Front. Microbiol. 2010, 1, 134. [Google Scholar] [CrossRef]
- Gao, W.; Chen, Y.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-based local antimicrobial drug delivery. Adv. Drug. Deliv. Rev. 2018, 127, 46–57. [Google Scholar] [CrossRef]
- Xiong, M.H.; Bao, Y.; Yang, X.Z.; Zhu, Y.H.; Wang, J. Delivery of antibiotics with polymeric particles. Adv. Drug Deliv. Rev. 2014, 78, 63–76. [Google Scholar] [CrossRef]
- Taccone, F.S.; Bond, O.; Cavicchi, F.Z.; Hites, M. Individualized antibiotic strategies. Curr. Opin. Anaesthesiol. 2016, 29, 166–171. [Google Scholar] [CrossRef]
- Gong, C.; Wu, Q.; Wang, Y.; Zhang, D.; Luo, F.; Zhao, X.; Wei, Y.; Qian, Z. A biodegradable hydrogel system containing curcumin encapsulated in micelles for cutaneous wound healing. Biomaterials 2013, 34, 6377–6387. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Zhang, H.; Guo, B. Conductive biomaterials as bioactive wound dressing for wound healing and skin tissue engineering. Nano-Micro Lett. 2022, 14, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.F.; Liu, X.; Zhang, S.; Pan, B.; Liu, M.L. Ciprofoxacin derivatives and their antibacterial activities. Eur. J. Med. Chem. 2018, 146, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, A.K.; Gupta, R. Magnetically mediated release of ciprofoxacin from polyvinyl alcohol based superparamagnetic nanocomposites. J. Mater. Sci. Mater. Med. 2011, 22, 357–369. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, L. Facile design and development of nano-clustery graphene-based macromolecular protein hydrogel loaded with ciprofoxacin to antibacterial improvement for the treatment of burn wound injury. Polym. Bull. 2021, 79, 7953–7968. [Google Scholar] [CrossRef]
- Pavlović, N.; Bogićević, I.A.; Zaklan, D.; Đanić, M.; Goločorbin-Kon, S.; Al-Salami, H.; Mikov, M. Infuence of bile acids in hydrogel pharmaceutical formulations on dissolution rate and permeation of clindamycin hydrochloride. Gels 2022, 8, 35. [Google Scholar] [CrossRef]
- Sadeghi, S.; Nourmohammadi, J.; Ghaee, A.; Soleimani, N. Carboxymethyl cellulose-human hair keratin hydrogel with controlled clindamycin release as antibacterial wound dressing. Int. J. Biol. Macromol. 2020, 147, 1239–1247. [Google Scholar] [CrossRef]
- Jiang, S.; Deng, J.; Jin, Y.; Qian, B.; Lv, W.; Zhou, Q.; Mei, E.; Neisiany, R.E.; Liu, Y.; You, Z.; et al. Breathable, antifreezing, mechanically skin-like hydrogel textile wound dressings with dual antibacterial mechanisms. Bioact. Mater. 2023, 21, 313–323. [Google Scholar] [CrossRef]
- Sanders, W.E., Jr.; Sanders, C.C. Toxicity of antibacterial agents: Mechanism of action on mammalian cells. Annu. Rev. Pharmacol. Toxicol. 1979, 19, 53–83. [Google Scholar] [CrossRef]
- Liao, C.H.; Chen, C.S.; Chen, Y.C.; Jiang, N.E.; Farn, C.J.; Shen, Y.S.; Hsu, M.L.; Chang, C.H. Vancomycin-loaded oxidized hyaluronic acid and adipic acid dihydrazide hydrogel: Bio-compatibility, drug release, antimicrobial activity, and bioflm model. J. Microbiol. Immunol. 2020, 53, 525–531. [Google Scholar] [CrossRef]
- Ingle, A.P.; Duran, N.; Rai, M. Bioactivity, mechanism of action, and cytotoxicity of copper-based nanoparticles: A review. Appl. Microbiol. Biot. 2014, 98, 1001–1009. [Google Scholar] [CrossRef]
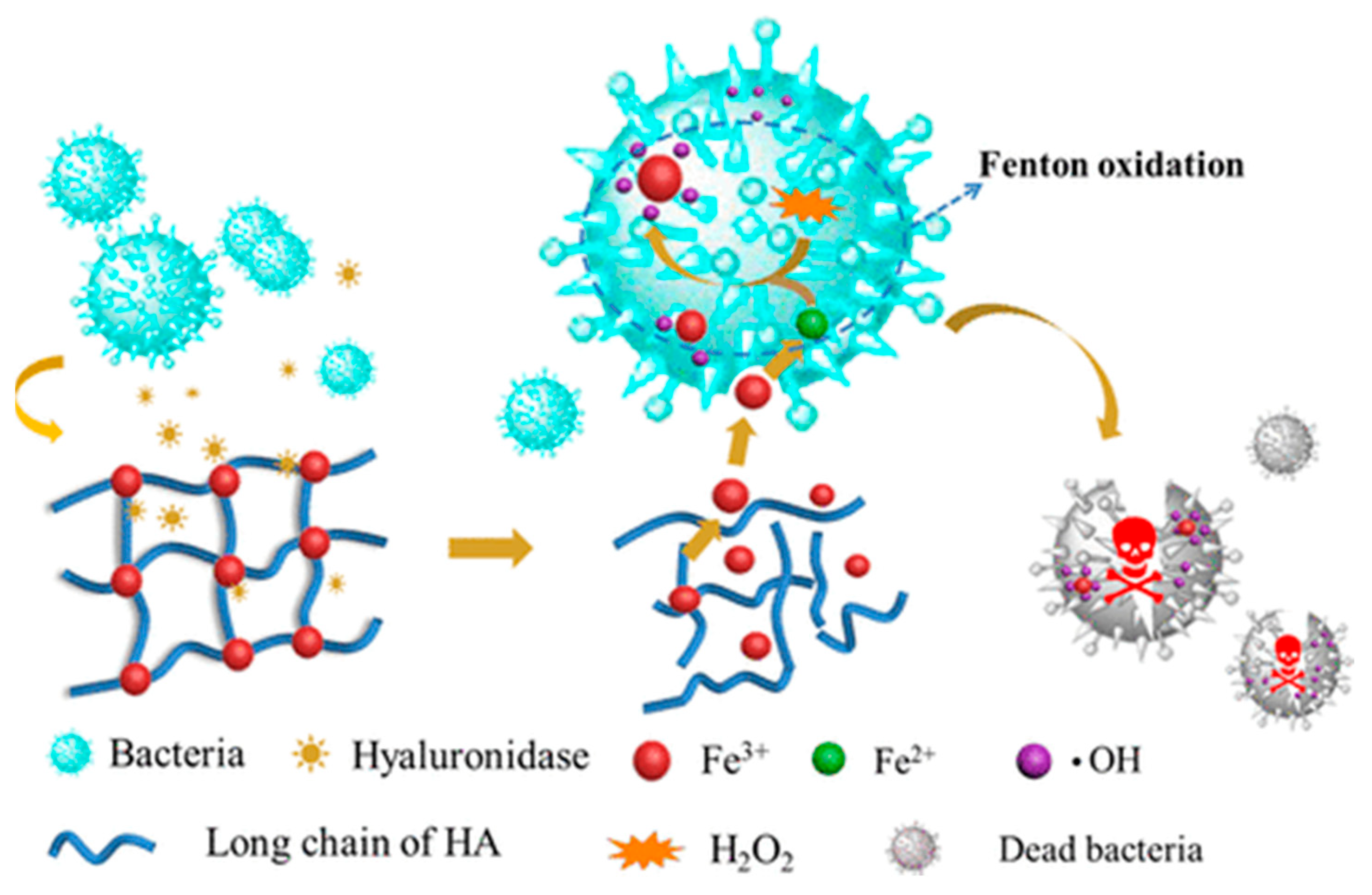
- Abdollahi, Z.; Zare, E.N.; Salimi, F.; Goudarzi, I.; Tay, F.R.; Makvandi, P. Bioactive carboxymethyl starch-based hydrogels decorated with CuO nanoparticles: Antioxidant and antimicrobial properties and accelerated wound healing in vivo. Int. J. Mol. Sci. 2021, 22, 2531. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xiang, J.; Sun, Y.; Wang, H.; Du, X.; Cheng, X.; Du, Z.; Wang, H. Skin-inspired nanofbrillated cellulose-reinforced hydrogels with high mechanical strength, long-term antibacterial, and self-recovery ability for wearable strain/pressure sensors. Carbohydr. Polym. 2021, 261, 117894. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, F.; Wei, Y.; Hu, Q.; Luo, Q.; Chen, C.; Wang, J.; Yang, L.; Luo, R.; Wang, Y. Dressing blood-contacting materials by a stable hydrogel coating with embedded antimicrobial peptides for robust antibacterial and antithrombus properties. ACS Appl. Mater. Inter. 2021, 13, 38947–38958. [Google Scholar] [CrossRef] [PubMed]
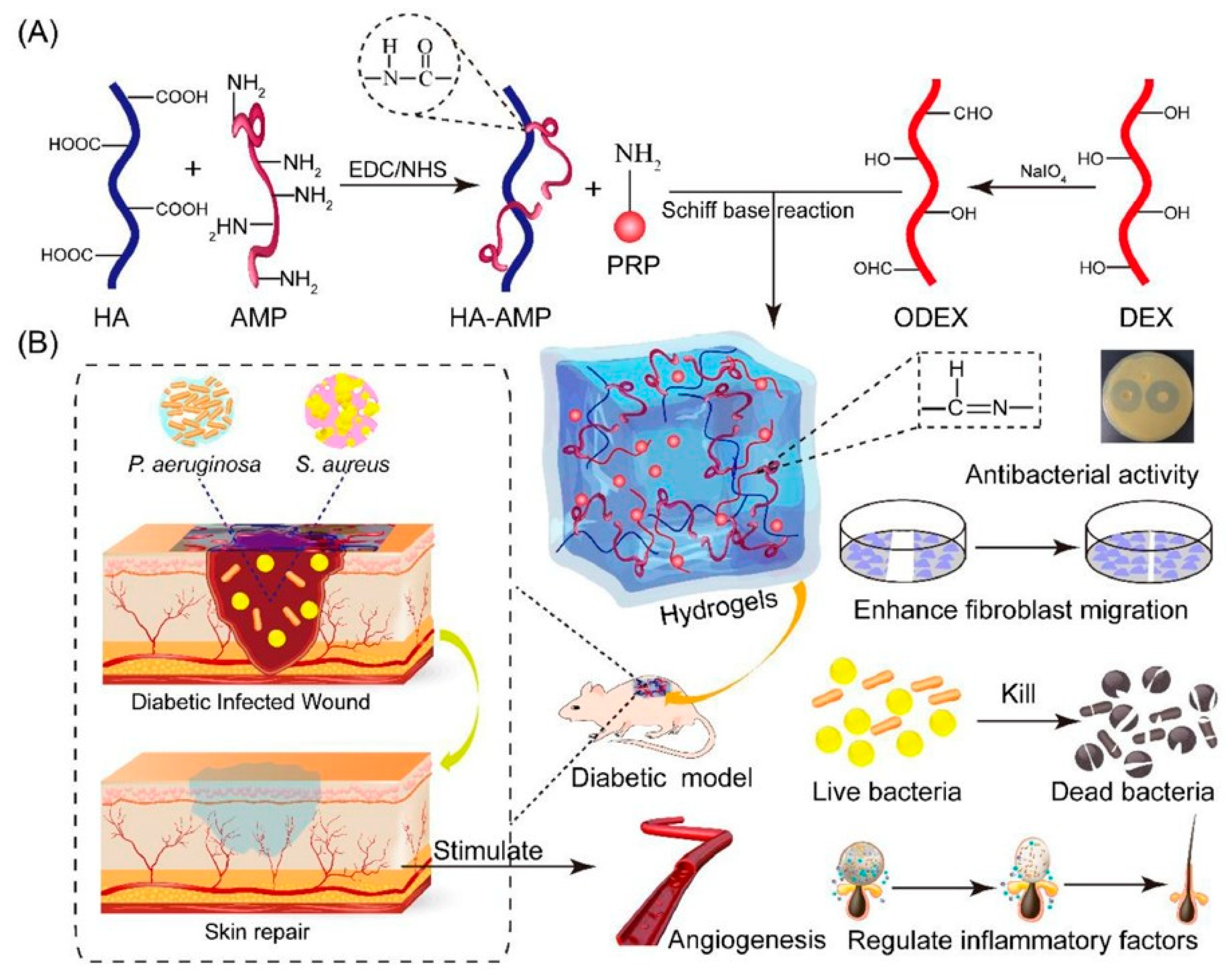
- Wei, S.; Xu, P.; Yao, Z.; Cui, X.; Lei, X.; Li, L.; Dong, Y.; Zhu, W.; Guo, R.; Cheng, B. A composite hydrogel with co-delivery of antimicrobial peptides and platelet-rich plasma to enhance healing of infected wounds in diabetes. Acta Biomater. 2021, 124, 205–218. [Google Scholar] [CrossRef]
- Cui, Q.; Yuan, H.; Bao, X.; Ma, G.; Wu, M.; Xing, C. Synergistic photodynamic and photothermal antibacterial therapy based on a conjugated polymer nanoparticle-doped hydrogel. ACS Appl. Bio Mater. 2020, 3, 4436–4443. [Google Scholar] [CrossRef]
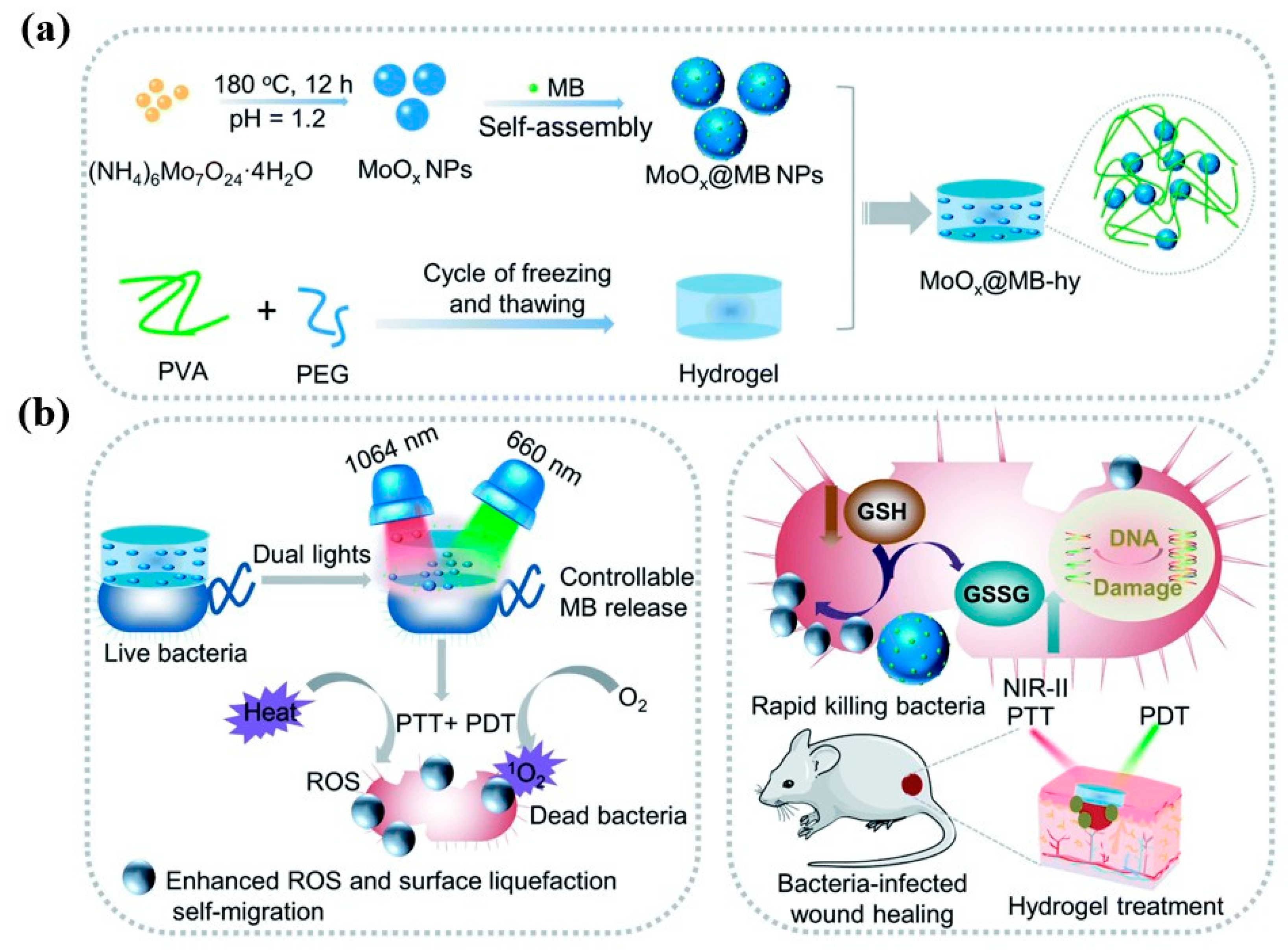
- Wang, Y.; Yao, H.; Zu, Y.; Yin, W. Biodegradable MoOx@ MB incorporated hydrogel as light-activated dressing for rapid and safe bacteria eradication and wound healing. RSC. Adv. 2022, 12, 8862–8877. [Google Scholar] [CrossRef]
- Pérez-Luna, V.H.; González-Reynoso, O. Encapsulation of biological agents in hydrogels for therapeutic applications. Gels 2018, 4, 61. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and their applications in targeted drug delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Forster, T.; Mayer, O.; Curtin, J.J.; Lehman, S.M.; Donlan, R.M. Bacteriophage cocktail for the prevention of biofilm formation by Pseudomonas aeruginosa on catheters in an in vitro model system. Antimicrob. Agents Chemother. 2010, 54, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Carson, L.; Gorman, S.P.; Gilmore, B.F. The use of lytic bacteriophages in the prevention and eradication of biofilms of Proteus mirabilis and Escherichia coli. FEMS Immunol. Med. Microbiol. 2010, 59, 447–455. [Google Scholar]
- Curtin, J.J.; Donlan, R.M. Using bacteriophages to reduce formation of catheter-associated biofilms by Staphylococcus epidermidis. Antimicrob. Agents Chemother. 2006, 50, 1268–1275. [Google Scholar] [CrossRef]
- Lehman, S.M.; Donlan, R.M. Bacteriophage-mediated control of a two-species biofilm formed by microorganisms causing catheterassociated urinary tract infections in an in vitro urinary catheter model. Antimicrob. Agents Chemother. 2015, 59, 1127–1137. [Google Scholar] [CrossRef]
- Rodney, M.; Donlan, S.M.L.; Andres, J. Garcia Controlled Covalent Attachment of Biactive Bacteriophage for Regulating Biofilm Development. U.S. Patent 9457132B2, 4 October 2016. [Google Scholar]
- Miao, J.; Wu, X.; Fang, Y.; Zeng, M.; Huang, Z.; Ouyang, M.; Wang, R. Multifunctional hydrogel coatings with high antimicrobial loading efficiency and pH-responsive properties for urinary catheter applications. J. Mater. Chem. B 2023, 11, 3373–3386. [Google Scholar] [CrossRef]
- Caplin, J.D.; García, A.J. Implantable antimicrobial biomaterials for local drug delivery in bone infection models. Acta Biomater. 2019, 93, 2–11. [Google Scholar] [CrossRef]
- Barros, J.A.R.; Melo, L.D.R.d.; Silva, R.A.R.d.; Ferraz, M.P.; Azeredo, J.C.V.d.R.; Pinheiro, V.M.d.C.; Colaço, B.J.A.; Fernandes, M.H.R.; Gomes, P.d.S.; Monteiro, F.J. Encapsulated bacteriophages in alginate-nanohydroxyapatite hydrogel as a novel delivery system to prevent orthopedic implant-associated infections. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102145. [Google Scholar] [CrossRef]
- Nicolle, L.E. Catheter associated urinary tract infections. Antimicrob. Resist. Infect. Control 2014, 3, 23. [Google Scholar] [CrossRef]
- Yao, C.; Teng, X.; Sun, D.; McCoy, C.P.; Zhang, S. Enhanced antifouling and anti-swarming properties poly (sulfobetaine methacrylate-co-2-hydroxy-3-phenoxypropyl acrylate) hydrogel coatings for urinary catheters. Colloids Surf. B Biointerfaces 2025, 245, 114277. [Google Scholar] [CrossRef] [PubMed]
- Moureau, N. Hydrophilic biomaterial intravenous hydrogel catheter for complication reduction in PICC and midline catheters. Expert. Rev. Med. Devices 2024, 21, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Gondil, V.S.; Chhibber, S. A novel wound dressing consisting of PVA-SA hybrid hydrogel membrane for topical delivery of bacteriophages and antibiotics. Int. J. Pharm. 2019, 572, 118779. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Harjai, K.; Chhibber, S. Topical treatment of Klebsiella pneumoniae B5055 induced burn wound infection in mice using natural products. J. Infect. Dev. Ctries 2010, 4, 367–377. [Google Scholar] [CrossRef]
- Kumari, S.; Harjai, K.; Chhibber, S. Bacteriophage versus antimicrobial agents for the treatment of murine burn wound infection caused by Klebsiella pneumoniae B5055. J. Med. Microbiol. 2011, 60, 205–210. [Google Scholar] [CrossRef]
- Merabishvili, M.; Monserez, R.; van Belleghem, J.; Rose, T.; Jennes, S.; De Vos, D.; Verbeken, G.; Vaneechoutte, M.; Pirnay, J.P. Stability of bacteriophages in burn wound care products. PLoS ONE 2017, 12, e0182121. [Google Scholar] [CrossRef]
- Tian, R.; Qiu, X.; Yuan, P.; Lei, K.; Wang, L.; Bai, Y.; Liu, S.; Chen, X. Fabrication of Self-Healing Hydrogels with On-Demand Antimicrobial Activity and Sustained Biomolecule Release for Infected Skin Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 17018–17027. [Google Scholar] [CrossRef]
- Li, Q.; Ai, R.; Fan, J.; Fu, X.; Zhu, L.; Zhou, Q.; Chen, L.; Ma, W.; Li, Y.; Liu, L. AgNPs-loaded chitosan/sodium alginate hydrogel film by in-situ green reduction with tannins for enhancing antibacterial activity. Mater. Today Commun. 2024, 38, 107927. [Google Scholar] [CrossRef]
- Jiang, Y.G.; Huang, J.J.; Wu, X.W.; Ren, Y.H.; Li, Z.A.; Ren, J.A. Controlled release of silver ions from AgNPs using a hydrogel based on konjac glucomannan and chitosan for infected wounds. Int. J. Biol. Macromol. 2020, 149, 148–157. [Google Scholar] [CrossRef]
- Rastegari, A.; Hasanshakir, F.; Mohammadi, Z.; Saadatpor, F.; Faghihi, H.; Moraffah, F. A chitosan-based hydrogel containing zinc oxide nanoparticles as a carrier for improving antibacterial activity and controlling the release of antibiotics. Micro Nano Lett. 2023, 18, 12172. [Google Scholar] [CrossRef]
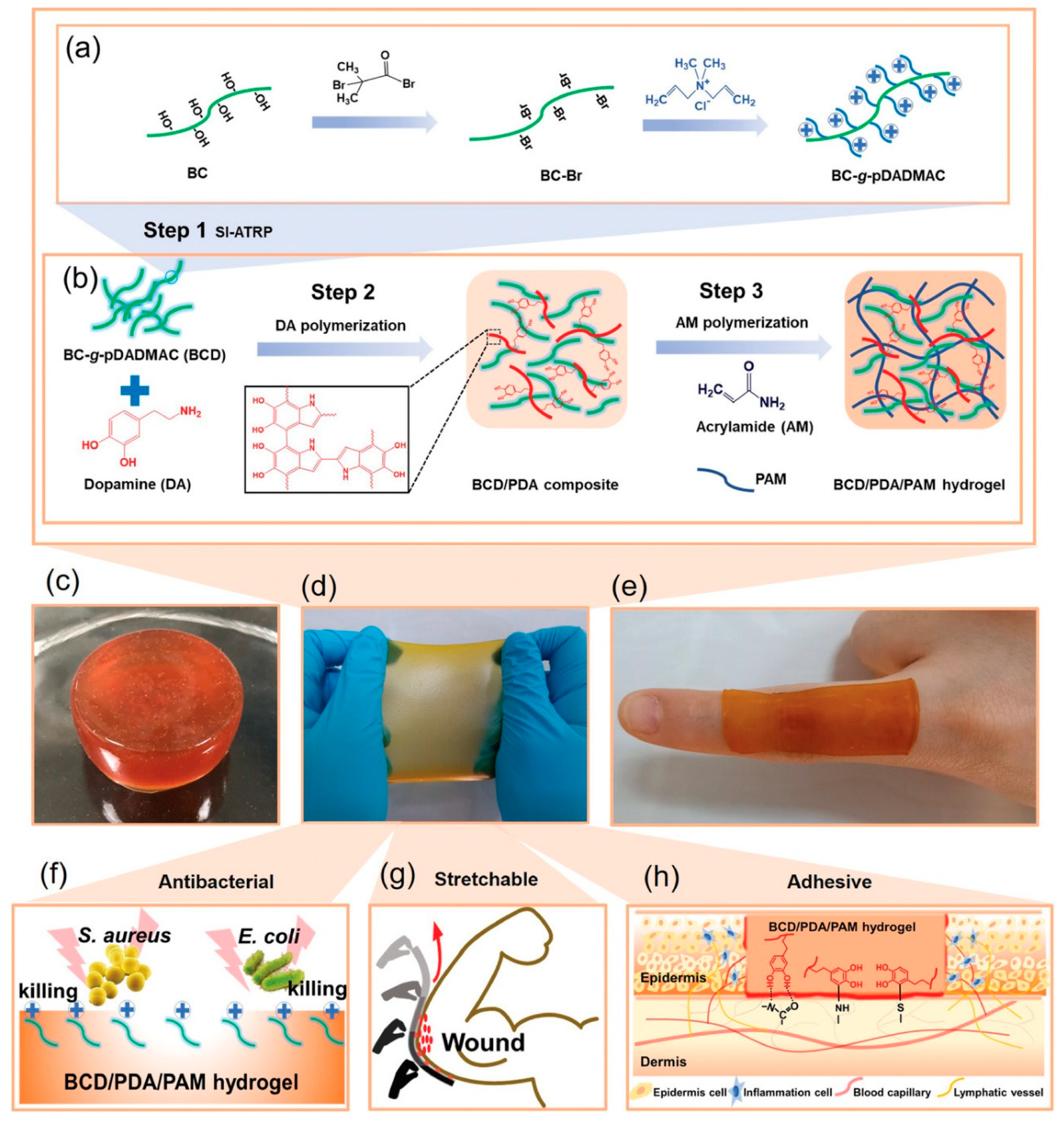
- Yang, Z.F.; Huang, R.K.; Zheng, B.N.; Guo, W.T.; Li, C.K.; He, W.Y.; Wei, Y.G.; Du, Y.; Wang, H.M.; Wu, D.C.; et al. Highly Stretchable, Adhesive, Biocompatible, and Antibacterial Hydrogel Dressings for Wound Healing. Adv. Sci. 2021, 8, 3627. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Wang, H.; Pan, X.; Zhang, C.; Zhang, K.; Chen, Z.; Dong, W.; Xie, A.; Qi, X. Dendritic Hydrogels with Robust Inherent Antibacterial Properties for Promoting Bacteria-Infected Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 11144–11155. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, W.; Xu, Q.; Zheng, Y. Progress in Antibacterial Hydrogel Dressing. Gels 2022, 8, 503. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Villanueva, M.E.; Cuestas, M.L.; Pérez, C.J.; Dall, V.C.; Copello, G.J. Smart release of antimicrobial ZnO nanoplates from a pH-responsive keratin hydrogel. J. Colloid Interface Sci. 2019, 536, 372–380. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Iqbal, I.; Ansari, M.N.M.; Razak, S.I.A.; Raza, M.A.; Sajjad, A.; Jabeen, F.; Riduan Mohamad, M.; Jusoh, N. Development of antibacterial, degradable and ph-responsive chitosan/guar gum/polyvinyl alcohol blended hydrogels for wound dressing. Molecules 2021, 26, 5937. [Google Scholar] [CrossRef]
- Sudarsan, S.; Selvi, M.S.; Chitra, G.; Sakthivel, S.; Franklin, D.S.; Guhanathan, S. Nontoxic pH-sensitive silver nanocomposite hydrogels for potential wound healing applications. Polym.-Plast. Technol. Mater. 2020, 60, 84–104. [Google Scholar] [CrossRef]
- Mi, L.; Xue, H.; Li, Y.; Jiang, S. A thermoresponsive antimicrobial wound dressing hydrogel based on a cationic betaine ester. Adv. Funct. Mater. 2011, 21, 4028–4034. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.G.; Chen, W.M.; Yu, A.X. Efficacy of thermosensitive chitosan/β-glycerophosphate hydrogel loaded with β-cyclodextrin-curcumin for the treatment of cutaneous wound infection in rats. Exp. Ther. Med. 2018, 15, 1304–1313. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Wang, F.; Ai, X.; Huang, D.; Liu, G.; Mi, P. 4-Enzyme-responsive polymers for drug delivery and molecular imaging. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Woodhead Publishing: Cambridge, UK, 2018; Volume 1, pp. 101–119. [Google Scholar]
- Zuo, Y.M.; Yan, X.; Xue, J.; Guo, L.-Y.; Fang, W.-W.; Sun, T.-C.; Li, M.; Zha, Z.; Yu, Q.; Wang, Y.; et al. Enzyme-Responsive Ag Nanoparticle Assemblies in Targeting Antibacterial against Methicillin-Resistant Staphylococcus aureus. ACS Appl. Mater. Interfaces 2020, 12, 4333–4342. [Google Scholar] [CrossRef]
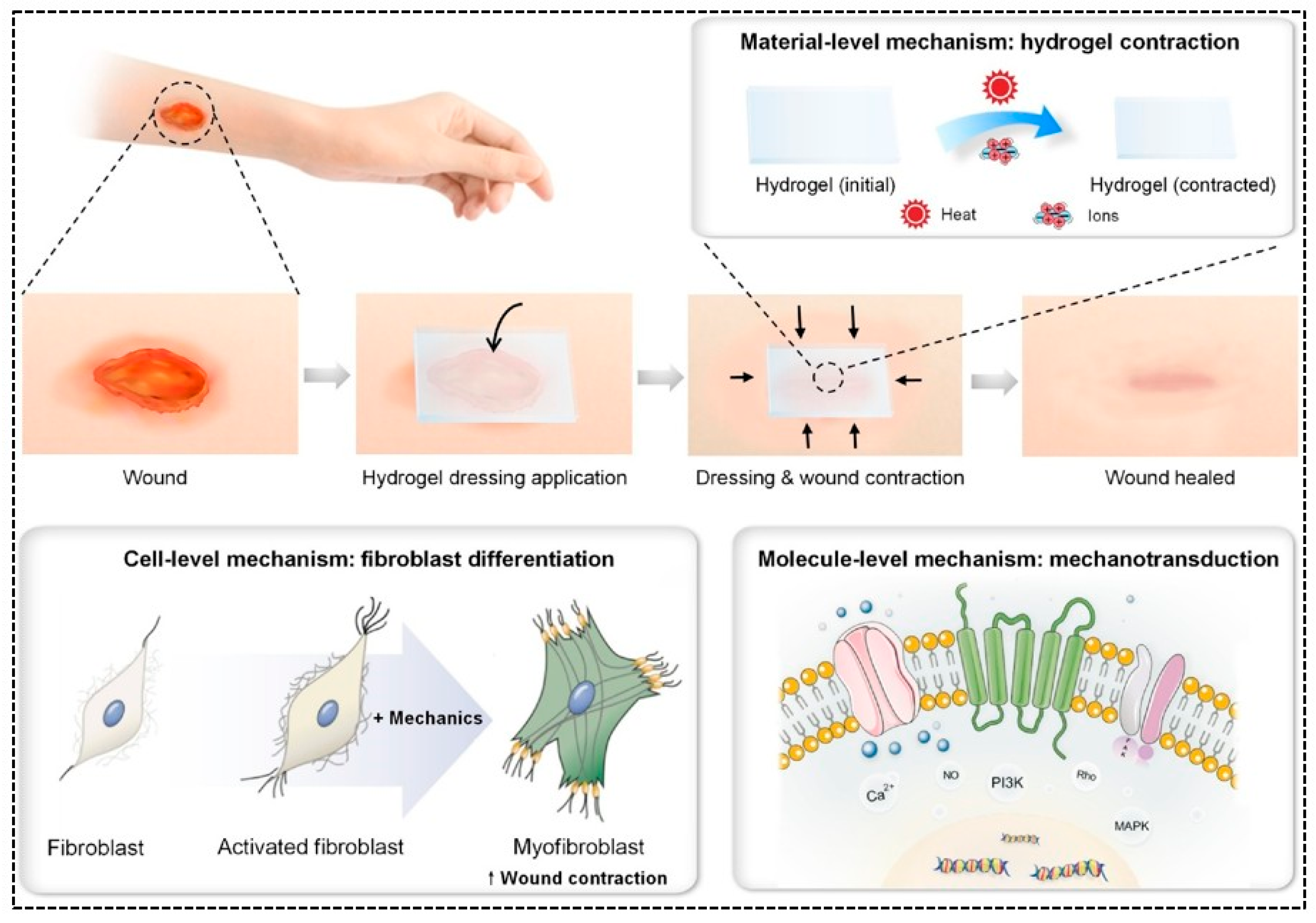
- Chang, L.; Du, H.; Xu, F.; Xu, C.; Liu, H. Hydrogel-enabled mechanically active wound dressings. Trends Biotechnol. 2024, 42, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Hoffmann, O.; Yu, K.; Lu, F.; Lan, G.; Dai, F.; Shang, S.; Xie, R. Self-contracting oxidized starch/gelatin hydrogel for noninvasive wound closure and wound healing. Mater. Des. 2020, 194, 108916. [Google Scholar] [CrossRef]
- Shi, Q.; Liu, H.; Tang, D.; Li, Y.; Li, X.J.; Xu, F. Bioactuators based on stimulus-responsive hydrogels and their emerging biomedical applications. NPG Asia Mater. 2019, 11, 64. [Google Scholar] [CrossRef]
- Van, W.A.; Pandhi, S.; Nixon, R.M.; Guyot, P.; Karabis, A.; Moore, R.A. Relative benefit-risk comparing diclofenac to other traditional non-steroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors in patients with osteoarthritis or rheumatoid arthritis: A network meta-analysis. Arthritis Res. Ther. 2015, 17, 66. [Google Scholar]
- Ghorbanzadeh, M.; Farhadian, N.; Golmohammadzadeh, S.; Karimi, M.; Ebrahimi, M. Formulation, clinical and histopathological assessment of microemulsion based hydrogel for UV protection of skin. Colloids Surf. B. Biointerfaces 2019, 179, 393–404. [Google Scholar] [CrossRef]
- Mehanna, M.M.; Mneimneh, A.T.; Abed El Jalil, K. Levofloxacin-loaded naturally occurring monoterpene-based nanoemulgel: A feasible efficient system to circumvent MRSA ocular infections. Drug Dev. Ind. Pharm. 2020, 46, 1787–1799. [Google Scholar] [CrossRef]
- Maitz, M.F.; Freudenberg, U.; Tsurkan, M.V.; Fischer, M.; Beyrich, T.; Werner, C. Bio-responsive polymer hydrogels homeostatically regulate blood coagulation. Nat. Commun. 2013, 4, 2168. [Google Scholar] [CrossRef]
- Aimetti, A.A.; Machen, A.J.; Anseth, K.S. Poly(ethylene glycol) hydrogels formed by thiol-ene photopolymerization for enzyme-responsive protein delivery. Biomaterials 2009, 30, 6048–6054. [Google Scholar] [CrossRef]
- Sivadas, N.; Cryan, S.A. Inhalable, bioresponsive microparticles for targeted drug delivery in the lungs. J. Pharm. Pharmacol. 2011, 63, 369–375. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kwon, D.Y.; Kwon, J.S.; Park, J.H.; Park, S.H.; Oh, H.J.; Kim, J.H.; Min, B.H.; Park, K.; Kim, M.S. Synergistic anti-tumor activity through combinational intratumoral injection of an in situ injectable drug depot. Biomaterials 2016, 85, 232–245. [Google Scholar] [CrossRef]
- Meng, Z.; Yang, S.; Yang, Y.; Zhang, L.; Cui, L. Synergistic chemotherapy and phototherapy based on red blood cell biomimetic nanomaterials. J. Control Release 2022, 352, 146–162. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Tachibana, T.; Yamana, K.; Kawasaki, R.; Yabuki, A. Simple Formation of cancer drug-containing self-assembled hydrogels with temperature and pH-responsive release. Langmuir 2021, 37, 11269–11275. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Bharadwaj, D.Y.; Podder, R.; Bubbly, S.G.; Gudennavar, S.B. Natural polymer-based hydrogels as prospective tissue equivalent materials for radiation therapy and dosimetry. Phys. Eng. Sci. Med. 2021, 44, 1107–1120. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zheng, X.; Ding, C.; Zou, Z.; Liang, Y.; Zhou, Y. Deciphering the biological effects of radiotherapy in cancer cells. Biomolecules 2022, 12, 1167. [Google Scholar] [CrossRef]
- Chinniah, S.; Stish, B.; Costello, B.A.; Pagliaro, L.; Childs, D.; Quevedo, F.; Lucien, F.; Bryce, A.; Park, S.S.; Orme, J.J. Radiation therapy in oligometastatic prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 684–692. [Google Scholar] [CrossRef]
- Odia, Y.; Gutierrez, A.N.; and Kotecha, R. Surgically targeted radiation therapy (STaRT) trials for brain neoplasms: A comprehensive review. Neuro Oncol. 2022, 24, S16–S24. [Google Scholar] [CrossRef]
- Sun, L.; Shen, F.; Tian, L.; Tao, H.; Xiong, Z.; Xu, J.; Liu, Z. ATP-responsive smart hydrogel releasing immune adjuvant synchronized with repeated chemotherapy or radiotherapy to boost antitumor immunity. Adv. Mater. 2021, 33, e2007910. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Z.; Suo, W.; Bao, Z.; Quan, H. Injectable hydrogel for synergetic low dose radiotherapy, chemodynamic therapy and photothermal therapy. Front. Bioeng. Biotechnol. 2021, 9, 757428. [Google Scholar] [CrossRef]
- Wang, N.; Gao, Q.; Tang, J.; Jiang, Y.; Yang, L.; Shi, X.; Chen, Y.; Zhang, Y.; Fu, S.; Lin, S. Anti-tumor effect of local injectable hydrogel-loaded endostatin alone and in combination with radiotherapy for lung cancer. Drug Deliv. 2021, 28, 183–194. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, L.; Huang, F.; Zhao, C.; Liu, J.; Zhang, Y.; Liu, J. Multifunctional Hybrid Hydrogel Enhanced Antitumor Therapy through Multiple Destroying DNA Functions by a Triple-Combination Synergistic Therapy. Adv. Healthc. Mater. 2021, 10, e2101190. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Z.; Qin, X.; Zhang, M.; Du, Q.; Li, Z.; Luan, Y. A Checkpoint-Regulatable Immune Niche Created by Injectable Hydrogel for Tumor Therapy. Adv. Funct. Mater. 2021, 31, 2104630. [Google Scholar] [CrossRef]
- Wang, W.; Song, H.; Zhang, J.; Li, P.; Li, C.; Wang, C.; Kong, D.; Zhao, Q. An injectable, thermosensitive and multicompartment hydrogel for simultaneous encapsulation and independent release of a drug cocktail as an effective combination therapy platform. J. Control. Release 2015, 203, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, H.; Wang, L.; Liu, T.; Yeh, J.; Lu, G.; Yang, L.; Mao, H. Delivery of therapeutic radioisotopes using nanoparticle platforms: Potential benefit in systemic radiation therapy. Nanotechnol. Sci. Appl. 2010, 3, 159–170. [Google Scholar] [PubMed]
- Liu, X.; Li, Z.; Loh, X.J.; Chen, K.; Li, Z.; Wu, Y.-L. Targeted and Sustained Corelease of Chemotherapeutics and Gene by Injectable Supramolecular Hydrogel for Drug-Resistant Cancer Therapy. Macromol. Rapid Commun. 2019, 40, 1800117. [Google Scholar] [CrossRef]
- El-Aneed, A. An overview of current delivery systems in cancer gene therapy. J. Control. Release 2004, 94, 1–14. [Google Scholar] [CrossRef]
- Dai, X.; Tan, C. Combination of microRNA therapeutics with small-molecule anticancer drugs: Mechanism of action and co-delivery nanocarriers. Adv. Drug Deliv. Rev. 2015, 81, 184–197. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.; Ma, C.; Tang, H.; Hao, H.; Li, M.; Luo, X.; Yang, M.; Gao, L.; Li, J. Advances in Hydrogels of Drug Delivery Systems for the Local Treatment of Brain Tumors. Gels 2024, 10, 404. [Google Scholar] [CrossRef]
- Murray, B.S. Microgels at fluid-fluid interfaces for food and drinks. Adv. Colloid Interface Sci. 2019, 271, 101990. [Google Scholar] [CrossRef]
- Lefroy, K.S.; Murray, B.S.; Ries, M.E. Advances in the use of microgels as emulsion stabilisers and as a strategy for cellulose functionalisation. Cellulose 2021, 28, 647–670. [Google Scholar] [CrossRef]
- Brézault, A.; Perrin, P.; Sanson, N. Multiresponsive Supramolecular Poly(N-isopropylacrylamide) Microgels. Macromolecules 2024, 57, 2651–2660. [Google Scholar] [CrossRef]
- Ruscito, A.; Chiessi, E.; Toumia, Y.; Oddo, L.; Domenici, F.; Paradossi, G. Microgel particles with distinct morphologies and common chemical compositions: A unified descrip-tion of the responsivity to temperature and osmotic stress. Gels 2020, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Green, J.J.; Elisseeff, J.H. Mimicking biological functionality with polymers for biomedical applications. Nature 2016, 540, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Suhail, M.; Shih, C.M.; Liu, J.Y.; Hsieh, W.C.; Lin, Y.W.; Wu, P.C. In-vitro and in-vivo evaluation of biocompatible polymeric microgels for pH- driven delivery of Ketorolac tromethamine. Int. J. Pharm. 2022, 626, 122194. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Li, D.; Li, Q.; Cao, X.; Dong, H. Microgel assembly: Fabrication, characteristics and application in tissue engineering and regenerative medicine. Bioact. Mater. 2022, 9, 105–119. [Google Scholar] [CrossRef]
- Agrawal, G.; Agrawal, R. Functional microgels: Recent advances in their biomedical applications. Small 2018, 14, e1801724. [Google Scholar] [CrossRef]
- Lenßen, P.; Hengsbach, R.; Frommelius, A.; Cammeraat, S.; Linssen, K.; Simon, U.; Wöll, D. Nanosized core-shell bio-hybrid microgels and their internal structure. Nanoscale 2024, 17, 4570. [Google Scholar] [CrossRef]
- Matalanis, A.; Jones, O.G.; McClements, D.J. Structured biopolymer-based delivery systems for encapsulation, protection, and release of lipophilic compounds. Food Hydrocoll. 2011, 25, 1865–1880. [Google Scholar] [CrossRef]
- Caldwell, A.S.; Aguado, B.A.; Anseth, K.S. Designing microgels for cell culture and controlled assembly of tissue microenvironments. Adv. Funct. Mater. 2020, 30, 1907670. [Google Scholar] [CrossRef]
- Hu, C.; van Bonn, P.; Demco, D.E.; Bolm, C.; Pich, A. Mechanochemical Synthesis of Stimuli Responsive Microgels. Angew. Chem.-Int. Ed. 2023, 62, e202305783. [Google Scholar] [CrossRef]
- Shewan, H.M.; Stokes, J.R. Review of techniques to manufacture micro-hydrogel particles for the food industry and their applications. J. Food Eng. 2013, 119, 781–792. [Google Scholar] [CrossRef]
- Yao, Y.J.; Wang, H.R.; Wang, R.R.; Chai, Y.; Ji, W.L. Fabrication and performance characterization of the membrane from self-dispersed gelatin-coupled cellulose microgels. Cellulose 2019, 26, 3255–3269. [Google Scholar] [CrossRef]
- Rovers, M.M.; Bakker, B.K.; Bakal, K.J. Using a Supramolecular Monomer Formulation Approach to Engineer Modular, Dynamic Microgels and Composite Macrogels. Adv. Mater. 2024, 36, 2405868. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Wang, Y.; Wang, S.; Zhao, C.; Gong, X. The dissociation of physical interaction clusters under tensile deformation of hybrid double network gels. Polymer 2020, 210, 122995. [Google Scholar] [CrossRef]
- Farjami, T.; Madadlou, A. Fabrication methods of biopolymeric microgels and microgel based hydrogels. Food Hydrocoll. 2017, 62, 262–272. [Google Scholar] [CrossRef]
- Alzanbaki, H.; Moretti, M.; Hauser, C.A.E. Engineered microgels—Their manufacturing and biomedical applications. Micromachines 2021, 12, 45. [Google Scholar] [CrossRef]
- Torres, O.; Murray, B.; Sarkar, A. Emulsion microgel particles: Novel encapsulation strategy for lipophilic molecules. Trends Food Sci. Technol. 2016, 55, 98–108. [Google Scholar] [CrossRef]
- Helgeson, M.E.; Chapin, S.C.; Doyle, P.S. Hydrogel microparticles from lithographic processes: Novel materials for fundamental and applied colloid science. Curr. Opin. Colloid Interface Sci. 2011, 16, 106–117. [Google Scholar] [CrossRef]
- Rolland, J.P.; Maynor, B.W.; Euliss, L.E.; Exner, A.E.; Denison, G.M.; DeSimone, J.M. Direct Fabrication and Harvesting of Monodisperse, Shape-Specific Nanobiomaterials. J. Am. Chem. Soc. 2005, 127, 10096–10100. [Google Scholar] [CrossRef]
- Tang, M.D.; Golden, A.P.; Tien, J. Molding of Three-Dimensional Microstructures of Gels. J. Am. Chem. Soc. 2003, 125, 12988–12989. [Google Scholar] [CrossRef]
- Pregibon, D.C.; Toner, M.; Doyle, P.S. Multifunctional Encoded Particles for High-Throughput Biomolecule Analysis. Science 2007, 315, 1393–1396. [Google Scholar] [CrossRef]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.-J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Lo, E.; Ali, S.; Khademhosseini, A. Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs. Proc. Natl. Acad. Sci. USA 2008, 105, 9522–9527. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Lee, J.B. Injectable Micro-Hydrogel for DNA Delivery: A Promising Therapeutic Platform. J. Funct. Biomater. 2024, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, M.; Yilmaz, A.S.; Ayyala, R.S.; Sahiner, N. Biocompatible Glycol Chitosan Microgels as Effective Drug Carriers. Gels 2023, 9, 398. [Google Scholar] [CrossRef]
- Sahiner, M.; Yilmaz, A.S.; Ayyala, R.S.; Sahiner, N. Carboxymethyl Chitosan Microgels for Sustained Delivery of Vancomycin and Long-Lasting Antibacterial Effects. Gels 2023, 9, 708. [Google Scholar] [CrossRef]
- Choi, Y.; Koh, H.Y.; Han, J.Y.; Seo, S. Synthesis of Hydrogel-Based Microgels and Nanogels Toward Therapeutic and Biomedical Applications. Appl. Sci. 2025, 15, 1368. [Google Scholar] [CrossRef]
- Rodriguez-Tellez, T.G.; Magana, H.; Cornejo-Bravo, J.M.; Palomino-Vizcaino, G.; Palomino-Vizcaino, K. Microgels of N-Isopropylacrylamide Copolymerized with an Amphiphilic Acid for the Delivery of Doxorubicin. Gels 2024, 10, 806. [Google Scholar] [CrossRef]
- Campora, S.; Mohsen, R.; Passaro, D.; Samir, H.; Ashraf, H.; Al-Mofty, S.E.-D.; Diab, A.A.; El-Sherbiny, I.M.; Snowden, M.J.; Ghersi, G. Functionalized Poly(N-isopropylacrylamide)-Based Microgels in Tumor Targeting and Drug Delivery. Gels 2021, 7, 203. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Wei, G.; Wang, Y.; Yang, G.; Wang, Y.; Ju, R. Recent progress in nanomedicine for enhanced cancer chemotherapy. Theranostics 2021, 11, 6370–6392. [Google Scholar] [CrossRef]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Wang, L.; Brey, E.M.; Uribe, G.R.; Tang, L. Smart Nanoparticles for Chemo-Based Combinational Therapy. Pharmaceutics 2021, 13, 853. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Zhu, J.; Xu, M.F.; Chen, J.; Gao, X.; Zhao, L.; Ding, F.; Wu, C.-Z. One-pot preparation of pH- and redox-responsive polymeric microgel as an efficient carrier for improved breast cancer therapy. Colloids Surf. A Physicochem. Eng. Asp. 2024, 685, 133320. [Google Scholar] [CrossRef]
- Khanal, A.; Ngoc Bui, M.P.; Seo, S.S. Microgel-encapsulated methylene blue for the treatment of breast cancer cells by photodynamic therapy. J. Breast Cancer 2014, 17, 18–24. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, W.; Zhang, R.; Zhang, C.; Yan, J.; Feng, J.; Rosenholm, J.M.; Shi, T.; Shen, X.; Zhang, H. Minimally invasive injection of biomimetic Nano@Microgel for in situ ovarian cancer treatment through enhanced photodynamic reactions and photothermal combined therapy. Mater. Today Bio 2023, 20, 100663. [Google Scholar] [CrossRef]
- Peña, O.A.; Martin, P. Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol. 2024, 25, 599–616. [Google Scholar] [CrossRef]
- Riley, L.; Schirmer, L.; Segura, T. Granular hydrogels: Emergent properties of jammed hydrogel microparticles and their applications in tissue repair and regeneration. Curr. Opin. Biotechnol. 2019, 60, 1–8. [Google Scholar] [CrossRef]
- Kang, Y.; Liu, X.; Wang, J.; Peng, P.; Liang, M.; Wang, Q.; Zheng, W.; Li, S.; Gao, C. Rod-Shaped Microgel Scaffolds with Interconnective Pores and Oxygen-generating Functions Promote Skin Wound Healing and Alleviate Hypertrophic Scar Formation. Adv. Funct. Mater. 2025, 35, 2413678. [Google Scholar] [CrossRef]
- Chen, G.; Wang, F.; Zhang, X.; Shang, Y.; Zhao, Y. Living microecological hydrogels for wound healing. Sci. Adv. 2023, 9, eadg3478. [Google Scholar] [CrossRef]
- Griffin, D.R.; Weaver, W.M.; Scumpia, P.O.; Di Carlo, D.; Segura, T. Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat. Mater. 2015, 14, 737–744. [Google Scholar] [CrossRef]
- Li, Y.; Song, W.; Kong, L.; He, Y.; Li, H. Injectable and Microporous Microgel-Fiber Granular Hydrogel Loaded with Bioglass and siRNA for Promoting Diabetic Wound Healing. Small 2024, 20, e2309599. [Google Scholar] [CrossRef] [PubMed]
- da Silva Campelo, M.; Saraiva, M.M.; Lima, A.B.N.; Dias, A.T.D.F.F.; Neto, J.F.C.; de Aguiar Soares, S.; Ricardo, N.M.P.S.; de Carvalho Leitão, R.F.; Ribeiro, M.E.N.P. Agaricus blazei Murill extract-loaded alginate/poly (vinyl alcohol)-based microgels as potential wound dressings. Colloids Surf. A Physicochem. Eng. Asp. 2024, 701, 134875. [Google Scholar] [CrossRef]
- Puiggalí-Jou, A.; Asadikorayem, M.; Maniura-Weber, K.; Zenobi-Wong, M. Growth factor-loaded sulfated microislands in granular hydrogels promote hMSCs migration and chon drogenic differentiation. Acta Biomater. 2023, 166, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Truong, V.X.; Fisch, P.; Levinson, C.; Glattauer, V.; Zenobi-Wong, M.; Thissen, H.; Forsythe, J.S.; Frith, J.E. Cartilage tissue formation through assembly of microgels containing mesenchymal stem cells. Acta. Biomater. 2018, 77, 48–62. [Google Scholar] [CrossRef]
- Wei, D.X.; Dao, J.W.; Chen, G.Q. A micro-ark for cells: Highly open porous polyhydroxyalkanoate microspheres as injectable scaffolds for tissue regeneration. Adv. Mater. 2018, 30, e1802273. [Google Scholar] [CrossRef]
- Bian, J.; Cai, F.; Chen, H.; Tang, Z.; Xi, K.; Tang, J.; Wu, L.; Xu, Y.; Deng, L.; Gu, Y.; et al. Modulation of local overactive inflammation via injectable hydrogel micro spheres. Nano. Lett. 2021, 21, 2690–2698. [Google Scholar] [CrossRef]
- He, J.; Chen, C.; Chen, L.; Cheng, R.; Sun, J.; Liu, X.; Wang, L.; Zhu, C.; Hu, S.; Xue, Y.; et al. Honeycomb like hydrogel microspheres for 3D bulk construction of tumor models. Research 2022, 2022, 9809763. [Google Scholar] [CrossRef]
- Wang, P.; Meng, X.; Wang, R.; Yang, W.; Yang, L.; Wang, J.; Wang, D.; Fan, C. Biomaterial scaffolds made of chemically cross-linked gelatin microsphere aggregates (C-GMSs) promote vascularized bone regeneration. Adv. Healthc. Mater. 2022, 11, e2102818. [Google Scholar] [CrossRef]
- Fang, J.; Koh, J.; Fang, Q.; Qiu, H.; Archang, M.M.; Hasani-Sadrabadi, M.M.; Miwa, H.; Zhong, X.; Sievers, R.; Gao, D.W.; et al. Drug delivery: Injectable drug-releasing microporous annealed par ticle scaffolds for treating myocardial infarction. Adv. Funct. Mater. 2020, 30, 2070289. [Google Scholar] [CrossRef]
- Nih, L.; Sideris, E.; Carmichael, S.; Segura, T. Injection of microporous annealing particle (MAP) hydrogels in the stroke cavity reduces gliosis and inflammation and promotes NPC migration to the lesion. Adv. Mater. 2017, 29, 1606471. [Google Scholar] [CrossRef]
- Dumont, C.; Carlson, M.; Munsell, M.; Ciciriello, A.; Strnadova, K.; Park, J.; Cummings, B.; Anderson, A.; Shea, L. Aligned hydrogel tubes guide regeneration following spinal cord injury. Acta Biomater. 2019, 86, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Mealy, J.; Chung, J.; Jeong, H.; Issadore, D.; Lee, D.; Atluri, P.; Burdick, J. Injectable granular hydrogels with multifunctional properties for biomedical applications. Adv. Mater. 2018, 30, 1705912. [Google Scholar] [CrossRef] [PubMed]
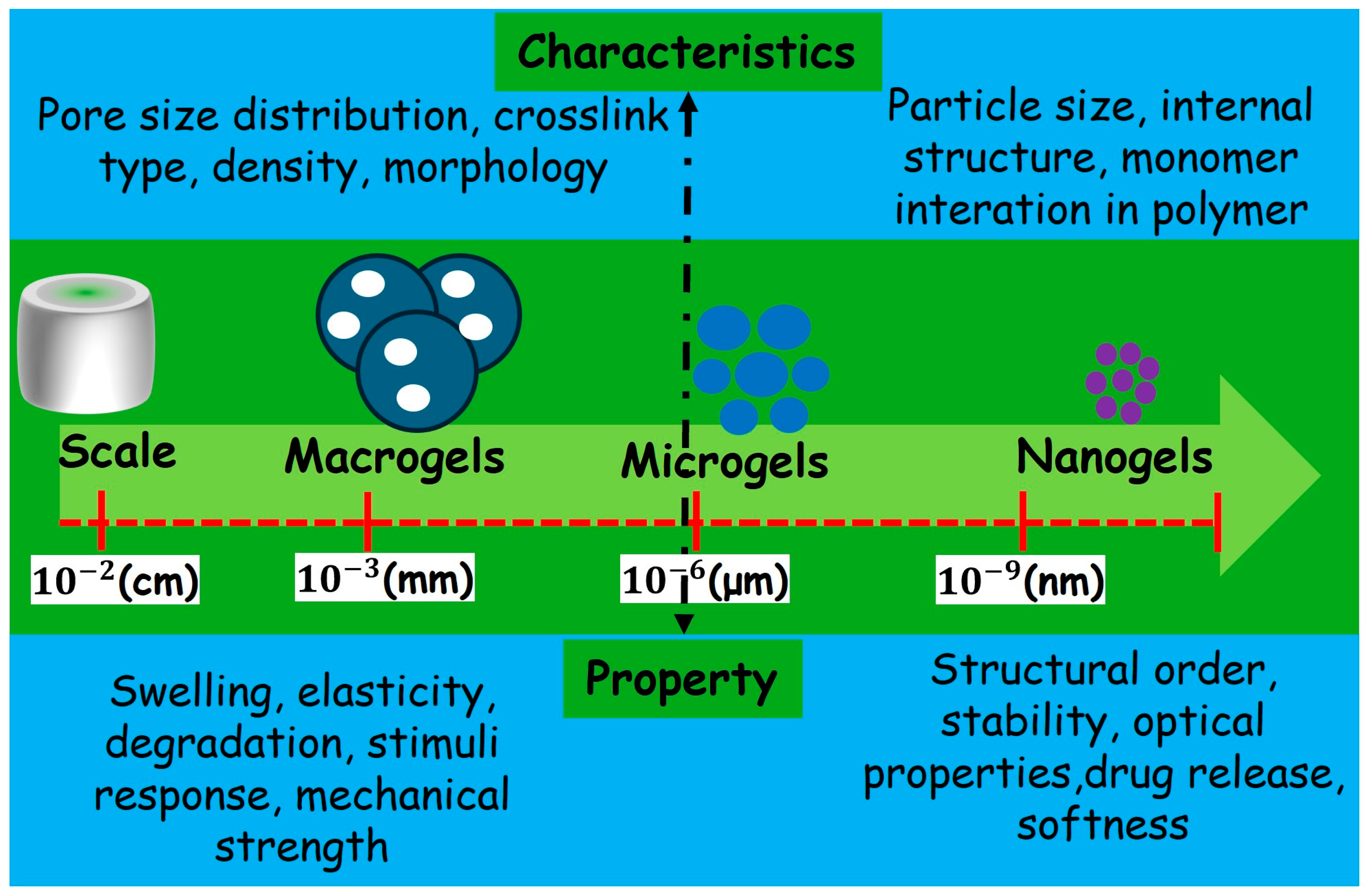



| Parameter | Hydrogel | Microgel | References |
|---|---|---|---|
| Particle Size | Macro-scale | Submicron to micron-scale | [12,14] |
| Water Absorption | High water content to form gel-like consistency | Absorb water but as individual particles | [8,29,38,56] |
| Stimuli Response | Responds to changes in pH, temperature | Highly responsive to environmental stimuli | [48,49,139,152,153,190] |
| Applications | Drug delivery, tissue engineering | Cancer treatment, Antibacterial applicaction | [11,18,121,122,175,180] |
| Synthesis | Often involves cross-linking polymers in bulk | Colloidal synthesis methods, such as emulsion or precipitation polymerization | [42,44,46,152,153,174] |
| Responsiveness | Typically responds uniformly throughout the structure | Exhibits rapid and reversible responses due to small size and surface area | [14,44,175] |
| Recent Advancements | Incorporation of bioactive molecules, improved mechanical properties | Use of metallo-supramolecular cross-linkers for core-shell structures | [48,49,156,157] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, M.S.; Yun, S.; Kim, H.-Y.; Ko, S.; Islam, M.; Nam, K.-W. Hydrogels and Microgels: Driving Revolutionary Innovations in Targeted Drug Delivery, Strengthening Infection Management, and Advancing Tissue Repair and Regeneration. Gels 2025, 11, 179. https://doi.org/10.3390/gels11030179
Ahmed MS, Yun S, Kim H-Y, Ko S, Islam M, Nam K-W. Hydrogels and Microgels: Driving Revolutionary Innovations in Targeted Drug Delivery, Strengthening Infection Management, and Advancing Tissue Repair and Regeneration. Gels. 2025; 11(3):179. https://doi.org/10.3390/gels11030179
Chicago/Turabian StyleAhmed, Md. Shahriar, Sua Yun, Hae-Yong Kim, Sunho Ko, Mobinul Islam, and Kyung-Wan Nam. 2025. "Hydrogels and Microgels: Driving Revolutionary Innovations in Targeted Drug Delivery, Strengthening Infection Management, and Advancing Tissue Repair and Regeneration" Gels 11, no. 3: 179. https://doi.org/10.3390/gels11030179
APA StyleAhmed, M. S., Yun, S., Kim, H.-Y., Ko, S., Islam, M., & Nam, K.-W. (2025). Hydrogels and Microgels: Driving Revolutionary Innovations in Targeted Drug Delivery, Strengthening Infection Management, and Advancing Tissue Repair and Regeneration. Gels, 11(3), 179. https://doi.org/10.3390/gels11030179







