Cyclodextrin–Hydrogel Hybrids in Advanced Drug Delivery
Abstract
1. Introduction
2. Cyclodextrin Composition and Functionalization
2.1. β-CD and Its Derivatives: Improving Drug Solubility and Controlled Release
2.2. HP-β-CD: A Versatile Drug Solubilizer and Delivery Enhancer
2.3. γ-CD: Expanding the Scope of Drug Complexation for Larger Molecules
2.4. Functionalized Cyclodextrins: Next-Generation Drug Delivery Systems
3. Hydrogel Polymers and Their Role in CD–Hydrogel Drug Delivery
3.1. Thermosensitive Hydrogels: Temperature-Responsive Drug Release Systems
3.2. pH-Responsive Hydrogels: Targeted Drug Release Based on pH Sensitivity
3.3. Mucoadhesive Hydrogels: Prolonging Drug Retention and Absorption
3.4. Hydrogels for Ophthalmic and Nasal Drug Delivery
3.5. Microneedle Hydrogel Systems and Bone Regeneration Hydrogels
3.6. Solvent-Exchange-Induced In Situ Gels (ISGs) and Microparticles (ISMs)
4. Preparation Methods for CD–Hydrogel Hybrids
4.1. CD Inclusion Complex Formation
4.2. Hydrogel Synthesis and Crosslinking Techniques
4.3. Nanogel and Emulsification-Based Formulations
4.4. Microneedle Patch Fabrication
4.5. Advanced Crosslinked and Bone Implant Hydrogels
5. Key Factors Influencing Drug Delivery Performance
5.1. Swelling and Drug Release Control
5.2. CD Type and Drug Complexation Ratio
5.3. Gelation Temperature and Thermosensitive Performance
5.4. Hydrogel Matrix Composition and Crosslinking Density
5.5. Environmental Sensitivity: pH, Temperature, and Mucoadhesion
5.6. Particle Size and Drug Permeation Efficiency
5.7. Delivery Route and Bioavailability Optimization
6. Evaluation and Characterization of CD–Hydrogel Systems
6.1. Physicochemical Characterization and Material Analysis
- Structural and Spectroscopic Analysis: Fourier-transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), nuclear magnetic resonance (1H NMR), and powder X-ray diffraction (PXRD) confirm the formation of drug–CD inclusion complexes, assess crystallinity, and evaluate molecular interactions within hydrogel matrices [1,2,4,14,18,34,50,67,68].
6.2. Rheological and Gelation Behavior
6.3. Drug Release and Permeation Studies
6.4. Pharmacokinetics and In Vivo Evaluations
6.5. Biocompatibility and Safety Evaluations
6.6. Optimization and Formulation Development
6.7. Biological Activity Studies
7. Therapeutic Applications of CD–Hydrogel Hybrids
7.1. Anti-Inflammatory and Pain-Relief Treatments
7.2. Antifungal Therapies
7.3. Infections, Inflammation, and Glaucoma
7.4. Wound Healing and Regenerative Medicine
7.5. Cancer Therapies
7.6. Neurological and Psychiatric Treatments
7.7. Diabetes and Metabolic Disorders
7.8. Inflammatory Bowel Disease, Cancer, and Biologic Therapies
8. Advancements in CD–Hydrogel Drug Delivery
8.1. Enhanced Drug Solubility and Bioavailability
8.2. Controlled and Sustained Drug Release
8.3. Enhanced Drug Retention, Permeation, and Therapeutic Efficacy
8.4. Biocompatibility and Reduced Toxicity
9. Challenges and Limitations of CD–Hydrogel Systems
9.1. Complex Formulation and Manufacturing Challenges
9.2. Stability and Variability in Drug Release Profiles
9.3. Limited In Vivo Validation and Clinical Translation
9.4. Regulatory Barriers and Commercialization Hurdles
10. Future Directions in CD–Hydrogel Research and Development
10.1. Development of Next-Generation Smart Hydrogels
10.2. Enhancing Drug Loading and Optimizing Release Kinetics
10.3. Expanding Applications in Neurology, Oncology, and Transdermal Therapy
10.4. Bridging the Gap Between Preclinical Research and Clinical Trials
10.5. Optimization of Oral Peptide and Hormone Delivery Systems
10.6. Enhancing Stability and Commercial Scalability
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ammar, H.O.; Ghorab, M.; Mahmoud, A.A.; Makram, T.S.; Noshi, S.H. Topical liquid crystalline gel containing lornoxicam/cyclodextrin complex. J. Incl. Phenom. Macrocycl. Chem. 2012, 73, 161–175. [Google Scholar] [CrossRef]
- Bilensoy, E.; Rouf, M.A.; Vural, I.; Sen, M.; Hincal, A.A. Mucoadhesive, thermosensitive, prolonged-release vaginal gel for clotrimazole:beta-cyclodextrin complex. AAPS PharmSciTech 2006, 7, E38. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Yang, J.; Song, F.; Pu, G.; Dong, F.; Liang, Z.; Zhang, J. Development of ion-triggered in situ gel containing ketoconazole/hydroxypropyl-beta-cyclodextrin for ocular delivery: In vitro and in vivo evaluation. Drug Deliv. 2024, 31, 2424217. [Google Scholar] [CrossRef] [PubMed]
- Kesavan, K.; Kant, S.; Singh, P.N.; Pandit, J.K. Effect of hydroxypropyl-beta-cyclodextrin on the ocular bioavailability of dexamethasone from a pH-induced mucoadhesive hydrogel. Curr. Eye Res. 2011, 36, 918–929. [Google Scholar] [CrossRef] [PubMed]
- Cirri, M.; Nerli, G.; Mennini, N.; Maestrelli, F.; Mura, P. Development and Characterization of Cyclodextrin-Based Nanogels as a New Ibuprofen Cutaneous Delivery System. Pharmaceutics 2022, 14, 15. [Google Scholar] [CrossRef]
- Deshkar, S.S.; Palve, V.K. Formulation and development of thermosensitive cyclodextrin-based in situ gel of voriconazole for vaginal delivery. J. Drug Deliv. Sci. Technol. 2019, 49, 277–285. [Google Scholar] [CrossRef]
- Fernandez-Ferreiro, A.; Fernandez Bargiela, N.; Varela, M.S.; Martinez, M.G.; Pardo, M.; Pineiro Ces, A.; Mendez, J.B.; Barcia, M.G.; Lamas, M.J.; Otero-Espinar, F. Cyclodextrin-polysaccharide-based, in situ-gelled system for ocular antifungal delivery. Beilstein J. Org. Chem. 2014, 10, 2903–2911. [Google Scholar] [CrossRef]
- Khalid, Q.; Ahmad, M.; Minhas, M.U. Synthesis of beta-cyclodextrin hydrogel nanoparticles for improving the solubility of dexibuprofen: Characterization and toxicity evaluation. Drug Dev. Ind. Pharm. 2017, 43, 1873–1884. [Google Scholar] [CrossRef]
- Omidian, H.; Akhzarmehr, A.; Chowdhury, S.D. Hydrogel Composites for Multifunctional Biomedical Applications. J. Compos. Sci. 2024, 8, 154. [Google Scholar] [CrossRef]
- Omidian, H.; Chowdhury, S.D.; Akhzarmehr, A. Hydrogels in biosensing and medical diagnostics. J. Bioact. Compat. Polym. 2024, 39, 480–506. [Google Scholar] [CrossRef]
- Omidian, H.; Chowdhury, S.D.; Cubeddu, L.X. Hydrogels for Neural Regeneration: Exploring New Horizons. Materials 2024, 17, 3472. [Google Scholar] [CrossRef] [PubMed]
- Omidian, H.; Wilson, R.L. Enhancing Hydrogels with Quantum Dots. J. Compos. Sci. 2024, 8, 203. [Google Scholar] [CrossRef]
- Filip, D.; Macocinschi, D.; Zaltariov, M.F.; Gafitanu, C.A.; Tuchilus, C.G.; Bele, A.; Ciubotaru, B.I.; Stoleru, E.; Bargan, A. Mucoadhesive and Antimicrobial Allantoin/beta Cyclodextrins-Loaded Carbopol Gels as Scaffolds for Regenerative Medicine. Gels 2022, 8, 23. [Google Scholar] [CrossRef]
- Mahmood, A.; Sharif, A.; Muhammad, F.; Sarfraz, R.M.; Abrar, M.A.; Qaisar, M.N.; Anwer, N.; Amjad, M.W.; Zaman, M. Development and in vitro evaluation of (beta-cyclodextrin-g-methacrylic acid)/Na(+)-montmorillonite nanocomposite hydrogels for controlled delivery of lovastatin. Int. J. Nanomed. 2019, 14, 5397–5413. [Google Scholar] [CrossRef]
- Doliwa, A.; Santoyo, S.; Ygartua, P. Transdermal lontophoresis and skin retention of piroxicam from gels containing piroxicam: Hydroxypropyl-beta-cyclodextrin complexes. Drug Dev. Ind. Pharm. 2001, 27, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Mahor, A.; Singh, G.; Bansal, K.; Singh, P.P.; Gupta, R.; Dutt, R.; Alanazi, A.M.; Khan, A.A.; Kesharwani, P. Formulation Development, In Vitro and In Vivo Evaluation of Topical Hydrogel Formulation of Econazole Nitrate-Loaded beta-Cyclodextrin Nanosponges. J. Pharm. Sci. 2021, 110, 3702–3714. [Google Scholar] [CrossRef]
- Li, S.T.; Long, M.; Li, J.Q.; Zhang, Y.T.; Feng, N.P.; Zhang, Z.C. Improved topical delivery of curcumin by hydrogels formed by composite carriers integrated with cyclodextrin metal-organic frameworks and cyclodextrin nanosponges. Int. J. Pharm. X 2024, 8, 11. [Google Scholar] [CrossRef]
- Khalid, Q.; Ahmad, M.; Minhas, M.U. Hydroxypropyl-β-cyclodextrin hybrid nanogels as nano-drug delivery carriers to enhance the solubility of dexibuprofen: Characterization, in vitro release, and acute oral toxicity studies. Adv. Polym. Technol. 2018, 37, 2171–2185. [Google Scholar] [CrossRef]
- Elmotasem, H.; Awad, G.E.A. A stepwise optimization strategy to formulate in situ gelling formulations comprising fluconazole-hydroxypropyl-beta-cyclodextrin complex loaded niosomal vesicles and Eudragit nanoparticles for enhanced antifungal activity and prolonged ocular delivery. Asian J. Pharm. Sci. 2020, 15, 617–636. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Chengqun, Y.; Zhenzhong, Z.; Weifeng, Z.; Yongmei, G. β-Cyclodextrin/chitosan-based (polyvinyl alcohol-co-acrylic acid) interpenetrating hydrogels for oral drug delivery. Int. J. Biol. Macromol. 2023, 242, 125149. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, W.C.; Huang, L.X.; Liu, J.L.; Yang, G. Nanocomposite pastes of gelatin and cyclodextrin-grafted chitosan nanoparticles as potential postoperative tumor therapy. Adv. Compos. Hybrid. Mater. 2023, 6, 14. [Google Scholar] [CrossRef]
- Basaran, B.; Bozkir, A. Thermosensitive and pH induced in situ ophthalmic gelling system for ciprofloxacin hydrochloride: Hydroxypropyl-β-cyclodextrin complex. Acta Pol. Pharm. 2012, 69, 1137–1147. [Google Scholar]
- Naeem, A.; Yu, C.; Zang, Z.; Zhu, W.; Deng, X.; Guan, Y. Synthesis and Evaluation of Rutin-Hydroxypropyl beta-Cyclodextrin Inclusion Complexes Embedded in Xanthan Gum-Based (HPMC-g-AMPS) Hydrogels for Oral Controlled Drug Delivery. Antioxidants 2023, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Filho, M.S.; Alvarez-Lorenzo, C.; Martinez-Pacheco, R.; Landin, M. Temperature-sensitive gels for intratumoral delivery of beta-lapachone: Effect of cyclodextrins and ethanol. Sci. World J. 2012, 2012, 126723. [Google Scholar] [CrossRef]
- Wang, L.L.; Zheng, W.S.; Chen, S.H.; Han, Y.X.; Jiang, J.D. Development of rectal delivered thermo-reversible gelling film encapsulating a 5-fluorouracil hydroxypropyl-beta-cyclodextrin complex. Carbohydr. Polym. 2016, 137, 9–18. [Google Scholar] [CrossRef]
- Cirri, M.; Maestrelli, F.; Nerli, G.; Mennini, N.; D’Ambrosio, M.; Luceri, C.; Mura, P.A. Development of a Cyclodextrin-Based Mucoadhesive-Thermosensitive In Situ Gel for Clonazepam Intranasal Delivery. Pharmaceutics 2021, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Slavkova, M.I.; Momekova, D.B.; Kostova, B.D.; Momekov, G.T.; Petrov, P.D. Novel dextran/β-cyclodextrin and dextran macroporous cryogels for topical delivery of curcumin in the treatment of cutaneous T-cell lymphoma. Bulg. Chem. Commun. 2017, 49, 792–799. [Google Scholar]
- Kulkarni, J.A.; Avachat, A.M. Pharmacodynamic and pharmacokinetic investigation of cyclodextrin-mediated asenapine maleate in situ nasal gel for improved bioavailability. Drug Dev. Ind. Pharm. 2017, 43, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.H.; Qiu, J.; Tan, H.P.; Li, D.; Ma, X.H. Synthesis and Characterization of Cyclodextrin-containing Hydrogel for Ophthalmic Drugs Delivery. J. Macromol. Sci. Part A-Pure Appl. Chem. 2013, 50, 983–990. [Google Scholar] [CrossRef]
- Sajeesh, S.; Bouchemal, K.; Marsaud, V.; Vauthier, C.; Sharma, C.P. Cyclodextrin complexed insulin encapsulated hydrogel microparticles: An oral delivery system for insulin. J. Control. Release 2010, 147, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Ou, G.; Li, Q.; Zhu, L.; Zhang, Y.; Liu, Y.; Li, X.; Du, L.; Jin, Y. Intranasal hydrogel of armodafinil hydroxypropyl-beta-cyclodextrin inclusion complex for the treatment of post-traumatic stress disorder. Saudi Pharm. J. 2022, 30, 265–282. [Google Scholar] [CrossRef]
- Mazet, R.; Choisnard, L.; Levilly, D.; Wouessidjewe, D.; Geze, A. Investigation of Combined Cyclodextrin and Hydrogel Formulation for Ocular Delivery of Dexamethasone Acetate by Means of Experimental Designs. Pharmaceutics 2018, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Werner, U.; Damge, C.; Maincent, P.; Bodmeier, R. Properties of in situ gelling nasal inserts containing estradiol/methyl β-cyclodextrin. J. Drug Deliv. Sci. Technol. 2004, 14, 275–284. [Google Scholar] [CrossRef]
- Saha, B.; Das, N.; Paul, P.; Barman, S.; Mondal, M.; Saha, S.; Choudhury, S.; Roy, N.; Ali, S.; Roy, M.N. Chemico-pharmaceutical investigations of an inclusion complex formed with valacyclovir and γ-cyclodextrin optimized by molecular docking for innovative applications as topical gel. New J. Chem. 2024, 48, 18187–18204. [Google Scholar] [CrossRef]
- Bilensoy, E.; Çirpanli, Y.; Sen, M.; Dogan, A.L.; Çalis, S. Thermosensitive mucoadhesive gel formulation loaded with 5-Fu: Cyclodextrin complex for HPV-induced cervical cancer. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 363–370. [Google Scholar] [CrossRef]
- Zou, L.; Zhang, Z.; Feng, J.; Ding, W.; Li, Y.; Liang, D.; Xie, T.; Li, F.; Li, Y.; Chen, J.; et al. Paclitaxel-Loaded TPGS(2k)/Gelatin-Grafted Cyclodextrin/Hyaluronic Acid-Grafted Cyclodextrin Nanoparticles for Oral Bioavailability and Targeting Enhancement. J. Pharm. Sci. 2022, 111, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, X.; Sun, F.; Cao, P. PVA hydrogels containing beta-cyclodextrin for enhanced loading and sustained release of ocular therapeutics. J. Biomater. Sci. Polym. Ed. 2010, 21, 1023–1038. [Google Scholar] [CrossRef] [PubMed]
- Mennini, N.; Casella, G.; Cirri, M.; Maestrelli, F.; Mura, P. Development of cyclodextrin hydrogels for vaginal delivery of dehydroepiandrosterone. J. Pharm. Pharmacol. 2016, 68, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Glisoni, R.J.; Garcia-Fernandez, M.J.; Pino, M.; Gutkind, G.; Moglioni, A.G.; Alvarez-Lorenzo, C.; Concheiro, A.; Sosnik, A. beta-Cyclodextrin hydrogels for the ocular release of antibacterial thiosemicarbazones. Carbohydr. Polym. 2013, 93, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tiwari, N.; Orellano, M.S.; Navarro, L.; Beiranvand, Z.; Adeli, M.; Calderon, M. Polyglycerol-Functionalized beta-Cyclodextrins as Crosslinkers in Thermoresponsive Nanogels for the Enhanced Dermal Penetration of Hydrophobic Drugs. Small 2024, 20, e2311166. [Google Scholar] [CrossRef] [PubMed]
- Giulbudagian, M.; Honzke, S.; Bergueiro, J.; Isik, D.; Schumacher, F.; Saeidpour, S.; Lohan, S.B.; Meinke, M.C.; Teutloff, C.; Schafer-Korting, M.; et al. Enhanced topical delivery of dexamethasone by beta-cyclodextrin decorated thermoresponsive nanogels. Nanoscale 2017, 10, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Sulistiawati, S.; Kristina Enggi, C.; Wiyulanda Iskandar, I.; Rachmad Saputra, R.; Sartini, S.; Rifai, Y.; Rahman, L.; Aswad, M.; Dian Permana, A. Bioavailability enhancement of sildenafil citrate via hydrogel-forming microneedle strategy in combination with cyclodextrin complexation. Int. J. Pharm. 2024, 655, 124053. [Google Scholar] [CrossRef] [PubMed]
- Raval, M.; Bagada, H. Formulation and Evaluation of Cyclodextrin-Based Thermosensitive In Situ Gel of Azithromycin for Periodontal Delivery. J. Pharm. Innov. 2019, 16, 67–84. [Google Scholar] [CrossRef]
- Sun, Y.; Du, L.; Liu, Y.; Li, X.; Li, M.; Jin, Y.; Qian, X. Transdermal delivery of the in situ hydrogels of curcumin and its inclusion complexes of hydroxypropyl-beta-cyclodextrin for melanoma treatment. Int. J. Pharm. 2014, 469, 31–39. [Google Scholar] [CrossRef]
- Ghanma, R.; Anjani, Q.K.; Naser, Y.A.; Sabri, A.H.B.; Hutton, A.R.J.; Vora, L.K.; Himawan, A.; Greer, B.; McCarthy, H.O.; Donnelly, R.F. Risperidone-cyclodextrin complex reservoir combined with hydrogel-forming microneedle array patches for enhanced transdermal delivery. Eur. J. Pharm. Biopharm. 2024, 202, 114415. [Google Scholar] [CrossRef] [PubMed]
- Hoang Thi, T.H.; Chai, F.; Lepretre, S.; Blanchemain, N.; Martel, B.; Siepmann, F.; Hildebrand, H.F.; Siepmann, J.; Flament, M.P. Bone implants modified with cyclodextrin: Study of drug release in bulk fluid and into agarose gel. Int. J. Pharm. 2010, 400, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Lopedota, A.; Denora, N.; Laquintana, V.; Cutrignelli, A.; Lopalco, A.; Tricarico, D.; Maqoud, F.; Curci, A.; Mastrodonato, M.; la Forgia, F.; et al. Alginate-Based Hydrogel Containing Minoxidil/Hydroxypropyl-beta-Cyclodextrin Inclusion Complex for Topical Alopecia Treatment. J. Pharm. Sci. 2018, 107, 1046–1054. [Google Scholar] [CrossRef]
- Moya-Ortega, M.D.; Alves, T.F.; Alvarez-Lorenzo, C.; Concheiro, A.; Stefansson, E.; Thorsteinsdottir, M.; Loftsson, T. Dexamethasone eye drops containing gamma-cyclodextrin-based nanogels. Int. J. Pharm. 2013, 441, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Huling, J.; Oschatz, S.; Lange, H.; Sterenczak, K.A.; Stahnke, T.; Markhoff, J.; Stachs, O.; Moller, S.; Undre, N.; Peil, A.; et al. gamma-Cyclodextrin hydrogel for the sustained release of josamycin for potential ocular application. Drug Deliv. 2024, 31, 2361168. [Google Scholar] [CrossRef] [PubMed]
- Khalid, F.M.; Ijaz, M.; Mahmood, A.; Waqas, M.K.; Hussain, T.; Asim, M.H.; Ahmad, N.; Arshad, S.; Rehman, M.U.; Nazir, I. Mucoadhesive, Fluconazole-Loaded Nanogels Complexed with Sulfhydryl-beta-cyclodextrin for Oral Thrush Treatment. AAPS PharmSciTech 2023, 24, 194. [Google Scholar] [CrossRef]
- Clemence, B.F.; Xiao, L.; Yang, G. Oral Administration of Berberine Hydrochloride Based on Chitosan/Carboxymethyl-beta-Cyclodextrin Hydrogel. Polymers 2024, 16, 27. [Google Scholar] [CrossRef]
- Badilli, U.; Amasya, G.; Sen, T.; Tarimci, N. Topical emulgel formulation containing inclusion complex of calcipotriol with cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2014, 78, 249–255. [Google Scholar] [CrossRef]
- Aswal, D.; Bisht, T. Transdermal Delivery of Fluconazole β-cyclodextrin Complex Incorporated in Aloe vera Gel for Fungal Therapy: Development, Characterization and in vitro Evaluation. Indian J. Pharm. Educ. Res. 2021, 55, s66–s74. [Google Scholar] [CrossRef]
- Malik, N.S.; Ahmad, M.; Alqahtani, M.S.; Mahmood, A.; Barkat, K.; Khan, M.T.; Tulain, U.R.; Rashid, A. beta-cyclodextrin chitosan-based hydrogels with tunable pH-responsive properties for controlled release of acyclovir: Design, characterization, safety, and pharmacokinetic evaluation. Drug Deliv. 2021, 28, 1093–1108. [Google Scholar] [CrossRef]
- Singh, R.M.; Kumar, A.; Pathak, K. Thermally triggered mucoadhesive in situ gel of loratadine: β-cyclodextrin complex for nasal delivery. AAPS PharmSciTech 2013, 14, 412–424. [Google Scholar] [CrossRef]
- Batool, N.; Sarfraz, R.M.; Mahmood, A.; Zaman, M.; Zafar, N.; Salawi, A.; Almoshari, Y.; Alshamrani, M. Orally Administered, Biodegradable and Biocompatible Hydroxypropyl-beta-Cyclodextrin Grafted Poly(methacrylic acid) Hydrogel for pH Sensitive Sustained Anticancer Drug Delivery. Gels 2022, 8, 21. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Wang, K.; Wang, L.; Yang, X.; Zhu, S. Cyclodextrin-containing hydrogels as an intraocular lens for sustained drug release. PLoS ONE 2017, 12, e0189778. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.M.; He, S.J.; Wang, H.; Zhang, S.; Yu, L.Z.; Zhang, Y.; Elshazly, E.H.; Ke, L.X.; Gong, R.M. Ionic gelated β-cyclodextrin-biotin-carboxymethyl chitosan nanoparticles prepared as carrier for oral delivery of protein drugs. J. Polym. Eng. 2020, 40, 440–447. [Google Scholar] [CrossRef]
- Rein, S.M.T.; Lwin, W.W.; Tuntarawongsa, S.; Phaechamud, T. Meloxicam-loaded solvent exchange-induced in situ forming beta-cyclodextrin gel and microparticle for periodontal pocket delivery. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 117, 111275. [Google Scholar] [CrossRef] [PubMed]
- Arslan, M.; Gevrek, T.N.; Sanyal, R.; Sanyal, A. Fabrication of poly(ethylene glycol)-based cyclodextrin containing hydrogels via thiol-ene click reaction. Eur. Polym. J. 2015, 62, 426–434. [Google Scholar] [CrossRef]
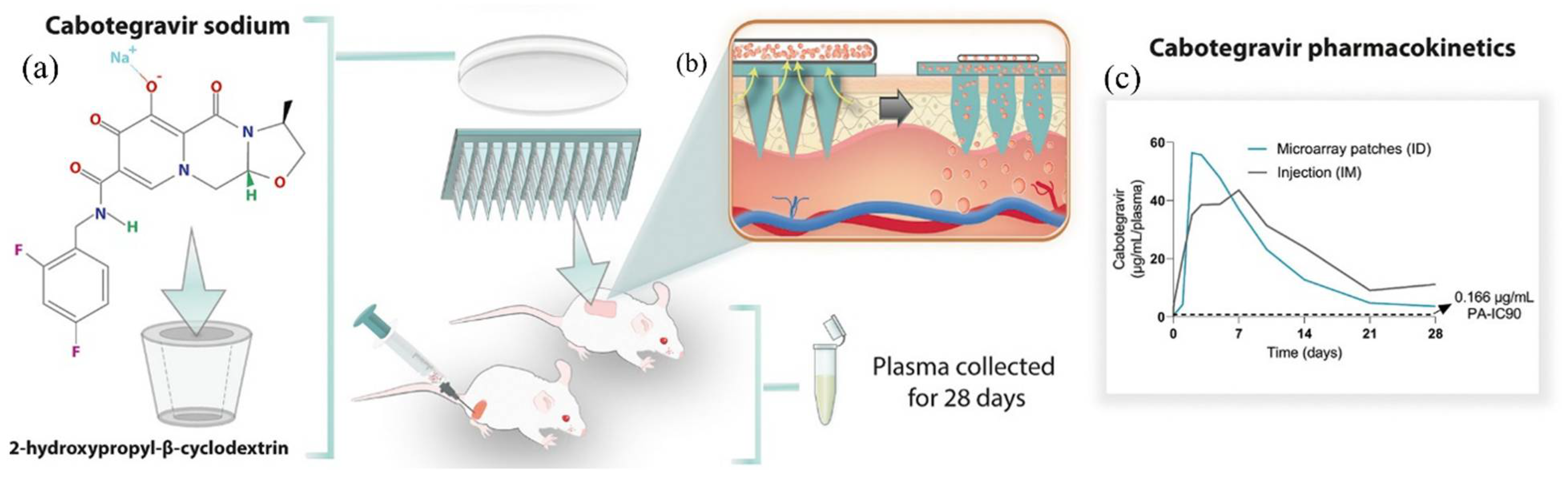
- Volpe-Zanutto, F.; Vora, L.K.; Tekko, I.A.; McKenna, P.E.; Permana, A.D.; Sabri, A.H.; Anjani, Q.K.; McCarthy, H.O.; Paredes, A.J.; Donnelly, R.F. Hydrogel-forming microarray patches with cyclodextrin drug reservoirs for long-acting delivery of poorly soluble cabotegravir sodium for HIV Pre-Exposure Prophylaxis. J. Control. Release 2022, 348, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Balakrishnan, P.; Park, E.K.; Song, K.W.; Hong, S.S.; Jang, T.Y.; Kim, K.S.; Chung, S.J.; Shim, C.K.; Kim, D.D. Poloxamer/cyclodextrin/chitosan-based thermoreversible gel for intranasal delivery of fexofenadine hydrochloride. J. Pharm. Sci. 2011, 100, 681–691. [Google Scholar] [CrossRef]
- Mahajan, H.S.; Shah, S.K.; Surana, S.J. Nasal in situ gel containing hydroxy propyl β-cyclodextrin inclusion complex of artemether: Development and in vitro evaluation. J. Incl. Phenom. Macrocycl. Chem. 2011, 70, 49–58. [Google Scholar] [CrossRef]
- Fang, G.; Wang, Q.; Yang, X.; Qian, Y.; Zhang, G.; Tang, B. γ-Cyclodextrin-based polypseudorotaxane hydrogels for ophthalmic delivery of flurbiprofen to treat anterior uveitis. Carbohydr. Polym. 2022, 277, 118889. [Google Scholar] [CrossRef] [PubMed]
- Pushpalatha, R.; Selvamuthukumar, S.; Kilimozhi, D. Cyclodextrin nanosponge based hydrogel for the transdermal co-delivery of curcumin and resveratrol: Development, optimization, in vitro and ex vivo evaluation. J. Drug Deliv. Sci. Technol. 2019, 52, 55–64. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, K.K.; Rao, R. Enhanced anti-psoriatic efficacy and regulation of oxidative stress of a novel topical babchi oil (Psoralea corylifolia) cyclodextrin-based nanogel in a mouse tail model. J. Microencapsul. 2019, 36, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Y.; Chen, S.; Cheong, K.L.; Teng, B. Carboxymethyl beta-cyclodextrin grafted carboxymethyl chitosan hydrogel-based microparticles for oral insulin delivery. Carbohydr. Polym. 2020, 246, 116617. [Google Scholar] [CrossRef]
- Lopedota, A.; Cutrignelli, A.; Denora, N.; Laquintana, V.; Lopalco, A.; Selva, S.; Ragni, L.; Tongiani, S.; Franco, M. New ethanol and propylene glycol free gel formulations containing a minoxidil-methyl-beta-cyclodextrin complex as promising tools for alopecia treatment. Drug Dev. Ind. Pharm. 2015, 41, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Iohara, D.; Okubo, M.; Anraku, M.; Uramatsu, S.; Shimamoto, T.; Uekama, K.; Hirayama, F. Hydrophobically Modified Polymer/alpha-Cyclodextrin Thermoresponsive Hydrogels for Use in Ocular Drug Delivery. Mol. Pharm. 2017, 14, 2740–2748. [Google Scholar] [CrossRef]
- Badr-El, S.M.; Al, H.M.; Kotta, S.; Abdulhafiz, N. Self-Assembled Supramolecular Hydrogel Based on α-Cyclodextrin/Poloxamer Polypseudorotaxanes for Ocular Delivery of Ciprofloxacin. Int. J. Pharmacol. 2021, 17, 15–27. [Google Scholar] [CrossRef]
- Choi, S.G.; Lee, S.E.; Kang, B.S.; Ng, C.L.; Davaa, E.; Park, J.S. Thermosensitive and mucoadhesive sol-gel composites of paclitaxel/dimethyl-beta-cyclodextrin for buccal delivery. PLoS ONE 2014, 9, e109090. [Google Scholar] [CrossRef]
- Szalai, B.; Budai-Szucs, M.; Kovacs, A.; Berko, S.; Grof, I.; Deli, M.A.; Katona, G.; Balogh, G.T.; Jojart-Laczkovich, O. The effect of mucoadhesive polymers on ocular permeation of thermoresponsive in situ gel containing dexamethasone-cyclodextrin complex. Int. J. Pharm. 2024, 667, 124848. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Yu, C.; Zhu, W.; Zang, Z.; Guan, Y. Study of Hydroxypropyl beta-Cyclodextrin and Puerarin Inclusion Complexes Encapsulated in Sodium Alginate-Grafted 2-Acrylamido-2-Methyl-1-Propane Sulfonic Acid Hydrogels for Oral Controlled Drug Delivery. Gels 2023, 9, 24. [Google Scholar] [CrossRef]
- Sherje, A.P.; Londhe, V. Development and Evaluation of pH-Responsive Cyclodextrin-Based in situ Gel of Paliperidone for Intranasal Delivery. AAPS PharmSciTech 2018, 19, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Oktay, A.N.; Celebi, N.; Ilbasmis-Tamer, S.; Kaplanoğlu, G.T. Cyclodextrin-based nanogel of flurbiprofen for dermal application: In vitro studies and in vivo skin irritation evaluation. J. Drug Deliv. Sci. Technol. 2023, 79, 8. [Google Scholar] [CrossRef]
- Enggi, C.K.; Sulistiawati, S.; Himawan, A.; Raihan, M.; Iskandar, I.W.; Saputra, R.R.; Rahman, L.; Yulianty, R.; Manggau, M.A.; Donelly, R.F.; et al. Application of Biomaterials in the Development of Hydrogel-Forming Microneedles Integrated with a Cyclodextrin Drug Reservoir for Improved Pharmacokinetic Profiles of Telmisartan. ACS Biomater. Sci. Eng. 2024, 10, 1554–1576. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Luo, Z.; Baidya, A.; Kim, H.J.; Wang, C.; Jiang, X.; Qu, M.; Zhu, J.; Ren, L.; Vajhadin, F.; et al. Biodegradable beta-Cyclodextrin Conjugated Gelatin Methacryloyl Microneedle for Delivery of Water-Insoluble Drug. Adv. Healthc. Mater. 2020, 9, e2000527. [Google Scholar] [CrossRef] [PubMed]
- Klaewklod, A.; Tantishaiyakul, V.; Hirun, N.; Sangfai, T.; Li, L. Characterization of supramolecular gels based on beta-cyclodextrin and polyethyleneglycol and their potential use for topical drug delivery. Mater. Sci. Eng. C-Mater. Biol. Appl. 2015, 50, 242–250. [Google Scholar] [CrossRef]
- Badshah, S.F.; Minhas, M.U.; Khan, K.U.; Barkat, K.; Abdullah, O.; Munir, A.; Suhail, M.; Malik, N.S.; Jan, N.; Chopra, H. Structural and in-vitro characterization of highly swellable β-cyclodextrin polymeric nanogels fabricated by free radical polymerization for solubility enhancement of rosuvastatin. Part. Sci. Technol. 2023, 41, 1131–1145. [Google Scholar] [CrossRef]
- Mahmood, A.; Ahmad, M.; Sarfraz, R.M.; Minhas, M.U. Development of Acyclovir Loaded-Cyclodextrin-g-Poly Methacrylic Acid Hydrogel Microparticles: An In Vitro Characterization. Adv. Polym. Technol. 2018, 37, 9. [Google Scholar] [CrossRef]
- Shalaby, E.S.; Shalaby, S.I.; AbouTaleb, S. Cyclodextrin nano-organogels as a delivery vehicle for peppermint essential oil to enhance its physico-chemical properties and skin photoprotective performance. J. Dispers. Sci. Technol. 2024, 1–13. [Google Scholar] [CrossRef]
- Catenacci, L.; Sorrenti, M.; Perteghella, S.; Mandracchia, D.; Torre, M.L.; Trapani, A.; Milanese, C.; Tripodo, G. Combination of inulin and beta-cyclodextrin properties for colon delivery of hydrophobic drugs. Int. J. Pharm. 2020, 589, 119861. [Google Scholar] [CrossRef] [PubMed]
- Kashapov, R.R.; Lykova, A.A.; Mamedova, V.L.; Kadyrova, S.F.; Sapunova, A.S.; Voloshina, A.D.; Mamedov, V.A.; Zakharova, L.Y. Solubility and biological activity enhancement of the highly lipophilic viridicatins via interaction with cyclodextrins. J. Drug Deliv. Sci. Technol. 2020, 59, 6. [Google Scholar] [CrossRef]
- Nasongkla, N.; Wiedmann, A.F.; Bruening, A.; Beman, M.; Ray, D.; Bornmann, W.G.; Boothman, D.A.; Gao, J. Enhancement of solubility and bioavailability of beta-lapachone using cyclodextrin inclusion complexes. Pharm. Res. 2003, 20, 1626–1633. [Google Scholar] [CrossRef]
- Pan, H.W.; Guo, J.; Zhu, L.; Leung, S.W.S.; Zhang, C.; Lam, J.K.W. Enhanced powder dispersion of dual-excipient spray-dried powder formulations of a monoclonal antibody and its fragment for local treatment of severe asthma. Int. J. Pharm. 2023, 644, 123272. [Google Scholar] [CrossRef] [PubMed]
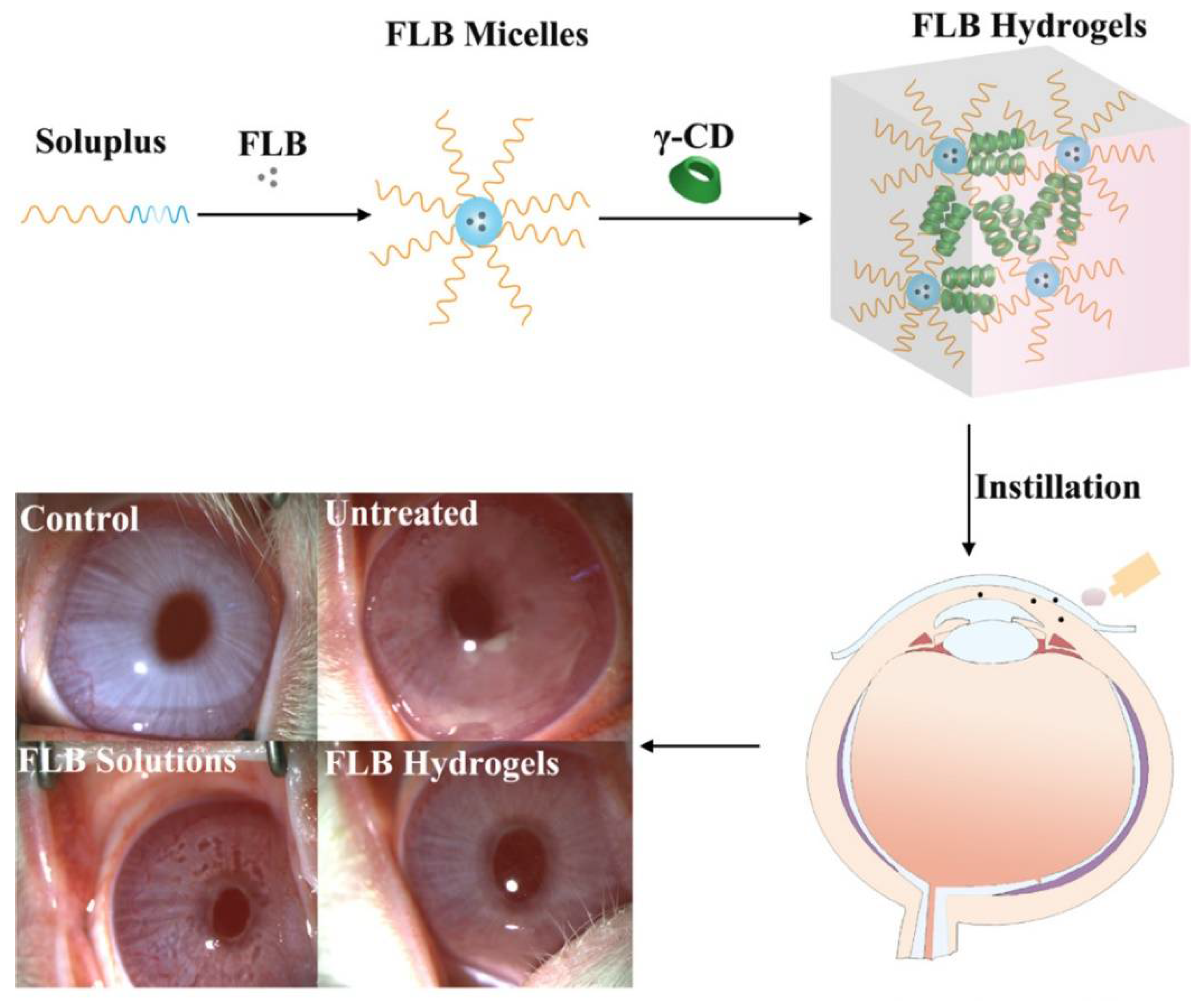

| Type of Cyclodextrin | Key Features and Applications | Key Formulation Characteristics | References |
|---|---|---|---|
| Beta-cyclodextrin (β-CD) | Most widely used; enhances drug solubility, stability, and controlled release; applied in wound healing, transdermal, and oral gels. | Forms strong inclusion complexes with hydrophobic drugs, improving solubility and stability. | [1,5,13,14,20,24,44,47,53,54,55,56,57,58,59,60] |
| Hydroxypropyl-beta-cyclodextrin (HP-β-CD) | Improves drug permeation, bioavailability, and mucoadhesion; widely used in thermosensitive, vaginal, ophthalmic, and nasal gels. | Provides higher aqueous solubility than β-CD, making it ideal for mucoadhesive and injectable hydrogels. | [3,4,15,19,22,23,25,28,31,38,43,45,61,62,63] |
| Gamma-cyclodextrin (γ-CD) | Effective in ophthalmic, nanogel, and sustained drug release formulations; improves solubility and stability for poorly soluble drugs. | Has a larger cavity size, allowing encapsulation of larger hydrophobic molecules. | [17,34,48,49,64] |
| Cyclodextrin nanosponge (CDNS) | Advanced crosslinked structures provide extended drug release, high encapsulation efficiency, and improved drug stability. | Forms nanostructured polymeric networks, allowing higher drug loading capacity. | [16,65,66] |
| Sulfobutyl ether-beta-cyclodextrin (SBECD) | Enhances solubility and stability in antifungal, ophthalmic, and systemic drug delivery applications. | Provides high aqueous solubility and is often used in parenteral formulations. | [7] |
| Carboxymethyl-beta-cyclodextrin (CMCD) | Used in pH-responsive oral and transdermal hydrogels; supports controlled drug release for antibacterial and protein therapies. | Forms pH-sensitive hydrogels, enabling controlled and site-specific drug release. | [51,67] |
| Methyl-beta-cyclodextrin (Mβ-CD) | Applied in nasal, topical, and transdermal drug formulations to enhance drug absorption. | Improves membrane permeability, making it effective for nasal and dermal applications. | [33,68] |
| Hydroxypropyl-gamma-cyclodextrin (HPγ-CD) | Primarily used in ophthalmic and vaginal hydrogels; increases solubility and drug bioavailability. | Similar to HP-β-CD, but with a larger cavity size, accommodating bigger molecules. | [32,48] |
| Alpha-cyclodextrin (α-CD) | Applied in supramolecular poloxamer-based hydrogels for ocular drug delivery and controlled release. | Has the smallest cavity size, making it suitable for small-molecule drug encapsulation. | [69,70] |
| Modified cyclodextrins (Captisol, RAMEB, etc.) | Specialized derivatives tailored for targeted drug release, solubility enhancement, and bioavailability improvement. | Designed for high-affinity drug binding and customized inclusion complexes. | [26,52,71] |
| Type of Hydrogel Polymer | Key Features and Applications | Key Formulation Characteristics | References |
|---|---|---|---|
| Poloxamer 407/Pluronic F127 | Most commonly used for thermosensitive hydrogels; provides mucoadhesion, in situ gelation, and enhanced drug release. | Gel forms liquid at room temperature and solidifies at body temperature. | [2,6,19,24,26,28,35,43,44,55,62,70,71,72] |
| Carbopol | Widely applied in mucoadhesive formulations; enables controlled release in ophthalmic, transdermal, and vaginal gels. | Forms highly viscous gels that enhance bioadhesion. | [2,4,13,16,35,43,50,55,66,68,74] |
| Hydroxypropyl methylcellulose (HPMC) | Enhances viscosity, gel strength, and bioadhesion; commonly used in ophthalmic, vaginal, and buccal applications. | Provides sustained drug release; often combined with thermogelling agents. | [6,19,23,25,33,35,63,69,72,74,75] |
| Chitosan | Improves mucoadhesion, controlled drug release, and bioavailability; frequently used in nasal, ocular, and oral delivery. | Forms pH-sensitive gels that dissolve at acidic pH. | [19,20,21,26,29,30,51,54,58,62,67] |
| Polyvinyl alcohol (PVA) | Provides biocompatibility, mechanical strength, and controlled release properties; used in ophthalmic and transdermal hydrogels. | Forms elastic, mechanically strong films for prolonged drug release. | [37,45,76] |
| Gelatin | Used for biodegradable and injectable formulations; applied in microneedles and self-healing hydrogels. | Crosslinked gelatin forms hydrogels that degrade in vivo. | [21,36,50,77] |
| CD-based nanosponges | Enhances drug stability and sustained release; often incorporated in topical and transdermal gels. | Forms crosslinked networks for extended drug entrapment. | [16,17,65] |
| Polyethylene glycol (PEG) | Applied in crosslinked hydrogels for extended drug release and controlled solubility. | Often chemically crosslinked to enhance stability and swelling. | [30,71,78] |
| Hydroxyethyl methacrylate (HEMA) | Used in contact lens drug delivery and ophthalmic formulations. | Provides high water content and oxygen permeability for ocular applications. | [29,39,57] |
| Methylcellulose (MC) | Provides temperature-sensitive properties; applied in ophthalmic and transdermal hydrogels. | Forms reversible thermogels that transition at physiological temperatures. | [31] |
| Process or Technique | Key Features and Applications | Common Cyclodextrins Used | Key Technical Data | References |
|---|---|---|---|---|
| Kneading | Frequently used for preparing drug–CD inclusion complexes; enhances solubility and bioavailability. | Beta-cyclodextrin, hydroxypropyl-beta-cyclodextrin | Drug–CD molar ratio of 1:1 to 1:4; kneading with minimal solvent (ethanol, water, or acetone); results in solid inclusion complex | [1,13,75] |
| Solvent evaporation | Produces stable inclusion complexes by dissolving CD and drug in a common solvent, followed by solvent removal. | Beta-cyclodextrin, sulfobutyl ether-beta-cyclodextrin | Solvent selection: ethanol, methanol, or acetone; evaporation temperature: 40–60 °C; complexation verified by FTIR, DSC, and PXRD | [48,53,75] |
| Freeze-drying (lyophilization) | Used for enhancing drug stability and solubility; commonly applied in ophthalmic and injectable hydrogels. | Hydroxypropyl-beta-cyclodextrin, methyl-beta-cyclodextrin | Lyophilization cycle: −50 °C to 25 °C; drug–CD complex dissolved in aqueous medium and frozen before sublimation | [33,68] |
| Spray-drying | Produces fine powders of drug–CD complexes, increasing dissolution and bioavailability. | Hydroxypropyl-beta-cyclodextrin, hydroxypropyl-gamma-cyclodextrin | Inlet temperature: 80–120 °C; solvent system: water–alcohol; particle size: 200–500 nm | [6] |
| Nanogel formation (free radical polymerization) | Used for crosslinking CD with polymeric networks, forming highly swellable drug-loaded nanogels. | Beta-cyclodextrin, cyclodextrin nanosponge | Crosslinkers: MBA, AMPS, and NVP; polymerization initiated by AIBN, KPS, and UV-radiation; particle size: 50–400 nm | [14,18,54,79] |
| Emulsification–solvent Evaporation | Applied for nano- and microgel preparation, allowing sustained drug release. | Gamma-cyclodextrin, hydroxypropyl-gamma-cyclodextrin | Emulsifier: PVA, Tween 80; droplet size control: ultrasonication; particle size: 100–500 nm | [48,75] |
| Crosslinking (chemical and physical methods) | Creates stable hydrogel matrices by polymerizing CD derivatives with crosslinkers. | Beta-cyclodextrin, carboxymethyl-beta-cyclodextrin | Chemical crosslinkers: glutaraldehyde, EPI, carbodiimide; physical methods: UV curing, freeze–thawing | [5,13,14,16,27,42,49,65] |
| pH-induced gelation | Utilized for forming pH-sensitive hydrogels that respond to acidic or basic environments. | Beta-cyclodextrin, hydroxypropyl-beta-cyclodextrin | pH-triggered drug release: swelling at pH 5–7; transition from sol to gel state at specific pH | [4,21,54,74] |
| Thermoresponsive gelation | Forms temperature-sensitive hydrogels that transition between liquid and gel states at physiological temperatures. | Hydroxypropyl-beta-cyclodextrin, randomly methylated beta-cyclodextrin | Gelation temperature: 28–37 °C; polymer systems: Pluronic, poloxamer, HPMC, PEO | [40,41,69,72] |
| Microneedle array technology | Used for transdermal drug delivery, allowing controlled drug release via microneedle patches. | Beta-cyclodextrin, hydroxypropyl-beta-cyclodextrin | Microneedle height: 300–600 µm; drug loading efficiency: 50–90%; patch swelling for controlled release | [42,76,77] |
| Key Factor | Effect on Drug Release | Common Cyclodextrins Involved | Key Technical Considerations | References |
|---|---|---|---|---|
| Cyclodextrin type and inclusion complex formation | Controls drug solubility and bioavailability, impacts release kinetics. | Beta-cyclodextrin, hydroxypropyl-beta-cyclodextrin, sulfobutyl ether-beta-cyclodextrin | Inclusion complex with molar ratio of 1:1 or higher, confirmed via FTIR, DSC, and PXRD | [1,3,4,7,13,16,19,22,43,61,68] |
| Hydrogel polymer composition and crosslinking density | Determines matrix porosity and drug diffusion rate, influences swelling capacity. | Beta-cyclodextrin-grafted polymers, carboxymethyl-beta-cyclodextrin, cyclodextrin nanosponges | Crosslinker concentration (0.1–2%) impacts gel stiffness, higher crosslinking → slower release | [27,40,51,65,77,80] |
| pH sensitivity of hydrogel | Regulates drug release in response to pH changes; important for oral and vaginal delivery. | Beta-cyclodextrin, hydroxypropyl-beta-cyclodextrin | pH-responsive swelling at pH 5–7 enhances release in physiological conditions | [4,21,80] |
| Temperature-sensitive gelation | Triggers drug release at physiological temperatures; suitable for ocular and transdermal applications. | Hydroxypropyl-beta-cyclodextrin, randomly methylated beta-cyclodextrin | Gelation occurs at 28–37 °C, modulated by Pluronic, poloxamer, and HPMC | [6,19,24,26,43] |
| Swelling and degradation of hydrogel matrix | Controls sustained release by gradual polymer erosion and swelling. | Cyclodextrin nanosponge, hydroxypropyl-gamma-cyclodextrin | Degradation time: hours to weeks, depending on hydrogel type | [14,20,23,42,56,73,79] |
| Drug hydrophobicity and molecular weight | Influences inclusion complex stability and diffusion rate in hydrogel. | Beta-cyclodextrin, hydroxypropyl-beta-cyclodextrin | Smaller hydrophobic drugs release faster, large molecules require hydrogel modifications | [20,40,60,79] |
| Ionic strength and osmotic effects | Affects gel structure and drug diffusion rate, particularly for mucoadhesive and ophthalmic gels. | Hydroxypropyl-beta-cyclodextrin, carboxymethyl-beta-cyclodextrin | High ionic strength delays release, osmotic balance crucial for in vivo stability | [30,32,58,78] |
| Microneedle patch swelling and penetration | Enhances transdermal drug diffusion, providing controlled long-term release. | Beta-cyclodextrin, hydroxypropyl-beta-cyclodextrin | Swelling time: 1–5 min, mechanical properties affect skin penetration | [42,76,77] |
| Emulsification and nanoparticle size | Smaller particle sizes enable faster diffusion, improving ocular and topical bioavailability. | Gamma-cyclodextrin, hydroxypropyl-gamma-cyclodextrin | Particle size: 100–500 nm enhances diffusion, emulsifiers stabilize nanogels | [8,19,48,75] |
| Release modulation via iontophoresis and electrostimulation | Enhances transdermal drug permeation, accelerates release in low-permeability drugs. | Hydroxypropyl-beta-cyclodextrin, beta-cyclodextrin | Electrical field: 0.1–1.0 mA/cm2 increases drug flux, used in pain management gels | [15,32,47,59] |
| Drug or Therapeutic Agent | Examples of Drugs Used | Cyclodextrin (CD) Types Used | Key Features and Applications | References |
|---|---|---|---|---|
| Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) | Ibuprofen, flurbiprofen, piroxicam | Beta-cyclodextrin, gamma-cyclodextrin, hydroxypropyl-beta-cyclodextrin | Used in topical, ophthalmic, and transdermal gels for pain relief and inflammation | [1,5,15,44,59,64,75,78] |
| Antifungal agents | Fluconazole, voriconazole, ketoconazole, clotrimazole, econazole | Sulfobutyl ether-beta-cyclodextrin, hydroxypropyl-beta-cyclodextrin, beta-cyclodextrin | Applied in mucoadhesive, thermosensitive, and ion-sensitive gels for vaginal, ocular, and systemic fungal infections | [2,3,6,7,19,50,53] |
| Ophthalmic drugs | Ciprofloxacin, dexamethasone, azithromycin, flurbiprofen, mitomycin | Alpha-cyclodextrin, hydroxypropyl-gamma-cyclodextrin, hydroxypropyl-beta-cyclodextrin | In situ gelling hydrogels used for sustained ocular release and improved bioavailability | [4,22,48,49,64,70,72] |
| Anticancer drugs | Curcumin, paclitaxel, 5-fluorouracil | Cyclodextrin nanosponge, randomly methylated beta-cyclodextrin, hydroxypropyl-beta-cyclodextrin | Applied in pH-sensitive, biodegradable, and thermoresponsive hydrogels for targeted chemotherapy | [17,21,27,35,56,65,71,77] |
| Antibiotics | Vancomycin, ciprofloxacin, azithromycin | Hydroxypropyl-beta-cyclodextrin, alpha-cyclodextrin, beta-cyclodextrin | Used in mucoadhesive and sustained-release hydrogels for periodontal, bone, and systemic infections | [22,43,46] |
| Antiviral drugs | Acyclovir, valacyclovir, cabotegravir | Beta-cyclodextrin grafted polymer, gamma-cyclodextrin, hydroxypropyl-beta-cyclodextrin | Studied in pH-responsive and transdermal hydrogels for herpes, HIV, and viral infections | [34,54,61,80] |
| Antipsychotic and neurological drugs | Clonazepam, asenapine, risperidone, armodafinil, paliperidone | Randomly methylated beta-cyclodextrin, hydroxypropyl-beta-cyclodextrin, hydroxypropyl-gamma-cyclodextrin | Used in nasal, buccal, and transdermal delivery for schizophrenia, epilepsy, and cognitive disorders | [26,28,31,45,74] |
| Cardiovascular and cholesterol-lowering Agents | Rosuvastatin, lovastatin, telmisartan | Beta-cyclodextrin polymeric nanogel, beta-cyclodextrin grafted hydrogel, beta-cyclodextrin microneedles | Applied in nanogel-based and mucoadhesive hydrogels for cholesterol reduction and blood pressure control | [14,76,79] |
| Hormone replacement and endocrine therapy | Insulin, estradiol, dehydroepiandrosterone | Methyl-beta-cyclodextrin, methyl-beta-cyclodextrin, hydroxypropyl-gamma-cyclodextrin | Used in vaginal and nasal gels for menopausal hormone therapy and insulin delivery | [30,33,38,67] |
| Antioxidants and natural therapeutics | Curcumin, rutin, resveratrol, gallic acid, babchi oil (Psoralea corylifolia), polyglycerol, peppermint | Cyclodextrin nanosponge, hydroxypropyl-beta-cyclodextrin, cyclodextrin nanosponge | Studied in topical and systemic hydrogels to enhance bioavailability and therapeutic efficacy | [20,27,40,65,66,81] |
| Category | Key Observations and Insights | References |
|---|---|---|
| Synergistic cyclodextrin use | - Co-utilization of multiple cyclodextrin derivatives (e.g., HP-β-CD and γ-CD) enhances solubility, bioavailability, and retention in formulations for voriconazole, curcumin, dehydroepiandrosterone, and dexamethasone. | [6,17,32,38] |
| - Sulfobutyl ether-beta-cyclodextrin (SBE-β-CD) reduces ocular irritation while improving drug permeation and retention in ion-sensitive in situ gels. | [7] | |
| - Gamma-CD metal–organic frameworks (γ-CD-MOFs) increase stability, photostability, and targeted hydrophobic drug delivery (e.g., curcumin, anticancer agents). | [17,27] | |
| Hybrid polymers and smart architectures | - Hybrid formulations combining natural (e.g., chitosan) and synthetic polymers (e.g., Pluronic F127, PEG) improve gelation, mechanical strength, and sustained release kinetics. | [6,27,43,51] |
| - Multi-responsive hydrogels (pH- and temperature-sensitive) facilitate multi-drug delivery, particularly in oncology and gastrointestinal treatments. | [14,22,43,56,67] | |
| Next-generation drug release control | - Stimuli-responsive hydrogels allow spatiotemporal drug release control, crucial for targeted cancer therapies and mucosal drug delivery. | [43,56,67,80] |
| - Ion-sensitive hydrogels enable precise ocular drug release, leveraging ionic fluctuations for on-demand administration. | [3] | |
| Advanced encapsulation strategies | - Cyclodextrin-based nanosponges and nanogels enhance stability, bioadhesion, and sustained release (e.g., curcumin nanogels). | [17,27,65] |
| - Gamma-CD metal–organic frameworks (MOFs) improve photostability and targeted drug localization, ideal for topical and anticancer applications. | [17,80] | |
| Multifunctional and bioactive properties | - Cyclodextrin hydrogels offer dual antimicrobial and antioxidant properties, suitable for wound healing, tissue repair, and infection prevention. | [20,51,53,66] |
| - Co-delivery of synergistic actives (e.g., curcumin and resveratrol) enhances cytotoxic efficacy and tissue penetration. | [27,65] | |
| Enhanced patient compliance and non-invasive therapies | - Microneedle-integrated hydrogels enable painless, transdermal drug delivery (e.g., sildenafil citrate for erectile dysfunction and curcumin for treating melanoma). | [42,77] |
| - Sustained-release formulations (7+ day drug profiles) improve compliance for chronic diseases (e.g., diabetes, schizophrenia). | [45,56,59,67] | |
| Scalability and sustainable production | - Spray-drying, freeze-drying, and emulsification techniques facilitate scalable hydrogel production with high reproducibility. | [6,17,33,68] |
| - Eco-friendly hydrogel development: green chemistry approaches, solvent-free processing, and sustainable crosslinkers align with regulatory trends. | [5,21,51] | |
| Regenerative medicine and tissue engineering | - Cyclodextrin hydrogels serve as bioactive scaffolds for wound healing, tissue regeneration, and drug-eluting implants. | [13,21,77] |
| - Integration of growth factors, peptides, and antimicrobial agents enhances regenerative capacity, particularly for inflammatory skin disorders, burns, chronic wounds, and post-surgical tumoral recovery. | [13,21,66] | |
| Comparative performance vs. commercial formulations | - Cyclodextrin-based hydrogels consistently outperform commercial counterparts, exhibiting superior drug solubility, permeability, and bioactivity. | [5,32,41,53] |
| - Example: fluconazole hydrogels demonrate larger antimicrobial inhibition zones (30 mm vs. 24 mm) compared to commercial antifungal formulations. | [19,53] | |
| Emerging frontiers in drug delivery | - Cutting-edge applications include gene therapy, HIV prevention, and neurodegenerative disease treatment (e.g., cabotegravir-loaded hydrogels for HIV prophylaxis). | [33,61,63] |
| - Microneedle-assisted cyclodextrin systems are being explored for targeted dermatological treatments, including melanoma therapy (curcumin patches). | [77] | |
| Optimized drug delivery for cancer | - Injectable hydrogels, pH-sensitive carriers, and CD-MOFs are transforming tumor-targeted therapy, reducing systemic toxicity while enhancing localized drug delivery. | [21,56] |
| - Combination therapies in hydrogel matrices (e.g., chemotherapeutics + antioxidants) significantly improve therapeutic outcomes. | [17,65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omidian, H.; Akhzarmehr, A.; Gill, E.J. Cyclodextrin–Hydrogel Hybrids in Advanced Drug Delivery. Gels 2025, 11, 177. https://doi.org/10.3390/gels11030177
Omidian H, Akhzarmehr A, Gill EJ. Cyclodextrin–Hydrogel Hybrids in Advanced Drug Delivery. Gels. 2025; 11(3):177. https://doi.org/10.3390/gels11030177
Chicago/Turabian StyleOmidian, Hossein, Arnavaz Akhzarmehr, and Erma J. Gill. 2025. "Cyclodextrin–Hydrogel Hybrids in Advanced Drug Delivery" Gels 11, no. 3: 177. https://doi.org/10.3390/gels11030177
APA StyleOmidian, H., Akhzarmehr, A., & Gill, E. J. (2025). Cyclodextrin–Hydrogel Hybrids in Advanced Drug Delivery. Gels, 11(3), 177. https://doi.org/10.3390/gels11030177







