Hybrid Systems of Gels and Nanoparticles for Cancer Therapy: Advances in Multifunctional Therapeutic Platforms
Abstract
1. Introduction
2. Gels and Nanoparticle Hybridization for Cancer Therapy
2.1. Enhanced Tumor Retention and Localized Drug Administration
2.2. Improvement of Stability and Biocompatibility of Nanoparticles
2.3. Controlled Drug Release
2.4. Active Cancer Targeting
2.5. Role of Nanoparticle Type in Hybrid Gel–Nanoparticle Systems
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cariolou, M.; Abar, L.; Aune, D.; Balducci, K.; Becerra-Tomas, N.; Greenwood, D.C.; Markozannes, G.; Nanu, N.; Vieira, R.; Giovannucci, E.L.; et al. Postdiagnosis recreational physical activity and breast cancer prognosis: Global Cancer Update Programme (CUP Global) systematic literature review and meta-analysis. Int. J. Cancer 2023, 152, 600–615. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Gao, P.; Wang, J.; Fang, Y.; Hwang, K.C. Advances of medical nanorobots for future cancer treatments. J. Hematol. Oncol. 2023, 16, 74. [Google Scholar] [CrossRef]
- Nikolaos, T. Obesity and Lung Cancer (Investigating the Relationship). EPRA Int. J. Multidiscip. Res. (IJMR) 2023, 9, 175–177. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.Z.; Jiang, Y.L.; Yang, B.; Long, F.X.; Yang, Z.; Tang, D.X. Traditional Chinese medicines and capecitabine-based chemotherapy for colorectal cancer treatment: A meta-analysis. Cancer Med. 2023, 12, 236–255. [Google Scholar] [CrossRef]
- Jin, E. Flexible nursing in patients with lung cancer who received chemotherapy. J. Cancer Res. Clin. Oncol. 2023, 149, 9959–9963. [Google Scholar] [CrossRef]
- Yan, M.; Wu, S.; Wang, Y.; Liang, M.; Wang, M.; Hu, W.; Yu, G.; Mao, Z.; Huang, F.; Zhou, J. Recent Progress of Supramolecular Chemotherapy Based on Host-Guest Interactions. Adv. Mater. 2024, 36, e2304249. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Shiranthika, C.; Wang, C.Y.; Chen, K.W.; Sumathipala, S. Reinforcement learning strategies in cancer chemotherapy treatments: A review. Comput. Methods Programs Biomed. 2023, 229, 107280. [Google Scholar] [CrossRef]
- Abodunrin, T.O.; Egharevba, G.s.O.; Owoeye, F.D.; Johnson, O.A.; Adeyemi, O.S. Metal Organic Frameworks in Photodynamic Therapy of Cancer: Functional Roles of Active Metal Centers, Integrated and Loaded Photosensitizers in the Framework. Mater. Adv. 2025. ahead of print. [Google Scholar] [CrossRef]
- Papa, V.; Furci, F.; Minciullo, P.L.; Casciaro, M.; Allegra, A.; Gangemi, S. Photodynamic Therapy in Cancer: Insights into Cellular and Molecular Pathways. Curr. Issues Mol. Biol. 2025, 47, 69. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xie, Y.; Zhang, J.; Li, Y.; Fan, Y.; Wang, H.; Wang, H.; Shen, Y.; Wang, K.; Teng, M. PEGylate engineering for preparing water-soluble deep/near-infrared red type I/II photosensitizer and its efficient photodynamic therapy of cancer cells. J. Mol. Struct. 2025, 1321, 139956. [Google Scholar] [CrossRef]
- Lee, K.W.; Wan, Y.; Huang, Z.; Zhao, Q.; Li, S.; Lee, C.S. Organic Optoelectronic Materials: A Rising Star of Bioimaging and Phototherapy. Adv. Mater. 2024, 36, e2306492. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Fontana, F.; Tapeinos, C.; Shahbazi, M.A.; Han, H.; Santos, H.A. Nanoparticles-based phototherapy systems for cancer treatment: Current status and clinical potential. Bioact. Mater. 2023, 23, 471–507. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Nie, W.; Dai, L.; Luo, R.; Lin, D.; Zhang, M.; Zhang, J.; Gao, F. Recent advances in natural polysaccharides-based controlled release nanosystems for anti-cancer phototherapy. Carbohydr. Polym. 2023, 301, 120311. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, S.H.; Freeman, G.J.; Dranoff, G.; Sharpe, A.H. Coinhibitory Pathways in Immunotherapy for Cancer. Annu. Rev. Immunol. 2016, 34, 539–573. [Google Scholar] [CrossRef]
- Borghaei, H.; Smith, M.R.; Campbell, K.S. Immunotherapy of cancer. Eur. J. Pharmacol. 2009, 625, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, J.M.; Butterfield, L.H.; Tarhini, A.A.; Zarour, H.; Kalinski, P.; Ferrone, S. Immunotherapy of cancer in 2012. CA Cancer J. Clin. 2012, 62, 309–335. [Google Scholar] [CrossRef]
- Franzese, C.; Stefanini, S.; Scorsetti, M. Radiation Therapy in the Management of Adrenal Metastases. Semin. Radiat. Oncol. 2023, 33, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Wedler, V.; Stiegler, L.M.S.; Gandziarowski, T.; Walter, J.; Peukert, W.; Distel, L.V.R.; Hirsch, A.; Klein, S. Shell-by-Shell functionalized nanoparticles as radiosensitizers and radioprotectors in radiation therapy of cancer cells and tumor spheroids. Colloids Surf. B Biointerfaces 2025, 245, 114276. [Google Scholar] [CrossRef]
- Yun, W.G.; Chae, Y.S.; Han, Y.; Jung, H.S.; Cho, Y.J.; Kang, H.C.; Kwon, W.; Park, J.S.; Chie, E.K.; Jang, J.Y. Efficacy of Neoadjuvant Radiotherapy After Chemotherapy and the Optimal Interval from Radiotherapy to Surgery for Borderline Resectable and Resectable Pancreatic Cancer. Ann. Surg. Oncol. 2025, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zou, Y.; Huang, L. Nano Delivery of Chemotherapeutic ICD Inducers for Tumor Immunotherapy. Small Methods 2023, 7, e2201307. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Q.; Xia, G.; Adilijiang, N.; Li, Y.; Hou, Z.; Fan, Z.; Li, J. Recent Advances in Targeted Drug Delivery Strategy for Enhancing Oncotherapy. Pharmaceutics 2023, 15, 2233. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Lin, S.; Wu, F.; Liu, J. Nanomaterial-based ophthalmic drug delivery. Adv. Drug Deliv. Rev. 2023, 200, 115004. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yu, Y.; Lin, R.; Liu, M.; Zhou, Q.; Liu, M.; Chen, L.; Wang, W.; Elzatahry, A.A.; Zhao, D.; et al. Camouflaged Virus-Like-Nanocarrier with a Transformable Rough Surface for Boosting Drug Delivery. Angew. Chem. Int. Ed. Engl. 2023, 62, e202216188. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, Y.; Zeng, H.; Wang, C.; Xu, C.; Wang, Q.; Wang, H.; Li, S.; Chen, J.; Xiao, C.; et al. Mechano-boosting nanomedicine antitumour efficacy by blocking the reticuloendothelial system with stiff nanogels. Nat. Commun. 2023, 14, 1437. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Jo, M.C.; Jeong, S.; Palanikumar, L.; Rotello, V.M.; Ryu, J.H.; Park, M.H. Externally controlled drug release using a gold nanorod contained composite membrane. Nanoscale 2016, 8, 11949–11955. [Google Scholar] [CrossRef]
- Di, L. Quantitative Translation of Substrate Intrinsic Clearance from Recombinant CYP1A1 to Humans. AAPS J. 2023, 25, 98. [Google Scholar] [CrossRef] [PubMed]
- Deyhim, T.; Cheifetz, A.S.; Papamichael, K. Drug Clearance in Patients with Inflammatory Bowel Disease Treated with Biologics. J. Clin. Med. 2023, 12, 7132. [Google Scholar] [CrossRef]
- Clark, P.; Kim, J.; Aphinyanaphongs, Y. Marketing and US Food and Drug Administration Clearance of Artificial Intelligence and Machine Learning Enabled Software in and as Medical Devices: A Systematic Review. JAMA Netw. Open 2023, 6, e2321792. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Luo, C.; Pang, Z.; Zhang, J.; Ruan, S.; Wu, M.; Wang, L.; Sun, T.; Li, N.; Han, L.; et al. Advances of nanoparticles as drug delivery systems for disease diagnosis and treatment. Chin. Chem. Lett. 2023, 34, 107518. [Google Scholar] [CrossRef]
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The Smart Drug Delivery System and Its Clinical Potential. Theranostics 2016, 6, 1306–1323. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qin, D.; Zou, J.; Li, X.; Guo, X.D.; Tang, Y.; Liu, C.; Chen, W.; Kong, N.; Zhang, C.Y.; et al. Living Leukocyte-Based Drug Delivery Systems. Adv. Mater. 2023, 35, e2207787. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Kim, S.; Oh, J.Y.; Kim, D.; Jin, S.; Choi, E.; Ryu, J.H. Surface protein-retractive and redox-degradable mesoporous organosilica nanoparticles for enhanced cancer therapy. J. Colloid. Interface Sci. 2023, 649, 1014–1022. [Google Scholar] [CrossRef]
- Palanikumar, L.; Jeena, M.T.; Kim, K.; Yong Oh, J.; Kim, C.; Park, M.H.; Ryu, J.H. Spatiotemporally and Sequentially-Controlled Drug Release from Polymer Gatekeeper-Hollow Silica Nanoparticles. Sci. Rep. 2017, 7, 46540. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Shi, S.; Goel, S.; Shen, X.; Xie, X.; Chen, Z.; Zhang, H.; Li, S.; Qin, X.; Yang, H.; et al. Recent advancements in mesoporous silica nanoparticles towards therapeutic applications for cancer. Acta Biomater. 2019, 89, 1–13. [Google Scholar] [CrossRef]
- Yang, S.; Palanikumar, L.; Jeong, S.; Kim, K.; Lee, J.; Jeoung, E.; Kim, C.; Ryu, J.H.; Park, M.H. Synergistic Effect of Photothermal Therapy and Chemotherapy Using Camptothecin-Conjugated Gold Nanorods. Part. Part. Syst. Charact. 2017, 35, 1700307. [Google Scholar] [CrossRef]
- He, C.; Lu, J.; Lin, W. Hybrid nanoparticles for combination therapy of cancer. J. Control Release 2015, 219, 224–236. [Google Scholar] [CrossRef]
- Beik, J.; Khateri, M.; Khosravi, Z.; Kamrava, S.K.; Kooranifar, S.; Ghaznavi, H.; Shakeri-Zadeh, A. Gold nanoparticles in combinatorial cancer therapy strategies. Coord. Chem. Rev. 2019, 387, 299–324. [Google Scholar] [CrossRef]
- Zhao, M.X.; Zhu, B.J. The Research and Applications of Quantum Dots as Nano-Carriers for Targeted Drug Delivery and Cancer Therapy. Nanoscale Res. Lett. 2016, 11, 207. [Google Scholar] [CrossRef] [PubMed]
- Zavareh, H.S.; Pourmadadi, M.; Moradi, A.; Yazdian, F.; Omidi, M. Chitosan/carbon quantum dot/aptamer complex as a potential anticancer drug delivery system towards the release of 5-fluorouracil. Int. J. Biol. Macromol. 2020, 165, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Pan, X.; Zhang, D.; Wu, Q.; Peng, J.; Hai, W. The therapeutic efficacy of CdTe and CdSe quantum dots for photothermal cancer therapy. Biomaterials 2012, 33, 7071–7083. [Google Scholar] [CrossRef] [PubMed]
- Masood, F. Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 569–578. [Google Scholar] [CrossRef]
- Brewer, E.; Coleman, J.; Lowman, A. Emerging Technologies of Polymeric Nanoparticles in Cancer Drug Delivery. J. Nanomater. 2011, 2011, 1–10. [Google Scholar] [CrossRef]
- Wuttke, S.; Braig, S.; Preiss, T.; Zimpel, A.; Sicklinger, J.; Bellomo, C.; Radler, J.O.; Vollmar, A.M.; Bein, T. MOF nanoparticles coated by lipid bilayers and their uptake by cancer cells. Chem. Commun. (Camb) 2015, 51, 15752–15755. [Google Scholar] [CrossRef]
- Sun, X.; He, G.; Xiong, C.; Wang, C.; Lian, X.; Hu, L.; Li, Z.; Dalgarno, S.J.; Yang, Y.W.; Tian, J. One-Pot Fabrication of Hollow Porphyrinic MOF Nanoparticles with Ultrahigh Drug Loading toward Controlled Delivery and Synergistic Cancer Therapy. ACS Appl. Mater. Interfaces 2021, 13, 3679–3693. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Sim, Y.; Yang, G.; Park, M.-H.; Kim, K.; Ryu, J.-H. Surface functionalization of metal–organic framework nanoparticle for overcoming biological barrier in cancer therapy. Inorg. Chem. Front. 2024, 11, 3119–3135. [Google Scholar] [CrossRef]
- Kim, K.; Lee, S.; Jin, E.; Palanikumar, L.; Lee, J.H.; Kim, J.C.; Nam, J.S.; Jana, B.; Kwon, T.H.; Kwak, S.K.; et al. MOF x Biopolymer: Collaborative Combination of Metal-Organic Framework and Biopolymer for Advanced Anticancer Therapy. ACS Appl. Mater. Interfaces 2019, 11, 27512–27520. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Xiang, J.; Zhou, Q.; Piao, Y.; Tang, J.; Shao, S.; Zhou, Z.; Bae, Y.H.; Shen, Y. The tumor EPR effect for cancer drug delivery: Current status, limitations, and alternatives. Adv. Drug Deliv. Rev. 2022, 191, 114614. [Google Scholar] [CrossRef]
- Subhan, M.A.; Yalamarty, S.S.K.; Filipczak, N.; Parveen, F.; Torchilin, V.P. Recent Advances in Tumor Targeting via EPR Effect for Cancer Treatment. J. Pers. Med. 2021, 11, 571. [Google Scholar] [CrossRef]
- Maeda, H. Macromolecular therapeutics in cancer treatment: The EPR effect and beyond. J. Control. Release 2012, 164, 138–144. [Google Scholar] [CrossRef]
- Fang, J.; Islam, W.; Maeda, H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv. Drug Deliv. Rev. 2020, 157, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Zamora, M.E.; Essien, E.O.; Bhamidipati, K.; Murthy, A.; Liu, J.; Kim, H.; Patel, M.N.; Nong, J.; Wang, Z.; Espy, C.; et al. Marginated Neutrophils in the Lungs Effectively Compete for Nanoparticles Targeted to the Endothelium, Serving as a Part of the Reticuloendothelial System. ACS Nano 2024, 18, 22275–22297. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wu, T.; Qin, Y.; Qi, Y.; Sun, Y.; Kong, M.; Jiang, X.; Qin, X.; Shen, Y.; Zhang, Z. A facile doxorubicin-dichloroacetate conjugate nanomedicine with high drug loading for safe drug delivery. Int. J. Nanomed. 2018, 13, 1281–1293. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, X.; Li, J.; Nie, Y.; Liao, G.; Yu, Y.; Li, C. Overcoming the Reticuloendothelial System Barrier to Drug Delivery with a “Don’t-Eat-Us” Strategy. ACS Nano 2019, 13, 13015–13026. [Google Scholar] [CrossRef]
- Vargas-Molinero, H.Y.; Serrano-Medina, A.; Palomino-Vizcaino, K.; López-Maldonado, E.A.; Villarreal-Gómez, L.J.; Pérez-González, G.L.; Cornejo-Bravo, J.M. Hybrid systems of nanofibers and polymeric nanoparticles for biological application and delivery systems. Micromachines 2023, 14, 208. [Google Scholar] [CrossRef]
- Soleymani, S.; Doroudian, M.; Soezi, M.; Beladi, A.; Asgari, K.; Mobarakshahi, A.; Aghaeipour, A.; Macloughlin, R. Engendered nanoparticles for treatment of brain tumors. Oncol. Res. 2024, 33, 15. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yeo, Y. Controlled Drug Release from Pharmaceutical Nanocarriers. Chem. Eng. Sci. 2015, 125, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Qu, H.; Lu, B.; Pan, Y.; Yang, J.; Duan, Z.; Wu, J.; Wang, Y.; Wang, C.; Hu, R.; et al. A metal-coordination stabilized small-molecule nanomedicine with high drug-loading capacity and synergistic photochemotherapy for cancer treatment. Nanoscale 2024, 16, 14734–14747. [Google Scholar] [CrossRef] [PubMed]
- Aina, O.H.; Sroka, T.C.; Chen, M.L.; Lam, K.S. Therapeutic cancer targeting peptides. Biopolymers 2002, 66, 184–199. [Google Scholar] [CrossRef]
- Adabi, M.; Naghibzadeh, M.; Adabi, M.; Zarrinfard, M.A.; Esnaashari, S.S.; Seifalian, A.M.; Faridi-Majidi, R.; Tanimowo Aiyelabegan, H.; Ghanbari, H. Biocompatibility and nanostructured materials: Applications in nanomedicine. Artif. Cells Nanomed. Biotechnol. 2017, 45, 833–842. [Google Scholar] [CrossRef]
- Katopodi, T.; Petanidis, S.; Floros, G.; Porpodis, K.; Kosmidis, C. Hybrid Nanogel Drug Delivery Systems: Transforming the Tumor Microenvironment through Tumor Tissue Editing. Cells 2024, 13, 908. [Google Scholar] [CrossRef] [PubMed]
- Farjadian, F.; Mirkiani, S.; Ghasemiyeh, P.; Kafshboran, H.R.; Mehdi-Alamdarlou, S.; Raeisi, A.; Esfandiarinejad, R.; Soleymani, S.; Goshtasbi, G.; Firouzabadi, N. Smart nanogels as promising platform for delivery of drug, gene, and vaccine; therapeutic applications and active targeting mechanism. Eur. Polym. J. 2024, 219, 113400. [Google Scholar] [CrossRef]
- Vishnubhakthula, S.; Elupula, R.; Duran-Lara, E.F. Recent Advances in Hydrogel-Based Drug Delivery for Melanoma Cancer Therapy: A Mini Review. J. Drug Deliv. 2017, 2017, 7275985. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Duan, W.; Xu, K.; Zheng, C. Three-dimensional network gel structure and viscosity reduction mechanism of heavy oil. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 130060. [Google Scholar] [CrossRef]
- Sato, N.; Aoyama, Y.; Yamanaka, J.; Toyotama, A.; Okuzono, T. Particle Adsorption on Hydrogel Surfaces in Aqueous Media due to van der Waals Attraction. Sci. Rep. 2017, 7, 6099. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, R.; Fukumoto, N.; Ise, K.; Shibasaki, K.; Hashizaki, K.; Fujii, M.; Taguchi, H. Preparation and Application of Decanoic Acid/Arginine Hydrogels to a Transdermal Formulation. Biol. Pharm. Bull. 2024, 47, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Peng, S.; Yang, D.; Roh, Y.H.; Funabashi, H.; Park, N.; Rice, E.J.; Chen, L.; Long, R.; Wu, M.; et al. A mechanical metamaterial made from a DNA hydrogel. Nat. Nanotechnol. 2012, 7, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Chelu, M.; Musuc, A.M. Polymer Gels: Classification and Recent Developments in Biomedical Applications. Gels 2023, 9, 161. [Google Scholar] [CrossRef] [PubMed]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Palomino, K.; Magana, H.; Bucio, E. Hydrogels Classification According to the Physical or Chemical Interactions and as Stimuli-Sensitive Materials. Gels 2021, 7, 182. [Google Scholar] [CrossRef]
- Wang, Z.; Ye, Q.; Yu, S.; Akhavan, B. Poly Ethylene Glycol (PEG)-Based Hydrogels for Drug Delivery in Cancer Therapy: A Comprehensive Review. Adv. Healthc. Mater. 2023, 12, e2300105. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Attama, A.A.; Nnamani, P.O.; Onokala, O.B.; Ugwu, A.A.; Onugwu, A.L. Nanogels as target drug delivery systems in cancer therapy: A review of the last decade. Front. Pharmacol. 2022, 13, 874510. [Google Scholar] [CrossRef] [PubMed]
- Solanki, R.; Bhatia, D. Stimulus-Responsive hydrogels for targeted cancer therapy. Gels 2024, 10, 440. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Kohane, D.S. Hybrid Nanoparticle-Hydrogel Systems for Drug Delivery Depots and Other Biomedical Applications. ACS Nano 2024, 18, 22780–22792. [Google Scholar] [CrossRef]
- Prabhakar, U.; Maeda, H.; Jain, R.K.; Sevick-Muraca, E.M.; Zamboni, W.; Farokhzad, O.C.; Barry, S.T.; Gabizon, A.; Grodzinski, P.; Blakey, D.C. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013, 73, 2412–2417. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bioconjug Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Chen, H.; Sun, L.N.; Zhang, B.; Yue, D.S.; Wang, C.L.; Zhang, Z.F. Injectable hydrogel with doxorubicin-loaded ZIF-8 nanoparticles for tumor postoperative treatments and wound repair. Sci. Rep. 2024, 14, 9983. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Guenneau, F.; Wang, X.; Helary, C.; Coradin, T. MnO2-gated Nanoplatforms with Targeted Controlled Drug Release and Contrast-Enhanced MRI Properties: From 2D Cell Culture to 3D Biomimetic Hydrogels. Nanotheranostics 2018, 2, 403–416. [Google Scholar] [CrossRef]
- Mikhail, A.S.; Morhard, R.; Mauda-Havakuk, M.; Kassin, M.; Arrichiello, A.; Wood, B.J. Hydrogel drug delivery systems for minimally invasive local immunotherapy of cancer. Adv. Drug Deliv. Rev. 2023, 202, 115083. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Gao, W.; Hu, H.; Ma, K.; He, B.; Dai, W.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. Novel thermo-sensitive hydrogel system with paclitaxel nanocrystals: High drug-loading, sustained drug release and extended local retention guaranteeing better efficacy and lower toxicity. J. Control Release 2014, 174, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-Hydrogel: A Hybrid Biomaterial System for Localized Drug Delivery. Ann. Biomed. Eng. 2016, 44, 2049–2061. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ding, J.; Chen, Z.; Zhang, Z.; Rong, Y.; Li, G.; He, C.; Chen, X. Injectable, Adhesive Albumin Nanoparticle-Incorporated Hydrogel for Sustained Localized Drug Delivery and Efficient Tumor Treatment. ACS Appl. Mater. Interfaces 2024, 16, 9868–9879. [Google Scholar] [CrossRef]
- Veloso, S.R.S.; Vázquez-González, M.; Spuch, C.; Freiría-Martínez, L.; Comís-Tuche, M.; Iglesias-Martínez-Almeida, M.; Rivera-Baltanás, T.; Hilliou, L.; Amorim, C.O.; Amaral, V.S.; et al. An Injectable Composite Co-Assembled Dehydropeptide-Based Magnetic/Plasmonic Lipogel for Multimodal Cancer Therapy. Adv. Funct. Mater. 2024, 34, 2402926. [Google Scholar] [CrossRef]
- Ito, S.; Nagasaka, K.; Komatsu, H.; Mamiya, H.; Takeguchi, M.; Nishiguchi, A.; Taguchi, T. Sprayable tissue adhesive microparticle-magnetic nanoparticle composites for local cancer hyperthermia. Biomater. Adv. 2024, 156, 213707. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhang, C.; Du, D.; Li, Y.; Sun, L.; Han, Y.; He, X.; Dai, J.; Shi, L. Metal-organic framework-based hydrogel with structurally dynamic properties as a stimuli-responsive localized drug delivery system for cancer therapy. Acta Biomater. 2022, 145, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.W.; Gao, G.; Hu, P.; Liu, J.B.; Guo, Y.; Zhang, X.; Yu, X.W.; Wu, F.G.; Lu, X. Palladium nanosheet-knotted injectable hydrogels formed via palladium-sulfur bonding for synergistic chemo-photothermal therapy. Nanoscale 2020, 12, 210–219. [Google Scholar] [CrossRef]
- Shen, S.; Wu, Y.; Liu, Y.; Wu, D. High drug-loading nanomedicines: Progress, current status, and prospects. Int. J. Nanomedicine 2017, 12, 4085–4109. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, G.; Jin, S.; Xu, L.; Zhao, C.X. Development of High-Drug-Loading Nanoparticles. Chempluschem 2020, 85, 2143–2157. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Kievit, F.M.; Veiseh, O.; Stephen, Z.R.; Wang, T.; Lee, D.; Ellenbogen, R.G.; Zhang, M. Fabrication of magnetic nanoparticles with controllable drug loading and release through a simple assembly approach. J. Control. Release 2012, 162, 233–241. [Google Scholar] [CrossRef]
- Kim, W.; Ly, N.K.; He, Y.; Li, Y.; Yuan, Z.; Yeo, Y. Protein corona: Friend or foe? Co-opting serum proteins for nanoparticle delivery. Adv. Drug Deliv. Rev. 2023, 192, 114635. [Google Scholar] [CrossRef]
- Gonzalez-Garcia, L.E.; MacGregor, M.N.; Visalakshan, R.M.; Lazarian, A.; Cavallaro, A.A.; Morsbach, S.; Mierczynska-Vasilev, A.; Mailander, V.; Landfester, K.; Vasilev, K. Nanoparticles Surface Chemistry Influence on Protein Corona Composition and Inflammatory Responses. Nanomaterials 2022, 12, 682. [Google Scholar] [CrossRef]
- Barz, M.; Parak, W.J.; Zentel, R. Concepts and Approaches to Reduce or Avoid Protein Corona Formation on Nanoparticles: Challenges and Opportunities. Adv. Sci. 2024, 11, e2402935. [Google Scholar] [CrossRef] [PubMed]
- Thalhauser, S.; Breunig, M. Considerations for efficient surface functionalization of nanoparticles with a high molecular weight protein as targeting ligand. Eur. J. Pharm. Sci. 2020, 155, 105520. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Kim, H.S.; Palanikumar, L.; Go, E.M.; Jana, B.; Park, S.A.; Kim, H.Y.; Kim, K.; Seo, J.K.; Kwak, S.K.; et al. Cloaking nanoparticles with protein corona shield for targeted drug delivery. Nat. Commun. 2018, 9, 4548. [Google Scholar] [CrossRef] [PubMed]
- Mout, R.; Moyano, D.F.; Rana, S.; Rotello, V.M. Surface functionalization of nanoparticles for nanomedicine. Chem. Soc. Rev. 2012, 41, 2539–2544. [Google Scholar] [CrossRef]
- Shen, J.; Lin, M.; Ding, M.; Yu, N.; Yang, C.; Kong, D.; Sun, H.; Xie, Z. Tumor immunosuppressive microenvironment modulating hydrogels for second near-infrared photothermal-immunotherapy of cancer. Mater. Today Bio 2022, 16, 100416. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Falahi, F.; Akbari-Birgani, S.; Maleki, A.; Nikfarjam, N. Chemo-Photothermal Therapy on Breast Cancer Cells in a 3D Coculture Hydrogel Model with In Situ Embedded Polydopamine Nanoparticle. ACS Appl. Eng. Mater. 2025. ahead of print. [Google Scholar] [CrossRef]
- Alioghli Ziaei, A.; Erfan-Niya, H.; Fathi, M.; Amiryaghoubi, N. In situ forming alginate/gelatin hybrid hydrogels containing doxorubicin loaded chitosan/AuNPs nanogels for the local therapy of breast cancer. Int. J. Biol. Macromol. 2023, 246, 125640. [Google Scholar] [CrossRef]
- Xin, H.; Naficy, S. Drug Delivery Based on Stimuli-Responsive Injectable Hydrogels for Breast Cancer Therapy: A Review. Gels 2022, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Wang, C. Magnetic iron oxide nanoparticle-loaded hydrogels for photothermal therapy of cancer cells. Front. Bioeng. Biotechnol. 2023, 11, 1130523. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Zhang, L.; Chen, X.; Yin, W.; Ni, L.; Wang, M. Photothermal nanohybrid hydrogels for biomedical applications. Front. Bioeng. Biotechnol. 2022, 10, 1066617. [Google Scholar] [CrossRef]
- Chakroborty, S.; Nath, N.; Mahal, A.; Barik, A.; Panda, A.R.; Fahaduddin; Bal, T.; Obaidullah, A.J.; Elawady, A. Stimuli-responsive nanogels: A smart material for biomedical applications. J. Mol. Liq. 2024, 403, 124828. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Malashkeevich, S.M.; Belogurova, N.G.; Kudryashova, E.V. Thermoreversible Gels Based on Chitosan Copolymers as ‘Intelligent’ Drug Delivery System with Prolonged Action for Intramuscular Injection. Pharmaceutics 2023, 15, 1478. [Google Scholar] [CrossRef]
- Timko, B.P.; Arruebo, M.; Shankarappa, S.A.; McAlvin, J.B.; Okonkwo, O.S.; Mizrahi, B.; Stefanescu, C.F.; Gomez, L.; Zhu, J.; Zhu, A.; et al. Near-infrared-actuated devices for remotely controlled drug delivery. Proc. Natl. Acad. Sci. USA 2014, 111, 1349–1354. [Google Scholar] [CrossRef]
- Mohammadi, Z.S.; Pourmadadi, M.; Abdouss, M.; Jafari, S.H.; Rahdar, A.; Díez-Pascual, A.M. pH-sensitive polyacrylic acid/Fe3O4@SiO2 hydrogel nanocomposite modified with agarose for controlled release of quercetin. Inorg. Chem. Commun. 2024, 163, 112338. [Google Scholar] [CrossRef]
- Liu, Y.; Ran, Y.; Ge, Y.; Raza, F.; Li, S.; Zafar, H.; Wu, Y.; Paiva-Santos, A.C.; Yu, C.; Sun, M.; et al. pH-Sensitive Peptide Hydrogels as a Combination Drug Delivery System for Cancer Treatment. Pharmaceutics 2022, 14, 14652. [Google Scholar] [CrossRef]
- Jia, Y.P.; Shi, K.; Yang, F.; Liao, J.F.; Han, R.X.; Yuan, L.P.; Hao, Y.; Pan, M.; Xiao, Y.; Qian, Z.Y.; et al. Multifunctional Nanoparticle Loaded Injectable Thermoresponsive Hydrogel as NIR Controlled Release Platform for Local Photothermal Immunotherapy to Prevent Breast Cancer Postoperative Recurrence and Metastases. Adv. Funct. Mater. 2020, 30, 2001059. [Google Scholar] [CrossRef]
- Wang, S.; Chen, B.; Ouyang, L.; Wang, D.; Tan, J.; Qiao, Y.; Ge, S.; Ruan, J.; Zhuang, A.; Liu, X.; et al. A Novel Stimuli-Responsive Injectable Antibacterial Hydrogel to Achieve Synergetic Photothermal/Gene-Targeted Therapy towards Uveal Melanoma. Adv. Sci. 2021, 8, e2004721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guan, X.; Xiao, X.; Chen, Z.; Zhou, G.; Fan, Y. Dual-phase injectable thermosensitive hydrogel incorporating Fe3O4@PDA with pH and NIR triggered drug release for synergistic tumor therapy. Eur. Polym. J. 2022, 176, 111424. [Google Scholar] [CrossRef]
- Manhas, P.; Sharma, R.; Wangoo, N.; Saini, R.; Saima; Agrewala, J.N.; Sharma, R.K. Dox-loaded TPU-PLGA nanoparticles embedded chitosan hydrogel formulation as an effective anti-cancer therapy. J. Mol. Liq. 2023, 383, 122170. [Google Scholar] [CrossRef]
- Kim, J.; Choi, Y.; Kim, D.H.; Yoon, H.Y.; Kim, K. Injectable Hydrogel-Based Combination Cancer Immunotherapy for Overcoming Localized Therapeutic Efficacy. Pharmaceutics 2022, 14, 1908. [Google Scholar] [CrossRef] [PubMed]
- Seidu, T.A.; Kutoka, P.T.; Asante, D.O.; Farooq, M.A.; Alolga, R.N.; Bo, W. Functionalization of Nanoparticulate Drug Delivery Systems and Its Influence in Cancer Therapy. Pharmaceutics 2022, 14, 1113. [Google Scholar] [CrossRef]
- Hong, L.; Li, W.; Li, Y.; Yin, S. Nanoparticle-based drug delivery systems targeting cancer cell surfaces. RSC Adv. 2023, 13, 21365–21382. [Google Scholar] [CrossRef]
- Prajapati, A.; Rangra, S.; Patil, R.; Desai, N.; Jyothi, V.G.S.S.; Salave, S.; Amate, P.; Benival, D.; Kommineni, N. Receptor-Targeted Nanomedicine for Cancer Therapy. Receptors 2024, 3, 323–361. [Google Scholar] [CrossRef]
- Muhamad, N.; Plengsuriyakarn, T.; Na-Bangchang, K. Application of active targeting nanoparticle delivery system for chemotherapeutic drugs and traditional/herbal medicines in cancer therapy: A systematic review. Int. J. Nanomed. 2018, 13, 3921–3935. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Mao, W.; Sun, J.; Liu, Z.; Shen, W.; Lee, H.K.; Tang, S. Targeting Hydrogel for Intelligent Recognition and Spatiotemporal Control in Cell-Based Therapeutics. Adv. Sci. 2024, 11, e2404172. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, G.; Li, M.; Xu, S.; Liu, H. Glucose Oxidase-Based pH/ROS Dual-Sensitive Nanoparticles for Tumor Starvation and Oxidative Therapy. ACS Appl. Nano Mater. 2024, 7, 18932–18942. [Google Scholar] [CrossRef]
- Lin, J.; Lin, J.-H.; Yeh, T.-Y.; Zheng, J.-H.; Cho, E.-C.; Lee, K.-C. Fabrication of hyaluronic acid with graphene quantum dot as a dual drug delivery system for cancer therapy. FlatChem 2024, 44, 100607. [Google Scholar] [CrossRef]
- Sheoran, S.; Arora, S.; Samsonraj, R.; Govindaiah, P.; Vuree, S. Lipid-based nanoparticles for treatment of cancer. Heliyon 2022, 8, e09403. [Google Scholar] [CrossRef] [PubMed]
- Waheed, I.; Ali, A.; Tabassum, H.; Khatoon, N.; Lai, W.F.; Zhou, X. Lipid-based nanoparticles as drug delivery carriers for cancer therapy. Front. Oncol. 2024, 14, 1296091. [Google Scholar] [CrossRef] [PubMed]
- Subhan, M.A.; Muzibur Rahman, M. Recent Development in Metallic Nanoparticles for Breast Cancer Therapy and Diagnosis. Chem. Rec. 2022, 22, e202100331. [Google Scholar] [CrossRef]
- Villalobos Gutierrez, P.T.; Munoz Carrillo, J.L.; Sandoval Salazar, C.; Viveros Paredes, J.M.; Gutierrez Coronado, O. Functionalized Metal Nanoparticles in Cancer Therapy. Pharmaceutics 2023, 15, 1932. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Feldmann, G.; Soni, S.; Ravi, R.; Karikar, C.; Maitra, A.; Maitra, A. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): A novel strategy for human cancer therapy. J. Nanobiotechnol. 2007, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.G.; Ghulam, M.; Lee, J.M. pH-Responsive Chitosan-Coated Porous Silica Nanoparticles for Drug Delivery. KR 10-1528197 B1, 2015. [Google Scholar]
- Omary, M.A.; Marpu, S. Facile Method for Making Non-Toxic Biomedical Compositions Comprising Hybrid Metal-Polymer Microparticles. US 9, 872, 916 B2, 2018. [Google Scholar]
- Zhao, Y.; Wan, J.; Yang, X.; Geng, S. Gold Nanocage-Loaded Thermosensitive Hydrogel System for Photothermal-Chemotherapy Synergistic Anti-Tumor Treatment. CN 106890332 B, 2020. [Google Scholar]
- Li, J.; Zheng, Y.; Tao, N.; Liu, Y.; Sun, X. Near-Infrared Light-Responsive Injectable Hybrid Hydrogel and Preparation Method and Application Thereof. CN 107456439 B, 2019. [Google Scholar]
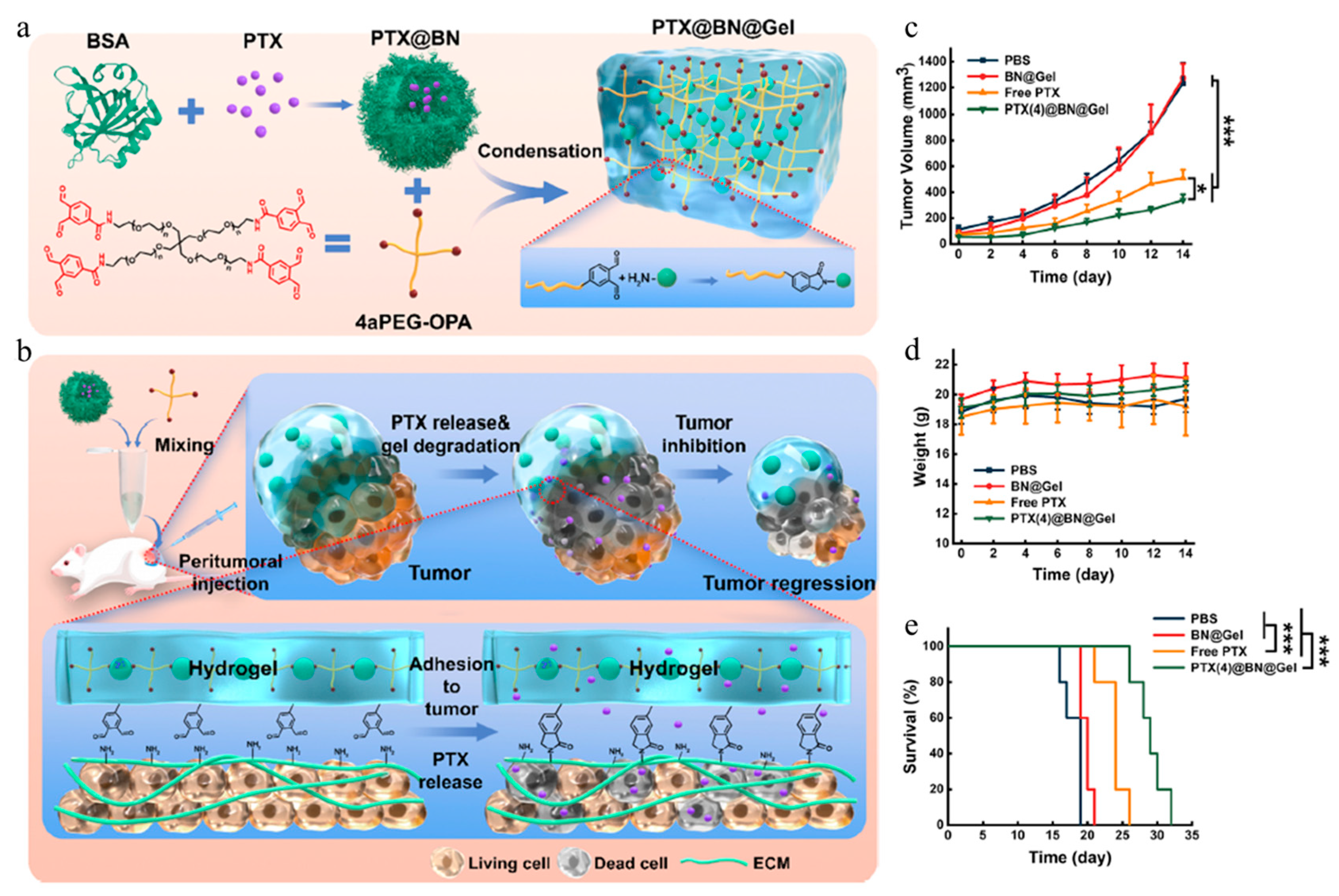
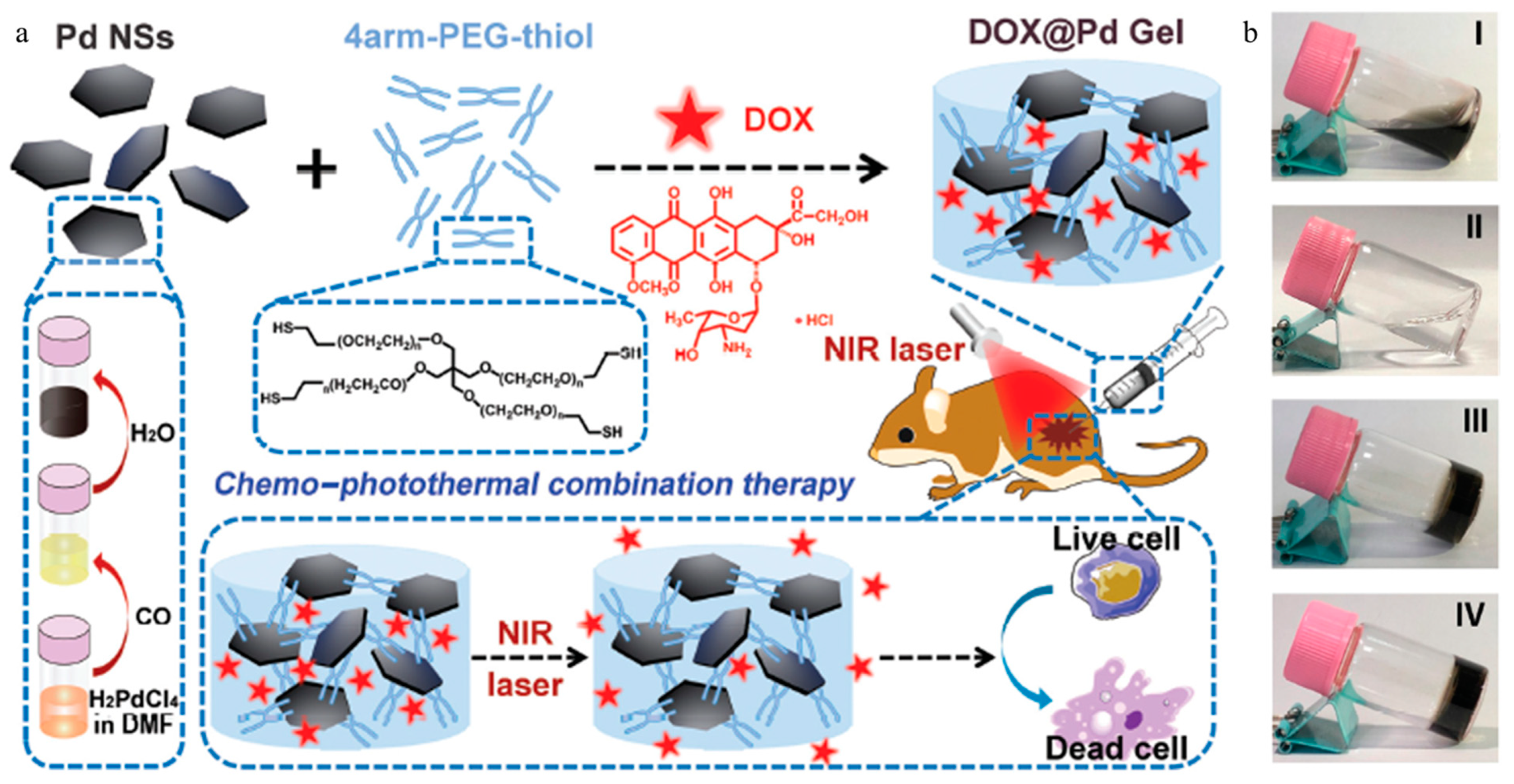

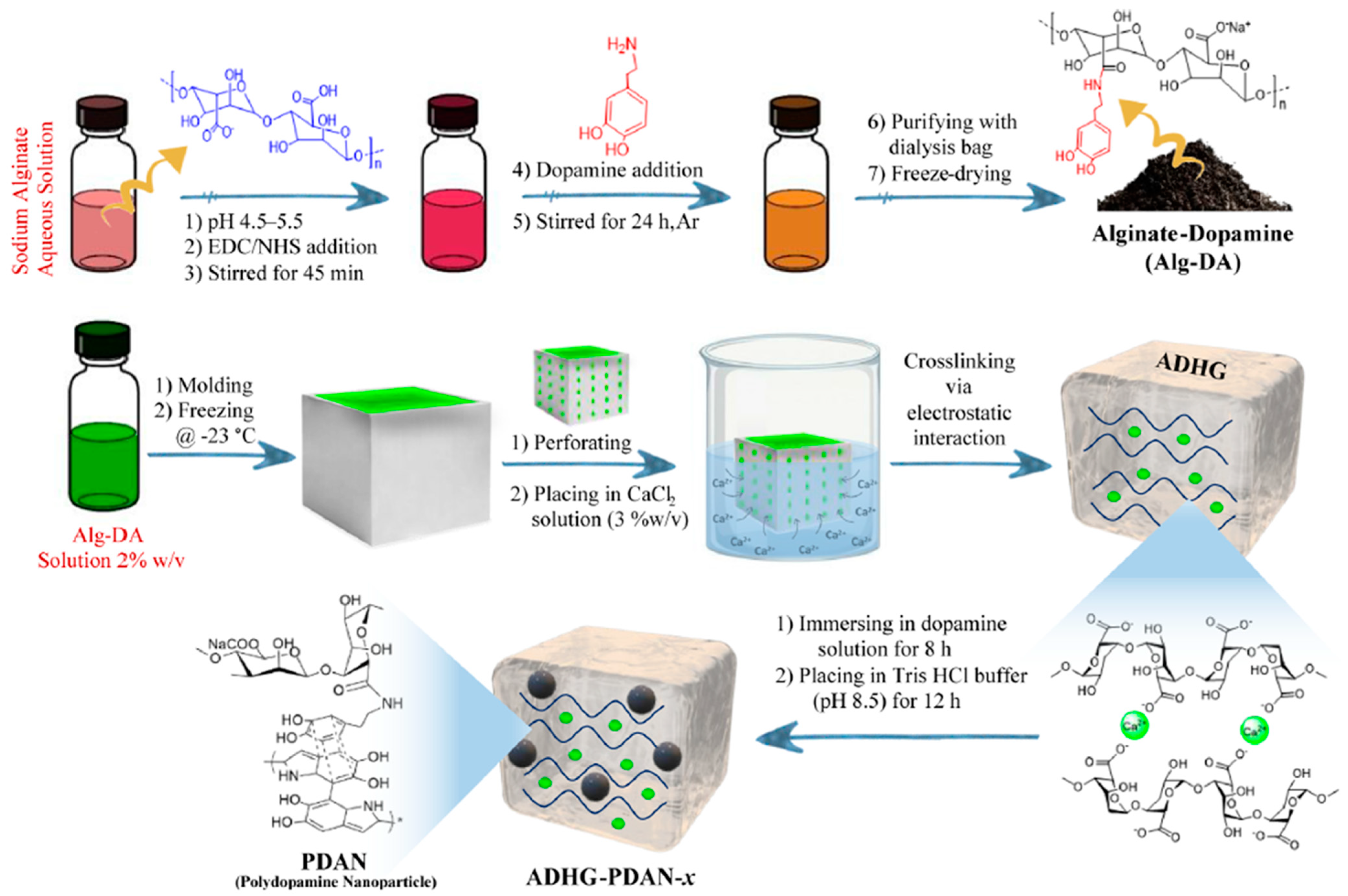
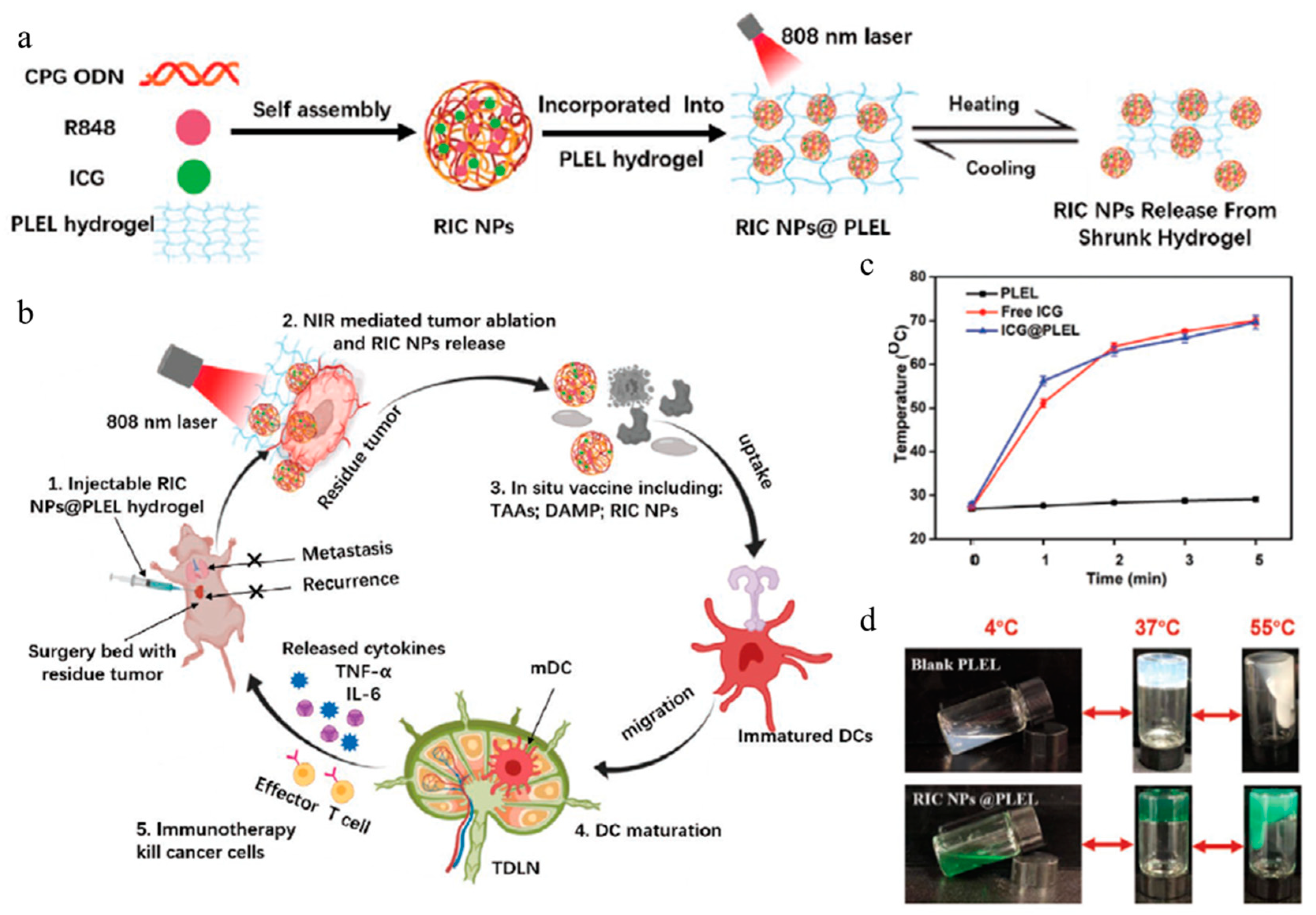

| Gel Type | Fabrication Method | Gelling Agent | Nanoparticle | Therapy Method | Drug | Ref |
|---|---|---|---|---|---|---|
| Hydrogel | Desolvation | BSA, branched PEG | BSA | Chemotherapy | PTX | [83] |
| Lipogel | Buffer switch method | 2-Naph-L-Phe-Z-ΔPhe-OH | MNP, MS-AuNR | Chemotherapy, photothermal therapy, magnetic hyperthermia | DOX, PTX | [84] |
| Colloidal gel | Coacervation andthermal crosslinking | C10-ApGltn | SPIONs | Magnetic hyperthermia | N/A | [85] |
| HA-BP-basedhydrogel | Dynamic coordination bonds | HA-BP, MOF | MOF | Chemotherapy | DOX, | [86] |
| Pd NS-knottedhydrogel | Dynamic Pd–S bonds | Pd NS, 4arm-PEG-thiol | Pd NS | Chemotherapy, photothermal therapy | DOX, | [87] |
| Alginate-basedhydrogel | Coordination | Alginate | SPIIN | Photothermal therapy, immunotherapy | CpG ODNs | [97] |
| Alginate-basedhydrogel | Electrostatic interaction | Alg-DA | PDA NP | Photothermal therapy | N/A | [98] |
| GelMA basedhydrogel | (Not specified in the paper) | GelMA | ZIF-8 | Chemotherapy | DOX | [78] |
| Alginate/gelatin-based hydrogel, Chitosan hydrogel | Schiff base reaction | CS, OAL, Gelatin, β-GP | AuNP | Chemotherapy | DOX | [99] |
| PLEL hydrogel | 3D micelle network | PLEL | RIC NP | Photothermal therapy, immunotherapy | ICG, R848, CpG ODNs | [108] |
| Chitosan–puerarin hydrogel | Grinding methodwith acetic acid | CS-puerarin, AuNRs | AuNRs | Photothermal therapy, gene-targeted therapy | DC_AC50, Puerarin | [109] |
| Chitosan–HPChydrogel | Temperature-inducedgelation | CS, GP, HPC | Fe3O4@PDA | Photothermal therapy, chemotherapy | Dox | [110] |
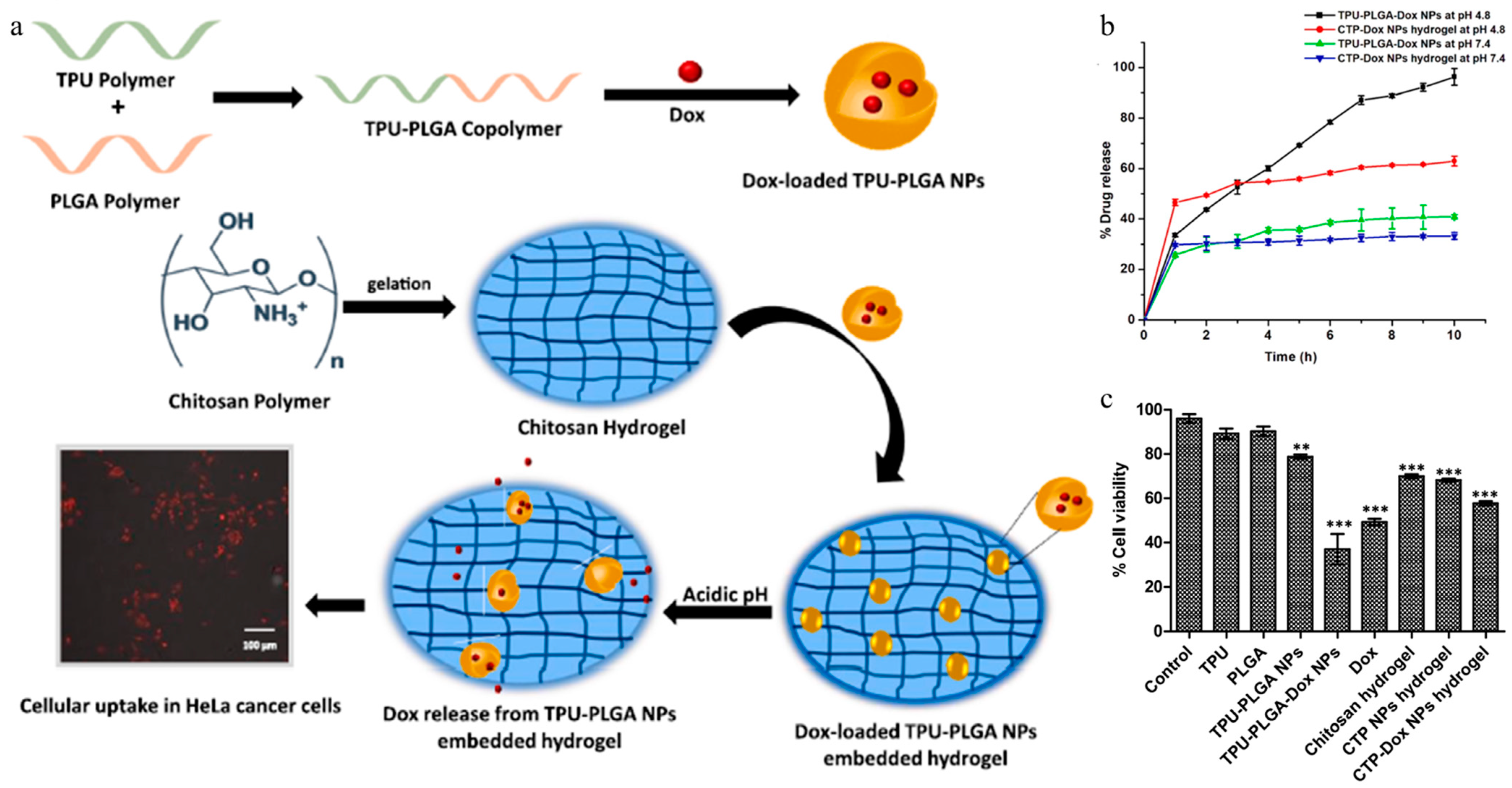
| Chitosan hydrogel | pH-induced gelation | CS, NaOH | TPU-PLGA-Dox | Chemotherapy | Dox | [111] |
| PVA/PEGhydrogel | Ionic crosslinking, hydrogen bonding | PVA, PEG | Fe3O4@Au/Mn-TCPP | Photothermal therapy, photodynamic therapy,immunotherapy | N/A | [117] |
| Hyaluronic acid hydrogel | Amide bond-mediated crosslinking | HA, TK | ZIF@HAgel-GOx | Starvation therapy, oxidative therapy | Dox, GOX | [118] |
| High molecular weight HA | Desolvation | HA | CQD-PEI /HA-PB | Dual-drug therapy(chemotherapy) | Dox, TAK-632 | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K. Hybrid Systems of Gels and Nanoparticles for Cancer Therapy: Advances in Multifunctional Therapeutic Platforms. Gels 2025, 11, 170. https://doi.org/10.3390/gels11030170
Kim K. Hybrid Systems of Gels and Nanoparticles for Cancer Therapy: Advances in Multifunctional Therapeutic Platforms. Gels. 2025; 11(3):170. https://doi.org/10.3390/gels11030170
Chicago/Turabian StyleKim, Kibeom. 2025. "Hybrid Systems of Gels and Nanoparticles for Cancer Therapy: Advances in Multifunctional Therapeutic Platforms" Gels 11, no. 3: 170. https://doi.org/10.3390/gels11030170
APA StyleKim, K. (2025). Hybrid Systems of Gels and Nanoparticles for Cancer Therapy: Advances in Multifunctional Therapeutic Platforms. Gels, 11(3), 170. https://doi.org/10.3390/gels11030170







