Antimicrobial Sponge: A Polyvinyl Alcohol, Tannic Acid and Curcumin-Loaded Nanolignin Hydrogel Composite Scaffold
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Organosolv Lignin–Curcumin Nanoparticles (Lig-Cur NPs)
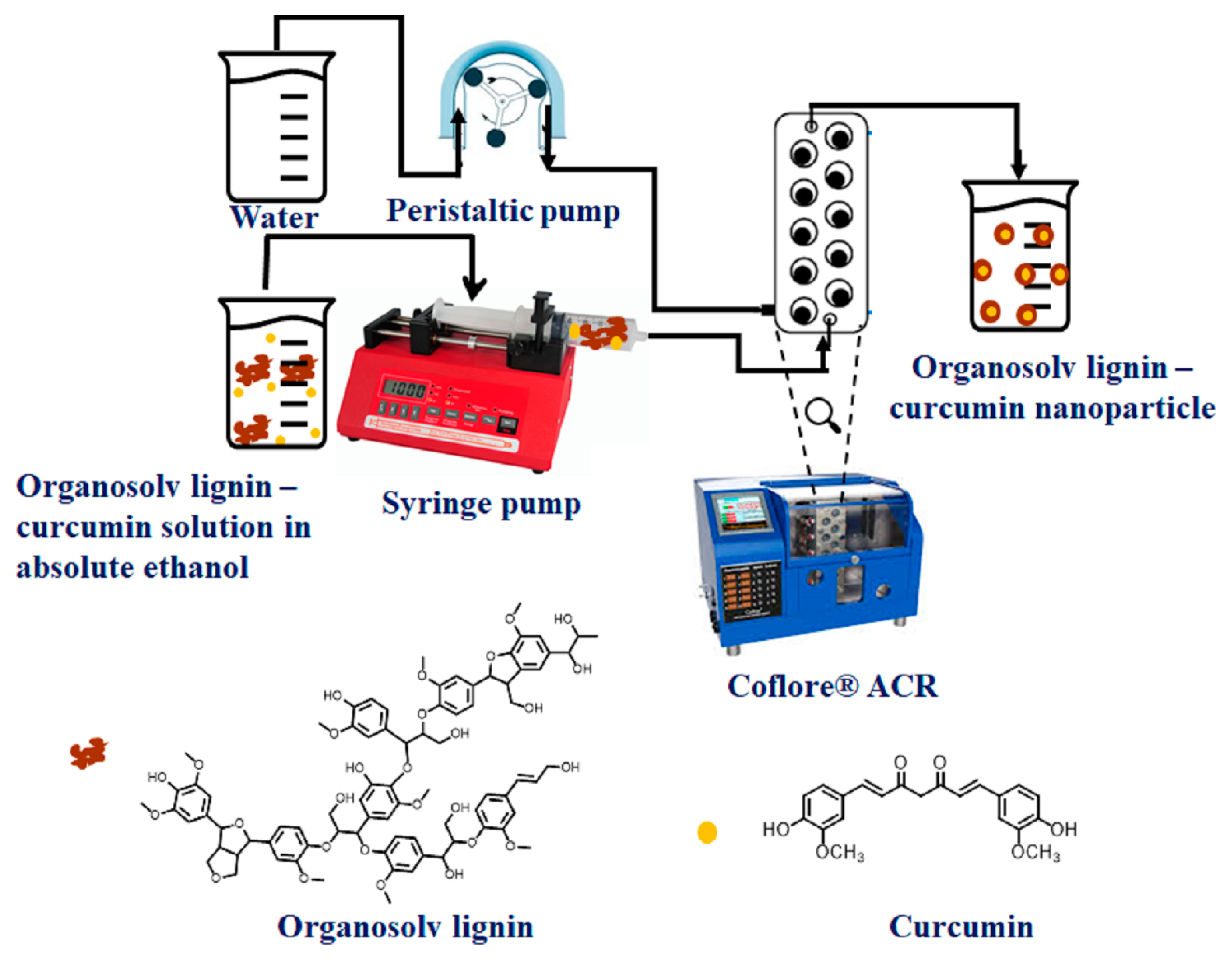
2.1.1. Synthesis of Lig-Cur NPs Using Continuous Flow Chemistry
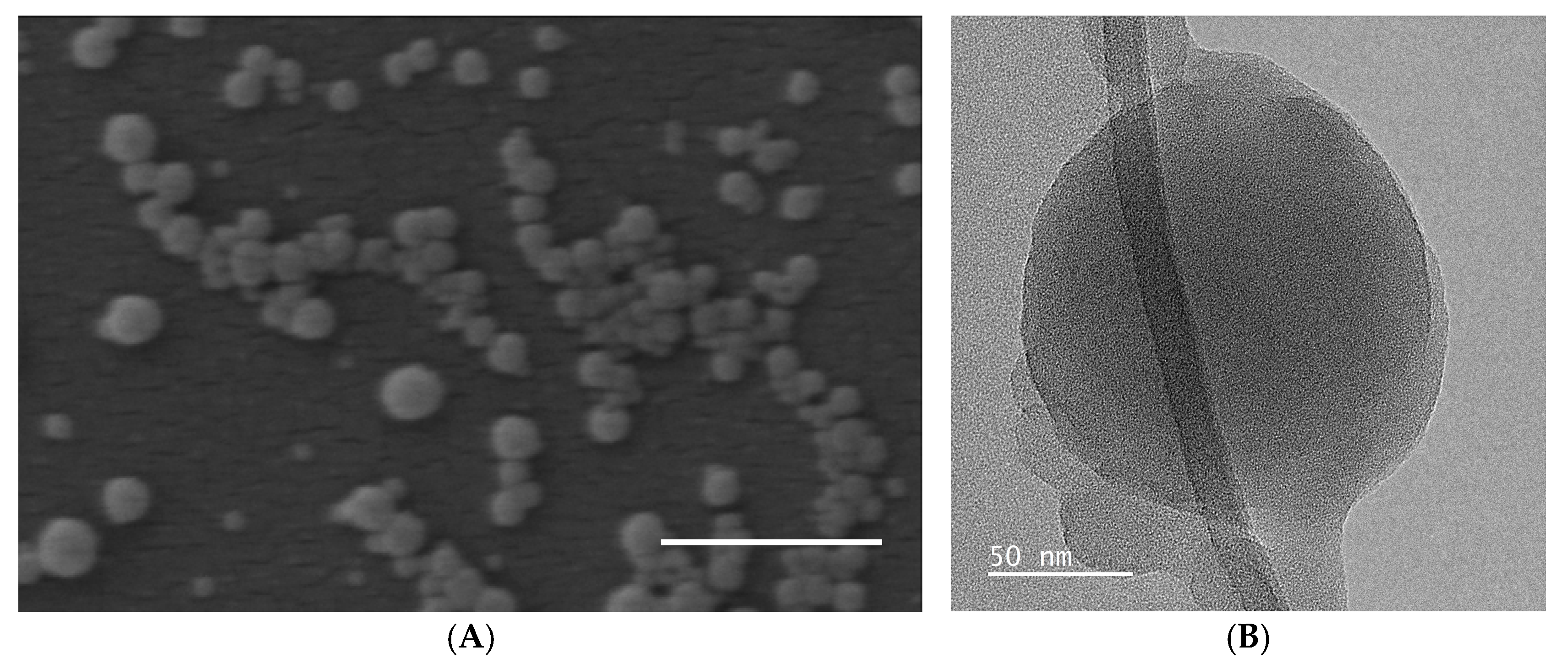
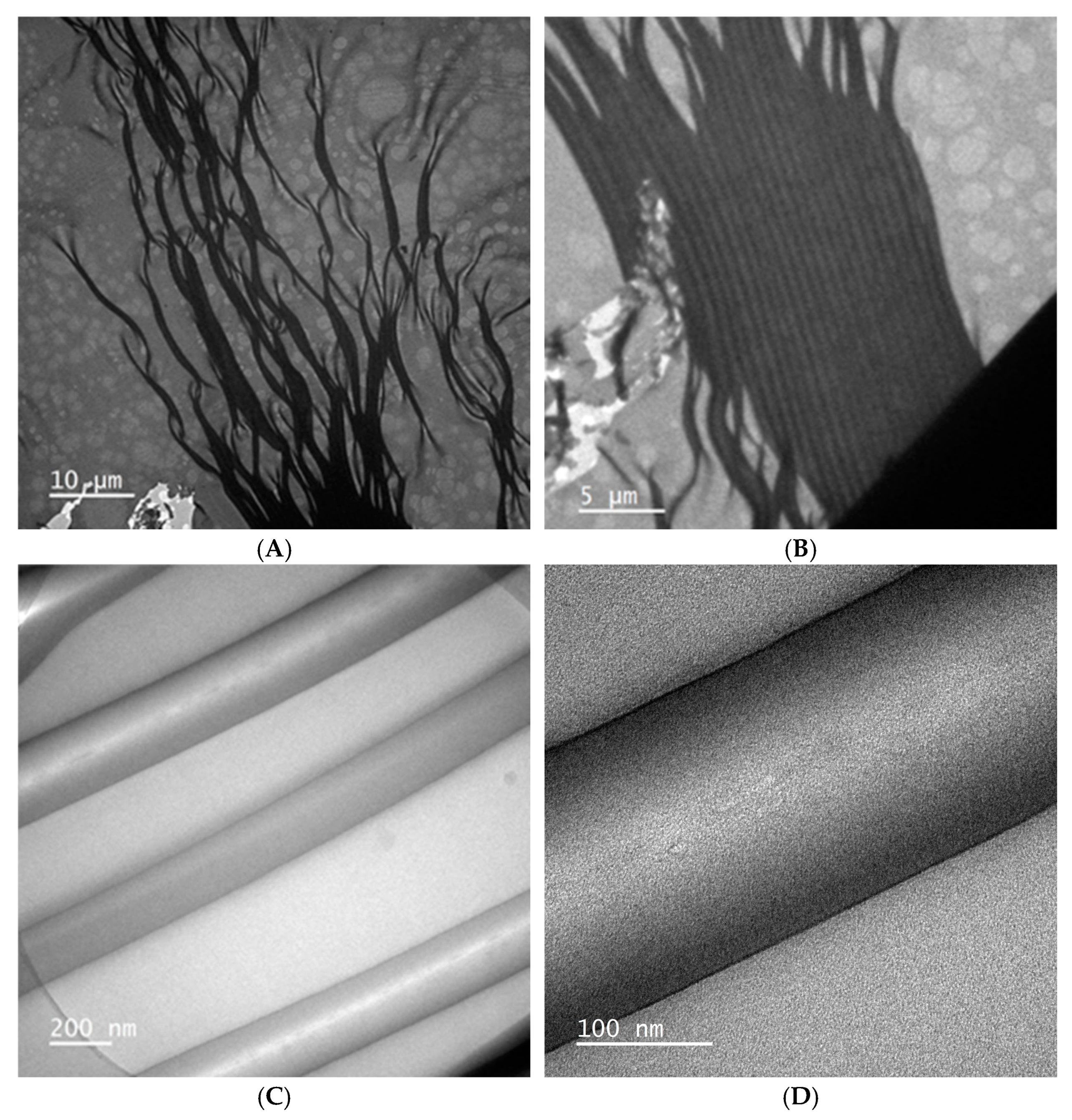
2.1.2. Hydrodynamic Size and Surface Morphology of Lig-Cur NPs
2.1.3. Structural Characterization of Lig-Cur NPs Using Fourier Transform Infrared Spectroscopy
2.2. Synthesis and Characterization of Tannic Acid-Crosslinked Polyvinyl Alcohol–Lig-Cur NPs with Hydrogel Composite Scaffolds (PVA-Lig-Cur-TA)
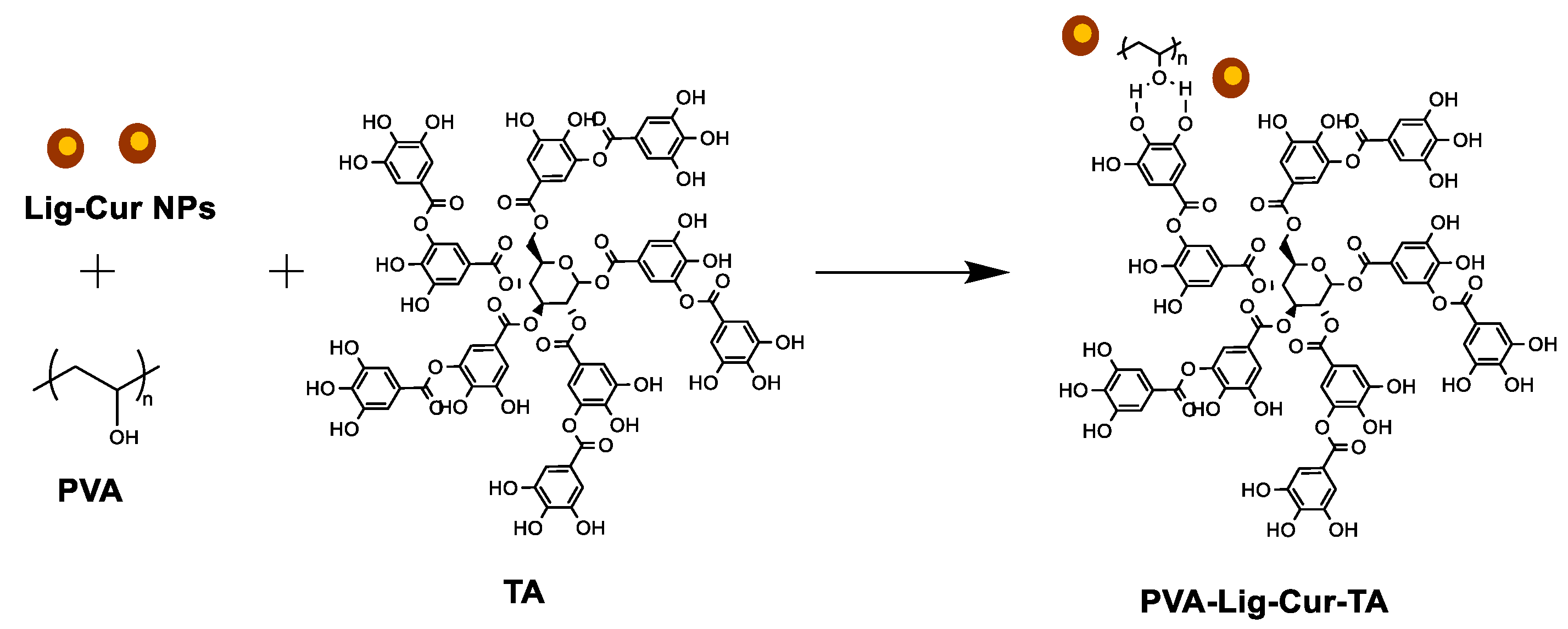
2.2.1. Synthesis of PVA-Lig-Cur-TA Hydrogel Scaffolds
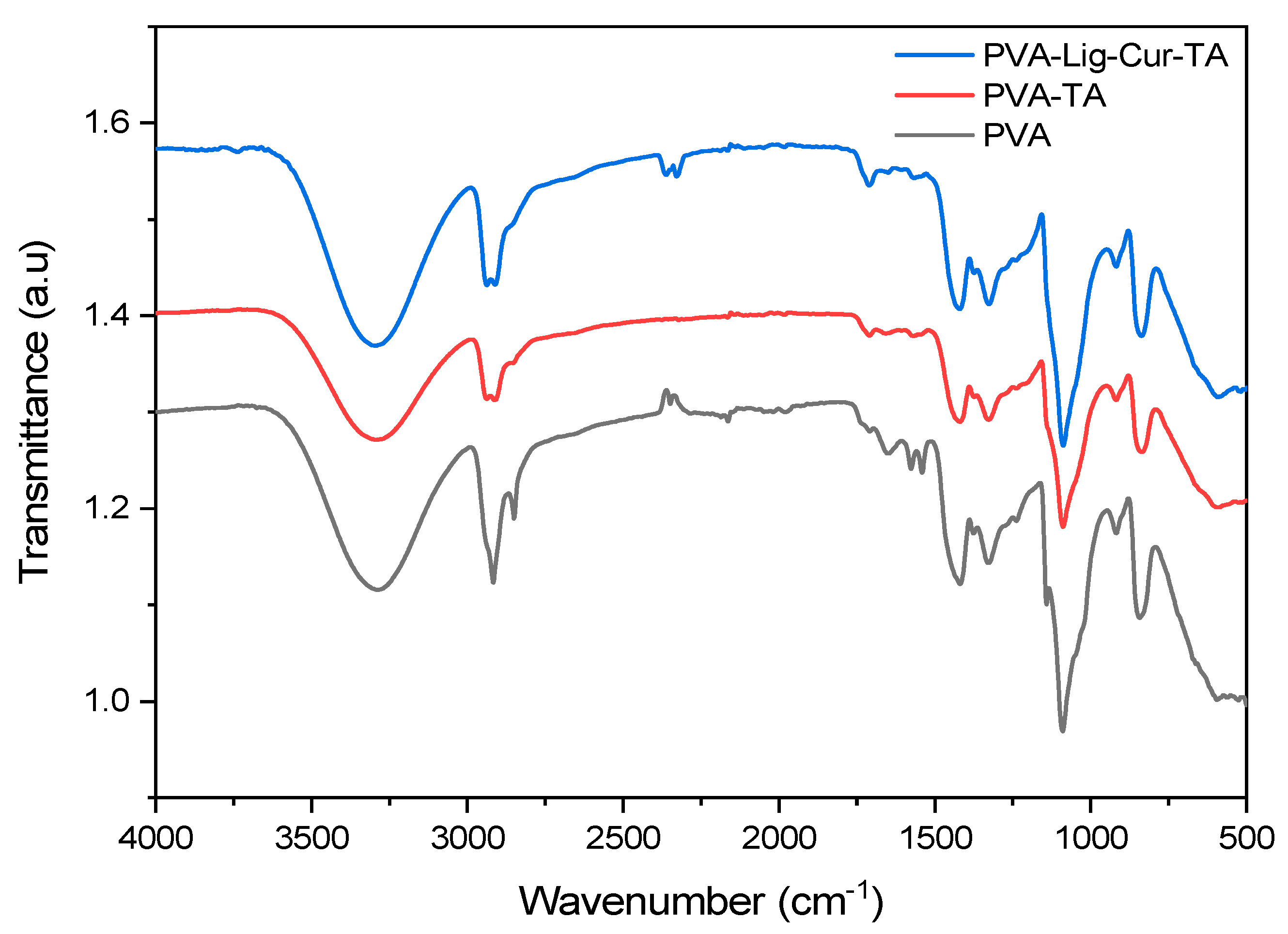
2.2.2. Characterization of PVA and PVA-Lig-Cur-TA Scaffold by FTIR and XRD
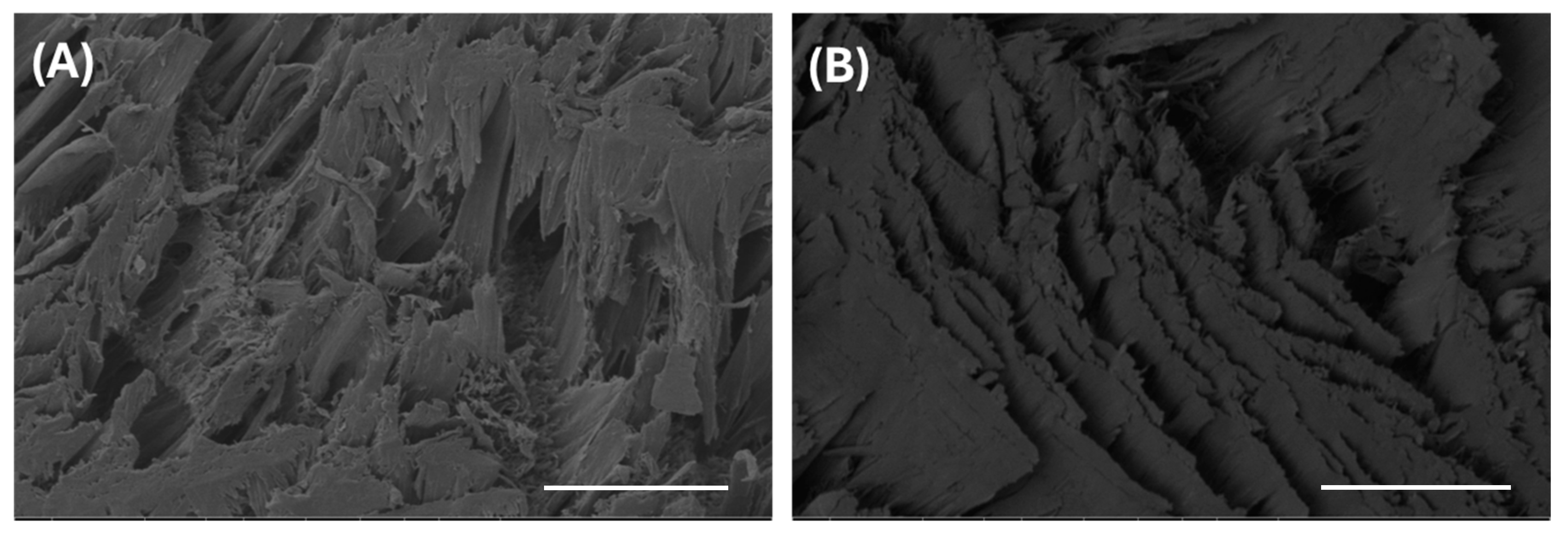
2.2.3. Surface Morphology of PVA-Lig-Cur-TA Hydrogel Scaffolds
2.2.4. Swelling and Degradation
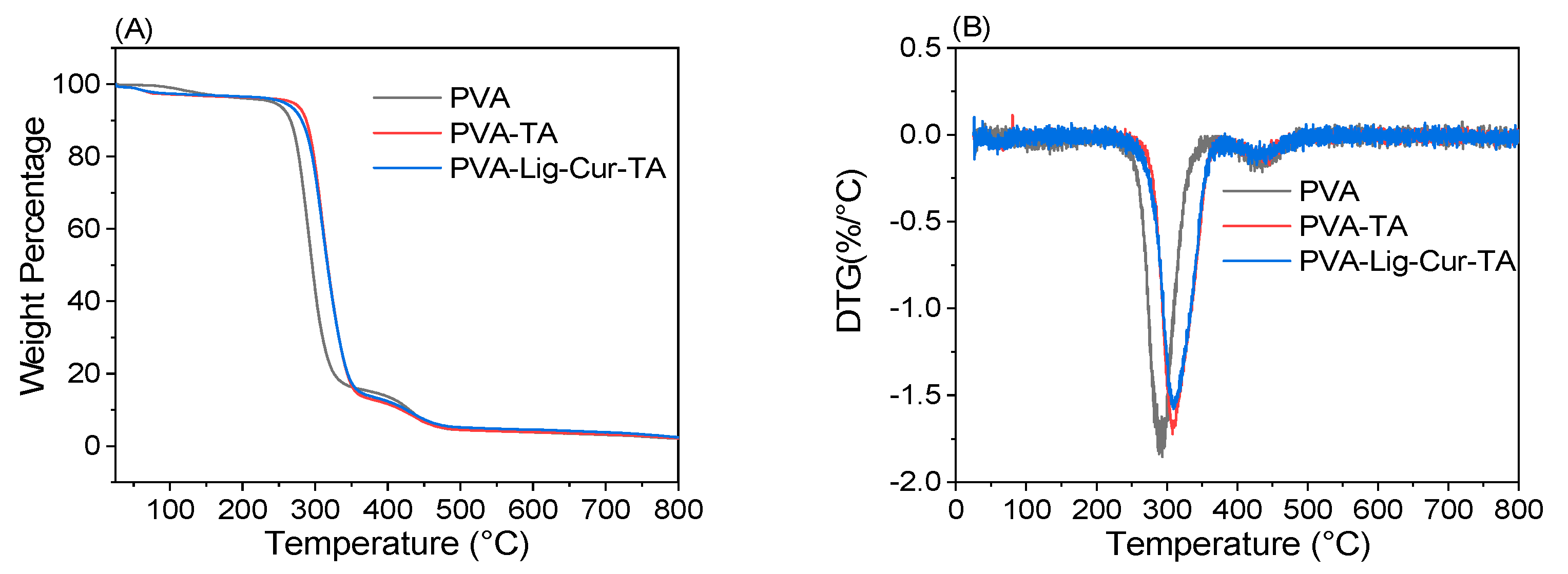
2.2.5. Thermal Stability
2.2.6. Antimicrobial Testing on the Scaffold
2.3. Further Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Synthesis of Organosolv Lignin–Curcumin Nanoparticles (Lig-Cur Nps)
4.2.2. Fabrication of Polyvinyl Alcohol–Lig-Cur Nps–Tannic Acid Composite Scaffold (PVA-Lig-Cur-TA)
4.2.3. Fabrication of Tannic Acid-Crosslinked Polyvinyl Alcohol (PVA-TA) Scaffold
4.2.4. Structural Characterization by Fourier Transform Infrared Spectroscopy (FTIR) and X-Ray Diffraction (XRD) Analysis
4.2.5. Particle Size and Zeta Potential Analysis Using Dynamic Light Scattering
4.2.6. Scanning Electron Microscopy (SEM)
4.2.7. Transmission Electron Microscopy (TEM)
4.2.8. Swelling and Degradation Studies
4.2.9. Thermogravimetric Analysis
4.2.10. Antimicrobial Testing
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sandy-Hodgetts, K.; Leslie, G.; Lewin, G.; Hendrie, D.; Carville, K. Surgical Wound Dehiscence in an Australian Community Nursing Service: Time and Cost to Healing. J. Wound Care 2016, 25, 377–383. [Google Scholar] [CrossRef]
- Feng, M.; Wu, Y.; Zhu, J.; Wu, X. The Application of the Modified Surgical Wound Dressing in Wound Care after Tracheotomy. Regen. Med. Res. 2017, 5, 1. [Google Scholar] [CrossRef]
- Ousey, K.; Rippon, M.G.; Rogers, A.A.; Totty, J.P. Considerations for an Ideal Post-Surgical Wound Dressing Aligned with Antimicrobial Stewardship Objectives: A Scoping Review. J. Wound Care 2023, 32, 334–347. [Google Scholar] [CrossRef] [PubMed]
- Panchatcharam, M.; Miriyala, S.; Gayathri, V.S.; Suguna, L. Curcumin Improves Wound Healing by Modulating Collagen and Decreasing Reactive Oxygen Species. Mol. Cell. Biochem. 2006, 290, 87–96. [Google Scholar] [CrossRef]
- Gayathri, K.; Bhaskaran, M.; Selvam, C.; Thilagavathi, R. Nano Formulation Approaches for Curcumin Delivery—A Review. J. Drug Deliv. Sci. Technol. 2023, 82, 104326. [Google Scholar] [CrossRef]
- Gao, H.; Seidi, F.; Cai, Y.; Sun, Z.; Bian, H.; Dai, H.; Xu, T. Construction of Curcumin-Conjugated PH-Responsive Lignin-Based Nanoparticles for Alleviating Oxidative Stress: Stability, Antioxidant Activity and Biocompatibility. Int. J. Biol. Macromol. 2025, 302, 140036. [Google Scholar] [CrossRef]
- Alqahtani, M.S.; Alqahtani, A.; Al-Thabit, A.; Roni, M.; Syed, R. Novel Lignin Nanoparticles for Oral Drug Delivery. J. Mater. Chem. B 2019, 7, 4461–4473. [Google Scholar] [CrossRef]
- Mahmud, M.M.; Zaman, S.; Perveen, A.; Jahan, R.A.; Islam, M.F.; Arafat, M.T. Controlled Release of Curcumin from Electrospun Fiber Mats with Antibacterial Activity. J. Drug Deliv. Sci. Technol. 2020, 55, 101386. [Google Scholar] [CrossRef]
- Jin, S.G. Production and Application of Biomaterials Based on Polyvinyl Alcohol (PVA) as Wound Dressing. Chem. Asian J. 2022, 17, e202200595. [Google Scholar] [CrossRef] [PubMed]
- Bercea, M. Recent Advances in Poly (Vinyl Alcohol)-Based Hydrogels. Polymers 2024, 16, 2021. [Google Scholar] [CrossRef] [PubMed]
- Iswariya, S.; Bhanukeerthi, A.V.; Velswamy, P.; Uma, T.S.; Perumal, P.T. Design and Development of a Piscine Collagen Blended Pullulan Hydrogel for Skin Tissue Engineering. RSC Adv. 2016, 6, 57863–57871. [Google Scholar] [CrossRef]
- Li, B.; Xia, X.; Chen, J.; Xia, D.; Xu, R.; Zou, X.; Wang, H.; Liang, C. Paclitaxel-Loaded Lignin Particle Encapsulated into Electrospun PVA/PVP Composite Nanofiber for Effective Cervical Cancer Cell Inhibition. Nanotechnology 2021, 32, 015101. [Google Scholar] [CrossRef]
- Rejmontová, P.; Kovalcik, A.; Humpolíček, P.; Capáková, Z.; Wrzecionko, E.; Sáha, P. The Use of Fractionated Kraft Lignin to Improve the Mechanical and Biological Properties of PVA-Based Scaffolds. RSC Adv. 2019, 9, 12346–12353. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-N.; Jiao, C.; Zhao, Y.; Zhang, J.; Wang, H. Self-Assembled Polyvinyl Alcohol–Tannic Acid Hydrogels with Diverse Microstructures and Good Mechanical Properties. ACS Omega 2018, 3, 11788–11795. [Google Scholar] [CrossRef] [PubMed]
- Si, C.; Tian, X.; Wang, Y.; Wang, Z.; Wang, X.; Lv, D.; Wang, A.; Wang, F.; Geng, L.; Zhao, J.; et al. A Polyvinyl Alcohol–Tannic Acid Gel with Exceptional Mechanical Properties and Ultraviolet Resistance. Gels 2022, 8, 751. [Google Scholar] [CrossRef]
- Kaczmarek, B. Tannic Acid with Antiviral and Antibacterial Activity as A Promising Component of Biomaterials—A Minireview. Materials 2020, 13, 3224. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef]
- Cui, Z.; Yao, L.; Ye, J.; Wang, Z.; Hu, Y. Solubility Measurement and Thermodynamic Modelling of Curcumin in Twelve Pure Solvents and Three Binary Solvents at Different Temperature (T = 278.15–323.15 K). J. Mol. Liq. 2021, 338, 116795. [Google Scholar] [CrossRef]
- Fontana, F.; Molinaro, G.; Moroni, S.; Pallozzi, G.; Ferreira, M.P.A.; Tello, R.P.; Elbadri, K.; Torrieri, G.; Correia, A.; Kemell, M.; et al. Biomimetic Platelet-Cloaked Nanoparticles for the Delivery of Anti-Inflammatory Curcumin in the Treatment of Atherosclerosis. Adv. Healthc. Mater. 2024, 13, 2302074. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zou, L.-Q.; Niu, J.; Liu, W.; Peng, S.-F.; Liu, C.-M. The Stability, Sustained Release and Cellular Antioxidant Activity of Curcumin Nanoliposomes. Molecules 2015, 20, 14293–14311. [Google Scholar] [CrossRef]
- Jõul, P.; Ho, T.T.; Kallavus, U.; Konist, A.; Leiman, K.; Salm, O.-S.; Kulp, M.; Koel, M.; Lukk, T. Characterization of Organosolv Lignins and Their Application in the Preparation of Aerogels. Materials 2022, 15, 2861. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.H. Polyvinyl Alcohol/Tannic Acid Hydrogel Prepared by a Freeze-Thawing Process for Wound Dressing Applications. Polym. Bull. 2017, 74, 2861–2872. [Google Scholar] [CrossRef]
- Chen, Y.-N.; Peng, L.; Liu, T.; Wang, Y.; Shi, S.; Wang, H. Poly (Vinyl Alcohol)–Tannic Acid Hydrogels with Excellent Mechanical Properties and Shape Memory Behaviors. ACS Appl. Mater. Interfaces 2016, 8, 27199–27206. [Google Scholar] [CrossRef] [PubMed]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P. FTIR Spectroscopy Characterization of Poly (Vinyl Alcohol) Hydrogel with Different Hydrolysis Degree and Chemically Crosslinked with Glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- ISO 20743:2021; Textiles—Determination of Antibacterial Activity of Textile Products. ISO: Geneva, Switzerland, 2021.
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity. Pharmaceuticals 2020, 13, 153. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Y.; Alam, W.; Ullah, H.; Dacrema, M.; Daglia, M.; Khan, H.; Arciola, C.R. Antimicrobial Potential of Curcumin: Therapeutic Potential and Challenges to Clinical Applications. Antibiotics 2022, 11, 322. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, H.; Niu, H.; Ma, X.; Yuan, Y.; Hong, H.; Liu, C. Tannic Acid-Loaded Mesoporous Silica for Rapid Hemostasis and Antibacterial Activity. Biomater. Sci. 2018, 6, 3318–3331. [Google Scholar] [CrossRef] [PubMed]
- Morena, A.G.; Bassegoda, A.; Natan, M.; Jacobi, G.; Banin, E.; Tzanov, T. Antibacterial Properties and Mechanisms of Action of Sonoenzymatically Synthesized Lignin-Based Nanoparticles. ACS Appl. Mater. Interfaces 2022, 14, 37270–37279. [Google Scholar] [CrossRef] [PubMed]




| Nanoparticles | Hydrodynamic Diameter (nm) | Polydispersity Index (PDI) | Zeta Potential (mV) |
|---|---|---|---|
| Lig-Cur NPs | 141 ± 6 | 0.2 | −45 ± 6 |
| Strain Designation | Gram Stain | Specimens (n = 3) | Contact Time = 0 h | After 24 h Incubation | Growth Value | Antibacterial Activity Value (Log10 CFU) |
|---|---|---|---|---|---|---|
| S. aureus ATCC 6538 | Gram-positive | Control PVA-TA | C0 (Log10 CFU) | Ct (Log10 CFU) | Ct-C0 (Log10 CFU) | 1.7 |
| 4.6 | 6.9 | 2.3 | ||||
| PVA-Lig-Cur-TA | T0 (Log10 CFU) | Tt (Log10 CFU) | Tt-T0 (Log10 CFU) | |||
| 4.2 | 4.8 | 0.6 | ||||
| P. aeruginosa ATCC 15442 | Gram-negative | Control PVA-TA | C0 (Log10 CFU) | Ct (Log10 CFU) | Ct-C0 (Log10 CFU) | >6.2 |
| 3.3 | 6.3 | 3 | ||||
| PVA-Lig-Cur-TA | T0 (Log10 CFU) | Tt (Log10 CFU) | Tt-T0 (Log10 CFU) | |||
| 3.2 | <0.1 | <−3.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anand, R.; Collard, D.; Thomann, J.-S.; Duday, D. Antimicrobial Sponge: A Polyvinyl Alcohol, Tannic Acid and Curcumin-Loaded Nanolignin Hydrogel Composite Scaffold. Gels 2025, 11, 168. https://doi.org/10.3390/gels11030168
Anand R, Collard D, Thomann J-S, Duday D. Antimicrobial Sponge: A Polyvinyl Alcohol, Tannic Acid and Curcumin-Loaded Nanolignin Hydrogel Composite Scaffold. Gels. 2025; 11(3):168. https://doi.org/10.3390/gels11030168
Chicago/Turabian StyleAnand, Resmi, Delphine Collard, Jean-Sébastien Thomann, and David Duday. 2025. "Antimicrobial Sponge: A Polyvinyl Alcohol, Tannic Acid and Curcumin-Loaded Nanolignin Hydrogel Composite Scaffold" Gels 11, no. 3: 168. https://doi.org/10.3390/gels11030168
APA StyleAnand, R., Collard, D., Thomann, J.-S., & Duday, D. (2025). Antimicrobial Sponge: A Polyvinyl Alcohol, Tannic Acid and Curcumin-Loaded Nanolignin Hydrogel Composite Scaffold. Gels, 11(3), 168. https://doi.org/10.3390/gels11030168








