Construction of PdCu Alloy Decorated on the N-Doped Carbon Aerogel as a Highly Active Electrocatalyst for Enhanced Oxygen Reduction Reaction
Abstract
1. Introduction
2. Results and Discussion
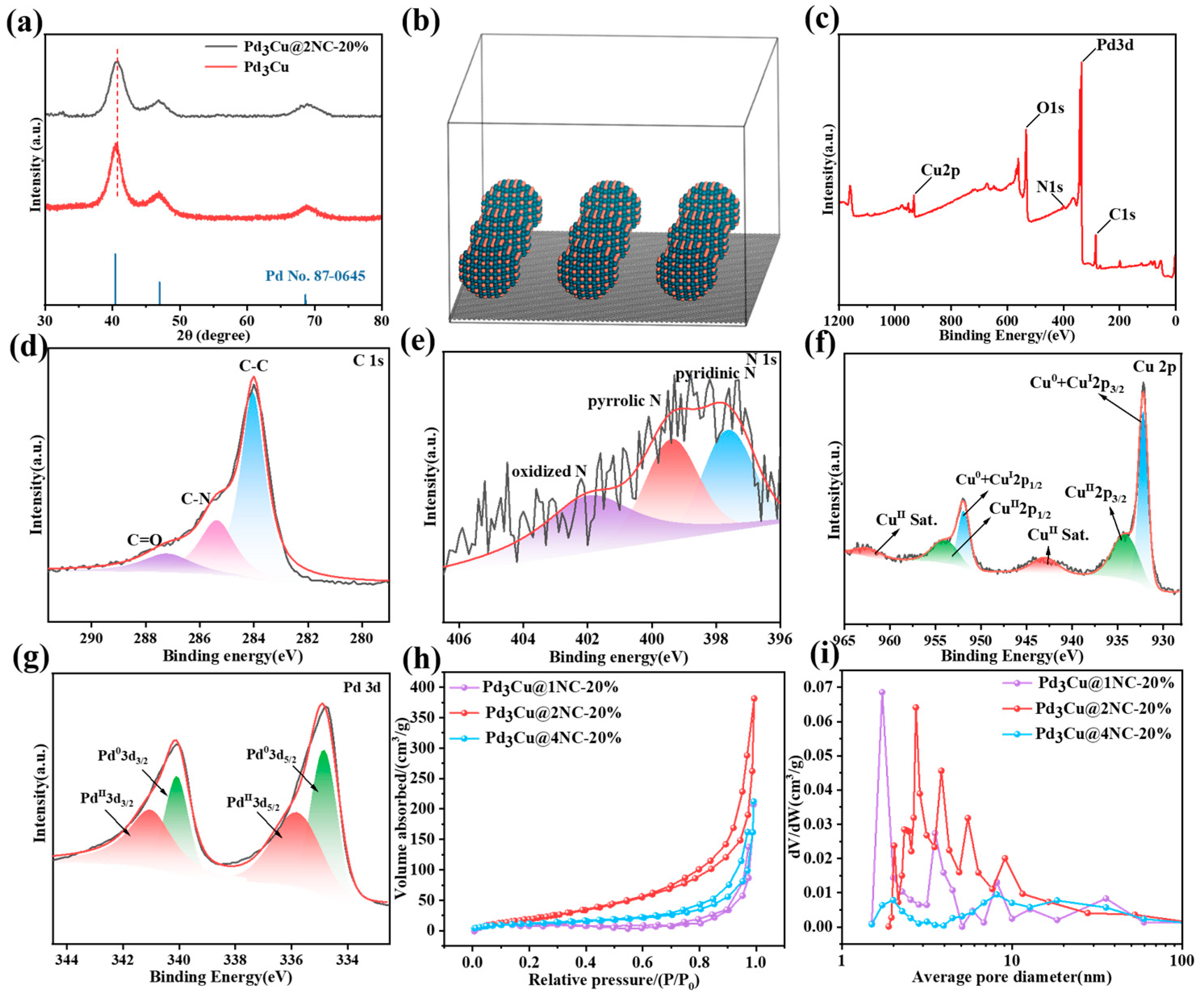
2.1. Chemical Composition and Structural Analysis
2.2. Electrocatalyst Preparation and Microstructure Analysis
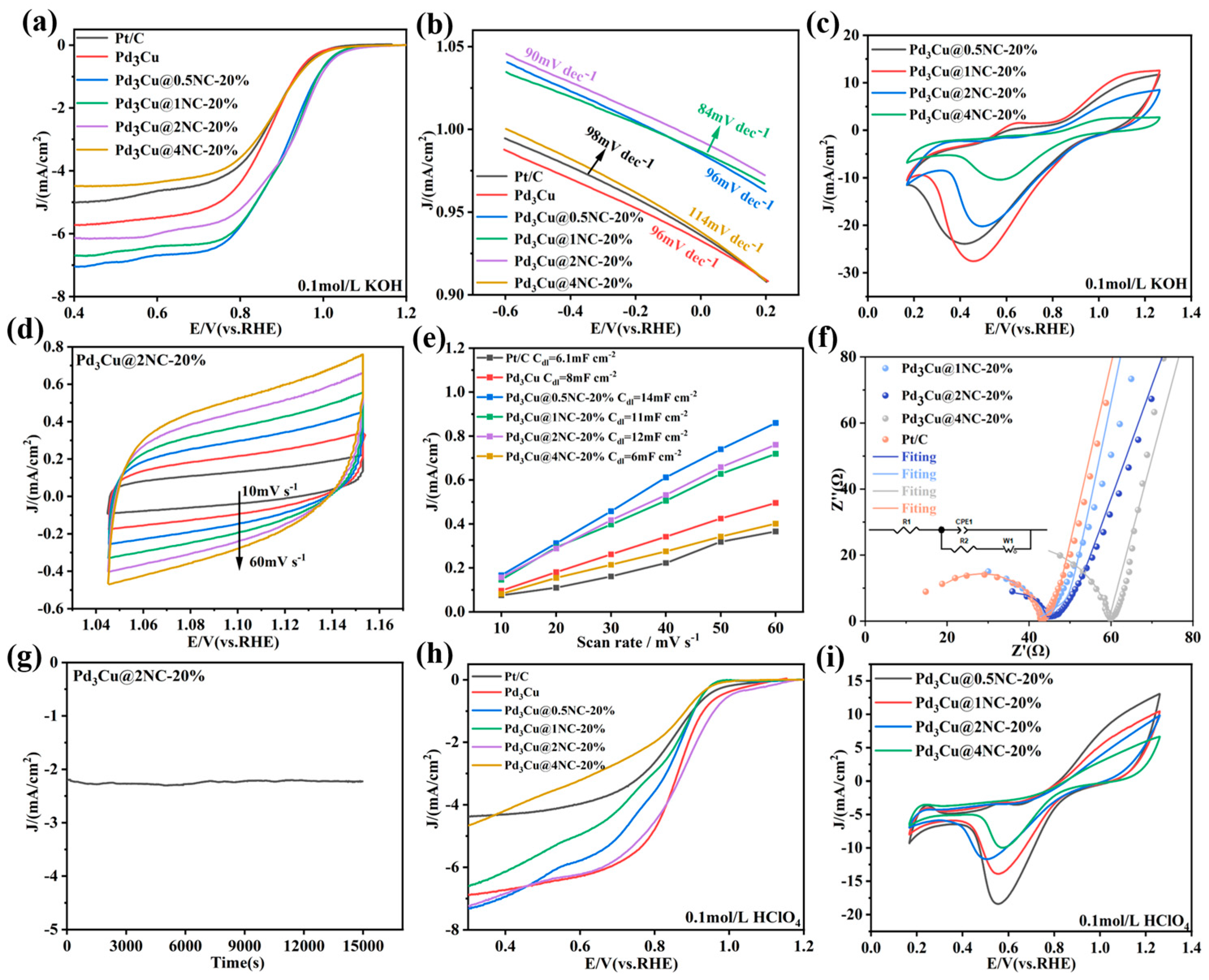
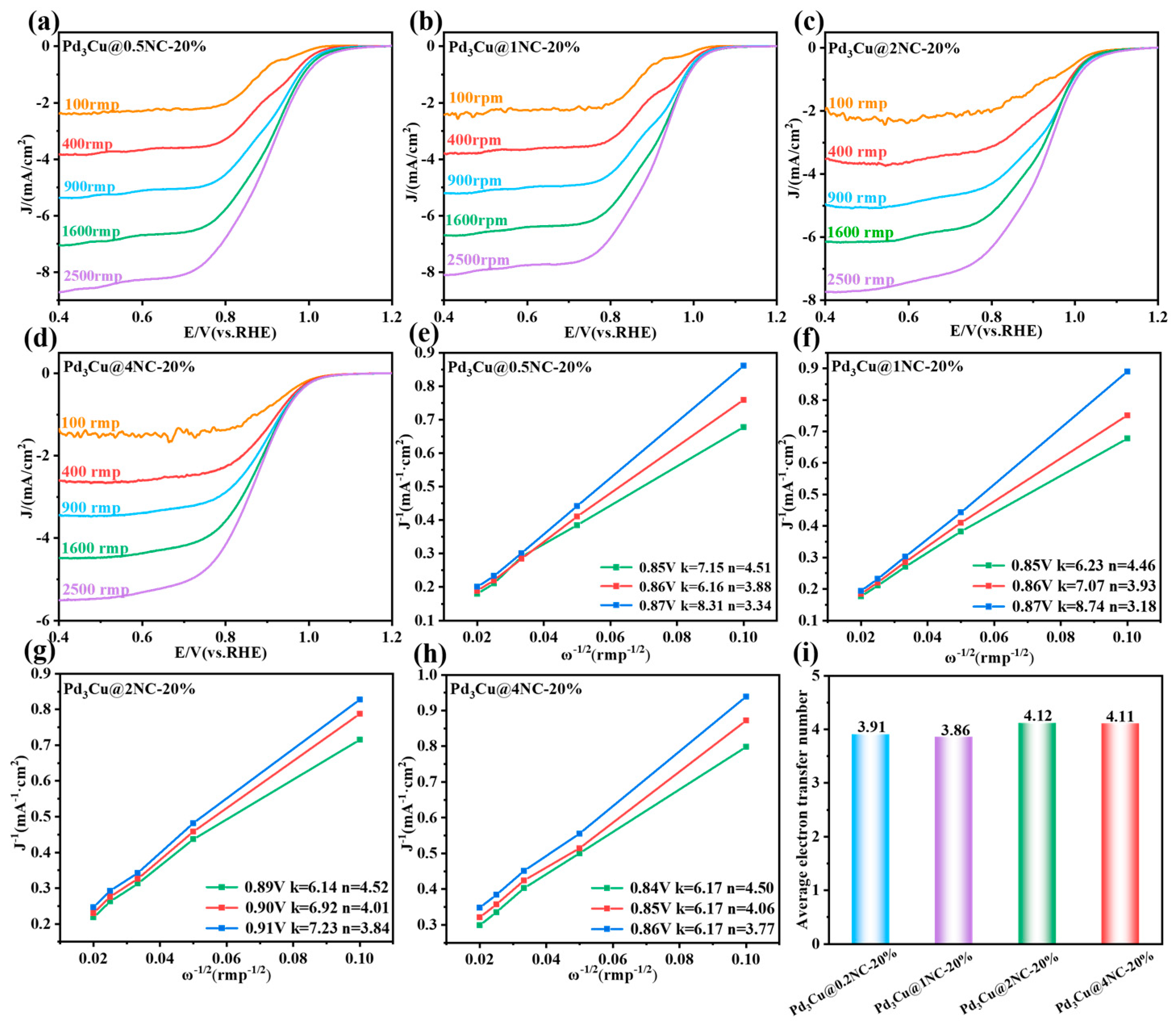
2.3. ORR Performance Analysis
2.4. OER Mechanism Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Method
4.2.1. Synthesis of N-Doped Carbon Aerogel
4.2.2. Synthesis of Pd3Cu@NC Aerogel
4.2.3. Characterizations
4.2.4. Electrochemical Measurements
4.2.5. Theoretical Calculations Details
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, L.; Ma, Y.; Vequizo, J.J.M.; Nakabayashi, M.; Gu, C.; Tao, X.; Yoshida, H.; Pihosh, Y.; Nishina, Y.; Yamakata, A.; et al. Efficient and stable visible-light-driven Z-scheme overall water splitting using an oxysulfide H2 evolution photocatalyst. Nat. Commun. 2024, 15, 397. [Google Scholar] [CrossRef] [PubMed]
- Slobodkin, I.; Davydova, E.; Sananis, M.; Breytus, A.; Rothschild, A. Electrochemical and chemical cycle for high-efficiency decoupled water splitting in a near-neutral electrolyte. Nat. Mater. 2024, 23, 398–405. [Google Scholar] [CrossRef]
- Yang, B.; Xiang, Z. Nanostructure engineering of cathode layers in proton exchange membrane fuel cells: From catalysts to membrane electrode assembly. ACS Nano 2024, 18, 11598–11630. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Trogadas, P.; Zhou, S.; Jiang, S.; Wu, Y.; Rasha, L.; Kockelmann, W.; Yang, J.D.; Neville, T.; Jervis, R.; et al. A scalable and robust water management strategy for PEMFCs: Operando electrothermal mapping and neutron imaging study. Adv. Sci. 2024, 11, 2404350. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Bai, J.; Zhang, N.; Jiang, Z.; Wang, Y.; Xiao, M.; Liu, C.; Zhu, S.; Xu, Z.J.; Ge, J.; et al. Rare earth evoked subsurface oxygen species in platinum alloy catalysts enable durable fuel cells. Angew. Chem. Int. Edit. 2024, 136, e202315119. [Google Scholar] [CrossRef]
- Shin, S.; Lee, E.; Nam, J.; Kwon, J.; Choi, Y.; Kim, B.J.; Ham, H.C.; Lee, H. Carbon-embedded Pt alloy cluster catalysts for proton exchange membrane fuel cells. Adv. Energy Mater. 2024, 14, 2400599. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, Y. Qualitative and quantitative electrochemiluminescence evaluation of trace Pt single-atom in MXenes. Nat. Commun. 2024, 15, 7086. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Li, S.; Cretu, O.; Kimoto, K.; Zhao, Y.; Zhu, L.; Wei, X.; Fu, L.; Jiang, D.; Wan, C.; et al. Mesoporous amorphous non-noble metals as versatile substrates for high loading and uniform dispersion of Pt-group single atoms. Sci. Adv. 2024, 10, eado2442. [Google Scholar] [CrossRef]
- Shi, K.; Si, D.; Teng, X.; Chen, L.; Shi, J. Pd/NiMoO4/NF electrocatalysts for the efficient and ultra-stable synthesis and electrolyte-assisted extraction of glycolate. Nat. Commun. 2024, 15, 2899. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, D.; Zhu, B.; Cheng, B.; Yu, J.; Yu, H. Enhancing photocatalytic H2O2 production with Au co-catalysts through electronic structure modification. Nat. Commun. 2024, 15, 3212. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Wang, J.; Ni, G.; Liu, K.; Liu, C.; Chen, S.; Wang, Q.; Chen, Y.; Luo, T.; Wang, X.; et al. Sustainable conversion of alkaline nitrate to ammonia at activities greater than 2 A cm−2. Nat. Commun. 2024, 15, 1264. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Chen, C.; Zhang, Y.; Wang, M.; Han, S.; Dong, X.; Yao, T.; Jia, S.; He, M.; Wu, H.; et al. Cooperation of different active sites to promote CO2 electroreduction to multi-carbon products at ampere-level. Angew. Chem. Int. Edit. 2024, 136, e202400439. [Google Scholar] [CrossRef]
- Do, V.H.; Prabhu, P.; Jose, V.; Yoshida, T.; Zhou, Y.; Miwa, H.; Kaneko, T.; Uruga, T.; Iwasawa, Y.; Lee, J.M. Pd–PdO nanodomains on amorphous Ru metallene oxide for high-performance multifunctional electrocatalysis. Adv. Mater. 2023, 35, 2208860. [Google Scholar] [CrossRef]
- Abdellah, A.M.; Ismail, F.; Siig, O.W.; Yang, J.; Andrei, C.M.; DiCecco, L.-A.; Rakhsha, A.; Salem, K.E.; Grandfield, K.; Bassim, N.; et al. Impact of palladium/palladium hydride conversion on electrochemical CO2 reduction via in-situ transmission electron microscopy and diffraction. Nat. Commun. 2024, 15, 938. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, W.; Yu, H.; Mao, Q.; Xu, Y.; Li, X.; Wang, Z.; Wang, L. Interface engineering of polyaniline-functionalized porous Pd metallene for alkaline oxygen reduction reaction. Appl. Catal. B Environ. Energy 2022, 307, 121172. [Google Scholar] [CrossRef]
- Zhong, M.; Xu, M.; Ren, S.; Li, W.; Wang, C.; Gao, M.; Lu, X. Modulating the electronic structure of Ni(OH)2 by coupling with low-content Pt for boosting the urea oxidation reaction enables significantly promoted energy-saving hydrogen production. Energy Environ. Sci. 2024, 17, 1984–1996. [Google Scholar] [CrossRef]
- Hu, S.; Qiao, P.; Liang, X.; Ba, G.; Zu, X.; Hu, H.; Ye, J.; Wang, D. Single-atom Pt–N4 active sites anchored on porous C3N4 nanosheet for boosting the photocatalytic CO2 reduction with nearly 100% CO selectivity. Appl. Catal. B Environ. Energy 2024, 346, 123737. [Google Scholar] [CrossRef]
- Wei, X.; Song, S.; Cai, W.; Kang, Y.; Fang, Q.; Ling, L.; Zhao, Y.; Wu, Z.; Song, X.; Xu, X.; et al. Pt nanoparticle–Mn single-atom pairs for enhanced oxygen reduction. ACS Nano 2024, 18, 4308–4319. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, L.; Liu, S.; Li, S.; Zhou, J.; Li, X.; Chen, X.; Sun, K.; Li, B.; Jiang, J.; et al. Fe, N-inducing interfacial electron redistribution in NiCo spinel on biomass-derived carbon for bi-functional oxygen conversion. Angew. Chem. Int. Edit. 2024, 136, e202319983. [Google Scholar] [CrossRef]
- Hua, Y.; Song, N.; Wu, Z.; Lan, Y.; Luo, H.; Song, Q.; Yang, J. Cu–Fe synergistic active sites boost kinetics of electrochemical nitrate reduction. Adv. Funct. Mater. 2024, 34, 2314461. [Google Scholar] [CrossRef]
- Snitkoff-Sol, R.Z.; Rimon, O.; Bond, A.M.; Elbaz, L. Direct measurement of the oxygen reduction reaction kinetics on iron phthalocyanine using advanced transient voltammetry. Nat. Catal. 2024, 7, 139–147. [Google Scholar] [CrossRef]
- Lin, Z.; Yang, A.; Zhang, B.; Liu, B.; Zhu, J.; Tang, Y.; Qiu, X. Coupling the atomically dispersed Fe-N3 sites with sub-5 nm Pd nanocrystals confined in N-doped carbon nanobelts to boost the oxygen reduction for microbial fuel cells. Adv. Funct. Mater. 2022, 32, 2107683. [Google Scholar] [CrossRef]
- Tian, Q.; Jing, L.; Du, H.; Yin, Y.; Cheng, X.; Xu, J.; Chen, J.; Liu, Z.; Wan, J.; Liu, J.; et al. Mesoporous carbon spheres with programmable interiors as efficient nanoreactors for H2O2 electrosynthesis. Nat. Commun. 2024, 15, 983. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Zhao, J.; Zhang, P.; Wang, S.; Wang, Y.; Zhang, Z.; Wen, N.; Ding, Z.; Yuan, R.; Wang, X.; et al. Highly selective photocatalytic reduction of CO2 to CH4 on electron-rich Fe species cocatalyst under visible light irradiation. Carbon Energy 2024, 6, e435. [Google Scholar] [CrossRef]
- Park, M.G.; Hwang, J.; Deng, Y.P.; Lee, D.U.; Fu, J.; Hu, Y.; Jang, M.J.; Choi, S.M.; Feng, R.; Jiang, G.; et al. Longevous cycling of rechargeable Zn-air battery enabled by “raisin-bread” cobalt oxynitride/porous carbon hybrid electrocatalysts. Adv. Mater. 2024, 36, 2311105. [Google Scholar] [CrossRef]
- Wu, X.; Ni, C.; Man, J.; Shen, X.; Cui, S.; Chen, X. A strategy to promote the ORR electrocatalytic activity by the novel engineering bunched three-dimensional Pd-Cu alloy aerogel. Chem. Eng. J. 2023, 454, 140293. [Google Scholar] [CrossRef]
- Yang, Z.; Li, Y.; Zhang, X.; Cui, X.; He, S.; Liang, H.; Ding, A. Sludge activated carbon-based CoFe2O4-SAC nanocomposites used as heterogeneous catalysts for degrading antibiotic norfloxacin through activating peroxymonosulfate. Chem. Eng. J. 2020, 384, 123319. [Google Scholar] [CrossRef]
- Hao, X.; Jiang, Z.; Zhang, B.; Tian, X.; Song, C.; Wang, L.; Maiyalagan, T.; Hao, X.; Jiang, Z.-J. N-doped carbon nanotubes de-rived from graphene oxide with embedment of FeCo nanoparticles as bifunctional air electrode for rechargeable liquid and flexible all-solid-state zinc–air batteries. Adv. Sci. 2021, 8, 2004572. [Google Scholar] [CrossRef]
- Keramatinia, M.; Ramezanzadeh, B.; Mahdavian, M.; Bahlakeh, G. Chemically controlled nitrogen-doped reduced-Graphene/Graphite oxide frameworks for aiding superior thermal/anti-corrosion performance: Integrated DFT-D & experimental evaluations. Chem. Eng. J. 2022, 437, 135241. [Google Scholar]
- Ahsan, M.A.; Puente Santiago, A.R.; Hong, Y.; Zhang, N.; Cano, M.; Rodriguez-Castellon, E.; Echegoyen, L.; Sreenivasan, S.T.; Noveron, J.C. Tuning of trifunctional NiCu bimetallic nanoparticles confined in a porous carbon network with surface com-position and local structural distortions for the electrocatalytic oxygen reduction, oxygen and hydrogen evolution reactions. J. Am. Chem. Soc. 2020, 142, 14688–14701. [Google Scholar] [CrossRef]
- Yan, R.; Yin, H.; Zuo, X.F.; Peng, W.; Zhu, X.; Shi, L.; Hou, J.; Wang, D.; Ye, F.; Li, J.; et al. Hollow PdCuCo medium-entropy alloy on reduced graphene oxide with proton-mediator boosted tandem catalysis for high-performance nitrate reduction. Appl. Catal. B Environ. Energy 2025, 361, 124609. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, G.; Liu, W.; Li, G.; He, T.; Zhang, H.; Yu, Y.; Peng, H. constructing highly active zeolite encapsulated PdAg alloy catalysts for typical VOCs deep oxidation: The role of electron-structure interaction. Appl. Catal. B Environ. Energy 2024, 352, 124051. [Google Scholar] [CrossRef]
- Zhu, C.; Shi, Q.; Fu, S.; Song, J.; Xia, H.; Du, D.; Lin, Y. Efficient synthesis of MCu (M = Pd, Pt, and Au) aerogels with accelerat-ed gelation kinetics and their high electrocatalytic activity. Adv. Mater. 2016, 28, 8779–8783. [Google Scholar] [CrossRef]
- Shi, Q.; Zhu, C.; Zhong, H.; Su, D.; Li, N.; Engelhard, M.H.; Xia, H.; Zhang, Q.; Feng, S.; Beckman, S.P.; et al. Nanovoid incor-porated IrxCu metallic aerogels for oxygen evolution reaction catalysis. ACS Energy Lett. 2018, 3, 2038–2044. [Google Scholar] [CrossRef]
- Naskar, S.; Freytag, A.; Deutsch, J.; Wendt, N.; Behrens, P.; Köckritz, A.; Bigall, N.C. Porous aerogels from shape-controlled metal nanoparticles directly from nonpolar colloidal solution. Chem. Mater. 2017, 29, 9208–9217. [Google Scholar] [CrossRef]
- Yuan, W.; Ding, T.; Mou, P.; Luo, Y.; Li, L.; Chen, Y.; Chen, X.; Shu, J.; Zhang, L. Semi-solid CNT@ NaK anode for potassium metal battery. Adv. Funct. Mater. 2023, 33, 2209774. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Z.; Wang, J.; Zhong, C.-J.; Liu, C.-J. Highly active and stable Pt–Pd alloy catalysts synthesized by room-temperature electron reduction for oxygen reduction reaction. Adv. Sci. 2017, 4, 1600486. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Huang, H.; Tai, J.; Wu, X.; Guo, Z.; Shen, X.; Cui, S.; Chen, X. The catalytic activity of reduced graphene aerogel anchored with CoFe2O4 spinel via self-assembly technique for enhanced oxygen evolution reaction. Carbon 2024, 219, 118847. [Google Scholar] [CrossRef]
- Mohamed, I.M.A.; Kanagaraj, P.; Yasin, A.S.; Iqbal, W.; Liu, C. Electrochemical impedance investigation of urea oxidation in alkaline media based on electrospun nanofibers towards the technology of direct-urea fuel cells. J. Alloys Compd. 2020, 816, 152513. [Google Scholar] [CrossRef]
- Qin, Y.; Ou, Z.; Guo, C.; Liu, Y.; Jin, R.; Xu, C.; Chen, H.; Si, Y.; Li, H. Phosphor-doping modulates the d-band center of Fe atoms in Fe-N4 catalytic sites to boost the activity of oxygen reduction. Appl. Catal. B Environ. 2025, 360, 124553. [Google Scholar] [CrossRef]
- Seong, H.; Min, K.; Lee, G.; Kwon, K.; Baeck, S.-H. Development of an efficient bifunctional electrocatalyst based on Co/CoSe2 nanoparticles embedded in N, Se co-doped carbon for AEMFC and rechargeable Zn-air battery. Appl. Catal. B Environ. 2025, 362, 124725. [Google Scholar] [CrossRef]
- Yin, X.; Xi, W.; Wang, P.; Wu, T.; Liu, P.; Gao, B.; Zheng, Y.; Lin, B. In-situ construction of 2D β-Co(OH)2 nanosheets hybridized with 1D N-doped carbon nanotubes as efficient bifunctional electrocatalyst for oxygen reduction and evolution reactions. Chem. Eng. J. 2025, 503, 158437. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, D.; Zhang, Y.; Yan, F.; Sun, L.; Jin, X.; Wang, Q.; Zheng, L.; Li, W. Regulation of the diatomic-site electronic structure with alloy nanoparticles in hollow carbon nanospheres for efficient oxygen reduction and evolution reactions. Chem. Eng. J. 2025, 503, 158579. [Google Scholar] [CrossRef]
- Wu, X.; Liu, L.; Yuan, K.; Shao, Y.; Shen, X.; Cui, S.; Chen, X. Modulating Electronic Structure and Atomic Insights into the Novel Hierarchically Porous PdCuFe Trimetallic Alloy Aerogel for Efficient Oxygen Reduction. Small 2024, 20, 202307243. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Lu, Q.; Wu, J.; Zhang, K.; Tang, M.; Wu, B.; She, S.; Zhang, X.; Shao, Z.; An, L. Screening Spinel Oxide Supports for RuO2 to Boost Bifunctional Electrocatalysts for Advanced Zn–Air Batteries. Adv. Funct. Mater. 2024, 34, 202401134. [Google Scholar] [CrossRef]
- Wang, M.; Xie, J.; Lu, Z.; Wang, J.; Yin, X.; Cao, Y. Biphase Alloy Nanoheterojunction Encapsulated within N-Doped Carbon Nanotubes as Bifunctional Oxygen Electrocatalyst for High-Performance Zn-Air and Mg-Air Batteries. Adv. Funct. Mater. 2025; 202423767, early view. [Google Scholar]
- Li, Y.; Luo, X.; Wei, Z.; Zhang, F.; Sun, Z.; Deng, Z.; Zhan, Z.; Zhao, C.; Sun, Q.; Zhang, L. Precisely constructing charge-asymmetric dual-atom Fe sites supported on hollow porous carbon spheres for efficient oxygen reduction. Energy Environ. Sci. 2024, 17, 4646–4657. [Google Scholar] [CrossRef]
- Chen, Z.; Cheng, W.; Cao, K.; Jin, M.; Rahali, S.; Chala, S.A.; Ebrahimi, E.; Ma, N.; Liu, R.; Lakshmanan, K. A Bifunctional Iron-Nickel Oxygen Reduction/Oxygen Evolution Catalyst for High-Performance Rechargeable Zinc-Air Batteries. Small 2024, 21, 202409161. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhang, L.; Huang, X.; Kong, Q.; Abdukayum, A.; Zhou, Y.; Cheng, G.; Gao, S.; Hu, G. Efficient Catalysis for Zinc-Air Batteries by Multiwalled Carbon Nanotubes-Crosslinked Carbon Dodecahedra Embedded with Co-Fe Nanoparticles. Small 2025, 21, 202409129. [Google Scholar] [CrossRef]
- Zou, J.; Bao, L.; Sun, Q.; Bao, C.; Chen, H.; Liu, H. Oxygen Reduction Reaction Catalysts for Zinc-Air Batteries Featuring Single Cobalt Atoms in a Nitrogen-Doped 3D-Interconnected Porous Graphene Framework. Small 2025, 21, 202409506. [Google Scholar] [CrossRef]
- Huang, S.; Tranca, D.; Rodríguez-Hernández, F.; Zhang, J.; Lu, C.; Zhu, J.; Liang, H.W.; Zhuang, X. Well-defined N3C1-anchored Single-Metal-Sites for Oxygen Reduction Reaction. Angew. Chem. Int. Edit. 2023, 63, e202314833. [Google Scholar] [CrossRef] [PubMed]
- Ban, J.; Wen, X.; Xu, H.; Wang, Z.; Liu, X.; Cao, G.; Shao, G.; Hu, J. Dual Evolution in Defect and Morphology of Single-Atom Dispersed Carbon Based Oxygen Electrocatalyst. Adv. Funct. Mater. 2021, 31, 2010472. [Google Scholar] [CrossRef]
- Jin, B.; Wang, Y.; Liu, Z.; France-Lanord, A.; Grossman, J.C.; Jin, C.; Tang, R. Revealing the cluster-cloud and its role in nano-crystallization. Adv. Mater. 2019, 31, 1808225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, Z.; Liu, F.; Yi, P.; Peng, L.; Chen, Y.; Wei, L.; Li, H. Unraveling the pH-dependent oxygen reduction perfor-mance on single-atom catalysts: From single- to dual-Sabatier optima. J. Am. Chem. Soc. 2024, 146, 3210–3219. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y.; Geng, S.; Wang, Y.; Song, S. High-temperature confinement synthesis of supported Pt–Ni nanoparticles for efficiently catalyzing oxygen reduction reaction. Adv. Funct. Mater. 2022, 32, 2113399. [Google Scholar] [CrossRef]
- Wu, J.; Han, Y.; Bai, Y.; Wang, X.; Zhou, Y.; Zhu, W.; He, T.; Wang, Y.; Huang, H.; Liu, Y.; et al. The electron transport regula-tion in carbon dots/In2O3 electrocatalyst enables 100% selectivity for oxygen reduction to hydrogen peroxide. Adv. Funct. Mater. 2022, 32, 2203647. [Google Scholar] [CrossRef]
- Shao, X.; Gan, R.; Rao, Y.; Nga, T.T.T.; Liang, M.; Dong, C.-L.; Ma, C.; Lee, J.Y.; Li, H.; Lee, H. Main Group SnN4O Single Sites with Optimized Charge Distribution for Boosting the Oxygen Reduction Reaction. ACS Nano 2024, 18, 14742–14753. [Google Scholar] [CrossRef] [PubMed]


| Samples | ΔGOH* | ΔGO* | ΔGOOH* | ΔG1 | ΔG2 | ΔG3 | ΔG4 |
|---|---|---|---|---|---|---|---|
| Site1 | 0.345 | 1.244 | 3.717 | −1.202 | −2.472 | −0.898 | −0.346 |
| Site2 | 0.386 | 1.431 | 3.965 | −0.954 | −2.534 | −1.044 | −0.387 |
| Site3 | −0.211 | 1.429 | 3.432 | −1.488 | −2.003 | −1.640 | 0.211 |
| Site4 | 1.232 | 1.394 | 4.110 | −0.809 | −2.716 | −0.161 | −1.232 |
| Site5 | 0.359 | 1.380 | 3.988 | −0.932 | −2.609 | −1.020 | −0.359 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Y.; Hao, W.; Altaf, A.; Lu, J.; Liu, L.; Zhu, C.; Gu, X.; Wu, X.; Shen, X.; Cui, S.; et al. Construction of PdCu Alloy Decorated on the N-Doped Carbon Aerogel as a Highly Active Electrocatalyst for Enhanced Oxygen Reduction Reaction. Gels 2025, 11, 166. https://doi.org/10.3390/gels11030166
Bai Y, Hao W, Altaf A, Lu J, Liu L, Zhu C, Gu X, Wu X, Shen X, Cui S, et al. Construction of PdCu Alloy Decorated on the N-Doped Carbon Aerogel as a Highly Active Electrocatalyst for Enhanced Oxygen Reduction Reaction. Gels. 2025; 11(3):166. https://doi.org/10.3390/gels11030166
Chicago/Turabian StyleBai, Yangxin, Wenke Hao, Aleeza Altaf, Jiaxin Lu, Liu Liu, Chuanyong Zhu, Xindi Gu, Xiaodong Wu, Xiaodong Shen, Sheng Cui, and et al. 2025. "Construction of PdCu Alloy Decorated on the N-Doped Carbon Aerogel as a Highly Active Electrocatalyst for Enhanced Oxygen Reduction Reaction" Gels 11, no. 3: 166. https://doi.org/10.3390/gels11030166
APA StyleBai, Y., Hao, W., Altaf, A., Lu, J., Liu, L., Zhu, C., Gu, X., Wu, X., Shen, X., Cui, S., & Chen, X. (2025). Construction of PdCu Alloy Decorated on the N-Doped Carbon Aerogel as a Highly Active Electrocatalyst for Enhanced Oxygen Reduction Reaction. Gels, 11(3), 166. https://doi.org/10.3390/gels11030166








