The Optimization of Culture Conditions for Injectable Recombinant Collagen Hydrogel Preparation Using Machine Learning
Abstract
1. Introduction
2. Results and Discussion
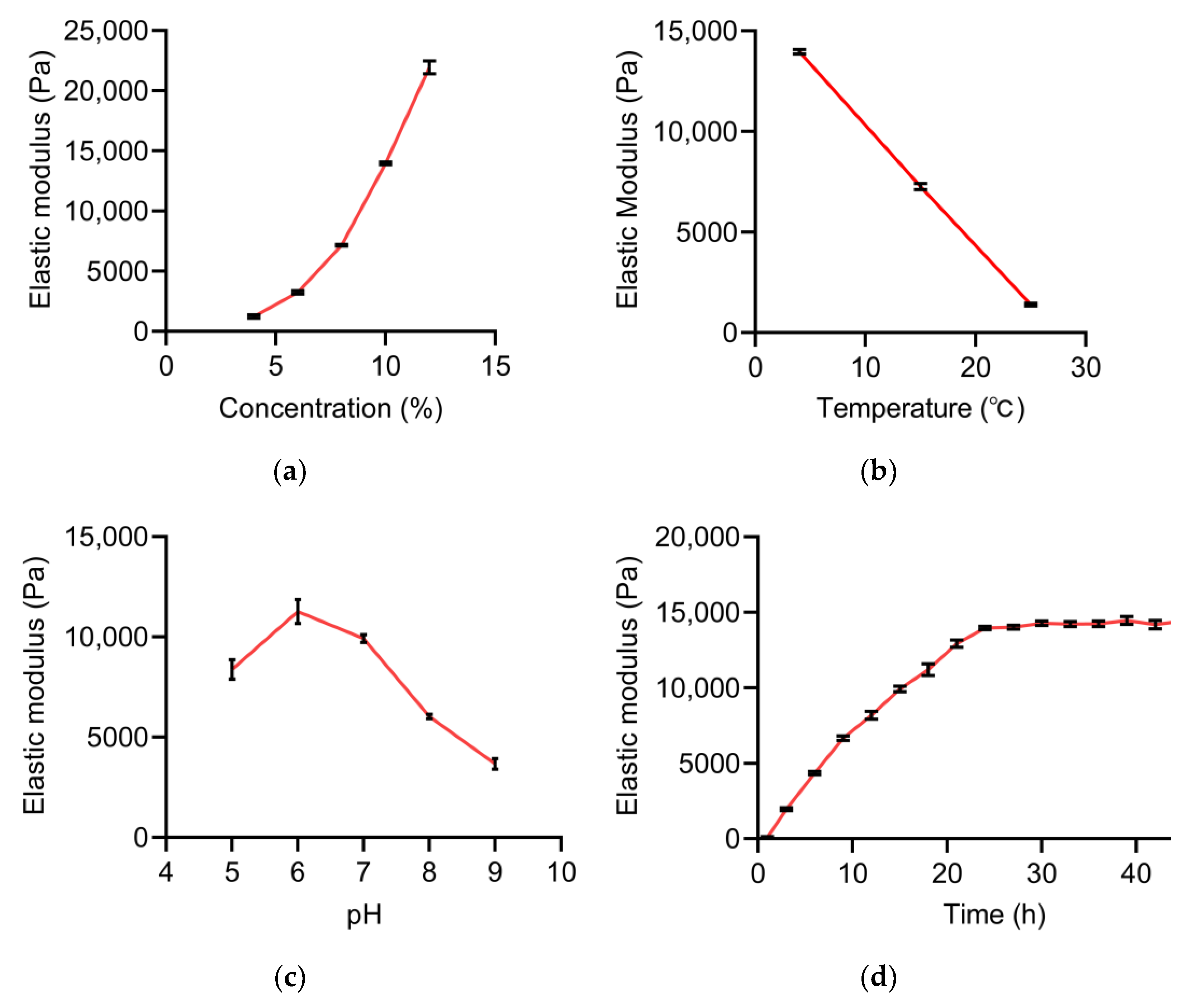
2.1. Single-Factor Experiment Analysis
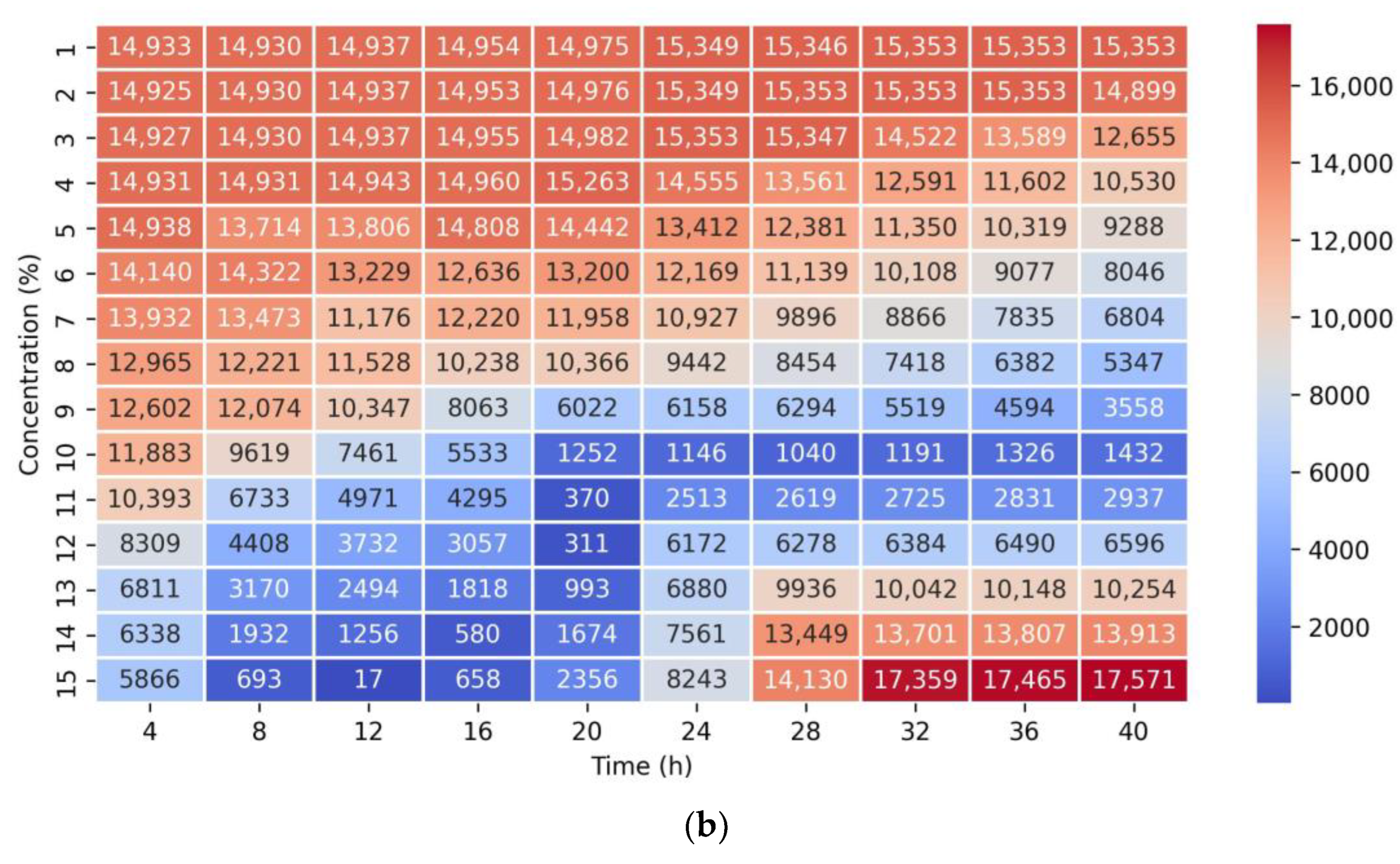
2.2. Model Analysis Results and Comparison
2.2.1. Multiple Linear Regression (ML)
2.2.2. Decision Tree (DT)
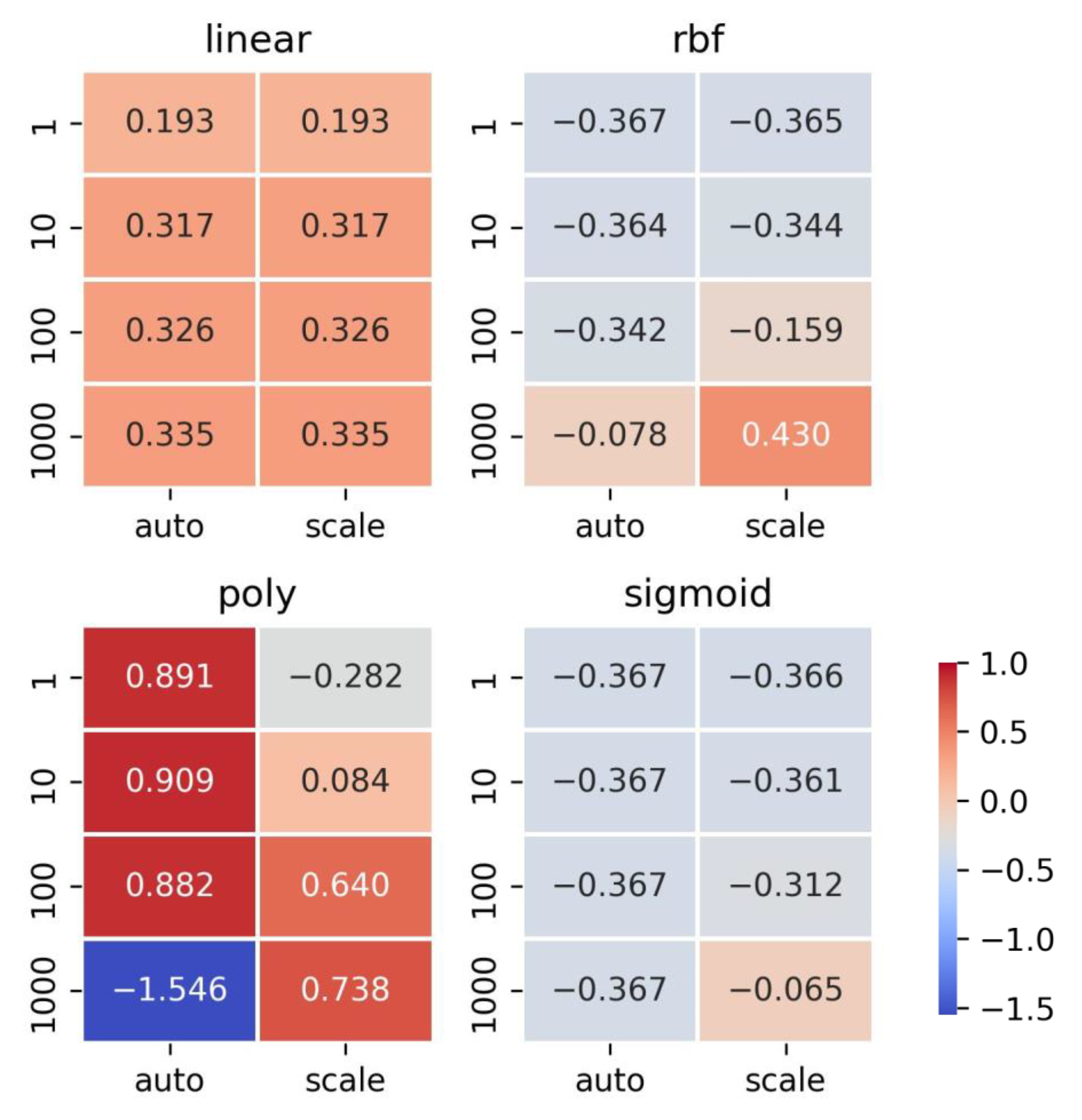
2.2.3. Support Vector Machine (SVM)
2.2.4. Neural Network (NN)
2.3. Model Evaluation and Prediction of Optimal Conditions
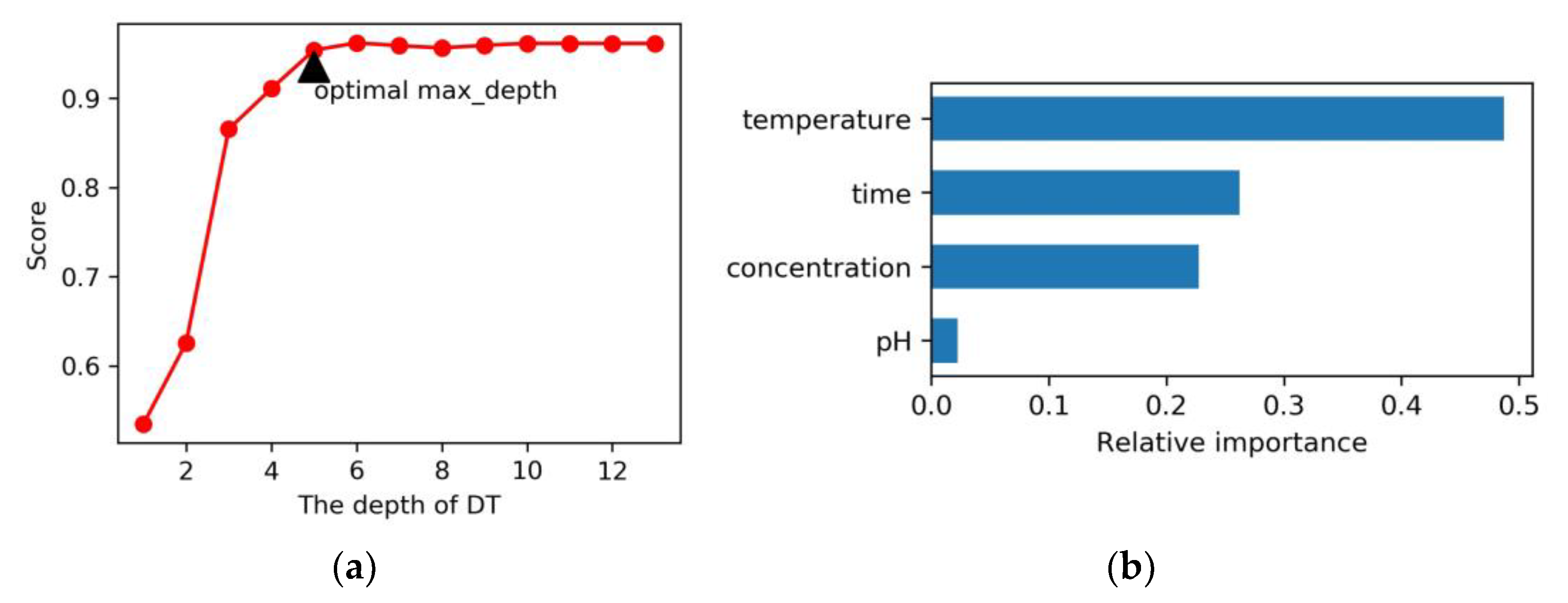
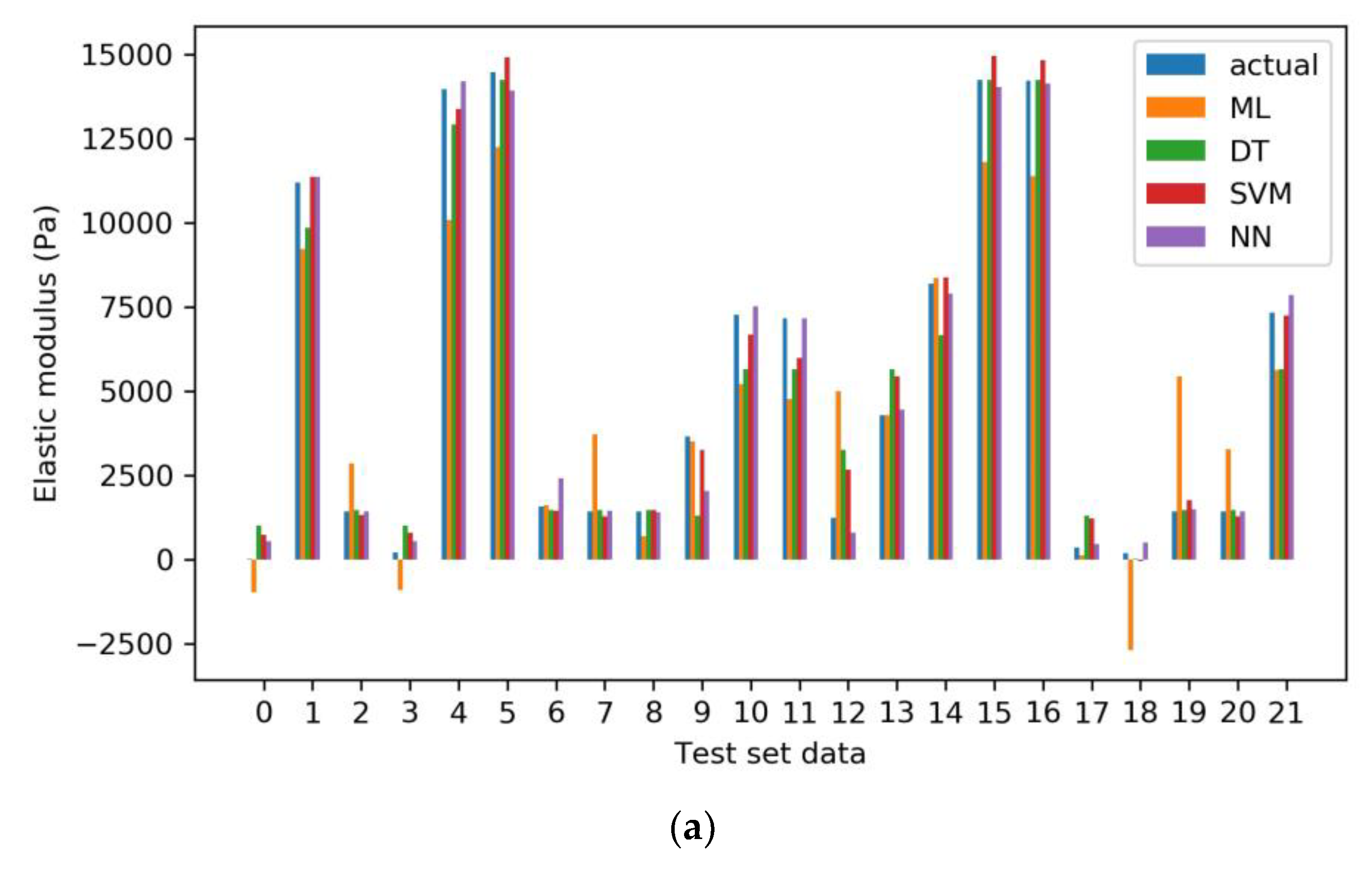
2.3.1. Model Evaluation
2.3.2. Prediction of Optimal Conditions
3. Conclusions
4. Materials and Methods
4.1. Single-Factor Experiments
4.2. Elastic Modulus Detection
4.3. Model Fitting and Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geckil, H.; Xu, F.; Zhang, X.H.; Moon, S.; Demirci, U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine 2010, 5, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Gosecka, M.; Bodaghi, M.; Crespy, D.; Youssef, G.; Dodda, J.M.; Wong, S.H.D.; Imran, A.B.; Gosecki, M.; Jobdeedamrong, A.; et al. Engineering multifunctional dynamic hydrogel for biomedical and tissue regenerative applications. Chem. Eng. J. 2024, 487, 150403. [Google Scholar] [CrossRef]
- Khattak, S.; Ullah, I.; Xie, H.; Tao, X.-D.; Xu, H.-T.; Shen, J. Self-healing hydrogels as injectable implants: Advances in translational wound healing. Coord. Chem. Rev. 2024, 509, 215790. [Google Scholar] [CrossRef]
- Nejati, S.; Mongeau, L. Injectable, pore-forming, self-healing, and adhesive hyaluronan hydrogels for soft tissue engineering applications. Sci. Rep. 2023, 13, 14303. [Google Scholar] [CrossRef]
- Tang, S.C.; Richardson, B.M.; Anseth, K.S. Dynamic covalent hydrogels as biomaterials to mimic the viscoelasticity of soft tissues. Prog. Mater. Sci. 2021, 120, 100738. [Google Scholar] [CrossRef]
- Li, C.; Deng, R.H.; Yang, M.; Yuan, F.Z.; Zhang, C.; Yu, J.K. Advanced Hydrogel Material for Meniscus Repair. Adv. Funct. Mater. 2024, 34, 12276. [Google Scholar] [CrossRef]
- Peng, Y.X.; Liang, S.; Meng, Q.F.; Liu, D.; Ma, K.S.; Zhou, M.L.; Yun, K.Q.; Rao, L.; Wang, Z.H. Engineered Bio-Based Hydrogels for Cancer Immunotherapy. Adv. Mater. 2024, 36, 13188. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.D.; Soranno, D.E.; Rodell, C.B.; Kim, I.L.; Burdick, J.A. Secondary Photocrosslinking of Injectable Shear-Thinning Dock-and-Lock Hydrogels. Adv. Healthc. Mater. 2013, 2, 1028–1036. [Google Scholar] [CrossRef]
- Parisi-Amon, A.; Mulyasasmita, W.; Chung, C.; Heilshorn, S.C. Protein-Engineered Injectable Hydrogel to Improve Retention of Transplanted Adipose-Derived Stem Cells. Adv. Healthc. Mater. 2013, 2, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.P.; Hsu, S.H. Self-healing hydrogel as an injectable implant: Translation in brain diseases. J. Biomed. Sci. 2023, 30, 43. [Google Scholar] [CrossRef]
- Ding, X.Y.; Li, G.; Zhang, P.; Jin, E.; Xiao, C.S.; Chen, X.S. Injectable Self-Healing Hydrogel Wound Dressing with Cysteine-Specific On-Demand Dissolution Property Based on Tandem Dynamic Covalent Bonds. Adv. Funct. Mater. 2021, 31, 11230. [Google Scholar] [CrossRef]
- Wu, J.W.; Chen, Q.; Deng, C.; Xu, B.P.; Zhang, Z.Y.; Yang, Y.; Lu, T.L. Exquisite design of injectable Hydrogels in Cartilage Repair. Theranostics 2020, 10, 9843–9864. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lin, W.; Astruc, D.; Gu, H.B. Syntheses and applications of dendronized polymers. Prog. Polym. Sci. 2019, 96, 43–105. [Google Scholar] [CrossRef]
- Volpi, M.; Paradiso, A.; Costantini, M.; Swie, W. Hydrogel-Based Fiber Biofabrication Techniques for Skeletal Muscle Tissue Engineering. ACS Biomater. Sci. Eng. 2022, 8, 379–405. [Google Scholar] [CrossRef]
- Fan, M.; Ma, Y.; Mao, J.H.; Zhang, Z.W.; Tan, H.P. Cytocompatible forming chitosan/hyaluronan hydrogels via a metal-free click chemistry for soft tissue engineering. Acta Biomater. 2015, 20, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Raia, N.R.; Partlow, B.P.; McGill, M.; Kimmerling, E.P.; Ghezzi, C.E.; Kaplan, D.L. Enzymatically crosslinked silk-hyaluronic acid hydrogels. Biomaterials 2017, 131, 58–67. [Google Scholar] [CrossRef]
- Feng, Y.; Xiao, K.C.; Chen, J.L.; Lin, J.J.; He, Y.Y.; He, X.L.; Cheng, F.Y.; Li, Z.; Li, J.H.; Luo, F.; et al. Immune-microenvironment modulatory polyurethane-hyaluronic acid hybrid hydrogel scaffolds for diabetic wound treatment. Carbohyd. Polym. 2023, 320, 121238. [Google Scholar] [CrossRef]
- Gwon, K.; Kim, E.; Tae, G. Heparin-hyaluronic acid hydrogel in support of cellular activities of 3D encapsulated adipose derived stem cells. Acta Biomater. 2017, 49, 284–295. [Google Scholar] [CrossRef]
- Yang, R.; Liu, X.; Ren, Y.H.; Xue, W.L.; Liu, S.; Wang, P.H.; Zhao, M.; Xu, H.; Chi, B. Injectable adaptive self-healing hyaluronic acid/poly (γ-glutamic acid) hydrogel for cutaneous wound healing. Acta Biomater. 2021, 127, 102–115. [Google Scholar] [CrossRef]
- Niemczyk-Soczynska, B.; Kolbuk, D.; Mikulowski, G.; Ciechomska, I.A.; Sajkiewicz, P. Methylcellulose/agarose hydrogel loaded with short electrospun PLLA/laminin fibers as an injectable scaffold for tissue engineering/3D cell culture model for tumour therapies. RSC Adv. 2023, 13, 11889–11902. [Google Scholar] [CrossRef]
- Su, T.; Zhang, M.Y.; Zeng, Q.K.; Pan, W.H.; Huang, Y.J.; Qian, Y.N.; Dong, W.; Qi, X.L.; Shen, J.L. Mussel-inspired agarose hydrogel scaffolds for skin tissue engineering. Bioact. Mater. 2021, 6, 579–588. [Google Scholar] [CrossRef]
- Miguel, S.P.; Ribeiro, M.P.; Brancal, H.; Coutinho, P.; Correia, I.J. Thermoresponsive chitosan-agarose hydrogel for skin regeneration. Carbohyd. Polym. 2014, 111, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Sukarto, A.; Yu, C.; Flynn, L.E.; Amsden, B.G. Co-delivery of Adipose-Derived Stem Cells and Growth Factor-Loaded Microspheres in RGD-Grafted N-Methacrylate Glycol Chitosan Gels for Focal Chondral Repair. Biomacromolecules 2012, 13, 2490–2502. [Google Scholar] [CrossRef]
- Wen, T.Y.; Zhang, C.; Ruan, D.; Li, F.; Ma, J. Constructing Tissue Engineered Nucleus Pulposus with Adipose-Derived Stem Cells and an Injectable Thermosensitive Chitosan Scaffold. J. Biomater. Tiss. Eng. 2014, 4, 1073–1079. [Google Scholar] [CrossRef]
- Zhao, X.; Li, P.; Guo, B.L.; Ma, P.X. Antibacterial and conductive injectable hydrogels based on quaternized chitosan-graft-polyaniline/oxidized dextran for tissue engineering. Acta Biomater. 2015, 26, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, X.X.; Fan, D.D.; Zhu, C.H. New suitable for tissue reconstruction injectable chitosan/collagen-based hydrogels. Soft Matter 2012, 8, 3781–3790. [Google Scholar] [CrossRef]
- Jaikumar, D.; Sajesh, K.M.; Soumya, S.; Nimal, T.R.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Injectable alginate-O-carboxymethyl chitosan/nano fibrin composite hydrogels for adipose tissue engineering. Int. J. Biol. Macromol. 2015, 74, 318–326. [Google Scholar] [CrossRef]
- Steinle, H.; Ionescu, T.M.; Schenk, S.; Golombek, S.; Kunnakattu, S.J.; Özbek, M.T.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Incorporation of Synthetic mRNA in Injectable Chitosan-Alginate Hybrid Hydrogels for Local and Sustained Expression of Exogenous Proteins in Cells. Int. J. Mol. Sci. 2018, 19, 1313. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.T.; Ye, S.; Ni, P.X.Z.; Zhong, M.; Shan, J.; Yuan, T.; Liang, J.; Fan, Y.J.; Zhang, X.D. Polyvinyl-alcohol, chitosan and graphene-oxide composed conductive hydrogel for electrically controlled fluorescein sodium transdermal release. Carbohyd. Polym. 2023, 319, 121172. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.J.; Li, S.H.; Guan, H.H.; Zhang, S.; Zhang, Y.X.; Zhang, M.; Yu, Y.; Chen, X.Y. Preparation and Properties of a Novel Biodegradable Composite Hydrogel Derived from Gelatin/Chitosan and Polylactic Acid as Slow-Release N Fertilizer. Polymers 2023, 15, 997. [Google Scholar] [CrossRef]
- Bharathi, R.; Harini, G.; Sankaranarayanan, A.; Shanmugavadivu, A.; Vairamani, M.; Selvamurugan, N. Nuciferine-loaded chitosan hydrogel-integrated 3D-printed polylactic acid scaffolds for bone tissue engineering: A combinatorial approach. Int. J. Biol. Macromol. 2023, 253, 127492. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.H.; Fan, D.D.; Duan, Z.Z.; Xue, W.J.; Shang, L.A.; Chen, F.L.; Luo, Y.E. Initial investigation of novel human-like collagen/chitosan scaffold for vascular tissue engineering. J. Biomed. Mater. Res. A 2009, 89a, 829–840. [Google Scholar] [CrossRef]
- Bidarra, S.J.; Barrias, C.C.; Granja, P.L. Injectable alginate hydrogels for cell delivery in tissue engineering. Acta Biomater. 2014, 10, 1646–1662. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.F.; Wang, T.T.; Feng, L.; Zhu, J.; Zhang, K.X.; Chen, X.S.; Cui, L.; Yin, J.B. Injectable In Situ Self-Cross-Linking Hydrogels Based on Poly(L-glutamic acid) and Alginate for Cartilage Tissue Engineering. Biomacromolecules 2014, 15, 4495–4508. [Google Scholar] [CrossRef] [PubMed]
- Jeon, O.; Samorezov, J.E.; Alsberg, E. Single and dual crosslinked oxidized methacrylated alginate/PEG hydrogels for bioadhesive applications. Acta Biomater. 2014, 10, 47–55. [Google Scholar] [CrossRef]
- Desai, R.M.; Koshy, S.T.; Hilderbrand, S.A.; Mooney, D.J.; Joshi, N.S. Versatile click alginate hydrogels crosslinked via tetrazine-norbornene chemistry. Biomaterials 2015, 50, 30–37. [Google Scholar] [CrossRef]
- Heidarian, P.; Kouzani, A.Z. A self-healing nanocomposite double network bacterial nanocellulose/gelatin hydrogel for three dimensional printing. Carbohyd. Polym. 2023, 313, 120879. [Google Scholar] [CrossRef]
- Gao, L.T.; Chen, Y.M.; Aziz, Y.; Wei, W.; Zhao, X.Y.; He, Y.; Li, J.H.; Li, H.P.; Miyatake, H.; Ito, Y. Tough, self-healing and injectable dynamic nanocomposite hydrogel based on gelatin and sodium alginate. Carbohyd. Polym. 2024, 330, 121812. [Google Scholar] [CrossRef]
- Sen, S.; Sharma, P.; Pal, V.K.; Roy, S. Designing Cardin-Motif Peptide and Heparin-Based Multicomponent Advanced Bioactive Hydrogel Scaffolds to Control Cellular Behavior. Biomacromolecules 2023, 24, 4923–4938. [Google Scholar] [CrossRef]
- Goh, M.; Kim, Y.; Gwon, K.; Min, K.; Hwang, Y.; Tae, G. In situ formation of injectable and porous heparin-based hydrogel. Carbohyd. Polym. 2017, 174, 990–998. [Google Scholar] [CrossRef]
- Fernández-Muiños, T.; Recha-Sancho, L.; López-Chicón, P.; Castells-Sala, C.; Mata, A.; Semino, C.E. Bimolecular based heparin and self-assembling hydrogel for tissue engineering applications. Acta Biomater. 2015, 16, 35–48. [Google Scholar] [CrossRef]
- Song, Y.H.; Shon, S.H.; Shan, M.R.; Stroock, A.D.; Fischbach, C. Adipose-derived stem cells increase angiogenesis through matrix metalloproteinase-dependent collagen remodeling. Integr. Biol. 2016, 8, 205–215. [Google Scholar] [CrossRef]
- Parmar, P.A.; Chow, L.W.; St-Pierre, J.P.; Horejs, C.M.; Peng, Y.Y.; Werkmeister, J.A.; Ramshaw, J.A.M.; Stevens, M.M. Collagen-mimetic peptide-modifiable hydrogels for articular cartilage regeneration. Biomaterials 2015, 54, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.H.; Wang, X.L.; Deng, C.; Teng, X.M.; Suuronen, E.J.; Shen, Z.Y.; Zhong, Z.Y. Injectable biodegradable hybrid hydrogels based on thiolated collagen and oligo(acryloyl carbonate)-poly(ethylene glycol)-oligo(acryloyl carbonate) copolymer for functional cardiac regeneration. Acta Biomater. 2015, 15, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Schnell, E.; Klinkhammer, K.; Balzer, S.; Brook, G.; Klee, D.; Dalton, P.; Mey, J. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-ε-caprolactone and a collagen/poly-ε-caprolactone blend. Biomaterials 2007, 28, 3012–3025. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.L.; Zhang, D.W.; Macedo, M.H.; Cui, W.G.; Sarmento, B.; Shen, G.F. Advanced Collagen-Based Biomaterials for Regenerative Biomedicine. Adv. Funct. Mater. 2019, 29, 4943. [Google Scholar] [CrossRef]
- Carriel, V.; Garrido-Gómez, J.; Hernández-Cortés, P.; Garzón, I.; García-García, S.; Sáez-Moreno, J.A.; Sánchez-Quevedo, M.D.C.; Campos, A.; Alaminos, M. Combination of fibrin-agarose hydrogels and adipose-derived mesenchymal stem cells for peripheral nerve regeneration. J. Neural Eng. 2013, 10, 026022. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.; Rytlewski, J.A.; Merchant, A.G.; Dhada, K.S.; Lewis, E.W.; Suggs, L.J. Fibrin-based 3D matrices induce angiogenic behavior of adipose-derived stem cells. Acta Biomater. 2015, 17, 78–88. [Google Scholar] [CrossRef]
- Hou, Q.H.; Liu, K.; Lian, C.X.; Liu, J.W.; Wei, W.Y.; Qiu, T.; Dai, H.L. A Gelatin-Based Composite Hydrogel with a “One Stone, Two Birds” Strategy for Photothermal Antibacterial and Vascularization of Infected Wounds. Biomacromolecules 2023, 24, 3397–3410. [Google Scholar] [CrossRef] [PubMed]
- Meco, E.; Lampe, K.J. Impact of Elastin-like Protein Temperature Transition on PEG-ELP Hybrid Hydrogel Properties. Biomacromolecules 2019, 20, 1914–1925. [Google Scholar] [CrossRef]
- Cipriani, F.; Krüger, M.; De Torre, I.G.; Sierra, L.Q.; Rodrigo, M.A.; Kock, L.; Rodriguez-Cabello, J.C. Cartilage Regeneration in Preannealed Silk Elastin-Like Co-Recombinamers Injectable Hydrogel Embedded with Mature Chondrocytes in an Ex Vivo Culture Platform. Biomacromolecules 2018, 19, 4333–4347. [Google Scholar] [CrossRef]
- Huang, Q.T.; Zou, Y.J.; Arno, M.C.; Chen, S.; Wang, T.; Gao, J.Y.; Dove, A.P.; Du, J.Z. Hydrogel scaffolds for differentiation of adipose-derived stem cells. Chem. Soc. Rev. 2017, 46, 6255–6275. [Google Scholar] [CrossRef]
- Li, X.M.; Kuang, Y.; Xu, B. “Molecular trinity” for soft nanomaterials: Integrating nucleobases, amino acids, and glycosides to construct multifunctional hydrogelators. Soft Matter 2012, 8, 2801–2806. [Google Scholar] [CrossRef]
- Li, Z.; Qiu, L.P.; Chen, Q.; Hao, T.N.; Qiao, M.X.; Zhao, H.X.; Zhang, J.; Hu, H.Y.; Zhao, X.L.; Chen, D.W.; et al. pH-sensitive nanoparticles of poly(L-histidine)-poly(lactide-co-glycolide)-tocopheryl polyethylene glycol succinate for anti-tumor drug delivery. Acta Biomater. 2015, 11, 137–150. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, D.N.; Alexander, P.G.; Yang, G.; Tan, J.; Cheng, A.W.M.; Tuan, R.S. Application of visible light-based projection stereolithography for live cell-scaffold fabrication with designed architecture. Biomaterials 2013, 34, 331–339. [Google Scholar] [CrossRef]
- Raic, A.; Rödling, L.; Kalbacher, H.; Lee-Thedieck, C. Biomimetic macroporous PEG hydrogels as 3D scaffolds for the multiplication of human hematopoietic stem and progenitor cells. Biomaterials 2014, 35, 929–940. [Google Scholar] [CrossRef]
- Wang, H.Y.; Cai, L.; Paul, A.; Enejder, A.; Heilshorn, S.C. Hybrid Elastin-like Polypeptide-Polyethylene Glycol (ELP-PEG) Hydrogels with Improved Transparency and Independent Control of Matrix Mechanics and Cell Ligand Density. Biomacromolecules 2014, 15, 3421–3428. [Google Scholar] [CrossRef]
- Daniele, M.A.; Adams, A.A.; Naciri, J.; North, S.H.; Ligler, F.S. Interpenetrating networks based on gelatin methacrylamide and PEG formed using concurrent thiol click chemistries for hydrogel tissue engineering scaffolds. Biomaterials 2014, 35, 1845–1856. [Google Scholar] [CrossRef] [PubMed]
- McGann, C.L.; Akins, R.E.; Kiick, K.L. Resilin-PEG Hybrid Hydrogels Yield Degradable Elastomeric Scaffolds with Heterogeneous Microstructure. Biomacromolecules 2016, 17, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Goktas, M.; Cinar, G.; Orujalipoor, I.; Ide, S.; Tekinay, A.B.; Guler, M.O. Self-Assembled Peptide Amphiphile Nanofibers and PEG Composite Hydrogels as Tunable ECM Mimetic Microenvironment. Biomacromolecules 2015, 16, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- Hruschka, V.; Saeed, A.; Slezak, P.; Al Ghanami, R.C.; Feichtinger, G.A.; Alexander, C.; Redl, H.; Shakesheff, K.; Wolbank, S. Evaluation of a Thermoresponsive Polycaprolactone Scaffold for In Vitro Three-Dimensional Stem Cell Differentiation. Tissue Eng. Pt A 2015, 21, 310–319. [Google Scholar] [CrossRef]
- Robinson, A.; Nkansah, A.; Bhat, S.; Karnik, S.; Jones, S.; Fairley, A.; Leung, J.; Wancura, M.; Sacks, M.S.; Dasi, L.P.; et al. Hydrogel-polyurethane fiber composites with enhanced microarchitectural control for heart valve replacement. J. Biomed. Mater. Res. A 2024, 112, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.H.; Liu, W.; Xie, Z.M.; Zhang, D.; Zhou, J.H.; Xu, Y.S.; Fu, B.P.; Zheng, S.Y.; Zhang, L.; Yang, J.T. In Situ Growth of Functional Hydrogel Coatings by a Reactive Polyurethane for Biomedical Devices. ACS Appl. Mater. Inter. 2023, 15, 56652–56664. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; Hsu, S.H. Cell reprogramming by 3D bioprinting of human fibroblasts in polyurethane hydrogel for fabrication of neural-like constructs. Acta Biomater. 2018, 70, 57–70. [Google Scholar] [CrossRef]
- Wang, D.F.; Cui, F.C.; Xi, L.Q.; Tan, X.Q.; Li, J.R.; Li, T.T. Preparation of a multifunctional non-stick tamarind polysaccharide-polyvinyl alcohol hydrogel immobilized with a quorum quenching enzyme for maintaining fish freshness. Carbohyd. Polym. 2023, 302, 120382. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Chen, L.Y.; Hu, L.C.; Feng, X.L.; Mao, Z.P.; Xu, H.; Wang, B.J.; Sui, X.F. Self-healing and acidochromic polyvinyl alcohol hydrogel reinforced by regenerated cellulose. Carbohyd. Polym. 2021, 255, 117331. [Google Scholar] [CrossRef]
- Zhu, W.C.; Jiang, L.; Wang, B.L.; Gu, S.L.; Hu, F.Y.; Wang, C.H.; Chen, Y.S. Rational Design of PMPC/PDMC/PEGDA Hydrogel Micropatterns onto Polylactic Acid with Enhanced Biological Activity. ACS Biomater. Sci. Eng. 2020, 6, 3799–3810. [Google Scholar] [CrossRef]
- Ilomuanya, M.O.; Okafor, P.S.; Amajuoyi, J.N.; Onyejekwe, J.C.; Okubanjo, O.O.; Adeosun, S.O.; Silva, B.O. Polylactic acid-based electrospun fiber and hyaluronic acid-valsartan hydrogel scaffold for chronic wound healing. Beni-Suef Univ. J. Basic 2020, 9, 31. [Google Scholar] [CrossRef]
- Saroia, J.; Wang, Y.E.; Wei, Q.H.; Zhang, K.; Lu, T.L.; Zhang, B. A review on biocompatibility nature of hydrogels with 3D printing techniques, tissue engineering application and its future prospective. Bio-Des. Manuf. 2018, 1, 265–279. [Google Scholar] [CrossRef]
- Pollot, B.E.; Rathbone, C.R.; Wenke, J.C.; Guda, T. Natural polymeric hydrogel evaluation for skeletal muscle tissue engineering. J. Biomed. Mater. Res. B 2017, 106, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Lynn, A.K.; Yannas, I.V.; Bonfield, W. Antigenicity and immunogenicity of collagen. J. Biomed. Mater. Res. B 2004, 71B, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Merle, C.; Perret, S.; Lacour, T.; Jonval, V.; Hudaverdian, S.; Garrone, R.; Ruggiero, F.; Theisen, M. Hydroxylated human homotrimeric collagen I in Agrobacterium tumefaciens-mediated transient expression and in transgenic tobacco plant. FEBS Lett. 2002, 515, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, P.R.; Galanis, M.; Richards, K.M.; Tebb, T.A.; Ramshaw, J.A.; Werkmeister, J.A. Production of recombinant hydroxylated human type III collagen fragment in Saccharomyces cerevisiae. DNA Cell Biol. 1998, 17, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Ma, X.; Gao, Y.; Fan, D.; Zhu, C.; Mi, Y.; Xue, W. Hydroxylation of Human Type III Collagen Alpha Chain by Recombinant Coexpression with a Viral Prolyl 4-Hydroxylase in Escherichia coli. Protein J. 2017, 36, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Mu, T.; Fan, D. Preparation of a low-cost minimal medium for engineered Escherichia coli with high yield of human-like collagen II. Pak. J. Pharm. Sci. 2014, 27, 663–669. [Google Scholar]
- Luo, Y.; Fan, D.D.; Ma, X.X.; Wang, D.W.; Mi, Y.; Hua, X.F.; Li, W.H. Process control for production of human-like collagen in fed-batch culture of Escherichia coli BL 21. Chin. J. Chem. Eng. 2005, 13, 276–279. [Google Scholar]
- Greener, J.G.; Kandathil, S.M.; Moffat, L.; Jones, D.T. A guide to machine learning for biologists. Nat. Rev. Mol. Cell Biol. 2022, 23, 40–55. [Google Scholar] [CrossRef]
- Tariq, A.; Yan, J.; Gagnon, A.S.; Khan, M.R.; Mumtaz, F. Mapping of cropland, cropping patterns and crop types by combining optical remote sensing images with decision tree classifier and random forest. Geo.-Spat. Inf. Sci. 2023, 26, 302–320. [Google Scholar] [CrossRef]
- Yang, C.; Liu, L.L.; Huang, F.; Huang, L.; Wang, X.-M. Machine learning-based landslide susceptibility assessment with optimized ratio of landslide to non-landslide samples. Gondwana Res. 2023, 123, 198–216. [Google Scholar] [CrossRef]
- Afolabi, O.J.; Mabuza-Hocquet, G.P.; Nelwamondo, F.V.; Paul, B.S. The Use of U-Net Lite and Extreme Gradient Boost (XGB) for Glaucoma Detection. IEEE Access 2021, 9, 47411–47424. [Google Scholar] [CrossRef]
- Kolev, M. XGB-COF: A machine learning software in Python for predicting the friction coefficient of porous Al-based composites with Extreme Gradient Boosting. Softw. Impacts 2023, 17, 100531. [Google Scholar] [CrossRef]
- Vapnik, V.N. An overview of statistical learning theory. IEEE Trans. Neural Netw. 1999, 10, 988–999. [Google Scholar] [CrossRef]
- Chapelle, O.; Haffner, P.; Vapnik, V.N. Support vector machines for histogram-based image classification. IEEE Trans. Neural Netw. 1999, 10, 1055–1064. [Google Scholar] [CrossRef]
- Bi, J.; Vapnik, V.N. Learning with rigorous support vector machines. Lect. Notes Artif. Int. 2003, 2777, 243–257. [Google Scholar] [CrossRef]
- Guyon, I.; Weston, J.; Barnhill, S.; Vapnik, V. Gene selection for cancer classification using support vector machines. Mach. Learn. 2002, 46, 389–422. [Google Scholar] [CrossRef]
- Chapelle, O.; Vapnik, V.; Bousquet, O.; Mukherjee, S. Choosing multiple parameters for support vector machines. Mach. Learn. 2002, 46, 131–159. [Google Scholar] [CrossRef]
- Vapnik, V.; Chapelle, O. Bounds on error expectation for support vector machines. Neural Comput. 2000, 12, 2013–2036. [Google Scholar] [CrossRef]
- Chapelle, O.; Vapnik, V. Model selection for support vector machines. Adv. Neural Inf. Process. Syst. 2000, 12, 230–236. [Google Scholar]
- Pu, X.; Fang, Z.; Liu, Y. Multilayer Perceptron networks training using particle swarm optimization with minimum velocity constraints. In Proceedings of the 4th International Symposium on Neural Networks (ISNN 2007), Nanjing, China, 3–7 June 2007. [Google Scholar]
- Lopez, R.; Onate, E. A variational formulation for the multilayer perceptron. In Artificial Neural Networks—Icann 2006, Pt 1; Kollias, S., Stafylopatis, A., Duch, W., Oja, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 4131, pp. 159–168. [Google Scholar]
- Zhang, Y.; Gao, J.; Zhou, H.; Assoc Comp, M. Breeds Classification with Deep Convolutional Neural Network. In Proceedings of the 12th International Conference on Machine Learning and Computing (ICMLC), Shenzhen, China, 15–17 February 2020. [Google Scholar]
- Tian, Y. Artificial Intelligence Image Recognition Method Based on Convolutional Neural Network Algorithm. IEEE Access 2020, 8, 125731–125744. [Google Scholar] [CrossRef]
- Li, P.; Li, J.; Wang, G. Application of convolutional neural network in natural language processing. In Proceedings of the 15th IEEE International Computer Conference on Wavelet Active Media Technology and Information Processing (ICCWAMTIP), Chengdu, China, 14–16 December 2018. [Google Scholar]
- Zhao, H.; Zeng, X.; Zhang, J.; Liu, Y.; Wang, X.; Li, T. A novel joint-processing adaptive nonlinear equalizer using a modular recurrent neural network for chaotic communication systems. Neural Netw. 2011, 24, 12–18. [Google Scholar] [CrossRef]
- Wang, J.; Wu, G. A multilayer recurrent neural network for solving continuous-time algebraic Riccati equations. Neural Netw. 1998, 11, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Solovyeva, E.B. Types of Recurrent Neural Networks for Non-linear Dynamic System Modelling. In Proceedings of the 20th IEEE International Conference on Soft Computing and Measurements (SCM), St Petersburg, Russia, 24–26 May 2017. [Google Scholar]
- Wu, S.; Sun, F.; Zhang, W.; Xie, X.; Cui, B. Graph Neural Networks in Recommender Systems: A Survey. ACM Comput. Surv. 2023, 55, 97. [Google Scholar] [CrossRef]
- Zhang, Z.; Cui, P.; Zhu, W. Deep Learning on Graphs: A Survey. IEEE Trans. Knowl. Data Eng. 2022, 34, 249–270. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, L.; Sun, J.; Zhao, Q.; Shuai, J. Predicting the potential human lncRNA-miRNA interactions based on graph convolution network with conditional random field. Brief. Bioinform. 2022, 23, bbac463. [Google Scholar] [CrossRef]
- Nie, J.; Xu, Y.; Huang, Y.; Li, J. The Review of Image Processing Based on Graph Neural Network. In Proceedings of the 14th International Conference on Intelligent Robotics and Applications (ICIRA), Yantai, China, 22–25 October 2021. [Google Scholar]
- Quan, P.; Shi, Y.; Lei, M.; Leng, J.; Zhang, T.; Niu, L. A Brief Review of Receptive Fields in Graph Convolutional Networks. In Proceedings of the 19th IEEE/WIC/ACM International Conference on Web Intelligence (WI), Thessaloniki, Greece, 13–17 October 2019. [Google Scholar]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Hao, J.G.; Ho, T.K. Machine Learning Made Easy: A Review of Scikit-learn Package in Python Programming Language. J. Educ. Behav. Stat. 2019, 44, 348–361. [Google Scholar] [CrossRef]
- Kuhn, M. Building Predictive Models in R Using the caret Package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Thompson, A.J.; Pillai, E.K.; Dimov, I.B.; Foster, S.K.; Franze, K. Rapid changes in tissue mechanics regulate cell behaviour in the developing embryonic brain. eLife 2019, 8, 39356. [Google Scholar] [CrossRef]
- Barriga, E.H.; Franze, K.; Charras, G.; Mayor, R. Tissue stiffening coordinates morphogenesis by triggering collective cell migration in vivo. Nature 2018, 554, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005, 8, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Yeh, W.C.; Li, P.C.; Jeng, Y.M.; Hsu, H.C.; Kuo, P.L.; Li, M.L.; Yang, P.M.; Lee, P.H. Elastic modulus measurements of human liver and correlation with pathology. Ultrasound Med. Biol. 2002, 28, 467–474. [Google Scholar] [CrossRef] [PubMed]

| Model | Test Set | Training Set | ||
|---|---|---|---|---|
| Score | RMSE | Score | RMSE | |
| ML | 0.824 | 2164 | 0.672 | 2872 |
| DT | 0.954 | 1108 | 0.977 | 757 |
| SVM | 0.985 | 625 | 0.980 | 714 |
| NN | 0.992 | 469 | 0.991 | 468 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Zhao, L.; Ren, Y.; Zuo, L.; Shen, Z.; Wu, J. The Optimization of Culture Conditions for Injectable Recombinant Collagen Hydrogel Preparation Using Machine Learning. Gels 2025, 11, 141. https://doi.org/10.3390/gels11020141
Li M, Zhao L, Ren Y, Zuo L, Shen Z, Wu J. The Optimization of Culture Conditions for Injectable Recombinant Collagen Hydrogel Preparation Using Machine Learning. Gels. 2025; 11(2):141. https://doi.org/10.3390/gels11020141
Chicago/Turabian StyleLi, Mengyu, Long Zhao, Yanan Ren, Linfei Zuo, Ziyi Shen, and Jiawei Wu. 2025. "The Optimization of Culture Conditions for Injectable Recombinant Collagen Hydrogel Preparation Using Machine Learning" Gels 11, no. 2: 141. https://doi.org/10.3390/gels11020141
APA StyleLi, M., Zhao, L., Ren, Y., Zuo, L., Shen, Z., & Wu, J. (2025). The Optimization of Culture Conditions for Injectable Recombinant Collagen Hydrogel Preparation Using Machine Learning. Gels, 11(2), 141. https://doi.org/10.3390/gels11020141






