A Review on the Rheological Properties of Single Amino Acids and Short Dipeptide Gels
Abstract
1. Introduction
2. Rheological Characterization Techniques
3. Overview of the Mechanical Properties of Single Amino Acids and Dipeptide-Based Gels
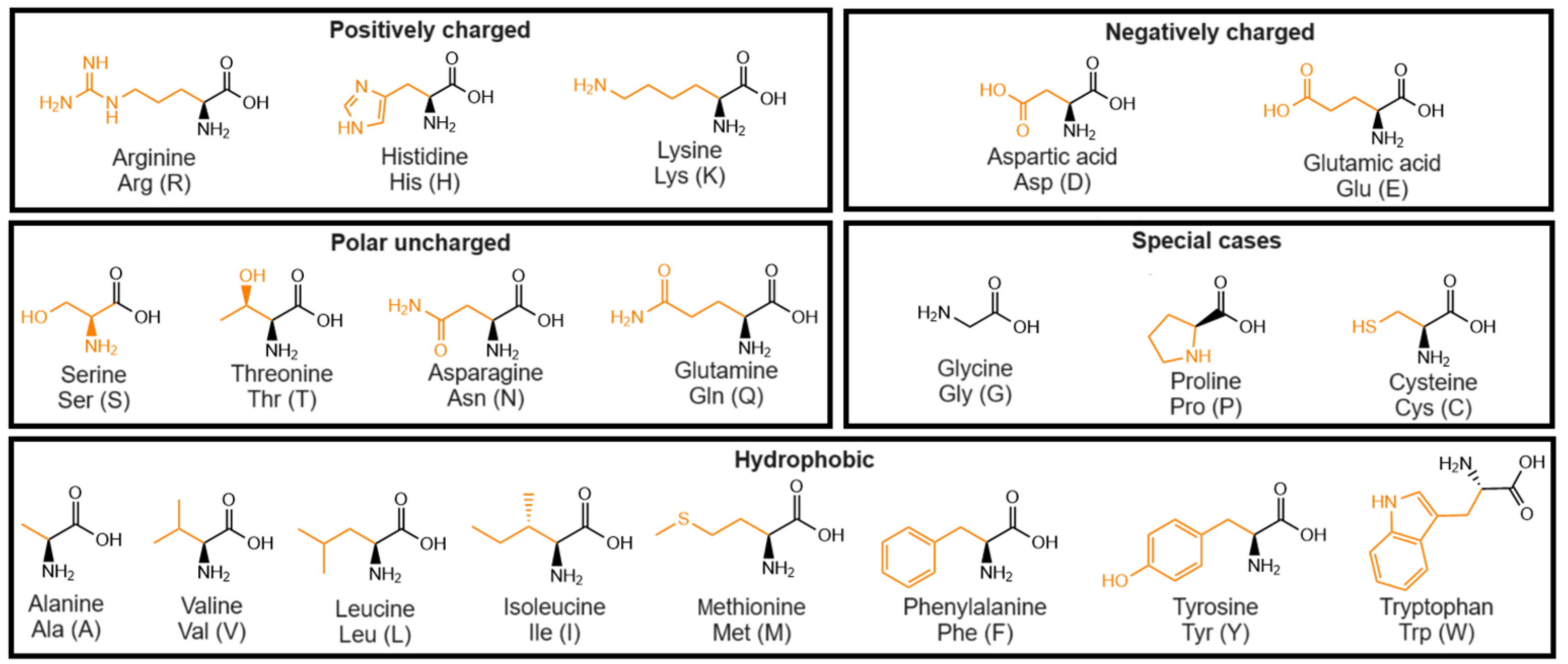
3.1. Single Amino Acids
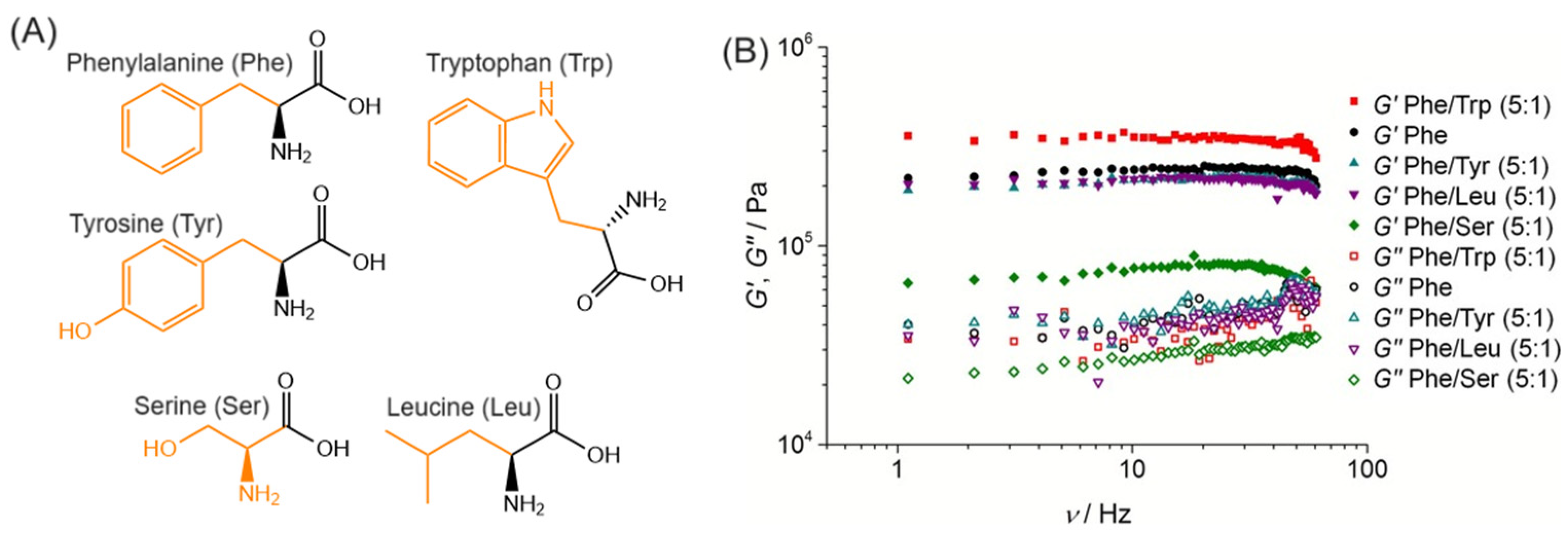
3.1.1. Uncapped Amino Acids
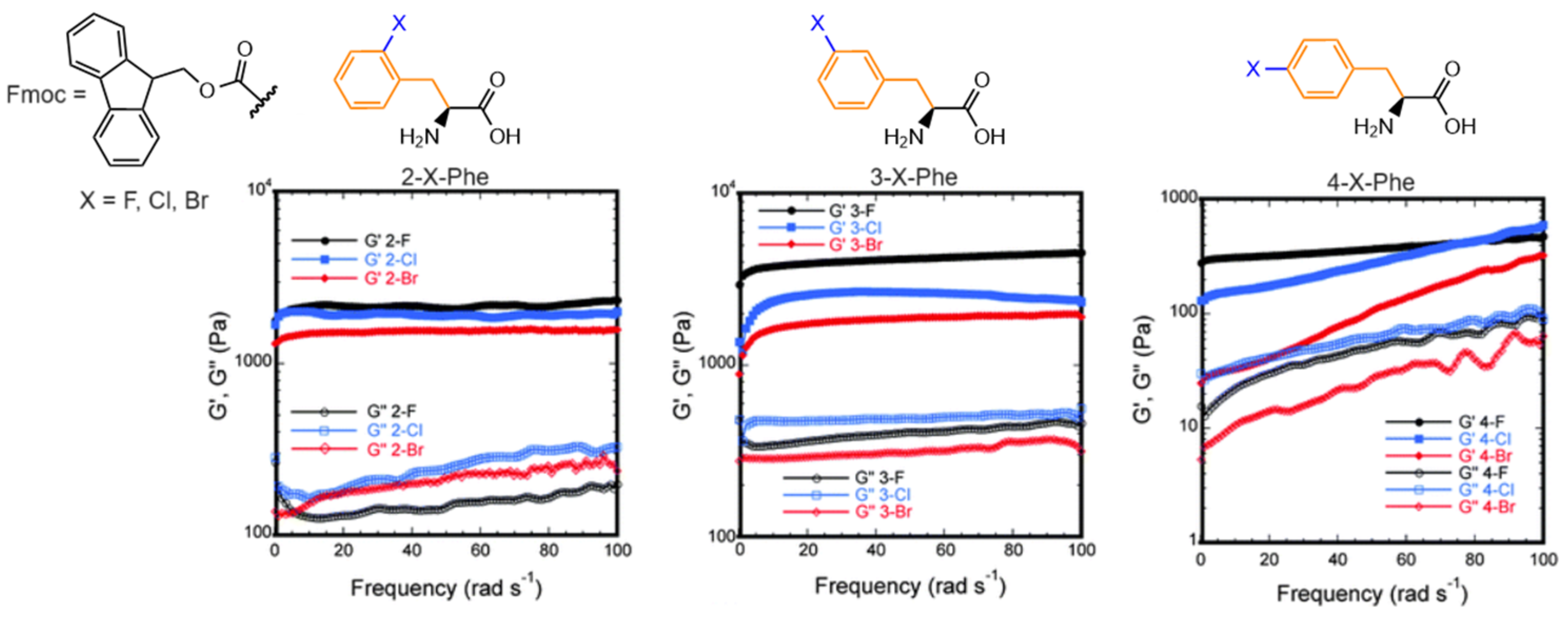
3.1.2. Fluorenylmethyloxycarbonyl N-Capped Phenylalanine (Fmoc-Phe) and Derivatives
3.1.3. Fluorenylmethyloxycarbonyl N-Capped Amino Acids (Fmoc-AAs)
3.1.4. Other N-Capped Amino Acids
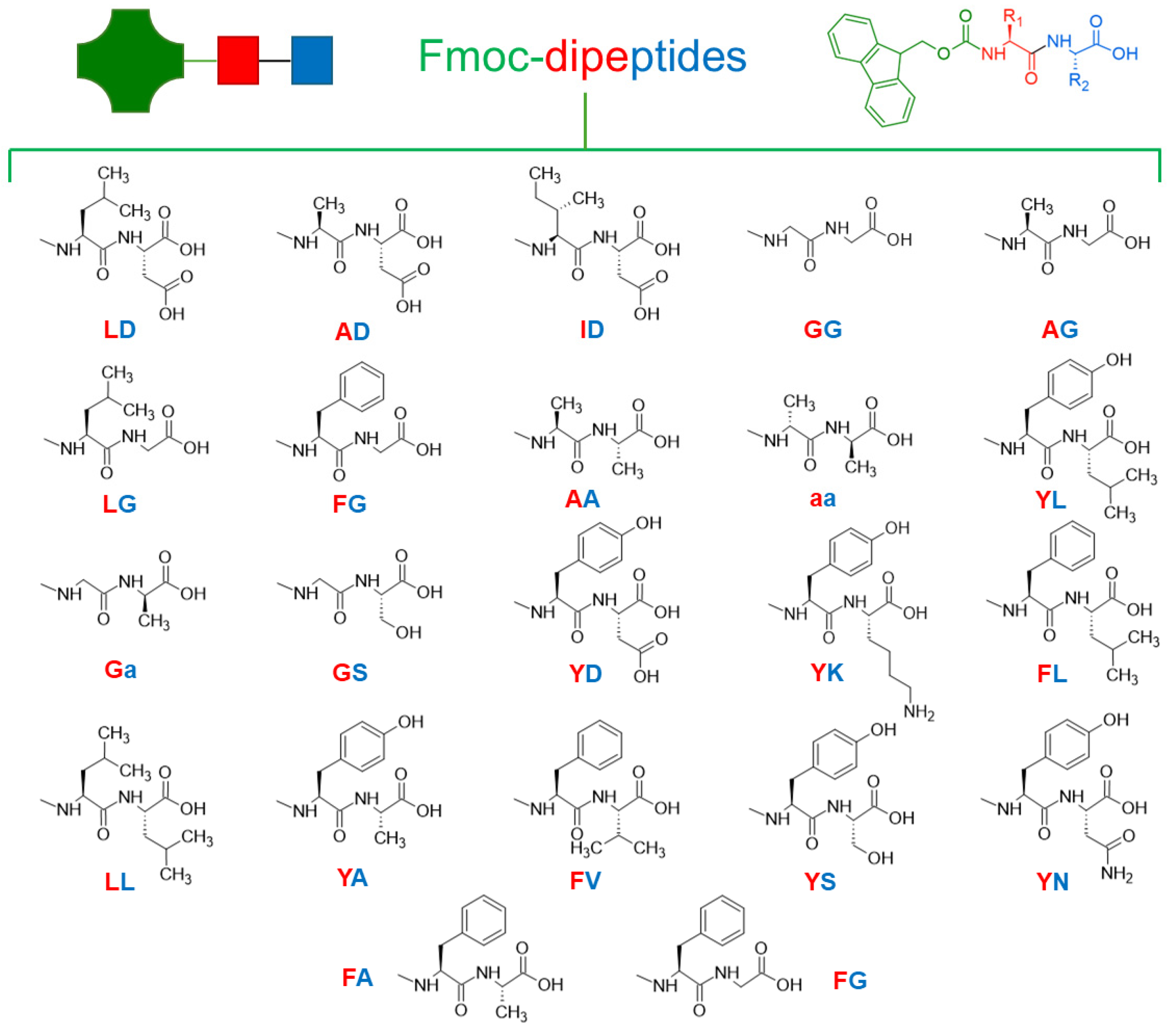
3.2. Dipeptides: Chemical Structure of Dipeptide-Based Hydrogels
3.2.1. Uncapped Dipeptides
| Gelator | Method | Media | pH | CGC (mM) | [Gel] (mM) | G′ (Pa) | G″ (Pa) | LVR (%) | γ (%) | Tm (°C) | Fibril (nm) | Highlights | Application | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L-Phe-Phe | pHE | Buffer | 7.3 | 20 | 20 | 22 | 3.7 | 10 | 10 | 46 | 200–1000 | - | - | [148] |
| SE + US | HFIP/ANS | 8 | 8 | 8 | 1780 | 102 | - | - | - | ~10 | - | - | [149] | |
| Phe-ΔPhe | HC | Buffer | 7 | 6.4 | 6.4 | 209,000 | 19,700 | - | - | - | 15–20 | - | Drug delivery | [151] |
| D-Phe-Phe | pHE | Buffer | 7.3 | 20 | 20 | 22.9 | 1.5 | 100 | 100 | 44 | 4.3 | Thermoreversible | - | [148] |
| 2-F-Phe-Phe | pHE | Buffer | 7.3 | 15 | 15 | 8 | 0.5 | 10 | 10 | 44 | 11.4 | Thermoreversible | - | [148] |
| 3-F-Phe-Phe | pHE | Buffer | 7.3 | 10 | 10 | 6.1 | 0.3 | 100 | 100 | 47 | 50–500 | Thermoreversible | - | [148] |
| 4-F-Phe-Phe | pHE | Buffer | 7.3 | 7 | 7 | 20.7 | 1.2 | 100 | 100 | 42 | 26.9 | Thermoreversible | - | [148] |
| 4-I-Phe-Phe | pHE | Buffer | 7.3 | 4 | 4 | 17.7 | 1.3 | 100 | 100 | 74 | 63 | Thermoreversible | - | [148] |
| Phe-Phe-NH2 | SE + US | HFIP/ANS | 8 | 4 | 4 | 30,100 | 103 | - | - | - | ~10 | - | - | [149] |
| Leu-ΔPhe | US | Buffer | 7 | 15.2 | 19.1 | 12,000 | 103 | - | - | - | >100 | - | - | [152] |
| L-Leu-Phe | HC | PBS | 7.4 | 40 | 40 | 104 | 103 | 10 | 100 | - | - | - | - | [150] |
| D-Leu-Phe | HC | PBS | 7.4 | 40 | 40 | 104 | 103 | 10 | 100 | - | 12 | - | - | [150] |
| D-Phe-Leu | HC | PBS | 7.4 | 20 | 20 | 103 | 102 | - | 0.1 | - | - | Not stable | - | [150] |
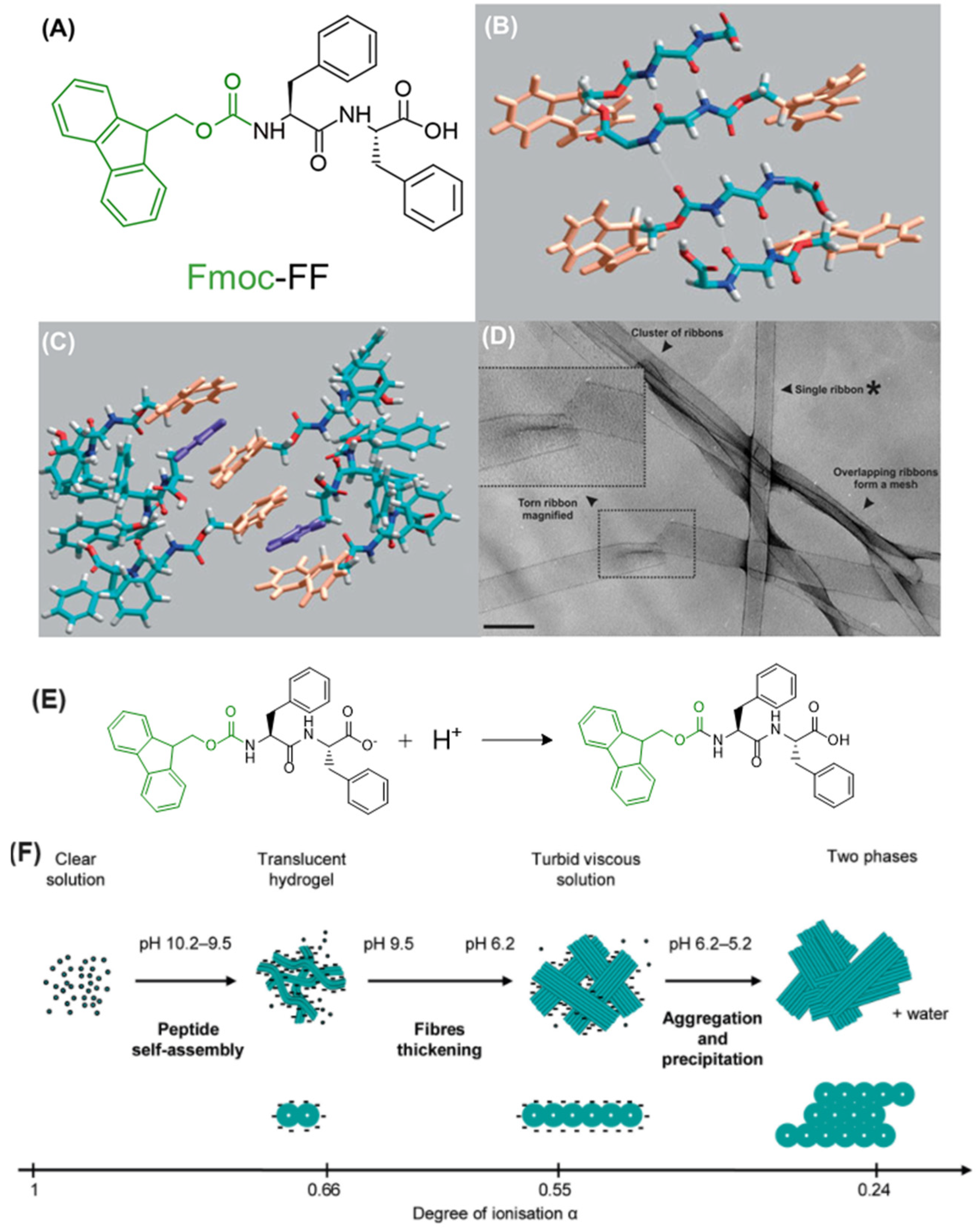
3.2.2. The Case of Fmoc-FF
3.2.3. Fmoc-Capped Dipeptides
3.2.4. Other N-Capped Dipeptides
| Gelator | Method | Media | pH | CGC (mM) | [Gel] (mM) | G′ (Pa) | G″ (Pa) | LVR (%) | γ (%) | Tm (°C) | Fibril (nm) | Highlights | Application | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fmoc-AA | pHE | Water | <4 | 1.6–16.9 | - | - | - | - | - | - | 68 ±1 8 | - | - | [160] |
| Fmoc-AD | HC | PBS | 7.4 | 142.3 | 10 | - | - | - | - | - | - | - | - | [173] |
| Fmoc-AG | pHE | Water | <4 | 4.3-17.9 | - | - | - | - | - | - | 30 ± 6 [d] | - | Cell growth | [160] |
| Fmoc-FA | GdL | Water | 3.75 | - | 14.6 | 95,600 | - | - | - | - | - | - | - | [65] |
| Fmoc-FG | pHE | Water | <4 | 4.0–17.8 | - | - | - | - | - | - | 25 ± 6 [d] | - | Cell growth | [160] |
| Fmoc-FG | GdL | Water | 3.75 | - | 14.6 | 41,000 | - | - | - | - | - | - | - | [65] |
| Fmoc-FL | D | PBS | ~8 | - | 20 | 11,500 | >1100 | <2 | ~6 | 100–150 | Fiber and straight rods | Fillers | [178] | |
| Fmoc-FV | pHE | Water | 7.4 | - | 20 | 800 | 650 | >100 | - | - | 30 [t] | - | 3D cell culture | [179] |
| Fmoc-GG | pHE | Water | <4 | 4.2–16.9 | - | - | - | - | - | - | 33 ± 8 [d] | - | Cell growth | [160] |
| Fmoc-ID | HC | PBS | 7.4 | 8.5 | 10 | - | - | - | - | - | - | - | - | [173] |
| Fmoc-K(Fmoc)-D | SE | Water/DMSO | 0.03 | 7 | - | - | <100 | - | - | - | Conductive gel | DNA binding | [181] | |
| Fmoc-LD | HC | PBS | 7.4 | 10.7 | 10 | 80 | ~15 | <0.3 | >10 | - | - | - | Drug delivery | [173] |
| Fmoc-LG | pHE | Water | <4 | 8.5–17.8 | - | - | - | - | - | - | 22 ± 5 [d] | - | Cell growth | [160] |
| pHE − HCl | Water | 3.75 | - | 14.6 | 5900 | - | - | - | - | - | - | - | [65] | |
| GdL | Water | 3.75 | - | 14.6 | 184,000 | - | - | - | - | - | - | - | [65] | |
| Fmoc-LL | D | PBS | ~8 | - | 20 | 1500 | ~300 | <1 | ~10 | - | 20–50 | - | Fillers | [178] |
| Fmoc-YA | D | PBS | ~8 | - | 20 | 800 | ~300 | <0.5 | ~5 | - | 20–50 | - | Fillers | [178] |
| Fmoc-YD | D | Water | - | - | 10 | ~4500 | ~2000 | <100 | ~50 | - | 18 [t] | Helical fibrils | 3D Bioprinting | [177] |
| Fmoc-YK | D | Water | - | - | 10 | 20 | 8 | <2 | ~30 | - | 5 [t] | Helical fibrils | 3D Bioprinting | [177] |
| Fmoc-YL | pHE | Water | ~7.3 | - | 10 | ~390 | ~190 | <0.1 | - | - | 40–200 | Stable ν = 0.1–15.8 Hz | [176] | |
| D | PBS | ~8 | - | 20 | 6000 | ~1000 | <2 | ~10 | - | - | Shear-thinning | Fillers | [178] | |
| Fmoc-YN | Enz/pHE | PBS | 8 | - | 10 | 3010 | 949 | - | - | - | - | - | - | [180] |
| Fmoc-YS | Enz/pHE | PBS | 8 | - | 10 | 3400 | 100 | - | - | - | - | - | - | [180] |
4. Perspective on the Structure-Property Relationship
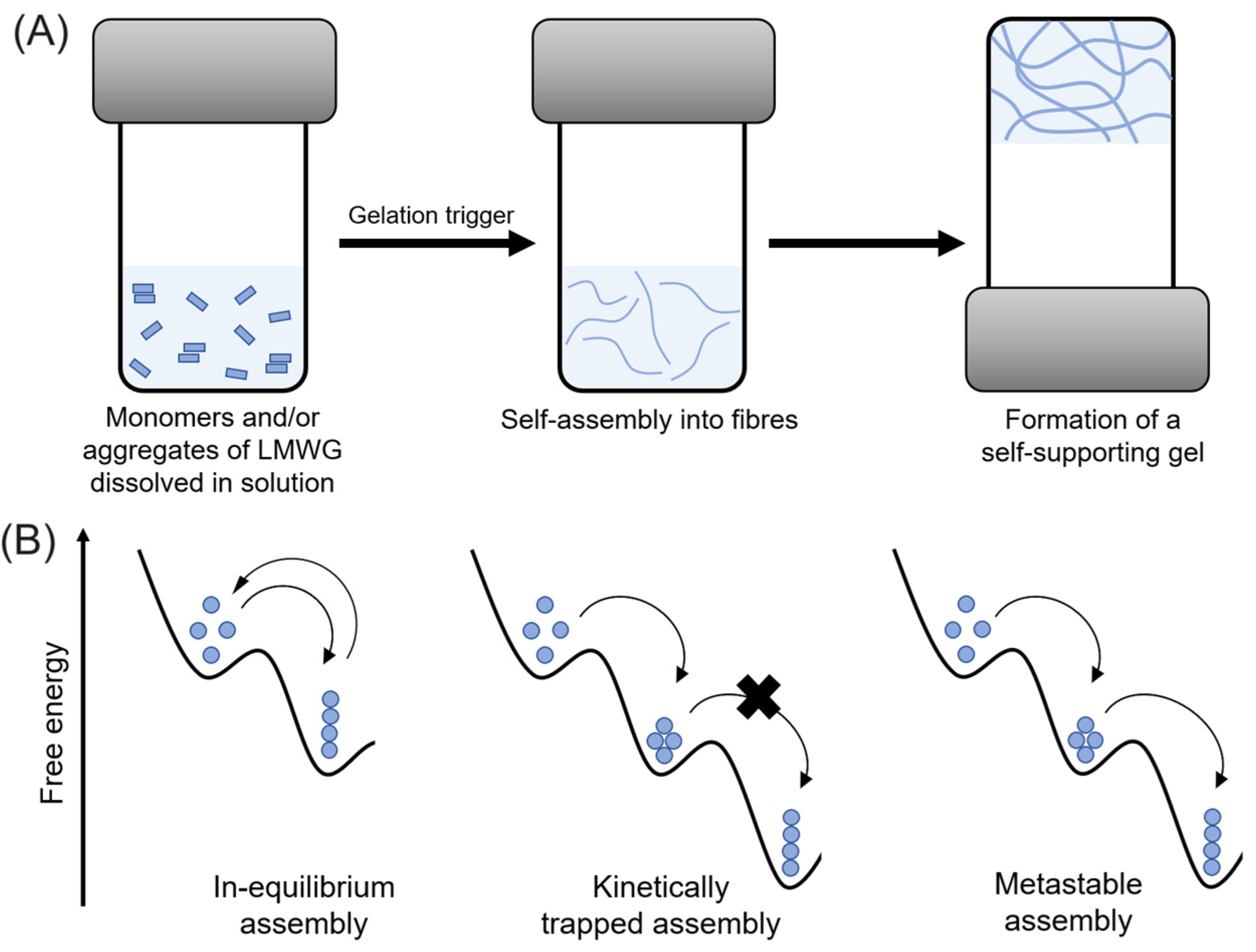
4.1. Influence of the Self-Assembly Pathway
4.2. Influence of the Chemical Structure
5. Conclusions and Challenges
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Z.; Ai, S.; Yang, Z.; Li, X. Peptide-Based Supramolecular Hydrogels for Local Drug Delivery. Adv. Drug Deliv. Rev. 2021, 174, 482–503. [Google Scholar] [CrossRef]
- Guan, T.; Li, J.; Chen, C.; Liu, Y. Self-Assembling Peptide-Based Hydrogels for Wound Tissue Repair. Adv. Sci. 2022, 9, e2104165. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, X.; Liang, G. Peptide-Based Supramolecular Hydrogels for Bioimaging Applications. Biomater. Sci. 2021, 9, 315–327. [Google Scholar] [CrossRef]
- Binaymotlagh, R.; Chronopoulou, L.; Haghighi, F.H.; Fratoddi, I.; Palocci, C. Peptide-Based Hydrogels: New Materials for Biosensing and Biomedical Applications. Materials 2022, 15, 5871. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiary, N.; Ghalandari, B.; Ghorbani, F.; Varma, S.N.; Liu, C. Advances in Peptide-Based Hydrogel for Tissue Engineering. Polymers 2023, 15, 1068. [Google Scholar] [CrossRef]
- Habibi, N.; Kamaly, N.; Memic, A.; Shafiee, H. Self-Assembled Peptide-Based Nanostructures: Smart Nanomaterials toward Targeted Drug Delivery. Nano Today 2016, 11, 41–60. [Google Scholar] [CrossRef]
- Ni, M.; Zhuo, S. Applications of Self-Assembling Ultrashort Peptides in Bionanotechnology. RSC Adv. 2019, 9, 844–852. [Google Scholar] [CrossRef]
- Das, S.; Das, D. Rational Design of Peptide-Based Smart Hydrogels for Therapeutic Applications. Front. Chem. 2021, 9, 770102. [Google Scholar] [CrossRef]
- Pramanik, B. Short Peptide-Based Smart Thixotropic Hydrogels. Gels 2022, 8, 569. [Google Scholar] [CrossRef]
- Draper, E.R.; Adams, D.J. Controlling the Assembly and Properties of Low-Molecular-Weight Hydrogelators. Langmuir 2019, 35, 6506–6521. [Google Scholar] [CrossRef]
- Raghavan, S.R.; Douglas, J.F. The Conundrum of Gel Formation by Molecular Nanofibers, Wormlike Micelles, and Filamentous Proteins: Gelation without Cross-Links? Soft Matter 2012, 8, 8539. [Google Scholar] [CrossRef]
- De Leon Rodriguez, L.M.; Hemar, Y.; Cornish, J.; Brimble, M.A. Structure-Mechanical Property Correlations of Hydrogel Forming β-Sheet Peptides. Chem. Soc. Rev. 2016, 45, 4797–4824. [Google Scholar] [CrossRef] [PubMed]
- Panja, S.; Fuentes-Caparrós, A.M.; Cross, E.R.; Cavalcanti, L.; Adams, D.J. Annealing Supramolecular Gels by a Reaction Relay. Chem. Mater. 2020, 32, 5264–5271. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Wang, J.; Zhao, S.; Chen, D.; Zhang, H.; Fang, Y.; Kong, N.; Zhou, Z.; Li, W.; Wang, H. Accelerating the Prediction and Discovery of Peptide Hydrogels with Human-in-the-Loop. Nat. Commun. 2023, 14, 3880. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.K.; Adams, D.J.; Berry, N.G. Will It Gel? Successful Computational Prediction of Peptide Gelators Using Physicochemical Properties and Molecular Fingerprints. Chem. Sci. 2016, 7, 4713–4719. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yuan, C.; Yan, X. Computational Approaches for Understanding and Predicting the Self-Assembled Peptide Hydrogels. Curr. Opin. Colloid Interface Sci. 2022, 62, 101645. [Google Scholar] [CrossRef]
- Ferguson, A.L.; Tovar, J.D. Evolution of π-Peptide Self-Assembly: From Understanding to Prediction and Control. Langmuir 2022, 38, 15463–15475. [Google Scholar] [CrossRef]
- Frederix, P.W.J.M.; Scott, G.G.; Abul-Haija, Y.M.; Kalafatovic, D.; Pappas, C.G.; Javid, N.; Hunt, N.T.; Ulijn, R.V.; Tuttle, T. Exploring the Sequence Space for (Tri-)Peptide Self-Assembly to Design and Discover New Hydrogels. Nat. Chem. 2015, 7, 30–37. [Google Scholar] [CrossRef]
- Toledano, S.; Williams, R.J.; Jayawarna, V.; Ulijn, R.V. Enzyme-Triggered Self-Assembly of Peptide Hydrogels via Reversed Hydrolysis. J. Am. Chem. Soc. 2006, 128, 1070–1071. [Google Scholar] [CrossRef]
- Ramachandran, S.; Tseng, Y.; Yu, Y.B. Repeated Rapid Shear-Responsiveness of Peptide Hydrogels with Tunable Shear Modulus. Biomacromolecules 2005, 6, 1316–1321. [Google Scholar] [CrossRef]
- Haines-Butterick, L.; Rajagopal, K.; Branco, M.; Salick, D.; Rughani, R.; Pilarz, M.; Lamm, M.S.; Pochan, D.J.; Schneider, J.P. Controlling Hydrogelation Kinetics by Peptide Design for Three-Dimensional Encapsulation and Injectable Delivery of Cells. Proc. Natl. Acad. Sci. USA 2007, 104, 7791–7796. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Chen, J.; Xu, W.; Wang, Q.; Wei, X.; Ma, Y.; Chen, F.; Zhang, G. Molecular Dynamics Study of Low Molecular Weight Gel Forming Salt-Triggered Dipeptide. Sci. Rep. 2023, 13, 6328. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Hakala, T.A.; Schnaider, L.; Bernardes, G.J.L.L.; Gazit, E.; Knowles, T.P.J.J. Biomimetic Peptide Self-Assembly for Functional Materials. Nat. Rev. Chem. 2020, 4, 615–634. [Google Scholar] [CrossRef]
- Jonker, A.M.; Löwik, D.W.P.M.; van Hest, J.C.M. Peptide- and Protein-Based Hydrogels. Chem. Mater. 2012, 24, 759–773. [Google Scholar] [CrossRef]
- Panja, S.; Adams, D.J. Stimuli Responsive Dynamic Transformations in Supramolecular Gels. Chem. Soc. Rev. 2021, 50, 5165–5200. [Google Scholar] [CrossRef]
- Raeburn, J.; Zamith Cardoso, A.; Adams, D.J.; Cardoso, A.Z.; Adams, D.J.; Zamith Cardoso, A.; Adams, D.J. The Importance of the Self-Assembly Process to Control Mechanical Properties of Low Molecular Weight Hydrogels. Chem. Soc. Rev. 2013, 42, 5143. [Google Scholar] [CrossRef] [PubMed]
- Ardoña, H.A.M.; Draper, E.R.; Citossi, F.; Wallace, M.; Serpell, L.C.; Adams, D.J.; Tovar, J.D. Kinetically Controlled Coassembly of Multichromophoric Peptide Hydrogelators and the Impacts on Energy Transport. J. Am. Chem. Soc. 2017, 139, 8685–8692. [Google Scholar] [CrossRef] [PubMed]
- Shao, T.; Falcone, N.; Kraatz, H.B. Supramolecular Peptide Gels: Influencing Properties by Metal Ion Coordination and Their Wide-Ranging Applications. ACS Omega 2020, 5, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- Raymond, D.M.; Nilsson, B.L. Multicomponent Peptide Assemblies. Chem. Soc. Rev. 2018, 47, 3659–3720. [Google Scholar] [CrossRef]
- Chivers, P.R.A.; Smith, D.K. Shaping and Structuring Supramolecular Gels. Nat. Rev. Mater. 2019, 4, 463–478. [Google Scholar] [CrossRef]
- Jervis, P.J.; Amorim, C.; Pereira, T.; Martins, J.A.; Ferreira, P.M.T.T. Exploring the Properties and Potential Biomedical Applications of NSAID-Capped Peptide Hydrogels. Soft Matter 2020, 16, 10001–10012. [Google Scholar] [CrossRef] [PubMed]
- Jervis, P.J.; Amorim, C.; Pereira, T.; Martins, J.A.; Ferreira, P.M.T.T. Dehydropeptide Supramolecular Hydrogels and Nanostructures as Potential Peptidomimetic Biomedical Materials. Int. J. Mol. Sci. 2021, 22, 2528. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Trinh, T.H.; Yoo, M.; Shin, J.; Lee, H.; Kim, J.; Hwang, E.; Lim, Y.-B.; Ryou, C. Self-Assembling Peptides and Their Application in the Treatment of Diseases. Int. J. Mol. Sci. 2019, 20, 5850. [Google Scholar] [CrossRef] [PubMed]
- Naskar, J.; Roy, S.; Joardar, A.; Das, S.; Banerjee, A. Self-Assembling Dipeptide-Based Nontoxic Vesicles as Carriers for Drugs and Other Biologically Important Molecules. Org. Biomol. Chem. 2011, 9, 6610. [Google Scholar] [CrossRef] [PubMed]
- Parween, S.; Misra, A.; Ramakumar, S.; Chauhan, V.S. Self-Assembled Dipeptide Nanotubes Constituted by Flexible β-Phenylalanine and Conformationally Constrained α,β-Dehydrophenylalanine Residues as Drug Delivery System. J. Mater. Chem. B 2014, 2, 3096. [Google Scholar] [CrossRef] [PubMed]
- James, J.; Mandal, A.B. Micelle Formation of Tyr-Phe Dipeptide and Val-Tyr-Val Tripeptide in Aqueous Solution and Their Influence on the Aggregation of SDS and PEO–PPO–PEO Copolymer Micelles. Colloids Surf. B Biointerfaces 2011, 84, 172–180. [Google Scholar] [CrossRef]
- Tanaka, W.; Shigemitsu, H.; Fujisaku, T.; Kubota, R.; Minami, S.; Urayama, K.; Hamachi, I. Post-Assembly Fabrication of a Functional Multicomponent Supramolecular Hydrogel Based on a Self-Sorting Double Network. J. Am. Chem. Soc. 2019, 141, 4997–5004. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Boyd-Moss, M.; Long, B.; Martel, A.; Parnell, A.; Dennison, A.J.C.; Barrow, C.J.; Nisbet, D.R.; Williams, R.J. Facile Control over the Supramolecular Ordering of Self-Assembled Peptide Scaffolds by Simultaneous Assembly with a Polysacharride. Sci. Rep. 2017, 7, 4797. [Google Scholar] [CrossRef]
- Du, E.Y.; Ziaee, F.; Wang, L.; Nordon, R.E.; Thordarson, P. The Correlations between Structure, Rheology, and Cell Growth in Peptide-Based Multicomponent Hydrogels. Polym. J. 2020, 52, 947–957. [Google Scholar] [CrossRef]
- Falcone, N.; Shao, T.; Andoy, N.M.O.; Rashid, R.; Sullan, R.M.A.; Sun, X.; Kraatz, H.B. Multi-Component Peptide Hydrogels-a Systematic Study Incorporating Biomolecules for the Exploration of Diverse, Tuneable Biomaterials. Biomater. Sci. 2020, 8, 5601–5614. [Google Scholar] [CrossRef]
- Gomes, V.; Veloso, S.R.S.; Correa-Duarte, M.A.; Ferreira, P.M.T.; Castanheira, E.M.S. Tuning Peptide-Based Hydrogels: Co-Assembly with Composites Driving the Highway to Technological Applications. Int. J. Mol. Sci. 2022, 24, 186. [Google Scholar] [CrossRef]
- Gila-Vilchez, C.; Rodriguez-Arco, L.; Mañas-Torres, M.C.; Álvarez de Cienfuegos, L.; Lopez-Lopez, M.T. Self-Assembly in Magnetic Supramolecular Hydrogels. Curr. Opin. Colloid Interface Sci. 2022, 62, 101644. [Google Scholar] [CrossRef]
- Veloso, S.R.S.; Vázquez-González, M.; Spuch, C.; Freiría-Martínez, L.; Comís-Tuche, M.; Iglesias-Martínez-Almeida, M.; Rivera-Baltanás, T.; Hilliou, L.; Amorim, C.O.; Amaral, V.S.; et al. An Injectable Composite Co-Assembled Dehydropeptide-Based Magnetic/Plasmonic Lipogel for Multimodal Cancer Therapy. Adv. Funct. Mater. 2024, 2402926. [Google Scholar] [CrossRef]
- Zanna, N.; Tomasini, C. Peptide-Based Physical Gels Endowed with Thixotropic Behaviour. Gels 2017, 3, 39. [Google Scholar] [CrossRef]
- Roth-Konforti, M.E.; Comune, M.; Halperin-Sternfeld, M.; Grigoriants, I.; Shabat, D.; Adler-Abramovich, L. UV Light-Responsive Peptide-Based Supramolecular Hydrogel for Controlled Drug Delivery. Macromol. Rapid Commun. 2018, 39, 1800588. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zheng, H.; Poh, P.S.P.; Machens, H.-G.G.; Schilling, A.F. Hydrogels for Engineering of Perfusable Vascular Networks. Int. J. Mol. Sci. 2015, 16, 15997–16016. [Google Scholar] [CrossRef]
- Amorim, C.; Veloso, S.R.S.; Castanheira, E.M.S.; Hilliou, L.; Pereira, R.B.; Pereira, D.M.; Martins, J.A.; Jervis, P.J.; Ferreira, P.M.T. Bolaamphiphilic Bis-Dehydropeptide Hydrogels as Potential Drug Release Systems. Gels 2021, 7, 52. [Google Scholar] [CrossRef]
- Yan, C.; Pochan, D.J. Rheological Properties of Peptide-Based Hydrogels for Biomedical and Other Applications. Chem. Soc. Rev. 2010, 39, 3528. [Google Scholar] [CrossRef] [PubMed]
- Sathaye, S.; Mbi, A.; Sonmez, C.; Chen, Y.; Blair, D.L.; Schneider, J.P.; Pochan, D.J. Rheology of Peptide- and Protein-based Physical Hydrogels: Are Everyday Measurements Just Scratching the Surface? WIREs Nanomed. Nanobiotechnol. 2015, 7, 34–68. [Google Scholar] [CrossRef]
- Adams, D.J. Personal Perspective on Understanding Low Molecular Weight Gels. J. Am. Chem. Soc. 2022, 144, 11047–11053. [Google Scholar] [CrossRef]
- Frith, W.J.; Donald, A.M.; Adams, D.J.; Aufderhorst-Roberts, A. Gels Formed from Amino-Acid Derivatives, Their Novel Rheology as Probed by Bulk and Particle Tracking Rheological Methods. J. Nonnewton. Fluid Mech. 2015, 222, 104–111. [Google Scholar] [CrossRef]
- Aufderhorst-Roberts, A.; Frith, W.J.; Kirkland, M.; Donald, A.M. Microrheology and Microstructure of Fmoc-Derivative Hydrogels. Langmuir 2014, 30, 4483–4492. [Google Scholar] [CrossRef] [PubMed]
- Draper, E.R.; Adams, D.J. How Should Multicomponent Supramolecular Gels Be Characterised? Chem. Soc. Rev. 2018, 47, 3395–3405. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Caparrós, A.M.; Dietrich, B.; Thomson, L.; Chauveau, C.; Adams, D.J. Using Cavitation Rheology to Understand Dipeptide-Based Low Molecular Weight Gels. Soft Matter 2019, 15, 6340–6347. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Aviñó, F.; Clegg, P.S. Non-Linear Dilational Rheology of Liquid-Liquid Interfaces Stabilized by Dipeptide Hydrogels. Rheol. Acta 2023, 62, 45–55. [Google Scholar] [CrossRef]
- Binaymotlagh, R.; Chronopoulou, L.; Palocci, C. Peptide-Based Hydrogels: Template Materials for Tissue Engineering. J. Funct. Biomater. 2023, 14, 233. [Google Scholar] [CrossRef] [PubMed]
- Mitrovic, J.; Richey, G.; Kim, S.; Guler, M.O. Peptide Hydrogels and Nanostructures Controlling Biological Machinery. Langmuir 2023, 39, 11935–11945. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xing, R.; Bai, S.; Yan, X. Recent Advances of Self-Assembling Peptide-Based Hydrogels for Biomedical Applications. Soft Matter 2019, 15, 1704–1715. [Google Scholar] [CrossRef] [PubMed]
- Haines, L.A.; Rajagopal, K.; Ozbas, B.; Salick, D.A.; Pochan, D.J.; Schneider, J.P. Light-Activated Hydrogel Formation via the Triggered Folding and Self-Assembly of a Designed Peptide. J. Am. Chem. Soc. 2005, 127, 17025–17029. [Google Scholar] [CrossRef]
- Ozbas, B.; Kretsinger, J.; Rajagopal, K.; Schneider, J.P.; Pochan, D.J. Salt-Triggered Peptide Folding and Consequent Self-Assembly into Hydrogels with Tunable Modulus. Macromolecules 2004, 37, 7331–7337. [Google Scholar] [CrossRef]
- Chen, L.; McDonald, T.O.; Adams, D.J. Salt-Induced Hydrogels from Functionalised-Dipeptides. RSC Adv. 2013, 3, 8714. [Google Scholar] [CrossRef]
- Stendahl, J.C.; Rao, M.S.; Guler, M.O.; Stupp, S.I. Intermolecular Forces in the Self-Assembly of Peptide Amphiphile Nanofibers. Adv. Funct. Mater. 2006, 16, 499–508. [Google Scholar] [CrossRef]
- Cardoso, A.Z.; Alvarez Alvarez, A.E.; Cattoz, B.N.; Griffiths, P.C.; King, S.M.; Frith, W.J.; Adams, D.J. The Influence of the Kinetics of Self-Assembly on the Properties of Dipeptide Hydrogels. Faraday Discuss. 2013, 166, 101. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, Z.; Adams, D.J. Controlling Peptidebased Hydrogelation. Mater. Today 2012, 15, 500–507. [Google Scholar] [CrossRef]
- Adams, D.J.; Butler, M.F.; Frith, W.J.; Kirkland, M.; Mullen, L.; Sanderson, P. A New Method for Maintaining Homogeneity during Liquid–Hydrogel Transitions Using Low Molecular Weight Hydrogelators. Soft Matter 2009, 5, 1856. [Google Scholar] [CrossRef]
- Fortunato, A.; Mba, M. A Peptide-Based Hydrogel for Adsorption of Dyes and Pharmaceuticals in Water Remediation. Gels 2022, 8, 672. [Google Scholar] [CrossRef] [PubMed]
- Ben Messaoud, G.; Le Griel, P.; Hermida-Merino, D.; Roelants, S.L.K.W.; Soetaert, W.; Stevens, C.V.; Baccile, N. PH-Controlled Self-Assembled Fibrillar Network Hydrogels: Evidence of Kinetic Control of the Mechanical Properties. Chem. Mater. 2019, 31, 4817–4830. [Google Scholar] [CrossRef]
- Vilaça, H.; Hortelão, A.C.L.; Castanheira, E.M.S.; Queiroz, M.-J.R.P.; Hilliou, L.; Hamley, I.W.; Martins, J.A.; Ferreira, P.M.T. Dehydrodipeptide Hydrogelators Containing Naproxen N -Capped Tryptophan: Self-Assembly, Hydrogel Characterization, and Evaluation as Potential Drug Nanocarriers. Biomacromolecules 2015, 16, 3562–3573. [Google Scholar] [CrossRef] [PubMed]
- Veloso, S.R.S.; Silva, J.F.G.; Hilliou, L.; Moura, C.; Coutinho, P.J.G.; Martins, J.A.; Testa-Anta, M.; Salgueiriño, V.; Correa-Duarte, M.A.; Ferreira, P.M.T.; et al. Impact of Citrate and Lipid-Functionalized Magnetic Nanoparticles in Dehydropeptide Supramolecular Magnetogels: Properties, Design and Drug Release. Nanomaterials 2021, 11, 16. [Google Scholar] [CrossRef]
- Thornton, K.; Smith, A.M.; Merry, C.L.R.; Ulijn, R.V. Controlling Stiffness in Nanostructured Hydrogels Produced by Enzymatic Dephosphorylation. Biochem. Soc. Trans. 2009, 37, 660–664. [Google Scholar] [CrossRef]
- Yang, Z.; Liang, G.; Ma, M.; Gao, Y.; Xu, B. In Vitro and In Vivo Enzymatic Formation of Supramolecular Hydrogels Based on Self-Assembled Nanofibers of a Β-Amino Acid Derivative. Small 2007, 3, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Coulter, S.M.; Pentlavalli, S.; Vora, L.K.; An, Y.; Cross, E.R.; Peng, K.; McAulay, K.; Schweins, R.; Donnelly, R.F.; McCarthy, H.O.; et al. Enzyme-Triggered L-α/D-Peptide Hydrogels as a Long-Acting Injectable Platform for Systemic Delivery of HIV/AIDS Drugs. Adv. Healthc. Mater. 2023, 12, 2203198. [Google Scholar] [CrossRef] [PubMed]
- Guilbaud, J.-B.; Rochas, C.; Miller, A.F.; Saiani, A. Effect of Enzyme Concentration of the Morphology and Properties of Enzymatically Triggered Peptide Hydrogels. Biomacromolecules 2013, 14, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, T.; Maeda, T.; Hotta, A. Effects of Salt Concentrations of the Aqueous Peptide-Amphiphile Solutions on the Sol–Gel Transitions, the Gelation Speed, and the Gel Characteristics. J. Phys. Chem. B 2014, 118, 11537–11545. [Google Scholar] [CrossRef] [PubMed]
- Fichman, G.; Schneider, J.P. Utilizing Frémy’s Salt to Increase the Mechanical Rigidity of Supramolecular Peptide-Based Gel Networks. Front. Bioeng. Biotechnol. 2021, 8, 594258. [Google Scholar] [CrossRef] [PubMed]
- Mañas-Torres, M.C.; Gila-Vilchez, C.; González-Vera, J.A.; Conejero-Lara, F.; Blanco, V.; Cuerva, J.M.; Lopez-Lopez, M.T.; Orte, A.; Álvarez de Cienfuegos, L. In Situ Real-Time Monitoring of the Mechanism of Self-Assembly of Short Peptide Supramolecular Polymers. Mater. Chem. Front. 2021, 5, 5452–5462. [Google Scholar] [CrossRef]
- Wychowaniec, J.K.; Patel, R.; Leach, J.; Mathomes, R.; Chhabria, V.; Patil-Sen, Y.; Hidalgo-Bastida, A.; Forbes, R.T.; Hayes, J.M.; Elsawy, M.A. Aromatic Stacking Facilitated Self-Assembly of Ultrashort Ionic Complementary Peptide Sequence: β-Sheet Nanofibers with Remarkable Gelation and Interfacial Properties. Biomacromolecules 2020, 21, 2670–2680. [Google Scholar] [CrossRef] [PubMed]
- Colquhoun, C.; Draper, E.R.; Schweins, R.; Marcello, M.; Vadukul, D.; Serpell, L.C.; Adams, D.J. Controlling the Network Type in Self-Assembled Dipeptide Hydrogels. Soft Matter 2017, 13, 1914–1919. [Google Scholar] [CrossRef] [PubMed]
- Zimberlin, J.A.; Sanabria-DeLong, N.; Tew, G.N.; Crosby, A.J. Cavitation Rheology for Soft Materials. Soft Matter 2007, 3, 763. [Google Scholar] [CrossRef]
- MacKintosh, F.C.; Käs, J.; Janmey, P.A. Elasticity of Semiflexible Biopolymer Networks. Phys. Rev. Lett. 1995, 75, 4425–4428. [Google Scholar] [CrossRef]
- Shih, W.-H.; Shih, W.Y.; Kim, S.-I.; Liu, J.; Aksay, I.A. Scaling Behavior of the Elastic Properties of Colloidal Gels. Phys. Rev. A 1990, 42, 4772–4779. [Google Scholar] [CrossRef]
- Wu, H.; Morbidelli, M. A Model Relating Structure of Colloidal Gels to Their Elastic Properties. Langmuir 2001, 17, 1030–1036. [Google Scholar] [CrossRef]
- Morse, D.C. Viscoelasticity of Concentrated Isotropic Solutions of Semiflexible Polymers. 2. Linear Response. Macromolecules 1998, 31, 7044–7067. [Google Scholar] [CrossRef]
- Veerman, C.; Rajagopal, K.; Palla, C.S.; Pochan, D.J.; Schneider, J.P.; Furst, E.M. Gelation Kinetics of β-Hairpin Peptide Hydrogel Networks. Macromolecules 2006, 39, 6608–6614. [Google Scholar] [CrossRef]
- Terech, P.; Sangeetha, N.M.; Maitra, U. Molecular Hydrogels from Bile Acid Analogues with Neutral Side Chains: Network Architectures and Viscoelastic Properties. Junction Zones, Spherulites, and Crystallites: Phenomenological Aspects of the Gel Metastability. J. Phys. Chem. B 2006, 110, 15224–15233. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Tang, C.; Elsawy, M.A.; Smith, A.M.; Miller, A.F.; Saiani, A. Controlling Self-Assembling Peptide Hydrogel Properties through Network Topology. Biomacromolecules 2017, 18, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Hashemnejad, S.M.; Kundu, S. Probing Gelation and Rheological Behavior of a Self-Assembled Molecular Gel. Langmuir 2017, 33, 7769–7779. [Google Scholar] [CrossRef]
- Adams, D.J.; Mullen, L.M.; Berta, M.; Chen, L.; Frith, W.J. Relationship between Molecular Structure, Gelation Behaviour and Gel Properties of Fmoc-Dipeptides. Soft Matter 2010, 6, 1971. [Google Scholar] [CrossRef]
- Fuentes-Caparrós, A.M.; McAulay, K.; Rogers, S.E.; Dalgliesh, R.M.; Adams, D.J. On the Mechanical Properties of N-Functionalised Dipeptide Gels. Molecules 2019, 24, 3855. [Google Scholar] [CrossRef]
- Li, D.; Shi, Y.; Wang, L. Mechanical Reinforcement of Molecular Hydrogel by Co-Assembly of Short Peptide-Based Gelators with Different Aromatic Capping Groups. Chin. J. Chem. 2014, 32, 123–127. [Google Scholar] [CrossRef]
- Boothroyd, S.; Saiani, A.; Miller, A.F. Controlling Network Topology and Mechanical Properties of Co-Assembling Peptide Hydrogels. Biopolymers 2014, 101, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Nagy, K.J.; Giano, M.C.; Jin, A.; Pochan, D.J.; Schneider, J.P. Enhanced Mechanical Rigidity of Hydrogels Formed from Enantiomeric Peptide Assemblies. J. Am. Chem. Soc. 2011, 133, 14975–14977. [Google Scholar] [CrossRef]
- Chen, G.; Li, J.; Song, M.; Wu, Z.; Zhang, W.; Wang, Z.; Gao, J.; Yang, Z.; Ou, C. A Mixed Component Supramolecular Hydrogel to Improve Mice Cardiac Function and Alleviate Ventricular Remodeling after Acute Myocardial Infarction. Adv. Funct. Mater. 2017, 27, 1701798. [Google Scholar] [CrossRef]
- Horgan, C.C.; Rodriguez, A.L.; Li, R.; Bruggeman, K.F.; Stupka, N.; Raynes, J.K.; Day, L.; White, J.W.; Williams, R.J.; Nisbet, D.R. Characterisation of Minimalist Co-Assembled Fluorenylmethyloxycarbonyl Self-Assembling Peptide Systems for Presentation of Multiple Bioactive Peptides. Acta Biomater. 2016, 38, 11–22. [Google Scholar] [CrossRef]
- Liyanage, W.; Nilsson, B.L. Substituent Effects on the Self-Assembly/Coassembly and Hydrogelation of Phenylalanine Derivatives. Langmuir 2016, 32, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Halperin-Sternfeld, M.; Ghosh, M.; Sevostianov, R.; Grigoriants, I.; Adler-Abramovich, L. Molecular Co-Assembly as a Strategy for Synergistic Improvement of the Mechanical Properties of Hydrogels. Chem. Commun. 2017, 53, 9586–9589. [Google Scholar] [CrossRef] [PubMed]
- Che, X.; Bai, B.; Zhang, T.; Zhang, C.; Zhang, C.; Zhang, P.; Wang, H.; Li, M. Gelation Behaviour and Gel Properties of Two-Component Organogels Containing a Photoresponsive Gelator. New J. Chem. 2017, 41, 8614–8619. [Google Scholar] [CrossRef]
- Xing, P.; Chu, X.; Li, S.; Xin, F.; Ma, M.; Hao, A. Switchable and Orthogonal Self-Assemblies of Anisotropic Fibers. New J. Chem. 2013, 37, 3949. [Google Scholar] [CrossRef]
- Draper, E.R.; Eden, E.G.B.; McDonald, T.O.; Adams, D.J. Spatially Resolved Multicomponent Gels. Nat. Chem. 2015, 7, 848–852. [Google Scholar] [CrossRef]
- Morris, K.L.; Chen, L.; Raeburn, J.; Sellick, O.R.; Cotanda, P.; Paul, A.; Griffiths, P.C.; King, S.M.; O’Reilly, R.K.; Serpell, L.C.; et al. Chemically Programmed Self-Sorting of Gelator Networks. Nat. Commun. 2013, 4, 1480. [Google Scholar] [CrossRef]
- Cornwell, D.J.; Daubney, O.J.; Smith, D.K. Photopatterned Multidomain Gels: Multi-Component Self-Assembled Hydrogels Based on Partially Self-Sorting 1,3:2,4-Dibenzylidene- d -Sorbitol Derivatives. Stoch. Process. Their Appl. 2016, 126, 337–359. [Google Scholar] [CrossRef]
- Singh, V.; Rai, R.K.; Arora, A.; Sinha, N.; Thakur, A.K. Therapeutic Implication of L-Phenylalanine Aggregation Mechanism and Its Modulation by D-Phenylalanine in Phenylketonuria. Sci. Rep. 2014, 4, 3875. [Google Scholar] [CrossRef]
- Hsu, W.-P.; Koo, K.-K.; Myerson, A.S. The Gel-Crystallization of 1-Phenylalanine and Aspartame from Aqueous Solutions. Chem. Eng. Commun. 2002, 189, 1079–1090. [Google Scholar] [CrossRef]
- Ramalhete, S.M.; Nartowski, K.P.; Sarathchandra, N.; Foster, J.S.; Round, A.N.; Angulo, J.; Lloyd, G.O.; Khimyak, Y.Z. Supramolecular Amino Acid Based Hydrogels: Probing the Contribution of Additive Molecules Using NMR Spectroscopy. Chem. Eur. J. 2017, 23, 8014–8024. [Google Scholar] [CrossRef] [PubMed]
- Bera, S.; Xue, B.; Rehak, P.; Jacoby, G.; Ji, W.; Shimon, L.J.W.; Beck, R.; Král, P.; Cao, Y.; Gazit, E. Self-Assembly of Aromatic Amino Acid Enantiomers into Supramolecular Materials of High Rigidity. ACS Nano 2020, 14, 1694–1706. [Google Scholar] [CrossRef] [PubMed]
- Babar, D.G.; Sarkar, S. Self-Assembled Nanotubes from Single Fluorescent Amino Acid. Appl. Nanosci. 2017, 7, 101–107. [Google Scholar] [CrossRef]
- Singh, P.; Pandey, S.K.; Grover, A.; Sharma, R.K.; Wangoo, N. Understanding the Self-Ordering of Amino Acids into Supramolecular Architectures: Co-Assembly-Based Modulation of Phenylalanine Nanofibrils. Mater. Chem. Front. 2021, 5, 1971–1981. [Google Scholar] [CrossRef]
- Ryan, D.M.; Anderson, S.B.; Senguen, F.T.; Youngman, R.E.; Nilsson, B.L. Self-Assembly and Hydrogelation Promoted by F5-Phenylalanine. Soft Matter 2010, 6, 475–479. [Google Scholar] [CrossRef]
- Ryan, D.M.; Doran, T.M.; Nilsson, B.L. Complementary π–π Interactions Induce Multicomponent Coassembly into Functional Fibrils. Langmuir 2011, 27, 11145–11156. [Google Scholar] [CrossRef]
- Ryan, D.M.; Doran, T.M.; Nilsson, B.L. Stabilizing Self-Assembled Fmoc–F5–Phe Hydrogels by Co-Assembly with PEG-Functionalized Monomers. Chem. Commun. 2011, 47, 475–477. [Google Scholar] [CrossRef]
- Liu, Y.; Kim, E.; Ulijn, R.V.; Bentley, W.E.; Payne, G.F. Reversible Electroaddressing of Self-assembling Amino-Acid Conjugates. Adv. Funct. Mater. 2011, 21, 1575–1580. [Google Scholar] [CrossRef]
- Xing, P.; Chu, X.; Li, S.; Ma, M.; Hao, A. Hybrid Gels Assembled from Fmoc–Amino Acid and Graphene Oxide with Controllable Properties. ChemPhysChem 2014, 15, 2377–2385. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Banerjee, A. Functionalized Single Walled Carbon Nanotube Containing Amino Acid Based Hydrogel: A Hybrid Nanomaterial. RSC Adv. 2012, 2, 2105. [Google Scholar] [CrossRef]
- Roy, S.; Banerjee, A. Amino Acid Based Smart Hydrogel: Formation, Characterization and Fluorescence Properties of Silver Nanoclusters within the Hydrogel Matrix. Soft Matter 2011, 7, 5300. [Google Scholar] [CrossRef]
- Draper, E.R.; Morris, K.L.; Little, M.A.; Raeburn, J.; Colquhoun, C.; Cross, E.R.; McDonald, T.O.; Serpell, L.C.; Adams, D.J. Hydrogels Formed from Fmoc Amino Acids. CrystEngComm 2015, 17, 8047–8057. [Google Scholar] [CrossRef]
- Shi, J.; Gao, Y.; Yang, Z.; Xu, B. Exceptionally Small Supramolecular Hydrogelators Based on Aromatic–Aromatic Interactions. Beilstein J. Org. Chem. 2011, 7, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Abraham, B.L.; Liyanage, W.; Nilsson, B.L. Strategy to Identify Improved N-Terminal Modifications for Supramolecular Phenylalanine-Derived Hydrogelators. Langmuir 2019, 35, 14939–14948. [Google Scholar] [CrossRef]
- Raymond, D.M.; Abraham, B.L.; Fujita, T.; Watrous, M.J.; Toriki, E.S.; Takano, T.; Nilsson, B.L. Low-Molecular-Weight Supramolecular Hydrogels for Sustained and Localized in Vivo Drug Delivery. ACS Appl. Bio Mater. 2019, 2, 2116–2124. [Google Scholar] [CrossRef]
- Quigley, E.; Johnson, J.; Liyanage, W.; Nilsson, B.L. Impact of Gelation Method on Thixotropic Properties of Phenylalanine-Derived Supramolecular Hydrogels. Soft Matter 2020, 16, 10158–10168. [Google Scholar] [CrossRef]
- Xie, Y.-Y.; Zhang, Y.-W.; Qin, X.-T.; Liu, L.-P.; Wahid, F.; Zhong, C.; Jia, S.-R. Structure-Dependent Antibacterial Activity of Amino Acid-Based Supramolecular Hydrogels. Colloids Surf. B Biointerfaces 2020, 193, 111099. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, B. A Simple Visual Assay Based on Small Molecule Hydrogels for Detecting Inhibitors of Enzymes. Chem. Commun. 2004, 21, 2424. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Misra, S.; Das, A.; Roy, S.; Datta, P.; Bhattacharjee, G.; Satpati, B.; Nanda, J. Supramolecular Hydrogel from an Oxidized Byproduct of Tyrosine. ACS Appl. Bio Mater. 2019, 2, 4881–4891. [Google Scholar] [CrossRef] [PubMed]
- Guilbaud-Chéreau, C.; Dinesh, B.; Schurhammer, R.; Collin, D.; Bianco, A.; Ménard-Moyon, C.; Guilbaud-che, C.; Bianco, A. Protected Amino Acid–Based Hydrogels Incorporating Carbon Nanomaterials for Near-Infrared Irradiation-Triggered Drug Release. ACS Appl. Mater. Interfaces 2019, 11, 13147–13157. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yuan, C.; Jiao, T.; Xing, R.; Yang, M.; Adams, D.J. Multifunctional Antimicrobial Biometallohydrogels Based on Amino Acid Coordinated Self-Assembly. Small 2020, 16, 1907309. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.M.M.; Augustine, G.; Ayyadurai, N.; Shanmugam, G. Biocytin-Based PH-Stimuli Responsive Supramolecular Multivariant Hydrogelator for Potential Applications. ACS Appl. Bio Mater. 2018, 1, 1382–1388. [Google Scholar] [CrossRef] [PubMed]
- Ramya, K.A.; Reddy, S.M.M.; Shanmugam, G.; Deshpande, A.P. Fibrillar Network Dynamics during Oscillatory Rheology of Supramolecular Gels. Langmuir 2020, 36, 13342–13355. [Google Scholar] [CrossRef] [PubMed]
- Arokianathan, J.F.; Ramya, K.A.; Janeena, A.; Deshpande, A.P.; Ayyadurai, N.; Leemarose, A.; Shanmugam, G. Non-Proteinogenic Amino Acid Based Supramolecular Hydrogel Material for Enhanced Cell Proliferation. Colloids Surf. B Biointerfaces 2020, 185, 110581. [Google Scholar] [CrossRef] [PubMed]
- Nanda, J.; Biswas, A.; Banerjee, A. Single Amino Acid Based Thixotropic Hydrogel Formation and PH-Dependent Morphological Change of Gel Nanofibers. Soft Matter 2013, 9, 4198. [Google Scholar] [CrossRef]
- Martí-Centelles, R.; Escuder, B. Morphology Diversity of L-Phenylalanine-Based Short Peptide Supramolecular Aggregates and Hydrogels. ChemNanoMat 2018, 4, 796–800. [Google Scholar] [CrossRef]
- Garcia, A.M.; Lavendomme, R.; Kralj, S.; Kurbasic, M.; Bellotto, O.; Cringoli, M.C.; Semeraro, S.; Bandiera, A.; De Zorzi, R.; Marchesan, S. Self-Assembly of an Amino Acid Derivative into an Antimicrobial Hydrogel Biomaterial. Chem. Eur. J. 2020, 26, 1880–1886. [Google Scholar] [CrossRef]
- Xing, P.; Chen, H.; Xiang, H.; Zhao, Y. Selective Coassembly of Aromatic Amino Acids to Fabricate Hydrogels with Light Irradiation-Induced Emission for Fluorescent Imprint. Adv. Mater. 2018, 30, 1705633. [Google Scholar] [CrossRef] [PubMed]
- Falcone, N.; Shao, T.; Rashid, R.; Kraatz, H.-B. Enzyme Entrapment in Amphiphilic Myristyl-Phenylalanine Hydrogels. Molecules 2019, 24, 2884. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.M.; Anderson, S.B.; Nilsson, B.L. The Influence of Side-Chain Halogenation on the Self-Assembly and Hydrogelation of Fmoc-Phenylalanine Derivatives. Soft Matter 2010, 6, 3220. [Google Scholar] [CrossRef]
- Ryan, D.M.; Doran, T.M.; Anderson, S.B.; Nilsson, B.L. Effect of C -Terminal Modification on the Self-Assembly and Hydrogelation of Fluorinated Fmoc-Phe Derivatives. Langmuir 2011, 27, 4029–4039. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Gu, H.; Fu, D.; Gao, P.; Lam, J.K.; Xu, B. Enzymatic Formation of Supramolecular Hydrogels. Adv. Mater. 2004, 16, 1440–1444. [Google Scholar] [CrossRef]
- Schnepp, Z.A.C.; Gonzalez-McQuire, R.; Mann, S. Hybrid Biocomposites Based on Calcium Phosphate Mineralization of Self-Assembled Supramolecular Hydrogels. Adv. Mater. 2006, 18, 1869–1872. [Google Scholar] [CrossRef]
- Yang, Z.; Liang, G.; Xu, B. Enzymatic Hydrogelation of Small Molecules. Acc. Chem. Res. 2008, 41, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.J.; Kumar, R.K.; Barron, N.J.; Mann, S. Cerium Oxide Nanoparticle-Mediated Self-Assembly of Hybrid Supramolecular Hydrogels. Chem. Commun. 2012, 48, 7934. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, S.; Wulf, V.; Bisker, G. Single-Walled Carbon Nanotubes as near-Infrared Fluorescent Probes for Bio-Inspired Supramolecular Self-Assembled Hydrogels. J. Colloid Interface Sci. 2024, 670, 439–448. [Google Scholar] [CrossRef]
- Koshti, B.; Swanson, H.W.A.; Wilson, B.; Kshtriya, V.; Naskar, S.; Narode, H.; Lau, K.H.A.; Tuttle, T.; Gour, N. Solvent-Controlled Self-Assembly of Fmoc Protected Aliphatic Amino Acids. Phys. Chem. Chem. Phys. 2023, 25, 11522–11529. [Google Scholar] [CrossRef]
- Yang, Z.; Gu, H.; Zhang, Y.; Wang, L.; Xu, B. Small Molecule Hydrogels Based on a Class of Antiinflammatory Agents. Chem. Commun. 2004, 2, 208. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.M.M.; Shanmugam, G.; Duraipandy, N.; Kiran, M.S.; Mandal, A.B. An Additional Fluorenylmethoxycarbonyl (Fmoc) Moiety in Di-Fmoc-Functionalized L-Lysine Induces PH-Controlled Ambidextrous Gelation with Significant Advantages. Soft Matter 2015, 11, 8126–8140. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowski, J.P.; Martin, A.D.; Mason, A.F.; Fife, C.M.; Sagnella, S.M.; Kavallaris, M.; Thordarson, P. Choice of Capping Group in Tripeptide Hydrogels Influences Viability in the Three-Dimensional Cell Culture of Tumor Spheroids. Chempluschem 2017, 82, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Truong, W.T.; Su, Y.; Gloria, D.; Braet, F.; Thordarson, P. Dissolution and Degradation of Fmoc-Diphenylalanine Self-Assembled Gels Results in Necrosis at High Concentrations in Vitro. Biomater. Sci. 2015, 3, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.; Wu, F.; Cheng, H.; Huang, Y.; Hsieh, Y.; Tseng, D.T.; Yeh, M.; Hung, S.; Lin, H. Functional Supramolecular Polymers: A Fluorescent Microfibrous Network in a Supramolecular Hydrogel for High-Contrast Live Cell-Material Imaging in 3D Environments. Adv. Healthc. Mater. 2016, 5, 2406–2412. [Google Scholar] [CrossRef] [PubMed]
- Koshti, B.; Kshtriya, V.; Naskar, S.; Narode, H.; Gour, N. Controlled Aggregation Properties of Single Amino Acids Modified with Protecting Groups. New J. Chem. 2022, 46, 4746–4755. [Google Scholar] [CrossRef]
- Torres-Gómez, N.; Nava, O.; Argueta-Figueroa, L.; García-Contreras, R.; Baeza-Barrera, A.; Vilchis-Nestor, A.R. Shape Tuning of Magnetite Nanoparticles Obtained by Hydrothermal Synthesis: Effect of Temperature. J. Nanomater. 2019, 2019, 7921273. [Google Scholar] [CrossRef]
- Kralj, S.; Bellotto, O.; Parisi, E.; Garcia, A.M.; Iglesias, D.; Semeraro, S.; Deganutti, C.; D’Andrea, P.; Vargiu, A.V.; Geremia, S.; et al. Heterochirality and Halogenation Control Phe-Phe Hierarchical Assembly. ACS Nano 2020, 14, 16951–16961. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.P.; Singh, N.; Sasselli, I.R.; Escuder, B.; Ulijn, R.V. Metastable Hydrogels from Aromatic Dipeptides. Chem. Commun. 2016, 52, 13889–13892. [Google Scholar] [CrossRef]
- Bellotto, O.; Kralj, S.; De Zorzi, R.; Geremia, S.; Marchesan, S. Supramolecular Hydrogels from Unprotected Dipeptides: A Comparative Study on Stereoisomers and Structural Isomers. Soft Matter 2020, 16, 10151–10157. [Google Scholar] [CrossRef]
- Panda, J.J.; Mishra, A.; Basu, A.; Chauhan, V.S. Stimuli Responsive Self-Assembled Hydrogel of a Low Molecular Weight Free Dipeptide with Potential for Tunable Drug Delivery. Biomacromolecules 2008, 9, 2244–2250. [Google Scholar] [CrossRef]
- Thota, C.K.; Yadav, N.; Chauhan, V.S. A Novel Highly Stable and Injectable Hydrogel Based on a Conformationally Restricted Ultrashort Peptide. Sci. Rep. 2016, 6, 31167. [Google Scholar] [CrossRef] [PubMed]
- Reches, M.; Gazit, E. Casting Metal Nanowires Within Discrete Self-Assembled Peptide Nanotubes. Science 2003, 300, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Görbitz, C.H. Nanotube Formation by Hydrophobic Dipeptides. Chem. Eur. J. 2001, 7, 5153–5159. [Google Scholar] [CrossRef] [PubMed]
- Reches, M.; Gazit, E. Designed Aromatic Homo-Dipeptides: Formation of Ordered Nanostructures and Potential Nanotechnological Applications. Phys. Biol. 2006, 3, S10–S19. [Google Scholar] [CrossRef] [PubMed]
- Diaferia, C.; Avitabile, C.; Leone, M.; Gallo, E.; Saviano, M.; Accardo, A.; Romanelli, A. Diphenylalanine Motif Drives Self-Assembling in Hybrid PNA-Peptide Conjugates. Chem. Eur. J. 2021, 27, 14307–14316. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Fei, J.; Li, Q.; Li, J. Covalently Assembled Dipeptide Nanospheres as Intrinsic Photosensitizers for Efficient Photodynamic Therapy in Vitro. Chem. Eur. J. 2016, 22, 6696. [Google Scholar] [CrossRef]
- Diaferia, C.; Gianolio, E.; Sibillano, T.; Mercurio, F.A.; Leone, M.; Giannini, C.; Balasco, N.; Vitagliano, L.; Morelli, G.; Accardo, A. Cross-Beta Nanostructures Based on Dinaphthylalanine Gd-Conjugates Loaded with Doxorubicin. Sci. Rep. 2017, 7, 307. [Google Scholar] [CrossRef]
- Reches, M.; Gazit, E. Self-assembly of Peptide Nanotubes and Amyloid-like Structures by Charged-termini-capped Diphenylalanine Peptide Analogues. Isr. J. Chem. 2005, 45, 363–371. [Google Scholar] [CrossRef]
- Jayawarna, V.; Ali, M.; Jowitt, T.A.; Miller, A.F.; Saiani, A.; Gough, J.E.; Ulijn, R.V. Nanostructured Hydrogels for Three-Dimensional Cell Culture Through Self-Assembly of Fluorenylmethoxycarbonyl–Dipeptides. Adv. Mater. 2006, 18, 611–614. [Google Scholar] [CrossRef]
- Tang, C.; Smith, A.M.; Collins, R.F.; Ulijn, R.V.; Saiani, A. Fmoc-Diphenylalanine Self-Assembly Mechanism Induces Apparent p K a Shifts. Langmuir 2009, 25, 9447–9453. [Google Scholar] [CrossRef] [PubMed]
- Rosa, E.; Diaferia, C.; Gallo, E.; Morelli, G.; Accardo, A. Stable Formulations of Peptide-Based Nanogels. Molecules 2020, 25, 3455. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.; Boháčová, K.; Fuentes-Caparrós, A.M.; Schweins, R.; Draper, E.R.; Adams, D.J.; Pujals, S.; Albertazzi, L. PAINT-ing Fluorenylmethoxycarbonyl (Fmoc)-Diphenylalanine Hydrogels. Chem. Eur. J. 2020, 26, 9869–9873. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Datta, H.K.; Roy, R.; Dastidar, P. Designing a Vehicle-Free Anti-Bacterial Topical Hydrogel from Fmoc-Diphenylalanine. Chem. Commun. 2023, 59, 9400–9403. [Google Scholar] [CrossRef] [PubMed]
- Nadernezhad, A.; Forster, L.; Netti, F.; Adler-Abramovich, L.; Teßmar, J.; Groll, J. Rheological Analysis of the Interplay between the Molecular Weight and Concentration of Hyaluronic Acid in Formulations of Supramolecular HA/FmocFF Hybrid Hydrogels. Polym. J. 2020, 52, 1007–1012. [Google Scholar] [CrossRef]
- Helen, W.; de Leonardis, P.; Ulijn, R.V.; Gough, J.; Tirelli, N. Mechanosensitive Peptidegelation: Mode of Agitation Controls Mechanical Properties and Nano-Scale Morphology. Soft Matter 2011, 7, 1732–1740. [Google Scholar] [CrossRef]
- Smith, A.M.; Williams, R.J.; Tang, C.; Coppo, P.; Collins, R.F.; Turner, M.L.; Saiani, A.; Ulijn, R.V. Fmoc-Diphenylalanine Self Assembles to a Hydrogel via a Novel Architecture Based on π–π Interlocked Β-Sheets. Adv. Mater. 2008, 20, 37–41. [Google Scholar] [CrossRef]
- Mahler, A.; Reches, M.; Rechter, M.; Cohen, S.; Gazit, E. Rigid, Self-Assembled Hydrogel Composed of a Modified Aromatic Dipeptide. Adv. Mater. 2006, 18, 1365–1370. [Google Scholar] [CrossRef]
- Gallo, E.; Diaferia, C.; Smaldone, G.; Rosa, E.; Pecoraro, G.; Morelli, G.; Accardo, A. Fmoc-FF Hydrogels and Nanogels for Improved and Selective Delivery of Dexamethasone in Leukemic Cells and Diagnostic Applications. Sci. Rep. 2024, 14, 9940. [Google Scholar] [CrossRef]
- Hirst, A.R.; Roy, S.; Arora, M.; Das, A.K.; Hodson, N.; Murray, P.; Marshall, S.; Javid, N.; Sefcik, J.; Boekhoven, J.; et al. Biocatalytic Induction of Supramolecular Order. Nat. Chem. 2010, 2, 1089–1094. [Google Scholar] [CrossRef]
- Johnson, T.; Bahrampourian, R.; Patel, A.; Mequanint, K. Fabrication of Highly Porous Tissue-Engineering Scaffolds Using Selective Spherical Porogens. Biomed. Mater. Eng. 2010, 20, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Orbach, R.; Adler-Abramovich, L.; Zigerson, S.; Mironi-Harpaz, I.; Seliktar, D.; Gazit, E. Self-Assembled Fmoc-Peptides as a Platform for the Formation of Nanostructures and Hydrogels. Biomacromolecules 2009, 10, 2646–2651. [Google Scholar] [CrossRef] [PubMed]
- Vegners, R.; Shestakova, I.; Kalvinsh, I.; Ezzell, R.M.; Janmey, P.A. Use of a Gel-forming Dipeptide Derivative as a Carrier for Antigen Presentation. J. Pept. Sci. 1995, 1, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Pappas, C.; Debnath, S.; Frederix, P.W.J.M.; Leckie, J.; Fleming, S.; Ulijn, R.V. Stable Emulsions Formed by Self-Assembly of Interfacial Networks of Dipeptide Derivatives. ACS Nano 2014, 8, 7005–7013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, H.; Yang, Z.; Xu, B. Supramolecular Hydrogels Respond to Ligand−Receptor Interaction. J. Am. Chem. Soc. 2003, 125, 13680–13681. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.; Debnath, S.; Frederix, P.W.J.M.; Hunt, N.T.; Ulijn, R.V. Insights into the Coassembly of Hydrogelators and Surfactants Based on Aromatic Peptide Amphiphiles. Biomacromolecules 2014, 15, 1171–1184. [Google Scholar] [CrossRef] [PubMed]
- Jian, H.; Wang, M.; Dong, Q.; Li, J.; Wang, A.; Li, X.; Ren, P.; Bai, S. Dipeptide Self-Assembled Hydrogels with Tunable Mechanical Properties and Degradability for 3D Bioprinting. ACS Appl. Mater. Interfaces 2019, 11, 46419–46426. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Li, J.; Zhao, L.; Wang, A.; Wang, M.; Li, J.; Jian, H.; Li, X.; Yan, X.; Bai, S. Dipeptide Self-Assembled Hydrogels with Shear-Thinning and Instantaneous Self-Healing Properties Determined by Peptide Sequences. ACS Appl. Mater. Interfaces 2020, 12, 21433–21440. [Google Scholar] [CrossRef]
- Najafi, H.; Tamaddon, A.M.; Abolmaali, S.; Borandeh, S.; Azarpira, N. Structural, Mechanical, and Biological Characterization of Hierarchical Nanofibrous Fmoc-Phenylalanine-Valine Hydrogels for 3D Culture of Differentiated and Mesenchymal Stem Cells. Soft Matter 2021, 17, 57–67. [Google Scholar] [CrossRef]
- Hughes, M.; Birchall, L.S.; Zuberi, K.; Aitken, L.A.; Debnath, S.; Javid, N.; Ulijn, R.V. Differential Supramolecular Organisation of Fmoc-Dipeptides with Hydrophilic Terminal Amino Acid Residues by Biocatalytic Self-Assembly. Soft Matter 2012, 8, 11565. [Google Scholar] [CrossRef]
- Chakraborty, P.; Tang, Y.; Yamamoto, T.; Yao, Y.; Guterman, T.; Zilberzwige-Tal, S.; Adadi, N.; Ji, W.; Dvir, T.; Ramamoorthy, A.; et al. Unusual Two-Step Assembly of a Minimalistic Dipeptide-Based Functional Hypergelator. Adv. Mater. 2020, 32, 1906043. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.; Lei, J.; Zhan, C.; Shimon, L.J.W.; Adler-Abramovich, L.; Wei, G.; Gazit, E. Structural Polymorphism in a Self-Assembled Tri-Aromatic Peptide System. ACS Nano 2018, 12, 3253–3262. [Google Scholar] [CrossRef] [PubMed]
- Veloso, S.R.S.; Jervis, P.J.; Silva, J.F.G.; Hilliou, L.; Moura, C.; Pereira, D.M.; Coutinho, P.J.G.; Martins, J.A.; Castanheira, E.M.S.; Ferreira, P.M.T. Supramolecular Ultra-Short Carboxybenzyl-Protected Dehydropeptide-Based Hydrogels for Drug Delivery. Mater. Sci. Eng. C 2021, 122, 111869. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.-Y.; Hsu, S.-M.; Cheng, H.; Hsu, L.-H.; Lin, H.-C. The Effect of Fluorine on Supramolecular Hydrogelation of 4-Fluorobenzyl-Capped Diphenylalanine. New J. Chem. 2015, 39, 4240–4243. [Google Scholar] [CrossRef]
- Dadhwal, S.; Fairhall, J.M.; Goswami, S.K.; Hook, S.; Gamble, A.B. Alkene–Azide 1,3-Dipolar Cycloaddition as a Trigger for Ultrashort Peptide Hydrogel Dissolution. Chem. Asian J. 2019, 14, 1143–1150. [Google Scholar] [CrossRef]
- Dadhwal, S.; Fairhall, J.M.; Hook, S.; Gamble, A.B. Tetrafluoroaryl Azide as an N-Terminal Capping Group for Click-to-Dissolve Diphenylalanine Hydrogels. RSC Adv. 2020, 10, 9234–9244. [Google Scholar] [CrossRef]
- Sitsanidis, E.D.; Kasapidou, P.M.; Hiscock, J.R.; Gubala, V.; Castel, H.; Popoola, P.I.A.; Hall, A.J.; Edwards, A.A. Probing the Self-Assembly and Anti-Glioblastoma Efficacy of a Cinnamoyl-Capped Dipeptide Hydrogelator. Org. Biomol. Chem. 2022, 20, 7458–7466. [Google Scholar] [CrossRef]
- Martin, A.D.; Robinson, A.B.; Mason, A.F.; Wojciechowski, J.P.; Thordarson, P. Exceptionally Strong Hydrogels through Self-Assembly of an Indole-Capped Dipeptide. Chem. Commun. 2014, 50, 15541–15544. [Google Scholar] [CrossRef]
- Martin, A.D.; Wojciechowski, J.P.; Warren, H.; in het Panhuis, M.; Thordarson, P. Effect of Heterocyclic Capping Groups on the Self-Assembly of a Dipeptide Hydrogel. Soft Matter 2016, 12, 2700–2707. [Google Scholar] [CrossRef]
- Martin, A.D.; Robinson, A.B.; Thordarson, P. Biocompatible Small Peptide Super-Hydrogelators Bearing Carbazole Functionalities. J. Mater. Chem. B 2015, 3, 2277–2280. [Google Scholar] [CrossRef]
- Aldilla, V.R.; Nizalapur, S.; Martin, A.; Marjo, C.E.; Rich, A.; Yee, E.; Suwannakot, P.; Black, D.S.; Thordarson, P.; Kumar, N. Design, Synthesis, and Characterisation of Glyoxylamide-Based Short Peptides as Self-Assembled Gels. New J. Chem. 2017, 41, 13462–13471. [Google Scholar] [CrossRef]
- Li, X.; Kuang, Y.; Lin, H.; Gao, Y.; Shi, J.; Xu, B. Supramolecular Nanofibers and Hydrogels of Nucleopeptides. Angew. Chem. Int. Ed. 2011, 50, 9365–9369. [Google Scholar] [CrossRef] [PubMed]
- Draper, E.R.; McDonald, T.O.; Adams, D.J. Photodimerisation of a Coumarin-Dipeptide Gelator. Chem. Commun. 2015, 51, 12827–12830. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Qiu, Z.; Xu, Y.; Shi, J.; Lin, H.; Zhang, Y. Supramolecular Hydrogels Based on Short Peptides Linked with Conformational Switch. Org. Biomol. Chem. 2011, 9, 2149. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Tanida, T.; Yoshii, T.; Hamachi, I. Rational Molecular Design of Stimulus-Responsive Supramolecular Hydrogels Based on Dipeptides. Adv. Mater. 2011, 23, 2819–2822. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liang, G.; Ma, M.; Gao, Y.; Xu, B. Conjugates of Naphthalene and Dipeptides Produce Molecular Hydrogelators with High Efficiency of Hydrogelation and Superhelical Nanofibers. J. Mater. Chem. 2007, 17, 850–854. [Google Scholar] [CrossRef]
- Awhida, S.; Draper, E.R.; McDonald, T.O.; Adams, D.J. Probing Gelation Ability for a Library of Dipeptide Gelators. J. Colloid Interface Sci. 2015, 455, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, R.; Qi, W.; Wu, Z.; Su, R.; He, Z. Kinetically Controlled Self-Assembly of Redox-Active Ferrocene–Diphenylalanine: From Nanospheres to Nanofibers. Nanotechnology 2013, 24, 465603. [Google Scholar] [CrossRef] [PubMed]
- Hilliou, L. Structure–Elastic Properties Relationships in Gelling Carrageenans. Polymers 2021, 13, 4120. [Google Scholar] [CrossRef]
- Lalitha Sridhar, S.; Vernerey, F.J. Mechanics of Transiently Cross-Linked Nematic Networks. J. Mech. Phys. Solids 2020, 141, 104021. [Google Scholar] [CrossRef]
- Nolan, M.C.; Fuentes Caparrós, A.M.; Dietrich, B.; Barrow, M.; Cross, E.R.; Bleuel, M.; King, S.M.; Adams, D.J. Optimising Low Molecular Weight Hydrogels for Automated 3D Printing. Soft Matter 2017, 13, 8426–8432. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Raeburn, J.; Sutton, S.; Spiller, D.G.; Williams, J.; Sharp, J.S.; Griffiths, P.C.; Heenan, R.K.; King, S.M.; Paul, A.; et al. Tuneable Mechanical Properties in Low Molecular Weight Gels. Soft Matter 2011, 7, 9721. [Google Scholar] [CrossRef]
- Veloso, S.R.S.; Tiryaki, E.; Spuch, C.; Hilliou, L.; Amorim, C.O.; Amaral, V.S.; Coutinho, P.J.G.; Ferreira, P.M.T.; Salgueiriño, V.; Correa-Duarte, M.A.; et al. Tuning the Drug Multimodal Release through a Co-Assembly Strategy Based on Magnetic Gels. Nanoscale 2022, 14, 5488–5500. [Google Scholar] [CrossRef] [PubMed]
- Veloso, S.R.S.; Martins, J.A.; Hilliou, L.; Amorim, C.O.; Amaral, V.S.; Almeida, B.G.; Jervis, P.J.; Moreira, R.; Pereira, D.M.; Coutinho, P.J.G.; et al. Dehydropeptide-Based Plasmonic Magnetogels: A Supramolecular Composite Nanosystem for Multimodal Cancer Therapy. J. Mater. Chem. B 2020, 8, 45–64. [Google Scholar] [CrossRef]
- Vilaça, H.; Carvalho, A.; Castro, T.; Castanheira, E.M.S.; Hilliou, L.; Hamley, I.; Melle-Franco, M.; Ferreira, P.M.T.; Martins, J.A. Unveiling the Role of Capping Groups in Naphthalene N-Capped Dehydrodipeptide Hydrogels. Gels 2023, 9, 464. [Google Scholar] [CrossRef]
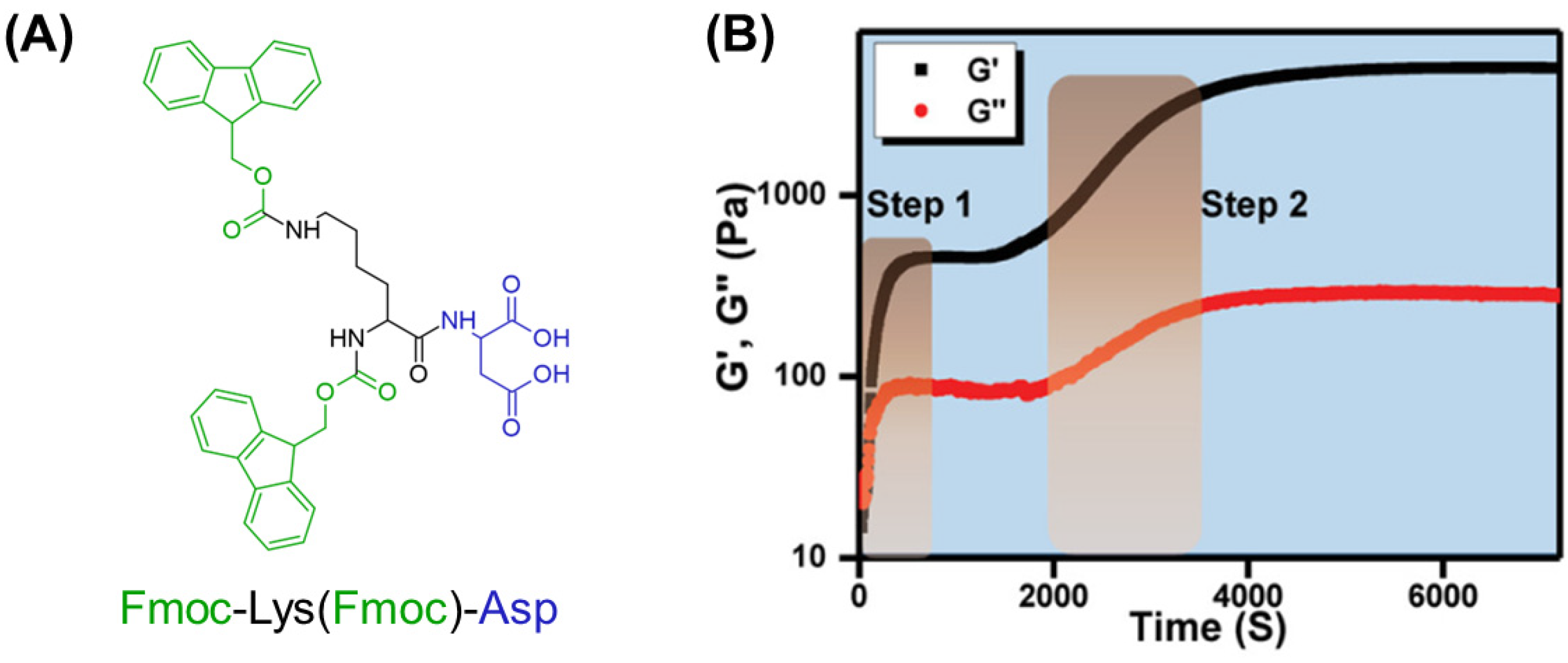
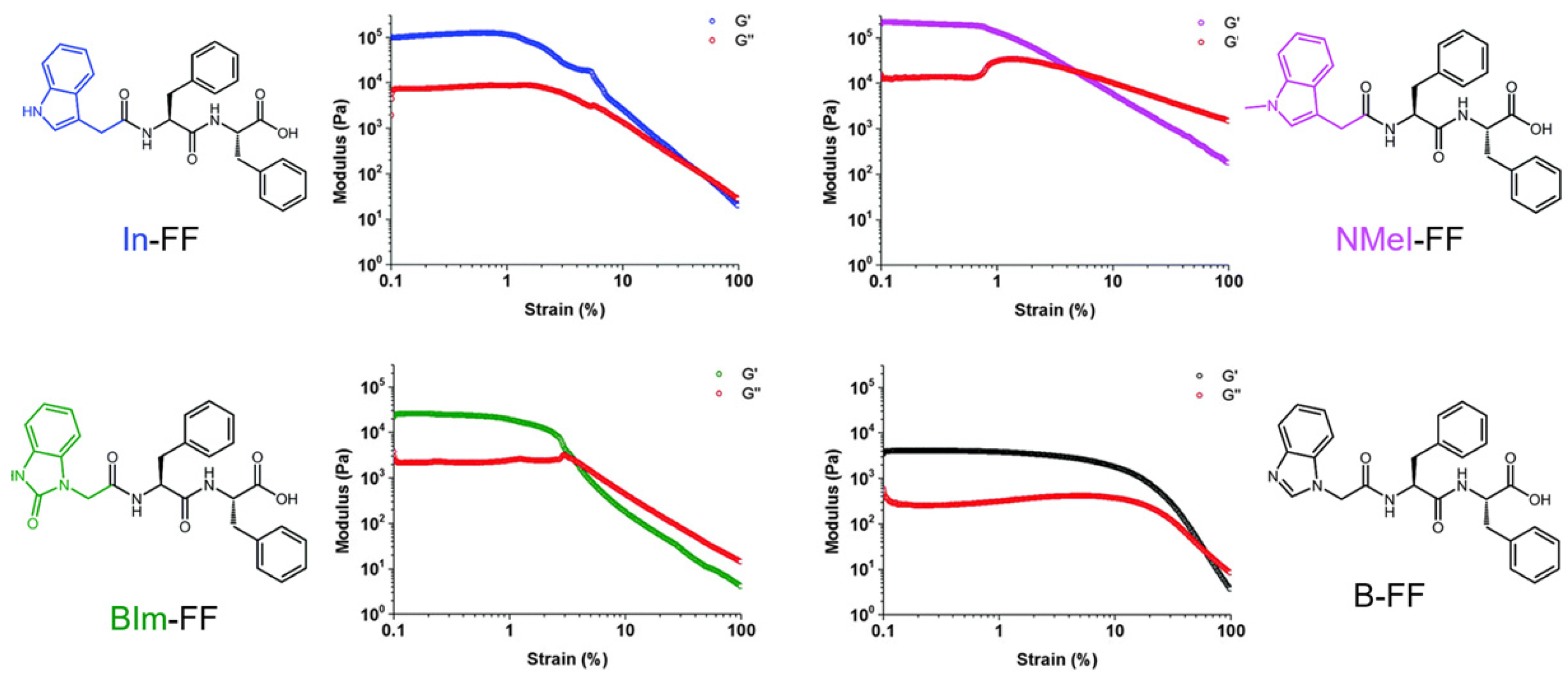
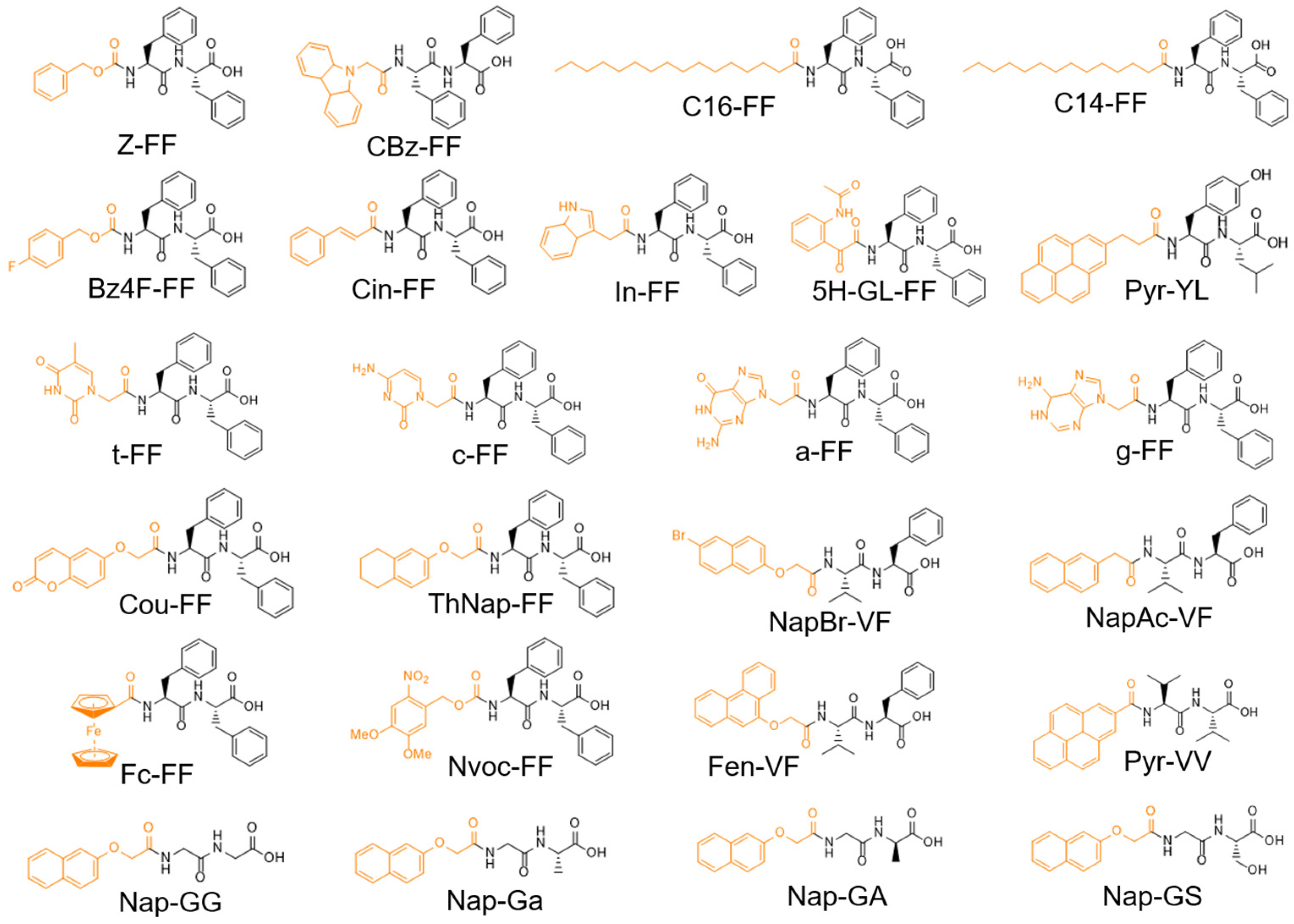
| Gelator | Method | Media | pH | CGC (mM) | [Gel] (mM) | G′ (Pa) | G″ (Pa) | LVR (%) | γ (%) | Tm (°C) | Fibril (nm) | Highlights | Application | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D-Phe | HC | PBS | - | 184 | 300 | - | - | - | - | - | - | - | - | [102] |
| L-Phe | HC | PBS | - | 99 | 300 | - | - | - | - | - | - | - | - | [102] |
| HC | Water | 6.45 | - | 303 | ~200,000 | ~45,000 | - | - | 323.6–326.6 | 437 | - | - | [104] | |
| Fmoc-Phe | HC | PB | 7.4 | 2.58 | - | ~103 | ~102 | - | - | 38 | 4.54–7.24 | Tuneable Tm | - | [113,114] |
| GdL | Water | 6.1 | 9.68 | 25.8 | ~104 | ~102 | 1 | 10 | - | - | - | - | [115] | |
| GdL | Water | 6.6 | 7.74 | 25.8 | 50,199 | ~2000 | 0.1 | 0.2 | 55 | - | Stringed nanoparticles | - | [116] | |
| SE | Water/DMSO (98:2 v/v%) | - | - | 4.9 | 39 | 5 | - | - | - | 295 | - | - | [95,117] | |
| Fmoc-Phe-DAP | NaCl + HC | Water | - | 20 | 33.7 | 383 | 59 | 1 | 1 | - | 500–1000 | Thixotropic fibrils and nanotubes | Drug delivery | [119] |
| Fmoc-4-NO2-Phe | SE | Water/DMSO (98:2 v/v%) | - | - | 4.9 | 410 | 66 | - | - | - | 12 | - | - | [95] |
| Fmoc-4-CN-Phe | SE | Water/DMSO (98:2 v/v%) | - | - | 4.9 | 140 | 17 | - | - | - | 25 | - | - | [95] |
| Fmoc-4-F-Phe | SE | Water/DMSO (98:2 v/v%) | - | - | 4.9 | 102 | 9 | - | - | - | 26 | - | - | [95] |
| Fmoc-4-NH2-Phe | SE | Water/DMSO (98:2 v/v%) | - | - | 4.9 | 527 | 61 | - | - | - | 11 | - | - | [95] |
| Fmoc-4-CH3-Phe | SE | Water/DMSO (98:2 v/v%) | - | - | 4.9 | 280 | 53 | - | - | - | 21 | - | - | [95] |
| Fmoc-3-F-Phe | GdL | Water | - | - | 7.5 | 3918 | 296 | - | - | - | 126 | - | - | [117] |
| GdL | Water | - | - | 5–15 | 102–103 | 101–102 | - | - | - | 11–19 | Thixotropic | - | [118] | |
| SE | Water/DMSO (98:2 v/v%) | - | - | 5 | 103–104 | ~104 | - | - | - | 22 | Thixotropic | - | [118] | |
| Fmoc-3F-Phe-DAP | NaCl + HC | Water | - | 20 | 33.7 | 21,311 | 3973 | 0.1 | 1 | - | 20–30 | Thixotropic fibrils and tapes | Drug delivery | [119] |
| Fmoc-F5-Phe | GdL | Water | - | - | 7.5 | 4786 | 449 | - | - | - | 13 | - | - | [117] |
| GdL | Water | - | - | 5–15 | 102–103 | 101–102 | - | - | - | 24–16 | Thixotropic | - | [118] | |
| SE | Water/DMSO (98:2 v/v%) | - | - | 5 | 103 | 102 | - | - | - | 15 | Thixotropic | - | [118] | |
| Fmoc-F5-Phe-DAP | NaCl + HC | Water | - | 20 | 33.7 | 10,776 | 2273 | 1 | 1 | - | 10–20 | Thixotropic Twisted fibers and tapes | Drug delivery | [119] |
| Fmoc-Tyr | SE | Water/DMSO (98:2 v/v%) | - | - | 4.9 | 506 | 59 | - | - | - | 13 | - | - | [95] |
| GdL | Water | 5.2 | <0.1 | 21.7 | 104–105 | ~104 | 1 | 1 | 80 | - | Thermoreversible | - | [115] | |
| HC | PB | 7.4 | 0.47 | 20 | ~3000 | ~800 | 1 | 1–10 | - | 20 | Flexible entangled fibers | Antimicrobial activity | [120] | |
| Fmoc-Tyr(PO4) | HC | Water | 2.5 | - | 40 | ~1000 | ~100 | - | - | - | 20–25 | Bundles (50–100 nm) | - | [121] |
| Enzyme | Water | 6 | - | 40 | ~5000 | ~2000 | - | - | - | 20–25 | Thermoreversible | - | [121] | |
| Fmoc-Tyr(3NO2) | HC | PB | 5 | 2 | 15.6 | ~7000 | ~800 | - | - | - | - | No recovery | Antimicrobial activity | [122] |
| HC | PB | 7 | 5.6 | 15.6 | ~1000 | ~500 | - | - | - | - | Thixotropic | Antimicrobial activity | [122] | |
| HC | PB | 8 | 11.2 | 15.6 | ~1000 | ~300 | - | - | - | - | Thixotropic | Antimicrobial activity | [122] | |
| Fmoc-Tyr /Fmoc-Tyr(Bzl) | SE | Water/DMSO (98:2 v/v%) | - | - | - | ~900 | ~300 | - | - | - | 10–50 & 50–80 | - | Photothermia Drug delivery | [123] |
| Fmoc-Phe/Fmoc-Tyr(Bzl) | SE | Water/DMSO (98:2 v/v%) | - | - | - | ~900 | ~200 | - | - | - | 10–100 | - | Photothermia Drug delivery | [123] |
| Fmoc-Trp | GdL | Water | 5.2 | 1.9 | 19 | ~104 | ~103 | 1 | 10 | 75 | - | - | - | [115] |
| HC | PB | 7.4 | 0.03 | - | 100 | 10 | 0.1 | 1 | - | ~20 | - | Antibacterial | [120] | |
| Fmoc-Met | GdL | Water | 5.2 | <0.13 | 27 | ~103 | ~102 | 1 | 10 | - | - | Syneresis | - | [115] |
| HC | PB | 7.4 | 0.12 | - | 1000 | 100 | 0.1 | 1 | - | ~20 | - | Antibacterial | [120] | |
| Fmoc-Gly | GdL | Water | 5.2 | 26.9 | 33.6 | ~102 | ~101 | 0.1 | 100 | - | - | - | - | [115] |
| Fmoc-Ile | GdL | Water | 5.2 | 19.8 | 28.3 | ~102 | ~101 | 1 | 100 | - | - | - | - | [115] |
| Fmoc-His | Metal | Tris-HNO3 | 9.1 | - | 10.6 | ~2000 | ~100 | 0.01 | 0.1 | - | ~20 | - | Antimicrobial activity | [124] |
| Fmoc-Pro | Metal | Tris-HNO3 | 9.1 | - | 11.9 | ~300 | ~10 | 0.01 | 0.1 | - | ~20 | - | Antimicrobial activity | [124] |
| Fmoc-Ala | Metal | Tris-HNO3 | 9.1 | - | 12.8 | ~1000 | ~100 | 0.01 | 0.1 | - | ~20 | - | Antimicrobial activity | [124] |
| Fmoc-Leu | Metal | Tris-HNO3 | 9.1 | - | 11.3 | ~2000 | ~400 | 0.01 | 0.1 | - | ~20 | - | Antimicrobial activity | [124] |
| Fmoc-Lys-Bct | US | PB | 7.4 | 5 | - | ~6000 | ~100 | - | - | 60 | - | Thixotropic | Antimicrobial activity | [125] |
| Fmoc-Lys(Fmoc) | SE | Water/DMSO (99:1 v/v%) | 6 | 5 | - | ~5000 | ~300 | 1 | 1–10 | - | - | Thixotropic | - | [126] |
| SE | Water/DMSO (99:1 v/v%) | 7.4 | 5 | - | ~500 | ~20 | 1 | 10 | - | - | Thixotropic | - | [126] | |
| Fmoc-Dap(Fmoc) | SE | Water/DMSO (97:3 v/v%) | 4.9 | 5.5 | - | 100 | 10 | 10 | 10 | - | 150–250 | Thixotropic | Drug delivery | [127] |
| SE | Water/DMSO (97:3 v/v%) | 7.4 | 18.2 | - | 10 | 1 | 1 | 10 | - | 250–300 | Thixotropic | Drug delivery | [127] | |
| SE | Water/DMSO (97:3 v/v%) | 9.1 | 23.7 | - | 1 | 1 | 0.1 | 10 | - | 250–600 | Thixotropic | Drug delivery | [127] | |
| 1-NapAc-Phe | GdL | Water | - | - | 7.5 | 941 | 82 | - | - | - | 11 | - | - | [117] |
| 1-NapAc-3F-Phe | GdL | Water | - | - | 7.5 | 1548 | 118 | - | - | - | 20 | - | - | [117] |
| 1-NapAc-F5-Phe | GdL | Water | - | - | 7.5 | 2522 | 336 | - | - | - | 13 | - | - | [117] |
| 2-NapAc-Phe | GdL | Water | 5.7 | 15 | 30 | 4849 | ~100 | 0.1 | 0.26 | 45 | - | - | - | [116] |
| 2-Nap-Phe | GdL | Water | 5.9 | 19 | 27 | 7820 | ~300 | 0.1 | 0.56 | 48 | - | - | - | [116] |
| Pyr-Phe | HC | PB | 7.4 | 0.85 | 118.3 | ~200 | ~60 | - | - | 66.4 | 30–55 | Thixotropic Helical fibers | Drug delivery | [128] |
| Cin-Phe | GdL | Water | 4.6 | 33.9 | 33.9 | 2519 | ~100 | 1 | 0.85 | 41 | - | - | - | [116] |
| Lauroyl-Phe | HC | Water | - | 43.2 | - | ~2000 | ~100 | 0.1 | 0.1–10 | - | - | Flat 2D sheets | - | [129] |
| Bz(4-NO2)-Phe | HC | PBS | 6 | 20 | 20 | 2000 | 200 | 40 | 100 | ~40 | - | 5 | Antimicrobial | [130] |
| BP-Phe | SE | CH4/H2O | - | 2 | 5 | 102–103 | 101–102 | 1 | 10–100 | - | - | 50 | Imprinting | [131] |
| Myr-L-Phe | HC | PB | 7 | 6.7 | - | 102 | 102 | - | - | 37 | 56 | Thixotropic | Enzyme entrapment | [132] |
| Myr-D-Phe | HC | PB | 7 | 6.7 | - | 102 | 10 | - | - | 37 | 58 | Thixotropic | Enzyme entrapment | [132] |
| Gelator | Method | Media | pH | CGC (mM) | [Gel] (mM) | G′ (Pa) | G″ (Pa) | LVR (%) | γ (%) | Tm (°C) | Fibril (nm) | Highlights | Application | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Z-FF | SE | Water/HFIP | - | <11.2 | 22.4 | >100,000 | <10,000 | - | - | - | - | Thixotropic | - | [182] |
| SE | Water/AA | - | - | 11.2 | <100,000 | ~1000 | - | - | - | - | - | - | [182] | |
| D | Water | - | 3.1 | - | 2000 | ~150 | <10 | <30 | - | - | - | - | [129] | |
| HC | Water | - | - | - | 300 | ~30 | <10 | >100 | - | - | - | - | [129] | |
| CBz-FF | pHE | PBS | 7.2 | 0.7 | 22.4 | 500 | 50 | - | - | - | 1.7 | - | - | [190] |
| C16-FF | D | Water | - | 1.8 | - | ~300 | ~70 | <1 | 10 | - | 20 [d] | Helical fibers | - | [129] |
| HC | Water | - | - | - | ~300 | ~150 | <1 | - | - | - | - | [129] | ||
| D | Water/salt | 11.7 | - | 9.1 | 2361 | 334 | >20 | ~80 | - | - | - | [61] | ||
| C14-FF | D/Ca2+ | Water/salt | 11.7 | - | 9.6 | 3400 | 732 | <10 | ~20 | - | - | - | - | [61] |
| Bz4F-FF | HC | Water | 7.89 | 33.5 | 44.8 | 5700 | ~1000 | >100- | - | - | 8 [d] | Tm dependence | Cell growth | [184] |
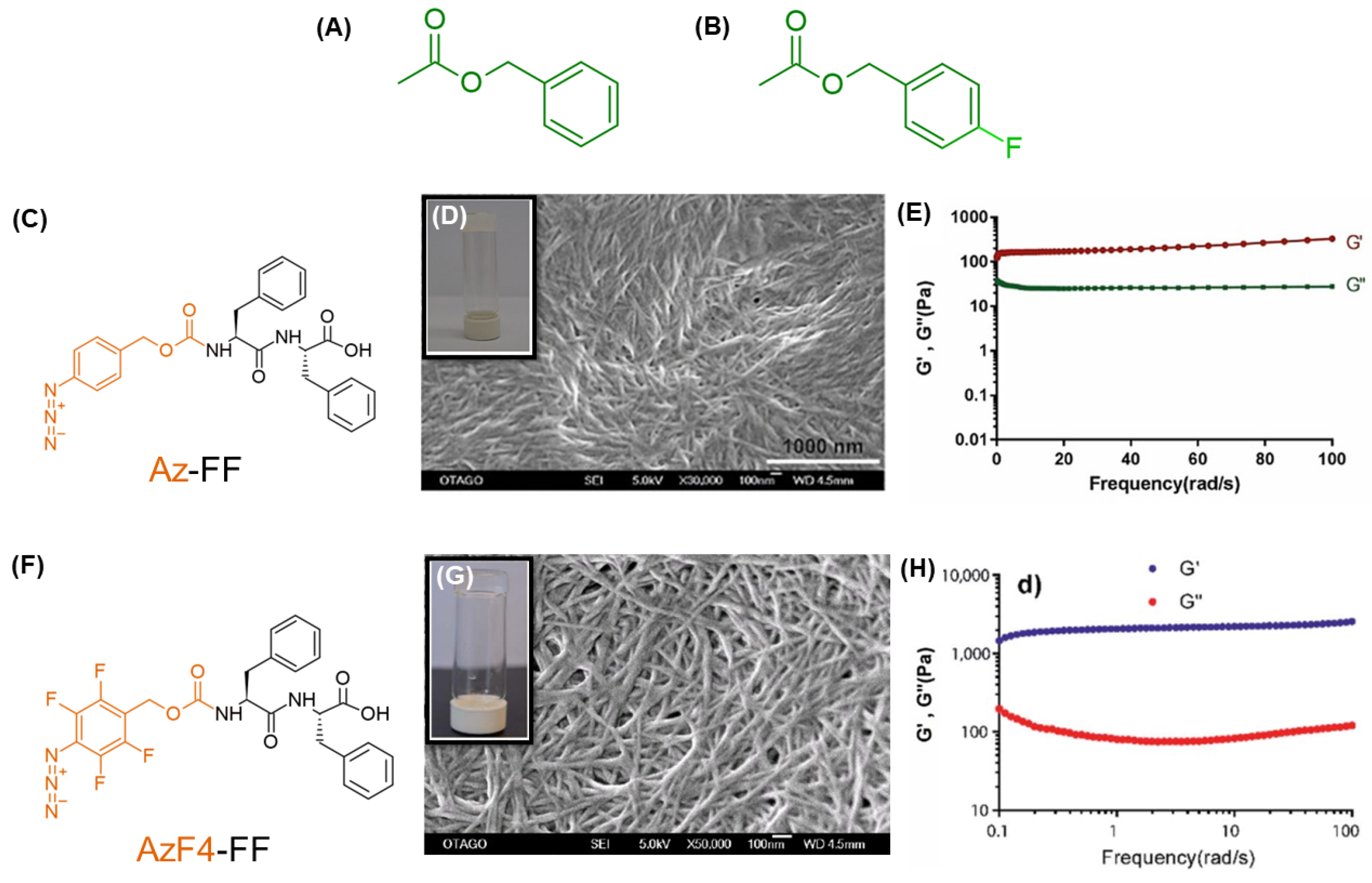
| Az-FF | SE | Water/DMSO | 3.4 | 10.2 | 10.2 | 200 | ~30 | - | - | - | - | - | Drug delivery | [185] |
| AzF4-FF | SE | Water/DMSO | 7.5 | 1.8 | 8.9 | 1500 | ~200 | - | - | - | - | - | Drug delivery | [185] |
| Cin-FF | HC | PBS | 7.4 | 4.5 | 4.5 | 226 | - | - | - | ~45 | Ribbon | Helical fibers | Cell growth | [187] |
| In-FF | pHE GdL | Water | - | 8.5 | 21.2 | 300,000 | ~5000 | - | - | - | 100–400 [t] | - | - | [188] |
| pHE GdL | Water | 4–5 | 6.4 | 12.8 | 100,000 | 10000 | <1 | >70 | - | - | Fiber dd | - | [189] | |
| NMeI-FF | pHE GdL | Water | 4–5 | 12.4 | 12.4 | 200,000 | 20000 | <1 | ~9 | - | - | Fiber dd | - | [189] |
| Bim-FF | pHE GdL | Water | 4–5 | 4.1 | 12.3 | 30,000 | 2000 | <3 | ~4 | - | - | Fiber dd | - | [189] |
| B-FF | pHE GdL | Water | 4–5 | 2.1 | 12.7 | 50,000 | 4000 | <10 | ~70 | - | - | Fiber dd | - | [189] |
| 5H-GL-FF | pHE GdL | Water | - | 2 | 4 | ~1000 | - | - | - | - | - | - | - | [191] |
| Pyr-YL | pHE | Water | ~7.3 | - | 10 | ~190 | ~45 | 0.1–1 | - | - | 40–200 | Stable ν 0.1–5.0 | - | [176] |
| ThNap-FF | D/Ca2+ | Water/salt | 11.7 | - | 10 | 54,944 | 8786 | <1 | >10 | - | - | - | - | [61] |
| SE | Water:DMSO (80:20 v/v) | ~4.3 | - | 4 | ~10,000 | ~1000 | >10 | - | - | - | Annealing | Molding | [13] | |
| a-FF | pHE | Water | 5 | 20.5 | 40.9 | 8090 | - | - | 1 | - | 16 [w] | - | - | [192] |
| g-FF | pHE | Water | 5 | 19.9 | 39.7 | 12,613 | - | - | 0.8 | - | 15 [w] | - | - | [192] |
| t-FF | pHE | Water | 5 | 20.9 | 41.8 | 6345 | - | - | 1.2 | - | 9 [w] | - | - | [192] |
| c-FF | pHE | Water | 5 | 21.6 | 43.2 | 26 | - | - | 0.6 | - | 10 [w] | - | - | [192] |
| Cou-FF | pHE GdL | Water | - | - | 9.7 | 82,000 | 10,000 | - | 1 | - | 42 [d] | - | - | [193] |
| Fc-FF | D | Water/MeOH (90:10 v/v) | - | - | 5.7 | ~1000 | ~40 | - | - | - | 40–90 [d] | - | -Redox | [198] |
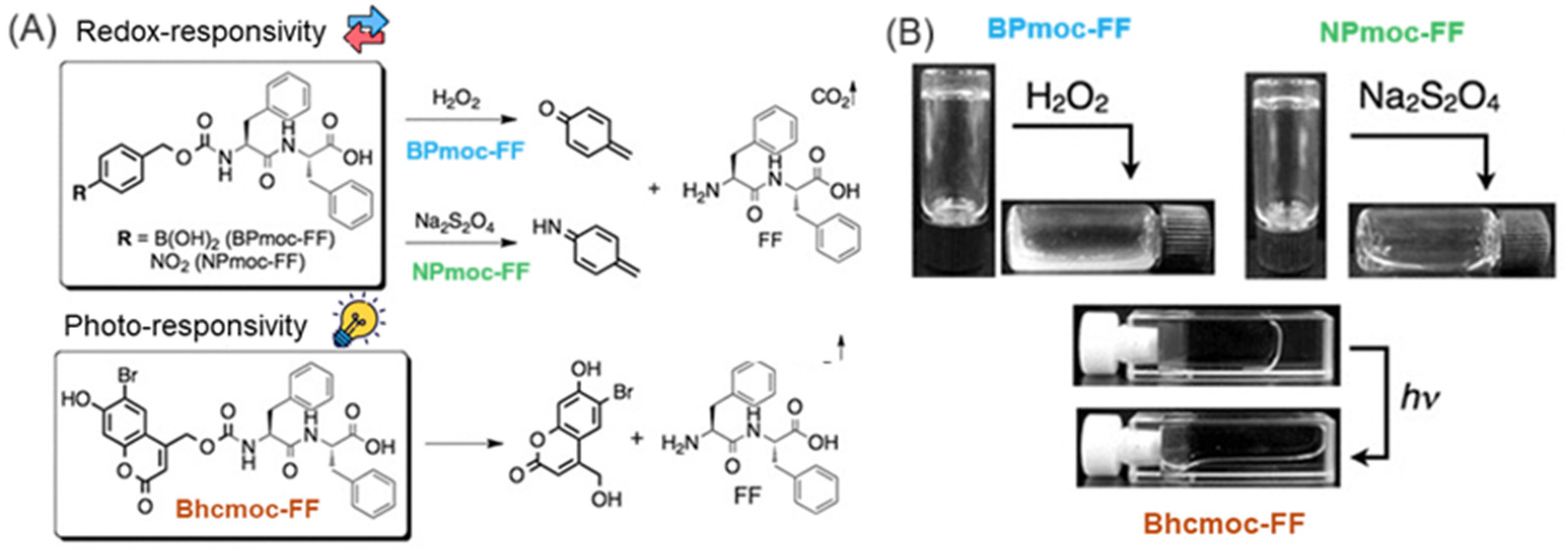
| BPmoc-FF | D | MES buffer | - | 1.0 | - | - | - | - | - | 43 | 10–30 [d] | Bundled tape-like | Stim. Resp | [195] |
| NPmoc-FF | D | MES buffer | - | >0.35 | - | - | - | - | - | - | - | - | Stim. Resp | [195] |
| Bhcmoc-FF | D | MES buffer | - | >0.40 | - | - | - | - | - | - | - | - | Stim. Resp | [195] |
| Nvoc-FF | SE | Water | 3.8 | - | 9 | 40,000 | - | <10 | - | - | - | - | Stim. Resp | [45] |
| Nap-GG | D | Water | ~2 | 3.2 | 15.8 | ~500 | ~40 | - | - | 46 | 30 [w] | - | - | [196] |
| Nap-Ga | D | Water | ~2 | 2.1 | 15.1 | ~5000 | ~450 | - | - | 51 | 30, 60 [p] | Left helical | - | [196] |
| Nap-GA | D | Water | ~2 | 2.1 | 15.1 | ~5000 | ~450 | - | - | 52 | 30, 60 [p] | Right helical | - | [196] |
| Nap-GS | D | Water | ~2 | 2.3 | 14.4 | ~5000 | ~450 | - | - | 50 | 50 [w] | - | - | [196] |
| NapBr-VF | pHE GdL SE | Water Water/DMSO (95/5) | 10–12 | - | 9.5 9.5 | 27,250 13,710 | 2610 1870 | - | - | - | - | - | - | [197] |
| NaAc-VF | pHE GdL SE | Water Water/DMSO (95/5) | 10–12 | - | 11.5 11.5 | 3610 455 | 495 510 | - | - | - | - | - | - | [197] |
| Fen-VF | pHE GdL SE | Water Water/DMSO (95/5) | 10–12 | - | 10 10 | 24,250 2460 | 2530 270 | - | - | - | - | - | - | [197] |
| Pyr-VV | pHE GdL SE | Water Water/DMSO (95/5) | 10–12 | - | 10.1 10.1 | 25,010 14,250 | 4760 2640 | - | - | - | - | - | - | [197] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veloso, S.R.S.; Rosa, M.; Diaferia, C.; Fernandes, C. A Review on the Rheological Properties of Single Amino Acids and Short Dipeptide Gels. Gels 2024, 10, 507. https://doi.org/10.3390/gels10080507
Veloso SRS, Rosa M, Diaferia C, Fernandes C. A Review on the Rheological Properties of Single Amino Acids and Short Dipeptide Gels. Gels. 2024; 10(8):507. https://doi.org/10.3390/gels10080507
Chicago/Turabian StyleVeloso, Sérgio R. S., Mariangela Rosa, Carlo Diaferia, and Célio Fernandes. 2024. "A Review on the Rheological Properties of Single Amino Acids and Short Dipeptide Gels" Gels 10, no. 8: 507. https://doi.org/10.3390/gels10080507
APA StyleVeloso, S. R. S., Rosa, M., Diaferia, C., & Fernandes, C. (2024). A Review on the Rheological Properties of Single Amino Acids and Short Dipeptide Gels. Gels, 10(8), 507. https://doi.org/10.3390/gels10080507









