Innovative Processing and Sterilization Techniques to Unlock the Potential of Silk Sericin for Biomedical Applications
Abstract
1. Introduction
2. Results and Discussion
2.1. Preliminary Optimization
2.1.1. Rotary Evaporation Concentration
2.1.2. Concentration Calibration
2.2. Physicochemical Properties
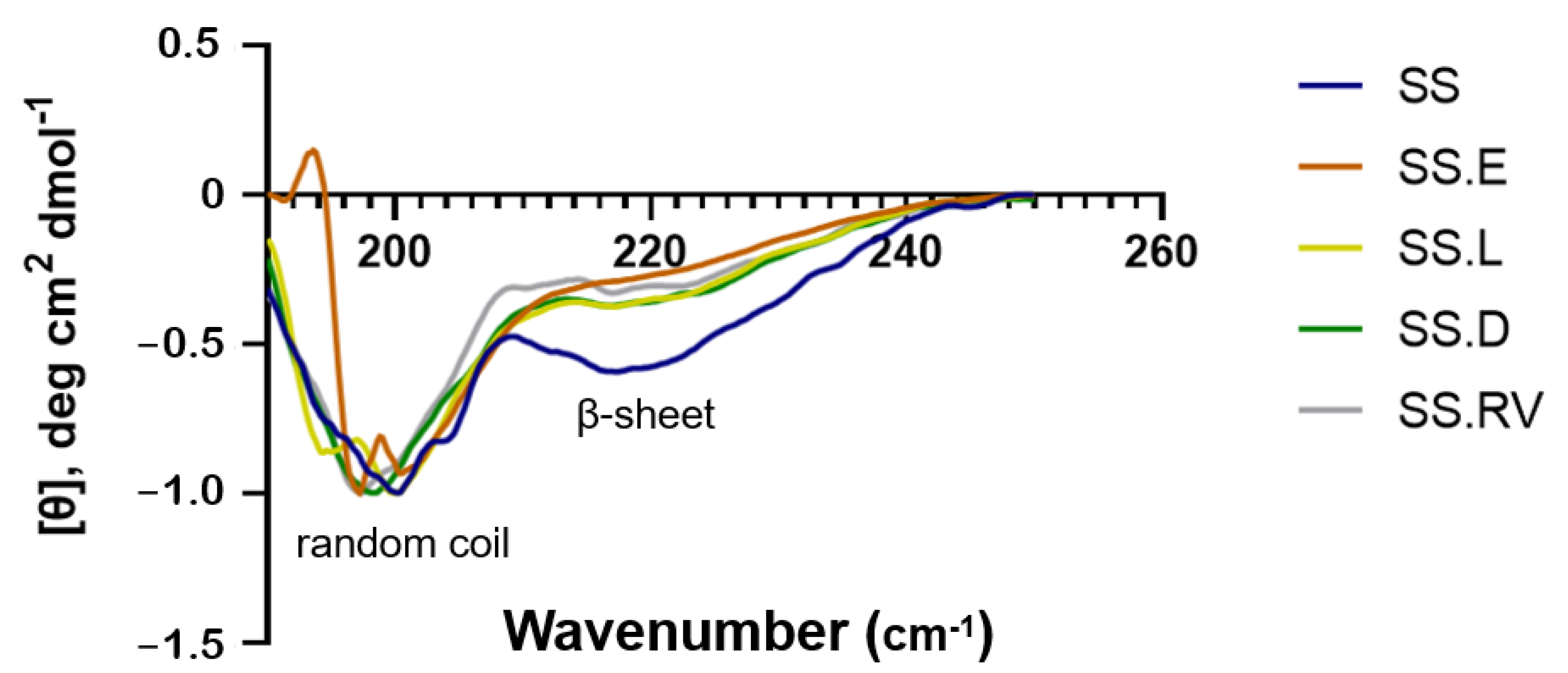
2.2.1. SS Extraction and Secondary Structure (CD, Cryo-SEM)
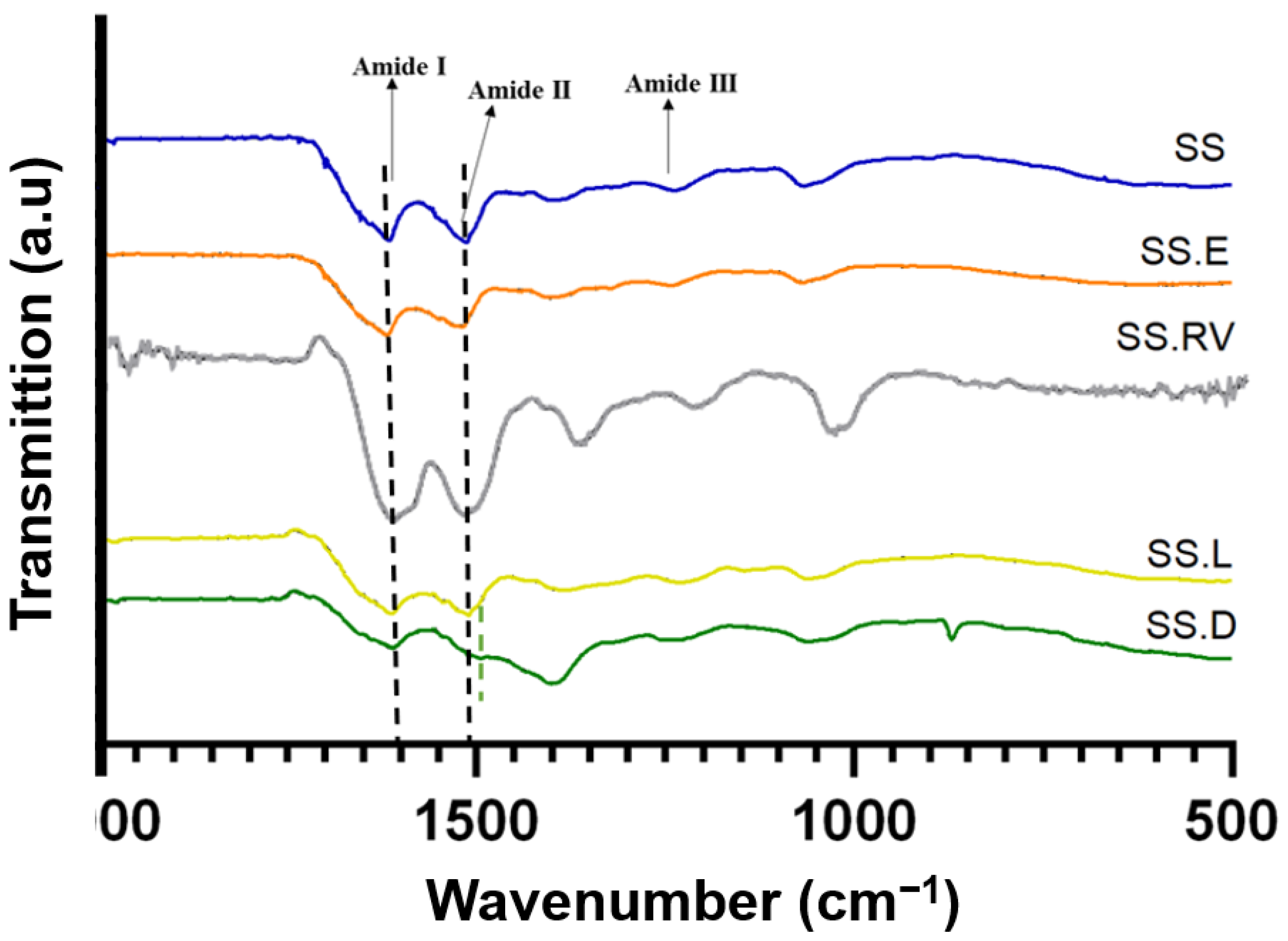
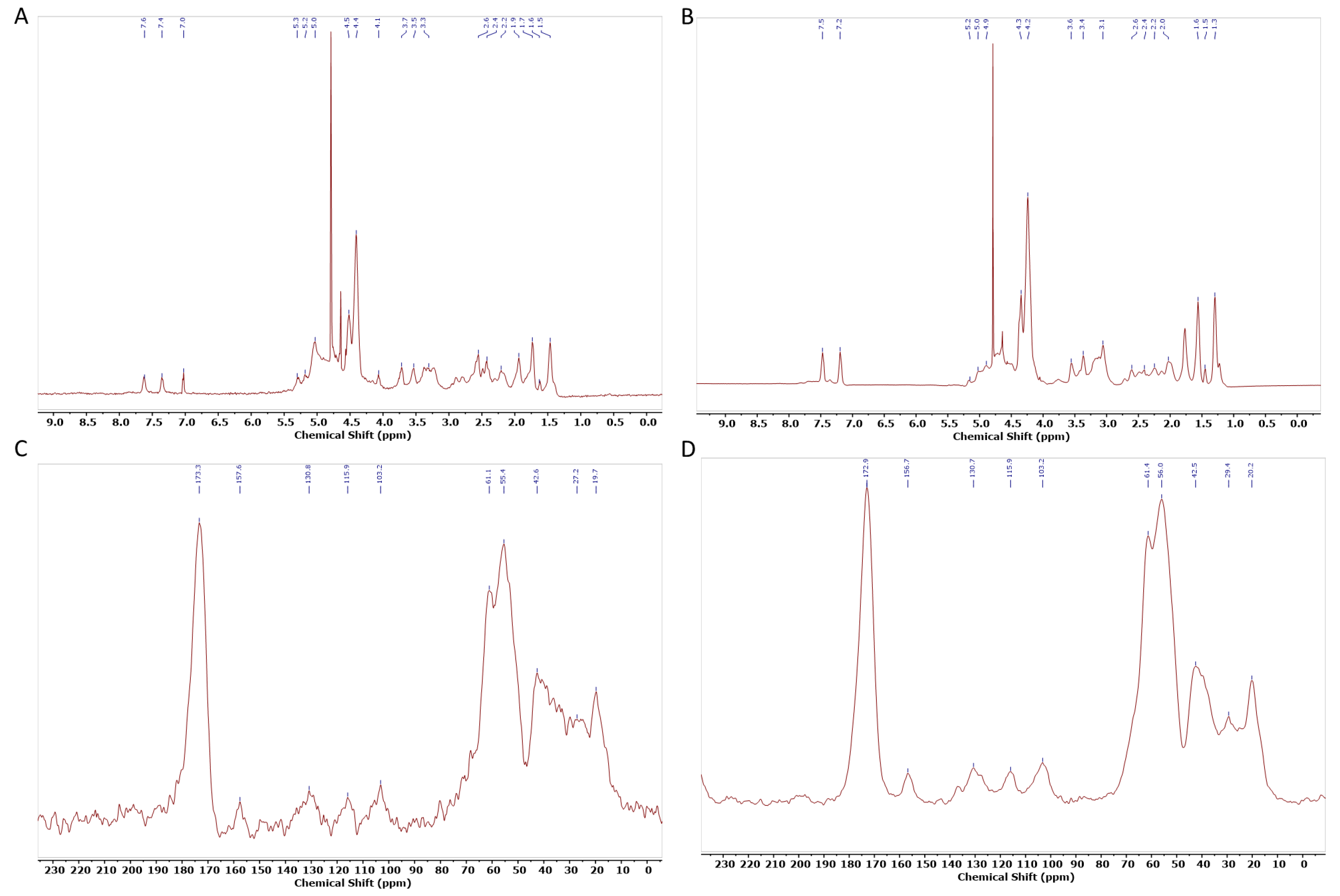
2.2.2. Spectroscopical Characterization (XRD, ATR-FTIR, Raman, NMR)
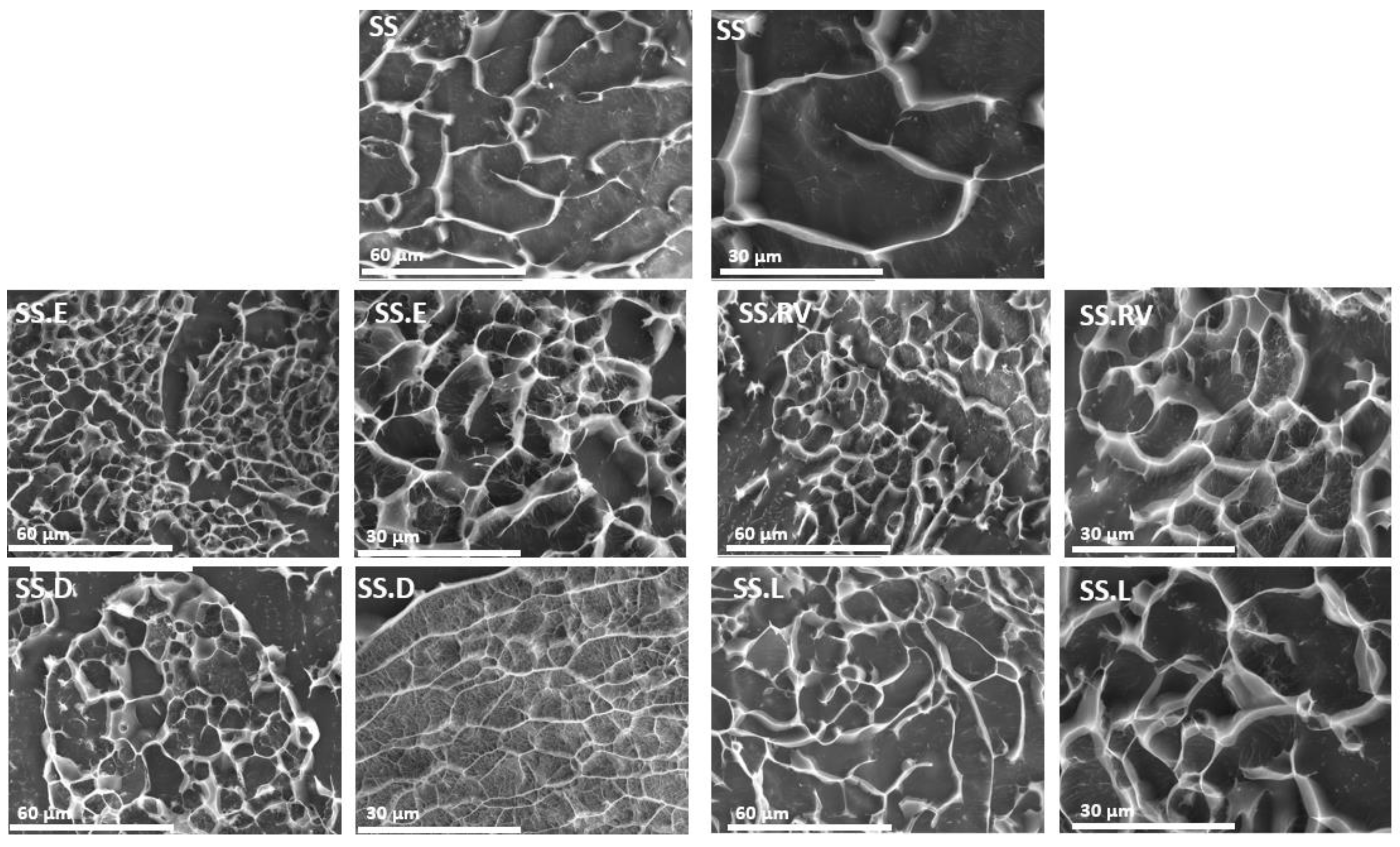
2.2.3. Morphology and Organization
2.2.4. Rheological Behavior
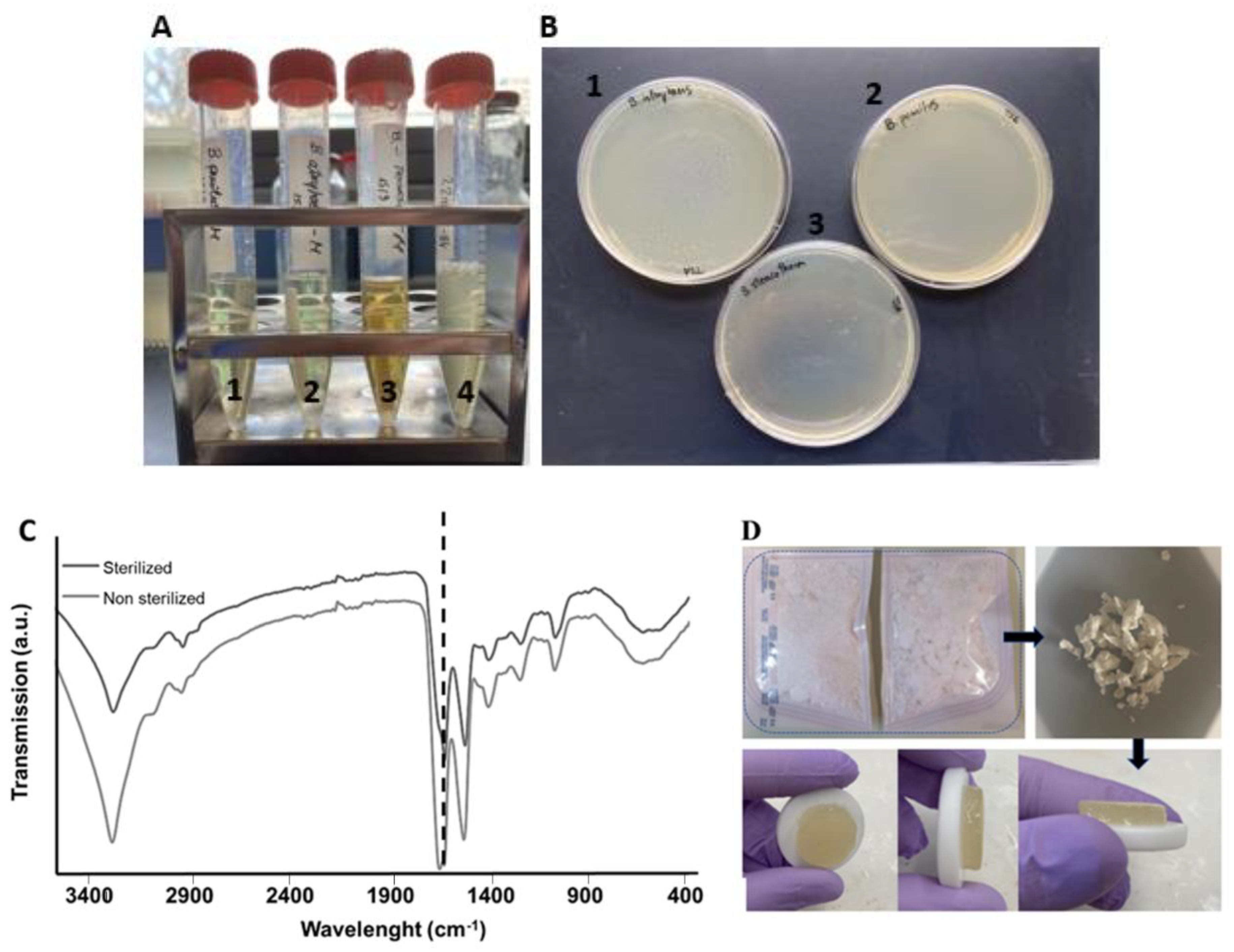
2.3. Validation of scCO2 Sterilization
Turbidity Tests and Protein Conformation
2.4. Lyophilized vs. Commercial Sericin
2.5. Biological Assessment
3. Conclusions
4. Materials and Methods
4.1. Sericin Extraction
4.2. Sericin Concentration Methods
4.2.1. Evaporation
4.2.2. Rotary Evaporation
4.2.3. Cryo-Lyophilization
4.2.4. Dialysis
4.2.5. Determination of Sericin Yield and Concentration
4.2.6. Supercritical Sterilization (scCO2)
4.3. Physicochemical Characterization
4.3.1. Circular Dichroism
4.3.2. X-Ray Diffraction
4.3.3. Fourier Transform Infrared Spectroscopy
4.3.4. Raman Spectroscopy
4.3.5. Nuclear Magnetic Resonance
4.3.6. Cryo-Scanning Electron Microscopy
4.3.7. Viscosity Measurements
4.3.8. Turbidity Tests
4.3.9. Produced Sericin vs. Commercial Sericin
4.3.10. Biological Behavior
4.3.11. Cytotoxicity and Cell Proliferation Assays
4.3.12. Scratch Assay
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Veiga, A.; Castro, F.; Rocha, F.; Oliveira, A.L. Recent Advances in Silk Sericin/Calcium Phosphate Biomaterials. Front. Mater. 2020, 7, 24. [Google Scholar] [CrossRef]
- Veiga, A.; Castro, F.; Rocha, F.; Oliveira, A. Protein-based Hydroxyapatite materials: Tuning composition towards biomedical applications. ACS Appl. Bio Mater. 2020, 3, 3441–3455. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.K.; Singh, M.K. Sericin—A unique biomaterial. IOSR J. Polym. Text. Eng. 2015, 2, 2348–3181. [Google Scholar] [CrossRef]
- Zhang, Y.Q. Applications of natural silk protein sericin in biomaterials. Biotechnol. Adv. 2002, 20, 91–100. [Google Scholar] [CrossRef]
- Aramwit, P.; Kanokpanont, S.; De-Eknamkul, W.; Srichana, T. Monitoring of inflammatory mediators induced by silk sericin. J. Biosci. Bioeng. 2009, 107, 556–561. [Google Scholar] [CrossRef]
- Dinescu, S.; Galateanu, B.; Albu, M.; Cimpean, A.; Dinischiotu, A.; Costache, M. Sericin Enhances the Bioperformance of Collagen-Based Matrices Preseeded with Human-Adipose Derived Stem Cells (hADSCs). Int. J. Mol. Sci. 2013, 14, 1870–1889. [Google Scholar] [CrossRef] [PubMed]
- Upadhayay, H.; Jahan, S.; Upreti, M. Cosmetotextiles: Emerging Trend in Technical Textiles. IOSR J. Polym. Text. Eng. 2016, 3, 8–14. [Google Scholar] [CrossRef]
- Veiga, A.; Castro, F.; Reis, C.C.; Sousa, A.; Oliveira, A.L.; Rocha, F. Hydroxyapatite/sericin composites: A simple synthesis route under near-physiological conditions of temperature and pH and preliminary study of the effect of sericin on the biomineralization process. Mater. Sci. Eng. C 2020, 108, 110400. [Google Scholar] [CrossRef]
- Isobe, T.; Ikebata, Y.; Onitsuka, T.; Wittayarat, M.; Sato, Y.; Taniguchi, M. Effect of sericin on preimplantation development of bovine embryos cultured individually. Theriogenology 2012, 78, 747–752. [Google Scholar] [CrossRef]
- Yanagihara, K.; Terada, S.; Miki, M.; Sasaki, M.; Yamada, H. Effect of the silk protein sericin on the production of adenovirus-based gene-therapy vectors. Biosci. Biotechnol. Biochem. 2006, 64, 59–64. [Google Scholar] [CrossRef]
- Tsubouchi, K.; Igarashi, Y.; Takasu, Y.; Yamada, H. Sericin Enhances Attachment of Cultured Human Skin Fibroblasts. Biosci. Biotechnol. Biochem. 2005, 69, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Toyosawa, T.; Terada, S.; Sasaki, M. Observation of individual cell behaviors to analyze mitogenic effects of sericin. In Animal Cell Technology: Basic & Applied Aspects; Springer: Berlin/Heidelberg, Germany, 2006; pp. 155–161. [Google Scholar] [CrossRef]
- SericinPlus. Sericin+. Available online: https://www.sericinplus.com/ (accessed on 25 November 2021).
- Sollice Biotech. Available online: https://www.sollicebiotech.com/ (accessed on 25 November 2021).
- SeriTech. From Silk Proteins to Medicinal Patches. Available online: https://www.seritech.co/our-company/ (accessed on 28 April 2021).
- Arbor, A.; States, U.; Berkeley, L. Properties and characterization of bone repair materials. In Bone Repair Biomaterials, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; pp. 65–102. [Google Scholar] [CrossRef]
- White, A.; Burns, D.; Christensen, T.W. Effective terminal sterilization using supercritical carbon dioxide. J. Biotechnol. 2006, 123, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Karajanagi, S.S.; Yoganathan, R.; Mammucari, R.; Park, H.; Cox, J.; Zeitels, S.M..; Langer, R.; Foster, N.R. Application of a dense gas technique for sterilizing soft biomaterials. Biotechnol. Bioeng. 2011, 108, 1716–1725. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.-J.; Das, G.; Shin, H.-S.; Patra, J.K. Silk Sericin Protein Materials: Characteristics and Applications in Food-Sector Industries. Int. J. Mol. Sci. 2023, 24, 4951. [Google Scholar] [CrossRef] [PubMed]
- Aramwit, P.; Kanokpanont, S.; Nakpheng, T.; Srichana, T. The Effect of Sericin from Various Extraction Methods on Cell Viability and Collagen Production. Int. J. Mol. Sci. 2010, 11, 2200–2211. [Google Scholar] [CrossRef] [PubMed]
- Aramwit, P.; Siritientong, T.; Srichana, T. Potential applications of silk sericin, a natural protein from textile industry by-products. Waste Manag. Res. 2012, 30, 217–224. [Google Scholar] [CrossRef]
- Oh, H.; Lee, J.Y.; Kim, M.K.; Um, I.C.; Lee, K.H. Refining hot-water extracted silk sericin by ethanol-induced precipitation. Int. J. Biol. Macromol. 2011, 48, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.J.; Um, I.C. Effect of sericin concentration and ethanol content on gelation behavior, rheological properties, and sponge characteristics of silk sericin. Eur. Polym. J. 2017, 93, 761–774. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, M.; Min, S.; Feng, Q.; Gao, X.; Zhu, L. Preparation and characterization of a novel spongy hydrogel from aqueous Bombyx mori sericin. J. Appl. Polym. Sci. 2008, 108, 1–10. [Google Scholar] [CrossRef][Green Version]
- Arango, M.C.; Álvarez-López, C. Effect of freezing temperature on the properties of lyophilized silk sericin scaffold. Mater. Res. Express 2019, 6, 095414. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Li, H.; Feng, Q.; Cui, F.; Xu, Y. Exploring natural silk protein sericin for regenerative medicine: An injectable, photoluminescent, cell-adhesive 3D hydrogel. Sci. Rep. 2015, 4, 7064. [Google Scholar] [CrossRef] [PubMed]
- Baptista-Silva, S.; Borges, S.; Costa-Pinto, A.R.; Costa, R.; Amorim, M.; Dias, J.R.; Ramos, O.; Alves, P.; Granja, P.L.; Soares, R.; et al. In Situ Forming Silk Sericin-Based Hydrogel: A Novel Wound Healing Biomaterial. ACS Biomater. Sci. Eng. 2021, 7, 1573–1586. [Google Scholar] [CrossRef] [PubMed]
- Kodama, K. The Preparation and Physico-chemical Properties of Sericin. Biochem. J. 1926, 20, 1208–1222. [Google Scholar] [CrossRef]
- Kweon, H.; Ha, H.C.; Kim, Y.S.; Chung, K.W.; Park, Y.H. Characteristics of Silk Sericin Extracted from Sericinjam. Int. J. Ind. Entomol. 2009, 18, 121–124. [Google Scholar]
- Zhang, M.; Yin, B.; Wang, Y.; Zhou, W.; Xu, H.; Zhang, W. Bioinspired Design of Sericin/Chitosan/Ag@MOF/GO Hydrogels for Efficiently Combating Resistant Bacteria, Rapid Hemostasis, and Wound Healing. Polymers 2021, 13, 2812. [Google Scholar] [CrossRef]
- Yang, M.; Shuai, Y.; Zhou, G.; Mandal, N.; Zhu, L.; Mao, C. Tuning molecular weights of Bombyx mori (B. mori) silk sericin to modify its assembly structures and materials formation. ACS Appl. Mater. Interfaces 2014, 6, 13782–13789. [Google Scholar] [CrossRef]
- Kim, M.K.; Kwak, H.W.; Lee, J.Y.; Yun, H.S.; Kim, M.H.; Lee, K.H. Effect of Lyoprotectant on the Solubility and Structure of Silk Sericin. Int. J. Ind. Entomol. 2012, 25, 133–137. [Google Scholar] [CrossRef]
- Ma, M.; Ayaz, P.; Jin, W.; Zhou, W. Improving the Color Stability of Naturally Colored Silk by Cross-Linking the Sericin with Phytic Acid. Int. J. Polym. Sci. 2019, 2019, 6936437. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, H.; Liu, F.; Li, Q.; Li, H. A highly transparent, elastic, injectable sericin hydrogel induced by ultrasound. Polym. Test. 2019, 77, 105890. [Google Scholar] [CrossRef]
- Ribeiro, N.; Soares, G.; Pinto, R.; Silva, J. A new era for sterilization based on supercritical CO2 technology. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 399–428. [Google Scholar] [CrossRef]
- Santos-Rosales, V.; Aguilar, M.; Lorenzo, J.; García-González, C. Supercritical CO2 technology for one-pot foaming and sterilization of polymeric scaffolds for bone regeneration. Int. J. Pharm. 2021, 605, 120801. [Google Scholar] [CrossRef]
- Soares, G.C.; Santos-Rosales, V.; Álvarez-López, C.; García-González, C.A. New Insights on Biopolymer Sterilization Using Supercritical CO2 Technology. In Proceedings of the 16th European Meeting on Supercritical Fluids, Lisbon, Portugal, 25–28 April 2017. [Google Scholar]
- David Eckersall, P. Proteins, Proteomics, and the Dysproteinemias. In Clinical Biochemistry of Domestic Animals; Elsevier: Amsterdam, The Netherlands, 2008; pp. 117–155. [Google Scholar] [CrossRef]
- Da Silva, T.L.; Da Silva, A.C.; Ribani, M.; Vieira, M.G.A.; Gimenes, M.L.; Da Silva, M.G.C. Evaluation of molecular weight distribution of sericin in solutions concentrated via precipitation by ethanol and precipitation by freezing/thawing. Chem. Eng. Trans. 2014, 38, 103–108. [Google Scholar] [CrossRef]
- Gupta, D.; Agrawal, A.; Chaudhary, H.; Gulrajani, M.; Gupta, C. Cleaner process for extraction of sericin using infrared. J. Clean. Prod. 2013, 52, 488–494. [Google Scholar] [CrossRef]
- Bascou, R.; Hardouin, J.; Ben Mlouka, M.A.; Guénin, E.; Nesterenko, A. Detailed investigation on new chemical-free methods for silk sericin extraction. Mater. Today Commun. 2022, 33, 104491. [Google Scholar] [CrossRef]
- Gulrajani, M.L.; Purwar, R.; Prasad, R.K.; Joshi, M. Studies on structural and functional properties of sericin recovered from silk degumming liquor by membrane technology. J. Appl. Polym. Sci. 2009, 113, 2796–2804. [Google Scholar] [CrossRef]
- Tsukada, M.; Komoto, T.; Kawai, T. Conformation of liquid silk sericin. Polym. J. 1979, 11, 503–507. [Google Scholar] [CrossRef]
- Cho, K.Y.; Lee, J.Y.; Cho, S.J.; Lee, K.H. Preparation of self-assembled silk sericin nanoparticles. Int. J. Biol. Macromol. 2003, 32, 36–42. [Google Scholar] [CrossRef]
- Dash, R.; Mukherjee, S.; Kundu, S.C. Isolation, purification and characterization of silk protein sericin from cocoon peduncles of tropical tasar silkworm, Antheraea mylitta. Int. J. Biol. Macromol. 2006, 38, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Nieves, D.; Chaikof, E.L. Collagen and Elastin Biomaterials for the Fabrication of Engineered Living Tissues. ACS Biomater. Sci. Eng. 2017, 3, 694–711. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.S.; Freitas, F.; Reis, M.A.M.; Oliveira, A.L. Silk Sericin: A Promising Sustainable Biomaterial for Biomedical and Pharmaceutical Applications. Polymers 2022, 14, 4931. [Google Scholar] [CrossRef]
- Kunz, R.I.; Brancalhão, R.M.C.; Ribeiro, L.F.C.; Natali, M.R.M. Silkworm sericin: Properties and biomedical applications. Biomed. Res. Int. 2016, 2016, 8175701. [Google Scholar] [CrossRef] [PubMed]
- Miles, A.J.; Ramalli, S.G.; Wallace, B.A. DichroWeb, a website for calculating protein secondary structure from circular dichroism spectroscopic data. Protein Sci. 2021, 30, pro.4153. [Google Scholar] [CrossRef]
- Whitmore, L.; Wallace, B.A. DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res. 2004, 32, W668–W673. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.L.; Marques, A.P.; Reis, R.L. Aligned silk-based 3-D architectures for contact guidance in tissue engineering. Acta Biomater. 2012, 8, 1530–1542. [Google Scholar] [CrossRef] [PubMed]
- Diana, C.C.; Lina, M.V.; Gustavo, A.H.; Catalina, A. Characterization of Colombian Silk Sericin Dehydrated by Spray Drying and Freeze Drying. Adv. J. Food Sci. Technol. 2018, 15, 5–14. [Google Scholar] [CrossRef]
- Whitmore, L.; Wallace, B.A. Protein secondary structure analyses from circular dichroism spectroscopy: Methods and reference databases. Biopolymers 2008, 89, 392–400. [Google Scholar] [CrossRef]
- Sah, M.K.; Pramanik, K. Preparation, characterization and in vitro study of biocompatible fibroin hydrogel. Afr. J. Biotechnol. 2011, 10, 7878–7892. [Google Scholar] [CrossRef][Green Version]
- Deen, I.; Rosei, F. Silk fibroin-derived polypeptides additives to promote hydroxyapatite nucleation in dense collagen hydrogels. PLoS ONE 2019, 14, e0219429. [Google Scholar] [CrossRef]
- Kwak, H.W.; Ju, J.E.; Shin, M.; Holland, C.; Lee, K.H. Sericin Promotes Fibroin Silk i Stabilization Across a Phase-Separation. Biomacromolecules 2017, 18, 2343–2349. [Google Scholar] [CrossRef] [PubMed]
- Manesa, K.C.; Kebede, T.G.; Dube, S.; Nindi, M.M. Profiling of silk sericin from cocoons of three southern African wild silk moths with a focus on their antimicrobial and antioxidant properties. Materials 2020, 13, 5706. [Google Scholar] [CrossRef] [PubMed]
- Saha, J.; Mondal, M.I.H.; Sheikh, M.R.K.; Habib, M.A. Extraction, Structural and Functional Properties of Silk Sericin Biopolymer from Bombyx mori Silk Cocoon Waste. J. Text. Sci. Eng. 2019, 9, 390. [Google Scholar] [CrossRef]
- Rocha, L.K.H.; Ferrarezi, M.M.F.; de Carvalho, F.C.; Chorilli, M. Sericin from Bombyx mori cocoons. Part I: Extraction and physicochemical-biological characterization for biopharmaceutical applications. Process Biochem. 2017, 61, 163–177. [Google Scholar] [CrossRef]
- Ji, Y.; Zhu, H.; Sun, S.; Li, Z. DFT-Calculated IR Spectrum Amide I, II, and III Band Contributions of N-Methylacetamide Fine Components. ACS Omega 2020, 5, 8572–8578. [Google Scholar] [CrossRef] [PubMed]
- Maddinedi, S.B.; Salim, M.; Sahoo, S.K.; Kumar, A. Silk sericin induced fabrication of reduced graphene oxide and its in-vitro cytotoxicity, photothermal evaluation. J. Photochem. Photobiol. B 2018, 186, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Radu, I.C.; Parteni, O.; Popa, O.; Oancea, F.; Vuluga, Z.; Ciocan, L.T. In Vitro Interaction of Doxorubicin-Loaded Silk Sericin Nanocarriers with MCF-7 Breast Cancer Cells Leads to DNA Damage. Polymers 2021, 13, 2047. [Google Scholar] [CrossRef] [PubMed]
- Myshakina, N.S.; Ahmed, Z.; Asher, S.A. Dependence of Amide Vibrations on Hydrogen Bonding. J. Phys. Chem. B 2008, 112, 11873–11877. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.P.; Griffiths, P.R. Comparison of the Amide I/II Intensity Ratio of Solution and Solid-State Proteins Sampled by Transmission, Attenuated Total Reflectance, and Diffuse Reflectance Spectrometry. Appl. Spectrosc. 1993, 47, 584–589. [Google Scholar] [CrossRef]
- Fellows, A.P.; Casford, M.T.L.; Davies, P.B. Spectral Analysis and Deconvolution of the Amide I Band of Proteins Presenting with High-Frequency Noise and Baseline Shifts. Appl. Spectrosc. 2020, 74, 597–615. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef]
- Benevides, J.M.; Overman, S.A.; Thomas, G.J. Raman Spectroscopy of Proteins. Curr. Protoc. Protein Sci. 2003, 33, 17.8.1–17.8.29. [Google Scholar] [CrossRef]
- Zuluaga-Vélez, A.; Cómbita-Merchán, D.F.; Buitrago-Sierra, R.; Santa, J.F.; Aguilar-Fernández, E.; Sepúlveda-Arias, J.C. Silk fibroin hydrogels from the Colombian silkworm Bombyx mori L: Evaluation of physicochemical properties. PLoS ONE 2019, 14, e0213303. [Google Scholar] [CrossRef]
- Du, L.; Khiari, Z.; Pietrasik, Z.; Betti, M. Physicochemical and functional properties of gelatins extracted from turkey and chicken heads. Poult. Sci. 2013, 92, 2463–2474. [Google Scholar] [CrossRef]
- Tao, W.; Li, M.; Xie, R. Preparation and structure of porous silk sericin materials. Macromol. Mater. Eng. 2005, 290, 188–194. [Google Scholar] [CrossRef]
- Sparkes, J.; Holland, C. The rheological properties of native sericin. Acta Biomater. 2018, 69, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Valluzzi, R.; Bini, E.; Vernaglia, B.; Kaplan, D.L. Cloning, Expression, and Assembly of Sericin-like Protein. J. Biol. Chem. 2003, 278, 46117–46123. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Sun, H.; Wang, Y.; Sánchez-Soto, M.; Schiraldi, D.A. The relation between the rheological properties of gels and the mechanical properties of their corresponding aerogels. Gels 2018, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Barajas-Gamboa, J.A.; Serpa-Guerra, A.M.; Restrepo-Osorio, A.; Álvarez-López, C. Sericin applications: A globular silk protein. Ing. Competitividad 2016, 18, 193–206. [Google Scholar] [CrossRef]
- Omar, A.; Gao, Y.; Wubulikasimu, A.; Arken, A.; Aisa, H.A.; Yili, A. Effects of trypsin-induced limited hydrolysis on the structural, functional, and bioactive properties of sericin. RSC Adv. 2021, 11, 25431–25440. [Google Scholar] [CrossRef] [PubMed]
- Baptista-Silva, S.; Borges, S.; Costa-Pinto, A.R.; Costa, R.; Amorim, M.; Dias, J.R.; Ramos, O.; Alves, P.; Granja, P.L.; Soares, R. Exploring Silk Sericin for Diabetic Wounds: An In Situ-Forming Hydrogel to Protect against Oxidative Stress and Improve Tissue Healing and Regeneration. Biomolecules 2022, 12, 801. [Google Scholar] [CrossRef]
- Siritientong, T.; Srichana, T.; Aramwit, P. The effect of sterilization methods on the physical properties of silk sericin scaffolds. AAPS PharmSciTech 2011, 12, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Kojthung, A.; Meesilpa, P.; Sudatis, B.; Treeratanapiboon, L.; Udomsangpetch, R.; Oonkhanond, B. Effects of gamma radiation on biodegradation of Bombyx mori silk fibroin. Int. Biodeterior. Biodegrad. 2008, 62, 487–490. [Google Scholar] [CrossRef]
- Rnjak-Kovacina, J.; DesRochers, T.M.; Burke, K.A.; Kaplan, D.L. The Effect of Sterilization on Silk Fibroin Biomaterial Properties. Macromol. Biosci. 2015, 15, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.; Stok, K.S.; Kohler, T.; Meinel, A.J.; Müller, R. Effect of sterilization on structural and material properties of 3-D silk fibroin scaffolds. Acta Biomater. 2014, 10, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Veiga, A.; Silva, I.V.; Duarte, M.M.; Oliveira, A.L. Current Trends on Protein Driven Bioinks for 3D Printing. Pharmaceutics 2021, 13, 1444. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Yao, Y.; Yim, E.K.F. The effects of surface topography modification on hydrogel properties. APL Bioeng 2021, 5, 036107. [Google Scholar] [CrossRef]
- Hu, X.; Park, S.-H.; Gil, E.S.; Xia, X.-X.; Weiss, A.S.; Kaplan, D.L. The influence of elasticity and surface roughness on myogenic and osteogenic differentiation of cells on silk-elastin biomaterials. Biomaterials 2011, 32, 8979–8989. [Google Scholar] [CrossRef] [PubMed]
- Bundi, A.; Wüthrich, K. 1H-NMR parameters of the common amino acid residues measured in aqueous solutions of the linear tetrapeptides H-Gly-Gly-X-L-Ala-OH. Biopolymers 1979, 18, 285–297. [Google Scholar] [CrossRef]
- Teramoto, H.; Kakazu, A.; Asakura, T. Native Structure and Degradation Pattern of Silk Sericin Studied by 13C NMR Spectroscopy. Macromolecules 2006, 39, 6–8. [Google Scholar] [CrossRef]
- Lanzarotti, E.; Defelipe, L.A.; Marti, M.A.; Turjanski, A.G. Aromatic clusters in protein–protein and protein–drug complexes. J. Cheminform. 2020, 12, 30. [Google Scholar] [CrossRef]
- Szilágyi, L.; Jardetzky, O. α-Proton chemical shifts and secondary structure in proteins. J. Magn. Reson. 1989, 83, 441–449. [Google Scholar] [CrossRef]
- Das, G.; Seo, S.-J.; Shin, H.-S.; Patra, J.K. Sericin-based nanoformulations: A comprehensive review on molecular mechanisms of interaction with organisms to biological applications. J. Nanobiotechnol. 2021, 19, 93. [Google Scholar] [CrossRef] [PubMed]
- Ting, T.; Yu, C.; Zhang, Q. The potential of silk sericin protein as a serum substitute or an additive in cell culture and cryopreservation. Amino Acids 2017, 49, 1029–1039. [Google Scholar] [CrossRef]
- Chirila, T.V.; Suzuki, S.; Bray, L.J.; Barnett, N.L.; Harkin, D.G. Evaluation of silk sericin as a biomaterial: In vitro growth of human corneal limbal epithelial cells on Bombyx mori sericin membranes. Prog. Biomater. 2013, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, B.G.; Veiga, A.; Barros, J.; García-González, C.A.; Oliveira, A.L. Sustainable Silk-Based Particulate Systems for the Controlled Release of Pharmaceuticals and Bioactive Agents in Wound Healing and Skin Regeneration. Int. J. Mol. Sci. 2024, 25, 3133. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Xu, J.; Wang, X.; Lin, X.; Yang, P. Electrospun green fluorescent-highly anisotropic conductive Janus-type nanoribbon hydrogel array film for multiple stimulus response sensors. Compos. B Eng. 2025, 288, 111933. [Google Scholar] [CrossRef]
- Veiga, A.; Silva, I.V.; Dias, J.R.; Alves, N.M.; Oliveira, A.L.; Ribeiro, V.P. Streamlining Skin Regeneration: A Ready-To-Use Silk Bilayer Wound Dressing. Gels 2024, 10, 439. [Google Scholar] [CrossRef]
- Xu, J.; Chang, L.; Chen, T.; Ren, T.; Zhang, Y.; Cai, Z. Study of the bending properties of variable stiffness chain mail fabrics. Compos. Struct. 2023, 322, 117369. [Google Scholar] [CrossRef]
- Veiga, A.; Foster, O.; Kaplan, D.L.; Oliveira, A.L. Expanding the boundaries of silk sericin biomaterials in biomedical applications. J. Mater. Chem. B 2024, 12, 7020–7040. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, X.; Lu, S.; Chen, G.; Xing, T. Prospects of Silk Sericin Membranes Fabricated with Tyrosinase. J. Fiber Bioeng. Inform. 2015, 8, 57–67. [Google Scholar] [CrossRef]
- Tzvetkova, M.; Hristova-Avakumova, N.G.; Atanasova, L.A.; Atanassova, S.L.; Panayotov, M.; Hadjimitova, V. Comparative evaluation of the radical scavenging activity of cocoon extracts from different silkworm breeds. Bulg. Chem. Commun. 2019, 51, 108–112. [Google Scholar]
- Gupta, D.; Agrawal, A.; Rangi, A. Extraction and characterization of silk sericin. Indian J. Fibre Text. Res. 2014, 39, 364–372. [Google Scholar]
- Greenfield, N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2007. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veiga, A.; Ramírez-Jiménez, R.A.; Santos-Rosales, V.; García-González, C.A.; Aguilar, M.R.; Rojo, L.; Oliveira, A.L. Innovative Processing and Sterilization Techniques to Unlock the Potential of Silk Sericin for Biomedical Applications. Gels 2025, 11, 114. https://doi.org/10.3390/gels11020114
Veiga A, Ramírez-Jiménez RA, Santos-Rosales V, García-González CA, Aguilar MR, Rojo L, Oliveira AL. Innovative Processing and Sterilization Techniques to Unlock the Potential of Silk Sericin for Biomedical Applications. Gels. 2025; 11(2):114. https://doi.org/10.3390/gels11020114
Chicago/Turabian StyleVeiga, Anabela, Rosa Ana Ramírez-Jiménez, Víctor Santos-Rosales, Carlos A. García-González, Maria Rosa Aguilar, Luis Rojo, and Ana L. Oliveira. 2025. "Innovative Processing and Sterilization Techniques to Unlock the Potential of Silk Sericin for Biomedical Applications" Gels 11, no. 2: 114. https://doi.org/10.3390/gels11020114
APA StyleVeiga, A., Ramírez-Jiménez, R. A., Santos-Rosales, V., García-González, C. A., Aguilar, M. R., Rojo, L., & Oliveira, A. L. (2025). Innovative Processing and Sterilization Techniques to Unlock the Potential of Silk Sericin for Biomedical Applications. Gels, 11(2), 114. https://doi.org/10.3390/gels11020114










