1. Introduction
The skin covers the entire external surface of the human body and can be considered its largest organ, and it plays a key role in both homeostasis and the prevention of invasion by microorganisms [
1]. However, certain traumatic events (for example, violent accidents/incidents, surgical operations, etc.) can result in severe skin injuries and (occasionally) full-thickness skin defects [
2]. If not treated properly, major skin defects may develop into chronic wounds that are difficult to heal. This can lead to a range of serious health complications, including inflammation, secondary damage, and bacterial infection, and (in extreme cases) disability or death [
3]. Additionally, uncontrollable bleeding caused by trauma is another major cause of death worldwide, and rapid hemostatic intervention improves the survival of injured patients [
4,
5]. To date, the most common method for treating skin wounds is surgical suturing [
6]. However, suturing procedures are usually performed by trained professionals, and these specialized medical interventions are often delayed in critical environments like battlefields. Consequently, there is an imperative to explore new wound treatment methods, and wound dressings with novel properties have subsequently been developed. In recent years, multifunctional dressings with antibacterial, hemostatic, and wound-healing properties have become a significant research focus of the medical community.
Recent research has revealed that wounds are more likely to heal in humid environments [
7]. Hence, wet wound dressings are gradually attracting more and more attention. Ideally, wound dressings should meet the following criteria: excellent biocompatibility without inducing inflammation or toxicity; superior hemostatic properties; effective inhibition of bacterial growth; and the ability to maintain a moist environment to support wound healing [
8,
9]. Hydrogels have been shown to exhibit outstanding swelling properties and biocompatibility, and they create a moist environment that facilitates wound healing. Hence, hydrogels are an ideal choice for wound dressings.
Marine polysaccharides are commonly utilized in hydrogels due to their excellent biocompatibility and biosafety [
10,
11,
12]. For example, carboxymethyl chitosan (CMCS) was demonstrated to exhibit enhanced solubility, biocompatibility, biodegradability, hemostatic and antibacterial effects [
13,
14]. Sodium alginate (SA) also exhibited excellent biocompatibility, and it was subsequently shown to facilitate wound healing [
12,
15]. Fucoidan (Fuc) was observed to exhibit procoagulant activity at low concentrations, although it did also exhibit anticoagulant activity at high concentrations [
16]. Moreover, Fuc can effectively regulate inflammation and accelerate tissue regeneration [
17]. Because of their favorable properties, hydrogels based on polysaccharides have garnered significant attention in tissue engineering and medical applications. Xie et al. [
14] reported the development of carboxymethyl chitosan/oxidized dextran/sodium alginate hydrogels that effectively prevented Staphylococcus aureus-induced wound infections. Intini et al. [
18] designed a 3D-printed hydrogel dressing based on chitosan that exhibited excellent cytocompatibility and biocompatibility, and this dressing was observed to promote wound healing in clinical evaluations. Liang et al. [
19] developed a multifunctional hyaluronic acid-based hydrogel dressing that exhibited antimicrobial and free radical scavenging properties. This innovative dressing enhanced the expression of CD31 growth factor, thereby promoting neovascularization in the wound area.
However, despite extensive research on hydrogels, several limitations remain to be addressed. The gelation time is currently too long, ranging from minutes to tens of minutes, which significantly compromises clinical operational efficiency [
20]. The slow gelation process also limits the in situ shaping capability at wound sites, thereby hindering the ability to conform closely to complex or irregular wound geometries [
21]. Additionally, the complex preparation process for hydrogels of interest often involves costly and toxic reagents, limiting the feasibility of industrial manufacturing [
22,
23]. More importantly, many of these products exhibit limited efficacy in promoting wound healing and achieving hemostasis [
24]. Therefore, there is an urgent need to develop new, high-performance, efficacious hydrogels.
In the present study, we aimed to address these challenges through the design of novel hydrogels. First, 16 polysaccharide mixtures (comprising CMCS, SA, and Fuc) with different ratios were designed using an orthogonal array. Their procoagulant activity was evaluated, and the mixture with the shortest coagulation time (named CAF) was selected. Hydrogels were then prepared using CAF as the primary material, with oxidized starch (OS)/tannic acid (TA) blends (OT) serving as the cross-linker. OS acts as a cross-linking agent by forming imine bonds with the amino groups of CAF via a Schiff base reaction, while TA further reinforces the network through multi-site hydrogen bonding and hydrophobic interactions with the polysaccharide chains. Through comprehensive evaluations, such as gelation time, swelling capacity, and antibacterial efficacy, an optimal hydrogel named COT was developed and selected for further analysis. These additional studies systematically assessed its biocompatibility, biosafety, hemostatic effects, and wound healing performance, and the results were compared with commercially available hydrogel products (Hydrosorb Gel, Paul Hartmann Co., Ltd., Heidenheim, Germany). Through these systematic studies, we demonstrated that COT was a high-performance, safe, and effective hydrogel wound dressing.
3. Conclusions
As the most extensive and exposed organ, the skin acts as a physical barrier, maintaining homeostasis and preventing pathogen invasion [
1]. Skin wounds can lead to numerous health complications, including inflammation and bacterial infection. If left untreated, these wounds can develop into chronic wounds that are difficult to heal. Additionally, the uncontrollable bleeding that accompanies a skin trauma is a major cause of death worldwide, and rapid hemostatic intervention can improve survival of the injured patients [
4,
5]. Therefore, the development of bioactive dressings with hemostatic properties, antibacterial capabilities, and wound healing activities has emerged as a significant research focus within the medical community.
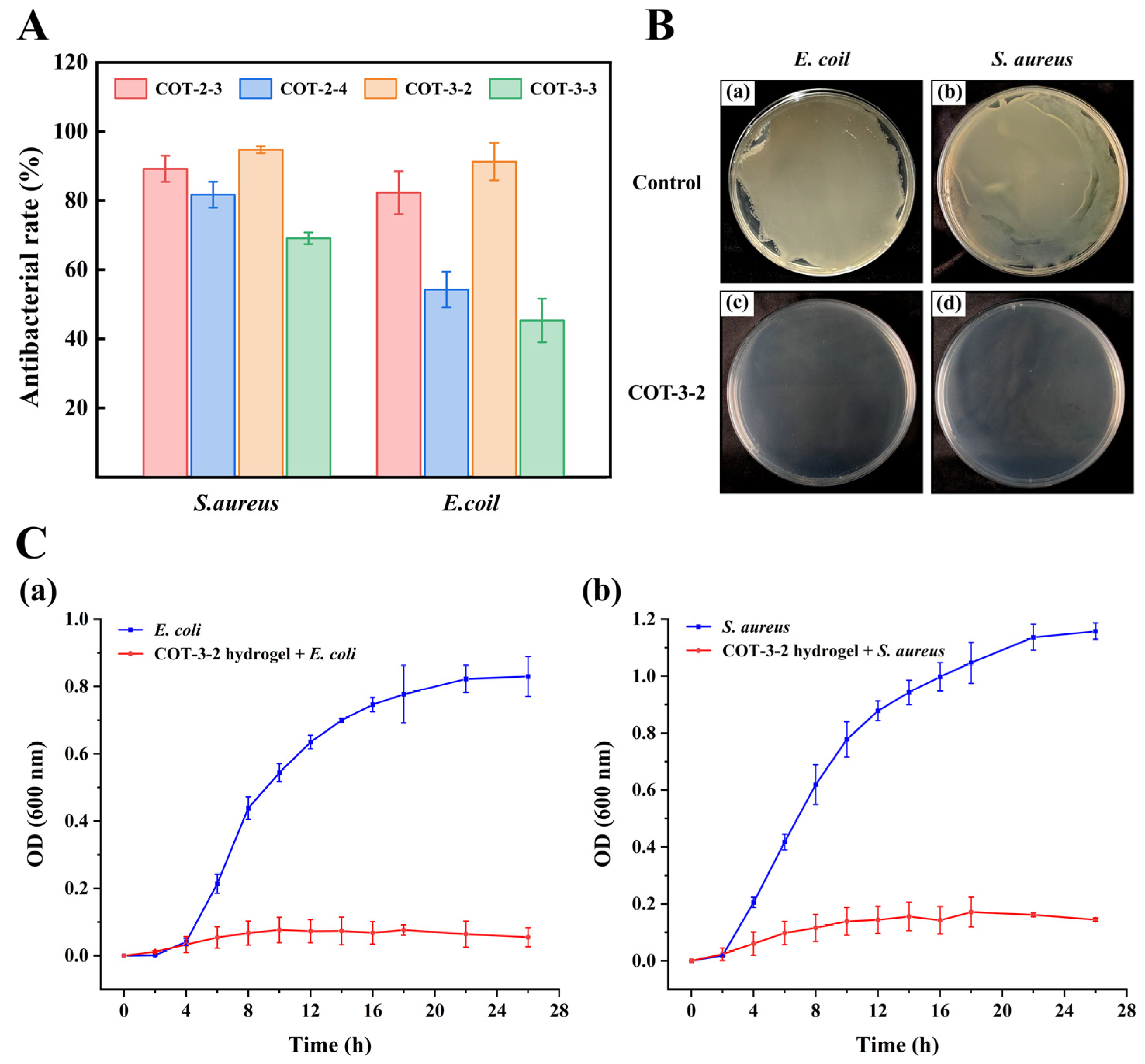
In this paper, a high-performance hydrogel wound dressing (COT hydrogel) was prepared and evaluated. COT hydrogel exhibited a rapid gelation process (within 13 s) and good self-healing and swelling properties. Moreover, the inhibition rates of COT hydrogel against E. coli and S. aureus exceeded 90%, effectively protecting wounds from pathogenic bacterial infections. The hemolysis rate of the COT hydrogel was also less than 5%, thereby meeting international biomaterial standards and demonstrating hemocompatibility. Cytocompatibility tests indicated that COT hydrogel exhibits low cytotoxicity on mice fibroblast cells (L929). Additionally, in vivo evaluations confirmed that COT hydrogel did not cause skin irritation and allergic reactions. Thus, COT hydrogel exhibits good biocompatibility and biosafety. Importantly, the hemostatic effect and wound healing performance of COT hydrogel were significantly better than those of a commercially available hydrogel (p < 0.001, p < 0.01, respectively). In summary, COT hydrogel is a safe, effective, and high-performance wound dressing with promising application prospects in the field of wound healing.
4. Materials and Methods
4.1. Materials
CMCS (arboxylation ≥ 80%; the average weight molecular mass, ~18 kDa), Tannic acid (TA) and 1-chloro-2, 4-dinitrobenzene (CNDNB) were purchased from Aladdin Reagent Co., Ltd. (Shanghai, China). SA (98%; the ratio of mannuronic and guluronic units (M/G), ~1.56; ~200 kDa) and soluble starch (solubility, 500 mg/mL in H2O; ~15 kDa) were obtained from Yuanye Bio-Technology Co., Ltd. (Shanghai, China). Fuc (fucose, 71.89 (mol%); Sulfate content, 28.24%; ~90 kDa) was from Rensheng Pharmaceutical Group Co. Ltd. (Weihai, China). Yunnan Baiyao was obtained from Yunnan Baiyao Group Co., Ltd. (Kunming, China). Chitosan hemostatic powder was from Qingdao biotemed Biomaterials Co., Ltd. (Qingdao, China). Sodium periodate was from Macklin Biochemical Co., Ltd. (Shanghai, China). Hydrosorb Gel (HG) was from Paul Hartmann Co., Ltd. (Heidenheim, Germany).
Rabbit blood and rabbit red blood cells (4%) were obtained from SenBeiJia Biological Technology Co., Ltd. (Nanjing, China). Dulbecco’s Modified Eagle’s Medium (DMEM) was obtained from Procell Life Science & Technology Co., Ltd. (Wuhan, China). The Live/Dead cell dichromatic stain was from Elabscience Biotechnology Co., Ltd. (Wuhan, China). The Cell counting kit-8 (CCK-8) was purchased from Ding Guo Chang Sheng Biotechnology Co., Ltd. (Beijing, China). Mice fibroblast cells (L929) were obtained from the Institute of Biochemistry and Cell Biology (Shanghai, China).
Kunming (KM) mice (4–5 weeks) were acquired from Pengyue Experimental Animal Breeding Co., Ltd. (Jinan, China).
4.2. Preperation of COT Hydrogels
4.2.1. Preparation of Polysaccharide Mixture CAF
CMCS, SA and Fuc were used as raw materials, and each was set at four different concentration levels (
Table 6). Polysaccharide mixtures with different ratios were designed using an orthogonal array L
16(4
5) (
Table 7). Specifically, CMCS, SA and Fuc were first dissolved at the corresponding concentrations, mixed in equal volumes, and then thoroughly stirred to ensure homogeneity. Thereafter, the resultant mixtures were freeze-dried and 16 polysaccharide mixtures were obtained.
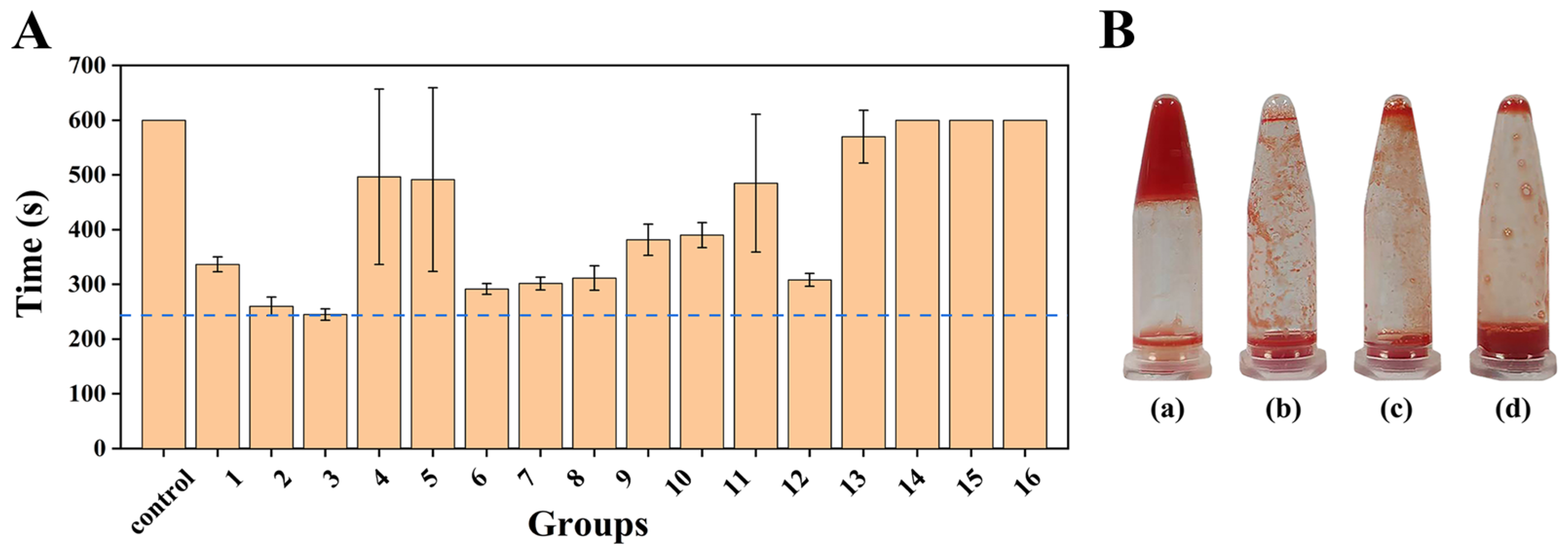
The procoagulant activity of polysaccharide mixtures was evaluated by measuring coagulation time. 100 μL of sodium citrate-anticoagulated rabbit blood was mixed with 100 μL polysaccharide mixture (10 mg/mL) and 100 μL CaCl2 solution (37 °C, 0.2 mol/L). The tube was tilted every 10 s to observe the fluidity, and timing was stopped upon complete coagulation. In the control group, the polysaccharide mixture was replaced with deionized water. The polysaccharide mixture with the shortest coagulation time was selected and compared with the commercially available hemostatic powder (Yunnan Baiyao and chitosan hemostatic powder).
4.2.2. Preparation of Oxidized Starch and OT Cross-Linking Agent
OS was prepared following the method by Zhang et al. [
47] with minor modifications [
38]. Briefly, soluble starch (10 g) was dissolved in deionized water, and 8.5 g NaIO
4 was then added. The resulting reaction was allowed to proceed at room temperature (avoiding direct light) for 4 h, and subsequently terminated by the addition of 1 mL ethylene glycol for another 30 min. The reaction solution was then dialyzed (molecular weight cutoff, 7000 Da) and lyophilized to obtain OS.
Next, the aldehyde group content of OS was determined using the rapid quantitative alkali consumption method [
48]. Dried OS (0.15 g) was suspended in 10 mL of 0.25 M NaOH solution in an Erlenmeyer flask. The flask was then swirled in a water bath at 70 °C for 2 min and rapidly cooled under running tap water for 1 min (with continuous swirling). Subsequently, 15 mL of 0.125 M H
2SO
4, 30 mL of deionized water, and 1 mL of 0.2% phenolphthalein indicator were added sequentially. Finally, the resulting acidic solution was titrated using 0.25 M NaOH solution. The percentage of dialdehyde units was calculated as follows:
where C
1 (M) and C
2 (M) represent the normality of NaOH and H
2SO
4, respectively. V
1 (mL) and V
2 (mL) represent the total volume of NaOH and H
2SO
4, respectively. W (g) is the dry weight of the OS sample, and 161 is the average molecular weight of the repeat unit in OS. The experiments were performed in triplicate.
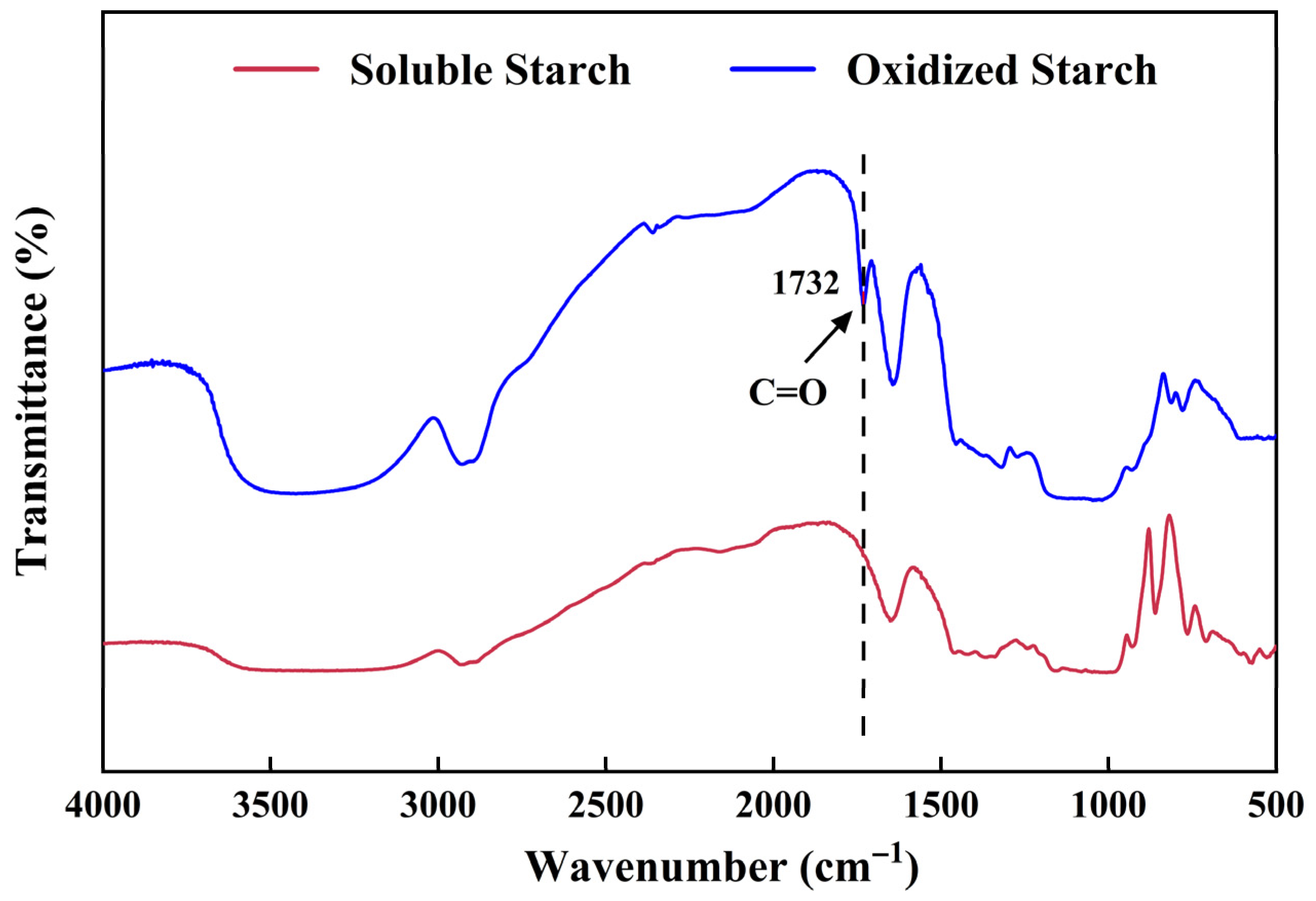
The FTIR spectra of soluble starch and OS were measured with a Nicolet Nexus 470 infrared spectrometer (Madison, WI, USA) in the scanning range of 400~4000 cm
−1 [
38].
A cross-linking agent OT solution, consisting of 2% OS and 5% TA (w/v), was then prepared.
4.2.3. Preparation of the Hydrogels
To prepare COT hydrogels for our preliminary investigations, 2% and 3% CAF solutions were thoroughly mixed with OT solution at ratios of 1:2, 1:1, 2:1, 3:1, 4:1, and 8:1 (v/v). The resulting mixtures were designated as COT-2-n and COT-3-n, respectively.
4.3. Characterization of COT Hydrogels
4.3.1. Determination of Gelation Time
The inverted test tube method was used to record the gelation time [
8]. Gelation times exceeding 3 min were not recorded.
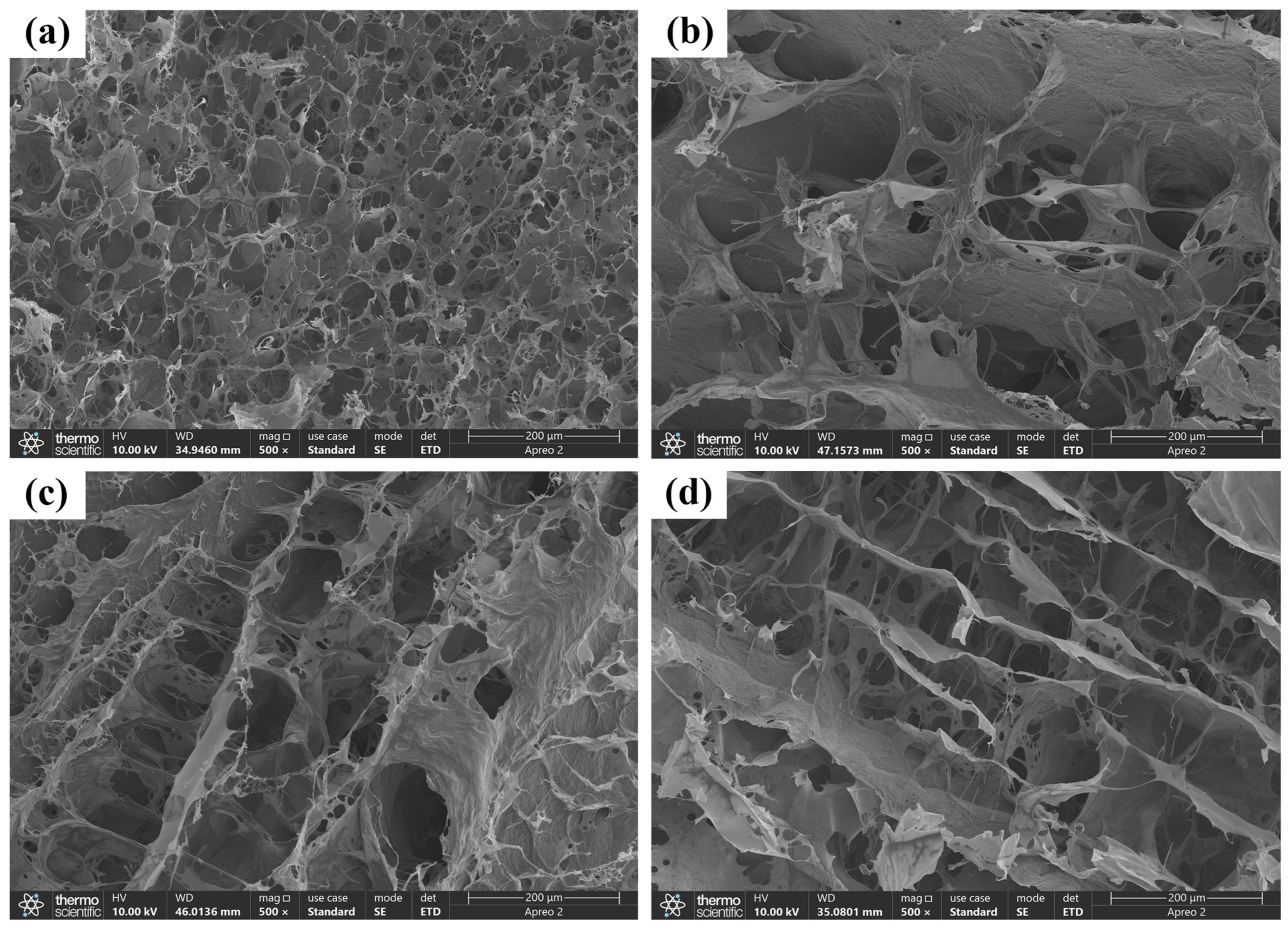
4.3.2. SEM Analysis
The microscopic network structures of the hydrogels were examined using SEM. First, the freeze-dried hydrogel samples were cut into thin sections. These sections were subsequently affixed to the sample holder using conductive adhesive and then sputter-coated with gold. All SEM images were acquired at an operating voltage of 5 kV.
4.3.3. FTIR Spectroscopy Analysis
The FTIR spectra of hydrogels were measured as mentioned above.
4.3.4. Rheological Properties Assay
Rheological properties of the hydrogels were characterized using a rheometer by monitoring the loss modulus (G″) and storage modulus (G′). All measurements were carried out at 37 °C using 20 mm parallel plates with a gap size of 1 mm. A volume of 0.3 mL of the hydrogel precursor solution was carefully dispensed between the parallel plates. Subsequently, frequency sweep analysis was conducted over a shear strain range of 1–500% and a shear frequency range of 0.1–100 rad/s.
4.3.5. Self-Healing Properties of COT Hydrogels
The self-healing properties of COT hydrogels were evaluated by staining two hydrogel sections and then observing their reconnection [
6]. The first heart-shaped hydrogel sample was stained with trypan blue, and the second heart-shaped hydrogel sample was stained with rhodamine B. Each hydrogel sample was then evenly divided into two halves. Finally, the differently stained halves were brought into contact and the self-healing characteristics of COT hydrogels were assessed through observations of the gel state.
4.3.6. Swelling Analysis
Freeze-dried COT hydrogels were weighed and subsequently immersed in PBS (pH, 7.2) at room temperature [
14]. At hourly intervals, the hydrogels were removed from the PBS solution, and excess surface moisture was carefully blotted using filter paper. The weight of the hydrogels was then recorded until a constant weight was achieved. The swelling rate was calculated as follows:
W0 and Wt represent the weights of initial and swelled hydrogels, respectively.
4.4. In Vitro Antibacterial Evaluation
The antibacterial activities of COT hydrogels were evaluated using
Escherichia coli (
E. coli) and
Staphylococcus aureus (
S. aureus) as representative species [
14,
49]. These bacterial strains were first cultured in Luria–Bertani (LB) liquid medium at 37 °C for 12 h. Next, 100 μL of COT hydrogel was dispensed into each well of a 48-well plate and allowed to fully solidify. The solidified hydrogels were then irradiated under ultraviolet (UV) light for 1 h to ensure complete sterilization. Next, 100 μL of bacterial suspension was added to each well. These samples were then incubated for an additional 12 h. As a control, we also incubated 100 μL of bacterial suspension without hydrogel. After 12 h, 900 μL of LB liquid medium was added to each well, and the cultures were further incubated for another 12 h. Finally, the optical density (OD) at 600 nm was measured for each well using a microplate reader (MK3, Thermo Scientific, Waltham, MA, USA). All antibacterial rates were calculated as follows:
where A
c and A
s represent the absorbances of the control group and the experimental group, respectively.
An additional batch was processed using COT-3-2 hydrogel following the same procedure. After the addition of 900 μL of LB medium, the surviving bacteria were re-suspended. 100 μL of the bacterial suspension was then plated onto an LB agar plate and incubated for 12 h, after which colony growth was observed.
The antibacterial activity of the hydrogel was further reflected by the bacterial growth curve [
50]. The hydrogel COT-3-2 was irradiated under UV light for 1 h to ensure complete sterilization and subsequently incubated in 10 mL LB medium for 12 h to prepare the leaching solution.
E. coli and
S. aureus were then inoculated into the leaching solution. Finally, the cultured broths were incubated at 37 °C and shaken at 200 rpm in a rotary shaker (MB-T610, Zhejiang Meibi Instrument Co., Ltd., Jiaxing, China). The turbidity of the culture was monitored by measuring the OD
600 at 2 h, 4 h, 6 h, 8 h, 10 h, 12 h, 14 h, 16 h, 18 h, 22 h, and 26 h.
4.5. Biocompatibility Performance Analyses
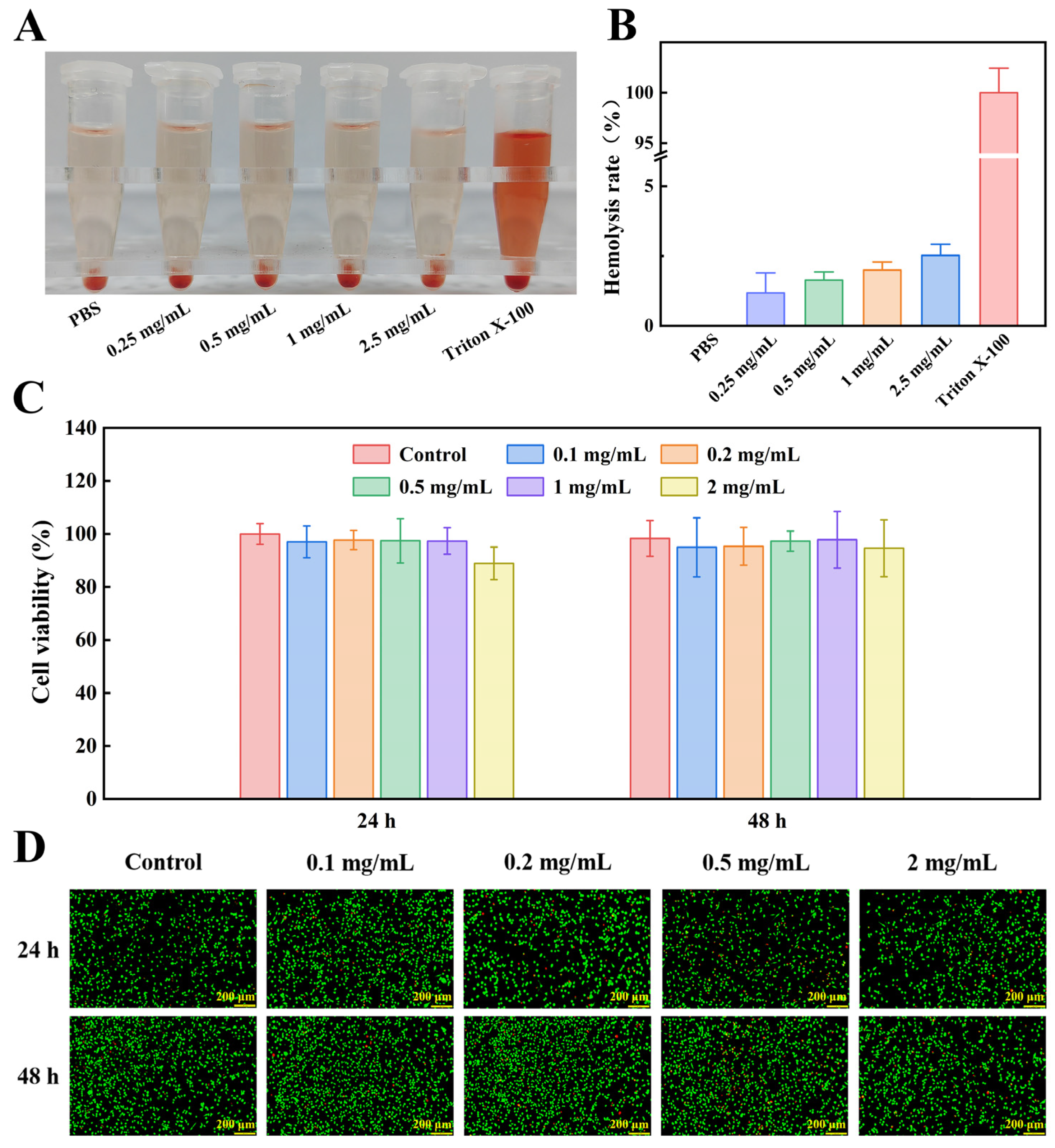
4.5.1. In Vitro Hemolysis Analysis
The blood compatibility of the optimal COT hydrogel was evaluated using a hemolysis test [
8,
43]. For the experiments, 1.2 mL of PBS, 300 μL of a 4% rabbit red blood cell suspension, and different masses of freeze-dried COT hydrogel were added into 2 mL test tubes (COT hydrogel was tested at final concentrations of 0.25, 0.5, 1, and 2.5 mg/mL). All samples were incubated at 37 °C for 2 h, and then centrifuged at 10,000 rpm for 15 min. Finally, OD
540nm readings were measured using a microplate reader (MK3, Thermo Scientific, Waltham, MA, USA). PBS and 0.1% Triton X-100 (which exhibits a pronounced hemolytic effect; Habibi et al. [
51] served as negative and positive controls, respectively. All hemolysis rates were calculated as follows:
where A
s, A
b, and A
t represented the absorbance of the COT hydrogel, the negative control, and the positive control, respectively.
4.5.2. In Vitro Cell Compatibility
First, the hydrogel was irradiated under UV light for 1 h to ensure complete sterilization. The sterilized COT hydrogel was then incubated in DMEM for 24 h to prepare the leaching solutions. Next, L929 cells were seeded in a 96-well plate at a density of 5000 cells per well. After an initial incubation period of 24 h, the medium in each well was replaced with hydrogel leaching solution. After co-incubation periods of 24 h and 48 h, the leaching solution was refreshed in each well. To assess cell viability, CCK-8 solution (10 wt%) was added to each well, and the plates were incubated for a further 1.5 h. The OD450nm was then measured using a microplate reader (MK3, Thermo Scientific). Cell viability was also assessed using a calcein-AM/PI double staining kit. The initial experimental procedure was as described above (for CCK-8). Next, L929 cells were incubated with calcein-AM (0.1 wt%) and PI (0.1 wt%) for an additional 30 min. Finally, the stained cells were observed and photographed using a fluorescence microscope (Nikon TS 100, Nikon, Tokyo, Japan).
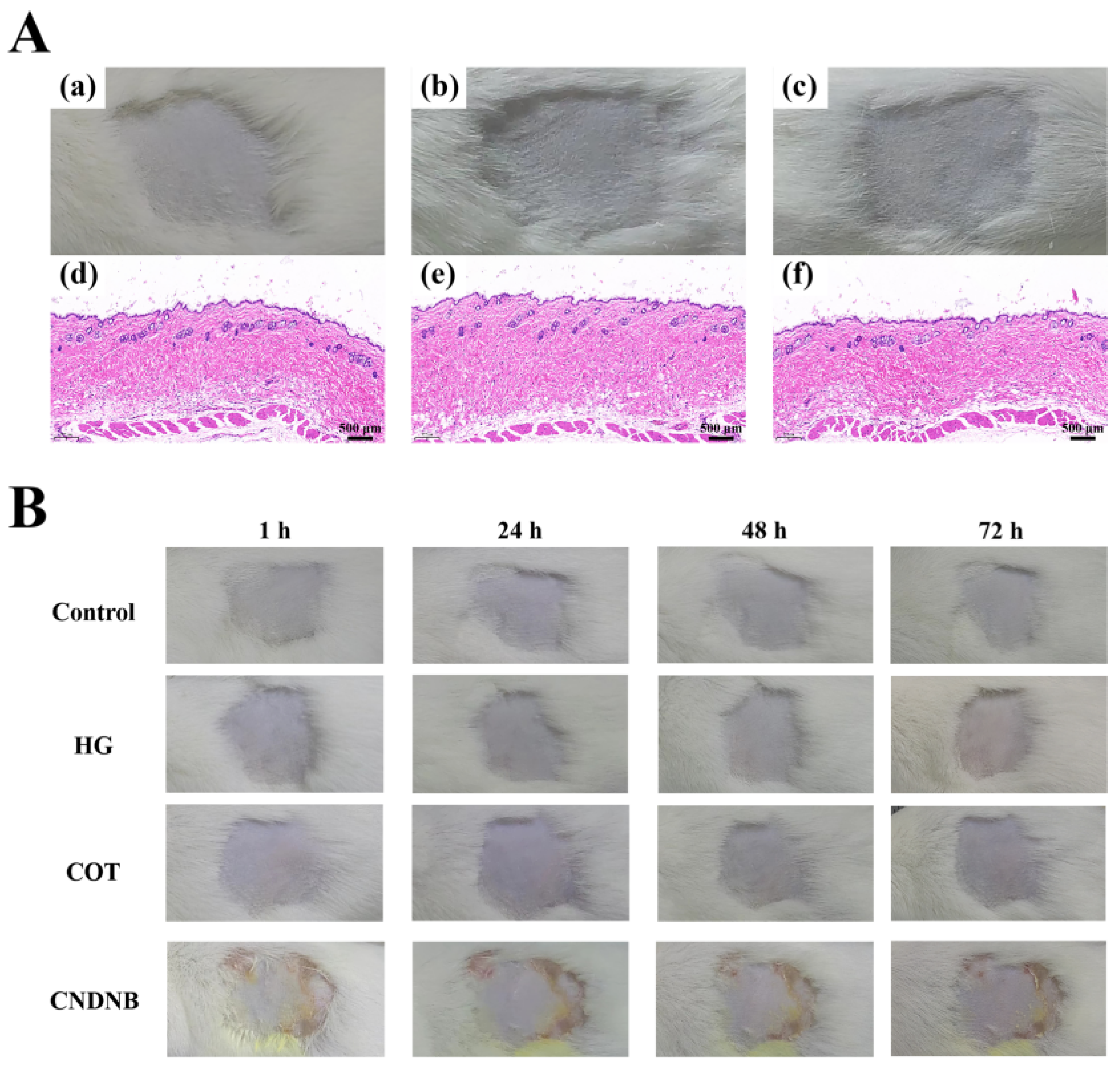
4.6. Skin Irritation Analysis
The skin irritation potential of COT hydrogel was evaluated following the method of Sallam et al. [
52] with minor modifications. A commercially available hydrogel (HG) was used for comparison. Male Kunming mice were housed in a controlled environment (23 ± 2 °C, 12/12 h light/dark cycles) with ad libitum access to food and water. After one week of acclimation, the mice were randomly divided into three groups: a control group; the HG group; and the COT hydrogel group. The dorsal flank area (2 × 2 cm
2) of each mouse was shaved 24 h prior to the start of testing. Next, individual hydrogel samples were applied to the hair-free skin, covering an area of 4 cm
2. In the control group, all mice were similarly treated with deionized water. The above application procedures were continued daily for 14 consecutive days. At 1 h post-application each day, skin assessments for erythema and edema were performed. Additionally, skin assessments for erythema and edema were performed at 24 h, 48 h, and 72 h following the final application. All skin reactions were assessed according to
Table 8 and
Table 9, and the degree of skin irritation in each mouse was quantified by calculating the Primary Skin Irritation Index (PII) as follows:
At the end of the 72 h observation period, skin and subcutaneous tissue at the administration site of each mouse were excised for fixation, dehydration, clearing, and paraffin embedding. The tissue sections were subsequently stained with hematoxylin and eosin (H&E) and then examined for histopathological changes.
4.7. Skin Allergenicity Analysis
To evaluate the potential allergenic and sensitizing effects of COT hydrogel, skin sensitivity tests were performed [
53]. First, mice were randomly divided into four groups: a control group (treated with deionized water); the HG group; the COT hydrogel group; and the CNDNB group (treated with 1% CNDNB, a potent sensitizer that effectively induces skin inflammatory responses). At 24 h before the start of testing, a 2 × 2 cm
2 area was shaved on the back (flank) of each mouse. The experiment consisted of two phases: an induction phase and a challenge phase. During the induction phase, the appropriate test substance was applied to the shaved area of each mouse on days 0, 7, and 14. After 6 h, the test substances were removed, and the application sites were cleaned with warm water. In the challenge phase (on day 21), the appropriate test substance was applied to the shaved area on the opposite side of each mouse to induce a contact response. After 6 h, the area was rinsed with warm water. The tested skin patches were then observed at 1 h, 24 h, 48 h, and 72 h post-application to assess the occurrence of allergic reactions (in each individual mouse). In each case, skin reactions were assessed and categorized based on the Magnusson and Kligman scales (
Table 10). Finally, allergenicity observation scores and sensitization rates were calculated.
4.8. Hemostatic Evaluation
The hemostatic efficacy of COT hydrogel was first evaluated by measuring the clotting time and BCI. The coagulation time was determined according to the procedure described in
Section 4.2.1 “Preparation of Polysaccharide Mixture CAF” with minor modifications. Briefly, 500 μL COT or HG hydrogel was added into 100 μL of sodium citrate-anticoagulated rabbit blood, followed by the addition of 100 μL CaCl
2 solution (37 °C, 0.02 mol/L). In the control group, an equal volume of blood was mixed directly with CaCl
2 solution. The coagulation time was recorded upon clot formation.
BCI was performed according to the following procedure with minor modifications [
54]: the COT or HG hydrogel (1 cm
2) was pre-incubated at 37 °C for 5 min. 100 μL of sodium citrate-anticoagulated rabbit blood was dropped onto each sample, followed by the addition of 10 μL of CaCl
2 solution (0.2 mol/L) at the same position. After incubating at 37 °C for 5 min, 10 mL of deionized water was added and incubated at 37 °C for another 10 min. The hemoglobin absorbance of non-adherent blood clots was measured at 542 nm (denoted as Abssample). The negative reference was sodium citrate-anticoagulated rabbit blood in deionized water (denoted as Absnegative). The BCI was calculated using this formula:
The hemostatic efficacy of COT hydrogel was further evaluated using a mouse liver injury model. First, the mice were randomly divided into three groups: the blank control group; the HG group; and the COT group. To generate the liver injury model, the upper abdomen of each mouse was surgically opened to expose the liver, and a 1 cm incision was made in the liver tissue. Subsequently, 1 mL of either COT hydrogel or HG was immediately applied to the wound site (the control group received no treatment). Immediately following completion of the procedure, the bleeding status was recorded at specified time points (5 s, 10 s, 30 s, and 60 s), and the final blood loss at 60 s was evaluated. Specifically, a pre-weighed filter paper was positioned under the liver, and the blood loss was determined by measuring the mass increase in the paper.
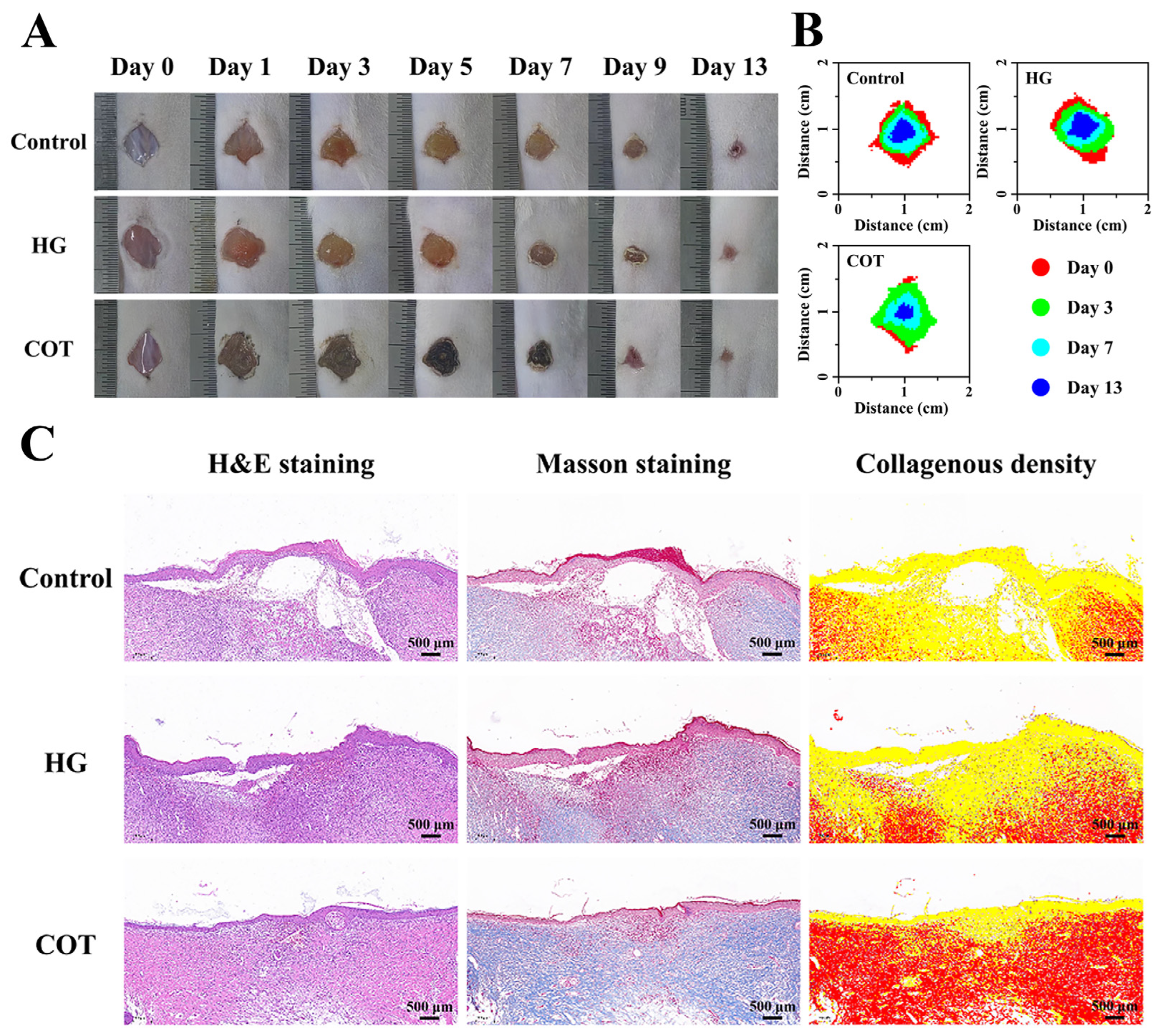
4.9. The Wound-Healing Analysis In Vivo
To assess wound-healing in vivo, the mice were randomly divided into three groups: a control group; the HG group; and the COT group. To establish a dorsal wound mouse model, mice with depilated backs were first anesthetized, and then full-thickness skin lesions measuring 10 mm in diameter were generated on their backs. The wound sites were then immediately covered with either COT hydrogel or HG (mice in the control group received no treatment). The hydrogels were refreshed daily, and the wound sizes were recorded on days 0, 1, 3, 5, 7, 9, and 13. Wound healing rates were calculated as follows:
where S
0 represents the original size of each wound site and S
t represents the area of each wound site on the day of recording.
After completion of the experiment, the skin and subcutaneous tissue at the wound healing site were excised for fixation, dehydration, clearing, and paraffin embedding. The tissue sections were subsequently stained with hematoxylin and eosin (H&E) and Masson’s trichrome stain, and histopathological changes were meticulously examined.
4.10. Statistical Analysis
All experimental data are expressed as mean ± standard deviation (SD). In all cases, one-way analysis of variance was used to evaluate the significance of differences in the mean (* p < 0.05, ** p < 0.01, *** p < 0.001).