Graphene Oxide Incorporated Nanohybrid Aerogels as New Generation Drug Carrier Platforms
Abstract
1. Introduction
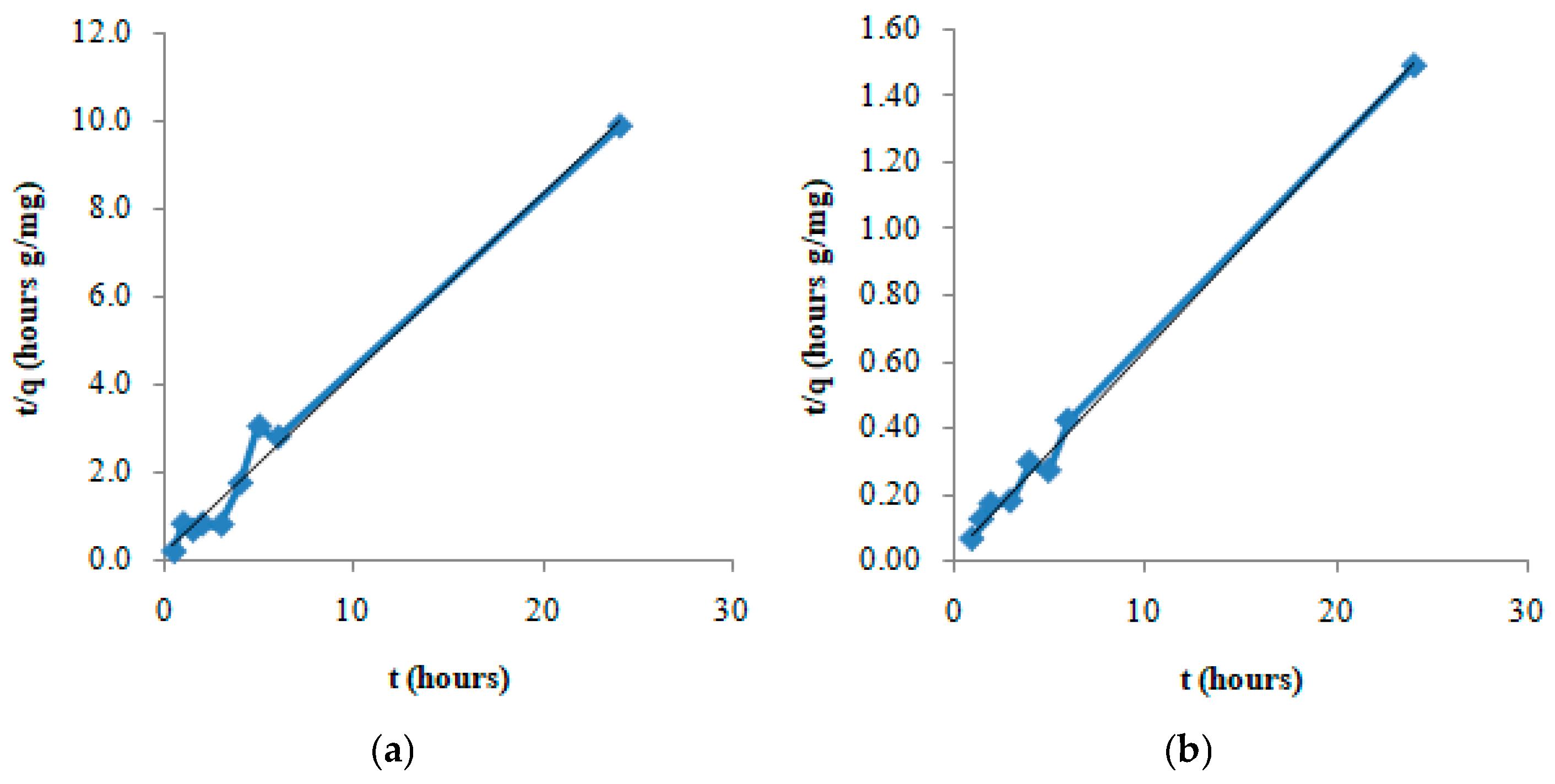
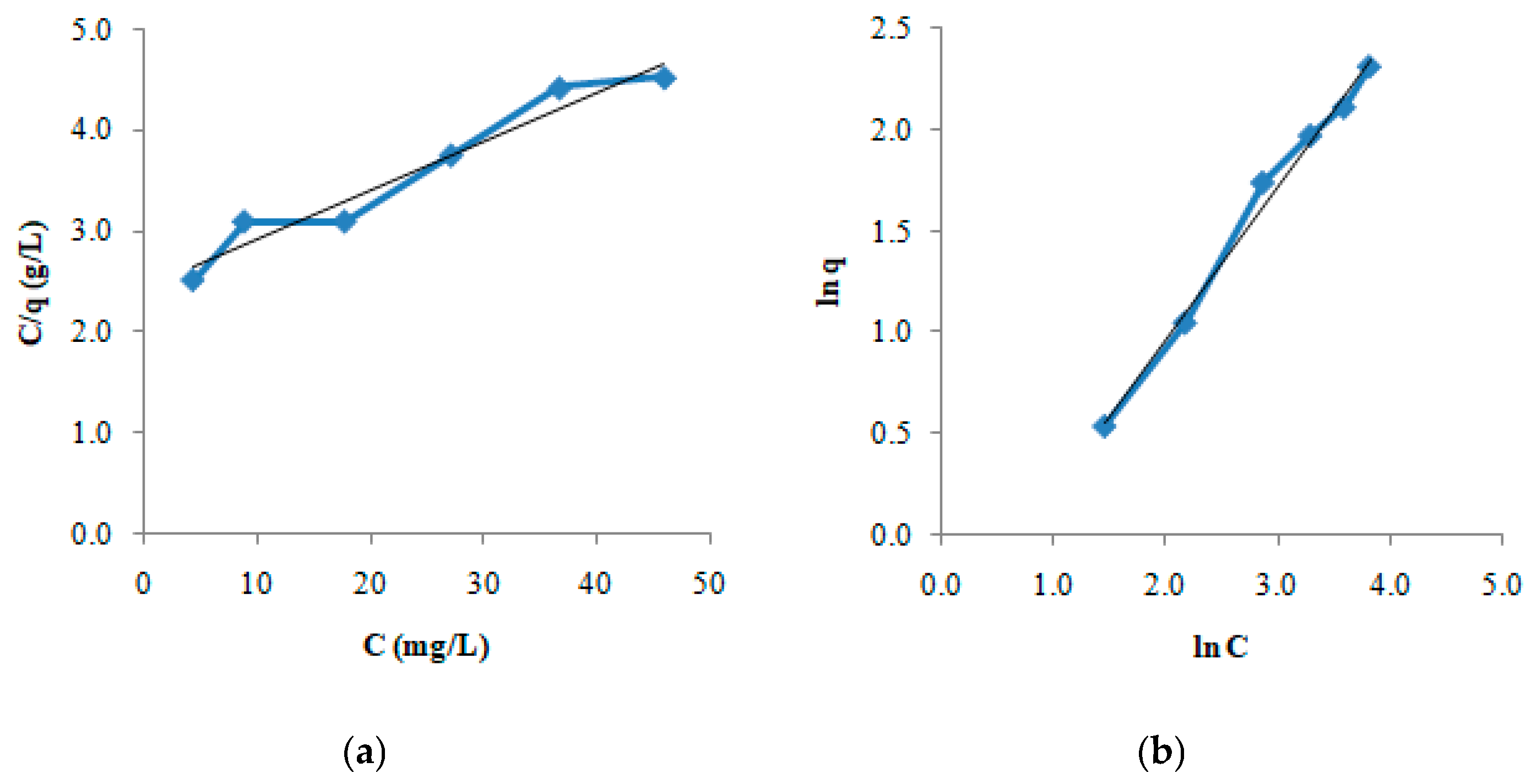
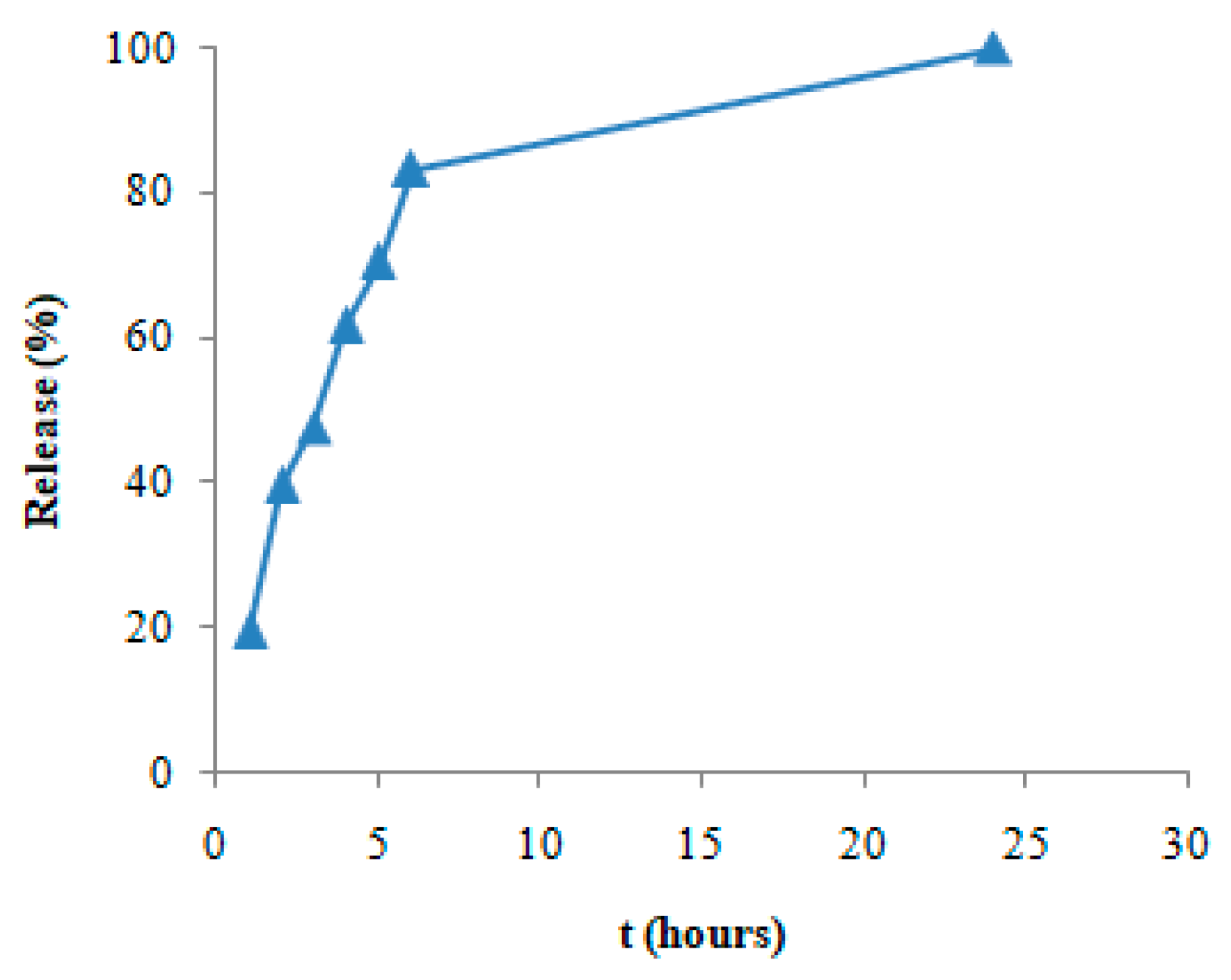
2. Results and Discussion
3. Conclusions
4. Materials and Methods
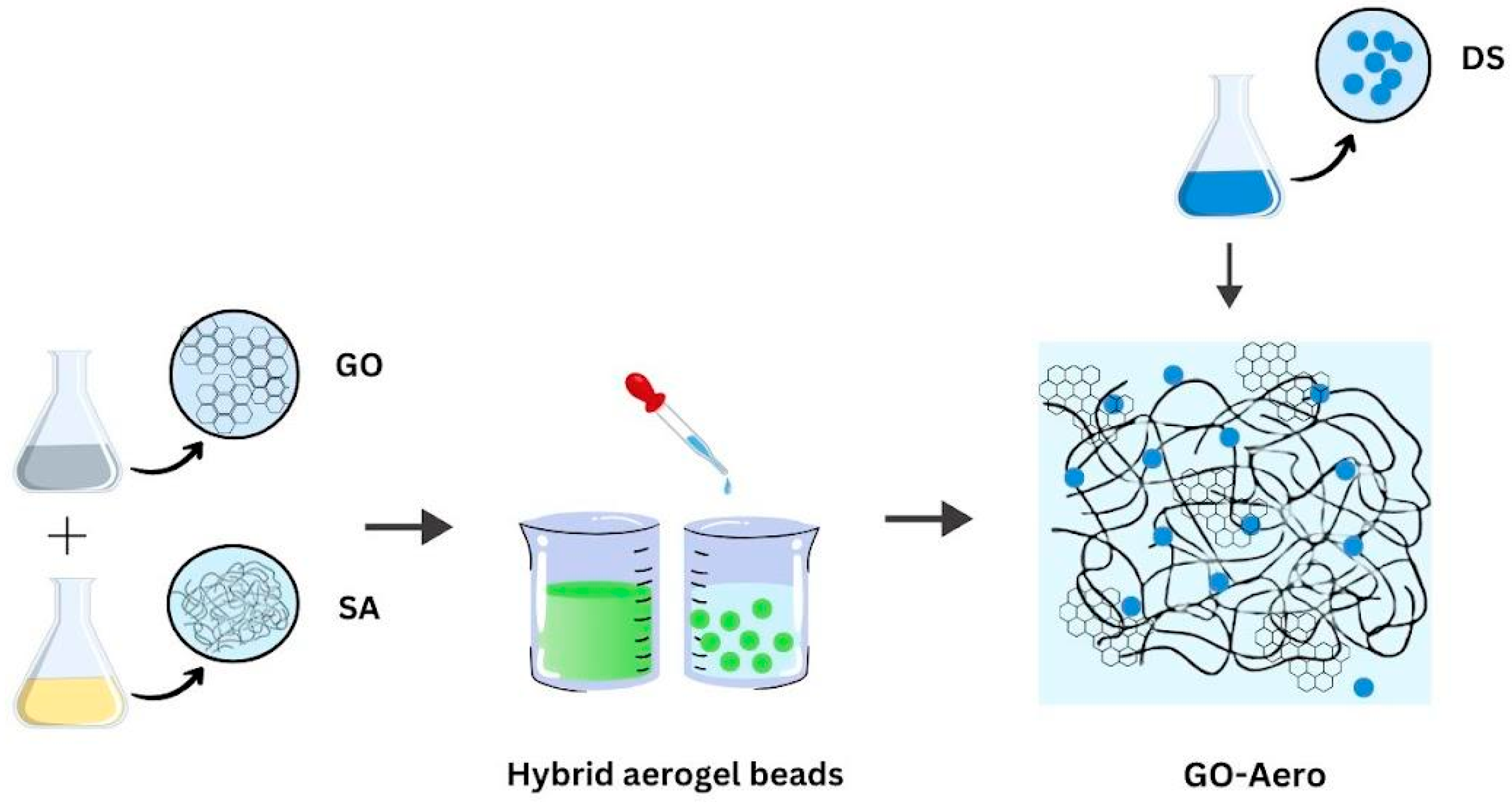
4.1. Production of the Graphene Oxide Incorporated Hybrid Aerogels
4.2. Characterization of the Nanohybrid Aerogels
4.3. Investigation of Drug Adsorption on Nanohybrid Aerogels
4.4. In Vitro Release Study of the Nanohybrid Aerogels
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GO | Graphene oxide |
| RGO | Reduced graphene oxide |
| XRD | X-ray diffraction |
| FTIR | Fourier transform InfraRed |
| PDI | Polydispersity index |
| SEM | Scanning Electron Microscope |
| EDX | Energy Dispersive X-ray analysis |
| DS | Diclofenac sodium |
| CNT | Carbon nanotubes |
| DLS | Dynamic light scattering |
References
- Wang, J.; Salihi, E.C.; Šiller, L. Green reduction of graphene oxide using alanine. Mater. Sci. Eng. C 2017, 72, 1–6. [Google Scholar] [CrossRef]
- Orsu, P.; Koyyada, A. Recent progresses and challenges in graphene-based nanomaterials for advanced therapeutical applications: A comprehensive review. Mater. Today Commun. 2020, 22, 100823. [Google Scholar] [CrossRef]
- Yamamoto, M.; Goto, S.; Tang, R.; Yamazaki, K. Toward three-dimensionally ordered nanoporous graphene materials: Template synthesis, structure, and applications. Chem. Sci. 2023, 15, 1953–1965. [Google Scholar] [CrossRef]
- Edirisinghe, M.; Tabish, T.A.; Gungordu, E.S. Graphene-based nanocomposites as antibacterial, antiviral and antifungal agents. Adv. Healthc. Mater. 2023, 12, e2201523. [Google Scholar] [CrossRef]
- Svigelj, R.; Toniolo, R.; Bertoni, C.; Fraleoni-Morgera, A. Synergistic applications of graphene-based materials and deep eutectic solvents in sustainable sensing: A comprehensive review. Sensors 2024, 24, 2403. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Gostaviceanu, A.; Gavrilaş, S.; Copolovici, L.; Copolovici, D.M. Graphene-oxide peptide-containing materials for biomedical applications. Int. J. Mol. Sci. 2024, 25, 10174. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.S.; Sangrizeh, F.H.; Jahani, N.; Abedin, M.S.; Chaleshgari, S.; Ardakan, A.K.; Baeelashaki, R.; Ranjbarpazuki, G.; Rahmanian, P.; Zandieh, M.A.; et al. Graphene oxide nanoarchitectures in cancer therapy: Drug and gene delivery, phototherapy, immunotherapy, and vaccine development. Environ. Res. 2023, 237 Pt 2, 117027. [Google Scholar] [CrossRef]
- Islam, M.S.; Roy, H.; Ahmed, T.; Firoz, S.H.; Chang, S.X. Surface-modified graphene oxide-based composites for advanced sequestration of basic blue 41 from aqueous solution. Chemosphere 2023, 340, 139827. [Google Scholar] [CrossRef]
- Singh, R.; Samuel, M.S.; Ravikumar, M.; Ethiraj, S.; Kumar, M. Graphene materials in pollution trace detection and environmental improvement. Environ. Res. 2024, 243, 117830. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhou, L.; Xiao, M.; Ma, S.; Cheng, G. Three-dimensional graphene promotes the proliferation of cholinergic neurons. Cells Tissues Organs 2024, 213, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Sohail Ahmad, M.; Inomata, Y.; Kida, T. Energy application of graphene-based membrane: Hydrogen separation. Chem. Rec. 2024, 24, e202300163. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yang, Z.; Li, F.; Xu, L.; Sun, Y. Cell-mediated targeting drug delivery systems. Drug Deliv. 2020, 27, 1425–1437. [Google Scholar] [CrossRef] [PubMed]
- Ashique, S.; Sandhu, N.K.; Chawla, V.; Chawla, P.A. Targeted drug delivery: Trends and perspectives. Curr. Drug Deliv. 2021, 18, 1435–1455. [Google Scholar] [CrossRef]
- Najm, A.; Moldoveanu, E.-T.; Niculescu, A.-G.; Grumezescu, A.M.; Beuran, M.; Gaspar, B.S. Advancements in drug delivery systems for the treatment of sarcopenia: An updated overview. Int. J. Mol. Sci. 2024, 25, 10766. [Google Scholar] [CrossRef]
- Trucillo, P. Drug carriers: Classification, administration, release profiles, and industrial approach. Processes 2021, 9, 470. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled drug delivery systems: Current status and future directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Zarrabi, A.; Bigham, A.; Taheriazam, A.; Saghari, Y.; Mirzaei, S.; Hashemi, M.; Hushmandi, K.; Karimi-Maleh, H.; NazarzadehZare, E.; et al. Nano platforms in breast cancer therapy: Drug/gene delivery, advanced nanocarriers, and immunotherapy. Med. Res. Rev. 2023, 43, 2115–2176. [Google Scholar] [CrossRef]
- Alavi, S.E.; Alharthi, S.; Alavi, S.Z.; Raza, A.; Ebrahimi Shahmabadi, H. Bioresponsive drug delivery systems. Drug Discov. Today 2024, 29, 103849. [Google Scholar] [CrossRef]
- Parvin, N.; Kumar, V.; Joo, S.W.; Mandal, T.K. Emerging trends in nanomedicine: Carbon-based nanomaterials for healthcare. Nanomaterials 2024, 14, 1085. [Google Scholar] [CrossRef]
- Johnson, A.P.; Jyothi, S.L.; Shahid, M.; Venkatesh, M.P.; Chidambaram, S.B.; Osmani, R.A.; Gangadharappa, H.V.; Pramod, K. Graphene oxide nanoribbons conjugated with 1, 2-distearoyl-sn-glycero-3-phosphoethanolamine-poly(ethylene glycol)-transferrin enhanced targeted delivery and cytotoxicity of raloxifene against breast cancer. Int. J. Biol. Macromol. 2024, 278 Pt 2, 134772. [Google Scholar] [CrossRef]
- Sreeharsha, N.; Chitrapriya, N.; Jang, Y.J.; Kenchappa, V. Evaluation of nanoparticle drug-delivery systems used in preclinical studies. Ther. Deliv. 2021, 12, 325–336. [Google Scholar] [CrossRef]
- Karki, N.; Tiwari, H.; Tewari, C.; Rana, A.; Pandey, N.; Basak, S.; Sahoo, N.G. Functionalized graphene oxide as a vehicle for targeted drug delivery and bioimaging applications. J. Mater. Chem. B 2020, 8, 8116–8148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, Y.; Li, K.; Li, J. Application of graphene oxide in tumor targeting and tumor therapy. J. Biomater. Sci. Polym. Ed. 2023, 34, 2551–2576. [Google Scholar] [CrossRef] [PubMed]
- Liao, R.; Zhang, Y.; Mao, W. Functionalized graphene oxide NPs as a nanocarrier for drug delivery system in quercetin/lurbinectedin as dual sensitive therapeutics for A549 lung cancer treatment. Heliyon 2024, 10, e31212. [Google Scholar] [CrossRef]
- Gu, Y.; Wu, X.; Gopalakrishna, T.Y.; Phan, H.; Wu, J. Graphene-like molecules with four zigzag edges. Angew. Int. Ed. 2018, 57, 6541–6545. [Google Scholar] [CrossRef]
- Demirhan, K.; BingolOzakpinar, O.; Salihi, E.C. Green and one step modification of graphene oxide using natural substances. Fuller. Nanotub. Carbon Nanostruct. 2021, 29, 716–723. [Google Scholar] [CrossRef]
- Skopinska-Wisniewska, J.; Tuszynska, M.; Kaźmierski, Ł.; Bartniak, M.; Bajek, A. Gelatin–Sodium Alginate Hydrogels Cross-Linked by Squaric Acid and Dialdehyde Starch as a Potential Bio-Ink. Polymers 2024, 16, 2560. [Google Scholar] [CrossRef]
- Tomić, S.L.; Radić, M.M.B.; Vuković, J.S.; Filipović, V.V.; Nikodinovic-Runic, J.; Vukomanović, M. Alginate-Based Hydrogels and Scaffolds for Biomedical Applications. Mar. Drugs 2023, 21, 177. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, A.K. Sodium Alginate: A Green Biopolymer Resource-Based Antimicrobial Edible Coating to Enhance Fruit Shelf-Life: A Review. Colloids Interfaces 2025, 9, 32. [Google Scholar] [CrossRef]
- Ayari, M.; Osfouri, S.; Azin, R.; Rostami, A. An environmentally friendly and low-cost alginate-based gel for water management in petroleum reservoirs: Characterization and efficacy investigation. Petroleum 2025, 11, 248–258. [Google Scholar] [CrossRef]
- Ding, X.; Pu, Y.; Tang, M.; Zhang, T. Environmental and health effects of graphene-family nanomaterials: Potential release pathways, transformation, environmental fate and health risks. Nano Today 2022, 42, 101379. [Google Scholar] [CrossRef]
- Kurapati, R.; Bianco, A. Peroxidase mimicking DNAZymes degrade graphene oxide. Nanoscale 2018, 10, 19316–19321. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wu, T.; Xia, F.; Li, Y.; Zhang, C.; Zhang, L.; Chen, M.; Li, X.; Zhang, L.; Liu, Y.; et al. Redox reaction between graphene oxide and In powder to prepare In2O3/reduced graphene oxide hybrids for supercapacitors. J. Power Sources 2014, 266, 282–290. [Google Scholar] [CrossRef]
- Huang, J.-J.; Yuan, Y.J. A Sedimentation Study of Graphene Oxide in Aqueous Solution Based on Gradient Differential Centrifugation. Phys. Chem. Chem. Phys. 2016, 18, 12312–12322. [Google Scholar] [CrossRef]
- Sha, M.S.; Anwar, H.; Musthafa, F.N.; Al-Lohedan, H.; Alfarwati, S.; Rajabathar, J.R.; Alahmad, J.K.; Cabibihan, J.-J.; Karnan, M.; Sadasivuni, K.K. Photocatalytic degradation of organic dyes using reduced graphene oxide (rGO). Sci. Rep. 2024, 14, 3608. [Google Scholar] [CrossRef]
- Tong, J.; Fu, Y.; Domaretskiy, D.; Della Pia, F.; Dagar, P.; Powell, L.; Bahamon, D.; Huang, S.; Xin, B.; Filho, R.N.C.; et al. Control of proton transport and hydrogenation in double-gated graphene. Nature 2024, 630, 619–624. [Google Scholar] [CrossRef]
- Zeng, Z.; Wang, Y.; Zhou, Q.; Yang, K.; Lin, D. New insight into the aggregation of graphene oxide in synthetic surface water: Carbonate nanoparticle formation on graphene oxide. Environ Pollut. 2019, 250, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Chen, L.; Li, W.; Zhou, Z.; Fang, Z.; Xu, Z.; Qian, X. Self-assembled three-dimensional double network graphene oxide/polyacrylic acid hybrid aerogel for removal of Cu2+ from aqueous solution. Environ. Sci. Pollut. Res. Int. 2018, 25, 34438–34447. [Google Scholar] [CrossRef]
- Wei, D.; Liu, X.; Lv, S.; Liu, L.; Wu, L.; Li, Z.; Hou, Y. Fabrication, structure, performance, and application of graphene-based composite aerogel. Materials 2021, 15, 299. [Google Scholar] [CrossRef]
- Kim, Y.; Patel, R.; Kulkarni, C.V.; Patel, M. Graphene-based aerogels for biomedical application. Gels 2023, 9, 967. [Google Scholar] [CrossRef]
- Alemán, J.; Chadwick, A.; He, J.; Hess, M.; Horie, K.; Jones, R.; Kratochvíl, P.; Meisel, I.; Mita, I.; Moad, G.; et al. Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials (IUPAC Recommendations 2007). Pure Appl. Chem. 2007, 79, 1801–1829. [Google Scholar] [CrossRef]
- Shi, W.; Ching, Y.C.; Chuah, C.H. Preparation of aerogel beads and microspheres based on chitosan and cellulose for drug delivery: A review. Int. J. Biol. Macromol. 2021, 170, 751–767. [Google Scholar] [CrossRef] [PubMed]
- Small, R.E. Diclofenac sodium. Clin. Pharm. 1989, 8, 545–558. [Google Scholar] [PubMed]
- Cooper, C.; Chapurlat, R.; Al-Daghri, N.; Herrero-Beaumont, G.; Bruyère, O.; Rannou, F.; Roth, R.; Uebelhart, D.; Reginster, J.Y. Safety of oral non-selective non-steroidal anti-inflammatory drugs in osteoarthritis: What does the literature say? Drugs Aging 2019, 36 (Suppl. S1), 15–24. [Google Scholar] [CrossRef]
- Bayram, D.; Vizdiklar, C.; Aydin, V.; İşli, F.; Akici, A. Investigation of prescribing trends and prescriptions for common diagnoses in primary care: Nationwide data of Turkey. Cukurova Med. J. 2020, 45, 695–708. [Google Scholar] [CrossRef]
- Madeo, L.; Curcio, M.; Iemma, F.; Fiore, N.; Hampel, S.; Cirillo, G. Release of bioactive molecules from graphene oxide-alginate hybrid hydrogels: Effect of crosslinking method. Coatings 2023, 9, 8. [Google Scholar] [CrossRef]
- Lee, K.; Rowley, J.A.; Eiselt, P.; Moy, E.M.; Bouhadir, K.H.; Mooney, D.J. Controlling mechanical and swelling properties of alginate hydrogels independently by cross-linker type and cross-linking density. Macromolecules 2000, 33, 9734–9739. [Google Scholar] [CrossRef]
- García-González, C.A.; Sosnik, A.; Kalmár, J.; De Marco, I.; Erkey, C.; Concheiro, A.; Alvarez-Lorenzo, C. Aerogels in drug delivery: From design to application. J. Control. Release 2021, 332, 40–63. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Wang, S.; Li, S.; Yang, H.; Luo, J. Progress in the functional modification of graphene/graphene oxide: A review. RSC Adv. 2020, 10, 15328–15345. [Google Scholar] [CrossRef]
- Yang, K.; Chen, B.; Zhu, X.; Xing, B. Aggregation, Adsorption, Morphological Transformation of Graphene Oxide in Aqueous Solutions Containing Different Metal Cations. Environ. Sci. Technol. 2016, 50, 11066–11075. [Google Scholar] [CrossRef] [PubMed]
- Pleuvry, B.J. Factors affecting drug absorption and distribution. Anaesth. Intensive Care Med. 2005, 6, 135–138. [Google Scholar] [CrossRef]
- Jiao, C.; Xiong, J.; Tao, J.; Xu, S.; Zhang, D.; Lin, H.; Chen, Y. Sodium alginate/graphene oxide aerogel with enhanced strength–toughness and its heavy metal adsorption study. Int. J. Biol. Macromol. 2016, 83, 133–141. [Google Scholar] [CrossRef]
- Taleb, M.A.; Kumar, R.; Barakat, M.A.; Almeelbi, T.B.; Al-Makishah, N.H. Facile synthesis of reduced graphene oxide/carbon/calcium alginate aerogel for the removal of pharmaceutical pollutants. Int. J. Biol. Macromol. 2025, 296, 139688. [Google Scholar] [CrossRef]
- Sun, L.; Fugetsu, B. Graphene oxide captured for green use: Influence on the structures of calcium alginate and macroporous alginic beads and their application to aqueous removal of acridine orange. Chem. Eng. J. 2014, 240, 565–573. [Google Scholar] [CrossRef]
- Çalışkan Salihi, E.; Wang, J.; Coleman, D.J.; Šiller, L. Enhanced removal of nickel (II) ions from aqueous solutions by SDS-functionalized graphene oxide. Sep. Sci. Technol. 2016, 51, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Tao, E.; Ma, D.; Yang, S.; Hao, X. Graphene oxide-montmorillonite/sodium alginate aerogel beads for selective adsorption of methylene blue in wastewater. J. Alloys Compd. 2020, 832, 154833. [Google Scholar] [CrossRef]
- Lentz, L.; Mayer, D.A.; Dogenski, M.; Ferreira, S.R.S. Hybrid aerogels of sodium alginate/graphene oxide as efficient adsorbents for wastewater treatment. Mater. Chem. Phys. 2022, 283, 125981. [Google Scholar] [CrossRef]
- Qamar, S.A.; Qamar, M.; Basharat, A.; Bilal, M.; Cheng, H.; Iqbal, H.M. Alginate-based nano-adsorbent materials–Bioinspired solution to mitigate hazardous environmental pollutants. Chemosphere 2022, 288, 132618. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Potts, J.R.; Velamakanni, A.; Murali, S.; Ruoff, R.S. Hydrazine-reduction of graphite-and graphene oxide. Carbon 2011, 49, 3019–3023. [Google Scholar] [CrossRef]
- G.V, Y.D.; Prabhu, A.; Anil, S.; Venkatesan, J. Preparation and characterization of dexamethasone-loaded sodium alginate-graphene oxide microspheres for bone tissue engineering. J. Drug Deliv. Sci. Technol. 2021, 64, 102624. [Google Scholar] [CrossRef]
- Çalışkan Salihi, E.; Gündüz, Z.; Baştuğ, A.S. Fast retention of isoniazid on organobentonite prepared using a green chemistry approach: Contribution of the π interactions. Sep. Sci. Technol. 2019, 54, 2695–2705. [Google Scholar] [CrossRef]
- Caliskan Salihi, E.; Berber, B.; İsanç, K. Kinetic adsorption of drugs using carbon nanofibers in simulated gastric and intestinal fluids. Int. J. Chem. Kinet. 2023, 55, 102–110. [Google Scholar] [CrossRef]
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 2001, 73, 121–136. [Google Scholar] [CrossRef]
- Lara-Pérez, C.; Leyva, E.; Zermeño, B.; Osorio, I.; Montalvo, C.; Moctezuma, E. Photocatalytic degradation of diclofenac sodium salt: Adsorption and reaction kinetic studies. Environ. Earth Sci. 2020, 79, 277. [Google Scholar] [CrossRef]
- Caliskan Salihi, E. Adsorption of metamizole sodium by activated carbon in simulated gastric and intestinal fluids. J. Turk. Chem. Soc. Sect. A Chem. 2017, 5, 237–246. [Google Scholar] [CrossRef]
- Çalışkan Salihi, E.; Cantürk Talman, R.Y.; Göktürk, S. Preparation and characterization of surfactant-loaded clays as drug adsorbents. J. Dispers. Sci. Technol. 2023, 44, 165–173. [Google Scholar] [CrossRef]
- Galarda, A.; Goscianska, J. Biocompatible Fe-based metal-organic frameworks as diclofenac sodium delivery systems for migraine treatment. Appl. Sci. 2023, 13, 12960. [Google Scholar] [CrossRef]
- Shamsudin, M.S.; Azha, S.F.; Sellaoui, L.; Badawi, M.; Bonilla-Petriciolet, A.; Ismail, S. Performance and interactions of diclofenac adsorption using alginate/carbon-based films: Experimental investigation and statistical physics modelling. Chem. Eng. J. 2022, 428, 131929. [Google Scholar] [CrossRef]
- CanoPlá, S.M.; D’Urso, A.; Fernández-Sánchez, J.F.; Colangelo, D.; Choquesillo-Lazarte, D.; Ferracini, R.; Bosetti, M.; Prat, M.; Gómez-Morales, J. Biomimetic citrate-coated luminescent apatite nanoplatforms for diclofenac delivery in inflammatory environments. Nanomaterials 2022, 12, 562. [Google Scholar] [CrossRef]
- Dias, C.S.; Franco, M.A.E.; Rodrigues, E.C.; Ferreira, J.L.; Viegas, B.M.; Féris, L.A.; Estumano, D.C.; Macêdo, E.N. Diclofenac sodium adsorption on activated carbon: Experimental, modeling and bayesian statistics. An. Acad. Bras. Cienc. 2024, 96 (Suppl. S1), e20231110. [Google Scholar] [CrossRef]
- Hu, D.; Huang, H.; Jiang, R.; Wang, N.; Xu, H.; Wang, Y.G.; Ouyang, X.K. Adsorption of diclofenac sodium on bilayer amino-functionalized cellulose nanocrystals/chitosan composite. J. Hazard. Mater. 2019, 369, 483–493. [Google Scholar] [CrossRef]
- Jauris, I.M.; Matos, C.F.; Saucier, C.; Lima, E.C.; Zarbin, A.J.G.; Fagan, S.B.; Zanella, I. Adsorption osodium diclofenac on graphene: A combined experimental and theoretical study. Phys. Chem. Chem. Phys. 2016, 18, 1526–1536. [Google Scholar] [CrossRef]
- Tran, T.V.; Nguyen, D.T.C.; Le, H.T.N.; Vo, D.N.; Nanda, S.; Nguyen, T.D. Optimization, equilibrium, adsorption behavior and role of surface functional groups on graphene oxide-based nanocomposite towards diclofenac drug. J. Environ. Sci. 2020, 93, 137–150. [Google Scholar] [CrossRef]
- Ghobadifar, V.; Bagheri Marandi, G.; Kurdtabar, M.; RezanejadeBardajee, G. Synthesis and characterization of MNPs hydrogel with pH-responsiveness properties to release diclofenac sodium as a model drug. Iran. J. Chem. Chem. Eng. 2023, 42, 875–889. [Google Scholar] [CrossRef]
- Rigueto, C.V.T.; Rosseto, M.; Nazari, M.T.; Ostwald, B.E.P.; Alessandretti, I.; Manera, C.; Dettmer, A. Adsorption of diclofenac sodium by composite beads prepared from tannery wastes-derived gelatin and carbon nanotubes. J. Environ. Chem. Eng. 2021, 9, 105030. [Google Scholar] [CrossRef]
- Smiljanić, D.; Daković, A.; Obradović, M.; Ožegović, M.; Marković, M.; Rottinghaus, G.E.; de Gennaro, B. Influence of the type and the amount of surfactant in Phillipsite on adsorption of diclofenac sodium. Catalysts 2023, 13, 71. [Google Scholar] [CrossRef]
- Salihi, E.Ç.; Zarrabi, A.; Zarepour, A.; Gürboğa, M.; Hasan Niari Niar, S.; Özakpınar, Ö.B.; Wang, J.; Daştan, H.; Khosravi, A.; Šiller, L. Ambient pressure dried graphene oxide-silica composite aerogels as pharmaceutical nanocarriers. J. Sol-Gel Sci. Technol. 2025, 113, 548–558. [Google Scholar] [CrossRef]
- Hiew, B.Y.Z.; Lee, L.Y.; Lai, K.C.; Gan, S.; Thangalazhy-Gopakumar, S.; Pan, G.-T.; Yang, T.C.-K. Adsorptive decontamination of diclofenac by three-dimensional graphene-based adsorbent: Response surface methodology, adsorption equilibrium, kinetic and thermodynamic studies. Environ. Res. 2019, 168, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Liang, Z.; Li, Y.; Chen, Y.; Liu, K.; Yang, G.; Liu, Y.; Lin, C.; Ye, X.; Shi, Y.; et al. Efficient adsorption of diclofenac sodium in water by a novel functionalized cellulose aerogel. Environ. Res. 2021, 194, 110652. [Google Scholar] [CrossRef]
- Hernández-Zamora, M.; Cruz-Castillo, L.M.; Martínez-Jerónimo, L.; Martínez-Jerónimo, F. Diclofenac Produces Diverse Toxic Effects on Aquatic Organisms of Different Trophic Levels, Including Microalgae, Cladocerans, and Fish. Water 2025, 17, 1489. [Google Scholar] [CrossRef]
- Hashem, H.M.; Motawea, A.; Kamel, A.H.; Bary, E.A.; Hassan, S.S. Fabrication and characterization of electrospun nanofibers using biocompatible polymers for the sustained release of venlafaxine. Sci. Rep. 2022, 12, 18037. [Google Scholar] [CrossRef]


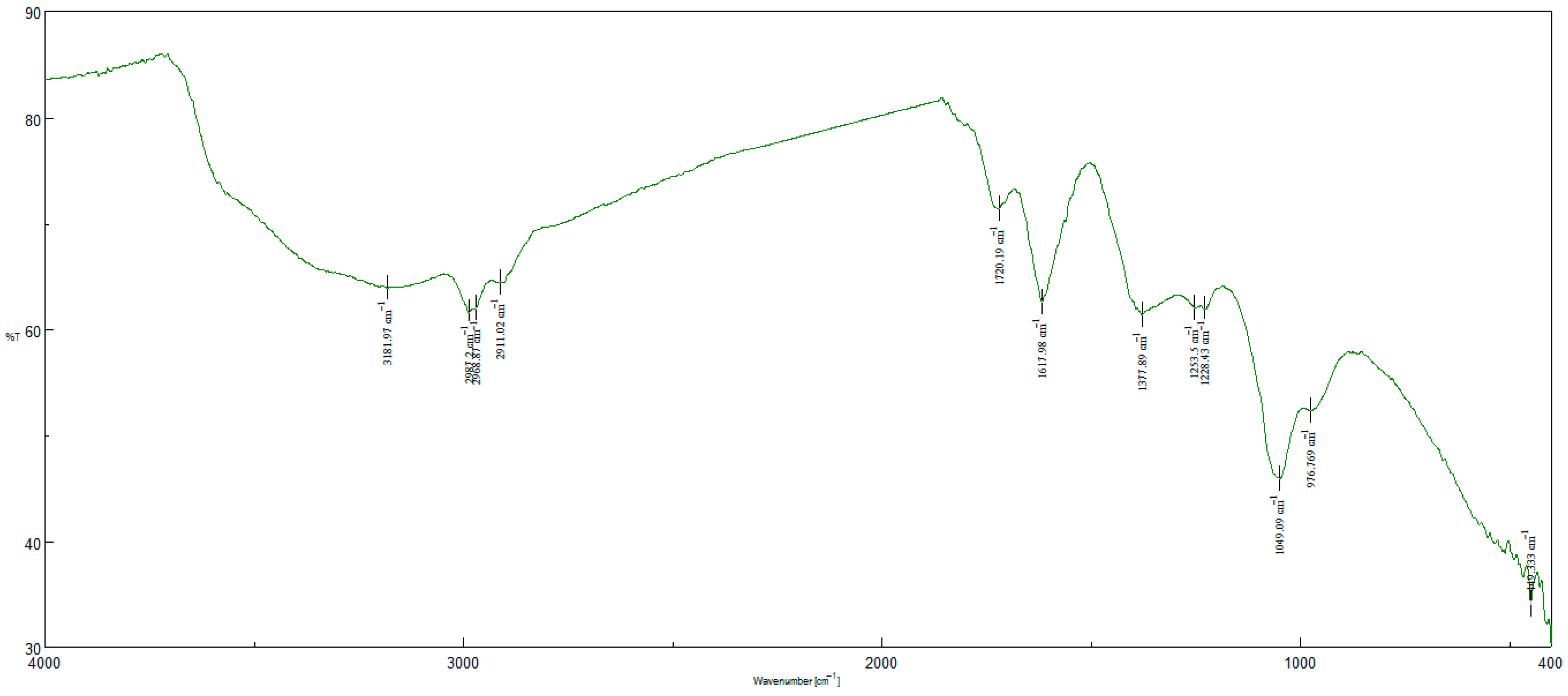
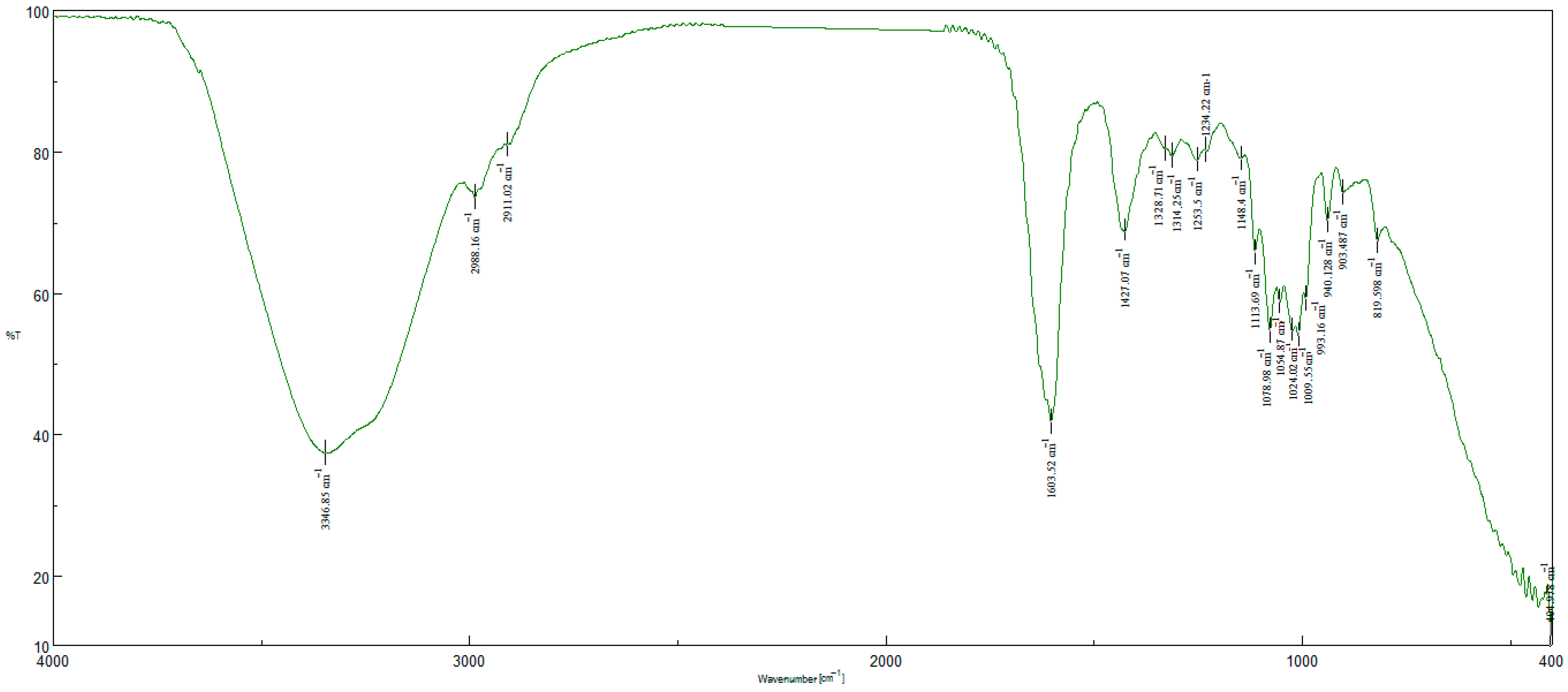
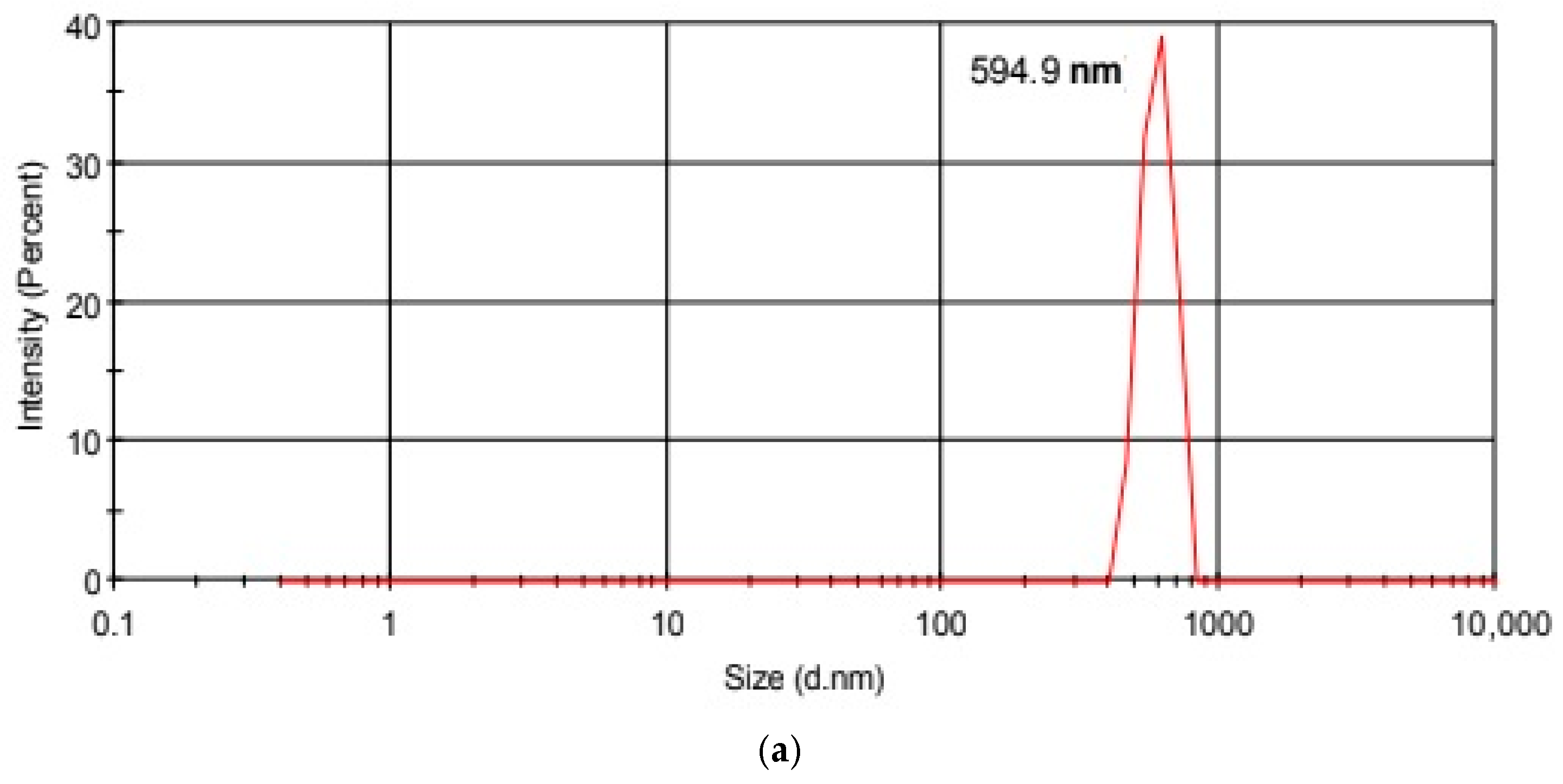
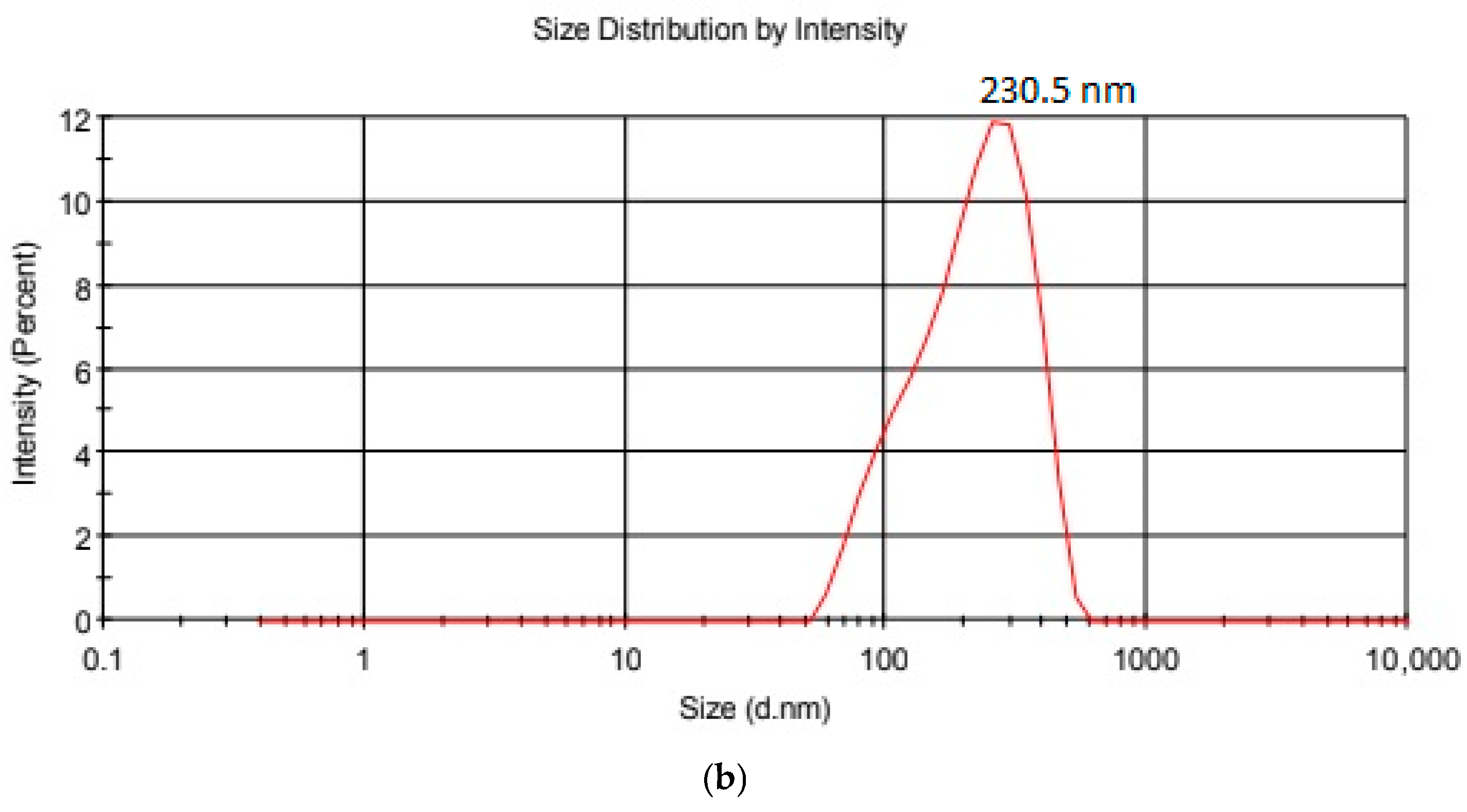
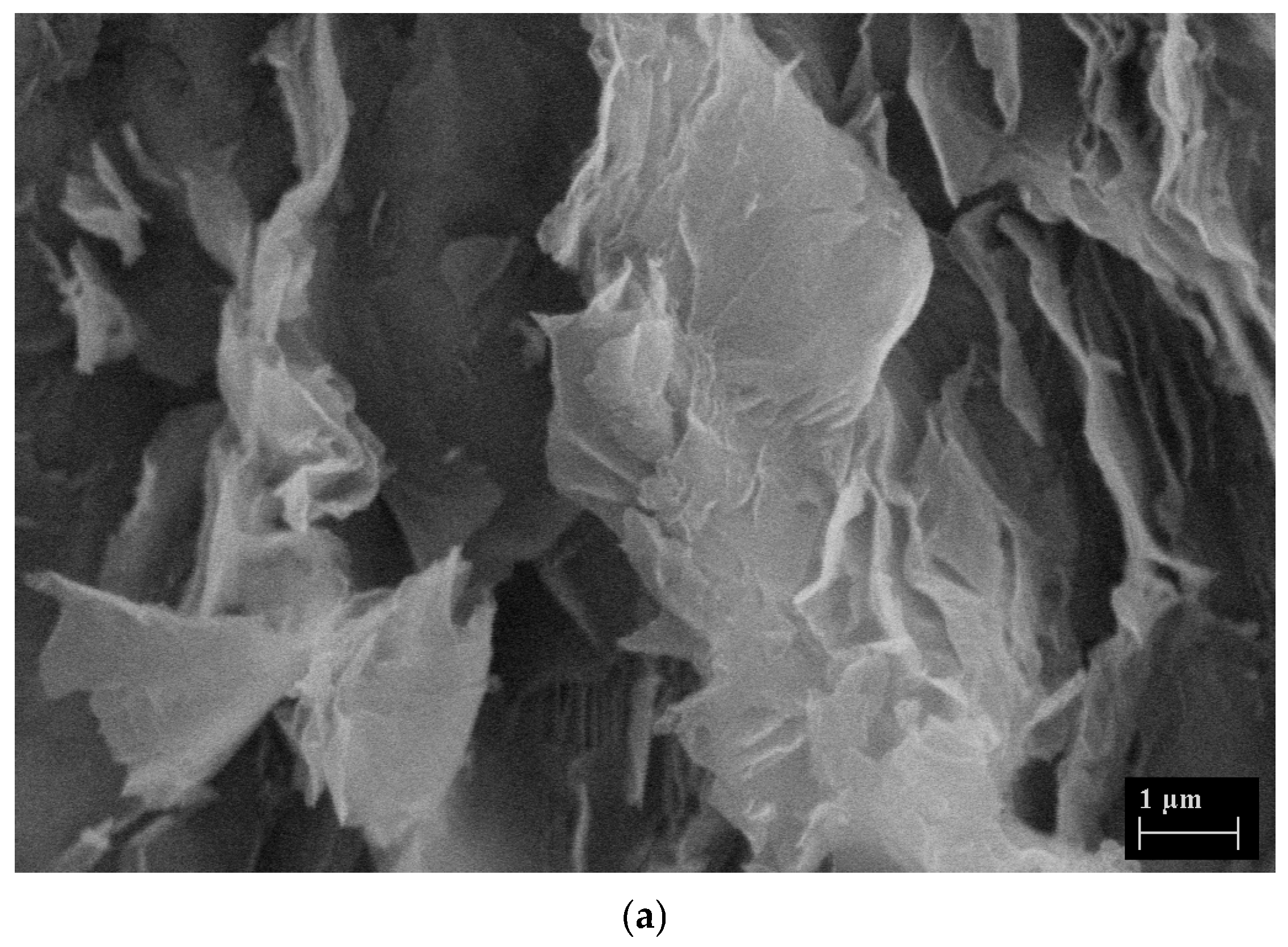
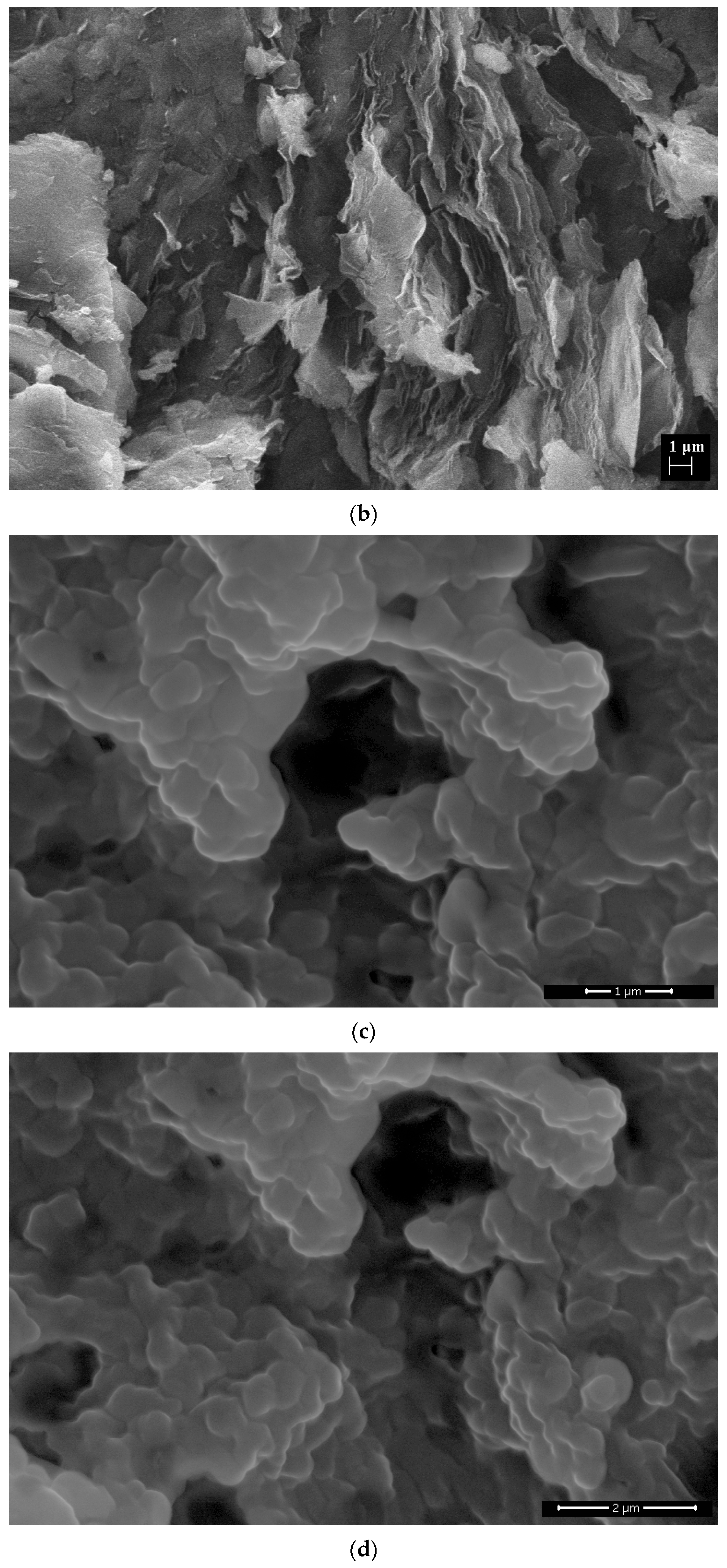
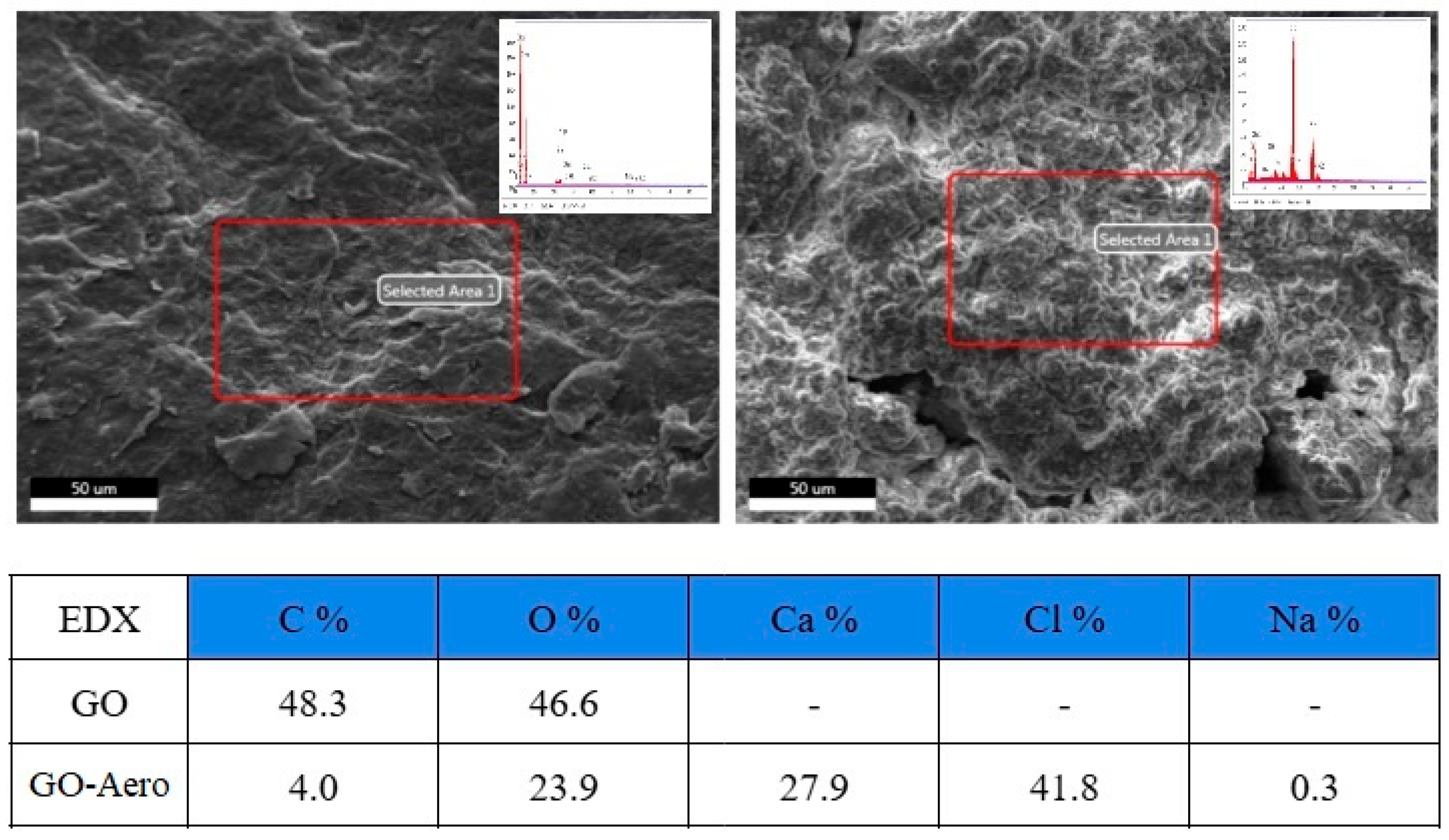
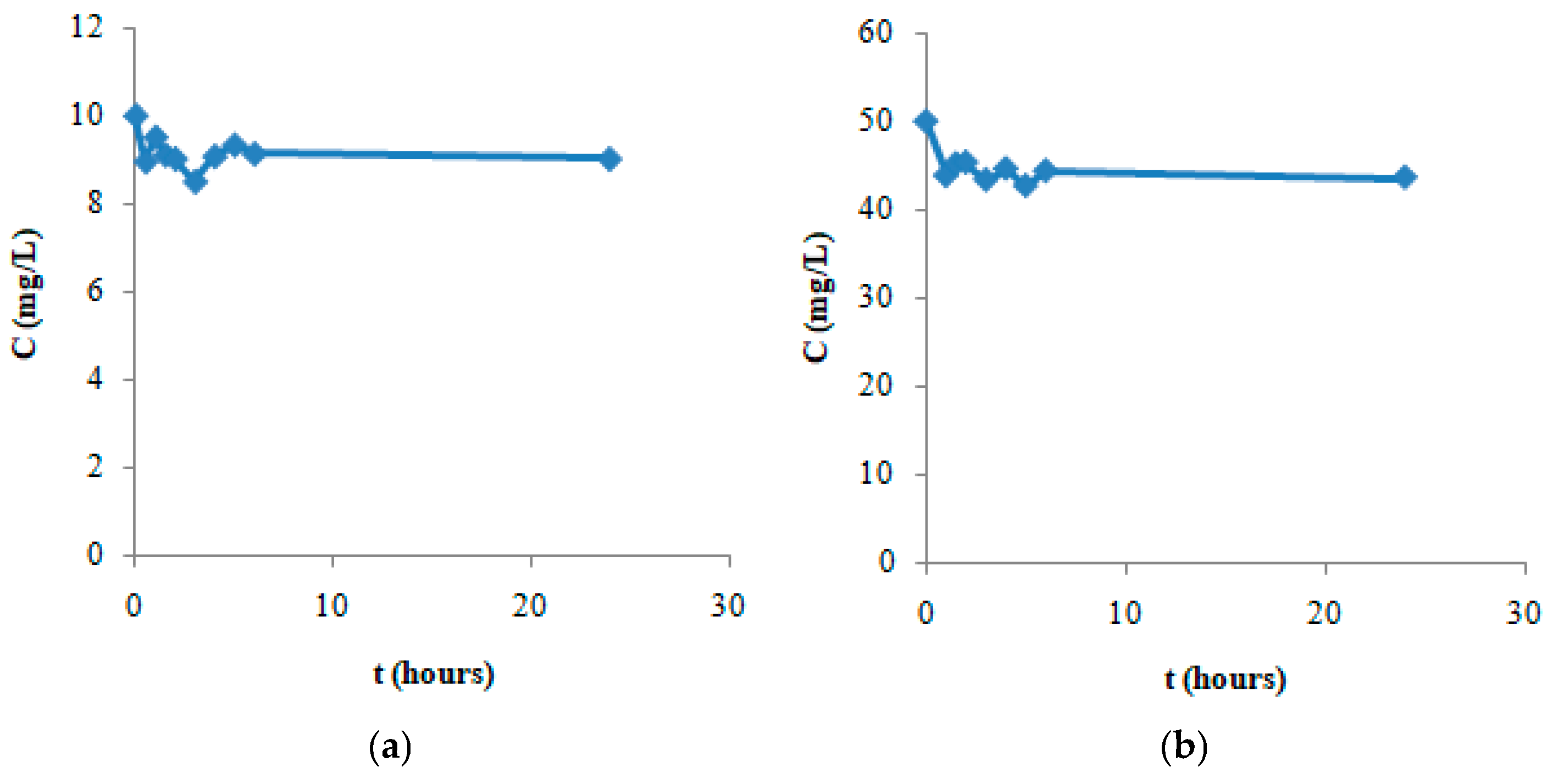
| Hydrodynamic Particle Size (nm) | PDI | Zeta Potential | pH of the Dispersion | |
|---|---|---|---|---|
| GO | 594.9 | 0.676 | −4.20 | 5.6 |
| GO-Aero | 230.5 | 0.461 | −22.6 | 6.2 |
| Pseudo 2nd Order Model | Lagergren 1st Order Model | |||||
|---|---|---|---|---|---|---|
| Concentration (for DS) | k2 | qe | R2 | k1 | qe | R2 |
| 10 mg/L | 0.967 | 2.44 | 0.983 | - | - | 0.347 |
| 50 mg/L | 0.186 | 16.39 | 0.995 | - | - | 0.077 |
| Langmuir Isotherm | Freundlich Isotherm | |||||
|---|---|---|---|---|---|---|
| Q (mg/g) | b (L/g) | R2 | 1/n | kF | R2 | |
| DS | 20.83 | 0.02 | 0.946 | 0.76 | 0.58 | 0.990 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çalışkan Salihi, E.; Hasan Niari Niar, S.; Ulucici, E.N.; Gedik, E.T.; Zengin, F.B.; Samur, F.; Şahin, S.D.; Taşkın, T. Graphene Oxide Incorporated Nanohybrid Aerogels as New Generation Drug Carrier Platforms. Gels 2025, 11, 949. https://doi.org/10.3390/gels11120949
Çalışkan Salihi E, Hasan Niari Niar S, Ulucici EN, Gedik ET, Zengin FB, Samur F, Şahin SD, Taşkın T. Graphene Oxide Incorporated Nanohybrid Aerogels as New Generation Drug Carrier Platforms. Gels. 2025; 11(12):949. https://doi.org/10.3390/gels11120949
Chicago/Turabian StyleÇalışkan Salihi, Elif, Shalaleh Hasan Niari Niar, Elif Nur Ulucici, Ebrar Tuğba Gedik, Fatma Betül Zengin, Fulya Samur, Seda Didem Şahin, and Turgut Taşkın. 2025. "Graphene Oxide Incorporated Nanohybrid Aerogels as New Generation Drug Carrier Platforms" Gels 11, no. 12: 949. https://doi.org/10.3390/gels11120949
APA StyleÇalışkan Salihi, E., Hasan Niari Niar, S., Ulucici, E. N., Gedik, E. T., Zengin, F. B., Samur, F., Şahin, S. D., & Taşkın, T. (2025). Graphene Oxide Incorporated Nanohybrid Aerogels as New Generation Drug Carrier Platforms. Gels, 11(12), 949. https://doi.org/10.3390/gels11120949





