Construction and Activity of Cisplatin-Loaded Chitosan–Zinc Amino-Porphyrin Photosensitizer Hydrogel
Abstract
1. Introduction
2. Results and Discussion
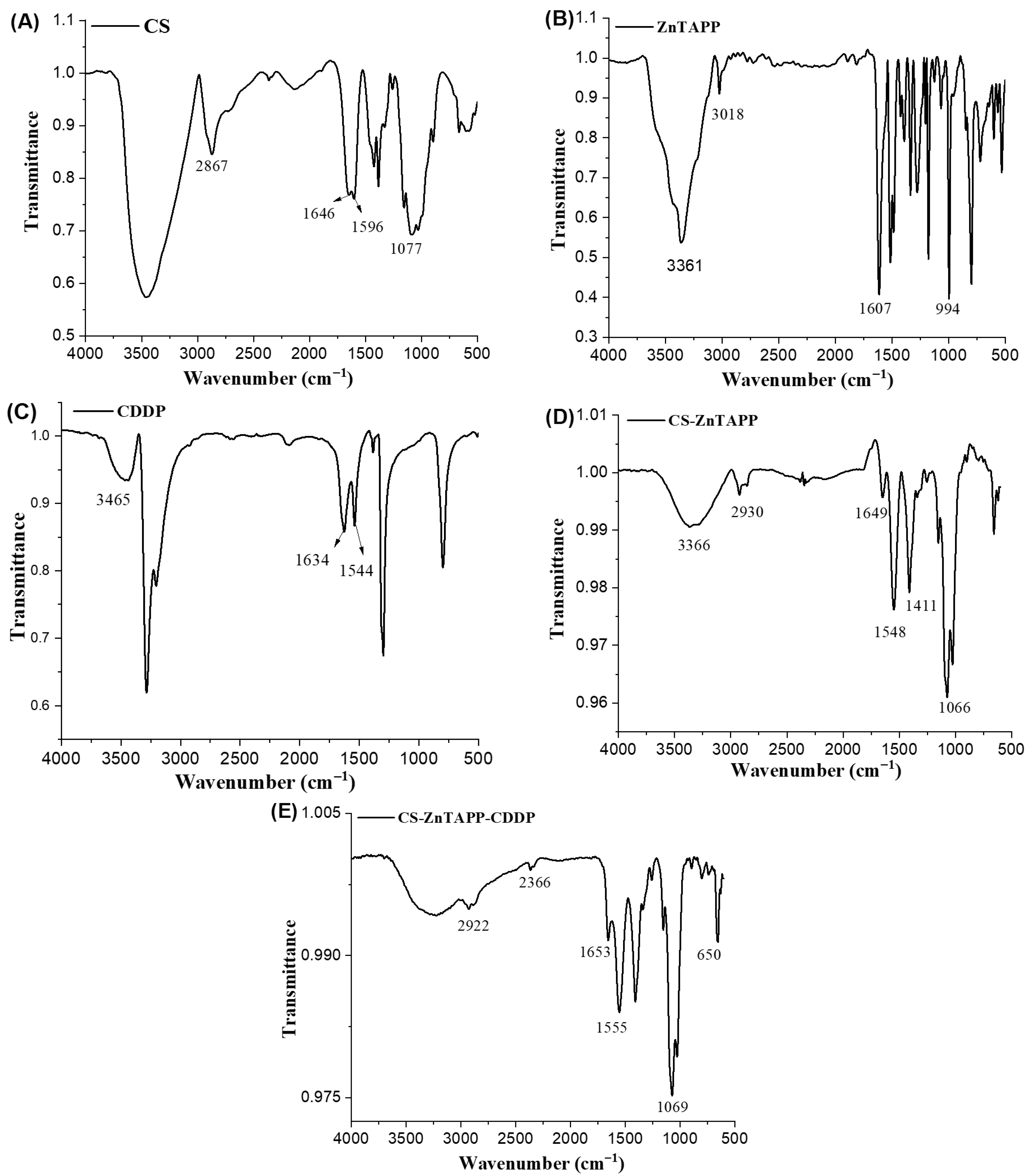
2.1. FTIR Spectral Analysis
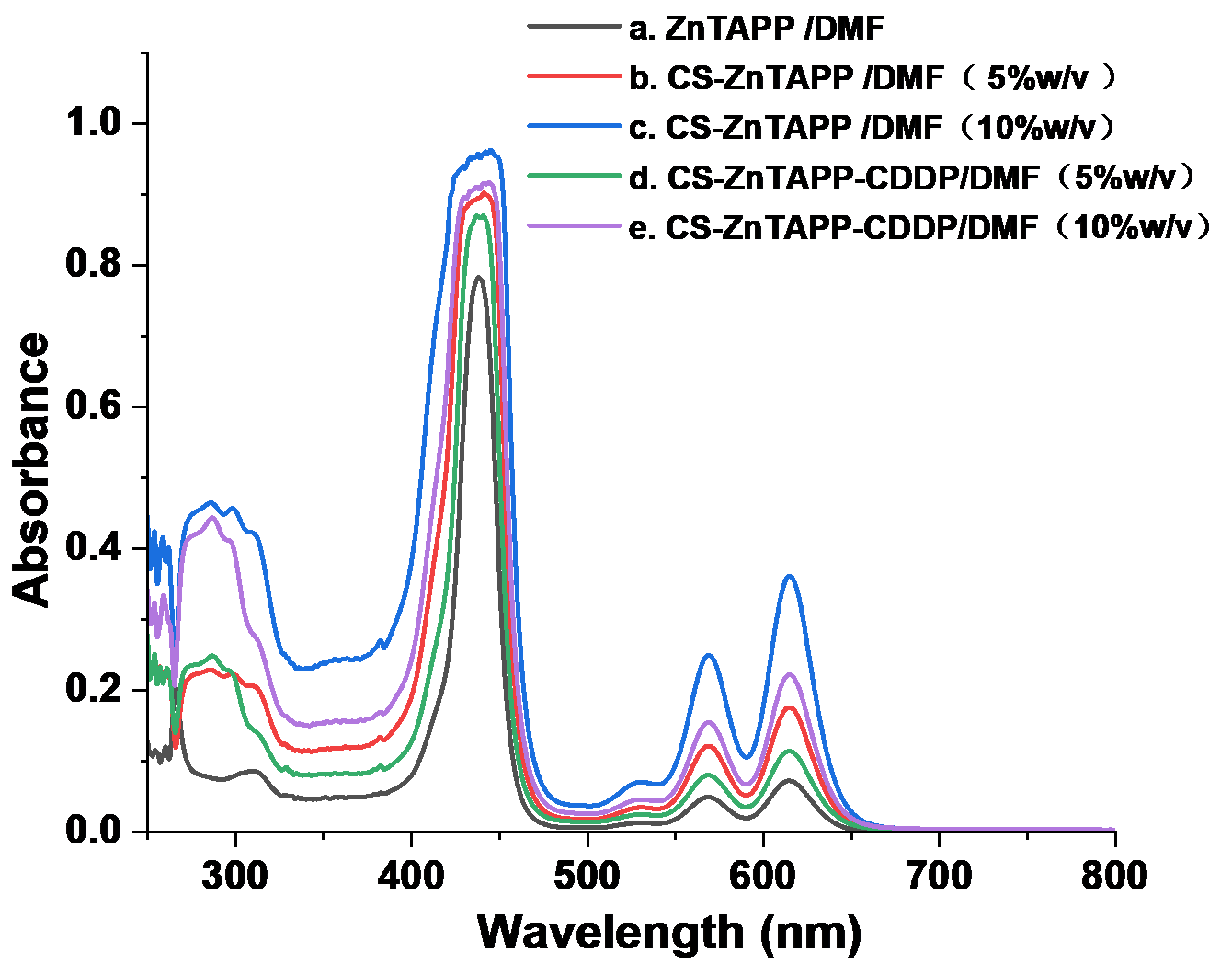
2.2. UV-Vis Spectral Analysis
2.3. Pt Content Determination and Analysis
2.4. ZnTAPP Content Determination and Analysis
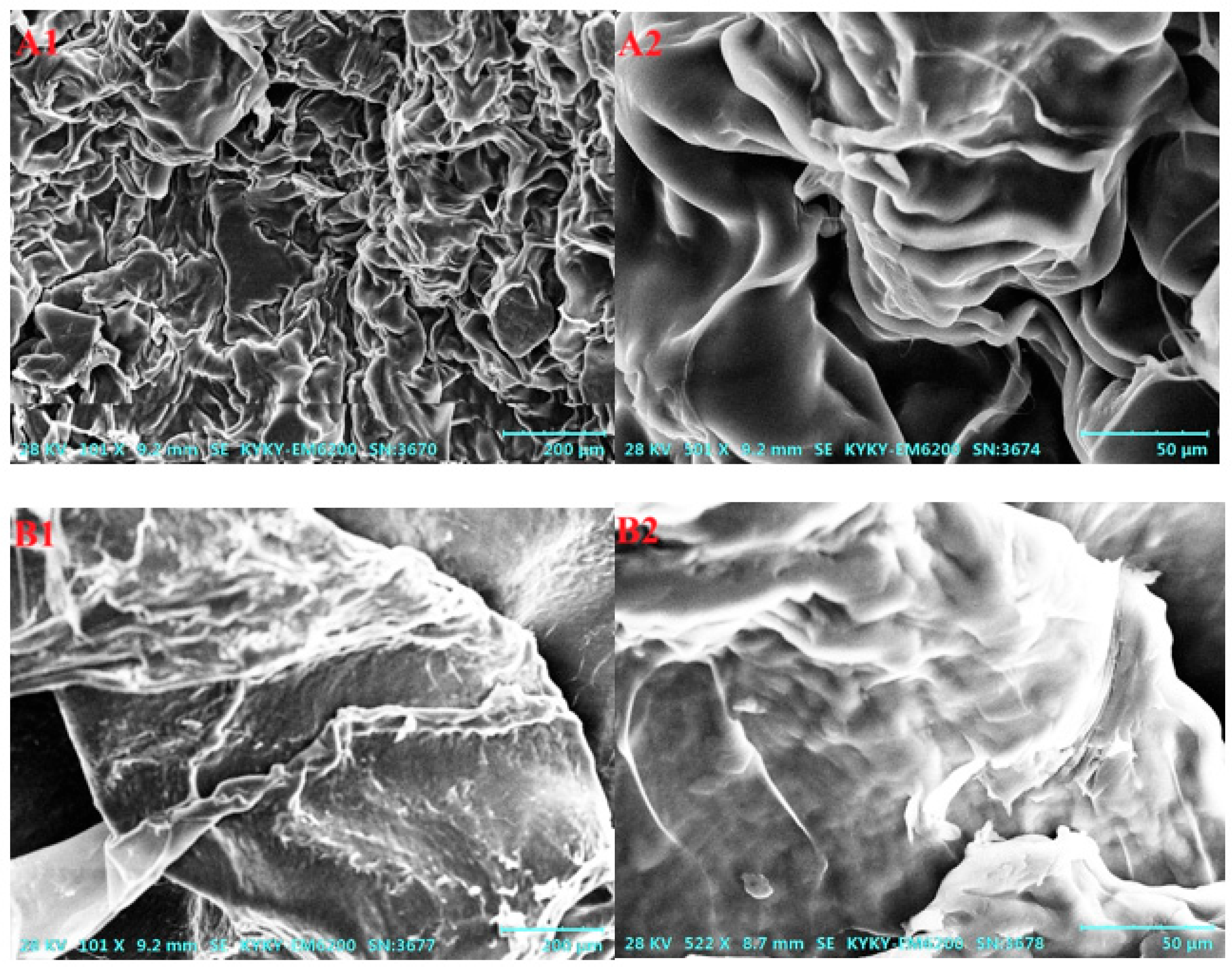
2.5. SEM Analysis
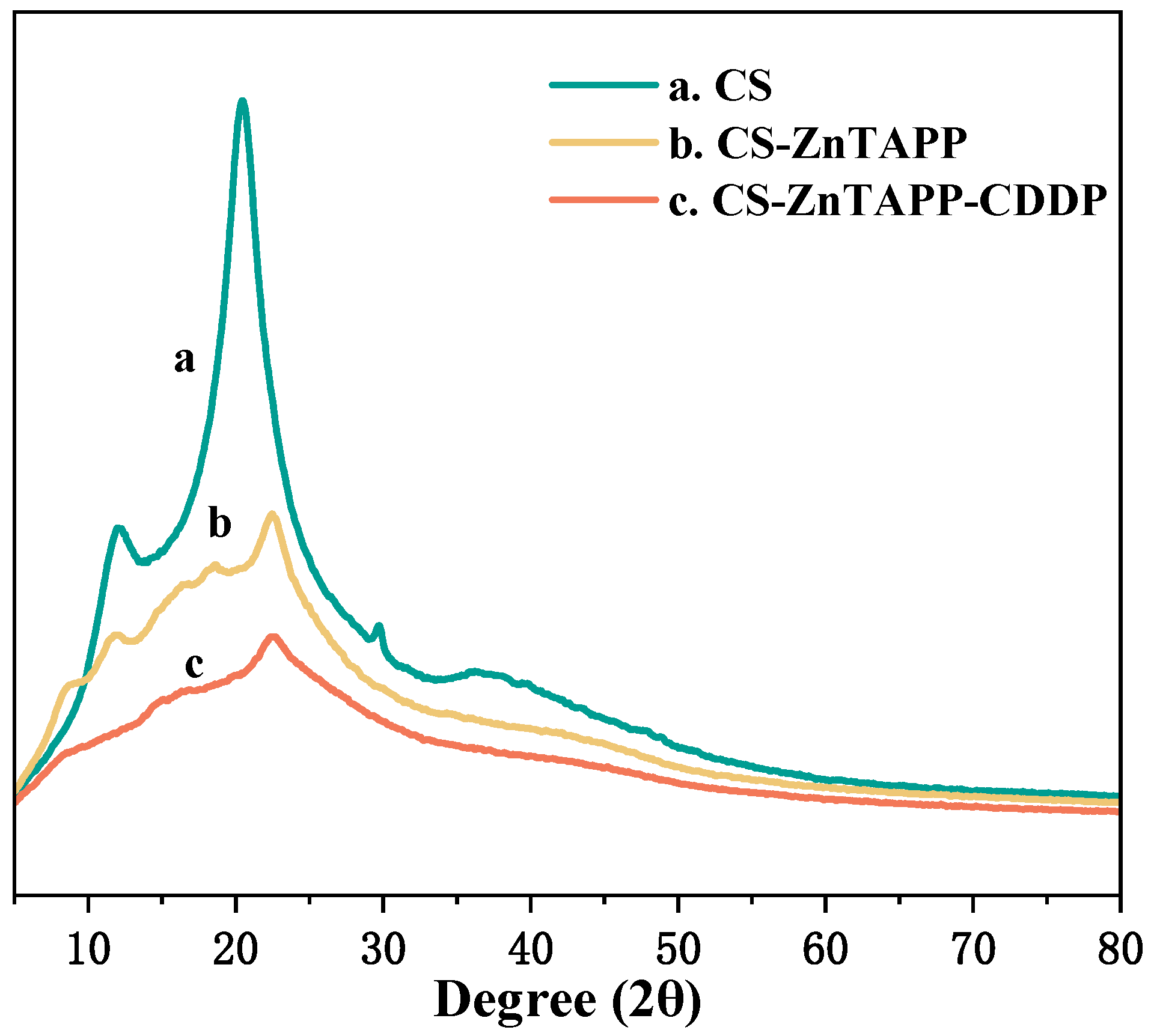
2.6. XRD Analysis
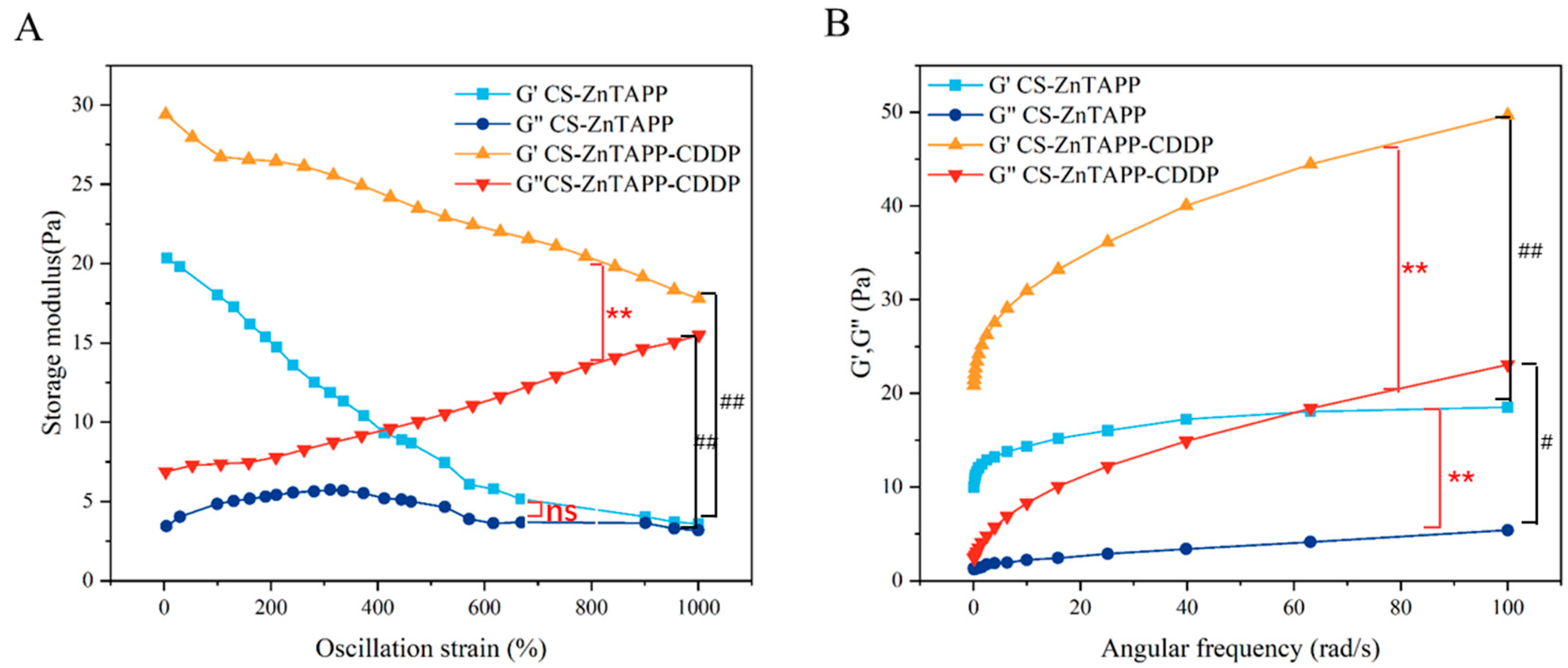
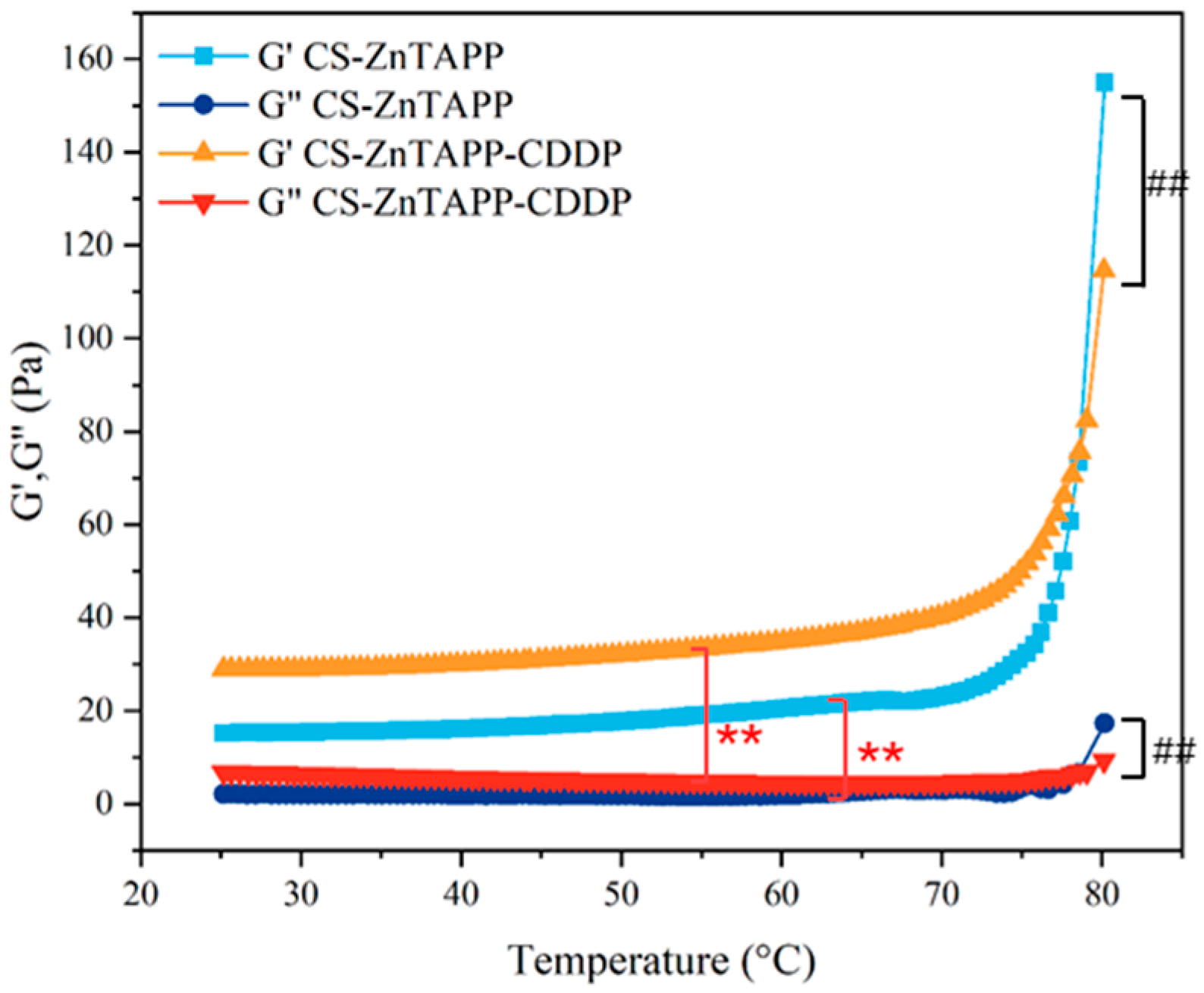
2.7. Rheological Behavior Analysis
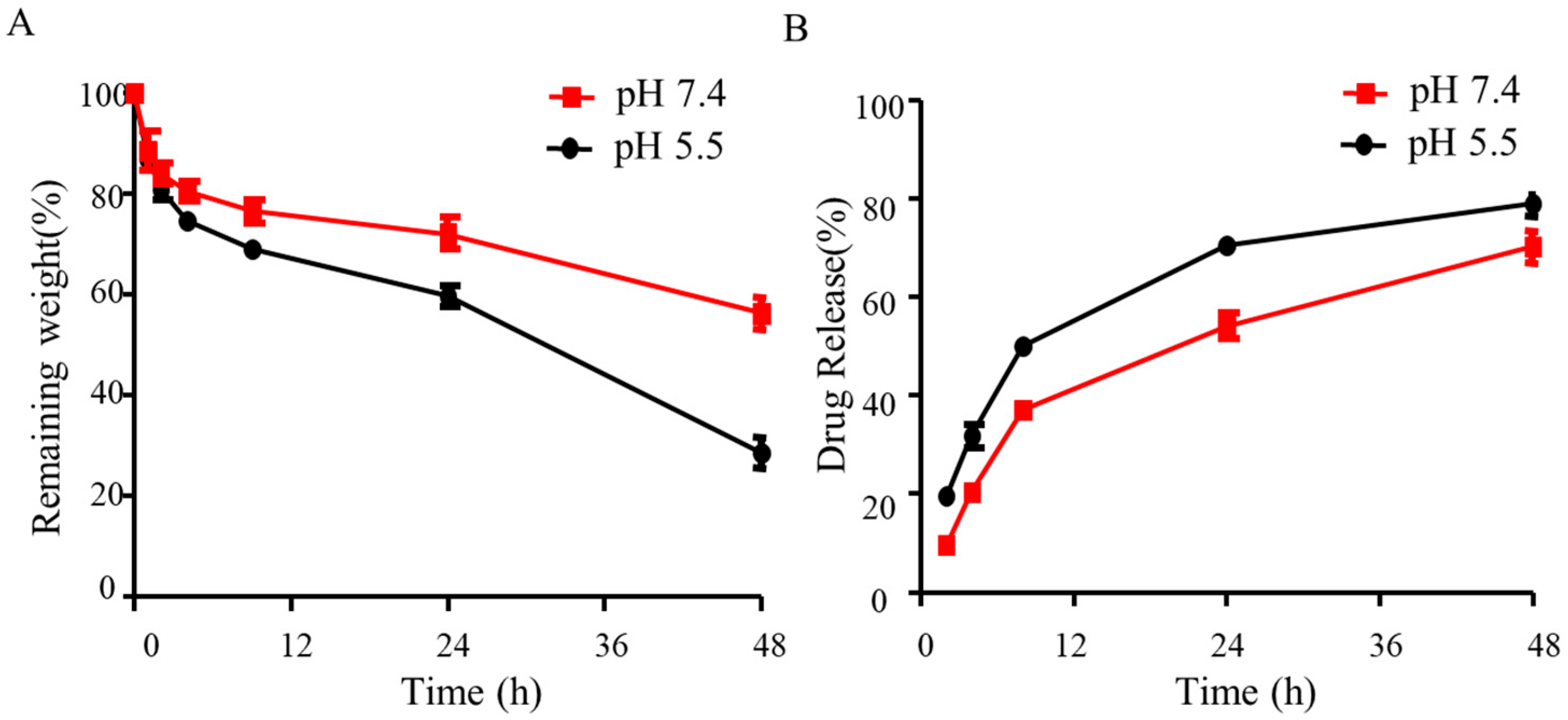
2.8. Degradation Properties of Hydrogels
2.9. Drug Release of Hydrogels
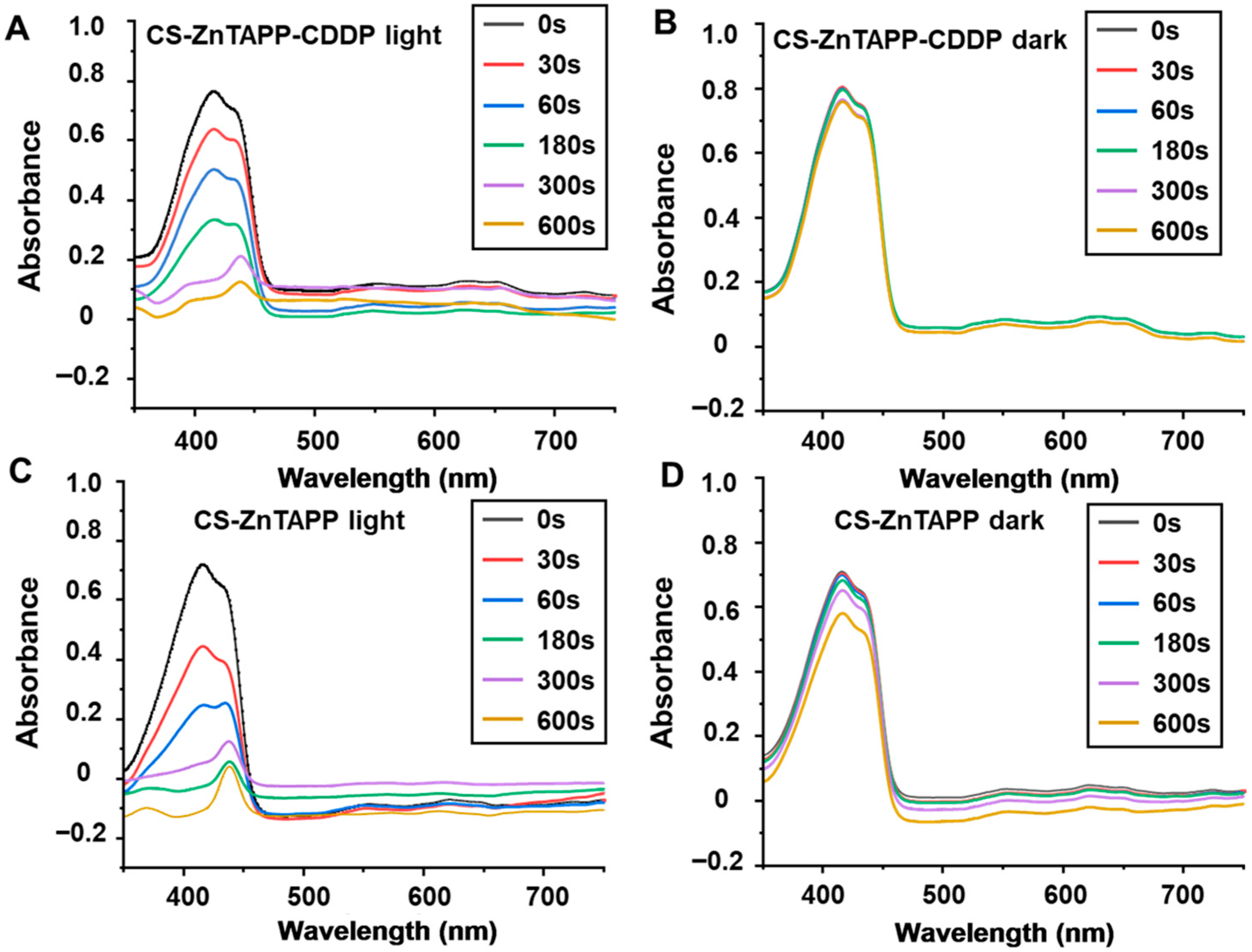
2.10. Singlet Oxygen (1O2) Production of Hydrogels
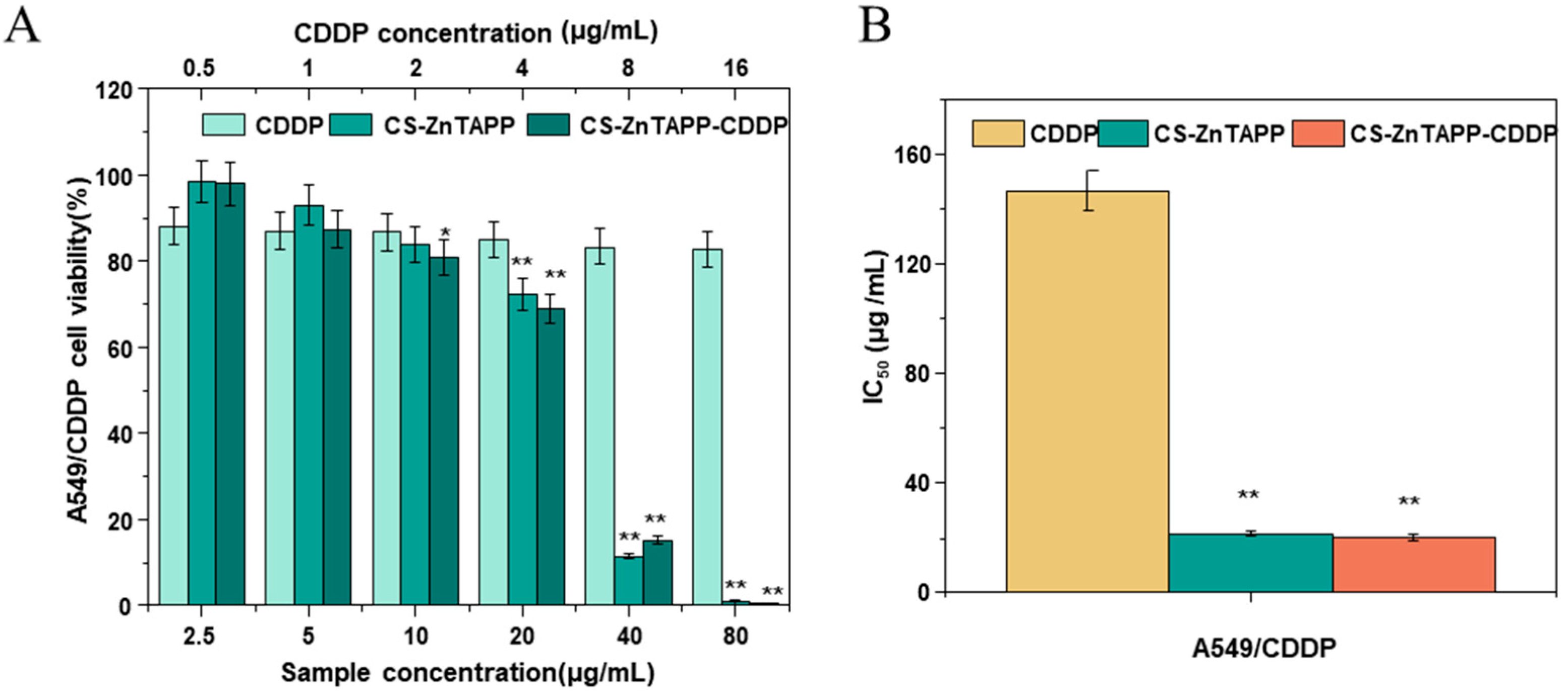
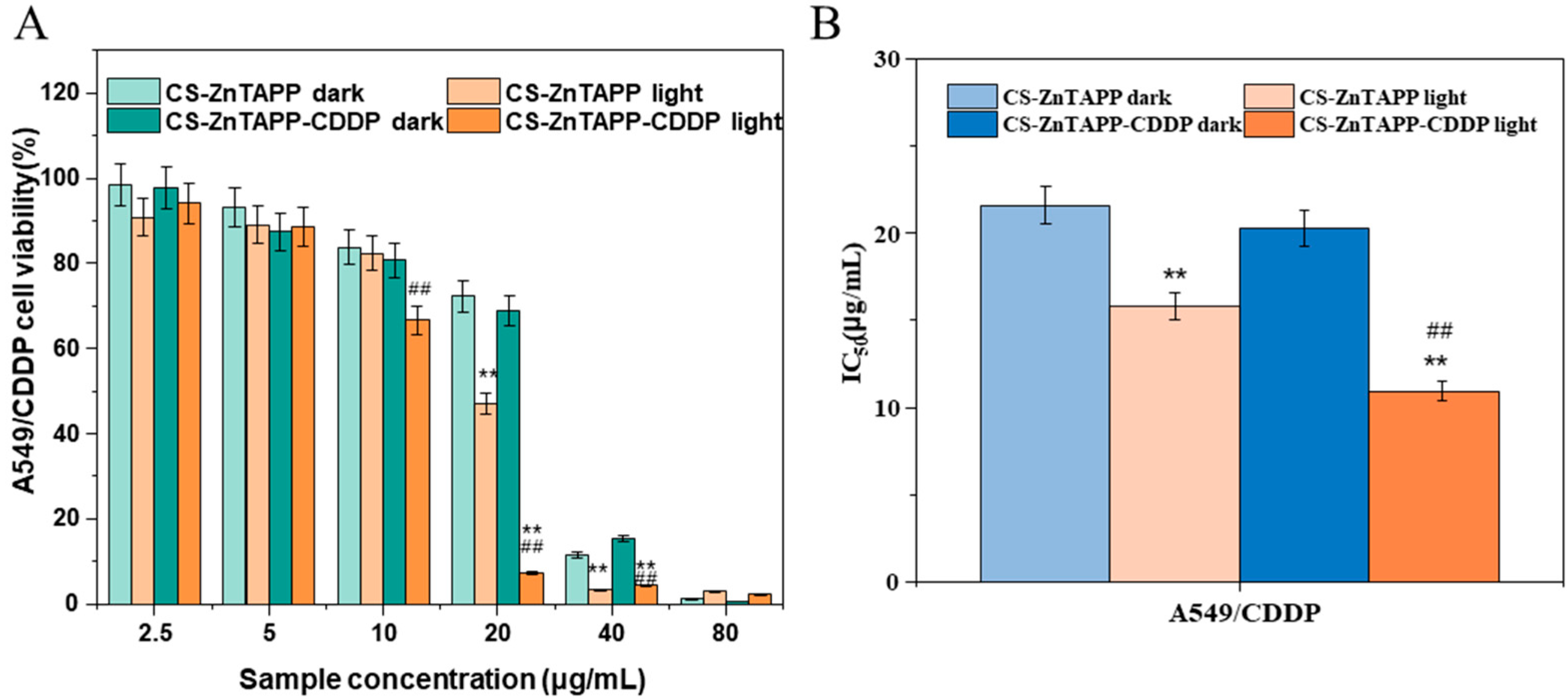
2.11. Anticancer Activity of Hydrogels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of CS-ZnTAPP-CDDP Hydrogel
4.3. Characterization of Hydrogels
4.4. Pt Content Measurement
4.5. ZnTAPP Content Measurement
4.6. Rheological Performance Testing
4.7. Measurement of Free Amino Groups
4.8. Degradation Determination of Hydrogels
4.9. In Vitro CDDP Release
4.10. Singlet Oxygen Generation
4.11. Cytotoxicity Studies
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, J.C.; Li, L.M.; Gao, J.Q. Biomaterials for local drug delivery in central nervous system. Int. J. Pharm. 2019, 560, 92–100. [Google Scholar] [CrossRef]
- Pornpitchanarong, C.; Aye, K.C.; Arunprasert, K.; Opanasopit, P.; Patrojanasophon, P. Computational designed and optimized liposomal curcumin-embedded bifunctional cross-linked hydrogels for wound healing. Gels 2024, 10, 598. [Google Scholar] [CrossRef]
- Ahmed, M.O.; Eman, M.A.E.; Abdelazeem, S.E.; Tamer, M.T.; Mohamed, S.M.E.; Ouyang, X.K.; Abolfazl, H. Advances in stimuli-responsive polymeric hydrogels for anticancer drug delivery: A review. J. Drug Deliv. Sci. Technol. 2024, 102, 106394. [Google Scholar] [CrossRef]
- Marambio, O.G.; Alvarez, L.; Diaz-Chamorro, H.; Sanchez, J.; Martin-Trasancos, R.; Palavecino, C.E.; Pizarro, G.D.C. Photoactive hydrogels as materials for biological applications: Preparation of thermally stable photoactive films. Gels 2025, 11, 663. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Zhang, X.; Shen, S.; Wu, B.; Peng, D.; Yin, J.; Wang, Y. A Succinoglycan-riclin-zinc-phthalocyanine-based composite hydrogel with enhanced photosensitive and antibacterial activity targeting biofilms. Gels 2025, 11, 672. [Google Scholar] [CrossRef] [PubMed]
- Harpreet, K.; Bishmita, G.; Ira, S.; Deepak, K.D.; Mohd, A.A.; Devlina, D.P.; Arindam, P. Hydrogels as a potential biomaterial for multimodal therapeutic applications. Mol. Pharm. 2024, 21, 4827–4848. [Google Scholar] [CrossRef]
- Suhail, M.; An, S.; Kiran, B.; Huang, S.; Wahab, A.; Yang, S.; Kong, X.; Iqbal, M.Z.; Wu, P. Formulation, characterization, anti-bacterial, anti-inflammatory, anti-cancer, and cytotoxicity assessment of collagen/gelatin-based hydrogels as controlled drug release agents. Int. J. Biol. Macromol. 2025, 328, 47653. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, L.; Xu, G.; Wang, M.; Li, J.; He, Z. Low-voltage/temperature double responsive N-isopropylacrylamide based shape-changing double network hydrogel with enhanced mechanical properties for controlled drug release and its mechanism. Mater. Des. 2025, 253, 113912. [Google Scholar] [CrossRef]
- Abdelghany, T.M.; Vukajlovic, N.V.D.; Smith, E.; Wong, J.Z.; Jackson, E.; Hilkens, C.M.U.; Lau, W.M.; Ng, K.W.; Novakovic, K. Engineering and in vitro evaluation of semi-dissolving, hydrogel-forming polymeric microneedles for sustained-release drug delivery. Int. J. Pharm. 2025, 682, 125932. [Google Scholar] [CrossRef]
- Edo, G.I.; Ndudi, W.; Ali, A.B.M.; Yousif, E.; Zainulabdeen, K.; Akpoghelie, P.O.; Isoje, E.F.; Igbuku, U.A.; Opiti, R.A.; Essaghah, A.E.A.; et al. Chitosan: An overview of its properties, solubility, functional technologies, food and health applications. Carbohydr. Res. 2025, 550, 109409. [Google Scholar] [CrossRef] [PubMed]
- Chellathurai, M.S.; Chung, L.Y.; Hilles, A.R.; Sofian, Z.M.; Singha, S.; Ghosal, K.; Mahmood, S. Pharmaceutical chitosan hydrogels: A review on its design and applications. Int. J. Biol. Macromol. 2024, 280, 135775. [Google Scholar] [CrossRef]
- Zhu, H.; Sun, H.; Dai, J.; Hao, J.; Zhou, B. Chitosan-based hydrogels in cancer therapy: Drug and gene delivery, stimuli-responsive carriers, phototherapy and immunotherapy. Int. J. Biol. Macromol. 2024, 282, 137047. [Google Scholar] [CrossRef] [PubMed]
- Mario, C.; Barbara, D.B.; Emilia, M. Hydrogel containing l-valine residues as a platform for cisplatin chemotherapy. Colloids Surf. B 2011, 88, 389–395. [Google Scholar]
- Anirudhan, T.S.; Mohan, M.; Rajeev, M.R. Modified chitosan-hyaluronic acid based hydrogel for the pH-responsive Co-delivery of cisplatin and doxorubicin. Int. J. Biol. Macromol. 2022, 201, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Salahshoori, I.; Ramezani, Z.; Cacciotti, I.; Yazdanbakhsh, A.; Hossain, M.K.; Hassanzadeganroudsari, M. Cisplatin uptake and release assessment from hydrogel synthesized in acidic and neutral medium: An experimental and molecular dynamics simulation study. J. Mol. Liq. 2021, 344, 117890. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Z.; Yang, S.; Zhang, R.; Guo, J.; Yang, D. Energy-storing DNA-based hydrogel remodels tumor microenvironments for laser-free photodynamic immunotherapy. Biomaterials 2024, 309, 122620. [Google Scholar] [CrossRef]
- Yin, W.; Li, P.Y.; Huang, H.H.; Feng, L.; Liu, S.H.; Liu, X.; Bai, F.Q. Porphyrin photosensitizer molecules as effective medicine candidates for photodynamic therapy: Electronic structure information aided design. RSC Adv. 2024, 14, 29368–29383. [Google Scholar] [CrossRef]
- Önal, H.T.; Aydemir, E.; Yetkin, D.; Tutuş, Ö.H.; Ayaz, F. Anti-cancer and immunomodulatory photodynamic therapy application of novel porphyrin derivatives. J. Drug Deliv. Sci. Technol. 2025, 104, 106513. [Google Scholar] [CrossRef]
- Quadrado, R.F.N.; Silvestri, S.; Souza, J.F.; Iglesias, B.A.; Fajardo, A.R. Advances in porphyrins and chlorins associated with polysaccharides and polysaccharides-based materials for biomedical and pharmaceutical applications. Carbohydr. Polym. 2024, 334, 122017. [Google Scholar] [CrossRef]
- Belali, S.; Karimi, A.R.; Hadizadeh, M. Cell-specific and pH- sensitive nanostructure hydrogel based on chitosan as a photosensitizer carrier for selective photodynamic therapy. Int. J. Biol. Macromol. 2018, 110, 437–448. [Google Scholar] [CrossRef]
- Poplăcean, I.C.; Mureșan-Pop, M.; Vasilescu, M.; Simion, A.; Simon, S. Synthesis and structural characterization of new chitosan-thiamine hydrochloride molecular complexes. J. Mol. Struct. 2025, 1321, 140094. [Google Scholar] [CrossRef]
- Giovanelli, L.; Lee, H.L.; Lacaze-Dufaure, C.; Koudia, M.; Clair, S.; Lin, Y.P.; Ksari, Y.; Themlin, J.M.; Abel, M.; Cafolla, A.A. Electronic structure of tetra (4-aminophenyl) porphyrin studied by photoemission, UV–Vis spectroscopy and density functional theory. J. Electron Spectrosc. Relat. Phenom. 2017, 218, 40–45. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, Y.; Han, Q.; Ji, L.; Zhang, H.; Fei, Z.; Wang, Y. Comparison of the physicochemical, rheological, and morphologic properties of chitosan from four insects. Carbohyd. Polym. 2019, 209, 266–275. [Google Scholar] [CrossRef]
- Luo, Q.; Han, Q.; Wang, Y.; Zhang, H.; Fei, Z.; Wang, Y. The thiolated chitosan: Synthesis, gelling and antibacterial capability. Int. J. Biol. Macromol. 2019, 139, 521–530. [Google Scholar] [CrossRef]
- Salehi, M.; Tabarsa, M.; Amraie, M.; Anvari, M.; Rezaei, M.; Smith, B.M. Characterization of rheological and structural properties of a gum from Balangu seeds. Int. J. Biol. Macromol. 2018, 117, 294–300. [Google Scholar] [CrossRef]
- Yang, Y.; Zhuo, Y.; Zhu, C.; Zhang, H.; Wang, Y. Characterization of gelatin- oxidized riclin cryogels and their applications as reusable ice cubes in shrimp preservation. Food Res. Int. 2024, 192, 114766. [Google Scholar] [CrossRef] [PubMed]
- Dehghan-Niri, M.; Vasheghani-Farahani, E.; Eslaminejad, M.B.; Tavakol, M.; Bagheri, F. Physicomechanical, rheological and in vitro cytocompatibility properties of the electron beam irradiated blend hydrogels of tyramine conjugated gum tragacanth and poly (vinyl alcohol). Mater. Sci. Eng. C Mater. 2020, 114, 111073. [Google Scholar] [CrossRef] [PubMed]
- Kopač, T.; Abrami, M.; Grassi, M.; Ručigaj, A.; Krajnc, M. Polysaccharide-based hydrogels crosslink density equation: A rheological and LF-NMR study of polymer-polymer interactions. Carbohyd. Polym. 2022, 277, 118895. [Google Scholar] [CrossRef]
- de Castro, R.; Kandhola, G.; Kim, J.-W.; Moore, Q.C.; Thompson, A.K. Fabrication of Chitosan/PEGDA Bionanocomposites for Enhanced Drug Encapsulation and Release Efficiency. Mol. Pharm. 2023, 20, 5532–5542. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jung, S. Biocompatible and self-recoverable succinoglycan dialdehyde-crosslinked alginate hydrogels for pH-controlled drug delivery. Carbohyd. Polym. 2020, 250, 116934. [Google Scholar] [CrossRef]
- Blebea, N.-M.; Pușcașu, C.; Vlad, R.-A.; Hancu, G. Chitosan-Based Gel Development: Extraction, Gelation Mechanisms, and Biomedical Applications. Gels 2025, 11, 275. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Lu, B.; Liu, X.; Meng, T.; Tan, Y.; Zhu, Y.; Liu, N.; Yuan, H.; Huang, X.; Hu, F. A pH-responsive glycolipid-like nanocarrier for optimising the time-dependent distribution of free chemical drugs in focal cells. Int. J. Pharm. 2017, 522, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Bayat, F.; Karimi, A.R. Design of photodynamic chitosan hydrogels bearing phthalocyanine-colistin conjugate as an antibacterial agent. Int. J. Biol. Macromol. 2019, 129, 927–935. [Google Scholar] [CrossRef]
- Swavey, S.; Yoho, J.; Wogensthal, K.; Bennett, T.L.; Gmeiner, W.H. Water-Soluble Zinc Porphyrin Capable of Light-Induced Photocleavage of DNA: Cell Localization Studies in Drosophila Melanogaster and Light Activated Treatment of Lung Cancer Cells. Eur. J. Inorg. Chem. 2016, 2017, 153–159. [Google Scholar]
- Karami, M.H.; Pourmadadi, M.; Abdouss, M.; Kalaee, M.R.; Moradi, O.; Rahdar, A.; Díez-Pascual, A.M. Novel chitosan/γ-alumina/carbon quantum dot hydrogel nanocarrier for targeted drug delivery. Int. J. Biol. Macromol. 2023, 251, 126280. [Google Scholar] [CrossRef]
- Mao, W.; Liao, Y.; Ma, D. A supramolecular assembly mediated by host-guest interactions for improved chemo-photodynamic combination therapy. Chem. Commun. 2020, 56, 4192–4195. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.; Ren, X.; Jia, R.; Si, L.; Bao, J.; Shi, Y.; Sun, J.; Zhong, Y.; Duan, P.-C.; et al. Microemulsion-Assisted Self-Assembly of Indium Porphyrin Photosensitizers with Enhanced Photodynamic Therapy. ACS Nano 2024, 18, 3161–3172. [Google Scholar] [CrossRef]
- Bayat, F.; Karimi, A.R.; Adimi, T. Design of nanostructure chitosan hydrogels for carrying zinc phthalocyanine as a photosensitizer and difloxacin as an antibacterial agent. Int. J. Biol. Macromol. 2020, 159, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, M.J.; Han, Q.; Fox, K.; Tran, N. Tuning Pluronic Hydrogel Networks: Effects of Vancomycin Loading on Gelation, Rheological Properties, and Micellar Structures. Gels 2025, 11, 688. [Google Scholar] [CrossRef]
- Yan, Y.; Guan, S.; Wang, S.; Xu, J.; Sun, C. Synthesis and characterization of protocatechuic acid grafted carboxymethyl chitosan with oxidized sodium alginate hydrogel through the Schiff’s base reaction. Int. J. Biol. Macromol. 2022, 222, 2581–2593. [Google Scholar] [CrossRef]
- Wang, Y.; Han, Q.; Bai, F.; Luo, Q.; Wu, M.L.; Song, G.; Zhang, H.M.; Wang, Y.Q. The assembly and antitumor activity of lycium barbarum polysaccharide-platinum- based conjugates. J. Inorg. Biochem. 2020, 205, 111001. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Xia, L.; Zheng, W.; Yin, G.-Q.; Yue, T.; Li, X.; Zhang, W.; Zhao, X.-L.; Yang, H.-B. Porphyrin-functionalized coordination star polymers and their potential applications in photodynamic therapy. Polym. Chem. 2019, 10, 6116–6121. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Li, D.; Wang, P.; Yang, Y.; Zhu, D.; Wang, Y. Construction and Activity of Cisplatin-Loaded Chitosan–Zinc Amino-Porphyrin Photosensitizer Hydrogel. Gels 2025, 11, 948. https://doi.org/10.3390/gels11120948
Zhang H, Li D, Wang P, Yang Y, Zhu D, Wang Y. Construction and Activity of Cisplatin-Loaded Chitosan–Zinc Amino-Porphyrin Photosensitizer Hydrogel. Gels. 2025; 11(12):948. https://doi.org/10.3390/gels11120948
Chicago/Turabian StyleZhang, Hongmei, Dongqing Li, Pengge Wang, Yunxia Yang, Daliang Zhu, and Yanqing Wang. 2025. "Construction and Activity of Cisplatin-Loaded Chitosan–Zinc Amino-Porphyrin Photosensitizer Hydrogel" Gels 11, no. 12: 948. https://doi.org/10.3390/gels11120948
APA StyleZhang, H., Li, D., Wang, P., Yang, Y., Zhu, D., & Wang, Y. (2025). Construction and Activity of Cisplatin-Loaded Chitosan–Zinc Amino-Porphyrin Photosensitizer Hydrogel. Gels, 11(12), 948. https://doi.org/10.3390/gels11120948





