Comparative Study of Collagen Gels Extracted from Different Sources
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Collagen Gels Extracted from Different Sources
2.1.1. Physical–Chemical Analysis
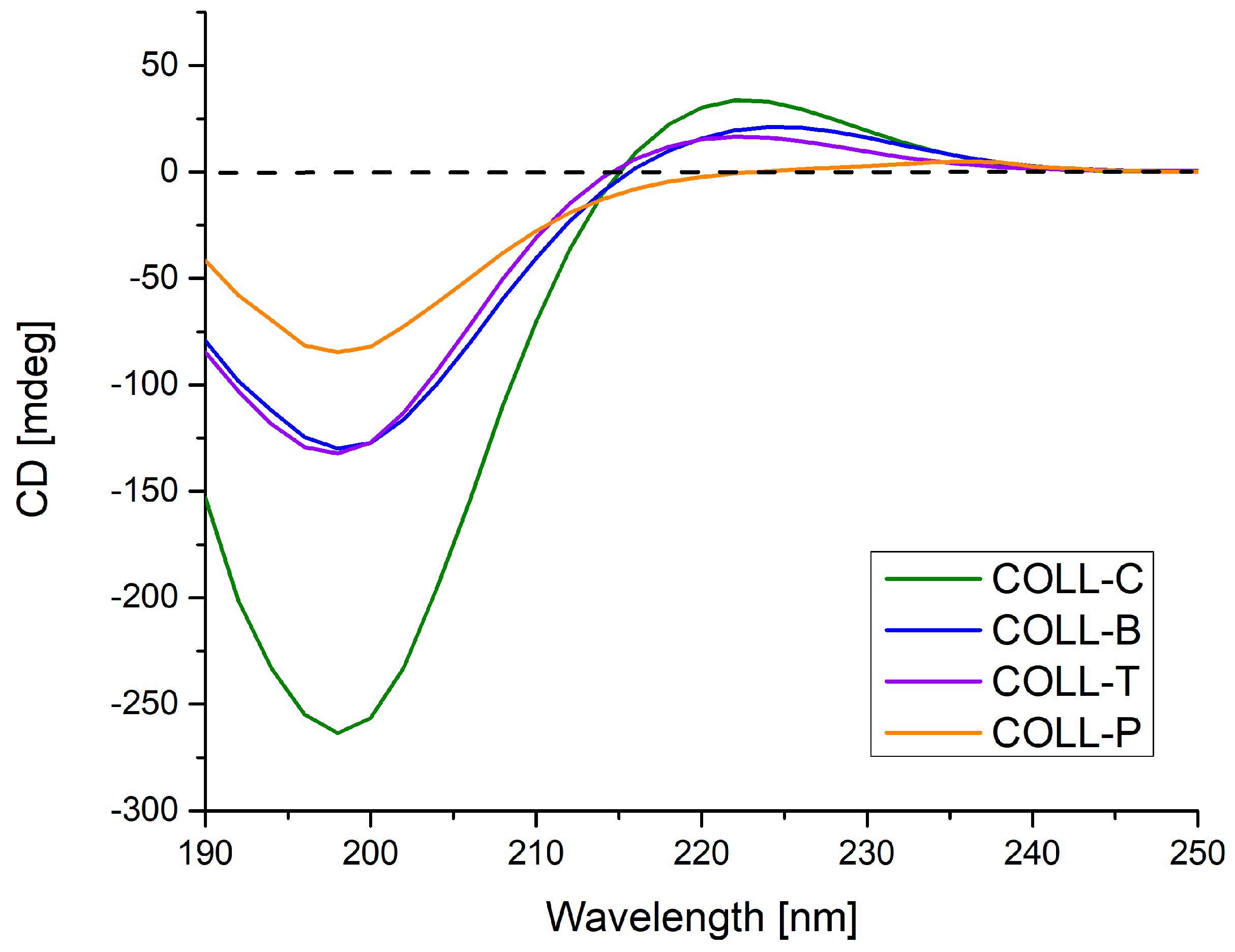
2.1.2. Circular Dichroism Spectroscopy (CD)
2.2. Characterization of Collagen Porous Matrices
2.2.1. Structural Analysis of Collagen Porous Matrices—FT-IR Spectra
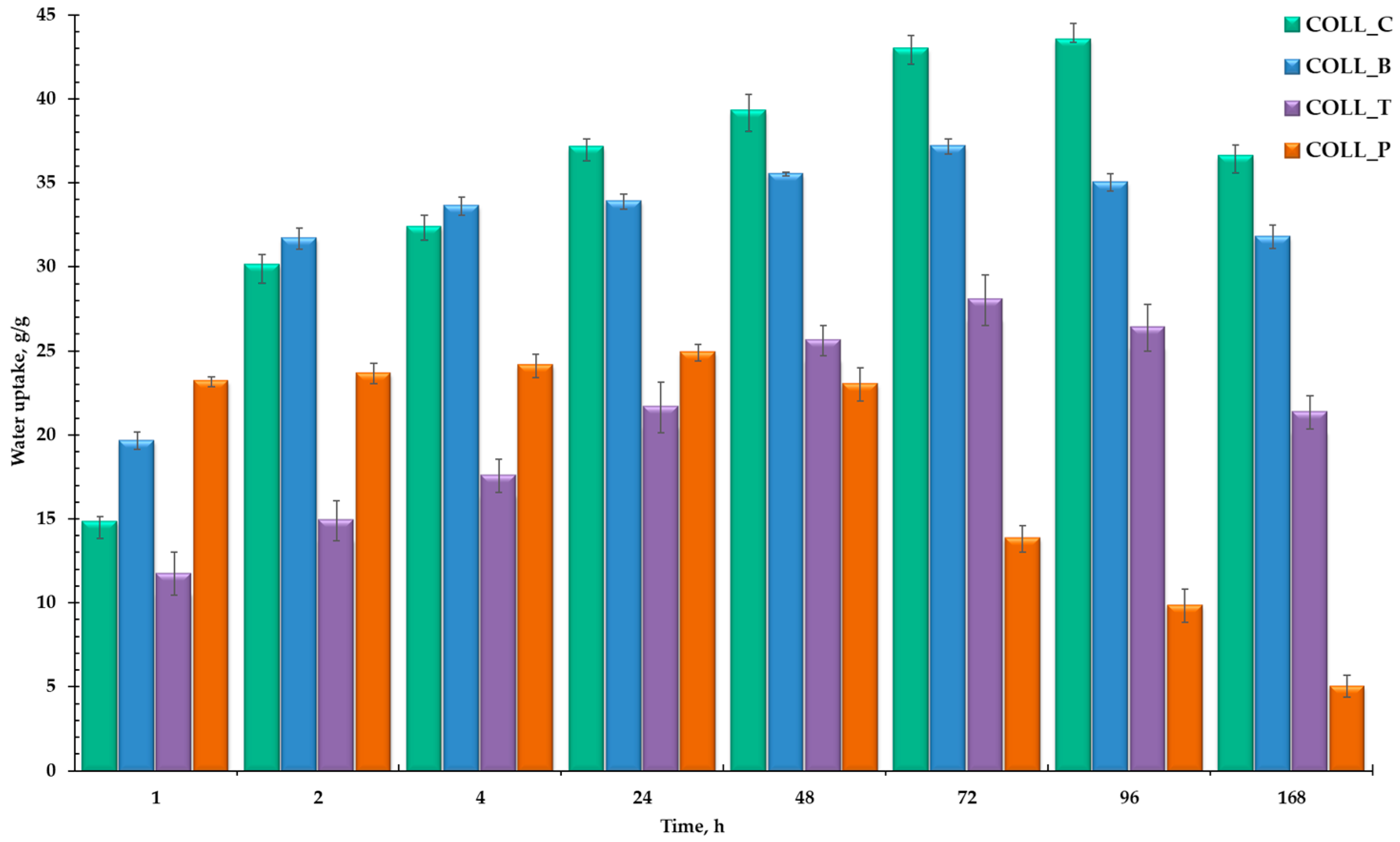
2.2.2. Swelling Behavior of Collagen Porous Matrices
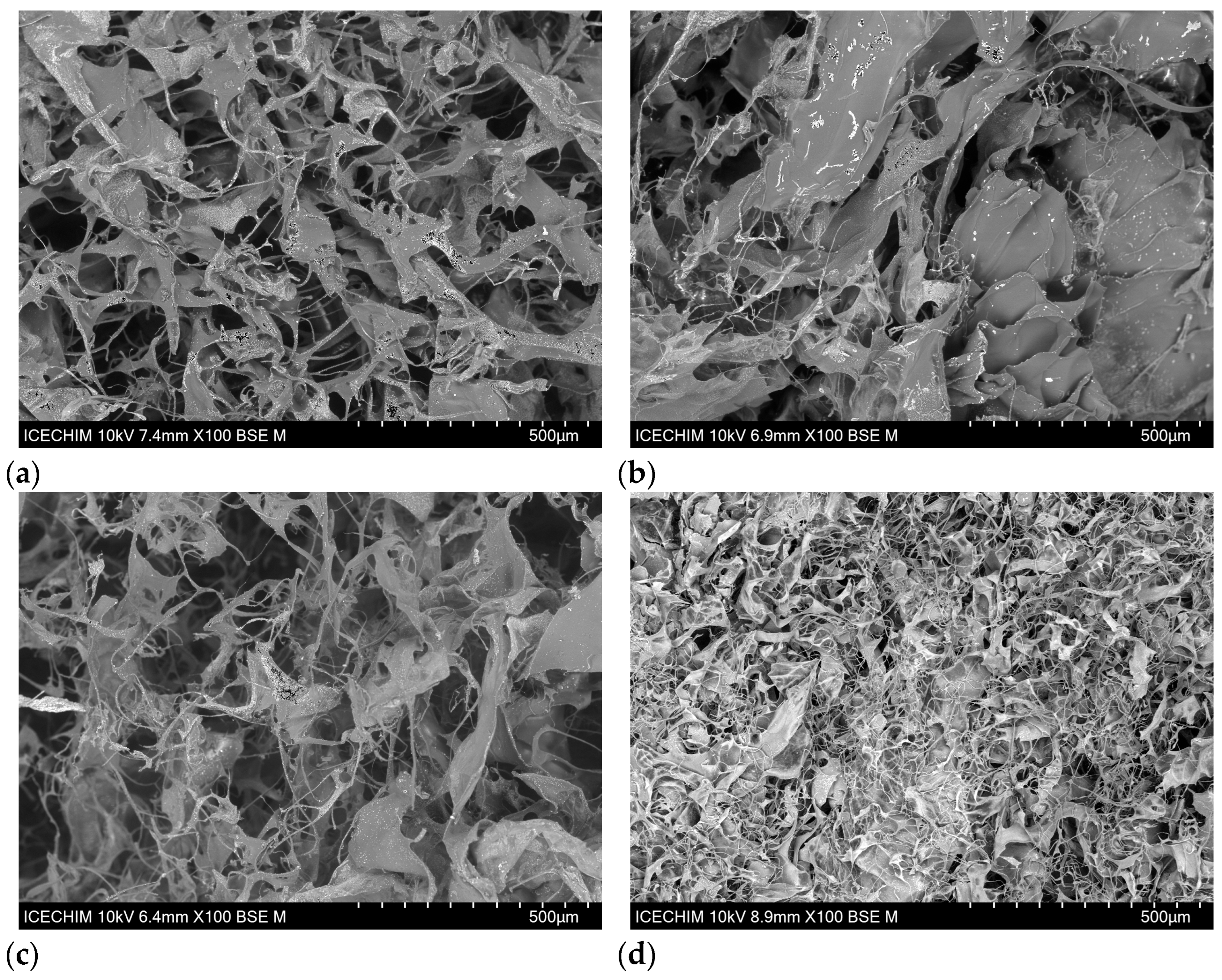
2.2.3. Morphological Analysis of Collagen Porous Matrices
2.2.4. Collagen Sponge Thermal Analysis
2.2.5. Collagen Porous Matrices Microbiological Analysis
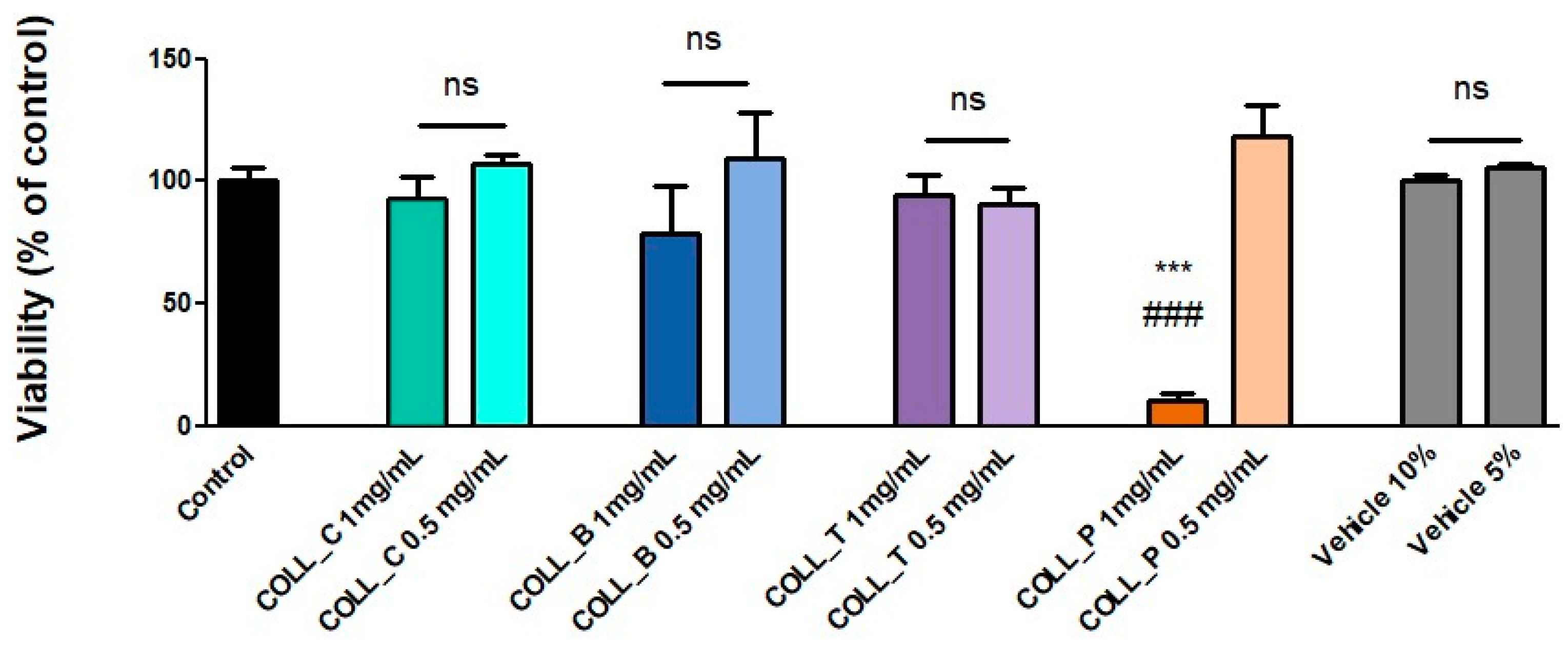
2.2.6. Evaluation of the Biocompatibility of Various Collagen Gels with MG63 Cells
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Extraction of Collagen Gels from Different Sources
4.2.2. Proximate Analysis of Extracted Gels
4.2.3. Structural Analysis of Extracted Gels
4.2.4. Preparation of Collagen Sponges
4.2.5. Structural Analysis of Collagen Porous Matrices
4.2.6. Swelling Behavior of Collagen Porous Matrices
4.2.7. Morphological Analysis of Collagen Porous Matrices
4.2.8. Collagen Sponges Thermal Analysis
4.2.9. Collagen Porous Matrices Microbiological Analysis
4.2.10. Assessment of Biocompatibility
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amirrah, I.N.; Lokanathan, Y.; Zulkiflee, I.; Wee, M.F.M.R.; Motta, A.; Fauzi, M.B. A Comprehensive Review on Collagen Type I Development of Biomaterials for Tissue Engineering: From Biosynthesis to Bioscaffold. Biomedicines 2022, 10, 2307. [Google Scholar] [CrossRef]
- Matinong, A.M.E.; Chisti, Y.; Pickering, K.L.; Haverkamp, R.G. Collagen Extraction from Animal Skin. Biology 2022, 11, 905. [Google Scholar] [CrossRef]
- Grønlien, K.G.; Pedersen, M.E.; Sanden, K.W.; Høst, V.; Karlsen, J.; Tønnesen, H.H. Collagen from Turkey (Meleagris Gallopavo) Tendon: A Promising Sustainable Biomaterial for Pharmaceutical Use. Sustain. Chem. Pharm. 2019, 13, 100166. [Google Scholar] [CrossRef]
- Albu, M.G. Collagen Gels and Matrices for Biomedical Applications: The Obtaining and Characterization of Collagen-Based Biomaterials as Support for Local Release; Lap Lambert Academic Publishing: Saarbrücken, Germany, 2011; ISBN 978-3-8443-3057-1. [Google Scholar]
- El-Fiqi, A.; Lee, J.H.; Lee, E.-J.; Kim, H.-W. Collagen Hydrogels Incorporated with Surface-Aminated Mesoporous Nanobioactive Glass: Improvement of Physicochemical Stability and Mechanical Properties Is Effective for Hard Tissue Engineering. Acta Biomater. 2013, 9, 9508–9521. [Google Scholar] [CrossRef] [PubMed]
- Coman, A.E.; Marin, M.M.; Roșca, A.M.; Albu Kaya, M.G.; Constantinescu, R.R.; Titorencu, I. Marine Resources Gels as Main Ingredient for Wound Healing Biomaterials: Obtaining and Characterization. Gels 2024, 10, 729. [Google Scholar] [CrossRef] [PubMed]
- Salim, N.V.; Madhan, B.; Glattauer, V.; Ramshaw, J.A.M. Comprehensive Review on Collagen Extraction from Food By-Products and Waste as a Value-Added Material. Int. J. Biol. Macromol. 2024, 278, 134374. [Google Scholar] [CrossRef]
- Albu, G.M.; Titorencu, I.; Ghica, M.V. Collagen-Based Drug Delivery Systems for Tissue Engineering. In Biomaterials Applications for Nanomedicine; Pignatello, R., Ed.; InTech: Rijeka, Croatia, 2011; ISBN 978-953-307-661-4. [Google Scholar]
- Zhou, Y.; Li, S.; Wang, D.; Zhao, Y.; Lei, X. Estimation of Type i Collagen Structure Dissolved in Inorganical Acids from Circular Dichroism Spectra. Biosci. J. 2018, 34, 778–789. [Google Scholar] [CrossRef]
- Al Hajj, W.; Salla, M.; Krayem, M.; Khaled, S.; Hassan, H.F.; El Khatib, S. Hydrolyzed Collagen: Exploring Its Applications in the Food and Beverage Industries and Assessing Its Impact on Human Health—A Comprehensive Review. Heliyon 2024, 10, e36433. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Li, R.; Bai, H.; Zhu, Z.; Zhu, L.; Zhu, C.; Che, Z.; Liu, H.; Wang, J.; et al. Collagen-Based Biomaterials for Bone Tissue Engineering. Mater. Des. 2021, 210, 110049. [Google Scholar] [CrossRef]
- Valcarcel, J.; Fraguas, J.; Hermida-Merino, C.; Hermida-Merino, D.; Piñeiro, M.M.; Vázquez, J.A. Production and Physicochemical Characterization of Gelatin and Collagen Hydrolysates from Turbot Skin Waste Generated by Aquaculture Activities. Mar. Drugs 2021, 19, 491. [Google Scholar] [CrossRef] [PubMed]
- Elgadir, M.A.; Mariod, A.A. Gelatin and Chitosan as Meat By-Products and Their Recent Applications. Foods 2022, 12, 60. [Google Scholar] [CrossRef]
- Wierzbicka, A.; Bartniak, M.; Waśko, J.; Kolesińska, B.; Grabarczyk, J.; Bociaga, D. The Impact of Gelatin and Fish Collagen on Alginate Hydrogel Properties: A Comparative Study. Gels 2024, 10, 491. [Google Scholar] [CrossRef] [PubMed]
- Correia, D.M.; Padrão, J.; Rodrigues, L.R.; Dourado, F.; Lanceros-Méndez, S.; Sencadas, V. Thermal and Hydrolytic Degradation of Electrospun Fish Gelatin Membranes. Polym. Test. 2013, 32, 995–1000. [Google Scholar] [CrossRef]
- Padilla, C.; Quero, F.; Pępczyńska, M.; Díaz-Calderon, P.; Acevedo, J.P.; Byres, N.; Blaker, J.J.; MacNaughtan, W.; Williams, H.E.L.; Enrione, J. Understanding the Molecular Conformation and Viscoelasticity of Low Sol-Gel Transition Temperature Gelatin Methacryloyl Suspensions. Int. J. Mol. Sci. 2023, 24, 7489. [Google Scholar] [CrossRef] [PubMed]
- Wiórek, A.; Mazur, P.K.; Żurawska, E.; Krzych, Ł.J. In Vivo Effects of Balanced Crystalloid or Gelatine Infusions on Functional Parameters of Coagulation and Fibrinolysis: A Prospective Randomized Crossover Study. J. Pers. Med. 2022, 12, 909. [Google Scholar] [CrossRef]
- Magramane, S.; Kállai-Szabó, N.; Farkas, D.; Süvegh, K.; Zelkó, R.; Antal, I. Comparative Evaluation of Gelatin and HPMC Inhalation Capsule Shells Exposed to Simulated Humidity Conditions. Pharmaceutics 2025, 17, 877. [Google Scholar] [CrossRef]
- Abuine, R.; Rathnayake, A.U.; Byun, H.-G. Biological Activity of Peptides Purified from Fish Skin Hydrolysates. Fish. Aquat. Sci. 2019, 22, 10. [Google Scholar] [CrossRef]
- Egner, P.; Pavlačková, J.; Sedlaříková, J.; Matošková, L.; Mokrejš, P.; Janalíková, M. Collagen Hydrolysates from Animal By-Products in Topical Cosmetic Formulations. Int. J. Mol. Sci. 2025, 26, 2776. [Google Scholar] [CrossRef]
- Park, S.-H.; Song, T.; Bae, T.S.; Khang, G.; Choi, B.H.; Park, S.R.; Min, B.-H. Comparative Analysis of Collagens Extracted from Different Animal Sources for Application of Cartilage Tissue Engineering. Int. J. Precis. Eng. Manuf. 2012, 13, 2059–2066. [Google Scholar] [CrossRef]
- Pires Figueiredo, M.; Rodríguez-Fernández, S.; Copes, F.; Mantovani, D. Review of Collagen Type I-Based Hydrogels: Focus on Composition-Structure-Properties Relationships. npj Biomed. Innov. 2025, 2, 16. [Google Scholar] [CrossRef]
- Schuetz, T.; Richmond, N.; Harmon, M.E.; Schuetz, J.; Castaneda, L.; Slowinska, K. The Microstructure of Collagen Type I Gel Cross-Linked with Gold Nanoparticles. Colloids Surf. B Biointerfaces 2013, 101, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Cross, V.L.; Zheng, Y.; Won Choi, N.; Verbridge, S.S.; Sutermaster, B.A.; Bonassar, L.J.; Fischbach, C.; Stroock, A.D. Dense Type I Collagen Matrices That Support Cellular Remodeling and Microfabrication for Studies of Tumor Angiogenesis and Vasculogenesis in Vitro. Biomaterials 2010, 31, 8596–8607. [Google Scholar] [CrossRef]
- Maistrenko, L.; Iungin, O.; Pikus, P.; Pokholenko, I.; Gorbatiuk, O.; Moshynets, O.; Okhmat, O.; Kolesnyk, T.; Potters, G.; Mokrousova, O. Collagen Obtained from Leather Production Waste Provides Suitable Gels for Biomedical Applications. Polymers 2022, 14, 4749. [Google Scholar] [CrossRef]
- Kristoffersen, K.A.; Afseth, N.K.; Böcker, U.; Dankel, K.R.; Rønningen, M.A.; Lislelid, A.; Ofstad, R.; Lindberg, D.; Wubshet, S.G. Post-Enzymatic Hydrolysis Heat Treatment as an Essential Unit Operation for Collagen Solubilization from Poultry by-Products. Food Chem. 2022, 382, 132201. [Google Scholar] [CrossRef]
- Wu, K.; Li, Y.; Chen, J. Effect of pH on the Structure, Functional Properties and Rheological Properties of Collagen from Greenfin Horse-Faced Filefish (Thamnaconus Septentrionalis) Skin. Mar. Drugs 2024, 22, 45. [Google Scholar] [CrossRef]
- Holmes, R.; Kirk, S.; Tronci, G.; Yang, X.; Wood, D. Influence of Telopeptides on the Structural and Physical Properties of Polymeric and Monomeric Acid-Soluble Type I Collagen. Mater. Sci. Eng. C 2017, 77, 823–827. [Google Scholar] [CrossRef]
- Feng, Y.; Melacini, G.; Taulane, J.P.; Goodman, M. Acetyl-Terminated and Template-Assembled Collagen-Based Polypeptides Composed of Gly-Pro-Hyp Sequences. 2. Synthesis and Conformational Analysis by Circular Dichroism, Ultraviolet Absorbance, and Optical Rotation. J. Am. Chem. Soc. 1996, 118, 10351–10358. [Google Scholar] [CrossRef]
- Tifany, M.L.; Krimm, S. Effwct of Temperature on the Circular Dichroism Spectra of Polypeptides in the Extended State. Biopolymers 1972, 11, 2309–2316. [Google Scholar] [CrossRef] [PubMed]
- Barth, D.; Milbradt, A.G.; Renner, C.; Moroder, L. A (4R)- or a (4S)-Fluoroproline Residue in Position Xaa of the (Xaa-Yaa-Gly) Collagen Repeat Severely Affects Triple-Helix Formation. ChemBioChem 2004, 5, 79–86. [Google Scholar] [CrossRef]
- Ghani, A.; Okada, M.; Sun, B.; Zhang, X.; Higuchi, I.; Takagi, Y. Comparative Study of the Gel-Forming Ability of Type I Collagens Extracted from Different Organs and Fish Species. Gels 2025, 11, 533. [Google Scholar] [CrossRef]
- Yue, C.; Ding, C.; Xu, M.; Hu, M.; Zhang, R. Self-Assembly Behavior of Collagen and Its Composite Materials: Preparation, Characterizations, and Biomedical Engineering and Allied Applications. Gels 2024, 10, 642. [Google Scholar] [CrossRef]
- Gopal, R.; Park, J.S.; Seo, C.H.; Park, Y. Applications of Circular Dichroism for Structural Analysis of Gelatin and Antimicrobial Peptides. Int. J. Mol. Sci. 2012, 13, 3229–3244. [Google Scholar] [CrossRef]
- Usha, R.; Ramasami, T. Role of Solvents in Stability of Collagen. J. Therm. Anal. Calorim. 2008, 93, 541–545. [Google Scholar] [CrossRef]
- Tronci, G.; Grant, C.A.; Thomson, N.H.; Russell, S.J.; Wood, D.J. Multi-Scale Mechanical Characterization of Highly Swollen Photo-Activated Collagen Hydrogels. J. R. Soc. Interface. 2015, 12, 20141079. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Melacini, G.; Taulane, J.P.; Goodman, M. Collagen-Based Structures Containing the Peptoid Residue N-Isobutylglycine (Nleu): Synthesis and Biophysical Studies of Gly-Pro-Nleu Sequences by Circular Dichroism, Ultraviolet Absorbance, and Optical Rotation. Biopolymers 1998, 39, 859–872. [Google Scholar] [CrossRef]
- Jenness, D.D.; Sprecher, C.; Johnson, W.C. Circular Dichroism of Collagen, Gelatin, and Poly(Proline) II in the Vacuum Ultraviolet. Biopolymers 1976, 15, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Jafari, H.; Lista, A.; Siekapen, M.M.; Ghaffari-Bohlouli, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Fish Collagen: Extraction, Characterization, and Applications for Biomaterials Engineering. Polymers 2020, 12, 2230. [Google Scholar] [CrossRef]
- Akita, M.; Nishikawa, Y.; Shigenobu, Y.; Ambe, D.; Morita, T.; Morioka, K.; Adachi, K. Correlation of Proline, Hydroxyproline and Serine Content, Denaturation Temperature and Circular Dichroism Analysis of Type I Collagen with the Physiological Temperature of Marine Teleosts. Food Chem. 2020, 329, 126775. [Google Scholar] [CrossRef]
- Fujii, K.K.; Taga, Y.; Takagi, Y.K.; Masuda, R.; Hattori, S.; Koide, T. The Thermal Stability of the Collagen Triple Helix Is Tuned According to the Environmental Temperature. Int. J. Mol. Sci. 2022, 23, 2040. [Google Scholar] [CrossRef]
- Rajabimashhadi, Z.; Gallo, N.; Salvatore, L.; Lionetto, F. Collagen Derived from Fish Industry Waste: Progresses and Challenges. Polymers 2023, 15, 544. [Google Scholar] [CrossRef]
- Albu, M.G.; Ferdes, M.; Kaya, D.A.; Ghica, M.V.; Titorencu, I.; Popa, L.; Albu, L. Collagen Wound Dressings with Anti-Inflammatory Activity. Mol. Cryst. Liq. Cryst. 2012, 555, 271–279. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Li, Y.; Yang, Z.; Jin, H. Physicochemical Properties of Collagen from Acaudina Molpadioides and Its Protective Effects against H2O2-Induced Injury in RAW264.7 Cells. Mar. Drugs 2020, 18, 370. [Google Scholar] [CrossRef]
- Lamers, D.L.; Rigueto, C.V.T.; Krein, D.D.C.; Loss, R.A.; Dettmer, A.; Gutterres, M. Sequential Extraction and Characterization of Gelatin from Turkey (Meleagris Gallopavo) Feet. Polym. Bull. 2024, 81, 12151–12167. [Google Scholar] [CrossRef]
- Wang, Y.; Song, L.; Guo, C.; Ji, R. Proteomic Identification and Characterization of Collagen from Bactrian Camel (Camelus Bactrianus) Hoof. Foods 2023, 12, 3303. [Google Scholar] [CrossRef] [PubMed]
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Muhammad, N.; Safi, S.Z.; Rahim, A.; Rizvi, S.A.A.; Rehman, I.U. FTIR Analysis of Natural and Synthetic Collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- Nashchekina, Y.A.; Starostina, A.A.; Trusova, N.A.; Sirotkina, M.Y.; Lihachev, A.I.; Nashchekin, A.V. Molecular and Fibrillar Structure Collagen Analysis by FTIR Spectroscopy. J. Phys. Conf. Ser. 2020, 1697, 012053. [Google Scholar] [CrossRef]
- Stani, C.; Vaccari, L.; Mitri, E.; Birarda, G. FTIR Investigation of the Secondary Structure of Type I Collagen: New Insight into the Amide III Band. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 118006. [Google Scholar] [CrossRef]
- Tudoroiu, E.-E.; Albu Kaya, M.G.; Titorencu, I.; Dinu-Pîrvu, C.E.; Marin, M.M.; Roșca, A.-M.; Popa, L.; Anuța, V.; Antoniac, A.; Chelaru, C.; et al. Design and Evaluation of New Wound Dressings Based on Collagen-Cellulose Derivatives. Mater. Des. 2023, 236, 112469. [Google Scholar] [CrossRef]
- Koochakzaei, A.; Ahmadi, H.; Mallakpour, S. An experimental comparative study of the effect of skin type on the stability of vegetable leather under acidic condition. J. Am. Leather Chem. Assoc. 2018, 113, 345–351. [Google Scholar]
- Tronci, G.; Doyle, A.; Russell, S.J.; Wood, D.J. Triple-Helical Collagen Hydrogels via Covalent Aromatic Functionalisation with 1,3-Phenylenediacetic Acid. J. Mater. Chem. B 2013, 1, 5478. [Google Scholar] [CrossRef]
- Ahmad, T.; Kumar, Y.; Muzaddadi, A.U.; Kumar, V.; Singhal, S.; Kumar, D.; Talukder, S.; Chand, S.; Devadason, I.P.; Biswas, A.K. Extraction and Characterization of Collagen Hydrolysate from Buffalo (Bubalus Bubalis) Skin Including Its Antioxidant Properties and Antiarthritic Effect. Waste Biomass Valor. 2024, 15, 4159–4173. [Google Scholar] [CrossRef]
- Luss, A.L.; Bobrova, M.M.; Kulikov, P.P.; Keskinov, A.A. Collagen-Based Scaffolds for Volumetric Muscle Loss Regeneration. Polymers 2024, 16, 3429. [Google Scholar] [CrossRef]
- Michalska-Sionkowska, M.; Warżyńska, O.; Kaczmarek-Szczepańska, B.; Łukowicz, K.; Osyczka, A.M.; Walczak, M. Preparation and Characterization of Fish Skin Collagen Material Modified with β-Glucan as Potential Wound Dressing. Materials 2021, 14, 1322. [Google Scholar] [CrossRef]
- Hannink, G.; Arts, J.J.C. Bioresorbability, Porosity and Mechanical Strength of Bone Substitutes: What Is Optimal for Bone Regeneration? Injury 2011, 42, S22–S25. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, T.; Tao, M.; Wang, Y.; Yao, X.; Zhu, C.; Xin, F.; Jiang, M. Development of Recombinant Human Collagen-Based Porous Scaffolds for Skin Tissue Engineering: Enhanced Mechanical Strength and Biocompatibility. Polymers 2025, 17, 303. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, N.; Khalili, A.; Sachlos, T.; Rezai, P. Fabrication of Porous Collagen Scaffolds Containing Embedded Channels with Collagen Membrane Linings. Micromachines 2024, 15, 1031. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhong, Y.; He, X.; Mai, S. A Collagen Membrane Pretreated with Citrate Promotes Collagen Mineralization and Bone Regeneration. J. Funct. Biomater. 2025, 16, 261. [Google Scholar] [CrossRef] [PubMed]
- Iafisco, M.; Foltran, I.; Sabbatini, S.; Tosi, G.; Roveri, N. Electrospun Nanostructured Fibers of Collagen-Biomimetic Apatite on Titanium Alloy. Bioinorg. Chem. Appl. 2012, 2012, 123953. [Google Scholar] [CrossRef]
- Yudaev, P.; Mezhuev, Y.; Chistyakov, E. Nanoparticle-Containing Wound Dressing: Antimicrobial and Healing Effects. Gels 2022, 8, 329. [Google Scholar] [CrossRef]
- Walczak, M.; Michalska-Sionkowska, M.; Kaczmarek, B.; Sionkowska, A. Surface and Antibacterial Properties of Thin Films Based on Collagen and Thymol. Mater. Today Commun. 2020, 22, 100949. [Google Scholar] [CrossRef]
- Ennaas, N.; Hammami, R.; Gomaa, A.; Bédard, F.; Biron, É.; Subirade, M.; Beaulieu, L.; Fliss, I. Collagencin, an Antibacterial Peptide from Fish Collagen: Activity, Structure and Interaction Dynamics with Membrane. Biochem. Biophys. Res. Commun. 2016, 473, 642–647. [Google Scholar] [CrossRef]
- European Pharmacopoeia. Microbiological Quality of Non-Sterile Pharmaceutical Preparations and Substances for Pharmaceutical Use. In European Directorate for the Quality of Medicines and HealthCare (EDQM), 11th ed.; Council of Europe: Strasbourg, France, 2023; Chapter 5.1.4. [Google Scholar]
- Tidswell, E.C.; Tirumalai, R.S.; Gross, D.D. Clarifications on the Intended Use of USP Microbiological Examination of Nonsterile Products: Microbial Enumeration Tests. PDA J. Pharm. Sci. Technol. 2024, 78, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Tyski, S.; Burza, M.; Laudy, A.E. Microbiological Contamination of Medicinal Products—Is It a Significant Problem? Pharmaceuticals 2025, 18, 946. [Google Scholar] [CrossRef] [PubMed]
- SR EN ISO 4684:2006; Leather and Leather Products—Determination of Volatile Matter Content. Romanian Standards Association (ASRO): Bucharest, Romania, 2006.
- SR EN ISO 4047:2002; Leather—Determination of Sulphated Total Ash and Sulphated Water-Soluble Ash. Romanian Standards Association (ASRO): Bucharest, Romania, 2002.
- SR ISO 5397:1996; Leather—Determination of Fat Content. Romanian Standards Association (ASRO): Bucharest, Romania, 1996.
- SR EN ISO 4045:2018; Leather—Chemical Tests—Determination of pH and Difference Figure. Romanian Standards Association (ASRO): Bucharest, Romania, 2018.
- Hulankova, R. Methods for Determination of Antimicrobial Activity of Essential Oils In Vitro—A Review. Plants 2024, 13, 2784. [Google Scholar] [CrossRef] [PubMed]






| Sample * | Dry Substance Content **, % | Total Nitrogen **, % | Protein Content **, ***, % | pH **, pH Units |
|---|---|---|---|---|
| COLL_C | 1.90 | 0.32 | 1.80 | 2 |
| COLL_B | 1.65 | 0.27 | 1.52 | 4.2 |
| COLL_T | 1.60 | 0.27 | 1.52 | 3.0 |
| COLL_P | 1.15 | 0.18 | 1.13 | 3.0 |
| Sample | Mass Loss, % | Residual Mass, % | Tmax (DTG), °C | ||
|---|---|---|---|---|---|
| 25–100, °C | 100–300, °C | 300–500, °C | |||
| Gel COLL_C | 8.51 | 11.97 | 45.46 | 34.06 | 322 |
| Gel COLL_B | 8.43 | 16.38 | 48.64 | 26.55 | 315 |
| Gel_COLL_T | 6.95 | 15.23 | 49.72 | 28.10 | 323 |
| Gel_COLL_P | 5.80 | 28.86 | 33.78 | 31.56 | 309 |
| Sample | TAMC * (CFU/g) | TYMC ** (CFU/g) | Detection of E. coli | Detection of S. aureus | Detection of P. aeruginosa |
|---|---|---|---|---|---|
| COLL_C | 1.9 × 101 | 1.2 × 101 | Absent | Absent | Absent |
| COLL_B | 2.2 × 101 | 1.2 × 101 | Absent | Absent | Absent |
| COLL_T | 2.4 × 101 | 1.5 × 101 | Absent | Absent | Absent |
| COLL_P | 1.1 × 101 | 1 × 101 | Absent | Absent | Absent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coman, A.E.; Marin, M.M.; Rosca, A.M.; Tutuianu, R.; Albu Kaya, M.G.; Ionita, A.; Constantinescu, R.R.; Titorencu, I. Comparative Study of Collagen Gels Extracted from Different Sources. Gels 2025, 11, 879. https://doi.org/10.3390/gels11110879
Coman AE, Marin MM, Rosca AM, Tutuianu R, Albu Kaya MG, Ionita A, Constantinescu RR, Titorencu I. Comparative Study of Collagen Gels Extracted from Different Sources. Gels. 2025; 11(11):879. https://doi.org/10.3390/gels11110879
Chicago/Turabian StyleComan, Alina Elena, Minodora Maria Marin, Ana Maria Rosca, Raluca Tutuianu, Madalina Georgiana Albu Kaya, Andreea Ionita, Rodica Roxana Constantinescu, and Irina Titorencu. 2025. "Comparative Study of Collagen Gels Extracted from Different Sources" Gels 11, no. 11: 879. https://doi.org/10.3390/gels11110879
APA StyleComan, A. E., Marin, M. M., Rosca, A. M., Tutuianu, R., Albu Kaya, M. G., Ionita, A., Constantinescu, R. R., & Titorencu, I. (2025). Comparative Study of Collagen Gels Extracted from Different Sources. Gels, 11(11), 879. https://doi.org/10.3390/gels11110879







