Dynamic Hydrogels in Breast Tumor Models
Abstract
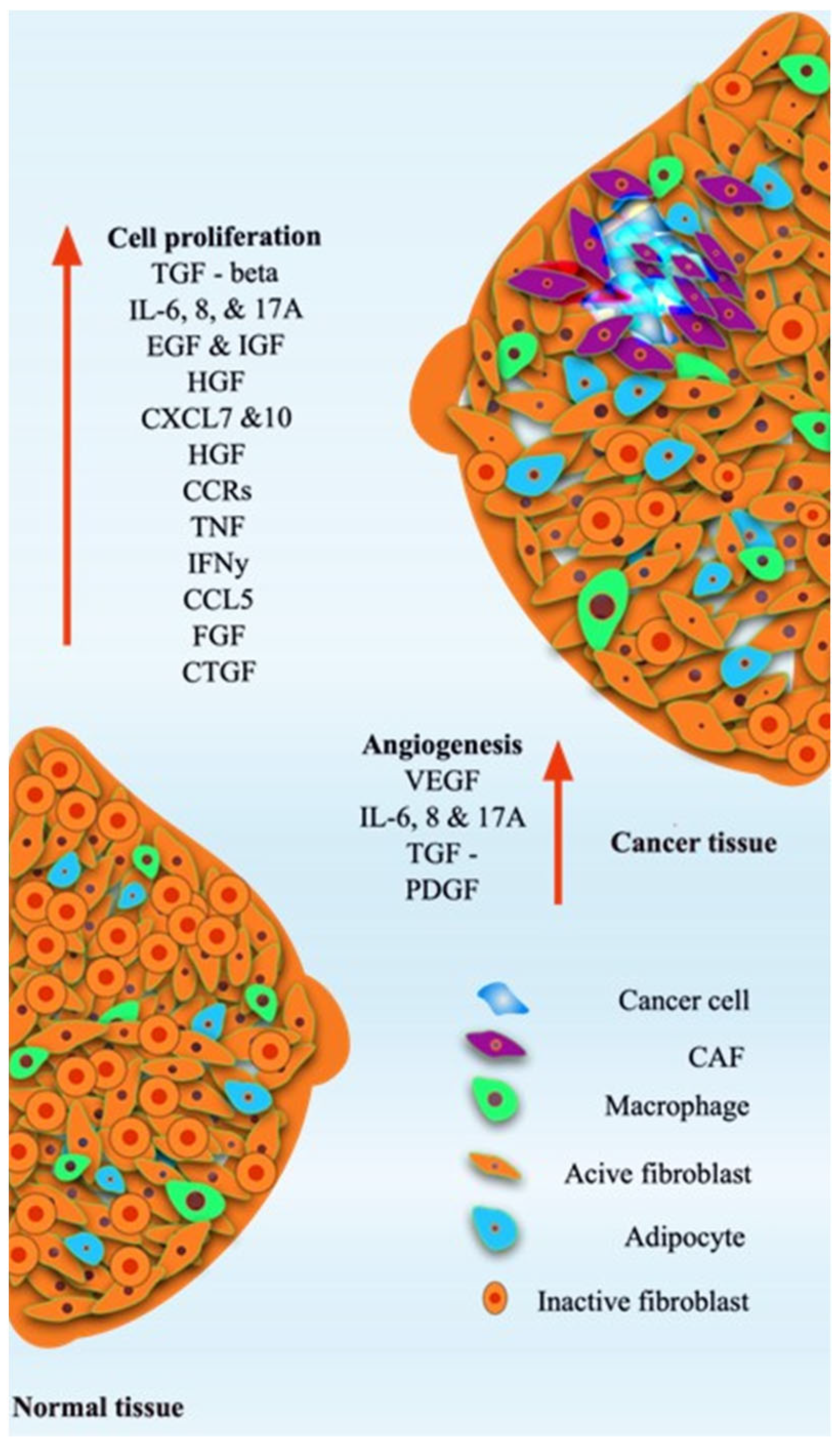
1. Introduction
2. Breast Tumor Models
| Hydrogel System | Material/Composition | Key Features | Applications in Breast Tumor Models | Limitations | References |
|---|---|---|---|---|---|
| Matrigel | Basement membrane extract (laminin, collagen IV, growth factors) | Mimics ECM, promotes cell adhesion, supports 3D organoid formation | Breast cancer spheroids/organoids, invasion studies, drug screening | Batch variability, animal-derived, poorly defined composition, limited mechanical tunability | [19,20,21] |
| Collagen-based hydrogels | Type I/III collagen | Biocompatible, fibrillar network, tunable stiffness | 3D breast tumor culture, migration/invasion assays, angiogenesis studies | Limited long-term stability, low reproducibility due to source variation | [20,22] |
| Alginate | Polysaccharide from algae | Tunable stiffness, ionic crosslinking, low immunogenicity | 3D breast spheroids, encapsulation of tumor and stromal cells, drug delivery studies | Poor cell adhesion unless modified, limited bioactive signals | [21,23,24] |
| Gelatin/GelMA (gelatin methacryloyl) | Denatured collagen, photopolymerizable | Cell-adhesive, tunable stiffness, light-crosslinkable | 3D breast tumor spheroids, co-culture with fibroblasts/endothelial cells, drug response studies | Requires UV or photo-initiator, mechanical properties may differ from native ECM | [25,26] |
| Hyaluronic acid (HA) hydrogels | HA, often crosslinked | Mimics tumor ECM, supports proliferation/migration, interacts with CD44 | Breast cancer invasion, CSC enrichment, drug testing | Mechanical properties may be limited; crosslinking can alter bioactivity | [27,28] |
| PEG-based hydrogels (polyethylene glycol) | Synthetic polymer, often functionalized | Chemically defined, tunable stiffness, degradable linkers | 3D breast cancer culture, controlled drug delivery, mechanotransduction studies | Bio-inert without modification; requires functionalization for cell adhesion | [29,30] |
| Fibrin hydrogels | Fibrinogen + thrombin | Supports angiogenesis, tumor–stroma interactions | Breast tumor spheroids, vascularized tumor models, metastasis assays | Rapid degradation, batch variability, limited long-term culture | [20,31] |
| Chitosan-based hydrogels | Chitosan polysaccharide, sometimes blended with collagen or gelatin | Biodegradable, modifiable, supports 3D culture | 3D breast tumor culture, drug screening, scaffold for co-culture | Poor mechanical strength alone; variable cell adhesion without modification | [20,32,33] |
| Synthetic hybrid hydrogels | Combinations: PEG + gelatin, HA + PEG, alginate + ECM proteins | Combines tunable mechanics with bioactivity | Personalized tumor organoids, mechanobiology studies, drug testing | More complex to fabricate; may require multi-step crosslinking | [34,35] |
3. General Characteristics of Hydrogels
3.1. Natural Hydrogels
3.2. Synthetic Hydrogels
3.3. Composite or Hybrid Hydrogels
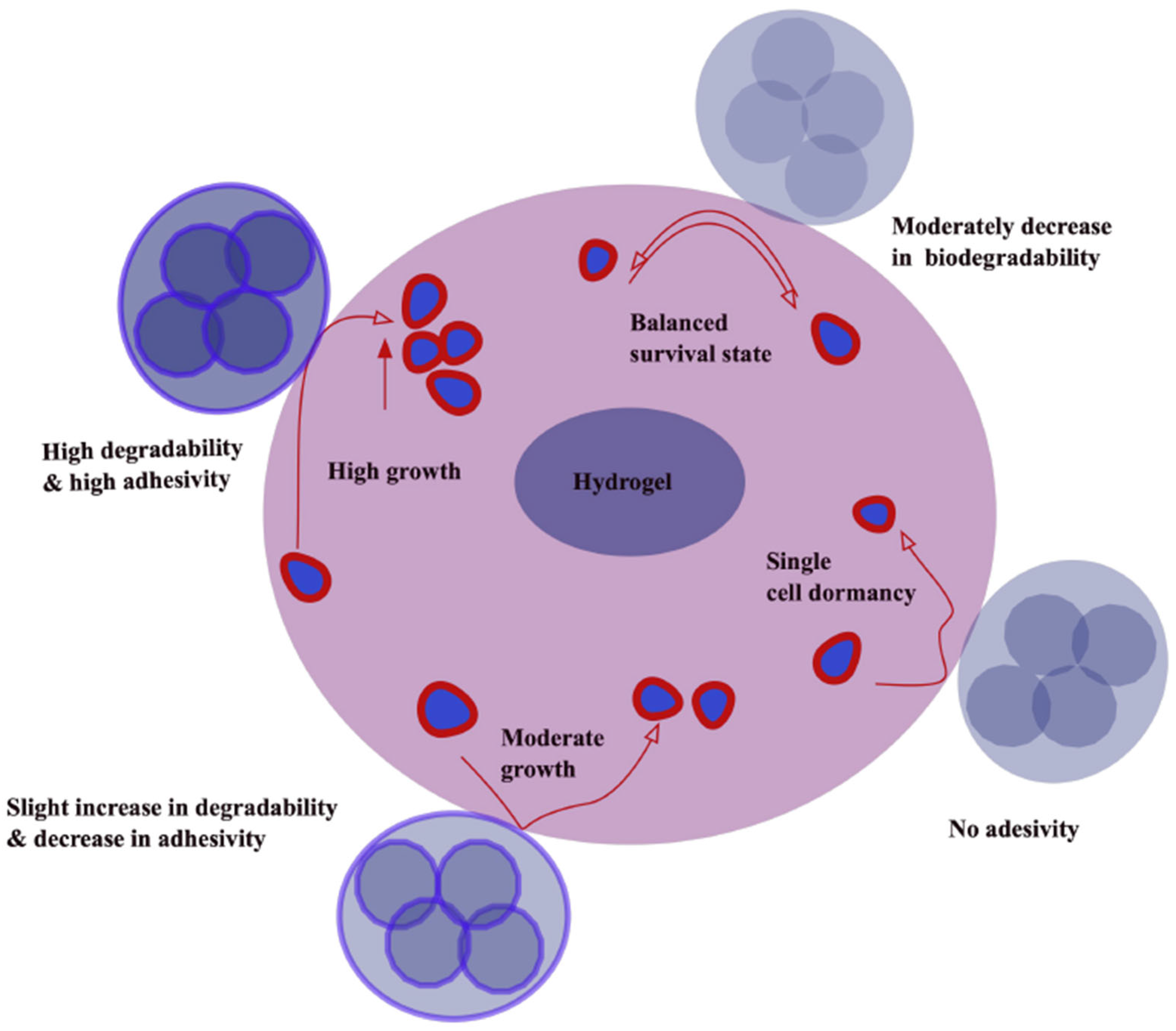
3.4. Static vs. Dynamic Hydrogels
4. Biomaterial Characteristics of Hydrogels
4.1. Biodegradability and Bioadhesion
| S.N. | Feature | Static Hydrogels | Dynamic Hydrogels | References |
|---|---|---|---|---|
| 1 | Mechanical properties | Stable, stiff, with limited adaptability | Tunable, can remodel or self-heal in response to stimuli | [74,75,76] |
| 2 | Crosslinking and examples | Permanent, Covalent: PEGGA/PEG-NB, polyacrylamide (2D), GElMA at fixed DoF Physical: Collagen I (neutralized), Matrigel (batch variable), alginate-Ca2+ | Reversible, dynamic covalent: hydrazone/oxime, boronate–diol, disulfide, thiol–ene with secondary light steps Supramolecular: B-cyclodextrin–adamantane, host–guest peptides, MMP-degradable PEG, photodegradable o-nitrobenzyl linkers, or weak supramolecular interactions | [77,78,79,80] |
| 3 | Mechanobiology | Good for static stiffness response curves (YAP/TAZ, focal adhesions). Limited stress relaxation control unless tailored | Stress relaxation and creep tunable, real-time stiffening (fibrosis) or softening (matrix degradation); supports durotaxis and mechanoadaptation studies | [81,82] |
| 4 | Biomimicry | Less biomimetic, static structure | Closer to natural ECM, adaptable and dynamic | [76,83] |
| 5 | ||||
| 6 | Stimuli responsiveness | Generally non-responsive | Response to pH, temperature, enzymes, light, redox, etc. | [76,84] |
| 7 | Self-healing ability | Absent (PEGDA/PAAm) or minimal (unless collagen/Matrigel) | Present, due to reversible crosslinking MMP-cleavable peptides (GPQGIWGQ, etc.) | [85,86,87] |
| 8 | Degradation | Controlled mainly by hydrolysis or enzymatic breakdown | Can degrade or restructure dynamically based on stimuli | [75,88] |
| 9 | Applications | Long-term implants, scaffolds needing stability | Drug delivery, tissue engineering, wound healing, 4D bioprinting | [89,90] |
| 10 | Advantages | High stability, mechanical robustness, simple, inexpensive, clear controls, batch-to-batch tunable (except Matrigel) | High adaptability, dynamic interactions, self-healing, physiologically closer to breast TME, captures progression, dormancy > reactivation, metastasis-like programs | [91,92] |
| 11 | Limitations | Lack of adaptability, no self-healing | Lower mechanical strength, sometimes unstable long term | [76,93] |
| 12 | Co-culture and TME complexity | Possible, but matrix lacks adaptive feedback to cells | Supports cell-driven desmoplasia, immune infiltration dynamics | [94,95] |
| 13 | Spatial/temporal patterning | Mostly pre-set, patterning requires multi-step fabrication | In situ photopatterning of stiffness/ligands; sequential cue delivery (e.g., EGF gradient after stiffening) | [96,97] |
| 14 | Drug testing | Stable baselines for screening doxorubicin, paclitaxel, tamoxifen, etc., good reproducibility | Can model acquire resistance by inducing progressive stiffening, HA accrual, or hypoxia formation, better for combination therapy timing studies | [98,99] |
4.2. Cell Aggregation Prevention
4.3. Control Release
4.4. Shear-Thinning Hydrogels
4.5. Mechanical Strength
4.6. Stimuli-Responsive and pH-Sensitive Hydrogels
4.7. Photosensitive Hydrogels
4.8. Magnetic and Ionic Strength Hydrogels
4.9. Dual-Responsive Hydrogels
5. Hydrogel Applications in Breast Tissue Regeneration
5.1. Scaffold Provision
5.2. Surgical Reconstruction Strategies
6. Hydrogel Application in Breast Tumor Models
6.1. Three-Dimensional Tumor Model
6.2. Emerging Technologies: 3D Bioprinting and Self-Folding Hydrogels
7. Hydrogels in Breast Cancer Therapy
7.1. Drug Delivery and Immunotherapy
7.2. Advanced Therapies and Photothermal Approaches
8. Translational Barrier from Bench to Bedside
9. Discussion
10. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, X.; Caruso, B.R.; Li, W. Advanced hydrogels in breast cancer therapy. Gels 2024, 10, 479. [Google Scholar] [CrossRef]
- McKernan, C.D.; Vorstenbosch, J.; Chu, J.J.; Nelson, J.A. Breast implant safety: An overview of current regulations and screening guidelines. J. Gen. Intern. Med. 2022, 37, 212–216. [Google Scholar] [CrossRef]
- Joshi, J.; Albers, C.; Smole, N.; Guo, S.; Smith, S.A. Human induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) for modeling cardiac arrhythmias: Strengths, challenges and potential solutions. Front. Physiol. 2024, 15, 1475152. [Google Scholar] [CrossRef] [PubMed]
- Pitton, M.; Urzì, C.; Farè, S.; Contessi Negrini, N. Visible light photo-crosslinking of biomimetic gelatin-hyaluronic acid hydrogels for adipose tissue engineering. J. Mech. Behav. Biomed. Mater. 2024, 158, 106675. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.F.; Martin, L.J.; Bronskill, M.; Yaffe, M.J.; Duric, N.; Minkin, S. Breast Tissue Composition and Susceptibility to Breast Cancer. JNCI J. Natl. Cancer Inst. 2010, 102, 1224–1237. [Google Scholar] [CrossRef]
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W.; et al. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes. Dis. 2018, 5, 77–106. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Luo, M.; Huang, J.; Zhang, K.; Zheng, S.; Zhang, S.; Zhou, J. Progression from ductal carcinoma in situ to invasive breast cancer: Molecular features and clinical significance. Signal Transduct. Target. Ther. 2024, 9, 83. [Google Scholar] [CrossRef]
- Aviv, A.; Anderson, J.J.; Shay, J.W. Mutations, Cancer and the Telomere Length Paradox. Trends Cancer 2017, 3, 253–258. [Google Scholar] [CrossRef]
- Caldon, C.E. Estrogen signaling and the DNA damage response in hormone dependent breast cancers. Front. Oncol. 2014, 4, 106. [Google Scholar] [CrossRef]
- Matza Porges, S.; Shamriz, O. Genetics of Immune Dysregulation and Cancer Predisposition: Two Sides of the Same Coin. Clin. Exp. Immunol. 2022, 210, 114–127. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Bahcecioglu, G.; Basara, G.; Ellis, B.W.; Ren, X.; Zorlutuna, P. Breast cancer models: Engineering the tumor microenvironment. Acta Biomater. 2020, 106, 1–21. [Google Scholar] [CrossRef]
- Guiro, K.; Arinzeh, T.L. Bioengineering Models for Breast Cancer Research. Breast Cancer 2015, 9, 57–70. [Google Scholar] [CrossRef]
- Hwangbo, H.; Chae, S.; Kim, W.; Jo, S.; Kim, G.H. Tumor-on-a-chip models combined with mini-tissues or organoids for engineering tumor tissues. Theranostics 2024, 14, 33–55. [Google Scholar] [CrossRef]
- Rijal, G.; Li, W. Native-mimicking in vitro microenvironment: An elusive and seductive future for tumor modeling and tissue engineering. J. Biol. Eng. 2018, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Zhou, X.; Mandal, K.; He, N.; Wennerberg, W.; Qu, M.; Jiang, X.; Sun, W.; Khademhosseini, A. Reconstructing the tumor architecture into organoids. Adv. Drug Deliv. Rev. 2021, 176, 113839. [Google Scholar] [CrossRef] [PubMed]
- Sievers, J.; Mahajan, V.; Welzel, P.B.; Werner, C.; Taubenberger, A. Precision Hydrogels for the Study of Cancer Cell Mechanobiology. Adv. Health Mater. 2023, 12, e2202514. [Google Scholar] [CrossRef] [PubMed]
- Dimou, P.; Trivedi, S.; Liousia, M.; D’Souza, R.R.; Klampatsa, A. Precision-Cut Tumor Slices (PCTS) as an Ex Vivo Model in Immunotherapy Research. Antibodies 2022, 11, 26. [Google Scholar] [CrossRef]
- Badea, M.A.; Balas, M.; Hermenean, A.; Ciceu, A.; Herman, H.; Ionita, D.; Dinischiotu, A. Influence of Matrigel on Single- and Multiple-Spheroid Cultures in Breast Cancer Research. SLAS Discov. 2019, 24, 563–578. [Google Scholar] [CrossRef]
- Shu, Y.; Li, B.; Ma, H.; Liu, J.; Cheng, Y.Y.; Li, X.; Liu, T.; Yang, C.; Ma, X.; Song, K. Three-dimensional breast cancer tumor models based on natural hydrogels: A review. J. Zhejiang Univ. Sci. B 2024, 25, 736–755. (In Chinese) [Google Scholar] [CrossRef]
- Cavo, M.; Caria, M.; Pulsoni, I.; Beltrame, F.; Fato, M.; Scaglione, S. A new cell-laden 3D Alginate-Matrigel hydrogel resembles human breast cancer cell malignant morphology, spread and invasion capability observed “in vivo”. Sci. Rep. 2018, 8, 5333. [Google Scholar] [CrossRef]
- Shi, W.; Mirza, S.; Kuss, M.; Liu, B.; Hartin, A.; Wan, S.; Kong, Y.; Mohapatra, B.; Krishnan, M.; Band, H.; et al. Embedded Bioprinting of Breast Tumor Cells and Organoids Using Low-Concentration Collagen-Based Bioinks. Adv. Health Mater. 2023, 12, e2300905. [Google Scholar] [CrossRef]
- Barros da Silva, P.; Zhao, X.; Bidarra, S.J.; Nascimento, D.S.; LaLone, V.; Lourenço, B.N.; Paredes, J.; Stevens, M.M.; Barrias, C.C. Tunable Hybrid Hydrogels of Alginate and Cell-Derived dECM to Study the Impact of Matrix Alterations on Epithelial-to-Mesenchymal Transition. Adv. Healthc. Mater. 2024, 13, 2401032. [Google Scholar] [CrossRef]
- Colak, B.; Ertas, Y.N. Implantable, 3D-Printed Alginate Scaffolds with Bismuth Sulfide Nanoparticles for the Treatment of Local Breast Cancer via Enhanced Radiotherapy. ACS Appl. Mater. Interfaces 2024, 16, 15718–15729. [Google Scholar] [CrossRef]
- Jiao, W.; Shan, J.; Gong, X.; Sun, Y.; Sang, L.; Ding, X.; Zhou, H.; Yu, M. GelMA hydrogel: A game-changer in 3D tumor modeling. Mater. Today Chem. 2024, 38, 102111. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Pahoff, S.; Bock, N.; Hutmacher, D.W. Gelatin Methacryloyl Hydrogels Control the Localized Delivery of Albumin-Bound Paclitaxel. Polymers 2020, 12, 501. [Google Scholar] [CrossRef] [PubMed]
- Alsharabasy, A.M.; Pandit, A. Hyaluronan-Based Hydrogels for 3D Modeling of Tumor Tissues. Tissue Eng. Part C Methods 2024, 30, 452–499. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, W.; Qian, J.; Wang, Y.; Hou, G.; Suo, A. Photo-crosslinked hyaluronic acid hydrogel as a biomimic extracellular matrix to recapitulate in vivo features of breast cancer cells. Colloids Surf. B Biointerfaces 2022, 209, 112159. [Google Scholar] [CrossRef]
- Wang, Z.; Ye, Q.; Yu, S.; Akhavan, B. Poly Ethylene Glycol (PEG)-Based Hydrogels for Drug Delivery in Cancer Therapy: A Comprehensive Review. Adv. Health Mater. 2023, 12, e2300105. [Google Scholar] [CrossRef]
- Koch, M.K.; Ravichandran, A.; Murekatete, B.; Clegg, J.; Joseph, M.T.; Hampson, M.; Jenkinson, M.; Bauer, H.S.; Snell, C.; Liu, C.; et al. Exploring the Potential of PEG-Heparin Hydrogels to Support Long-Term Ex Vivo Culture of Patient-Derived Breast Explant Tissues. Adv. Healthc. Mater. 2023, 12, 2202202. [Google Scholar] [CrossRef]
- Heilala, M.; Lehtonen, A.; Arasalo, O.; Peura, A.; Pokki, J.; Ikkala, O.; Nonappa; Klefström, J.; Munne, P.M. Fibrin Stiffness Regulates Phenotypic Plasticity of Metastatic Breast Cancer Cells. Adv. Healthc. Mater. 2023, 12, 2301137. [Google Scholar] [CrossRef]
- Li, T.; Ashrafizadeh, M.; Shang, Y.; Nuri Ertas, Y.; Orive, G. Chitosan-functionalized bioplatforms and hydrogels in breast cancer: Immunotherapy, phototherapy and clinical perspectives. Drug Discov. Today 2024, 29, 103851. [Google Scholar] [CrossRef]
- Tsao, C.T.; Kievit, F.M.; Wang, K.; Erickson, A.E.; Ellenbogen, R.G.; Zhang, M. Chitosan-based thermoreversible hydrogel as an in vitro tumor microenvironment for testing breast cancer therapies. Mol. Pharm. 2014, 11, 2134–2142. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.-H.; Chen, X.-Y.; Fu, L.-Q.; Du, W.-L.; Yang, X.; Mou, X.-Z.; Hu, P.-Y. Design and Development of Hybrid Hydrogels for Biomedical Applications: Recent Trends in Anticancer Drug Delivery and Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 630943. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ma, X.; Liu, J.; Zhang, H.; Fu, D. Advances in the application of natural/synthetic hybrid hydrogels in tissue engineering and delivery systems: A comprehensive review. Int. J. Pharm. 2025, 672, 125323. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, W.; Cai, C.; Zhang, H.; Shen, H.; Han, Y. Patient-derived xenograft models in cancer therapy: Technologies and applications. Signal Transduct. Target. Ther. 2023, 8, 160. [Google Scholar] [CrossRef]
- Lonberg, N. The Problem with Syngeneic Mouse Tumor Models. Cancer Immunol. Res. 2025, 13, 456–462. [Google Scholar] [CrossRef]
- Kersten, K.; de Visser, K.E.; van Miltenburg, M.H.; Jonkers, J. Genetically engineered mouse models in oncology research and cancer medicine. EMBO Mol. Med. 2017, 9, 137–153. [Google Scholar] [CrossRef]
- Liu, S.; Jin, P. Advances and Challenges in 3D Bioprinted Cancer Models: Opportunities for Personalized Medicine and Tissue Engineering. Polymers 2025, 17, 948. [Google Scholar] [CrossRef]
- Coughlin, M.F.; Kamm, R.D. The Use of Microfluidic Platforms to Probe the Mechanism of Cancer Cell Extravasation. Adv. Health Mater. 2020, 9, e1901410. [Google Scholar] [CrossRef]
- Baumgartner, C. Computational modeling and simulation in oncology. Clin. Transl. Med. 2025, 15, e70456. [Google Scholar] [CrossRef]
- Rijal, G. The Decellularized Extracellular Matrix in Regenerative Medicine. Regen. Med. 2017, 12, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Papanicolaou, M.; Parker, A.L.; Yam, M.; Filipe, E.C.; Wu, S.Z.; Chitty, J.L.; Wyllie, K.; Tran, E.; Mok, E.; Nadalini, A.; et al. Temporal profiling of the breast tumour microenvironment reveals collagen XII as a driver of metastasis. Nat. Commun. 2022, 13, 4587. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.R.; Ruud, K.F.; Martinez, S.R.; Li, W. Identification of the Collagen Types Essential for Mammalian Breast Acinar Structures. Gels 2022, 8, 837. [Google Scholar] [CrossRef] [PubMed]
- Rijal, G.; Li, W. A versatile 3D tissue matrix scaffold system for tumor modeling and drug screening. Sci. Adv. 2017, 3, e1700764. [Google Scholar] [CrossRef]
- Weng, B.; Li, M.; Zhu, W.; Peng, J.; Mao, X.; Zheng, Y.; Zhang, C.; Pan, S.; Mao, H.; Zhao, J. Distinguished biomimetic dECM system facilitates early detection of metastatic breast cancer cells. Bioeng. Transl. Med. 2024, 9, e10597. [Google Scholar] [CrossRef]
- Blanco-Fernandez, B.; Rey-Vinolas, S.; Bağcı, G.; Rubi-Sans, G.; Otero, J.; Navajas, D.; Perez-Amodio, S.; Engel, E. Bioprinting Decellularized Breast Tissue for the Development of Three-Dimensional Breast Cancer Models. ACS Appl. Mater. Interfaces 2022, 14, 29467–29482. [Google Scholar] [CrossRef]
- Rhee, S. Fibroblasts in three dimensional matrices: Cell migration and matrix remodeling. Exp. Mol. Med. 2009, 41, 858–865. [Google Scholar] [CrossRef]
- Yang, S.; Zheng, L.; Chen, Z.; Jiao, Z.; Liu, T.; Nie, Y.; Kang, Y.; Pan, B.; Song, K. Decellularized Pig Kidney with a Micro-Nano Secondary Structure Contributes to Tumor Progression in 3D Tumor Model. Materials 2022, 15, 1935. [Google Scholar] [CrossRef]
- Wu, M.; Zhou, H.; Hu, J.; Wang, Z.; Xu, Y.; Wu, Y.; Xiang, Y.; Yin, J.; Wei, P.; Xu, K.; et al. Decellularized porcine kidney-incorporated hydrogels for cell-laden bioprinting of renal cell carcinoma model. J. Bioprinting 2024, 10. [Google Scholar] [CrossRef]
- Rijal, G.; Li, W. 3D scaffolds in breast cancer research. Biomaterials 2016, 81, 135–156. [Google Scholar] [CrossRef]
- Xu, J.; Yang, S.; Su, Y.; Hu, X.; Xi, Y.; Cheng, Y.Y.; Kang, Y.; Nie, Y.; Pan, B.; Song, K. A 3D bioprinted tumor model fabricated with gelatin/sodium alginate/decellularized extracellular matrix bioink. Int. J. Bioprint 2023, 9, 630. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Satchell, S.C.; Wertheim, J.A.; Shah, R.N. Poly(ethylene glycol)-crosslinked gelatin hydrogel substrates with conjugated bioactive peptides influence endothelial cell behavior. Biomaterials 2019, 201, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Bupphathong, S.; Quiroz, C.; Huang, W.; Chung, P.F.; Tao, H.Y.; Lin, C.H. Gelatin Methacrylate Hydrogel for Tissue Engineering Applications-A Review on Material Modifications. Pharmaceuticals 2022, 15, 171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zeng, Z.; Chen, Y.; Deng, L.; Zhang, Y.; Que, Y.; Jiao, Y.; Chang, J.; Dong, Z.; Yang, C. 3D-printed GelMA/CaSiO(3) composite hydrogel scaffold for vascularized adipose tissue restoration. Regen. Biomater. 2023, 10, rbad049. [Google Scholar] [CrossRef]
- Tytgat, L.; Van Damme, L.; Van Hoorick, J.; Declercq, H.; Thienpont, H.; Ottevaere, H.; Blondeel, P.; Dubruel, P.; Van Vlierberghe, S. Additive manufacturing of photo-crosslinked gelatin scaffolds for adipose tissue engineering. Acta Biomater. 2019, 94, 340–350. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, F.; Dong, Z. Strategies for Constructing Tissue-Engineered Fat for Soft Tissue Regeneration. Tissue Eng. Regen. Med. 2024, 21, 395–408. [Google Scholar] [CrossRef]
- Tatsis, D.; Vasalou, V.; Kotidis, E.; Anestiadou, E.; Grivas, I.; Cheva, A.; Koliakos, G.; Venetis, G.; Pramateftakis, M.G.; Ouzounidis, N.; et al. The Combined Use of Platelet-Rich Plasma and Adipose-Derived Mesenchymal Stem Cells Promotes Healing. A Review of Experimental Models and Future Perspectives. Biomolecules 2021, 11, 1403. [Google Scholar] [CrossRef]
- Kim, J.R.; Cho, Y.S.; Park, J.H.; Kim, T.H. Poly(HEMA-co-MMA) Hydrogel Scaffold for Tissue Engineering with Controllable Morphology and Mechanical Properties Through Self-Assembly. Polymers 2024, 16, 3014. [Google Scholar] [CrossRef]
- Sanz-Horta, R.; Matesanz, A.; Gallardo, A.; Reinecke, H.; Jorcano, J.L.; Acedo, P.; Velasco, D.; Elvira, C. Technological advances in fibrin for tissue engineering. J. Tissue Eng. 2023, 14, 20417314231190288. [Google Scholar] [CrossRef]
- Ullah, A.; Kim, D.Y.; Lim, S.I.; Lim, H.R. Hydrogel-Based Biointerfaces: Recent Advances, Challenges, and Future Directions in Human-Machine Integration. Gels 2025, 11, 232. [Google Scholar] [CrossRef]
- Tordi, P.; Ridi, F.; Samorì, P.; Bonini, M. Cation-Alginate Complexes and Their Hydrogels: A Powerful Toolkit for the Development of Next-Generation Sustainable Functional Materials. Adv. Funct. Mater. 2025, 35, 2416390. [Google Scholar] [CrossRef]
- Feyissa, Z.; Edossa, G.D.; Gupta, N.K.; Negera, D. Development of double crosslinked sodium alginate/chitosan based hydrogels for controlled release of metronidazole and its antibacterial activity. Heliyon 2023, 9, e20144. [Google Scholar] [CrossRef] [PubMed]
- Bedell, M.L.; Torres, A.L.; Hogan, K.J.; Wang, Z.; Wang, B.; Melchiorri, A.J.; Grande-Allen, K.J.; Mikos, A.G. Human gelatin-based composite hydrogels for osteochondral tissue engineering and their adaptation into bioinks for extrusion, inkjet, and digital light processing bioprinting. Biofabrication 2022, 14, 045012. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.M.; De la Hoz Siegler, H. Evolution of Hybrid Hydrogels: Next-Generation Biomaterials for Drug Delivery and Tissue Engineering. Gels 2024, 10, 216. [Google Scholar] [CrossRef]
- Yin, B.; Gosecka, M.; Bodaghi, M.; Crespy, D.; Youssef, G.; Dodda, J.M.; Wong, S.H.D.; Imran, A.B.; Gosecki, M.; Jobdeedamrong, A.; et al. Engineering multifunctional dynamic hydrogel for biomedical and tissue regenerative applications. Chem. Eng. J. 2024, 487, 150403. [Google Scholar] [CrossRef]
- Han, Y.; Cao, Y.; Lei, H. Dynamic Covalent Hydrogels: Strong yet Dynamic. Gels 2022, 8, 577. [Google Scholar] [CrossRef]
- Condò, I.; Giannitelli, S.M.; Lo Presti, D.; Cortese, B.; Ursini, O. Overview of Dynamic Bond Based Hydrogels for Reversible Adhesion Processes. Gels 2024, 10, 442. [Google Scholar] [CrossRef]
- Wu, M.; Han, L.; Yan, B.; Zeng, H. Self-healing hydrogels based on reversible noncovalent and dynamic covalent interactions: A short review. Supramol. Mater. 2023, 2, 100045. [Google Scholar] [CrossRef]
- Parvin, N.; Joo, S.W.; Mandal, T.K. Injectable Biopolymer-Based Hydrogels: A Next-Generation Platform for Minimally Invasive Therapeutics. Gels 2025, 11, 383. [Google Scholar] [CrossRef]
- Soni, S.S.; D’Elia, A.M.; Alsasa, A.; Cho, S.; Tylek, T.; O’Brien, E.M.; Whitaker, R.; Spiller, K.L.; Rodell, C.B. Sustained release of drug-loaded nanoparticles from injectable hydrogels enables long-term control of macrophage phenotype. Biomater. Sci. 2022, 10, 6951–6967. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Ma, Y.; Wang, M.; Pan, G. Nano-crosslinked dynamic hydrogels for biomedical applications. Mater. Today Bio 2023, 20, 100640. [Google Scholar] [CrossRef]
- Thai, V.L.; Ramos-Rodriguez, D.H.; Mesfin, M.; Leach, J.K. Hydrogel degradation promotes angiogenic and regenerative potential of cell spheroids for wound healing. Mater. Today Bio 2023, 22, 100769. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Huang, J.; A, Y.; Yuan, N.; Chen, C.; Lin, D. Research Advances in Mechanical Properties and Applications of Dual Network Hydrogels. Int. J. Mol. Sci. 2022, 23, 15757. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Hakim Khalili, M.; Zhang, R.; Wilson, S.; Goel, S.; Impey, S.A.; Aria, A.I. Additive Manufacturing and Physicomechanical Characteristics of PEGDA Hydrogels: Recent Advances and Perspective for Tissue Engineering. Polymers 2023, 15, 2341. [Google Scholar] [CrossRef]
- Miao, Z.; Lu, Z.; Wu, H.; Liu, H.; Li, M.; Lei, D.; Zheng, L.; Zhao, J. Collagen, agarose, alginate, and Matrigel hydrogels as cell substrates for culture of chondrocytes in vitro: A comparative study. J. Cell Biochem. 2018, 119, 7924–7933. [Google Scholar] [CrossRef]
- García, F.; Smulders, M.M.J. Dynamic covalent polymers. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3551–3577. [Google Scholar] [CrossRef]
- Kakuta, T.; Takashima, Y.; Nakahata, M.; Otsubo, M.; Yamaguchi, H.; Harada, A. Preorganized hydrogel: Self-healing properties of supramolecular hydrogels formed by polymerization of host-guest-monomers that contain cyclodextrins and hydrophobic guest groups. Adv. Mater. 2013, 25, 2849–2853. [Google Scholar] [CrossRef]
- Scott, K.E.; Fraley, S.I.; Rangamani, P. A spatial model of YAP/TAZ signaling reveals how stiffness, dimensionality, and shape contribute to emergent outcomes. Proc. Natl. Acad. Sci. USA 2021, 118, e2021571118. [Google Scholar] [CrossRef]
- Narasimhan, B.N.; Fraley, S.I. Degradability tunes ECM stress relaxation and cellular mechanics. bioRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Florit, M.; Pardo, A.; Domingues, R.M.A.; Graça, A.L.; Babo, P.S.; Reis, R.L.; Gomes, M.E. Natural-Based Hydrogels for Tissue Engineering Applications. Molecules 2020, 25, 5858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, B.M. Current Advances in Stimuli-Responsive Hydrogels as Smart Drug Delivery Carriers. Gels 2023, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.N.; Singh, A.; Rothenberg, A.R.; Elisseeff, J.H.; Ewald, A.J. The independent roles of mechanical, structural and adhesion characteristics of 3D hydrogels on the regulation of cancer invasion and dissemination. Biomaterials 2013, 34, 9486–9495. [Google Scholar] [CrossRef]
- Xue, L.; An, R.; Zhao, J.; Qiu, M.; Wang, Z.; Ren, H.; Yu, D.; Zhu, X. Self-Healing Hydrogels: Mechanisms and Biomedical Applications. MedComm 2025, 6, e70181. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, Z.; Chen, D.; Li, Y.; Zhang, Q.; Su, J. Bone Regeneration Using MMP-Cleavable Peptides-Based Hydrogels. Gels 2021, 7, 199. [Google Scholar] [CrossRef]
- Neumann, M.; di Marco, G.; Iudin, D.; Viola, M.; van Nostrum, C.F.; van Ravensteijn, B.G.P.; Vermonden, T. Stimuli-Responsive Hydrogels: The Dynamic Smart Biomaterials of Tomorrow. Macromolecules 2023, 56, 8377–8392. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pr. 2013, 2013, 316–342. [Google Scholar] [CrossRef]
- Tran, T.S.; Balu, R.; Mettu, S.; Roy Choudhury, N.; Dutta, N.K. 4D Printing of Hydrogels: Innovation in Material Design and Emerging Smart Systems for Drug Delivery. Polymers 2022, 15, 1282. [Google Scholar] [CrossRef]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef]
- Pradhan, S.; Slater, J.H. Tunable hydrogels for controlling phenotypic cancer cell states to model breast cancer dormancy and reactivation. Biomaterials 2019, 215, 119177. [Google Scholar] [CrossRef]
- Liang, C.; Dudko, V.; Khoruzhenko, O.; Hong, X.; Lv, Z.-P.; Tunn, I.; Umer, M.; Timonen, J.V.I.; Linder, M.B.; Breu, J.; et al. Stiff and self-healing hydrogels by polymer entanglements in co-planar nanoconfinement. Nat. Mater. 2025, 24, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.A.; Faull, P.A.; Seymour, A.J.; Yu, T.T.L.; Loaiza, S.; Auner, H.W.; Snijders, A.P.; Gentleman, E. Neighboring cells override 3D hydrogel matrix cues to drive human MSC quiescence. Biomaterials 2018, 176, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Flores-Torres, S.; Dimitriou, N.M.; Pardo, L.A.; Kort-Mascort, J.; Pal, S.; Peza-Chavez, O.; Kuasne, H.; Berube, J.; Bertos, N.; Park, M.; et al. Bioprinted Multicomponent Hydrogel Co-culture Tumor-Immune Model for Assessing and Simulating Tumor-Infiltrated Lymphocyte Migration and Functional Activation. ACS Appl. Mater. Interfaces 2023, 15, 33250–33262. [Google Scholar] [CrossRef] [PubMed]
- Leijten, J.; Seo, J.; Yue, K.; Santiago, G.T.; Tamayol, A.; Ruiz-Esparza, G.U.; Shin, S.R.; Sharifi, R.; Noshadi, I.; Álvarez, M.M.; et al. Spatially and Temporally Controlled Hydrogels for Tissue Engineering. Mater. Sci. Eng. R. Rep. 2017, 119, 1–35. [Google Scholar] [CrossRef]
- Stowers, R.S.; Allen, S.C.; Suggs, L.J. Dynamic phototuning of 3D hydrogel stiffness. Proc. Natl. Acad. Sci. USA 2015, 112, 1953–1958. [Google Scholar] [CrossRef]
- Zang, C.; Tian, Y.; Tang, Y.; Tang, M.; Yang, D.; Chen, F.; Ghaffarlou, M.; Tu, Y.; Ashrafizadeh, M.; Li, Y. Hydrogel-based platforms for site-specific doxorubicin release in cancer therapy. J. Transl. Med. 2024, 22, 879. [Google Scholar] [CrossRef]
- Park, K.M.; Gerecht, S. Hypoxia-inducible hydrogels. Nat. Commun. 2014, 5, 4075. [Google Scholar] [CrossRef]
- Morwood, A.J.; El-Karim, I.A.; Clarke, S.A.; Lundy, F.T. The Role of Extracellular Matrix (ECM) Adhesion Motifs in Functionalised Hydrogels. Molecules 2023, 28, 4616. [Google Scholar] [CrossRef]
- Weber, L.M.; Hayda, K.N.; Haskins, K.; Anseth, K.S. The effects of cell–matrix interactions on encapsulated β-cell function within hydrogels functionalized with matrix-derived adhesive peptides. Biomaterials 2007, 28, 3004–3011. [Google Scholar] [CrossRef]
- Bal-Ozturk, A.; Cecen, B.; Avci-Adali, M.; Topkaya, S.N.; Alarcin, E.; Yasayan, G.; Ethan, Y.C.; Bulkurcuoglu, B.; Akpek, A.; Avci, H.; et al. Tissue Adhesives: From Research to Clinical Translation. Nano Today 2021, 36. [Google Scholar] [CrossRef]
- Bej, R.; Haag, R. Mucus-Inspired Dynamic Hydrogels: Synthesis and Future Perspectives. J. Am. Chem. Soc. 2022, 144, 20137–20152. [Google Scholar] [CrossRef] [PubMed]
- Jawadi, Z.; Yang, C.; Haidar, Z.S.; Santa Maria, P.L.; Massa, S. Bio-Inspired Muco-Adhesive Polymers for Drug Delivery Applications. Polymers 2022, 14, 5459. [Google Scholar] [CrossRef] [PubMed]
- Pak, S.; Chen, F. Functional Enhancement of Guar Gum-Based Hydrogel by Polydopamine and Nanocellulose. Foods 2023, 12, 1304. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Zhao, Q.; Zhang, W.; Wu, J.; Shi, Y.; Wang, K.; Jiang, J.; Duan, J. High mechanical, self-adhesive oxidized guar gum/chitosan hydrogel prepared at room temperature based on a nickel-urushiol catalytic system for wireless wearable sensors. Int. J. Biol. Macromol. 2024, 282, 136899. [Google Scholar] [CrossRef]
- Zhou, Y.; Kang, L.; Yue, Z.; Liu, X.; Wallace, G.G. Composite Tissue Adhesive Containing Catechol-Modified Hyaluronic Acid and Poly-l-lysine. ACS Appl. Bio Mater. 2020, 3, 628–638. [Google Scholar] [CrossRef]
- Juan, C.-Y.; Zhang, Y.-S.; Cheng, J.-K.; Chen, Y.-H.; Lin, H.-C.; Yeh, M.-Y. Lysine-Triggered Polymeric Hydrogels with Self-Adhesion, Stretchability, and Supportive Properties. Polymers 2024, 16, 1388. [Google Scholar] [CrossRef]
- Michalicha, A.; Belcarz, A.; Giannakoudakis, D.A.; Staniszewska, M.; Barczak, M. Designing Composite Stimuli-Responsive Hydrogels for Wound Healing Applications: The State-of-the-Art and Recent Discoveries. Materials 2024, 17, 278. [Google Scholar] [CrossRef]
- Muthiah Pillai, N.S.; Eswar, K.; Amirthalingam, S.; Mony, U.; Kerala Varma, P.; Jayakumar, R. Injectable Nano Whitlockite Incorporated Chitosan Hydrogel for Effective Hemostasis. ACS Appl. Bio Mater. 2019, 2, 865–873. [Google Scholar] [CrossRef]
- Liu, Z.; Tang, Y.; Chen, Y.; Lu, Z.; Rui, Z. Dynamic covalent adhesives and their applications: Current progress and future perspectives. Chem. Eng. J. 2024, 497, 154710. [Google Scholar] [CrossRef]
- Ribeiro, M.M.; Simões, M.; Vitorino, C.; Mascarenhas-Melo, F. Physical crosslinking of hydrogels: The potential of dynamic and reversible bonds in burn care. Coord. Chem. Rev. 2025, 542, 216868. [Google Scholar] [CrossRef]
- Bovone, G.; Dudaryeva, O.Y.; Marco-Dufort, B.; Tibbitt, M.W. Engineering Hydrogel Adhesion for Biomedical Applications via Chemical Design of the Junction. ACS Biomater. Sci. Eng. 2021, 7, 4048–4076. [Google Scholar] [CrossRef]
- Shao, H.; Deng, J.; Xu, Z.; Zhu, J.; Jian, W.; Zhang, P.; Zhou, X.; Zhang, X.; She, H.; Ma, J.; et al. A Janus hydrogel that enables wet tissue adhesion and resists abdominal adhesions. Mater. Today Bio 2024, 28, 101248. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng. Part B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Hua, M.; Zhang, C.W.; Hong, W.; Yan, Y.; Jazzar, A.; Chen, C.; Shi, P.; Si, M.; Wu, D.; et al. Noncovalent Aggregation for Diverse Properties in Hydrogels: A Comprehensive Review. Chem. Rev. 2025. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Chen, M.H.; Wang, L.L.; Chung, J.J.; Kim, Y.H.; Atluri, P.; Burdick, J.A. Methods To Assess Shear-Thinning Hydrogels for Application As Injectable Biomaterials. ACS Biomater. Sci. Eng. 2017, 3, 3146–3160. [Google Scholar] [CrossRef]
- Sun, Y.; Nie, Y.; Wang, L.; Gong, J.P.; Tanaka, S.; Tsuda, M. Tumor-mimetic hydrogel stiffness regulates cancer stemness properties in H-Ras-transformed cancer model cells. Biochem. Biophys. Res. Commun. 2025, 743, 151163. [Google Scholar] [CrossRef]
- Moghaddam, A.S.; Dunne, K.; Breyer, W.; Wu, Y.; Pashuck, E.T. Hydrogels with multiple RGD presentations increase cell adhesion and spreading. Acta Biomater. 2025, 199, 142–153. [Google Scholar] [CrossRef]
- Elosegui-Artola, A.; Trepat, X.; Roca-Cusachs, P. Control of Mechanotransduction by Molecular Clutch Dynamics. Trends Cell Biol. 2018, 28, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Gautam, B.; Veenbrink, V.A.; Rugina, C.; Sturm, M.; Nastaseanu, V.; Eijsbouts, T.; Kiljański, M.; Esteves, A.C.C. Hybrid CNCs/PNIPAAm Thermoresponsive Hydrogels Showing Enhanced Mechanical Strength and Biocompatibility. J. Polym. Sci. 2025, 63, 4320–4333. [Google Scholar] [CrossRef]
- Mai, Z.; Lin, Y.; Lin, P.; Zhao, X.; Cui, L. Modulating extracellular matrix stiffness: A strategic approach to boost cancer immunotherapy. Cell Death Dis. 2024, 15, 307. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Ooi, H.S.; Bian, L.; Ouyang, L.; Sun, W. Dynamic hydrogels for biofabrication: A review. Biomaterials 2025, 320, 123266. [Google Scholar] [CrossRef]
- Andrade, F.; Roca-Melendres, M.M.; Durán-Lara, E.F.; Rafael, D.; Schwartz, S. Stimuli-Responsive Hydrogels for Cancer Treatment: The Role of pH, Light, Ionic Strength and Magnetic Field. Cancers 2021, 13, 1164. [Google Scholar] [CrossRef]
- Ji, W.; Wu, Q.; Han, X.; Zhang, W.; Wei, W.; Chen, L.; Li, L.; Huang, W. Photosensitive hydrogels: From structure, mechanisms, design to bioapplications. Sci. China Life Sci. 2020, 63, 1813–1828. [Google Scholar] [CrossRef]
- Rajasekar, M.; Kavyashree, V.; Sangamithra, E.; Baskaran, P.; Faustina Maria, M.; Mary, J.; Sivakumar, M.; Selvam, M. Review on biomaterial applications of photoresponsive based chromophore Hydrogels: Recent developments and future perspectives. Results Chem. 2024, 7, 101462. [Google Scholar] [CrossRef]
- Sacco, P.; Piazza, F.; Marsich, E.; Abrami, M.; Grassi, M.; Donati, I. Ionic Strength Impacts the Physical Properties of Agarose Hydrogels. Gels 2024, 10, 94. [Google Scholar] [CrossRef]
- Xuan, X.; Li, Y.; Xu, X.; Pan, Z.; Li, Y.; Luo, Y.; Sun, L. Three-Dimensional Printable Magnetic Hydrogels with Adjustable Stiffness and Adhesion for Magnetic Actuation and Magnetic Hyperthermia Applications. Gels 2025, 11, 67. [Google Scholar] [CrossRef]
- Luo, K.; Hu, W. A dual thermo/pH-sensitive hydrogel as 5-Fluorouracil carrier for breast cancer treatment. Anti-Cancer Drugs 2025, 36, 220–231. [Google Scholar] [CrossRef]
- Lee, M.; Lee, M.; Kim, S.; Park, N. Stimuli-Responsive DNA Hydrogel Design Strategies for Biomedical Applications. Biosensors 2025, 15, 355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, W.; Zhou, X.; Lin, H.; Zhu, X.; Lou, Y.; Zheng, L. CRISPR-Responsive RCA-Based DNA Hydrogel Biosensing Platform with Customizable Signal Output for Rapid and Sensitive Nucleic Acid Detection. Anal. Chem. 2024, 96, 15998–16006. [Google Scholar] [CrossRef] [PubMed]
- Unnikrishnan, K.; Thomas, L.V.; Ram Kumar, R.M. Advancement of Scaffold-Based 3D Cellular Models in Cancer Tissue Engineering: An Update. Front. Oncol. 2021, 11, 733652. [Google Scholar] [CrossRef]
- Giraudo, M.V.; Di Francesco, D.; Catoira, M.C.; Cotella, D.; Fusaro, L.; Boccafoschi, F. Angiogenic Potential in Biological Hydrogels. Biomedicines 2020, 8, 436. [Google Scholar] [CrossRef]
- Reddy, M.S.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Steiner, D.; Mutschall, H.; Winkler, S.; Horch, R.E.; Arkudas, A. The Adipose-Derived Stem Cell and Endothelial Cell Coculture System-Role of Growth Factors? Cells 2021, 10, 2074. [Google Scholar] [CrossRef]
- Ni, R.; Luo, C.; Ci, H.; Sun, D.; An, R.; Wang, Z.; Yang, J.; Li, Y.; Sun, J. Construction of vascularized tissue-engineered breast with dual angiogenic and adipogenic micro-tissues. Mater. Today Bio 2023, 18, 100539. [Google Scholar] [CrossRef]
- Blanco-Fernandez, B.; Ibañez-Fonseca, A.; Orbanic, D.; Ximenes-Carballo, C.; Perez-Amodio, S.; Rodríguez-Cabello, J.C.; Engel, E. Elastin-like Recombinamer Hydrogels as Platforms for Breast Cancer Modeling. Biomacromolecules 2023, 24, 4408–4418. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Kankala, R.K.; Jiang, M.; Long, L.; Li, W.; Zou, L.; Chen, A.; Liu, Y. Decellularized extracellular matrix-based disease models for drug screening. Mater. Today Bio 2024, 29, 101280. [Google Scholar] [CrossRef]
- Shi, Y.; Guan, Z.; Cai, G.; Nie, Y.; Zhang, C.; Luo, W.; Liu, J. Patient-derived organoids: A promising tool for breast cancer research. Front. Oncol. 2024, 14, 1350935. [Google Scholar] [CrossRef]
- Tzeng, Y.-D.T.; Hsiao, J.-H.; Tseng, L.-M.; Hou, M.-F.; Li, C.-J. Breast cancer organoids derived from patients: A platform for tailored drug screening. Biochem. Pharmacol. 2023, 217, 115803. [Google Scholar] [CrossRef]
- Thorel, L.; Perréard, M.; Florent, R.; Divoux, J.; Coffy, S.; Vincent, A.; Gaggioli, C.; Guasch, G.; Gidrol, X.; Weiswald, L.-B.; et al. Patient-derived tumor organoids: A new avenue for preclinical research and precision medicine in oncology. Exp. Mol. Med. 2024, 56, 1531–1551. [Google Scholar] [CrossRef]
- Papaioannou, T.G.; Manolesou, D.; Dimakakos, E.; Tsoucalas, G.; Vavuranakis, M.; Tousoulis, D. 3D Bioprinting Methods and Techniques: Applications on Artificial Blood Vessel Fabrication. Acta Cardiol. Sin. 2019, 35, 284–289. [Google Scholar] [CrossRef]
- Mao, Y.; Yu, K.; Isakov, M.S.; Wu, J.; Dunn, M.L.; Jerry Qi, H. Sequential Self-Folding Structures by 3D Printed Digital Shape Memory Polymers. Sci. Rep. 2015, 5, 13616. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Xu, Z.; Li, L.; Guo, K.; Mi, J.; Wu, H.; Li, Y.; Xie, C.; Jin, J.; Xu, J.; et al. Hydrogels with programmed spatiotemporal mechanical cues for stem cell-assisted bone regeneration. Nat. Commun. 2025, 16, 3633. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, V.; Atapour-Mashhad, H.; Shahvali, S.; Salehi, B.; Shaban, M.; Shirzad, M.; Salahvarzi, A.; Mohammadi, M. Hydrogels as advanced drug delivery platforms for cancer immunotherapy: Promising innovations and future outlook. J. Nanobiotechnol. 2025, 23, 545. [Google Scholar] [CrossRef] [PubMed]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications. Mater. Today Bio 2022, 13, 100186. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, B.; Sun, H.; Liu, D.; Zhu, Y.; Zhu, Q.; Liu, X. The Dual Effect of 3D-Printed Biological Scaffolds Composed of Diverse Biomaterials in the Treatment of Bone Tumors. Int. J. Nanomed. 2023, 18, 293–305. [Google Scholar] [CrossRef]
- de Melo Santana, B.; Pieretti, J.C.; Gomes, R.N.; Cerchiaro, G.; Seabra, A.B. Cytotoxicity towards Breast Cancer Cells of Pluronic F-127/Hyaluronic Acid Hydrogel Containing Nitric Oxide Donor and Silica Nanoparticles Loaded with Cisplatin. Pharmaceutics 2022, 14, 2837. [Google Scholar] [CrossRef]
- Cabral, F.V.; Santana, B.D.; Lange, C.N.; Batista, B.L.; Seabra, A.B.; Ribeiro, M.S. Pluronic F-127 Hydrogels Containing Copper Oxide Nanoparticles and a Nitric Oxide Donor to Treat Skin Cancer. Pharmaceutics 2023, 15, 1971. [Google Scholar] [CrossRef]
- Valentino, A.; Yazdanpanah, S.; Conte, R.; Calarco, A.; Peluso, G. Smart Nanocomposite Hydrogels as Next-Generation Therapeutic and Diagnostic Solutions. Gels 2024, 10, 689. [Google Scholar] [CrossRef]
- Kass, L.E.; Nguyen, J. Nanocarrier-hydrogel composite delivery systems for precision drug release. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol 2022, 14, e1756. [Google Scholar] [CrossRef] [PubMed]
- Lungu, A.; Ghitman, J.; Cernencu, A.I.; Serafim, A.; Florea, N.M.; Vasile, E.; Iovu, H. POSS-containing hybrid nanomaterials based on thiol-epoxy click reaction. Polymer 2018, 145, 324–333. [Google Scholar] [CrossRef]
- Bai, Y.; Han, B.; Zhang, Y.; Zhang, Y.; Cai, Y.; Shen, L.; Jia, Y. Advancements in Hydrogel Application for Ischemic Stroke Therapy. Gels 2022, 8, 777. [Google Scholar] [CrossRef] [PubMed]
- Beheshtizadeh, N.; Gharibshahian, M.; Bayati, M.; Maleki, R.; Strachan, H.; Doughty, S.; Tayebi, L. Vascular endothelial growth factor (VEGF) delivery approaches in regenerative medicine. Biomed. Pharmacother. 2023, 166, 115301. [Google Scholar] [CrossRef]
- Raina, N.; Pahwa, R.; Bhattacharya, J.; Paul, A.K.; Nissapatorn, V.; de Lourdes Pereira, M.; Oliveira, S.M.R.; Dolma, K.G.; Rahmatullah, M.; Wilairatana, P.; et al. Drug Delivery Strategies and Biomedical Significance of Hydrogels: Translational Considerations. Pharmaceutics 2022, 14, 574. [Google Scholar] [CrossRef]
- Delgado-Pujol, E.J.; Martínez, G.; Casado-Jurado, D.; Vázquez, J.; León-Barberena, J.; Rodríguez-Lucena, D.; Torres, Y.; Alcudia, A.; Begines, B. Hydrogels and Nanogels: Pioneering the Future of Advanced Drug Delivery Systems. Pharmaceutics 2025, 17, 215. [Google Scholar] [CrossRef]
- Yu, H.; Gao, R.; Liu, Y.; Fu, L.; Zhou, J.; Li, L. Stimulus-Responsive Hydrogels as Drug Delivery Systems for Inflammation Targeted Therapy. Adv. Sci. 2024, 11, e2306152. [Google Scholar] [CrossRef]
- Nazli, A.; Irshad Khan, M.Z.; Rácz, Á.; Béni, S. Acid-sensitive prodrugs; a promising approach for site-specific and targeted drug release. Eur. J. Med. Chem. 2024, 276, 116699. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, W.; Suo, Y.; Liu, Y.; Zhan, X.; Zhou, J.; Chen, Z.; Wu, X.; Yin, X.; Bao, B. Nanomaterials engineered for photothermal therapy in neural tumors and neurodegenerative diseases: Biomaterial design, clinical mechanisms and applications. Front. Bioeng. Biotechnol. 2025, 13, 1631627. [Google Scholar] [CrossRef]
- Nag, S.; Mitra, O.; Tripathi, G.; Adur, I.; Mohanto, S.; Nama, M.; Samanta, S.; Gowda, B.H.J.; Subramaniyan, V.; Sundararajan, V.; et al. Nanomaterials-assisted photothermal therapy for breast cancer: State-of-the-art advances and future perspectives. Photodiagnosis Photodyn. Ther. 2024, 45, 103959. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Teng, L.; Gao, L.; Su, T.; Fu, L.; Qiu, Z.; Bi, Y. Advances in Multiple Stimuli-Responsive Drug-Delivery Systems for Cancer Therapy. Int. J. Nanomed. 2021, 16, 1525–1551. [Google Scholar] [CrossRef] [PubMed]
- Mi, P. Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics. Theranostics 2020, 10, 4557–4588. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Sahandi Zangabad, P.; Ghasemi, A.; Amiri, M.; Bahrami, M.; Malekzad, H.; Ghahramanzadeh Asl, H.; Mahdieh, Z.; Bozorgomid, M.; Ghasemi, A.; et al. Temperature-Responsive Smart Nanocarriers for Delivery Of Therapeutic Agents: Applications and Recent Advances. ACS Appl. Mater. Interfaces 2016, 8, 21107–21133. [Google Scholar] [CrossRef]
- Gheysoori, P.; Paydayesh, A.; Jafari, M.; Peidayesh, H. Thermoresponsive nanocomposite hydrogels based on Gelatin/poly (N–isopropylacrylamide) (PNIPAM) for controlled drug delivery. Eur. Polym. J. 2023, 186, 111846. [Google Scholar] [CrossRef]
- Kar, A.; Jain, D.; Kumar, S.; Rajput, K.; Pal, S.; Rana, K.; Kar, R.; Jha, S.K.; Medatwal, N.; Yavvari, P.S.; et al. A localized hydrogel-mediated chemotherapy causes immunogenic cell death via activation of ceramide-mediated unfolded protein response. Sci. Adv. 2023, 9, eadf2746. [Google Scholar] [CrossRef]
- Rached, L.; Laparra, A.; Sakkal, M.; Danlos, F.-X.; Barlesi, F.; Carbonnel, F.; De Martin, E.; Ducreux, M.; Even, C.; Le Pavec, J.; et al. Toxicity of immunotherapy combinations with chemotherapy across tumor indications: Current knowledge and practical recommendations. Cancer Treat. Rev. 2024, 127, 102751. [Google Scholar] [CrossRef]
- Tsai, J.S.; Wei, S.H.; Chen, C.W.; Yang, S.C.; Tseng, Y.L.; Su, P.L.; Lin, C.C.; Su, W.C. Pembrolizumab and Chemotherapy Combination Prolonged Progression-Free Survival in Patients with NSCLC with High PD-L1 Expression and Low Neutrophil-to-Lymphocyte Ratio. Pharmaceuticals 2022, 15, 1407. [Google Scholar] [CrossRef]
- Meng, D.; Lei, H.; Zheng, X.; Han, Y.; Sun, R.; Zhao, D.; Liu, R. A temperature-sensitive phase-change hydrogel of tamoxifen achieves the long-acting antitumor activation on breast cancer cells. Onco Targets Ther. 2019, 12, 3919–3931. [Google Scholar] [CrossRef]
- Matai, I.; Kaur, G.; Soni, S.; Sachdev, A.; Vikas; Mishra, S. Near-infrared stimulated hydrogel patch for photothermal therapeutics and thermoresponsive drug delivery. J. Photochem. Photobiol. B Biol. 2020, 210, 111960. [Google Scholar] [CrossRef]
- Liu, C.; Liao, Y.; Liu, L.; Xie, L.; Liu, J.; Zhang, Y.; Li, Y. Application of injectable hydrogels in cancer immunotherapy. Front. Bioeng. Biotechnol. 2023, 11, 1121887. [Google Scholar] [CrossRef]
- Li, Q.; Wen, J.; Liu, C.; Jia, Y.; Wu, Y.; Shan, Y.; Qian, Z.; Liao, J. Graphene-Nanoparticle-Based Self-Healing Hydrogel in Preventing Postoperative Recurrence of Breast Cancer. ACS Biomater. Sci. Eng. 2019, 5, 768–779. [Google Scholar] [CrossRef]
- Xu, R.; Wang, S.; Guo, Q.; Zhong, R.; Chen, X.; Xia, X. Anti-Tumor Strategies of Photothermal Therapy Combined with Other Therapies Using Nanoplatforms. Pharmaceutics 2025, 17, 306. [Google Scholar] [CrossRef] [PubMed]
- Rajabpour, M.; Pourmadadi, M.; Yazdian, F.; Hallajisani, A.; Fazeli, A. Treatment of breast cancer with curcumin-loaded hydrogel nanocomposite containing starch/agarose/zinc oxide. Int. J. Biol. Macromol. 2025, 315, 144492. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, K.; Nguyen, C.H.; Rijal, G. Asporin increases the extracellular matrix cross-links and inhibits the cancer cell migration. Tumour Biol. 2025, 47, 10104283241313441. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ren, Y.; Song, W.; Yu, B.; Liu, H. Rational design in functional hydrogels towards biotherapeutics. Mater. Des. 2022, 223, 111086. [Google Scholar] [CrossRef]
- Gouveia, B.G.; Rijo, P.; Gonçalo, T.S.; Reis, C.P. Good manufacturing practices for medicinal products for human use. J. Pharm. Bioallied Sci. 2015, 7, 87–96. [Google Scholar] [CrossRef]
- Segneanu, A.E.; Bejenaru, L.E.; Bejenaru, C.; Blendea, A.; Mogoşanu, G.D.; Biţă, A.; Boia, E.R. Advancements in Hydrogels: A Comprehensive Review of Natural and Synthetic Innovations for Biomedical Applications. Polymers 2025, 17, 2026. [Google Scholar] [CrossRef]
- Luo, Y.; Zhou, X.; Liu, C.; Lu, R.; Jia, M.; Li, P.; Zhang, S. Scavenging ROS and inflammation produced during treatment to enhance the wound repair efficacy of photothermal injectable hydrogel. Biomater. Adv. 2022, 141, 213096. [Google Scholar] [CrossRef]
- Ren, J.; Jiang, Z.; He, J.; Wang, X.; Jin, W.; Yu, Z. Current status and perspectives on design, fabrication, surface modification, and clinical applications of biodegradable magnesium alloys. J. Magnes. Alloys 2025, 13, 3564–3595. [Google Scholar] [CrossRef]
- Catoira, M.C.; González-Payo, J.; Fusaro, L.; Ramella, M.; Boccafoschi, F. Natural hydrogels R&D process: Technical and regulatory aspects for industrial implementation. J. Mater. Sci. Mater. Med. 2020, 31, 64. [Google Scholar] [CrossRef]
- Wu, L.; Zhao, J.; Huang, J.; Huang, P.; Zhao, H. Advances and challenges in three-dimensional bioprinting of bone organoids: Materials, techniques, and functionalization strategies. Int. J. Bioprinting 2025, 11, 1–22. [Google Scholar] [CrossRef]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the potential of hydrogels for advanced therapeutic applications: Current achievements and future directions. Signal Transduct. Target. Ther. 2024, 9, 166. [Google Scholar] [CrossRef]
- Vats, K.; Benoit, D.S. Dynamic manipulation of hydrogels to control cell behavior: A review. Tissue Eng. Part B Rev. 2013, 19, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Micalet, A.; Moeendarbary, E.; Cheema, U. 3D In Vitro Models for Investigating the Role of Stiffness in Cancer Invasion. ACS Biomater. Sci. Eng. 2023, 9, 3729–3741. [Google Scholar] [CrossRef] [PubMed]
- Ayad, N.M.E.; Kaushik, S.; Weaver, V.M. Tissue mechanics, an important regulator of development and disease. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180215. [Google Scholar] [CrossRef] [PubMed]
- Acciaretti, F.; Vesentini, S.; Cipolla, L. Fabrication Strategies Towards Hydrogels for Biomedical Application: Chemical and Mechanical Insights. Chem. Asian J. 2022, 17, e202200797. [Google Scholar] [CrossRef]
- Salemme, V.; Centonze, G.; Avalle, L.; Natalini, D.; Piccolantonio, A.; Arina, P.; Morellato, A.; Ala, U.; Taverna, D.; Turco, E.; et al. The role of tumor microenvironment in drug resistance: Emerging technologies to unravel breast cancer heterogeneity. Front. Oncol. 2023, 13, 1170264. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rijal, G.; Park, I.-W. Dynamic Hydrogels in Breast Tumor Models. Gels 2025, 11, 855. https://doi.org/10.3390/gels11110855
Rijal G, Park I-W. Dynamic Hydrogels in Breast Tumor Models. Gels. 2025; 11(11):855. https://doi.org/10.3390/gels11110855
Chicago/Turabian StyleRijal, Girdhari, and In-Woo Park. 2025. "Dynamic Hydrogels in Breast Tumor Models" Gels 11, no. 11: 855. https://doi.org/10.3390/gels11110855
APA StyleRijal, G., & Park, I.-W. (2025). Dynamic Hydrogels in Breast Tumor Models. Gels, 11(11), 855. https://doi.org/10.3390/gels11110855








