Dialdehyde Starch Cross-Linked Collagen with Heparin Conjugation: Characterization and Feasibility Study for Osteochondral Tissue Repair
Abstract
1. Introduction
2. Results and Discussion
2.1. Compressive Stiffness Changes in DAS-COL by Conditional Neutralization Buffer
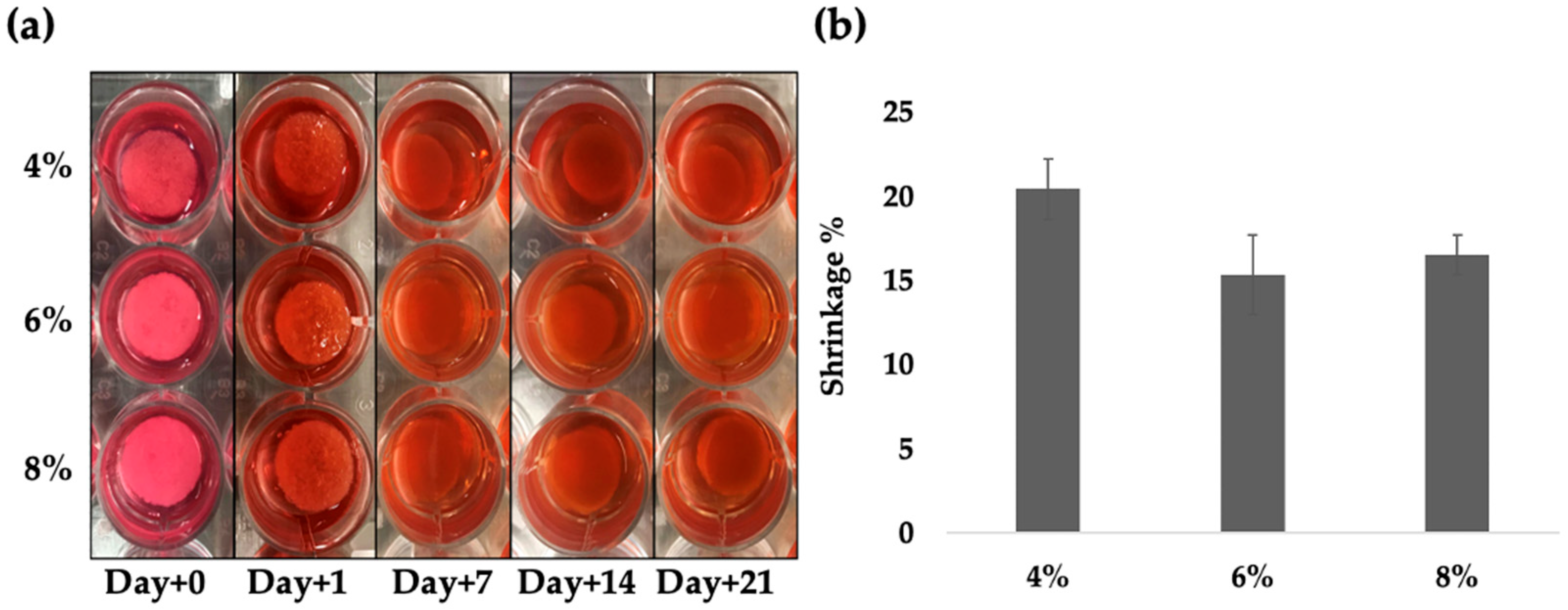
2.2. Degradation Test of DAS-COL Hydrogels
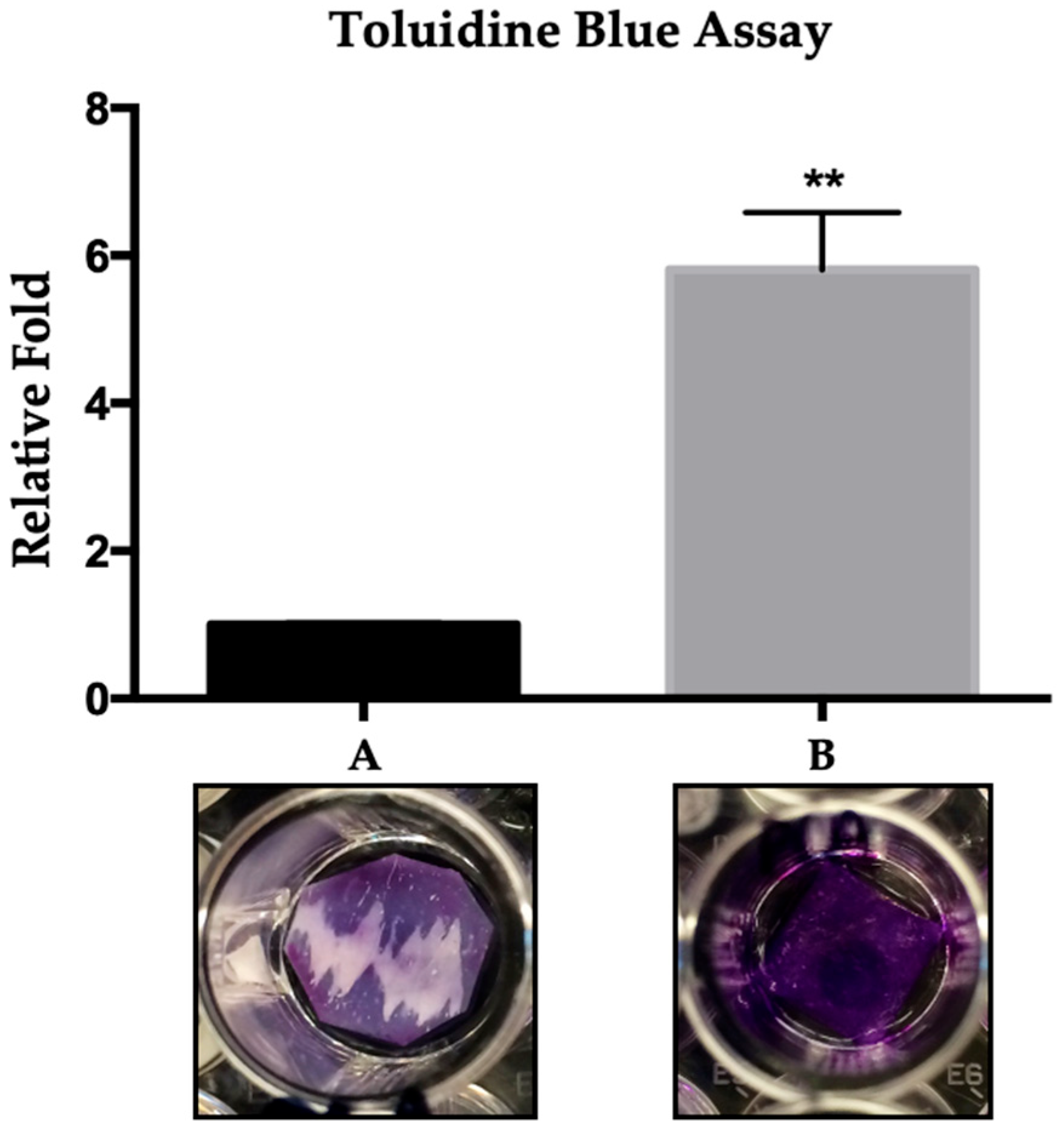
2.3. Toluidine Blue Assay for Heparin Conjugated DAS-COL (DAS-COL-HEP)
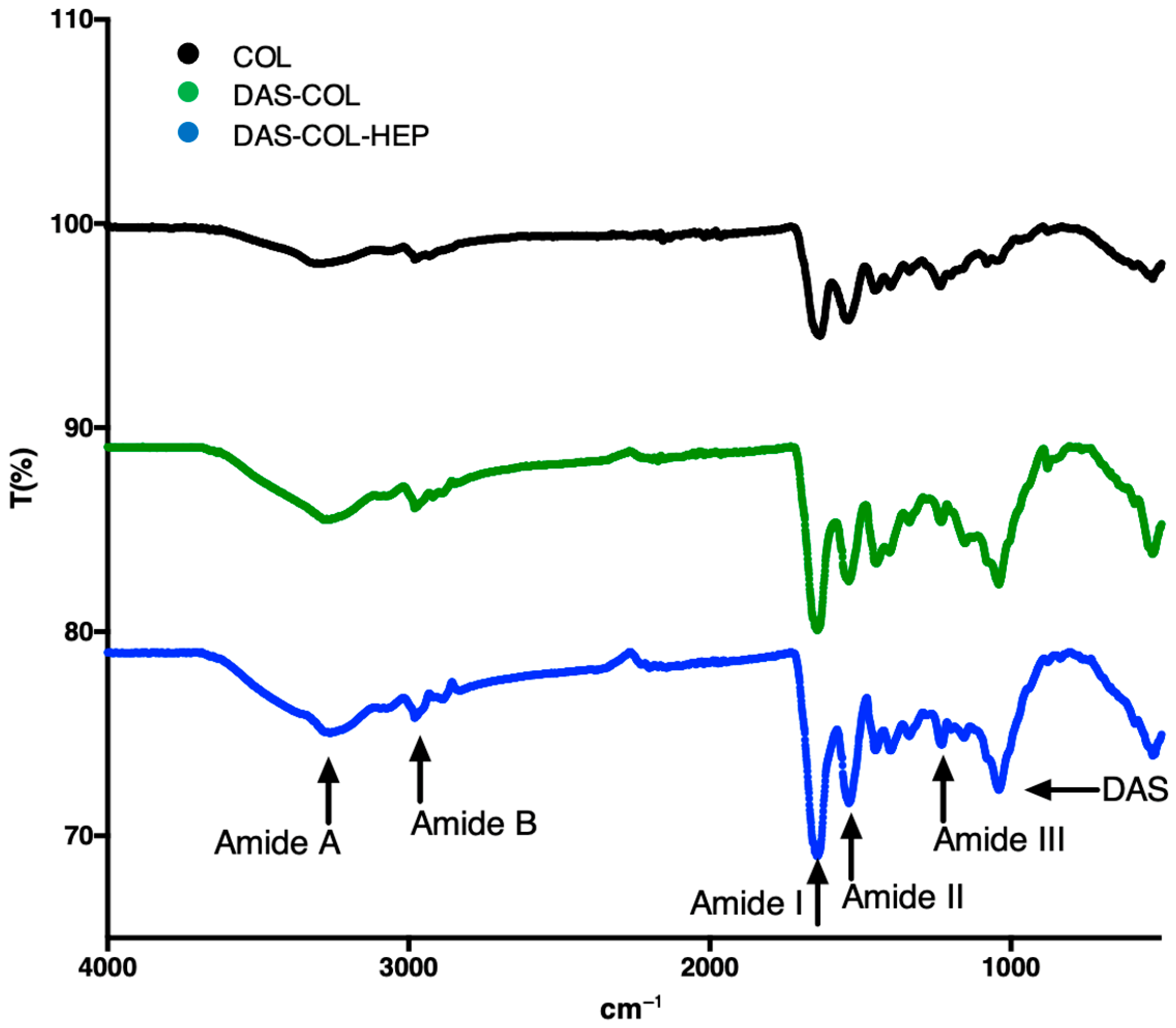
2.4. FT-IR Analysis of DAS-Crosslinked and Heparin-Conjugated Collagen
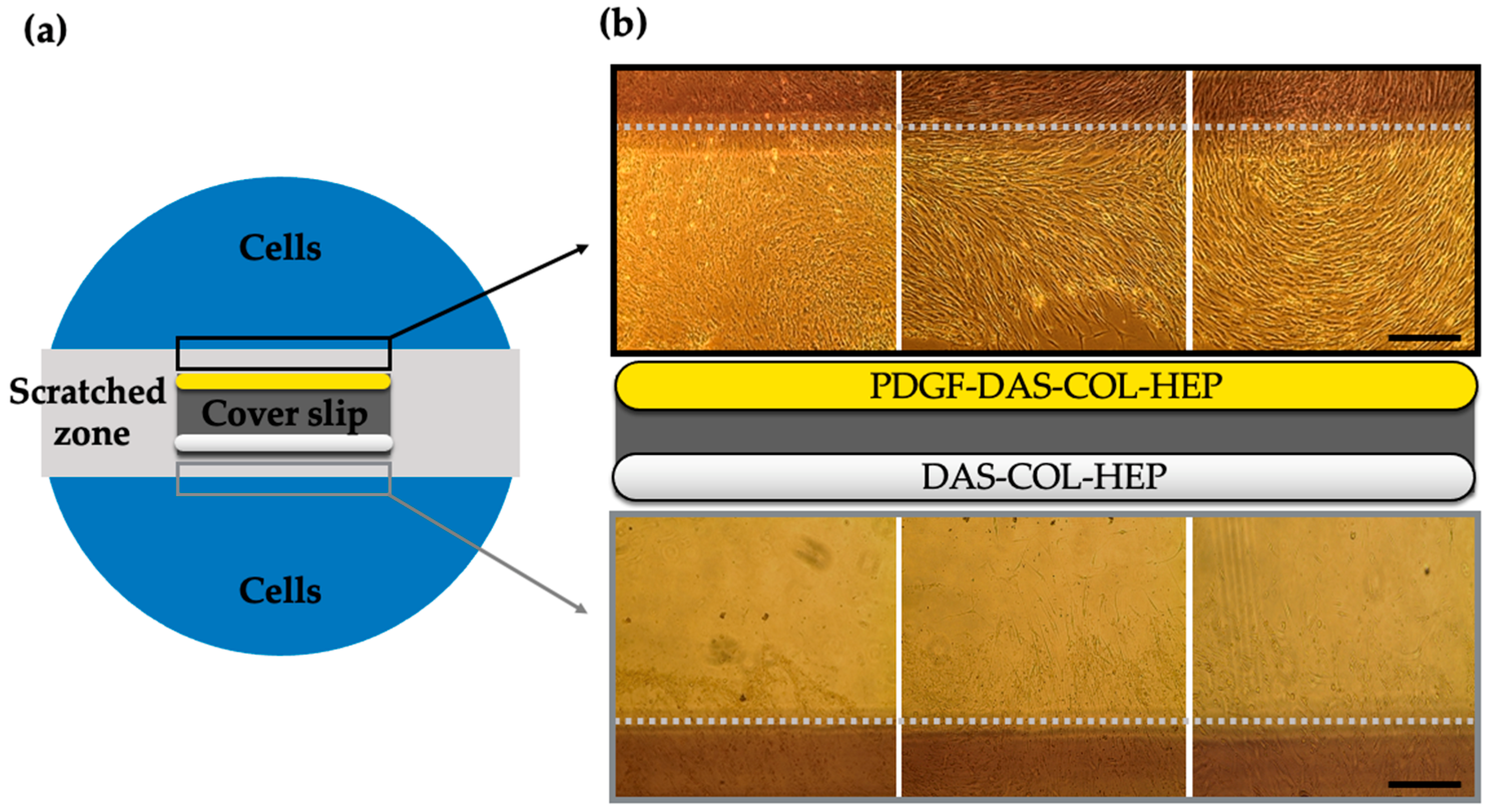
2.5. Chondrocyte Migration Test of PDGF-BB Conjugated DAS-COL-HEP
2.6. Chondrocyte Viability and Proliferation Test of TGF-β3 Conjugated DAS-COL-HEP
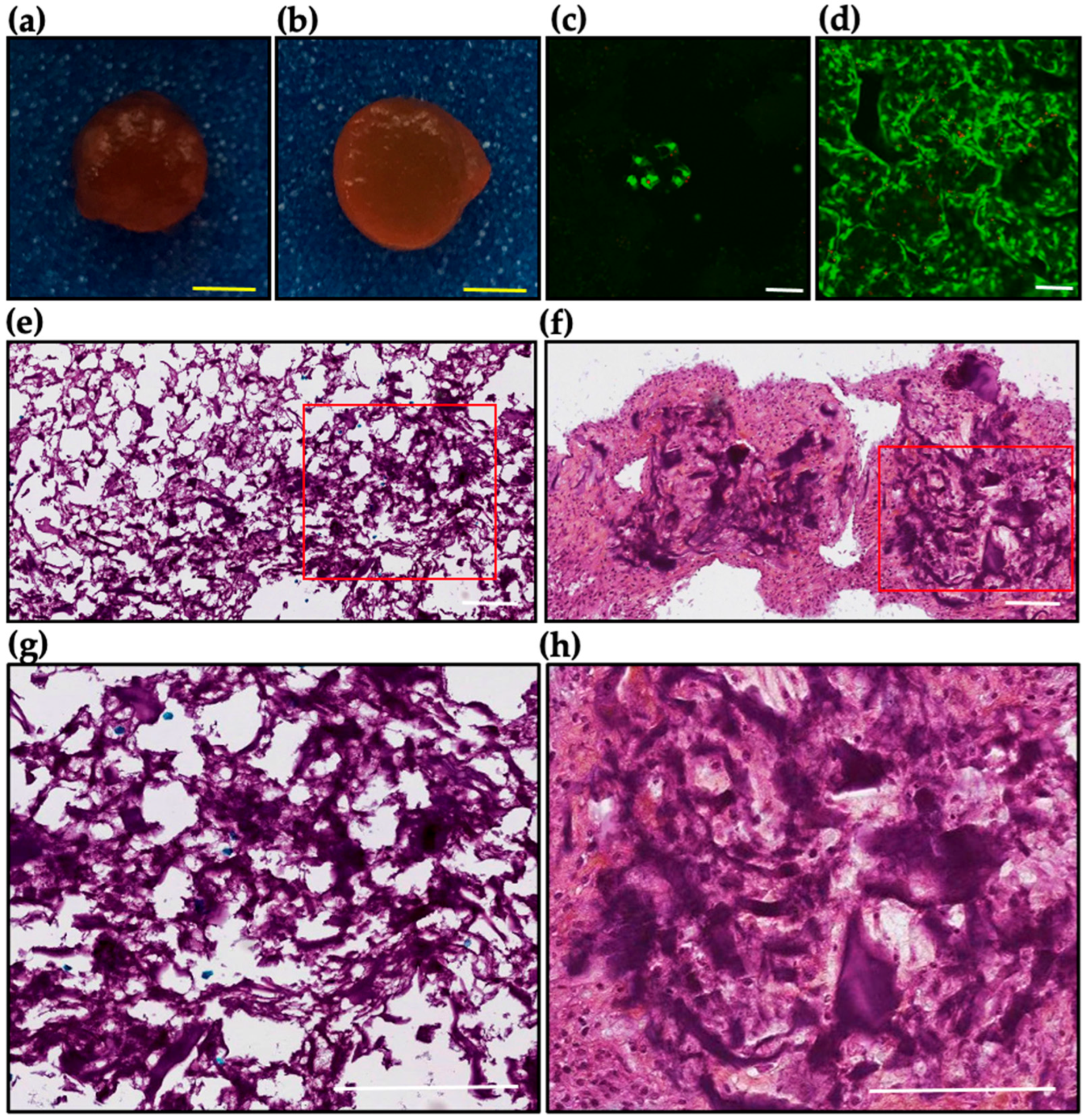
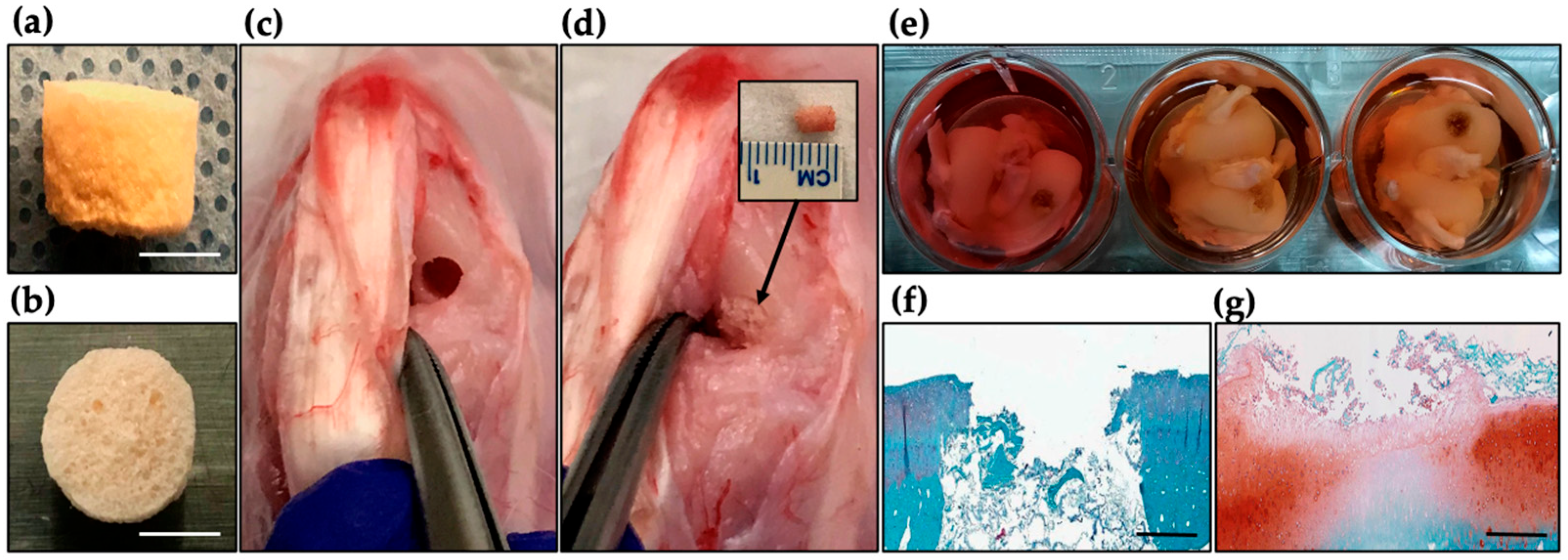
2.7. Ex Vivo Evaluation of DAS-COL-HEP Blocks in a Rabbit Osteochondral Knee Model
2.8. Discussion
3. Conclusions
4. Materials and Methods
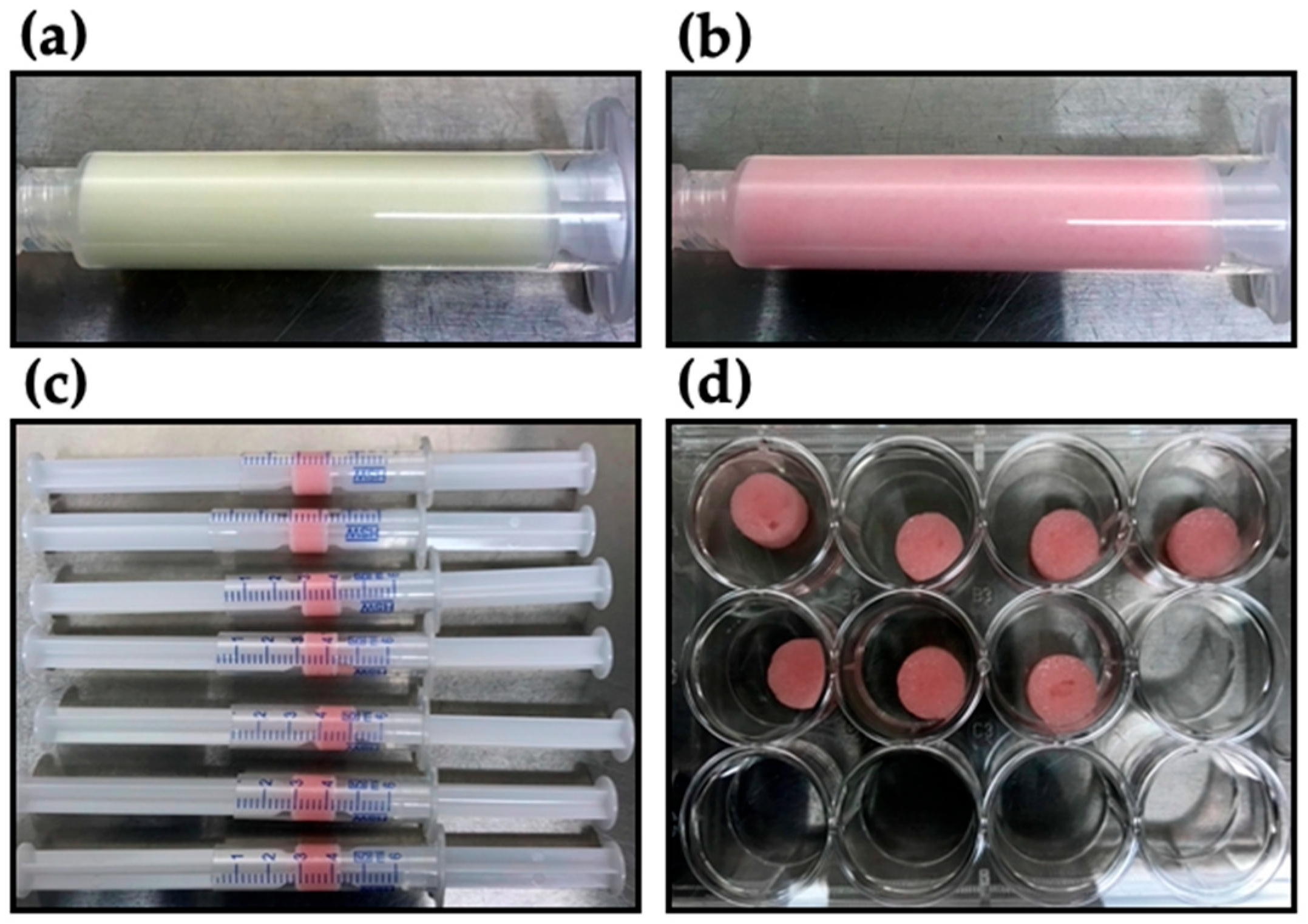
4.1. Preparation of Dialdehyde Starch Crosslinked Collagen (DAS-COL)
4.2. Compression Test of DAS-COL Gel Blocks
4.3. Degradation Test of DAS-COL Gel Blocks
4.4. Toluidine Blue Assay of Heparin-Conjugated DAS-COL (DAS-COL-HEP)
4.5. FT-IR of COL, DAS-COL, and DAS-COL-HEP
4.6. Cell Scratch Assay of PDBF-BB-Conjugated DAS-COL-HEP
4.7. Cell Encapsulated DAS-COL-HEP in the TGF- β3 Growth Media
4.8. Ex Vivo Culture of DAS-COL-HEP Gel Block Implanted Rabbit Osteochondral Knee
4.9. Statistical Analysis
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DAS | Dialdehyde Starch |
| COL | Collagen |
| HEP | Heparin |
| FT-IR | Fourier Transform Infrared (FT-IR) spectrometry |
| EDC | 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride |
| NHS | N-hydroxy succinimide |
| PDGF | Platelet-Derived Growth Factor |
| TGF | Transforming Growth Factor |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| GAG | Glycosaminoglycan |
| TBD | Decalcifier |
References
- Zhu, J.; Li, Z.; Zou, Y.; Lu, G.; Ronca, A.; D’Amora, U.; Liang, J.; Fan, Y.; Zhang, X.; Sun, Y. Advanced application of collagen-based biomaterials in tissue repair and restoration. J. Leather Sci. Eng. 2022, 4, 30. [Google Scholar] [CrossRef]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef]
- Wang, H. The Potential of Collagen Treatment for Comorbid Diseases. Polymers 2023, 15, 3999. [Google Scholar] [CrossRef]
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef]
- Gajbhiye, S.; Wairkar, S. Collagen fabricated delivery systems for wound healing: A new roadmap. Biomater. Adv. 2022, 142, 213152. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The development of collagen based composite scaffolds for bone regeneration. Bioact. Mater. 2018, 3, 129–138. [Google Scholar] [CrossRef]
- Zheng, L.; Tseomashko, N.; Voronova, A.; Vasil’kov, A.; Hu, X.; Wang, X. Recent advances of collagen composite biomaterials for biomedical engineering: Antibacterial functionalization and 3D-printed architecturalization. Collagen Leather 2024, 6, 22. [Google Scholar] [CrossRef]
- Sarrigiannidis, S.O.; Rey, J.M.; Dobre, O.; González-García, C.; Dalby, M.J.; Salmeron-Sanchez, M. A tough act to follow: Collagen hydrogel modifications to improve mechanical and growth factor loading capabilities. Mater. Today Bio 2021, 10, 100098. [Google Scholar] [CrossRef]
- Li, T.; Zhou, Z.; Xie, Y.; Cai, W.; Zhu, X.; Jia, Y.; Zhang, Z.; Xu, F.; Huang, G. Engineering strong and tough collagen hydrogels and tissue constructs via twisting and crosslinking. Cell Rep. Phys. Sci. 2025, 6, 102454. [Google Scholar] [CrossRef]
- Ye, B.; Wu, B.; Su, Y.; Sun, T.; Guo, X. Recent Advances in the Application of Natural and Synthetic Polymer-Based Scaffolds in Musculoskeletal Regeneration. Polymers 2022, 14, 4566. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Lou, Y.Y.; Li, T.H.; Liu, B.Z.; Chen, K.; Zhang, D.; Li, T. Cross-linking methods of type I collagen-based scaffolds for cartilage tissue engineering. Am. J. Transl. Res. 2022, 14, 1146–1159. [Google Scholar] [PubMed]
- Islam, M.M.; AbuSamra, D.B.; Chivu, A.; Argüeso, P.; Dohlman, C.H.; Patra, H.K.; Chodosh, J.; González-Andrades, M. Optimization of Collagen Chemical Crosslinking to Restore Biocompatibility of Tissue-Engineered Scaffolds. Pharmaceutics 2021, 13, 832. [Google Scholar] [CrossRef]
- Angele, P.; Abke, J.; Kujat, R.; Faltermeier, H.; Schumann, D.; Nerlich, M.; Kinner, B.; Englert, C.; Ruszczak, Z.; Mehrl, R.; et al. Influence of different collagen species on physico-chemical properties of crosslinked collagen matrices. Biomaterials 2004, 25, 2831–2841. [Google Scholar] [CrossRef] [PubMed]
- Powell, H.M.; Boyce, S.T. EDC cross-linking improves skin substitute strength and stability. Biomaterials 2006, 27, 5821–5827. [Google Scholar] [CrossRef]
- Adamiak, K.; Sionkowska, A. Current methods of collagen cross-linking: Review. Int. J. Biol. Macromol. 2020, 161, 550–560. [Google Scholar] [CrossRef]
- Parhi, R. Cross-Linked Hydrogel for Pharmaceutical Applications: A Review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Gough, J.E.; Scotchford, C.A.; Downes, S. Cytotoxicity of glutaraldehyde crosslinked collagen/poly(vinyl alcohol) films is by the mechanism of apoptosis. J. Biomed. Mater. Res. 2002, 61, 121–130. [Google Scholar] [CrossRef]
- St Clair, M.B.; Bermudez, E.; Gross, E.A.; Butterworth, B.E.; Recio, L. Evaluation of the genotoxic potential of glutaraldehyde. Environ. Mol. Mutagen. 1991, 18, 113–119. [Google Scholar] [CrossRef]
- Shepherd, D.V.; Shepherd, J.H.; Ghose, S.; Kew, S.J.; Cameron, R.E.; Best, S.M. The process of EDC-NHS Cross-linking of reconstituted collagen fibres increases collagen fibrillar order and alignment. APL Mater. 2015, 3, 014902. [Google Scholar] [CrossRef]
- Kawamura, T.; Yunoki, S.; Ohyabu, Y.; Uraoka, T.; Muramatsu, K. Crosslinking Efficacy and Cytotoxicity of Genipin and Its Activated Form Prepared by Warming It in a Phosphate Buffer: A Comparative Study. Materials 2021, 14, 6600. [Google Scholar] [CrossRef]
- Wang, H.; Wan, J.; Zhang, Z.; Hou, R. Recent advances on 3D-bioprinted gelatin methacrylate hydrogels for tissue engineering in wound healing: A review of current applications and future prospects. Int. Wound J. 2024, 21, e14533. [Google Scholar] [CrossRef] [PubMed]
- Zennifer, A.; Manivannan, S.; Sethuraman, S.; Kumbar, S.G.; Sundaramurthi, D. 3D bioprinting and photocrosslinking: Emerging strategies & future perspectives. Biomater. Adv. 2022, 134, 112576. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Xu, J.; Yu, Q.; Zhang, J.; Hu, X.; Sun, C.; Zhang, H. Photo-Crosslinkable Hydrogels for 3D Bioprinting in the Repair of Osteochondral Defects: A Review of Present Applications and Future Perspectives. Micromachines 2022, 13, 1038. [Google Scholar] [CrossRef]
- Klotz, B.J.; Gawlitta, D.; Rosenberg, A.; Malda, J.; Melchels, F.P.W. Gelatin-Methacryloyl Hydrogels: Towards Biofabrication-Based Tissue Repair. Trends Biotechnol. 2016, 34, 394–407. [Google Scholar] [CrossRef]
- Ali, S.M.; Patrawalla, N.Y.; Kajave, N.S.; Brown, A.B.; Kishore, V. Species-Based Differences in Mechanical Properties, Cytocompatibility, and Printability of Methacrylated Collagen Hydrogels. Biomacromolecules 2022, 23, 5137–5147. [Google Scholar] [CrossRef]
- Duymaz, D.; Karaoğlu, İ.C.; Kizilel, S. Effect of Photoinitiation Process on Photo-Crosslinking of Gelatin Methacryloyl Hydrogel Networks. bioRxiv 2025, 46, e00376. [Google Scholar] [CrossRef]
- Brinkman, W.T.; Nagapudi, K.; Thomas, B.S.; Chaikof, E.L. Photo-cross-linking of type I collagen gels in the presence of smooth muscle cells: Mechanical properties, cell viability, and function. Biomacromolecules 2003, 4, 890–895. [Google Scholar] [CrossRef]
- Alizadehgharib, S.; Östberg, A.K.; Dahlgren, U. Effects of the methacrylate/acrylate monomers HEMA, TEGDMA, DEGDA, and EMA on the immune system. Clin. Exp. Dent. Res. 2017, 3, 227–234. [Google Scholar] [CrossRef]
- Lugović-Mihić, L.; Filija, E.; Varga, V.; Premuž, L.; Parać, E.; Tomašević, R.; Barac, E.; Špiljak, B. Unwanted Skin Reactions to Acrylates: An Update. Cosmetics 2024, 11, 127. [Google Scholar] [CrossRef]
- Xia, D.; Chen, J.; Zhang, Z.; Dong, M. Emerging polymeric biomaterials and manufacturing techniques in regenerative medicine. Aggregate 2022, 3, e176. [Google Scholar] [CrossRef]
- Shiroud Heidari, B.; Ruan, R.; Vahabli, E.; Chen, P.; De-Juan-Pardo, E.M.; Zheng, M.; Doyle, B. Natural, synthetic and commercially-available biopolymers used to regenerate tendons and ligaments. Bioact. Mater. 2023, 19, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Sionkowska, A. Current research on the blends of natural and synthetic polymers as new biomaterials: Review. Prog. Polym. Sci. 2011, 36, 1254–1276. [Google Scholar] [CrossRef]
- Suamte, L.; Tirkey, A.; Babu, P.J. Design of 3D smart scaffolds using natural, synthetic and hybrid derived polymers for skin regenerative applications. Smart Mater. Med. 2023, 4, 243–256. [Google Scholar] [CrossRef]
- Bansal, P.; Katiyar, D.; Prakash, S.; Rao, N.G.R.; Saxena, V.; Kumar, V.; Kumar, A. Applications of some biopolymeric materials as medical implants: An overview. Mater. Today Proc. 2022, 65, 3377–3381. [Google Scholar] [CrossRef]
- Jie, I.W.K.; Lee, K.W.A.; Yoon, S.E.; Song, J.K.; Chan, L.K.W.; Lee, C.H.; Jeong, E.; Kim, J.H.; Yi, K.H. Advancements in Clinical Utilization of Recombinant Human Collagen: An Extensive Review. Life 2025, 15, 582. [Google Scholar] [CrossRef]
- Ning, C.; Li, P.; Gao, C.; Fu, L.; Liao, Z.; Tian, G.; Yin, H.; Li, M.; Sui, X.; Yuan, Z.; et al. Recent advances in tendon tissue engineering strategy. Front. Bioeng. Biotechnol. 2023, 11, 1115312. [Google Scholar] [CrossRef]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17 (Suppl. 4), 467–479. [Google Scholar] [CrossRef]
- Delgado, L.M.; Bayon, Y.; Pandit, A.; Zeugolis, D.I. To cross-link or not to cross-link? Cross-linking associated foreign body response of collagen-based devices. Tissue Eng. Part B Rev. 2015, 21, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Mohd Roslan, M.R.; Mohd Kamal, N.L.; Abdul Khalid, M.F.; Mohd Nasir, N.F.; Cheng, E.M.; Beh, C.Y.; Tan, J.S.; Mohamed, M.S. The State of Starch/Hydroxyapatite Composite Scaffold in Bone Tissue Engineering with Consideration for Dielectric Measurement as an Alternative Characterization Technique. Materials 2021, 14, 1960. [Google Scholar] [CrossRef]
- Koski, C.; Onuike, B.; Bandyopadhyay, A.; Bose, S. Starch-Hydroxyapatite Composite Bone Scaffold Fabrication Utilizing a Slurry Extrusion-Based Solid Freeform Fabricator. Addit. Manuf. 2018, 24, 47–59. [Google Scholar] [CrossRef]
- Duan, Q.; Liu, H.; Zheng, L.; Cai, D.; Huang, G.; Liu, Y.; Guo, R. Novel resorbable bone wax containing β-TCP and starch microspheres for accelerating bone hemostasis and promoting regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1105306. [Google Scholar] [CrossRef]
- Ali Akbari Ghavimi, S.; Ebrahimzadeh, M.H.; Shokrgozar, M.A.; Solati-Hashjin, M.; Abu Osman, N.A. Effect of starch content on the biodegradation of polycaprolactone/starch composite for fabricating in situ pore-forming scaffolds. Polym. Test. 2015, 43, 94–102. [Google Scholar] [CrossRef]
- Lee, C.-S.; Hwang, H.S. Starch-Based Hydrogels as a Drug Delivery System in Biomedical Applications. Gels 2023, 9, 951. [Google Scholar] [CrossRef]
- Waghmare, V.S.; Wadke, P.R.; Dyawanapelly, S.; Deshpande, A.; Jain, R.; Dandekar, P. Starch based nanofibrous scaffolds for wound healing applications. Bioact. Mater. 2018, 3, 255–266. [Google Scholar] [CrossRef]
- Mei, S.; Roopashree, R.; Altalbawy, F.M.A.; Hamid, J.A.; Ahmed, H.H.; Naser, B.K.; Rizaev, J.; AbdulHussein, A.H.; Saud, A.; Hammoodi, H.A.; et al. Synthesis, characterization, and applications of starch-based nano drug delivery systems for breast cancer therapy: A review. Int. J. Biol. Macromol. 2024, 280, 136058. [Google Scholar] [CrossRef]
- Shapi’i, R.A.; Othman, S.H.; Nordin, N.; Kadir Basha, R.; Nazli Naim, M. Antimicrobial properties of starch films incorporated with chitosan nanoparticles: In vitro and in vivo evaluation. Carbohydr. Polym. 2020, 230, 115602. [Google Scholar] [CrossRef] [PubMed]
- Sarhadi, H.; Shahdadi, F.; Salehi Sardoei, A.; Hatami, M.; Ghorbanpour, M. Investigation of physio-mechanical, antioxidant and antimicrobial properties of starch–zinc oxide nanoparticles active films reinforced with Ferula gummosa Boiss essential oil. Sci. Rep. 2024, 14, 5789. [Google Scholar] [CrossRef] [PubMed]
- Skopinska-Wisniewska, J.; Wegrzynowska-Drzymalska, K.; Bajek, A.; Maj, M.; Sionkowska, A. Is dialdehyde starch a valuable cross-linking agent for collagen/elastin based materials? J. Mater. Sci. Mater. Med. 2016, 27, 67. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.I.; Olmer, M.; Baek, J.; D’Lima, D.D.; Lotz, M.K. Platelet-derived growth factor-coated decellularized meniscus scaffold for integrative healing of meniscus tears. Acta Biomater. 2018, 76, 126–134. [Google Scholar] [CrossRef]
- Baek, J.; Lee, K.I.; Ra, H.J.; Lotz, M.K.; D’Lima, D.D. Collagen fibrous scaffolds for sustained delivery of growth factors for meniscal tissue engineering. Nanomedicine 2022, 17, 77–93. [Google Scholar] [CrossRef]
- Lee, K.I.; Gamini, R.; Olmer, M.; Ikuta, Y.; Hasei, J.; Baek, J.; Alvarez-Garcia, O.; Grogan, S.P.; D’Lima, D.D.; Asahara, H.; et al. Mohawk is a transcription factor that promotes meniscus cell phenotype and tissue repair and reduces osteoarthritis severity. Sci. Transl. Med. 2020, 12, eaan7967. [Google Scholar] [CrossRef] [PubMed]
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Muhammad, N.; Safi, S.Z.; Rahim, A.; Rizvi, S.A.A.; Rehman, I.U. FTIR analysis of natural and synthetic collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- Salthouse, D.; Goulding, P.D.; Reay, S.L.; Jackson, E.L.; Xu, C.; Ahmed, R.; Mearns-Spragg, A.; Novakovic, K.; Hilkens, C.M.U.; Ferreira, A.M. Amine-reactive crosslinking enhances type 0 collagen hydrogel properties for regenerative medicine. Front. Bioeng. Biotechnol. 2024, 12, 1391728. [Google Scholar] [CrossRef]
- Brudzyńska, P.; Kulka-Kamińska, K.; Piwowarski, Ł.; Lewandowska, K.; Sionkowska, A. Dialdehyde Starch as a Cross-Linking Agent Modifying Fish Collagen Film Properties. Materials 2024, 17, 1475. [Google Scholar] [CrossRef]
- Tian, R.; Zhao, Y.; Fu, Y.; Yang, S.; Jiang, L.; Sui, X. Sacrificial hydrogen bonds enhance the performance of covalently crosslinked composite films derived from soy protein isolate and dialdehyde starch. Food Chem. 2024, 456, 140055. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Sun, Y.; Wu, Y.; Wang, J.; Ding, Y.; Cheng, J.; Guo, M. Mechanical, microstructural, and rheological characterization of gelatin-dialdehyde starch hydrogels constructed by dual dynamic crosslinking. LWT 2022, 161, 113374. [Google Scholar] [CrossRef]
- Yamaoka, H.; Yamaoka, K.; Watanabe, S.; Tanaka, H.; Hosoyamada, M.; Komuro, Y. Lactose Stabilization Prolongs In Vivo Retention of Cross-linked Fish Collagen Subcutaneous Grafts in Nude Mice. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4601. [Google Scholar] [CrossRef]
- Mori, H.; Shimizu, K.; Hara, M. Dynamic viscoelastic properties of collagen gels with high mechanical strength. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 3230–3236. [Google Scholar] [CrossRef]
- Omobono, M.A.; Zhao, X.; Furlong, M.A.; Kwon, C.-H.; Gill, T.J.; Randolph, M.A.; Redmond, R.W. Enhancing the stiffness of collagen hydrogels for delivery of encapsulated chondrocytes to articular lesions for cartilage regeneration. J. Biomed. Mater. Res. Part A 2015, 103, 1332–1338. [Google Scholar] [CrossRef]
- Lin, H.; Zhao, Y.; Sun, W.; Chen, B.; Zhang, J.; Zhao, W.; Xiao, Z.; Dai, J. The effect of crosslinking heparin to demineralized bone matrix on mechanical strength and specific binding to human bone morphogenetic protein-2. Biomaterials 2008, 29, 1189–1197. [Google Scholar] [CrossRef]
- Nazarzadeh Zare, E.; Khorsandi, D.; Zarepour, A.; Yilmaz, H.; Agarwal, T.; Hooshmand, S.; Mohammadinejad, R.; Ozdemir, F.; Sahin, O.; Adiguzel, S.; et al. Biomedical applications of engineered heparin-based materials. Bioact. Mater. 2024, 31, 87–118. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, Y.; Mizumachi, H.; Yoshida, K.; Ijima, H. Heparin-conjugated collagen as a potent growth factor-localizing and stabilizing scaffold for regenerative medicine. Regen. Ther. 2020, 15, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Sapin-Minet, A.; Stefan, L.; Perrin, J.; Raeth-Fries, I.; Gaucher, C. Heparinized collagen-based hydrogels for tissue engineering: Physical, mechanical and biological properties. Int. J. Pharm. 2025, 670, 125126. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Rigueur, D.; Lyons, K.M. TGFβ signaling in cartilage development and maintenance. Birth Defects Res. Part C Embryo Today Rev. 2014, 102, 37–51. [Google Scholar] [CrossRef]
- Du, X.; Cai, L.; Xie, J.; Zhou, X. The role of TGF-beta3 in cartilage development and osteoarthritis. Bone Res. 2023, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mehmood, K.; Chang, Y.-F.; Tang, Z.; Li, Y.; Zhang, H. The molecular mechanisms of glycosaminoglycan biosynthesis regulating chondrogenesis and endochondral ossification. Life Sci. 2023, 335, 122243. [Google Scholar] [CrossRef]
- Zhao, G.Z.; Zhang, L.Q.; Liu, Y.; Fang, J.; Li, H.Z.; Gao, K.H.; Chen, Y.Z. Effects of platelet-derived growth factor on chondrocyte proliferation, migration and apoptosis via regulation of GIT1 expression. Mol. Med. Rep. 2016, 14, 897–903. [Google Scholar] [CrossRef]
- Tamaddon, M.; Wang, L.; Liu, Z.; Liu, C. Osteochondral tissue repair in osteoarthritic joints: Clinical challenges and opportunities in tissue engineering. Bio-Des. Manuf. 2018, 1, 101–114. [Google Scholar] [CrossRef]
- Chiang, K.-Y.; Matsumura, F.; Yu, C.-C.; Qi, D.; Nagata, Y.; Bonn, M.; Meister, K. True Origin of Amide I Shifts Observed in Protein Spectra Obtained with Sum Frequency Generation Spectroscopy. J. Phys. Chem. Lett. 2023, 14, 4949–4954. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.K.; Baek, J.; Grogan, S.P.; Koo, T.-H.; D’Lima, D.D. Dialdehyde Starch Cross-Linked Collagen with Heparin Conjugation: Characterization and Feasibility Study for Osteochondral Tissue Repair. Gels 2025, 11, 850. https://doi.org/10.3390/gels11110850
Lee JK, Baek J, Grogan SP, Koo T-H, D’Lima DD. Dialdehyde Starch Cross-Linked Collagen with Heparin Conjugation: Characterization and Feasibility Study for Osteochondral Tissue Repair. Gels. 2025; 11(11):850. https://doi.org/10.3390/gels11110850
Chicago/Turabian StyleLee, Jason K., Jihye Baek, Shawn P. Grogan, Tae-Hoon Koo, and Darryl D. D’Lima. 2025. "Dialdehyde Starch Cross-Linked Collagen with Heparin Conjugation: Characterization and Feasibility Study for Osteochondral Tissue Repair" Gels 11, no. 11: 850. https://doi.org/10.3390/gels11110850
APA StyleLee, J. K., Baek, J., Grogan, S. P., Koo, T.-H., & D’Lima, D. D. (2025). Dialdehyde Starch Cross-Linked Collagen with Heparin Conjugation: Characterization and Feasibility Study for Osteochondral Tissue Repair. Gels, 11(11), 850. https://doi.org/10.3390/gels11110850






