Composite Hydrogels with Embedded Electrospun Fibers as Drug Delivery Systems
Abstract
1. Introduction
2. Electrospinning Technique
2.1. Overview
2.2. ES Fundamentals
2.3. Incorporation of Drug into Nanofibers
2.4. Emerging Challenges in ES
| System Processing | Bioactive Agent | Polymer | Aim | Main Focus | Application Area | Ref. |
|---|---|---|---|---|---|---|
| Uniaxial/coaxial electrospinning | hydrocortisone | Polycaprolactone (PCL) | Alternative to cream-based therapies for 24 h release | In vitro release, ex vivo penetration, and permeation on porcine skin | Skin disease therapy | [66] |
| Twin-screw melt granulation of loaded-electrospun fibers | itraconazole | poly-vinylpyrrolidone (PVP)-vinyl acetate, hydroxypropyl methylcellulose (HPMC) | To improve the dissolution of poorly water-soluble drugs | Post-processing, stability | Biopharmaceutics industrial processing | [67] |
| Uniaxial electrospinning | pregabalin | PVP, HPMC, polyvinyl alcohol (PVA) | Drug release and solubility control | Release from water-soluble polymers | Drug delivery systems | [68] |
| Uniaxial electrospinning | budesonide | PCL, poly(D,L-lactide-co-glycolide) (PLGA) | Morphology control, fiber diameter prediction | Processing parameters, release | Drug delivery systems | [69] |
| Nanoparticles in nano/microfibers | Gentamicin, dexamethasone | Poly(lactic acid) (PLA) fibers embedded with halloysite nanotubes | Dual hydrophilic/hydrophobic release | Drug release | Drug delivery systems | [70] |
| Uniaxial/coaxial electrospinning | captopril | Ethyl cellulose (EC) | Up-scaling | Bench-top and scale-up method, release | Drug delivery systems | [71] |
| Uniaxial electrospinning as coating | vancomycin | PVA | Antibacterial implant coating | Antibacterial properties | Biomaterial devices | [72] |
| Uniaxial electrospinning (flat/drum collectors) | melatonin | PCL | Fabrication optimization for up-scaling | Formulations | Wound healing | [73] |
| Microsphere suspension electrospinning | ampicilin, rhodamine | PVP | To increase the load capacity, to eliminate the burst effect | Manufacturing method | Drug delivery | [74] |
| Mono-, bi-, and tri-layer fibers | Acetaminophen | Cellulose acetate (CA) | Gradient drug distribution | Release profiles | Drug delivery | [75] |
| Uniaxial electrospinning | sulfamethoxazole | PVP, PVA, HPMC | Drug encapsulation | Release profiles, solubility | Drug delivery | [76] |
| Uniaxial, coaxial and layer-by-layer electrospinning | tofacitinib | PCL | 3 days- release | Morphology, release, permeation | Skin disease therapy | [77] |
| High-speed electrospinning | doxycycline-hyclate | 2-hydroxypropyl-β-cyclodextrin (HP-β-CD) | Quality assurance system, upscaling | Load, morphology, monitoring methods | Drug delivery | [78] |
| Electrospun fiber-in/on -film composites | phenytoin | Ethyl cellulose | Tailorable in vitro drug release | Release profiles | Wound healing | [79] |
| Film based on electrospun fibers | quercetin | Eudragit EPO/sodium hyaluronate | Antioxidant and antiperoxidation strategies | Antioxidant properties | Drug encapsulation | [80] |
| Core–shell electrospun nanofibers coated with silver nanoparticles | rifampicin | PCL | Antibacterial effect | Morphology, antibacterial properties | Tissue engineering | [81] |
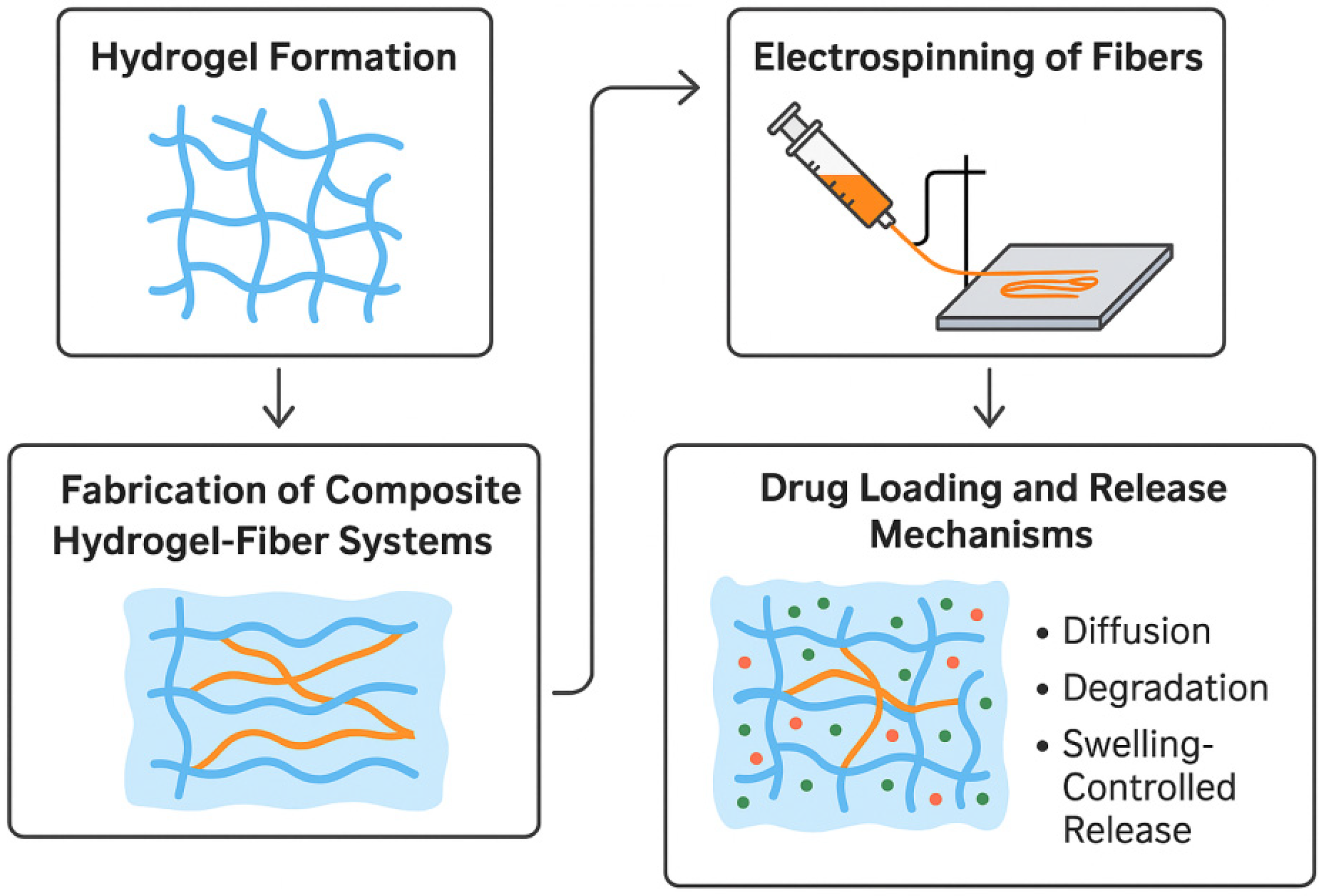
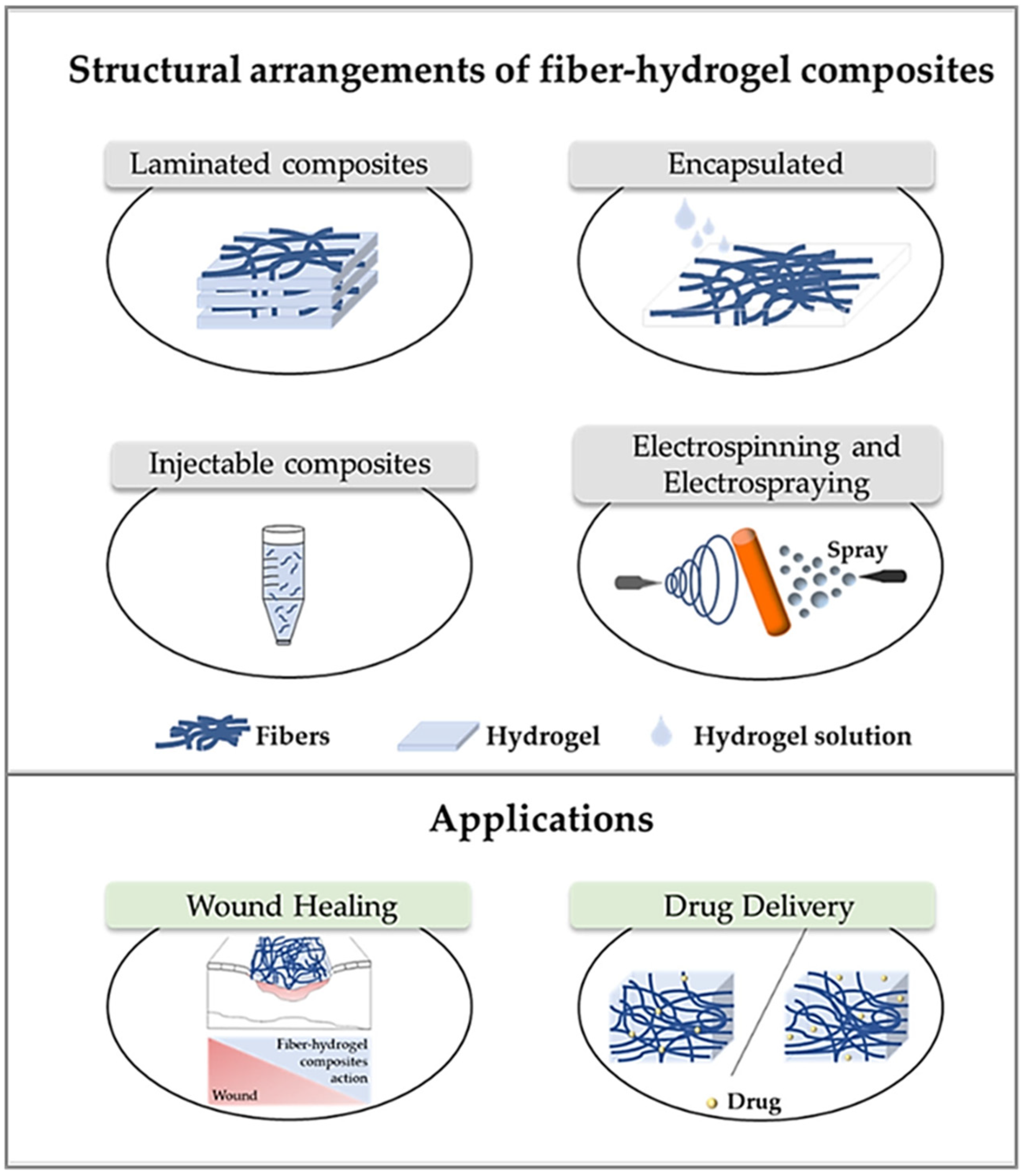
3. Composite Materials Based on ES Nanofibrous Mats and Hydrogels
Platforms Combining Hydrogel 3D Printing and Electrospinning
4. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.; Xu, S.; Wang, R.; Che, Y.; Han, C.; Feng, W.; Wang, C.; Zhao, W. Electrospun nanofiber/hydrogel composite materials and their tissue engineering applications. J. Mater. Sci. Technol. 2023, 162, 157. [Google Scholar] [CrossRef]
- Patel, D.K.; Won, S.-Y.; Jung, E.; Han, S.S. Recent progress in biopolymer-based electrospun nanofibers and their potential biomedical applications: A review. Int. J. Biol. Macromol. 2025, 239, 139426. [Google Scholar] [CrossRef]
- Shabani, A.; Al, G.A.; Berri, N.; Castro-Dominguez, B.; Leese, H.S.; Martinez-Hernandez, U. Electrospinning technology, machine learning, and control approaches: A review. Adv. Eng. Mater. 2025, 27, 2401353. [Google Scholar] [CrossRef]
- Choi, C.; Yun, E.; Cha, C. Emerging technology of nanofiber-composite hydrogels for biomedical applications. Macromol. Biosci. 2023, 23, e2300222. [Google Scholar] [CrossRef] [PubMed]
- Nanda, D.; Behera, D.; Pattnaik, S.S.; Sahoo, D.; Panigrahi, S.K.; Mallick, B.C. Advances in natural polymer-based hydrogels: Synthesis, applications, and future directions in biomedical and environmental fields. Discov. Polym. 2025, 2, 6. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, X.; Liu, J.; Zhang, H.; Fu, D. Advances in the application of natural/synthetic hybrid hydrogels in tissue engineering and delivery systems: A comprehensive review. Int. J. Pharm. 2025, 672, 125323. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.A.; Aslam, M.A.; Abdullah, M.F.B.; Al-Arjan, W.S.; Stojanovic, G.M.; Hasan, A. Hydrogels: Classifications, fundamental properties, applications, and scopes in recent advances in tissue engineering and regenerative medicine—A comprehensive review. Arab. J. Chem. 2024, 17, 105968. [Google Scholar] [CrossRef]
- Protsak, I.S.; Morozov, Y.M. Fundamentals and advances in stimuli-responsive hydrogels and their applications: A review. Gels 2025, 11, 30. [Google Scholar] [CrossRef]
- Santhamoorthy, M.; Kim, S.-C. A review of the development of biopolymer hydrogel-based scaffold materials for drug delivery and tissue engineering applications. Gels 2025, 11, 178. [Google Scholar] [CrossRef]
- Ghosh, T.; Das, T.; Purwar, R. Review of electrospun hydrogel nanofiber system: Synthesis, properties and applications. Polym. Eng. Sci. 2021, 61, 1887–1911. [Google Scholar] [CrossRef]
- Sheffield, C.; Meyers, K.; Johnson, E.; Rajachar, R.M. Application of composite hydrogels to control physical properties in tissue engineering and regenerative medicine. Gels 2018, 4, 51. [Google Scholar] [CrossRef]
- Mele, E. Chapter 3: Biomimetic electrospun composites: From fundamental insights to commercialization. In Electrospinning: From Basic Research to Commercialization; Kny, E., Ghosal, K., Thomas, S., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2018; pp. 55–78. [Google Scholar]
- Thenmozhi, S.; Dharmaraj, N.; Kadirvelu, K.; Kim, H.Y. Electrospun nanofibers: New generation materials for advanced applications. Mater. Sci. Eng. B 2017, 217, 36–48. [Google Scholar] [CrossRef]
- Kong, B.; Liu, R.; Guo, J.; Lu, L.; Zhou, Q.; Zhao, Y. Tailoring micro/nano-fibers for biomedical applications. Bioact. Mater. 2023, 19, 328–347. [Google Scholar] [CrossRef] [PubMed]
- Ahadian, S.; Obregón, R.; Ramón-Azcón, J.; Salazar, G.; Ramalingam, M. Clinical/preclinical aspects of nanofiber composites. In Nanofiber Composites for Biomedical Applications; Ramalingam, M., Ramakrishna, S., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 507–528. [Google Scholar]
- Murugupandian, R.; Suresh, A.S.; Vijaylal, L.; John, A.E.; Uthirapathy, V. A review on nanofibrous scaffolding technique for potential tissue engineering applications. Trends Biomater. Artif. Organs 2022, 36, 27–36. [Google Scholar]
- Rodríguez-Tobías, H.; Morales, G.; Grande, D. Comprehensive review on electrospinning techniques as versatile approaches toward antimicrobial biopolymeric composite fibers. Mater. Sci. Eng. C 2019, 101, 306–322. [Google Scholar] [CrossRef]
- Aldana, A.A.; Abraham, G.A. Current advances in electrospun gelatin-based scaffolds for tissue engineering applications. Int. J. Pharm. 2017, 523, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Popov Pereira da Cunha, M.D.; Caracciolo, P.C.; Abraham, G.A. Latest advances in electrospun plant-derived protein scaffolds for biomedical applications. Curr. Opin. Biomed. Eng. 2021, 18, 100243. [Google Scholar] [CrossRef]
- Montini-Ballarin, F.; Calvo, D.; Caracciolo, P.C.; Rojo, F.; Frontini, P.M.; Abraham, G.A.; Guinea, G.V. Mechanical behavior of bilayered small-diameter nanofibrous structures as biomimetic vascular grafts. J. Mech. Behav. Biomed. Mater. 2016, 60, 220–233. [Google Scholar] [CrossRef]
- Sridhar, R.; Lakshminarayanan, R.; Madhaiyan, K.; Amutha Barathi, V.; Lim, K.H.C.; Ramakrishna, S. Electrosprayed nanoparticles and electrospun nanofibers based on natural materials: Applications in tissue regeneration, drug delivery and pharmaceuticals. Chem. Soc. Rev. 2015, 44, 790–814. [Google Scholar] [CrossRef]
- Chen, K.; Hu, H.; Zeng, Y.; Pan, H.; Wang, S.; Zhang, Y.; Shi, L.; Tan, G.; Pan, W.; Liu, H. Recent advances in electrospun nanofibers for wound dressing. Eur. Polym. J. 2022, 178, 111490. [Google Scholar] [CrossRef]
- Ghosal, K.; Agatemor, C.; Špitálsky, Z.; Thomas, S.; Kny, E. Electrospinning tissue engineering and wound dressing scaffolds from polymer–titanium dioxide nanocomposites. Chem. Eng. J. 2019, 358, 1262–1278. [Google Scholar] [CrossRef]
- Rimoli, I.H.; Unalan, I.; Mutlu, N.; Michálek, M.; Abraham, G.A.; Liverani, L.; Boccaccini, A.R. Cotton wool-like ion-doped bioactive glass nanofibers: Investigation of Zn and Cu combined effect. Biomed. Mater. 2024, 19, 065001. [Google Scholar]
- Kajdič, S.; Planinšek, O.; Gašperlin, M.; Kocbek, P. Electrospun nanofibers for customized drug-delivery systems. J. Drug Deliv. Sci. Technol. 2019, 51, 672–681. [Google Scholar] [CrossRef]
- Thakkar, S.; Misra, M. Electrospun polymeric nanofibers: New horizons in drug delivery. Eur. J. Pharm. Sci. 2017, 107, 148–167. [Google Scholar] [CrossRef] [PubMed]
- Sonzogni, A.; Rivero, G.; González, V.; Abraham, G.A.; Calderón, M.; Minari, R. Nano-in-nano enteric protein delivery system: Coaxial Eudragit® L100-55 fibers containing poly(N-vinylcaprolactam) nanogels. Biomater. Sci. 2024, 12, 335. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Sagor, M.M.H.; Arafat, M.T. Functional electrospun polymeric materials for bioelectronic devices: A review. Mater. Adv. 2022, 3, 6753–6772. [Google Scholar] [CrossRef]
- Halicka, K.; Cabaj, J. Electrospun nanofibers for sensing and biosensing applications—A review. Int. J. Mol. Sci. 2021, 22, 6357. [Google Scholar] [CrossRef]
- Silva, J.A.; De Gregorio, P.R.; Rivero, G.; Abraham, G.A.; Nader-Macías, M.E.F. Immobilization of vaginal Lactobacillus in polymeric nanofibers for its incorporation in vaginal probiotic products. Eur. J. Pharm. Sci. 2021, 156, 105563. [Google Scholar] [CrossRef]
- Rather, A.H.; Khan, R.S.; Wani, T.U.; Beigh, M.A.; Sheikh, F.A. Overview on immobilization of enzymes on synthetic polymeric nanofibers fabricated by electrospinning. Biotechnol. Bioeng. 2022, 119, 9–33. [Google Scholar] [CrossRef]
- Liu, Z.; Ramakrishna, S.; Liu, X. Electrospinning and emerging healthcare and medicine possibilities. APL Bioeng. 2020, 4, 030901. [Google Scholar] [CrossRef]
- Reddy, V.S.; Tian, Y.; Zhang, C.; Ye, Z.; Roy, K.; Chinnappan, A.; Ramakrishna, S.; Liu, W.; Ghosh, R. A review on electrospun nanofibers based advanced applications: From health care to energy devices. Polymers 2021, 13, 3746. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Cerviño, M.C.; Fuioaga, C.P.; Atanase, L.I.; Abraham, G.A.; Rivero, G. Electrohydrodynamic techniques for the manufacture and/or immobilization of vesicles. Polymers 2023, 15, 795. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhang, J.; Li, J.; Li, D.; Xiao, C.; Xiao, H.; Yang, H.; Zhuang, X.; Chen, X. Electrospun polymer biomaterials. Prog. Polym. Sci. 2019, 90, 1–34. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Zeng, L.; Qiao, Z.; Liu, X.; Liu, H.; Zhang, J.; Ding, J. Fabrication of electrospun polymer nanofibers with diverse morphologies. Molecules 2019, 24, 834. [Google Scholar] [CrossRef]
- Yoo, H.S.; Kim, T.G.; Park, T.G. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Deliv. Rev. 2009, 61, 1033–1042. [Google Scholar] [CrossRef]
- Bongiovanni Abel, S.; Montini Ballarin, F.; Abraham, G.A. Combination of electrospinning with other techniques for the fabrication of 3D polymeric and composite nanofibrous scaffolds with improved cellular interactions. Nanotechnology 2020, 31, 172002. [Google Scholar] [CrossRef]
- Persano, L.; Camposeo, A.; Tekmen, C.; Pisignano, D. Industrial upscaling of electrospinning and applications of polymer nanofibers: A review. Macromol. Mater. Eng. 2013, 298, 504–520. [Google Scholar] [CrossRef]
- Kim, I.G.; Lee, J.-H.; Unnithan, A.R.; Park, C.-H.; Kim, C.S. A comprehensive electric field analysis of cylinder-type multi-nozzle electrospinning system for mass production of nanofibers. J. Ind. Eng. Chem. 2015, 31, 251–256. [Google Scholar] [CrossRef]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef]
- Bock, N.; Dargaville, T.R.; Woodruff, M.A. Electrospraying of polymers with therapeutic molecules: State of the art. Top. Issue Polym. Biomater. 2012, 37, 1510–1551. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Functional self-assembled nanofibers by electrospinning. Adv. Polym. Sci. 2008, 219, 107–171. [Google Scholar]
- Avossa, J.; Herwig, G.; Toncelli, C.; Itel, F.; Rossi, R.M. Electrospinning based on benign solvents: Current definitions, implications and strategies. Green Chem. 2022, 24, 2347–2375. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- De Vrieze, S.; Van Camp, T.; Nelvig, A.; Hagström, B.; Westbroek, P.; De Clerck, K. The effect of temperature and humidity on electrospinning. J. Mater. Sci. 2009, 44, 1357–1362. [Google Scholar] [CrossRef]
- Mailley, D.; Hébraud, A.; Schlatter, G. A review on the impact of humidity during electrospinning: From the nanofiber structure engineering to the applications. Macromol. Mater. Eng. 2021, 306, 2100115. [Google Scholar] [CrossRef]
- Williams, G.R.; Raimi-Abraham, B.T.; Luo, C.J. Monoaxial electrospinning. In Nanofibres in Drug Delivery; UCL Press: London, UK, 2018; pp. 60–105. [Google Scholar]
- Kenry; Lim, C.T. Nanofiber technology: Current status and emerging developments. Prog. Polym. Sci. 2017, 70, 1–17. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef]
- Cornejo Bravo, J.M.; Villarreal Gómez, L.J.; Serrano Medina, A. Electrospinning for drug delivery systems: Drug incorporation techniques. In Electrospinning—Material, Techniques, and Biomedical Applications; Haider, S., Haider, A., Eds.; IntechOpen: London, UK, 2016; Chapter 7; pp. 141–155. [Google Scholar]
- Giannetti, R.; Abraham, G.A.; Rivero, G. The role of emulsion parameters in tramadol sustained-release from electrospun mats. Mater. Sci. Eng. C 2019, 99, 1493–1501. [Google Scholar] [CrossRef]
- Rivero, G.; Aldana, A.A.; Frontini Lopez, Y.R.; Liverani, L.; Boccaccini, A.R.; Bustos, D.M.; Abraham, G.A. 14-3-3ε protein-immobilized PCL-HA electrospun scaffolds with enhanced osteogenicity. J. Mater. Sci. Mater. Med. 2019, 30, 99. [Google Scholar] [CrossRef]
- Chou, S.-F.; Carson, D.; Woodrow, K.A. Current strategies for sustaining drug release from electrospun nanofibers. J. Control. Release 2015, 220, 584–591. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.-J. Drug delivery applications of core-sheath nanofibers prepared by coaxial electrospinning: A review. Pharmaceutics 2019, 11, 305. [Google Scholar] [CrossRef]
- Meinel, A.; Germershaus, O.; Luhmann, T.; Merkle, H.; Meinel, L. Electrospun matrices for localized drug delivery: Current technologies and selected biomedical applications. Eur. J. Pharm. Biopharm. 2012, 81, 1–13. [Google Scholar] [CrossRef]
- Shabanloo, R.; Montazer, M.; Farahani, A.; Karimi, N. A review on surface modification of nanofibrous textiles for diverse applications: Focus on medical uses. Heliyon 2025, 11, e41863. [Google Scholar] [CrossRef] [PubMed]
- Elfawal, G.F.; Šišková, A.O.; Andicsová, A.E. Electrospinning: A game-changer in fiber production and practical applications. Fibers Polym. 2025, 26, 4133–4160. [Google Scholar] [CrossRef]
- Ejiohuo, O. A perspective on the synergistic use of 3D printing and electrospinning to improve nanomaterials for biomedical applications. Nano Trends 2023, 4, 100025. [Google Scholar] [CrossRef]
- Yeo, M.; Kim, G. Micro/nano-hierarchical scaffold fabricated using a cell electrospinning/3D printing process for co-culturing myoblasts and HUVECs to induce myoblast alignment and differentiation. Acta Biomater. 2020, 107, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Dalton, P.D.; Woodfield, T.B.F.; Mironov, V.; Groll, J. Advances in hybrid fabrication toward hierarchical tissue constructs. Adv. Sci. 2020, 7, 1902953. [Google Scholar] [CrossRef]
- Khrystonko, O.; Rimpelová, S.; Burianová, T.; Švorčík, V.; Lyutakov, O.; Elashnikov, R. Smart multi stimuli-responsive electrospun nanofibers for on-demand drug release. J. Colloid Interface Sci. 2023, 648, 338. [Google Scholar] [CrossRef]
- Tayebi-Khorrami, V.; Rahmanian-Devin, P.; Reza Fadaei, M.; Movaffagh, J.; Reza Askari, V. Advanced applications of smart electrospun nanofibers in biomedical fields. Mater. Sci. Eng. R Rep. 2024, 145, 100037. [Google Scholar]
- Vannaladsaysy, V.; Choudhury, S.; Datta, S.; Chatterjee, K. NIR-responsive shape memory composite nanofibers as deployable matrices for biomedical applications. Smart Mater. Struct. 2025, 34, 055004. [Google Scholar] [CrossRef]
- Ferraris, S.; Spriano, S.; Calogero Scalia, A.; Cochis, A.; Rimondini, L.; Cruz-Maya, I.; Guarino, V.; Varesano, A.; Vineis, C. Topographical and biomechanical guidance of electrospun fibers for biomedical applications. Polymers 2020, 12, 2896. [Google Scholar] [CrossRef] [PubMed]
- Gürtler, A.; Lang, J.; Czyrski, G.; Sirois, J.; Melican, K.; Rades, T.; Heinz, A. Electrospun fiber patches for inflammatory skin diseases—Correlating in vitro drug release with ex vivo permeation. Biomater. Adv. 2025, 166, 214068. [Google Scholar] [CrossRef] [PubMed]
- Záhonyi, P.; Müncz, A.G.; Péter-Haraszti, A.; Nagy, Z.; Csontos, I.; Marosi, G.; Szabó, E. Continuous twin-screw melt granulation of drug-loaded electrospun fibers. Eur. J. Pharm. Biopharm. 2025, 206, 114580. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Shi, L.; Móczó, J.; Pukánszky, B. Encapsulation of a drug into electrospun fibers spun from water soluble polymers to control solubility and release. Heliyon 2024, 10, e38935. [Google Scholar] [CrossRef]
- Joy, N.; Venugopal, D.; Gopinath, A.; Samavedi, S. Connecting in situ cone/jet length in electrospinning to fiber diameter and drug release for the rational design of electrospun drug carriers. Chem. Eng. Sci. 2024, 295, 120168. [Google Scholar] [CrossRef]
- Carvalho, G.; Coimbra, P. Electrospun composite fibers of poly(lactic acid) and halloysite for the sustained co-delivery of drugs with opposite water affinities. Appl. Clay Sci. 2023, 245, 107155. [Google Scholar] [CrossRef]
- Geng, Y.; Williams, G. Developing and scaling up captopril-loaded electrospun ethyl cellulose fibers for sustained-release floating drug delivery. Int. J. Pharm. 2023, 648, 123557. [Google Scholar] [CrossRef]
- Rajabi, T.; Naffakh-Moosavy, H.; Bagheri, F. The synergic effect of nanosecond fiber laser and drug-loaded electrospun PVA coating on metallurgical and biological characteristics of Ti-6Al-4V alloy. Appl. Surf. Sci. Adv. 2024, 22, 100619. [Google Scholar] [CrossRef]
- Azizoğlu, G.; Azizoğlu, E.; Barker, T.; Özer, O. Single and multi-dose drug loaded electrospun fiber mats for wound healing applications. J. Drug Deliv. Sci. Technol. 2023, 81, 104168. [Google Scholar] [CrossRef]
- Mirek, A.; Grzeczkowicz, M.; Belaid, H.; Bartkowiak, A.; Barranger, F.; Abid, M.; Wasyłeczko, M.; Pogorielov, M.; Bechelany, M.; Lewińska, D. Electrospun UV-cross-linked polyvinylpyrrolidone fibers modified with polycaprolactone/polyethersulfone microspheres for drug delivery. Biomater. Adv. 2023, 147, 213330. [Google Scholar] [CrossRef]
- Wang, M.; Ge, R.; Zhang, F.; Yu, D.; Liu, Z.; Li, X.; Shen, H.; Williams, G. Electrospun fibers with blank surface and inner drug gradient for improving sustained release. Biomater. Adv. 2023, 150, 213404. [Google Scholar] [CrossRef]
- Yi, L.; Hegyesi, N.; Móczó, J.; Pukánszky, B. Improved release of sulfamethoxazole from electrospun water soluble fibers. Polymer 2024, 308, 127407. [Google Scholar] [CrossRef]
- Gürtler, A.; Maltschik, A.; Güler Yildiz, S.; Vangelofski, K.; Gade, L.; Grohganz, H.; Rades, T.; Heinz, A. Advancing inflammatory skin disease therapy: Sustained tofacitinib release via electrospun fiber dressings. Eur. J. Pharm. Biopharm. 2024, 202, 114423. [Google Scholar] [CrossRef] [PubMed]
- Fazekas, B.; Péterfi, O.; Galata, D.; Nagy, Z.; Hirsch, E. Process analytical technology based quality assurance of API concentration and fiber diameter of electrospun amorphous solid dispersions. Eur. J. Pharm. Biopharm. 2024, 204, 114529. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.; Chou, S. Physicomechanical characterizations and in vitro release studies of electrospun ethyl cellulose fibers, solvent cast carboxymethyl cellulose films, and their composites. Int. J. Biol. Macromol. 2024, 267, 131374. [Google Scholar] [CrossRef]
- Cobarrubias-Carapia, S.; Cervantes-Chávez, J.; Rojas-Avelizapa, N.; Luna-Barcenas, G.; Amaro-Reyes, A.; Pool, H.; Villaseñor-Ortega, F. Development of an antioxidant and anti-lipid peroxidation film based on quercetin-loaded Eudragit EPO®/sodium hyaluronate electrospun fibers. Mater. Lett. 2025, 378, 137589. [Google Scholar] [CrossRef]
- Musciacchio, L.; Mardirossian, M.; Marussi, G.; Crosera, M.; Turco, G.; Porrelli, D. Core-shell electrospun polycaprolactone nanofibers, loaded with rifampicin and coated with silver nanoparticles, for tissue engineering applications. Biomater. Adv. 2025, 166, 214036. [Google Scholar] [CrossRef]
- Elyasifar, N.; Samani, S.; Beheshtizadeh, N.; Farzin, A.; Samadikuchaksaraei, A.; Ai, J.; Ebrahimi-Barough, S.; Milan, P.B.; Haramshahi, S.M.A.; Azami, M.; et al. Bi-layered photocrosslinkable chitosan-curcumin hydrogel/soy protein nanofibrous mat skin substitute. Materialia 2023, 32, 101923. [Google Scholar]
- De France, K.J.; Xu, F.; Toufanian, S.; Chan, K.J.; Said, S.; Stimpson, T.C.; González-Martínez, E.; Moran-Mirabal, J.M.; Cranston, E.D.; Hoare, T. Multi-scale structuring of cell-instructive cellulose nanocrystal composite hydrogel sheets via sequential electrospinning and thermal wrinkling. Acta Biomater. 2021, 128, 250–261. [Google Scholar] [CrossRef]
- Alvandi, H.; Jaymand, M.; Eskandari, M.; Aghaz, F.; Hosseinzadeh, L.; Heydari, M.; Arkan, E. A sandwich electrospun nanofibers/Tragacanth hydrogel composite containing Aloe vera extract and silver sulfadiazine as a wound dressing. Polym. Bull. 2023, 80, 11235–11248. [Google Scholar]
- Niemczyk-Soczynska, B.; Zaszczyńska, A.; Zabielski, K.; Sajkiewicz, P. Hydrogel, electrospun and composite materials for bone/cartilage and neural tissue engineering. Materials 2021, 14, 6899. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Song, M.; Li, X.; Du, Y.; Gao, Z.; Zhao, Y.-Q.; Li, C.; Yan, H.; Mo, X.; Wang, C.; et al. Temperature-responsive self-contraction nanofiber/hydrogel composite dressing facilitates the healing of diabetic-infected wounds. Mater. Today Bio 2024, 28, 101214. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Yang, J.; Zu, Y.; Chi, J.; Shi, K. Aligned electrospun fiber film loaded with multi-enzyme mimetic iridium nanozymes for wound healing. J. Nanobiotechnol. 2022, 20, 478. [Google Scholar] [CrossRef] [PubMed]
- Liguori, A.; Gino, M.; Panzavolta, S.; Torricelli, P.; Maglio, M.; Parrilli, A.; Gualandi, C.; Griffoni, C.; Brodano, G.B.; Fini, M.; et al. Tantalum nanoparticles enhance the osteoinductivity of multiscale composites based on poly(lactide-co-glycolide) electrospun fibers embedded in a gelatin hydrogel. Mater. Today Chem. 2022, 24, 100804. [Google Scholar] [CrossRef]
- Hong, Y.; Huber, A.; Takanari, K.; Amoroso, N.J.; Hashizume, R.; Badylak, S.F.; Wagner, W.R. Mechanical properties and in vivo behavior of a biodegradable synthetic polymer microfiber-extracellular matrix hydrogel biohybrid scaffold. Biomaterials 2011, 32, 3387–3394. [Google Scholar] [CrossRef]
- Zhao, X.; Ding, M.; Xu, C.; Zhang, X.; Liu, S.; Lin, X.; Wang, L.; Xia, Y. A self-reinforcing strategy enables the intimate interface for anisotropic alginate composite hydrogels. Carbohydr. Polym. 2021, 251, 117054. [Google Scholar] [CrossRef]
- Ilomuanya, M.O.; Okafor, P.S.; Amajuoyi, J.N.; Onyejekwe, J.C.; Okubanjo, O.O.; Adeosun, S.O.; Silva, B.O. Polylactic acid-based electrospun fiber and hyaluronic acid-valsartan hydrogel scaffold for chronic wound healing. Beni Suef Univ. J. Basic Appl. Sci. 2020, 9, 57. [Google Scholar] [CrossRef]
- Riaz, T.; Gull, N.; Islam, A.; Dilshad, M.R.; Atanase, L.I.; Delaite, C. Needleless electrospinning of poly(ε-caprolactone) nanofibers deposited on gelatin film for controlled release of Ibuprofen. Chem. Pap. 2023, 77, 2657–2669. [Google Scholar] [CrossRef]
- Zare, P.; Pezeshki-Modaress, M.; Davachi, S.M.; Zare, P.; Yazdian, F.; Simorgh, S.; Ghanbari, H.; Rashedi, H.; Bagher, Z. Alginate sulfate-based hydrogel/nanofiber composite scaffold with controlled Kartogenin delivery for tissue engineering. Carbohydr. Polym. 2021, 266, 118123. [Google Scholar] [CrossRef]
- Riaz, T.; Khenoussi, N.; Rata, D.M.; Atanase, L.I.; Adolphe, D.C.; Delaite, C. Blend electrospinning of poly(ϵ-caprolactone) and poly(ethylene glycol-400) nanofibers loaded with ibuprofen as a potential drug delivery system for wound dressings. Autex Res. J. 2023, 23, 66–76. [Google Scholar] [CrossRef]
- Rehman, N.; Dilshad, M.R.; Islam, A.; Gull, N.; Riaz, T.; Khan, S.M.; Khan, R.U. Novel graphene oxide loaded sodium alginate hydrogels cross-linked with tetraethyl orthosilicate for cephradine release analysis. J. Drug Deliv. Sci. Technol. 2021, 66, 102784. [Google Scholar] [CrossRef]
- Ghauri, Z.H.; Islam, A.; Qadir, M.A.; Gull, N.; Haider, B.; Khan, R.U.; Riaz, T. Development and evaluation of pH-sensitive biodegradable ternary blended hydrogel films (chitosan/guar gum/PVP) for drug delivery application. Sci. Rep. 2021, 11, 452. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef] [PubMed]
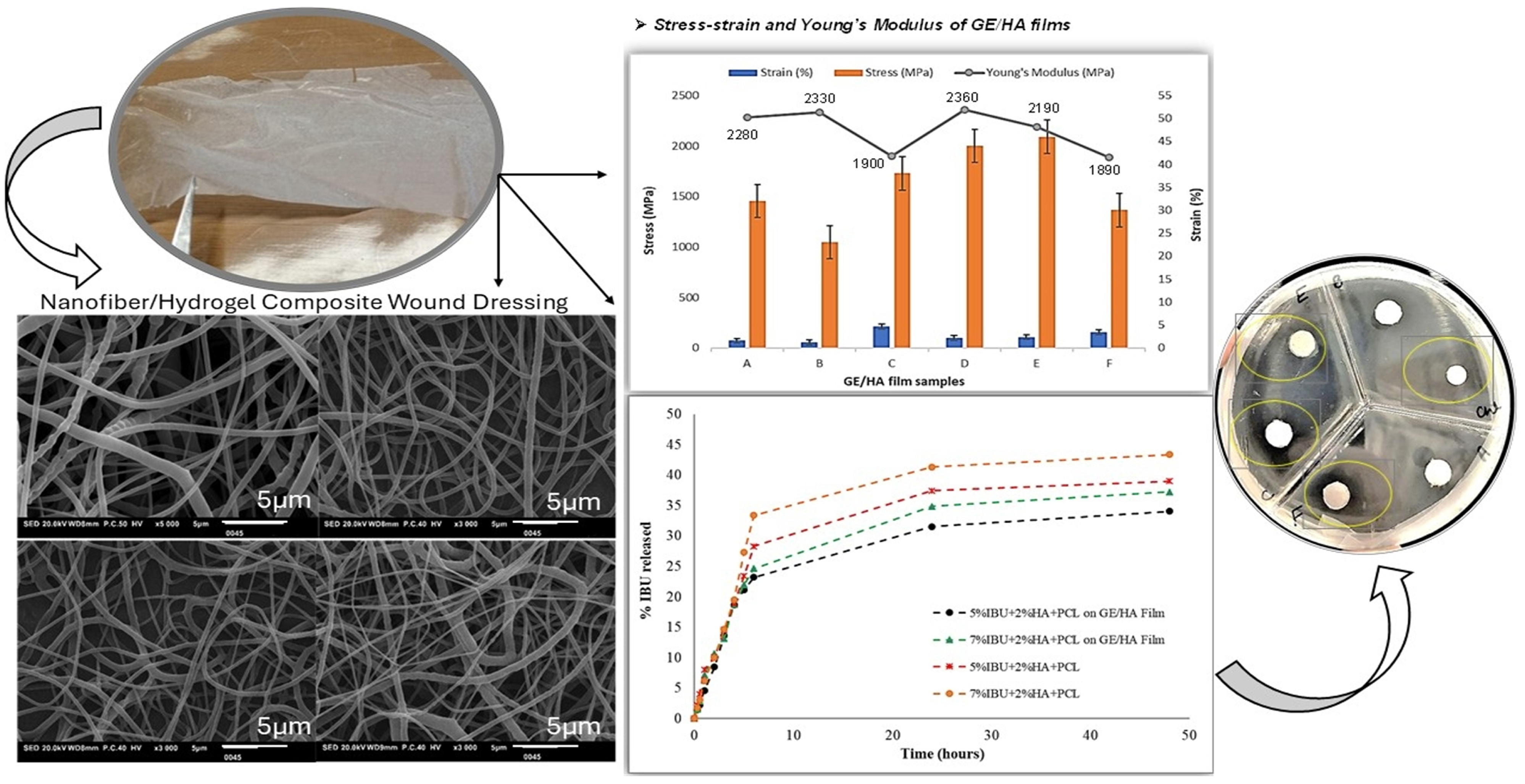
- Xu, S.; Deng, L.; Zhang, J.; Yin, L.; Dong, A. Composites of electrospun-fibers and hydrogels: A potential solution to current challenges in biological and biomedical field. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 640–656. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Huang, J.; Luo, M.; Fang, Y.; Zeng, X.; Wu, J.; Du, J. Near-field electrospun PCL fibers/GelMA hydrogel composite dressing with controlled deferoxamine-release ability and retiform surface for diabetic wound healing. Nano Res. 2023, 16, 599–612. [Google Scholar] [CrossRef]
- Shehab-ElDin, A.N.; Sobh, R.A.; Rabie, A.M.; Mohamed, W.S.; Nasr, H.E. Polyacrylamide grafted electrospun polyamide 6 nanocomposite fibers for drug delivery application. Egypt J. Chem. 2022, 65, 33–47. [Google Scholar] [CrossRef]
- Chen, S.; Yao, W.; Wang, H.; Wang, T.; Xiao, X.; Sun, G.; Yang, J.; Guan, Y.; Zhang, Z.; Xia, Z.; et al. Injectable electrospun fiber-hydrogel composite sequentially releasing clonidine and ropivacaine for prolonged and walking regional analgesia. Theranostics 2022, 12, 4904–4921. [Google Scholar] [CrossRef]
- Attia, M.F.; Montaser, A.S.; Arifuzzaman, M.; Pitz, M.; Jlassi, K.; Alexander-Bryant, A.; Kelly, S.S.; Alexis, F.; Whitehead, D.C. In situ photopolymerization of acrylamide hydrogel to coat cellulose acetate nanofibers for drug delivery system. Polymers 2021, 13, 1863. [Google Scholar] [CrossRef]
- Bandiera, A.; Passamonti, S.; Dolci, L.S.; Focarete, M.L. Composite of elastin-based matrix and electrospun poly(L-lactic acid) fibers: A potential smart drug delivery system. Front. Bioeng. Biotechnol. 2018, 6, 127. [Google Scholar] [CrossRef]
- Irantash, S.; Gholipour-Kanani, A.; Najmoddin, N.; Varsei, M. A hybrid structure based on silk fibroin/PVA nanofibers and alginate/gum tragacanth hydrogel embedded with cardamom extract. Sci. Rep. 2024, 14, 63061. [Google Scholar] [CrossRef]
- Darwesh, A.Y.; Helmy, A.M.; Abdelhakk, H.M.; Giri, B.; Maniruzzaman, M. 3D-printed short nanofibers/hydrogel-based vaginal films as a novel system for the delivery of anti-HIV microbicide drugs. J. Drug Deliv. Sci. Technol. 2024, 97, 105775. [Google Scholar] [CrossRef]
- Teixeira, M.O.; Antunes, J.C.; Felgueiras, H.P. Recent advances in fiber–hydrogel composites for wound healing and drug delivery systems. Antibiotics 2021, 10, 248. [Google Scholar] [CrossRef]
- McMurtrey, R.J. Patterned and functionalized nanofiber scaffolds in three-dimensional hydrogel constructs enhance neurite outgrowth and directional control. J. Neural Eng. 2014, 11, 066009. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.; Yun, E.; Song, M.; Kim, J.; Son, J.S.; Cha, C. Multiscale control of nanofiber-composite hydrogel for complex 3D cell culture by extracellular matrix composition and nanofiber alignment. Biomater. Res. 2024, 28, 32. [Google Scholar] [CrossRef] [PubMed]
- Hiraki, H.L.; Matera, D.L.; Rose, M.J.; Kent, R.N.; Todd, C.W.; Stout, M.E.; Wank, A.E.; Schiavone, M.C.; DePalma, S.J.; Zarouk, A.A.; et al. Magnetic alignment of electrospun fiber segments within a hydrogel composite guides cell spreading and migration phenotype switching. Front. Bioeng. Biotechnol. 2021, 9, 679165. [Google Scholar] [CrossRef] [PubMed]
- Atila, D.; Keskin, D.; Lee, Y.L.; Lin, F.H.; Hasirci, V.; Tezcaner, A. Injectable methacrylated gelatin/thiolated pectin hydrogels carrying melatonin/tideglusib-loaded core/shell PMMA/silk fibroin electrospun fibers for vital pulp regeneration. Colloids Surf. B Biointerfaces 2023, 222, 113078. [Google Scholar] [CrossRef]
- Estrada-Villegas, G.M.; Del Rio-De Vicente, J.I.; Argueta-Figueroa, L.; Gonzalez-Perez, G. UV-initiated crosslinking of electrospun chitosan/poly(ethylene oxide) nanofibers doped with ZnO-nanoparticles: Development of antibacterial nanofibrous hydrogel. MRS Commun. 2020, 10, 642–651. [Google Scholar] [CrossRef]
- Han, N.; Rao, S.S.; Johnson, J.; Parikh, K.S.; Bradley, P.A.; Lannutti, J.J.; Winter, J.O. Hydrogel-electrospun fiber mat composite coatings for neural prostheses. Front. Neuroeng. 2011, 4, 2. [Google Scholar] [CrossRef]
- Hsieh, A.; Zahir, T.; Lapitsky, Y.; Amsden, B.; Wan, W.; Shoichet, M.S. Hydrogel/electrospun fiber composites influence neural stem/progenitor cell fate. Soft Matter 2010, 6, 2227–2237. [Google Scholar] [CrossRef]
- Kim, J.S.; Choi, J.; Ki, C.S.; Lee, K.H. 3D silk fiber construct embedded dual-layer PEG hydrogel for articular cartilage repair—In vitro assessment. Front. Bioeng. Biotechnol. 2021, 9, 653509. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.S.; Yoo, H.S. Electrospun nanofibrils embedded hydrogel composites for cell cultivation in a biomimetic environment. RSC Adv. 2017, 7, 54246–54253. [Google Scholar] [CrossRef]
- Lee, C.-H.; Huang, W.-Y.; Lee, K.-Y.; Kuan, C.-H.; Wu, T.-C.; Sun, J.-S.; Wang, T.-W. Bioinspired adhesive nanofibrous hydrogel promotes immune infiltration through effective immunochemotherapy for osteosarcoma treatment. Chem. Eng. J. 2024, 486, 150236. [Google Scholar] [CrossRef]
- Li, J.; Zhang, T.; Pan, M.; Xue, F.; Lv, F.; Ke, Q.; Xu, H. Nanofiber/hydrogel core–shell scaffolds with three-dimensional multilayer patterned structure for accelerating diabetic wound healing. J. Nanobiotechnol. 2022, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ge, J.; Guo, Q.; Wang, J.; Wu, J.; Yan, Z.; Špitalský, Z.; Liu, Y. Polyvinyl alcohol/collagen composite scaffold reinforced with biodegradable polyesters/gelatin nanofibers for adipose tissue engineering. Int. J. Biol. Macromol. 2024, 263, 130237. [Google Scholar] [CrossRef]
- Macor, L.P.; Colombi, S.; Tamarit, J.-L.; Engel, E.; Pérez-Madrigal, M.M.; García-Torres, J.; Alemán, C. Immediate-sustained lactate release using alginate hydrogel assembled to proteinase K/polymer electrospun fibers. Int. J. Biol. Macromol. 2023, 238, 124117. [Google Scholar] [CrossRef]
- Martin, A.; Nyman, J.N.; Reinholdt, R.; Cai, J.; Schaedel, A.L.; van der Plas, M.J.A.; Malmsten, M.; Rades, T.; Heinz, A. In situ transformation of electrospun nanofibers into nanofiber-reinforced hydrogels. Nanomaterials 2022, 12, 2437. [Google Scholar] [CrossRef]
- Song, Y.; Hu, Q.; Liu, S.; Wang, Y.; Zhang, H.; Chen, J.; Yao, G. Electrospinning/3D printing drug-loaded antibacterial polycaprolactone nanofiber/sodium alginate-gelatin hydrogel bilayer scaffold for skin wound repair. Int. J. Biol. Macromol. 2024, 275, 129705. [Google Scholar] [CrossRef]
- Mohabatpour, F.; Karkhaneh, A.; Sharifi, A.M. A hydrogel/fiber composite scaffold for chondrocyte encapsulation in cartilage tissue regeneration. RSC Adv. 2016, 6, 83135–83145. [Google Scholar] [CrossRef]
- Najafi, R.; Chahsetareh, H.; Pezeshki-Modaress, M.; Aleemardani, M.; Simorgh, S.; Davachi, S.M.; Alizadeh, R.; Asghari, A.; Hassanzadeh, S.; Bagher, Z. Alginate sulfate/ECM composite hydrogel containing electrospun nanofiber with encapsulated human adipose-derived stem cells for cartilage tissue engineering. Int. J. Biol. Macromol. 2023, 238, 124098. [Google Scholar] [CrossRef]
- Nakielski, P.; Pawłowska, S.; Rinoldi, C.; Ziai, Y.; De Sio, L.; Urbanek, O.; Zembrzycki, K.; Pruchniewski, M.; Lanzi, M.; Salatelli, E.; et al. Multifunctional platform based on electrospun nanofibers and plasmonic hydrogel: A smart nanostructured pillow for near-infrared light-driven biomedical applications. ACS Appl. Mater. Interfaces 2020, 12, 54328–54342. [Google Scholar] [CrossRef]
- Toftdal, M.S.; Christensen, N.P.; Kadumudi, F.B.; Dolatshahi-Pirouz, A.; Grunnet, L.G.; Chen, M. Mechanically reinforced hydrogel vehicle delivering angiogenic factor for beta cell therapy. J. Colloid Interface Sci. 2024, 667, 54–63. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Cheraga, N.; Huang, N.P. 3D cell/scaffold model based on aligned-electrospun-nanofiber film/hydrogel multi-layers for construction of anisotropic engineered tissue. Biointerphases 2022, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Megone, W.; Peijs, T.; Gautrot, J.E. Functionalization of electrospun PLA fibers using amphiphilic block copolymers for use in carboxymethyl-cellulose hydrogel composites. Nanocomposites 2020, 6, 85–98. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.T.; Jamshidi, A. A new strategy for fabrication of bone scaffolds using electrospun nano-HAp/PHB fibers and protein hydrogels. Chem. Eng. J. 2016, 289, 38–47. [Google Scholar] [CrossRef]
- Barzegar, A.; Ebrahimzadeh, S.; Vahdani, V.; Tohidifar, N.; Zarrini, G.; Hatami, H.; Nikzad, B.; Warda, M.; Hacimuftuoglu, A. Engineering bi-layered skin-like nanopads with electrospun nanofibers and chitosan films for promoting fibroblast infiltration in tissue regeneration and wound healing. Int. J. Biol. Macromol. 2024, 277, 134398. [Google Scholar] [CrossRef]
- Karimizade, A.; Hasanzadeh, E.; Abasi, M.; Enderami, S.E.; Mirzaei, E.; Annabi, N.; Mellati, A. Collagen short nanofiber-embedded chondroitin sulfate–hyaluronic acid nanocomposite: A cartilage-mimicking in situ-forming hydrogel with fine-tuned properties. Int. J. Biol. Macromol. 2024, 266, 131051. [Google Scholar] [CrossRef]
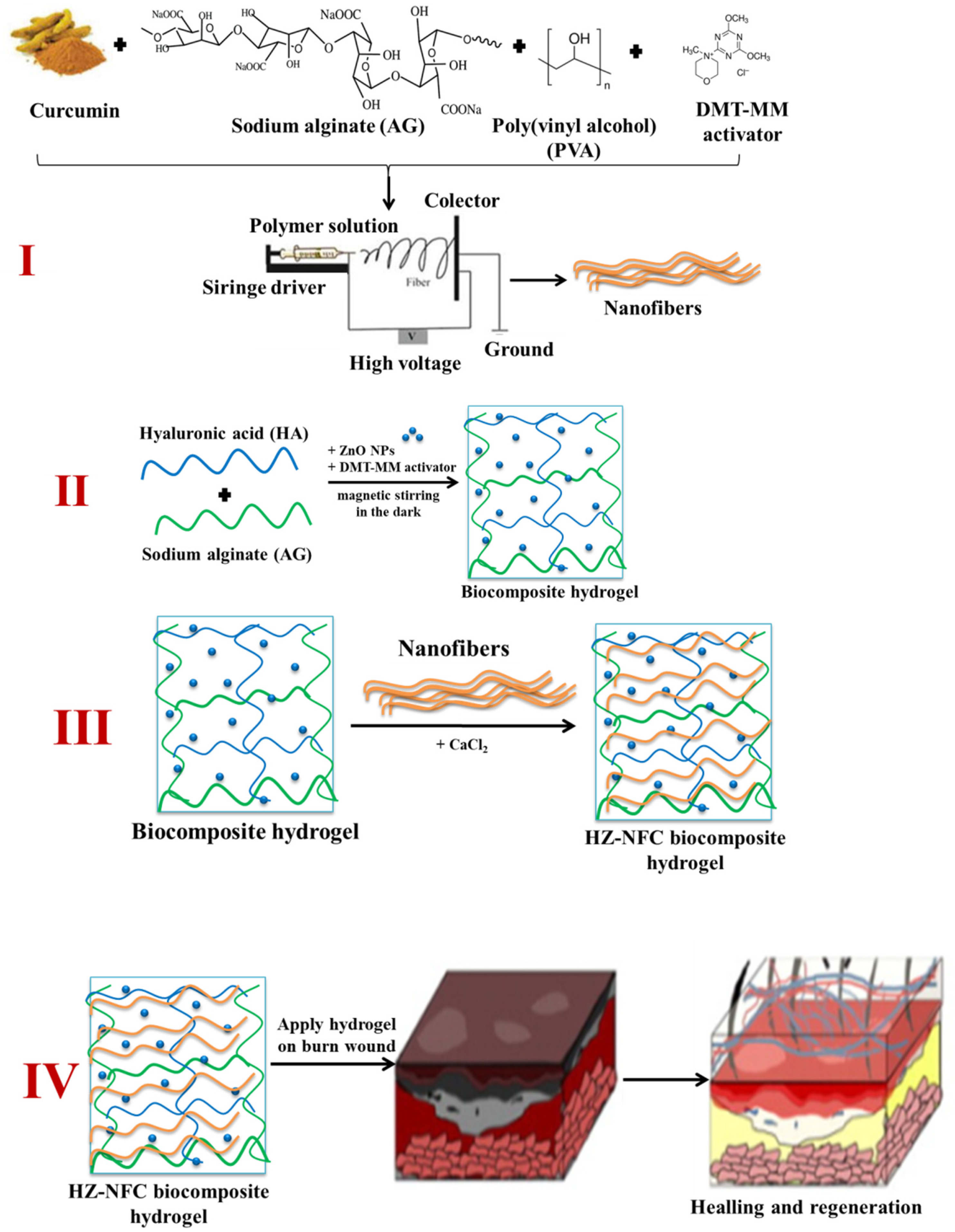
- Rată, D.M.; Cadinoiu, A.N.; Vochița, G.; Gherghel, D.; Lakkaboyana, S.K.; Fuioagă, C.P. Biocomposite complex hydrogels with antimicrobial activity suitable for wound healing. J. Polym. Sci. 2025, 63, 1878–1890. [Google Scholar] [CrossRef]
| Technique | Advantages | Disadvantages | Key Factors | References |
|---|---|---|---|---|
| Blending | Simple, straightforward, single step Highest loading rate | Requires a solvent for both the polymer and the drug Burst release is generally observed | Drug/polymer interactions HLB/solubility may compromise homogeneity Diffusion-dependent release profiles | [50,51] |
| Emulsion electrospinning | Minimizes the contact of the solvent with (sensitive) drugs Standard setup Core–sheath (protective) structures | Additional compositional parameters to optimize (surfactant(s) type(s), concentrations, etc.). | Emulsions must be stable during processing Relative viscosity | [37,52,53,54] |
| Coaxial electrospinning | Different polymer/drug combinations in core–sheath fibers Modular platform is achievable for multi-drug systems Longer release profiles (when the drug is in the core) | Specific coaxial nozzle required Additional compositional and processing parameters to tune Limited scalability | Processing optimization (core/shell ratio, relative flow rates, etc.) Core–shell thickness allows for fine-tuning of release profiles | [55] |
| Post-processing surface modification: Physical absorption, coatings, etc. | Simple Avoids contact of the drug with the polymer solvent | Non-covalent (weak) bond Fast release Limited drug incorporation | Polymer-drug interactions | [56] |
| Post-processing surface modification: Grafting, crosslinking, etc. | Covalent (stronger) bond Readily exposed drugs Avoids contact of the drug with the polymer solvent | Limited drug incorporation Multiple steps | Chemical paths | [57] |
| Supramolecular-loaded sub-compartments within electrospun fibers | Different protective physical barriers for longer release profiles Suitable for sensitive drugs | Multiple steps | Supramolecular carrier/polymer compatibility | [27] |
| Nanofibers | Hydrogel | Drug/ Cytokine | Technique | Major Findings | Application |
|---|---|---|---|---|---|
| Soy protein isolate (SPI) | Methacrylated Chitosan (CS) | Curcumin, Riboflavin | Electrospinning, photo-crosslinking | Anti-inflammatory response of curcumin, fibroblast formation, and epidermal formation due to SPI nanofibers, complete wound closure in 20 days | Epidermal regeneration and wound healing [82] |
| Poly(oligoethylene glycol methacrylate) (POEGMA) | A-POEGMA and H-POEGMA suspensions | Cellulose nano crystals (CNC) | Electrospinning, spin coating, thermal wrinkling | Enhanced mechanical strength and decreased protein adsorption, tunable nanofiber orientation and density, cell growth, and proliferation | In vivo cell screening and in vivo tissue regeneration [83] |
| Polyacrylonitrile (PAN) | Tragacanth gum | Silver Sulfadiazine (SSD), Aloe vera | Electrospinning and spraying | Increased swelling, good mechanical properties, 70% SSD release in 72 hrs., cytocompatibility | Wound healing [84] |
| Poly-(lactic acid-co-trimethylene carbonate) (PLATMC) | Methacrylate Gelatin (GelMa) | Epinecidin-1@chitosan nanoparticles (Epi-1@CS) | Electrospinning, layer stacking, UV irradiation | Cytocompatibility, antioxidant, anti-inflammatory, and antibacterial properties, promoting collagen deposition and angiogenesis | Temperature-responsive composite hydrogel for diabetic-infected wounds [86] |
| Poly(ester urethane)urea (PEUU) | dECM gel (Porcine dermal tissue) | --- | Concurrent electrospinning, electrospraying | Flexible, good mechanical strength, high degree of cellular infiltration | Tissue regeneration [89] |
| Polylactic acid (PLA) | Hyaluronic acid (HA) | Valsartan, ascorbic acid | Electrospinning, layered deposition | Graded release of VA, increased re-epithelialization, and enhanced collagen deposition | Chronic wound healing [91] |
| Polyacrylamide6 (PA6) | Grafted PA6 | Tallow modified Clay (TMC), Doxycycline hydrochloride drug | Electrospinning, free radical polymerization | Enhanced mechanical strength and swelling ratio, burst release of drug in 2 hrs., antibacterial activity against E. coli and S. aureus | Drug delivery [100] |
| Poly-ε-caprolactone (PCL) | F127 hydrogel | Ropivacaine (Rop), Clonidine (Clo) | Electrospinning, mixing | Good in vivo biodegradability and biosafety, sustained release of Rop, and sensorimotor segregation effect achieved | Injectable composite for prolonged walking analgesia [101] |
| Cellulose Acetate (CA) | Poly(acrylamide) (poly-Aam) | Ibuprofen (Ib) | Electrospinning, coating, and photopolymerization | Sustained release of Ib, biocompatibility with 3T3 adipose cells in vitro | Drug delivery system [102] |
| Poly(L-lactic acid) (PLLA) | Human Elastin-like Polypeptides (HELPs) | --- | Electrospinning, deposition | Enhanced wettability, stable HELP moiety | Drug delivery system [103] |
| Poly-ε-caprolactone (PCL) | Chitosan (CS) | --- | Electrospinning, layer-by-layer assembly | Improved porosity and water retention, good mechanical properties, cytocompatibility, cell attachment, proliferation, and infiltration | Skin tissue regeneration and wound healing [104] |
| Collagen short nanofibers (CSNFs) | Hyaluronic acid (HA), Chondroitin sulfate (CS) | --- | Electrospinning, Schiff-base reaction | Cytocompatible, biodegradable, showed chondrogenic differentiation, and no inflammatory response | Tissue engineering and cartilage repair [105] |
| Silk fibroin/PVA | Sodium Alginate/gum tragacanth (SA/GT) | Cardamom extract | Electrospinning, layered deposition | Good swelling ratio, sustained release of Cardamom extract, biocompatibility, and cell proliferation, antibacterial activity against E. coli and S. aureus | Wound healing and skin tissue regeneration [106] |
| Poly-ε-caprolactone (PCL) | EFZ/HG (glycerin) hydrogel matrix | Ritonavir (RIT), Efavirenz (EFZ) | Electrospinning, cryocutting, 3D printing | Good mechanical stability and elongation, in vitro rapid release of EFZ in 45 min, slow RIT release during 72 hrs | Anti-HIV drug delivery system [107] |
| Methacrylated Gelatin (MGel) | Methacrylated Gelatin (MGel) and Methacrylated Hyaluronic acid (MHA) | Superparamagnetic iron oxide (Fe3O4) nanoparticles as MNPs | Electrospinning, magnetic field-induced nanofiber alignment in hydrogel, in situ crosslinking | Anisotropic morphology, hydrogel promoted myofibroblast differentiation | ECM composition, tissue engineering [108] |
| Dextran Vinyl Sulfone (DVS) | Dextran Vinyl Sulfone (DVS) | PVP-coated Superparamagnetic iron oxide nanoparticles (SPIONs) | Electrospinning, magnetic field-induced nanofiber alignment in hydrogel, UV-crosslinking | Orthogonal tunability of fiber length, density, alignment, and controlled multidirectional cellular migration | Tissue repair and controlling cell behavior [109] |
| Core/shell PMMA/silk fibroin nanofibers | Methacrylated gelatin and thiolated pectin | Melatonin (Mel) and Tideglusib (Td) | Coaxial electrospinning | Controlled release of Mel and Td to induce dental pulp stem cell proliferation and odontogenic differentiation | Injectable hydrogels for dental pulp regeneration [110] |
| Poly(ethylene) oxide (PEO)/Chitosan (CS) | Nanofibrous hydrogel | ZnO-NPs, Pentaerythritol triacrylate (PETA) | Nanospinner electrospinning setup, UV irradiation | High swelling ratio, antibacterial activity against S. aureus, E. coli, S. epidermidis, and P. aeruginosa | Antibacterial applications [111] |
| Poly-ε-caprolactone (PCL) | Poly(ethylene glycol)-poly(e-caprolactone) (PEGPCL) | Nerve growth factor (NGF) | Electrospinning, photo-polymerization | Sustained release of NGF for two weeks, cytocompatibility | Neural prostheses [112] |
| Collagen or Poly(ε-caprolactone-co-D,L-lactide) (P(CL:DLLA)) | Hyaluronan (HA)/Methylcellulose (MC) | Neural stem/progenitor cells (NSPCs) | Electrospinning, dispersion in cell culture | Cytocompatible, in vitro NSPC survival and differentiation | Injectable neural cell distribution and delivery [113] |
| 3D Silk fiber, nylon monofilament, PGLA monofilament | LD- and HD- PEG | --- | Thiol-norbornene photoclick Chemistry for hydrogel synthesis, dual-layered stacking | Strong interfacial adhesion, good mechanical strength, controllable degradability, and showed successful chondrogenesis | Articulate cartilage repair [114] |
| Polycaprolactone (PCL) nanofibrils by hydrolysis | Gelatin and Alginate | Murine fibroblast cell line (NIH3T3) | Electrospinning of PCL nanofibrils | Enhanced mechanical properties, fibroblast showed superior adhesion behavior, and collagen synthesis | Cell cultivation for ECM regeneration [115] |
| Hyaluronic acid (HA)/Polycaprolactone (PCL) | Gelatin (catechol modified) | Doxorubicin (DOX), cytokines-loaded polyelectrolyte complex nanoparticles (PCNs) | Dual source/dual power electrospinning | Controlled release of DOX and cytokines, inhibition of cancer cell growth | Targeted drug delivery system for osteosarcoma cancer treatment [116] |
| Poly (D, L-lactic acid) (PDLLA) | Methacrylated Gelatin (GelMA) | --- | Core/shell composite produced by coaxial electrospinning | High porosity and water retention promoted endothelial cell proliferation, migration, adhesion, infiltration, and angiogenic differentiation | Diabetic wound healing [117] |
| PLGA, LA: GA/PCL/Gelatin (PPG) | Polyvinyl alcohol (PVA), collagen | Dopamine | Electrospinning, freeze–thawing | Enhanced mechanical stability during compression, high water absorption and swelling ratio, cell proliferation, adhesion, and growth on porous scaffold | Adipose tissue engineering [118] |
| Polylactic acid (PLA) | Alginate/Sodium L-lactate | Proteinase K | Electrospinning followed by Plasma treatment | Hydrophilic nanofibers, burst release of lactate followed by sustained release for ten days | Controlled drug release [119] |
| Polydopamine (PDA)/Polyethylene oxide (PEO), Zein | Gelatin, Polyethylene imine (PEI), PDA, Zein | Tetracycline hydrochloride (T) | Core–shell nanofibers by coaxial electrospinning | Water retention, swell ability, burst release of T, antibacterial resistance against E. coli and S. aureus | Wound healing [120] |
| Ethyl cellulose (EC) nanofibers | Carboxymethyl cellulose (CMC) film | Phenytoin (PHT), Tetracycline hydrochloride (TCH) | Electrospinning, solvent casting, fiber-on-film, and fiber-in-film | Fiber-in-film composite showed stage release of TCH and PHT in 8 hrs., fiber-on-film composite showed simultaneous release of TCH and PHT | Modulated drug delivery system [79] |
| Polycaprolactone (PCL) | Sodium Alginate-Gelatin | Amoxicillin (AMX), Epidermal growth factor (rhEGF) | Electrospinning, 3D printing | Showed good mechanical properties, both hydrophobic outer and hydrophilic inner, good cell adhesion, and proliferation | Wound healing applications [121] |
| Poly(lactic acid) (PLA) | Alginate-graft-hyaluronate (Alg-g-HA) | Chondrocytes | Electrospinning, hydrogel with nanofiber suspension | Higher compressive modulus, cytocompatible, produced cartilage matrix | Cartilage tissue regeneration [122] |
| Polycaprolactone/Gelatin | Alginate sulfate | Human adipose-derived stem cells (hASCs), powdered ECM | Electrospinning | Enhanced cell proliferation and chondrogenic differentiation | Cartilage tissue engineering [123] |
| Poly(L-lactide) (PLLA) | P(NIPAAm-co-NIPMAAm) | Rhodamine B, Gold nanorods (AuNRs) | Electrospinning, UV irradiation for crosslinking | NIR thermoresponse of hydrogel, sustained drug release, and penetration | Thermoresponsive hydrogel for controlled drug delivery [124] |
| Poly-(γ-benzyl-L-glutamate) (PBLG) Poly(l-lactide-co-ε-caprolactone) (PLCL)/gelatin methacryloyl (GelMA)/alginate | PRONOVA SLG100 Alginate (NovaMatrix, Norway) | Vascular endothelial growth factor (VEGF) | Core/shell coaxial electrospinning | Enhanced mechanical strength, great cell viability, and VEGF release in two weeks | Angiogenic factor delivery for beta cell therapy to treat diabetes mellitus [125] |
| Poly(lactic-co-glycolic acid) (PLGA) ANFs | Collagen and GelMA-PEO | Cardiac fibroblasts (CFs), HL-1 Cardiomyocytes | Aligned electrospinning | Highly oriented nanofibers, uniform length and diameter, high cell viability, aligned tissue growth | Anisotropic engineered tissue [126] |
| Polylactic acid (PLA), PLA-b- PDMAEMA | Carboxy-methylcellulose (CMC) | --- | Spin coating and electrospinning, UV crosslinking | Increase in storage modulus, good reinforcement effect, improved hydrophilicity | Injectable composite systems for biomedical applications [127] |
| Polyhydroxy butyrate (PHB) | Methacrylated Gelatin | Bioactive HAp nanoparticles (bone mineral) | Electrospinning, UV crosslinking | Good mechanical properties, bone cell viability, and infiltration for 14 days | Bone tissue regeneration [128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuioaga, P.C.; Rata, D.M.; Riaz, T.; Rivero, G.; Abraham, G.A.; Atanase, L.I. Composite Hydrogels with Embedded Electrospun Fibers as Drug Delivery Systems. Gels 2025, 11, 826. https://doi.org/10.3390/gels11100826
Fuioaga PC, Rata DM, Riaz T, Rivero G, Abraham GA, Atanase LI. Composite Hydrogels with Embedded Electrospun Fibers as Drug Delivery Systems. Gels. 2025; 11(10):826. https://doi.org/10.3390/gels11100826
Chicago/Turabian StyleFuioaga, Paul Codrin, Delia Mihaela Rata, Tabinda Riaz, Guadalupe Rivero, Gustavo A. Abraham, and Leonard Ionut Atanase. 2025. "Composite Hydrogels with Embedded Electrospun Fibers as Drug Delivery Systems" Gels 11, no. 10: 826. https://doi.org/10.3390/gels11100826
APA StyleFuioaga, P. C., Rata, D. M., Riaz, T., Rivero, G., Abraham, G. A., & Atanase, L. I. (2025). Composite Hydrogels with Embedded Electrospun Fibers as Drug Delivery Systems. Gels, 11(10), 826. https://doi.org/10.3390/gels11100826









